Chemical Constituents from Coleus strobilifer and Their Xanthine Oxidase Inhibitory Activity
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
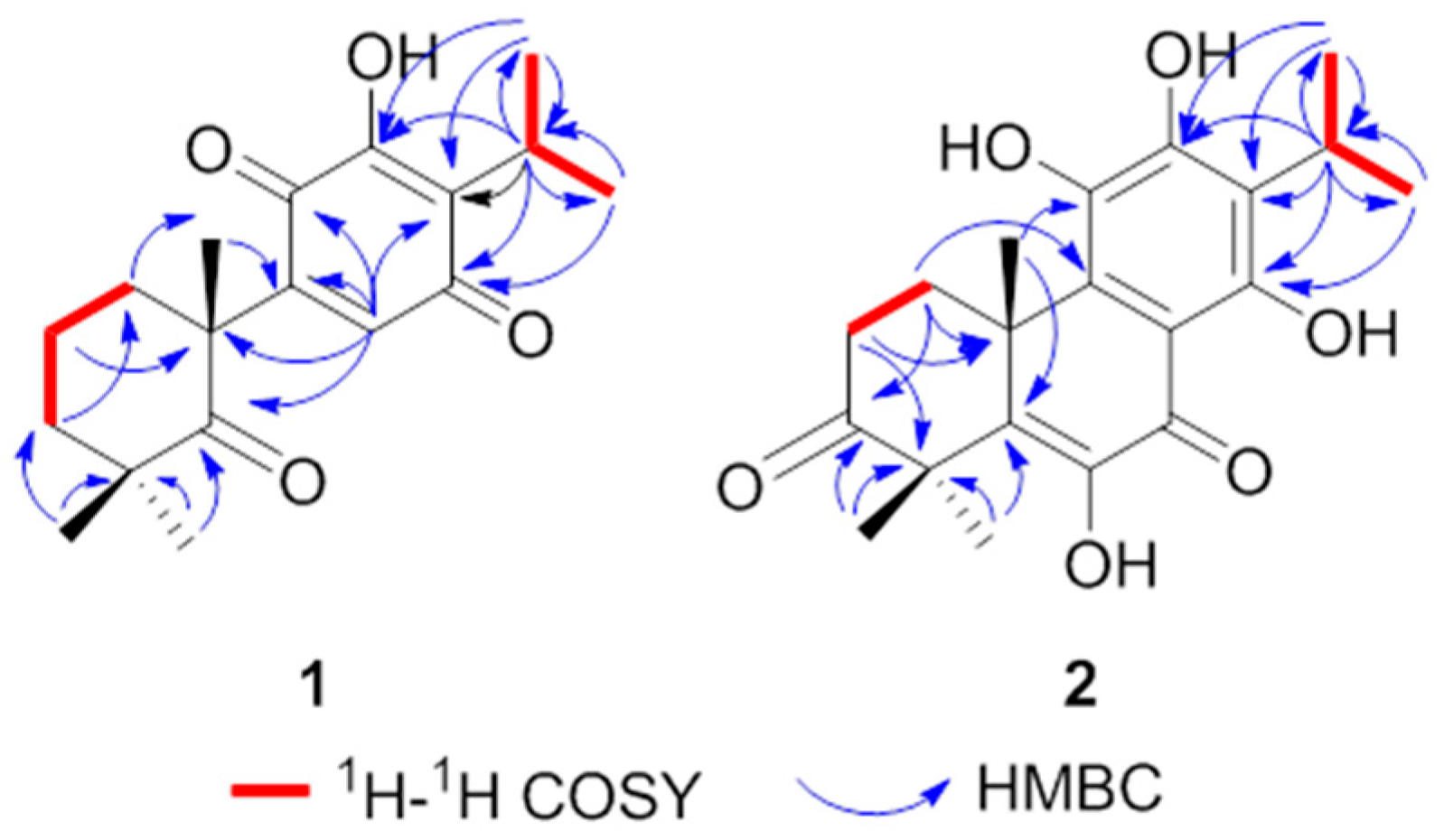
4.3.1. 10R-Carnosuain
4.3.2. 10R-Coleon U-3-One
4.3.3. X-Ray Crystal Structure Analysis
4.4. Determination of Xanthine Oxidase Inhibitory Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z.; Gao, J. Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Zhao, Z.; Li, X.; Pang, J.; Chen, J. Recent Progress and Future Perspectives on Anti-Hyperuricemic Agents. J. Med. Chem. 2024, 67, 19966–19987. [Google Scholar] [CrossRef]
- Piani, F.; Agnoletti, D.; Borghi, C. Advances in pharmacotherapies for hyperuricemia. Expert. Opin. Pharmacother. 2023, 24, 737–745. [Google Scholar] [CrossRef]
- Otani, N.; Ouchi, M.; Kudo, H.; Tsuruoka, S.; Hisatome, I.; Anzai, N. Recent approaches to gout drug discovery: An update. Expert Opin. Drug Discov. 2020, 15, 943–954. [Google Scholar] [CrossRef]
- Senatore, F.; Lentini, F.; Venza, F.; Bruno, M.; Napolitano, F. Composition and antibacterial activity of the essential oil of Anisochilus carnosus (Linn. fil.) Benth., a Tamil plant acclimatized in Sicily. Flavour Fragr. J. 2003, 18, 202–204. [Google Scholar] [CrossRef]
- National Administration of Traditional Chinese Medicine. Chinese Materia Medica; Shanghai Scientific & Technical Publishers: Shanghai, China, 1998; Herbal Volume, p. 6012. [Google Scholar]
- Shang, C.L.; Li, Y.X.; Duan, S.Q.; Zhang, N.; Lu, Y. Study on quality standard of Anisochilus carnosus Fructus. Chin. Mod. Med. 2016, 23, 12–15. [Google Scholar]
- Gandhi, R.R.; Kopare, N.P.; Rathod, S.A.; Shirsat, R.P.; Koche, D.K. Physico-chemical, Fluorescent and Phytochemical analysis of Anisochilus carnosus (Lf) Wall: A Lamiaceae herb from Maharashtra, India. Indian J. Appl. Pure Biol. 2022, 37, 1–12. [Google Scholar]
- Bhagat, J.; Lobo, R.; Parmar, V.; Ballal, M. In vitro Free Radical Scavenging Potential of Indian Habitant Anisochilus carnosus (L.F.) Wall. Chin. J. Nat. Med. 2011, 9, 456–460. [Google Scholar]
- Gupta, N.; Lobo, R.; Kumar, N.; Bhagat, J.; Mathew, J. Identity-based high-performance thin layer chromatography fingerprinting profile and tumor inhibitory potential of Anisochilus carnosus (L.f.) wall against ehrlich ascites carcinoma. Pharmacogn. Mag. 2015, 11, 474. [Google Scholar]
- Shirsat, R.P.; Imran, S.; Deepak, K.K. A report on identification of a unique hygrine like compound from chloroform extract of Anisochilus carnosus (Lf) Wall. Drug Discov. 2020, 14, 130–134. [Google Scholar]
- Bhagat, J.; Lobo, R.; Kumar, N.; Mathew, J.E.; Pai, A. Cytotoxic potential of Anisochilus carnosus (Lf) wall and estimation of luteolin content by HPLC. BMC Complement. Altern. Med. 2014, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.N.; Li, B.; Li, T.Z. Compositions and Methods for Inhibiting Xanthine Oxidase. CN Patent CN202211017599.0, 2 November 2022. [Google Scholar]
- Hijazi, M.; Hijazi, K.; Bouhadir, K.; Fatfat, Z.; Aboul-Ela, M.; Gali-Muhtasib, H.; El-Lakany, A. Anticancer activity of abietane diterpenoids from Salvia libanoticum grown in Lebanon. Pharmacogn. Mag. 2021, 17, 127. [Google Scholar] [CrossRef]
- Ntungwe, E.N.; Stojanov, S.J.; Duarte, N.M.; Candeias, N.R.; Díaz-Lanza, A.M.; Vágvölgyi, M.; Hunyadi, A.; Pešić, M.; Rijo, P. C20-nor-Abietane and Three Abietane Diterpenoids from Plectranthus mutabilis Leaves as P-Glycoprotein Modulators. ACS Med. Chem. Lett. 2022, 13, 674–680. [Google Scholar] [CrossRef]
- Kusumoto, N.; Ashitani, T.; Hayasaka, Y.; Murayama, T.; Ogiyama, K.; Takahashi, K. Antitermitic Activities of Abietane-type Diterpenes from Taxodium distichum Cones. J. Chem. Ecol. 2009, 35, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.C.; Rüdi, P.; Eugster, H. Drüsenfarbstoffe aus Labiaten: Die polaren Diterpenoide aus Plectranthus argentatus S. T. BLAKE. Helv. Chim. Acta 1984, 67, 1523–1530. [Google Scholar] [CrossRef]
- Urones, J.G.; Marcos, I.S.; Cubillo, I.; Garrido, N.M.; Basabe, P. Terpenoid compounds from Parentucellia latifolia. Phytochemistry 1990, 29, 2223–2228. [Google Scholar] [CrossRef]
- Huo, L.N.; Wang, W.; Liu, Y.; Liu, X.H.; Zhang, L.; Cheng, K.; Liu, K.; Gao, H. Chemical constituents from leaves of Perilla frutescens. Chin. Tradit. Herb. Drugs 2016, 47, 26–31. [Google Scholar]
- Hung, C.Y.; Tsai, Y.C.; Li, K.Y. Phenolic Antioxidants Isolated from the Flowers of Osmanthus fragrans. Molecules 2012, 17, 10724–10737. [Google Scholar] [CrossRef]
- Chen, C.L.; Hu, S.J.; Zhu, L.L.; Yao, L.M.; Wang, X.X.; Zhang, A.N.; Zhang, Q.Y.; Qin, L.P.; Wu, J.J. Isolation and identification of chemical constituents of Rubi Fructus, screening novel cannabinoid CB2 receptor agonist and evaluation of its anti-osteoporosis effect. Chin. Tradit. Herb. Drugs 2024, 55, 386–401. [Google Scholar]
- Zhao, J.; Wei, F.; Liu, H.; Qin, R.; Yang, X. Two aromatic acid derivatives and a xanthone from Hypericum hengshanense. Nat. Prod. Res. 2024, 38, 1537–1544. [Google Scholar] [CrossRef]
- Games, E.; Guerreiro, M.; Santana, F.; Pinheiro, N.; De Oliveira, E.; Lopes, F.; Olivo, C.; Tibério, I.; Martins, M.; Lago, J.; et al. Structurally Related Monoterpenes p-Cymene, Carvacrol and Thymol Isolated from Essential Oil from Leaves of Lippia sidoides Cham. (Verbenaceae) Protect Mice against Elastase-Induced Emphysema. Molecules 2016, 21, 1390. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Dong, M.H.; Zhou, L.; Zhao, C.L.; Ye, J.H.; Zhang, J. Chemical constituents from Isodon amethystoides distributed in Libo. Chin. Tradit. Herb. Drugs 2020, 51, 4405–4410. [Google Scholar]
- Acebedo, S.L.; Alonso, F.; Ramírez, J.A.; Galagovsky, L.R. Synthesis of aromatic stigmastanes: Application to the synthesis of aromatic analogs of brassinosteroids. Tetrahedron 2012, 68, 3685–3691. [Google Scholar] [CrossRef]
- Dube, N.P.; Tembu, V.J.; Nyemba, G.R.; Davison, C.; Rakodi, G.H.; Kemboi, D.; De La Mare, J.A.; Siwe-Noundou, X.; Manicum, A.-L.E. In vitro cytotoxic effect of stigmasterol derivatives against breast cancer cells. BMC Complement. Med. Ther. 2023, 23, 316. [Google Scholar] [CrossRef]
- Wang, M.C.; Kong, W.Z.; Yang, G.C.; Wang, C.H.; Zhang, L.H.; Gao, J.M.; Zhang, X.Y. Structure, anti-inflammatory and anti-bacterial activities of novel pentacyclic triterpenoids and other constituents from the leaves of Pittosporum elevaticostatum. Fitoterapia 2024, 177, 106142. [Google Scholar] [CrossRef]
- Brown, G.D.; Liang, G.Y.; Sy, L.K. Terpenoids from the seeds of Artemisia annua. Phytochemistry 2003, 64, 303–323. [Google Scholar] [CrossRef]
- Duan, W.L.; Lou, J.H.; Wang, Q.; Zhao, Z.Y.; Lai, Q.; Pei, Q.; Pei, S.F.; Zeng, G.Z.; Yin, J.L. Study on the chemical constituents of Cyperus papyrus. Nat. Prod. Res. Dev. 2021, 33, 1129–1136. [Google Scholar]
- Jin, H.Z.; Mao, S.H.; Zhou, T.X.; Wang, R.; Tang, X.C.; Qin, G.W. Alkaloids from Stems of Sinomenium acutum. Chin. J. Nat. Med. 2007, 5, 35–37. [Google Scholar]
- Zhang, X.; Wang, Y.; Qin, Q.; Wang, Y.; Xu, J.; He, X. Pronounced anti-neuroinflammatory jasmonates and terpenes isolated from lychee seeds. Fitoterapia 2021, 152, 104924. [Google Scholar] [CrossRef]
- Yang, P.W.; Zhang, P.L.; Han, Z.J.; Yang, Y.B.; Wu, T. Two new sulfur-containing derivatives from Raphani Semen. Chin. Tradit. Herb. Drugs 2023, 54, 3408–3416. [Google Scholar]
- Collin, G.; Garneau, F.X.; Gagnon, H.; Pichette, A.; Lavoie, S. Analysis of Cymenes in Essential Oils: The Case of Lepechinia meyeni (Walp.) Epling. J. Essent. Oil Res. 2010, 22, 310–313. [Google Scholar] [CrossRef]
- Carman, R.M.; Garner, A.C.; Klika, K.D. 2,9-Dihydroxy- and 2,10-Dihydroxy-1,8-cineole. Two New Possum Urinary Metabolites. Aust. J. Chem. 1994, 47, 1509–1521. [Google Scholar] [CrossRef]
- Li, M.X.; Cui, X.L.; Huang, D.D.; Ji, R.F.; Yang, J.; He, X. Chemical constituents from Tripterygium wilfordii and antioxidant activities offlavanols. Chin. Tradit. Herb. Drugs 2023, 54, 6220–6227. [Google Scholar]
- Gáborová, M.; Šmejkal, K.; Kubínová, R. Abietane diterpenes of the genus Plectranthus sensu lato. Molecules 2021, 27, 166. [Google Scholar] [CrossRef]
- Grayer, R.J.; Paton, A.J.; Simmonds, M.S.; Howes, M.J.R. Differences in diterpenoid diversity reveal new evidence for separating the genus Coleus from Plectranthus. Nat. Prod. Rep. 2021, 38, 1720–1728. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Sarkar, D.; Shetty, K. Metabolic stimulation of plant phenolics for food preservation and health. Annu. Rev. Food Sci. Technol. 2014, 5, 395–413. [Google Scholar] [CrossRef]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the roles of plant sterols in development and stress responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the biosynthesis of terpenoids and their ecological functions in plant resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef]
- Zhong, H.; Abdulla; Zhang, Y.; Deng, L.; Zhao, M.; Tang, J.; Zhang, H.; Feng, F.; Wang, J. Exploring the potential of novel xanthine oxidase inhibitory peptide (ACECD) derived from Skipjack tuna hydrolysates using affinity-ultrafiltration coupled with HPLC-MALDI-TOF/TOF-MS. Food Chem. 2021, 347, 129068. [Google Scholar] [CrossRef]


| No. | Compound 1 1 | Compound 2 2 | ||
|---|---|---|---|---|
| δC (ppm) | δH (ppm, J in Hz) | δC (ppm) | δH (ppm, J in Hz) | |
| 1 | 38.8 | 1.59 (dq, J = 12.7, 2.8 Hz, 1H) 2.02, overlap | 28.6 | 3.45 (1H, m) 1.88 (1H, dt, J = 13.7, 9.8 Hz) |
| 2 | 18.4 | 1.92, overlap 1.76, overlap | 34.2 | 2.80 (1H, ddd, J = 19.0, 9.3, 1.4 Hz) 2.64 (1H, ddd, J = 19.0, 10.5, 9.1 Hz) |
| 3 | 38.8 | 1.97, overlap 1.73, overlap | 217.7 | |
| 4 | 44.7 | 50.0 | ||
| 5 | 218.0 | 140.5 | ||
| 6 | 142.1 | |||
| 7 | 182.8 | |||
| 8 | 134.7 | 6.51 (1H, s, H-8) | 106.9 | |
| 9 | 150.1 | 135.5 | ||
| 10 | 49.9 | 40.2 | ||
| 11 | 183.3 | 136.7 | ||
| 12 | 151.2 | 154.4 | ||
| 13 | 125.5 | 121.1 | ||
| 14 | 187.3 | 159.4 | ||
| 15 | 34.1 | 3.16 (1H, hept, J = 7.1 Hz, H-13) | 26.0 | 3.48 (1H, m, H-15) |
| 16 | 19.9 | 1.20 (3H, s, H-14) | 20.5 | 1.34 (3H, s, H-16) |
| 17 | 19.9 | 1.20 (3H, s, H-15) | 20.6 | 1.36 (3H, s, H-17) |
| 18 | 28.0 | 1.22 (3H, s, H-16) | 21.6 | 1.55 (3H, s, H-18) |
| 19 | 28.4 | 1.21 (3H, s, H-17) | 25.1 | 1.55 (3H, s, H-19) |
| 20 | 23.4 | 1.38 (3H, s, H-18) | 21.6 | 1.42 (3H, s, H-20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Qin, J.-X.; Hong, Y.; Gan, X.-N.; Li, T.-Z.; Wang, M.-Q.; Zheng, X.-W.; Li, B.; Fang, X.; Liang, S. Chemical Constituents from Coleus strobilifer and Their Xanthine Oxidase Inhibitory Activity. Molecules 2026, 31, 30. https://doi.org/10.3390/molecules31010030
Qin J-X, Hong Y, Gan X-N, Li T-Z, Wang M-Q, Zheng X-W, Li B, Fang X, Liang S. Chemical Constituents from Coleus strobilifer and Their Xanthine Oxidase Inhibitory Activity. Molecules. 2026; 31(1):30. https://doi.org/10.3390/molecules31010030
Chicago/Turabian StyleQin, Jia-Xu, Yang Hong, Xiao-Na Gan, Ting-Zhao Li, Meng-Qi Wang, Xiang-Wei Zheng, Bo Li, Xin Fang, and Shuang Liang. 2026. "Chemical Constituents from Coleus strobilifer and Their Xanthine Oxidase Inhibitory Activity" Molecules 31, no. 1: 30. https://doi.org/10.3390/molecules31010030
APA StyleQin, J.-X., Hong, Y., Gan, X.-N., Li, T.-Z., Wang, M.-Q., Zheng, X.-W., Li, B., Fang, X., & Liang, S. (2026). Chemical Constituents from Coleus strobilifer and Their Xanthine Oxidase Inhibitory Activity. Molecules, 31(1), 30. https://doi.org/10.3390/molecules31010030






