Microalgae and Macroalgae as Advanced Sources of Tyrosinase Inhibitors
Abstract
1. Introduction
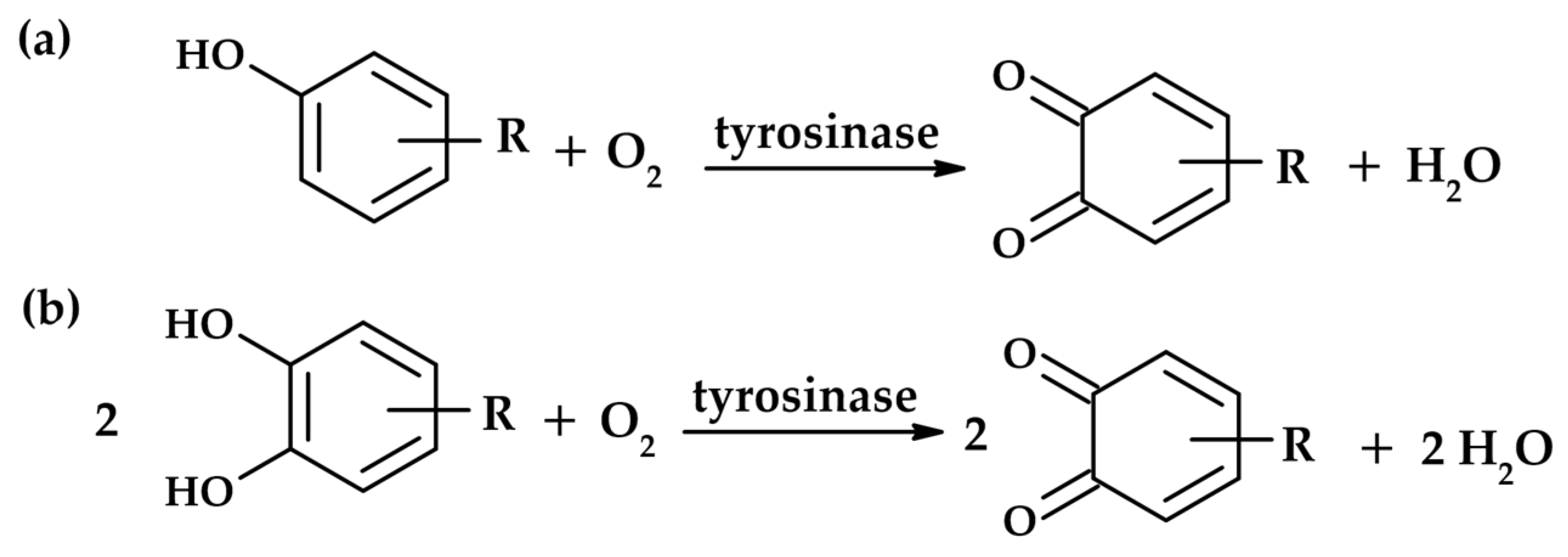
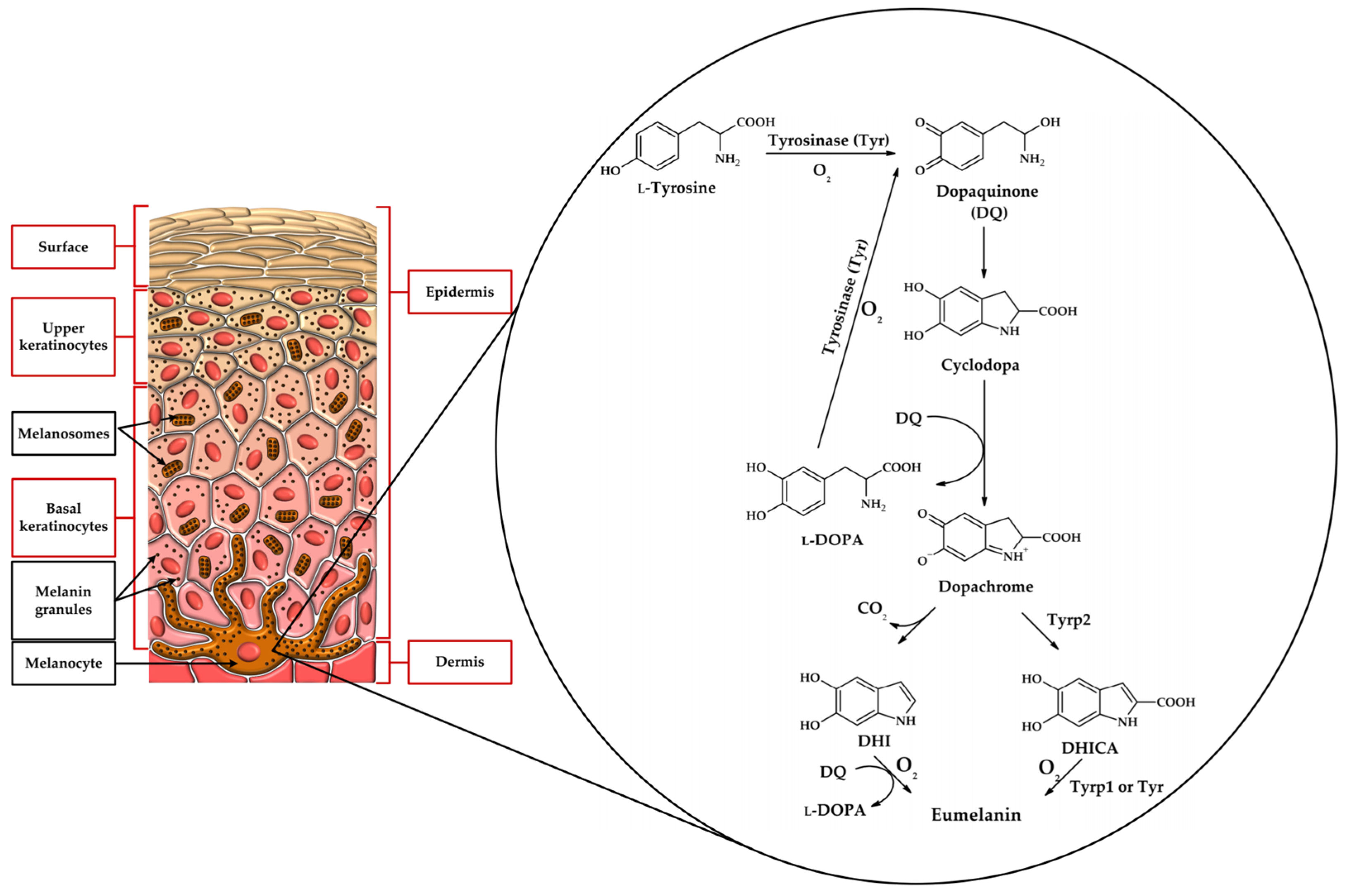
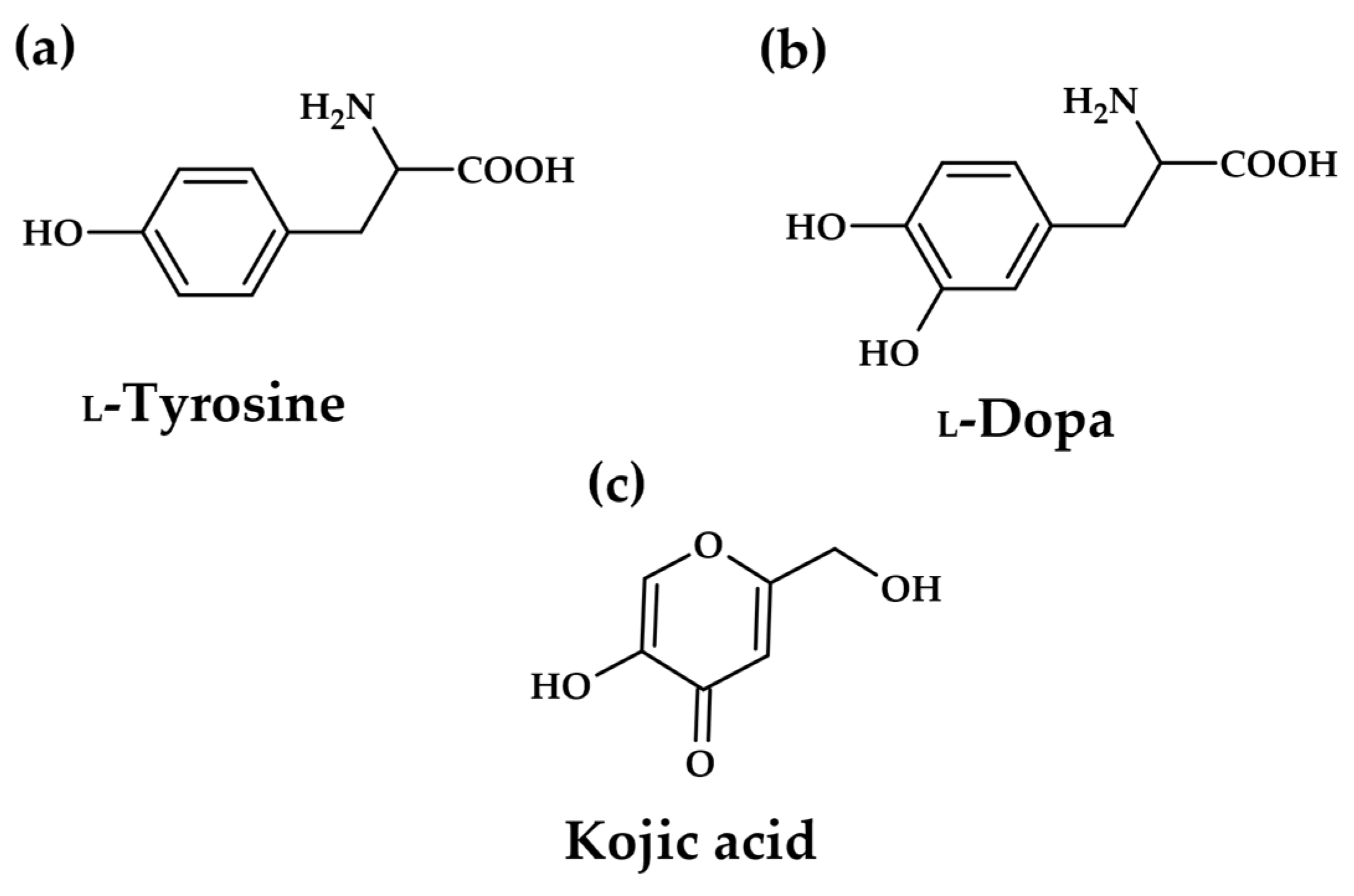
1.1. Tyrosinase
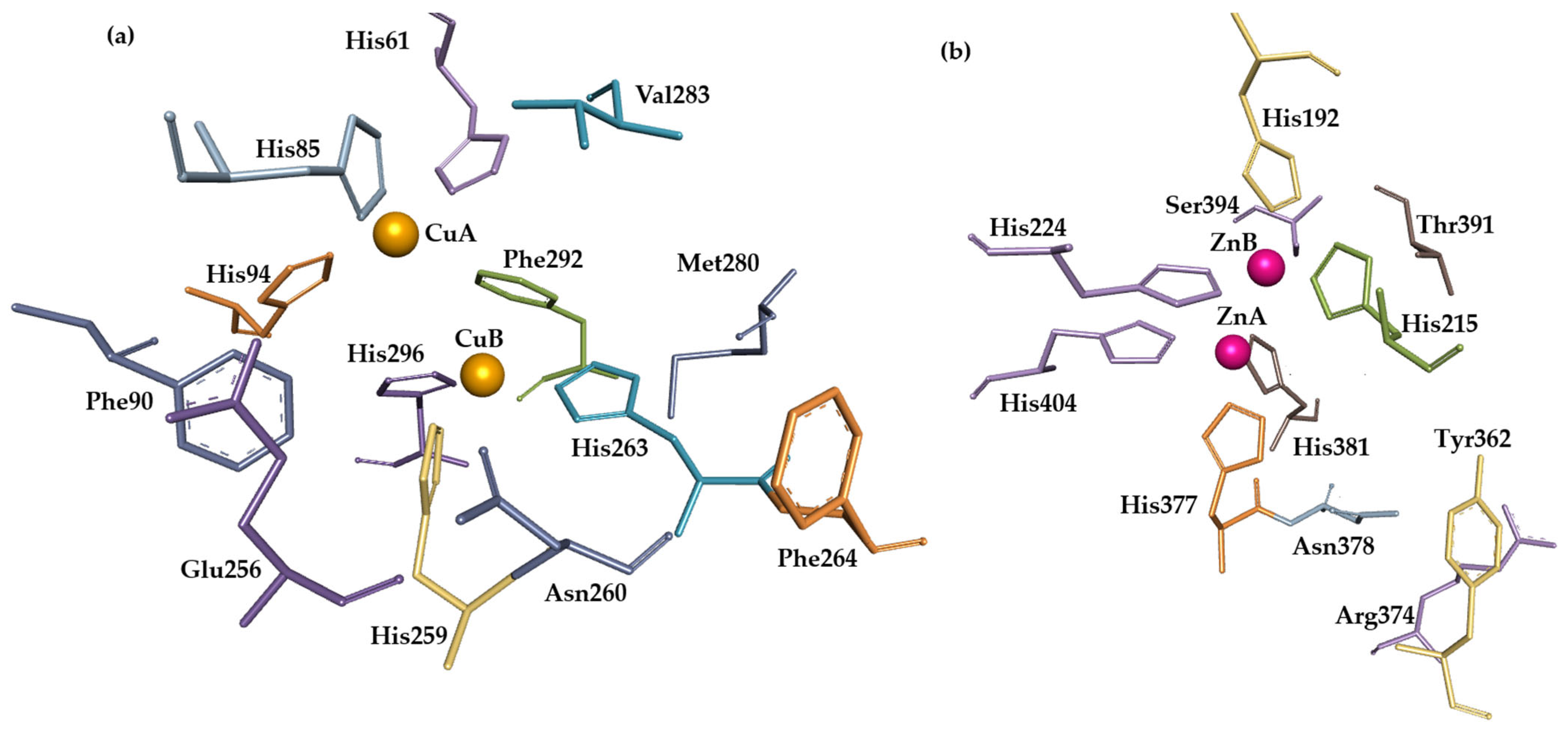
1.2. Tyrosinase Active Site
1.3. Marine Phycochemicals
2. Algal Sources of Tyrosinase Inhibitors
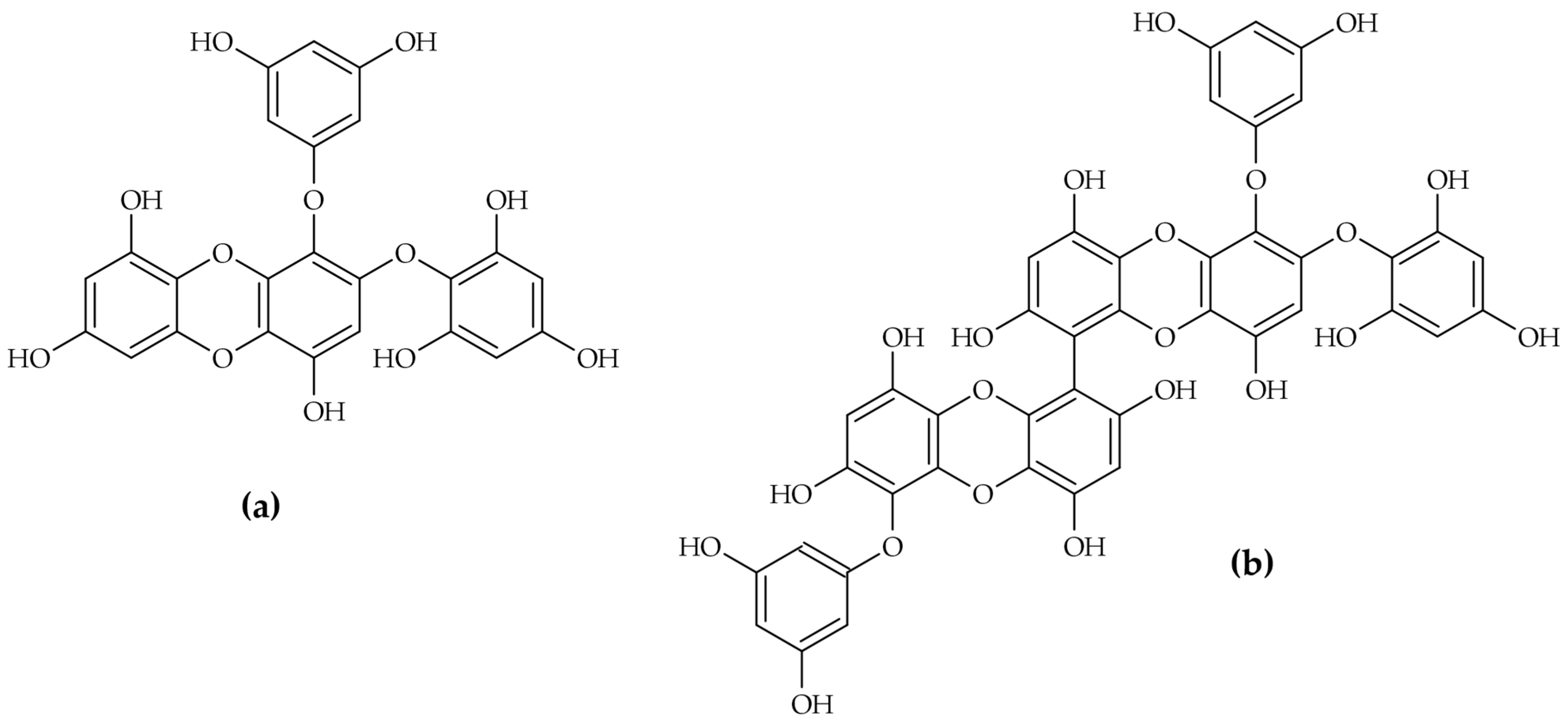
2.1. Macroalgae (Seaweeds)
2.2. Microalgae and Cyanobacteria
3. Biochemical Mechanisms and Enzyme Kinetics of Algal Inhibitors
- Non-competitive inhibitors: These compounds bind to the enzyme at an allosteric site distinct from the active centre. They interact with both the free enzyme and the E-S complex with the same equilibrium constant [34]. Interestingly, crude extracts of brown algae like Ecklonia cava and Eisenia bicyclis often exhibit non-competitive inhibition kinetics [13].
- Allosteric inhibitors: Some enzymes possess more than one site able to bind ligands. A ligand that binds at one site induces structural changes in the protein that are transmitted via the polypeptide chain to the other active site, diminishing the binding ability of the substrate to its active site, is called an allosteric inhibitor [34]. An example of such an inhibitor is phlorotannins, sourced from Ecklonia stolonifera phlorofucofuroeckol-A [35]. Carotenoids, such as apocarotenoids (e.g., bixin), are also reported to inhibit tyrosinase allosterically through hydrophobic interactions [29].
- 2-phloroeckol: Single-step mechanism (mechanism A). This molecule exhibits a competitive, slow-binding profile characterized by a single-step association, where the ligand slowly binds directly to the active site to form a stable encounter complex [12].
- 2-O-(2,4,6-trihydroxyphenyl)-6,6′-bieckol: Two-step mechanism (mechanism B). This compound, which has a higher molecular weight and more complex structure, follows a more complex mechanism. It involves an initial rapid interaction followed by a slower enzyme isomerisation that results in a new, long-lived conformational state of the enzyme [12]. This ability to induce a structural shift and persistently inactivate the enzyme imparts a significant pharmacological advantage.
4. Sustainable Extraction and Green Chemistry
5. Commercial Application Landscape and Future Biorefineries
5.1. Cosmeceutical and Skin-Whitening Applications
5.2. Medical and Agricultural Potential
5.3. Comparative Sustainability Assessment (LCA)
5.4. Algal Safety
6. Conclusions and Critical Outlook
- Standardization and comparative metrics—Researchers must adopt rigorous, standardized kinetic assays and consistently report Relative Inhibitory Activity (RA) normalized against a positive control to allow for scientifically robust comparison across the highly variable literature [31].
- Technological integration—The adoption of green extraction technologies, such as Natural Deep Eutectic Solvents (NADESs), is vital for achieving high yield, low cost, and maximal stability of sensitive compounds like phlorotannins during scale-up [51]. Concurrently, microencapsulation and targeted delivery systems are necessary to ensure the clinical efficacy of labile bioactives.
- Translational safety assessment—Future research must prioritize comprehensive in vivo and ADMET/toxicology evaluations, similar to those performed for stigmasterol, to validate the safety and selectivity of new inhibitors before clinical integration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solomon, E.I.; Baldwin, M.J.; Lowery, M.D. Electronic structures of active sites in copper proteins: Contributions to reactivity. Chem. Rev. 1992, 92, 521–542. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M. The catalytic cycle of tyrosinase: Peroxide attack on the phenolate ring followed by O-O bond cleavage. J. Biol. Inorg. Chem. 2003, 8, 567–576. [Google Scholar] [CrossRef]
- Claus, H.; Decker, H. Bacterial tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Schurink, M.; Boeriu, C.G.; Wichers, H.; Dijkstra, B.W. Crystallization and preliminary X-ray crystallographic analysis of tyrosinase from the mushroom Agaricus bisporus. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2011, 67, 575–578. [Google Scholar] [CrossRef]
- D’Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galvan, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and melanogenesis: From pigment cells to human health and technological applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; Ozeki, H. Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigment Cell Res. 2000, 13, 103–109. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef]
- Land, E.J.; Ramsden, C.A.; Riley, P.A. The mechanism of suicide-inactivation of tyrosinase: A substrate structure investigation. Tohoku J. Exp. Med. 2007, 212, 341–348. [Google Scholar] [CrossRef]
- Beltran, E.; Serafini, M.R.; Alves, I.A.; Aragón Novoa, D.M. Novel Synthesized Tyrosinase Inhibitors: A Systematic Patent Review (2012-Present). Curr. Med. Chem. 2024, 31, 308–335. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Suyama, T.L.; Kim, H.; Glukhov, E.; Gerwick, W.H. Discovery of Novel Tyrosinase Inhibitors from Marine Cyanobacteria. Front. Microbiol. 2022, 13, 912621. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Kim, Y.; Kim, Y.T. A Study on the Tyrosinase Inhibitory and Antioxidant Effect of Microalgae Extracts. Microbiol. Biotechnol. Lett. 2021, 49, 167–173. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Park, S.; Park, J.S.; Kim, Y.H.; Yang, S.Y. Slow-Binding Inhibition of Tyrosinase by Ecklonia cava Phlorotannins. Mar. Drugs 2019, 17, 359. [Google Scholar] [CrossRef]
- Ryu, J.; Yim, M.; Kim, J.; Lee, J.M.; Lee, M.S.; Lee, D.; Hwang, J.-Y.; Kim, K.T.; Kim, Y.-M.; Eom, S. Tyrosinase inhibition effects of korean edible brown, green, and red seaweed extracts. Fish. Aquat. Sci. 2024, 27, 468–473. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of Human Tyrosinase Related Protein 1 Reveals a Binuclear Zinc Active Site Important for Melanogenesis. Angew. Chem. Int. Ed. 2017, 56, 9812–9815. [Google Scholar] [CrossRef] [PubMed]
- Ghani, U. Azole Inhibitors of Mushroom and Human Tyrosinases: Current Advances and Prospects of Drug Development for Melanogenic Dermatological Disorders. Eur. J. Med. Chem. 2022, 239, 114525. [Google Scholar] [CrossRef]
- Thong-Olran, A.; Sermsakulwat, S.; Napiroon, T.; Jaikaew, P.; Kongkiatpaiboon, S.; Tayana, N.; Wichachucherd, B.; Charoenrat, T.; Traijitt, T.; Chittapun, S. Seaweed-derived bioactives with anti-tyrosinase activity: A potential for skin-whitening cosmetics with in silico and in vitro approaches. Biotechnol. Rep. 2025, 47, e00910. [Google Scholar] [CrossRef] [PubMed]
- Golshany, H.; Siddiquy, M.; Elbarbary, A.; Seddiek, A.S.; Kamal, A.; Yu, Q.; Fan, L. Exploring fucus vesiculosus phlorotannins: Insights into chemistry, extraction, purification, identification and bioactivity. Food Biosci. 2024, 61, 104769. [Google Scholar] [CrossRef]
- Ayub, A.; Rahayu, F.; Khamidah, A.; Antarlina, S.S.; Iswari, K.; Supriyadi, K.; Mufidah, E.; Singh, A.; Chopra, C.; Wani, A.K. Harnessing microalgae as a bioresource for nutraceuticals: Advancing bioactive compound exploration and shaping the future of health and functional food innovation. Discov. Appl. Sci. 2025, 7, 389. [Google Scholar] [CrossRef]
- Jayawardhana, H.A.C.K.H.; Jayawardena, U.T.; Sanjeewa, K.K.A.; Liyanage, N.M.; Nagahawatta, D.P.; Lee, H.G.; Kim, J.; Jeon, Y.J. Marine algal polyphenols as skin protective agents: Current status and future prospectives. Mar. Drugs 2023, 21, 285. [Google Scholar] [CrossRef]
- Je, J.G.; Jiang, Y.; Heo, J.H.; Li, X.; Jeon, Y.J.; Ryu, B.M. Mitigative Effects of PFF-A Isolated from Ecklonia cava on Pigmentation in a Zebrafish Model and Melanogenesis in B16F10 Cells. Mar. Drugs 2022, 20, 123. [Google Scholar] [CrossRef]
- Haldys, K.; Latajka, R. Thiosemicarbazones with tyrosinase inhibitory activity. MedChemComm 2019, 10, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Gunasekara, D.M.N.M.; Wang, L.; Herath, K.H.I.N.M.; Sanjeewa, K.K.A. Cosmeceutical Applications of Phlorotannins from Brown Seaweeds. Phycology 2025, 5, 15. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Z.; Zhang, J.; Xu, Y. Bromophenols from the red alga Sargassum latiuscula inhibit tyrosinase activity and melanin synthesis. Mar. Drugs 2019, 17, 295. [Google Scholar] [CrossRef]
- Ferreira, L.L.G.; Andricopulo, A.D. ADMET Modeling Approaches in Drug Discovery. Drug Discov. Today 2019, 24, 1157–1165. [Google Scholar] [CrossRef]
- Muruganandam, A.R.; Venkatasubramanian, S.; Jagmag, S.A.; Veerichetty, V. Antityrosinase activity of phycocyanin and cream formulation for hyperpigmentation. IOP Conf. Ser. Mater. Sci. Eng. 2023, 1291, 012039. [Google Scholar] [CrossRef]
- Anantharaman, A.; Hemachandran, H.; Priya, R.R.; Sankari, M.; Gopalakrishnan, M.; Palanisami, N.; Siva, R. Inhibitory effect of apocarotenoids on the activity of tyrosinase: Multi-spectroscopic and docking studies. J. Biosci. Bioeng. 2016, 121, 13–20. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids: Distribution, Function in Nature, and Analysis Using LC-Photodiode Array Detector (DAD)-MS and MS/MS System. Mass Spectrom. 2023, 12, A0133. [Google Scholar] [CrossRef]
- Proteau, P.J.; Gerwick, W.H.; Garcia-Pichel, F.; Castenholz, R.W. The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 1993, 49, 825–829. [Google Scholar] [CrossRef]
- Baeza-Morales, A.; Medina-García, M.; Martínez-Peinado, P.; Pascual-García, S.; Pujalte-Satorre, C.; López-Jaén, A.B.; Martínez-Espinosa, R.M.; Sempere-Ortells, J.M. The Antitumour Mechanisms of Carotenoids: A Comprehensive Review. Antioxidants 2024, 13, 1060. [Google Scholar] [CrossRef]
- Seo, J.P.; Kim, M.S.; Han, M.H.; Oh, B.K.; Cho, Y.J. The Composition for Tyrosinase Inhibitory Activity and Melanin Inhibitory. Korean Patent No. KR20150058715A, 29 May 2015. [Google Scholar]
- Silva, T.; SSalomon, P.; Hamerski, L.; Walter, J.; BMenezes, R.; Siqueira, J.E.; Santos, A.; Santos, J.A.M.; Ferme, N.; Guimarães, T.; et al. Inhibitory effect of microalgae and cyanobacteria extracts on influenza virus replication and neuraminidase activity. PeerJ 2018, 6, e5716. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-D.; Choi, H.; Abekura, F.; Park, J.-Y.; Yang, W.-S.; Yang, S.-H.; Kim, C.-H. Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation. Int. J. Mol. Sci. 2023, 24, 8226. [Google Scholar] [CrossRef]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis; WILEY: Hoboken, NJ, USA, 2004. [Google Scholar]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.R.; Jung, H.A.; Choi, J.S. Phlorotannins with Potential Anti-Tyrosinase and Antioxidant Activity Isolated from the Marine Seaweed Ecklonia Stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Matoba, Y.; Kumagai, T.; Yamamoto, A.; Yoshitsu, H.; Sugiyama, M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J. Biol. Chem. 2006, 281, 8981–8990. [Google Scholar] [CrossRef]
- Catarino, M.D.; Costa, B.S.B.; Circuncisão, A.R.; Silva, A.M.S.; Cardoso, S.M.; Braga, S.S. γ-Cyclodextrin Inclusion of Phloroglucinol: Solid State Studies and Antioxidant Activity throughout the Digestive Tract. Appl. Sci. 2022, 12, 2340. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant potential of extracts obtained from macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and micro-algae (Chlorella vulgaris and Spirulina platensis) assisted by ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef]
- Steevensz, A.J.; Mackinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.B.; Melanson, J.E. Profiling phlorotannins in brown macroalgae by liquid chromatography-high resolution mass spectrometry. Phytochem. Anal. 2020, 23, 547–553. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of extraction of phlorotannins from the Arctic Fucus vesiculosus using natural deep eutectic solvents and their HPLC profiling with tandem high-resolution mass spectrometry. Mar. Drugs 2023, 21, 263. [Google Scholar] [CrossRef]
- Rivera-Tovar, P.R.; Contreras-Contreras, G.; Rivas-Reyes, P.I.; Pérez-Jiménez, J.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S. Sustainable Recovery of Phlorotannins from Durvillaea incurvata: Integrated Extraction and Purification with Advanced Characterization. Antioxidants 2025, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.T.; Tran, D.P.; Dam, D.T.; Nguyen, M.L.; Nguyen, T.M.A.; Pham, T.H.M.; Le, X.D.; Do, H.N.; Pham, T.H.H.; Pham, T.N.; et al. Optimization of microwave-assisted extraction of phlorotannin from Sargassum swartzii (Turn.) C. Ag. Ethanol/Water. Nat. Prod. Commun. 2021, 16, 1934578X21996184. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate composition, phenolic content and in vitro antioxidant activity of aqueous extracts of the seaweeds Ascophyllum nodosum, Bifurcaria bifurcata and Fucus vesiculosus. effect of addition of the extracts on the oxidative stability of canola oil under accelerated storage conditions. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yi, M.; Ding, L.; He, S.; Yan, X. Isolation and purification of a neuroprotective phlorotannin from the marine algae Ecklonia maxima by size exclusion and high-speed counter-current chromatography. Mar. Drugs 2019, 17, 212. [Google Scholar] [CrossRef]
- Montero, L.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Separation and characterization of phlorotannins from brown algae Cystoseira abies-marina by comprehensive two-dimensional liquid chromatography. Electrophoresis 2014, 35, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.H.; Mäki-Arvela, P.; Mikkola, J.P.; Lienqueo, M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2017, 16, 201–208. [Google Scholar] [CrossRef]
- Ammari, N.; Rae, M.; Khatib, N.E.; Islam, S.; Ranade, V.V.; Walker, G.; Singh, M. Sustainable and novel approaches for bioactive compound extraction: Development of hydrodynamic cavitation and coupled machine learning-spline techniques for Ascophyllum nodosum and Fucus vesiculosus. Food Chem. 2025, 495, 146210. [Google Scholar] [CrossRef]
- Kurihara, H.; Kujira, K. Phlorotannins derived from the brown alga colpomenia bullosa as tyrosinase inhibitors. Nat. Prod. Commun. 2021, 16, 1934578X211021317. [Google Scholar] [CrossRef]
- Martínez, J.H.; Castañeda, H.G. Preparation and chromatographic analysis of phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef]
- Ford, L.; Theodoridou, K.; Sheldrake, G.N.; Walsh, P.J. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef]
- Fu, W.; Magnúsdóttir, M.; Brynjólfson, S.; Palsson, B.Ø.; Paglia, G. UPLC-UV-MSE analysis for quantification and identification of major carotenoid and chlorophyll species in algae. Anal. Bioanal. Chem. 2012, 404, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef]
- Ryu, N.H.; Lim, Y.; Park, J.E.; Kim, J.; Kim, J.Y.; Kwon, S.W.; Kwon, O. Impact of daily Chlorella consumption on serum lipid and carotenoid profiles in mildly hypercholesterolemic adults: A double-blinded, randomized, placebo-controlled study. Nutr. J. 2014, 13, 57. [Google Scholar] [CrossRef]
- Tello, P.; Santos, J.; Calero, N.; Trujillo-Cayado, L.A. Formulation and Characterization of Sustainable Algal-Derived Nanoemulgels: A Green Approach to Minimize the Dependency on Synthetic Surfactants. Polymers 2024, 16, 194. [Google Scholar] [CrossRef]
- Sahil, S.; Bodh, S.; Verma, P. Spirulina platensis: A comprehensive review of its nutritional value, antioxidant activity and functional food potential. J. Cell. Biotechnol. 2024, 10, 159–172. [Google Scholar] [CrossRef]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Liu, X.; Yu, C. Extraction and Nano-Sized Delivery Systems for Phlorotannins to Improve Its Bioavailability and Bioactivity. Mar. Drugs 2021, 19, 625. [Google Scholar] [CrossRef]
- Okeke, E.S.; Nweze, E.J.; Chibuogwu, C.C.; Anaduaka, E.G.; Chukwudozie, K.I.; Ezeorba, T.P.C. Aquatic phlorotannins and human health: Bioavailability, toxicity, and future prospects. Nat. Prod. Commun. 2021, 16, 1934578X211056144. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, H.; Yu, X. Improving potential strategies for biological activities of phlorotannins derived from seaweeds. Crit. Rev. Food Sci. Nutr. 2023, 64, 9832–9853. [Google Scholar] [CrossRef]
- Mirchandani, Y.; Patravale, V.B. Solid lipid nanoparticles for hydrophilic drugs. J. Control. Release Off. J. Control. Release Soc. 2021, 335, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Alğın Yapar, E. Skin Whiteners an Overview. Marmara Pharm. J. 2016, 21, 48–53. [Google Scholar] [CrossRef]
- Liu, H.; Li, G.; Wang, H. Skin Whitening Complex Prepared from Algae Serving as Main Raw Materials. Chinese Patent No. CN101904804A, 9 June 2010. [Google Scholar]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Grand View Research. Skin lightening Products Market Size, Share & Trends Analysis Report by Product (Creams, Lotions), by Nature (Synthetic, Natural), by Region, and Segment Forecasts, 2022–2030. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/skin-lightening-products-market (accessed on 28 November 2025).
- Nirmal, N.P.; Benjakul, S. Inhibition of melanosis formation in pacific white shrimp by the extract of lead (Leucaena leucocephala) seed. Food Chem. 2011, 128, 427–432. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Alavi, S.N.; Sarvestani, F.S.; Shaabani, S.; Ibáñez, E. Seaweed phenolic compounds: Relationship with antioxidant activity and application in different food products. Food Chem. X 2025, 32, 103242. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Valenzuela-Soto, E.; Lizardi-Mendoza, J.; Goycoolea, F.; Martínez-Téllez, M.A.; Villegas-Ochoa, M.A.; Monroy-García, I.N.; Ayala-Zavala, J.F. Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol’. J. Sci. Food Agric. 2009, 89, 15–23. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, S.; Su, J.; Zhu, M.; Zhou, M.; Chen, T.; Han, Y. Recent advances in carrageenan-based films for food packaging applications. Front. Nutr. 2022, 9, 1004588. [Google Scholar] [CrossRef]
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Biotechnol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Sijil, P.V.; Cherita, C.; Jethani, H.; Chauhan, V.S. Chapter 1. microalgae as a renewable and sustainable source of high value metabolites. In Microalgae for Sustainable Products; Royal Society of Chemistry: London, UK, 2022; pp. 1–26. [Google Scholar] [CrossRef]
- Lerat, Y.; Cornish, M.L.; Critchley, A.T. Applications of algal biomass in global food and feed markets: From traditional usage to the potential for functional products. In Blue Biotechnology; Wiley-VCH: Weinheim, Germany, 2018; pp. 143–189. [Google Scholar] [CrossRef]
- Salehipour-Bavarsad, F.; Nematollahi, M.A.; Pistocchi, R.; Pezzolesi, L. Algal Food Safety: Possible Contaminations, Challenges of Harmonized Quality Assessments, and Suggested Recommendations for the Nascent Industry of Microalgae-Based Products. Algal Res. 2024, 81, 103579. [Google Scholar] [CrossRef]
- Wu, G.; Zhuang, D.; Chew, K.W.; Ling, T.C.; Khoo, K.S.; Van Quyen, D.; Feng, S.; Show, P.L. Current Status and Future Trends in Removal, Control, and Mitigation of Algae Food Safety Risks for Human Consumption. Molecules 2022, 27, 6633. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final report of the safety assessment of kojic acid as used in cosmetics. Int. J. Toxicol. 2010, 29, 244S–273S. [Google Scholar] [CrossRef]
- Zilles, J.C.; Benvenutti, R.; Matte, U.; Ferrari, M.; Carvalho, T. Biological activities and safety data of kojic acid and its derivatives: A review. Exp. Dermatol. 2022, 31, 1640–1652. [Google Scholar] [CrossRef]
- Owolabi, J.O.; Fabiyi, O.S.; Adelakin, L.A.; Ekwerike, M.C. Effects of Skin Lightening Cream Agents—Hydroquinone and Kojic Acid, on the Skin of Adult Female Experimental Rats. Clin. Cosmet. Investig. Dermatol. 2020, 13, 283–289. [Google Scholar] [CrossRef] [PubMed]


| Source Organism (Species) | Compound/ Extract | Chemical Class | IC50 Value (μg/mL or μM) | Kojic Acid Reference IC50 (μM) | Inhibition Type | Ref. |
|---|---|---|---|---|---|---|
| Cyanobacteria | scytonemin monomer (ScyM) | apocarotenoid | 4.90 µM | 11.31 µM | single molecule | [10] |
| Ecklonia cava (brown alga) | total phenolic extract | phlorotannins | 4.38 ± 0.08 µg/mL | N/A | non-competitive (extract) | [13] |
| Ecklonia cava (brown alga) | 2-phloroeckol | phlorotannin | 7.0 ± 0.2 µM | N/A | competitive, slow-binding | [12] |
| Eisenia bicyclis (brown alga) | extract | phlorotannins | 4.46 ± 0.52 µg/mL | N/A | non-competitive (extract) | [13] |
| Spirulina platensis (microalga) | phycocyanin | phycobiliprotein | 30.88 µg/mL (cell-based) | 34.06 µg/mL (Cell-based) | N/A | [20] |
| Gracilaria fisheri (red alga) | stigmasterol (isolated) | sterol | 3.38 ± 0.28 µg/mL (cell viability) | N/A | highly selective | [13] |
| Compound/Extract | Source (Algal Division) | Inhibition Type | Key Binding Features /Mechanism | Ref. |
|---|---|---|---|---|
| 2-O-(2,4,6-trihydroxyphenyl)-6,6′-bieckol | Phaeophyceae (E. cava) | Competitive, slow-binding | Two-step enzyme isomerisation, extensive H-bonding (Lys79, His85) | [12] |
| Dieckol | Phaeophyceae (E. stolonifera) | Non-competitive | Allosteric binding site, high binding affinity (Ki = 15 μM) | [11,32] |
| Dimeric Bromophenol (Comp. 3) | Rhodophyceae (S. latiuscula) | Competitive | Direct active site binding, H-bonding to Arg268 and Per404 | [22] |
| Scytonemin Monomer (ScyM) | Cyanobacteria | Slowly reversible mixed-type | Binds E and E-S complex; phenol moiety indispensable | [10] |
| Peptide DER | Microalgae (Spirulina) | Competitive/active site | H-bonding with His244, His259, His260, Asn260 (MD confirmed) | [39] |
| Algal Group (Class) | Representative Species | Primary Active Compound Class | Specific Bioactive Examples | Commercial Advantage/Sustainability Note | Ref. |
|---|---|---|---|---|---|
| Cyanobacteria | Arthrospira platensis (Spirulina) | Phycobiliproteins | C-Phycocyanin | High biomass productivity, GRAS status, NLC potential for stability | [23] |
| Cyanobacteria | Marine Cyanobacteria | Apocarotenoids | Scytonemin, Monomer (ScyM) | Ultra-high potency, novel small molecular scaffold | [10] |
| Chlorophyceae | Dunaliella tertiolecta | Carotenoids/phenolics | β-carotene, Zeaxanthin, phenolic acids | Efficient cultivation, dual antioxidant role | [11] |
| Bacillariophyceae | Nitzschia sp. | Phenolics/extracts | Undefined extract | Patented application for melanin inhibition | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Harasym, J.; Hałdys, K. Microalgae and Macroalgae as Advanced Sources of Tyrosinase Inhibitors. Molecules 2026, 31, 20. https://doi.org/10.3390/molecules31010020
Harasym J, Hałdys K. Microalgae and Macroalgae as Advanced Sources of Tyrosinase Inhibitors. Molecules. 2026; 31(1):20. https://doi.org/10.3390/molecules31010020
Chicago/Turabian StyleHarasym, Joanna, and Katarzyna Hałdys. 2026. "Microalgae and Macroalgae as Advanced Sources of Tyrosinase Inhibitors" Molecules 31, no. 1: 20. https://doi.org/10.3390/molecules31010020
APA StyleHarasym, J., & Hałdys, K. (2026). Microalgae and Macroalgae as Advanced Sources of Tyrosinase Inhibitors. Molecules, 31(1), 20. https://doi.org/10.3390/molecules31010020







