Monocyte-Derived miRNA-1914-5p Attenuates IL-1β–Induced Monocyte Adhesion and Transmigration
Abstract
1. Introduction
2. Results
2.1. IL-1β Exposure Enhanced Human Monocyte Mac-1 Expression and Adhesion to Human Endothelial Cells
2.2. IL-1β Exposure Enhanced Expression of MCP-1 by Human Monocytes and Their Transmigration through a Human Endothelial Cell Layer
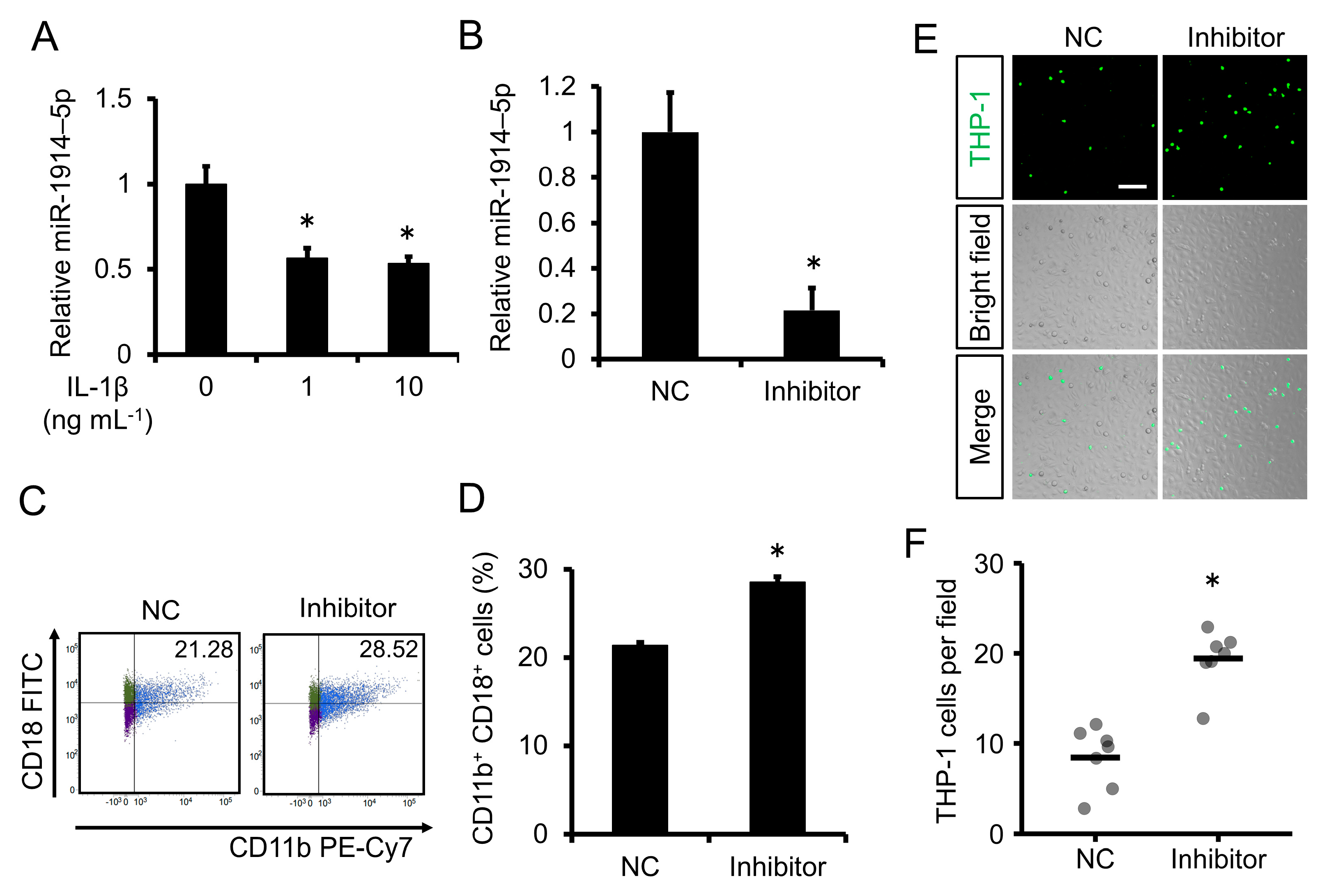
2.3. Expression of miR-1914-5p Was Downregulated by IL-1β Exposure in Human Monocytes; Inhibition of miR-1914-5p Expression Induced Monocyte Adhesion to Human Endothelial Cells
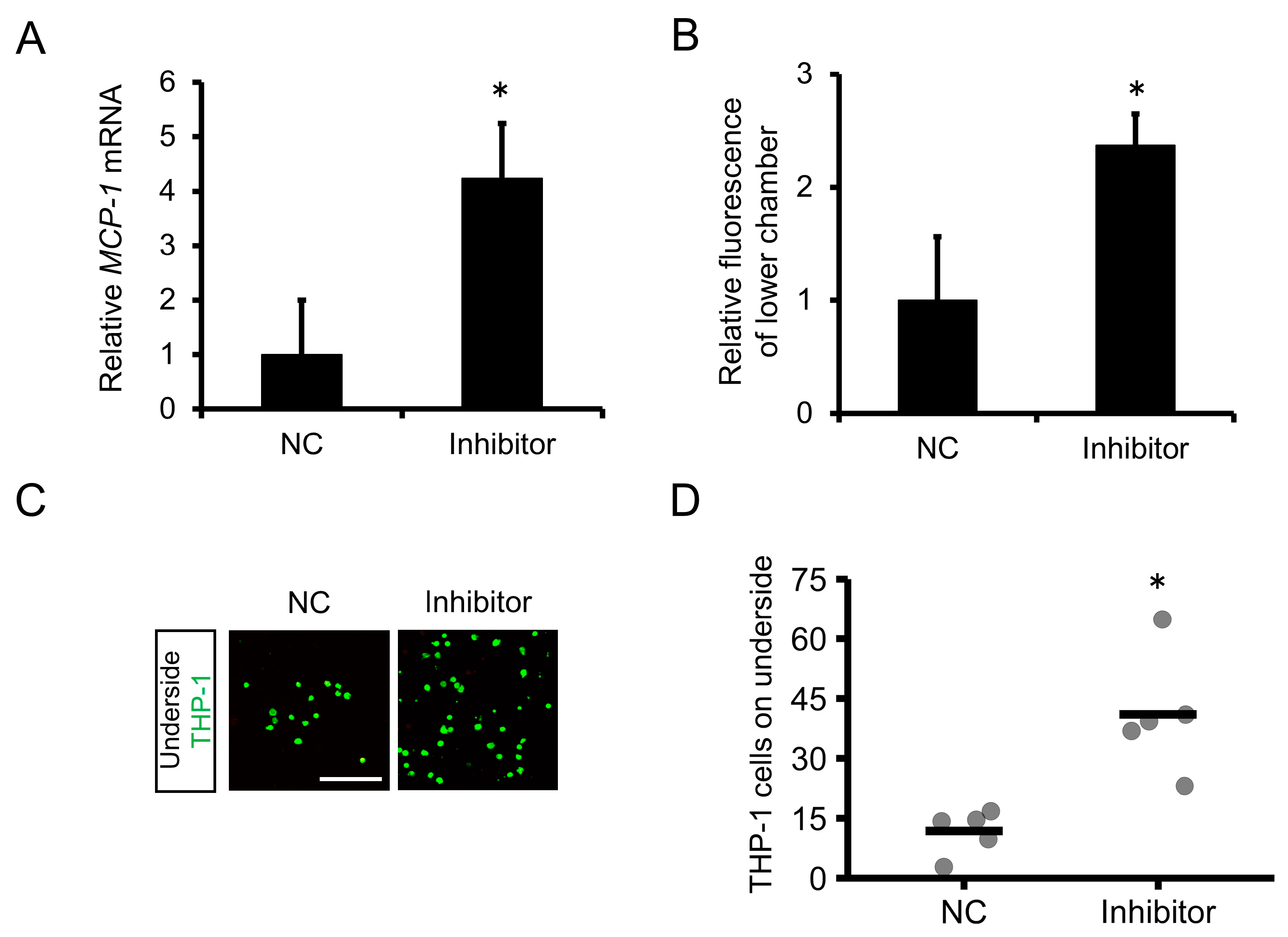
2.4. Inhibition of miR-1914-5p Expression in Human Monocytes Upregulated the Expression of MCP-1 and Increased Monocyte Transmigration through a Human Endothelial Cell Layer
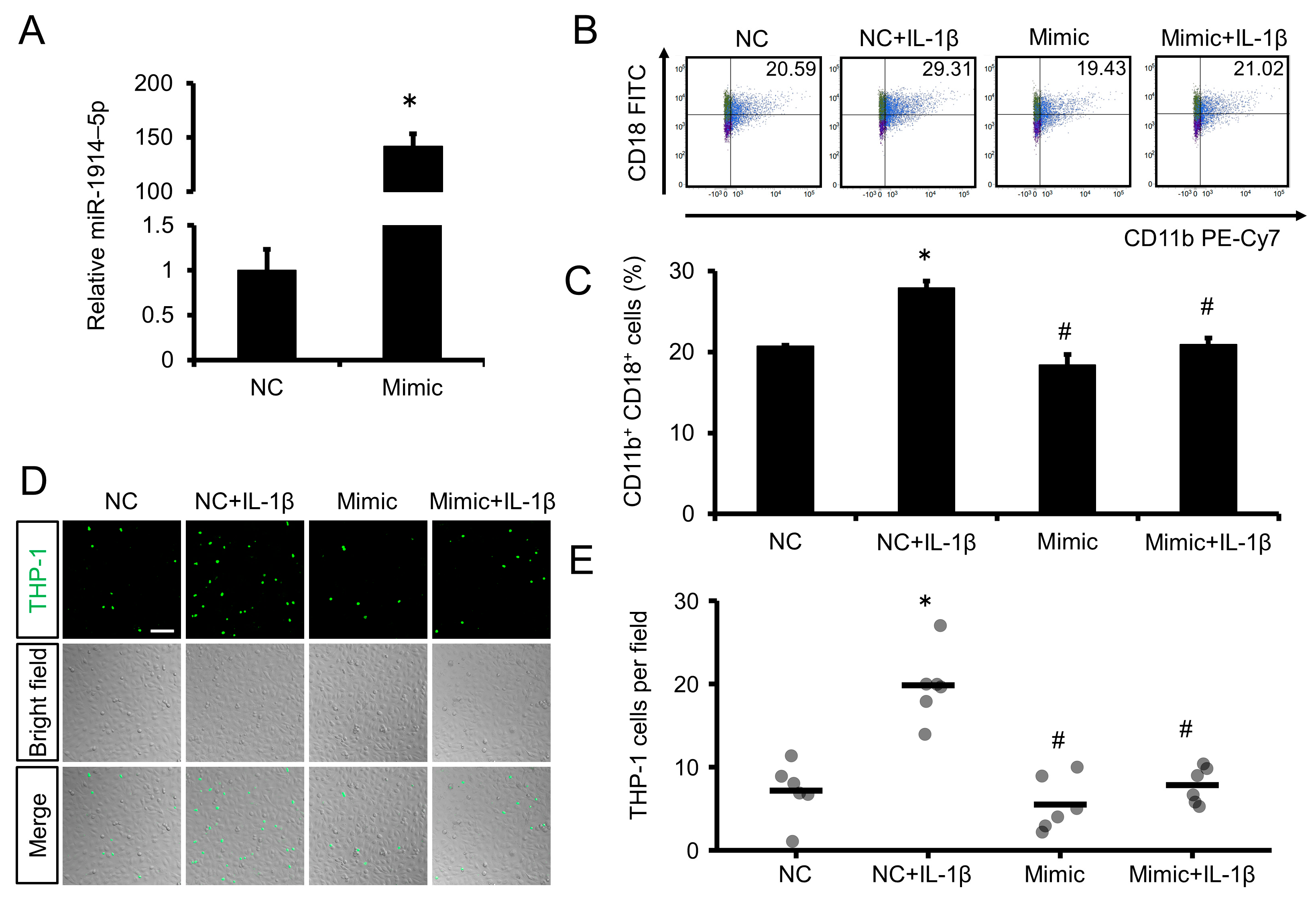
2.5. Overexpression of miR-1914-5p Suppressed the Adhesion of IL-1β–Exposed Human Monocytes to Human Endothelial Cells
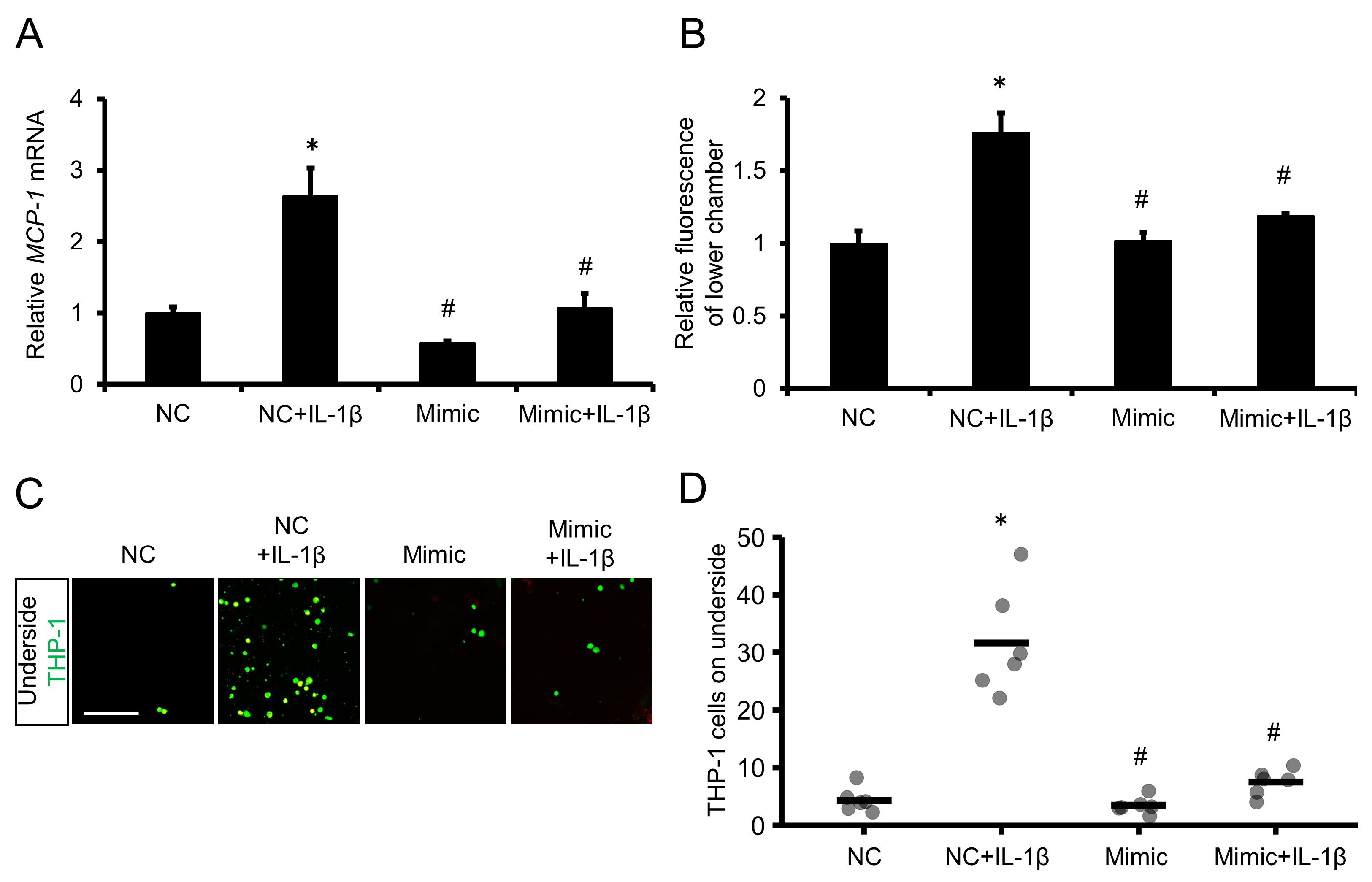
2.6. Overexpression of miR-1914-5p in IL-1β–Exposed Human Monocytes Suppressed MCP-1 Expression and Transmigration through a Human Endothelial Cell Layer
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. miRNA Isolation and Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
4.3. qRT-PCR
4.4. Transfection
4.5. Reporter Plasmid Construction and Luciferase Reporter Assay
4.6. Flow Cytometry
4.7. Cell Adhesion Assay
4.8. Transmigration Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansson, G.K.; Robertson, A.K.L.; Söderberg-Nauclér, C. Inflammation and Atherosclerosis. Annu. Rev. Pathol. 2006, 1, 297–329. [Google Scholar] [CrossRef] [PubMed]
- Li, A.C.; Glass, C.K. The Macrophage Foam Cell as a Target for Therapeutic Intervention. Nat. Med. 2002, 8, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in Atherosclerosis: A Dynamic Balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Modarai, B.; Humphries, J.; Mattock, K.; Waltham, M.; Burnand, K.G.; Smith, A. The Monocyte/Macrophage as a Therapeutic Target in Atherosclerosis. Curr. Opin. Pharmacol. 2009, 9, 109–118. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the Site of Inflammation: The Leukocyte Adhesion Cascade Updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Winter, C.; Silvestre-Roig, C.; Ortega-Gomez, A.; Lemnitzer, P.; Poelman, H.; Schumski, A.; Winter, J.; Drechsler, M.; de Jong, R.; Immler, R.; et al. Chrono-Pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab. 2018, 28, 175–182.e5. [Google Scholar] [CrossRef]
- Makó, V.; Czúcz, J.; Weiszhár, Z.; Herczenik, E.; Matkó, J.; Prohászka, Z.; Cervenak, L. Proinflammatory Activation Pattern of Human Umbilical Vein Endothelial Cells Induced by IL-1β, TNF-α, and LPS. Cytom. Part A 2010, 77, 962–970. [Google Scholar] [CrossRef]
- Geovanini, G.R.; Libby, P. Atherosclerosis and Inflammation: Overview and Updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, E.K.; Seo, K.W.; Bae, J.U.; Park, S.Y.; Kim, C.D. TLR4-Mediated Expression of Mac-1 in Monocytes Plays a Pivotal Role in Monocyte Adhesion to Vascular Endothelium. PLoS ONE 2014, 9, e104588. [Google Scholar] [CrossRef]
- Lee, S.J.; Baek, S.E.; Jang, M.A.; Kim, C.D. SIRT1 Inhibits Monocyte Adhesion to the Vascular Endothelium by Suppressing Mac-1 Expression on Monocytes. Exp. Mol. Med. 2019, 51. [Google Scholar] [CrossRef]
- Weber, C.; Noels, H. Atherosclerosis: Current Pathogenesis and Therapeutic Options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Schenkel, A.R.; Mamdouh, Z.; Muller, W.A. Locomotion of Monocytes on Endothelium Is a Critical Step during Extravasation. Nat. Immunol. 2004, 5, 393–400. [Google Scholar] [CrossRef]
- Phillipson, M.; Heit, B.; Colarusso, P.; Liu, L.; Ballantyne, C.M.; Kubes, P. Intraluminal Crawling of Neutrophils to Emigration Sites: A Molecularly Distinct Process from Adhesion in the Recruitment Cascade. J. Exp. Med. 2006, 203, 2569–2575. [Google Scholar] [CrossRef]
- Keiper, T.; Al-Fakhri, N.; Chavakis, E.; Athanasopoulos, A.N.; Isermann, B.; Herzog, S.; Saffrich, R.; Hersemeyer, K.; Bohle, R.M.; Haendeler, J.; et al. The Role of Junctional Adhesion Molecule-C (JAM-C) in Oxidized LDL-mediated Leukocyte Recruitment. FASEB J. 2005, 19, 2078–2080. [Google Scholar] [CrossRef]
- Lopez-Pedrera, C.; Barbarroja, N.; Patiño-Trives, A.M.; Luque-Tévar, M.; Torres-Granados, C.; Aguirre-Zamorano, M.A.; Collantes-Estevez, E.; Pérez-Sánchez, C. Role of Micrornas in the Development of Cardiovascular Disease in Systemic Autoimmune Disorders. Int. J. Mol. Sci. 2020, 21, 2012. [Google Scholar] [CrossRef]
- Zhong, L.; Simard, M.J.; Huot, J. Endothelial MicroRNAs Regulating the NF-KB Pathway and Cell Adhesion Molecules during Inflammation. FASEB J. 2018, 32, 4070–4084. [Google Scholar] [CrossRef]
- Kihara, T.; Toriuchi, K.; Aoki, H.; Kakita, H.; Yamada, Y.; Aoyama, M. Interleukin-1β Enhances Cell Adhesion in Human Endothelial Cells via MicroRNA-1914–5p Suppression. Biochem. Biophys. Rep. 2021, 27, 101046. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef]
- Mai, W.; Liao, Y. Targeting IL-1β in the Treatment of Atherosclerosis. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting Inflammation in Atherosclerosis - from Experimental Insights to the Clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Peng, J.; Ren, Z.; He, N.Y.; Li, Q.; Zhao, X.S.; Wang, M.M.; Wen, H.Y.; Tang, Z.H.; Jiang, Z.S.; et al. Functional Regulatory Roles of MicroRNAs in Atherosclerosis. Clin. Chim. Acta 2016, 460, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.B.; Kapoor, P.; Howard, J.K.; Shah, A.M.; Lord, G.M. An Update on the Roles of Immune System-Derived MicroRNAs in Cardiovascular Diseases. Cardiovasc. Res. 2021, 117, 2434–2449. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Yang, Z.; Xia, Q.; Gao, S.; Sun, S.; Luo, X.; Li, Z.; Zhang, X.; Li, X. ACADSB Regulates Ferroptosis and Affects the Migration, Invasion, and Proliferation of Colorectal Cancer Cells. Cell Biol. Int. 2020, 44, 2334–2343. [Google Scholar] [CrossRef]
- Nomura, M.; Liu, J.; Yu, Z.X.; Yamazaki, T.; Yan, Y.; Kawagishi, H.; Rovira, I.I.; Liu, C.; Wolfgang, M.J.; Mukouyama, Y.S.; et al. Macrophage Fatty Acid Oxidation Inhibits Atherosclerosis Progression. J. Mol. Cell. Cardiol. 2019, 127, 270–276. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Zhang, H.; Zhou, H.; Jiao, T.; Feng, M.; Na, F.; Sun, M.; Zhao, M.; Xue, L.; et al. RBMS1 Promotes Gastric Cancer Metastasis through Autocrine IL-6/JAK2/STAT3 Signaling. Cell Death Dis. 2022, 13, 287. [Google Scholar] [CrossRef]
- Abe, S.; Tokoro, F.; Matsuoka, R.; Arai, M.; Noda, T.; Watanabe, S.; Horibe, H.; Fujimaki, T.; Oguri, M.; Kato, K.; et al. Association of Genetic Variants with Dyslipidemia. Mol. Med. Rep. 2015, 12, 5429–5436. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, F.; Li, L.; Gao, H.; Arckacki, S.; Wang, I.Z.; Barnard, J.; Ellis, S.; Hubbard, C.; Topol, E.J.; et al. Genome-Wide Linkage Analysis of Large Multiple Multigenerational Families Identifies Novel Genetic Loci for Coronary Artery Disease. Sci. Rep. 2017, 7, 5472. [Google Scholar] [CrossRef]
- Vromman, A.; Ruvkun, V.; Shvartz, E.; Wojtkiewicz, G.; Santos Masson, G.; Tesmenitsky, Y.; Folco, E.; Gram, H.; Nahrendorf, M.; Swirski, F.K.; et al. Stage-Dependent Differential Effects of Interleukin-1 Isoforms on Experimental Atherosclerosis. Eur. Heart J. 2019, 40, 2482–2491. [Google Scholar] [CrossRef]
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as Potential Biomarkers in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 5547. [Google Scholar] [CrossRef]
- Freedman, J.E.; Ercan, B.; Morin, K.M.; Liu, C.T.; Tamer, L.; Ayaz, L.; Kanadasi, M.; Cicek, D.; Seyhan, A.I.; Akilli, R.E.; et al. The Distribution of Circulating MicroRNA and Their Relation to Coronary Disease. F1000Research 2012, 1, 1–12. [Google Scholar] [CrossRef]
- Emini Veseli, B.; Perrotta, P.; De Meyer, G.R.A.; Roth, L.; Van der Donckt, C.; Martinet, W.; De Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017, 816, 3–13. [Google Scholar] [CrossRef]
- Hyun, Y.M.; Choe, Y.H.; Park, S.A.; Kim, M. LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) Distinctly Regulate Neutrophil Extravasation through Hotspots I and II. Exp. Mol. Med. 2019, 51. [Google Scholar] [CrossRef]
- Li, N.; Mao, D.; Lü, S.; Tong, C.; Zhang, Y.; Long, M. Distinct Binding Affinities of Mac-1 and LFA-1 in Neutrophil Activation. J. Immunol. 2013, 190, 4371–4381. [Google Scholar] [CrossRef]
- Ding, Z.M.; Babensee, J.E.; Simon, S.I.; Lu, H.; Perrard, J.L.; Bullard, D.C.; Dai, X.Y.; Bromley, S.K.; Dustin, M.L.; Entman, M.L.; et al. Relative Contribution of LFA-1 and Mac-1 to Neutrophil Adhesion and Migration. J. Immunol. 1999, 163, 5029–5038. [Google Scholar] [CrossRef]
- Gerhardt, T.; Ley, K. Monocyte Trafficking across the Vessel Wall. Cardiovasc. Res. 2015, 107, 321–330. [Google Scholar] [CrossRef]
- Sumagin, R.; Prizant, H.; Lomakina, E.; Waugh, R.E.; Sarelius, I.H. LFA-1 and Mac-1 Define Characteristically Different Intralumenal Crawling and Emigration Patterns for Monocytes and Neutrophils In Situ. J. Immunol. 2010, 185, 7057–7066. [Google Scholar] [CrossRef]
- Ostermann, G.; Weber, K.S.C.; Zernecke, A.; Schröder, A.; Weber, C. JAM-I Is a Ligand of the Β2 Integrin LFA-I Involved in Transendothelial Migration of Leukocytes. Nat. Immunol. 2002, 3, 151–158. [Google Scholar] [CrossRef]
- Meerschaert, J.; Furie, M.B. The Adhesion Molecules Used by Monocytes for Migration across Endothelium Include CD11a/CD18, CD11b/CD18, and VLA-4 on Monocytes and ICAM-1, VCAM-1, and Other Ligands on Endothelium. J. Immunol. 1995, 154. [Google Scholar] [CrossRef]
- Shimono, Y.; Zabala, M.; Cho, R.W.; Lobo, N.; Dalerba, P.; Qian, D.; Diehn, M.; Liu, H.; Panula, S.P.; Chiao, E.; et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009, 138, 592–603. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toriuchi, K.; Kihara, T.; Aoki, H.; Kakita, H.; Takeshita, S.; Ueda, H.; Inoue, Y.; Hayashi, H.; Shimono, Y.; Yamada, Y.; et al. Monocyte-Derived miRNA-1914-5p Attenuates IL-1β–Induced Monocyte Adhesion and Transmigration. Int. J. Mol. Sci. 2023, 24, 2829. https://doi.org/10.3390/ijms24032829
Toriuchi K, Kihara T, Aoki H, Kakita H, Takeshita S, Ueda H, Inoue Y, Hayashi H, Shimono Y, Yamada Y, et al. Monocyte-Derived miRNA-1914-5p Attenuates IL-1β–Induced Monocyte Adhesion and Transmigration. International Journal of Molecular Sciences. 2023; 24(3):2829. https://doi.org/10.3390/ijms24032829
Chicago/Turabian StyleToriuchi, Kohki, Toshie Kihara, Hiromasa Aoki, Hiroki Kakita, Satoru Takeshita, Hiroko Ueda, Yasumichi Inoue, Hidetoshi Hayashi, Yohei Shimono, Yasumasa Yamada, and et al. 2023. "Monocyte-Derived miRNA-1914-5p Attenuates IL-1β–Induced Monocyte Adhesion and Transmigration" International Journal of Molecular Sciences 24, no. 3: 2829. https://doi.org/10.3390/ijms24032829
APA StyleToriuchi, K., Kihara, T., Aoki, H., Kakita, H., Takeshita, S., Ueda, H., Inoue, Y., Hayashi, H., Shimono, Y., Yamada, Y., & Aoyama, M. (2023). Monocyte-Derived miRNA-1914-5p Attenuates IL-1β–Induced Monocyte Adhesion and Transmigration. International Journal of Molecular Sciences, 24(3), 2829. https://doi.org/10.3390/ijms24032829






