L-Fucose-Rich Sulfated Glycans from Edible Brown Seaweed: A Promising Functional Food for Obesity and Energy Expenditure Improvement
Abstract
1. Introduction
2. Results
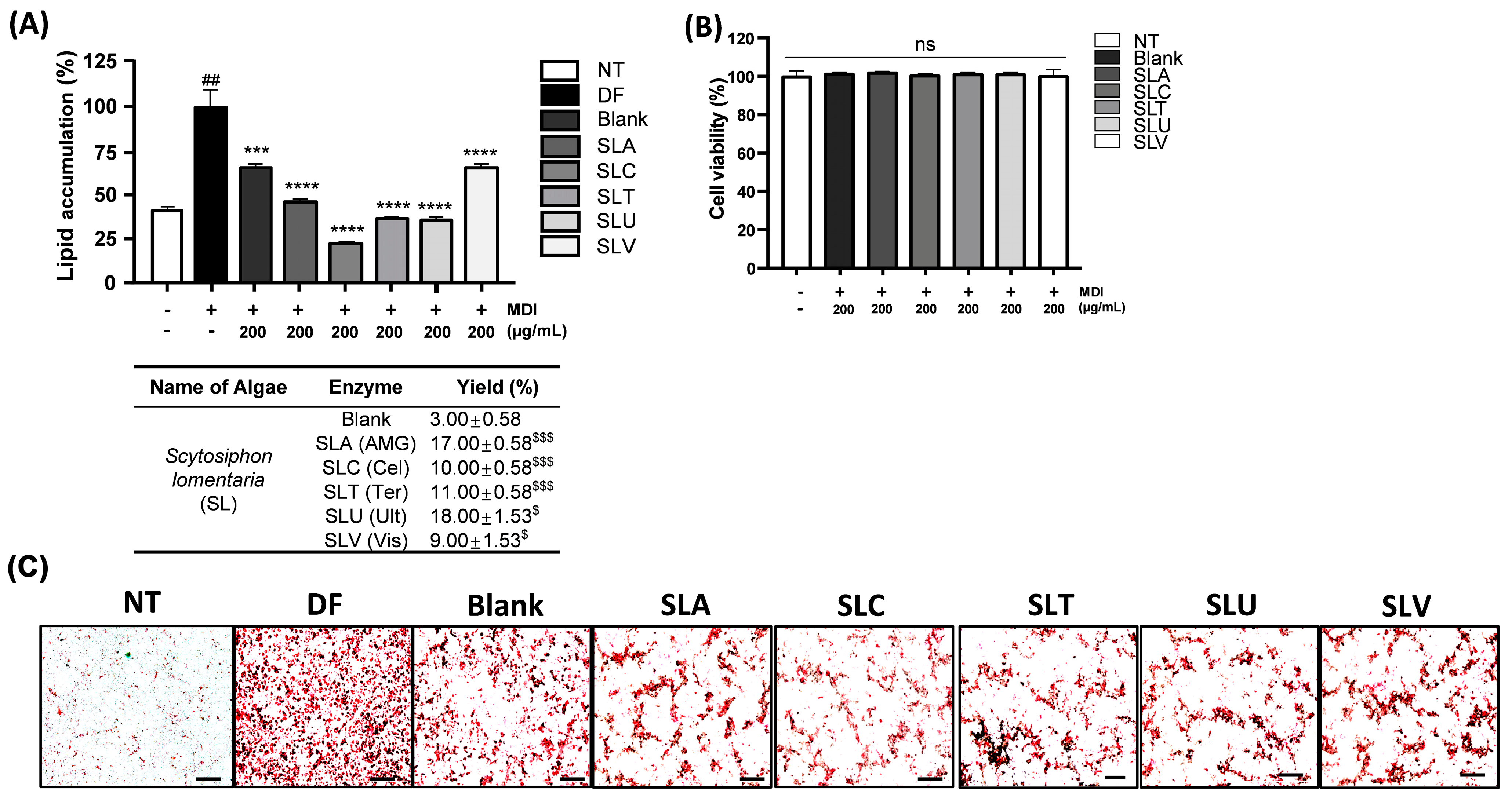
2.1. Anti-Lipid Accumulation Effect of Polysaccharides Extracting Enzymatic SL Hydrolysates
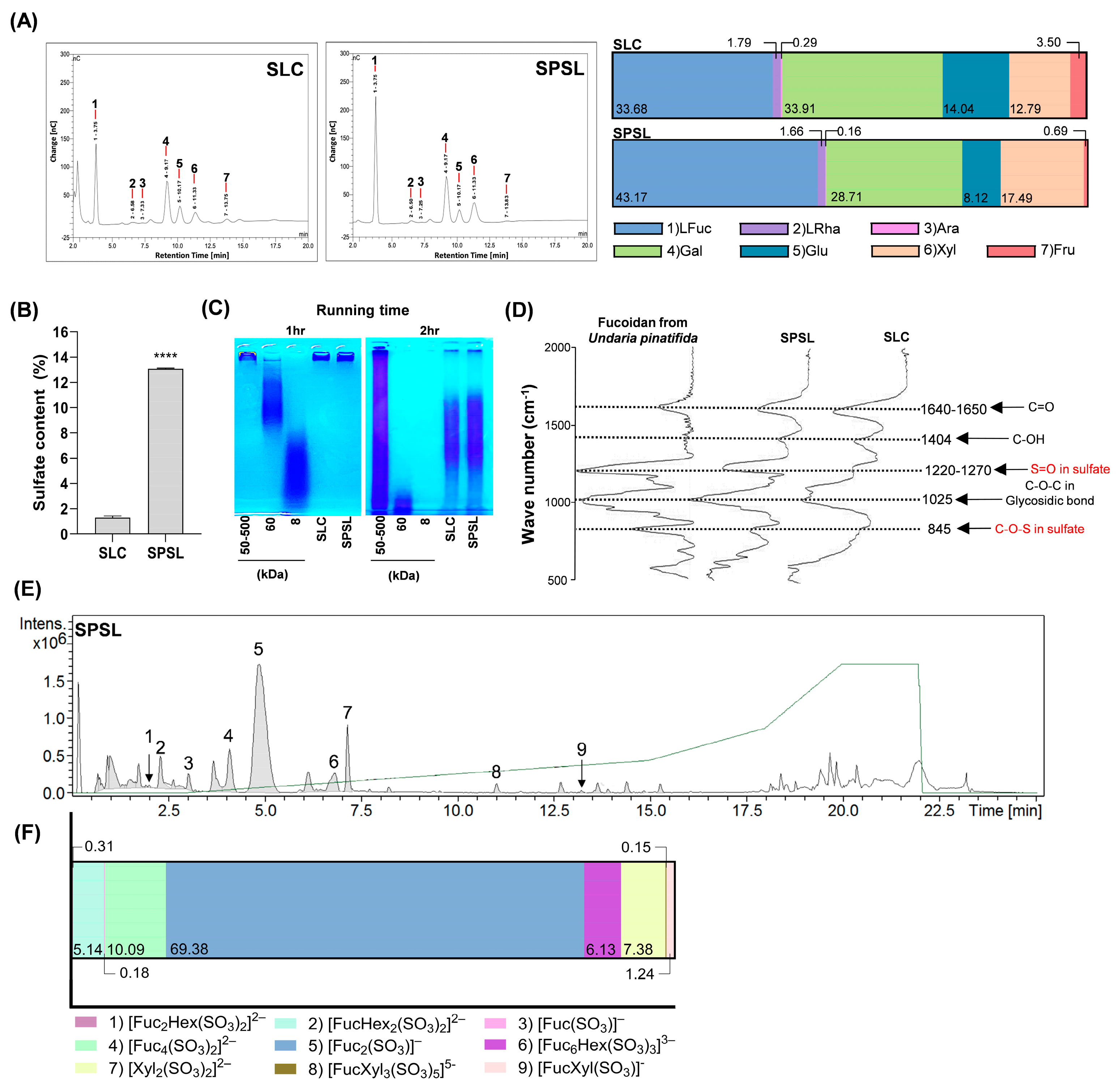
2.2. Characterization of Sulfated Polysaccharide Fraction Isolated from SL via Ethanol Precipitation
2.3. Anti-Lipogenesis Effect of SPSL in 3T3-L1 Adipocytes
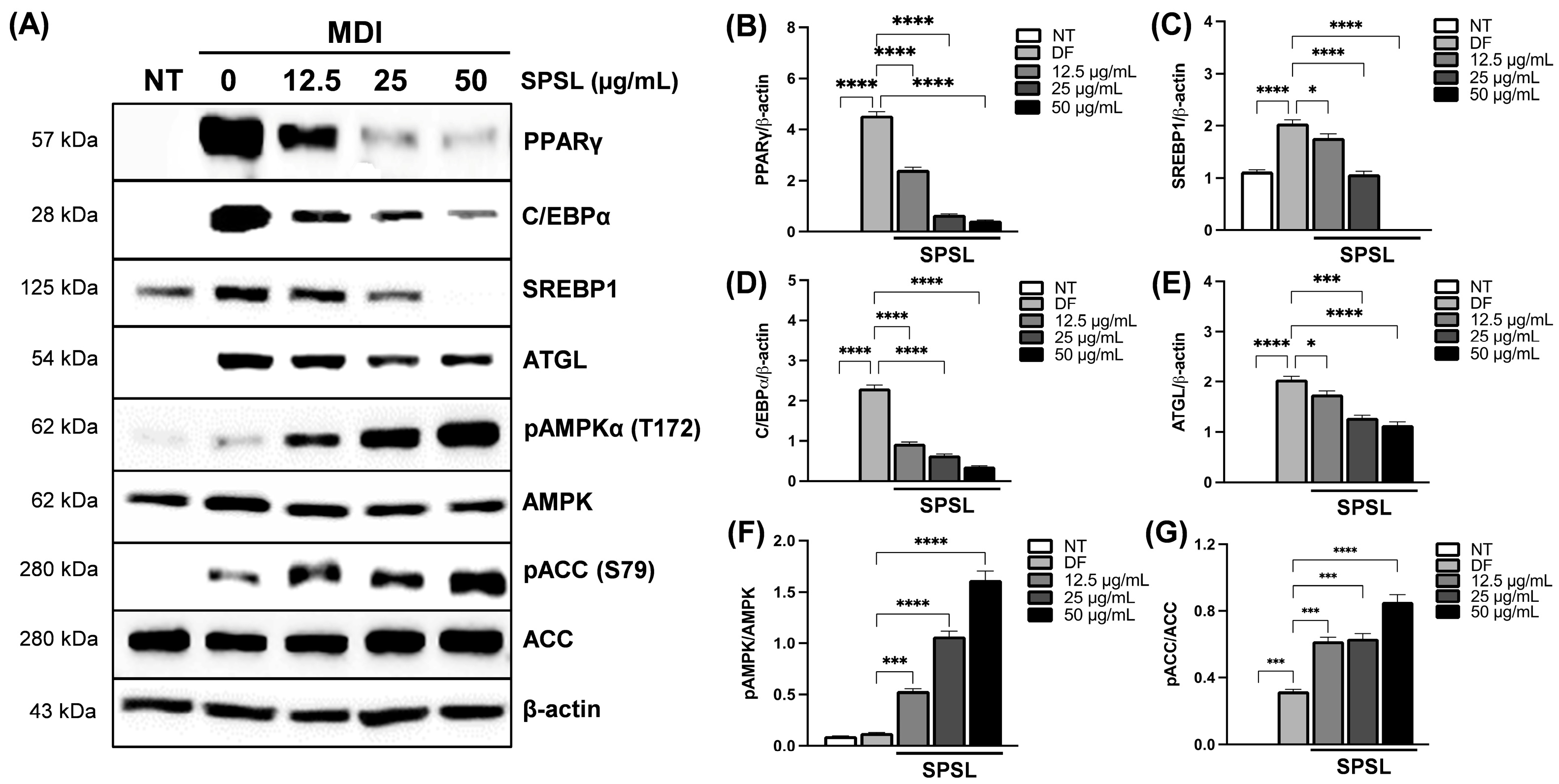
2.4. SPSL Blunted Adipogenesis by Regulating Key Adipogenic Modulators
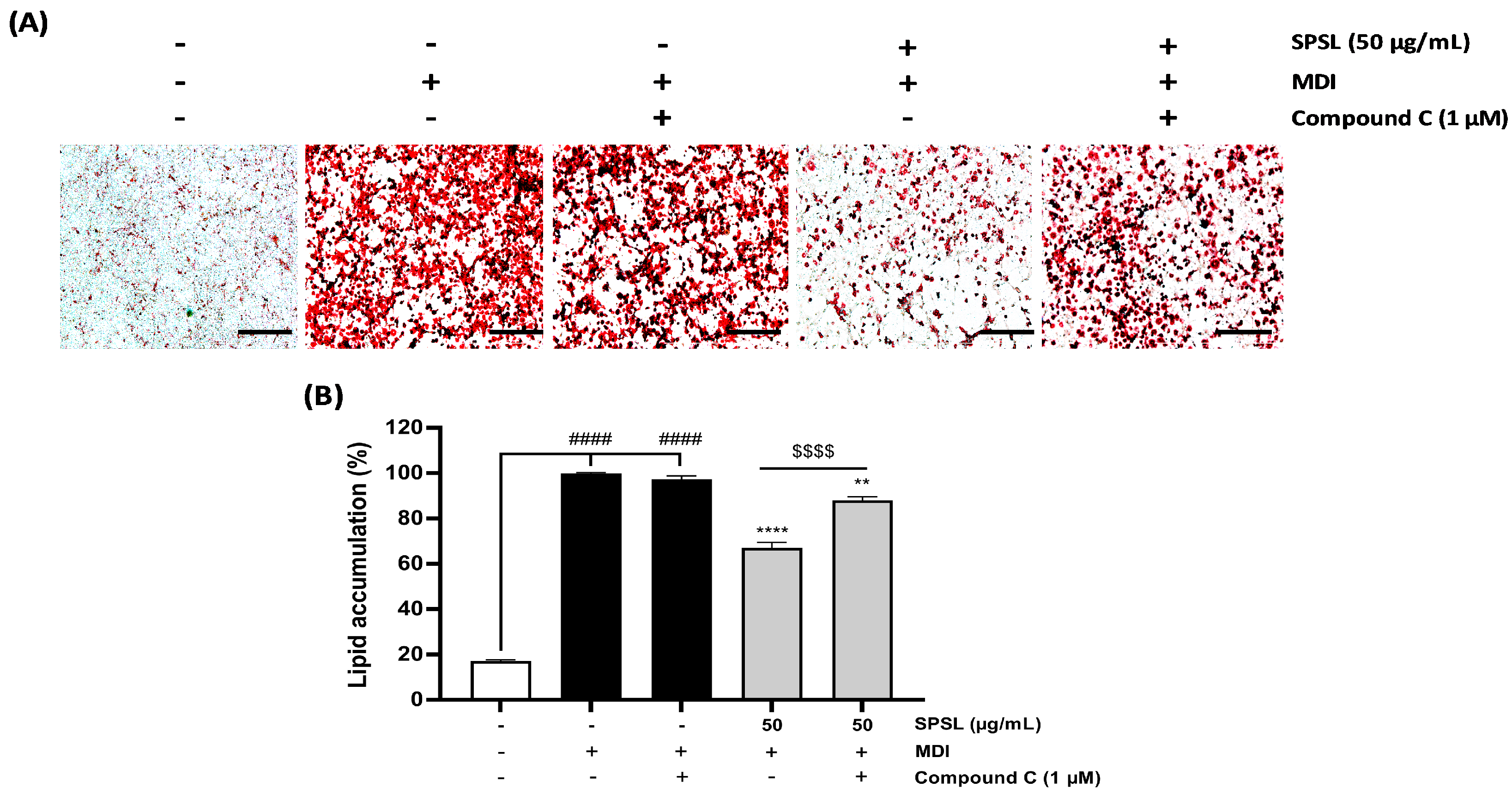
2.5. Inhibiting AMPK Activation Disturbed the Anti-Lipogenic Effect of SPSL
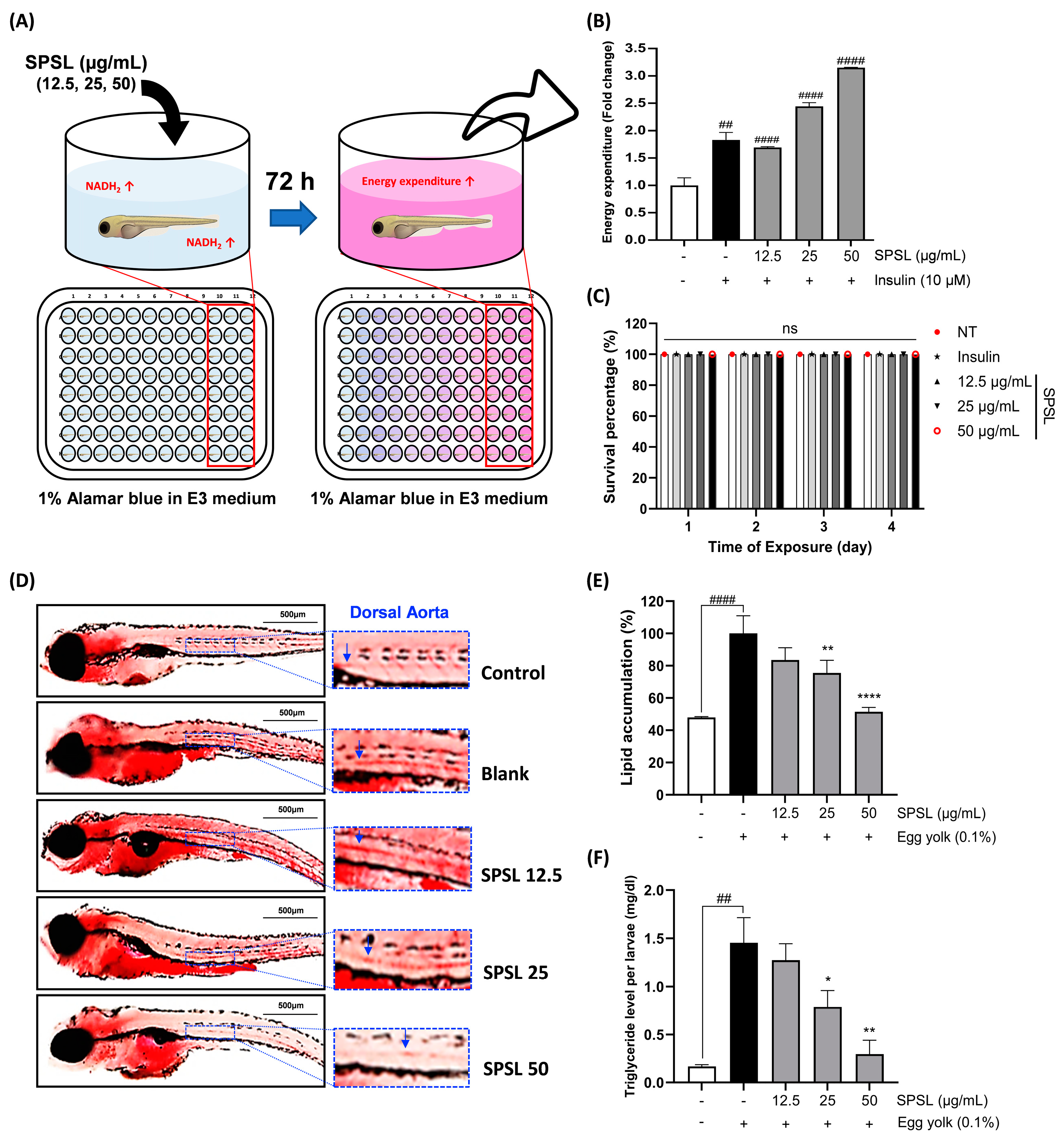
2.6. SPSL Enhanced the Energy Expenditure Rate and Lipid Accumulation in Zebrafish
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Celluclast-Assisted SL Hydrolysate and Sulfated Polysaccharides from SLC
4.3. Proximate Composition Analysis
4.4. Monosaccharide Composition Analysis Protocol
4.5. Estimation of Molecular Weight Distribution of Sulfated Polysaccharide
4.6. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
4.7. Identification of Sulfated Glycans in SPSL Using UPLC-Q-TOF MS/MS Based Fucoidan Library
4.8. Cell Culture and Determination of Adipocyte Differentiation
4.9. Immunoblotting Assay
4.10. Zebrafish Maintenance
4.11. Energy Expenditure Assay Using Zebrafish Embryo
4.12. High Cholesterol Diet Assay Using Zebrafish Embryo
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, I.; Maity, P. COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci. Total Environ. 2020, 728, 138882. [Google Scholar] [CrossRef]
- Naja, F.; Hamadeh, R. Nutrition amid the COVID-19 pandemic: A multi-level framework for action. Eur. J. Clin. Nutr. 2020, 74, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.M.; Gribsholt, S.B.; Andersen, A.L.; Richelsen, B.; Bruun, J.M. Obesity augments the disease burden in COVID-19: Updated data from an umbrella review. Clin. Obes. 2022, 12, e12508. [Google Scholar] [CrossRef] [PubMed]
- Puska, P.; Nishida, C.; Porter, D.; World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2003; pp. 1–2. [Google Scholar]
- Grajek, M.; Gdańska, A.; Krupa-Kotara, K.; Głogowska-Ligus, J.; Kobza, J. Global Self-Esteem, Physical Activity, and Body Composition Changes Following a 12-Week Dietary and Physical Activity Intervention in Older Women. Int. J. Environ. Res. Public Health 2022, 19, 13220. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.-Y. Anti-obesity drugs: A review about their effects and safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef]
- Purnell, J.Q.; le Roux, C.W. Hypothalamic control of body fat mass by food intake: The key to understanding why obesity should be treated as a disease. Diabetes Obes. Metab. 2024, 26, 3–12. [Google Scholar] [CrossRef]
- Gham, M.; Um, M.; Kye, S. Evaluation of dietary quality and nutritional status based on nutrition quotient and health functional food intake in the Korea elderly. J. Korean Soc. Food Cult. 2019, 34, 474–485. [Google Scholar]
- Farid, M.; Kodama, K.; Arato, T.; Okazaki, T.; Oda, T.; Ikeda, H.; Sengoku, S. Comparative study of functional food regulations in Japan and globally. Glob. J. Health Sci 2019, 11, 132. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Ryu, B.; Ahn, G.; Yeo, I.-K.; Jeon, Y.-J. Therapeutic potential of algal natural products against metabolic syndrome: A review of recent developments. Trends Food Sci. Technol. 2020, 97, 286–299. [Google Scholar] [CrossRef]
- Kaur, M.; Saini, K.C.; Ojah, H.; Sahoo, R.; Gupta, K.; Kumar, A.; Bast, F. Abiotic stress in algae: Response, signaling and transgenic approaches. J. Appl. Phycol. 2022, 34, 1843–1869. [Google Scholar] [CrossRef]
- Ould, E.; Caldwell, G.S. The Potential of Seaweed for Carbon Capture; CABI Review: São Paulo, Brazil, 2022. [Google Scholar]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wang, S.-H.; Huang, C.-Y.; Dong, C.-D.; Huang, C.-Y.; Chang, C.-C.; Chang, J.-S. Effect of molecular mass and sulfate content of fucoidan from Sargassum siliquosum on antioxidant, anti-lipogenesis, and anti-inflammatory activity. J. Biosci. Bioeng. 2021, 132, 359–364. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Li, J.; Chen, K.; Li, S.; Liu, T.; Wang, F.; Xia, Y.; Lu, J.; Zhou, Y.; Guo, C. Pretreatment with Fucoidan from Fucus vesiculosus Protected against ConA-Induced Acute Liver Injury by Inhibiting Both Intrinsic and Extrinsic Apoptosis. PLoS ONE 2016, 11, e0152570. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Karpe, F.; Fielding, B.A.; Macdonald, I.A.; Coppack, S.W. Integrative physiology of human adipose tissue. Int. J. Obes. 2003, 27, 875–888. [Google Scholar] [CrossRef]
- Lyons, C.L.; Roche, H.M. Nutritional Modulation of AMPK-Impact upon Metabolic-Inflammation. Int. J. Mol. Sci. 2018, 19, 3092. [Google Scholar] [CrossRef]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanisms of adipogenesis: The anti-adipogenic role of AMP-activated protein kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, K.K. Hypothalamic AMPK as a Mediator of Hormonal Regulation of Energy Balance. Int. J. Mol. Sci. 2018, 19, 3552. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.I. A Revised Checklist and Preliminary Assessment of the Macrobenthic Marine Algae and Seagrasses of Oregon; Native Plant Society of Oregon: Eugene, OR, USA, 1997. [Google Scholar]
- Miller, K. Seaweeds of California; Updates of California Seaweed Species List; University of California Jepson Herbarium: Berkeley, CA, USA, 2012; pp. 1–59. [Google Scholar]
- Lee, O.H.; Yoon, K.Y.; Kim, K.J.; You, S.; Lee, B.Y. Seaweed extracts as a potential tool for the attenuation of oxidative damage in obesity-related pathologies 1. J. Phycol. 2011, 47, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Wynne, M.; Taylor, W. Common Seaweeds of China; English Edition; Taylor & Francis: Abingdon, UK, 1984. [Google Scholar]
- Matsumoto, K.; Ichihara, K.; Shimada, S. Taxonomic reinvestigation of Petalonia (Phaeophyceae, Ectocarpales) in southeast of Honshu, Japan, with a description of Petalonia tenuis sp. nov. Phycologia 2014, 53, 127–136. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Flores, M.L.; Pujol, C.A.; Becerra, M.B.; Navarro, D.A.; Córdoba, O.; Damonte, E.B.; Stortz, C.A. Fucoidans from the phaeophyta Scytosiphon lomentaria: Chemical analysis and antiviral activity of the galactofucan component. Carbohydr. Res. 2019, 478, 18–24. [Google Scholar] [CrossRef]
- Jung, J.G.; Kim, J.H.; Kim, J.H.; Kim, Y.S.; Jin, D.-H.; Jin, H.-J. Effect of Scytosiphon lomentaria Ethanol Extracts on Myostatin Activity and Zebrafish Obesity Induced by High Feeding. J. Life Sci. 2021, 31, 699–709. [Google Scholar]
- Hyun, J.; Yang, H.-W.; Je, J.-G.; Lee, H.-G.; Kim, G.H.; Jeon, Y.-J. The potent antioxidant effect of Neutrase-assisted hydrolysate from heat-resistant Pyropia yezoensis by molecular weight change. Algal Res. 2023, 69, 102894. [Google Scholar] [CrossRef]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Choi, S.S.; Park, J.; Choi, J.H. Revisiting PPARγ as a target for the treatment of metabolic disorders. BMB Rep. 2014, 47, 599–608. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Liu, M.; Zhou, Y.; Zhang, L.; Li, Y. The New Role of AMP-Activated Protein Kinase in Regulating Fat Metabolism and Energy Expenditure in Adipose Tissue. Biomolecules 2021, 11, 1757. [Google Scholar] [CrossRef]
- Pollard, A.E.; Martins, L.; Muckett, P.J.; Khadayate, S.; Bornot, A.; Clausen, M.; Admyre, T.; Bjursell, M.; Fiadeiro, R.; Wilson, L.; et al. AMPK activation protects against diet-induced obesity through Ucp1-independent thermogenesis in subcutaneous white adipose tissue. Nat. Metab. 2019, 1, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Samuels, J.S.; Shashidharamurthy, R.; Rayalam, S. Novel anti-obesity effects of beer hops compound xanthohumol: Role of AMPK signaling pathway. Nutr. Metab. 2018, 15, 42. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.E.; Han, J.S. Fucoidan Stimulates Glucose Uptake via the PI3K/AMPK Pathway and Increases Insulin Sensitivity in 3T3-L1 Adipocytes. J. Life Sci. 2021, 31, 1–9. [Google Scholar]
- Lee, H.-G.; Jayawardena, T.U.; Song, K.-M.; Choi, Y.-S.; Jeon, Y.-J.; Kang, M.-C. Dietary fucoidan from a brown marine algae (Ecklonia cava) attenuates lipid accumulation in differentiated 3T3-L1 cells and alleviates high-fat diet-induced obesity in mice. Food Chem. Toxicol. 2022, 162, 112862. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Pang, G.; Xie, J.; Chen, Q.; Hu, Z. How functional foods play critical roles in human health. Food Sci. Hum. Wellness 2012, 1, 26–60. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, S.; Cheon, J.; Lee, J.; Kim, B.K.; Lee, S.-H.; Kong, C.; Kim, Y.Y.; Kim, M. Effects of Scytosiphon lomentaria on osteoblastic proliferation and differentiation of MC3T3-E1 cells. Nutr. Res. Pract. 2016, 10, 148–153. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, N.; Tian, Y.; Chang, Y.; Wang, J. Fucoidans from Thelenota ananas with 182.4 kDa Exhibited Optimal Anti-Adipogenic Activities by Modulating the Wnt/β-Catenin Pathway. J. Ocean Univ. China 2021, 20, 921–930. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef]
- Yajun, Z.; Qingtong, X.; Lijun, Y.; Peter Chi-Keung, C.; Zhengang, Z. Behavior of Non-Digestible Polysaccharides in Gastrointestinal Tract: A Mechanistic Review of its Anti-Obesity Effect. eFood 2021, 2, 59–72. [Google Scholar] [CrossRef]
- Gurpilhares, D.d.B.; Cinelli, L.P.; Simas, N.K.; Pessoa Jr, A.; Sette, L.D. Marine prebiotics: Polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chem. 2019, 280, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Lee, H.-G.; Hyun, J.; Jayawardhana, H.H.A.C.K.; Liyanage, N.M.; Nagahawatta, D.P.; Kang, M.-C.; Jeon, Y.-J. Enzyme-assisted hydrolyzation, chemical characterization, and lipid-lowering activity of crude sulfated polysaccharides from Sargassum coreanum. J. Funct. Foods 2023, 107, 105627. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Y.; Wu, K.; Wang, X. Dual effects of metformin on adipogenic differentiation of 3T3-L1 preadipocyte in AMPK-dependent and independent manners. Int. J. Mol. Sci. 2018, 19, 1547. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, J.; Kim, H.L.; Sim, J.E.; Youn, D.H.; Kang, J.; Lim, S.; Jeong, M.Y.; Yang, W.M.; Lee, S.G. Vanillic acid attenuates obesity via activation of the AMPK pathway and thermogenic factors in vivo and in vitro. FASEB J. 2018, 32, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Kajita, K.; Mune, T.; Ikeda, T.; Matsumoto, M.; Uno, Y.; Sugiyama, C.; Matsubara, K.; Morita, H.; Takemura, M.; Seishima, M.; et al. Effect of fasting on PPARgamma and AMPK activity in adipocytes. Diabetes Res. Clin. Pract. 2008, 81, 144–149. [Google Scholar] [CrossRef]
- Zuo, J.; Zhang, Y.; Wu, Y.; Liu, J.; Wu, Q.; Shen, Y.; Jin, L.; Wu, M.; Ma, Z.; Tong, H. Sargassum fusiforme fucoidan ameliorates diet-induced obesity through enhancing thermogenesis of adipose tissues and modulating gut microbiota. Int. J. Biol. Macromol. 2022, 216, 728–740. [Google Scholar] [CrossRef]
- Zou, Y.; Ju, X.; Chen, W.; Yuan, J.; Wang, Z.; Aluko, R.E.; He, R. Rice bran attenuated obesity via alleviating dyslipidemia, browning of white adipocytes and modulating gut microbiota in high-fat diet-induced obese mice. Food Funct. 2020, 11, 2406–2417. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kang, M.-C.; Jeon, Y.-J.; Kim, Y.-S. Inhibitory effect of sulfated polysaccharide from Sargassum thunbergii in PA-induced lipid accumulation in vitro. Food Biosci. 2023, 51, 102144. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; de Mello, J.C. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, M.A. A Rapid Method for Determination of Sulfate in Water Samples. Environ. Lett. 1974, 7, 237–243. [Google Scholar] [CrossRef]
- Je, J.-G.; Kim, C.-Y.; Sim, J.; Roh, Y.; Kurera, M.J.M.S.; Liyanage, N.M.; Jung, S.; Jeon, Y.-J.; Ryu, B. Anti-inflammatory activity and structural analysis of fucoidan extracted from Sargassum fusiforme via ESI-TOF MS spectrometry. Food Biosci. 2024, 60, 104414. [Google Scholar] [CrossRef]
- Hyun, J.; Park, M.H.; Lee, Y.H.; Lee, Y.; Jeong, S.J.; Choi, S.S.; Khim, K.W.; Eom, H.J.; Hur, J.-H.; Park, C.Y.; et al. Vernicia fordii (Hemsl.) Airy Shaw extract stimulates insulin secretion in pancreatic β-cells and improves insulin sensitivity in diabetic mice. J. Ethnopharmacol. 2021, 278, 114238. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Lin, C.-Y.; Hsu, C.-C.; Yeh, K.-Y.; Her, G.M. Liver-directed microRNA-7a depletion induces nonalcoholic fatty liver disease by stabilizing YY1-mediated lipogenic pathways in zebrafish. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2018, 1863, 844–856. [Google Scholar] [CrossRef]

| Proximate Composition (%) | |||
|---|---|---|---|
| Sample | Polysaccharides | Proteins | Polyphenols |
| SLC | 12.49 ± 0.26 | 9.89 ± 0.38 | 0.85 ± 0.04 |
| SPSL | 41.99 ± 0.65 ** | 12.10 ± 1.65 * | 0.48 ± 0.04 |
| No. | Predicted Glycan Structure | Area | m/z | Exact Mass (g·mol−1) |
|---|---|---|---|---|
| 1 | [Fuc2Hex(SO3)2]2− | 174,865 | 315.0391 | 630.0783 |
| 2 | [FucHex2(SO3)2]2− | 2,863,978 | 323.0366 | 646.0732 |
| 3 | [Fuc(SO3)]− | 97,920 | 243.018 | 243.018 |
| 4 | [Fuc4(SO3)2]2− | 5,618,608 | 380.0706 | 760.1413 |
| 5 | [Fuc2(SO3)]− | 38,633,162 | 389.0759 | 389.0759 |
| 6 | [Fuc6Hex(SO3)3]3− | 3,410,449 | 431.0865 | 1293.2595 |
| 7 | [Xyl2(SO3)2]2− | 4,109,871 | 219.9971 | 439.9942 |
| 8 | [FucXyl3(SO3)5]5− | 83,677 | 190.9886 | 954.9429 |
| 9 | [FucXyl(SO3)]− | 691,885 | 375.0602 | 375.0603 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, J.; Lee, H.-G.; Je, J.-G.; Choi, Y.-S.; Song, K.-M.; Kim, T.-K.; Ryu, B.; Kang, M.-C.; Jeon, Y.-J. L-Fucose-Rich Sulfated Glycans from Edible Brown Seaweed: A Promising Functional Food for Obesity and Energy Expenditure Improvement. Int. J. Mol. Sci. 2024, 25, 9738. https://doi.org/10.3390/ijms25179738
Hyun J, Lee H-G, Je J-G, Choi Y-S, Song K-M, Kim T-K, Ryu B, Kang M-C, Jeon Y-J. L-Fucose-Rich Sulfated Glycans from Edible Brown Seaweed: A Promising Functional Food for Obesity and Energy Expenditure Improvement. International Journal of Molecular Sciences. 2024; 25(17):9738. https://doi.org/10.3390/ijms25179738
Chicago/Turabian StyleHyun, Jimin, Hyo-Geun Lee, Jun-Geon Je, Yun-Sang Choi, Kyung-Mo Song, Tae-Kyung Kim, Bomi Ryu, Min-Cheol Kang, and You-Jin Jeon. 2024. "L-Fucose-Rich Sulfated Glycans from Edible Brown Seaweed: A Promising Functional Food for Obesity and Energy Expenditure Improvement" International Journal of Molecular Sciences 25, no. 17: 9738. https://doi.org/10.3390/ijms25179738
APA StyleHyun, J., Lee, H.-G., Je, J.-G., Choi, Y.-S., Song, K.-M., Kim, T.-K., Ryu, B., Kang, M.-C., & Jeon, Y.-J. (2024). L-Fucose-Rich Sulfated Glycans from Edible Brown Seaweed: A Promising Functional Food for Obesity and Energy Expenditure Improvement. International Journal of Molecular Sciences, 25(17), 9738. https://doi.org/10.3390/ijms25179738








