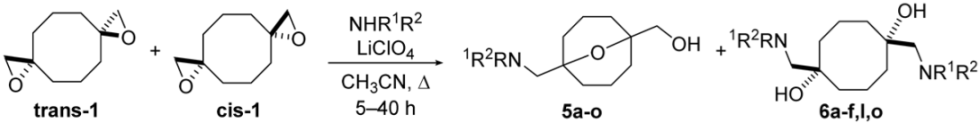
Abstract
Nucleophilic ring-opening of bis(oxiranes), containing several reactive centers, can be used to elaborate straightforward atom-economy and stereoselective approaches to polyfunctionalized compounds. In the present work, ring-opening of cis- and trans-diastereomers of a spirocyclic bis(oxirane), containing a cyclooctane core (namely, 1,8-dioxadispiro[2.3.2.3]dodecane), upon treatment with various amines, was studied. Trans-isomer afforded aminoalcohols with 9-oxabicyclo[3.3.1]nonane moiety, formed via domino-process, including opening of an oxirane ring followed by intramolecular cyclization. Ring-opening of cis-isomer gave aminosubstituted cis-cyclooctane-1,5-diols, derived from independent reaction of two oxirane moieties. Activation of oxirane rings by the addition of LiClO4, acting as a Lewis acid, allowed the involvement of a number of primary and secondary aliphatic amines as well as aniline derivatives in the reaction. Scope and limitations of the reaction were studied and a series of aminoalcohols with a 9-oxabicyclo[3.3.1]nonane core and symmetric diaminodiols with a cyclooctane core were obtained.
1. Introduction
Oxiranes represent versatile intermediates, finding application in the synthesis of medicinal drugs, natural compounds, polymers, and other products with practicable properties [1,2,3,4,5]. Synthetically useful transformations of these strained rings are characterized by predictability, regio- and stereoselectivity [6,7], mild conditions, and broad reaction scope [8,9,10,11,12]. Bis(oxiranes), containing several reactive centers, are structures of particular interest. Generally, they are used to elaborate straightforward approaches to polyfunctionalized compounds [13,14,15], though examples of formation of saturated O-heterocycles via intramolecular cyclization are reported as well [16,17]. An eight-membered ring present in the molecule opens additional synthetic opportunities due to transannular transformations leading to polycyclic structures [18,19].
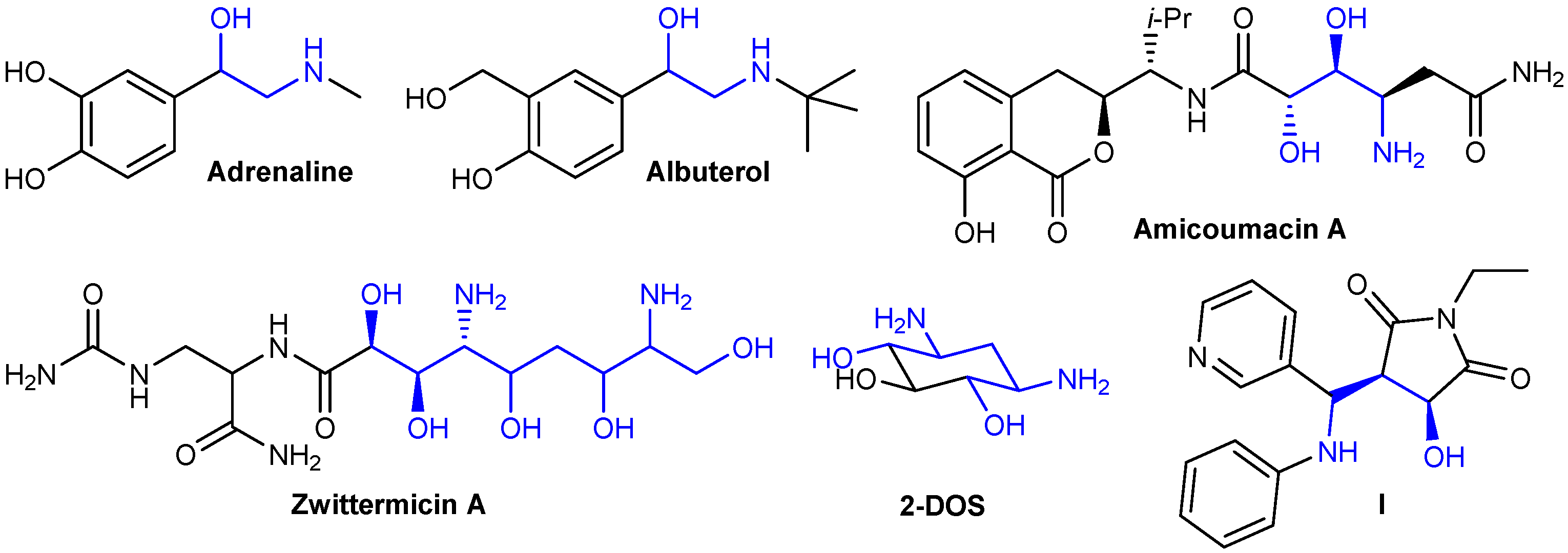
Aminoalcohols and diaminodiols are of interest as scaffolds occurring in a number of bioactive and natural compounds (Figure 1). For instance, adrenalin represents a β-aminoalcohol, as well as a number of adrenergic drugs, such as a short-acting bronchodilator albuterol [20]. Linear aminodiol or aminopolyol moieties are present in natural antibiotics such as amicoumacin A [21] and zwittermicin A [22]. 2-Deoxystreptamine (2-DOS), containing a cyclohexane core, is of importance as a key aminocyclitol scaffold of a number of clinically used aminoglycoside antibiotics (gentamicin, neomycin, etc.) [23]. Conformationally constrained aminoalcohols, such as compound I, represent rigid peptidomimetics [24].
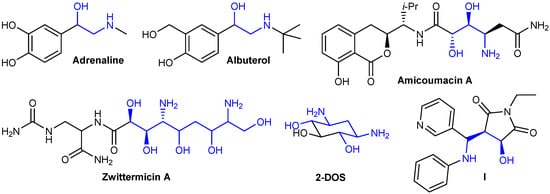
Figure 1.
Examples of bioactive compounds containing aminoalcohol scaffolds.
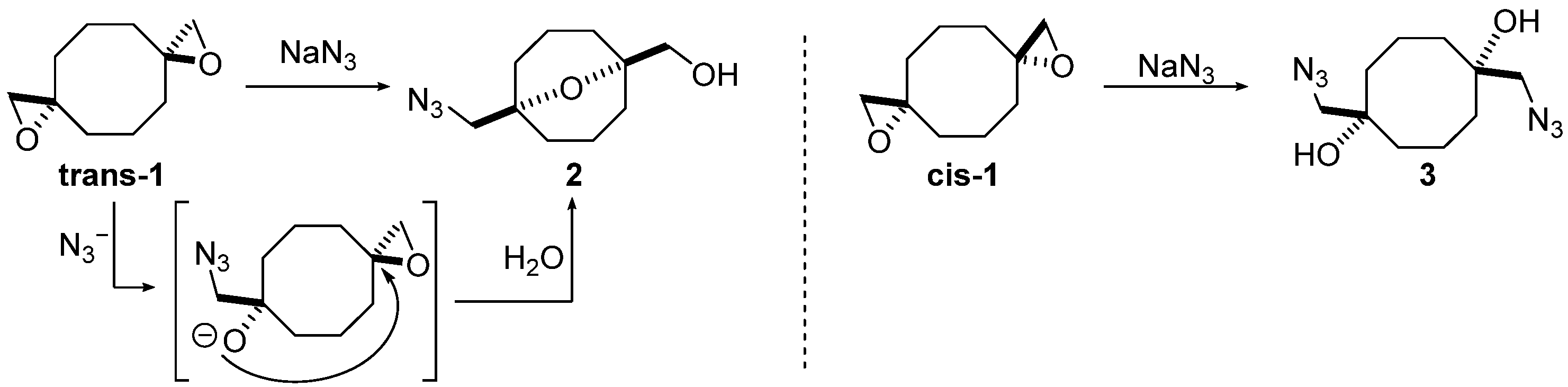
Previously, we have reported the first example of transannular reactions of spirocyclic bis(oxirane) trans-1 as a nucleophile (Scheme 1) [25], which was later expanded for a tetrakis(oxirane) [26]. The reactivity of two diastereomers of bis(oxirane) trans-1 and cis-1 towards NaN3 was investigated and it was shown to be determined by the configuration of oxirane moieties: while trans-1 afforded 9-oxabicyclo[3.3.1]nonane 2, formed via domino process, including opening of one oxirane ring followed by intramolecular cyclization, diastereomer cis-1 gave the sole isomer of diazidodiol 3, derived from independent ring-opening of two oxirane moieties.
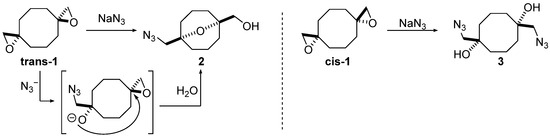
Scheme 1.
Ring-opening of bis(oxiranes) trans-1 and cis-1 with sodium azide [18].
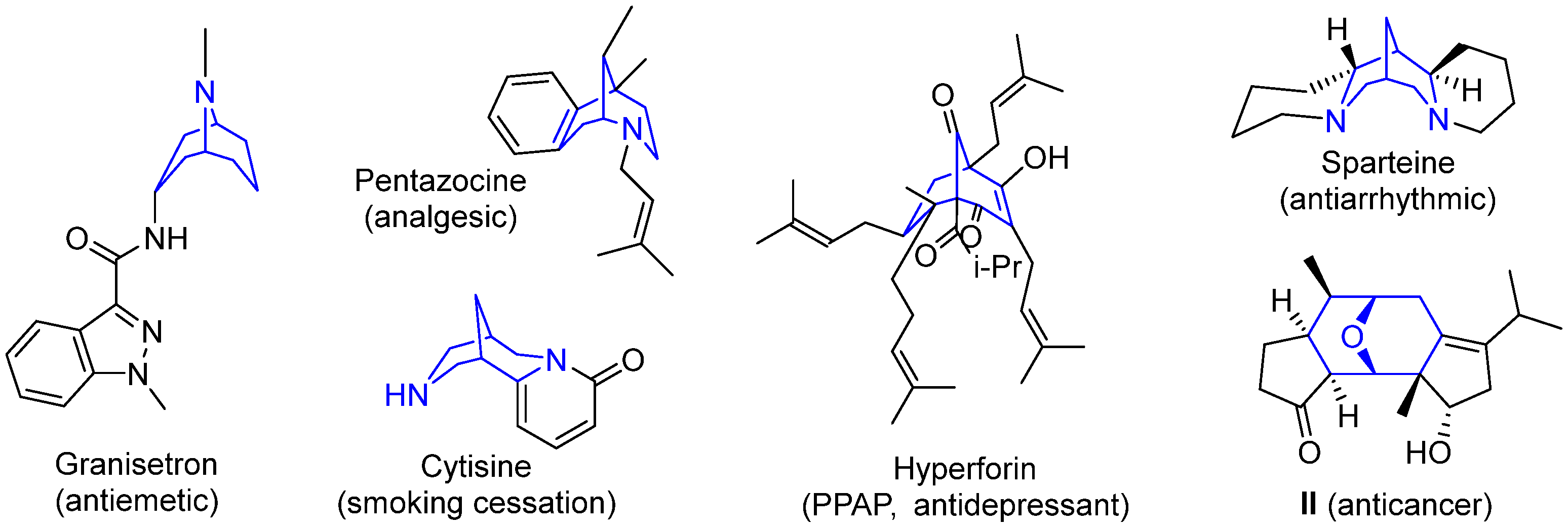
It should be mentioned that molecules containing cleft-shaped frameworks such as bicyclo[3.3.1]nonanes and their aza-derivatives have found application as conformationally restricted molecules for the purposes of medicinal chemistry, metal complex catalysis, and the detection of metal ions and small molecules [27]. Natural and synthetic derivatives of aza- and diazabicyclo[3.3.1]nonanes (granisetron, pentazocine, cytisine) are used as medicinal drugs (Figure 2) [28,29,30]. Numerous natural compounds containing fragments of bicyclo[3.3.1]nonane (for example, polycyclic polyprenylated acylphloroglucinols (PPAPs) [31,32]), 2-azabicyclo[3.3.1]nonane (morphine alkaloids [33]), 9-azabicyclo[3.3.1]nonane (granate [34] and bis(indole) alkaloids [35]), 3,7-diazabicyclo[3.3.1]nonane (bispidine-based alkaloids [36]), as well as synthetic bicyclo[3.3.1]nonanes, reveal a broad spectrum of biological activity, including anticancer properties [37,38,39,40].
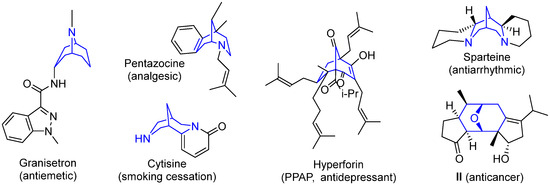
Figure 2.
Examples of medicinal drugs and bioactive natural compounds containing bicyclo[3.3.1]nonane and related scaffolds.
9-Oxabicyclo[3.3.1]nonane moiety, as well as similar frameworks, represents a conformationally restricted 3D-scaffold attractive for drug design and occurring in bioactive compound. For example, diterpenoid II (Figure 2) exhibits antiproliferative activity towards cancer cells with good selectivity compared to normal cell lines [41]. At the same time, in contrast to the above-mentioned bicyclononane derivatives, 9-oxabicyclo[3.3.1]nonanes are rather hard to synthesize and much less examples of them are described; that makes the search for straightforward approaches to these compounds a challenging task.
As for diazidodiol 3, it represents an attractive structure to be used as a linker moiety for construction of conjugates for biomedical applications using azide–alkyne cycloaddition strategies [42,43]. It was successfully used to obtain bis(triazoles) [44] and bis(steroid) derivatives with anticancer activity [45].
In the present work, we aimed to study the nucleophilic ring-opening of bis(oxiranes) trans-1 and cis-1 upon treatment with various amines in order to elaborate preparative approaches to aminoalcohols and diaminodiols of previously unknown structural types.
2. Results and Discussion
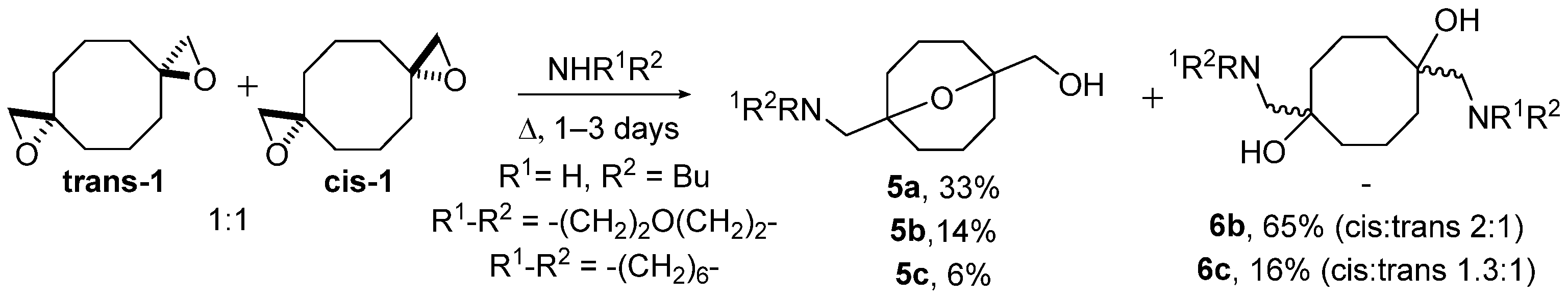
A diastereomeric mixture of bis(oxiranes) trans-1 and cis-1 was studied upon the treatment with n-butylamine, morpholine, azepane, and aniline (Scheme 2). The reactions were performed under reflux in the medium of an amine without a solvent. In the case of n-butylamine, 9-oxabicyclo[3.3.1]nonane 5a, derived from transannular reaction of trans-1, was the only product. Cis-bis(oxirane) did not interact with butylamine. Reaction of trans-1 and cis-1 with more nucleophilic morpholine and azepane besides oxabicyclononane derivatives 5b,c afforded symmetric diaminodiols 6b,c, the products of independent ring-opening. Diaminodiols 6b,c were obtained as diastereomeric mixtures where cis-isomers prevailed. The configuration of isomers 6b,c was established based on different symmetries of molecules as previously performed for the starting bis(oxiranes) [25]. Aniline did not interact with bis(oxiranes) trans-1 and cis-1 in these conditions. The obtained preliminary results were in good correlation with the nucleophilicity of amines: secondary amines interacted with both trans-1 and cis-1; less nucleophilic butylamine interacted only with trans-1, able to undergo quick intramolecular reaction; the least nucleophilic aniline reacted with neither of the diastereomers.
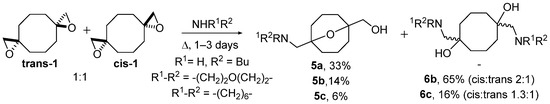
Scheme 2.
Ring-opening of bis(oxiranes) trans-1 and cis-1 with n-butylamine, morpholine, and azepane; amines used as solvent.
In order to involve a broader scope of amines into the reaction and to improve its yield and selectivity, the conditions of nucleophilic opening were optimized on the examples of morpholine and butylamine. The solvent, temperature, time, and reagent ratios were varied (see Table S1). In the absence of basic or acidic additives, the reaction proceeded slowly even in acetonitrile under reflux, and an attempt to employ K2CO3 as a base led to complete decomposition of organic material. The use of LiClO4, chosen because of its ability to promote oxiranes’ ring-opening by acting as Lewis acid with non-nucleophilic anion [46], provided almost complete conversion of trans-1 and cis-1 at room temperature, though it required three days in the case of morpholine. Optimal reaction conditions were found to be reflux in acetonitrile for 5 h. Various quantities of LiClO4 were required, depending on the structure of amine: reactions with butylamine required 10-fold excess per oxirane ring, while for more nucleophilic morpholine, 1.2-fold excess per oxirane ring was enough for the complete conversion of starting bis(oxiranes). It should be mentioned that in optimized conditions, only cis-isomers of diaminodiols 6 were formed, i.e., only transannular reaction proceeded for trans-1a.
Bis(oxiranes) trans-1 and cis-1 were treated with various amines under optimized conditions (Table 1). In most cases, the diastereomeric mixture of starting bis(oxiranes) was involved into the reaction, with the ratio of starting diastereomers trans-1:cis-1 varying from 1:0.8 to 1:0.9; in some cases, when the products were hard to separate, either pure trans-1 or cis-1 was used as the starting material. Bis(oxirane) trans-1 smoothly reacted with primary and secondary amines, affording aminoalcohols 5a–k of 9-oxabicyclo[3.3.1]nonane series. Aniline, as well as EDG-substituted p-toluidine and p-anisidine, afforded the products of transannular reaction 5l–n after reflux for 5–10 h, while the reaction with p-bromoaniline gave the product 5o only after reflux for 30 h. Bis(oxirane) cis-1 also interacted with aliphatic and aromatic amines; yet, unfortunately, the resulting diaminodiols 6 were hard to isolate due to low chromatographic mobility and the tendency to form salts. Nevertheless, diaminodiols 6a–f could be isolated via column chromatography in moderate to high yields. Reaction of cis-1 with p-bromoaniline even after 40 h afforded an equimolar mixture of diaminodiol 6o and 7-{[(4-bromophenyl)amino]methyl}-1-oxaspiro[2.7]decan-7-ol (7o), which was the product of ring-opening of one oxirane moiety. Amines with stronger electron-acceptor substituents, namely, p-nitroaniline, sulfanilamide, and methanesulfonamide, did not act as nucleophiles towards bis(oxiranes) trans-1 and cis-1 in the described conditions.

Table 1.
Ring-opening of bis(oxiranes) trans-1 and cis-1 with various amines.
To summarize, a preparative method of ring-opening of spirocyclic bis(oxiranes) trans-1 and cis-1 upon treatment with various amines in the presence of LiClO4 was elaborated to yield a series of aminoalcohols with 9-oxabicyclo[3.3.1]nonane core and substituted cis-cyclooctane-1,5-diols, containing two fragments of amine. The products of the ring-opening of bis(oxirane) trans-1, functionalized 9-oxabicyclo[3.3.1]nonanes, represent a valuable conformationally restricted scaffold for drug design, while the ring-opening of bis(oxirane) cis-1 with bioactive amines may be used as a stereoselective approach to bivalent ligands with a hydrophobic linker.
3. Materials and Methods
3.1. General
1H and 13C NMR spectra were recorded on a 400 MHz spectrometer Agilent 400-MR (Agilent Technologies, Santa Clara, CA, USA; 400.0, 100.6 or 376.3 MHz for 1H, 13C or 19F, respectively) at r.t. in CDCl3, if not stated otherwise; chemical shifts δ were measured with reference to CDCl3 (δH = 7.26 ppm, δC = 77.16 ppm) or to CFCl3. When necessary, assignments of signals in NMR spectra were made using 2D techniques. Accurate mass measurements (HRMS) were obtained on Bruker micrOTOF II (Bruker Daltonik GmbH, Bremen, Germany) or G3 QTof quadrupole-time-of-flight (Waters, Milford, MA, USA) with electrospray ionization (ESI). Analytical thin layer chromatography was carried out with silica gel plates supported on aluminum (ALUGRAM® Xtra SIL G/UV254, Macherey-Nagel, Duren, Germany); the detection was performed by UV lamp (254 nm). Column chromatography was performed on silica gel (Silica 60, 0.015–0.04 mm, Macherey-Nagel, Duren, Germany). Bis(oxiranes) trans-1 and cis-1 [25] were obtained via the described method. All other starting materials were commercially available. All reagents except commercial products of satisfactory quality were purified according to literature procedures prior to use.
3.2. Reaction of Bis(oxiranes) trans-1 and cis-1 with Amines (General Method)
To a solution of bis(oxirane) (0.1 mmol, 17 mg) in dry CH3CN (3 mL), LiClO4 (0.5–2 mmol) and corresponding amine (0.22 mmol) were added. The mixture was stirred at 80 °C for 5–40 h. The solvent was evaporated under reduced pressure. The product was isolated via preparative column chromatography (SiO2).
{5-[(Butylamino)methyl]-9-oxabicyclo[3.3.1]nonan-1-yl}methanol (5a). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 81% (20 mg), yellow oil, Rf 0.20 (light petrol:EtOAc:MeOH 3:1:0.1). 1H NMR (δ, ppm, J, Hz): 0.91 (t, 3H, 3J = 7.3, CH3), 1.27–1.39 (m, 4H, 2CH2, cy-Oct + CH2, Bu), 1.39–1.57 (m, 4H, 2CH2, cy-Oct + CH2, Bu), 1.58–1.75 (m, 6H, 6CH2, cy-Oct), 1.91–2.08 (m, 2H, 2CH2, cy-Oct), 2.54 (s, 2H, CH2N), 2.61–2.69 (m, 2H, CH2N, Bu), 3.11 (br.s, 2H, OH + NH), 3.31 (s, 2H, CH2OH). 13C NMR (δ, ppm): 14.1 (CH3), 18.5 (2CH2, cy-Oct), 20.6 (CH2, Bu), 29.8 (2CH2, cy-Oct), 31.4 (CH2, Bu), 31.8 (2CH2, cy-Oct), 50.2 (CH2N, Bu), 61.2 (CH2N), 71.4 (CH2OH), 71.7 (C-CH2N), 72.5 (C-CH2O). HRMS (ESI+, m/z): calculated for C14H27NO2 [M + H]+: 242.2115, found: 242.2122.
[5-(Morpholin-4-ylmethyl)-9-oxabicyclo[3.3.1]nonan-1-yl]methanol (5b). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:5. Yield 88% (22 mg), orange oil, Rf 0.20 (light petrol:EtOAc:MeOH 3:1:0.1). 1H NMR (δ, ppm): 1.28–1.46 (m, 4H, 4CH2, cy-Oct), 1.57–1.78 (m, 6H, 6CH2, cy-Oct), 1.94–2.11 (m, 2H, 2CH2, cy-Oct), 2.23 (s, 2H, CH2N), 2.37 (br.s, 1H, OH), 2.51–2.62 (m, 4H, 2CH2N, morpholine), 3.28 (s, 2H, CH2OH), 3.64–3.73 (m, 4H, 2CH2O, morpholine). 13C NMR (δ, ppm): 18.8 (2CH2, cy-Oct), 29.8 (2CH2, cy-Oct), 31.9 (2CH2, cy-Oct), 55.7 (2CH2N, morpholine), 67.2 (2CH2O, morpholine), 70.0 (CH2N), 71.6 (CH2OH), 72.1 (C-CH2O), 73.7 (C-CH2N). HRMS (ESI+, m/z): calculated for C14H25NO3 [M + H]+: 256.1907, found: 256.1906.
[5-(Azepan-1-ylmethyl)-9-oxabicyclo[3.3.1]nonan-1-yl]methanol (5c). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:5. Yield 54% (15 mg), yellow oil, Rf 0.26 (light petrol:DCM:MeOH 1:3:1). 1H NMR (δ, ppm): 1.31–1.47 (m, 4H, 4CH2, cy-Oct), 1.51–1.73 (m, 14H, 6CH2, cy-Oct + 4CH2, azepane), 1.93–2.09 (m, 2H, 2CH2, cy-Oct), 2.41 (s, 2H, CH2N), 2.73–2.83 (m, 4H, 2CH2N, azepane), 3.29 (s, 2H, CH2OH), 4.62 (br.s, 1H, OH). 13C NMR (δ, ppm): 18.9 (2CH2, cy-Oct), 27.5 (2CH2, azepane), 29.1 (2CH2, azepane), 30.0 (2CH2, cy-Oct), 31.7 (2CH2, cy-Oct), 57.9 (2CH2N, azepane), 69.2 (CH2N), 71.79 (CH2OH), 71.83 (C-CH2OH), 74.4 (C-CH2N). HRMS (ESI+, m/z): calculated for C16H29NO2 [M + H]+: 268.2271, found: 268.2272.
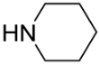
[5-(Piperidin-1-ylmethyl)-9-oxabicyclo[3.3.1]nonan-1-yl]methanol (5d). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:5. Yield 78% (13 mg), yellow oil, Rf 0.14 (light petrol: DCM:MeOH 1:3:0.5). 1H NMR (δ, ppm): 1.29–1.48 (m, 4H, 4CH2, cy-Oct + 2H, CH2, piperidine), 1.49–1.81 (m, 6H, 6CH2, cy-Oct + 4H, 2CH2, piperidine), 1.95–2.11 (m, 2H, 2CH2, cy-Oct), 2.14–2.34 (m, 2H, CH2N), 2.54 (br.s, 4H, 2CH2N, piperidine), 3.29 (s, 2H, CH2O). 13C NMR (δ, ppm): 18.9 (2CH2, cy-Oct), 24.1 (CH2, piperidine), 26.1 (2CH2, piperidine), 29.9 (2CH2, cy-Oct), 32.0 (2CH2, cy-Oct), 56.8 (2CH2N, piperidine), 70.2 (CH2N), 71.7 (CH2OH), 72.0 (C-CH2OH), 73.6 (C-CH2N). HRMS (ESI+, m/z): calculated for C15H27NO2 [M + H]+: 254.2115, found: 254.2119.
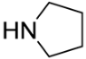
[5-(Pyrrolidin-1-ylmethyl)-9-oxabicyclo[3.3.1]nonan-1-yl]methanol (5e). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:5. Yield 83% (20 mg), yellow oil, Rf 0.24 (light petrol:DCM:MeOH 1:4:1). 1H NMR (δ, ppm): 1.32–1.40 (m, 2H, 2CH2, cy-Oct), 1.44–1.52 (m, 2H, 2CH2, cy-Oct), 1.58–1.76 (m, 6H, 6CH2, cy-Oct), 1.78–1.92 (m, 4H, 2CH2, pyrrolidine), 1.96–2.13 (m, 2H, 2CH2, cy-Oct), 2.56 (br.s, 2H, CH2N), 2.82 (br.s, 4H, 2CH2N, pyrrolidine), 3.32 (br.s, 2H, 2CH2OH, pyrrolidine). 13C NMR (δ, ppm): 18.8 (2CH2, cy-Oct), 23.8 (2CH2, pyrrolidine), 29.6 (2CH2, cy-Oct), 32.1 (2CH2, cy-Oct), 56.5 (2CH2, pyrrolidine), 68.1 (CH2N), 71.5 (CH2OH), 72.3 (C-CH2N), 72.8 (C-CH2OH). HRMS (ESI+, m/z): calculated for C14H25NO2 [M + H]+:240.1958, found: 240.1961.
1,5-Bis[(dibutylamino)methyl]cyclooctane-1,5-diol (5f). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:5. Yield 83% (25 mg), yellow oil, Rf 0.16 (EtOAc). 1H NMR (δ, ppm, J, Hz): 0.89 (t, 3J = 7.2, 6H, 2CH3, Bu), 1.20–1.31 (m, 4H, 2CH2, Bu), 1.31–1.45 (m, 8H, 4CH2, cy-Oct + 2CH2, Bu), 1.56–1.77 (m, 6H, 6CH2, cy-Oct), 1.95–2.08 (m, 2H, 2CH2, cy-Oct), 2.30 (s, 2H, CH2N), 2.44–2.57 (m, 4H, 2CH2N, Bu), 3.28 (s, 2H, CH2O). 13C NMR (δ, ppm): 14.3 (2CH3, Bu), 18.9 (2CH2, cy-Oct), 20.8 (2CH2, Bu), 29.5 (2CH2, Bu), 29.8 (2CH2, cy-Oct), 31.8 (2CH2, cy-Oct), 56.1 (2CH2N, Bu), 66.3 (CH2N), 71.7 (CH2O), 72.1 (C-CH2O), 73.8 (C-CH2N). HRMS (ESI+, m/z): calculated for C18H35NO2 [M + H]+: 298.2741, found: 298.2725.
{5-[(Propargylamino)methyl]-9-oxabicyclo[3.3.1]nonan-1-yl}methanol (5g). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 70% (16 mg), yellow oil, Rf 0.32 (light petrol:EtOAc:MeOH 1:1:1). 1H NMR (δ, ppm, J, Hz): 1.30–1.40 (m, 2H, 2CH2, cy-Oct), 1.41–1.52 (m, 2H, 2CH2, cy-Oct), 1.57–1.74 (m, 6H, 6CH2, cy-Oct), 1.95–2.10 (m, 2H, 2CH2, cy-Oct), 2.28 (t, 4J = 2.4, 1H, CH, propargyl), 2.67 (s, 2H, CH2N), 3.06 (br.s, 2H, NH, OH), 3.35 (s, 2H, CH2O), 3.54 (d, 4J = 2.4 Hz, CH2, propargyl). 13C NMR (δ, ppm): 18.4 (2CH2, cy-Oct), 29.7 (2CH2, cy-Oct), 31.6 (2CH2, cy-Oct), 38.5 (CH2, propargyl), 59.8 (CH2N), 71.3 (CH2OH), 71.7 (C-CH2N), 72.6 (C-CH2O), 72.8 (CH, propargyl), 80.9 (C, propargyl). HRMS (ESI+, m/z): calculated for C13H21NO2 [M + H]+: 224.1645, found: 224.1649.
{5-[(Benzylamino)methyl]-9-oxabicyclo[3.3.1]nonan-1-yl}methanol (5h). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 80% (22 mg), yellow oil, Rf 0.39 (light petrol:EtOAc:MeOH 1:1:0.5). 1H NMR (δ, ppm): 1.27–1.36 (m, 2H, 2CH2, cy-Oct), 1.37–1.50 (m, 2H, 2CH2, cy-Oct), 1.54–1.71 (m, 6H, 4CH2, cy-Oct), 1.89–2.03 (m, 2H, 2CH2, cy-Oct), 2.57 (s, 2H, CH2N), 3.31 (s, 2H, CH2O), 4.00 (s, 2H, PhCH2N), 4.10 (br.s, 2H, NH+OH), 7.28–7.41 (m, 5H, 5CH, Ph). 13C NMR (δ, ppm): 18.5 (2CH2, cy-Oct), 29.7 (2CH2, cy-Oct), 31.7 (2CH2, cy-Oct), 53.7 (PhCH2N), 60.0 (CH2N), 71.5 (CH2OH), 71.8 (C), 72.6 (C), 127.5 (CH, Ph), 128.7 (4CH, Ph), 138.7 (C, Ph). HRMS (ESI+, m/z): calculated for C17H25NO2 [M + H]+: 276.1958, found: 276.1965.
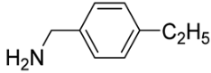
(5-{[(4-Ethylbenzyl)amino]methyl}-9-oxabicyclo[3.3.1]nonan-1-yl)methanol (5i). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 65% (20 mg), yellow oil, Rf 0.78 (light petrol:DCM:MeOH 1:1:0.5). 1H NMR (δ, ppm, J, Hz): 1.23 (t, 3H, 3J 7.6, CH3), 1.25–1.34 (m, 2H, 2CH2, cy-Oct), 1.38–1.49 (m, 2H, 2CH2, cy-Oct), 1.56–1.74 (m, 6H, 6CH2, cy-Oct), 1.91–2.06 (m, 2H, 2CH2, cy-Oct), 2.55 (s, 2H, CH2N), 2.64 (q, 2H, 3J 7.6, CH2, Et), 3.26 (s, 2H, CH2O), 3.77 (br.s, 2H, NH+OH), 3.93 (s, 2H, ArCH2N), 7.15–7.20 (m, 2H, 2CH, Ar), 7.30–7.36 (m, 2H, 2CH, Ar). 13C NMR (δ, ppm): 15.7 (CH3), 18.4 (2CH2, cy-Oct), 28.7 (CH2, Et), 29.6 (2CH2, cy-Oct), 31.7 (2CH2, cy-Oct), 53.3 (ArCH2N), 59.5 (CH2N), 71.2 (CH2OH), 71.3 (C-CH2N), 72.8 (C-CH2OH), 128.3 (2CH, Ar), 129.0 (2CH, Ar), 134.4 (C, Ar), 143.9 (C, Ar). HRMS (ESI+, m/z): calculated for C19H29NO2 [M + H]+: 304.2271, found: 304.2272.
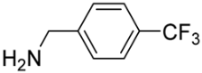
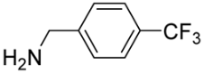
[5-({[4-(Trifluoromethyl)benzyl])amino}methyl)-9-oxabicyclo[3.3.1]nonan-1-yl]methanol (5j). Reaction time—10 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 40% (13 mg), yellow oil, Rf 0.24 (light petrol:EtOAc 1:1). 1H NMR (δ, ppm, J, Hz): 1.31–1.46 (m, 4H, 4CH2, cy-Oct), 1.55–1.78 (m, 6H, 6CH2, cy-Oct), 1.93–2.09 (m, 2H, 2CH2, cy-Oct), 2.47 (s, 2H, CH2N), 3.31 (s, 2H, CH2O), 3.87 (s, 2H, ArCH2N), 7.41–7.50 (m, 2H, 2CH, Ar), 7.54–7.60 (m, 2H, 2CH, Ar). 13C NMR (δ, ppm, J, Hz): 18.6 (2CH2, cy-Oct), 29.9 (2CH2, cy-Oct), 31.8 (2CH2, cy-Oct), 53.7 (ArCH2N), 61.0 (CH2N), 71.6 (CH2OH), 72.3 (C), 72.4 (C), 124.5 (q, 1JCF 272), 125.4 (q, 3JCF 4, 2CH, Ar), 128.3 (2CH, Ar), 129.3 (q, 2JCF 32, C, Ar), 145.0 (C, Ar). 19F NMR (δ, ppm): −62.37 (s, 3F). HRMS (ESI+, m/z): calculated for C18H24F3NO2 [M + H]+: 344.1832, found: 344.1836.
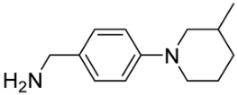
[5-({[4-(3-Methylpiperidin-1-yl)benzyl]amino}methyl)-9-oxabicyclo[3.3.1]non-1-yl]methanol (5k). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 16% (6 mg), yellow oil, Rf 0.78 (light petrol:DCM:MeOH 1:1:0.5). 1H NMR (δ, ppm, J, Hz): 0.94 (d, 3H, 3J 6.6, CH3), 0.99–1.13 (m, 1H, CH2, piperidine), 1.19–1.35 (m, 2H, cy-Oct), 1.40–1.51 (m, 2H, 2CH2, cy-Oct), 1.50–1.87 (m, 10H, 6CH2, cy-Oct + 2CH2 piperidine + CH, piperidine), 1.88–2.09 (m, 2H, 2CH2, cy-Oct), 2.31–2.41 (m, 1H, CH2N, piperidine), 2.60–2.73 (m, 1H, CH2N, piperidine), 2.76 (s, 2H, CH2N), 3.34 (s, 2H, CH2O), 3.54–3.69 (m, 2H, 2CH2N, piperidine), 4.26 (s, 2H, ArCH2N), 6.86–6.96 (m, 2H, 2CH, Ar), 7.36–7.38 (d, 2H, 2CH, Ar), 7.89 (br.s, 2H, NH+OH). 13C NMR (δ, ppm): 18.1 (2CH2, cy-Oct), 19.6 (CH3), 25.2 (CH2, piperidine), 28.9 (2CH2, cy-Oct), 30.9 (CH, piperidine), 31.3 (2CH2, cy-Oct), 33.0 (CH2, piperidine), 49.2 (CH2, piperidine), 51.7 (ArCH2N), 55.7 (CH2N), 56.9 (CH2, piperidine), 69.4 (C-CH2N), 70.7 (CH2OH), 73.8 (C-CH2OH), 116.1 (2CH, Ar), 118.9 (C, Ar), 131.6 (2CH, Ar), 152.5 (C, Ar). HRMS (ESI+, m/z): calculated for C23H36N2O2 [M + H]+: 373.2850, found: 373.2852.
{5-[(Phenylamino)methyl]-9-oxabicyclo[3.3.1]nonan-1-yl}methanol (5l). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 36% (9 mg), yellow oil, Rf 0.77 (light petrol:EtOAc 1:2). 1H NMR (δ, ppm): 1.35–1.43 (m, 2H, 2CH2, cy-Oct), 1.44–1.53 (m, 2H, 2CH2, cy-Oct), 1.59–1.79 (m, 6H, 4CH2, cy-Oct), 1.97–2.11 (m, 2H, 2CH2, cy-Oct), 3.03 (s, 2H, CH2N), 3.35 (s, 2H, CH2O), 6.58–6.73 (m, 3H, 3CH, Ph), 7.12–7.19 (m, 2H, 2CH, Ph). 13C NMR (101 MHz, CDCl3) δ, ppm: 18.5 (2CH2, cy-Oct), 29.8 (2CH2, cy-Oct), 31.6 (2CH2, cy-Oct), 55.4 (CH2N), 71.5 (CH2OH), 72.4 (C), 72.5 (C), 113.0 (2CH, Ph), 117.3 (CH, Ph), 129.3 (2CH, Ph), 148.9 (C, Ph). HRMS (ESI+, m/z): calculated for C16H23NO2 [M + H]+: 262.1802, found: 262.1806.
{5-[(4-Methoxyphenylamino)methyl]-9-oxabicyclo[3.3.1]nonan-1-yl}methanol (5m). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 95% (27 mg), yellow oil, Rf 0.32 (EtOAc:DCM 1:5). 1H NMR (δ, ppm): 1.34–1.42 (m, 2H, 2CH2, cy-Oct), 1.43–1.53 (m, 2H, 2CH2, cy-Oct), 1.59–1.81 (m, 6H, 6CH2, cy-Oct), 1.96–2.10 (m, 2H, 2CH2, cy-Oct), 2.97 (s, 2H, CH2N), 3.35 (s, 2H, CH2O), 3.74 (s, 3H, OMe), 6.56–6.64 (m, 2H, 2CH, Ar), 6.74–6.80 (m, 2H, 2CH, Ar). 13C NMR (δ, ppm): 18.5 (2CH2, cy-Oct), 29.9 (2CH2, cy-Oct), 31.7 (2CH2, cy-Oct), 56.0 (OMe), 56.5 (CH2N), 71.6 (CH2O), 72.4 (C-CH2NH), 72.5 (C-CH2OH), 114.3 (2CH, Ar), 115.1 (2CH, Ar), 143.3 (C-NH, Ar), 152.1 (C-O, Ar). HRMS (ESI+, m/z): calculated for C17H25NO3 [M + H]+: 292.1907, found: 292.1911.
{5-[(4-Methylphenylamino)methyl]-9-oxabicyclo[3.3.1]nonan-1-yl}methanol (5n). Reaction time—10 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 26% (7 mg), yellow oil, Rf 0.67 (light petrol:EtOAc 1:1). 1H NMR (δ, ppm): 1.32–1.42 (m, 2H, 2CH2, cy-Oct), 1.43–1.53 (m, 2H, 2CH2, cy-Oct), 1.57–1.82 (m, 6H, 4CH2, cy-Oct), 1.95–2.11 (m, 2H, 2CH2, cy-Oct), 2.23 (s, 3H, CH3), 3.00 (s, 2H, CH2N), 3.35 (s, 2H, CH2O), 6.52–6.58 (m, 2H, 2CH, Ar), 6.93–7.01 (m, 2H, 2CH, Ar). 13C NMR (δ, ppm): 18.5 (2CH2, cy-Oct), 20.5 (CH3), 29.8 (2CH2, cy-Oct), 31.6 (2CH2, cy-Oct), 55.7 (CH2N), 71.6 (CH2OH), 72.4 (C), 72.5 (C), 113.1 (2CH, Ar), 126.4 (C, Ar), 129.8 (2CH, Ar), 146.7 (C, Ar). HRMS (ESI+, m/z): calculated for C17H25NO2 [M + H]+: 276.1958, found: 276.1966.
{5-[(4-Bromophenylamino)methyl]-9-oxabicyclo[3.3.1]nonan-1-yl}methanol (5o). Reaction time—30 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 17% (6 mg), yellow oil, Rf 0.58 (light petrol:EtOAc 1:1). 1H NMR (δ, ppm): 1.34–1.43 (m, 2H, 2CH2, cy-Oct), 1.43–1.52 (m, 2H, 2CH2, cy-Oct), 1.58–1.75 (m, 6H, 4CH2, cy-Oct), 1.97–2.12 (m, 2H, 2CH2, cy-Oct), 2.98 (s, 2H, CH2N), 3.35 (s, 2H, CH2O), 6.45–6.54 (m, 2H, 2CH, Ar), 7.19–7.26 (m, 2H, 2CH, Ar). 13C NMR (δ, ppm): 18.5 (2CH2, cy-Oct), 29.8 (2CH2, cy-Oct), 31.6 (2CH2, cy-Oct), 55.3 (CH2N), 71.6 (CH2OH), 72.4 (C), 72.6 (C), 108.7 (C, Ar), 114.5 (2CH, Ar), 132.0 (2CH, Ar), 148.0 (C, Ar). HRMS (ESI+, m/z): calculated for C16H22BrNO2 [M + H]+: 340.0907, found: 340.0916.
1,5-Bis[(butylamino)methyl]cyclooctane-1,5-diol (6a). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 56% (19 mg), yellow oil, Rf 0.05 (CH3CN). 1H NMR (δ, ppm, J, Hz): 0.90 (t, 6H, 3J 7.2, 2CH3), 1.29–1.38 (m, 4H, 2CH2, Bu), 1.39–1.50 (m, 10H, 6CH2, cy-Oct + 2CH2, Bu), 1.77–1.90 (m, 6H, 6CH2, cy-Oct), 2.46 (s, 4H, 2CH2N), 2.62 (t, 4H, 3J 7.1, 2CH2N, Bu). 13C NMR (δ, ppm): 14.1 (2CH3, Bu), 18.8 (2CH2, cy-Oct), 20.5 (2CH2, Bu), 32.6 (2CH2, Bu), 37.5 (4CH2, cy-Oct), 50.5 (2CH2N, Bu), 60.5 (2CH2N), 72.8 (2C). HRMS (ESI+, m/z): calculated for C18H38N2O2 [M + H]+: 315.3006, found: 315.3015.
1,5-Bis(morpholin-4-ylmethyl)cyclooctane-1,5-diol (6b). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:5. Yield 97% (33 mg), yellow oil, Rf = 0.61 (light petrol:EtOAc:MeOH 3:1:1). 1H NMR (δ, ppm): 1.37–1.54 (m, 6H, 6CH2, cy-Oct), 1.79–1.95 (m, 6H, 6CH2, cy-Oct), 2.26 (s, 4H, 2CH2N), 2.60–2.63 (m, 8H, 4CH2N, morpholine), 2.99 (br.s, 2H, 2OH), 3.68–3.71 (m, 8H, 4CH2O, morpholine). 13C NMR (δ, ppm): 18.4 (2CH2, cy-Oct), 37.9 (4CH2, cy-Oct), 56.3 (4CH2N, morpholine), 67.5 (4CH2O, morpholine), 69.0 (2CH2N), 74.0 (2C). HRMS (ESI+, m/z): calculated for C18H34N2O4 [M + H]+: 343.2591, found: 343.2596.
1,5-Bis(azepan-4-ylmethyl)cyclooctane-1,5-diol (6c). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:5. Yield 19% (7 mg), yellow oil, Rf 0.08 (light petrol:DCM:MeOH 1:3:1). 1H NMR (δ, ppm): 1.38–1.52 (m, 6H, 6CH2, cy-Oct), 1.53–1.71 (m, 16H, 8CH2, azepane), 1.78–1.96 (m, 6H, 6CH2, cy-Oct), 2.43 (s, 4H, 2CH2N), 2.74–2.86 (m, 8H, 4CH2N, azepane). 13C NMR (δ, ppm): 18.7 (2CH2, cy-Oct), 27.1 (4CH2, azepane), 29.1 (4CH2, azepane), 37.8 (4CH2, cy-Oct), 59.2 (4CH2N, azepane), 68.8 (2CH2N), 73.7 (2C). HRMS (ESI+, m/z): calculated for C22H42N2O2 [M + H]+: 367.3319, found: 367.3319.
1,5-Bis(piperidin-4-ylmethyl)cyclooctane-1,5-diol (6d). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:5. Yield 97% (30 mg), yellow oil, Rf 0.09 (light petrol:DCM:MeOH 1:3:2). 1H NMR (δ, ppm): 1.35–1.53 (m, 6H, 6CH2, cy-Oct + 4H, 2CH2, piperidine), 1.53–1.65 (m, 8H, 4CH2, piperidine), 1.79–1.95 (m, 6H, 6CH2, cy-Oct), 2.29 (s, 4H, 2CH2N), 2.61 (br.s, 8H, 4CH2N, piperidine). 13C NMR (δ, ppm): 18.5 (2CH2, cy-Oct), 23.9 (2CH2, piperidine), 26.3 (4CH2, piperidine), 38.0 (4CH2, cy-Oct), 57.4 (4CH2N, piperidine), 68.6 (2CH2N), 73.3 (2C). HRMS (ESI+, m/z): calculated for C20H38N2O2 [M + H]+: 339.3006, found 339.3003.
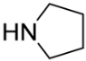
1,5-Bis(pyrrolidin-4-ylmethyl)cyclooctane-1,5-diol (6e). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:5. Yield 61% (23 mg), yellow oil, Rf 0.1 (light petrol:DCM:MeOH 1:1:1). 1H NMR (δ, ppm): 1.40–1.57 (m, 6H, 6CH2, cy-Oct), 1.68–1.79 (m, 8H, 4CH2, pyrrolidine), 1.80–1.94 (m, 6H, 6CH2, cy-Oct), 2.45 (s, 4H, 2CH2N), 2.62–2.74 (m, 8H, 4CH2N, pyrrolidine). 13C NMR (δ, ppm): 18.6 (2CH2, cy-Oct), 24.3 (4CH2, pyrrolidine), 38.0 (4CH2, cy-Oct), 57.0 (4CH2, pyrrolidine), 67.2 (2CH2N), 73.4 (2C). HRMS (ESI+, m/z): calculated for C18H34N2O2 [M + H]+: 311.2693, found: 311.2701.
1,5-Bis[(dibutylamino)methyl]cyclooctane-1,5-diol (6f). Reaction time—20 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:5. Yield 65% (28 mg), yellow oil, Rf 0.24 (light petrol:DCM:MeOH 1:4:1). 1H NMR (CD3OD; δ, ppm, J, Hz): 0.96 (t, 3J = 7.3, 12H, 4CH3, Bu), 1.25–1.42 (m, 8H, 4CH2, Bu), 1.46–1.64 (m, 14H, 6CH2, cy-Oct + 4CH2, Bu), 1.85–1.98 (m, 6H, 6CH2, cy-Oct), 2.64 (br.s, 4H, 2CH2N), 2.79 (br.s, 8H, 4CH2N, Bu). 13C NMR (CD3OD; δ, ppm): 14.3 (4CH3, Bu), 18.8 (2CH2, cy-Oct), 21.4 (4CH2, Bu), 29.0 (4CH2, Bu), 38.4 (4CH2, cy-Oct), 57.2 (4CH2N, Bu), 66.5 (2CH2N), 74.6 (2C). HRMS (ESI+, m/z): calculated for C26H54N2O2 [M + H]+: 427.4258, found: 427.4282.
1,5-Bis[(phenylamino)methyl]cyclooctane-1,5-diol (6l). Reaction time—5 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 6% (2 mg), yellow oil, Rf 0.58 (light petrol:EtOAc 2:1). 1H NMR (δ, ppm): 1.56–1.71 (m, 6H, 6CH2, cy-Oct), 1.83–1.93 (m, 2H, 2CH2, cy-Oct), 1.93–2.04 (m, 4H, 4CH2, cy-Oct), 3.05 (s, 4H, 2CH2N), 6.59–6.77 (m, 6H, 6CH, Ph), 7.12–7.20 (m, 4H, 4CH, Ph). 13C NMR (δ, ppm): 18.5 (2CH2, cy-Oct), 37.3 (4CH2, cy-Oct), 55.9 (2CH2, CH2N), 74.2 (2C), 113.4 (4CH, Ar), 118.0 (2CH, Ar), 129.4 (4CH, Ar), 148.9 (2C, Ar). HRMS (ESI+, m/z): calculated for C22H30N2O2 [M + H]+: 355.2380, found: 355.2388.
1,5-Bis[(4-bromophenylamino)methyl]cyclooctane-1,5-diol (6o). Reaction time—40 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 4% (2 mg), yellow oil, Rf 0.32 (light petrol:EtOAc 1:2). 1H NMR (δ, ppm): 1.50–1.69 (m, 6H, 6CH2, cy-Oct), 1.79–1.91 (m, 2H, 2CH2, cy-Oct), 1.91–2.02 (m, 4H, 4CH2, cy-Oct), 3.00 (s, 4H, 2CH2N), 6.49–6.59 (m, 4H, 4CH, Ar), 7.20–7.29 (m, 4H, 4CH, Ph). 13C NMR (δ, ppm): 18.4 (2CH2, cy-Oct), 37.3 (4CH2, cy-Oct), 55.8 (2CH2, CH2N), 74.2 (2C), 109.4 (2C, Ar), 114.9 (4CH, Ar), 132.1 (4CH, Ar), 147.9 (C, Ar). HRMS (ESI+, m/z): calculated for C22H28Br2N2O2 [M + H]+: 511.0590, found: 511.0587.
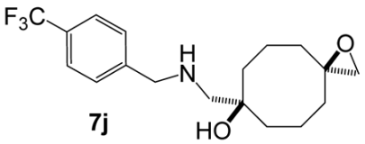
7-({[(4-Trifluoromethyl)benzyl]amino}methyl)-1-oxaspiro[2.7]decan-7-ol (7j). Reaction time—10 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 7% (2 mg), yellow oil, Rf 0.16 (EtOAc). 1H NMR (δ, ppm): 1.38–1.49 (m, 4H, 2CH2, cy-Oct), 1.56–1.87 (m, 8H, 6CH2, cy-Oct), 2.55 (s, 2H, CH2O), 2.60 (s, 2H, CH2N), 3.90 (s, 2H, ArCH2N), 7.37–7.48 (m, 2H, 2CH, Ar), 7.54–7.64 (m, 2H, 2CH, Ar). 13C NMR (δ, ppm): 19.3 (2CH2, cy-Oct), 34.9 (2CH2, cy-Oct), 35.9 (2CH2, cy-Oct), 54.1 (CH2N), 55.3 (CH2O), 58.1 (ArCH2N), 59.1 (C, epoxy), 73.8 (C-OH), 125.5 (q, 3JCF 4, 2CH, Ar), 128.4 (2CH, Ar). Signals of CF3-group and quaternary carbon atoms were not observed due to low concentration of the compound. 19F NMR (δ, ppm): −62.44 (c, 3F). HRMS (ESI+, m/z): calculated for C18H24F3NO2 [M + H]+: 344.1832, found: 344.1827.
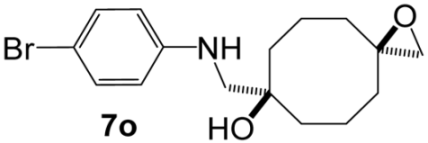
7-{[(4-Bromophenyl)amino]methyl}-1-oxaspiro[2.7]decan-7-ol (7o). Reaction time—40 h. Reagent ratio (bis(oxirane):amine:LiClO4)—1:2.2:20. Yield 6% (2 mg), yellow oil, Rf 0.39 (light petrol:EtOAc 1:1). 1H NMR (δ, ppm): 1.47–1.92 (m, 12H, 6CH2, cy-Oct), 2.63 (s, 2H, CH2O), 3.04 (s, 2H, CH2N), 7.48–7.58 (m, 2H, 2CH, Ar), 7.19–7.26 (m, 2H, 2CH, Ar). 13C NMR (δ, ppm): 19.4 (2CH2, cy-Oct), 34.9 (2CH2, cy-Oct), 35.8 (2CH2, cy-Oct), 53.5 (CH2N), 55.5 (CH2O), 59.0 (C, epoxy), 75.1 (C-OH), 109.2 (C, Ar), 114.8 (2CH, Ar), 132.1 (2CH, Ar), 147.9 (C, Ar). HRMS (ESI+, m/z): calculated for C16H22BrNO2 [M + H]+: 340.0907, found: 340.0915.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules31020252/s1; Table S1: Optimization of ring-opening conditions; copies of NMR spectra of the obtained compounds.
Author Contributions
Conceptualization, E.B.A. and K.N.S.; methodology, E.B.A. and K.N.S.; validation, K.N.S. and Y.K.G.; investigation, O.V.R., D.V.S., S.V.K., Y.K.G. and O.A.M.; data curation, K.N.S.; writing—original draft preparation, K.N.S.; writing—review and editing, E.B.A.; visualization, K.N.S. and Y.K.G.; supervision, E.B.A.; project administration, E.B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed within the framework of the state assignment (No. 121021000105-7, Molecular design, synthesis, and study of physiologically active compounds, advancing the methodology of medicinal chemistry, chemoinformatics, and targeted chemical synthesis).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Acknowledgments
The research was carried out using the NMR spectrometer Agilent 400-MR and mass-spectrometer G3 QTof quadrupole-time-of-flight purchased under the program of M.V. Lomonosov Moscow State University development.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mamedova, V.L.; Khikmatova, G.Z.; Korshin, D.E.; Mamedova, S.V.; Gavrilova, E.L.; Mamedov, V.A. Epoxides: Methods of synthesis, reactivity, practical significance. Russ. Chem. Rev. 2022, 91, RCR5049. [Google Scholar] [CrossRef]
- Meninno, S.; Lattanzi, A. Epoxides: Small Rings to Play with under Asymmetric Organocatalysis. ACS Org. Inorg. Au 2022, 2, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Moschona, F.; Savvopoulou, I.; Tsitopoulou, M.; Tataraki, D.; Rassias, G. Epoxide syntheses and ring-opening reactions in drug development. Catalysts 2020, 10, 1117. [Google Scholar] [CrossRef]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F.R.; Frey, H. Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef]
- Yang, G.-W.; Xie, R.; Zhang, Y.-Y.; Xu, C.-K.; Wu, G.-P. Evolution of Copolymers of Epoxides and CO2: Catalysts, Monomers, Architectures, and Applications. Chem. Rev. 2024, 124, 12305–12380. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, J. Regioselective Nucleophilic Ring Opening Reactions of Unsymmetric Oxiranes. Prog. Chem. 2011, 23, 165–180. [Google Scholar]
- Li, X.; Yang, Z.; Xu, J. Comprehensive Theoretical Investigation on the Regioselectivity in the Nucleophilic Ring Opening of Epoxides. Curr. Org. Synth. 2013, 10, 169–177. [Google Scholar] [CrossRef]
- Saddique, F.A.; Zahoor, A.F.; Faiz, S.; Ali, S.; Naqvi, R.; Usman, M.; Ahmad, M. Recent trends in ring opening of epoxides by amines as nucleophiles. Synth. Commun. 2016, 46, 831–868. [Google Scholar] [CrossRef]
- Meninno, S.; Lattanzi, A. Organocatalytic Asymmetric Reactions of Epoxides: Recent Progress. Chem. A Eur. J. 2016, 22, 3632–3642. [Google Scholar] [CrossRef] [PubMed]
- Faiz, S.; Zahoor, A.F. Ring opening of epoxides with C-nucleophiles. Mol. Divers. 2016, 20, 969–987. [Google Scholar] [CrossRef]
- Chen, Z.; Nasr, S.M.; Kazemi, M.; Mohammadi, M. A Mini-Review: Achievements in the Thiolysis of Epoxides. Mini-Rev. Org. Chem. 2020, 17, 352–362. [Google Scholar] [CrossRef]
- Talukdar, R. Synthetically important ring opening reactions by alkoxybenzenes and alkoxynaphthalenes. RSC Adv. 2020, 10, 31363–31376. [Google Scholar] [CrossRef]
- Kumar, A.B.; Anderson, J.M.; Melendez, A.L.; Manetsch, R. Synthesis and Structure–Activity Relationship Studies of 1,3-Disubstituted 2-Propanols as BACE-1 Inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 4740–4744. [Google Scholar] [CrossRef]
- Brediļņina, J.; Villo, P.; Andersons, K.; Toom, L.; Vares, L. Hydrolytic and Aminolytic Kinetic Resolution of Terminal Bis-Epoxides. J. Org. Chem. 2013, 78, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Zolfigol, M.A.; Moosavi-Zare, A.R.; Zarei, M.; Zare, A.; Noroozizadeh, E.; Karamian, R.; Asadbegy, M. Synthesis of β-Phthalimido-Alcohols via Regioselective Ring Opening of Epoxide by Using Reusable Basic Magnetic Nano Particles and Their Biological Investigation. RSC Adv. 2016, 6, 62460–62466. [Google Scholar] [CrossRef]
- Veidenberg, I.; Toom, L.; Villo, P.; Vares, L. An Efficient and Highly Stereoselective Approach to trans-2,5-Disubstituted-Tetrahydrofuran and trans-2,6-Disubstituted-Tetrahydropyran Derivatives. Tetrahedron Lett. 2014, 55, 3569–3571. [Google Scholar] [CrossRef]
- Qayed, W.S.; Luzzio, F.A. Chiral Pool/Henry/Enzymatic Routes to Acetogenin Synthons. Lett. Org. Chem. 2015, 12, 622–630. [Google Scholar] [CrossRef]
- Cope, A.C.; Martin, M.M.; McKervey, M.A. Transannular reactions in medium-sized rings. Q. Rev. Chem. Soc. 1966, 20, 119–152. [Google Scholar] [CrossRef]
- Reyes, E.; Uria, U.; Carrillo, L.; Vicario, J.L. Transannular reactions in asymmetric total synthesis. Tetrahedron 2014, 70, 9461–9484. [Google Scholar] [CrossRef]
- Vardanyan, R. Adrenergic (Sympathomimetic) Drugs. In Synthesis of Best-Seller Drugs; Vardanyan, R., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 189–196. [Google Scholar] [CrossRef]
- Prokhorova, I.V.; Akulich, K.A.; Makeeva, D.S.; Osterman, I.A.; Skvortsov, D.A.; Sergiev, P.V.; Dontsova, O.A.; Yusupova, G.; Yusupov, M.M.; Dmitriev, S.E. Amicoumacin A Induces Cancer Cell Death by Targeting the Eukaryotic Ribosome. Sci. Rep. 2016, 6, 27720. [Google Scholar] [CrossRef]
- Sansinenea, E.; Ortiz, A. Zwittermicin A: A Promising Aminopolyol Antibiotic from Biocontrol Bacteria. Curr. Org. Chem. 2012, 16, 978–987. [Google Scholar] [CrossRef]
- Takahashi, Y.; Igarashi, M. Destination of Aminoglycoside Antibiotics in the ‘Post-Antibiotic Era’. J. Antibiot. 2018, 71, 4–14. [Google Scholar] [CrossRef]
- Singh, G.; Gupta, N.; Sethi, N.; Gupta, V.; Raj, T.; Ishar, M.P.S. Facile Synthesis of Some New Peptidomimetic β3-and β2,3-Amino Alcohols Possessing Pyridyl Moiety via Reductive Ring Opening of Pyridyl-isoxazolidines. Chem. Biodivers. 2024, 21, e202301323. [Google Scholar] [CrossRef] [PubMed]
- Sedenkova, K.N.; Ryzhikova, O.V.; Stepanova, S.A.; Averin, A.D.; Kositov, S.V.; Grishin, Y.K.; Gloriozov, I.P.; Averina, E.B. Bis(oxiranes) containing cyclooctane core: Synthesis and reactivity towards NaN3. Molecules 2022, 27, 6889. [Google Scholar] [CrossRef]
- Sedenkova, K.N.; Savchenkova, D.V.; Ryzhikova, O.V.; Grishin, Y.K.; Averina, E.B. Stereo-Dependent Nucleophilic Ring-Opening of 1,6,10,14-Tetraoxatetraspiro[2.1.25.1.29.1.213.13]Hexadecane upon Treatment with Sodium Azide. Russ. Chem. Bull. 2024, 73, 2105–2109. [Google Scholar] [CrossRef]
- Roy, N.; Das, R.; Paira, R.; Paira, P. Different Routes for the Construction of Biologically Active Diversely Functionalized Bicyclo[3.3.1]nonanes: An Exploration of New Perspectives for Anticancer Chemotherapeutics. RSC Adv. 2023, 13, 22389–22480. [Google Scholar] [CrossRef]
- Seynaeve, C.; Verweij, J.; de Mulder, P.H.M. 5-HT3 Receptor Antagonists, a New Approach in Emesis: A Review of Ondansetron, Granisetron and Tropisetron. Anti-Cancer Drugs 1991, 2, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Brogden, R.N.; Speight, T.M.; Avery, G.S. Pentazocine: A Review of its Pharmacological Properties, Therapeutic Efficacy and Dependence Liability. Drugs 1973, 5, 6–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Huang, P.; Wang, D.; Zhang, Z.; Zhou, Z.; Liang, L.; Yao, R.; Yang, L. Cytisine: State of the art in pharmacological activities and pharmacokinetics. Biomed. Pharmacother. 2024, 171, 116210. [Google Scholar] [CrossRef]
- Ciochina, R.; Grossman, R.B. Polycyclic Polyprenylated Acylphloroglucinols. Chem. Rev. 2006, 106, 3963–3986. [Google Scholar] [CrossRef]
- Phang, Y.; Wang, X.; Lu, Y.; Fu, W.; Zheng, C.; Xu, H. Bicyclic polyprenylated acylphloroglucinols and their derivatives: Structural modification, structure-activity relationship, biological activity and mechanism of action. Eur. J. Med. Chem. 2020, 205, 112646. [Google Scholar] [CrossRef]
- Bonjoch, J.; Diaba, F.; Bradshaw, B. Synthesis of 2-Azabicyclo[3.3.1]nonanes. Synthesis 2011, 2011, 993–1018. [Google Scholar] [CrossRef]
- Kim, N.; Estrada, O.; Chavez, B.; Stewart, C., Jr.; D’Auria, J.C. Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis. Molecules 2016, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.P.; Rahman, M.T.; Cook, J.M. Bisindole Alkaloids from the Alstonia Species: Recent Isolation, Bioactivity, Biosynthesis, and Synthesis. Molecules 2021, 26, 3459. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, A.; Rossetti, A. Synthesis of Natural Compounds Based on the [3,7]-Diazabicyclo[3.3.1]nonane (Bispidine) Core. Eur. J. Org. Chem. 2021, 2021, 1491–1507. [Google Scholar] [CrossRef]
- Nurieva, E.V.; Zefirov, N.A.; Mamaeva, A.V.; Grishin, Y.K.; Kuznetsov, S.A.; Zefirova, O.N. Synthesis of Non-Steroidal 2-Methoxyestradiol Mimetics Based on the Bicyclo[3.3.1]nonane Structural Motif. Mendeleev Commun. 2017, 27, 240–242. [Google Scholar] [CrossRef]
- Nurieva, E.V.; Semenova, I.S.; Nuriev, V.N.; Shishov, D.V.; Baskin, I.I.; Zefirova, O.N.; Zefirov, N.S. The Diels–Alder Reaction as an Approach to the Synthesis of Bicyclo[3.3.1]nonane Analogues of Colchicine. Russ. J. Org. Chem. 2010, 46, 1877–1880. [Google Scholar] [CrossRef]
- Abate, C.; Perrone, R.; Berardi, F. Classes of Sigma2 (σ2) Receptor Ligands: Structure Affinity Relationship (SAfiR) Studies and Antiproliferative Activity. Curr. Pharm. Des. 2012, 18, 938–949. [Google Scholar] [CrossRef]
- Fallica, A.N.; Pittalà, V.; Modica, M.N.; Salerno, L.; Romeo, G.; Marrazzo, A.; Helal, M.A.; Intagliata, S. Recent Advances in the Development of Sigma Receptor Ligands as Cytotoxic Agents: A Medicinal Chemistry Perspective. J. Med. Chem. 2021, 64, 7926–7962. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lin, S.; Zhang, S.; Pan, L.; Chai, C.; Su, J.-C.; Yang, B.; Liu, J.; Wang, J.; Hu, Z.; et al. Modified Fusicoccane-Type Diterpenoids from Alternaria brassicicola. J. Nat. Prod. 2020, 83, 1931–1938. [Google Scholar] [CrossRef]
- Tsyrenova, B.D.; Lemport, P.S.; Nenajdenko, V.G. Di- and Polyazides: Synthesis, Chemical Transformations and Practical Applications. Russ. Chem. Rev. 2023, 92, RCR5066. [Google Scholar] [CrossRef]
- Kaur, J.; Saxena, M.; Rishi, N. An Overview of Recent Advances in Biomedical Applications of Click Chemistry. Bioconjugate Chem. 2021, 32, 2049–2069. [Google Scholar] [CrossRef] [PubMed]
- Ryzhikova, O.V.; Sedenkova, K.N.; Kositov, S.V.; Tafeenko, V.A.; Grishin, Y.K.; Averina, E.B. Stereoselective approach to hydroxyalkyl-1,2,3-triazoles containing cyclooctane core and their use for CuAAC catalysis. Catalysts 2023, 13, 835. [Google Scholar] [CrossRef]
- Ryzhikova, O.V.; Churkina, A.S.; Sedenkova, K.N.; Savchenkova, D.V.; Shakhov, A.S.; Lavrushkina, S.V.; Grishin, Y.K.; Zefirov, N.A.; Zefirova, O.N.; Gracheva, Y.A.; et al. Mono- and bis(steroids) containing a cyclooctane core: Synthesis, antiproliferative activity, and action on cell cytoskeleton microtubules. Arch. Pharm. 2024, 357, 2400483. [Google Scholar] [CrossRef]
- Hansen, T.; Vermeeren, P.; Yoshisada, R.; Filippov, D.V.; van der Marel, G.A.; Codée, J.D.C.; Hamlin, T.A. How Lewis Acids Catalyze Ring-Openings of Cyclohexene Oxide. J. Org. Chem. 2021, 86, 3565–3573. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.