Advances in MXene-Based Electrochemical Sensors for Multiplexed Detection in Biofluids
Abstract
1. Introduction
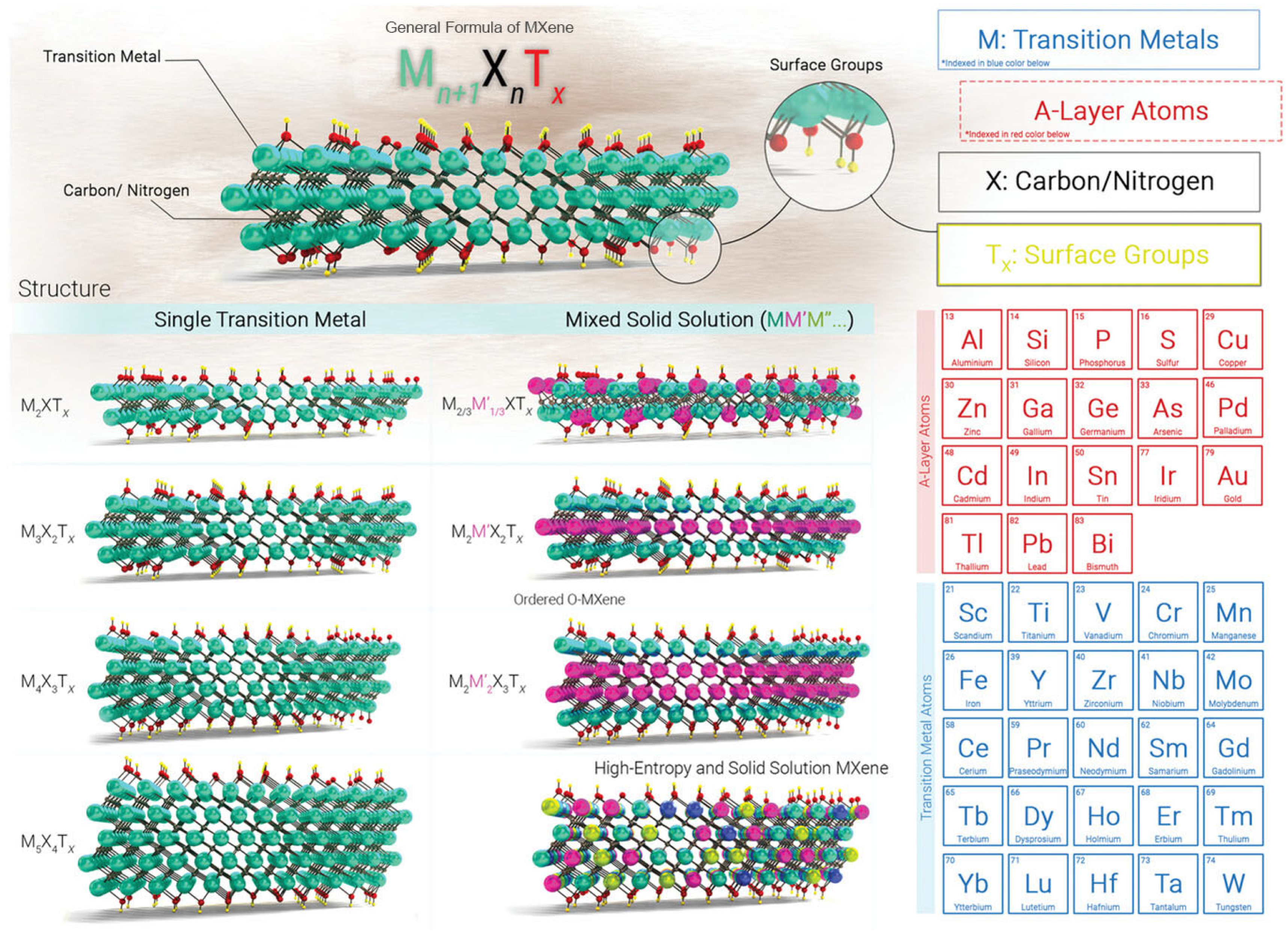
2. Brief Introduction to MXene
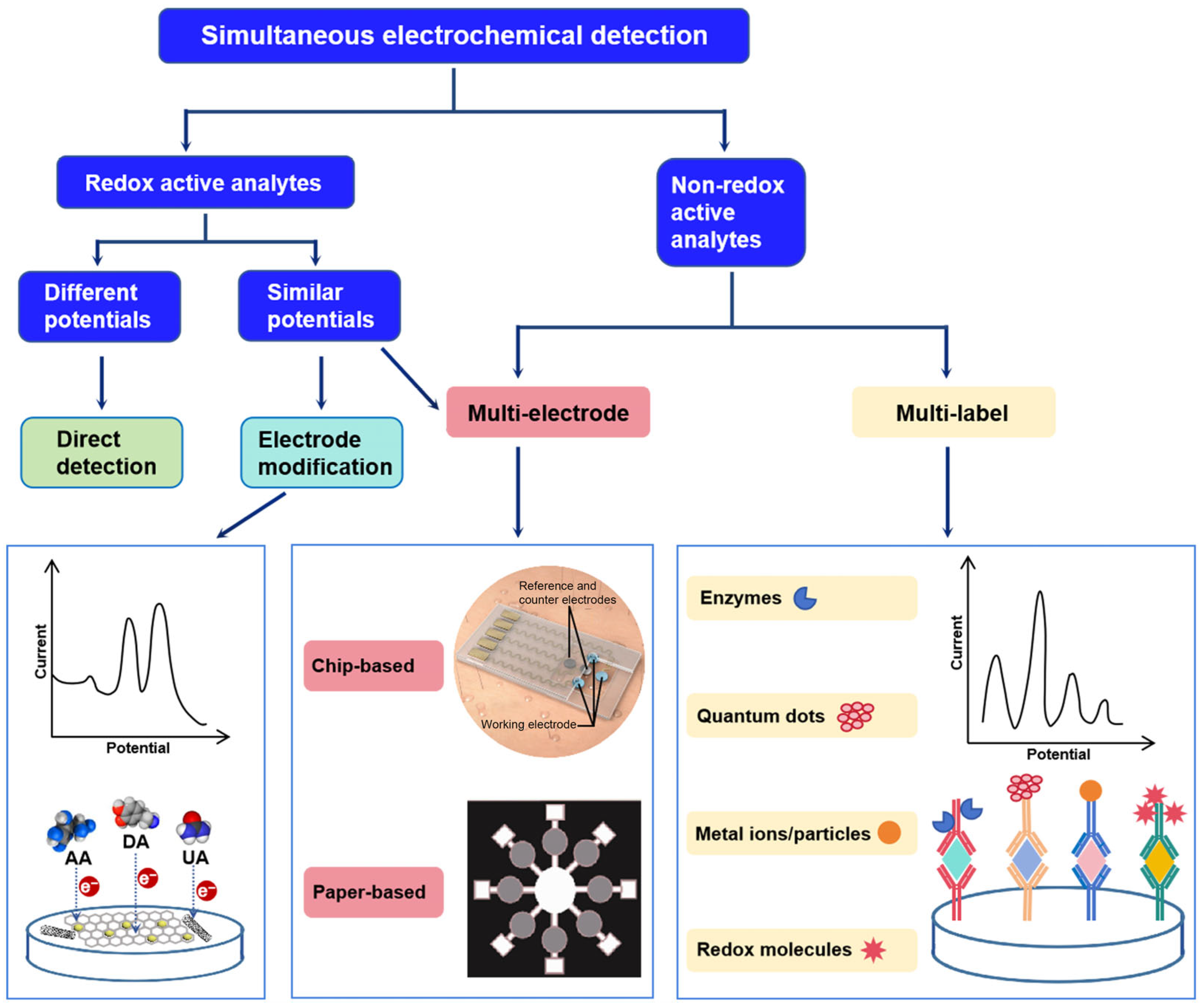
3. Strategies for Discriminating Different Target Signals in Simultaneous Electrochemical Detection
4. MXene-Based Electrochemical Sensors for Multiplexed Detection in Biofluids
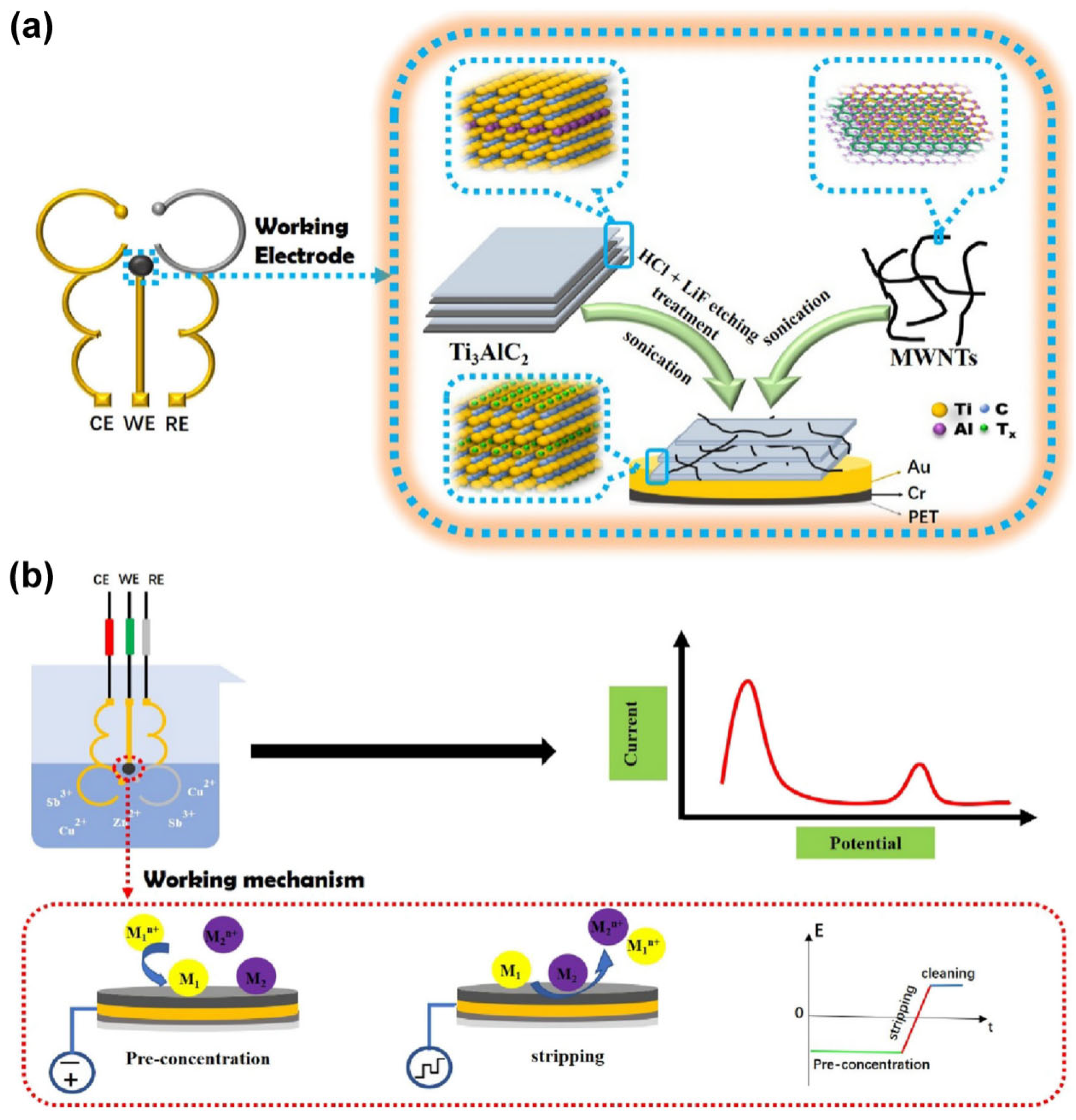
4.1. Heavy Metal Ions
4.2. Biomarkers
4.2.1. Small Molecules
4.2.2. Macromolecules
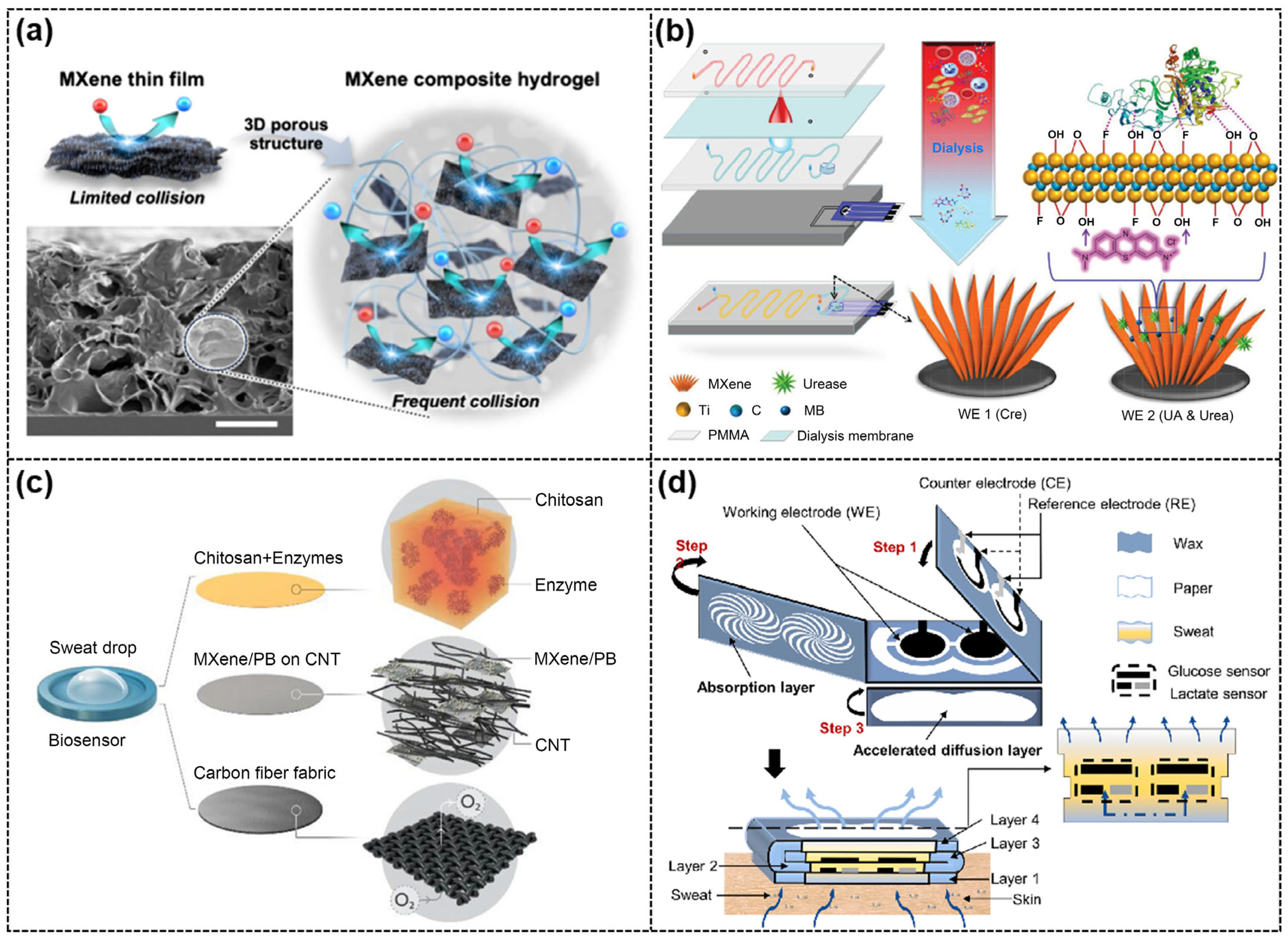
4.2.3. Multi-Type Analytes
4.3. Drugs
5. Conclusions and Outlook
- (1)
- MXene is mainly synthesized through HF etching and in situ HF etching, which results in the formation of -F, -O, and -OH surface groups. These non-uniform terminations are not favorable for the effective immobilization of biorecognition elements. Furthermore, F-terminated MXene exhibit low electrical conductivity [103]. In the future, some strategies can be employed to modulate the surface groups of synthesized MXene to enhance its electrochemical properties [104]. In addition, other fluoride-free synthesis methods for MXene can be explored.
- (2)
- The existing studies focus on the multiplexed detection of sweat, blood, urine, and saliva, while other important biofluids, such as tears and interstitial fluid, have not yet been explored. In the future, detection methods for other biofluids should be developed to extend the application of MXene-based, multiplexed electrochemical sensors.
- (3)
- The current targets mainly cover electrolytes, metabolites, neurotransmitters, proteins, nucleic acids, etc., while the detection of pathogens (e.g., bacteria and viruses) is lacking. In the future, the MXene-based electrochemical sensors for the simultaneous detection of pathogens in biofluids can be further developed.
- (4)
- Real-time monitoring of biofluids is currently focused on SPE-based wearable sensors for sweat detection. However, real-time monitoring of other biofluids, such as interstitial fluid and blood, remains an important area for breakthrough. In the future, wearable microneedle arrays or even implantable sensors are expected to be developed for real-time monitoring of other biofluids [105].
- (5)
- The existing sensors are mostly confined to the simultaneous detection of two to three targets, while the high-throughput detection of more than five targets remains to be achieved. In the future, higher throughput detection can be realized through the fabrication of microelectrode arrays or integration with microfluidic technologies.
- (6)
- The accurate analysis of complex high-throughput data from signal interference in high-throughput electrochemical detection is a great challenge, and the introduction of machine learning techniques provides an effective solution to this problem [106]. In the future, MXene-based, high-throughput electrochemical platforms can be developed in combination with machine learning techniques for more accurate biofluid detection.
- (7)
- Integration of MXene-based, electrochemical multiplexed sensors into clinical POC diagnostic platforms also need to systematically address the regulatory, biocompatibility, and ethical issues [107]. Rigorous quality control and standardized procedures need to be established to ensure the reliability of the sensors in real biofluidic environments, which set high standards for long-term material performance and manufacturing processes. Biocompatibility assessment is crucial, and the potential toxicity, inflammatory response, and long-term in vivo retention effects of MXene-based materials in the target biofluidic environments must be comprehensively examined to ensure their biosafety. In addition, ethical issues related to the protection of user data privacy, the acquisition of informed consent, and the accessibility and fairness of these advanced diagnostic tools also need to be proactively addressed.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Yang, G.; Qu, F. Advances of aptamer-based small-molecules sensors in body fluids detection. Talanta 2024, 268, 125348. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gu, S.; Zhang, Q.; Song, E.; Kuang, T.; Chen, F.; Yu, X.; Chang, L. Recent advances in biofluid detection with micro/nanostructured bioelectronic devices. Nanoscale 2021, 13, 3436–3453. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Krämer, J.; Kang, R.; Grimm, L.M.; De Cola, L.; Picchetti, P.; Biedermann, F. Molecular probes, chemosensors, and nanosensors for optical detection of biorelevant molecules and ions in aqueous media and biofluids. Chem. Rev. 2022, 122, 3459–3636. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, Y.; Lin, Z.; Ouyang, Z. High-precision quantitation of biofluid samples using direct mass spectrometry analysis. Anal. Chem. 2019, 91, 6986–6990. [Google Scholar] [CrossRef]
- Lu, L. Recent advances in synthesis of three-dimensional porous graphene and its applications in construction of electrochemical (bio)sensors for small biomolecules detection. Biosens. Bioelectron. 2018, 110, 180–192. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Klebes, A.; Ates, H.C.; Verboket, R.D.; Urban, G.A.; Von Stetten, F.; Dincer, C.; Früh, S.M. Emerging multianalyte biosensors for the simultaneous detection of protein and nucleic acid biomarkers. Biosens. Bioelectron. 2024, 244, 115800. [Google Scholar] [CrossRef]
- Pakchin, P.S.; Fathi, F.; Samadi, H.; Adibkia, K. Recent advances in receptor-based optical biosensors for the detection of multiplex biomarkers. Talanta 2025, 281, 126852. [Google Scholar] [CrossRef]
- Li, B.; Xie, X.; Meng, T.; Guo, X.; Li, Q.; Yang, Y.; Jin, H.; Jin, C.; Meng, X.; Pang, H. Recent advance of nanomaterials modified electrochemical sensors in the detection of heavy metal ions in food and water. Food Chem. 2024, 440, 138213. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Review on applications of carbon nanomaterials for simultaneous electrochemical sensing of environmental contaminant dihydroxybenzene isomers. Arab. J. Chem. 2020, 13, 6092–6105. [Google Scholar] [CrossRef]
- Amali, R.K.A.; Lim, H.N.; Ibrahim, I.; Huang, N.M.; Zainal, Z.; Ahmad, S.A.A. Significance of nanomaterials in electrochemical sensors for nitrate detection: A review. Trends Environ. Anal. Chem. 2021, 31, e00135. [Google Scholar] [CrossRef]
- Liu, T.; Chu, Z.; Jin, W. Electrochemical mercury biosensors based on advanced nanomaterials. J. Mater. Chem. B 2019, 7, 3620–3632. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Y.; Ying, Y.; Ping, J. Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection. TrAC Trends Anal. Chem. 2019, 115, 187–202. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Fouad, F.H.; Fayek, G.R.; El Sayed, K.M.; Ahmed, M.N.; Mahmoud, R.Z.; El Nashar, R.M. Recent advances in electrochemical sensors based on nanomaterials for detection of red dyes in food products: A review. Food Chem. 2023, 435, 137656. [Google Scholar] [CrossRef]
- Uçar, A.; Aydoğdu Tığ, G.; Er, E. Recent advances in two dimensional nanomaterial-based electrochemical (bio)sensing platforms for trace-level detection of amino acids and pharmaceuticals. TrAC Trends Anal. Chem. 2023, 162, 117027. [Google Scholar] [CrossRef]
- Min, X.; Liu, F.; Wang, Y.; Yan, Y.; Wang, H. Synthesis and electrochemical behavior of monolayer-Ti3C2Tx for capacitive deionization. J. Cent. South Univ. 2022, 29, 359–372. [Google Scholar] [CrossRef]
- Jia, L.; Zhou, S.; Ahmed, A.; Yang, Z.; Liu, S.; Wang, H.; Li, F.; Zhang, M.; Zhang, Y.; Sun, L. Tuning MXene electrical conductivity towards multifunctionality. Chem. Eng. J. 2023, 475, 146361. [Google Scholar] [CrossRef]
- Zeraati, A.S.; Mirkhani, S.A.; Sun, P.; Naguib, M.; Braun, P.V.; Sundararaj, U. Improved synthesis of Ti3C2Tx MXenes resulting in exceptional electrical conductivity, high synthesis yield, and enhanced capacitance. Nanoscale 2021, 13, 3572–3580. [Google Scholar] [CrossRef]
- Mathew, M.; Rout, C.S. Electrochemical biosensors based on Ti3C2Tx MXene: Future perspectives for on-site analysis. Curr. Opin. Electrochem. 2021, 30, 100782. [Google Scholar] [CrossRef]
- Mohanapriya, D.; Satija, J.; Senthilkumar, S.; Ponnusamy, V.K.; Thenmozhi, K. Design and engineering of 2D MXenes for point-of-care electrochemical detection of bioactive analytes and environmental pollutants. Coord. Chem. Rev. 2024, 507, 215746. [Google Scholar] [CrossRef]
- Rhouati, A.; Berkani, M.; Vasseghian, Y.; Golzadeh, N. MXene-based electrochemical sensors for detection of environmental pollutants: A comprehensive review. Chemosphere 2022, 291, 132921. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, P.-S.; Kim, S.-Y. Electrochemical sensing interfaces based on novel 2D-MXenes for monitoring environmental hazardous toxic compounds: A concise review. J. Ind. Eng. Chem. 2022, 109, 52–67. [Google Scholar] [CrossRef]
- Pengsomjit, U.; Alabdo, F.; Olawale, S.H.; Choowongkomon, K.; Sharma, V.K.; Darwish, I.A.; Kraiya, C. Recent advances and analytical perspectives in MXene-based electrochemical miniaturized sensors for environmental analysis and monitoring. Microchem. J. 2024, 206, 111433. [Google Scholar] [CrossRef]
- Umapathi, R.; Raju, C.V.; Safarkhani, M.; Haribabu, J.; Lee, H.U.; Rani, G.M.; Huh, Y.S. Versatility of MXene based materials for the electrochemical detection of phenolic contaminants. Coord. Chem. Rev. 2025, 525, 216305. [Google Scholar] [CrossRef]
- Seitak, A.; Luo, S.; Cai, N.; Liao, K.; Pappa, A.-M.; Lee, S.; Chan, V. Emergence of MXene-based electrochemical biosensors for biomolecule and pathogen detection. Sens. Actuators Rep. 2023, 6, 100175. [Google Scholar] [CrossRef]
- Karadurmus, L.S.; Kaya, I.; Cetinkaya, A.; Ozkan, S.A. New brand MXene-based electrochemical point-of-care sensors as novel diagnostic devices. TrAC Trends Anal. Chem. 2023, 165, 117145. [Google Scholar] [CrossRef]
- Li, T.; Shang, D.; Gao, S.; Wang, B.; Kong, H.; Yang, G.; Shu, W.; Xu, P.; Wei, G. Two-dimensional material-based electrochemical sensors/biosensors for food safety and biomolecular detection. Biosensors 2022, 12, 314. [Google Scholar] [CrossRef]
- Lorencova, L.; Kasak, P.; Kosutova, N.; Jerigova, M.; Noskovicova, E.; Vikartovska, A.; Barath, M.; Farkas, P.; Tkac, J. MXene-based electrochemical devices applied for healthcare applications. Microchim. Acta 2024, 191, 88. [Google Scholar] [CrossRef]
- Ganesan, S.; Ramajayam, K.; Kokulnathan, T.; Palaniappan, A. Recent advances in two-dimensional MXene-based electrochemical biosensors for sweat analysis. Molecules 2023, 28, 4617. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Gadhari, N.S.; Li, X.; Rao, Z.; Navale, S.T.; Shen, Y.; Patil, V.R.; Huang, Y. Recent advances in MXene–based electrochemical sensors and biosensors. TrAC Trends Anal. Chem. 2019, 120, 115643. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Qamar, M.Z.; Khalid, Z.; Chamanehpour, E.; Mishra, Y.K. Two-dimensional MXenes: A route from synthesis to applications in self-powered IoT devices. Chem. Eng. J. 2024, 490, 151600. [Google Scholar] [CrossRef]
- Mohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar]
- Patil, A.M.; Jadhav, A.A.; Chodankar, N.R.; Avatare, A.T.; Hong, J.; Dhas, S.D.; Patil, U.M.; Jun, S.C. Recent progress of MXene synthesis, properties, microelectrode fabrication techniques for microsupercapacitors and microbatteries energy storage devices and integration: A comprehensive review. Coord. Chem. Rev. 2024, 517, 216020. [Google Scholar] [CrossRef]
- Seidi, F.; Shamsabadi, A.A.; Firouzjaei, M.D.; Elliott, M.; Saeb, M.R.; Huang, Y.; Li, C.; Xiao, H.; Anasori, B. MXenes antibacterial properties and applications: A review and perspective. Small 2023, 19, 2206716. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.-Å.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent conductive two-dimensional titanium carbide epitaxial thin films. Chem. Mater. 2014, 26, 2374. [Google Scholar] [CrossRef]
- Sun, W.; Shah, S.A.; Chen, Y.; Tan, Z.; Gao, H.; Habib, T.; Radovic, M.; Green, M.J. Electrochemical etching of Ti2AlC to Ti2CTX (MXene) in low-concentration hydrochloric acid solution. J. Mater. Chem. A 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-free synthesis of high-purity Ti3C2Tx (T=OH, O) via alkali treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, A.; Hassan, A.; Neher, G.; Nepal, D.; Pachter, R.; Kennedy, W.J.; Ramakrishnan, S.; Vaia, R.A. Halogen etch of Ti3AlC2 MAX phase for MXene fabrication. ACS Nano 2021, 15, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.-L.; Cheng, H.M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Z.; Wang, H.; Chan, C.H.; Chan, N.Y.; Chen, X.X.; Dai, J.-Y. Plasma-enhanced pulsed-laser deposition of single-crystalline Mo2C ultrathin superconducting films. Phys. Rev. Mater. 2017, 1, 34002. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, H.; Jin, H.; Wu, M.; Fang, Y.; Sun, J.; Hu, Z.; Li, T.; Wu, J.; Huang, L.; et al. Salt-templated synthesis of 2D metallic MoN and other nitrides. ACS Nano 2017, 11, 2180–2186. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Wang, Y.; Wang, T.; Shang, S.; Ren, W. Magnetron-sputtering deposited molybdenum carbide MXene thin films as a saturable absorber for passively Q-switched lasers. J. Mater. Chem. C 2020, 8, 1608–1613. [Google Scholar] [CrossRef]
- Gürbüz, B.; Ciftci, F. Bio-electric-electronics and tissue engineering applications of MXenes wearable materials: A review. Chem. Eng. J. 2024, 489, 151230. [Google Scholar] [CrossRef]
- Gul, I.; Sayed, M.; Saeed, T.; Rehman, F.; Naeem, A.; Gul, S.; Khan, Q.; Naz, K.; Rehman, M. Unveiling cutting-edge progress in the fundamentals of MXene: Synthesis strategies, energy and bio-environmental applications. Coord. Chem. Rev. 2024, 511, 215870. [Google Scholar] [CrossRef]
- Thakur, A.; Chandran, B.S.N.; Davidson, K.; Bedford, A.; Fang, H.; Im, Y.; Kanduri, V.; Wyatt, B.C.; Nemani, S.K.; Poliukhova, V.; et al. Step-by-step guide for synthesis and delamination of Ti3C2Tx MXene. Small Methods 2023, 7, 2300030. [Google Scholar] [CrossRef]
- Pakchin, P.S.; Nakhjavani, S.A.; Saber, R.; Ghanbari, H.; Omidi, Y. Recent advances in simultaneous electrochemical multi-analyte sensing platforms. TrAC Trends Anal. Chem. 2017, 92, 32–41. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, N. Design of immunoprobes for electrochemical multiplexed tumor marker detection. Expert Rev. Mol. Diag. 2015, 15, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, U.; Rainbow, J.; Flynn, C.; Aidoo-Brown, J.; Estrela, P.; Moschou, D. Strategies for multiplexed electrochemical sensor development. In Modern Techniques in Biosensors. Studies in Systems, Decision and Control; Dutta, G., Biswas, A., Chakrabarti, A., Eds.; Springer: Singapore, 2021; Volume 327, pp. 63–93. [Google Scholar]
- Noviana, E.; Henry, C.S. Simultaneous electrochemical detection in paper-based analytical devices. Curr. Opin. Electrochem. 2020, 23, 1–6. [Google Scholar] [CrossRef]
- Feng, S.; Yu, L.; Yan, M.; Ye, J.; Huang, J.; Yang, X. Holey nitrogen-doped graphene aerogel for simultaneously electrochemical determination of ascorbic acid, dopamine and uric acid. Talanta 2021, 224, 121851. [Google Scholar] [CrossRef] [PubMed]
- Nantaphol, S.; Kava, A.A.; Channon, R.B.; Kondo, T.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Janus electrochemistry: Simultaneous electrochemical detection at multiple working conditions in a paper-based analytical device. Anal. Chim. Acta 2019, 1056, 88–95. [Google Scholar] [CrossRef]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef]
- Karimzadeh, Z.; Hasanzadeh, M.; Isildak, I.; Khalilzadeh, B. Multiplex bioassaying of cancer proteins and biomacromolecules: Nanotechnological, structural and technical perspectives. Int. J. Biol. Macromol. 2020, 165, 3020–3039. [Google Scholar] [CrossRef]
- Grabowska, I.; Zapotoczny, S.; Chlopicki, S. Multiplex electrochemical aptasensors for detection of endothelial dysfunction: Ready for prime time? TrAC Trends Anal. Chem. 2023, 169, 117372. [Google Scholar] [CrossRef]
- Feng, J.; Chu, C.; Ma, Z. Electrochemical signal substance for multiplexed immunosensing interface construction: A mini review. Molecules 2022, 27, 267. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Kausaite-Minkstimiene, A.; Ramanaviciene, A. Metal nanoparticle and quantum dot tags for signal amplification in electrochemical immunosensors for biomarker detection. Chemosensors 2021, 9, 85. [Google Scholar] [CrossRef]
- Kondzior, M.; Grabowska, I. Antibody-electroactive probe conjugates based electrochemical immunosensors. Sensors 2020, 20, 2014. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Nanostructures for nonlabeled and labeled electrochemical immunosensors: Simultaneous electrochemical detection of cancer markers: A review. Talanta 2019, 205, 120153. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, M.A.; Shamsipur, M.; Saber, R.; Sarkar, S. Simultaneous determination of CYC and VEGF165 tumor markers based on immobilization of flavin adenine dinucleotide and thionine as probes on reduced graphene oxide-poly(amidoamine)/gold nanocomposite modified dual working screen-printed electrode. Sens. Actuators B Chem. 2017, 240, 1174–1181. [Google Scholar] [CrossRef]
- Wardani, N.I.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Molecularly imprinted polymer dual electrochemical sensor for the one-step determination of albuminuria to creatinine ratio (ACR). Talanta 2023, 265, 124769. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Sharifuzzaman, M.; Sharma, S.; Xuan, X.; Zhang, S.; Ko, S.G.; Yoon, S.H.; Park, J.Y. High-performance flexible electrochemical heavy metal sensor based on layer-by-layer assembly of Ti3C2Tx/MWNTs nanocomposites for noninvasive detection of copper and zinc ions in human biofluids. ACS Appl. Mater. Interfaces 2020, 12, 48928–48937. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, P.; Gao, B.; Yuan, M.; Yu, J.; Wang, Z.; Chen, X. Self-reduction of bimetallic nanoparticles on flexible MXene-graphene electrodes for simultaneous detection of ascorbic acid, dopamine, and uric acid. Microchem. J. 2023, 185, 108177. [Google Scholar] [CrossRef]
- Jia, D.; Yang, T.; Wang, K.; Zhou, L.; Wang, E.; Chou, K.-C.; Wang, H.; Hou, X. Facile in-situ synthesis of Ti3C2Tx/TiO2 nanowires toward simultaneous determination of ascorbic acid, dopamine and uric acid. J. Alloys Compd. 2024, 985, 173392. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, Q.; Li, X.; Wu, L.; Yu, A.; Lai, G.; Fu, L.; Wei, Q.; Dai, D.; Jiang, N.; et al. A double-deck structure of reduced graphene oxide modified porous Ti3C2Tx electrode towards ultrasensitive and simultaneous detection of dopamine and uric acid. Biosensors 2021, 11, 462. [Google Scholar] [CrossRef]
- Shang, L.; Li, R.; Li, H.; Yu, S.; Sun, X.; Yu, Y.; Ren, Q. The simultaneous detection of dopamine and uric acid in vivo based on a 3D reduced graphene oxide–MXene composite electrode. Molecules 2024, 29, 1936. [Google Scholar] [CrossRef]
- Mun, T.J.; Yang, E.; Moon, J.; Kim, S.; Park, S.G.; Kim, M.; Choi, N.; Lee, Y.; Kim, S.J.; Seong, H. Silane-functionalized MXene-PEGDA hydrogel for enhanced electrochemical sensing of neurotransmitters and antioxidants. ACS Appl. Polym. Mater. 2024, 6, 9533–9544. [Google Scholar] [CrossRef]
- Elumalai, S.; Mani, V.; Jeromiyas, N.; Ponnusamy, V.K.; Yoshimura, M. A composite film prepared from titanium carbide Ti3C2Tx (MXene) and gold nanoparticles for voltammetric determination of uric acid and folic acid. Microchim. Acta 2020, 187, 33. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.A.; Filik, H. Synthesis and characteristics of Cu/N-codoped Ti3C2Tx MXene using copper-aniline solid complex and application to simultaneous electrochemical sensing of adenine and guanine in artificial sweat. Anal. Lett. 2024, 57, 2972–2993. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, X.T.; Zhang, R.Y.; Zhang, Y.; Wu, L.M.; Lu, W.; Li, J.Q.; Li, Y.C.; Zhang, H. MXene-enabled electrochemical microfluidic biosensor: Applications toward multicomponent continuous monitoring in whole blood. Adv. Funct. Mater. 2019, 29, 1807326. [Google Scholar] [CrossRef]
- Ji, G.; Wang, J.; Wang, Z.; Zhang, S.; Fang, Z.; Wang, Y.; Gao, Z. Transient paper-based electrochemical biosensor fabricated by superadditive Cu-TCPP(Fe)/MXene for multipathway non-invasive, highly sensitive detection of bodily metabolites. Biosens. Bioelectron. 2024, 261, 116509. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.-H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-based wearable biosensor system for high-performance in vitro perspiration analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Liu, R.; Li, J.; Zhang, Q.; Shi, G.; Li, Y.; Hou, C.; Wang, H. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 2021, 174, 112828. [Google Scholar] [CrossRef]
- Sharifuzzaman, M.; Barman, S.C.; Zahed, M.A.; Sharma, S.; Yoon, H.; Nah, J.S.; Kim, H.; Park, J.Y. An electrodeposited MXene-Ti3C2Tx nanosheets functionalized by task-specific ionic liquid for simultaneous and multiplexed detection of bladder cancer biomarkers. Small 2020, 16, 2002517. [Google Scholar] [CrossRef]
- Zhang, W.; Du, J.; Wang, K.; Li, Y.; Chen, C.; Yang, L.; Kan, Z.; Dong, B.; Wang, L.; Xu, L. Integrated dual-channel electrochemical immunosensor for early diagnosis and monitoring of periodontitis by detecting multiple biomarkers in saliva. Anal. Chim. Acta 2023, 1247, 340878. [Google Scholar] [CrossRef]
- Reza, M.S.; Sharifuzzaman, M.; Asaduzzaman, M.; Islam, Z.; Lee, Y.; Kim, D.; Park, J.Y. Polyaromatic hydrocarbon-functionalized 2D MXene-based 3D porous antifouling nanocomposite with long shelf life for high-performance electrochemical immunosensor applications. ACS Appl. Mater. Interfaces 2024, 16, 31610–31623. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Li, Z.; Li, Z.; Zhou, S.; Xia, Y.; Mou, L. Fast and in-situ electrodeposition of MXene/AuNPs composite for multiplexed and sensitive detection of infectious biomarkers using an electrochemical biosensor. Microchem. J. 2024, 207, 112064. [Google Scholar] [CrossRef]
- Mohammadniaei, M.; Koyappayil, A.; Sun, Y.; Min, J.; Lee, M.-H. Gold nanoparticle/MXene for multiple and sensitive detection of oncomiRs based on synergetic signal amplification. Biosens. Bioelectron. 2020, 159, 112208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; He, C.; Wu, W.; Yang, H.; Peng, L.; Wen, L.; Hu, Z.; Hou, C.; Huo, D. MXene-MoS2 carbon-fiber-based flexible electrochemical interface for multiple bioanalysis in biofluids. Chem. Eng. J. 2022, 446, 136841. [Google Scholar] [CrossRef]
- Kalasin, S.; Sangnuang, P. Multiplex wearable electrochemical sensors fabricated from sodiated polymers and Mxene nanosheet to measure sodium and creatinine levels in sweat. ACS Appl. Nano Mater. 2023, 6, 18209–18221. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Han, J.; Zhang, M.; Li, F.; Yang, D. High-sensitivity flexible electrochemical sensor for real-time multi-analyte sweat analysis. Talanta 2025, 287, 127644. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Zhang, J.; Zhang, H.; Li, Y. Simultaneous voltammetric determination of acetaminophen and isoniazid using MXene modified screen-printed electrode. Biosens. Bioelectron. 2019, 130, 315–321. [Google Scholar] [CrossRef]
- Mari, E.; Duraisamy, M.; Eswaran, M.; Sellappan, S.; Won, K.; Chandra, P.; Tsai, P.-C.; Huang, P.-C.; Chen, Y.-H.; Lin, Y.-C.; et al. Highly electrochemically active Ti3C2Tx MXene/MWCNT nanocomposite for the simultaneous sensing of paracetamol, theophylline, and caffeine in human blood samples. Microchim. Acta 2024, 191, 212. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Lu, Y.; Zhang, Y. ZIF-8 nanoparticles densely covered MXene as functionalized platform for electrochemical detection of medical small molecules. Electrochim. Acta 2024, 502, 144872. [Google Scholar] [CrossRef]
- Rajaji, U.; Ganesh, P.S.; Kim, S.Y.; Govindasamy, M.; Alshgari, R.A.; Liu, T.Y. MoS2 sphere/2D S-Ti3C2 MXene nanocatalysts on laser-induced graphene electrodes for hazardous aristolochic acid and roxarsone electrochemical detection. ACS Appl. Nano Mater. 2022, 5, 3252–3264. [Google Scholar] [CrossRef]
- Devi, R.K.; Ganesan, M.; Chen, T.-W.; Chen, S.-M.; Abbasi, A.M.; Ali, M.A.; Elshikh, M.S.; Yu, J.; Chuang, H.-Y.; Xu, B.; et al. MXene-interdigitated holey-graphene oxide nanocomposite for simultaneous detection of antibiotic and anticancer drugs with ultra-high sensitivity. Chem. Eng. J. 2023, 474, 145693–145706. [Google Scholar] [CrossRef]
- Ravipati, M.; Ramasamy, D.; Badhulika, S. Highly selective cobalt-MOF/vanadium carbide MXene hydrogel for simultaneous electrochemical determination of levothyroxine and carbamazepine in simulated blood serum. Electrochim. Acta 2025, 525, 146154. [Google Scholar] [CrossRef]
- Yin, H.; Truskewycz, A.; Cole, I.S. Quantum dot (QD)-based probes for multiplexed determination of heavy metal ions. Microchim. Acta 2020, 187, 336. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; An, J.M.; Kim, J.; Kim, D. Review: Recent progress in fluorescent molecular systems for the detection of disease-related biomarkers in biofluids. Coord. Chem. Rev. 2023, 494, 215336. [Google Scholar] [CrossRef]
- Mandal, S.; Li, Z.; Chatterjee, T.; Khanna, K.; Montoya, K.; Dai, L.; Petersen, C.; Li, L.; Tewari, M.; Johnson-Buck, A.; et al. Direct kinetic fingerprinting for high-accuracy single-molecule counting of diverse disease biomarkers. Acc. Chem. Res. 2021, 54, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wu, S.; Ye, C.; Liu, X.; Gao, J.; Cui, N.; Guo, P.; Lai, G.; Wei, Q.; Yang, M.; et al. Sensitivity enhancement of potassium ion (K+) detection based on graphene field-effect transistors with surface plasma pretreatment. Sens. Actuators B Chem. 2019, 285, 333–340. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, L.; Duan, H. Nanomaterial based electrochemical sensors for in vitro detection of small molecule metabolites. Biotechnol. Adv. 2016, 34, 234–249. [Google Scholar] [CrossRef]
- Sangubotla, R.; Kim, J. Recent trends in analytical approaches for detecting neurotransmitters in Alzheimer’s disease. Trends Anal. Chem. 2018, 105, 240–250. [Google Scholar] [CrossRef]
- Xu, T.-Q.; Zhang, Q.-L.; Zheng, J.-N.; Lv, Z.-Y.; Wei, J.; Wang, A.-J.; Feng, J.-J. Simultaneous determination of dopamine and uric acid in the presence of ascorbic acid using Pt nanoparticles supported on reduced graphene oxide. Electrochim. Acta 2014, 115, 109–115. [Google Scholar] [CrossRef]
- Liang, X.-H.; Yu, A.-X.; Bo, X.-J.; Du, D.-Y.; Su, Z.-M. Metal/covalent-organic frameworks-based electrochemical sensors for the detection of ascorbic acid, dopamine and uric acid. Coord. Chem. Rev. 2023, 497, 215427. [Google Scholar] [CrossRef]
- Hussain, S.; Zaidi, S.A.; Vikraman, D.; Kim, H.-S.; Jung, J. Facile preparation of molybdenum carbide (Mo2C) nanoparticles and its effective utilization in electrochemical sensing of folic acid via imprinting. Biosens. Bioelectron. 2019, 140, 111330. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Xiao, S.; Liu, Y.; Bai, M.; Gong, L.; Zhao, J.; Chen, D. Revolutionizing precision medicine: Exploring wearable sensors for therapeutic drug monitoring and personalized therapy. Biosensors 2023, 13, 726. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Noiphung, J.; Rodthongkum, N.; Larpant, N.; Thirabowonkitphithan, P.; Rojanarata, T.; Hasan, M.; Huang, Y.; Laiwattanapaisal, W. Nanomaterials-based electrochemical sensors and biosensors for the detection of non-steroidal anti-inflammatory drugs. TrAC Trends Anal. Chem. 2021, 143, 116403. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, N.; Seo, Y. MXenes: Versatile 2D materials with tailored surface chemistry and diverse applications. J. Energy Chem. 2024, 90, 253–293. [Google Scholar] [CrossRef]
- Peng, M.; Dong, M.; Wei, W.; Xu, H.; Liu, C.; Shen, C. The introduction of amino termination on Ti3C2 MXene surface for its flexible film with excellent property. Carbon 2021, 179, 400–407. [Google Scholar] [CrossRef]
- Ye, C.; Lukas, H.; Wang, M.; Lee, Y.; Gao, W. Nucleic acid-based wearable and implantable electrochemical sensors. Chem. Soc. Rev. 2024, 53, 7960–7982. [Google Scholar] [CrossRef]
- Gao, Z.-W.; Yu, Y.; Chen, S.-H.; Li, Y.-Y.; Liu, Z.-H.; Yang, M.; Li, P.-H.; Song, Z.-Y.; Huang, X.-J. Machine learning-driven simultaneous quantification of Cd(II) and Cu(II) on Co2P/CoP heterostructure: Enhanced electrochemical signals via activated Co-P electron bridge. J. Hazard. Mater. 2025, 491, 138030. [Google Scholar] [CrossRef]
- Alagarsamy, K.N.; Saleth, L.R.; Diedkova, K.; Zahorodna, V.; Gogotsi, O.; Pogorielov, M.; Dhingra, S. MXenes in healthcare: Transformative applications and challenges in medical diagnostics and therapeutics. Nanoscale 2025, 17, 11785–11811. [Google Scholar] [CrossRef]

| Strategy | Advantages | Disadvantages |
|---|---|---|
| Electrode modification |
|
|
| Multi-electrode |
|
|
| Multi-label |
|
|
| Working Electrodes [Ref.] | Signal Separation Strategies | Analytes | Biofluids * | Analytical Methods | Sensitivity | Limit of Detection | Linear Range |
|---|---|---|---|---|---|---|---|
| Ti3C2Tx/MWCNTs/Au [65] | / | Cu2+ Zn2+ | Urine and sweat | SWASV | / | Cu2+: 0.1 ppb Zn2+: 1.5 ppb | Cu2+: 10–600 ppb Zn2+: 350–830 ppb |
| Ti-C-Tx/GCE [66] | Electrode modification | DA UA AA | Urine | DPV | / | AA: 4.64 μM DA: 0.06 μM UA: 0.075 μM | AA: 100–1000 μM DA: 0.5–50 μM UA: 0.5–4 μM & 100–1500 μM |
| Au-Pd/Ti3C2Tx/LSG [67] | Electrode modification | AA DA UA | Urine and sweat | DPV | / | AA: 3 μM DA: 0.13 μM UA: 1.47 μM | AA: 10–1600 μM DA: 12–240 μM UA: 8–100 μM & 200–800 μM |
| Ti3C2Tx/TiO2 NWs/GCE [68] | Electrode modification | AA DA UA | Urine | DPV | / | AA: 6.61 μM DA: 0.093 μM UA: 0.038 μM | AA: 300–1800 μM DA: 2–33 μM UA: 2–33 μM |
| Ti3C2Tx/rGO/GCE [69] | Electrode modification | DA UA | Serum | DPV | / | DA: 9.5 nM UA: 0.3 μM | DA: 0.1–100 μM UA: 1–1000 μM |
| 3D rGO-Ti3C2Tx/Cu wire [70] | Electrode modification | DA UA | FBS+rat striatum | DPV | DA: 0.74 µA/µM·cm2 UA: 2.96 µA/µM·cm2; 0.81 µA/µM·cm2 | DA: 0.061 μM UA: 0.085 μM | DA: 0.5–500 μM UA: 0.5–60 μM; 80–450 μM |
| Ti3C2Tx-PEGDA hydrogel/Au [71] | Electrode modification | DA 5-HT UA | Serum | DPV | / | DA: 2.55 μM 5-HT: 0.83 μM UA: 25.11 μM | DA: 2.5–200 μM 5-HT: 1–100 μM UA: 10–100 μM |
| AuNP@Ti3C2Tx/GCE [72] | Electrode modification | UA FA | Serum | Amperometry (i–t) | UA: 0.530 µA/µM·cm2 FA: 0.494 µA/µM·cm2 | UA: 11.5 nM FA: 6.20 nM | UA: 0.03–1520 μM FA: 0.02–3580 μM |
| Cu@N-Ti3C2Tx/GCE [73] | / | Adenine Guanine | Artificial sweat | DPV | / | adenine: 0.01 μM guanine: 0.02 μM | 0.1–10 μM |
| Urease-MB/Ti3C2Tx/SPE for Urea and UA; Ti3C2Tx/SPE for Cre [74] | Electrode modification and multi-electrode | Urea UA Cre | Whole blood | SWV | / | Urea: 0.02 mM UA: 5 μM Cre: 1.2 μM | Urea: 0.1–3 mM UA: 30–500 μM Cre: 10–400 μM |
| GOx(UOx)/Cu-TCPP(Fe)/Ti3C2Tx/paper-based electrode [75] | Multi-electrode | Glu UA | Artificial sweat, urine, and saliva | CV | / | Glu: 1.88 aM UA: 5.80 pM | Glu: 0.001 nM–5 mM UA: 0.025 nM–5 mM |
| GOx(LOx)/CNTs/Ti3C2Tx/PB/CFMs [76] | Multi-electrode | Glu Lac | Sweat | CA | Glu: 35.3 µA/mM·cm2 Lac: 11.4 µA/mM·cm2 | Glu: 0.33 μM Lac: 0.67 μM | Glu: 10–1500 μM Lac: 0–22 mM |
| GOX(LOX)/MB/Ti3C2Tx/SPCE [77] | Multi-electrode | Glu Lac | Sweat | CA & DPV | Glu: 2.4 nA/μM Lac: 0.49 μA/mM | Glu: 17.05 μM Lac: 3.73 μM | Glu: 0.08–1.25 mM Lac: 0.3–20.3 mM |
| DIDμEs/MXNSs-AFBPB [78] | Multi-electrode | Apo-A1 NMP 22 | Urine | DPV | / | Apo-A1: 0.3 pg/mL NMP 22: 0.7 pg/mL | 0.1 pg/mL–50 ng/mL |
| IrOx/Ti3C2Tx/SPE [79] | Multi-electrode | IL-1β MMP-8 | Artificial saliva and clinicopathological saliva | DPV | / | IL-1β: 0.014 ng/mL MMP-8: 0.13 ng/ mL | IL-1β: 0.1–100 ng/mL MMP-8: 1–200 ng/mL |
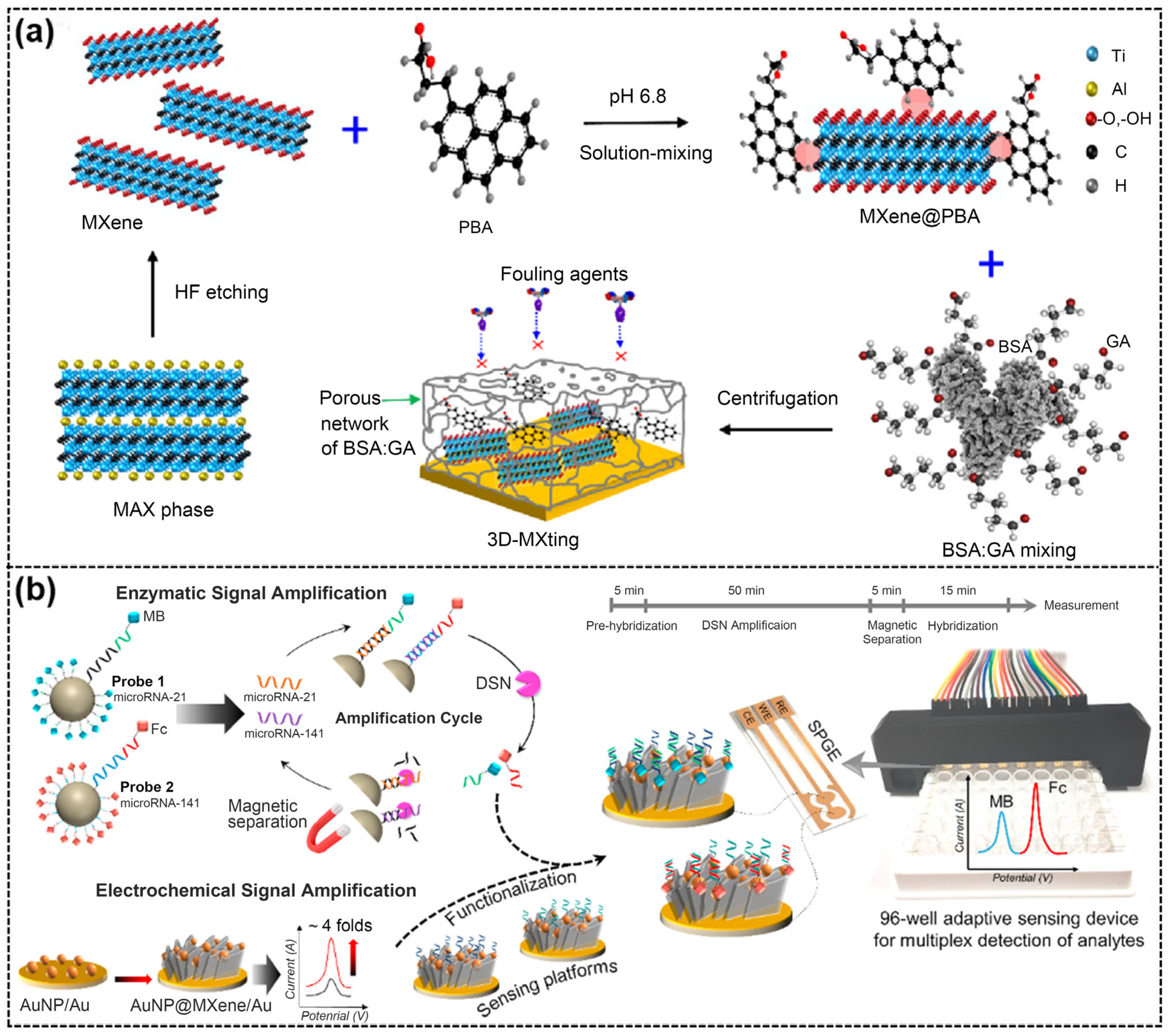
| 3D-MXting/Au [80] | Multi-electrode | CRP Ferritin | Serum | CV | / | CRP: 6.2 pg/mL Ferritin: 4.2 pg/mL | 0.01–100 ng/mL |
| AuNPs and Ti3C2Tx-SPE [81] | Multi-electrode | HBsAg Anti-HIV Anti-TP | Serum | DPV | / | HBsAg: 0.01 ng/mL Anti-HIV: 0.10 ng/mL Anti-TP: 0.11 ng/mL | HBsAg: 0.05–1000 ng/mL Anti-HIV: 0.25–100 ng/mL Anti-TP: 0.35–140 ng/mL |
| AuNP@Ti3C2Tx/SPGE [82] | Multi-electrode and multi-label | MicroRNA-21 MicroRNA-141 | Plasma | DPV | / | microRNA-21: 204 aM microRNA-141: 138 aM | 500 aM–50 nM |
| CFP-Ti3C2Tx-MoS2 for AA, DA and UA; CFP-Ti3C2Tx-MoS2-AuNPs for microRNA [83] | Electrode modification and multi-electrode | AA DA UA MicroRNA | Urine (AA, DA, UA) and serum (microRNA) | DPV | / | AA: 0.89 μM DA: 0.23 μM UA: 0.35 μM MicroRNA: 3.16 aM | AA: 10–1000 μM DA: 0.5–200 μM UA: 0.5–150 μM MicroRNA: 0.1 fM–10 fM; 10 fM to 10 nM |
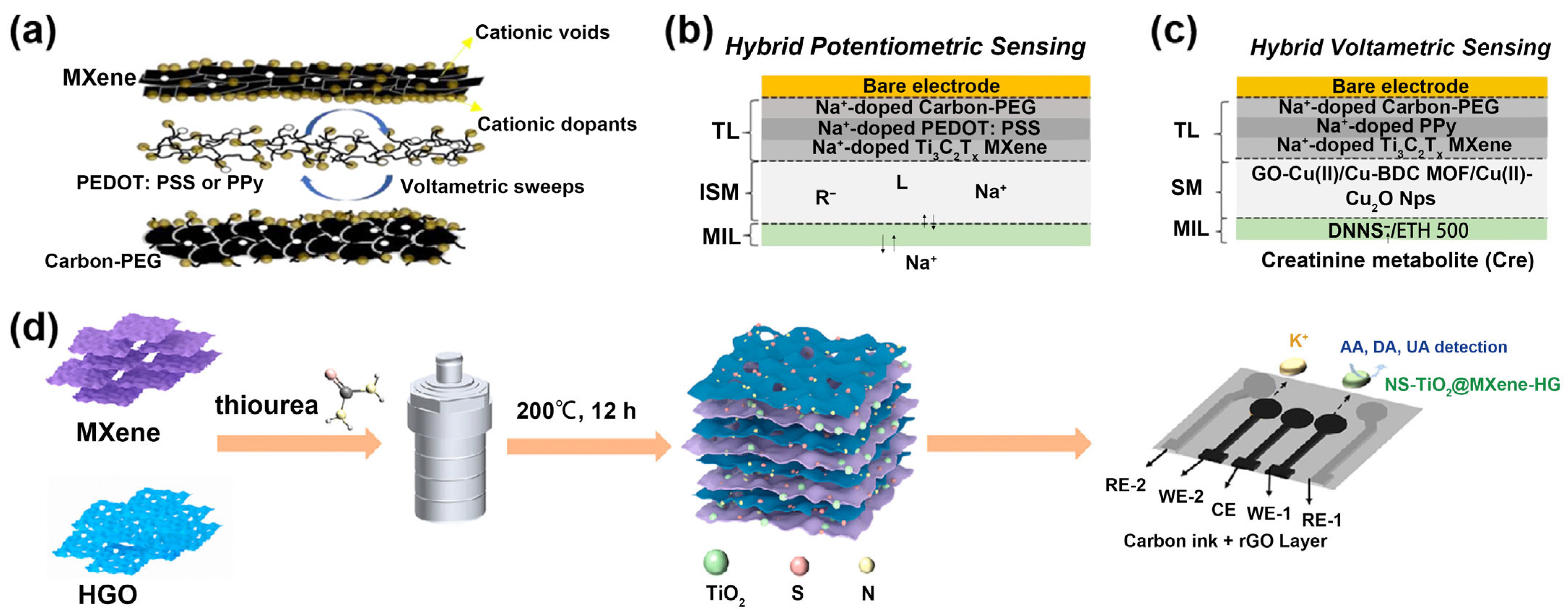
| TE/carbon-PEG/PEDOT:PSS/Ti3C2Tx/ISM/MIL for Na+; TE/carbon-PEG/PPy/Ti3C2Tx/SM/MIL for Cre [84] | Multi-electrode | Na+ Cre | Sweat | Potentiometry DPV | Na+: –58.9 mV/dec Cre: 0.014 ± 0.001 μA/μM | Na+: 10–6.2 M Cre: 0.12 μM | Na+: 10–6–10–1 M Cre: 0.6–2800 μM |
| NS-TiO2@MXene-HG/rGSPE for AA, DA and UA; ISM/rGSPE for K+ [85] | Electrode modification and multi-electrode | AA DA UA K+ | Sweat | i–t OCPT | AA: 20.78 µA/µM·cm2 DA: 32.78 µA/µM·cm2 / / | AA: 0.025 μM DA: 0.1 μM UA: 0.14 μM / | AA: 0.1–2200 μM DA: 0.25–100 μM; 100–400 μM UA: 0.25–100 μM; 100–225 μM K+: 0.19–24 mM; 24–125 mM |
| Ti3C2Tx/SPE [86] | / | ACOP INZ | Serum | DPV | / | ACOP: 0.048 μM INZ: 0.064 mM | ACOP: 0.25–2000 μM INZ: 0.1–4.6 mM |
| Ti3C2Tx-MWCNT/SPE [87] | Electrode modification | PA TP CF | Serum | DPV | PA: 2.194 µA/µM·cm2 TP: 2.179 µA/µM·cm2 CF: 5.035 µA/µM·cm2 | PA: 0.23 µM TP: 0.57 µM CF: 0.43 µM | PA: 1.0–90.1 µM TP: 2.0–62.0 µM CF: 2.0–90.9 µM |
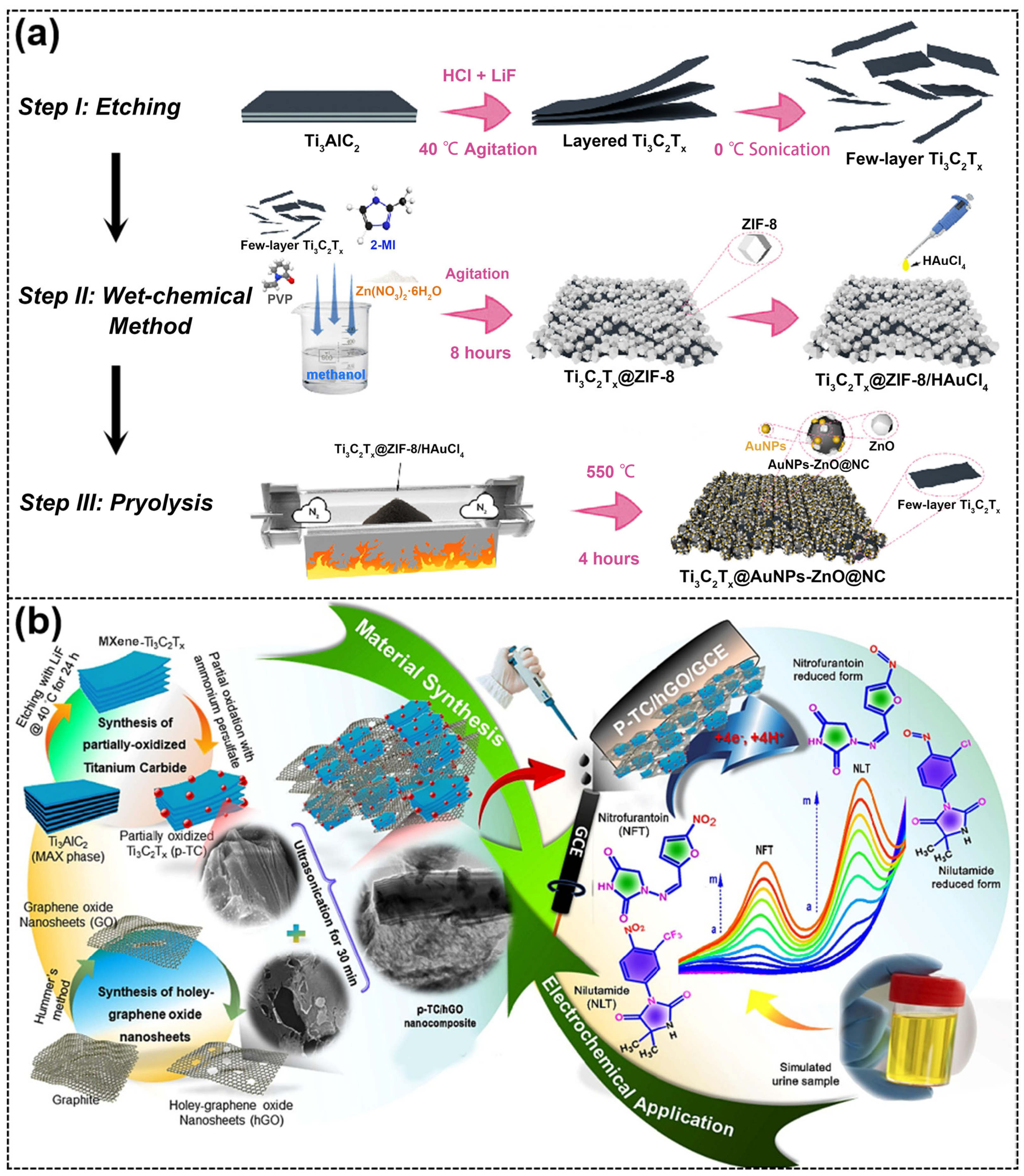
| Ti3C2Tx@AuNPs-ZnO@NC/GCE [88] | / | DA ACOP XA | Blood | DPV | / | DA: 0.041 μM AC: 0.059 μM XA: 0.067 μM | DA: 3–200 μM AC: 15–500 μM XA: 8–350 μM |
| MoS2/S-Ti3C2/LGE [89] | / | AA’ ROX | Urine and serum | DPV | AA’: 69.955 µA/µM·cm2; 32.488 µA/µM·cm2 ROX: 56.972 µA/µM·cm2; 19.688 µA/µM·cm2 | AA’: 1.65 nM ROX: 2.31 nM | 0.01–875.01 μM |
| p-TC/hGO/GCE [90] | Electrode modification | NFT NLT | Artificial urine | DPV | NFT: 52.8 µA/µM·cm2 NLT: 19.5 µA/µM·cm2 | NFT: 1.2 nM NLT: 1.9 nM | NFT: 0.05–135 μM NLT: 0.05–158 μM |
| MOF-71/V2C MXene–hydrogel [91] | / | LT4 CBZ | Simulated serum | DPV | / | LT4: 5.6 nM CBZ: 6.7 nM | LT4: 10 nM–100 μM CBZ: 10 nM–500 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Xie, C.; Lu, H. Advances in MXene-Based Electrochemical Sensors for Multiplexed Detection in Biofluids. Int. J. Mol. Sci. 2025, 26, 5368. https://doi.org/10.3390/ijms26115368
Yang M, Xie C, Lu H. Advances in MXene-Based Electrochemical Sensors for Multiplexed Detection in Biofluids. International Journal of Molecular Sciences. 2025; 26(11):5368. https://doi.org/10.3390/ijms26115368
Chicago/Turabian StyleYang, Meiqing, Congkai Xie, and Haozi Lu. 2025. "Advances in MXene-Based Electrochemical Sensors for Multiplexed Detection in Biofluids" International Journal of Molecular Sciences 26, no. 11: 5368. https://doi.org/10.3390/ijms26115368
APA StyleYang, M., Xie, C., & Lu, H. (2025). Advances in MXene-Based Electrochemical Sensors for Multiplexed Detection in Biofluids. International Journal of Molecular Sciences, 26(11), 5368. https://doi.org/10.3390/ijms26115368






