Abstract
Periodontal disease is a chronic inflammatory disease caused by periodontal bacteria. Recently, periodontal phototherapy, treatment using various types of lasers, has attracted attention. Photobiomodulation, the biological effect of low-power laser irradiation, has been widely studied. Although many types of lasers are applied in periodontal phototherapy, molecular biological effects of laser irradiation on cells in periodontal tissues are unclear. Here, we have summarized the molecular biological effects of diode, Nd:YAG, Er:YAG, Er,Cr:YSGG, and CO2 lasers irradiation on cells in periodontal tissues. Photobiomodulation by laser irradiation enhanced cell proliferation and calcification in osteoblasts with altering gene expression. Positive effects were observed in fibroblasts on the proliferation, migration, and secretion of chemokines/cytokines. Laser irradiation suppressed gene expression related to inflammation in osteoblasts, fibroblasts, human periodontal ligament cells (hPDLCs), and endothelial cells. Furthermore, recent studies have revealed that laser irradiation affects cell differentiation in hPDLCs and stem cells. Additionally, some studies have also investigated the effects of laser irradiation on endothelial cells, cementoblasts, epithelial cells, osteoclasts, and osteocytes. The appropriate irradiation power was different for each laser apparatus and targeted cells. Thus, through this review, we tried to shed light on basic research that would ultimately lead to clinical application of periodontal phototherapy in the future.
1. Introduction
Periodontal tissue consists of “gingiva, periodontal ligament, cementum, and alveolar bone” [1]. Periodontal diseases cause a wide range of inflammatory conditions that affect the periodontal tissue, which could lead to loss of teeth and contribute to systemic inflammation [2]. Basic periodontal therapy eliminates etiological factors for periodontal disease and relieves inflammation in periodontal tissues [3]. Recently, periodontal therapy using a laser, “periodontal phototherapy,” has attracted much attention [4]. Many reports have been published on the methods recommended for periodontal therapy using lasers [5,6]. In addition, laser irradiation is also applied to treat pressure ulcers [7] and pain associated with temporomandibular dysfunction [8]. However, basic research related to clinical research is inadequate. We propose that it is necessary to understand the molecular biological effects of periodontal phototherapy for periodontal therapy. Photobiomodulation (PBM) is a treatment method based on research findings suggesting that irradiation with specific wavelengths of red or infrared light produces a wide range of physiological effects in cells, tissues, animals, and humans [9]. A previous study reported that the effect was a nonthermal process involving endogenous chromophores eliciting photophysical and photochemical phenomena at various biological scales, resulting in beneficial therapeutic outcomes [10].
The purpose of this review was to investigate cytological responses against laser irradiation for periodontal regeneration. We tried to provide insights on basic research that would subsequently lead to clinical research on periodontal phototherapy in the future.
2. Interaction with Tissues
When laser energy reaches a tissue surface, it can be reflected, scattered, absorbed, or transmitted to the surrounding tissues. The performance of a laser is determined by the degree of absorption. In particular, absorption in biological tissues is strongly influenced by the absorption coefficient in water, which is inherent to each wavelength [11,12]. Thus, lasers are clinically classified into two types depending on their wavelength: (1) a deeply penetrating type where the laser light penetrates and scatters into the tissue more deeply, such as the neodymium-doped yttrium-aluminum-garnet (Nd:YAG) (1064 nm) and diode lasers (810–980 nm available for clinical application and (2) a superficially absorbed type (shallowly penetrating type) where the laser light is absorbed in the superficial layer and does not penetrate or scatter deeply, such as the carbon dioxide (CO2) (10,600 nm), erbium-doped yttrium-aluminum-garnet (Er:YAG) (2940 nm), and erbium, chromium: yttrium –scandium-gallium-garnet (Er,Cr:YSGG) (2780 nm) lasers [4,13].
3. Effects of Laser Irradiation on Osteoblasts
The number of reports about the effects of laser irradiation on osteoblasts is increasing. For periodontal regeneration, osteoblasts play essential roles in bone formation and remodeling [14]. Therefore, the laser irradiation of osteoblasts is an important focus of research.
Most reports on laser-irradiated osteoblasts in vitro used diode lasers, including a blue diode laser (λ = 450 nm), a red diode laser (λ = 635–660 nm), and a Ga-Al-As laser (λ = 780–980 nm). Several studies have reported the effects of the Nd:YAG laser (λ = 1064 nm) on osteoblasts in vitro. A few reports on the effects of CO2 and Er:YAG lasers (λ = 2940 nm) have been published.
3.1. Diode Lasers
Various kinds of osteoblasts or osteoblast-like cells were used in previous studies to assess the effects of diode lasers. Most studies used cell lines such as MC3T3-E1 cells, an osteoblastic cell line derived from mouse, in 12 reports [15,16,17,18,19,20,21,22,23,24,25,26]. The effects of laser irradiation on Saos-2 [27,28,29,30,31,32,33,34], MG-63 [17,35,36,37,38,39,40], and human osteoblastic cell lines [32,41,42,43,44,45,46,47] were investigated in 8, 7, and 8 studies, respectively. In addition, primary osteoblasts from rat calvaria or human bone were also used in 8 and 5 studies, respectively.
Many studies have reported on the proliferation of cells irradiated by diode lasers. Diode laser irradiation significantly increased cell proliferation 1–3 days after irradiation [17,20,21,24,25,26,28,29,33,34,42,48,49,50,51]. Most of the effective energy density (fluence) ranges were from 1 to 10 J/cm2. In a previous study, irradiation at a total energy of 45.9–137.6 J/cm2 significantly increased the proliferation of human fetal osteoblasts (hFOB 1.19) [42]. Laser irradiation using various fluences (0.48–3.84, 5.0–8.3, and 45.9–137.6 J/cm2) also significantly enhanced cell proliferation at a later period of observation (e.g., on day 4–12) [29,42,48,52,53,54,55,56]. The proliferation of hypoxic-cultured osteoblasts was increased at 24 and 72 h after irradiation at 1.2 and 3.6 J/cm2, respectively [41]. In contrast, diode laser irradiation at similar fluences did not show a significant increase in osteoblast proliferation in some studies [17,27,35,38,57,58]. However, fluorescence-activated cell sorting (FACS) analysis of the cell cycle revealed that the percentage of cells in G2/M phase was significantly greater in rat calvarial osteoblastic cells by diode laser irradiation at 3.8 J/cm2 at 12 h after irradiation compared to nonirradiated control cells [49]. Cell viability and migration have also been evaluated in many studies. They were significantly increased by irradiation at 0.5–12 J/cm2 [30,32,36,43,44,45,47,56,59,60] Irradiation at fluences greater than 20 J/cm2 significantly decreased cell viability [30]. Previously, in most studies, it has been reported that diode laser irradiation at 1–12 J/cm2 tended to enhance the proliferation and viability of osteoblasts. However, effective irradiation protocols of diode lasers on the migration of osteoblasts have not been specifically determined.
Calcification of osteoblasts promoted by diode laser irradiation at 0.4–8.3 J/cm2 has been demonstrated in several studies [23,26,29,33,35,46,49,50,52,53,54,56,59,61,62,63]. Significantly enhanced mineralization in osteoblasts was observed at 7 days at the earliest [23], and in many cases, at around 20 days after irradiation [26,33,35,49,52,53,54,56,59,63]. Both single irradiation and multiple irradiations significantly promoted the calcification of osteoblasts.
Diode laser irradiation has been reported to affect gene and protein expression related to osteogenic differentiation, including alkaline phosphatase (ALP), osteocalcin, type Ⅰ collagen, Runt-related protein transcription factor 2 (Runx2), osterix, bone morphogenetic proteins (BMPs), transforming growth factor-β1 and β2 (TGF-β1 and β2), osteopontin, receptor activator of NF-κB ligand (RANKL), and osteoprotegerin (OPG).
At 1–14 days after irradiation, mRNA expression of ALP was significantly increased by irradiation at 0.4–6.7 J/cm2 [26,33,35,39,40,46,54,57]. ALP activity was also significantly enhanced at day 1–18 after irradiation at 1–10 J/cm2 in a number of studies [17,22,23,26,28,29,37,46,49,52,53,56,62,64]. Meanwhile, a previous study revealed that irradiation at 2 J/cm2 significantly decreased ALP activity in osteoblasts at 48 and 72 h [34]. Some studies have reported that diode laser irradiation did not increase ALP activity of primary osteoblasts after irradiation at 1.5 or 3 J/cm2 [57,58].
Osteocalcin, a marker of osteoblast terminal differentiation [65], has been investigated in relation to calcification of osteoblasts by diode laser irradiation. Ozawa et al. [52] reported that 830 nm diode irradiation at 3.8 J/cm2 on day 1 significantly increased the number of osteocalcin mRNA-positive cells and cell masses on day 2 and 4, respectively. Moreover, diode laser irradiation at 3 J/cm2 significantly enhanced osteocalcin synthesis in primary human osteoblast-like cells cultured on titanium implant material on day 10 [55]. Osteocalcin activity in hFOB 1.19 was significantly increased at 7 days after 940 nm laser irradiation at considerably high fluences in the range of 22.9–137.6 J/cm2 [42]. Even in hypoxic-cultured human osteoblasts, mRNA Bglap was increased on day 1–3 by diode laser irradiation at 1.2–3.6 J/cm2 [41]. The expression of Bglap on day 14 was significantly decreased in a human osteoblast cell line irradiated at 0.5, 1, and 2 J/cm2 [45]; however, indocyanine green (ICG)-mediated PBM significantly increased Bglap expression on day 7 following irradiation at 0.5 J/cm2 [46]. ICG-mediated PBM is a PBM with a photosensitizer, a light-activated molecule, and shares similar mechanisms with photodynamic therapy [46]. The effects of diode laser irradiation on osteoblasts have been investigated in the expression of type I collagen [31,34,35,40,41,45,46,47]. Most reports have shown that low-level irradiation at 0.5–3.6 J/cm2 significantly increased type I collagen expression in human osteoblastic cells at 1–20 days after irradiation [34,35,40,41,45,46,47]. Irradiation at higher fluences (5 and 15 J/cm2) also significantly increased Col1a1 expression at 24, 48, and 72 h in a previous study [31]. Irradiation at 1.2–3.6 J/cm2 significantly increased the mRNA expression of type I collagen in hFOB 1.19 at 24 h after irradiation compared to that in hypoxic-cultured osteoblasts. However, at 48 and 72 h, type I collagen mRNA expression was significantly lower than that in hypoxic-cultured osteoblasts upon irradiation [41].
Several studies have reported the effect of diode laser irradiation on the expression of Runx2, an essential transcription factor for osteoblast differentiation [18,26,33,57,62,66]. Laser irradiation at 808 nm and 0.4 J/cm2 at continuous wave mode and 1.9 J/cm2 of 2 Hz pulsed mode at 830 nm significantly increased the expression of Runx2 [18,33]. Ultrahigh-frequency and ultrashort-pulse 405 nm blue laser irradiation at 5.6 J/cm2 on osteoblasts significantly increased Runx2 expression on day 3 in MC3T3-E1 cells [26]. Some reports showed that irradiation at 3 J/cm2 decreased Runx2 expression in primary human osteoblast-like cells from alveolar bone [57,62].
Osterix is generally required for Bglap activation and bone formation [67] and is mutually regulated with Runx2 for the proliferation and differentiation of osteoblast-lineage cells and their progenitors [66]. Irradiation at 1.9–5.9 J/cm2 significantly increased the expression of Osx at 9 h on day 3 in osteoblasts [18,23,26,64]. In contrast, downregulation of Osx at 3, 6, and 12 h in primary human osteoblast-like cells from the alveolar bone after irradiation at 3 J/cm2 was reported [62].
BMPs, factors for bone formation, induce various genes, including Runx2 and Osterix (Sp7) [68]. The effects of diode laser irradiation on BMP expression in osteoblasts have been studied previously [18,35,40,41,47,57]. The expression of Bmp2, Bmp4, and Bmp7 was significantly increased at 6, 9, and 12 h after irradiation at 0.9–2.8 J/cm2 in MC3T3-E1 cells [18]. At later time points, on day 1–20, BMP mRNA expression was also significantly increased by irradiation at 1.2–6.7 J/cm2 [35,40,41]. Regarding bisphosphonate (BP)-related osteonecrosis of the jaw, a combined application of rhBMP-2 and irradiation at 1.2 J/cm2 was more effective in enhancing osteoblastic activity and bone formation activity in alendronate-treated hFOB 1.19 than the application of either modality alone [47].
BMPs belong to the TGF-β family, which is a prototype of a large family of cytokines involved in the growth and remodeling of bone [69]. TGF-β1 mRNA expression in osteoblasts was significantly increased at day 1–3, 10, and 20 after irradiation at 1.2–6.7 J/cm2 [35,40,41]. Laser irradiation at 830 nm and 3 J/cm2 significantly promoted TGF-β1 production, as measured by an enzyme-linked immunosorbent assay [55]. The expression of TGF-β1 suppressed by alendronate was recovered following a combined application of rhBMP-2 and irradiation at 1.2 J/cm2 in hFOB1.19 cells [47]. However, irradiation at 5–10 J/cm2 significantly decreased the expression of TGFB1 in Saos-2 cells at 48 and 72 h [31].
Several previous studies have reported the expression of osteopontin [33,34,47,57]. Osteopontin, a bone matrix noncollagenous glycophosphoprotein, is secreted by osteoblasts during bone mineralization and remodeling [70]. Tani et al. [33] reported that red diode laser irradiation at 0.4 J/cm2 (λ = 635 nm) significantly increased osteopontin expression by densitometric analysis of the fluorescence intensity of immunostained osteopontin. Another study indicated that 808 nm laser irradiation at 1.2 J/cm2 had a greater effect on osteopontin expression in alendronate-treated hFOB 1.19 cells than in rhBMP-treated cells by Western blotting analysis [47]. However, 670 nm laser irradiation at 2 J/cm2 significantly decreased osteopontin mRNA expression in Saos-2 cells at 24 h compared to cells irradiated at 1 J/cm2 [34]. Irradiation at 3 J/cm2 also decreased osteopontin mRNA expression in primary human osteoblast-like cells at 14 days [57].
To investigate the effects of diode laser irradiation on bone remodeling, osteoclast-related markers (e.g., RANKL and OPG) have been studied [30,44,47,51,57]. A previous study reported a significant downregulation of RANKL, a significant upregulation of OPG, and a significant decrease in the RANKL/OPG ratio in primary rat calvarial cells irradiated at 1.1 J/cm2 [51], whereas irradiation with a dose of 3 J/cm2 increased the RANKL/OPG ratio in primary human osteoblast-like cells on titanium disks [57]. Irradiation at 5, 10, and 50 J/cm2 tended to increase the RANKL/OPG ratio, but no significant differences were observed [30]. Ga-Al-As laser irradiation with 808 or 920 nm at 1.2 J/cm2 increased RANKL and OPG expression in hFOB 1.19 cells [44,47].
Other factors related to bone formation are affected by diode laser irradiation. Smad1/5/8, which are activated by BMPs and referred to as BMP-specific receptor-regulated Smads [68,71], exhibited significantly enhanced phosphorylation by 805 nm laser irradiation at 5.9 J/cm2 [64]. The expression of phospho-extracellular signal-regulated kinase (ERK) was significantly increased by irradiation at 5 and 10 J/cm2 [36], and phosphorylated ERK1/2 was also increased at 15 min after irradiation at 2.9 J/cm2 [25]. The expression of distal-less homeobox 5 (Dlx5), which stimulates osteoblast differentiation [72], and Msh homeobox 2 (Msx2), which promotes osteoprogenitor proliferation but prevents differentiation, was significantly enhanced by irradiation at 1.9 J/cm2 in MC3T3-E1 cells [18]. Diode laser irradiation at 7.6 J/cm2 significantly increased osteoglycin gene expression at 2 h after irradiation [16]. Osteoglycin was reported to increase osteoblast differentiation in some studies [73], whereas other studies reported that osteoglycin decreases osteoblast differentiation [74].
3.2. Nd:YAG Laser
Nd:YAG laser is the second-most studied laser for its effect on osteoblasts or osteoblast-like cells after diode lasers. The effects of Nd:YAG laser on Saos-2 human osteoblast-like cells have been investigated in many studies [75,76,77,78,79]. Nd:YAG laser irradiation has been reported to have positive effects on osteoblasts or osteoblast-like cells. Irradiation at 10 Hz and 20 mJ for 10 s had a stimulatory effect on the cell viability and proliferation of Saos-2 cells at 7, 14, and 21 days [75]. Cell proliferation of Saos-2 was also significantly increased at 48 h following irradiation at 50 or 70 Hz and 20 mJ/pulse for 10 s [76]. Another study reported that irradiation 3 times at 0.5–2 W enhanced cell proliferation rates of Saos-2 cells on day 4 compared to one-time irradiation [77]. Irradiation at 10.3 J/cm2 accelerated cell migration until 24 h after irradiation and significantly enhanced ATP production in Saos-2 cells at 24 h following irradiation [78]. However, a Q-switched Nd:YAG laser irradiation at 1.5, 3, or 5 J/cm2 significantly decreased proliferation in MC3T3-E1 cells [80].
Nd: YAG laser irradiation showed various effects regarding gene and protein expression related to osteogenic differentiation or bone remodeling. ALP activity was significantly increased in MC3T3-E1 cells at 3, 7, and 14 days after irradiation at 1.5, 3, or 5 J/cm2, with or without nonglycosylated human recombinant BMP-2 (100 ng/mL) treatment [80]. ALP gene (Alpl) expression was significantly increased in Saos-2 cells at 24 h and 7 days after irradiation at 17.3 and 1.5 J/cm2, respectively [76,79]. An increase in Runx2 and osteopontin mRNA expression was also significantly induced at 7 days after irradiation at 1.5 J/cm2 in Saos-2 cells [76]. Expression of BMP2 was significantly increased in MC3T3-E1 cells 2 days after irradiation at 3 J/cm2 [80]. Irradiation at 17.3 J/cm2 significantly increased mRNA expression of RANKL and OPG in Saos-2 cells at 24 h [79]. In a previous study, highly intensified calcium deposition on day 12 and significantly enhanced mineralization on day 21 were observed in MC3T3-E1 cells by Nd:YAG laser irradiation at 1.5–5 J/cm2 with or without rhBMP-2 treatment [80]. In addition, intracellular Ca2+ in Saos-2 cells was increased by irradiation at 50 Hz with a fluence of 1.5 J/cm2 through the activation of the transient receptor potential 1 (TRPC1) ion channels [76]. Gene expression of insulin-like growth factor-1 (IGF-1; IGF1), an important regulator of bone formation [81], was significantly enhanced in MC3T3-E1 cells on day 2 after irradiation at 3 J/cm2 with or without rhBMP-2 treatment [80].
3.3. Er:YAG Laser
Three previous studies reported the effects of Er:YAG laser irradiation on osteoblasts or osteoblast-like cells in vitro [82,83,84]. Er:YAG laser irradiation at 5.1–12.7 J/cm2 significantly reduced mitochondrial activity in Saos-2 cells compared to nonirradiated cells. However, mitochondrial activity was significantly increased with decreasing energy settings and/or increasing the distance between the laser application tip and the bottom of the culture plate [82]. Er:YAG laser irradiation at a fluence of 1.0–4.3 J/cm2 significantly increased MC3T3-E1 cell proliferation by irradiation in the absence of a culture medium. When irradiated at higher fluences (6.7 and 8.6 J/cm2), cell cytotoxicity of MC3T3-E1 was significantly increased. In the presence of a culture medium during irradiation, Er:YAG laser irradiation at much higher fluences (12.9 and 15.1 J/cm2) significantly increased MC3T3-E1 cell proliferation on day 1 and 3 without increasing cell cytotoxicity. The effect of Er:YAG laser on cell proliferation seemed to be induced by the activation of ERK [83], which plays a central role in the control of cell proliferation [85]. Another study reported that Er:YAG laser irradiation did not affect cell proliferation but significantly enhanced calcification of primary osteoblast-like cells from rat calvaria [84]. Irradiation at 3.3 J/cm2 significantly promoted mineralization of primary osteoblast-like cells on day 7, possibly via enhanced Bglap expression, without major thermal effects. Microarray analysis revealed that irradiation at 3.3 J/cm2 caused an upregulation of inflammation-related genes and downregulation of Wisp2, which plays an important role in the differentiation and mineralization of osteoblasts [86]. Gene set enrichment analysis showed that Er:YAG laser irradiation enriched Notch signaling, which plays a critical role in various cellular functions, including the promotion of osteogenic differentiation of osteoblasts in synergy with BMP [87].
3.4. Er,Cr:YSGG Laser
There are no reports on the direct effects of Er,Cr:YSGG laser irradiation on osteoblasts or osteoblast-like cells. Hence, the molecular biological effects of Er,Cr:YSGG laser irradiation on osteoblasts remains unclear.
3.5. CO2 Laser
The effect of CO2 laser irradiation on osteoblast-like cells was reported in a previous study [88]. The study investigated the effect of CO2 laser irradiation on rat osteoblast-like ROS 17/2.8 cells at 0.5–2 W for 20 s, resulting in a power density of 0.4–1.43 J/cm2. CO2 laser irradiation at 1.43 J/cm2 enhanced the mRNA expression of bone sialoprotein (BSP) at 12 h after irradiation. Transcription of BSP (IBSP) gene was also enhanced via the tyrosine kinase, Src tyrosine kinase, and ERK 1/2 signaling pathways, and fibroblast growth factor 2 response element in the rat IBSP gene promoter by CO2 laser irradiation.
3.6. Summary
The contents of this section are summarized in Table 1. Several reports have revealed the favorable effects of laser irradiation on osteoblasts or osteoblast-like cells. Laser irradiation enhances or increases cell proliferation, viability, migration, calcification, and expression of genes and proteins related to osteogenic differentiation, thereby promoting bone formation. These effects were observed in many studies using various types of lasers with different wavelengths; however, most of the effective energy fluences were low (under 6.0 J/cm2). In some studies, high power as well as low power irradiation was reported to have biological effects on osteoblasts or osteoblast-like cells. However, in vitro evidence related to Nd:YAG, CO2, Er:YAG, and Er,Cr:YSGG lasers are still limited. Further research is needed to elucidate the molecular biological effects of laser irradiation on osteoblasts.

Table 1.
Summary of the effects of laser irradiation on osteoblasts.
4. Effects of Laser Irradiation on Fibroblasts
Fibroblasts are components of the connective tissue, which migrate to a lesion from the late inflammatory phase until epithelialization is completed [89]. Fibroblasts play an essential role in supporting other cells, are associated with wound healing or regeneration, and function to break down blood clots, thereby secreting various growth factors and cytokines and creating new extracellular matrix (ECM) and collagen structures [90]. Additionally, fibroblasts play a critical role in wound contraction [91]. Therefore, fibroblasts are essential for effective wound healing and tissue regeneration.
Since various types of lasers have been shown to enhance wound healing through tissue repair and anti-inflammatory effects in previous studies [92,93], the biological and molecular mechanism of this event has been pursued over the years. In particular, the effect of lasers on fibroblasts has been focused on this field. In this section, we focus on gingival fibroblasts.
4.1. Diode Laser
Diode lasers are representative lasers used in PBM for wound healing, and their biostimulatory effects, such as anti-inflammatory effects, have been reported in previous studies [4]. To determine the physiological mechanisms related to the biological effects, lipopolysaccharide (LPS)-challenged human gingival fibroblasts (HGFs) were irradiated using an 830 nm diode laser, with a total energy of 1.9–12.6 J corresponding to 3–20 min exposure, and PGE2 production and cyclooxygenase (COX)-1 (COX1) and COX-2 (COX2) gene expression were analyzed. The results suggested that PGE2 production and COX-2 mRNA levels were significantly suppressed in a dose-dependent manner upon laser exposure [94]. Additionally, dramatic downregulation of plasminogen activator (PA) activity, implicated in the degradation of extracellular matrix and synthesis of kinin in the process of inflammation, and downregulation of tissue PA mRNA levels were observed in the HGFs irradiated with 830 nm laser at 7.9 J/cm2 compared to that in the control group [95]. In addition, under similar conditions, interleukin (IL)-1β production was reduced, and further investigation by RT-PCR showed that mRNA expression of IL-1β was inhibited, whereas that of IL-1β-converting enzyme (ICE) was invariable [96].
The effect of diode laser on the proliferation and migration of fibroblasts has been reported previously [97,98,99], and the exposure time is more relevant to cell proliferation and cell survival than to power output [97]. Moreover, the cell proliferation rates in the single-dose and double-dose groups were compared using a 685 nm diode laser. Although cell proliferation was enhanced in both groups, no significant difference was observed between the two laser-irradiated groups. In addition, a single dose of 2.0 J/cm2 in the irradiated group resulted in a higher proliferation and viability rate than the nonirradiated control group. They also evaluated the secretion of growth factors such as basic fibroblast growth factor (bFGF), IGF-1, and the receptor of IGF-1 (IGFBP3). Single-dose irradiation significantly increased the secretion of bFGF and IGF-1 in irradiated cells, but the secretion of IGFBP3 was not significantly increased compared to that in control cells. All growth factors were significantly increased in the double-dose group compared to the nonirradiated group [100]. Similar to this result, the upregulation of mRNA expression for other growth factors such as IGF, VEGF, and TGF-β has also been confirmed [101,102,103]. In contrast, irradiation with an 810 nm laser caused a dramatic reduction in HGF cell numbers in vitro, with variable parameters, i.e., fluence of 24.6–492.8 J/cm2 [104].
Diode lasers have also been reported to have positive effects on collagen synthesis [102]. Investigation of the effect of a 904 nm diode laser on cell growth and procollagen synthesis of NIH-3T3 fibroblasts was approximately three- to sixfold after irradiation with 3 and 4 J/cm2, although no significant increase in procollagen synthesis was observed. However, neither cell growth nor procollagen synthesis was observed at 5 J/cm2 [105]. In contrast, in another study, gene expression of collagen type 1 was upregulated in the HGF cell line (HGF3-PI 53) 3 days after irradiation (4 J/cm2) [99].
To examine the laser’s effect on fibroblast-myofibroblast differentiation, NIH/3T3 fibroblasts were irradiated with a 635 nm diode laser at 0.3 J/cm2, and morphological, biochemical, and electrophysiological assays were conducted. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 (MMPs play a pivotal role in physiological processes such as tissue remodeling) was upregulated, whereas tissue inhibitors of MMPs (TIMP)-1 and TIMP-2 were suppressed. Additionally, TGF-β1/Smad3‒mediated fibroblast-myoblast transition was inhibited. These results suggest that the diode laser modulates the TRPC1 ion channel, which in turn contributes to an antifibrotic effect by interfering with TGF-β1 signaling [106].
Bisphosphonate treatment is known to have a negative effect on wound healing [107]. In a study, diode laser irradiation tended to increase the viability of HGFs, although no significant difference was observed compared to that of nonirradiated control HGFs. However, when HGFs were cultured in a bisphosphonate-conditioned medium, laser irradiation significantly increased cell viability. Furthermore, laser irradiation on cell-free bisphosphonate-conditioned medium before culturing HGFs had no significant effect on cell viability, which indicated that laser irradiation directly affected the HGFs rather than suppressing the medicinal effect of bisphosphonate [43]. Thus, diode lasers may have the potential to become a supportive tool for preventing and treating of bisphosphonate-related diseases, such as osteonecrosis of the jaw.
However, adverse effects of lasers have also been reported. Diode laser irradiation (904 nm) at 3 J/cm2 on fibroblast cell line changed the ultrastructure of the cells’ cytoplasmic organelles; concurrently, a significant reduction in protein synthesis was observed [108]. Therefore, the cytotoxicity of the lasers should also be investigated for their safe usage.
4.2. Nd:YAG Laser
Previous studies have indicated that Nd:YAG lasers have various biological effects on cells, both in vivo and in vitro [4]. Nd:YAG laser (wavelength: 1060 nm) reduced collagen synthesis in human skin fibroblasts at energy levels as low as 1.1 × 103 J/cm2, without altering DNA replication or cell viability [109]. Furthermore, DNA replication and collagen synthesis in human skin fibroblasts have been compared between Nd:YAG laser irradiation at 1.2–4.7 × 103 J/cm2 for 3–12 s and under halogen lamp heat. Marked inhibition of DNA replication and collagen production was observed in the laser-irradiated fibroblasts, although no such decrease was noted in the halogen lamp-heated fibroblasts. Therefore, several characteristics other than the thermal effect may be critical in altering the biological functions of fibroblasts [110].
Nevertheless, histological analysis of laser-treated skin areas showed new collagen formation and increased the number of fibroblasts [111]. In addition, many other studies showed an increase in procollagen and collagen type-1 levels after Nd:YAG laser irradiation [76,112,113,114,115]. Nd:YAG laser downregulated the expression of MMP-1 and MMP-2 enzymes in the injured skin [112]. The reduction in MMP-1 was observed in keratinocyte-fibroblasts after Q-switched Nd:YAG laser irradiation at 8 J/cm2 [113]. Moreover, the effects of different wavelengths (532 nm and 1064 nm) of a Q-switched Nd:YAG laser on human skin fibroblasts were investigated. Both the lasers significantly increased the expression of type I and III procollagen and tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 and decreased MMP-2 and MMP-3 expression. Higher increased/decreased rates were observed in the 1064 nm Nd:YAG laser irradiation. Additionally, the 532 nm Nd:YAG laser increased Hsp70 and IL-6 expression, whereas the 1064 nm Nd:YAG laser upregulated TGF-β expression, suggesting that the molecular biological effects of Nd:YAG laser irradiation may differ according to the wavelengths used [115].
4.3. Er:YAG and Er,Cr:YSGG Lasers
The specific absorption characteristics of Er:YAG and Er,Cr:YSGG lasers have been reported to be beneficial for wound healing after soft tissue ablation [116,117]. During ablation, cells underlying the surface layer, including fibroblasts, indirectly receive low energy of the Er:YAG/Er,Cr:YSGG laser irradiation, which has been shown to promote wound healing and tissue regeneration [10]. Therefore, recently, a direct effect of low-level irradiation of Er:YAG/Er,Cr:YSGG laser on fibroblasts has been investigated in vitro. Pourzarandian et al. [118] showed that low-level laser therapy using an Er:YAG laser enhanced the proliferation of cultured HGFs and identified the optimal stimulative energy density of 3.4 J/cm2. They also observed a significant increase in PGE2 production and COX-2 mRNA expression after irradiation, and laser-induced PGE2 synthesis was completely inhibited by the COX-2 inhibitor, NS398 [119]. Proteomic analysis has been performed to investigate differentially expressed proteins in HGFs induced by low-level Er:YAG laser irradiation. On day 1 after irradiation at 2.1 J/cm2, significant cell proliferation without cell damage was observed. In addition, a total of 377 differentially expressed proteins were identified by mass spectrometry, 59 of which were upregulated and 15 were downregulated in laser-irradiated HGFs. Among the upregulated differentially expressed proteins, galectin-7, which is one of the essential proteins in the wound-healing process, was validated by quantitative PCR, Western blotting analysis, and enzyme-linked immunosorbent assay. To confirm the effect of galectin-7, HGFs were treated with recombinant human galectin-7, and cell proliferation was assessed in a dose-dependent manner, which suggested that alteration in protein expression and upregulation of galectin-7 may partly contribute to proliferation in HGFs [120]. Kong et al. [121] observed maximal cell proliferation at 6.3 J/cm2 on day 3 after irradiation, although it was accompanied by an increase in lactate dehydrogenase (LDH) release. An increase in ATP level, Ki-67 staining, and cyclin-A2 mRNA expression was confirmed, and it was observed that the increase in cell proliferation was due to the effect of Er:YAG laser irradiation on the cell cycle. However, alterations in the mitochondria and ribosomal endoplasmic reticulum (ER) were observed at 3 h postirradiation at 6.3 J/cm2; the changes subsided after 24 h, suggesting the occurrence of transient cellular injury. Furthermore, as the surface temperature of laser-irradiated cells reached 40.9 °C, nonirradiated cells were treated with a medium warmed at 40 °C, which also increased cell proliferation. In addition, laser-induced cell proliferation was suppressed by inhibitors of the thermosensory transient receptor potential channels (TRPV-1), capsazepine, or SKF96365. Finally, 21 genes involved in heat-related biological responses and endoplasmic reticulum-associated degradation were identified by microarray analysis. Therefore, 6.3 J/cm2 laser irradiation on HGFs may enhance cell proliferation through photothermal effects, despite transient cellular damage.
The effects of Er:YAG and Er,Cr:YSGG irradiation on cultured fibroblast cell lines (NCBI:C-165) were compared at different fluences: 1 W power output (10 Hz and 100 mJ) and 0.5 W power output (10 Hz and 150 mJ), respectively. Cell proliferation was upregulated in both groups compared to that in the control, but Er,Cr:YSGG laser irradiation tended to be more effective in cell proliferation than Er:YAG laser irradiation [122].
The mechanical effects of Er:YAG laser irradiation on fibroblasts have also been studied. As the number of primary human gingival fibroblasts significantly decreased after 3.0 W irradiation, gene expression analysis was conducted for cells irradiated at 0.6, 1.0, and 1.2 W. Cells were divided into four groups: control cells (not undergoing any procedures), cells undergoing only Er:YAG laser irradiation, cells undergoing only centrifugal loading, and a cells undergoing both Er:YAG laser irradiation and centrifugal force loading. Gene expression of COX2, IL1B, TNFA, BMP2, and BMP4 was significantly increased in laser-irradiated cells (in a dose-dependent manner) compared to the control cells at 24 h after irradiation. Additionally, only COX2 gene expression showed a significant increase in the centrifugal-loaded cells compared to control cells. In contrast, gene expression of COX2, IL1B, TNFA, BMP2, and BMP4 was significantly higher in the laser-loaded and centrifugally loaded cells than in the centrifugally loaded cells. These results suggest that bone metabolism genes may be regulated by mechanical stimulation and laser irradiation combined [123].
4.4. CO2 Laser
High-power CO2 laser irradiation is mainly used in various surgical procedures as an alternative to traditional scalpel procedures [124]. Recently, low-level laser irradiation with a CO2 laser has gained attention in dentistry due to its promotive effect on wound healing [125,126,127]. The secretion of TGF-β1 was downregulated, whereas that of bFGF was upregulated by high-frequency CO2 laser irradiation, which occurred maximally at 4.7 J/cm2 in both normal and keloid dermal fibroblasts in vitro, resulting in enhancement of cell replication [128]. Thus, CO2 laser irradiation may have the ability to balance collagen organization in fibrosis. Furthermore, PBM with a CO2 laser on proliferation and migration was examined at the cellular level. Promotion of cell proliferation and migration of cultured human dermal fibroblasts (HDFs) were examined by MTS assay and cell migration assay, respectively, with irradiation of 1.0 J/cm2. In addition, with the same power, Western blotting analysis showed activation of Akt, ERK, and JNK signaling pathways. However, suppression of Akt, ERK, or JNK signaling pathways significantly inhibited both the proliferation and migration of laser-irradiated HDFs. The study indicated that low-level laser irradiation with a CO2 laser might promote proliferation and migration of fibroblasts via activation of Akt, ERK, or JNK signaling pathways [129].
From another perspective, as the clinical use of CO2 lasers has increased, the safety of laser irradiation has been investigated. Apfelberg et al. [130] exposed cultured fibroblasts to CO2 laser irradiation before the occurrence of malignancy was examined. The results showed that CO2 laser-irradiated cells did not exhibit a greater incidence of malignancy compared to controls, indicating that CO2 laser seems to be noncarcinogenic in laboratory cells.
4.5. Summary
The contents of this section are summarized in Table 2. The effects of PBM by laser irradiation on fibroblasts appear to be comparable despite the different wavelengths. Proliferation, migration, and secretion of cytokines/chemokines are the main functions affected by laser irradiation; which may lead to early wound healing; although, the biological/molecular evidence to support this phenomenon is still partial and inadequate. Moreover, excessive power or irradiation time results in cell damage and ineffective treatment. However, concurrently, lasers have the potential to regulate collagen synthesis through fibroblast stimulation depending on the target disease. Thus, further investigation on optimal configuration, which is consistent in vivo and in vitro, and more profound bioinformatic studies are required in the future to clarify the critical mechanisms of the effects of lasers on fibroblasts.

Table 2.
Summary of the effects of laser irradiation on fibroblasts.
5. Effects of Laser Irradiation on Periodontal Ligament Cells
The periodontal ligament is the only ligament in the body that connects two distinct hard tissues. It is a fibrous, complex, and soft connective tissue that attaches the tooth root to the inner wall of the alveolar bone. The periodontal ligament thickness decreases with age. It is functionally essential for tooth support and for allowing teeth to withstand the forces generated during mastication [131].
5.1. Diode Laser
Diode laser irradiation has been reported to have positive effects on human periodontal ligament cells (hPDLCs). PBM at energy doses of 2 and 4 J/cm2 upregulated gene expression related to osteogenic differentiation, including BMP2, OC (BGLAP), RUNX2, and ALPL in hPDLCs. PBM enhanced the osteogenic differentiation of hPDLCs via cAMP regulation [132]. Additionally, it significantly increased cellular viability, decreased cellular inflammatory marker expression, and increased OC activity in hPDLCs at two energy densities (5 and 10 J/cm2) [133]. Suppression of inflammation is one of the positive effects of laser irradiation. After 670 nm Ga-Al-As laser irradiation (5 and 10 J/cm2), the mRNA expression of inducible NO synthase (INOS), COX2, and IL1B were decreased compared to that in nonirradiated control cells.
Another study using an 830 nm laser showed that laser irradiation at 3.8 J/cm2 decreased COX-2 and cytosolic phospholipase A2-α mRNA expression after 24 h in mechanically stretched hPDLCs [134]. The increase in PGE2 production was significantly inhibited by diode laser irradiation at 346–1152 J/cm2 in a dose-dependent manner. The increase in IL-1β production was also significantly inhibited by diode laser irradiation, although the inhibition was observed only with high-power irradiation [135]. Diode laser irradiation (4.0–7.9 J/cm2) significantly inhibited a marked increase in plasminogen activator activity in hPDLCs in response to stretching [136]. Additionally, Huang et al. [137] reported that the gene expression levels of INOS, TNFA, and IL1B in LPS-exposed periodontal ligament cells were decreased after irradiation, and phospho-ERK expression was significantly increased in the laser-irradiated cells compared to that in nonirradiated cells.
hPDLCs irradiated with an 810 nm diode laser, showed promotion of proliferation and differentiation. Irradiation at 3.9 J/cm2 increased proliferation of human periodontal ligament fibroblasts (PDLFs) between 24 and 48 h, and ALP activity at 48 and 72 h. The phosphorylated ERK level was also more prominent after irradiation at 3.9 J/cm2 energy fluency [138]. Additionally, the protein expression of MMP-8 in hPDLFs was decreased by 810 nm diode laser irradiation at 10 J/cm2 [139]. Moreover, the 809 nm diode laser irradiation at 2.0–7.8 J/cm2 of PDLFs significantly upregulated their proliferation up to 72 h [140].
5.2. Er:YAG Laser
Er:YAG laser irradiation at 4.2 J/cm2 on hPDLFs promoted cell proliferation, migration, and invasion abilities. The report also revealed that the silencing of galectin-7 abrogated the effects of Er:YAG laser on cell proliferation, migration, and invasion, suggesting that the Er:YAG laser promoted these effects through the induction of galectin-7 [141].
5.3. Nd:YAG Laser, Er,Cr:YSGG Laser, and CO2 Laser
There are no reports on the effects of Nd:YAG laser, Er,Cr:YSGG laser, and CO2 laser irradiation on periodontal ligament cells.
5.4. Summary
The contents of this section are summarized in Table 3. Laser irradiation on PDLs enhanced cell proliferation, migration, calcification, and differentiation. In addition, gene expression was altered by laser irradiation, especially with suppression of inflammatory products. However, the effects of laser irradiation on hPDLCs and hPDLFs were only investigated using diode and Er:YAG lasers. It is necessary to generate more evidence and reveal the mechanisms by which laser irradiation affects hPDLCs.

Table 3.
Summary of the effects of laser irradiation on human periodontal ligament cells.
6. Effects of Laser Irradiation on Endothelial Cells
The mechanisms related to the effects of laser irradiation on wound healing are not completely clear. However, some studies reported that laser treatment could accelerate wound healing, especially in acute, chronic, and impaired wound-healing conditions [142] as well as in periodontal disease [143]. Endothelial cells play important roles in the process of wound healing and regeneration of periodontal tissue [144]. Hence, in this section, we summarized the direct effects of laser irradiation on endothelial cells.
6.1. Diode Laser
Some researchers have investigated the effect of diode laser irradiation on endothelial cells. The human vascular endothelial cell line (HECV) irradiated with an 808 nm diode laser (60 J/cm2) demonstrated no significant difference in viability but demonstrated higher proliferation than non-treated cells. Moreover, the study reported that diode laser stimulated mitochondrial oxygen consumption and ATP synthesis in HECV [145]. Another study using the 808 nm diode laser reported that CD54, CD62E, monocyte chemotactic protein-1 (MCP-1) expression, and von Willebrand factor release were altered in human umbilical vein endothelial cells (HUVECs) stimulated with IL-1β followed by laser irradiation. MCP-1 expression in HUVECs was significantly lower 6 h after 4.5 J/cm2 stimulation than in IL-1β stimulated cells. In addition, both 1.5 and 4.5 J/cm2 of laser irradiation inhibited IL-1β-induced increase in CD54 and CD62E concentration in the supernatant. Therefore, this study suggested that low-power laser irradiation decreased the pro-inflammatory and procoagulant activity of IL-1β-stimulated endothelial cells [146]. Moreover, a 670 nm diode laser irradiation caused a stimulatory effect on the proliferation of HUVECs [147] and increased their viability [43]. Using a 635 nm diode laser at different doses (2, 4, and 8 J/cm2), all doses of irradiation significantly increased the proliferation of HUVECs and significantly reduced the concentration of soluble vascular endothelial growth factor (sVEGFR-1), an inhibitor of vascular endothelial growth factor (VEGF), compared with nonirradiated cells [148]. A study using rhesus macaque choroid-retinal endothelial cells (RF/6A) reported that an 810 nm diode laser (over 84.0 J/cm2) irradiation caused significant cell death, and irradiation at a fluence of 45.9–76.4 J/cm2 induced Hsp70 hyperexpression at 12–18 h postirradiation [149].
6.2. Nd:YAG Laser
Some studies have reported the effect of Nd:YAG laser irradiation on endothelial cells. A significant induction in vinculin expression, a focal adhesion protein involved in cell adhesion and migration, in human endothelial H-end cells was observed in the Nd:YAG-irradiated (fluence, 1.5 J/cm2) cells. Moreover, this study showed that Nd:YAG laser irradiation did not affect cell viability and stimulated cell growth [76]. In another study, cultured rat aortic endothelial cells at 5 h after Nd:YAG (1.6 J/cm2) laser irradiation were examined using a DNA array chip. This study showed that 20 genes in laser-treated cells were upregulated by more than four-fold compared to those in the control, and Nd:YAG laser irradiation also upregulated gene expression related to cell migration, cell structure neurotransmission, and inflammation [150]. Moreover, Nd:YAG laser irradiation (1.5 J/cm2) of HUVECs cultured on titanium disks coated with Porphyromonas gingivalis LPS caused downregulation of endothelial adhesion molecules, including that of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM) levels compared to nonirradiated HUVECs [151].
6.3. Er:YAG, Er,Cr:YSGG, and CO2 Lasers
There are no reports on the direct effects of irradiation on endothelial cells. Therefore, the molecular biological effects of Er:YAG, Er,Cr:YSGG, and CO2 laser irradiation on endothelial cells are not apparent to date.
6.4. Summary
The contents of this section are summarized in Table 4. In conclusion, studies on the molecular biological effects on endothelial cells using lasers have not been so much reported and are inconclusive. Further research is needed to reveal the effects of diode lasers on endothelial cells.

Table 4.
Summary of the effects of laser irradiation on endothelial cells.
7. Effects of Laser Irradiation on Cementoblasts
Cementum is a unique, avascular, and mineralized tissue formed by cementoblasts [152]. Only one study has reported the effects of laser irradiation on cementoblasts. Diode laser irradiation at 940 nm was performed on root plate- or microplate-seeded cementoblasts at a fluence of 18 J/cm2. Cell proliferation was not different until 96 h, but laser irradiation significantly retarded the decrease in cell proliferation after 96 h compared to the untreahted control group. Additionally, Ibsp and Bglap, which are transcripts required for cementum formation, were significantly increased in laser-irradiated cells compared to nonirradiated cells. Moreover, the expression levels of Bmp-2,3,6,7 were significantly increased. These results indicate that biostimulation can be used during regenerative periodontal therapies to trigger cells with a periodontal attachment apparatus [153]. The contents of this section are summarized in Table 5.

Table 5.
Summary of the effects of laser irradiation on cementoblasts.
8. Effects of Laser Irradiation on Epithelial Cells
Epithelial cells are found on the surfaces of tissues and organs. Although they share some common characteristics, they vary in size, shape, and general appearance, according to their location [154]. Moreover, they protect deeper tissues against the external environment and possess secretory and supportive functions, thus contributing to homeostasis maintenance. Epithelial cells play an important role in wound healing [155]. There are many studies on the effects of laser irradiation on epithelial cells associated with various tissues [156,157,158], with the exception of oral tissues. Herein, we summarize the effects of laser irradiation on oral epithelial cells. To our knowledge, no research has been published regarding the effects of Nd:YAG, Er:YAG, Er,Cr:YSGG, or CO2 lasers on oral epithelial cells. Although some studies have reported increased proliferation in Nd:YAG laser-irradiated epithelial cells [77], there are no studies on oral tissues.
8.1. Diode Laser
Diode laser irradiation has been previously reported to enhance wound healing [159]. Diode lasers can penetrate superficial tissues to exert their effects in deeper tissues. However, epithelial cells are the first cells that receive laser energy. Thus, epithelial cells absorb the highest amount of energy compared to other underlying cells. Therefore, the effects of diode lasers have been studied in vitro on various epithelial cells, including keratinocytes [160], a keratinocyte cell line (Hacat) [161,162], epithelial adenocarcinoma (HeLa) cells [163,164], pigment epithelial cells [165,166,167], and human breast epithelial cell lines (SVCT and Bre80hTERT) [168]. In this section, we focus on epithelial cells or cell lines related to oral tissues.
To examine the effect of a low level diode laser irradiation on oral epithelial cells, cultured, normal human oral keratinocyte (NOKSI) cells were irradiated using an 810 nm diode laser in continuous wave mode for 5 min, at a distance of 14.5 cm. Upregulated gene and protein expression of human β defensin-2 (HBD-2), a potent antimicrobial and wound-healing factor, were confirmed by qPCR, Western blotting, and immunostaining. Increased expression of HBD-2 was mediated by laser-activation of the TGF-β1 pathway [169].
A pulsed diode laser has also been used for in vitro studies on epithelial cells. In a study by Ejiri et al. [170], the effect of low-level diode laser irradiation was examined on primary human gingival epithelial cells (HGECs). Using a 904–910 nm diode laser applied at a high frequency of 30 kHz for 1–10 min, at 5.7–56.7 J/cm2, a significant increase in the proliferation of laser-irradiated cells was detected by WST-8 assay, 24 h after laser irradiation. The maximum proliferative effect was observed after 5 min of laser irradiation. The in vitro wound-healing assay showed a dramatic increase in migration among the laser-irradiated cells. Moreover, phosphorylation of MAPK/ERK was observed at 5, 15, 60, and 120 min after irradiation, whereas stress-activated protein kinases/c-Jun N-terminal kinase and p38 MAPK remained unphosphorylated. These results indicate that proliferation and migration of HGECs may be promoted via activation of MAPK/ERK.
The antimicrobial effects of diode lasers have been reported previously [171]. To investigate the cellular mechanisms of these effects, human oral squamous epithelial carcinoma cell lines (Ca9-22 and SCC-25) were treated with LPS. An 805 nm diode laser was then used to irradiate cells in a repeated pulse mode for 60 s at a 1 cm distance. The expression of DEL1, which encodes a protein with anti-inflammatory effects, was significantly increased following laser irradiation. In contrast, LPS-induced IL-6 and IL-8 expression was significantly suppressed following laser irradiation. A significant increase in migration was also observed in laser-irradiated cells [172]. These results indicate a suppressive effect of laser irradiation on the inflammatory response.
8.2. Summary
The contents of this section are summarized in Table 6. Many studies have examined the effect of laser irradiation on the epithelium in vivo [156,157,158]. However, despite the importance of epithelial cells in wound healing, in vitro studies are limited, especially with regard to epithelial cells associated with oral tissues. Epithelial cells have different characteristics based on their location and associated tissues, and further investigation of oral epithelial cells is required to enable the advancement of laser therapies for use in oral wound healing.

Table 6.
Summary of the effects of laser irradiation on epithelial cells.
9. Effects of Laser Irradiation on Osteocytes
Only a few studies have reported the effects of laser irradiation on osteocytes. Suppression of Sost expression was observed in primary osteocyte-like cells isolated from rat calvaria after CO2 laser irradiation at 0.7–2.8 J/cm2 without an increase in temperature. The study reported an increase in Dmp1 expression was after CO2 laser irradiation at 1.4–2.8 J/cm2 [173]. Ohsugi et al. [174] reported that Sost expression in osteogenic cells (osteoblast-like cells isolated from rat calvaria cultured with osteoinduction medium for 21 days) was decreased at 6 h after Er:YAG laser irradiation (2940 nm) at energy densities of 1.5 and 3.1 J/cm2. Following the results obtained by quantitative PCR, sclerostin (coded by Sost) expression in the cultured supernatant was significantly decreased. As sclerostin produced by osteocytes can inhibit osteoblast activity and suppress bone formation, it is thought that Er:YAG laser irradiation may promote bone formation via the suppression of Sost expression. The contents of this section are summarized in Table 7.

Table 7.
Summary of the effects of laser irradiation on osteocytes.
10. Effects of Laser Irradiation on Osteoclasts
Osteoclasts are multinucleated giant cells that have the capacity to resorb mineralized tissues [175]. The development of osteoclasts proceeds within the local microenvironment of the bone.
Only a single report has been published on the effect of laser irradiation on osteoclasts in vitro. Rat osteoclast precursor cells (osteoclast-like cells) purified from rat bone marrow were subjected to laser irradiation with 810 nm diode and a maximum power output of 50 mW at exposure times of 1, 3, 6, or 10 min/day, which corresponded to 9.3, 28.0, 56.0, or 93.3 J/cm2, respectively. Laser irradiation at 9.3–56.0 J/cm2 increased the number of tartrate-resistant, acid phosphatase-positive multinucleate cells. Furthermore, osteoclasts appeared on day 2 in the laser-irradiated groups but not until day 3 in the nonirradiated control groups. Receptor activator of NF-kappaB (RANK) in the laser-irradiated groups showed significantly greater staining compared to the control group on day 2 and 3 by immunohistochemistry, and the mRNA expression of RANK was upregulated, consistent with the immunohistochemistry results. The study suggested that irradiation with an 810 nm diode laser facilitated the differentiation and activation of osteoclasts via RANK expression [176]. The contents of this section are summarized in Table 8.

Table 8.
Summary of the effects of laser irradiation on osteoclasts.
11. Effects of Laser Irradiation on Stem Cells
In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more numbers of the same stem cells. They are the earliest type of cells in the cell lineage [177]. Mesenchymal stem cells (MSCs) are multipotent cells found in adult tissues. Adult MSCs were isolated from almost every type of connective tissue, such as adipose [178], bone marrow, periodontal ligament [179], and dental pulp tissues [180]. Stem cell therapy is the use of stem cells to treat or prevent diseases. Stem cell therapy is applied to many types of treatments, including regeneration and wound healing [181]. Recently, the PBM effects of laser irradiation on MSCs have attracted much attention. In the next paragraph, we have reviewed the effect of laser irradiation on MSCs.
11.1. Diode Laser
Although studies on PBM’s effect on MSCs are limited, several studies using diode lasers with different wavelengths have been reported. PBM (using a combination of 630 and 810 nm lasers) stimulated the viability of human adipose-derived stem cells (hASCs) and human bone marrow mesenchymal stem cells (hBM-MSCs). In addition, PBM (irradiation once or twice at 630 nm and 0.6 and 1.2 J/cm2) increased the viability of hASCs compared to the control and laser-treated hBM-MSCs. Furthermore, PBM (using a combination of 630 and 810 nm lasers, 3 times irradiation at 2.4 J/cm2) increased hASC viability compared to control and laser-treated hBM-MSCs [182]. Some studies have reported the effect of laser irradiation at around 630 or 810 nm on MSCs. Diode laser irradiation at 635 nm in MSCs derived from femurs and tibias in rats caused an increase in the expression levels of v-akt murine thymoma viral oncogene homolog 1 (Akt1), cyclin D1 gene (Ccnd1), phosphatidylinositol 3-kinase, catalytic alpha polypeptide gene (Pik3ca), in addition to a decrease in protein tyrosine phosphatase nonreceptor type 6 (Ptpn6), and serine/threonine kinase 17b (Stk17b) expression. Microarray analysis was also performed in this study, which revealed that 119 genes were differentially expressed, and various genes involved in cell proliferation, apoptosis, and the cell cycle were affected. The study suggested that the increase in MSC proliferation was mediated through the PI3K/Akt/mTOR/eIF4E pathway [183]. In addition, cytotoxicity evaluated by LDH assay did not show a significant difference between nonirradiated and 635 nm diode laser-irradiated (0.5–5.0 J/cm2) MSCs obtained from rat bone marrow. Diode laser irradiation at 0.5 J/cm2 was found to be an optimal energy density to stimulate the proliferation of bone marrow stromal cells (BMSCs); additionally, irradiation at 5.0 J/cm2 significantly stimulated the secretion of VEGF and NGF. Furthermore, after 5-aza induction, myogenic differentiation was observed in all the groups, and diode laser irradiation at 5.0 J/cm2 dramatically facilitated the differentiation [184]. The effect of 635 nm diode laser irradiation on the osteogenic differentiation of MSCs has also been reported. Laser irradiation (0.4 J/cm2) on human mesenchymal stromal cells (hMSCs) increased vinculin-rich clusters, osteogenic expression of markers (e.g., Runx-2, alkaline phosphatase, osteopontin), and mineralized bone-like nodule structure deposition as well as induced stress fiber formation and upregulated the expression of the proliferation marker Ki67. The study suggested that 635 nm diode laser irradiation may be a potentially effective option for promoting/improving bone regeneration [33]. In addition, 635 nm diode laser irradiation (0.3 J/cm2) on MSCs derived from the femora and tibia of male C2F1 mice significantly enhanced MSC proliferation, without a change in cell viability. They also found that the increase in proliferation after 635 nm diode laser irradiation was associated with the upregulation and activation of the Notch-1 pathway and increased membrane conductance through voltage-gated K+, BK, and Kir channels and T- and L-type Ca2+ channels [185].
Recently, studies on diode laser irradiation at 808 nm related to MSCs have also been reported. At 0.5-4.0 J/cm2, irradiation of human gingival mesenchymal stem cells (HGMSCs) promoted their migration but not proliferation. Furthermore, diode laser irradiation could activate mitochondrial ROS, which could elevate the phosphorylation levels of JNK and IKB in HGMSCs, further activating NF-κB concomitantly with the elevation of the nuclear translocation of p65. Taken together, these results indicate that PBM may promote cell migration via the ROS/JNK/NF-κB pathway [186]. High power 808 nm diode laser irradiation (64 J/cm2) enhanced osteogenesis. Laser irradiation of BMSCs from 3-old female BALB/c mice increased the protein expression of Runx2 and Osterix and suppressed PPARγ, a pivotal transcription factor in adipogenic differentiation. Positive areas of ALP and Alizarin Red S histochemical staining were significantly increased after laser irradiation [187].
Regarding the 606 nm diode laser irradiation, irradiation with 1.9 J/cm2 enhanced the proliferation of BMSCs, although irradiation with 11.7 J/cm2 suppressed the proliferation. The cytotoxic effect of 50 µg/mL carboplatin was eliminated, and the inhibitory effect of 0.1 µg/mL vincristine was attenuated by laser irradiation at 1.9 J/cm2 [188]. In addition, 660 nm diode laser irradiation (5 J/cm2) on stem cells from human exfoliated deciduous teeth (SHEDs) increased cell proliferation and expression of mesenchymal stem cell markers, including OCT4, Nestin, and CD90 [189]. However, another study showed that human dental pulp stem cells (hDPSCs) irradiated at 660 nm and 5 J/cm2 showed signs of apoptosis and necrosis as observed by transmission electron microscopy (TEM). Diode laser irradiation at 3 J/cm2 increased fibronectin production in hDPSCs [190]. The effects of 660 nm diode laser irradiation (1.6 J/cm2) were also evaluated in hDPSCs. Gene expression of brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GNDF), matrix-associated protein 2 (MAP2), nuclear receptor-related 1 protein (NURR1), and dopamine transporter (DAT) were increased, especially in the first 7 days of dopaminergic induction. However, the hDPSCs were not able to differentiate into functional dopaminergic neurons either in nonirradiated control or laser-irradiated groups [191].
11.2. Nd:YAG Laser
There are some reports on the effects of Nd:YAG laser irradiation on MSCs. Nd:YAG laser irradiation (2 and 4 J/cm2) on hBMSCs promoted proliferation and osteogenesis, although irradiation at an energy density of 16 J/cm2 significantly suppressed the proliferation and osteogenesis of hBMSCs [192]. In addition, Nd:YAG laser irradiation at 9.8 J/cm2 on MSCs obtained from horses did not show a difference in viability between irradiated and control MSCs. However, laser-irradiated MSCs exhibited slightly lower proliferation and significantly increased expression of IL-10 and VEGF compared to nonirradiated control MSCs [193]. Frequency-doubled Nd:YAG laser irradiation (532 nm) of human adipose tissue-derived stem cells (hADSCs) was performed at densities of 5–45 J/cm2 for 30–300 s. Mitochondrial activity of hADSCs was evaluated by autofluorescence emission at wavelengths associated with nicotinamide adenine dinucleotide (NADH) and flavoproteins. Laser irradiation at 5–9.2 J/cm2 significantly increased the proliferation of hADSCs, which was attributed to an increase in mitochondrial activity, although hADSCs irradiated at 28 and 45 J/cm2 showed a significant decrease in proliferation and autofluorescence [194].
11.3. CO2 Laser
We found only one report that mentioned the effect of CO2 laser irradiation on MSCs in an in vitro study. CO2 laser irradiation (9 W, exposure time 4 ms/shot and a medium pattern of the spots) on hADSCs increased their proliferation when cultured under nutrient-deprived conditions (0.5% FBS) and reduced cell proliferation in a medium supplemented with 10% FBS. CO2 laser irradiation caused a transient increase in mitochondrial ROS and the capacity to restore Δψm after rotenone-induced depolarization, and increased the secretion of MMP-2 in conditioned media comprising MMP-9, VEGF, and adiponectin, which have the capacity to support the angiogenesis of endothelial progenitor cells. The study concluded that CO2 laser irradiation on ADSCs might activate the redox pathways that increase cell proliferation and enhance the secretion of angiogenic molecules [195].
11.4. Summary
The contents of this section are summarized in Table 9. Reports on the effects of laser irradiation on MSCs are limited. However, some studies have shown that laser irradiation, especially low-power irradiation, causes cell proliferation and favorable gene expression changes in MSCs. MSCs are already clinically applied for periodontal regeneration. MSC sheets transplanted to root surfaces can induce regeneration of periodontal tissue [196]. Although further research is required to clarify the effects of laser irradiation on MSCs, laser irradiation may enhance MSCs regenerative capabilities in periodontal tissues.

Table 9.
Summary of the effects of laser irradiation on mesenchymal stem cells.
12. Conclusions
This review summarizes the effects of laser irradiation on cells related to periodontal tissues (Figure 1) and clearly shows that laser irradiation can have many positive effects on various cell types in periodontal tissues. Numerous studies have reported that laser irradiation enhances cell proliferation, migration, viability, calcification, gene expression, and protein expression. Additionally, the favorable effects on cells vary depending on the fluences and type of lasers. Irradiation using diodes or Nd:YAG lasers is clinically feasible and can be applied in association with any periodontal procedure, since it reaches deep tissues due to its deeply penetrating wavelength. By contrast, Er:YAG and CO2 lasers, which are only superficially absorbed, are only effective on epithelial cells and connective tissue surfaces, during nonsurgical periodontal treatments, or exposed bone and connective tissues, during periodontal surgeries. Although the purposes of periodontal treatment include anti-inflammation, tissue repair, and tissue regeneration, a single laser irradiation under a single specific irradiation condition cannot achieve all desired positive effects. Furthermore, a certain irradiation condition might have negative effects on some cells in periodontal tissues, since appropriate irradiation conditions vary with cell type. When applying a laser to regenerate periodontal tissues in the clinic, it is necessary to consider which cells need to be targeted and activated and then select the suitable laser type and energy fluence to match the cell type. However, there is still insufficient basic research on the PBM of lasers from this review. Because the type of lasers, irradiation time, distance, and fluence are quite varied, it is difficult to critically determine the optimal criteria for laser usage. From this review, we realize the promising PBM effects of lasers in periodontal therapy, and we can gain insights regarding the appropriate fluences to be utilized in laser applications to osteoblasts, fibroblasts, and MSCs. We will continue to research lasers for periodontal phototherapy, including regeneration of periodontal tissues in the future.
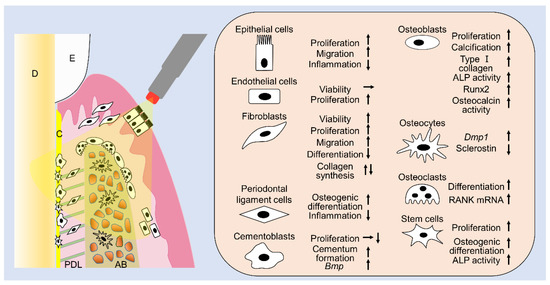
Figure 1.
The summary of this review. Laser irradiation has various effects on cells related to periodontal tissues. E: enamel, D: dentin, PDL: periodontal ligament, AB: alveolar bone.
Author Contributions
Literature collection, Y.O., H.N., T.S., M.H., and S.K.; writing, Y.O., H.N., T.S., M.H., S.K., and A.A.; revision, S.K. and T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by JSPS KAKENHI, Grant Number JP20K21670, to T.I. and JP20K18501, to Y.O.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lang, N.P.; Lindhe, J. Clinical Periodontology and Implant Dentistry; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 2. [Google Scholar]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 155–175. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontoloy 2000 2015, 68, 217–269. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Aoki, A.; Coluzzi, D.; Yukna, R.; Wang, C.Y.; Pavlic, V.; Izumi, Y. Lasers in minimally invasive periodontal and peri-implant therapy. Periodontoloy 2000 2016, 71, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Asefi, S.; Hooshyarfard, A.; Sculean, A.; Romanos, G.E.; Aoki, A.; Fekrazad, R. Photobiomodulation in Periodontology and Implant Dentistry: Part 1. Photobiomodul. Photomed. Laser Surg. 2019, 37, 739–765. [Google Scholar] [CrossRef]
- Ruh, A.C.; Frigo, L.; Cavalcanti, M.; Svidnicki, P.; Vicari, V.N.; Lopes-Martins, R.A.B.; Leal Junior, E.C.P.; De Isla, N.; Diomede, F.; Trubiani, O.; et al. Laser photobiomodulation in pressure ulcer healing of human diabetic patients: Gene expression analysis of inflammatory biochemical markers. Lasers Med. Sci. 2018, 33, 165–171. [Google Scholar] [CrossRef]
- Cavalcanti, M.F.; Silva, U.H.; Leal-Junior, E.C.; Lopes-Martins, R.A.; Marcos, R.L.; Pallotta, R.C.; Diomede, F.; Trubiani, O.; De Isla, N.; Frigo, L. Comparative Study of the Physiotherapeutic and Drug Protocol and Low-Level Laser Irradiation in the Treatment of Pain Associated with Temporomandibular Dysfunction. Photomed. Laser Surg. 2016, 34, 652–656. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-µm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- Niemz, M.H. Laser-Tissue Interaction. In Fundamentals and Applications; Springer: Berlin, Germany, 1996. [Google Scholar]
- Aoki, A.; Sasaki, K.; Watanabe, H.; Ishikawa, I. Lasers in non-surgical periodontal therapy. Periodontology 2000 2004, 36, 59–97. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Tamura, K.; Hiratsuka, K.; Abiko, Y. Stimulation of MCM3 gene expression in osteoblast by low level laser irradiation. Lasers Med. Sci. 2001, 16, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hamajima, S.; Hiratsuka, K.; Kiyama-Kishikawa, M.; Tagawa, T.; Kawahara, M.; Ohta, M.; Sasahara, H.; Abiko, Y. Effect of low-level laser irradiation on osteoglycin gene expression in osteoblasts. Lasers Med. Sci. 2003, 18, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Renno, A.C.; McDonnell, P.A.; Parizotto, N.A.; Laakso, E.L. The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro. Photomed. Laser Surg. 2007, 25, 275–280. [Google Scholar] [CrossRef]
- Fujimoto, K.; Kiyosaki, T.; Mitsui, N.; Mayahara, K.; Omasa, S.; Suzuki, N.; Shimizu, N. Low-intensity laser irradiation stimulates mineralization via increased BMPs in MC3T3-E1 cells. Lasers Surg. Med. 2010, 42, 519–526. [Google Scholar] [CrossRef]
- Kanenari, M.; Zhao, J.; Abiko, Y. Enhancement of microtubule-associated protein-1 Alpha gene expression in osteoblasts by low level laser irradiation. Laser 2011, 20, 47–51. [Google Scholar] [CrossRef][Green Version]
- Migliario, M.; Pittarella, P.; Fanuli, M.; Rizzi, M.; Reno, F. Laser-induced osteoblast proliferation is mediated by ROS production. Lasers Med. Sci. 2014, 29, 1463–1467. [Google Scholar] [CrossRef]
- Pagin, M.T.; de Oliveira, F.A.; Oliveira, R.C.; Sant’Ana, A.C.; de Rezende, M.L.; Greghi, S.L.; Damante, C.A. Laser and light-emitting diode effects on pre-osteoblast growth and differentiation. Lasers Med. Sci. 2014, 29, 55–59. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Matos, A.A.; Matsuda, S.S.; Buzalaf, M.A.; Bagnato, V.S.; Machado, M.A.; Damante, C.A.; Oliveira, R.C.; Peres-Buzalaf, C. Low level laser therapy modulates viability, alkaline phosphatase and matrix metalloproteinase-2 activities of osteoblasts. J. Photochem. Photobiol. B 2017, 169, 35–40. [Google Scholar] [CrossRef]
- Son, J.H.; Park, B.S.; Kim, I.R.; Sung, I.Y.; Cho, Y.C.; Kim, J.S.; Kim, Y.D. A novel combination treatment to stimulate bone healing and regeneration under hypoxic conditions: Photobiomodulation and melatonin. Lasers Med. Sci. 2017, 32, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, Y.; Dong, S.; Liu, S.; Zhang, X.; Si, X.; Zhou, Y. Laser irradiation promotes the proliferation of mouse pre-osteoblast cell line MC3T3-E1 through hedgehog signaling pathway. Lasers Med. Sci. 2017, 32, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, R.; Gunji, H.; Tsuka, Y.; Yoshimi, Y.; Awada, T.; Sumi, K.; Nakajima, K.; Kimura, A.; Hiraki, T.; Abe, T.; et al. Effects of high-frequency near-infrared diode laser irradiation on the proliferation and migration of mouse calvarial osteoblasts. Lasers Med. Sci. 2018, 33, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Mikami, R.; Mizutani, K.; Aoki, A.; Tamura, Y.; Aoki, K.; Izumi, Y. Low-level ultrahigh-frequency and ultrashort-pulse blue laser irradiation enhances osteoblast extracellular calcification by upregulating proliferation and differentiation via transient receptor potential vanilloid 1. Lasers Surg. Med. 2018, 50, 340–352. [Google Scholar] [CrossRef]
- Coombe, A.R.; Ho, C.T.; Darendeliler, M.A.; Hunter, N.; Philips, J.R.; Chapple, C.C.; Yum, L.W. The effects of low level laser irradiation on osteoblastic cells. Clin. Orthod. Res. 2001, 4, 3–14. [Google Scholar] [CrossRef]
- Bayram, H.; Kenar, H.; Tasar, F.; Hasirci, V. Effect of low level laser therapy and zoledronate on the viability and ALP activity of Saos-2 cells. Int. J. Oral. Maxillofac. Surg. 2013, 42, 140–146. [Google Scholar] [CrossRef]
- Bloise, N.; Ceccarelli, G.; Minzioni, P.; Vercellino, M.; Benedetti, L.; De Angelis, M.G.; Imbriani, M.; Visai, L. Investigation of low-level laser therapy potentiality on proliferation and differentiation of human osteoblast-like cells in the absence/presence of osteogenic factors. J. Biomed. Opt. 2013, 18, 128006. [Google Scholar] [CrossRef]
- Incerti Parenti, S.; Checchi, L.; Fini, M.; Tschon, M. Different doses of low-level laser irradiation modulate the in vitro response of osteoblast-like cells. J. Biomed. Opt. 2014, 19, 108002. [Google Scholar] [CrossRef]
- Tschon, M.; Incerti-Parenti, S.; Cepollaro, S.; Checchi, L.; Fini, M. Photobiomodulation with low-level diode laser promotes osteoblast migration in an in vitro micro wound model. J. Biomed. Opt. 2015, 20, 78002. [Google Scholar] [CrossRef]
- Heymann, P.G.; Ziebart, T.; Kammerer, P.W.; Mandic, R.; Saydali, A.; Braun, A.; Neff, A.; Draenert, G.F. The enhancing effect of a laser photochemotherapy with cisplatin or zolendronic acid in primary human osteoblasts and osteosarcoma cells in vitro. J. Oral. Pathol. Med. 2016, 45, 803–809. [Google Scholar] [CrossRef]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular in vitro Study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.; Koehn, J.; Sutter, W.; Wendtlandt, G.; Wanschitz, F.; Thurnher, D.; Baghestanian, M.; Turhani, D. Initial effects of low-level laser therapy on growth and differentiation of human osteoblast-like cells. Wien Klin Wochenschr 2008, 120, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Saracino, S.; Mozzati, M.; Martinasso, G.; Pol, R.; Canuto, R.A.; Muzio, G. Superpulsed laser irradiation increases osteoblast activity via modulation of bone morphogenetic factors. Lasers Surg. Med. 2009, 41, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Lu, Y.C.; Kao, C.T. Low-level diode laser therapy reduces lipopolysaccharide (LPS)-induced bone cell inflammation. Lasers Med. Sci. 2012, 27, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Huertas, R.M.; Luna-Bertos, E.D.; Ramos-Torrecillas, J.; Leyva, F.M.; Ruiz, C.; Garcia-Martinez, O. Effect and clinical implications of the low-energy diode laser on bone cell proliferation. Biol. Res. Nurs. 2014, 16, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Incerti Parenti, S.; Panseri, S.; Gracco, A.; Sandri, M.; Tampieri, A.; Alessandri Bonetti, G. Effect of low-level laser irradiation on osteoblast-like cells cultured on porous hydroxyapatite scaffolds. Ann. Ist. Super Sanita 2013, 49, 255–260. [Google Scholar] [PubMed]
- Medina-Huertas, R.; Manzano-Moreno, F.J.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Garcia-Martinez, O.; Ruiz, C. The effects of low-level diode laser irradiation on differentiation, antigenic profile, and phagocytic capacity of osteoblast-like cells (MG-63). Lasers Med. Sci. 2014, 29, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Moreno, F.J.; Medina-Huertas, R.; Ramos-Torrecillas, J.; Garcia-Martinez, O.; Ruiz, C. The effect of low-level diode laser therapy on early differentiation of osteoblast via BMP-2/TGF-beta1 and its receptors. J. Craniomaxillofac. Surg. 2015, 43, 1926–1932. [Google Scholar] [CrossRef]
- Pyo, S.J.; Song, W.W.; Kim, I.R.; Park, B.S.; Kim, C.H.; Shin, S.H.; Chung, I.K.; Kim, Y.D. Low-level laser therapy induces the expressions of BMP-2, osteocalcin, and TGF-beta1 in hypoxic-cultured human osteoblasts. Lasers Med. Sci. 2013, 28, 543–550. [Google Scholar] [CrossRef]
- Jawad, M.M.; Husein, A.; Azlina, A.; Alam, M.K.; Hassan, R.; Shaari, R. Effect of 940 nm low-level laser therapy on osteogenesis in vitro. J. Biomed. Opt. 2013, 18, 128001. [Google Scholar] [CrossRef]
- Walter, C.; Pabst, A.M.; Ziebart, T. Effects of a low-level diode laser on oral keratinocytes, oral fibroblasts, endothelial cells and osteoblasts incubated with bisphosphonates: An in vitro study. Biomed. Rep. 2015, 3, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Kim, K.H.; Choi, N.R.; Kim, I.R.; Park, B.S.; Kim, Y.D.; Kim, U.K.; Kim, C.H. Effect of low-level laser therapy on bisphosphonate-treated osteoblasts. Maxillofac. Plast Reconstr. Surg. 2016, 38, 48. [Google Scholar] [CrossRef] [PubMed]
- Bolukbasi Ates, G.; Ak Can, A.; Gulsoy, M. Investigation of photobiomodulation potentiality by 635 and 809 nm lasers on human osteoblasts. Lasers Med. Sci. 2017, 32, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Ates, G.B.; Ak, A.; Garipcan, B.; Gulsoy, M. Indocyanine green-mediated photobiomodulation on human osteoblast cells. Lasers Med. Sci. 2018, 33, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Hong, J.U.; Song, J.M.; Kim, I.R.; Park, B.S.; Kim, C.H.; Shin, S.H. Combined effect of recombinant human bone morphogenetic protein-2 and low level laser irradiation on bisphosphonate-treated osteoblasts. J. Korean Assoc. Oral. Maxillofac. Surg. 2018, 44, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, N.A.; Hiraki, K.R.; Marques, M.M. Irradiation at 780 nm increases proliferation rate of osteoblasts independently of dexamethasone presence. Lasers Surg. Med. 2006, 38, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, E.; Goto, T.; Matayoshi, T.; Kobayashi, S.; Takahashi, T. Optimal low-energy laser irradiation causes temporal G2/M arrest on rat calvarial osteoblasts. Calcif. Tissue Int. 2006, 79, 443–450. [Google Scholar] [CrossRef]
- Shimizu, N.; Mayahara, K.; Kiyosaki, T.; Yamaguchi, A.; Ozawa, Y.; Abiko, Y. Low-intensity laser irradiation stimulates bone nodule formation via insulin-like growth factor-I expression in rat calvarial cells. Lasers Surg. Med. 2007, 39, 551–559. [Google Scholar] [CrossRef]
- Xu, M.; Deng, T.; Mo, F.; Deng, B.; Lam, W.; Deng, P.; Zhang, X.; Liu, S. Low-intensity pulsed laser irradiation affects RANKL and OPG mRNA expression in rat calvarial cells. Photomed. Laser Surg. 2009, 27, 309–315. [Google Scholar] [CrossRef]
- Ozawa, Y.; Shimizu, N.; Kariya, G.; Abiko, Y. Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 1998, 22, 347–354. [Google Scholar] [CrossRef]
- Ueda, Y.; Shimizu, N. Pulse irradiation of low-power laser stimulates bone nodule formation. J. Oral. Sci. 2001, 43, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Shimizu, N. Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J. Clin. Laser Med. Surg. 2003, 21, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Khadra, M.; Lyngstadaas, S.P.; Haanaes, H.R.; Mustafa, K. Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 2005, 26, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; do Vale Placa, R.; Sant’Ana, A.C.P.; Greghi, S.L.A.; Zangrando, M.S.R.; de Rezende, M.L.R.; Oliveira, R.C.; Damante, C.A. Laser and LED photobiomodulation effects in osteogenic or regular medium on rat calvaria osteoblasts obtained by newly forming bone technique. Lasers Med. Sci. 2020. [Google Scholar] [CrossRef]
- Petri, A.D.; Teixeira, L.N.; Crippa, G.E.; Beloti, M.M.; de Oliveira, P.T.; Rosa, A.L. Effects of low-level laser therapy on human osteoblastic cells grown on titanium. Braz. Dent J. 2010, 21, 491–498. [Google Scholar] [CrossRef]
- Emes, Y.A.K.; Aybar, B. Low-level laser therapy vs. pulsed electromagnetic field on neonatal rat calvarial osteoblast-like cells. Lasers Med. Sci. 2013, 28, 901–909. [Google Scholar] [CrossRef]
- Morsoleto, M.; Sella, V.; Machado, P.; Bomfim, F.D.; Fernandes, M.H.; Morgado, F.; Lopes Filho, G.J.; Plapler, H. Effect of low power laser in biomodulation of cultured osteoblastic cells of Wistar rats1. Acta Cir. Bras 2019, 34, e201900210. [Google Scholar] [CrossRef]
- Pires Oliveira, D.A.; de Oliveira, R.F.; Zangaro, R.A.; Soares, C.P. Evaluation of low-level laser therapy of osteoblastic cells. Photomed Laser Surg. 2008, 26, 401–404. [Google Scholar] [CrossRef]
- Dortbudak, O.; Haas, R.; Mallath-Pokorny, G. Biostimulation of bone marrow cells with a diode soft laser. Clin. Oral. Implant. Res. 2000, 11, 540–545. [Google Scholar] [CrossRef]
- Grassi, F.R.; Ciccolella, F.; D’Apolito, G.; Papa, F.; Iuso, A.; Salzo, A.E.; Trentadue, R.; Nardi, G.M.; Scivetti, M.; De Matteo, M.; et al. Effect of low-level laser irradiation on osteoblast proliferation and bone formation. J. Biol. Regul. Homeost. Agents 2011, 25, 603–614. [Google Scholar]
- Mergoni, G.; Vescovi, P.; Belletti, S.; Uggeri, J.; Nammour, S.; Gatti, R. Effects of 915 nm laser irradiation on human osteoblasts: A preliminary in vitro study. Lasers Med. Sci. 2018, 33, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Kitamura, C.; Fukushima, H.; Nakamichi, I.; Abiko, Y.; Terashita, M.; Jimi, E. Low-level laser irradiation enhances BMP-induced osteoblast differentiation by stimulating the BMP/Smad signaling pathway. J. Cell Biochem. 2010, 111, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Nomura, S.; Yamaguchi, A.; Suda, T.; Yoshiki, S. In situ hybridization of bone matrix proteins in undecalcified adult rat bone sections. J. Histochem. Cytochem. 1992, 40, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Molecular Mechanism of Runx2-Dependent Bone Development. Mol. Cells 2020, 43, 168–175. [Google Scholar]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010, 147, 35–51. [Google Scholar] [CrossRef]
- Heldin, C.H.; Miyazono, K.; ten Dijke, P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471. [Google Scholar] [CrossRef]
- Fujihara, S.; Yokozeki, M.; Oba, Y.; Higashibata, Y.; Nomura, S.; Moriyama, K. Function and regulation of osteopontin in response to mechanical stress. J. Bone Min. Res. 2006, 21, 956–964. [Google Scholar] [CrossRef]
- Miyazono, K.; Kusanagi, K.; Inoue, H. Divergence and convergence of TGF-beta/BMP signaling. J. Cell Physiol. 2001, 187, 265–276. [Google Scholar] [CrossRef]
- Ryoo, H.M.; Hoffmann, H.M.; Beumer, T.; Frenkel, B.; Towler, D.A.; Stein, G.S.; Stein, J.L.; van Wijnen, A.J.; Lian, J.B. Stage-specific expression of Dlx5 during osteoblast differentiation: Involvement in regulation of osteocalcin gene expression. Mol. Endocrinol. 1997, 11, 1681–1694. [Google Scholar] [CrossRef]
- Deckx, S.; Heymans, S.; Papageorgiou, A.P. The diverse functions of osteoglycin: A deceitful dwarf, or a master regulator of disease? Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Starup-Linde, J.; Viggers, R.; Handberg, A. Osteoglycin and Bone-a Systematic Review. Curr. Osteoporos. Rep. 2019, 17, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Arisu, H.D.; Turkoz, E.; Bala, O. Effects of Nd:Yag laser irradiation on osteoblast cell cultures. Lasers Med. Sci. 2006, 21, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Chellini, F.; Sassoli, C.; Nosi, D.; Deledda, C.; Tonelli, P.; Zecchi-Orlandini, S.; Formigli, L.; Giannelli, M. Low pulse energy Nd:YAG laser irradiation exerts a biostimulative effect on different cells of the oral microenvironment: “an in vitro study”. Lasers Surg. Med. 2010, 42, 527–539. [Google Scholar] [CrossRef]
- Kara, C.; Selamet, H.; Gokmenoglu, C.; Kara, N. Low level laser therapy induces increased viability and proliferation in isolated cancer cells. Cell Prolif. 2018, 51, e12417. [Google Scholar] [CrossRef]
- Tsuka, Y.; Kunimatsu, R.; Gunji, H.; Nakajima, K.; Kimura, A.; Hiraki, T.; Nakatani, A.; Tanimoto, K. Effects of Nd:YAG low-level laser irradiation on cultured human osteoblasts migration and ATP production: In vitro study. Lasers Med. Sci. 2019, 34, 55–60. [Google Scholar] [CrossRef]
- Tsuka, Y.; Kunimatsu, R.; Gunji, H.; Abe, T.; Medina, C.C.; Nakajima, K.; Kimura, A.; Hiraki, T.; Nakatani, A.; Tanimoto, K. Examination of the Effect of the Combined Use of Nd: YAG Laser Irradiation and Mechanical Force Loading on Bone Metabolism Using Cultured Human Osteoblasts. J. Lasers Med. Sci. 2020, 11, 138–143. [Google Scholar] [CrossRef]
- Kim, I.S.; Cho, T.H.; Kim, K.; Weber, F.E.; Hwang, S.J. High power-pulsed Nd:YAG laser as a new stimulus to induce BMP-2 expression in MC3T3-E1 osteoblasts. Lasers Surg. Med. 2010, 42, 510–518. [Google Scholar] [CrossRef]
- Middleton, J.; Arnott, N.; Walsh, S.; Beresford, J. Osteoblasts and osteoclasts in adult human osteophyte tissue express the mRNAs for insulin-like growth factors I and II and the type 1 IGF receptor. Bone 1995, 16, 287–293. [Google Scholar] [CrossRef]
- Schwarz, F.; Rothamel, D.; Herten, M.; Bieling, K.; Scherbaum, W.; Becker, J. Effects of an Er:YAG laser on mitochondrial activity of human osteosarcoma-derived osteoblasts in vitro. Lasers Med. Sci. 2004, 19, 37–40. [Google Scholar] [CrossRef]
- Aleksic, V.; Aoki, A.; Iwasaki, K.; Takasaki, A.A.; Wang, C.Y.; Abiko, Y.; Ishikawa, I.; Izumi, Y. Low-level Er:YAG laser irradiation enhances osteoblast proliferation through activation of MAPK/ERK. Lasers Med. Sci. 2010, 25, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Niimi, H.; Ohsugi, Y.; Katagiri, S.; Watanabe, K.; Hatasa, M.; Shimohira, T.; Tsuchiya, Y.; Maekawa, S.; Hirota, T.; Kadokura, H.; et al. Effects of Low-Level Er:YAG Laser Irradiation on Proliferation and Calcification of Primary Osteoblast-Like Cells Isolated From Rat Calvaria. Front. Cell Dev. Biol. 2020, 8, 459. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Kawaki, H.; Kubota, S.; Suzuki, A.; Suzuki, M.; Kohsaka, K.; Hoshi, K.; Fujii, T.; Lazar, N.; Ohgawara, T.; Maeda, T.; et al. Differential roles of CCN family proteins during osteoblast differentiation: Involvement of Smad and MAPK signaling pathways. Bone 2011, 49, 975–989. [Google Scholar] [CrossRef]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef]
- Sasaki, Y.; Wang, S.; Ogata, Y. Transcriptional regulation of bone sialoprotein gene by CO(2) laser irradiation. J. Oral. Sci. 2011, 53, 51–59. [Google Scholar] [CrossRef][Green Version]
- Dekoninck, S.; Blanpain, C. Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 2019, 21, 18–24. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–411. [Google Scholar]
- Grinnell, F. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 1994, 124, 401–404. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. Aims Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Solmaz, H.; Dervisoglu, S.; Gulsoy, M.; Ulgen, Y. Laser biostimulation of wound healing: Bioimpedance measurements support histology. Lasers Med. Sci. 2016, 31, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Yamaguchi, M.; Abiko, Y. Inhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 production and cyclooxygenase-2 in human gingival fibroblasts. Eur. J. Oral. Sci. 2000, 108, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Takema, T.; Yamaguchi, M.; Abiko, Y. Reduction of Plasminogen Activator Activity Stimulated by Lipopolysaccharide from Periodontal Pathogen in Human Gingival Fibroblasts by Low-energy Laser Irradiation. Lasers Med. Sci. 2000, 15, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Yamaguchi, M.; Abiko, Y. Inhibition of interleukin-1beta production and gene expression in human gingival fibroblasts by low-energy laser irradiation. Lasers Med. Sci. 2001, 16, 218–223. [Google Scholar] [CrossRef]
- Almeida-Lopes, L.; Rigau, J.; Zângaro, R.A.; Guidugli-Neto, J.; Jaeger, M.M. Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg. Med. 2001, 29, 179–184. [Google Scholar] [CrossRef]
- Basso, F.G.; Pansani, T.N.; Turrioni, A.P.; Bagnato, V.S.; Hebling, J.; de Souza Costa, C.A. In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. Int. J. Dent 2012, 2012, 719452. [Google Scholar] [CrossRef]
- Frozanfar, A.; Ramezani, M.; Rahpeyma, A.; Khajehahmadi, S.; Arbab, H.R. The Effects of Low Level Laser Therapy on the Expression of Collagen Type I Gene and Proliferation of Human Gingival Fibroblasts (Hgf3-Pi 53): In vitro Study. Iran J. Basic Med. Sci. 2013, 16, 1071–1074. [Google Scholar]
- Saygun, I.; Karacay, S.; Serdar, M.; Ural, A.U.; Sencimen, M.; Kurtis, B. Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival fibroblasts. Lasers Med. Sci. 2008, 23, 211–215. [Google Scholar] [CrossRef]
- Basso, F.G.; Pansani, T.N.; Soares, D.G.; Scheffel, D.L.; Bagnato, V.S.; de Souza Costa, C.A.; Hebling, J. Biomodulation of Inflammatory Cytokines Related to Oral Mucositis by Low-Level Laser Therapy. Photochem. Photobiol. 2015, 91, 952–956. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, S.B. Effects of different setting of diode laser on the mRNA expression of growth factors and type I collagen of human gingival fibroblasts. Lasers Med. Sci. 2012, 27, 325–331. [Google Scholar] [CrossRef]
- Damante, C.A.; De Micheli, G.; Miyagi, S.P.; Feist, I.S.; Marques, M.M. Effect of laser phototherapy on the release of fibroblast growth factors by human gingival fibroblasts. Lasers Med. Sci. 2009, 24, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Kreisler, M.; Daubländer, M.; Willershausen-Zönnchen, B.; d’Hoedt, B. Effect of diode laser irradiation on the survival rate of gingival fibroblast cell cultures. Lasers Surg. Med. 2001, 28, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.N.; Eduardo Cde, P.; Matson, E.; Marques, M.M. Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg. Med. 2002, 31, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Chellini, F.; Squecco, R.; Tani, A.; Idrizaj, E.; Nosi, D.; Giannelli, M.; Zecchi-Orlandini, S. Low intensity 635 nm diode laser irradiation inhibits fibroblast-myofibroblast transition reducing TRPC1 channel expression/activity: New perspectives for tissue fibrosis treatment. Lasers Surg. Med. 2016, 48, 318–332. [Google Scholar] [CrossRef]
- Kaibuchi, N.; Iwata, T.; Yamato, M.; Okano, T.; Ando, T. Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater. 2016, 42, 400–410. [Google Scholar] [CrossRef]
- Marques, M.M.; Pereira, A.N.; Fujihara, N.A.; Nogueira, F.N.; Eduardo, C.P. Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts. Lasers Surg. Med. 2004, 34, 260–265. [Google Scholar] [CrossRef]
- Castro, D.J.; Abergel, R.P.; Meeker, C.; Dwyer, R.M.; Lesavoy, M.A.; Uitto, J. Effects of the Nd:YAG laser on DNA synthesis and collagen production in human skin fibroblast cultures. Ann. Plast Surg. 1983, 11, 214–222. [Google Scholar] [CrossRef]
- Abergel, R.P.; Meeker, C.A.; Dwyer, R.M.; Lesavoy, M.A.; Uitto, J. Nonthermal effects of ND:YAG laser on biological functions of human skin fibroblasts in culture. Lasers Surg. Med. 1984, 3, 279–284. [Google Scholar] [CrossRef]
- Cisneros, J.L.; Río, R.; Palou, J. The Q-switched neodymium (Nd):YAG laser with quadruple frequency. Clinical histological evaluation of facial resurfacing using different wavelengths. Derm. Surg. 1998, 24, 345–350. [Google Scholar] [CrossRef]
- Jansen, P.L.; Rosch, R.; Jansen, M.; Binnebösel, M.; Junge, K.; Alfonso-Jaume, A.; Klinge, U.; Lovett, D.H.; Mertens, P.R. Regulation of MMP-2 gene transcription in dermal wounds. J. Investig. Derm. 2007, 127, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, A.; Perfetto, B.; Guerrera, L.P.; Oliviero, G.; Baroni, A. Q-switched 1064 nm Nd-Yag nanosecond laser effects on skin barrier function and on molecular rejuvenation markers in keratinocyte-fibroblasts interaction. Lasers Med. Sci. 2019, 34, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, L.; Dang, Y.; Liu, B.; Zhao, D. Investigation of the 1064 nm Q-switched Nd:YAG laser on collagen expression in an animal model. Photomed. Laser Surg. 2012, 30, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Ye, X.; Weng, Y.; Tong, Z.; Ren, Q. Effects of the 532-nm and 1064-nm Q-switched Nd: YAG lasers on collagen turnover of cultured human skin fibroblasts: A comparative study. Lasers Med. Sci. 2010, 25, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Ishikawa, I.; Suzuki, M.; Hasegawa, K. Clinical assessments of the erbium:YAG laser for soft tissue surgery and scaling. J. Clin. Laser Med. Surg. 1996, 14, 67–75. [Google Scholar] [CrossRef]
- Aoki, A.; Mizutani, K.; Takasaki, A.A.; Sasaki, K.M.; Nagai, S.; Schwarz, F.; Yoshida, I.; Eguro, T.; Zeredo, J.L.; Izumi, Y. Current status of clinical laser applications in periodontal therapy. Gen Dent 2008, 56, 674–687, quiz 688-9, 767. [Google Scholar]
- Pourzarandian, A.; Watanabe, H.; Ruwanpura, S.M.; Aoki, A.; Ishikawa, I. Effect of low-level Er:YAG laser irradiation on cultured human gingival fibroblasts. J. Periodontol. 2005, 76, 187–193. [Google Scholar] [CrossRef]
- Pourzarandian, A.; Watanabe, H.; Ruwanpura, S.M.; Aoki, A.; Noguchi, K.; Ishikawa, I. Er:YAG laser irradiation increases prostaglandin E production via the induction of cyclooxygenase-2 mRNA in human gingival fibroblasts. J. Periodontal Res. 2005, 40, 182–186. [Google Scholar] [CrossRef]
- Ogita, M.; Tsuchida, S.; Aoki, A.; Satoh, M.; Kado, S.; Sawabe, M.; Nanbara, H.; Kobayashi, H.; Takeuchi, Y.; Mizutani, K.; et al. Increased cell proliferation and differential protein expression induced by low-level Er:YAG laser irradiation in human gingival fibroblasts: Proteomic analysis. Lasers Med. Sci. 2015, 30, 1855–1866. [Google Scholar] [CrossRef]
- Kong, S.; Aoki, A.; Iwasaki, K.; Mizutani, K.; Katagiri, S.; Suda, T.; Ichinose, S.; Ogita, M.; Pavlic, V.; Izumi, Y. Biological effects of Er:YAG laser irradiation on the proliferation of primary human gingival fibroblasts. J. Biophotonics 2018, 11, 201700157. [Google Scholar] [CrossRef]
- Talebi-Ardakani, M.R.; Torshabi, M.; Karami, E.; Arbabi, E.; Rezaei Esfahrood, Z. In Vitro Study of Er:YAG and Er, Cr:YSGG Laser Irradiation on Human Gingival Fibroblast Cell Line. Acta Med. Iran 2016, 54, 251–255. [Google Scholar]
- Tsuka, Y.; Kunimatsu, R.; Gunji, H.; Abe, T.; Medina, C.C.; Hiraki, T.; Nakatani, A.; Sakata, S.; Rikitake, K.; Aisyah, P.N.; et al. Examination of the effect of combined use of Er:YAG laser irradiation and mechanical force loading on bone metabolism using primary human gingival fibroblasts. Lasers Med. Sci. 2020, 35, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.J.; Wylie, J. Interventional laser surgery: An effective surgical and diagnostic tool in oral precancer management. Int. J. Oral. Maxillofac. Surg. 2002, 31, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Iwasaka, K.; Hemmi, E.; Tomita, K.; Ishihara, S.; Katayama, T.; Sakagami, H. Effect of CO₂ laser irradiation on hormesis induction in human pulp and periodontal ligament fibroblasts. In Vivo 2011, 25, 787–793. [Google Scholar] [PubMed]
- Pant, V.; Dixit, J.; Agrawal, A.K.; Seth, P.K.; Pant, A.B. Behavior of human periodontal ligament cells on CO2 laser irradiated dentinal root surfaces: An in vitro study. J. Periodontal Res. 2004, 39, 373–379. [Google Scholar] [CrossRef]
- Yamasaki, A.; Tamamura, K.; Sakurai, Y.; Okuyama, N.; Yusa, J.; Ito, H. Remodeling of the rat gingiva induced by CO2 laser coagulation mode. Lasers Surg. Med. 2008, 40, 695–703. [Google Scholar] [CrossRef]
- Nowak, K.C.; McCormack, M.; Koch, R.J. The effect of superpulsed carbon dioxide laser energy on keloid and normal dermal fibroblast secretion of growth factors: A serum-free study. Plast Reconstr. Surg. 2000, 105, 2039–2048. [Google Scholar] [CrossRef]
- Shingyochi, Y.; Kanazawa, S.; Tajima, S.; Tanaka, R.; Mizuno, H.; Tobita, M. A Low-Level Carbon Dioxide Laser Promotes Fibroblast Proliferation and Migration through Activation of Akt, ERK, and JNK. PLoS ONE 2017, 12, e0168937. [Google Scholar] [CrossRef]
- Apfelberg, D.B.; Mittelman, H.; Chadi, B. Carcinogenic potential of in vitro carbon dioxide laser exposure of fibroblast. Obs. Gynecol. 1983, 61, 493–496. [Google Scholar]
- Barczyk, M.; Bolstad, A.I.; Gullberg, D. Role of integrins in the periodontal ligament: Organizers and facilitators. Periodontoloy 2000 2013, 63, 29–47. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, C.H.; Yeh, L.Y.; Yeh, M.L.; Ting, C.C.; Wang, Y.H. Low-power laser irradiation promotes the proliferation and osteogenic differentiation of human periodontal ligament cells via cyclic adenosine monophosphate. Int. J. Oral. Sci. 2013, 5, 85–91. [Google Scholar] [CrossRef]
- Huang, T.H.; Chen, C.C.; Liu, S.L.; Lu, Y.C.; Kao, C.T. A low-level diode laser therapy reduces the lipopolysaccharide (LPS)-induced periodontal ligament cell inflammation. Laser Phys. Lett. 2014, 11, 075602. [Google Scholar] [CrossRef]
- Mayahara, K.; Yamaguchi, A.; Sakaguchi, M.; Igarashi, Y.; Shimizu, N. Effect of Ga-Al-As laser irradiation on COX-2 and cPLA2-alpha expression in compressed human periodontal ligament cells. Lasers Surg. Med. 2010, 42, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Yamaguchi, M.; Goseki, T.; Shibata, Y.; Takiguchi, H.; Iwasawa, T.; Abiko, Y. Inhibition of prostaglandin E2 and interleukin 1-beta production by low-power laser irradiation in stretched human periodontal ligament cells. J. Dent Res. 1995, 74, 1382–1388. [Google Scholar] [CrossRef]
- Ozawa, Y.; Shimizu, N.; Abiko, Y. Low-energy diode laser irradiation reduced plasminogen activator activity in human periodontal ligament cells. Lasers Surg. Med. 1997, 21, 456–463. [Google Scholar] [CrossRef]
- Huang, T.H.; Liu, S.L.; Chen, C.L.; Shie, M.Y.; Kao, C.T. Low-level laser effects on simulated orthodontic tension side periodontal ligament cells. Photomed. Laser Surg. 2013, 31, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Yim, J.Y.; Koo, K.T.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; Chung, C.P.; Kim, T.I. Biological effects of a semiconductor diode laser on human periodontal ligament fibroblasts. J. Periodontal. Implant Sci. 2010, 40, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Dehdashtizadeh, A.; Esnaashari, N.; Farhad, S.Z.; Ejeian, F.; Amini, S. The effect of laser irradiation and doxycycline application on the production of matrix metalloproteinase-8 and collagen I from cultured human periodontal ligament cells. Dent. Res. J. Isfahan 2020, 17, 213–218. [Google Scholar] [PubMed]
- Kreisler, M.; Christoffers, A.B.; Willershausen, B.; d’Hoedt, B. Effect of low-level GaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: An in vitro study. J. Clin. Periodontol. 2003, 30, 353–358. [Google Scholar] [CrossRef]
- Lin, T.; Yu, C.C.; Liu, C.M.; Hsieh, P.L.; Liao, Y.W.; Yu, C.H.; Chen, C.J. Er:YAG laser promotes proliferation and wound healing capacity of human periodontal ligament fibroblasts through Galectin-7 induction. J. Med. Assoc. 2020. [Google Scholar] [CrossRef]
- Peplow, P.V.; Chung, T.Y.; Baxter, G.D. Laser photobiomodulation of wound healing: A review of experimental studies in mouse and rat animal models. Photomed. Laser Surg. 2010, 28, 291–325. [Google Scholar] [CrossRef]
- Schwarz, F.; Aoki, A.; Sculean, A.; Becker, J. The impact of laser application on periodontal and peri-implant wound healing. Periodontology 2000 2009, 51, 79–108. [Google Scholar] [CrossRef] [PubMed]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Baldini, F.; Benedicenti, S.; Panfoli, I.; Vergani, L. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med. Sci. 2019, 34, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Protasiewicz, M.; Kuliczkowski, W.; Woznicka, A.K.; Szymkiewicz, P.; Derkacz, A.; Andrzej, M.; Dziegiel, P. Influence of low-power laser illumination on pro-inflammatory response in human endothelial cells stimulated with interleukin-1a. In Vivo 2013, 27, 313–319. [Google Scholar]
- Schindl, A.; Merwald, H.; Schindl, L.; Kaun, C.; Wojta, J. Direct stimulatory effect of low-intensity 670 nm laser irradiation on human endothelial cell proliferation. Br. J. Derm. 2003, 148, 334–336. [Google Scholar] [CrossRef]
- Góralczyk, K.; Szymańska, J.; Łukowicz, M.; Drela, E.; Kotzbach, R.; Dubiel, M.; Michalska, M.; Góralczyk, B.; Zając, A.; Rość, D. Effect of LLLT on endothelial cells culture. Lasers Med. Sci. 2015, 30, 273–278. [Google Scholar] [CrossRef]
- Du, S.; Zhang, Q.; Zhang, S.; Wang, L.; Lian, J. Heat shock protein 70 expression induced by diode laser irradiation on choroid-retinal endothelial cells in vitro. Mol. Vis. 2012, 18, 2380–2387. [Google Scholar]
- Masuda, Y.; Yokose, S.; Sakagami, H. Gene Expression Analysis of Cultured Rat-Endothelial Cells after Nd:YAG Laser Irradiation by Affymetrix GeneChip Array. In Vivo 2017, 31, 51–54. [Google Scholar] [CrossRef][Green Version]
- Giannelli, M.; Bani, D.; Tani, A.; Pini, A.; Margheri, M.; Zecchi-Orlandini, S.; Tonelli, P.; Formigli, L. In vitro evaluation of the effects of low-intensity Nd:YAG laser irradiation on the inflammatory reaction elicited by bacterial lipopolysaccharide adherent to titanium dental implants. J. Periodontol. 2009, 80, 977–984. [Google Scholar] [CrossRef]
- Saygin, N.E.; Giannobile, W.V.; Somerman, M.J. Molecular and cell biology of cementum. Periodontoloy 2000 2000, 24, 73–98. [Google Scholar] [CrossRef]
- Bozkurt, S.B.; Hakki, E.E.; Kayis, S.A.; Dundar, N.; Hakki, S.S. Biostimulation with diode laser positively regulates cementoblast functions, in vitro. Lasers Med. Sci. 2017, 32, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Chan, F.K. Inflammasome, Inflammation, and Tissue Homeostasis. Trends Mol. Med. 2018, 24, 304–318. [Google Scholar] [CrossRef] [PubMed]
- de Farias Gabriel, A.; Wagner, V.P.; Correa, C.; Webber, L.P.; Pilar, E.F.S.; Curra, M.; Carrard, V.C.; Martins, M.A.T.; Martins, M.D. Photobiomodulation therapy modulates epigenetic events and NF-kappaB expression in oral epithelial wound healing. Lasers Med. Sci. 2019, 34, 1465–1472. [Google Scholar] [CrossRef]
- Luomanen, M.; Rauhamaa-Makinen, R.; Meurman, J.H.; Kosloff, T.; Tiitta, O. Healing of rat mouth mucosa after irradiation with CO2, Nd:YAG, and CO2-Nd:YAG combination lasers. Scand. J. Dent. Res. 1994, 102, 223–228. [Google Scholar] [CrossRef]
- Sawabe, M.; Aoki, A.; Komaki, M.; Iwasaki, K.; Ogita, M.; Izumi, Y. Gingival tissue healing following Er:YAG laser ablation compared to electrosurgery in rats. Lasers Med. Sci. 2015, 30, 875–883. [Google Scholar] [CrossRef]
- Posten, W.; Wrone, D.A.; Dover, J.S.; Arndt, K.A.; Silapunt, S.; Alam, M. Low-level laser therapy for wound healing: Mechanism and efficacy. Derm. Surg. 2005, 31, 334–340. [Google Scholar] [CrossRef]
- Grossman, N.; Schneid, N.; Reuveni, H.; Halevy, S.; Lubart, R. 780 nm low power diode laser irradiation stimulates proliferation of keratinocyte cultures: Involvement of reactive oxygen species. Lasers Surg. Med. 1998, 22, 212–218. [Google Scholar] [CrossRef]
- Basso, F.G.; Oliveira, C.F.; Kurachi, C.; Hebling, J.; Costa, C.A. Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med. Sci. 2013, 28, 367–374. [Google Scholar] [CrossRef]
- Donnarumma, G.; De Gregorio, V.; Fusco, A.; Farina, E.; Baroni, A.; Esposito, V.; Contaldo, M.; Petruzzi, M.; Pannone, G.; Serpico, R. Inhibition of HSV-1 replication by laser diode-irradiation: Possible mechanism of action. Int. J. Immunopathol. Pharm. 2010, 23, 1167–1176. [Google Scholar] [CrossRef]
- Yang, H.Q.; Wang, Y.H.; Chen, J.X.; Zheng, L.Q.; Xie, S.S. [Low level laser irradiation in the visible spectra induces HeLa cells proliferation]. Spectrosc. Spectr. Anal. 2012, 32, 1024–1027. [Google Scholar]
- Mognato, M.; Squizzato, F.; Facchin, F.; Zaghetto, L.; Corti, L. Cell growth modulation of human cells irradiated in vitro with low-level laser therapy. Photomed. Laser Surg. 2004, 22, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Varga, V.; Toimela, T.; Hiitelä, K.; Tähti, H.; Oja, S.S.; Süveges, I.; Salminen, L. Laser treatment of cultured retinal pigment epithelial cells-evaluation of the cellular damage in vitro. J. Ocul. Pharm. 2004, 20, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Barak, A.; Goldkorn, T.; Morse, L.S. Laser induces apoptosis and ceramide production in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Hattenbach, L.O.; Beck, K.F.; Pfeilschifter, J.; Koch, F.; Ohrloff, C.; Schacke, W. Pigment-epithelium-derived factor is upregulated in photocoagulated human retinal pigment epithelial cells. Ophthalmic Res. 2005, 37, 341–346. [Google Scholar] [CrossRef]
- Powell, K.; Low, P.; McDonnell, P.A.; Laakso, E.L.; Ralph, S.J. The effect of laser irradiation on proliferation of human breast carcinoma, melanoma, and immortalized mammary epithelial cells. Photomed. Laser Surg. 2010, 28, 115–123. [Google Scholar] [CrossRef]
- Tang, E.; Khan, I.; Andreana, S.; Arany, P.R. Laser-activated transforming growth factor-β1 induces human β-defensin 2: Implications for laser therapies for periodontitis and peri-implantitis. J. Periodontal. Res. 2017, 52, 360–367. [Google Scholar] [CrossRef]
- Ejiri, K.; Aoki, A.; Yamaguchi, Y.; Ohshima, M.; Izumi, Y. High-frequency low-level diode laser irradiation promotes proliferation and migration of primary cultured human gingival epithelial cells. Lasers Med. Sci. 2014, 29, 1339–1347. [Google Scholar] [CrossRef]
- Nagahara, A.; Mitani, A.; Fukuda, M.; Yamamoto, H.; Tahara, K.; Morita, I.; Ting, C.C.; Watanabe, T.; Fujimura, T.; Osawa, K.; et al. Antimicrobial photodynamic therapy using a diode laser with a potential new photosensitizer, indocyanine green-loaded nanospheres, may be effective for the clearance of Porphyromonas gingivalis. J. Periodontal. Res. 2013, 48, 591–599. [Google Scholar] [CrossRef]
- Fujimura, T.; Mitani, A.; Fukuda, M.; Mogi, M.; Osawa, K.; Takahashi, S.; Aino, M.; Iwamura, Y.; Miyajima, S.; Yamamoto, H.; et al. Irradiation with a low-level diode laser induces the developmental endothelial locus-1 gene and reduces proinflammatory cytokines in epithelial cells. Lasers Med. Sci 2014, 29, 987–994. [Google Scholar] [CrossRef]
- Yokose, S.K.H. Low-power carbon dioxide laser irradiation reduces sclerostin expression, but stimulates Dmp-1 expression in osteocyte-like cells of rats. J. Bio-Integ. 2013, 3, 8. [Google Scholar] [CrossRef]
- Ohsugi, Y.; Katagiri, S.; Hirota, T.; Niimi, H.; Hatasa, M.; Watanabe, K.; Shimohira, T.; Mizutani, K.; Kitazawa, M.; Matsuzawa, A.; et al. Laser irradiation decreases sclerostin expression in bone and osteogenic cells. Faseb. J. 2020. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Aihara, N.; Yamaguchi, M.; Kasai, K. Low-energy irradiation stimulates formation of osteoclast-like cells via RANK expression in vitro. Lasers Med. Sci. 2006, 21, 24–33. [Google Scholar] [CrossRef]
- Atala, A.L.R. Handbook of Stem Cells; Academic Press: Cambridge, MA, USA, 2012; Volume 452. [Google Scholar]
- Mvula, B.; Mathope, T.; Moore, T.; Abrahamse, H. The effect of low level laser irradiation on adult human adipose derived stem cells. Lasers Med. Sci. 2008, 23, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Callaghan, M.J.; Longaker, M.T. Progress and potential for regenerative medicine. Annu. Rev. Med. 2007, 58, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Zare, F.; Moradi, A.; Fallahnezhad, S.; Ghoreishi, S.K.; Amini, A.; Chien, S.; Bayat, M. Photobiomodulation with 630 plus 810nm wavelengths induce more in vitro cell viability of human adipose stem cells than human bone marrow-derived stem cells. J. Photochem. Photobiol. BBiol. 2019, 201, 111658. [Google Scholar] [CrossRef]
- Wu, Y.H.; Wang, J.; Gong, D.X.; Gu, H.Y.; Hu, S.S.; Zhang, H. Effects of low-level laser irradiation on mesenchymal stem cell proliferation: A microarray analysis. Lasers Med. Sci. 2012, 27, 509–519. [Google Scholar] [CrossRef]
- Hou, J.F.; Zhang, H.; Yuan, X.; Li, J.; Wei, Y.J.; Hu, S.S. In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: Proliferation, growth factors secretion and myogenic differentiation. Lasers Surg. Med. 2008, 40, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Chellini, F.; Sassoli, C.; Francini, F.; Pini, A.; Squecco, R.; Nosi, D.; Bani, D.; Zecchi-Orlandini, S.; Formigli, L. Photoactivation of bone marrow mesenchymal stromal cells with diode laser: Effects and mechanisms of action. J. Cell. Physiol. 2013, 228, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, X.; Zhu, S.; Xie, Y.; Du, J.; Ge, H.; Bai, Y.; Liu, Y.; Guo, L. Photobiomodulation with 808-nm diode laser enhances gingival wound healing by promoting migration of human gingival mesenchymal stem cells via ROS/JNK/NF-kappaB/MMP-1 pathway. Lasers Med. Sci. 2020, 35, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Agas, D.; Laus, F.; Cuteri, V.; Hanna, R.; Sabbieti, M.G.; Benedicenti, S. The Effects of Photobiomodulation of 808 nm Diode Laser Therapy at Higher Fluence on the in Vitro Osteogenic Differentiation of Bone Marrow Stromal Cells. Front. Physiol. 2018, 9, 123. [Google Scholar] [CrossRef]
- Horvat-Karajz, K.; Balogh, Z.; Kovacs, V.; Drrernat, A.H.; Sreter, L.; Uher, F. In vitro effect of carboplatin, cytarabine, paclitaxel, vincristine, and low-power laser irradiation on murine mesenchymal stem cells. Lasers Surg. Med. 2009, 41, 463–469. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Diniz, I.M.A.; Maranduba, C.M.S.; Miyagi, S.P.H.; Rodrigues, M.; Moura-Netto, C.; Marques, M.M. Short-term evaluation of photobiomodulation therapy on the proliferation and undifferentiated status of dental pulp stem cells. Lasers Med. Sci. 2019, 34, 659–666. [Google Scholar] [CrossRef]
- Garrido, P.R.; Pedroni, A.C.F.; Cury, D.P.; Moreira, M.S.; Rosin, F.; Sarra, G.; Marques, M.M. Effects of photobiomodulation therapy on the extracellular matrix of human dental pulp cell sheets. J. Photochem. Photobiol. BBiol. 2019, 194, 149–157. [Google Scholar] [CrossRef]
- Yurtsever, M.C.; Kiremitci, A.; Gumusderelioglu, M. Dopaminergic induction of human dental pulp stem cells by photobiomodulation: Comparison of 660 nm laser light and polychromatic light in the nir. J. Photochem. Photobiol. BBiol. 2020, 204, 111742. [Google Scholar] [CrossRef]
- Wang, L.; Wu, F.; Liu, C.; Song, Y.; Guo, J.; Yang, Y.; Qiu, Y. Low-level laser irradiation modulates the proliferation and the osteogenic differentiation of bone marrow mesenchymal stem cells under healthy and inflammatory condition. Lasers Med. Sci. 2019, 34, 169–178. [Google Scholar] [CrossRef]
- Peat, F.J.; Colbath, A.C.; Bentsen, L.M.; Goodrich, L.R.; King, M.R. In Vitro Effects of High-Intensity Laser Photobiomodulation on Equine Bone Marrow-Derived Mesenchymal Stem Cell Viability and Cytokine Expression. Photomed. Laser Surg. 2018, 36, 83–91. [Google Scholar] [CrossRef]
- Anwer, A.G.; Gosnell, M.E.; Perinchery, S.M.; Inglis, D.W.; Goldys, E.M. Visible 532 nm laser irradiation of human adipose tissue-derived stem cells: Effect on proliferation rates, mitochondria membrane potential and autofluorescence. Lasers Surg. Med. 2012, 44, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Constantin, A.; Dumitrescu, M.; Mihai Corotchi, M.C.; Jianu, D.; Simionescu, M. CO2 laser increases the regenerative capacity of human adipose-derived stem cells by a mechanism involving the redox state and enhanced secretion of pro-angiogenic molecules. Lasers Med. Sci. 2017, 32, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamato, M.; Washio, K.; Yoshida, T.; Tsumanuma, Y.; Yamada, A.; Onizuka, S.; Izumi, Y.; Ando, T.; Okano, T.; et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets—A safety and efficacy study in ten patients. Regen 2018, 9, 38–44. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).