Abstract
Fermentation remains central to food manufacturing and to the bio-based production of organic acids, solvents, and functional metabolites. This review integrates the biochemical pathways, key microorganisms, and application space of five major industrial fermentations—alcoholic, acetic, butyric, lactic, and propionic. We summarize the principal metabolic routes (EMP/ED glycolysis; oxidative ethanol metabolism; butyrate-forming pathways; and the Wood–Werkman, acrylate, and 1,2-propanediol routes to propionate) and relate them to the dominant microbial groups involved, including yeasts, acetic acid bacteria, lactic acid bacteria, clostridia, and propionibacteria. We highlight how the resulting metabolite spectra—ethanol, acetic acid, butyrate, lactate, propionate, and associated secondary metabolites—underpin product quality and safety in fermented foods and beverages and enable the industrial synthesis of platform chemicals, polymers, and biofuels. Finally, we discuss current challenges and opportunities for sustainable fermentation, including waste stream valorization, process intensification, and the integration of systems biology and metabolic engineering within circular economy frameworks.
1. Introduction
Fermentation is one of the oldest biotechnological processes, in which microorganisms, through their metabolic activity, transform plant- or animal-based raw materials into products with modified physicochemical and sensory properties [1,2,3,4]. From a biochemical standpoint, fermentation is a metabolic process in which energy is generated from organic compounds in the absence of an exogenous electron acceptor [5]. During fermentation, microorganisms catabolize carbohydrates into alcohols, carbon dioxide and/or organic acids, while simultaneously utilizing nutrients present in raw materials, such as carbon, nitrogen, vitamins and trace elements, for growth and reproduction [1]. As a result of microbial enzymatic activity, complex organic molecules undergo chemical and physical transformations and are converted into simpler, bioactive, functional and nutritionally beneficial compounds [6]. The metabolic reactions occurring during fermentation not only alter food composition but also enhance its nutritional, sensory and functional characteristics [7]. This biochemical capacity has been exploited empirically since antiquity, long before the microbial basis of fermentation was understood.
The origins of fermentation can be traced back to early human societies, with archaeological evidence indicating its use as early as the Late Epipaleolithic period. Residues of fermented cereal-based beverages discovered at Raqefet Cave in present-day Israel provide the earliest known proof of deliberate fermentation, highlighting its role in early food processing practices. Over subsequent millennia, fermentation became a fundamental method of food production and preservation across ancient civilizations, including Mesopotamia, Egypt, and China, where it was used for the manufacture of bread, beer, wine, and other fermented products. Initially, fermentation relied on spontaneous processes driven by indigenous microbiota associated with raw materials and the environment. Through empirical observation, humans gradually learned to manipulate fermentation conditions to improve product quality and stability. The biological basis of fermentation remained unknown until the 19th century, when Louis Pasteur demonstrated its microbial nature. This discovery enabled intentional strain selection and process optimization, ultimately laying the foundation for modern industrial biotechnology [8,9,10]. Today, fermentation is increasingly integrated with systems biology and metabolic engineering to improve productivity, robustness, and sustainability of bioprocesses. In practice, these tools enable the identification of metabolic bottlenecks and stress-response limitations and support rational strain and process design to improve yields, robustness, and substrate utilization.
Accordingly, modern fermentation practice is often distinguished by the degree of microbial and process control applied during production. Two primary approaches to food fermentation are recognized. The first is spontaneous fermentation, driven by autochthonous microorganisms naturally present in raw materials or the processing environment [3,11]. Due to the undefined composition and dynamics of the microbial communities involved, spontaneous fermentation requires strict monitoring and stabilization [6]. The second approach involves the use of starter cultures, which dominate contemporary industrial practice. Starter cultures consist of selected and well-characterized strains of bacteria, yeasts or molds that initiate and guide fermentation in a controlled, reproducible manner, suppressing undesirable microorganisms and enhancing food safety [12]. These cultures accelerate fermentation, convert available carbohydrates into alcohols and organic acids that act as natural preservatives, and impart desirable sensory attributes. Their application standardizes product quality, extends shelf life and reduces technological variability. In addition, many strains exhibit antagonistic activity toward pathogens, supporting microbial safety. For these reasons, ongoing research focuses on isolating and characterizing strains with optimal technological and probiotic potential for use as functional starter cultures [6,12].
While spontaneous fermentations are increasingly valued for their contribution to product diversity, regional identity, and complex sensory profiles, their inherent microbial variability limits their applicability in large-scale industrial production. In contrast, defined starter cultures enable reproducible fermentation kinetics, improved safety through rapid acidification or ethanol production, and consistent product quality. Consequently, modern food fermentation often combines traditional practices with controlled starter cultures or selected mixed consortia to balance authenticity with industrial reliability, particularly in processes requiring scalability and compliance with safety standards [9].
Fermentation technologies therefore continue to play a key role in the food sector, improving preservation, ensuring microbiological safety, enhancing sensory quality, and enabling the transformation of perishable raw materials into stable, shelf-stable, and nutritionally valuable products [4]. Because these pathways are metabolically connected and often occur sequentially or in mixed-culture systems, a comparative overview is essential to capture fermentation as an integrated biotechnological platform rather than isolated processes. Depending on the substrates and microorganisms involved, five major types of fermentation can be distinguished: alcoholic, acetic, butyric, lactic and propionic.
The aim of this review is to provide a comprehensive and integrative overview of the biochemical pathways, microbial ecology, and industrial applications of these five major fermentation types. The article synthesizes current knowledge on the metabolic mechanisms underlying each process, the microorganisms involved, their technological roles and their relevance in the food, pharmaceutical and biotechnological industries. Additionally, the review highlights recent advances, emerging trends and future prospects in fermentation-based bioprocessing, with particular emphasis on sustainability, functional food development and the valorization of biological raw materials. Key barriers to sustainable implementation include heterogeneous and seasonally variable waste streams, inhibitory compounds in low-cost substrates, and the need for robust strains and scalable downstream processing. In addition to summarizing classical pathways and applications, this review addresses cross-cutting industrial challenges such as process scale-up, yield optimization, and economic feasibility, and discusses emerging trends in sustainable and data-driven fermentation technologies. In the following sections, each fermentation type is discussed within a consistent framework, progressing from core biochemical reactions to the principal microorganisms involved and, finally, to representative industrial applications. By jointly analyzing five archetypal fermentation types rather than a single product, this review highlights fermentation as a modular and interconnected biotechnological platform, in which distinct pathways collectively support food production, platform chemicals, biofuels, and functional biomaterials.
2. Alcoholic Fermentation
2.1. Biochemical Basis
Alcoholic (ethanolic) fermentation is an anaerobic biochemical process in which sugars such as glucose, fructose, and sucrose are converted into ethyl alcohol (ethanol) and carbon dioxide [1,3,8,10,13,14]. The process begins with glycolysis—a series of enzyme-catalyzed reactions that degrade one molecule of glucose into two molecules of pyruvate, accompanied by the formation of two molecules of ATP and two molecules of NADH. In the next step, pyruvate undergoes decarboxylation catalyzed by pyruvate decarboxylase, producing acetaldehyde and releasing carbon dioxide. Subsequently, acetaldehyde is reduced to ethanol in a reaction catalyzed by alcohol dehydrogenase, during which NADH is oxidized back to NAD+. The regeneration of NAD+ enables the continuation of glycolysis and energy production under anaerobic conditions [7,8,13,15,16,17]. The overall reaction can be expressed as follows:
C6H12O6 → 2 C2H5OH + 2 CO2
While the overall stoichiometry is conserved, ethanol yield, productivity, and stress tolerance depend strongly on the microorganism and the glycolytic route used, as outlined below.
2.2. Microorganisms and Metabolic Pathways
Alcoholic fermentation is carried out primarily by yeasts and, to a lesser extent, by certain bacterial species [18,19,20]. The most commonly employed microorganism is Saccharomyces cerevisiae, which ferments glucose via the classical glycolytic pathway known as the Embden–Meyerhof–Parnas (EMP) pathway. In this route, each molecule of glucose is converted into two molecules of pyruvate and subsequently—according to the mechanism described above—into ethanol and carbon dioxide [15,20,21,22].
Certain bacteria, such as Zymomonas mobilis, utilize an alternative sugar catabolic pathway—the Entner–Doudoroff (ED) pathway. Similarly to the EMP pathway, it leads to the formation of pyruvate but is characterized by a lower energetic yield, producing only one ATP molecule per molecule of glucose, and by reduced biomass formation, thereby channeling a higher proportion of sugar toward ethanol production [15,18,19,21].
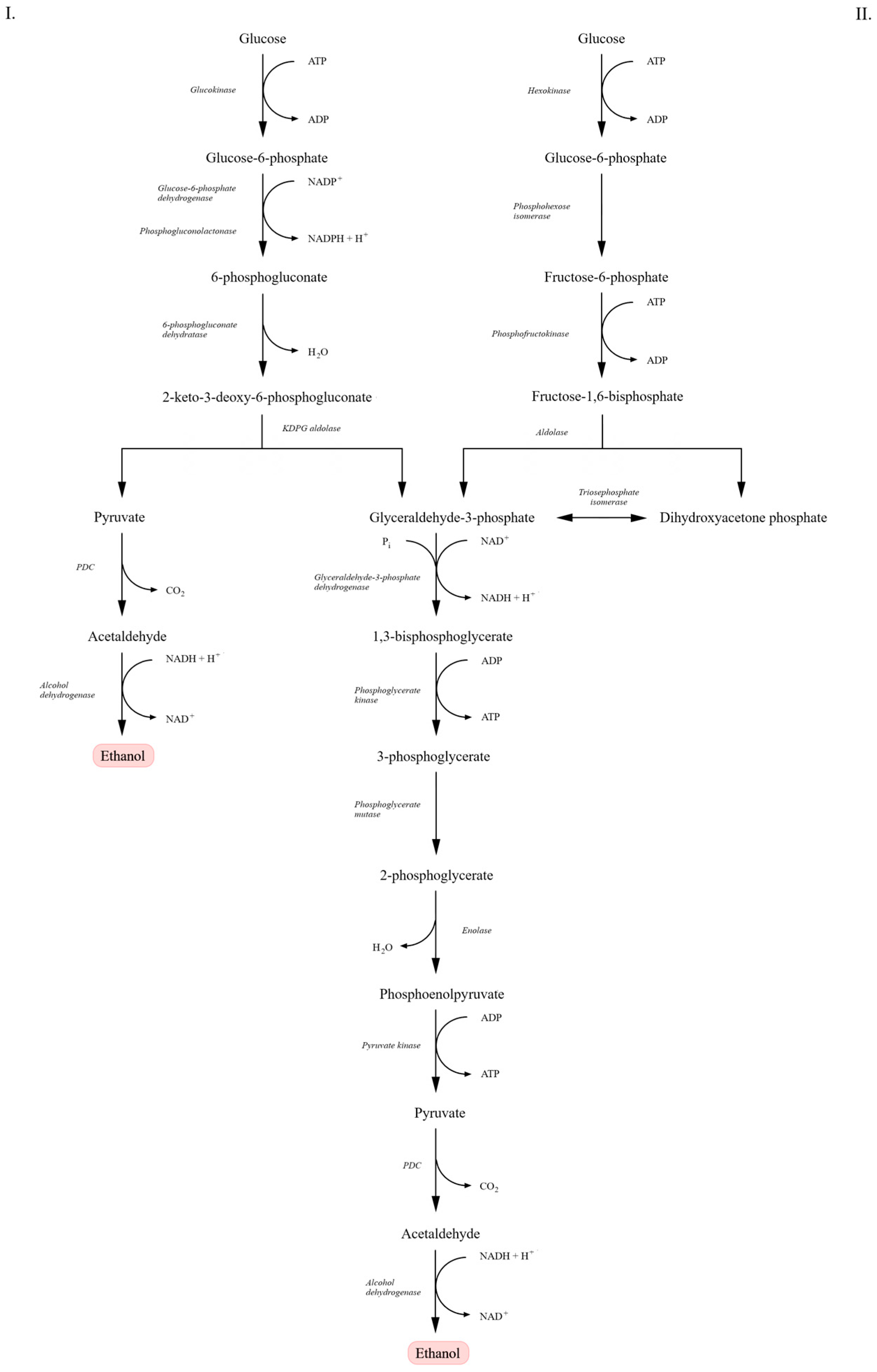
As illustrated in Figure 1, the ED pathway differs from the classical EMP route in both reaction sequence and enzymatic machinery. In the EMP pathway, glucose is metabolized to pyruvate with the net production of two ATP and two NADH molecules. A key enzyme, fructose-1,6-bisphosphate aldolase, cleaves fructose-1,6-bisphosphate into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. In contrast, in the ED pathway, glucose-6-phosphate is converted into 6-phosphogluconate and subsequently into 2-keto-3-deoxy-6-phosphogluconate (KDPG), which is cleaved by KDPG aldolase directly into pyruvate and glyceraldehyde-3-phosphate—yielding lower energy output [21,23,24]. Despite differences in the early steps of glucose metabolism, the downstream reactions—conversion of glyceraldehyde-3-phosphate to pyruvate—proceed identically in both pathways, involving the same enzymes and resulting in the same end products. In each case, pyruvate is further converted to ethanol and carbon dioxide [15,22].
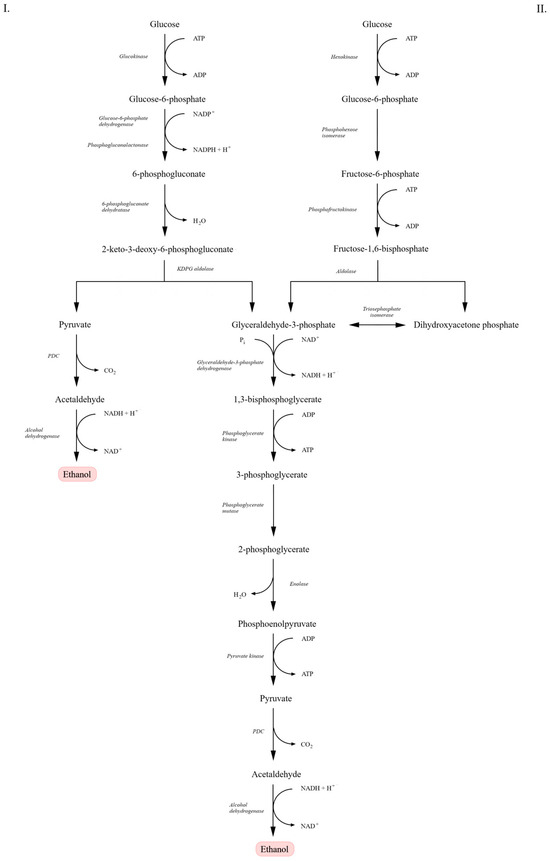
Figure 1.
Comparison of the Entner–Doudoroff (ED) and Embden–Meyerhof–Parnas (EMP) pathways leading to alcoholic fermentation. In the ED pathway (I), glucose is converted through glucose-6-phosphate, 6-phosphogluconate, and 2-keto-3-deoxy-6-phosphogluconate (KDPG), yielding pyruvate and glyceraldehyde-3-phosphate. In the EMP pathway (II), glucose is metabolized through glucose-6-phosphate, fructose-1,6-bisphosphate, and glyceraldehyde-3-phosphate. In both pathways, pyruvate is decarboxylated to acetaldehyde by pyruvate decarboxylase (PDC), followed by reduction to ethanol by alcohol dehydrogenase (ADH). Scheme adapted from [21].
Although the EMP pathway yields more ATP per mole of glucose than the ED pathway, this higher energy conservation in S. cerevisiae is generally associated with increased biomass formation and stress tolerance rather than maximal ethanol yield [16,23,25]. In contrast, the lower ATP yield of the ED pathway in Z. mobilis can limit biomass synthesis and direct a higher fraction of carbon toward ethanol production, resulting in higher specific ethanol productivity despite lower energetic efficiency [18,23,24].
The choice of microorganism and metabolic route depends on physiology, ethanol yield, fermentation rate, substrate spectrum and tolerance to inhibitory compounds. Yeasts such as S. cerevisiae are valued for their high ethanol tolerance, robustness under industrial conditions, and capacity to withstand osmotic, thermal, and inhibitory stresses. These features, together with its genetic stability, extensive industrial know-how, and availability of advanced molecular tools, explain why S. cerevisiae remains the dominant microorganism in industrial alcoholic fermentation [25,26]. In contrast, Z. mobilis exhibits high ethanol productivity, rapid fermentation rates, and low biomass formation due to the lower ATP yield of the ED pathway, making strain selection strongly dependent on the specific requirements of a given bioprocess [19]. These metabolic features directly translate into technological performance, which explains why specific strains are preferred for beverage, baking, and biofuel applications.
In recent years, alcoholic fermentation has become a major target of metabolic engineering and synthetic biology aimed at improving ethanol yield, productivity, and stress tolerance under industrial conditions [25,27,28]. In S. cerevisiae, engineering strategies have focused on redirecting carbon flux toward ethanol by minimizing by-product formation (e.g., glycerol and organic acids), enhancing cofactor balance, and increasing tolerance to ethanol, temperature, and osmotic stress [16,25,29]. Genome-scale metabolic models and CRISPR/Cas-based genome editing have enabled systematic modification of glycolytic regulation, redox metabolism, and membrane composition, resulting in strains with improved robustness and fermentation performance [25,30,31,32].
In parallel, Z. mobilis has been extensively engineered to expand its substrate spectrum beyond glucose to pentoses such as xylose and arabinose, facilitating the efficient conversion of lignocellulosic hydrolysates into ethanol [24,33]. Synthetic biology approaches, including pathway modularization, dynamic gene regulation, and adaptive laboratory evolution, are increasingly integrated with process engineering to optimize large-scale bioethanol production and reduce overall process costs [18,34,35]. These advances highlight the transition of alcoholic fermentation from a classical bioprocess to a data-driven and design-oriented platform for sustainable biofuel and biochemical production [25,27].
2.3. Industrial Applications
Alcoholic fermentation is a biotechnological process with a wide range of industrial applications, encompassing the food, biotechnological, chemical, and energy sectors (Table 1). Its ability to convert carbohydrates into ethanol and other bioactive metabolites makes it a cornerstone of both traditional and modern bioprocessing technologies [9,26].
This type of fermentation represents one of the oldest and most fundamental biotechnological processes applied in the food industry [7,36,37,38]. The classical examples include the production of wine, beer, cider, mead, and distilled beverages such as whisky, rum, and vodka. In these processes, yeasts—primarily S. cerevisiae—convert sugars present in raw materials such as grape must, barley mash, apple juice, or honey solutions into ethanol and carbon dioxide. For distilled beverages, fermentation serves as a preliminary stage before distillation, which increases ethanol concentration. In addition to ethanol, numerous aromatic compounds, such as esters, aldehydes, and higher alcohols are generated, contributing to the sensory complexity and quality of the final beverages. For example, ethyl esters (e.g., ethyl acetate, ethyl hexanoate) impart fruity and floral notes, higher alcohols contribute complexity and mouthfeel, and aldehydes influence freshness and balance, collectively shaping the characteristic sensory profiles of fermented beverages [8,39,40,41,42].
Alcoholic fermentation also plays an important role in the production of low-alcohol fermented beverages, including water kefir, kombucha, kvass, and ginger beer. In the initial stage of these processes, yeasts carry out alcoholic fermentation, resulting in small amounts of ethanol, which is subsequently oxidized to acetic acid by acetic acid bacteria. The final product therefore contains only trace amounts of alcohol, and its sensory and nutritional value arises from fermentation metabolites and microorganisms with potential probiotic properties [9,37].
Beyond beverage production, alcoholic fermentation has significant applications in biotechnology for the biosynthesis of natural flavors, enzymes, and aromatic components. During fermentation, yeasts generate ethyl esters, aldehydes, and ketones that impart characteristic aroma profiles and are widely utilized in the dairy, bakery, and confectionery industries [16,36,43,44]. In bakery processes, S. cerevisiae metabolizes sugars in flour into ethanol and carbon dioxide, leading to dough leavening and crumb formation. Although ethanol evaporates during baking, intermediate metabolites formed during fermentation contribute to the desirable flavor and aroma of baked goods [45,46].
In the chemical and pharmaceutical sectors, ethanol obtained via yeast fermentation is a valuable raw material and universal solvent. It serves as a substrate for the synthesis of various organic compounds such as ethylene, ethyl acetate, diethyl ether, acetic acid, glycerol, and organic acids (e.g., lactic and succinic), which are employed in the production of pharmaceuticals, cosmetics, plastics, solvents, and preservatives [7,29,47,48]. In pharmacy, ethanol functions as a carrier for active substances, a preservative, a disinfectant, and a technological agent in extraction and stabilization processes of bioactive compounds [36,43,49].
In modern bioenergy technologies, alcoholic fermentation is used for the production of bioethanol—a renewable and low-emission fuel that serves as a sustainable alternative to fossil fuels [14,19,25,48,50]. Fermentation-derived ethanol also plays an important role in green chemistry, acting as a feedstock for the synthesis of bioplastics, bioesters, and other bio-based fuel components, thus supporting the development of a circular and sustainable economy [9,19].
Alcoholic fermentation further holds cultural and historical importance. In many regions worldwide, it forms part of culinary heritage and serves as the basis for traditional beverages such as Japanese sake, Eastern European kvass, South American chicha, and Asian plant-based drinks such as pulque, tuba, and toddy. These processes, often carried out by wild yeasts or naturally occurring microbial consortia, give products unique sensory and cultural characteristics [36,51,52,53,54].


Table 1.
Representative industrial applications of alcoholic fermentation in the food, chemical and energy sectors.
Table 1.
Representative industrial applications of alcoholic fermentation in the food, chemical and energy sectors.
| Application Area | Example Products | Main Microorganisms | Fermentation Products/Effects | Technological Significance | Typical Product Concentration | Representative References |
|---|---|---|---|---|---|---|
| Alcoholic beverages | Wine, beer, cider, mead, spirits (whisky, rum, vodka) | Saccharomyces cerevisiae, S. bayanus, Schizosaccharomyces pombe | Ethanol, CO2, esters, higher alcohols, aldehydes | Alcohol production; development of aroma and flavor; natural preservation | Beer: 3–8% (v/v); Wine: 10–15% (v/v); Spirits (after distillation): >40% (v/v) | [8,26,36,46,55,56,57] |
| Low-alcohol fermented drinks | Kombucha, kvass, water kefir, ginger beer | Saccharomyces, Zygosaccharomyces, Acetobacter, Lactobacillus | Ethanol → acetic acid, CO2, organic acids | Light carbonation; improved flavor and probiotic potential | <1% (v/v) ethanol | [9,12,58,59,60,61,62,63,64,65,66] |
| Baking and confectionery | Bread, rolls, yeast doughs | Saccharomyces cerevisiae | CO2, volatile ethanol, esters | Dough leavening; improved texture, flavor, and aroma | Ethanol transient; CO2 for leavening (ethanol evaporates during baking) | [14,36,43,46,67,68,69] |
| Flavor and aroma production | Natural flavors, fruit and dairy aroma compounds | Saccharomyces, Kluyveromyces, Torulaspora spp. | Esters, aldehydes, ketones, higher alcohols | Development of natural flavoring ingredients for food and beverages | Trace levels (mg·L−1 range) | [8,36,40,43,57,70,71,72,73] |
| Pharmaceutical and chemical industry | Solvents, acetic acid, ethyl acetate, diethyl ether, glycerol | S. cerevisiae, Candida, Kluyveromyces spp. | Ethanol, organic acids, secondary metabolites | Production of solvents, preservatives, and pharmaceutical intermediates | Ethanol typically >95% (v/v) after purification | [7,14,25,36,47,74] |
| Bioenergy sector | Bioethanol fuel, bioplastics, bioesters | S. cerevisiae, Zymomonas mobilis | Ethanol, CO2 | Renewable energy generation; sustainable fuel and biocomponent production | 80–120 g·L−1 ethanol | [14,25,33,36,47,74,75] |
| Cultural and traditional fermentation | Sake, chicha, kvass, pulque, toddy, tuba | Wild yeasts, mixed consortia (Saccharomyces, Lactobacillus) | Ethanol, CO2, esters, organic acids | Preservation of traditional food heritage; unique sensory profiles | Typically 2–8% (v/v) ethanol | [26,36,39,46,53,54,76,77,78,79] |
Typical ethanol concentrations range from approximately 3–8% (v/v) in beer, 10–15% (v/v) in wine, <1% (v/v) in low-alcohol fermented beverages, and >80–100 g·L−1 in industrial bioethanol fermentations, reflecting differences in microbial physiology, pathway utilization (EMP versus ED), and process objectives [9,18,24,25,26,41,64].
3. Acetic Fermentation
Following alcoholic fermentation, acetic fermentation represents a distinct yet functionally connected process in which ethanol is further oxidized by specialized bacteria under aerobic conditions, linking primary sugar catabolism with organic acid production.
3.1. Biochemical Pathway and Key Microorganisms
The production of acetic acid represents a complex, two-step metabolic pathway involving different groups of microorganisms and specialized enzymatic systems. The first stage comprises alcoholic fermentation, occurring under anaerobic conditions, during which simple sugars, such as glucose are converted into ethanol and carbon dioxide by the action of yeasts, mainly of the genus Saccharomyces. The subsequent stage, proceeding in the presence of oxygen, involves acetic acid bacteria (AAB) that oxidize ethanol to acetic acid and water [80], according to the reaction:
Both stages require microorganisms with high adaptive capacity, enabling their activity under variable and often unfavorable environmental conditions. During alcoholic fermentation, the use of yeast strains with high alcohol tolerance is essential to ensure efficient ethanol production. In the acetic acid fermentation phase, the selection of bacterial strains capable of withstanding both high ethanol concentrations and the accumulation of acetic acid is crucial, as this process demands dual tolerance to both compounds [81].
The most important acetic acid–producing bacteria used in biotechnological vinegar production belong to the genera Acetobacter, Gluconacetobacter, Gluconobacter, and Komagataeibacter. These strains exhibit high efficiency in ethanol oxidation to acetic acid and substantial tolerance to product accumulation in the fermentation environment [81,82].
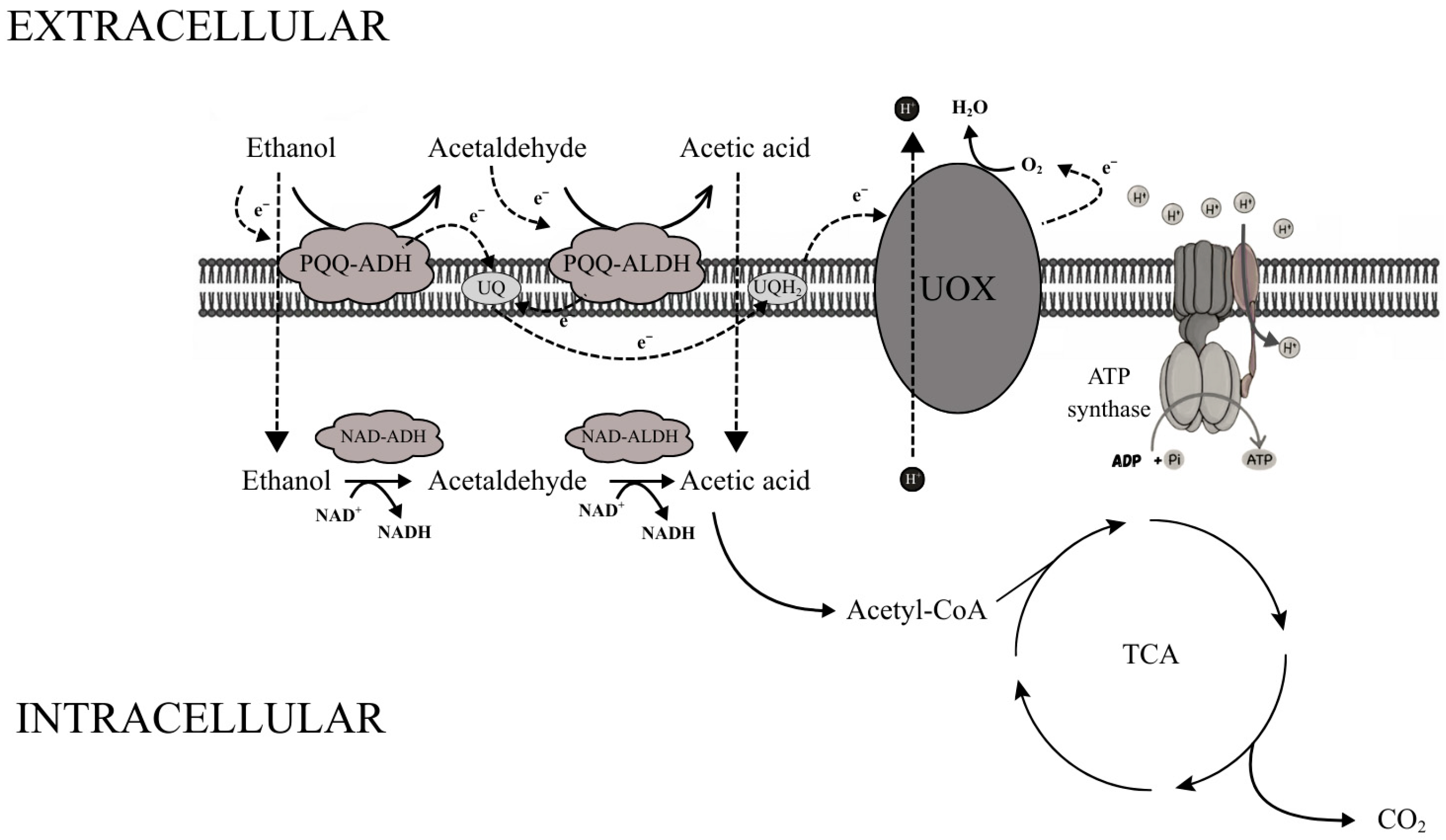
The mechanism of acetic acid fermentation is based on a specialized membrane-bound enzymatic system located in the cytoplasmic membrane of acetic acid bacteria. Two key dehydrogenases are involved in this process: the pyrroloquinoline quinone (PQQ)-dependent alcohol dehydrogenase (ADH) and the acetaldehyde dehydrogenase (ALDH). The first enzyme catalyzes the oxidation of ethanol to acetaldehyde, while the second converts acetaldehyde into acetic acid. Both dehydrogenases are membrane-bound and oriented toward the periplasmic space, allowing direct oxidation of substrates located outside the cytoplasm and the release of products into the extracellular medium [83,84,85,86].
Electrons released during ethanol and acetaldehyde oxidation are transferred from PQQ to ubiquinone (UQ), which acts as a mobile electron carrier. Reduced ubiquinone (ubiquinol, UQH2) donates electrons to ubiquinol oxidase (UOX), which transfers them to molecular oxygen, the terminal electron acceptor. This reaction yields water, while proton translocation across the membrane generates a proton motive force that drives ATP synthesis via ATP synthase [83,84,85,86].
In addition to the membrane-bound system, AAB possess a cytoplasmic dehydrogenase complex using NAD+/NADP+ as cofactors. NAD-dependent alcohol dehydrogenase (NAD-ADH) and NADP-dependent acetaldehyde dehydrogenase (NADP-ALDH) oxidize ethanol that diffuses into the cytoplasm to acetic acid; the latter can be converted into acetyl-CoA, which enters the tricarboxylic acid (TCA) cycle and is fully oxidized to CO2 and water (Figure 2) [23,83,87]. Thus, the coupling of periplasmic ethanol oxidation to proton motive force generation enables efficient ATP synthesis under high ethanol and acidic conditions, while the combined ethanol and acid tolerance of yeasts and acetic acid bacteria directly underpins their robustness and efficiency in industrial acetic fermentation systems [83,87,88,89].
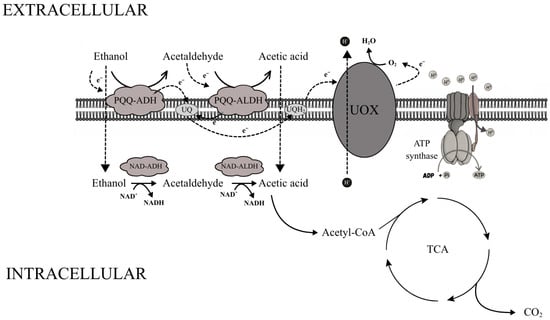
Figure 2.
Schematic representation of the membrane-bound and cytosolic respiratory chains involved in ethanol oxidation in acetic acid bacteria. In this system, periplasmic PQQ-dependent dehydrogenases catalyze the oxidation of ethanol and acetaldehyde, transferring electrons through ubiquinone to the terminal ubiquinol oxidase (UOX), which drives proton translocation and ATP formation. Intracellularly, acetate is further converted to acetyl-CoA and enters the tricarboxylic acid (TCA) cycle. Scheme adapted from [83].
Under conditions relevant for acetic acid production, the membrane-bound PQQ-ADH and ALDH dominate, whereas cytoplasmic NAD-ADH and NADP-ALDH are largely inhibited. High ethanol concentrations favor the membrane pathway. As ethanol is depleted, cytoplasmic dehydrogenase activity increases and the contribution of the membrane system declines. This dynamic regulation allows AAB to optimize energy conservation and adapt to shifting environmental conditions [83,85,87,90]. Together, these metabolic adaptations explain why acetic acid bacteria are uniquely suited for industrial vinegar production and other acetic acid-based bioprocesses.
Moreover, with traditional strain selection, acetic acid bacteria have increasingly become targets of metabolic engineering and systems-level optimization aimed at improving acetic acid productivity, acid tolerance, and process robustness [83,87,91,92]. Recent studies have focused on enhancing ethanol and acetic acid tolerance through modification of membrane lipid composition, overexpression of stress response regulators, and optimization of respiratory chain components involved in periplasmic ethanol oxidation [83,85,90]. Advances in genome sequencing and comparative genomics of Acetobacter, Komagataeibacter, and Gluconacetobacter species have enabled the identification of key genes associated with acid resistance, oxidative stress defense, and efficient energy conservation [93].
Emerging synthetic biology approaches, including targeted gene deletions, controlled overexpression of PQQ-dependent dehydrogenases, and adaptive laboratory evolution, have further improved strain performance under high-acidity and high-ethanol conditions relevant to industrial vinegar production [94]. Coupled with bioprocess intensification strategies and real-time monitoring of dissolved oxygen and redox status, these developments highlight the transition of acetic fermentation from a largely empirical process to a rationally engineered biotechnological platform [84,95].
3.2. Industrial Applications
Building on the biochemical mechanisms described above, acetic fermentation has been widely exploited in both food and non-food industries due to its ability to generate acetic acid and related metabolites with preservative, sensory, and functional properties. While vinegar production remains the most recognized application, acetic fermentation should be viewed as a platform bioprocess supporting multiple industrial sectors.
Acetic fermentation plays a crucial role across the food industry, extending beyond conventional vinegar production. The most widespread application is the manufacture of edible vinegars, which are aqueous acetic acid solutions typically containing 4–15% (w/v) acetic acid. Depending on the raw material and technology used, spirit vinegar (from ethanol), wine vinegar, apple cider vinegar, and rice vinegar can be distinguished, each with characteristic sensory and nutritional properties [61,82]. Acetic acid content determines preservative, antimicrobial and organoleptic attributes. Vinegar functions as both a condiment and a natural preservative due to its ability to lower pH and inhibit undesirable microflora. Commercial vinegar production commonly involves a two-stage fermentation: alcoholic fermentation by yeasts (e.g., S. cerevisiae), followed by acetic oxidation by AAB [36,54]. A summary of the major industrial applications of acetic fermentation and acetic acid across different sectors is provided in Table 2.
Acetic fermentation also underpins the production of low-alcohol fermented beverages such as kombucha, water kefir, and kvass. In these systems, AAB form symbiotic consortia with yeasts (SCOBY), producing not only acetic and gluconic acids but also bacterial cellulose (e.g., Komagataeibacter xylinus). These metabolites contribute to acidity, carbonation, antioxidant capacity, and microbiota-modulating effects, enhancing the functional value of these beverages [9,54].
In the food industry, acetic fermentation is further exploited for the biosynthesis of natural flavors and esters. Acetic acid, acetaldehyde, and ethyl esters derived from fermentation are widely used in seasoning, dairy, and bakery applications as natural aromatic components. Acidification through acetic acid production also sup-ports biopreservation by inhibiting spoilage and pathogenic microorganisms, extending the shelf life of pickles, sauces, dressings, and marinades [43,61,81,82,96]. In this context, acetic fermentation provides a natural alternative to synthetic preservatives, aligning with consumer demand for clean-label and minimally processed foods.
At the industrial scale, acetic acid represents a high-volume platform chemical with global production exceeding 15 million tons annually, primarily for the synthesis of vinyl acetate monomer (VAM), purified terephthalic acid (PTA), and acetic anhydride [86,97]. While bulk acetic acid is still predominantly produced via petrochemical methanol carbonylation, food-grade and specialty acetic acid is exclusively obtained through microbial fermentation, and bio-based acetic acid is increasingly explored as a renewable alternative for polymer, textile, and pharmaceutical applications [86,98]. In the chemical industry, acetic acid serves as a precursor for the synthesis of polyethylene terephthalate (PET), cellulose acetate, and polyvinyl acetate, which are used in beverage packaging, photographic materials, coatings, and adhesives [99,100,101].
In the textile industry, acetic acid is used in both fiber production and fabric processing. Owing to its chemical properties, it plays a key role in dyeing processes, acting as a mordant that enhances color fastness and resistance to fading. It also functions as a pH regulator, adjusting the dye bath environment to meet the specific requirements of dyes and fiber types. Additionally, it is used in fabric cleaning, degreasing, and textile printing processes, where it facilitates dye binding and enhances pattern intensity and durability [24,34,87,90,102].
In cosmetics, acetic acid and its derivatives (esters and acetate salts) are incorporated into shampoos, conditioners, perfumes, and skincare formulations as pH adjusters, antimicrobial agents, and fragrance components [1,72,90,97]. In the pharmaceutical industry, acetic acid is used as a solvent, antiseptic, and synthetic intermediate in esterification and acetylation reactions, enabling the production of compounds with therapeutic properties. Due to its antimicrobial activity, it is also applied in wound cleansing and treatment of superficial infections [1,90].


Table 2.
Industrial applications of acetic fermentation and acetic acid in the food, chemical, textile, cosmetic and pharmaceutical sectors.
Table 2.
Industrial applications of acetic fermentation and acetic acid in the food, chemical, textile, cosmetic and pharmaceutical sectors.
| Application Area | Example Products/Applications | Main Microorganisms | Fermentation Products/Effects | Technological Significance/Outcomes | Representative References |
|---|---|---|---|---|---|
| Vinegar production | Spirit vinegar, wine vinegar, apple cider vinegar, rice vinegar | Acetobacter aceti, Komagataeibacter xylinus, Gluconacetobacter europaeus | Acetic acid, water | Conversion of ethanol to acetic acid; pH reduction; flavor and aroma development; natural food preservation | [36,61,81,84,85,103,104] |
| Low-alcohol fermented beverages | Kombucha, water kefir, kvass | Symbiotic consortia of Acetobacter, Komagataeibacter, Saccharomyces, Zygosaccharomyces | Acetic acid, gluconic acid, bacterial cellulose | Functional beverages with antioxidant, detoxifying, and probiotic properties; SCOBY formation | [9,12,58,59,60,61,62,63,65,66] |
| Flavor and ester biosynthesis | Natural flavor concentrates, fruit and dairy flavoring compounds | Acetobacter, Gluconobacter, Saccharomyces spp. | Acetic acid, acetaldehyde, ethyl acetate, ethyl lactate | Generation of natural aromatic compounds for the dairy, seasoning, and bakery industries | [8,36,40,43,70,72,105] |
| Food preservation and bioprotection | Pickles, sauces, salad dressings, marinades | Acetobacter spp., Lactobacillus spp. | Acetic acid (acidification) | Growth inhibition of spoilage and pathogenic microorganisms; natural biopreservation; shelf-life extension | [1,4,6,36,65,106,107,108,109,110] |
| Chemical industry | Synthesis of polyethylene terephthalate (PET), cellulose acetate, polyvinyl acetate | Industrial oxidation systems using Acetobacter spp. or catalytic pathways | Acetic acid | Precursor for plastics, adhesives, and synthetic fibers; solvent in esterification and acetylation processes | [14,47,75,111,112] |
| Textile industry | Fiber dyeing and finishing, textile printing, degreasing | Industrial-grade acetic acid (chemical product) | Acetic acid | pH regulation in dye baths; mordant improving color fixation and fastness; cleaning and degreasing agent | [7,61,113] |
| Cosmetic industry | Hair care products, perfumes, skincare formulations | (industrial acetic acid and its esters) | Acetic acid, acetate esters, salts | Ingredient in shampoos and conditioners; pH adjuster; perfume component; antimicrobial additive | [47,61,95,104,110] |
| Pharmaceutical industry | Antiseptics, drug synthesis intermediates, solvents | (industrial acetic acid) | Acetic acid, acetyl derivatives | Reactant in acetylation and esterification; disinfectant and antimicrobial agent in medicinal preparations | [7,47,74,95,103,112] |
| Household and cleaning products | Window cleaners, dishwashing liquids, descalers | — | Diluted acetic acid | Removal of limescale, grease, and mineral deposits; eco-friendly cleaning and descaling agent | [61,75,95,103] |
In household and institutional applications, diluted acetic acid is widely used as an eco-friendly cleaning agent. It is a common component of window cleaners, dishwashing liquids, and descaling formulations, where it effectively removes grease, mineral deposits, limescale, and rust [61,90,98].
Taken together, these examples demonstrate that acetic fermentation is not limited to vinegar production but constitutes a versatile biotechnological platform linking food processing with chemical manufacturing, materials science, pharmaceuticals, and sustainable consumer products.
4. Butyric Fermentation
In contrast to acetic fermentation, which relies on aerobic ethanol oxidation, butyric fermentation proceeds strictly under anaerobic conditions and redirects carbohydrate-derived carbon toward reduced short-chain fatty acids.
4.1. Biochemical Pathway and Key Microorganisms
Butyric fermentation is an anaerobic pathway of carbohydrate degradation in which the main end product is butyric acid (butanoic acid, CH3CH2CH2COOH), a short-chain volatile fatty acid. This process is carried out primarily by bacteria of the genera Clostridium, Butyrivibrio and Butyribacterium. Butyrate production has also been described in Coprococcus, Eubacterium, Fusobacterium, Megasphaera, Roseburia and Sarcina. Among these, Clostridium species are of particular importance due to their high fermentative potential and capacity to synthesize butyric acid from diverse substrates, including glucose, xylose, lactose and glycerol [114,115,116].
Metabolically, butyric fermentation begins with glycolysis via the EMP pathway, yielding pyruvate. Under strict anaerobic conditions, pyruvate is oxidatively decarboxylated to acetyl-CoA by pyruvate:ferredoxin oxidoreductase (PFOR), which functionally replaces the pyruvate dehydrogenase complex. This conversion is accompanied by the release of CO2 and the generation of reduced ferredoxin, with excess reducing equivalents dissipated as molecular hydrogen (H2). Acetyl-CoA serves as a central branching point. A fraction is converted to acetic acid via phosphotransacetylase (PTA) and acetate kinase (AK), generating ATP through substrate-level phosphorylation. The remaining acetyl-CoA condenses to acetoacetyl-CoA and is subsequently reduced to butyryl-CoA via β-hydroxybutyryl-CoA and crotonyl-CoA. Key enzymes include thiolase, β-hydroxybutyryl-CoA dehydrogenase, crotonase and butyryl-CoA dehydrogenase (linked to an electron-transferring flavoprotein, ETF) [114,115,116,117].
In the terminal step, butyryl-CoA is converted to butyric acid either via phosphotransbutyrylase (PTB) and butyrate kinase (BUK), through a butyryl phosphate intermediate, or via a CoA-transferase (CTF) that transfers CoA to acetate, producing butyrate and regenerating acetyl-CoA (Figure 3). Only some Clostridium strains possess both routes, and their relative contributions to total butyrate formation remain incompletely resolved [115,116,118]. Up to three moles of ATP per mole of glucose can be generated in butyric fermentation, which is relatively high for an anaerobic process. Most Clostridium species are heterofermentative, producing butyrate, acetate and, in smaller quantities, ethanol or isopropanol [117,118]. These metabolic characteristics explain both the technological potential and the process-related challenges associated with butyric fermentation in industrial systems.
Recent advances in metabolic engineering and synthetic biology have substantially expanded the biotechnological potential of butyric fermentation, particularly in solventogenic and butyrate-producing Clostridium species. Engineering efforts have focused on redirecting carbon flux toward butyrate and butanol by modulating key nodes such as the acetyl-CoA branch point (acetate vs. butyrate formation), strengthening reducing equivalent availability (ferredoxin/NADH balance), and reducing competing pathways that lead to lactate, acetone, or excessive acetate formation [115,116,119,120]. At the same time, CRISPR/Cas-based genome editing, together with systems-level analyses (transcriptomics, proteomics, and genome-scale metabolic models), has enabled targeted modification of acidogenesis/solventogenesis regulation, improved tolerance to butyric acid and butanol, and enhanced utilization of diverse substrates, including lignocellulosic sugars and glycerol [121,122]. Adaptive laboratory evolution and dynamic pathway control are increasingly combined with in situ product removal strategies to mitigate end-product inhibition and improve process productivity, supporting the transition of butyric fermentation from a classical anaerobic pathway to a rationally optimized platform for the sustainable production of short-chain fatty acids and bio-based solvents [123,124,125].
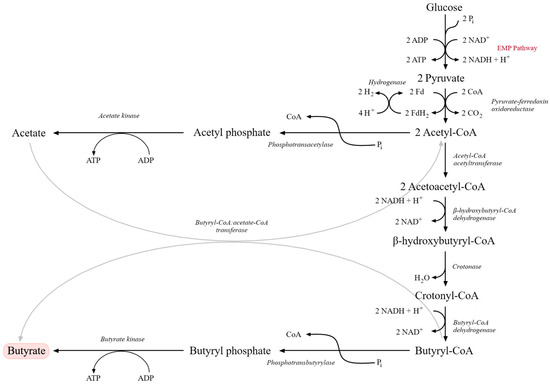
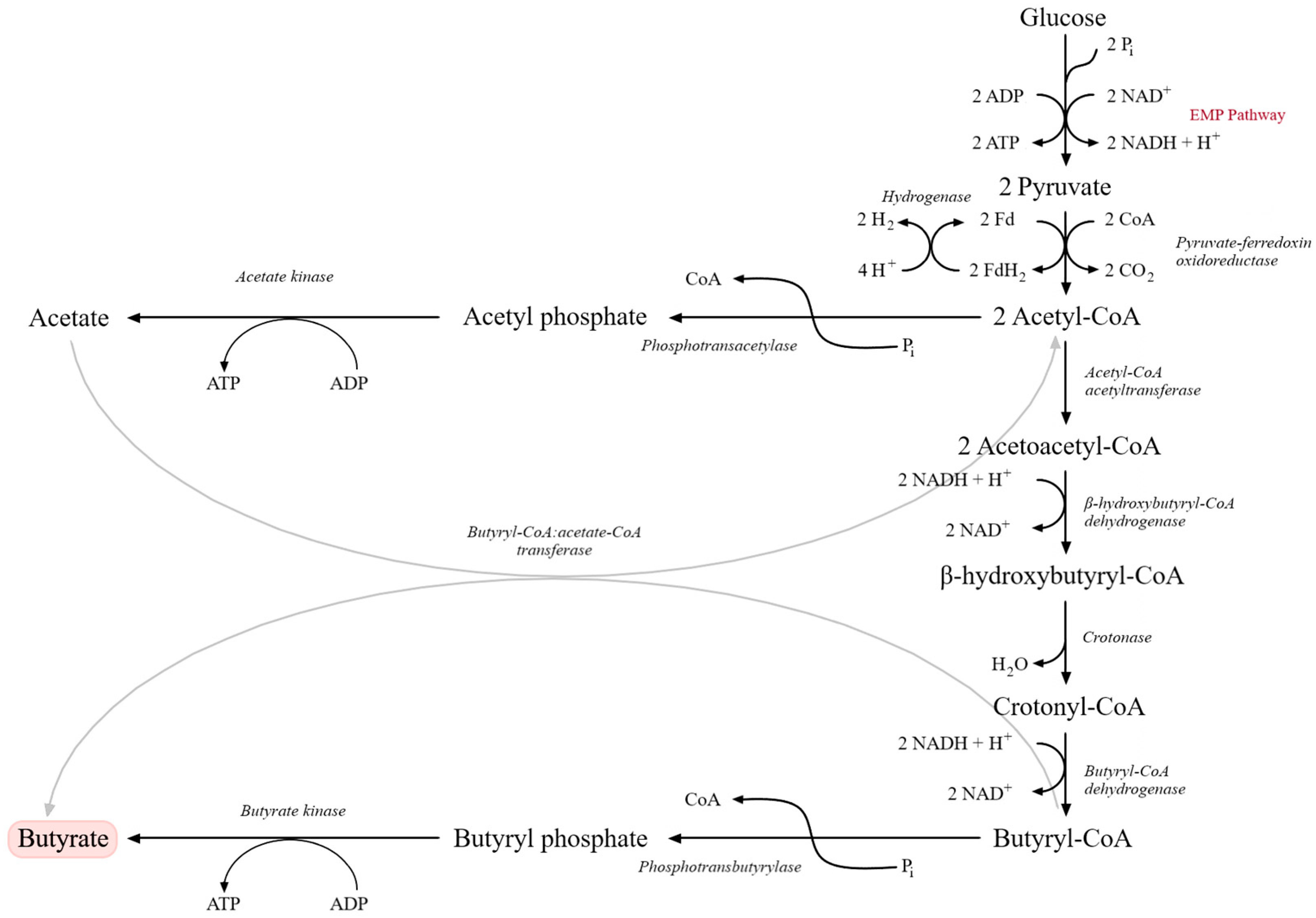

Figure 3.
Metabolic pathway of butyric acid fermentation originating from glucose catabolism via the Embden–Meyerhof–Parnas (EMP) pathway. Glucose is converted to pyruvate, which is subsequently transformed into acetyl-CoA and further metabolized through acetoacetyl-CoA, β-hydroxybutyryl-CoA, crotonyl-CoA, and butyryl-CoA intermediates. Final conversion into butyrate occurs via butyryl phosphate. Parallel reactions involving acetate formation and ATP generation through substrate-level phosphorylation are also shown. Key enzymatic steps and electron transfer reactions involved in NADH oxidation and hydrogen production are indicated (adapted from [120]).
Figure 3.
Metabolic pathway of butyric acid fermentation originating from glucose catabolism via the Embden–Meyerhof–Parnas (EMP) pathway. Glucose is converted to pyruvate, which is subsequently transformed into acetyl-CoA and further metabolized through acetoacetyl-CoA, β-hydroxybutyryl-CoA, crotonyl-CoA, and butyryl-CoA intermediates. Final conversion into butyrate occurs via butyryl phosphate. Parallel reactions involving acetate formation and ATP generation through substrate-level phosphorylation are also shown. Key enzymatic steps and electron transfer reactions involved in NADH oxidation and hydrogen production are indicated (adapted from [120]).

4.2. Industrial Applications
In industrial practice, butyric fermentation has been exploited across the food, chemical, energy, and pharmaceutical sectors due to its capacity to generate butyrate, solvents, and other value-added metabolites. Although pure butyric acid has a pungent odor, its esters exhibit pleasant fruity aromas and are widely used as flavoring agents in food, confectionery and perfumery. In dairy technology, uncontrolled butyric fermentation by Clostridium tyrobutyricum can cause late blowing in cheese, leading to quality defects and economic losses. Conversely, controlled butyrate formation contributes to characteristic flavor profiles in some traditional cheeses [126,127,128]. Representative industrial applications of butyric fermentation and butyric acid are summarized in Table 3.
In the chemical and materials industries, butyric acid and its derivatives are important intermediates in the synthesis of plastics and textile fibers, improving resistance to heat, light and mechanical stress. Cellulose acetate butyrate (CAB), a thermoplastic material derived from butyric acid, combines flexibility, UV stability and solubility in organic solvents, making it suitable for durable coatings, films and specialty polymers [47,114,116].
Butyric fermentation also underlies the acetone—butanol—ethanol (ABE) process, in which Clostridium acetobutylicum and C. beijerinckii produce solvents such as butanol, acetone and ethanol. Butanol is regarded as a promising advanced biofuel due to its high energy density and favorable combustion properties, emphasizing the importance of butyric fermentation in renewable energy systems [7,34,116].
Butyrate and its salts (e.g., sodium and calcium butyrate) are widely used in pharmaceuticals, animal nutrition, cosmetics and the synthesis of bioplastics and bioesters. In animal feed, butyrate-based additives serve as alternatives to antibiotic growth promoters, supporting gut health, nutrient absorption and immune function [75,114,115,129].
In environmental biotechnology, butyrate-producing bacteria participate in the degradation of biomass and organic waste, contributing to biogas generation and the production of organic acids that can be upgraded to biofuels or biochemical intermediates [75,123,130]. Clostridium butyricum is also considered a probiotic capable of producing butyrate in the colon, where it supports epithelial cell metabolism, exerts anti-inflammatory and anticarcinogenic effects, and promotes a beneficial microbiota composition [11,129,131].
In medicine, butyric acid has therapeutic potential in gastrointestinal diseases, colorectal cancer and hemoglobinopathies [132,133,134]. It induces differentiation and reduces proliferation of cancer cells, promotes regulatory T cell (Treg) generation and modulates immune responses [133,135,136]. By enhancing mitochondrial function, oxidative phosphorylation and fatty acid β-oxidation, butyrate also shows neuroprotective properties [137,138]. Several butyrate derivatives with antithyroid, anesthetic and vasoconstrictive activities have been developed, and new acyloxyalkyl butyrate prodrugs are under clinical investigation [139,140].


Table 3.
Representative applications of butyric fermentation and butyric acid in food, energy, chemical and pharmaceutical industries.
Table 3.
Representative applications of butyric fermentation and butyric acid in food, energy, chemical and pharmaceutical industries.
| Application Area | Example Products/Applications | Main Microorganisms | Fermentation Products/Effects | Technological Significance/Outcomes | Representative References |
|---|---|---|---|---|---|
| Food and flavor industry | Butter flavoring, cheese production, aroma compounds, fruit esters | Clostridium butyricum, Clostridium tyrobutyricum, Butyrivibrio fibrisolvens | Butyric acid, ethyl butyrate, butyl butyrate | Natural flavor generation (buttery, fruity notes); improvement of aroma profiles; undesirable in cheese spoilage (“late blowing”) | [43,114,115,117,128,141,142,143,144,145] |
| Chemical and materials industry | Cellulose acetate butyrate (CAB), plasticizers, textile fibers | Clostridium acetobutylicum, Clostridium beijerinckii | Butyric acid, butanol, acetone, esters | Synthesis of thermoplastics, coatings, and resins; improved material flexibility, UV stability, and solvent resistance | [25,33,47,74,75,111,114,143] |
| Biofuel and solvent production (ABE process) | Butanol, acetone, ethanol | Clostridium acetobutylicum, C. beijerinckii, C. pasteurianum | Butanol, acetone, ethanol | Production of renewable solvents and biofuels; butanol as a high-energy, low-volatility gasoline substitute | [14,33,47,75,111,112,114,143] |
| Animal nutrition and feed additives | Livestock feed, poultry supplements | Clostridium butyricum, Butyrivibrio fibrisolvens | Sodium butyrate, calcium butyrate | Replacement for antibiotic growth promoters; enhancement of gut health, nutrient absorption, and immunity | [45,100,112,115,128,134,146,147] |
| Pharmaceutical and medical applications | Therapeutics for gut disorders, cancer, hemoglobinopathies | Clostridium butyricum | Butyric acid, butyrate derivatives | Anti-inflammatory, anticarcinogenic, and neuroprotective effects; induction of cell differentiation; modulation of immune response | [43,100,115,128,134,146,148,149,150] |
| Probiotic and microbiome modulation | Probiotic supplements, intestinal health products | Clostridium butyricum, Butyrivibrio fibrisolvens | Butyric acid (SCFA) | Regulation of gut microbiota; stimulation of epithelial regeneration; trophic effect on colonocytes | [45,100,110,112,115,134,146,149,151,152] |
| Environmental biotechnology | Biogas and biohydrogen production, waste valorization | Clostridium butyricum, C. pasteurianum | H2, CO2, volatile fatty acids | Conversion of organic waste into biogas and organic acids; sustainable bioenergy recovery and waste reduction | [33,50,75,109,111,114,123,130,143,153] |
| Cosmetic industry | Skin and hair care formulations | Industrially derived butyric esters | Butyric esters, butyrate salts | Use in fragrance formulations; moisturizing and conditioning properties; pH regulation | [95,100,110,115,134,146] |
5. Lactic Fermentation
Unlike butyric fermentation, which primarily directs carbon toward reduced short-chain fatty acids and solvents, lactic fermentation channels carbohydrate metabolism toward lactate formation and rapid acidification of the environment.
5.1. Metabolic Pathways and Key Microbial Groups
Lactic acid fermentation is carried out by both homo- and heterofermentative lactic acid bacteria (LAB) [4,54,154]. Homolactic LAB metabolize hexoses (primarily glucose) via the EMP pathway (Figure 4), yielding two molecules of lactate per molecule of glucose [7,36,155,156]. Glucose is converted to pyruvate, which is reduced to lactate by lactate dehydrogenase with concomitant re-oxidation of NADH to NAD+, resulting in a net gain of two ATP per mole of glucose [157]. Strict homolactic fermentation of glucose does not generate CO2 [158]. Representative homolactic LAB include Lactiplantibacillus plantarum (formerly Lactobacillus plantarum), Pediococcus pentosaceus, Lactococcus lactis and Streptococcus thermophilus [112,142,156,159].
Homolactic pathway:
C6H12O6 (glucose) → 2 CH3-CHOH-COOH (lactic acid)
In contrast, heterofermentative LAB can ferment both hexoses and pentoses. For hexoses, they employ the phosphoketolase branch of the pentose phosphate pathway (PPP; Figure 3), which requires ribulose-5-phosphate 3-epimerase and phosphoketolase. This route typically yields equimolar lactate, ethanol (or acetate) and CO2 [106,157,158,160,161]. Obligate heterofermenters lack key EMP enzymes—most notably fructose-bisphosphate aldolase and triose phosphate isomerase—thus channeling carbon exclusively through the phosphoketolase pathway. Typical heterofermentative genera include Leuconostoc, Oenococcus, Levilactobacillus (e.g., L. brevis) and Limosilactobacillus (e.g., L. fermentum) [142,158,159,161].
Heterofermentative phosphoketolase pathway:
C6H12O6 (glucose) → CH3-CHOH-COOH (lactic acid) + C2H5OH (ethanol) + CO2 [106,157,161].
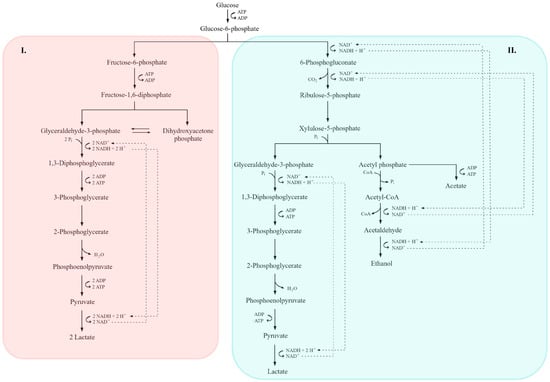
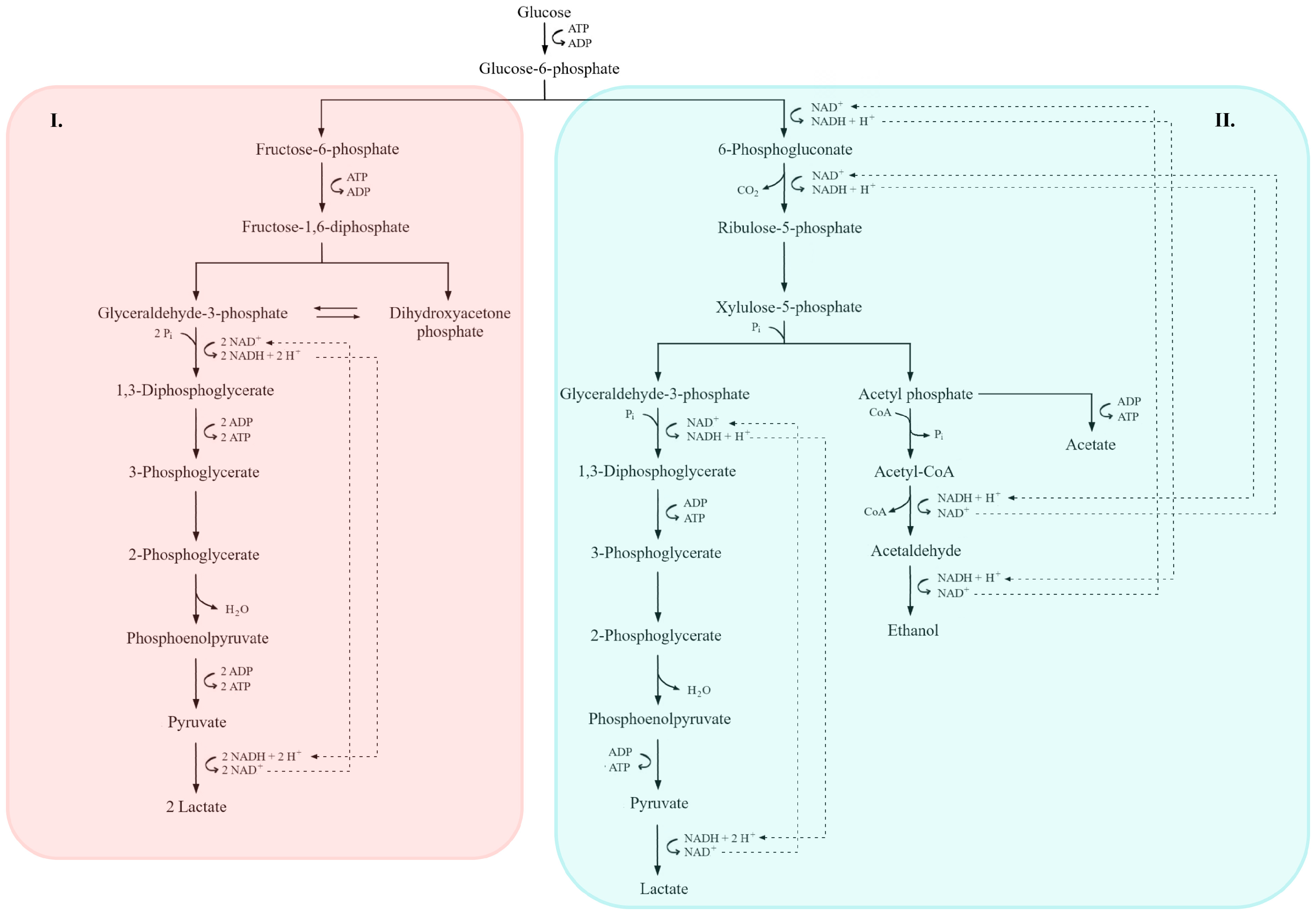

Figure 4.
Comparative overview of homofermentative (I) and heterofermentative (II) lactic acid fermentation pathways. In the homofermentative route (I), glucose is metabolized via the Embden–Meyerhof–Parnas (EMP) pathway, yielding pyruvate and subsequently lactate as the main end product, along with ATP generation. In the heterofermentative route (II), glucose is processed through the 6-phosphogluconate/phosphoketolase pathway, leading to the formation of lactate, ethanol or acetate, and CO2. Key intermediates, redox reactions, and ATP-yielding steps are indicated. Dashed lines denote NADH/NAD+ electron transfer pathways, while the shaded areas highlight the distinct metabolic branches of each fermentation type (adapted from [7,36,156]).
Figure 4.
Comparative overview of homofermentative (I) and heterofermentative (II) lactic acid fermentation pathways. In the homofermentative route (I), glucose is metabolized via the Embden–Meyerhof–Parnas (EMP) pathway, yielding pyruvate and subsequently lactate as the main end product, along with ATP generation. In the heterofermentative route (II), glucose is processed through the 6-phosphogluconate/phosphoketolase pathway, leading to the formation of lactate, ethanol or acetate, and CO2. Key intermediates, redox reactions, and ATP-yielding steps are indicated. Dashed lines denote NADH/NAD+ electron transfer pathways, while the shaded areas highlight the distinct metabolic branches of each fermentation type (adapted from [7,36,156]).

(In the presence of external electron acceptors, ethanol can be replaced by acetate with additional ATP formation) [158,161]. The balance between homo- and heterofermentative metabolism, ATP yield, and by-product formation directly influences both the technological performance of lactic acid bacteria and the characteristics of fermented products [106,142,160,161].
In recent years, lactic acid bacteria have become prominent targets of metabolic engineering and synthetic biology aimed at improving lactic acid yield, optical purity, robustness, and industrial scalability [155,156,158]. Engineering strategies have focused on controlling the stereospecificity of lactate dehydrogenases to selectively produce optically pure L- or D-lactic acid, which is critical for high-performance polylactic acid (PLA) synthesis [162]. Additional approaches include redirection of carbon flux toward lactate by minimizing by-product formation (e.g., ethanol, acetate, or CO2), enhancement of acid tolerance through membrane and stress-response engineering, and optimization of redox balance [112,157,159].
Advances in genome sequencing, CRISPR/Cas-based genome editing, and systems-level analyses have enabled precise modification of industrial LAB strains such as Lactiplantibacillus plantarum, Lactococcus lactis, and Streptococcus thermophilus. These tools have facilitated the development of strains with improved substrate utilization, resistance to bacteriophages, and stable performance under low-pH and high-product conditions [27,158,163]. Together with adaptive laboratory evolution and process-integrated control strategies, these innovations support the transition of lactic fermentation from a traditional food process to a rationally engineered platform for sustainable production of organic acids, biopolymers, and functional food ingredients [123,130].
5.2. Industrial and Health-Related Applications
From an industrial and technological perspective, lactic fermentation has been widely applied in food processing, health-oriented products, and industrial biotechnology due to its efficiency, safety, and functional metabolite profile. LAB convert carbohydrates, mainly glucose and lactose, into lactic acid and secondary metabolites such as diacetyl, acetaldehyde, hydrogen peroxide and bacteriocins [7,36,157,158]. These compounds contribute to the sensory profile, stability and microbiological safety of fermented foods by lowering pH and inhibiting pathogenic and spoilage microorganisms [110,142]. The major applications of lactic fermentation and lactic acid across food, medical, and materials-related sectors are summarized in Table 4.
Lactic fermentation underlies the production of numerous fermented foods, including dairy products (yogurt, kefir, buttermilk, cheese), vegetables (sauerkraut, kimchi, pickled cucumbers), meats (dry-cured sausages, salami), cereal-based products (sourdough) and plant-based beverages (soy, oat and beetroot drinks) [9,79]. LAB such as Lactobacillus delbrueckii subsp. bulgaricus, S. thermophilus, L. plantarum and Leuconostoc mesenteroides are essential for improving quality, nutritional value and sensory characteristics [129,142,159].
Beyond traditional fermentation, LAB are central to the development of functional and probiotic foods. Strains such as Lactobacillus rhamnosus GG, L. casei and Bifidobacterium bifidum are associated with health benefits, including support of the intestinal microbiota, enhanced mineral absorption, synthesis of B-group vitamins and modulation of immune responses [110,129,146,149,154,164,165]. In the food industry, lactic acid acts as a natural preservative, acidulant, pH regulator, flavor enhancer, cryoprotectant and prebiotic component [106,166]. Owing to its antibacterial and antioxidant properties, it is also used as a disinfectant and stabilizer in fermented food production [71,102,110,155,158].
In the chemical industry, lactic acid is a renewable platform chemical for the production of biopolymers, particularly polylactic acid (PLA), which is an environmentally friendly alternative to petroleum-based plastics. PLA is used in biodegradable films, fibers and packaging materials, contributing to green chemistry and circular economy strategies [7,14,47,50]. Lactic acid is also used in the synthesis of lactate esters, acrylic acid, propylene oxide, acetaldehyde and propylene glycol, which are widely applied in cosmetic, pharmaceutical and chemical products [47,155].
In dermatology and cosmetology, lactic acid is valued for its moisturizing, exfoliating and brightening properties. It is incorporated into anti-acne, anti-ageing and hydrating formulations as well as intimate hygiene products. Acting as a humectant, it binds water in the stratum corneum and improves skin elasticity and appearance [49,155,166].
In medicine and pharmacy, lactic acid and its salts (lactates) are widely used due to their biocompatibility, low toxicity and involvement in natural metabolic pathways [7,155]. They form key components of infusion solutions such as Ringer’s lactate, which help maintain acid–base balance and restore electrolytes in patients with dehydration or metabolic acidosis [7]. Calcium, magnesium and zinc lactates are used in dietary supplements and pharmaceutical formulations due to their high bioavailability and stabilizing properties [110,146,165].
A major pharmaceutical application of lactic acid is its role as a monomer for biodegradable polymers, including PLA and its copolymers such as poly(lactide-co-glycolide) (PLGA). These materials are used in biodegradable implants, surgical sutures and controlled drug delivery systems, enabling gradual and targeted release of active compounds, particularly in oncology, endocrinology and immunotherapy [7,14,36,47]. Lactic acid-derived materials are also used to fabricate biodegradable scaffolds for tissue engineering, supporting bone, muscle and skin regeneration [7,47,167]. Their biodegradability and renewable origin align with sustainable development principles and offer an eco-friendly alternative to conventional synthetic polymers [9,14,50,99,101].


Table 4.
Lactic fermentation and lactic acid: major applications in food, cosmetics, pharmaceuticals and materials science.
Table 4.
Lactic fermentation and lactic acid: major applications in food, cosmetics, pharmaceuticals and materials science.
| Application Area | Function/Role of Lactic Fermentation or Lactic Acid | Key Microorganisms/ Compounds | Industrial or Health Relevance | Selected References |
|---|---|---|---|---|
| Food Fermentation and Preservation | Conversion of carbohydrates (mainly glucose and lactose) into lactic acid, diacetyl, acetaldehyde, hydrogen peroxide, and bacteriocins; acidification of the environment inhibits spoilage and pathogenic microorganisms. | Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus, Lactiplantibacillus plantarum, Leuconostoc mesenteroides | Production of fermented foods (yogurt, kefir, cheese, sauerkraut, kimchi, sourdough); enhanced safety, shelf-life, and sensory quality. | [5,13,27,36,71,77,79,106,155,156,159,168,169,170,171,172,173,174] |
| Functional and Probiotic Foods | Support of intestinal microbiota; synthesis of B-group vitamins; enhancement of mineral absorption; immune modulation. | Lactobacillus rhamnosus GG, L. casei, Bifidobacterium bifidum | Development of health-promoting foods with probiotic activity; improvement of gastrointestinal and immune health. | [6,11,96,110,129,131,143,146,147,149,151,152,154,165,175,176,177,178,179,180,181,182,183,184,185] |
| Food Industry (Technological Additive) | Acts as a natural preservative, acidulant, pH regulator, flavor enhancer, cryoprotectant, and prebiotic component; provides antimicrobial and antioxidant protection. | Lactic acid; bacteriocins (e.g., nisin); hydrogen peroxide | Improves food quality, safety, and texture; stabilizes emulsions; inhibits spoilage flora. | [4,17,22,28,36,71,72,106,127,155,157,158,159,161,186,187,188] |
| Biopolymer Production | Precursor for polylactic acid (PLA) synthesis, an eco-friendly biodegradable polymer replacing petrochemical plastics. | Lactic acid (from Lactobacillus fermentation) | PLA used in films, fibers, and packaging materials; supports circular economy and green chemistry. | [7,14,25,33,43,47,74,75,124,161] |
| Chemical Industry | Intermediate for synthesis of lactate esters, acrylic acid, propylene oxide, acetaldehyde, and propylene glycol. | Lactic acid and its derivatives | Production of solvents, adhesives, surfactants, and coatings for cosmetics, pharmaceuticals, and polymers. | [7,14,47,74,75,155,161,166] |
| Cosmetics and Dermatology | Humectant, exfoliant, and brightening agent in skincare; promotes hydration, elasticity, and renewal of the stratum corneum. | Lactic acid and its salts | Used in anti-aging, anti-acne, and moisturizing formulations; improves skin tone and appearance. | [107,110,146,148,155] |
| Medicine and Pharmacy | Ingredient in infusion solutions (e.g., Ringer’s lactate); component of mineral supplements; stabilizer in drug formulations. | Calcium, magnesium, and zinc lactates | Rehydrates and maintains acid–base balance; enhances bioavailability of minerals. | [7,47,148,155] |
| Pharmaceutical Biotechnology | Building block for biodegradable polymers such as PLA and PLGA used in drug delivery systems, sutures, and implants. | Lactic acid monomers and copolymers | Enables controlled drug release, tissue compatibility, and gradual biodegradation in vivo. | [7,33,43,47,112,161] |
| Tissue Engineering and Regenerative Medicine | Source material for biodegradable scaffolds supporting bone, muscle, and skin regeneration. | PLA and its copolymers; lactic acid-based composites | Sustainable alternative to petrochemical polymers; biocompatible matrices for tissue growth. | [7,9,14,33,43,47,74,161] |
6. Propionic Fermentation
In contrast to lactic fermentation, propionic fermentation represents a metabolically linked process in which lactate and other reduced substrates are further converted into propionic acid, acetate, and CO2 under anaerobic conditions.
6.1. Wood–Werkman (Dicarboxylic Acid) Pathway and Key Microorganisms
Propionic acid fermentation is an anaerobic metabolic process in which substrates such as glucose, glycerol or lactic acid are converted into propionic acid, accompanied by acetic acid and CO2 formation [111,119]. This process is characteristic of several groups of anaerobic bacteria, among which Propionibacterium species have the highest industrial relevance. These microorganisms exhibit high fermentative efficiency and a broad substrate spectrum (Table 5) [189]. Other bacteria capable of propionic fermentation include Clostridium propionicum and selected species of Veillonella, Selenomonas, Megasphaera, Fusobacterium and Bacteroides, which typically show lower yields and a narrower substrate range [111,119].

Table 5.
Propionate-producing microorganisms, their primary substrates and fermentation products.
Stoichiometrically, propionic acid fermentation can be expressed as follows:
For glucose,
C6H12O6 → 4/3 CH3CH2COOH + 2/3 CH3COOH + 2/3 CO2 + 4/3 H2O + 4 ATP
For lactic acid,
CH3CHOHCOOH → 2/3 CH3CH2COOH + 1/3 CH3COOH + 1/3 CO2 + 2/3 H2O + ATP
Propionic acid can be produced via three main metabolic routes: (1) the Wood–(dicarboxylic acid) cycle, (2) the acrylate pathway and (3) the 1,2-propanediol pathway [189]. In Propionibacterium spp., the predominant route is the Wood– pathway. It starts from pyruvate, generated via glycolysis, which can follow two alternative routes:
(i) Carboxylation to oxaloacetate: this initiates the Wood–Werkman cycle and leads to propionic acid. (ii) Decarboxylation to acetyl-CoA: this serves as the precursor for acetic acid.
In the first route, pyruvate is converted to oxaloacetate in a biotin-dependent carboxyl transfer reaction catalyzed by methylmalonyl-CoA carboxytransferase, which transfers a carboxyl group from methylmalonyl-CoA to pyruvate, yielding oxaloacetate and propionyl-CoA. Oxaloacetate is then reduced to malate, dehydrated to fumarate and reduced to succinate. Succinate is converted to succinyl-CoA by succinyl-CoA synthetase and rearranged via a vitamin B12-dependent methylmalonyl-CoA mutase to methylmalonyl-CoA and back to propionyl-CoA. Finally, propionyl-CoA is converted to propionic acid with the release of CoA by a CoA-transferase [74,88,98,118,189,201].
Parallel to this, a portion of pyruvate may be converted into acetyl-CoA, followed by the formation of acetic acid. This occurs through pyruvate dehydrogenase complex–catalyzed decarboxylation of pyruvate to acetyl-CoA, which is subsequently converted into acetate via phosphotransacetylase (PTA) and acetate kinase (AK) reactions, yielding ATP as a byproduct. Consequently, acetic acid is produced as a secondary metabolite of propionic acid fermentation [88,98,118,189,201].
A schematic representation of the propionic acid fermentation pathway and the key enzymes involved is shown in Figure 5 [74,88,98,118,189]. While the Wood–Werkman pathway predominates in Propionibacterium species, alternative metabolic routes have evolved in other anaerobic bacteria to support propionate formation under different physiological and ecological conditions.
In recent years, propionibacteria have increasingly been explored as targets for metabolic engineering and systems-guided strain improvement to enhance propionic acid productivity, reduce by-product formation (especially acetate), and improve tolerance to product inhibition [111,119,189,201]. A major limitation of industrial propionate production is the strong inhibitory effect of propionic acid on cell growth and redox homeostasis; therefore, contemporary strategies focus on improving acid tolerance and export capacity, as well as optimizing intracellular redox balance and ATP conservation in the Wood–Werkman cycle [89,121,193]. In parallel, pathway-level interventions aimed at shifting carbon flux toward propionyl-CoA (and away from acetyl-CoA/acetate) have been investigated, including regulation of key nodes such as pyruvate utilization, succinate conversion steps, and CoA-transferase reactions [192,194,202].
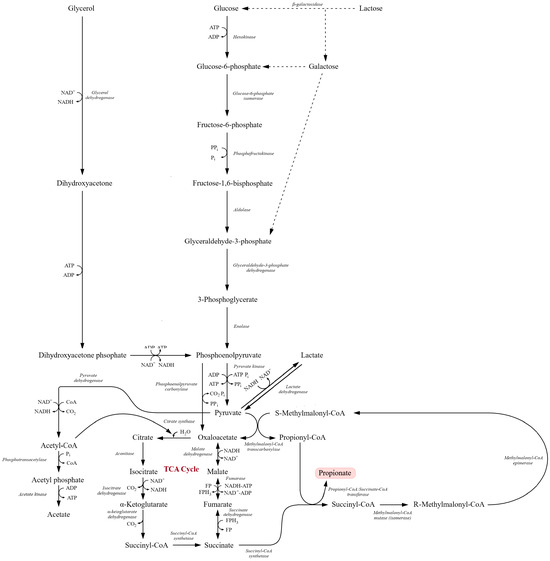
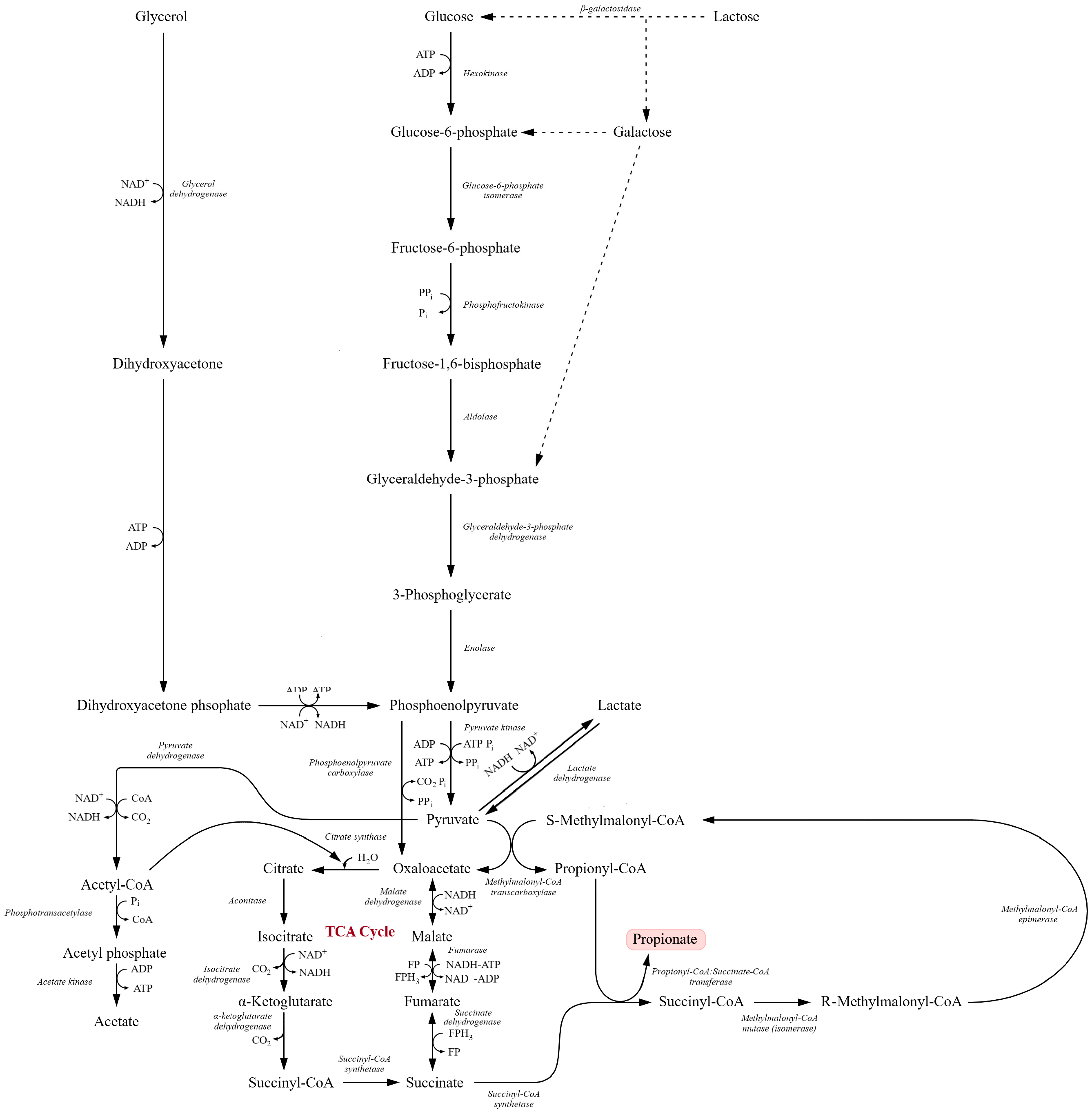

Figure 5.
Integrated metabolic pathways involved in the conversion of carbohydrates and glycerol into propionate via the succinate–propionate pathway. Glucose, galactose, and glycerol are converted to dihydroxyacetone phosphate and subsequently processed through glycolysis to phosphoenolpyruvate and pyruvate. Pyruvate is then metabolized through the tricarboxylic acid (TCA) cycle to oxaloacetate, malate, fumarate, succinate, and succinyl-CoA. Further reactions lead to the formation of methylmalonyl-CoA intermediates and ultimately propionyl-CoA and propionate. Key enzymatic steps, redox reactions, ATP-generating conversions, and metabolic branch points linking glycolysis, glycerol utilization, and the TCA cycle are indicated (adapted from [88,189,201]).
Figure 5.
Integrated metabolic pathways involved in the conversion of carbohydrates and glycerol into propionate via the succinate–propionate pathway. Glucose, galactose, and glycerol are converted to dihydroxyacetone phosphate and subsequently processed through glycolysis to phosphoenolpyruvate and pyruvate. Pyruvate is then metabolized through the tricarboxylic acid (TCA) cycle to oxaloacetate, malate, fumarate, succinate, and succinyl-CoA. Further reactions lead to the formation of methylmalonyl-CoA intermediates and ultimately propionyl-CoA and propionate. Key enzymatic steps, redox reactions, ATP-generating conversions, and metabolic branch points linking glycolysis, glycerol utilization, and the TCA cycle are indicated (adapted from [88,189,201]).

Advances in genome sequencing, metabolic modeling, and multi-omics analyses have improved understanding of propionibacterial physiology and facilitated rational process optimization, particularly for Propionibacterium freudenreichii and P. acidipropionici, which are also major industrial producers of vitamin B12 [189,191,203]. Emerging synthetic biology approaches—including targeted genome editing, adaptive laboratory evolution, and engineered co-cultures that couple lactate-producing LAB with propionate producers—are increasingly used to improve stability, yields, and substrate flexibility, supporting the development of propionic fermentation as a sustainable platform for food preservation and bio-based chemicals [28,123,204].
6.2. Alternative Propionate Pathways (Acrylate and 1,2-Propanediol)
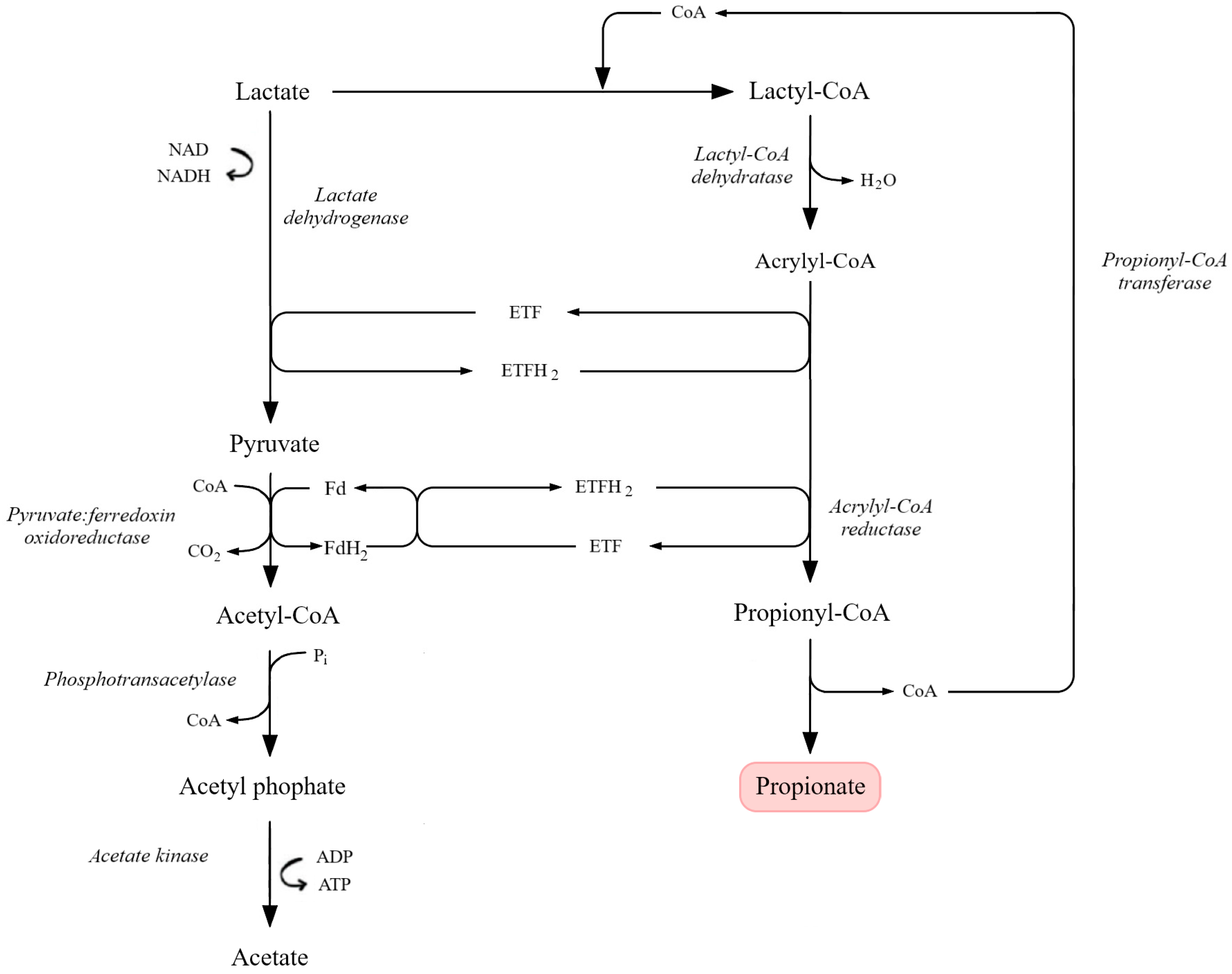
The acrylate pathway is an alternative propionic acid fermentation route used by some obligate anaerobes, notably C. propionicum. It begins with the conversion of pyruvate to lactate via L-lactate dehydrogenase. Propionyl-CoA transferase then transfers CoA from propionyl-CoA to lactate, producing lactoyl-CoA and releasing propionic acid, the main product of this pathway. Lactoyl-CoA is dehydrated by lactoyl-CoA dehydratase to acryloyl-CoA, a toxic intermediate. To minimize toxicity, acryloyl-CoA is rapidly reduced back to propionyl-CoA by acryloyl-CoA reductase. The pathway thus functions as a cyclic process with internal CoA recycling, in which propionyl-CoA transferase plays a central dual role (Figure 6) [88,189,193,201].
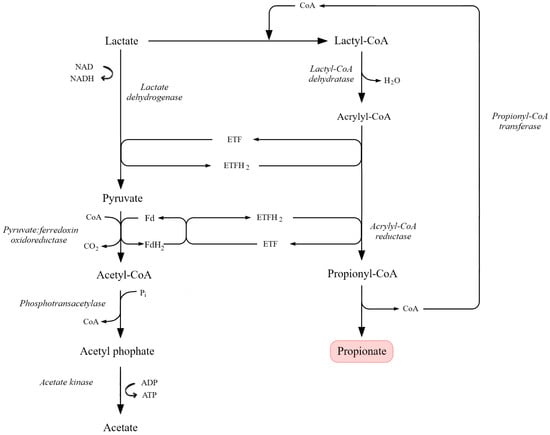
Figure 6.
Lactate-based acrylate pathway leading to propionate formation. Lactate is oxidized to pyruvate via lactate dehydrogenase, with subsequent conversion to acetyl-CoA by pyruvate:ferredoxin oxidoreductase. In the acrylate branch, lactyl-CoA is dehydrated to acrylyl-CoA, which is further reduced to propionyl-CoA by acrylyl-CoA reductase in an electron-transferring flavoprotein (ETF/ETFH2)-dependent system. Propionyl-CoA is then converted to propionate through CoA transferase activity. Parallel conversion of acetyl-CoA to acetate via acetyl phosphate also contributes to ATP generation. Key enzymatic reactions, redox cycles, and metabolite flow are indicated (adapted from [201]).
Because acryloyl-CoA is cytotoxic, bacteria must maintain its intracellular concentration at very low levels. This becomes particularly challenging at elevated pH, when redox balance is disturbed. Under such conditions, microorganisms redirect carbon flux toward acetic acid formation at the expense of propionic acid, rendering the acrylate pathway less efficient. The metabolic burden associated with rapid detoxification of acryloyl intermediates further reduces overall fermentation yield [89,189,201,202,205].
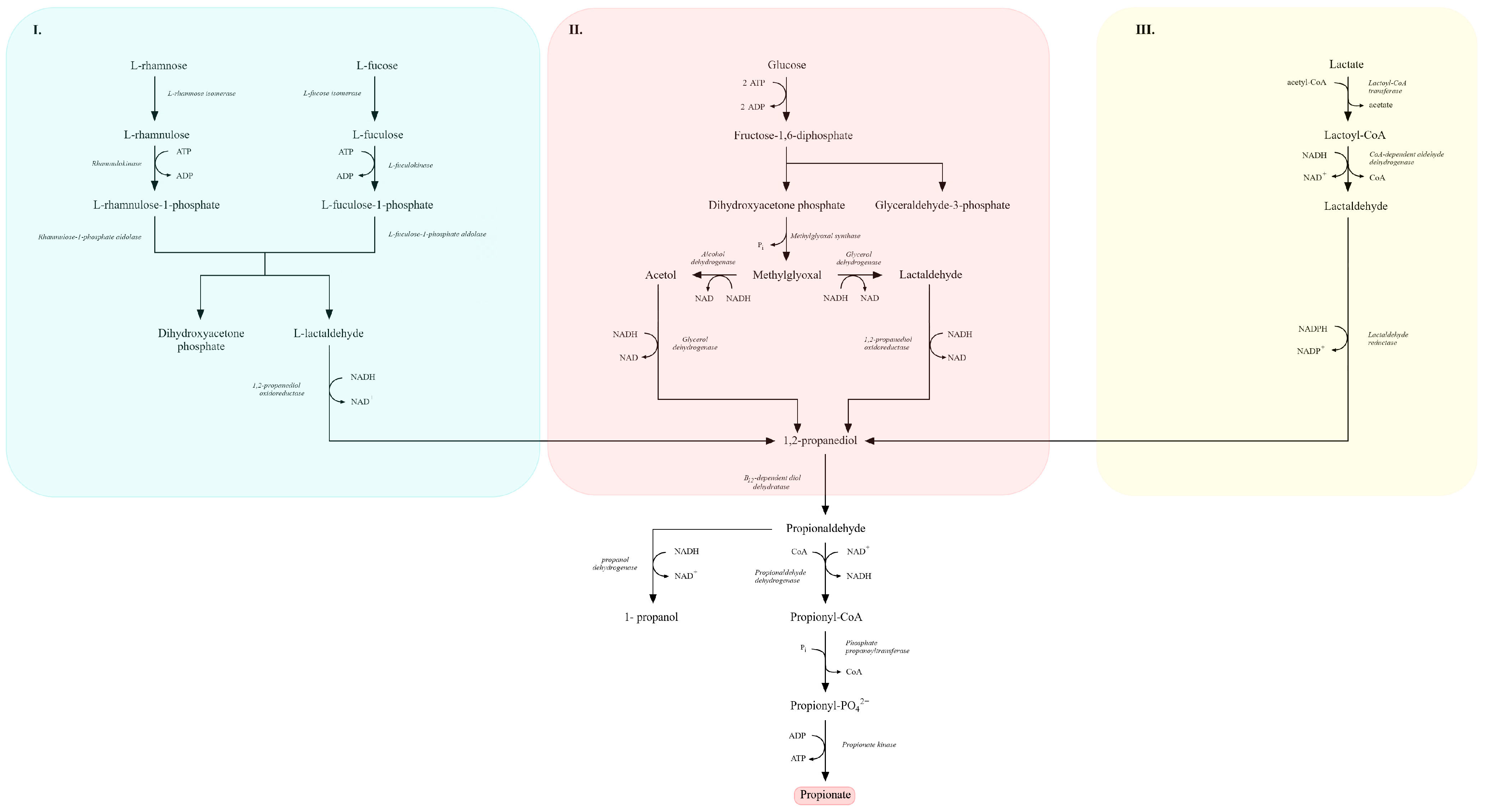
The 1,2-propanediol fermentation pathway constitutes another route to propionic acid. Several bacteria, including Salmonella enterica, Roseburia inulinivorans and representatives of Lactobacillus, Ruminococcus obeum and Bacteroides thetaiotaomicron, can synthesize 1,2-propanediol from various carbon sources such as deoxy sugars (e.g., fucose and rhamnose), dihydroxyacetone or lactic acid [122,206,207].
Three main microbial routes for 1,2-propanediol biosynthesis are described (Figure 7):
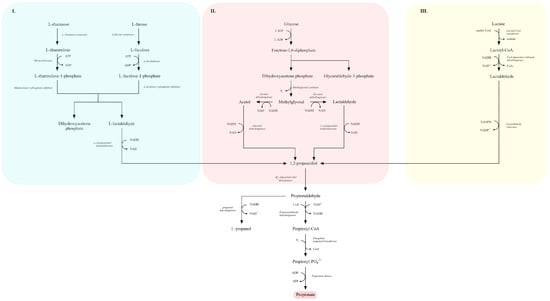
Figure 7.
Metabolic pathways leading to the formation of 1,2-propanediol and propionate from sugars, sugar-derived deoxyhexoses, and lactate. Panel (I) shows the catabolism of L-rhamnose and L-fucose to dihydroxyacetone phosphate and L-lactaldehyde via L-rhamnulose-1-phosphate and L-fuculose-1-phosphate intermediates. Panel (II) presents the conversion of glucose-derived triose phosphates into acetol, methylglyoxal, and lactaldehyde, followed by their reduction to 1,2-propanediol. Panel (III) illustrates the lactate-dependent pathway in which lactoyl-CoA and lactaldehyde serve as precursors for 1,2-propanediol formation. The central pathway depicts the downstream conversion of 1,2-propanediol to propionaldehyde, propionyl-CoA, propionyl-phosphate, and finally propionate. Key enzymatic steps, redox reactions, and metabolic branch points between carbohydrate, deoxyhexose, and lactate catabolism are indicated (adapted from [189,201]).
I. Deoxyhexose pathway—L-rhamnose or L-fucose is metabolized to 1,2-propanediol. For example,
L-fucose → L-fuculose → fuculose-1-phosphate → L-lactaldehyde → 1,2-propanediol, via L-fucose isomerase, L-fuculokinase, L-fuculose-1-phosphate aldolase and lactaldehyde reductase [122,207].
II. Methylglyoxal pathway—dihydroxyacetone phosphate (DHAP) is converted to methylglyoxal and subsequently reduced to 1,2-propanediol. Due to the cytotoxicity of methylglyoxal, this route is less favored [122,208].
III. Lactic acid pathway—lactic acid is converted to lactoyl-CoA, reduced to lactaldehyde and then to 1,2-propanediol. This pathway, often active under acidic conditions, is considered most attractive for industrial applications because it does not generate toxic intermediates and does not require expensive substrates [200,204].
After synthesis, 1,2-propanediol can be further converted to propionic acid. It is dehydrated to propionaldehyde by propanediol dehydratase, then oxidized to propionyl-CoA and reduced to propanol by propionaldehyde and propanol dehydrogenases, respectively. Propionyl-CoA is finally converted to propionyl phosphate and then to propionic acid by phosphotransacylase and propionate kinase [189,192,200].
The diversity of propionate-forming pathways, their energetic efficiency, and their dependence on substrate availability collectively determine the industrial and technological relevance of propionic fermentation.
6.3. Industrial Applications
Building on the metabolic frameworks described above, propionic fermentation has found widespread application in food processing, biotechnology, and chemical industries due to the preservative, functional, and platform-chemical properties of propionic acid [7,54,203]. The best-known example is the ripening of Swiss-type cheeses (e.g., Emmental, Maasdam, Gruyère), in which Propionibacterium freudenreichii subsp. shermanii converts lactic acid into propionic and acetic acids and CO2. Gas evolution forms the characteristic eyes in the cheese, while organic acids contribute to the nutty aroma and mild flavor. Propionic acid also inhibits molds and spoilage bacteria, extending shelf life [36,144]. An overview of the major industrial applications of propionic fermentation and propionic acid is provided in Table 6.

Table 6.
Industrial applications of propionic fermentation and propionic acid across food, chemical, cosmetic, polymer and pharmaceutical sectors.
In the wider food industry, propionic acid and its salts—sodium, calcium, potassium and ammonium propionates—are used as natural preservatives due to strong antibacterial and antifungal properties. They inhibit species such as Aspergillus flavus, Bacillus spp., Salmonella spp. and yeasts, and in combination with lactic and acetic acids effectively suppress Listeria monocytogenes and other foodborne pathogens [74,98,189]. Propionates are therefore used in bakery products, confectionery, cheeses and silage to improve microbial stability and safety.
Propionic acid (CH3CH2COOH) is a three-carbon carboxylic acid, miscible with water and many organic solvents, and has been granted GRAS status by the U.S. Food and Drug Administration. Beyond food, it is used in chemical, cosmetic, polymer and pharmaceutical industries [111,119]. In biotechnology, propionic fermentation serves as the basis for industrial vitamin B12 (cobalamin) production by P. freudenreichii and P. acidipropionici. Microbial cobalamin is widely used in pharmaceuticals and functional foods [79,189].
In the chemical industry, propionic acid acts as an important intermediate in the synthesis of various organic compounds, including herbicides and pesticides used in crop protection. Due to its chemical reactivity, it is also used as a precursor for the synthesis of propionate esters, which serve as components in protective coatings, industrial varnishes, and organic solvents. These esters are valued for their volatility, film-forming properties, and durability, making them crucial in the paint and coatings industry [74,98].
In the construction materials and cleaning products sector, propionic acid is applied for its antimicrobial activity. It is added to paints, adhesives, detergents, and impregnating agents to prevent the growth of bacteria, molds, and fungi on surfaces, thereby enhancing material durability and improving hygienic safety [119].
In the plastics industry, propionic acid and its esters are key intermediates for cellulose-based fibers and biodegradable polymers. Cellulose propionate combines favorable mechanical, optical and moisture-resistance properties, making it suitable for films, packaging, photographic layers and decorative components, and it is considered a next-generation biopolymer [100,111,209].
In the cosmetic industry, propionate salts are used as fragrance bases and natural preservatives in skincare formulations. When combined with butyl rubber, they improve the texture, elasticity, and stability of cosmetic emulsions, while their antimicrobial activity reduces the need for synthetic preservatives [119].
In pharmaceutical and veterinary applications, propionic acid and its salts—especially sodium propionate—are used as auxiliary and therapeutic substances. Due to their antibacterial and anti-inflammatory properties, they are applied in the treatment of skin infections, fungal diseases, and mucosal inflammations. Propionates are also components of ophthalmic preparations used to treat conjunctivitis and are included in antiseptic and anti-inflammatory drugs. In veterinary medicine, they are used in hygiene preparations for livestock, protecting against infections of the skin and hooves [74,98,119]. The multifunctionality of propionic acid and its derivatives makes them a crucial link between biotechnology, chemical, pharmaceutical, and material industries. Owing to their potential integration into sustainable bioproduction processes, propionic acid is considered a key biocomponent of the future, aligning with the principles of green chemistry and the circular economy [9,111].
7. Comparative Roles of Key Microorganisms Across Fermentation Systems
Having reviewed the biochemical pathways, microbial ecology, and industrial applications of alcoholic, acetic, butyric, lactic, and propionic fermentations, it becomes evident that although certain microorganisms occur across multiple fermentation systems, their metabolic roles and technological relevance differ substantially depending on ecological context and process conditions. S. cerevisiae exemplifies this functional plasticity, acting as the primary biocatalyst of alcoholic fermentation by converting sugars into ethanol and CO2 via fermentative glycolysis, while simultaneously generating a wide spectrum of aroma-active secondary metabolites that define beverage quality [8,20,26,57]. In contrast, in lactic and mixed-culture fermentations, yeasts such as S. cerevisiae, Kluyveromyces spp., and Torulaspora spp. typically play auxiliary roles, contributing to redox balance, oxygen scavenging, vitamin release, and the formation of volatile compounds that support lactic acid bacteria growth and enhance sensory complexity rather than serving as dominant fermentative agents [46,54,142,173].
Lactic acid bacteria represent the principal acidifying microorganisms in lactic fermentation, where they drive rapid pH reduction, microbial stabilization, and product safety through lactic acid and bacteriocin production [37,106,157,159]. However, in alcoholic and acetic fermentations, LAB generally constitute secondary microbiota, influencing microbial succession, flavor development, and maturation processes, particularly in wine, beer, and traditional fermented beverages [41,57,64,87]. A further context-dependent functional shift is observed for acetic acid bacteria, which dominate ethanol oxidation during acetic fermentation but appear as minor or late-stage contributors in alcoholic or mixed fermentations, where they modulate acidity and aroma rather than serving as primary producers [83,87,95].
In propionic fermentation, Propionibacterium species assume a specialized role by converting lactate into propionic and acetic acids and CO2 via the Wood–Werkman pathway, thereby linking lactic and propionic fermentations both metabolically and technologically, as exemplified by Swiss-type cheese ripening [126,189,193,195]. Collectively, these examples demonstrate that microbial functionality in fermentation cannot be interpreted solely at the species level but must be evaluated within a dynamic ecological framework shaped by substrate availability, interspecies interactions, and process design [27,54,173]. This integrative perspective provides a conceptual synthesis of microbial roles across fermentation systems and underpins the diverse applications discussed throughout this review.
8. Conclusions and Future Perspectives
Fermentation, one of humanity’s oldest biotechnological tools, remains central to both traditional food production and cutting-edge industrial biotechnology. This review has summarized the biochemical foundations, microbial ecology and industrial relevance of the five major fermentation types: alcoholic, acetic, butyric, lactic and propionic fermentation. Despite their diversity, these processes share common features: they enable energy conservation under anaerobic or microaerophilic conditions, generate organic acids and alcohols as key metabolites, and transform raw materials into products with enhanced shelf life, safety, sensory quality and functional value.
The diversity of microbial communities plays a decisive role in shaping the sensory properties, quality, and consistency of fermented products. In spontaneous and mixed-culture fermentations, complex consortia of yeasts, lactic acid bacteria, acetic acid bacteria, and other microorganisms engage in cross-feeding, metabolic cooperation, and competitive interactions that generate rich and distinctive flavor profiles. At the same time, this microbial diversity introduces variability and challenges for process standardization and reproducibility. Understanding and managing community dynamics therefore represents a key frontier in balancing product authenticity with industrial consistency.
Despite their long history and technological maturity, fermentation-based processes face several challenges onat industrialon an industrial scale. Key limitations include product inhibition by organic acids and solvents, trade-offs between yield and productivity, high downstream processing costs, and variability associated with complex feedstocks. Scale-up from laboratory to industrial bioreactors further requires careful control of mass transfer, redox balance, and microbial stability. Addressing these challenges relies on integrated strategies combining strain improvement, process optimization, in situ product recovery, and techno-economic assessment to ensure competitiveness with petrochemical alternatives. Many of these challenges are fermentation-specific and were highlighted in the individual sections above [28,33,121].
Recent advances in metabolic engineering and synthetic biology are reshaping classical fermentation processes. Engineered S. cerevisiae strains with optimized redox balance, inhibitor tolerance, and expanded substrate spectra are increasingly applied in alcoholic and mixed fermentations. Specifically, systems biology tools such as genome-scale metabolic models, flux balance analysis, and multi-omics integration have enabled the identification of metabolic bottlenecks, redox imbalances, and by-product formation pathways, which can then be rationally targeted by metabolic engineering. In lactic and propionic fermentations, genome-scale metabolic models and CRISPR-based tools enable targeted modulation of acid yields, cofactor regeneration, and by-product formation. Similarly, engineered Clostridium species are being developed to improve solvent selectivity and butyrate productivity while reducing acid toxicity. These approaches, supported by multi-omics integration and adaptive laboratory evolution, transform traditional fermentations into controllable microbial cell factories [24,25,156].
Alcoholic fermentation underpins the production of fermented beverages, baked goods and bioethanol, linking cultural traditions with modern energy and chemical industries. Acetic fermentation is essential for vinegar and low-alcohol beverages and provides acetic acid as a versatile platform chemical for polymers, textiles, cosmetics and pharmaceuticals. Butyric fermentation is both a challenge in some fermented foods and a powerful biotechnological route to short-chain fatty acids, solvents and advanced biofuels. Lactic fermentation remains the backbone of fermented foods and functional/probiotic products and supplies lactic acid for biodegradable polymers and medical biomaterials. Propionic fermentation closes the circle by supporting cheese ripening, vitamin B12 production and a wide range of chemical, cosmetic and pharmaceutical applications.
Looking forward, several trends are likely to shape the future of fermentation-based technologies:
- Strain engineering and synthetic biology will enable the design of microbial cell factories with enhanced productivity, substrate flexibility and stress tolerance, including engineered LAB, Saccharomyces, Zymomonas, Clostridium and Propionibacterium strains.
- Multi-omics and systems biology approaches will deepen understanding of microbial consortia in spontaneous and mixed-culture fermentations, allowing rational manipulation of community structure and function to optimize product profiles.
- Valorization of agro-industrial by-products will expand, using fermentation to convert low-value streams into high-value compounds such as organic acids, biopolymers, aroma compounds and biofuels, supporting circular bioeconomy strategies.
- From a sustainability perspective, fermentation technologies increasingly support circular and zero-waste bioeconomy concepts. Alcoholic and lactic fermentations are widely applied to valorize agro-industrial by-products such as whey, molasses, fruit pomace, and cereal residues into ethanol, lactic acid, and functional metabolites. Acetic acid bacteria enable the upgrading of ethanol-rich side streams into vinegar and gluconic acid, while propionic and butyric fermentations convert lactate- or glycerol-rich wastes into preservatives, biofuels, and platform chemicals. Integrated biorefineries combining multiple fermentation steps exemplify how classical processes can be coupled to achieve near-complete carbon utilization, reduced waste generation, and lower environmental impact. Key challenges in transitioning toward sustainable bioprocessing include the heterogeneity and seasonal variability of waste streams, the presence of inhibitory compounds, logistical constraints in feedstock collection, and the need for robust strains capable of efficiently utilizing mixed and low-cost substrates.
- Development of functional and personalized foods will increasingly rely on tailored fermentations and starter cultures with targeted health effects, including modulation of the gut microbiota, immune system and metabolic homeostasis.
In parallel, technological innovation and digitalization are transforming fermentation monitoring and control. The integration of real-time sensing technologies for pH, redox potential, dissolved gases, and key metabolites, combined with soft sensors, digital twins, machine learning, and artificial intelligence-driven control systems, enables predictive modeling, early fault detection, and dynamic optimization of fermentation conditions. Such data-driven approaches are particularly valuable for large-scale and mixed-culture fermentations, where they support improved reproducibility, scalability, resource efficiency, and economic performance. Together with process intensification strategies such as membrane bioreactors, in situ product recovery, and high-pressure processing, these tools contribute to more sustainable, robust, and competitive fermentation-based bioprocesses [27,33,173].
Author Contributions
Conceptualization, A.R. and W.G.; methodology, A.R.; investigation, A.R. and W.G.; data curation, W.G.; writing—original draft preparation, A.R. and W.G.; writing—review and editing, A.R.; visualization, W.G.; supervision, A.R.; project administration, A.R.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education, Poland, within the program “Nauka dla Społeczeństwa II”, project number NdS-II/SN/0476/2023/01, with a total funding amount of 1,811,910 PLN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An Overview of Fermentation in the Food Industry—Looking Back from a New Perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef]
- Todorovic, S.; Akpinar, A.; Assunção, R.; Bär, C.; Bavaro, S.L.; Berkel Kasikci, M.; Domínguez-Soberanes, J.; Capozzi, V.; Cotter, P.D.; Doo, E.-H.; et al. Health Benefits and Risks of Fermented Foods—The PIMENTO Initiative. Front. Nutr. 2024, 11, 1458536. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Antoniadou, Μ.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2020, 10, 69. [Google Scholar] [CrossRef]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Ray, R.C.; Joshi, V.K. Fermented Foods: Past, Present and Future; Fermented Foods (CRC Press); CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781466585769. [Google Scholar]
- Mannaa, M.; Han, G.; Seo, Y.-S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
- El-Mansi, M.; Nielsen, J.; Mousdale, D.M.; Allman, T.; Carlson, R. (Eds.) Fermentation Microbiology and Biotechnology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-1-138-58102-9. [Google Scholar]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Praveen, M.; Brogi, S. Microbial Fermentation in Food and Beverage Industries: Innovations, Challenges, and Opportunities. Foods 2025, 14, 114. [Google Scholar] [CrossRef]
- Taveira, I.C.; Nogueira, K.M.V.; Oliveira, D.L.G.D.; Silva, R.D.N. Fermentation: Humanity’s Oldest Biotechnological Tool. Front. Young Minds 2021, 9, 568656. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Schwan, R.F.; Bressani, A.P.P.; Martinez, S.J.; Batista, N.N.; Dias, D.R. The Essential Role of Spontaneous and Starter Yeasts in Cocoa and Coffee Fermentation. FEMS Yeast Res. 2023, 23, foad019. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A Review on Mechanisms and Commercial Aspects of Food Preservation and Processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Ruane, J.; Sonnino, A.; Agostini, A. Bioenergy and the Potential Contribution of Agricultural Biotechnologies in Developing Countries. Biomass Bioenergy 2010, 34, 1427–1439. [Google Scholar] [CrossRef]
- Boumba, V.A.; Ziavrou, K.S.; Vougiouklakis, T. Biochemical Pathways Generating Post-Mortem Volatile Compounds Co-Detected during Forensic Ethanol Analyses. Forensic Sci. Int. 2008, 174, 133–151. [Google Scholar] [CrossRef]
- Eknikom, S.; Nasuno, R.; Takagi, H. Molecular Mechanism of Ethanol Fermentation Inhibition via Protein Tyrosine Nitration of Pyruvate Decarboxylase by Reactive Nitrogen Species in Yeast. Sci. Rep. 2022, 12, 4664. [Google Scholar] [CrossRef]
- Malakar, S.; Paul, S.K.; Jolvis Pou, K.R. Biotechnological Interventions in Beverage Production. In Biotechnological Progress and Beverage Consumption; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–37. ISBN 978-0-12-816678-9. [Google Scholar]
- Aminian, A.; Motamedian, E. Investigating Ethanol Production Using the Zymomonas Mobilis Crude Extract. Sci. Rep. 2023, 13, 1165. [Google Scholar] [CrossRef]
- Yan, S. The Biochemical Basis of Ethanol Fermentation and Its Industrial Applications. Biol. Evid. 2024, 14, 238–249. [Google Scholar] [CrossRef]
- Zamora, F. Biochemistry of Alcoholic Fermentation. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 3–26. ISBN 978-0-387-74116-1. [Google Scholar]
- Guragain, Y.N.; Probst, K.V.; Vadlani, P.V. Fuel Alcohol Production. In Encyclopedia of Food Grains; Elsevier: Amsterdam, The Netherlands, 2016; pp. 235–244. ISBN 978-0-12-394786-4. [Google Scholar]
- Kopp, D.; Sunna, A. Alternative Carbohydrate Pathways—Enzymes, Functions and Engineering. Crit. Rev. Biotechnol. 2020, 40, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Ward, B. Bacterial Energy Metabolism. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 201–233. ISBN 978-0-12-397169-2. [Google Scholar]
- Xia, J.; Yang, Y.; Liu, C.-G.; Yang, S.; Bai, F.-W. Engineering Zymomonas Mobilis for Robust Cellulosic Ethanol Production. Trends Biotechnol. 2019, 37, 960–972. [Google Scholar] [CrossRef]
- Nielsen, J.; Larsson, C.; Van Maris, A.; Pronk, J. Metabolic Engineering of Yeast for Production of Fuels and Chemicals. Curr. Opin. Biotechnol. 2013, 24, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Chambers, P.J.; Pretorius, I.S. Fermenting Knowledge: The History of Winemaking, Science and Yeast Research. EMBO Rep. 2010, 11, 914–920. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Parente, E.; Ercolini, D. Metagenomics Insights into Food Fermentations. Microb. Biotechnol. 2017, 10, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, S.R.; Bernstein, H.C.; Song, H.-S.; Fredrickson, J.K.; Fields, M.W.; Shou, W.; Johnson, D.R.; Beliaev, A.S. Engineering Microbial Consortia for Controllable Outputs. ISME J. 2016, 10, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Maslanka, R.; Zadrag-Tecza, R. Reproductive Potential of Yeast Cells Depends on Overall Action of Interconnected Changes in Central Carbon Metabolism, Cellular Biosynthetic Capacity, and Proteostasis. Int. J. Mol. Sci. 2020, 21, 7313. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-J.; Qi, L.; Zhu, Y.-X.; He, M.; Xiang, Q.; Zheng, D.-Q. Spontaneous and Environment Induced Genomic Alterations in Yeast Model. Cell Insight 2025, 4, 100209. [Google Scholar] [CrossRef]
- Wan, Z.; Hu, H.; Liu, K.; Qiao, Y.; Guo, F.; Wang, C.; Xin, F.; Zhang, W.; Jiang, M. Engineering Industrial Yeast for Improved Tolerance and Robustness. Crit. Rev. Biotechnol. 2024, 44, 1461–1477. [Google Scholar] [CrossRef]
- Yook, S.; Alper, H.S. Recent Advances in Genetic Engineering and Chemical Production in Yeast Species. FEMS Yeast Res. 2025, 25, foaf009. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; Da Silva, S.S. The Path Forward for Lignocellulose Biorefineries: Bottlenecks, Solutions, and Perspective on Commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef]
- Haldar, D.; Shabbirahmed, A.M.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D.; Ponnusamy, V.K.; Patel, A.K. Understanding the Management of Household Food Waste and Its Engineering for Sustainable Valorization-A State-of-the-Art Review. Bioresour. Technol. 2022, 358, 127390. [Google Scholar] [CrossRef]
- Hu, M.; Bao, W.; Peng, Q.; Hu, W.; Yang, X.; Xiang, Y.; Yan, X.; Li, M.; Xu, P.; He, Q.; et al. Metabolic Engineering of Zymomonas Mobilis for Co-Production of D-Lactic Acid and Ethanol Using Waste Feedstocks of Molasses and Corncob Residue Hydrolysate. Front. Bioeng. Biotechnol. 2023, 11, 1135484. [Google Scholar] [CrossRef]
- Hui, Y.H.; Meunier-Goddik, L.; Josephsen, J.; Nip, W.-K.; Stanfield, P.S. (Eds.) Handbook of Food and Beverage Fermentation Technology; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-203-91355-0. [Google Scholar]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef]
- Tamang, J.P.; Kailasapathy, K. Fermented Foods and Beverages of the World; Food Science and Technology; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-9495-4. [Google Scholar]
- Estreicher, S.K. Wine and France: A Brief History. Eur. Rev. 2023, 31, 91–179. [Google Scholar] [CrossRef]
- Kaur, P.; Ghoshal, G.; Banerjee, U.C. Traditional Bio-Preservation in Beverages: Fermented Beverages. In Preservatives and Preservation Approaches in Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–113. ISBN 978-0-12-816685-7. [Google Scholar]
- Mas, A.; Portillo, M.C. Strategies for Microbiological Control of the Alcoholic Fermentation in Wines by Exploiting the Microbial Terroir Complexity: A Mini-Review. Int. J. Food Microbiol. 2022, 367, 109592. [Google Scholar] [CrossRef] [PubMed]
- Tzamourani, A.P.; Taliadouros, V.; Paraskevopoulos, I.; Dimopoulou, M. Developing a Novel Selection Method for Alcoholic Fermentation Starters by Exploring Wine Yeast Microbiota from Greece. Front. Microbiol. 2023, 14, 1301325. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive Phenolic Compounds: Production and Extraction by Solid-State Fermentation. A Review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Wittwer, A.; Howell, K. Rising Stars in the Bakery: Novel Yeasts for Modern Bread. Microbiol. Aust. 2022, 43, 75–78. [Google Scholar] [CrossRef]
- Chen, A.; Pan, C.; Chen, J. Comparative Analysis of Bread Quality Using Yeast Strains from Alcoholic Beverage Production. Microorganisms 2024, 12, 2609. [Google Scholar] [CrossRef]
- Lahue, C.; Madden, A.A.; Dunn, R.R.; Smukowski Heil, C. History and Domestication of Saccharomyces Cerevisiae in Bread Baking. Front. Genet. 2020, 11, 584718. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, H.U.; Choi, S.; Yi, J.; Lee, S.Y. Microbial Production of Building Block Chemicals and Polymers. Curr. Opin. Biotechnol. 2011, 22, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Vasileiadi, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces Cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Saha Turna, N.; Chung, R.; McIntyre, L. A Review of Biogenic Amines in Fermented Foods: Occurrence and Health Effects. Heliyon 2024, 10, e24501. [Google Scholar] [CrossRef]
- Nadar, C.G.; Fletcher, A.; Moreira, B.R.D.A.; Hine, D.; Yadav, S. Waste to Protein: A Systematic Review of a Century of Advancement in Microbial Fermentation of Agro-industrial Byproducts. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13375. [Google Scholar] [CrossRef]
- Subandi, S.; Ratih, N.K.; Soka, S.; Suwanto, A. Effect of Tempeh Supplementation on the Profiles of Human Intestinal Immune System and Gut Microbiota. Microbiol. Indones. 2017, 11, 11–17. [Google Scholar] [CrossRef]
- Ohya, Y.; Kashima, M. History, Lineage and Phenotypic Differentiation of Sake Yeast. Biosci. Biotechnol. Biochem. 2019, 83, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Romulo, A.; Surya, R. Tempe: A Traditional Fermented Food of Indonesia and Its Health Benefits. Int. J. Gastron. Food Sci. 2021, 26, 100413. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef] [PubMed]
- Le Jeune, C.; Erny, C.; Demuyter, C.; Lollier, M. Evolution of the Population of Saccharomyces Cerevisiae from Grape to Wine in a Spontaneous Fermentation. Food Microbiol. 2006, 23, 709–716. [Google Scholar] [CrossRef]
- Pretorius, I.S.; Bauer, F.F. Meeting the Consumer Challenge through Genetically Customized Wine-Yeast Strains. Trends Biotechnol. 2002, 20, 426–432. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Kievit, R.L.; Siebert, T.; Lattey, K.A.; Bramley, B.R.; Francis, I.L.; King, E.S.; Pretorius, I.S. The Influence of Yeast on the Aroma of Sauvignon Blanc Wine. Food Microbiol. 2009, 26, 204–211. [Google Scholar] [CrossRef]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling Microbial Ecology of Industrial-Scale Kombucha Fermentations by Metabarcoding and Culture-Based Methods. FEMS Microbiol. Ecol. 2017, 93, fix048. [Google Scholar] [CrossRef]
- Cousin, F.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.-M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The Evaluation of Chemical, Antioxidant, Antimicrobial and Sensory Properties of Kombucha Tea Beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; El-Shabasy, R.M.; Tahir, H.E.; Abo-Atya, D.M.; Saeed, A.; Abolibda, T.Z.; Guo, Z.; Zou, X.; Zhang, D.; Du, M.; et al. Vinegar—A Beneficial Food Additive: Production, Safety, Possibilities, and Applications from Ancient to Modern Times. Food Funct. 2024, 15, 10262–10282. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-Based Analysis of the Bacterial and Fungal Compositions of Multiple Kombucha (Tea Fungus) Samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Oliveira, Í.A.C.L.D.; Rolim, V.A.D.O.; Gaspar, R.P.L.; Rossini, D.Q.; De Souza, R.; Bogsan, C.S.B. The Technological Perspectives of Kombucha and Its Implications for Production. Fermentation 2022, 8, 185. [Google Scholar] [CrossRef]
- Roselli, G.E.; Kerruish, D.W.M.; Crow, M.; Smart, K.A.; Powell, C.D. The Two Faces of Microorganisms in Traditional Brewing and the Implications for No- and Low-Alcohol Beers. Front. Microbiol. 2024, 15, 1346724. [Google Scholar] [CrossRef]
- Tefon Öztürk, B.E.; Eroğlu, B.; Delik, E.; Çiçek, M.; Çiçek, E. Comprehensive Evaluation of Three Important Herbs for Kombucha Fermentation. Food Technol. Biotechnol. 2023, 61, 127–137. [Google Scholar] [CrossRef]
- Yuniarto, A.; Anggadiredja, K.; Annisa Nur Aqidah, R. Antifungal Activity of Kombucha Tea against Human Pathogenic Fungi. Asian J. Pharm. Clin. Res. 2016, 9, 253. [Google Scholar] [CrossRef]
- Brancoli, P.; Gmoser, R.; Taherzadeh, M.J.; Bolton, K. The Use of Life Cycle Assessment in the Support of the Development of Fungal Food Products from Surplus Bread. Fermentation 2021, 7, 173. [Google Scholar] [CrossRef]
- Lau, S.W.; Chong, A.Q.; Chin, N.L.; Talib, R.A.; Basha, R.K. Sourdough Microbiome Comparison and Benefits. Microorganisms 2021, 9, 1355. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, M.; Zare, L.; Khalili Sadrabad, E.; Hosseini Sharif Abad, A.; Mollakhalili-Meybodi, N.; Abedi, A.-S. The Impacts of Salt Reduction Strategies on Technological Characteristics of Wheat Bread: A Review. J. Food Sci. Technol. 2022, 59, 4141–4151. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of Phenolic Compounds by Lactobacillus Spp. during Fermentation of Cherry Juice and Broccoli Puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Nsogning Dongmo, S.; Procopio, S.; Sacher, B.; Becker, T. Flavor of Lactic Acid Fermented Malt Based Beverages: Current Status and Perspectives. Trends Food Sci. Technol. 2016, 54, 37–51. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.; Sachan, S. Enzymes Used in the Food Industry: Friends or Foes? In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 827–843. ISBN 978-0-12-813280-7. [Google Scholar]
- Zhao, G.; Ding, L.-L.; Yao, Y.; Cao, Y.; Pan, Z.-H.; Kong, D.-H. Extracellular Proteome Analysis and Flavor Formation During Soy Sauce Fermentation. Front. Microbiol. 2018, 9, 1872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ling, C.; Chen, Y.; Jiang, X.; Chen, G.-Q. Microbial Engineering for Easy Downstream Processing. Biotechnol. Adv. 2019, 37, 107365. [Google Scholar] [CrossRef] [PubMed]
- Thi, N.B.D.; Lin, C.-Y.; Kumar, G. Waste-to-Wealth for Valorization of Food Waste to Hydrogen and Methane towards Creating a Sustainable Ideal Source of Bioenergy. J. Clean. Prod. 2016, 122, 29–41. [Google Scholar] [CrossRef]
- Kusumoto, K.-I.; Yamagata, Y.; Tazawa, R.; Kitagawa, M.; Kato, T.; Isobe, K.; Kashiwagi, Y. Japanese Traditional Miso and Koji Making. J. Fungi 2021, 7, 579. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Rosenberg, D.; Zhao, H.; Lengyel, G.; Nadel, D. Fermented Beverage and Food Storage in 13,000 y-Old Stone Mortars at Raqefet Cave, Israel: Investigating Natufian Ritual Feasting. J. Archaeol. Sci. Rep. 2018, 21, 783–793. [Google Scholar] [CrossRef]
- Nout, M.J.R.; Kiers, J.L. Tempe Fermentation, Innovation and Functionality: Update into the Third Millenium. J. Appl. Microbiol. 2005, 98, 789–805. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef]
- Deshmukh, G.; Manyar, H. Production Pathways of Acetic Acid and Its Versatile Applications in the Food Industry. In Biotechnological Applications of Biomass; Peixoto Basso, T., Olitta Basso, T., Carlos Basso, L., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83881-180-8. [Google Scholar]
- Ge, Y.; Wu, Y.; Aihaiti, A.; Wang, L.; Wang, Y.; Xing, J.; Zhu, M.; Hong, J. The Metabolic Pathways of Yeast and Acetic Acid Bacteria During Fruit Vinegar Fermentation and Their Influence on Flavor Development. Microorganisms 2025, 13, 477. [Google Scholar] [CrossRef]
- Gomes, R.J.; Borges, M.D.F.; Rosa, M.D.F.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139–151. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xie, Z.; Zhang, H.; Liebl, W.; Toyama, H.; Chen, F. Oxidative Fermentation of Acetic Acid Bacteria and Its Products. Front. Microbiol. 2022, 13, 879246. [Google Scholar] [CrossRef]
- Mota, J.; Vilela, A. Aged to Perfection: The Scientific Symphony behind Port Wine, Vinegar, and Acetic Acid Bacteria. Fermentation 2024, 10, 200. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Hong, H. Classification of Acetic Acid Bacteria and Their Acid Resistant Mechanism. AMB Express 2021, 11, 29. [Google Scholar] [CrossRef]
- Vidra, A.; Németh, Á. Bio-Produced Acetic Acid: A Review. Period. Polytech. Chem. Eng. 2017, 62, 245–256. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of Acetic Acid Bacteria and Their Role in Vinegar and Fermented Beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef] [PubMed]
- Müller, V. Energy Conservation in Acetogenic Bacteria. Appl. Environ. Microbiol. 2003, 69, 6345–6353. [Google Scholar] [CrossRef]
- Buckel, W. Energy Conservation in Fermentations of Anaerobic Bacteria. Front. Microbiol. 2021, 12, 703525. [Google Scholar] [CrossRef]
- Shinjoh, M.; Toyama, H. Industrial Application of Acetic Acid Bacteria (Vitamin C and Others). In Acetic Acid Bacteria; Matsushita, K., Toyama, H., Tonouchi, N., Okamoto-Kainuma, A., Eds.; Springer: Tokyo, Japan, 2016; pp. 321–338. ISBN 978-4-431-55931-3. [Google Scholar]
- Härer, L.; Hilgarth, M.; Ehrmann, M.A. Comparative Genomics of Acetic Acid Bacteria within the Genus Bombella in Light of Beehive Habitat Adaptation. Microorganisms 2022, 10, 1058. [Google Scholar] [CrossRef]
- Nakano, S.; Ebisuya, H. Physiology of Acetobacter and Komagataeibacter spp.: Acetic Acid Resistance Mechanism in Acetic Acid Fermentation. In Acetic Acid Bacteria; Matsushita, K., Toyama, H., Tonouchi, N., Okamoto-Kainuma, A., Eds.; Springer: Tokyo, Japan, 2016; pp. 223–234. ISBN 978-4-431-55931-3. [Google Scholar]
- Pelicaen, R.; Weckx, S.; Gonze, D.; De Vuyst, L. Application of Comparative Genomics of Acetobacter Species Facilitates Genome-Scale Metabolic Reconstruction of the Acetobacter ghanensis LMG 23848T and Acetobacter Senegalensis 108B Cocoa Strains. Front. Microbiol. 2022, 13, 1060160. [Google Scholar] [CrossRef]
- Song, J.; Wang, J.; Wang, X.; Zhao, H.; Hu, T.; Feng, Z.; Lei, Z.; Li, W.; Zheng, Y.; Wang, M. Improving the Acetic Acid Fermentation of Acetobacter pasteurianus by Enhancing the Energy Metabolism. Front. Bioeng. Biotechnol. 2022, 10, 815614. [Google Scholar] [CrossRef]
- Román-Camacho, J.J.; García-García, I.; Santos-Dueñas, I.M.; García-Martínez, T.; Mauricio, J.C. Latest Trends in Industrial Vinegar Production and the Role of Acetic Acid Bacteria: Classification, Metabolism, and Applications—A Comprehensive Review. Foods 2023, 12, 3705. [Google Scholar] [CrossRef]
- Chakraborty, R.; Roy, S. Exploration of the Diversity and Associated Health Benefits of Traditional Pickles from the Himalayan and Adjacent Hilly Regions of Indian Subcontinent. J. Food Sci. Technol. 2018, 55, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Budiman, A.W.; Nam, J.S.; Park, J.H.; Mukti, R.I.; Chang, T.S.; Bae, J.W.; Choi, M.J. Review of Acetic Acid Synthesis from Various Feedstocks Through Different Catalytic Processes. Catal. Surv. Asia 2016, 20, 173–193. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, Z.; Jiang, L. Acetic and Propionic Acids. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 189–199. ISBN 978-0-08-088504-9. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Ngai, T. Recent Advances in Chemically Modified Cellulose and Its Derivatives for Food Packaging Applications: A Review. Polymers 2022, 14, 1533. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of Microbial Enzymes in Food Industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef]
- Johnston, C.S.; Steplewska, I.; Long, C.A.; Harris, L.N.; Ryals, R.H. Examination of the Antiglycemic Properties of Vinegar in Healthy Adults. Ann. Nutr. Metab. 2010, 56, 74–79. [Google Scholar] [CrossRef]
- Yanagihara, N.; Mayumi, M.; Yoshikawa, J.; Akuzawa, S.; Fujii, A.; Nagano, M.; Koizumi, Y.; Maehashi, K. Flavor Assessment of a Lactic Fermented Vinegar Described in Japanese Books from the Edo Period (1603–1867). Heliyon 2024, 10, e32344. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Simpson, D.J.; Gänzle, M.G. A Phylogenetic Perspective on the Performance of Lactococcus Lactis as Starter Culture in Milk and Plant Milks. Food Res. Int. 2025, 221, 117562. [Google Scholar] [CrossRef]
- Caplice, E. Food Fermentations: Role of Microorganisms in Food Production and Preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented Foods: An Update on Evidence-Based Health Benefits and Future Perspectives. Food Res. Int. 2022, 156, 111133. [Google Scholar] [CrossRef]
- Raak, C.; Ostermann, T.; Boehm, K.; Molsberger, F. Regular Consumption of Sauerkraut and Its Effect on Human Health: A Bibliometric Analysis. Glob. Adv. Health Med. 2014, 3, 12–18. [Google Scholar] [CrossRef]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products Through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, R.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.; Marcellin, E. Microbial Propionic Acid Production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Awad, H.M.; Diaz, R.; Malek, R.A.; Othman, N.Z.; Aziz, R.A.; El Enshasy, H.A. Efficient Production Process for Food Grade Acetic Acid by Acetobacter Aceti in Shake Flask and in Bioreactor Cultures. J. Chem. 2012, 9, 2275–2286. [Google Scholar] [CrossRef]
- Huang, J.; Tang, W.; Zhu, S.; Du, M. Biosynthesis of Butyric Acid by Clostridium Tyrobutyricum. Prep. Biochem. Biotechnol. 2018, 48, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Fu, H.; Yang, H.K.; Xu, W.; Wang, J.; Yang, S.-T. Butyric Acid: Applications and Recent Advances in Its Bioproduction. Biotechnol. Adv. 2018, 36, 2101–2117. [Google Scholar] [CrossRef]
- Zigová, J.; Šturdík, E. Advances in Biotechnological Production of Butyric Acid. J. Ind. Microbiol. Biotechnol. 2000, 24, 153–160. [Google Scholar] [CrossRef]
- Wong, P.Y.Y.; Kitts, D.D. Chemistry of Buttermilk Solid Antioxidant Activity. J. Dairy Sci. 2003, 86, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F.; Mannazzu, I. Fermentation. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 310–321. ISBN 978-0-444-64130-4. [Google Scholar]
- Ahmadi, N.; Khosravi-Darani, K.; Mortazavian, A.M. An Overview of Biotechnological Production of Propionic Acid: From Upstream to Downstream Processes. Electron. J. Biotechnol. 2017, 28, 67–75. [Google Scholar] [CrossRef]
- Bao, T.; Feng, J.; Jiang, W.; Fu, H.; Wang, J.; Yang, S.-T. Recent Advances in N-Butanol and Butyrate Production Using Engineered Clostridium Tyrobutyricum. World J. Microbiol. Biotechnol. 2020, 36, 138. [Google Scholar] [CrossRef]
- Folch, P.L.; Bisschops, M.M.M.; Weusthuis, R.A. Metabolic Energy Conservation for Fermentative Product Formation. Microb. Biotechnol. 2021, 14, 829–858. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Bu, C.; Zou, L.; Hu, Y.; Zheng, Z.-J.; Ouyang, J. A Comprehensive Review on Microbial Production of 1,2-Propanediol: Micro-Organisms, Metabolic Pathways, and Metabolic Engineering. Biotechnol. Biofuels 2021, 14, 216. [Google Scholar] [CrossRef]
- Torres-León, C.; Chávez-González, M.L.; Hernández-Almanza, A.; Martínez-Medina, G.A.; Ramírez-Guzmán, N.; Londoño-Hernández, L.; Aguilar, C.N. Recent Advances on the Microbiological and Enzymatic Processing for Conversion of Food Wastes to Valuable Bioproducts. Curr. Opin. Food Sci. 2021, 38, 40–45. [Google Scholar] [CrossRef]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid State Fermentation (SSF): Diversity of Applications to Valorize Waste and Biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Azimi, H.; Tezel, H.; Thibault, J. Optimization of the in Situ Recovery of Butanol from ABE Fermentation Broth via Membrane Pervaporation. Chem. Eng. Res. Des. 2019, 150, 49–64. [Google Scholar] [CrossRef]
- Oštarić, F.; Antunac, N.; Cubric-Curik, V.; Curik, I.; Jurić, S.; Kazazić, S.; Kiš, M.; Vinceković, M.; Zdolec, N.; Špoljarić, J.; et al. Challenging Sustainable and Innovative Technologies in Cheese Production: A Review. Processes 2022, 10, 529. [Google Scholar] [CrossRef]
- Puri, R.; Bot, F.; Singh, U.; O’Mahony, J.A. Influence of Transglutaminase Crosslinking on Casein Protein Fractionation during Low Temperature Microfiltration. Foods 2021, 10, 3146. [Google Scholar] [CrossRef]
- Salque, M.; Bogucki, P.I.; Pyzel, J.; Sobkowiak-Tabaka, I.; Grygiel, R.; Szmyt, M.; Evershed, R.P. Earliest Evidence for Cheese Making in the Sixth Millennium Bc in Northern Europe. Nature 2013, 493, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Kok, C.R.; Hutkins, R. Yogurt and Other Fermented Foods as Sources of Health-Promoting Bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef]
- Tropea, A. Food Waste Valorization. Fermentation 2022, 8, 168. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeong, D.; Kim, H.; Seo, K.-H. Modern Perspectives on the Health Benefits of Kefir in next Generation Sequencing Era: Improvement of the Host Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 1782–1793. [Google Scholar] [CrossRef]
- Canani, R.B. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review Article: The Role of Butyrate on Colonic Function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Kuchta-Noctor, A.M.; Murray, B.A.; Stanton, C.; Devery, R.; Kelly, P.M. Anticancer Activity of Buttermilk Against SW480 Colon Cancer Cells Is Associated with Caspase-Independent Cell Death and Attenuation of Wnt, Akt, and ERK Signaling. Nutr. Cancer 2016, 68, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, Neuroepigenetics and the Gut Microbiome: Can a High Fiber Diet Improve Brain Health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Van De Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The Neuropharmacology of Butyrate: The Bread and Butter of the Microbiota-Gut-Brain Axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef] [PubMed]
- Blank-Porat, D.; Gruss-Fischer, T.; Tarasenko, N.; Malik, Z.; Nudelman, A.; Rephaeli, A. The Anticancer Prodrugs of Butyric Acid AN-7 and AN-9, Possess Antiangiogenic Properties. Cancer Lett. 2007, 256, 39–48. [Google Scholar] [CrossRef]
- Han, A.; Bennett, N.; Ahmed, B.; Whelan, J.; Donohoe, D.R. Butyrate Decreases Its Own Oxidation in Colorectal Cancer Cells through Inhibition of Histone Deacetylases. Oncotarget 2018, 9, 27280–27292. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017; ISBN 978-1-4899-7679-6. [Google Scholar]
- Gobbetti, M. The Sourdough Microflora: Interactions of Lactic Acid Bacteria and Yeasts. Trends Food Sci. Technol. 1998, 9, 267–274. [Google Scholar] [CrossRef]
- Huang, W.; Dong, A.; Pham, H.T.; Zhou, C.; Huo, Z.; Wätjen, A.P.; Prakash, S.; Bang-Berthelsen, C.H.; Turner, M.S. Evaluation of the Fermentation Potential of Lactic Acid Bacteria Isolated from Herbs, Fruits and Vegetables as Starter Cultures in Nut-Based Milk Alternatives. Food Microbiol. 2023, 112, 104243. [Google Scholar] [CrossRef]
- Thierry, A.; Maillard, M.-B. Production of Cheese Flavour Compounds Derived from Amino Acid Catabolism by Propionibacterium freudenreichii. Le Lait 2002, 82, 17–32. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, X.; Wang, B. A Review on the General Cheese Processing Technology, Flavor Biochemical Pathways and the Influence of Yeasts in Cheese. Front. Microbiol. 2021, 12, 703284. [Google Scholar] [CrossRef]
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceño-Casarrubias, C.E.; Sánchez Pardo, M.E.; Bermúdez-Humarán, L.G. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef]
- Turkmen, N.; Akal, C.; Özer, B. Probiotic Dairy-Based Beverages: A Review. J. Funct. Foods 2019, 53, 62–75. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.; Wang, C.; Ding, L.; Zhao, F.; Wang, M.; Fu, J.; Wang, H. Megasphaera Elsdenii Lactate Degradation Pattern Shifts in Rumen Acidosis Models. Front. Microbiol. 2019, 10, 162. [Google Scholar] [CrossRef]
- Soemarie, Y.B.; Milanda, T.; Barliana, M.I. Fermented Foods as Probiotics: A Review. J. Adv. Pharm. Technol. Res. 2021, 12, 335–339. [Google Scholar] [CrossRef]
- Thomas, T.A.; Deters, A.; Miller, A.C.; Loh, H.Y.; Engle, T.E.; Nagaraja, T.G. PSXII-29 Development of an in Vitro Rumen Fermentation Model to Assess Fusobacterium necrophorum Growth Inhibition. J. Anim. Sci. 2023, 101, 641–642. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Lisko, D.; Johnston, G.; Johnston, C. Effects of Dietary Yogurt on the Healthy Human Gastrointestinal (GI) Microbiome. Microorganisms 2017, 5, 6. [Google Scholar] [CrossRef]
- Tropea, A.; Potortì, A.G.; Lo Turco, V.; Russo, E.; Vadalà, R.; Rando, R.; Di Bella, G. Aquafeed Production from Fermented Fish Waste and Lemon Peel. Fermentation 2021, 7, 272. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Hashemi, S.M.B. Lactic Acid Production—Producing Microorganisms and Substrates Sources-State of Art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Aguirre-Garcia, Y.L.; Nery-Flores, S.D.; Campos-Muzquiz, L.G.; Flores-Gallegos, A.C.; Palomo-Ligas, L.; Ascacio-Valdés, J.A.; Sepúlveda-Torres, L.; Rodríguez-Herrera, R. Lactic Acid Fermentation in the Food Industry and Bio-Preservation of Food. Fermentation 2024, 10, 168. [Google Scholar] [CrossRef]
- Carr, F.J.; Chill, D.; Maida, N. The Lactic Acid Bacteria: A Literature Survey. Crit. Rev. Microbiol. 2002, 28, 281–370. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Lactic Acid Bacteria as Starter Cultures: An Update in Their Metabolism and Genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. Lactic Acid Bacteria as Functional Starter Cultures for the Food Fermentation Industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic Metabolism Revisited: Metabolism of Lactic Acid Bacteria in Food Fermentations and Food Spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Liu, S. Practical Implications of Lactate and Pyruvate Metabolism by Lactic Acid Bacteria in Food and Beverage Fermentations. Int. J. Food Microbiol. 2003, 83, 115–131. [Google Scholar] [CrossRef]
- Zheng, Z.; Sheng, B.; Ma, C.; Zhang, H.; Gao, C.; Su, F.; Xu, P. Relative Catalytic Efficiency of ldhL- and ldhD-Encoded Products Is Crucial for Optical Purity of Lactic Acid Produced by Lactobacillus Strains. Appl. Environ. Microbiol. 2012, 78, 3480–3483. [Google Scholar] [CrossRef]
- Leenay, R.T.; Vento, J.M.; Shah, M.; Martino, M.E.; Leulier, F.; Beisel, C.L. Genome Editing with CRISPR-Cas9 in Lactobacillus plantarum Revealed That Editing Outcomes Can Vary Across Strains and Between Methods. Biotechnol. J. 2019, 14, 1700583. [Google Scholar] [CrossRef]
- Leite, A.M.D.O.; Miguel, M.A.L.; Peixoto, R.S.; Rosado, A.S.; Silva, J.T.; Paschoalin, V.M.F. Microbiological, Technological and Therapeutic Properties of Kefir: A Natural Probiotic Beverage. Braz. J. Microbiol. 2013, 44, 341–349. [Google Scholar] [CrossRef]
- Rastogi, Y.R.; Thakur, R.; Thakur, P.; Mittal, A.; Chakrabarti, S.; Siwal, S.S.; Thakur, V.K.; Saini, R.V.; Saini, A.K. Food Fermentation—Significance to Public Health and Sustainability Challenges of Modern Diet and Food Systems. Int. J. Food Microbiol. 2022, 371, 109666. [Google Scholar] [CrossRef]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A Boon for Production of Bioactive Compounds by Processing of Food Industries Wastes (By-Products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef]
- Gunatillake, P.; Adhikari, R. Biodegradable Synthetic Polymers for Tissue Engineering. Eur. Cell. Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Delgado, S.; Guadamuro, L.; Flórez, A.B.; Vázquez, L.; Mayo, B. Fermentation of Commercial Soy Beverages with Lactobacilli and Bifidobacteria Strains Featuring High β-Glucosidase Activity. Innov. Food Sci. Emerg. Technol. 2019, 51, 148–155. [Google Scholar] [CrossRef]
- Fisberg, M.; Machado, R. History of Yogurt and Current Patterns of Consumption. Nutr. Rev. 2015, 73, 4–7. [Google Scholar] [CrossRef]
- Pame, K.; Laskar, S.; Borah, S. Utilization of Processed Animal Byproducts as a Raw Material to Develop Shelf-Stable and Cost Effective Pet Food. Int. J. Vet. Sci. Anim. Husb. 2023, 8, 31–34. [Google Scholar] [CrossRef]
- Read, J. Living Fermented Foods and Drinks. In Oxford Research Encyclopedia of Food Studies; Oxford University Press: Oxford, UK, 2024; ISBN 978-0-19-776253-0. [Google Scholar]
- Robinson, R.K. (Ed.) Modern Dairy Technology; Springer: Boston, MA, USA, 1993; ISBN 978-1-4684-8174-7. [Google Scholar]
- Smid, E.J.; Lacroix, C. Microbe–Microbe Interactions in Mixed Culture Food Fermentations. Curr. Opin. Biotechnol. 2013, 24, 148–154. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.-M.; G. Biliaderis, C. Fermented Cereal-Based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally Fermented Pickles: How the Microbial Diversity Associated with Their Nutritional and Health Benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Cárdenas, N.; Calzada, J.; Peirotén, Á.; Jiménez, E.; Escudero, R.; Rodríguez, J.M.; Medina, M.; Fernández, L. Development of a Potential Probiotic Fresh Cheese Using Two Lactobacillus salivarius Strains Isolated from Human Milk. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Conway, V.; Couture, P.; Gauthier, S.; Pouliot, Y.; Lamarche, B. Effect of Buttermilk Consumption on Blood Pressure in Moderately Hypercholesterolemic Men and Women. Nutrition 2014, 30, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Cuamatzin-García, L.; Rodríguez-Rugarcía, P.; El-Kassis, E.G.; Galicia, G.; Meza-Jiménez, M.D.L.; Baños-Lara, M.D.R.; Zaragoza-Maldonado, D.S.; Pérez-Armendáriz, B. Traditional Fermented Foods and Beverages from around the World and Their Health Benefits. Microorganisms 2022, 10, 1151. [Google Scholar] [CrossRef]
- EL-Sayed, A.I.M.; El-Borai, A.M.; Akl, S.H.; EL-Aassar, S.A.; Abdel-Latif, M.S. Identification of Lactobacillus Strains from Human Mother Milk and Cottage Cheese Revealed Potential Probiotic Properties with Enzymatic Activity. Sci. Rep. 2022, 12, 22522. [Google Scholar] [CrossRef]
- Kim, B.; Mun, E.-G.; Kim, D.; Kim, Y.; Park, Y.; Lee, H.-J.; Cha, Y.-S. A survey of research papers on the health benefits of kimchi and kimchi lactic acid bacteria. J. Nutr. Health 2018, 51, 1–13. [Google Scholar] [CrossRef]
- Koehler, K.; Drenowatz, C. Integrated Role of Nutrition and Physical Activity for Lifelong Health. Nutrients 2019, 11, 1437. [Google Scholar] [CrossRef]
- Santos, A.; San Mauro, M.; Sanchez, A.; Torres, J.M.; Marquina, D. The Antimicrobial Properties of Different Strains of Lactobacillus Spp. Isolated from Kefir. Syst. Appl. Microbiol. 2003, 26, 434–437. [Google Scholar] [CrossRef]
- Sasaki, M.; Oba, C.; Nakamura, K.; Takeo, H.; Toya, H.; Furuichi, K. Milk-Based Culture of Penicillium Camemberti and Its Component Oleamide Affect Cognitive Function in Healthy Elderly Japanese Individuals: A Multi-Arm Randomized, Double-Blind, Placebo-Controlled Study. Front. Nutr. 2024, 11, 1357920. [Google Scholar] [CrossRef]
- Shankar, V.; Mahboob, S.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Govindarajan, M. A Review on Microbial Degradation of Drinks and Infectious Diseases: A Perspective of Human Well-Being and Capabilities. J. King Saud Univ. Sci. 2021, 33, 101293. [Google Scholar] [CrossRef]
- Tapsell, L.C. Fermented Dairy Food and CVD Risk. Br. J. Nutr. 2015, 113, S131–S135. [Google Scholar] [CrossRef] [PubMed]
- Csatlos, N.-I.; Simon, E.; Teleky, B.-E.; Szabo, K.; Diaconeasa, Z.M.; Vodnar, D.-C.; Ciont, C.; Pop, O.-L. Development of a Fermented Beverage with Chlorella Vulgaris Powder on Soybean-Based Fermented Beverage. Biomolecules 2023, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Dullius, A.; Rama, G.R.; Giroldi, M.; Goettert, M.I.; Lehn, D.N.; Volken De Souza, C.F. Bioactive Peptide Production in Fermented Foods. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–72. ISBN 978-0-12-823506-5. [Google Scholar]
- Kregiel, D. Health Safety of Soft Drinks: Contents, Containers, and Microorganisms. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kieliszek, M.; Ścibisz, I. Propionibacterium Spp.—Source of Propionic Acid, Vitamin B12, and Other Metabolites Important for the Industry. Appl. Microbiol. Biotechnol. 2018, 102, 515–538. [Google Scholar] [CrossRef]
- Dumas, E.; Feurtey, A.; Rodríguez De La Vega, R.C.; Le Prieur, S.; Snirc, A.; Coton, M.; Thierry, A.; Coton, E.; Le Piver, M.; Roueyre, D.; et al. Independent Domestication Events in the Blue-cheese Fungus Penicillium roqueforti. Mol. Ecol. 2020, 29, 2639–2660. [Google Scholar] [CrossRef]
- Miyamoto, J.; Ando, Y.; Yamano, M.; Nishida, A.; Murakami, K.; Kimura, I. Acidipropionibacterium Acidipropionici, a Propionate-Producing Bacterium, Contributes to GPR41 Signaling and Metabolic Regulation in High-Fat Diet-Induced Obesity in Mice. Front. Nutr. 2025, 12, 1542196. [Google Scholar] [CrossRef] [PubMed]
- Baur, T.; Dürre, P. New Insights into the Physiology of the Propionate Producers Anaerotignum Propionicum and Anaerotignum Neopropionicum (Formerly Clostridium Propionicum and Clostridium Neopropionicum). Microorganisms 2023, 11, 685. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, S.; Janssen, P.H.; Schink, B. Energetics and Kinetics of Lactate Fermentation to Acetate and Propionate via Methylmalonyl-CoA or Acrylyl-CoA. FEMS Microbiol. Lett. 2002, 211, 65–70. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic Distribution of Three Pathways for Propionate Production within the Human Gut Microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Hespell, R.B. Microbial Rumen Fermentation. J. Dairy Sci. 1981, 64, 1153–1169. [Google Scholar] [CrossRef]
- Zhang, S.-M.; Huang, S.-L. The Commensal Anaerobe Veillonella Dispar Reprograms Its Lactate Metabolism and Short-Chain Fatty Acid Production during the Stationary Phase. Microbiol. Spectr. 2023, 11, e03558-22. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kim, Y.-K.; Nguyen, T.V.T.; Kwon, J.; Bang, Y.-J. Metabolic Profiling and Genetic Tool Development in the Mucosal Bacterium Selenomonas Sputigena. Genes Genomics 2025, 47, 997–1009. [Google Scholar] [CrossRef]
- Dighe, A.S.; Shouche, Y.S.; Ranade, D.R. Selenomonas lipolytica Sp. Nov., an Obligately Anaerobic Bacterium Possessing Lipolytic Activity. Int. J. Syst. Bacteriol. 1998, 48, 783–791. [Google Scholar] [CrossRef]
- Cabral, L.D.S.; Weimer, P.J. Megasphaera Elsdenii: Its Role in Ruminant Nutrition and Its Potential Industrial Application for Organic Acid Biosynthesis. Microorganisms 2024, 12, 219. [Google Scholar] [CrossRef]
- Li, B.; Gao, W.; Pan, Y.; Yao, Y.; Liu, G. Progress in 1,3-Propanediol Biosynthesis. Front. Bioeng. Biotechnol. 2024, 12, 1507680. [Google Scholar] [CrossRef] [PubMed]
- Vidra, A.; Németh, Á. Bio-Produced Propionic Acid: A Review. Period. Polytech. Chem. Eng. 2017, 62, 57–67. [Google Scholar] [CrossRef]
- Selmer, T.; Willanzheimer, A.; Hetzel, M. Propionate CoA-transferase from Clostridium propionicum: Cloning of the Gene and Identification of Glutamate 324 at the Active Site. Eur. J. Biochem. 2002, 269, 372–380. [Google Scholar] [CrossRef]
- Tripathi, A.; Pandey, V.K.; Panesar, P.S.; Taufeeq, A.; Mishra, H.; Rustagi, S.; Malik, S.; Kovács, B.; Suthar, T.; Shaikh, A.M. Fermentative Production of Vitamin B12 by Propionibacterium shermanii and Pseudomonas denitrificans and Its Promising Health Benefits: A Review. Food Sci. Nutr. 2024, 12, 8675–8691. [Google Scholar] [CrossRef]
- Oude Elferink, S.J.W.H.; Krooneman, J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Schink, B.; Pfennig, N. Propionigenium modestum Gen. Nov. Sp. Nov. a New Strictly Anaerobic, Nonsporing Bacterium Growing on Succinate. Arch. Microbiol. 1982, 133, 209–216. [Google Scholar] [CrossRef]
- Jakobson, C.M.; Slininger, M.F.; Tullman-Ercek, D.; Mangan, N.M. A Systems-Level Model Reveals That 1,2-Propanediol Utilization Microcompartments Enhance Pathway Flux Through Intermediate Sequestration. PLoS Comput. Biol. 2017, 13, e1005525. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Campbell, G.; Mayer, C.-D.; Flint, H.J. Whole-Genome Transcription Profiling Reveals Genes Up-Regulated by Growth on Fucose in the Human Gut Bacterium “Roseburia inulinivorans”. J. Bacteriol. 2006, 188, 4340–4349. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Booth, I.R. Importance of Glutathione for Growth and Survival of Escherichia Coli Cells: Detoxification of Methylglyoxal and Maintenance of Intracellular K+. J. Bacteriol. 1998, 180, 4314–4318. [Google Scholar] [CrossRef] [PubMed]
- Furlan Sandrini, D.M.; Morgado, D.L.; De Oliveira, A.J.A.; De Moraes, D.A.; Varanda, L.C.; Frollini, E. Cellulose Esters: Synthesis for Further Formation of Films with Magnetite Nanoparticles Incorporated. Int. J. Biol. Macromol. 2024, 264, 130594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.