Molecular Hydrogen Neuroprotection in Post-Ischemic Neurodegeneration in the Form of Alzheimer’s Disease Proteinopathy: Underlying Mechanisms and Potential for Clinical Implementation—Fantasy or Reality?
Abstract
:1. Introduction
2. Search Criteria and Data Collection
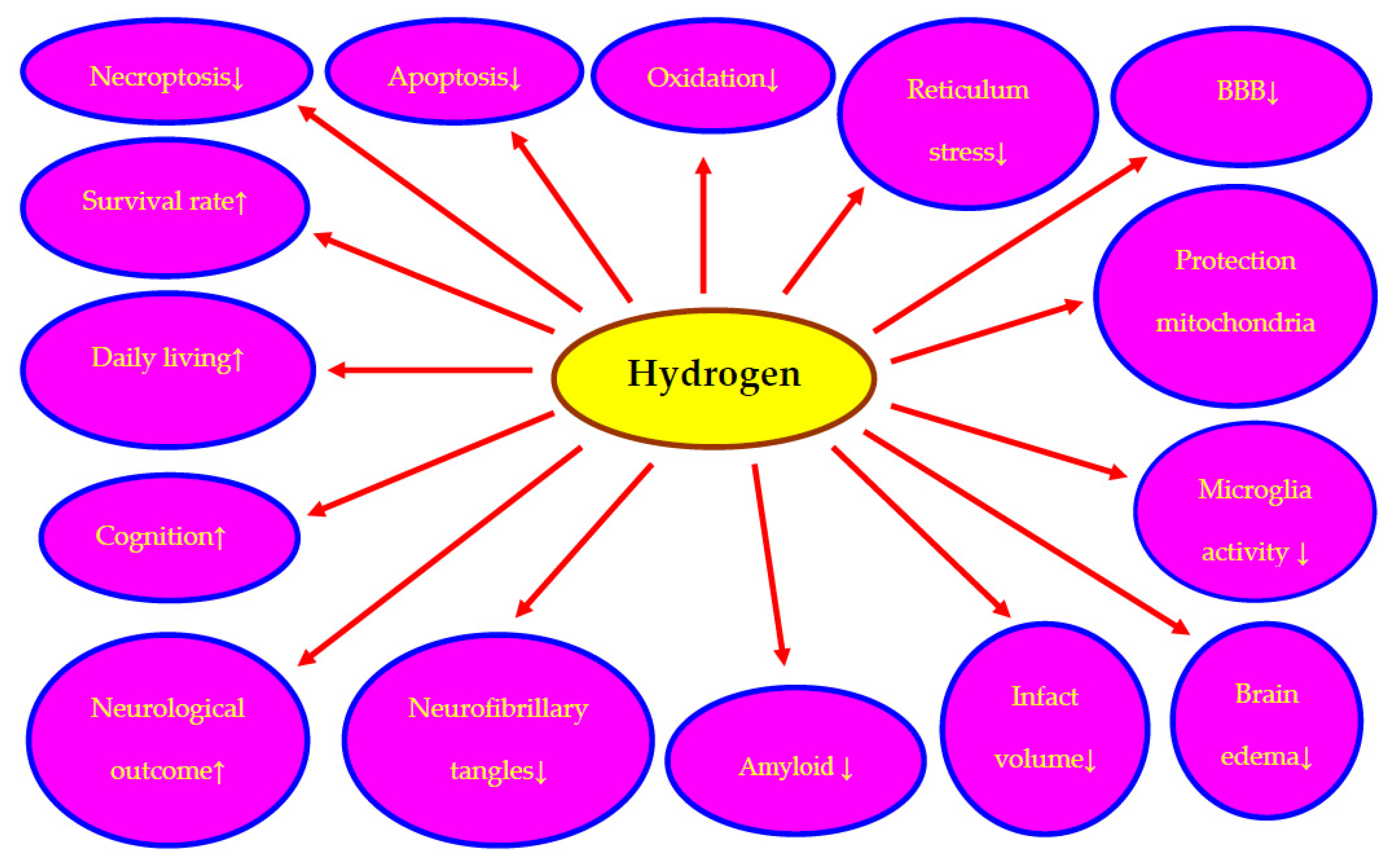
3. Molecular Hydrogen Neuroprotection in Post-Ischemic Brain Injury
3.1. In Animals
3.2. In Humans
4. Molecular Hydrogen versus Amyloid and Tau Protein Modification
5. Molecular Hydrogen Bioavailability
6. Conclusions
7. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta 2012, 1820, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, P.-F.; Wang, F.; Chen, J.-G. Targeting gaseous molecules to protect against cerebral ischaemic injury: Mechanisms and prospects. Clin. Exp. Pharmacol. Physiol. 2012, 39, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Htun, Y.; Nakamura, S.; Kusaka, T. Hydrogen and therapeutic gases for neonatal hypoxic-ischemic encephalopathy: Potential neuroprotective adjuncts in translational research. Pediatr. Res. 2021, 89, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Lanphier, E.H. Human respiration under increased pressures. Symp. Soc. Exp. Biol. 1972, 26, 379–394. [Google Scholar] [PubMed]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef]
- Gharib, B.; Hanna, S.; Abdallahi, O.M.; Lepidi, H.; Gardette, B.; De Reggi, M. Anti-inflammatory properties of molecular hydrogen: Investigation on parasite-induced liver inflammation. Comptes Rendus L’académie Sci.-Ser. III-Sci. Vie 2001, 324, 719–724. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Huo, T.-T.; Zeng, Y.; Liu, X.-N.; Sun, L.; Han, H.-Z.; Chen, H.-G.; Lu, Z.-H.; Huang, Y.; Nie, H.; Dong, H.-L.; et al. Hydrogen rich saline improves survival and neurological outcome after cardiac arrest and cardiopulmonary resuscitation in rats. Anesth. Analg. 2014, 119, 368–380. [Google Scholar] [CrossRef]
- Takeuchi, S.; Nagatani, K.; Otani, N.; Nawashiro, H.; Sugawara, T.; Wada, K.; Mori, K. Hydrogen improves neurological function through attenuation of blood-brain barrier disruption in spontaneously hypertensive stroke-prone rats. BMC Neurosci. 2015, 16, 22. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Wu, F.; Sun, X.; Xiang, Z.; Yang, L.; Huang, S.; Lu, Z.; Sun, Y.; Yu, W.-F. Intrathecal infusion of hydrogen-rich normal saline attenuates neuropathic pain via inhibition of activation of spinal astrocytes and microglia in rats. PLoS ONE 2014, 9, e97436. [Google Scholar] [CrossRef]
- Iketani, M.; Ohsawa, I. Molecular hydrogen as a neuroprotective agent. Curr. Neuropharmacol. 2017, 15, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Qin, S. Different types of molecular hydrogen donors and their pharmacokinetics in vivo. Sheng Li Xue Bao 2019, 71, 371–377. [Google Scholar] [PubMed]
- Ono, H.; Nishijima, Y.; Adachi, N.; Sakamoto, M.; Kudo, Y.; Nakazawa, J.; Nakao, A. Hydrogen (H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers. Med. Gas Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, M.; Satoh, Y.; Otsubo, Y.; Kazama, T. Molecular hydrogen attenuates neuropathic pain in mice. PLoS ONE 2014, 9, e100352. [Google Scholar]
- Li, Q.; Yu, P.; Zeng, Q.; Luo, B.; Cai, S.; Hui, K.; Yu, G.; Zhu, C.; Chen, X.; Duan, M.; et al. Neuroprotective effect of hydrogen-rich saline in global cerebral ischemia/reperfusion rats: Up-regulated tregs and down-regulated miR-21, miR-210 and NF-jB expression. Neurochem. Res. 2016, 41, 2655–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, H.; Nishijima, Y.; Ohta, S.; Sakamoto, M.; Kinone, K.; Horikosi, T.; Tamaki, M.; Takeshita, H.; Futatuki, T.; Ohishi, W.; et al. Hydrogen gas inhalation treatment in acute cerebral infarction: A randomized controlled clinical study on safety and neuroprotection. J. Stroke Cerebrovasc. Dis. 2017, 26, 2587–2594. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Peng, Y.; Qin, C.; Fan, F.; Liu, J.; Long, J. Hydrogen-rich water improves cognitive impairment gender-dependently in APP/PS1 mice without affecting Ab clearance. Free. Radic. Res. 2018, 52, 1311–1322. [Google Scholar] [CrossRef]
- Wang, H.; Huo, X.; Chen, H.; Li, B.; Liu, J.; Ma, W.; Wang, X.; Xie, K.; Yu, Y.; Shi, K. Hydrogen rich saline activated autophagy via HIF-1a pathways in neuropathic pain model. BioMed Res. Int. 2018, 2018, 4670834. [Google Scholar]
- Huang, J.; Liu, W.; Manaenko, A.; Sun, X.; Mei, Q.; Hu, Q. Hydrogen inhibits microglial activation and regulates microglial phenotype in a mouse middle cerebral artery occlusion model. Med. Gas Res. 2019, 9, 127–132. [Google Scholar]
- Huang, L.; Applegate, R.L., II; Applegate, P.M.; Gong, L.; Ocak, U.; Boling, W.; Zhang, J.H. Inhalation of high-concentration hydrogen gas attenuates cognitive deficits in a rat model of asphyxia induced cardiac arrest. Med. Gas Res. 2019, 9, 122–126. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Li, T.-T.; Cao, H.-L.; Yang, W.-C. Recent advances in the neuroprotective effects of medical gases. Med. Gas Res. 2019, 9, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Fang, Y.-J.; Luo, Y.-J.; Lenahan, C.; Zhang, J.-M.; Chen, S. The role of medical gas in stroke: An updated review. Med. Gas Res. 2019, 9, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, P.; Yue, C.; Jin, Z.; Liu, Q.; Du, X.; He, Q. Sustained release of bioactive hydrogen by Pd hydride nanoparticles overcomes Alzheimer’s disease. Biomaterials 2019, 197, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishijima, Y.; Adachi, N.; Sakamoto, M.; Kudo, Y.; Kaneko, K.; Nakao, A.; Imaoka, T. A basic study on molecular hydrogen (H2) inhalation in acute cerebral ischemia patients for safety check with physiological parameters and measurement of blood H2 level. Med. Gas Res. 2012, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Hayashida, K.; Sano, M.; Suzuki, M.; Shibusawa, T.; Yoshizawa, J.; Kobayashi, Y.; Suzuki, T.; Ohta, S.; Morisaki, H.; et al. Feasibility and safety of hydrogen gas inhalation for post-cardiac arrest syndrome—First in human pilot study. Circ. J. 2016, 80, 1870–1873. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Hayashida, K.; Sano, M.; Onuki, S.; Suzuki, M. Efficacy of inhaled hydrogen on neurological outcome following brain ischemia during post-cardiac arrest care (HYBRID II trial): Study protocol for a randomized controlled trial. Trials 2017, 18, 488. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Suzuki, M.; Hayashida, K.; Kobayashi, Y.; Yoshizawa, J.; Shibusawa, T. Hydrogen gas inhalation alleviates oxidative stress in patients with post-cardiac arrest syndrome. J. Clin. Biochem. Nutr. 2020, 67, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhang, H.-T.; Qin, S.-C. Neuroprotective effects of molecular hydrogen: A critical review. Neurosci. Bull. 2021, 37, 389–404. [Google Scholar] [CrossRef]
- Sano, M.; Tamura, T. Hydrogen gas therapy: From preclinical studies to clinical trials. Curr. Pharm. Des. 2021, 27, 650–665. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.; Yang, P.; Liu, J. Hydrogen as a complementary therapy against ischemic stroke: A review of the evidence. J. Neurol. Sci. 2019, 396, 240–246. [Google Scholar] [CrossRef]
- Hayashida, K.; Sano, M.; Kamimura, N.; Yokota, T.; Suzuki, M.; Ohta, S.; Fukuda, K.; Hori, S. Hydrogen inhalation during normoxic resuscitation improves neurological outcome in a rat model of cardiac arrest independently of targeted temperature management. Circulation 2014, 130, 2173–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a novel therapeutic molecule, regulates oxidative stress, inflammation, and apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Bao, C.; He, Y.; Tian, X.; Yang, Y.; Zhang, T. Hydrogen gas (XEN) inhalation ameliorates airway inflammation in asthma and COPD patients. QJM Int. J. Med. 2020, 113, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-G.; Sun, W.-Z.; Hu, J.-Y.; Jie, Z.-J.; Xu, J.-F.; Cao, J.; Song, Y.-L.; Wang, C.-H.; Wang, J.; Zhao, H.; et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: Results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir. Res. 2021, 22, 149. [Google Scholar] [CrossRef]
- Guan, W.-J.; Chen, R.-C.; Zhong, N.-S. Strategies for the prevention and management of coronavirus disease 2019. Eur. Respir. J. 2020, 55, 2000597. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.-J.; Wei, C.-H.; Chen, A.-L.; Sun, X.-C.; Guo, G.-Y.; Zou, X.; Shi, J.-D.; Lai, P.-Z.; Zheng, Z.-G.; Zhong, N.-S. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J. Thorac. Dis. 2020, 12, 3448–3452. [Google Scholar] [CrossRef]
- Pluta, R.; Kida, E.; Lossinsky, A.S.; Golabek, A.A.; Mossakowski, M.J.; Wisniewski, H.M. Complete cerebral ischemia with short-term survival in rats induced by cardiac arrest. I. Extracellular accumulation of Alzheimer’s β-amyloid protein precursor in the brain. Brain Res. 1994, 649, 323–328. [Google Scholar] [CrossRef]
- Kiryk, A.; Pluta, R.; Figiel, I.; Mikosz, M.; Ułamek, M.; Niewiadomska, G.; Jabłoński, M.; Kaczmarek, L. Transient brain ischemia due to cardiac arrest causes irreversible long-lasting cognitive injury. Behav. Brain Res. 2011, 219, 1–7. [Google Scholar] [CrossRef]
- Sekeljic, V.; Bataveljic, D.; Stamenkovic, S.; Ułamek, M.; Jabłoński, M.; Radenovic, L.; Pluta, R.; Andjus, P.R. Cellular markers of neuroinflammation and neurogenesis after ischemic brain injury in the long-term survival rat model. Brain Struct. Funct. 2012, 217, 411–420. [Google Scholar] [CrossRef]
- Pluta, R.; Bogucka-Kocka, A.; Ułamek-Kozioł, M.; Bogucki, J.; Czuczwar, S.J. Ischemic tau protein gene induction as an additional key factor driving development of Alzheimer’s phenotype changes in CA1 area of hippocampus in an ischemic model of Alzheimer’s disease. Pharmacol. Rep. 2018, 70, 881–884. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Tau protein dysfunction after brain ischemia. J. Alzheimer’s Dis. 2018, 66, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, R. Brain Ischemia: Alzheimer’s Disease Mechanisms; Nova Science Publishers, Inc.: New York, NY, USA, 2019; p. 311. [Google Scholar]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Amyloid pathology in the brain after ischemia. Folia Neuropathol. 2019, 57, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Radenovic, L.; Nenadic, M.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J.; Andjus, P.R.; Pluta, R. Heterogeneity in brain distribution of activated microglia and astrocytes in a rat ischemic model of Alzheimer’s disease after 2 years of survival. Aging 2020, 12, 12251–12267. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. The role of gut microbiota in an ischemic stroke. Int. J. Mol. Sci. 2021, 22, 915. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Brain ischemia as a prelude to Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 636653. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Czuczwar, S.J.; Januszewski, S.; Jabłoński, M. The many faces of post-ischemic tau protein in brain neurodegeneration of the Alzheimer’s disease type. Cells 2021, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Neuroinflammation in post-ischemic neurodegeneration of the brain: Friend, foe, or both? Int. J. Mol. Sci. 2021, 22, 4405. [Google Scholar] [CrossRef]
- Pluta, R. Brain ischemia as a bridge to Alzheimer’s disease. Neural Regen. Res. 2022, 17, 791–792. [Google Scholar] [CrossRef]
- Hossmann, K.A.; Schmidt-Kastner, R.; Ophoff, B.G. Recovery of integrative central nervous function after one hour global cerebro-circulatory arrest in normothermic cat. J. Neurol. Sci. 1987, 77, 305–320. [Google Scholar] [CrossRef]
- Snowdon, D.A.; Greiner, L.H.; Mortimer, J.A.; Riley, K.P.; Greiner, P.A.; Markesbery, W.R. Brain infarction and the clinical expression of Alzheimer disease: The Nun Study. JAMA 1997, 277, 813–817. [Google Scholar] [CrossRef]
- Pluta, R. The role of apolipoprotein E in the deposition of β-amyloid peptide during ischemia–reperfusion brain injury. A model of early Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2000, 903, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Ułamek, M.; Jabłoński, M. Alzheimer’s mechanisms in ischemic brain degeneration. Anat. Rec. 2009, 292, 1863–1881. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Jabłoński, M.; Ułamek, M. Factors in creepy delayed neuronal death in hippocampus following brain ischemia-reperfusion injury with long-term survival. Acta Neurochir. 2010, 106, 37–41. [Google Scholar]
- Pluta, R.; Ułamek, M.; Jabłoński, M. Consideration of the ischaemic basis and treatment of Alzheimer’s disease. Folia Neuropathol. 2010, 48, 11–26. [Google Scholar] [PubMed]
- Pluta, R.; Barcikowska, M.; Debicki, G.; Ryba, M.; Januszewski, S. Changes in amyloid precursor protein and apolipoprotein E immunoreactivity following ischemic brain injury in rat with long-term survival: Influence of idebenone treatment. Neurosci. Lett. 1997, 232, 95–98. [Google Scholar] [CrossRef]
- Kocki, J.; Ułamek-Kozioł, M.; Bogucka-Kocka, A.; Januszewski, S.; Jabłonski, M.; Gil-Kulik, P.; Brzozowska, J.; Petniak, A.; Furmaga-Jabłonska, W.; Bogucki, J.; et al. Dysregulation of amyloid precursor protein, β-secretase, presenilin 1 and 2 genes in the rat selectively vulnerable CA1 subfield of hippocampus following transient global brain ischemia. J. Alzheimer’s Dis. 2015, 47, 1047–1056. [Google Scholar] [CrossRef] [Green Version]
- Pluta, R.; Kocki, J.; Ułamek-Kozioł, M.; Petniak, A.; Gil-Kulik, P.; Januszewski, S.; Bogucki, J.; Jabłoński, M.; Brzozowska, J.; Furmaga-Jabłońska, W.; et al. Discrepancy in expression of β-secretase and amyloid-β protein precursor in Alzheimer-related genes in the rat medial temporal lobe cortex following transient global brain ischemia. J. Alzheimer’s Dis. 2016, 51, 1023–1031. [Google Scholar] [CrossRef] [Green Version]
- Pluta, R.; Kocki, J.; Ułamek-Kozioł, M.; Bogucka-Kocka, A.; Gil-Kulik, P.; Januszewski, S.; Jabłoński, M.; Petniak, A.; Brzozowska, J.; Bogucki, J.; et al. Alzheimer-associated presenilin 2 gene is dysregulated in rat medial temporal lobe cortex after complete brain ischemia due to cardiac arrest. Pharmacol. Rep. 2016, 68, 155–161. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Kocki, J.; Bogucka-Kocka, A.; Petniak, A.; Gil-Kulik, P.; Januszewski, S.; Bogucki, J.; Jabłoński, M.; Furmaga-Jabłońska, W.; Brzozowska, J.; et al. Dysregulation of autophagy, mitophagy and apoptotic genes in the medial temporal lobe cortex in an ischemic model of Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 54, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Ułamek-Kozioł, M.; Kocki, J.; Bogucka-Kocka, A.; Januszewski, S.; Bogucki, J.; Czuczwar, S.J.; Pluta, R. Autophagy, mitophagy and apoptotic gene changes in the hippocampal CA1 area in a rat ischemic model of Alzheimer’s disease. Pharmacol. Rep. 2017, 69, 1289–1294. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Kocki, J.; Januszewski, S.; Bogucki, J.; Bogucka-Kocka, A.; Pluta, R. Dysregulation of autophagy, mitophagy, and apoptosis genes in the CA3 region of the hippocampus in the ischemic model of Alzheimer’s disease in the rat. J. Alzheimer’s Dis. 2019, 72, 1279–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, R.; Ułamek-Kozioł, M.; Kocki, J.; Bogucki, J.; Januszewski, S.; Bogucka-Kocka, A.; Czuczwar, S.J. Expression of the tau protein and amyloid protein precursor processing genes in the CA3 area of the hippocampus in the ischemic model of Alzheimer’s disease in the rat. Mol. Neurobiol. 2020, 57, 1281–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Participation of amyloid and tau protein in neuronal death and neurodegeneration after brain ischemia. Int. J. Mol. Sci. 2020, 21, 4599. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Kiś, J.; Januszewski, S.; Jabłoński, M.; Czuczwar, S.J. Cross-talk between amyloid, tau protein and free radicals in post-ischemic brain neurodegeneration in the form of Alzheimer’s disease proteinopathy. Antioxidants 2022, 11, 146. [Google Scholar] [CrossRef]
- Pluta, R.; Furmaga-Jabłońska, W.; Januszewski, S.; Czuczwar, S.J. Post-ischemic brain neurodegeneration in the form of Alzheimer’s disease proteinopathy: Possible therapeutic role of curcumin. Nutrients 2022, 14, 248. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Post-ischemic neurodegeneration of the hippocampus resembling Alzheimer’s disease proteinopathy. Int. J. Mol. Sci. 2022, 23, 306. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Sun, X. Hydrogen inhalation improves mouse neurological outcomes after cerebral ischemia/reperfusion independent of anti-necroptosis. Med. Gas Res. 2018, 8, 1–5. [Google Scholar]
- Chen, L.; Chao, Y.; Cheng, P.; Li, N.; Zheng, H.; Yang, Y. UPLCQTOF/MS-based metabolomics reveals the protective mechanism of hydrogen on mice with ischemic stroke. Neurochem. Res. 2019, 44, 1950–1963. [Google Scholar] [CrossRef]
- Huang, L.; Applegate, R.L., II; Applegate, P.M.; Boling, W.; Zhang, J.H. Inhalation of high concentration hydrogen gas improves shortterm outcomes in a rat model of asphyxia induced-cardiac arrest. Med. Gas Res. 2018, 8, 73–78. [Google Scholar] [CrossRef]
- Chen, G.; Chen, B.; Dai, C.; Wang, J.; Wang, J.; Huang, Y.; Li, Y. Hydrogen inhalation is superior to mild hypothermia for improving neurological outcome and survival in a cardiac arrest model of spontaneously hypertensive rat. Shock 2018, 50, 689–695. [Google Scholar] [CrossRef]
- Wang, P.; Jia, L.; Chen, B.; Zhang, L.; Liu, J.; Long, J.; Li, Y. Hydrogen inhalation is superior to mild hypothermia in improving cardiac function and neurological outcome in an asphyxial cardiac arrest model of rats. Shock 2016, 46, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Manaenko, A.; Zhan, Y.; Liu, W.W.; Ostrowki, R.P.; Tang, J.; Zhang, J.H. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischaemia rat model. Neuroscience 2010, 169, 402–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Q.; Hui, K.; Zhang, L.; Sun, X.; Li, W.; Duan, M. The effect of hydrogen-rich saline on the brain of rats with transient ischemia. J. Surg. Res. 2011, 168, e95–e01. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Choi, J.-I. Hydrogen-rich water improves cognitive ability and induces antioxidative, antiapoptotic, and anti-inflammatory effects in an acute ischemia-reperfusion injury mouse model. BioMed Res. Int. 2021, 9956938, 12p. [Google Scholar] [CrossRef]
- Jiang, Z.; Alamuri, T.T.; Muir, E.R.; Choi, D.W.; Duong, T.Q. Longitudinal multiparametric MRI study of hydrogen-enriched water with minocycline combination therapy in experimental ischemic stroke in rats. Brain Res. 2020, 1748, 147122. [Google Scholar] [CrossRef]
- Nagatani, K.; Wada, K.; Takeuchi, S.; Kobayashi, H.; Uozumi, Y.; Otani, N.; Fujita, M.; Tachibana, S.; Nawashiro, H. Effect of hydrogen gas on the survival rate of mice following global cerebral ischemia. Shock 2012, 37, 645–652. [Google Scholar] [CrossRef]
- Ge, P.; Zhao, J.; Li, S.; Ding, Y.; Yang, F.; Luo, Y. Inhalation of hydrogen gas attenuates cognitive impairment in transient cerebral ischemia via inhibition of oxidative stress. Neurol. Res. 2012, 34, 187–194. [Google Scholar] [CrossRef]
- Hayashida, K.; Sano, M.; Kamimura, N.; Yokota, T.; Suzuki, M.; Maekawa, Y.; Kawamura, A.; Abe, T.; Ohta, S.; Fukuda, K.; et al. H2 gas improves functional outcome after cardiac arrest to an extent comparable to therapeutic hypothermia in a rat model. J. Am. Heart Assoc. 2012, 1, e003459. [Google Scholar] [CrossRef] [Green Version]
- Hayashida, K.; Miyara, S.J.; Shinozaki, K.; Takegawa, R.; Yin, T.; Rolston, D.M.; Choudhary, R.C.; Guevara, S.; Molmenti, E.P.; Becker, L.B. Inhaled gases as therapies for post-cardiac arrest syndrome: A narrative review of recent developments. Front. Med. 2021, 7, 586229. [Google Scholar] [CrossRef]
- Yin, T.; Becker, L.B.; Choudhary, R.C.; Takegawa, R.; Shoaib, M.; Shinozaki, K.; Endo, Y.; Homma, K.; Rolston, D.M.; Eguchi, S.; et al. Hydrogen gas with extracorporeal cardiopulmonary resuscitation improves survival after prolonged cardiac arrest in rats. J. Transl. Med. 2021, 19, 462. [Google Scholar] [CrossRef]
- Gong, X.; Fan, X.; Yin, X.; Xu, T.; Li, J.; Guo, J.; Zhao, X.; Wei, S.; Yuan, Q.; Wang, J.; et al. Hydrogen therapy after resuscitation improves myocardial injury involving inhibition of autophagy in an asphyxial rat model of cardiac arrest. Exp. Ther. Med. 2022, 23, 376. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhou, J.; Zhan, W.; Xiong, Y.; Hu, C.; Li, X.; Li, X.; Li, Y.; Liao, X. The neuroprotective effects of intraperitoneal injection of hydrogen in rabbits with cardiac arrest. Resuscitation 2013, 84, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Tian, R.; Yan, H.; Pei, L.; Hou, Z.; Hao, S.; Li, Y.V.; Tian, Q.; Liu, B.; Zhang, Q. Hydrogen-rich water protects against ischemic brain injury in rats by regulating calcium buffering proteins. Brain Res. 2015, 1615, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, C.; Manuel, M.L.; Yuan, S.; Kevil, C.G.; McCarter, K.D.; Lu, W.; Sun, H. Role of hydrogen sulfide in early blood-brain barrier disruption following transient focal cerebral ischemia. PLoS ONE 2015, 10, e0117982. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, X.; Shi, J.; Shi, D.; Ye, Z.; Liu, W.; Li, M.; Wang, Q.; Kang, Z.; Bi, H.; et al. Lactulose ameliorates cerebral ischemia–reperfusion injury in rats by inducing hydrogen by activating Nrf2 expression. Free Radic. Biol. Med. 2013, 65, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.; Sun, X.; Li, R.; Sun, Q.; Cai, J.; Kang, Z.; Lv, S.; Zhang, J.H.; Zhang, W. Hydrogen saline offers neuroprotection by reducing oxidative stress in a focal cerebral ischemia-reperfusion rat model. Med. Gas Res. 2011, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ai, Y. Hydrogen saline suppresses neuronal cell apoptosis and inhibits the p38 mitogen-activated protein kinase-caspase-3 signaling pathway following cerebral ischemia-reperfusion injury. Mol. Med. Rep. 2017, 16, 5321–5325. [Google Scholar] [CrossRef] [Green Version]
- Nagatani, K.; Nawashiro, H.; Takeuchi, S.; Tomura, S.; Otani, N.; Osada, H.; Wada, K.; Katoh, H.; Tsuzuki, N.; Mori, K. Safety of intravenous administration of hydrogen-enriched fluid in patients with acute cerebral ischemia: Initial clinical studies. Med. Gas Res. 2013, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Hugyecz, M.; Mracskó, E.; Hertelendy, P.; Farkas, E.; Domoki, F.; Bari, F. Hydrogen supplemented air inhalation reduces changes of prooxidant enzyme and gap junction protein levels after transient global cerebral ischemia in the rat hippocampus. Brain Res. 2011, 1404, 31–38. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Ji, M.; Jia, M.; Chen, H.; Yang, J.; Duan, M. Hydrogen-rich saline attenuates neuronal ischemia-reperfusion injury by protecting mitochondrial function in rats. J. Surg. Res. 2014, 192, 564–572. [Google Scholar] [CrossRef]
- Chen, K.; Wang, N.; Diao, Y.; Dong, W.; Sun, Y.; Liu, L.; Wu, X. Hydrogen-rich saline attenuates brain injury induced by cardiopulmonary bypass and inhibits microvascular endothelial cell apoptosis via the PI3K/Akt/GSK3b signaling pathway in rats. Cell. Physiol. Biochem. 2017, 43, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gui, Q.; Jin, L.; Yu, P.; Wu, L.; Cao, L.; Wang, Q.; Duan, M. Hydrogen-rich saline attenuates hippocampus endoplasmic reticulum stress after cardiac arrest in rats. Neurosci. Lett. 2017, 640, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.R.; Perry, D.A.; Raza, A.; Nedder, A.P.; Pollack, E.; Regan, W.L.; van den Bosch, S.J.; Polizzotti, B.D.; Yang, E.; Davila, D.; et al. Perioperatively inhaled hydrogen gas diminishes neurologic injury following experimental circulatory arrest in swine. JACC Basic Transl. Sci. 2019, 4, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Jendroska, K.; Poewe, W.; Daniel, S.E.; Pluess, J.; Iwerssen-Schmidt, H.; Paulsen, J.; Barthel, S.; Schelosky, L.; Cervos-Navarro, J.; DeArmond, S.J. Ischemic stress induces deposition of amyloid beta immunoreactivity in human brain. Acta Neuropathol. 1995, 90, 461–466. [Google Scholar] [CrossRef]
- Wiśniewski, H.M.; Maślińska, D. Beta-protein immunoreactivity in the human brain after cardiac arrest. Folia Neuropathol. 1996, 34, 65–71. [Google Scholar]
- Jendroska, K.; Hoffmann, O.M.; Patt, S. Amyloid β peptide and precursor protein (APP) in mild and severe brain ischemia. Ann. N. Y. Acad. Sci. 1997, 826, 401–405. [Google Scholar] [CrossRef]
- Van Groen, T.; Puurunen, K.; Maki, H.M.; Sivenius, J.; Jolkkonen, J. Transformation of diffuse beta-amyloid precursor protein and beta-amyloid deposits to plaques in the thalamus after transient occlusion of the middle cerebral artery in rats. Stroke 2005, 36, 1551–1556. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Wu, H.; Yang, Y.; Wand, D.; Chen, Y.; Gu, Y.; Liu, T. Cerebral ischemia and Alzheimer’s disease: The expression of amyloid-β and apolipoprotein E in human hippocampus. J. Alzheimer’s Dis. 2007, 12, 335–341. [Google Scholar] [CrossRef]
- Kato, T.; Hirano, A.; Katagiri, T.; Sasaki, H.; Yamada, S. Neurofibrillary tangle formation in the nucleus basalis of Meynert ipsilateral to a massive cerebral infarct. Ann. Neurol. 1988, 23, 620–623. [Google Scholar] [CrossRef]
- Hatsuta, H.; Takao, M.; Nogami, A.; Uchino, A.; Sumikura, H.; Takata, T.; Morimoto, S.; Kanemaru, K.; Adachi, T.; Arai, T.; et al. Tau and TDP-43 accumulation of the basal nucleus of Meynert in individuals with cerebral lobar infarcts or hemorrhage. Acta Neuropathol. Commun. 2019, 7, 49. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Shi, Q.-Q.; Zhang, L.; Yue, C.-P.; He, Z.-J.; Li, X.-X.; He, Q.-J.; Liu, Q.; Du, X.-B. Hydrogen-rich water ameliorates neuropathological impairments in a mouse model of Alzheimer’s disease through reducing neuroinflammation and modulating intestinal microbiota. Neural Regen. Res. 2022, 17, 409–417. [Google Scholar] [PubMed]
- Lin, C.-L.; Huang, W.-N.; Li, H.-H.; Huang, C.-N.; Hsieh, S.; Lai, C.; Lu, F.-J. Hydrogen-rich water attenuates amyloid-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem. Biol. Interact. 2015, 240, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Zhang, G.; Zhang, T.; Lou, J.; Liu, J. L-arabinose elicits gut-derived hydrogen production and ameliorates metabolic syndrome in C57BL/6J mice on high-fat-diet. Nutrients 2019, 11, 3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Goshi, E.; He, Q. Micro/nanomaterials-augmented hydrogen therapy. Adv. Healthc. Mater. 2019, 8, e1900463. [Google Scholar] [CrossRef]
- Shimouchi, A.; Nose, K.; Shirai, M.; Kondo, T. Estimation of molecular hydrogen consumption in the human whole body after the ingestion of hydrogen-rich water. Adv. Exp. Med. Biol. 2012, 737, 245–250. [Google Scholar]
- Liu, C.; Kurokawa, R.; Fujino, M.; Hirano, S.; Sato, B.; Li, X. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4, 5485. [Google Scholar] [CrossRef] [Green Version]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef] [Green Version]
| Ischemia | Animal | Strain | Treatment | Benefits | References |
|---|---|---|---|---|---|
| Focal | Mice | C57B/L | Inhalation of 66.7% hydrogen/33.3% oxygen for 90 min post-ischemia. | Inhibition of microglial activity and regulation of microglial phenotype. Improvement of neurological outcome. | [19,68] |
| Global | Mice | C57BL/6J | Inhalation hydrogen (1.3%), oxygen (30%), and nitrogen (68.7%). 45 min of ischemia and 180 min of reperfusion, and 3 h/d, from 1 to 3 days post-ischemia. | Improved survival. Attenuation of neuronal injury, autophagy and brain edema. | [77] |
| Global | Rat | Wistar | 2.1% hydrogen supplemented by room air ventilation for 4 h after ischemia. | Reduction changes of prooxidant enzyme and gap junction protein levels. | [90] |
| Global | Rat | Sprague-Dawley | Hydrogen-rich saline (5 mL/kg) was injected immediately post-ischemia. | Significant improvement of surviving cells. Reduction tissue damage, the degree of mitochondrial swelling, and the loss of mitochondrial membrane potential but also preservation the mitochondrial cytochrome c content. | [91] |
| Global | Rat | Sprague-Dawley | I.V. hydrogen-rich saline (1 mL/kg, 4 mL/kg, or 6 mL/kg), HRS was given before hypoxia and during reoxygenation. | Inhibition of hippocampus endoplasmic reticulum stress and microvascular endothelial cells apoptosis via PI3K/Akt/GSK3β signaling pathway. | [92] |
| Global | Rat | Sprague-Dawley | Hydrogen-rich saline 5 mL/kg was intraperitoneally injected immediately and 6 h post-ischemia. | Significant improvement survival rate and neurological function. The beneficial effects associated with decreased levels of oxidative products, as well as the increased levels of antioxidant enzymes and accompanied by the increased activity of glucose-regulated protein 78, the decreased activity of cysteinyl aspartate specific proteinase-12 (caspase-12). | [93] |
| Global | Rat | Wistar | Inhalation of 2% hydrogen started immediately at the end of ischemia and lasted for 3 h. | Attenuation of cognitive impairment. Decreased pyramidal neuronal death in CA1 region of hippocampus. | [78] |
| Global | Rat | Sprague-Dawley | Hydrogen-rich saline was administered i.v. at 1 min before end of ischemia, followed by injections at 6 and 12 h post-ischemia. | Improves survival and neurological outcome. | [8] |
| Focal | Rat | Sprague-Dawley | 6 mL/kg i.p. per rat before and after ischemia. | Reduction brain infarct volume and improvement of neurological function. Prevention the ischemia-induced reduction of parvalbumin and hippocalcin levels and also reduced the glutamate toxicity-induced death of neurons. Attenuation the glutamate toxicity-induced by elevate in intracellular calcium. | [84] |
| Focal | Rat | Sprague-Dawley | 0.5 mL/kg/day saturated hydrogen saline (0.6 mmol/L) i.p. 3 days prior to ischemia and immediately during 24 h of reperfusion. | Significantly reduction the number of apoptotic cells, and the protein expression of p38 MAPK and caspase-3. These effects may be associated with the p38MAPK signaling pathway. | [88] |
| Focal | Rat | Sprague-Dawley | Hydrogen saline was injected i.p. (1 mL/100 g body weight) at designed time points 0, 3 or 6 h after reperfusion onset. | Reduction 8-hydroxyl-2′-deoxyguanosine, malondidehyde, interleukin-1β, tumor necrosis factor-α, and suppressed caspase 3 activity in ischemic brain. | [87] |
| Global | Rabbit | White | Before ischemia i.p. injection of hydrogen low dose (10 mL/kg) or high dose (20 mL/kg). | Improvement survival and neurological outcomes, reduction of neuronal damage and inhibition of neuronal apoptosis. Reduction indicators of oxidative stress in the blood and the hippocampus and increased activity of antioxidant enzyme. | [83] |
| Global | Swine | Yorkshire | Inhalation of hydrogen (2.40%) for a 24-h period during and after the ischemic injury. | Reduced neurological injury. | [94] |
| Ischemia | Number of Participants | Treatment | Benefits | Study | References |
|---|---|---|---|---|---|
| Focal | 50 patients | Inhalation 3% hydrogen gas (1 h twice a day) for initial 7 days. | Reduced infarct size, improved neurological outcome and daily living activity. | Randomized | [16] |
| Global | 5 patients | 2% hydrogen with oxygen was supplied via a respirator after admission to the intensive care unit for 18 h. | 4 patients survived 90 days with a favorable neurological outcome. | Pilot study | [25] |
| Global | 360 patients | 2% hydrogen with 24 to 50% oxygen was supplied via mechanical ventilation after admission for 18 h. | The first multicenter randomized trial is underway to confirm the efficacy of hydrogen on neurological outcomes in comatose out-of-hospital cardiac arrest survivors. | Randomized, double-blind, placebo-controlled trial. | [26] |
| Global | 5 patients | Inhalation 2% hydrogen with titrated oxygen was initiated upon admission for 18 h. | Oxidative stress markers were reduced in cardiogenic post-cardiac arrest patients but were slightly elevated in the patient with sepsis. Inflammatory cytokine levels remained unchanged in cardiogenic post-cardiac arrest patients, whereas a dramatic reduction was observed in one patient with sepsis. | Pilot study | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluta, R.; Januszewski, S.; Czuczwar, S.J. Molecular Hydrogen Neuroprotection in Post-Ischemic Neurodegeneration in the Form of Alzheimer’s Disease Proteinopathy: Underlying Mechanisms and Potential for Clinical Implementation—Fantasy or Reality? Int. J. Mol. Sci. 2022, 23, 6591. https://doi.org/10.3390/ijms23126591
Pluta R, Januszewski S, Czuczwar SJ. Molecular Hydrogen Neuroprotection in Post-Ischemic Neurodegeneration in the Form of Alzheimer’s Disease Proteinopathy: Underlying Mechanisms and Potential for Clinical Implementation—Fantasy or Reality? International Journal of Molecular Sciences. 2022; 23(12):6591. https://doi.org/10.3390/ijms23126591
Chicago/Turabian StylePluta, Ryszard, Sławomir Januszewski, and Stanisław J. Czuczwar. 2022. "Molecular Hydrogen Neuroprotection in Post-Ischemic Neurodegeneration in the Form of Alzheimer’s Disease Proteinopathy: Underlying Mechanisms and Potential for Clinical Implementation—Fantasy or Reality?" International Journal of Molecular Sciences 23, no. 12: 6591. https://doi.org/10.3390/ijms23126591
APA StylePluta, R., Januszewski, S., & Czuczwar, S. J. (2022). Molecular Hydrogen Neuroprotection in Post-Ischemic Neurodegeneration in the Form of Alzheimer’s Disease Proteinopathy: Underlying Mechanisms and Potential for Clinical Implementation—Fantasy or Reality? International Journal of Molecular Sciences, 23(12), 6591. https://doi.org/10.3390/ijms23126591








