Abstract
Melanoma treatment comprised a few treatment choices with insufficient efficacy before the emergence of molecularly targeted medication and immune checkpoint inhibitors, which dramatically improved patient outcomes. B-Rapidly Accelerated Fibrosarcoma (BRAF) and Mitogen-Activated Protein Kinase (MAPK) Kinase (MEK) inhibitors significantly improved survival in BRAF-mutant melanoma and immune checkpoint inhibitors, such as anti-programmed cell death 1 (PD-1) and Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) agents, established new standards of care. Challenges remain, however, including the existence of resistance mechanisms and the reduced efficacy of immune-based therapies in Asian populations, particularly for acral and mucosal subtypes. This review highlights historical and current therapeutic advancements, discusses regional considerations, and explores emerging strategies aiming at globally optimizing melanoma management.
1. Introduction
Chemotherapy for melanoma before 2011, the year during which vemurafenib and ipilimumab were approved by the FDA (Food and Drug Administration) in the U.S., provided only few choices for treatment, which had insufficient efficacy. At that time, melanoma was recognized as an untreatable malignancy when advanced. In the 1970s, dacarbazine (DTIC) was established as the standard treatment; however, its efficacy was limited, with poor response rates and a minimal impact on overall survival [1]. Subsequently, combination chemotherapy regimens were investigated in an attempt to enhance therapeutic outcomes. Despite these efforts, no survival advantage over single-agent therapies could be demonstrated, leaving melanoma treatment with unmet clinical needs [2].
The therapeutic landscape shifted dramatically with the advent of molecularly targeted therapies and immune checkpoint inhibitors. Targeted agents such as BRAF inhibitors (the first BRAF inhibitor, vemurafenib, was approved by the FDA in 2011 [3]), particularly when combined with MEK inhibitors (the first combination of BRAF and MEK was approved by the FDA for the first time in 2014 (dabrafenib + trametinib) [4]), showed substantial clinical benefit in patients harboring BRAF mutations. Concurrently, the introduction of immune checkpoint inhibitors (the first anti-CTLA antibody ipilimumab was approved in 2011 [5]), and notably of anti-PD-1 antibodies (nivolumab [6] and pembrolizumab [7] were approved in 2014 by the FDA), revolutionized the management of advanced melanoma, establishing these agents as cornerstone therapies and setting new standards of care.
In this review, we summarize the historical evolution of melanoma treatments, from conventional chemotherapy to the current era of precision medicine and immunotherapy. Additionally, we discuss emerging therapeutic strategies and potential directions for the future, aiming to address ongoing challenges and optimize outcomes for patients with melanoma. Importantly, we focus on the unique characteristics and clinical features of melanoma in Asian populations, which often differ from those observed in Caucasian patients. By highlighting these distinctions, this review aims to underscore the need for region-specific clinical evidence and to contribute to the development of more tailored treatment strategies for Asian patients.
2. Historical Approaches to Melanoma Treatment
2.1. Chemotherapeutic Agents
Dacarbazine (DTIC) had been a standard treatment for metastatic melanoma for over 30 years (Figure 1), despite low response rates of 5–12% [8,9,10,11] (Table 1). Adding interferon or tamoxifen to DTIC did not significantly improve outcomes but increased toxicity [12].
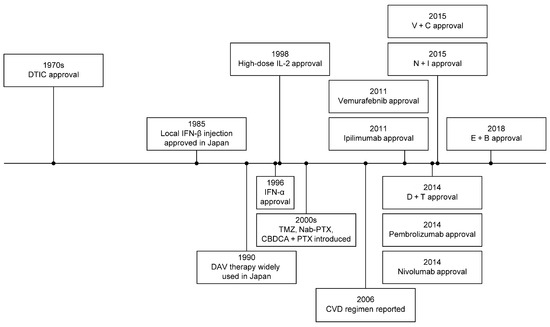
Figure 1.
Historical development of melanoma treatment.

Table 1.
Summary of historical therapeutic approaches in melanoma and associated response rates [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32].
Temozolomide has shown no significant differences in response rates or survival outcomes compared to DTIC, with a reported response rate of 13.4% [13,14] (Table 1).
nab-Paclitaxel (nab-PTX, ABI-007, Abraxane) is a 130 nm albumin-bound particle formulation of paclitaxel designed to enhance drug delivery without the need for solvent-based carriers [15]. This solvent-free formulation improves bioavailability and facilitates higher intratumoral drug concentrations, with preclinical studies reporting a 33% increase compared to solvent-based paclitaxel at equivalent doses [16]. Clinical trials have demonstrated its efficacy and safety, establishing its potential advantages in drug delivery and therapeutic outcomes. Notably, a phase III trial of nab-PTX reported a response rate of 15%, with no significant differences in response rate or OS compared to DTIC [17] (Table 1).
No studies have directly compared the efficacy of the carboplatin plus paclitaxel (CBDCA + PTX) regimen with dacarbazine using statistical methods. However, the CBDCA + PTX regimen demonstrated an objective response rate of 18% in a phase III trial for metastatic malignant melanoma [18] (Table 1). Although this was not a direct comparison, it is considered that there may be no significant difference in efficacy between dacarbazine and the CBDCA + PTX regimen.
The CVD regimen is a combination therapy comprising cisplatin, vindesine, and dacarbazine. Phase III clinical trials have reported response rates ranging from 20% to 35% [19] (Table 1). However, despite its moderate response rates, the CVD regimen did not contribute to an improvement in overall survival (OS) [19]. Consequently, it has been reported that the regimen fails to demonstrate significant superiority over dacarbazine monotherapy in clinical outcomes [19].
The regimens mentioned above, including DTIC, temozolomide, nab-PTX, CBDCA+PTX, and CVD therapy, are listed in the latest NCCN Guidelines and remain potential options for chemotherapy in current clinical practice [20]. Although not included in the NCCN Guidelines, DAV therapy has been widely used in Japan. DAV therapy, consisting of dacarbazine (DTIC), nimustine hydrochloride (ACNU), and vincristine (VCR), has been employed as an adjuvant treatment for advanced melanoma for several decades, despite the absence of dedicated clinical trials validating its efficacy [21]. In addition, a modified version, DAV-feron therapy, which includes the incorporation of interferon-beta (IFN-β), has been utilized. These therapeutic approaches are hypothesized to exert anti-melanoma immune effects by modulating tumor-associated macrophages (TAMs) and influencing the tumor microenvironment [21].
Cytotoxic chemotherapy, while its role has markedly diminished with the advent of immune checkpoint inhibitors and targeted therapies, remains a potential therapeutic option in selected clinical scenarios and warrants consideration in tailored treatment strategies.
2.2. Treatments Involving the Use of IL-2 or IFN
High-dose interleukin-2 (HD IL-2) monotherapy demonstrated an overall response rate (ORR) of approximately 15–20% in advanced melanoma [22,23], while combination approaches, such as stereotactic body radiotherapy (SBRT), have been reported to improve the ORR to 54% [24] (Table 1). The therapy has the unique ability to induce durable complete responses in a subset of patients, a feature less commonly observed with cytotoxic agents like dacarbazine. However, its clinical adoption remains limited due to significant toxicities, including vascular leak syndrome and systemic inflammatory responses, which require intensive management and restrict its use to specialized centers (Table 1). In Japan, high-dose IL-2 has not been approved for use in the National Health Insurance system.
Interferon-α (IFN-α) therapy has shown limited efficacy. In a randomized trial comparing interferon-α (IFN-α) combined with dacarbazine (DTIC) versus DTIC alone, no significant differences in response rate or overall survival were observed [25,26]. In addition, IFN-α therapy is associated with both hematologic and non-hematologic toxicities, including bone marrow suppression, fever, and myalgia. While these adverse effects are generally manageable and reversible, they often necessitate dose adjustments or treatment delays [14]. Despite its potential immunomodulatory and antiproliferative benefits, the balance between efficacy and tolerability remains a critical consideration in its clinical application [14,27]. Interferon-α has been approved for use as an adjuvant therapy after surgery under the National Health Insurance system in Japan; however, its use has become extremely limited in recent years (Table 1).
By contrast, the local injection of interferon-beta (IFN-β) demonstrated effectiveness in several studies in the literature for cutaneous metastasis and in-transit metastases [28,29,30,31,32]. Several studies have reported the complete or partial remission of melanoma lesions following intralesional IFN-β administration [28,29] (Table 1). Adverse events associated with local IFN-β injection were limited to mild localized swelling, erythema, and inflammation at the injection site [30]. Overall, the treatment demonstrated a favorable tolerability profile. The combination of immune checkpoint inhibitors and interferon-β has shown potential therapeutic benefits [32]. Under the National Health Insurance system in Japan, IFN-β was combined with DAV therapy until recent advancements in molecularly targeted therapies were made. However, clinical evidence supporting its efficacy remains limited, necessitating further investigation through well-designed studies to validate its clinical utility.
3. Current Advances in Melanoma Therapy
3.1. Molecular Classification of Melanoma (The Cancer Genome Atlas Project)
Genomic profiling has revealed that cutaneous melanoma can be divided into four major molecular subtypes, characterized by their dominant driver mutations. The Cancer Genome Atlas (TCGA) project (2015) classified 333 melanomas into BRAF-mutant, RAS-mutant, NF1-mutant, and Triple Wild-Type (Triple-WT) groups [33]. In this schema, approximately 50–60% of tumors carry activating BRAF mutations (predominantly V600E/K), ~20–30% have RAS mutations (mostly NRAS codon 61), ~10–15% harbor loss-of-function mutations in NF1, and the remainder lack alterations in all three (Triple-WT) [33]. Notably, the NF1 subgroup frequently shows a very high mutation burden and NF1 loss serves as an alternative route to MAPK activation. The Triple-WT group (comprising about 15% of cases) is defined by the absence of hot-spot BRAF/NRAS/NF1 mutations and is genetically heterogeneous; some Triple-WT tumors instead contain other oncogenic events (e.g., mutations or amplifications in KIT, PDGFRA, GNAQ/GNA11, etc.). In TCGA’s analysis, these four subtypes did not show clear differences in overall survival but did underline the centrality of MAPK-pathway deregulation in melanoma—in fact, >90% of the BRAF, RAS, and NF1 subtypes showed UV-signature mutational spectra. Collectively, the TCGA classification highlights that most melanomas harbor mutations converging on the RAS–RAF–MEK–ERK (MAPK) signaling cascade (Figure 2).
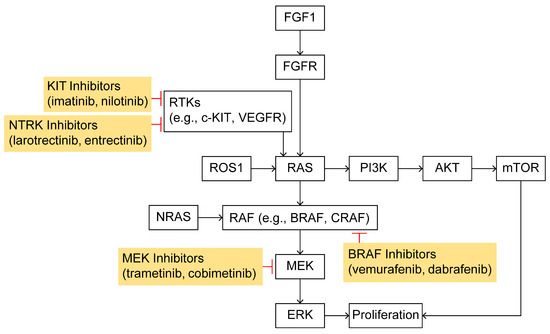
Figure 2.
Pathogenic mechanisms and therapeutic targets in melanoma: the figure illustrates the RAS–RAF–MEK–MAPK signaling pathway activated by growth factor receptors, highlighting how specific genetic mutations contribute to melanoma development and how these mutations serve as therapeutic targets for currently available treatments.
3.2. Molecularly Targeted Therapies
The development of molecularly targeted therapies has dramatically changed the treatment landscape for malignant melanoma, particularly for cases harboring actionable genetic mutations. These therapies specifically target the molecular drivers of tumor progression, offering superior efficacy compared to traditional cytotoxic agents with less adverse events.
3.2.1. BRAF V600 Mutation
B-Rapidly Accelerated Fibrosarcoma (BRAF) mutations, most commonly V600E and V600K, are key drivers of melanoma, occurring in approximately 60% of cutaneous melanoma worldwide [34,35]. These mutations activate the downstream Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Signaling Pathway (MEK-MAPK), promoting cell proliferation and survival [35]. Targeted therapies have focused on BRAF inhibitors and their combinations with MEK inhibitors, which have consistently demonstrated enhanced efficacy compared to monotherapy. The combination of dabrafenib and trametinib (D + T) achieves an overall response rate (ORR) of 68%, with a median progression-free survival (PFS) of 11.1 months and a median overall survival (OS) of 25.9 months [36]. In contrast, vemurafenib monotherapy yields lower outcomes, with a PFS of 7.3 months and OS of 16.9 months [37]. Another effective combination, encorafenib and binimetinib (E + B), reported an ORR of 64%, a PFS of 14.9 months, and an OS of 33.6 months in the COLUMBUS trial [38]. Additionally, vemurafenib combined with cobimetinib (V + C) demonstrated an ORR of 70%, a PFS of 12.3 months, and an OS of 22.3 months [39]. The superior efficacy of combination therapies involving BRAF and MEK inhibitors is attributed to their ability to more effectively suppress the MAPK signaling pathway [40]. This dual inhibition strategy not only prolongs PFS and overall survival OS but also mitigates the emergence of resistance mechanisms commonly observed with BRAF inhibitor monotherapy. For example, BRAF inhibitors alone can lead to the compensatory reactivation of the MAPK pathway through upstream receptor tyrosine kinases or secondary MEK mutations, which drive continued tumor progression [41]. The addition of MEK inhibitors counteracts these mechanisms by ensuring sustained pathway inhibition, resulting in improved clinical outcomes [42]. Moreover, the safety profile of combination therapy is favorable, as it allows for lower doses of each agent, reducing dose-dependent toxicities while maintaining efficacy [43]. Among the approved combinations, D + T, V + C, and E + B have demonstrated significant improvements in objective response rates (ORRs) and survival metrics compared to monotherapy in BRAF V600-mutant melanoma [35,37,38]. However, the therapeutic efficacy of BRAF + MEK inhibitors may vary depending on the specific mutation subtype, such as V600E or V600K [44,45]. Clinically, V600K mutations are more prevalent in older males and are associated with chronic sun damage, which contrasts with the typical characteristics of V600E melanoma [46]. In contrast, V600E mutations are more commonly observed in younger patients and occur predominantly in non-sun-damaged skin [46]. These tumors are often linked to a lower mutational burden compared to V600K and demonstrate a stronger dependency on the ERK pathway, which may explain their relatively higher responsiveness to BRAF+MEK inhibitor therapies [44,45]. These molecular differences are reflected in clinical outcomes, with patients harboring V600K mutations showing lower response rates and shorter progression-free survival compared to those with V600E mutations [45]. For example, the median PFS has been reported as 5.7 months for V600K compared to 7.1 months for V600E [44,45]. This reduced efficacy is likely due to the lower dependency of V600K melanomas on the ERK pathway, limiting the impact of MAPK-targeted therapies [44]. Additionally, V600K melanomas are associated with worse overall prognosis, including higher rates of metastasis to the brain and lungs and shorter intervals from diagnosis to disease progression [45].
Given the higher mutational burden and unique tumor microenvironment of V600K melanomas, these patients may benefit more from immunotherapy compared to those with V600E. Indeed, studies have demonstrated that V600K patients treated with anti-PD-1 therapies exhibit superior outcomes, with longer median progression-free survival (19 months vs. 2.7 months for V600E) and a trend toward improved overall survival (20.4 months vs. 11.7 months for V600E) [44]. These findings suggest that therapeutic strategies for BRAF-mutant melanomas should consider the specific mutation subtype to optimize clinical outcomes.
Both BRAF V600E and V600K mutations are known to confer sensitivity to molecularly targeted therapies such as BRAF inhibitors. However, resistance to these treatments is a prevalent and significant challenge in clinical practice. Mechanistically, resistance to BRAF inhibitors is largely driven by the reactivation or bypassing of the MAPK signaling pathway. This process is often facilitated by mechanisms such as the upregulation of receptor tyrosine kinases, including platelet-derived growth factor receptor beta (PDGFRβ), or genetic alterations in NRAS [47]. These adaptive changes enable tumor cells to evade MAPK pathway inhibition, thereby contributing to therapeutic resistance. Studies indicate that resistance emerges in a substantial proportion of patients, with estimates suggesting that up to 50% of cases experience resistance within 6 to 12 months following the initiation of therapy [48,49].
Efforts to mitigate resistance have led to the exploration of several therapeutic strategies. Combination therapy with BRAF inhibitors and MEK inhibitors has shown promise in delaying the onset of resistance by simultaneously targeting multiple nodes within the MAPK pathway [43,48,49]. Additionally, the integration of immune checkpoint inhibitors (ICIs), such as anti-PD-1 and anti-CTLA-4 antibodies, into treatment regimens has emerged as a compelling approach. This combination leverages the synergistic potential of immune activation alongside molecularly targeted therapy, potentially enhancing treatment efficacy and providing a durable response [50,51]. While the combination of immune checkpoint inhibitors with BRAF and MEK inhibitors has emerged as a promising therapeutic strategy for patients with BRAF-mutant melanoma, alternative approaches to overcoming resistance are currently under investigation. Among these, the inhibition of the glucocorticoid receptor (GCR) has been explored as a potential means to counteract resistance by modulating tumor cell survival pathways and mitigating the effects of stress hormone signaling. Estrela et al. demonstrated that GCR antagonism overcomes resistance to BRAF inhibition in BRAF V600E-mutated metastatic melanoma through the modulation of stress response pathways [52]. Additionally, fibroblast growth factor 1 (FGF1) inhibitors have been studied in this context, as the upregulation of FGF1 has been implicated in resistance to BRAF and MEK inhibition, and its blockade may help to resensitize melanoma cells to targeted therapy. Wang et al. reported that the activation of the FGFR cascade leads to sustained ERK signaling, and adding FGF1 inhibitors to BRAF/MEK inhibitors may restore treatment sensitivity [53]. Moreover, the inhibition of p90 ribosomal S6 kinase (RSK) has demonstrated potential in suppressing protein synthesis and tumor proliferation in melanoma cells that have developed dual resistance to BRAF and MEK inhibitors. BI-D1870 and BRD7389, two RSK inhibitors, were found to significantly reduce tumor growth and protein synthesis in melanoma cell lines exhibiting dual resistance [54].
Furthermore, histone deacetylase (HDAC) inhibitors have gained attention due to their ability to downregulate the microphthalmia-associated transcription factor (MITF), which plays a crucial role in melanoma progression and therapeutic resistance. Aida et al. demonstrated that MITF suppression via HDAC inhibition enhances the apoptotic response of melanoma cells to BRAF inhibitors in preclinical models [55].
These alternative strategies hold promise for overcoming acquired resistance to BRAF and MEK inhibitors, but further research and clinical validation are warranted to establish their efficacy and safety in clinical settings.
3.2.2. KIT Mutation
KIT mutations occur in approximately 9.5% of melanomas, with notable variation among subtypes [56]. Acral melanomas exhibit KIT mutations in 8.3–23% of cases, while mucosal melanomas have the highest reported frequency, ranging from 15.6% to 50% [57,58]. Unlike BRAF mutations, KIT mutations are rare in cutaneous melanoma, with a reported frequency ranging from 1.7% to 4.3% [57,58].
These mutations, primarily affecting exons 11 and 13, lead to constitutive activation of tyrosine kinase signaling pathways, contributing to melanoma progression [57]. Among these, mutations in exon 11 (e.g., L576P) and exon 13 (e.g., K642E) are the most prevalent, accounting for approximately 40–55% of mutations, and are associated with the activation of MAPK and PI3K/AKT pathways [57,58].
Clinical studies demonstrate an ORR to KIT inhibitors, such as imatinib, nilotinib, and dasatinib, ranging from 15 to 40%, with the highest efficacy observed in cases with specific mutations like L576P and K642E [59,60]. For example, imatinib achieved an ORR of 24.4% and a disease control rate (DCR) of 66.7% in patients with exon 11 or 13 mutations [61]. Nilotinib showed comparable outcomes, with an ORR of 26% and DCR of 73.8%, particularly in patients with the aforementioned mutations [61]. However, data on the efficacy of KIT inhibitors in patients with mutations outside these exons are limited [59]. Furthermore, in the absence of KIT mutations but in the presence of gene amplification, the therapeutic efficacy of these agents is considerably limited [59,62].
Despite these advancements, resistance to KIT inhibitors remains a significant challenge, often driven by secondary mutations, activation of alternative pathways, and microenvironmental factors. Mechanisms of resistance include the reactivation of downstream signaling pathways, such as MAPK and PI3K/AKT, and contributions from tumor-associated macrophages and angiogenic factors [61]. To counter these, combinatorial therapeutic approaches targeting multiple pathways have been explored. Preclinical studies have shown that the dual inhibition of PI3K and MAPK pathways enhances antitumor efficacy [57,61]. Furthermore, novel agents like ponatinib, approved by the FDA for CML and Ph + ALL but not yet for melanoma, which targets KIT and angiogenic pathways, have demonstrated potential in overcoming resistance [61]. Preclinical studies of ponatinib reported promising results, demonstrating potent antitumor effects in KIT-mutant melanoma models [63].
3.2.3. NRAS Mutations
Neuroblastoma RAS viral oncogene homolog (NRAS) mutations occur in 15–20% of melanomas and are associated with aggressive behavior and poor prognosis [64]. These mutations activate multiple signaling pathways, including PI3K/Akt and MEK-ERK, promoting cancer growth [65]. While targeting NRAS directly has been ineffective, inhibiting downstream effectors or KRAS shows promising results [66]. MEK inhibitors, particularly binimetinib, have demonstrated activity in NRAS-mutant melanoma [64,67]. Combining MEK inhibitors with CDK4/6 inhibitors appears to be a promising strategy [64,68]; however, it has not yet been approved by the FDA for melanoma. Other potential combinations include MEK inhibitors with FAK inhibitors, autophagy inhibitors, or pan-RAF inhibitors [69]; however, these treatments have not been approved for melanoma treatment by the FDA so far. The FAK inhibitor defactinib, together with avutometinib (an MEK/RAF inhibitor), was put forward for FDA approval in 2025 for ovarian cancer. Tovorafenib, which inhibits multiple RAF family kinases, was approved for pediatric low-grade glioma in 2024. Immunotherapies, especially immune checkpoint inhibitors, may be particularly effective in NRAS-mutant melanoma [66]. Despite these advances, the ideal treatment for NRAS-mutant melanoma remains elusive, necessitating further research [70,71].
3.2.4. ROS1 Fusions and Mutations
ROS1 fusions have been identified across various cancer types, with particular clinical relevance in non-small cell lung cancer (NSCLC) [72,73], where targeted therapies such as crizotinib [74] and entrectinib [75] have demonstrated significant efficacy. In contrast, the pathogenic significance of ROS1 mutations in NSCLC remains unclear, as their role in tumorigenesis and response to therapy has yet to be well-defined [76].
In melanoma, both ROS1 mutations and ROS1 fusions have been reported, though their prevalence and implications differ. ROS1 mutations are more frequently observed, occurring in approximately 14.8% to 25.0% of cases [76], while ROS1 fusions are rare and detected in only around 1% of melanomas [77]. Emerging evidence suggests that melanomas harboring ROS1 mutations may exhibit heightened sensitivity to immune checkpoint inhibitors (ICIs), including PD-1 and CTLA-4 inhibitors [76]. This is thought to be associated with a significantly higher tumor mutational burden (TMB) in ROS1-mutated melanomas, potentially enhancing neoantigen presentation and immune system activation.
On the other hand, ROS1 fusions in melanoma may represent a therapeutic target akin to NSCLC. A case of melanoma harboring GOPC-ROS1 fusion has been reported, where treatment with crizotinib resulted in a favorable clinical response [78]. Similarly, entrectinib has shown potential efficacy in ROS1 fusion-positive melanomas [77], suggesting that ROS1 fusion-directed therapies could be a viable treatment strategy in select cases.
Despite these promising findings, further investigation is warranted to elucidate the precise role of ROS1 mutations and fusions in melanoma. Future studies should focus on clarifying their oncogenic mechanisms, therapeutic implications, and potential as predictive biomarkers for both immunotherapy and targeted therapy.
3.2.5. NTRK Fusions and Mutations
Similarly to ROS1 gene fusions, Neutrophic tropomyosin receptor kinase (NTRK) alterations in melanoma encompass both gene fusions and somatic mutations. While NTRK gene fusions are rare, they are recognized as oncogenic drivers across multiple cancer types, including melanoma [79,80]. The prevalence of NTRK gene fusions varies among melanoma subtypes, being notably higher in spitzoid melanoma (21–29%) compared to other subtypes, where the frequency is reported to be < 1–2.5% [79]. Identified NTRK fusion partners in melanoma include TRIM63-NTRK1, DDR2-NTRK1, GON4L-NTRK1, TP53-NTRK1, LMNA-NTRK1, TRAF2-NTRK2, ETV6-NTRK3, MYO5A-NTRK3, and MYH9-NTRK3.
In contrast, somatic NTRK mutations appear to be more common than gene fusions in cutaneous melanoma, with a reported frequency of 19.5% (86/440 cases) [81]. Notably, patients with NTRK mutations have demonstrated a significantly higher ORR to immune checkpoint inhibitors (ICIs) compared to those without NTRK mutations (42.8% vs. 23.5%, p = 0.002) [81]. These findings suggest a potential role of NTRK mutations as predictive biomarkers for ICI therapy response in melanoma.
Given the therapeutic implications of NTRK fusions, molecular screening for these alterations is recommended in BRAF, NRAS, and KIT wild-type melanomas [80]. Targeted therapies with TRK inhibitors, such as larotrectinib and entrectinib, have shown high response rates (>75%) in NTRK fusion-positive cancers across different histologies [82,83]. These inhibitors are generally well-tolerated, with on-target adverse events being rare [82]. However, acquired resistance through NTRK kinase domain mutations has been reported, which may be addressed by second-generation TRK inhibitors such as selitrectinib (LOXO-195) and repotrectinib (TPX-0005) [82].
The FDA granted accelerated approval to larotrectinib in 2018 and to entrectinib in 2019 for the treatment of solid tumors harboring NTRK gene fusions, with entrectinib also receiving approval for pediatric patients. Clinical trials have suggested the efficacy of TRK inhibitors in melanoma, although further studies are warranted to establish their role in this malignancy [84,85].
3.3. Immunotherapy
Immune checkpoint inhibitors (ICIs) represent a cornerstone in the management of unresectable and metastatic melanoma, with two primary classes initially approved for clinical use: CTLA-4 inhibitors and PD-1 inhibitors. The CTLA-4 inhibitor, ipilimumab, enhances T cell activity by disrupting the immunosuppressive interaction between CTLA-4 and B7 [86,87,88]. Since its approval by the FDA in 2011 and in Japan in 2015, ipilimumab has been associated with prolonged overall survival (OS) [5]. A phase III trial (CA184-024) demonstrated that ipilimumab combined with dacarbazine improved OS compared to dacarbazine alone [89], although this combination was limited by an increased risk of hepatotoxicity [90]. Consequently, ipilimumab monotherapy or its combination with PD-1 inhibitors has become the preferred strategy in advanced melanoma.
PD-1 inhibitors, such as pembrolizumab and nivolumab, function by blocking the PD-1 receptor on T cells, thereby enhancing the immune system’s capacity to recognize and eliminate cancer cells [91,92]. Nivolumab was approved by the FDA and by Japanese authorities in 2014, while pembrolizumab was approved by the FDA in 2014 and for use in Japan in 2016. Pembrolizumab has demonstrated superior efficacy over ipilimumab in both treatment-naive patients with metastatic or unresectable melanoma (KEYNOTE-006 trial [93,94]) and in previously treated patients with metastatic or unresectable disease following chemotherapy (KEYNOTE-002 [95]). Similarly, nivolumab has demonstrated significant efficacy as monotherapy and in combination with ipilimumab, as shown in the CHECKMATE-037 trial [6], which compared nivolumab to chemotherapy, the CHECKMATE-066 trial [96], which evaluated nivolumab against dacarbazine, and the CHECKMATE-067 trial [97,98], which evaluated the nivolumab and ipilimumab combination against nivolumab and ipilimumab. Data from these pivotal clinical trials underscore the efficacy of ICIs in improving clinical outcomes in melanoma patients. These findings support the use of pembrolizumab and nivolumab as key agents in the therapeutic arsenal for unresectable melanoma. It remains unclear precisely which patients with melanoma benefit from ICIs. PD-1 inhibitors, such as pembrolizumab and nivolumab, exert therapeutic effects primarily by blocking the interaction between the PD-1 receptor on T cells and its ligand, PD-L1, expressed on tumor cells. Initially, tumor PD-L1 expression was anticipated to correlate positively with clinical response. However, the clinical implementation of PD-L1 as a reliable biomarker has been challenging due to its dynamic variability and intratumoral heterogeneity, as well as the observation that some patients with PD-L1-negative tumors also respond favorably to PD-1 inhibitors [99].
Recent studies have demonstrated that PD-1, traditionally considered exclusive to immune cells, is also expressed on melanoma cells [100,101]. In melanoma, the binding of PD-1 onto tumor cells with PD-L1 expressed by the surrounding tumor or stromal cells can activate the mTOR signaling pathway, thereby directly promoting tumor growth independently of immune cell interactions [101]. Notably, PD-1 inhibitors may exert their therapeutic efficacy not only through immune modulation but also by directly interacting with PD-1 expressed on melanoma cells [101]. Furthermore, recent evidence indicates that androgen receptor activation in melanoma cells may interfere with the efficacy of ICIs [100]. Detailed biomarkers for treatment response will be discussed in a separate section; however, numerous studies are currently underway to identify reliable biomarkers predictive of response to ICIs.
Lymphocyte-activation gene 3 (LAG-3) inhibitors represent the third class of immune checkpoint inhibitors (ICIs) and have been approved by the FDA for the treatment of melanoma in combination with nivolumab since 2022.
LAG-3 is an inhibitory molecule expressed on the surface of T cells, where it negatively regulates T cell activation. Its blockade enhances immune responses by facilitating T cell proliferation and function [102]. Notably, LAG-3 interacts with major histocompatibility complex (MHC) class II molecules, further modulating immune activation [103,104].
The phase II/III RELATIVITY-047 trial demonstrated that the combination of nivolumab and relatlimab, an anti-LAG-3 monoclonal antibody, significantly prolonged progression-free survival (PFS) compared to nivolumab monotherapy in patients with advanced melanoma (median PFS: 10.1 months vs. 4.6 months, respectively) [105]. While this combination has been incorporated into clinical practice in the United States, it has not yet been approved in Japan. Nevertheless, it is listed in the National Comprehensive Cancer Network (NCCN) guidelines, indicating its potential for future adoption in real-world clinical settings.
Clinical trials investigating the immune checkpoint inhibitors discussed above are summarized in Table 2.

Table 2.
Summary of clinical trials evaluating immune checkpoint inhibitors (ICIs) in advanced melanoma (non-adjuvant settings).
3.4. Talimogene Laherparepvec (T-VEC)
Talimogene laherparepvec (T-VEC) is an oncolytic virus therapy derived from the herpes simplex virus type 1 (HSV-1) that has been genetically engineered to selectively replicate within tumor cells and enhance systemic anti-tumor immunity. T-VEC is administered via intralesional injection and functions by inducing direct tumor cell lysis and promoting an immunogenic response, which includes the upregulation of the granulocyte-macrophage colony-stimulating factor (GM-CSF) to recruit antigen-presenting cells and stimulate cytotoxic T lymphocytes [106].
A pivotal phase III trial (OPTiM) compared T-VEC with recombinant GM-CSF in patients with unresectable stage IIIB-IV melanoma [5]. The study demonstrated that T-VEC significantly improved the durable response rate (DRR) compared to GM-CSF (16.3% vs. 2.1%, p < 0.001) and exhibited greater efficacy in patients with earlier-stage disease (stage IIIB-IIIC: DRR 33.0%) [5]. Furthermore, the therapy elicited systemic effects, leading to tumor regression in non-injected lesions, indicative of an abscopal immune response [5,107].
In efforts to enhance therapeutic outcomes, a phase II study evaluated the combination of T-VEC with ipilimumab, a CTLA-4 checkpoint inhibitor, in unresectable melanoma [108]. The combination demonstrated superior overall response rates (ORR: 39% vs. 18%, p = 0.002) compared to ipilimumab monotherapy [108]. Despite the promising response rate, progression-free survival (PFS) and overall survival (OS) benefits remain under investigation.
T-VEC is primarily recommended for patients with stage IIIB-IV melanoma with injectable cutaneous, subcutaneous, or nodal metastases. However, its efficacy diminishes in stage IV-M1b/M1c disease, where systemic immunotherapy or targeted therapy is often preferred [20]. Additionally, data suggest that T-VEC is most effective in treatment-naïve patients, as prior exposure to systemic therapies may attenuate the immunogenic response [109]. This may be due to the immunosuppressive alterations in the tumor microenvironment following previous systemic interventions [107].
The superior efficacy of T-VEC in initial treatment settings may be attributed to its mechanism of action, which involves both direct viral-mediated oncolysis and the stimulation of a systemic immune response. In patients previously treated with immunosuppressive agents, the tumor microenvironment may develop resistance mechanisms that limit T-VEC’s immune-stimulatory effects [107].
T-VEC represents a novel approach to melanoma treatment by leveraging local oncolysis and systemic immune activation. While its clinical utility is predominantly within early metastatic disease, ongoing studies are exploring its integration with immune checkpoint inhibitors to optimize therapeutic efficacy in advanced melanoma cases.
4. Specific Considerations for Melanoma Treatment in Asian Populations
4.1. Immune Checkpoint Inhibitor Therapy in Asian Populations
Acral lentiginous melanoma (ALM) and mucosal melanoma (MM) are predominant melanoma subtypes in Asian populations, contrasting with the higher prevalence of cutaneous melanoma (CM) in Caucasians. ALM accounts for approximately 40–71% of melanomas in Asian populations [110,111,112], and MM constitutes about 15–27% [110,113,114]. In contrast, ALM is relatively rare among Caucasians, comprising only 1–3% of melanomas [114,115], whereas MM accounts for approximately 1% [116].
These subtypes are biologically distinct, exhibiting a lower tumor mutational burden (TMB) and different oncogenic drivers compared to CM [117].
The efficacy of immune checkpoint inhibitors (ICIs) is notably reduced in ALM and MM. While the objective response rates (ORRs) for anti-PD-1 therapy in CM range between 30% and 40% [6,93,96,118,119], the ORRs for ALM and MM are significantly lower, ranging from 14% to 19% [117]. The reduced tumor mutational burden (TMB) in ALM and MM, attributable to their occurrence in UV-shielded regions, is a primary factor contributing to the diminished efficacy of ICIs [120,121,122].
Even for CM, recent studies suggest that Asian patients may experience lower ICI efficacy than Caucasians. This observation is attributed to lower TMB in Asian populations. For instance, Huang et al. noted that Asian cutaneous melanoma (CM) exhibits a median TMB of 5.1 mutations per megabase (mut/Mb), which is significantly lower than that observed in Caucasian populations [123]. Similarly, Hida et al. reported that the mean TMB of Asian CM is 4.6 mut/Mb, suggesting that the TMB range for Asian CM can be approximated as 4.6–5.1 mut/Mb [124]. In contrast, Caucasian CM is characterized by a markedly higher mean TMB of 49.17 mut/Mb [125], highlighting a significant disparity in the mutational landscape between these populations.
These findings suggest that the efficacy of ICIs may vary among different ethnic groups, even within the same subtype of malignant melanoma. To address this potential variability, prospective studies evaluating the efficacy of ICIs across diverse ethnic populations may be warranted in the future.
4.2. Monotherapy vs. Combination Therapy for Asian Melanoma Patients
The question of whether combination therapy of different-class ICIs offers superior benefits compared to monotherapy in treating ALM and MM remains a crucial concern. Retrospective studies and clinical trials have produced mixed results. For ALM, combination therapy has demonstrated higher ORR in nail apparatus melanoma (e.g., 61% with nivolumab plus ipilimumab vs. 10% with monotherapy) but has shown no significant survival advantage for lesions on other acral sites, such as palms and soles [126]. Similarly, studies on MM have reported improved ORRs with combination therapy compared to monotherapy (43% vs. 26%, respectively), but recent analyses indicate no statistically significant differences in overall survival (OS) or progression-free survival (PFS) between the two approaches [127].
Given these findings, the routine use of combination therapy for Asian patients with ALM or MM may not be warranted in all cases. While combination therapy may be considered for selected subtypes, such as nail apparatus ALM, or in cases of monotherapy failure, its benefits in broader contexts appear limited. Importantly, clinical decisions should be guided by individual patient characteristics, including anatomical site, molecular features, and performance status, to maximize therapeutic outcomes. Further prospective studies are essential to clarify the optimal therapeutic strategies for these challenging melanoma subtypes.
4.3. Therapeutic Approaches for BRAF-Positive Cases in Asian Populations
Recent studies, including the B-CHECK-RWD study conducted in Japan, indicate significant differences in the efficacy of immune checkpoint inhibitors (ICIs) between Asian and Caucasian populations with advanced BRAF V600-mutant melanoma. While ICIs such as nivolumab and the combination of nivolumab plus ipilimumab demonstrate promising outcomes in Western populations, their efficacy appears comparatively reduced in Asian cohorts [128].
In contrast, BRAF/MEK inhibitors maintain comparable efficacy between Asian and Caucasian populations. Data show ORRs of 68% and 67% in Caucasian and Japanese cohorts, respectively, when treated with dabrafenib plus trametinib [128]. Despite this equivalence, the sequencing of BRAF/MEK inhibitors and ICIs remains a pivotal consideration. The DREAMseq and SECOMBIT trials in Western populations advocate for upfront ICI therapy due to its potential for durable survival benefits [129,130]. However, the suboptimal response of ICIs in Asian patients complicates the direct adoption of this strategy.
The B-CHECK-RWD study highlights that salvage therapy with ICIs following disease progression on BRAF/MEK inhibitors yields diminished outcomes, whereas BRAF/MEK inhibitors retain efficacy irrespective of treatment line [128]. Consequently, the choice of first-line therapy in Asian patients necessitates a case-by-case approach, balancing disease characteristics, patient tolerance, and access to therapies.
5. Future Perspectives on Melanoma Management
Recent advancement in immunology, molecular biology and tumor immunology enabled us several possible ways to treat melanoma.
First, as aforementioned, to target potential pro-proliferating signaling molecules, such as BRAF, KIT, NRAS, TERT, and CCND1, most of which harbor constitutive active mutations in these molecules, treatments should target signaling pathways leading to tumor cell proliferation [123]. TERT promoter mutations, which promote telomerase activity and enable the indefinite replication of tumor cells, are particularly common in acral melanomas and represent potential therapeutic targets [117,131]. Amplifications in CCND1, which drive uncontrolled cell cycle progression via CDK4/6 activation, are another hallmark of acral melanoma, highlighting a distinct pathway compared to UV-induced melanomas [123].
The second way to combat melanoma is to control the tumor microenvironment, with tumor-infiltrating lymphocytes (TILs) playing a key role in this approach. Lifileucel, an autologous TIL therapy, has shown efficacy even in patients who have failed immune checkpoint inhibitors (ICIs) and BRAF/MEK-targeted therapies, as demonstrated in the Phase II C-144-01 trial, which reported an objective response rate (ORR) of 31.4% [132]. Having received FDA approval in the United States, lifileucel is now included in the NCCN guidelines as a promising treatment strategy [20], though it remains unapproved in Japan. The treatment involves the surgical resection of tumor tissue, followed by a 22-day ex vivo expansion of TILs, lymphodepleting chemotherapy with cyclophosphamide and fludarabine, and the subsequent administration of high-dose IL-2 to support TIL persistence. However, significant challenges remain, including the complexity and cost of treatment, the risk of severe adverse events such as thrombocytopenia, capillary leak syndrome, and hypotension, and the need for specialized centers to administer therapy [132]. Despite these limitations, TIL therapy represents a valuable addition to the evolving landscape of melanoma treatment.
The third method is chimeric antigen receptor T (CAR-T) cell therapy, an innovative immunotherapeutic strategy that has demonstrated remarkable efficacy in hematologic malignancies, leading to its FDA approval for specific leukemia and lymphoma subtypes [133,134,135,136]. While CAR-T therapy for melanoma is not yet widely established, ongoing clinical investigations suggest its potential in targeting melanoma-specific antigens [137].
One promising target in melanoma is tyrosinase-related protein 1 (TYRP1), which is highly expressed in tumor cells but has limited expression in normal tissues [138,139]. Preclinical studies have provided compelling evidence of the efficacy and safety of TYRP1-directed CAR-T cells, showing robust antitumor activity in both in vitro and in vivo models [140].
Although the application of CAR-T therapy in solid tumors remains in its early stages, ongoing clinical trials and advancements in antigen selection, CAR engineering, and immunosuppressive microenvironment modulation hold promise for expanding its therapeutic potential in melanoma.
A fourth strategy involves combining immune checkpoint inhibitors (ICIs) with other therapeutic modalities to enhance antitumor efficacy and overcome resistance (Table 3). While ICIs have significantly improved outcomes in melanoma, intrinsic and acquired resistance remains a challenge [141]. To address this, a number of combination strategies have been developed to target complementary immune-inhibitory pathways.
Among these, LAG-3 has gained particular attention. LAG-3 is an inhibitory receptor co-expressed with PD-1 on exhausted T cells, and its blockade has been shown to reinvigorate antitumor immunity. The combination of the anti-PD-1 antibody nivolumab with the anti-LAG-3 antibody relatlimab demonstrated improved efficacy compared to anti-PD-1 monotherapy, leading to an FDA approval of the combination regimen in 2022 [105]. This dual-checkpoint blockade represents a rational approach to enhance immune activation by targeting synergistic inhibitory signals.
Radiotherapy and certain chemotherapeutic agents can induce immunogenic cell death (ICD), enhancing tumor antigen presentation and promoting anti-tumor immunity [141,142]. Clinical studies suggest that concurrent or sequential radiotherapy with ICIs may augment systemic responses, particularly in patients with brain metastases [143,144]. Recent clinical trials are investigating the combination of ICIs with radiation therapy or cytotoxic agents [105,145,146,147,148,149,150,151,152,153].
Oncolytic viruses represent another promising strategy. T-VEC, an intralesional herpesvirus expressing GM-CSF, has shown synergistic effects with ICIs in early-phase studies [154]. However, a phase III trial (MASTERKEY-265) of T-VEC combined with pembrolizumab did not demonstrate superiority over pembrolizumab alone, highlighting the importance of patient selection [155]. Novel oncolytic agents, such as RP1, are being tested in anti-PD-1-refractory melanoma and have demonstrated encouraging response rates (IGNYTE-3) [156].
Therapeutic cancer vaccines, particularly personalized neoantigen vaccines, are emerging as valuable partners for ICIs. In the phase II KEYNOTE-942 trial, the mRNA-4157 vaccine combined with pembrolizumab significantly improved recurrence-free survival in patients with resected high-risk melanoma [157]. Additional studies, including KEYNOTE-D36, are exploring peptide- and dendritic cell-based vaccines in advanced settings [158].
Adoptive cell therapy, particularly tumor-infiltrating lymphocyte (TIL) therapy, is also being investigated in combination with ICIs to improve treatment durability. TIL therapy involves harvesting and expanding autologous T cells from resected tumors, then reinfusing them into the patient following lymphodepletion. This approach has shown promising results in heavily pretreated melanoma patients. In the ongoing phase III TILVANCE-301 trial, the combination of lifileucel (an autologous TIL product) with pembrolizumab is being compared to pembrolizumab monotherapy in treatment-naïve patients with advanced melanoma [159]. This study aims to determine whether the addition of TIL therapy can enhance the efficacy of first-line immunotherapy. Early-phase data have already suggested durable responses, particularly in patients with prior resistance to checkpoint blockade.
Collectively, these combination approaches aim to overcome immune resistance by promoting T cell priming, antigen release, and favorable remodeling of the tumor microenvironment, and they are supported by a growing body of preclinical and clinical evidence.

Table 3.
Summary of clinical trials evaluating combination strategies with immune checkpoint inhibitors in melanoma.
Table 3.
Summary of clinical trials evaluating combination strategies with immune checkpoint inhibitors in melanoma.
| Phase | Trial Name | Intervention vs. Control | Patient Population | Reference |
|---|---|---|---|---|
| II/III | RELATIVITY-047 | Nivolumab + Relatlimab vs. Nivolumab | Treatment-naive advanced melanoma | [105,145] |
| II | RadVax | Nivolumab + Ipilimumab + HFRT vs. Nivolumab + Ipilimumab alone | Unresectable stage IV melanoma | [146] |
| I | NCT02858869 | Pembrolizumab + SRS (single arm) | Melanoma or NSCLC with untreated brain metastases | [147] |
| I | NCT02716948 | Nivolumab + SRS (single arm) | Melanoma or NSCLC with untreated brain and/or spine metastases | [148] |
| II | NIRVANA | Nivolumab + multisite HFRT (single arm) | Metastatic melanoma; no prior systemic therapy | [150] |
| II | CHEERS | Standard-of-care ICI therapy combined with SBRT vs. standard-of-care ICI therapy | Advanced or metastatic melanoma | [152,153] |
| II | NCT02617849 | Pembrolizumab in combination with carboplatin/paclitaxel (single arm) | Metastatic malignant melanoma | [153] |
| I/II | IGNYTE-3 | Nivolumab + RP1 oncolytic HSV-1 or Rp1 alone | Advanced solid tumor (including melanoma) refractory to anti-PD-1 therapy | [156] |
| II | KEYNOTE-D36 | Pembrolizumab + EVX-01 vs. historical control (no concurrent control arm) | Treatment-naive advanced melanoma | [158] |
| III | TILVANCE-301 | Pembrolizumab + Lifileucel TIL vs. Pembrolizumab alone | Treatment-naive advanced melanoma | [159] |
| II | ABC-X | Nivolumab + Ipilimumab + SRS vs. Nivolumab + Ipilimumab alone | Melanoma brain metastases; treatment-naïve | [160] |
| III | MASTERKEY-265 | Pembrolizumab + T-VEC vs. Pembrolizumab + placebo | Unresectable stage IIIB–IVM1c melanoma, anti-PD-1 naïve, injectable lesions required | [161] |
| II | KEYNOTE-942 | mRNA-4157 (V940) + Pembrolizumab vs. Pembrolizumab alone | Resected high-risk cutaneous melanoma | [162] |
Note: HFRT: hypofractionated radiotherapy; SRS: stereotactic radio surgery; SBRT: stereotactic body radiotherapy; ICI: immune checkpoint inhibitors.
The fifth emerging avenue in melanoma immunotherapy focuses on natural killer (NK) cells, which are increasingly recognized as key effectors in antitumor immunity [163,164]. Recent evidence highlights the role of androgen receptor (AR) signaling in impairing NK cell-mediated cytotoxicity [100]. In melanoma cells, ligand-activated AR promotes the shedding of the NKG2D ligand MICA via the upregulation of ADAM10, a disintegrin and metalloprotease [100]. AR forms a functional complex with β1 integrin and ADAM10, leading to the loss of surface MICA and reduced NK cell recognition. This mechanism facilitates immune evasion, and high serum levels of soluble MICA correlate with a poor response to anti-PD-1 therapy [100]. Sex-based disparities in NK cell function may further compound this immune escape. Females generally exhibit more robust NK activity, while androgens in males may dampen innate immune responses, contributing to worse melanoma outcomes [165,166,167,168,169]. Consequently, the therapeutic blockade of AR represents a compelling strategy. Preclinical models suggest that AR antagonists, such as bicalutamide or enzalutamide, can restore NK-mediated cytotoxicity and synergize with immune checkpoint inhibitors [100]. These findings warrant the future clinical evaluation of AR-targeted therapies in melanoma, particularly in male patients or those with high AR expression.
As a seventh emerging strategy, the identification and validation of biomarkers closely associated with therapeutic response and prognosis have become critical components of melanoma management. Recent studies have identified multiple molecular biomarkers in melanoma associated with therapeutic response and prognosis in the context of immune checkpoint inhibitors (ICIs). PD-L1 expression, while a logical predictor for anti-PD-1/PD-L1 therapies, has shown limited utility in melanoma [170]. Many patients with PD-L1-negative tumors still respond to anti-PD-1 therapy [171], underscoring the need for better biomarkers. High tumor mutational burden (TMB), a hallmark of UV-induced melanoma, is associated with a greater likelihood of response to ICIs [172]. The IFN-γ signature, characterized by an elevated expression of interferon-γ-responsive genes such as CXCL9, CXCL10, IDO1, IFNG, and HLA-DR within the tumor microenvironment, reflects an inflamed immune state enriched with activated T cells. This gene expression profile strongly predicts therapeutic responsiveness to anti-PD-1 therapy [173]. Conversely, tumors with low IFN-γ signature (non-inflamed) rarely respond to single-agent immunotherapy; these “cold” tumors might require combination strategies (e.g., adding CTLA-4 blockade, or novel agents to induce T cell infiltration). Overall, the T cell-inflamed GEP is a robust biomarker to predict benefit from PD-1/L1 inhibitors [173]. Tumor-Infiltrating Lymphocytes (TILs) and immune cells represent essential biomarkers reflecting the tumor immune environment. High densities of CD8+ cytotoxic T cells within tumors correlate with better responsiveness to ICIs, particularly in the presence of PD-L1 expression [174,175]. A higher CD8:FOXP3 T cell ratio, indicative of increased effector T cells relative to regulatory T cells, also predicts favorable responses [176]. Conversely, immunosuppressive cell populations, such as PD-L1⁻ M2-polarized macrophages, myeloid-derived suppressor cells (MDSCs) [176], and elevated inflammatory markers (IL-6, CRP, neutrophil-to-lymphocyte ratio) [177] are associated with a poor prognosis and resistance to ICIs. Indoleamine 2,3-dioxygenase (IDO1), an enzyme that contributes to immune suppression by degrading tryptophan, is typically associated with a poor prognosis; however, paradoxically, elevated IDO1 expression in tumors might indicate a favorable response to CTLA-4 blockade, as it suggests a regulatory mechanism that checkpoint inhibitors can overcome [173].
Emerging Immune Targets such as LAG-3 are increasingly recognized biomarkers. LAG-3, commonly co-expressed with PD-1 on exhausted T cells, has shown potential as both a predictive biomarker and therapeutic target. The combination of anti-LAG-3 (relatlimab) and nivolumab recently demonstrated clinical efficacy in advanced melanoma [105].
Host Factors, notably specific HLA genotypes and the gut microbiome, significantly impact immunotherapy outcomes. Certain HLA class I alleles (e.g., HLA-B44 supertype, HLA heterozygosity) correlate with improved ICI responses [178]. Furthermore, the gut microbiome composition, particularly the enrichment of Akkermansia and Bifidobacterium, has emerged as a novel biomarker predictive of immunotherapy efficacy [178].
Beyond immunotherapy, the molecular profile of melanoma also informs the application of targeted therapies. The presence of a BRAF V600 mutation remains a pivotal predictive biomarker for targeted treatment, with combined BRAF and MEK inhibitor therapy yielding objective response rates of approximately 70% [179]. Additionally, mutations in NRAS have been associated with poorer prognoses, characterized by increased tumor aggressiveness, higher metastatic potential, and reduced melanoma-specific survival [180].
However, no single biomarker is sufficient; thus, integrative multi-biomarker approaches (combining TMB, gene expression signatures, and immune contexture) are being explored to improve predictive accuracy [178]. This multifaceted strategy holds promise for better personalizing therapy and prognostication in malignant melanoma.
Table 4 outlines the future directions discussed in this section. While many of these strategies remain investigational, they reflect the dynamic and rapidly progressing field of melanoma research and offer a foundation for future therapeutic innovations.

Table 4.
Feature perspectives for melanoma treatment.
Author Contributions
S.K. and M.K. searched the literature and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank all the members of Department of Dermatology, Jichi Medical University, who were involved in the treatment of melanoma patients.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| DTIC | Dacarbazine |
| DAV | Dacarbazine, ACNU, Vincristine |
| TMZ | Temozolomide |
| IFN-α | Interferon-alpha |
| IFN-β | Interferon-beta |
| nab-PTX | nab-Paclitaxel |
| CBDCA | Carboplatin |
| PTX | Paclitaxel |
| V + C | Vemurafenib + Cobimetinib |
| BRAF + MEK | BRAF inhibitor + MEK inhibitor |
| E + B | Encorafenib + Binimetinib |
| D + T | Dabrafenib + Trametinib |
| N + I | Nivolumab + Ipilimumab |
| RAS | Rat sarcoma |
| NRAS | Neuroblastmoa RAS |
| HRAS | Harvey RAS |
| KRAS | Kristen RAS |
| NF1 | Neurofibromin 1 |
| ERK | Extracellular signal-regulated kinase |
| RAF | Rapidly accelerated fibrosarcoma |
| MAPK | Mitogen activated protein kinase |
| MAPKK | Mitogen activated protein kinase kinase (MEK) |
| FAK | Focal adhesion kinase |
| PI3K | Phosphatidyl inositol 3 kinase |
| CDK | Cyclin dependent kinase |
References
- Kushnir, I.; Merimsky, O. The evolution in melanoma treatment as a reflection of precision-oriented medicine. Oncol. Lett. 2013, 5, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.M.; Heise, R.; Merk, H.F.; Abuzahra, F. Current and future directions in the treatment of metastatic malignant melanoma. Curr. Med. Chem. Anti-Cancer Agents 2003, 3, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Garbe, C.; Eigentler, T.K.; Keilholz, U.; Hauschild, A.; Kirkwood, J.M. Systematic review of medical treatment in melanoma: Current status and future prospects. Oncologist 2011, 16, 5–24. [Google Scholar] [CrossRef]
- Avril, M.; Aamdal, S.; Grob, J.; Hauschild, A.; Mohr, P.; Bonerandi, J.; Weichenthal, M.; Neuber, K.; Bieber, T.; Gilde, K.; et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: A phase III study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 1118–1125. [Google Scholar] [CrossRef]
- Bedikian, A.Y.; Millward, M.; Pehamberger, H.; Conry, R.; Gore, M.; Trefzer, U.; Pavlick, A.C.; DeConti, R.; Hersh, E.M.; Hersey, P.; et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: The Oblimersen Melanoma Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 4738–4745. [Google Scholar] [CrossRef]
- Schadendorf, D.; Ugurel, S.; Schuler-Thurner, B.; Nestle, F.O.; Enk, A.; Bröcker, E.-B.; Grabbe, S.; Rittgen, W.; Edler, L.; Sucker, A.; et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: A randomized phase III trial of the DC study group of the DeCOG. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2006, 17, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Falkson, C.I.; Ibrahim, J.; Kirkwood, J.M.; Coates, A.S.; Atkins, M.B.; Blum, R.H. Phase III trial of dacarbazine versus dacarbazine with interferon alpha-2b versus dacarbazine with tamoxifen versus dacarbazine with interferon alpha-2b and tamoxifen in patients with metastatic malignant melanoma: An Eastern Cooperative Oncology Group study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998, 16, 1743–1751. [Google Scholar]
- Kim, C.; Lee, C.W.; Kovacic, L.; Shah, A.; Klasa, R.; Savage, K.J. Long-term survival in patients with metastatic melanoma treated with DTIC or temozolomide. Oncologist 2010, 15, 765–771. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaufmann, R.; Spieth, K.; Leiter, U.; Mauch, C.; von den Driesch, P.; Vogt, T.; Linse, R.; Tilgen, W.; Schadendorf, D.; Becker, J.C.; et al. Temozolomide in combination with interferon-alfa versus temozolomide alone in patients with advanced metastatic melanoma: A randomized, phase III, multicenter study from the Dermatologic Cooperative Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 9001–9007. [Google Scholar] [CrossRef]
- Gradishar, W.J. Albumin-bound paclitaxel: A next-generation taxane. Expert Opin. Pharmacother. 2006, 7, 1041–1053. [Google Scholar] [CrossRef]
- Desai, N.; Trieu, V.; Yao, Z.; Louie, L.; Ci, S.; Yang, A.; Tao, C.; De, T.; Beals, B.; Dykes, D.; et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 1317–1324. [Google Scholar] [CrossRef]
- Hersh, E.M.; Del Vecchio, M.; Brown, M.P.; Kefford, R.; Loquai, C.; Testori, A.; Bhatia, S.; Gutzmer, R.; Conry, R.; Haydon, A.; et al. A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma. Ann. Oncol. 2015, 26, 2267–2274. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Lee, S.J.; Zhao, F.; Schuchter, L.M.; Flaherty, L.; Kefford, R.; Atkins, M.B.; Leming, P.; Kirkwood, J.M. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 373–379. [Google Scholar] [CrossRef]
- Bajetta, E.; Del Vecchio, M.; Nova, P.; Fusi, A.; Daponte, A.; Sertoli, M.R.; Queirolo, P.; Taveggia, P.; Bernengo, M.G.; Legha, S.S.; et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2006, 17, 571–577. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Melanoma: Cutaneous, Version 3.2024. Published 23 September 2024. Available online: https://www.nccn.org (accessed on 18 December 2024).
- Fujimura, T.; Kambayashi, Y.; Ohuchi, K.; Muto, Y.; Aiba, S. Treatment of Advanced Melanoma: Past, Present and Future. Life Basel Switz. 2020, 10, 208. [Google Scholar] [CrossRef]
- Du Bois, J.S.; Trehu, E.G.; Mier, J.W.; Shapiro, L.; Epstein, M.; Klempner, M.; Dinarello, C.; Kappler, K.; Ronayne, L.; Rand, W.; et al. Randomized placebo-controlled clinical trial of high-dose interleukin-2 in combination with a soluble p75 tumor necrosis factor receptor immunoglobulin G chimera in patients with advanced melanoma and renal cell carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.O.; Downey, S.G.; Klapper, J.A.; Yang, J.C.; Sherry, R.M.; Royal, R.E.; Kammula, U.S.; Hughes, M.S.; Restifo, N.P.; Levy, C.L.; et al. Treatment of Metastatic Melanoma Using Interleukin-2 Alone or in Conjunction with Vaccines. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 5610–5618. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.; Crittenden, M.; Seung, S.K.; Fountain, C.B.; Payne, R.; Chang, S.; Fleser, J.; Phillips, K.; Malkasian, I.; Dobrunick, L.B.; et al. Randomized phase II study of stereotactic body radiotherapy and interleukin-2 versus interleukin-2 in patients with metastatic melanoma. J. Immunother. Cancer 2020, 8, e000773. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Garbe, C.; Stolz, W.; Ellwanger, U.; Seiter, S.; Dummer, R.; Ugurel, S.; Sebastian, G.; Nashan, D.; Linse, R.; et al. Dacarbazine and interferon alpha with or without interleukin 2 in metastatic melanoma: A randomized phase III multicentre trial of the Dermatologic Cooperative Oncology Group (DeCOG). Br. J. Cancer 2001, 84, 1036–1042. [Google Scholar] [CrossRef]
- Young, A.M.; Marsden, J.; Goodman, A.; Burton, A.; Dunn, J.A. Prospective randomized comparison of dacarbazine (DTIC) versus DTIC plus interferon-alpha (IFN-alpha) in metastatic melanoma. Clin. Oncol. R. Coll. Radiol. 2001, 13, 458–465. [Google Scholar]
- Egberts, F.; Gutzmer, R.; Ugurel, S.; Becker, J.C.; Trefzer, U.; Degen, A.; Schenck, F.; Frey, L.; Wilhelm, T.; Hassel, J.C.; et al. Sorafenib and pegylated interferon-α2b in advanced metastatic melanoma: A multicenter phase II DeCOG trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1667–1674. [Google Scholar] [CrossRef]
- Takahara, Y.; Kan, T.; Teshima, Y.; Matsubara, D.; Takahagi, S.; Tanaka, A.; Hide, M. Malignant melanoma with in-transit metastases refractory to programmed cell death-1 inhibitor successfully treated with local interferon-β injections: A case report. Mol. Clin. Oncol. 2021, 15, 212. [Google Scholar] [CrossRef]
- Aoyagi, S.; Hata, H.; Homma, E.; Shimizu, H. Sequential local injection of low-dose interferon-beta for maintenance therapy in stage II and III melanoma: A single-institution matched case-control study. Oncology 2012, 82, 139–146. [Google Scholar] [CrossRef]
- Fujimura, T.; Okuyama, R.; Ohtani, T.; Ito, Y.; Haga, T.; Hashimoto, A.; Aiba, S. Perilesional treatment of metastatic melanoma with interferon-beta. Clin. Exp. Dermatol. 2009, 34, 793–799. [Google Scholar] [CrossRef]
- Kakizaki, A.; Fujimura, T.; Furudate, S.; Kambayashi, Y.; Yamauchi, T.; Yagita, H.; Aiba, S. Immunomodulatory effect of peritumorally administered interferon-beta on melanoma through tumor-associated macrophages. Oncoimmunology 2015, 4, e1047584. [Google Scholar] [CrossRef]
- Fujimura, T.; Hidaka, T.; Kambayashi, Y.; Furudate, S.; Kakizaki, A.; Tono, H.; Tsukada, A.; Haga, T.; Hashimoto, A.; Morimoto, R.; et al. Phase I study of nivolumab combined with IFN-β for patients with advanced melanoma. Oncotarget 2017, 8, 71181–71187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- The Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Smalley, K.S.M. Understanding melanoma signaling networks as the basis for molecular targeted therapy. J. Investig. Dermatol. 2010, 130, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, E.B.; Tsao, H. Melanoma: Clinical features and genomic insights. Cold Spring Harb Perspect Med. 2014, 4, a015388. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1315–1327. [Google Scholar] [CrossRef]
- Kroeze, S.G.; Fritz, C.; Hoyer, M.; Lo, S.S.; Ricardi, U.; Sahgal, A.; Stahel, R.; Stupp, R.; Guckenberger, M. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: A systematic review. Cancer Treat. Rev. 2017, 53, 25–37. [Google Scholar] [CrossRef]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib combined with vemurafenib in advanced BRAF (V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef]
- Eroglu, Z.; Ribas, A. Combination therapy with BRAF and MEK inhibitors for melanoma: Latest evidence and place in therapy. Ther. Adv. Med. Oncol. 2016, 8, 48–56. [Google Scholar] [CrossRef]
- Nazarian, R.; Shi, H.; Wang, Q.; Kong, X.; Koya, R.C.; Lee, H.; Chen, Z.; Lee, M.-K.; Attar, N.; Sazegar, H.; et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010, 468, 973–977. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.P.; Wang, K.Y.; Wilmott, J.S.; Holst, J.; Carlino, M.S.; Park, J.J.; Quek, C.; Wongchenko, M.; Yan, Y.; Mann, G.; et al. Distinct Molecular Profiles and Immunotherapy Treatment Outcomes of V600E and V600K BRAF-Mutant Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Zengarini, C.; Mussi, M.; Veronesi, G.; Alessandrini, A.; Lambertini, M.; Dika, E. BRAF V600K vs. BRAF V600E: A comparison of clinical and dermoscopic characteristics and response to immunotherapies and targeted therapies. Clin. Exp. Dermatol. 2022, 47, 1131–1136. [Google Scholar] [CrossRef]
- Goto, K.; Yoshikawa, S.; Takai, T.; Tachibana, K.; Honma, K.; Isei, T.; Kukita, Y.; Oishi, T. Clinicopathologic and genetic characterization of invasive melanoma with BRAF V600K mutation: A study of 16 cases. J. Cutan. Pathol. 2023, 50, 739–747. [Google Scholar] [CrossRef]
- Patel, M.; Eckburg, A.; Gantiwala, S.; Hart, Z.; Dein, J.; Lam, K.; Puri, N. Resistance to Molecularly Targeted Therapies in Melanoma. Cancers 2021, 13, 1115. [Google Scholar] [CrossRef]
- Welsh, S.J.; Rizos, H.; Scolyer, R.A.; Long, G.V. Resistance to combination BRAF and MEK inhibition in metastatic melanoma: Where to next? Eur. J. Cancer Oxf. Engl. 1990 2016, 62, 76–85. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Flaherty, K.T. Resistance to BRAF-targeted therapy in melanoma. Eur. J. Cancer 2013, 49, 1297–1304. [Google Scholar] [CrossRef]
- Haist, M.; Stege, H.; Kuske, M.; Bauer, J.; Klumpp, A.; Grabbe, S.; Bros, M. Combination of immune-checkpoint inhibitors and targeted therapies for melanoma therapy: The more, the better? Cancer Metastasis Rev. 2023, 42, 481–505. [Google Scholar] [CrossRef]
- Ribas, A.; Lawrence, D.; Atkinson, V.; Agarwal, S.; Miller, W.H.; Carlino, M.S.; Fisher, R.; Long, G.V.; Hodi, F.S.; Tsoi, J.; et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat. Med. 2019, 25, 936–940. [Google Scholar] [CrossRef]
- Estrela, J.M.; Salvador, R.; Marchio, P.; Valles, S.L.; Lopez-Blanch, R.; Rivera, P.; Benlloch, M.; Alcacer, J.; Perez, C.L.; Pellicer, J.A.; et al. Glucocorticoid receptor antagonism overcomes resistance to BRAF inhibition in BRAFV600E-mutated metastatic melanoma. Am. J. Cancer Res. 2019, 9, 2580–2598. [Google Scholar] [PubMed]
- Wang, V.E.; Xue, J.Y.; Frederick, D.T.; Cao, Y.; Lin, E.; Wilson, C.; Urisman, A.; Carbone, D.P.; Flaherty, K.T.; Bernards, R.; et al. Adaptive Resistance to Dual BRAF/MEK Inhibition in BRAF-Driven Tumors through Autocrine FGFR Pathway Activation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 7202–7217. [Google Scholar] [CrossRef] [PubMed]
- Theodosakis, N.; Micevic, G.; Langdon, C.G.; Ventura, A.; Means, R.; Stern, D.F.; Bosenberg, M.W. p90RSK Blockade Inhibits Dual BRAF and MEK Inhibitor-Resistant Melanoma by Targeting Protein Synthesis. J. Investig. Dermatol. 2017, 137, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Aida, S.; Sonobe, Y.; Tanimura, H.; Oikawa, N.; Yuhki, M.; Sakamoto, H.; Mizuno, T. MITF suppression improves the sensitivity of melanoma cells to a BRAF inhibitor. Cancer Lett. 2017, 409, 116–124. [Google Scholar] [CrossRef]
- Gong, H.Z.; Zheng, H.Y.; Li, J. The clinical significance of KIT mutations in melanoma: A meta-analysis. Melanoma Res. 2018, 28, 259–270. [Google Scholar] [CrossRef]
- Millán-Esteban, D.; García-Casado, Z.; Manrique-Silva, E.; Virós, A.; Kumar, R.; Furney, S.; López-Guerrero, J.A.; Requena, C.; Bañuls, J.; Traves, V.; et al. Distribution and clinical role of KIT gene mutations in melanoma according to subtype: A study of 492 Spanish patients. Eur. J. Dermatol. EJD 2021, 31, 830–838. [Google Scholar] [CrossRef]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Harlow, A.; Cruz, F.; Azar, S.; et al. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef]
- Steeb, T.; Wessely, A.; Petzold, A.; Kohl, C.; Erdmann, M.; Berking, C.; Heppt, M.V. c-Kit inhibitors for unresectable or metastatic mucosal, acral or chronically sun-damaged melanoma: A systematic review and one-arm meta-analysis. Eur. J. Cancer 2021, 157, 348–357. [Google Scholar] [CrossRef]
- Kim, K.B.; Alrwas, A. Treatment of KIT-mutated metastatic mucosal melanoma. Chin. Clin. Oncol. 2014, 3, 35. [Google Scholar]
- Pham, D.D.M.; Guhan, S.; Tsao, H. KIT and Melanoma: Biological Insights and Clinical Implications. Yonsei Med. J. 2020, 61, 562–571. [Google Scholar] [CrossRef]
- Hodi, F.S.; Corless, C.L.; Giobbie-Hurder, A.; Fletcher, J.A.; Zhu, M.; Marino-Enriquez, A.; Fisher, D.E. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gu, Z.; Wu, J.; Huang, X.; Zhou, R.; Shi, C.; Tao, W.; Wang, L.; Wang, Y.; Zhou, G.; et al. Repurposing Ponatinib as a Potent Agent against KIT Mutant Melanomas. Theranostics 2019, 9, 1952–1964. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Puzanov, I. Treatment of NRAS-mutant melanoma. Curr. Treat. Options Oncol. 2015, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Sullivan, R.J. NRAS mutant melanoma: An overview for the clinician for melanoma management. Melanoma Manag. 2016, 3, 47–59. [Google Scholar] [CrossRef]
- Kelleher, F.C.; McArthur, G.A. Targeting NRAS in melanoma. Cancer J. Sudbury Mass. 2012, 18, 132–136. [Google Scholar] [CrossRef]
- Dummer, R.; Schadendorf, D.; Ascierto, P.A.; Arance, A.; Dutriaux, C.; Di Giacomo, A.M.; Rutkowski, P.; Del Vecchio, M.; Gutzmer, R.; Mandala, M.; et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 435–445. [Google Scholar] [CrossRef]
- Muñoz-Couselo, E.; Adelantado, E.Z.; Ortiz, C.; García, J.S.; Perez-Garcia, J. NRAS-mutant melanoma: Current challenges and future prospect. OncoTargets Ther. 2017, 10, 3941–3947. [Google Scholar] [CrossRef]
- Zhao, J.; Galvez, C.; Beckermann, K.E.; Johnson, D.B.; Sosman, J.A. Novel insights into the pathogenesis and treatment of NRAS mutant melanoma. Expert Rev. Precis. Med. Drug Dev. 2021, 6, 281–294. [Google Scholar] [CrossRef]
- Randic, T.; Kozar, I.; Margue, C.; Utikal, J.; Kreis, S. NRAS mutant melanoma: Towards better therapies. Cancer Treat. Rev. 2021, 99, 102238. [Google Scholar] [CrossRef]
- Vu, H.L.; Aplin, A.E. Targeting mutant NRAS signaling pathways in melanoma. Pharmacol. Res. 2016, 107, 111–116. [Google Scholar] [CrossRef]
- Bergethon, K.; Shaw, A.T.; Ou, S.-H.I.; Katayama, R.; Lovly, C.M.; McDonald, N.T.; Massion, P.P.; Siwak-Tapp, C.; Gonzalez, A.; Fang, R.; et al. ROS1 rearrangements define a unique molecular class of lung cancers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.D.; Doebele, R.C. Molecular pathways: ROS1 fusion proteins in cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 4040–4045. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Ou, S.H.; Bang, Y.J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M.G.; Shaw, A.T.; de Braud, F.; Rolfo, C.; Ahn, M.-J.; Wolf, J.; et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 261–270. [Google Scholar] [CrossRef]
- Ma, S.-C.; Zhu, H.-B.; Wang, J.; Zhang, Y.-P.; Guo, X.-J.; Long, L.-L.; Guo, Z.-Q.; Wu, D.-H.; Dong, Z.-Y.; Bai, X. De Novo Mutation in Non-Tyrosine Kinase Domain of ROS1 as a Potential Predictor of Immune Checkpoint Inhibitors in Melanoma. Front. Oncol. 2021, 11, 666145. [Google Scholar] [CrossRef]
- Couts, K.L.; McCoach, C.E.; Murphy, D.; Christiansen, J.; Turner, J.; Lewis, K.D.; Robinson, W.A.; Doebele, R.C. Acral Lentiginous Melanoma Harboring a ROS1 Gene Fusion with Clinical Response to Entrectinib. JCO Precis. Oncol. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Y.; Zhou, Y.; Ji, Q.; Qian, W.; Jia, D.; Jin, G.; Qi, Y.; Li, X.; Li, N.; et al. Case report: Complete remission with crizotinib in ROS1 fusion-positive sinonasal mucosal melanoma. Front. Oncol. 2022, 12, 942258. [Google Scholar] [CrossRef]
- Lezcano, C.; Shoushtari, A.N.; Ariyan, C.; Hollmann, T.J.; Busam, K.J. Primary and Metastatic Melanoma with NTRK Fusions. Am. J. Surg. Pathol. 2018, 42, 1052–1058. [Google Scholar] [CrossRef]
- Forschner, A.; Forchhammer, S.; Bonzheim, I. NTRK gene fusions in melanoma: Detection, prevalence and potential therapeutic implications. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2020, 18, 1387–1392. [Google Scholar] [CrossRef]
- Yan, J.; Deng, L.; Yu, J.; Wu, X.; Wang, S.; Cang, S. Multi-cohort analysis identifies somatic NTRK mutations as a biomarker for immune checkpoint inhibitor use in cutaneous melanoma. Clin. Transl. Med. 2023, 13, e1478. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Kheder, E.S.; Hong, D.S. Emerging Targeted Therapy for Tumors with NTRK Fusion Proteins. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5807–5814. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Siena, S.; Ou, S.-H.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; Dubois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Linsley, P.S.; Greene, J.L.; Tan, P.; Bradshaw, J.; Ledbetter, J.A.; Anasetti, C.; Damle, N.K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J. Exp. Med. 1992, 176, 1595–1604. [Google Scholar] [CrossRef]
- Walunas, T.L.; Bakker, C.Y.; Bluestone, J.A. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 1996, 183, 2541–2550. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Yamazaki, N.; Uhara, H.; Fukushima, S.; Uchi, H.; Shibagaki, N.; Kiyohara, Y.; Tsutsumida, A.; Namikawa, K.; Okuyama, R.; Otsuka, Y.; et al. Phase II study of the immune-checkpoint inhibitor ipilimumab plus dacarbazine in Japanese patients with previously untreated, unresectable or metastatic melanoma. Cancer Chemother. Pharmacol. 2015, 76, 969–975. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Carlino, M.S.; McNeil, C.; Ribas, A.; Grob, J.-J.; Schachter, J.; Nyakas, M.; Kee, D.; Petrella, T.M.; Blaustein, A.; et al. Seven-Year Follow-Up of the Phase III KEYNOTE-006 Study: Pembrolizumab Versus Ipilimumab in Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 3998–4003. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Kitano, S.; Nakayama, T.; Yamashita, M. Biomarkers for Immune Checkpoint Inhibitors in Melanoma. Front. Oncol. 2018, 8, 270. [Google Scholar] [CrossRef]
- Di Donato, M.; Cristiani, C.M.; Capone, M.; Garofalo, C.; Madonna, G.; Passacatini, L.C.; Ottaviano, M.; Ascierto, P.A.; Auricchio, F.; Carbone, E.; et al. Role of the androgen receptor in melanoma aggressiveness. Cell Death Dis. 2025, 16, 34. [Google Scholar] [CrossRef]
- Kleffel, S.; Posch, C.; Barthel, S.R.; Mueller, H.; Schlapbach, C.; Guenova, E.; Elco, C.P.; Lee, N.; Juneja, V.R.; Zhan, Q.; et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell 2015, 162, 1242–1256. [Google Scholar] [CrossRef]
- Workman, C.J.; Cauley, L.S.; Kim, I.J.; Blackman, M.A.; Woodland, D.L.; Vignali, D.A.A. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J. Immunol. 2004, 172, 5450–5455. [Google Scholar] [CrossRef] [PubMed]
- Baixeras, E.; Huard, B.; Miossec, C.; Jitsukawa, S.; Martin, M.; Hercend, T.; Auffray, C.; Triebel, F.; Piatier-Tonneau, D. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J. Exp. Med. 1992, 176, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Huard, B.; Prigent, P.; Tournier, M.; Bruniquel, D.; Triebel, F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol. 1995, 25, 2718–2721. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Ruby, C.E.; Hughes, T.; Slingluff, C.L. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer 2014, 2, 11. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with oncolytic viruses: Progress and challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination with Ipilimumab Versus Ipilimumab Alone in Patients with Advanced, Unresectable Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1658–1667. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Chi, Z.; Li, S.; Sheng, X.; Si, L.; Cui, C.; Han, M.; Guo, J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: A study of 522 consecutive cases. BMC Cancer 2011, 11, 85. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Yoshikawa, S.; Minagawa, A.; Takenouchi, T.; Yokota, K.; Uchi, H.; Noma, N.; Nakamura, Y.; Asai, J.; Kato, J.; et al. Clinical and histopathological characteristics and survival analysis of 4594 Japanese patients with melanoma. Cancer Med. 2019, 8, 2146–2156. [Google Scholar] [CrossRef]
- Roh, M.R.; Kim, J.; Chung, K.Y. Treatment and outcomes of melanoma in acral location in Korean patients. Yonsei Med. J. 2010, 51, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Kong, Y.; Chi, Z.; Sheng, X.; Cui, C.; Wang, X.; Mao, L.; Tang, B.; Li, S.; Lian, B.; et al. MAPK Pathway and TERT Promoter Gene Mutation Pattern and Its Prognostic Value in Melanoma Patients: A Retrospective Study of 2,793 Cases. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6120–6127. [Google Scholar] [CrossRef] [PubMed]
- Tomizuka, T.; Namikawa, K.; Higashi, T. Characteristics of melanoma in Japan: A nationwide registry analysis 2011–2013. Melanoma Res. 2017, 27, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Pt, B.; Am, G.; Ml, M.; Ma, T. Acral lentiginous melanoma: Incidence and survival patterns in the United States, 1986–2005. Arch. Dermatol. 2009, 145, 427–434. [Google Scholar]
- Bishop, K.D.; Olszewski, A.J. Epidemiology and survival outcomes of ocular and mucosal melanomas: A population-based analysis. Int. J. Cancer 2014, 134, 2961–2971. [Google Scholar] [CrossRef]
- Mori, T.; Izumi, T.; Doi, R.; Kamimura, A.; Takai, S.; Teramoto, Y.; Nakamura, Y. Immune checkpoint inhibitor-based therapy for advanced acral and mucosal melanoma. Exp. Dermatol. 2023, 32, 276–289. [Google Scholar] [CrossRef]
- Ribas, A.; Hamid, O.; Daud, A.; Hodi, F.S.; Wolchok, J.D.; Kefford, R.; Joshua, A.M.; Patnaik, A.; Hwu, W.-J.; Weber, J.S.; et al. Association of Pembrolizumab with Tumor Response and Survival Among Patients with Advanced Melanoma. JAMA 2016, 315, 1600–1609. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Rawson, R.V.; Johansson, P.A.; Hayward, N.K.; Waddell, N.; Patch, A.-M.; Lo, S.; Pearson, J.V.; Thompson, J.F.; Mann, G.J.; Scolyer, R.A.; et al. Unexpected UVR and non-UVR mutation burden in some acral and cutaneous melanomas. Lab. Investig. J. Tech. Methods Pathol. 2017, 97, 130–145. [Google Scholar] [CrossRef]
- Park, S.E.; Park, K.; Lee, E.; Kim, J.-Y.; Ahn, J.S.; Im, Y.-H.; Lee, C.; Jung, H.; Cho, S.Y.; Park, W.-Y.; et al. Clinical implication of tumor mutational burden in patients with HER2-positive refractory metastatic breast cancer. Oncoimmunology 2018, 7, e1466768. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, J.; Wen, X.; Zhu, B.; Liu, W.; Wang, J.; Jiang, H.; Ding, Y.; Li, D.; Zhang, X. Next-generation sequencing in advanced Chinese melanoma reveals therapeutic targets and prognostic biomarkers for immunotherapy. Sci. Rep. 2022, 12, 9559. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Idogawa, M.; Kato, J.; Kiniwa, Y.; Horimoto, K.; Sato, S.; Sawada, M.; Tange, S.; Okura, M.; Okuyama, R.; et al. Genetic Characteristics of Cutaneous, Acral, and Mucosal Melanoma in Japan. Cancer Med. 2024, 13, e70360. [Google Scholar] [CrossRef] [PubMed]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Nakamura, Y.; Namikawa, K.; Kiniwa, Y.; Kato, H.; Yamasaki, O.; Yoshikawa, S.; Maekawa, T.; Matsushita, S.; Takenouchi, T.; Inozume, T.; et al. Efficacy comparison between anti-PD-1 antibody monotherapy and anti-PD-1 plus anti-CTLA-4 combination therapy as first-line immunotherapy for advanced acral melanoma: A retrospective, multicenter study of 254 Japanese patients. Eur. J. Cancer 2022, 176, 78–87. [Google Scholar] [CrossRef]
- Bhave, P.; Ahmed, T.; Lo, S.N.; Shoushtari, A.; Zaremba, A.; Versluis, J.M.; Mangana, J.; Weichenthal, M.; Si, L.; Lesimple, T.; et al. Efficacy of anti-PD-1 and ipilimumab alone or in combination in acral melanoma. J. Immunother. Cancer 2022, 10, e004668. [Google Scholar] [CrossRef]
- Namikawa, K.; Ito, T.; Yoshikawa, S.; Yoshino, K.; Kiniwa, Y.; Ohe, S.; Isei, T.; Takenouchi, T.; Kato, H.; Mizuhashi, S.; et al. Systemic therapy for Asian patients with advanced BRAF V600-mutant melanoma in a real-world setting: A multi-center retrospective study in Japan (B-CHECK-RWD study). Cancer Med. 2023, 12, 17967–17980. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lee, S.J.; Chmielowski, B.; Tarhini, A.A.; Cohen, G.I.; Truong, T.-G.; Moon, H.H.; Davar, D.; O’Rourke, M.; Stephenson, J.J.; et al. Combination Dabrafenib and Trametinib Versus Combination Nivolumab and Ipilimumab for Patients with Advanced BRAF-Mutant Melanoma: The DREAMseq Trial-ECOG-ACRIN EA6134. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 186–197. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Mandalà, M.; Ferrucci, P.F.; Guidoboni, M.; Rutkowski, P.; Ferraresi, V.; Arance, A.; Guida, M.; Maiello, E.; Gogas, H.; et al. Sequencing of Ipilimumab Plus Nivolumab and Encorafenib Plus Binimetinib for Untreated BRAF-Mutated Metastatic Melanoma (SECOMBIT): A Randomized, Three-Arm, Open-Label Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 212–221. [Google Scholar] [CrossRef]
- Wang, M.; Fukushima, S.; Sheen, Y.-S.; Ramelyte, E.; Cruz-Pacheco, N.; Shi, C.; Liu, S.; Banik, I.; Aquino, J.D.; Acosta, M.S.; et al. The genetic evolution of acral melanoma. Nat. Commun. 2024, 15, 6146. [Google Scholar] [CrossRef]
- Chesney, J.; Lewis, K.D.; Kluger, H.; Hamid, O.; Whitman, E.; Thomas, S.; Wermke, M.; Cusnir, M.; Domingo-Musibay, E.; Phan, G.Q.; et al. Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies: Pooled analysis of consecutive cohorts of the C-144-01 study. J. Immunother. Cancer 2022, 10, e005755. [Google Scholar] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Shah, P.D.; Huang, A.C.; Xu, X.; Orlowski, R.; Amaravadi, R.K.; Schuchter, L.M.; Zhang, P.; Tchou, J.; Matlawski, T.; Cervini, A.; et al. Phase I Trial of Autologous RNA-electroporated cMET-directed CAR T Cells Administered Intravenously in Patients with Melanoma and Breast Carcinoma. Cancer Res. Commun. 2023, 3, 821–829. [Google Scholar] [CrossRef]
- Murisier, F.; Beermann, F. Genetics of pigment cells: Lessons from the tyrosinase gene family. Histol. Histopathol. 2006, 21, 567–578. [Google Scholar]
- Murisier, F.; Guichard, S.; Beermann, F. A conserved transcriptional enhancer that specifies Tyrp1 expression to melanocytes. Dev. Biol. 2006, 298, 644–655. [Google Scholar] [CrossRef][Green Version]
- Jilani, S.; Saco, J.D.; Mugarza, E.; Pujol-Morcillo, A.; Chokry, J.; Ng, C.; Abril-Rodriguez, G.; Berger-Manerio, D.; Pant, A.; Hu, J.; et al. CAR-T cell therapy targeting surface expression of TYRP1 to treat cutaneous and rare melanoma subtypes. Nat. Commun. 2024, 15, 1244. [Google Scholar] [CrossRef]
- Ozbay Kurt, F.G.; Lasser, S.; Arkhypov, I.; Utikal, J.; Umansky, V. Enhancing immunotherapy response in melanoma: Myeloid-derived suppressor cells as a therapeutic target. J Clin Investig. 2023, 133, e170762. [Google Scholar] [CrossRef]
- Procureur, A.; Simonaggio, A.; Bibault, J.E.; Oudard, S.; Vano, Y.A. Enhance the Immune Checkpoint Inhibitors Efficacy with Radiotherapy Induced Immunogenic Cell Death: A Comprehensive Review and Latest Developments. Cancers 2021, 13, 678. [Google Scholar] [CrossRef] [PubMed]
- Knisely, J.P.S.; Yu, J.B.; Flanigan, J.; Sznol, M.; Kluger, H.M.; Chiang, V.L.S. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J. Neurosurg. 2012, 117, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kiess, A.P.; Wolchok, J.D.; Barker, C.A.; Postow, M.A.; Tabar, V.; Huse, J.T.; Chan, T.A.; Yamada, Y.; Beal, K. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: Safety profile and efficacy of combined treatment. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Bristol-Myers Squibb. A Randomized, Double-Blind Phase 2/3 Study of Relatlimab Combined with Nivolumab Versus Nivolumab in Participants with Previously Untreated Metastatic or Unresectable Melanoma [Internet]. Clinicaltrials.gov; March 2025. Report No.: NCT03470922. Available online: https://clinicaltrials.gov/study/NCT03470922 (accessed on 27 April 2025).
- Abramson Cancer Center at Penn Medicine. A Randomized Phase 2 Trial of Ipilumumab and Nivolumab with or Without Hypofractionated Radiotherapy in Patients with Metastatic Melanoma [Internet]. Clinicaltrials.gov; February 2025. Report No.: NCT03646617. Available online: https://clinicaltrials.gov/study/NCT03646617 (accessed on 25 April 2025).
- Khan, M.K. Pilot Study of Pembrolizumab and Stereotactic Radio-Surgery (SRS) for Patients with Melanoma or Non-Small Cell Lung Cancer (NSCLC) Brain Metastases (BM) [Internet]. Clinicaltrials.gov; May 2024. Report No.: NCT02858869. Available online: https://clinicaltrials.gov/study/NCT02858869 (accessed on 25 April 2025).
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. A Pilot Study of Stereotactic Radiosurgery Combined with Nivolumab in Patients with Newly Diagnosed Melanoma Metastases in the Brain and Spine [Internet]. Clinicaltrials.gov; November 2021. Report No.: NCT02716948. Available online: https://clinicaltrials.gov/study/NCT02716948 (accessed on 25 April 2025).
- Gérard, A.; Doyen, J.; Cremoni, M.; Bailly, L.; Zorzi, K.; Ruetsch-Chelli, C.; Brglez, V.; Picard-Gauci, A.; Troin, L.; Esnault, V.L.; et al. Baseline and early functional immune response is associated with subsequent clinical outcomes of PD-1 inhibition therapy in metastatic melanoma patients. J. Immunother. Cancer 2021, 9, e002512. [Google Scholar] [CrossRef]
- Centre Hospitalier Universitaire de Nice. Nivolumab in Combination with High Dose Radiotherapy at Varied Tumor Sites in Advanced Melanoma and no Prior Antitumoral Treatment [Internet]. Clinicaltrials.gov; November 2023. Report No.: NCT02799901. Available online: https://clinicaltrials.gov/study/NCT02799901 (accessed on 27 April 2025).
- Spaas, M.; Sundahl, N.; Kruse, V.; Rottey, S.; De Maeseneer, D.; Duprez, F.; Lievens, Y.; Surmont, V.; Brochez, L.; Reynders, D.; et al. Checkpoint Inhibitors in Combination with Stereotactic Body Radiotherapy in Patients with Advanced Solid Tumors: The CHEERS Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1205–1213. [Google Scholar] [CrossRef]
- University Hospital, Ghent. CHEckpoint Inhibition in Combination with an Immunoboost of External Beam Radiotherapy in Solid Tumors: CHEERS-Trial [Internet]. Clinicaltrials.gov; January 2024. Report No.: NCT03511391. Available online: https://clinicaltrials.gov/study/NCT03511391 (accessed on 25 April 2025).
- Miller, W. A Phase II Study of Pembrolizumab with Carboplatin/Paclitaxel in Patients with Metastatic Melanoma [Internet]. clinicaltrials.gov; February 2021. Report No.: NCT02617849. Available online: https://clinicaltrials.gov/study/NCT02617849 (accessed on 27 April 2025).
- Robinson, C.; Xu, M.M.; Nair, S.K.; Beasley, G.M.; Rhodin, K.E. Oncolytic viruses in melanoma. Front. Biosci. Landmark Ed. 2022, 27, 63. [Google Scholar] [CrossRef]
- Ribas, A.; Chesney, J.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. 1037O MASTERKEY-265: A phase III, randomized, placebo (Pbo)-controlled study of talimogene laherparepvec (T) plus pembrolizumab (P) for unresectable stage IIIB–IVM1c melanoma (MEL). Ann. Oncol. 2021, 32, S868–S869. [Google Scholar] [CrossRef]
- Wong, M.K.; Sacco, J.J.; Robert, C.; Michels, J.; Bowles, T.L.; In, G.K.; Tsai, K.K.; Lebbe, C.; Gaudy-Marqueste, C.; Couselo, E.M.; et al. Efficacy and safety of RP1 combined with nivolumab in patients with anti–PD-1–failed melanoma from the IGNYTE clinical trial. J. Clin. Oncol. 2024, 42 (Suppl S16), 9517. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Long, G.V.; Ferrucci, P.F.; Khattak, A.; Meniawy, T.M.; Ott, P.A.; Chisamore, M.; Trolle, T.; Hyseni, A.; Heegaard, E. KEYNOTE—D36: Personalized immunotherapy with a neoepitope vaccine, EVX-01 and pembrolizumab in advanced melanoma. Future Oncol. 2022, 18, 3473–3480. [Google Scholar] [CrossRef]
- Iovance Biotherapeutics, Inc. A Phase 3, Multicenter, Randomized, Open-label, Parallel Group, Treatment Study to Assess the Efficacy and Safety of the Lifileucel (LN-144, Autologous Tumor Infiltrating Lymphocytes [TIL]) Regimen in Combination with Pembrolizumab Compared with Pembrolizumab Monotherapy in Participants with Untreated, Unresectable or Metastatic Melanoma [Internet]. Clinicaltrials.gov; April 2025. Report No.: NCT05727904. Available online: https://clinicaltrials.gov/study/NCT05727904 (accessed on 22 April 2025).
- University of California, San Francisco. Immunotherapy with or Without Radiation Therapy in Treating Patients with Recurrent or Second Primary Head and Neck Cancer [Internet]. Clinicaltrials.gov; February 2024. Report No.: NCT03340129. Available online: https://clinicaltrials.gov/study/NCT03340129 (accessed on 25 April 2025).
- A Phase 1b/3, Multicenter, Trial of Talimogene Laherparepvec in Combination with Pembrolizumab (MK-3475) for Treatment of Unresectable Stage IIIB to IVM1c Melanoma (MASTERKEY-265) [Internet]. ClinicalTrials.gov; April 2021. Report No.: NCT02263508. Available online: https://clinicaltrials.gov/study/NCT02263508 (accessed on 25 April 2025).
- AstraZeneca. Study of Durvalumab and/or Tremelimumab in Combination with Radiotherapy in Head and Neck Squamous Cell Carcinoma (HNSCC) [Internet]. Clinicaltrials.gov; April 2024. Report No.: NCT03897881. Available online: https://clinicaltrials.gov/study/NCT03897881 (accessed on 25 April 2025).
- Garofalo, C.; De Marco, C.; Cristiani, C.M. NK Cells in the Tumor Microenvironment as New Potential Players Mediating Chemotherapy Effects in Metastatic Melanoma. Front. Oncol. 2021, 11, 754541. [Google Scholar] [CrossRef] [PubMed]
- Cristiani, C.M.; Turdo, A.; Ventura, V.; Apuzzo, T.; Capone, M.; Madonna, G.; Mallardo, D.; Garofalo, C.; Giovannone, E.D.; Grimaldi, A.M.; et al. Accumulation of Circulating CCR7+ Natural Killer Cells Marks Melanoma Evolution and Reveals a CCL19-Dependent Metastatic Pathway. Cancer Immunol. Res. 2019, 7, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Dobos, J.; Mohos, A.; Tóvári, J.; Rásó, E.; Lőrincz, T.; Zádori, G.; Tímár, J.; Ladányi, A. Sex-dependent liver colonization of human melanoma in SCID mice--role of host defense mechanisms. Clin. Exp. Metastasis 2013, 30, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Artomov, M.; Goggins, W.; Daly, M.; Tsao, H. Gender Disparity and Mutation Burden in Metastatic Melanoma. J. Natl. Cancer Inst. 2015, 107, djv221. [Google Scholar] [CrossRef]
- Joosse, A.; de Vries, E.; Eckel, R.; Nijsten, T.; Eggermont, A.M.; Hölzel, D.; Coebergh, J.W.W.; Engel, J. Gender differences in melanoma survival: Female patients have a decreased risk of metastasis. J. Investig. Dermatol. 2011, 131, 719–726. [Google Scholar] [CrossRef]
- Robinson, J.K.; Mallett, K.A.; Turrisi, R.; Stapleton, J. Engaging patients and their partners in preventive health behaviors: The physician factor. Arch. Dermatol. 2009, 145, 469–473. [Google Scholar] [CrossRef][Green Version]
- Swetter, S.M.; Johnson, T.M.; Miller, D.R.; Layton, C.J.; Brooks, K.R.; Geller, A.C. Melanoma in middle-aged and older men: A multi-institutional survey study of factors related to tumor thickness. Arch. Dermatol. 2009, 145, 397–404. [Google Scholar] [CrossRef]
- Nebhan, C.A.; Johnson, D.B. Predictive biomarkers of response to immune checkpoint inhibitors in melanoma. Expert Rev. Anticancer. Ther. 2020, 20, 137–145. [Google Scholar] [CrossRef]
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef]
- Ning, B.; Liu, Y.; Wang, M.; Li, Y.; Xu, T.; Wei, Y. The Predictive Value of Tumor Mutation Burden on Clinical Efficacy of Immune Checkpoint Inhibitors in Melanoma: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 748674. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Sarda, C.; Pala, V.; Ribero, S.; Quaglino, P. Prognostic biomarkers in melanoma: A 2023 update from clinical trials in different therapeutic scenarios. Expert Rev. Mol. Diagn. 2024, 24, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, D.; Ramsay, L.; Lajoie, M.; Anderson-Trocme, L.; Lingrand, M.; Berry, D.; Perus, L.J.; Wei, Y.; Moraes, C.; Alkallas, R.; et al. Spatially mapping the immune landscape of melanoma using imaging mass cytometry. Sci. Immunol. 2022, 7, eabi5072. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.; Giraldo, N.A.; Green, B.F.; Cottrell, T.R.; Stein, J.E.; Engle, E.L.; Xu, H.; Ogurtsova, A.; Roberts, C.; Wang, D.; et al. Analysis of multispectral imaging with the AstroPath platform informs efficacy of PD-1 blockade. Science 2021, 372, eaba2609. [Google Scholar] [CrossRef]
- Laino, A.S.; Woods, D.; Vassallo, M.; Qian, X.; Tang, H.; Wind-Rotolo, M.; Weber, J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 2020, 8, e000842. [Google Scholar] [CrossRef]
- Reschke, R.; Enk, A.H.; Hassel, J.C. Prognostic Biomarkers in Evolving Melanoma Immunotherapy. Am. J. Clin. Dermatol. 2025, 26, 213–223. [Google Scholar] [CrossRef]
- Imani, S.; Roozitalab, G.; Emadi, M.; Moradi, A.; Behzadi, P.; Jabbarzadeh Kaboli, P. The evolution of BRAF-targeted therapies in melanoma: Overcoming hurdles and unleashing novel strategies. Front. Oncol. 2024, 14, 1504142. [Google Scholar] [CrossRef]
- Revythis, A.; Shah, S.; Kutka, M.; Moschetta, M.; Ozturk, M.A.; Pappas-Gogos, G.; Ioannidou, E.; Sheriff, M.; Rassy, E.; Boussios, S. Unraveling the Wide Spectrum of Melanoma Biomarkers. Diagnostics 2021, 11, 1341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).