Abstract
The expression of genes of various proinflammatory chemokines and cytokines is controlled, among others, by the signaling pathway of the nuclear factor kappaB (NF-κB) superfamily of proteins, providing an impact on immune system functioning. The present review addresses the influence and role of the NF-κB pathway in the development and progression of most vital endometrial diseases in human and animal species. Immune modulation by NF-κB in endometritis, endometrosis, endometriosis, and carcinoma results in changes in cell migration, proliferation, and inflammation intensity in both the stroma and epithelium. In endometrial cells, the NF-κB signaling pathway may be activated by multiple stimuli, such as bacterial parts, cytokines, or hormones binding to specific receptors. The dysregulation of the immune system in response to NF-κB involves aberrant production of chemokines and cytokines, which plays a role in endometritis, endometriosis, endometrosis, and endometrial carcinoma. However, estrogen and progesterone influence on the reproductive tract always plays a major role in its regulation. Thus, sex hormones cannot be overlooked in endometrial disease physiopathology. While immune system dysregulation seems to be NF-κB-dependent, the hormone-independent and hormone-dependent regulation of NF-κB signaling in the endometrium should be considered in future studies. Future goals in this research should be a step up into clinical trials with compounds affecting NF-κB as treatment for endometrial diseases.
1. Introduction
The nuclear factor kappaB (NF-κB) is one of the transcription factors responsible for cellular chemoresistance [1]. The NF-κB signaling pathway controls the expression of genes of proinflammatory chemokines and cytokines, adhesion molecules, chemoattractants for inflammatory cells, and antigen receptors on immune cells [2]. It remains a critical regulator of immune systems in most multicellular organisms. It can be found in most animal cell types, involved in multiple cellular responses [3,4,5]. Proinflammatory chemokines and cytokines act under both physiological conditions and pathological processes, and their aberrant production leads to immune system dysregulation, which plays a role in some endometrial diseases’ initiation and progression [6]. In the case of endometritis, NF-κB is strongly involved in the activation of proinflammatory genes which are critical for uterine response to infection and inflammation [7]. In the case of endometriosis, NF-κB stimulates the expression of genes that regulate endometriotic cell adhesion, migration, and proliferation, as well as extracellular matrix (ECM) remodeling and inflammation intensity in ectopic endometrium [1,6], whereas in the case of endometrosis, NF-κB seems to take part in the mediation of the expression of genes that stimulate proinflammatory chemokines and inhibit anti-inflammatory cytokine in fibrotic endometrium, as well as stimulating ECM remodeling and chemotaxis [8,9]. Moreover, NF-κB is also associated with chronic inflammation in neoplastic development, as well as with the mediation of insensitivity to growth inhibitory signals, avoidance of apoptosis, angiogenesis, and metastasis [10]. As the endometrium undergoes cyclic changes in all species, its cells have to be constantly supplied with signal molecules, which can lead to increased susceptibility to actions of the NF-κB pathway [11].
Animal models are used widely in studies regarding the NF-κB pathway, especially using genetically modified mice. Especially in cancer and chronic inflammatory diseases it is an important object of interest, with the possible development of new therapeutic approaches [5]. Additionally, in endometriosis studies mice and rats are being used [1]. While the prevalence and importance of endometrial diseases varies between species, most of the mechanisms leading to their development are alike [4,5]. This indicates that we can learn about the NF-κB pathway in endometrial diseases on both sides—using animal data to improve human studies, and using human data in animal research.
In this review, the role of NF-κB in the pathogenesis of endometrial diseases in humans and animals is described. As NF-κB signaling has a close relationship with estrogen and progesterone, which are the key regulatory factors for the initiation and progression of endometrial diseases, the hormone-dependent regulation of NF-κB pathways is also summarized.
2. NF-κB Signaling in Endometrial Disease
Many groups have reported that the activation of NF-κB plays, through complex mechanisms [12,13,14,15,16], a vital role in the regulation of the initiation and progression of many endometrial diseases such as endometritis [7,17,18,19], endometriosis [6,19,20], endometrosis [8,9,11], and endometrial carcinoma [16,21,22,23]. As the referred endometrial diseases have a marked impact on reproductive health, causing subfertility and infertility, the selected recent research on pathways, targets, and mechanisms are summarized in Table 1.

Table 1.
The selected recent research on pathways, targets, and mechanisms of the most common endometrial diseases (endometritis, endometriosis, endometrosis, and endometrial carcinoma) was carried out on humans and animals (cattle, mouse, and horse) specimens.
2.1. NF-κBa Activation
The superfamily of NF-κB proteins consists of five known members: protein RelA of NF-κB superfamily (RelA (p65)), protein RelB of NF-κB superfamily (RelB), protein cRel of NF-κB superfamily (cRel), protein NF-κB1 of NF-κB superfamily (NF-κB1 (p50/p105)), and protein NF-κB2 (NF-κB2 (p52/p100)) [11]. Each of the five NF-κB members interacts with suitable inhibitory factors belonging to the family of inhibitors of κB (IκB) (IκBα, IκBβ, IκBε, or Bcl-3) or the C-terminal sequences of the NF-κB precursor proteins (p105 and p100) [12]. The RelA, RelB, and cRel proteins share a homology domain Rel, which is a transcription activation domain, allowing control of the transcription of DNA molecules, whereas NF-κB1 and NF-κB2 proteins are precursor proteins, which need proteolytic activation and forming dimers with suitable Rel protein [13]. Heterodimers of NF-κB are present in the cytoplasm bound to IκB proteins, which are phosphorylated by IκB kinases (IKKs) when NF-κB signaling is activated [1]. This activation takes place through canonical and noncanonical signaling pathways, which engage RelA/NF-κB1 and RelB/NF-κB2 subunits, respectively [15]. However, regardless of the pathway, this IκB-related activation by the IKK complex allows NF-κB dimers to perform nuclear translocation and activation of the transcription of target genes [1,16].
In endometrial cells, both stroma and epithelium, the NF-κB signaling pathway may be activated by multiple stimuli, such as bacterial parts, cytokines, or hormones binding to specific receptors [1,17,22]. Each stimulus may activate the IKK complex, which phosphorylates IκB proteins and thus allows the cytoplasmic heterodimer to be released and translocated to the nucleus [25] on a canonical (RelA/NF-κB1) [7,8,9,16,18,19,21,22] or noncanonical (RelB/NF-κB2) [8,9,16] pathway. This translocation of a transcription activation domain elicits the transcriptional activity of genes [1] of several receptors, enzymes, cytokines/chemokines, and other signaling proteins, indicating the key role of NF-κB signaling in the pathogenesis of the most common endometrial diseases [6,7,8,9,16,17,18,19,20,21,22,23,24]. In this way, NF-κB signaling can regulate the endometrial cells’ reaction to infection and inflammation [7]; immune cells’ chemotaxis [6]; cellular behaviors of endometrial cells including cells proliferation, adhesion, migration [6], apoptosis [21], and invasion [23]; as well as ECM remodeling [19], which is illustrated in detail in the following subsections.
2.2. Endometritis
Endometrial infections are the most common reproductive diseases in dairy cows [4,26] and breeding mares [27,28], whereas the clinical significance of chronic endometritis has rarely been a concern in human clinical practice [29]. In the course of endometritis, the infiltration of immune cells into the endometrial stroma is observed, while the type and intensity of infiltration varies between the subtype of endometritis [29,30]. Suppurative endometritis, which is mostly caused by a bacterial infection, is characterized by the recruitment of neutrophils and T cells, whereas nonsuppurative endometritis is characterized by the infiltration of lymphocytes and plasma cells [30], while macrophages, eosinophils [28], and mast cells [31] are uncommon. In response to the intrauterine bacterial infection, Toll-like receptor 4 (TLR4) on the endometrial cells of luminal epithelium recognizes lipopolysaccharide (LPS) derived from Gram-negative bacteria and activates NF-κB and mitogen-activated protein kinase (MAPK) pathways [7].
In human [17] and bovine endometrium [7,18], activation of these pathways stimulates the release of interleukins (ILs): IL-1β, IL-6, and IL-8, as well as monocyte chemoattractant protein 1 (MCP-1), tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (COX-2), and inducible NO synthase (iNOS). IL-1β, IL-6, and TNF-α act as the proinflammatory cytokines and immediately cause an inflammatory response in affected endometrium [24]. Furthermore, together with MCP-1, they act as chemokines. These chemokines are involved in the recruitment and activation of macrophages, neutrophils, eosinophils, basophils, monocytes, and natural killer cells (NK-cell) to the inflamed endometrium [32,33], whereas the NF-κB signaling pathway protects a survival of immune cells in the face of bacterial infections or irritation [34] (Figure 1).
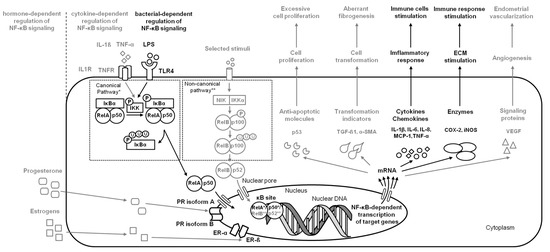
Figure 1.
A simplified schema of the NF-κB signaling pathway in the endometrium in the case of endometritis. Studied pathways are marked in black. Pathways requiring further research are marked in grey. Both canonical pathway * and noncanonical pathway ** are considered.
2.3. Endometriosis
Endometriosis is a quite common benign gynecological human disease, affecting up to 50% of women with infertility [1]. In the course of endometriosis, the ectopic growth of the endometrium, predominantly glands, and stroma outside the uterus is observed [6]. The ectopic endometrium appears mainly in the ovaries and pelvic peritoneum, causing chronic pain, dysmenorrhea, and infertility [1].
In response to multiple factors such as IL-1β, TNF-α [1], and TLR4 [19], endometriotic cells activate the NF-κB signaling pathway, affecting endometriosis initiation and progression. In the initial step of endometriosis, the NF-κB pathway induces upregulation of adhesion molecules, including Decoy receptor 3 (DcR3) [35], homing cell adhesion molecule (CD44), intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) [36], which affect the aberrant adhesion of endometriotic cells in the ectopic locations [35]. On the progression step of endometriosis, the activation of the NF-κB pathway stimulates the release of IL-1β, IL-6, IL-8, TNF-α, interferon γ (IFN-γ), eotaxin, regulated on activation, normal T-cell expressed and secreted (RANTES) [6], and proliferating cell nuclear antigen (PCNA) [1], which promote a chronic inflammatory environment in endometriotic foci; enhances angiogenesis via increased production of vascular endothelial growth factor (VEGF) [6]; and avoidance of apoptosis via activation of the antiapoptotic molecules X-linked inhibitor of apoptosis (XIAP), B-cell lymphoma 2 protein (Bcl-2), and B-cell lymphoma-extra large protein (Bcl-XL) [1]. Moreover, the NF-κB pathway stimulates transcription of endopeptidases—matrix metalloproteinases (MMPs): MMP-2 and MMP-9; which are responsible for ECM degradation, and thus increasing endometriotic cell migration and invasion [20,36]. One may conclude that the ability of endometriotic cells to migrate and invade is crucial for the implantation and extension of the ectopic endometrium [1] (Figure 2).
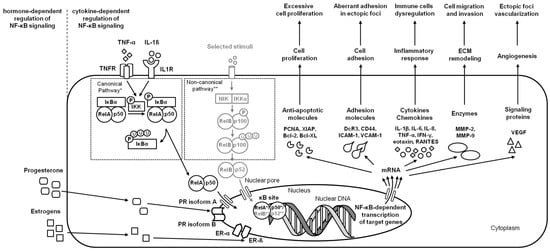
Figure 2.
A simplified schema of the NF-κB signaling pathway in the endometrium in the case of endometriosis. Studied pathways are marked in black. Pathways requiring further research are marked in grey. Both canonical pathway * and noncanonical pathway ** are considered.
2.4. Endometrosis
Endometrosis is a common benign gynecological equine disease, affecting up to 50% of older mares [30]. In the course of endometrosis, chronic degenerative changes in the endometrium are observed [28]. In affected endometrium, alterations of the epithelial and stromal cells lead to periglandular fibrosis and degeneration, dilatation, and atypical differentiation of the endometrial glands [30,37,38].
On the initial step of endometrosis, increased release of pro-fibrotic cytokines and chemokines (IL-1α, IL-1ß, IL-6, IL-10, and TNF-α) [39,40] stimulates immune cells infiltration and, via transforming growth factor- ß1 (TGF-ß1), up-regulates alpha smooth muscle actin (α-SMA) transcripts in resident fibroblasts, promoting their differentiation into myofibroblasts and thus leading to alternated secretion of ECM components and fibrogenesis [40,41,42]. On the next step of endometriosis, dysregulated immune cells synthesize profibrotic cytokines (IL-6, MCP-1, TNF-α, and TGF-ß1) that participate in remodeling of the endometrial ECM mediated by the modified activity of MMPs (up-regulated MMP-2 and MMP-9 transcription and downregulated MMP-13 transcription), decrease the activity of the tissue inhibitors of metalloproteinases (TIMP) [40,41,42,43,44], and increase activity of hyaluronan synthases (HASs) [8,9]. One may observe that the cascade of endometrial changes in endometrosis is similar to those described in other endometrial diseases; however, the knowledge of the role of NF-κB in its signaling pathway is limited. The similarities found in the signs of immune cell dysregulation and ECM remodeling [8,40,41,42] and the genetic background [45] of equine endometriosis with other endometrial diseases provide a rationale for further in-depth investigations. So far, with the increased progression of endometrosis, the increased activation of canonical (RelA/NF-κB1) and noncanonical (RelB/NF-κB2) signaling pathways, resulting in downregulation of IL-6 releasing and up-regulation of HAS 1 and HAS 3 activity, were demonstrated [8]. Moreover, the increased activation of canonical (RelA/NF-κB1) signaling pathways resulted in up-regulation of IL-6 and MCP-1 release as well as up-regulation of HAS 2 activity, which was noted in destructive histopathological types of endometrosis [9]. While other potentially relevant receptors, enzymes, cytokines, chemokines, or other signaling proteins have not yet been studied in the context of the NF-κB signaling pathway, which does not rule out their involvement in the pathogenesis of endometrosis, the need for further study of NF-κB signaling in endometrosis is indicated by the results of the latest research, according to which advanced endometrial fibrosis may be transformed into cancer [45]. In the case of endometrosis, changes in the expression of both metabolism-related genes (CYP1B1, COX4I1, COX3, and UQCRFS1) and immune response genes (MMP7, JCHAIN, PIGR, CALR, B2M, and FCGRT) may alter the metabolic-immune microenvironment in affected endometrium, predisposing fibrotic tissue to carcinogenesis [45]. Especially since ECM deposition, remodeling, and cross-linking driving fibrosis to stiffen the endometrial stroma are characteristic of human endometrial carcinoma [21,23,46] (Figure 3).
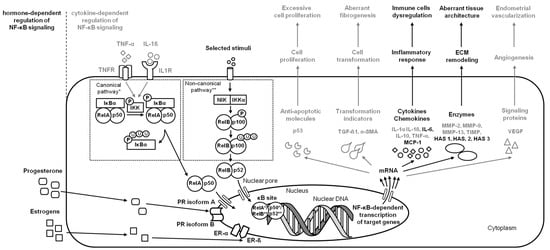
Figure 3.
A simplified schema of the NF-κB signaling pathway in the endometrium in the case of endometrosis. Studied pathways are marked in black. Pathways requiring further research are marked in grey. Both canonical pathway * and noncanonical pathway ** are considered.
2.5. Endometrial Carcinoma
Endometrial carcinoma is one of the most common forms of malignant gynecological disease in humans [47], and at the same time the second most common gynecological neoplasm and the fourth most frequent cancer in women worldwide [22]. In contrast to women, endometrial carcinoma is rarely reported as single case reports in mares [48,49,50,51,52], and even less often in cattle [53].
In the course of endometrial carcinoma, the two main clinico-pathological variants of endometrial carcinoma are considered—type 1 representing low-grade and estrogen-dependent endometrioid carcinomas, and type 2 representing high-grade, aggressive, and estrogen-independent nonendometrioid carcinomas [54]. Type 1 usually coexists with or is preceded by endometrial hyperplasia, whereas type 2 arises occasionally in endometrial polyps or from precancerous lesions developing in atrophic endometrium [16]. Both types of carcinogenesis are related to the NF-κB signaling pathway by the regulation of genes expression involved in apoptosis, the cell cycle, differentiation, and cell migration. The activity of NF-κB pathways may provide a survival advantage for tumor cells by the suppression of apoptosis via regulation of target genes Bcl-XL [16] and caspase-3 (CASP3) [47]. Moreover, the activity of NF-κB in endometrial carcinoma promotes tumor cell migration, invasion, and metastasis via stimulation of Ras-related C3 botulinum toxin substrate 1 (Rac1), MMP-2 [24], and MMP-9 [21,23] transcription, and thus the basement membranes’ and ECM degradation, respectively [21,23]. Interestingly, in endometrial cancer cells both Rac1—an indicator of tumor cell migration and invasion, and MMP-2—an indicator of tumor metastasis and primary tumor growth—were downregulated by IL-37 [24]. IL-37 is an anti-inflammatory cytokine [55] involved in immune responses [56] and tumorigenesis [57] through the downregulation of pro-inflammatory cytokines and inhibition of metastasis, respectively. Moreover, the activity of NF-κB pathways may also decrease the expression of IL-37 protein in cancer cells, confirming the inhibitory roles of IL-37 on metastasis in endometrial carcinoma [23] (Figure 4).
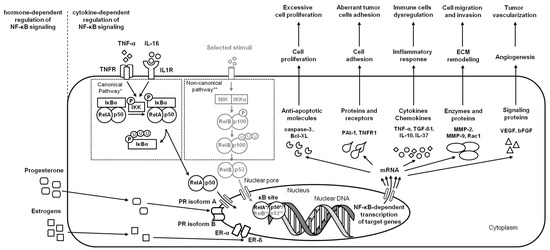
Figure 4.
A simplified schema of the NF-κB signaling pathway in the endometrium in the case of endometrial carcinoma. Studied pathways are marked in black. Pathways requiring further research are marked in grey. Both canonical pathway * and noncanonical pathway ** are considered.
3. Regulation of NF-κB Signaling in Endometrium
It has been evidenced that the hormone-dependent immune system dysregulation in response to the aberrant production of chemokines and cytokines plays a role in the initiation and progression of endometrial diseases, such as endometritis [7,17], endometriosis [1,6], endometrosis [11], and endometrial carcinoma [23]. In both humans and animals, hormonal therapies, based on the creation of hypoestrogenic (GnRH agonists), hyperandrogenic (danazol and gestrinone), or hyperprogestogenic (progesterone and progestins) environments are commonly used [6,58,59], often causing systemic side effects due to the suppression of endometrial cell proliferation and endogenous steroid hormone concentrations [6,60]. Therefore, the hormone-dependent regulation of NF-κB signaling in the endometrium is summarized here to highlight the hormone-NF-κB interaction.
3.1. Progesterone’s Regulation
In both, humans [61,62] and livestock animals [7], the uterus is resistant to infections with decreasing blood progesterone concentration and is susceptible to infections with increasing blood progesterone concentration. In human endometrium, progesterone was proven to inhibit TLR4 expression, NF-κB activation, as well as IL-6 and IL-8 productions, limiting the effectiveness of inflammation in response to bacterial infection [17]. Similarly, in bovine endometrium, progesterone was found to decrease the expressions of IL-1β, IL-6, IL-8, and TNF-α via the NF-κB and MAPK signaling pathways [7]. The progesterone-related suppression of the NF-κB pathway was observed in endometrial cells with high progesterone receptors (PR) expression, contrary to weak PR expression, suggesting the PR-mediated action [17]. Moreover, it has been proven that NF-κB (RelA (p65)) and PR can repress each other through direct contact [63].
Progesterone has two isoforms of PRs (PR isoform A and PR isoform B) encoded by the same gene, whereas PR isoform A is predominant in the uterus and ovaries [64,65]. In endometrial epithelium and stroma, progesterone passes through the cells and binds to an intranuclear receptor, then regulates cell development and differentiation by inducing multiple genes transcription [65,66]. Progesterone binds to PR isoform A and acts as an antagonist for estrogen-induced epithelial cell proliferation. Moreover, progesterone downregulates PR expression, and thus regulates both its biological effects and receptor abundance [30,67]. In human endometrium, these biological alterations during the ovarian cycle are also regulated via NF-κB (RelA (p65))/PR repression [68]; however, in animals, the NF-κB-PR interaction in the ovarian cycle requires confirmation. So far, in equine endometrium, no correlations were found between genes expression of RelA (p65), NF-kB1, NF-kB2, and PR in both the follicular and luteal phases of the ovarian cycle [11]. In human endometrium, the NF-κB-PR interaction was suggested to be involved in pathophysiologic processes, such as irregular uterine bleeding [69] and endometriosis [1,70,71]. In the case of recurrence of ovarian endometriosis, both inverse [70] and direct [71] relations between RelA (p65) and PR were reported; however, both researchers considered PR isoform B as predominant [70,71]. In equine endometrium, the initial signs of NF-κB-PR interaction were indicated in mild endometrosis by the strong positive correlation between RelA and PR genes transcription [11]. In the case of endometrial carcinoma, progesterone exertion was suspected to inhibit anti-inflammatory cytokines production, which was confirmed for IL-10 [62] and excluded for IL-37 [23]. The recent result suggests some signs of hormone-dependent immune system dysregulation in endometrial carcinogenesis. However, regarding the role of the NF-κB-PR interaction, this requires further research.
3.2. Estrogen’s Regulation
Contrary to the blood progesterone concentration in both humans [61,62] and livestock animals [7], the uterus is susceptible to infections with the decreasing of blood estrogen concentration and is resistant to infections with the increasing of blood estrogen concentration. In endometrial epithelium and stroma, estrogen passes through the cells, binds to an intranuclear receptor, and regulates cell development and differentiation by inducing multiple genes transcription [65,66]. In the canonical pathway, estrogen has two specific estrogen receptors (ERs) (ER-α and ER-ß) encoded by different genes. ER-α is predominant in the uterus [65,72]. The binding of estradiol to ER-α and ER-ß has contradictory uterotrophic effects. The activation of ER-α stimulates the proliferation of the epithelial cells and stromal cells, whereas the activation of ER- ß inhibits it. Moreover, the activation of ER-α upregulates PR expression, whereas the activation of ER- ß downregulates it [30,67]. In healthy human endometrium, the negative crosstalk between ER-α and NF-κB was confirmed as a part of the regulatory process of normal physiological responses [73]. In horses, the NF-κB-ER-α interaction has not been confirmed so far, since no correlations were found between genes expression of RelA (p65), NF-kB1, NF-kB2, and ER-α and ER-ß in both phases of the ovarian cycle [18]. Considering endometriosis as an estrogen-dependent disease, the NF-κB-ER interaction was suggested to be involved in its pathogenesis. It can be observed that estrogen promotes the implantation of endometrial tissue to ectopic foci, and thus is necessary but not sufficient for sustaining endometriosis [20]. In the ectopic endometrial cells, ER-α and ER-β were reported as able to increase NF-κB activity [74] by activating several proinflammatory pathways (CXCL12/CXCR4, PI3K/Akt), and thus promote the viability and proliferation of endometriotic cells [75]. In other studies on endometriosis, the inhibitory effect of estrogen signaling on NF-κB has been reported [76,77]. Estrogen was able to reduce the expression of a gene that encodes the angiotensin II receptor (AGTR1), and thus activates NF-κB signaling [77]. Moreover, in the eutopic endometrium, ER-β was able to downregulate TNFα/NF-κB signaling [76]. Therefore, the effects of estrogen signaling on NF-κB in endometriosis are controversial [1]. In equine endometrium, the initial signs of NF-κB-ER interaction were indicated in inactive nondestructive, active nondestructive, and active destructive types of endometrosis by moderate to strong negative correlations between RelA, NF-kB1, and ER-β genes transcription [11]. In the case of endometrial carcinoma, estradiol action was suspected to inhibit IL-10 [62], but not IL-37 [23] anti-inflammatory cytokines production. Moreover, in the endometrial carcinoma model, the NF-κB activity was induced by different concentrations of estradiol in a rapid, non-genomic, and non-receptor manner. It was confirmed that activation of NF-κB plays a role in estradiol-induced angiogenesis by up-regulator basic fibroblast growth factor (bFGF) and VEGF, a major angiogenic factor that induces endothelial cell proliferation and thus promotes tumor-induced angiogenesis [78]. Therefore, the role of the NF-κB–estrogen interaction, both receptor and non-receptor, in the hormone-dependent immune system dysregulation in endometrial diseases requires further research.
4. Perspectives for NF-κB Signaling in Endometrium
Over 700 compounds were identified as inhibitors of the NF-κB pathway, used to decrease transcription of genes [1] of several receptors (TLR4, ER-α, ER-ß, PGR, and TNFR1), enzymes (COX-2, iNOS, MMP-2, MMP-9, HAS 1, HAS 2, and HAS 3), cytokines/chemokines (IL-1α, IL-1β, IL-4, IL-6, IL-7, IL-8, IL-13, IL-15, IL-17, IL-18, IL-37, MCP-1, TNF-α, TGF-ß1, eotaxin, INF-γ, IP-10, MIP-1α, MIP-1β, and RANTES), and other signaling proteins (FGF, G-CSF, VEGF, Bcl-XL, caspase-3, PAI-1, and Rac1), respectively [6,7,8,9,15,16,17,18,19,20,21,22,23,24].
The biggest interest regarding influencing NF-κB signaling regards cancer treatment. In human medicine plenty of compounds of different origin have been found to inhibit this pathway; however, they are still in development and research. Most studies are conducted in isolated endometrial cells, cancer cells, or in mice models, hence clinical effectiveness is yet unknown.
Dehydroxymethylepoxyquinomicin (DHMEQ) is a specific inhibitor of NF-κB which binds to Rel proteins. It was found to induce apoptosis, stop cell cycles, and decrease proinflammatory cytokines secretion in cancer cell lines [79].
Mushroom-produced compounds are widely evaluated in the context of cancer therapy. Polysaccharide produced by Ganoderma lucidum is capable of inhibiting classical activation of NF-κB, which decreases P-glycoprotein expression in cancer cells. P-glycoprotein is one of the factors responsible for drug resistance, as it decreases transport of chemotherapeutic agents. In that way, this polysaccharide enables the effectiveness of anticancer drugs. Another polysaccharide decreasing levels of NF-κB is produced by Phellinus linteus. This species produces another compound, caffeic acid phenethyl ester (CAPE), which inhibites NF-κB binding to DNA. Another mechanism influencing NF-κB activation is promoting TNF-α, and thus suppressing anti-apoptotic action of NF-κB, which can be achieved with cordycepin produced by Cordyceps militaris, a parasitic fungus [2].
Another group of active substances is produced by plants. Parthenolide, acquired from feverfew (Tanacetum parthenium) was proven to inhibit NF-κB both in human and canine cancer cells [80]. Curcumin is already a quite well-known compound with a plethora of biological activities. It was found that in endometrial cancer liposomal curcumin decreased NF-κB expression in correlation with curcumin concentration [21].
Curcumin was also found to repress activation of NF-κB induced by TNF-α and IL-1β in endometriosis, with effects such as reduced production of proinflammatory cytokines, chemokines, and cell adhesion molecules. Other plant extracts were also evaluated in the context of endometrosis, such as andrographolide from Andrographis paniculate. Its mechanism is to inhibit NF-κB binding with DNA, and thus decrease the proliferation of endometriotic cells, resulting in lesion size reduction. Another compound with anti-inflammatory action in endometriosis is astragaloside IV, produced by Astragalus membranaceus by inhibition of the TLR4/NF-κB pathway [1,22].
Synthetic compound BAY 11-7085 prevents the phosphorylation of IκBα and release of NF-κB. A result of this is increased endometriotic cell apoptosis and reduced proliferation. To a similar effect, suppression of IκBα phosphorylation can be achieved with pyrrolidine dithiocarbamate, a chelating agent. Endometriotic cell apoptosis can also be induced with BV6, an antagonist of inhibitors of apoptosis proteins. On the other hand, thalidomide and pioglitazone, apart from their main mechanisms of action, can also decrease IL-8 secretion by inhibition of NF-κB activation by TNF-α in endometriosis [1].
Reverbα is a gene responsible for the circadian clock. Its activation inhibits activation of NF-κB by LPS, giving future perspectives for endometritis treatment. However, currently there is not much interest in NF-κB inhibition in endometritis. In endometrosis in mares NF-κB influence is only being evaluated; however, some results suggest that in future it can be considered as a therapeutic target [20].
Despite the conducted research, NF-κB inhibitors are still under development as treatment for endometrial diseases. As there is huge variability in active substances and their mechanisms of action it is extremely challenging to develop efficient and safe drugs. Proving the biological effect of a substance is an obvious initial step, but because of variety of the pathways activated by NF-κB, sometimes with contradictory actions, other possible consequences of NF-κB inhibition have to established. It is important to evaluate if NF-κB inhibition would be beneficial considering its role in cellular senescence. Usually it is safer to use more specific drugs, affecting a narrower range of cell and tissue physiology [1,78,81,82].
As NF-kB is composed of the same proteins in mammals, animal models can be relatively easily adapted for research and clinical trials. Additionally, it gives the opportunity to transfer data and knowledge between species. However, it is important to exclude compound activity in other physiological pathways, which would cause side effects [3,78,81]. Clinical trials are an important next step in future studies, even using animal models more closely related to humans than mice. On the other hand, animal species are still a step behind regarding drug development, and more fundamental research has yet to be conducted [4].
5. Conclusions
The aberrant NF-κB signaling pathway, causing immune system dysregulation, is involved in several aspects of endometrial disease pathogenesis in both humans and animals. This dysregulation in response to the aberrant production of chemokines and cytokines plays a role in the initiation and progression of endometritis, endometriosis, endometrosis, and endometrial carcinoma. Moreover, this immune system dysregulation seems to be both NF-κB-dependent and hormone-dependent; therefore, future studies should be focused on the hormone-dependent regulation of NF-κB signaling in the endometrium, as the detailed identification of hormone–NF-κB interaction is an important step toward understanding the pathogenesis of these conditions and developing effective strategies for the treatment of common endometrial diseases. Future perspectives in studies regarding the NF-κB pathway in endometrial diseases regard performing clinical trials for the development of treatment.
Author Contributions
Conceptualization, Ł.Z. and M.D.; writing—original draft preparation, Ł.Z., T.J. and M.D.; writing—review and editing, Ł.Z., T.J., G.F.-D., B.P. and M.D.; visualization, M.D.; supervision, G.F.-D. and B.P.; project administration, M.D.; funding acquisition, Ł.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Science Centre, Poland, “PRELUDIUM-20” Project, No. 2021/41/N/NZ5/04384.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This study is the result of the MD internship “Molecular mechanism of equine endometrosis: the current state of knowledge on the fibrosis pathway involving growth factors and fibroblast transformation” in the Departmento de Morfologia e Função, CIISA, Faculdade de Medicina Veterinária, Universidade de Lisboa from 1 July to 30 September 2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Wang, J.; Zhang, X. An Update on the Multifaceted Role of NF-kappaB in Endometriosis. Int. J. Biol. Sci. 2022, 18, 4400–4413. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J. Current uses of mushrooms in cancer treatment and their anticancer mechanisms. Int. J. Mol. Sci. 2022, 23, 10502. [Google Scholar] [CrossRef] [PubMed]
- Mothes, J.; Busse, D.; Kofahl, B.; Wolf, J. Sources of dynamic variability in NF-κB signal transduction: A mechanistic model. BioEssays 2015, 37, 452–462. [Google Scholar] [CrossRef]
- García-García, V.A.; Alameda, J.P.; Page, A.; Casanova, M.L. Role of NF-κB in ageing and age-related diseases: Lessons from genetically modified mouse models. Cells 2021, 10, 1906. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, E.; Hoffmann, A. NF-κB signaling. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Banerjee, S.; Driss, A.; Xu, W.; Mehrabi, S.; Nezhat, C.; Sidell, N.; Taylor, R.N.; Thompson, W.E. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-κB signaling pathway. J. Cell. Physiol. 2019, 234, 6298–6312. [Google Scholar] [CrossRef]
- Cui, L.; Wang, H.; Lin, J.; Wang, Y.; Dong, J.; Li, J.; Li, J. Progesterone inhibits inflammatory response in E. coli-or LPS-Stimulated bovine endometrial epithelial cells by NF-κB and MAPK pathways. Dev. Comp. Immunol. 2020, 105, 103568. [Google Scholar] [CrossRef]
- Domino, M.; Jasinski, T.; Kautz, E.; Juszczuk-Kubiak, E.; Ferreira-Dias, G.; Zabielski, R.; Sady, M.; Gajewski, Z. Expression of genes involved in the NF-κB-dependent pathway of the fibrosis in the mare endometrium. Theriogenology 2020, 147, 18–24. [Google Scholar] [CrossRef]
- Jasiński, T.; Zdrojkowski, Ł.; Kautz, E.; Juszczuk-Kubiak, E.; Ferreira-Dias, G.; Domino, M. Equine endometrosis pathological features: Are they dependent on NF-κB Signaling pathway? Animals 2021, 11, 3151. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Jasiński, T.; Zdrojkowski, Ł.; Ferreira-Dias, G.; Kautz, E.; Juszczuk-Kubiak, E.; Domino, M. Molecular Mechanism of Equine Endometrosis: The NF-κB-Dependent Pathway Underlies the Ovarian Steroid Receptors’ Dysfunction. Int. J. Mol. Sci. 2022, 23, 7360. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [PubMed]
- May, M.J.; Ghosh, S. Signal transduction through NF-κB. Trends Immunol. 1998, 19, 80–88. [Google Scholar] [CrossRef]
- Umezawa, K. Possible role of peritoneal NF-kB in peripheral inflammation and cancer: Lessons from the inhibitor DHMEQ. Biomed. Pharm. 2011, 65, 252–259. [Google Scholar] [CrossRef]
- Pallares, J.; Martínez-Guitarte, J.L.; Dolcet, X.; Llobet, D.; Rue, M.; Palacios, J.; Prat, J.; Matias-Guiu, X. Abnormalities in the NF-κB family and related proteins in endometrial carcinoma. J. Pathol. 2004, 204, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Mita, S.; Shimizu, Y.; Notsu, T.; Imada, K.; Kyo, S. Dienogest inhibits Toll-like receptor 4 expression induced by costimulation of lipopolysaccharide and high-mobility group box 1 in endometrial epithelial cells. Fertil. Steril. 2011, 96, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Qu, Y.; Li, J.; Cui, L.; Wang, Y.; Lin, J.; Wang, H. Cortisol inhibits NF-κB and MAPK pathways in LPS activated bovine endometrial epithelial cells. Int. Immunopharmacol. 2018, 56, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cui, L.; Huang, X.; Wang, S.; Li, D.; Li, L.; Sun, Y.; Du, M. Activation of Rev-erbα attenuates lipopolysaccharide-induced inflammatory reactions in human endometrial stroma cells via suppressing TLR4-regulated NF-κB activation. Acta Biochim. Biophys. Sin. 2019, 51, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Wang, X.; Wan, L.; Liu, Y.; Shi, Y.; Zhang, L.; Fang, Z.; Wei, Z. PDCD4 suppresses proliferation, migration, and invasion of endometrial cells by inhibiting autophagy and NF-κB/MMP2/MMP9 signal pathway. Biol. Reprod. 2018, 99, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gong, Z.; Zhou, S.; Yang, S.; Wang, D.; Chen, X.; Wu, J.; Liu, L.; Zhong, S.; Zhao, J.; et al. Liposomal curcumin targeting endometrial Cancer through the NF-κB pathway. Cell. Physiol. Biochem. 2018, 48, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Besso, M.J.; Rosso, M.; Lapyckyj, L.; Moiola, C.P.; Matos, M.L.; Mercogliano, M.F.; Schilacci, R.; Reventos, J.; Colas, E.; Gil-Moreno, A.; et al. FXYD5/Dysadherin, a biomarker of endometrial cancer myometrial invasion and aggressiveness: Its relationship with TGF-β1 and NF-κB pathways. Front. Oncol. 2019, 9, 1306. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Z.; Tang, Z.; Xue, C.; Yu, H.; Zhang, D.; Li, Y.; Liu, X.; Shi, Y.; Zhang, L.; et al. IL-37bΔ1-45 suppresses the migration and invasion of endometrial cancer cells by targeting the Rac1/NF-κB/MMP2 signal pathway. Lab. Investig. 2021, 101, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, O.; Zhang, W.; Liu, L.; Xu, C. Astragaloside IV exerts anti-inflammatory role in endometriosis by downregulating TLR4/NF-κB pathway. Trop. J. Pharm. Res. 2019, 18, 539–545. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecular basis of NF-κB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef]
- Elweza, A.E.; Ezz, M.A.; Acosta, T.J.; Talukder, A.K.; Shimizu, T.; Hayakawa, H.; Shimada, M.; Imakawa, K.; Zaghlouls, A.H.; Miyamoto, A. A proinflammatory response of bovine endometrial epithelial cells to active sperm in vitro. Mol. Reprod. Dev. 2018, 85, 215–226. [Google Scholar] [CrossRef]
- Morris, L.H.A.; McCue, P.M.; Aurich, C. Equine endometritis: A review of challenges and new approaches. Reproduction 2020, 160, 95–110. [Google Scholar] [CrossRef]
- Katila, T.; Ferreira-Dias, G. Evolution of the Concepts of Endometrosis, Post Breeding Endometritis, and Susceptibility of Mares. Animals 2022, 12, 779. [Google Scholar] [CrossRef]
- Kimura, F.; Takebayashi, A.; Ishida, M.; Nakamura, A.; Kitazawa, J.; Morimune, A.; Hirata, K.; Takahashi, A.; Tsuji, S.; Takashima, A.; et al. Review: Chronic endometritis and its effect on reproduction. J. Obstet. Gynaecol. Res. 2019, 45, 951–960. [Google Scholar] [CrossRef]
- Schöniger, S.; Schoon, H.A. The healthy and diseased equine endometrium: A review of morphological features and molecular analyses. Animals 2020, 10, 625. [Google Scholar] [CrossRef]
- Witkowski, M.; Katkiewicz, M.; Zając, S.; Kochan, J. Effect of Long-term Hyperimmunization on the Presence of Mast Cells in the Endometrium of the Mare. J. Equine Vet. Sci. 2015, 35, 569–572. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Bromfield, J.J. Tolerance and innate immunity shape the development of postpartum uterine disease and the impact of endometritis in dairy cattle. Annu. Rev. Anim. Biosci. 2019, 7, 361–384. [Google Scholar] [CrossRef]
- Hedia, M.; Ibrahim, S.; Mahmoud, K.; Ahmed, Y.; Ismail, S.; El-Belely, M. Hemodynamic changes in cytokines, chemokines, acute phase proteins and prostaglandins in mares with subclinical endometritis. Theriogenology 2021, 171, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, G.; Karin, M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.W.; Huang, M.T.; Wang, P.H.; Huang, B.S.; Chen, Y.J.; Hsieh, S.L. Decoy receptor 3 promotes cell adhesion and enhances endometriosis development. J. Pathol. 2018, 244, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Babaei, S.; Moini, A.; Eftekhari-Yazdi, P. Controlling Semi-Invasive Activity of Human Endometrial Stromal Cells by Inhibiting NF-kB Signaling Pathway Using Aloe-emodin and Aspirin. J. Reprod. Fertil. 2021, 22, 227–240. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Galvão, A.; Szóstek, A.; Amaral, A.; Mateus, L.; Skarzynski, D.J.; Ferreira-Dias, G. Physiopathologic mechanisms involved in mare endometrosis. Reprod. Dom. Anim. 2014, 49, 82–87. [Google Scholar] [CrossRef]
- Alpoim-Moreira, J.; Fernandes, C.; Rebordão, M.R.; Amaral, A.; Pinto-Bravo, P.; Bliebernicht, M.; Skarzynski, D.J.; Ferreira-Dias, G. Collagens and DNA methyltransferases in mare endometrosis. Reprod. Domest. Anim. 2019, 54, 46–52. [Google Scholar] [CrossRef]
- Szóstek, A.Z.; Lukasik, K.; Galvão, A.M.; Ferreira-Dias, G.M.; Skarzynski, D.J. Impairment of the interleukin system in equine endometrium during the course of endometrosis. Biol. Reprod. 2013, 89, 1–13. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.Z.; Lukasik, K.; Skarzynski, D.J.; Okuda, K. Effect of transforming growth factor-β1 on α-smooth muscle actin and collagen expression in equine endometrial fibroblasts. Theriogenology 2019, 124, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Szóstek-Mioduchowska, A.Z.; Baclawska, A.; Okuda, K.; Skarzynski, D.J. Effect of proinflammatory cytokines on endometrial collagen and metallopeptidase expression during the course of equine endometrosis. Cytokine 2019, 123, 154767. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.; Słowinska, M.; Pacewicz, J.; Skarzynski, D.J.; Okuda, K. Matrix metallopeptidase expression and modulation by transforming growth factor-1 in equine endometrosis. Sci. Rep. 2020, 10, 1119. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.; Leciejewska, N.; Zelmanska, B.; Staszkiewicz-Chodor, J.; Ferreira-Dias, G.; Skarzynski, D. Lysophosphatidic acid as a regulator of endometrial connective tissue growth factor and prostaglandin secretion during estrous cycle and endometrosis in the mare. BMC Vet. Res. 2020, 16, 343. [Google Scholar] [CrossRef]
- Skarzynski, D.J.; Szóstek-Mioduchowska, A.Z.; Rebordão, M.R.; Jalali, B.M.; Piotrowska-Tomala, K.K.; Leciejewska, N.; Łazarczyk, M.; Ferreira-Dias, G.M. Neutrophils, monocytes and other immune components in the equine endometrium: Friends or foes? Theriogenology 2020, 150, 150–157. [Google Scholar] [CrossRef]
- Witkowskia, M.; Duliban, M.; Rak, A.; Profaska-Szymik, M.; Gurgul, A.; Arent, Z.J.; Galuszka, A.; Kotula-Balak, M. Next-Generation Sequencing analysis discloses genes implicated in equine endometrosis that may lead to tumorigenesis. Theriogenology 2022, 189, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, T.; Yan, H.; Guo, K.; Liu, Z.; Wei, L.; Qiu, C.; Liang, J. Fatostatin reverses progesterone resistance by inhibiting the SREBP1-NF-κB pathway in endometrial carcinoma. Cell Death Dis. 2021, 12, 544. [Google Scholar] [CrossRef] [PubMed]
- Gunson, D.E.; Gillette, D.M.; Beech, J.; Orsini, J. Endometrial adenocarcinoma in a mare. Vet. Pathol. 1980, 17, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.K.; Fuentealba, I.C.; Schmitz, D.G.; Read, W.K. Endometrial adenocarcinoma in a mare. Cornell Vet. 1990, 80, 65–73. [Google Scholar]
- Thompson, R.; Armién, A.G.; Rasmussen, J.M.; Wolf, T.M. Uterine adenocarcinoma in a Przewalski’s wild horse (Equus ferus przewalskii). J. Zoo Wildl. Med. 2014, 45, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Katkiewicz, M.; Witkowski, M. Gruczolakorak błony śluzowej macicy klaczy-opis przypadku. Życie Wet. 2016, 91, 852–853. [Google Scholar]
- Lopez, C.; Ciccarelli, M.; Gold, J.R.; Tibary, A. Uterine adenocarcinoma in Quarter horse mare. Equine Vet. Educ. 2017, 30, 640–644. [Google Scholar] [CrossRef]
- Garcia-Iglesias, M.J.; Bravo-Moral, A.M.; Perez-Martinez, C.; Ferreras-Estrada, M.C.; Martinez-Rodriguez, J.M.; Escudero-Diez, A. Incidence and pathomorphology of uterine tumours in the cow. J. Vet. Med. Sci. 1995, 42, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Bockman, J.V. Two pathogenic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; McDonnell, P.C.; Lehr, R.; Tierney, L.; Tzimas, M.N.; Griswold, D.E.; Capper, E.A.; Tal-Singer, R.; Wells, G.I.; Doyle, M.L.; et al. Identification and initial characterization of four novel members of the interleukin-1 family. J. Biol. Chem. 2000, 275, 10308–10314. [Google Scholar] [CrossRef] [PubMed]
- Ding, V.A.; Zhu, Z.; Mantz, A.A.; Xiao, H.; Wakefield, M.R.; Bai, Q.; Fang, Y. The role of IL-37 in non-cancerous diseases. Pathol. Oncol. Res. 2017, 23, 463–470. [Google Scholar] [CrossRef]
- Ding, V.A.; Zhu, Z.; Xiao, H.; Wakefield, M.R.; Bai, Q.; Fang, Y. The role of IL-37 in cancer. Med. Oncol. 2016, 33, 68. [Google Scholar] [CrossRef]
- Bhatnagar, P.C.; Chaudhary, J.L.; Bhardwaj, B.; Shakhar, C.; Gupta, L.; Sharma, D.K. Effect of different hormonal protocols and nutrient supplementation on reproductive performance of cattle under different field conditions. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1511–1522. [Google Scholar] [CrossRef]
- Crabtree, J. Update on the management of anoestrus and transitional phase in horses. In Pract. 2021, 43, 457–466. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Beagley, K.W.; Gockel, C.M. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 2003, 38, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Bommer, I.; Muzzio, D.O.; Zygmunt, M.; Jensen, F. Progesterone and estradiol exert an inhibitory effect on the production of anti-inflammatory cytokine IL-10 by activated MZ B cells. J. Reprod. Immunol. 2016, 116, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoven, E.; Wissink, S.; van der Saag, P.T.; van der Burg, B. Negative interaction between the RelA (p65) subunit of NF-kappaB and the progesterone receptor. J. Biol. Chem. 1996, 271, 6217–6224. [Google Scholar] [CrossRef]
- Mote, P.A.; Arnett-Mansfield, R.L.; Gava, N.; Defazio, A.; Mulac-Jericevic, B.; Conneely, O.M.; Clarke, C.L. Overlapping and distinct expression of progesterone receptors A and B in mouse uterus and mammary gland during the estrus cycle. Endocrinology 2006, 147, 5503–5512. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.S.M.; Scoggin, K.E.; Canisso, I.F.; Troedsson, M.H.T.; Squires, E.L.; Ball, B.A. Expression of receptors for ovarian steroids and prostaglandin E2 in the endometrium and myometrium of mares during estrus, diestrus and early pregnancy. Anim. Reprod. Sci. 2014, 151, 169–181. [Google Scholar] [CrossRef]
- DeFranco, D.B. Navigating steroid hormone receptors through the nuclear compartment. Mol. Endocrinol. 2002, 16, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Hartt, L.S.; Carling, S.J.; Joyce, M.M.; Johnson, G.A.; Vanderwall, D.K.; Ott, T.L. Temporal and spatial associations of oestrogen receptor alpha and progesterone receptor in the endometrium of cyclic and early pregnant mares. Reproduction 2005, 130, 241–250. [Google Scholar] [CrossRef]
- González-Ramos, R.; Rocco, J.; Rojas, C.; Sovino, H.; Poch, A.; Kohen, P.; Alvarado-Diaz, C.; Devoto, L. Physiologic activation of nuclear factor kappa-B in the endometrium during the menstrual cycle is altered in endometriosis patients. Fertil. Steril. 2012, 97, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Cui, L.J.; Li, A.Y.; Zhang, J.P.; Liu, Y.; Zhao, J.S.; Xu, X.B.; He, B.; Wang, J.D.; Chu, L.; et al. Endometrial breakdown with sustained progesterone release involves NF-κB-mediated functional progesterone withdrawal in a mouse implant model. Mol. Reprod. Dev. 2016, 83, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Wang, Y.; Lu, Y.; Yuan, L.; Liu, X.; Guo, S.W. Immunoreactivity of progesterone receptor isoform B and nuclear factor kappa-B as biomarkers for recurrence of ovarian endometriomas. Am. J. Obstet. Gynecol. 2008, 199, 486.e1–486.e10. [Google Scholar] [CrossRef]
- Han, A.R.; Lee, T.H.; Kim, S.; Lee, H.Y. Risk factors and biomarkers for the recurrence of ovarian endometrioma: About the immunoreactivity of progesterone receptor isoform B and nuclear factor kappa B. Gynecol. Endocrinol. 2017, 33, 70–74. [Google Scholar] [CrossRef]
- Enmark, E.; Pelto-Huikko, M.; Grandien, K.; Lagercrantz, S.; Lagercrantz, J.; Fried, G.; Nordenskjold, M.; Gustafsson, J.A. Human estrogen receptor b-gene structure, chromosomal localization, and expression pattern. J. Clin. Endocr. Metab. 1997, 82, 4258–4265. [Google Scholar] [CrossRef] [PubMed]
- Kayisli, U.A.; Guzeloglu-Kayisli, O.; Arici, A. Endocrine-immune interactions in human endometrium. Ann. N. Y. Acad. Sci. 2004, 1034, 50–63. [Google Scholar] [CrossRef]
- Guzeloglu-Kayisli, O.; Halis, G.; Taskiran, S.; Kayisli, U.A.; Arici, A. DNA-binding ability of NF-kappaB is affected differently by ERalpha and ERbeta and its activation results in inhibition of estrogen responsiveness. Reprod. Sci. 2008, 15, 493–505. [Google Scholar] [CrossRef]
- Mei, J.; Zhu, X.Y.; Jin, L.P.; Duan, Z.L.; Li, D.J.; Li, M.Q. Estrogen promotes the survival of human secretory phase endometrial stromal cells via CXCL12/CXCR4 up-regulation-mediated autophagy inhibition. Hum. Reprod. 2015, 30, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Lee, J.E.; Cho, Y.J.; Park, M.J.; O’Malley, B.W. Genomic Function of Estrogen Receptor β in Endometriosis. Endocrinology 2019, 160, 2495–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, Y.; He, L.; Yao, X.; Chen, J. Involvement of angiotensin II receptor type 1/NF-κB signaling in the development of endometriosis. Exp. Ther. Med. 2020, 20, 3269–3277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, H.; Lu, Y.; Chen, H.; Jiang, S.; Li, L. Effects of estradiol on VEGF and bFGF by Akt in endometrial cancer cells are mediated through the NF-κB pathway. Oncol. Rep. 2016, 36, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Yaguchi, T.; Sugiyama, J.; Sumimoto, H.; Umezawa, K.; Iwata, T.; Susumu, N.; Fujii, T.; Kawamura, N.; Kobayashi, A.; et al. Immunosuppression through constitutively activated NF-κB signalling in human ovarian cancer and its reversal by an NF-κB inhibitor. Br. J. Cancer 2014, 110, 2965–2974. [Google Scholar] [CrossRef]
- Schlein, L.J.; Thamm, D.H. NF-kB activation in canine cancer. Vet. Pathol. 2022, 59, 724–732. [Google Scholar] [CrossRef]
- Jing, H.; Lee, S. NF-κB in cellular senescence and cancer treatment. Mol. Cells 2014, 37, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).