Abstract
Phenanthrenes, which are polycyclic aromatic hydrocarbons comprising three benzene rings, exhibit a diverse range of functions. These compounds are utilized in the synthesis of resins, plant growth hormones, reducing dyes, tannins and other products. Notably, phenanthrenes possess significant pharmacological properties, including anti-tumor, anti-inflammatory and antioxidant activities, offering broad prospects for development, particularly in the fields of medicine and health. Interestingly, although aristolochic acid (AA) is a potent carcinogen, its lactam analogs can kill cancer cells and exhibit therapeutic effects against cancer. This provides a promising strategy for the toxicity-effect transformation of phenanthrenes. In this paper, we reviewed 137 articles to systematically review the anti-tumor potential and toxic effects of natural phenanthrenes isolated from the 19th century to the present, thus offering references and laying a foundation for their further research, development and utilization.
1. Introduction
Phenanthrenes are a type of aromatic hydrocarbon metabolite formed through the aromatic epoxidation coupling of styrene. Figure 1 illustrates the parent structure of phenanthrenes. Over the past decade, the incidence and mortality rates of malignant tumors in China have continued to rise. Currently, the 5-year relative survival rate for malignant tumors stands at approximately 40.5%. This represents an increase of about ten percentage points compared to ten years ago. In recent years, research into naturally occurring anticancer agents has gained popularity. The phenanthrenes reviewed are predominantly found in nature, with over 250 types identified, mostly oxygen-containing analogs derived from advanced plants. A substantial body of research indicates that the majority of phenanthrenes originate from higher plants, particularly within the Orchidaceae family, which includes 49 species such as Dendrobium Cymbidium, Elia, Jaws, Bletilla, Coelogyna, Cymbidium, Mayfly and Epidermis [1]. Phenanthrenes have also been identified in the Dioscoreaceae, Asteraceae and Betulaceae families. Typically, phenanthrenes are isolated from the entire plant, and research has also occasionally focused on the cortex, tubers or stems. In recent years, numerous studies have been conducted at home and abroad on the anti-tumor activity of phenanthrenes. For example, chrysotoxol and nudol extracted and isolated from Dendrobium officinale have inhibitory effects on liver cancer and osteosarcoma; the 7-methoxy-8-methyl-5-vinyl-9,10-dihydro-phenanthren-2-ol isolated from J.effususus showed inhibitory effects on A2780, A2780 cis, KCR, MCF-7, HeLa, HTB-26 and T47D human tumor cells; and the 5,5′,7,7′-tetramethoxy-9,9′,10,10′-tetrahydro-[3,3′-biphenanthrene]-2,2′-diol isolated from the orchid plant Panlongshen has moderate anticancer activity against liver cancer.
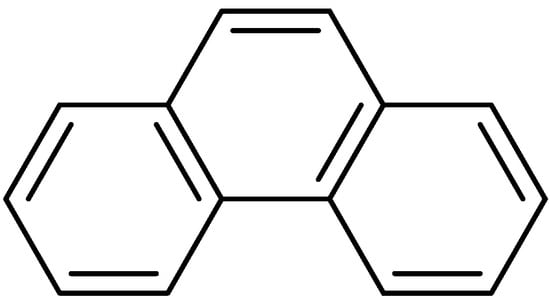
Figure 1.
The parent structure of phenanthrenes.
Although phenanthrenes themselves are not carcinogenic, they serve as prototype molecules for the biological studies of carcinogenic compounds such as benzo[a]anthracene, dibenzo[a,h]anthracene and benzo[a]pyrene. These compounds were classified as carcinogens by the World Health Organization in 2017 [2]. For instance, the metabolism of deuterated phenanthrene in humans has been used as a marker to assess the potential susceptibility of smokers to lung cancer [3]. Aristolochic acid, a nitrophenic acid compound, can cause various cancers such as urothelial, renal cell, hepatocellular, biliary tract and gastrointestinal cancers when ingested through Chinese herbal medicines, skin contact or the inhalation of herbal powder [4]. AAs comprise a group of nitrophenanthrene organic acids naturally present in Aristolochia plants such as Radix Aristolochiae fangchi and Asarum caudigerellum extensively utilized in traditional Chinese medicine as raw materials [5]. AAI, the most prevalent compound, is found in nearly all Aristolochia plants, often co-existing with aristolactams [4]. The primary toxic components in these compounds are AAI and AAII [6]. Catalyzed by nitroreductase, some of these compounds are reduced to aristolochic lactam, while others interact with DNA during the reduction process to form adducts. Nitro groups are critically toxic in AA derivatives, with methoxy and hydroxyl groups further enhancing toxicity. AAI is notably the most toxic element in Aristolochia plants. Further studies have confirmed the strong nephrotoxicity of AA and its metabolite, aristolochic lactam. The mutation and carcinogenic toxicity of AA are linked to its metabolite, AA lactam nitrogen ion, due to its strong electrophilic nature. It can bind to the extracyclic amino groups of DNA bases, forming adducts that may mutate the RAS and p53 genes, subsequently inducing tumors [7,8]. In fact, researchers found the tumor inhibitory activity of AA in the early stage of the study but eventually slowed down due to the toxicity of AA [9,10,11]. In this paper, we summarize the research on the antitumor activity and toxicity of phenanthrenes. To further explore their potential of toxicity-effect transformation, future strategies may include structural modification, traditional Chinese medicine processing techniques or other biological approaches to achieve. In addition, we can gain a more comprehensive understanding through more advanced research design and methods.
2. Antitumor Activities of Phenanthrene Alkaloids from Natural Products
Phenanthrene and dihydrophenanthrene, isolated from various plants, have demonstrated cytotoxic properties against specific tumor cell lines [12]. In vitro effects may be mediated by several potential mechanisms: cell membrane rupture, impaired cell metabolism, DNA damage or a combination of these. Inducing apoptosis in tumor cells is a primary anticancer strategy and a common mechanism of various anticancer drugs, involving multiple apoptotic mediators that lead to programmed cell death (Figure 2).
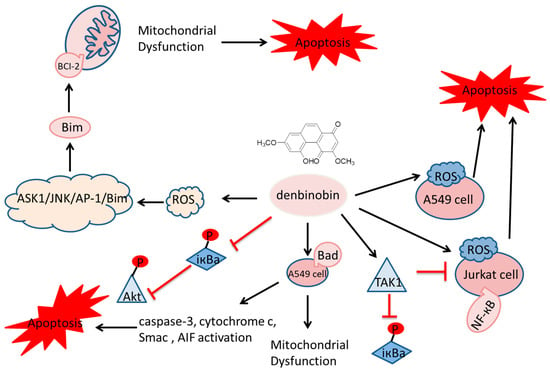
Figure 2.
Cytotoxic properties against specific tumor cell lines.  indicate the promotional effects,
indicate the promotional effects,  indicate the inhibitory effects.
indicate the inhibitory effects.
2.1. Antitumor Phenanthrenes
Orchids are the principal family and the most abundant source of natural phenanthrenes. Twenty-four phenanthrenes have been isolated from Dendrobium genus [12], and there are 12 phenanthrenes in Dendrobium officinale Kimura & Migo alone: chrysotoxene (1), confusarin (2), nudol (3), 2,5-Dihydroxy-3,4-dimethoxyphenylene (4), 2,3,4,7-tetramethoxy phenylene (5), 2,7-Diarboxy-1,5,6-trimethoxyphenanthrene (6), 2,5-Dicarboxy-3,4–dimethoxy phenyl (7), 3,5-Dicarboxy-2,4-dimethoxyphenyl (8) and denbinobin (9) (Figure 3). Recent pharmacological studies have revealed their diverse physiological activities, including antitumor, antioxidant and anti-inflammatory effects. Compounds 1-3 have exhibited inhibitory effects on liver [13,14], lung [15] and osteosarcoma [16] cancers in vitro. The cytotoxic activity of compound 1 against HepG2 cells has an IC50 of 19.64 µM. The IC50 values for compound 2 on HI-60 and THP-1 cells are 18.95 ± 0.70 and 11.51 ± 0.12 µM, respectively. For compound 3, IC50 values against the MG63 cell line were 21.86 ± 0.17 µM (24 h), 14.58 ± 0.24 µM (48 h) and 12.97 ± 0.28 µM (72 h); for the U2OS cell line, the values were 21.52 ± 0.08 µM (24 h), 13.99 ± 0.16 µM (48 h) and 11.29 ± 0.21 µM (72 h). Compound 9 has been shown to inhibit NF-κB signaling, exerting anti-inflammatory effects [17,18]. Additionally, compound 2 has been found to promote the growth of neural synapses [19].
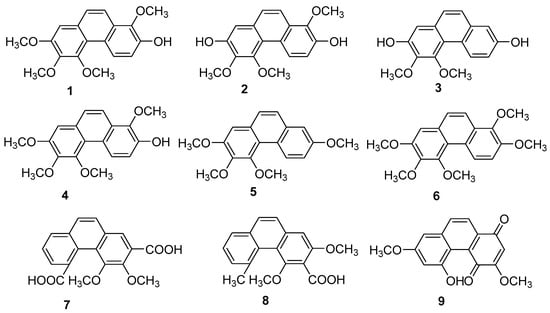
Figure 3.
The phenanthrenes in Dendrobium officinale.
Tamus communis, a member of the Dioscoreaceae family, is native to Asia, Africa and Europe. Five phenanthrenes (10–14) (Figure 4) were isolated from the fresh rhizomes of Tamus communis by Réthy. They are 7-hydroxy-2,3,4-trimethoxy-phenanthrene (10), 2,7-dihydroxy-3,4-dimethoxy-phenanthrene (11), 7-dihydroxy-3,4,8-trimethoxyphenan threne (12), 3,7-dihydroxy-2,4,8-trimethoxyphenanthrene (13) and 3,7-dihydroxy-2,4-dimethoxy phenanthrene (14). Compound 10 is a newly discovered natural product, and compounds 11–14 were isolated from Tamus communis for the first time. An in vitro MTT assay assessing the cytotoxicity against the HeLa cell line revealed that compound 12 has significant activity, with an IC50 value of 0.97 μM [20].
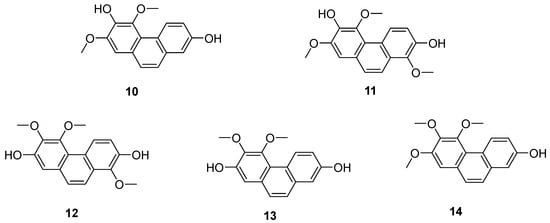
Figure 4.
The phenanthrenes in Tamus communis.
Jinchai Dendrobium Lindl. (Dendrobium nobile Lindl.), a valuable medicinal herb within the Orchidaceae family, is part of the Dendrobium genus. Recent research has highlighted phenanthrenes in Dendrobium officinale Lindl. as critical compounds for investigating its anticancer properties, with several exhibiting varying degrees of antitumor activity. Zhou et al. extracted, isolated and purified these natural products from Dendrobium officinale extracts. Subsequently, four phenanthrenes were separated using diverse chromatographic techniques: dentiflor B (15), cypripedin (16), moscatin (17) and 2,4,8-trimethoxyphenanthene-3,7-diol (18) (Figure 5). These compounds were tested on human breast cancer MCF-7 cells, demonstrating significant inhibitory effects, which provide robust support for the antitumor research of phenanthrenes in Dendrobium nobile [21]. The IC50 values for compound 12 on MCF-7, A549 and SW480 cells were 23.75 ± 0.82, 16.29 ± 0.25 and 18.97 ± 1.04 μM [22]. Liang et al. [23] isolated 1,5,6-trimethoxy-2,7-dihydroxy-phenanthrene (19) (Figure 5) from Dendrobium officinale, which displayed significant cytotoxic effects on HeLa and HepG2 cells, with IC50 values of 0.42 and 0.20 µM.
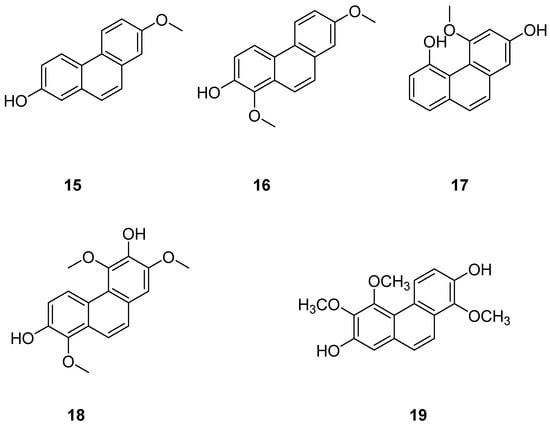
Figure 5.
The phenanthrenes in Dendrobium nobile.
Phenanthrenes are prevalent secondary metabolites in plants of the Lamiaceae family. In 2002, Italian scholar Della Greca et al. isolated two new phenanthrenes (20–21) (Figure 6) and one pyrene compound from Juncus acutus L. [24]. In 2020, Ma’s team isolated nine compounds, including coumarins and eight phenanthrenes, from non-polar n-hexane and CH2Cl2 fractions of Luzula sylvatica. Among them, four compounds were newly discovered: hydrojuncinol (22) (Figure 6), sylvatin A, sylvatin B and sylvatin C. Phenanthrene has demonstrated promising in vitro anti proliferative activity against various cancer cell lines [25,26,27]. Consequently, the cytotoxicity of these compounds was evaluated for THP-1 (a monocytic leukemia cell line) using a diazazoline assay. The IC50 values for compound 22 and hydrojuncuenin (23) (Figure 6) were found to be 3 and 5 μM. Moreover, compound 22 significantly inhibited the production of reactive oxygen species (ROS) in a dose-dependent manner, showing moderate cytotoxicity on THP-1 cells with an IC50 lower than 6 µM [28]. Further research will focus on the most active compounds, particularly the newly identified phenanthrene dehydrogenated nepenthol, aiming to explore its inhibitory effects on other tumor cells and delve deeper into its mechanism of action. Wen ming Liu et al. isolated and purified 2,7-dihydroxy-1-methyl-5-vinylphenanthrene, named dehydrogenated rush alcohol (24) (Figure 6), from traditional Chinese herbal medicine rush, finding that this compound effectively inhibited gastric cancer cell-mediated angiogenesis mimicry with very low toxicity. The activity of SGC-7901 and AGS cells was studied using this compound, and their IC50 values were 35.89 µM and 32.92 µM. DHE has demonstrated an inhibitory effect on the growth of gastric cancer cells [29] by inducing tumor-suppressing endoplasmic reticulum (ER) stress responses and reducing tumor-adaptive ER responses [30]. Two compounds, 5-(1-methoxyethyl)-1-methyl-phenanthrene-2,7-diol (25) and Dehydroeffusal (26) (Figure 6), were isolated from the ethanol extract of Juncus effuses by Ma. Cell activity experiments were conducted on SHSY-5Y, SMMC-7721, HepG-2, Hela and MCF-7 cancer cells. These compounds exhibited selective inhibitory activity against MCF-7 cells, with an IC50 of 10.9 μM. Compound 26 inhibited the growth of HepG2 and HeLa cells, with very similar IC50 values of 12.4 and 13.1 μM, respectively [25]. Denbinobin (27), fimbriol B (28) and 2,3,5-trihydroxy-4,9-dimethoxy phenanthrene (29) (Figure 6) showed a strong ability to induce apoptosis in hepatocytes [31]. Nam et al. studied the cytotoxic effects of nine phenanthrenes and 9,10-dihydro-phenanthrenes on human hypopharyngeal squamous cell carcinoma cell lines, concluding that methylation of the phenolic group or its oxidation to an oxygen-containing group enhances cytotoxic activity [32].
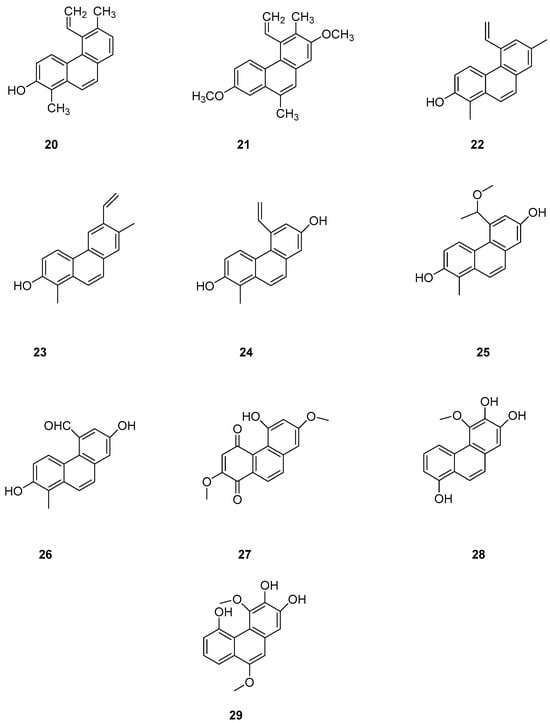
Figure 6.
The phenanthrenes in L. sylvatica.
Batatasins, endogenous plant hormones first isolated from the leftover seeds of yam by Hashimoto et al. in 1972 [33], belong to the homodiene class. These molecules feature a variety of hydroxyl and methoxy groups on the A/B ring, with the A ring containing several functional and methoxy groups in the ortho and para-positions. The B ring, however, only contains substitutions in the ortho and meta-positions, lacking oxygen groups [34]. The molecular structure of yam compounds resembles that of resveratrol, which is known for its anti-inflammatory, antioxidant, and anti-tumor properties [35,36]. Consequently, the physiological functions of yam compounds have garnered increasing attention in recent years. Yam Batatasins I (30) (Figure 7) was isolated by Min Hye Yang et al. from the chloroform phase of a crude yam extract and found to inhibit both type I and type II cyclooxygenases, with IC50 values of 3.0 ± 0.4 and 2.7 ± 0.7 μg/mL [37]. Simultaneously, members of Yue’s research group isolated the same compound from the dichloromethane phase of a crude yam extract, discovering its ability to suppress the production of prostaglandin D2 and leukotriene C4 in mouse bone marrow cells, achieving an IC50 value of 1.78 μM [38].
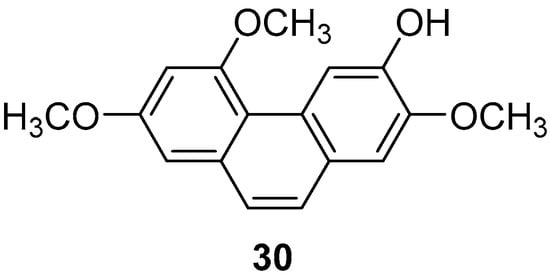
Figure 7.
The phenanthrene in yam.
2.2. Antitumor 9,10-Dihydrophenanthrene
The search for eco-friendly algae removal agents to manage algal blooms in eutrophic habitats is a prominent area of natural product chemistry. Among these, 9,10-dihydrophenanthrene isolated from the wetland plant Juncus effusus is noted for its allelopathic effects on harmful seaweed organisms. In further in-depth research, Marina Della Greca et al. also investigated Juncus acutus, which was another wetland cordyceps species from the Mediterranean region, where 9,10-dihydrophenanthrene compounds are significant active ingredients [24]. They reported the separation of nine types of 9,10-dihydrophenanthrene (31–39) (Figure 8), three phenanthrenes, and one related pyrene compound. Some phenanthrenes isolated from Juncus effusus have shown promising cytotoxic and in vitro antitumor activities [39,40]. In 1992, Dellagreca et al. isolated many 9,10-dihydrophenanthrene compounds with in vitro antitumor activity. Csaba Bús et al. assessed these compounds across seven human tumor cell lines (A2780, A2780 cis, KCR, MCF-7, HeLa, HTB-26 and T47D) and one normal cell line (MRC-5) using an MTT assay. The IC50 values of compound 22 against these cell lines were 22.3 ± 2.7, 16.9 ± 4.7, 24.2 ± 2.1, 12.9 ± 0.2, 24.7 ± 0.3, 22.8 ± 0.2, 14.2 ± 1.1 and 18.9 ± 4.0 μM, respectively. The IC50 values of compound 24 were 23.8 ± 1.3, 37.1 ± 2.8, 35.8 ± 1.7, 37.1 ± 1.1, 0.5 ± 0.0, 41.7 ± 3.5, 25.0 ± 0.4 and 40.9 μM, respectively [41]. Over recent years, several phenanthrenes from the Juncus genus have been tested for their in vitro cytotoxicity against various cancer cell lines using different test systems, exhibiting promising activities. For instance, Su et al. conducted a chemical study on the ethyl acetate-soluble fraction of the ethanol extract from the medulla of rush grass, isolating three new compounds, 9,10-dihydro phenanthrene and rush grass extracts E–G (40–42) (Figure 8); two new phenanthrene types, dehydrogenated rush grass extracts D–E; a new ferulic glycoside; and a known 9,10-dihydrophenanthrene: 4,7-dihydroxy-2-methoxy-9,10-dihydrophenanthrene (43) (Figure 8). They evaluated the in vitro cytotoxic activity of compounds 40–43 on seven human cancer cell lines (A549, MCF-7, BEL-7402, HeLa, COLO 205, BGC-823 and SK-OV-3). Among them, compound 40 exhibited weak cytotoxicity on MCF-7 and HeLa cell lines with IC50 values of 21.3 and 60.5 μM, respectively. Compound 43 showed moderate cytotoxicity to MCF-7 and HeLa cell lines, with IC50 values of 9.17 and 19.6 μM [42]. Lee et al. studied the cytotoxicity of compound 43 and denbinobin, finding that the synthesized methylated and acetylated derivatives did not exhibit antitumor activity in vitro and in vivo [43].
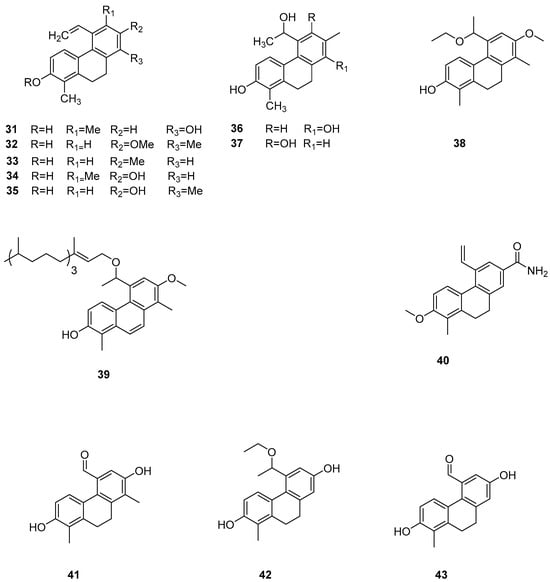
Figure 8.
The 9,10-dihydrophenanthrens in Juncus effuses.
Lusianthridin (44) (Figure 9), isolated from Dendrobium officinale, has been demonstrated to exert cytotoxic effects both in vitro and in vivo. This compound shows significant activity against A549 human lung cancer, SK-OV-3 human ovarian adenocarcinoma cells and HL-60 human promyelocytic leukemia cells, with ED50 values of 7.7, 9.4 and 9.5 μM [44].
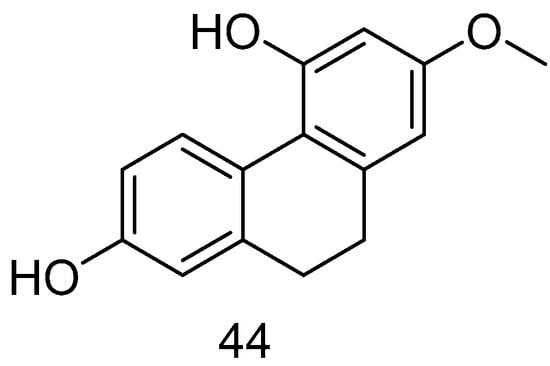
Figure 9.
The 9,10-dihydrophenanthrene in Lusianthridin.
Zhao et al. conducted studies on various phenanthrene monomers isolated from the rhizomes of Dendrobium officinale, revealing significant cytotoxicity against human leukemia cell lines (HI60, THP-1). Orchinol (45) (Figure 10) demonstrated substantial cytotoxicity against HI-60 and THP-1 cells, with IC50 values of 11.96 and 8.92 μM [13]. Liu et al. isolated 13 phenanthrenes from the orchid plant Panlongshen (Spiranthes sinensis) and conducted in vitro cytotoxicity studies on mouse melanoma B16-F10 cells, human gastric cancer SGC-790 cells and human liver cancer HepG2 cells [45]. Their results indicated that spiranthesphenanthrine A (46) (Figure 10) exhibited greater cytotoxicity than the control compound cisplatin on B16-F10 cells, with an IC50 value of 19.0 ± 7.3 μM. Western blots showed that spiranthesphenanthrine A inhibits the migration of B16-F10 cells, potentially due to the inhibition of epithelial cell apoptosis. This compound may be a potential candidate for preventing tumor metastasis.
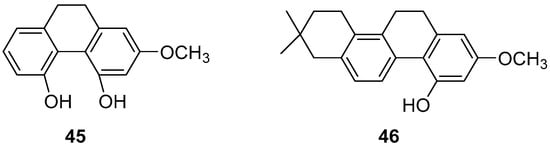
Figure 10.
The 9,10-dihydrophenanthrenes in Dendrobium officinale.
Bai Ji (Bletilla striata), a traditional Chinese medicine, comprises the dried tubers of the orchid plant Bletilla striata (Thunb.) Reichb. f., a prized medicinal variety mainly found in East China, Central South, Southwest, Gansu, Shaanxi and other regions. Guizhou is noted for the largest production and best quality. Annually from September to October, as the stems and leaves wither, the tubers are harvested, roots removed, washed, boiled or steamed until no white core remains, and then sun-dried until semi-dry, after which the outer skin is removed, and further sun-drying is conducted. As of December 2020, over 261 compounds have been isolated and identified from this genus. These isolates include stilbenes (benzyl and phenanthrene), flavonoids, triterpenoids, steroids, simple phenolic compounds and glucosoxybenzyl 2-isobutyl malate compounds. The 9,10-dihydro-4,7-dimethoxyphenanthrene-2,8-diol (47) (Figure 11) has shown inhibitory effects on LPS-induced NO production in RAW 264.7 cells, with an IC50 value ranging from 25.0 to 87.2 μM [46].
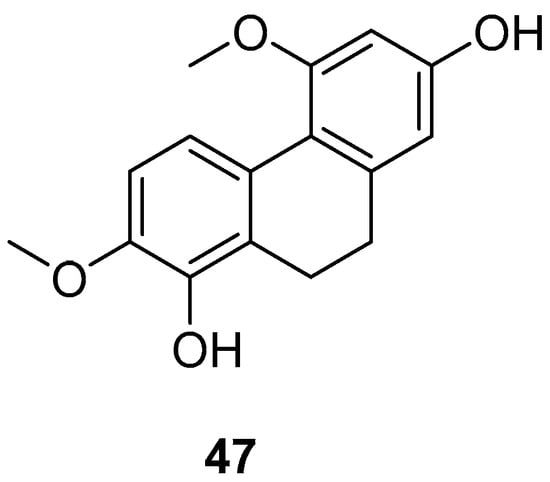
Figure 11.
The 9,10-dihydrophenanthrene in Bai Ji.
Combretum, a genus in the Loricaceae family, comprises approximately 250 species distributed across both tropical hemispheres, excluding Oceania, with a significant presence in tropical Africa. About 12 species are found in China, primarily south of the Yangtze River. The family Serranidae, to which it belongs, is known for yielding a variety of bioactive chemical components. Eder Bisoli et al. conducted chemical analyses on the roots and stems of Combretum laxum, isolating a new dihydrostilbene derivative, two phenanthrenes, three dihydrophenanthrenes (48–50) (Figure 12), along with a lignan, a triterpene, an orange ketone, a flavone, a naphthoquinone and two benzoic acid derivatives [47]. The appearance of these compounds in the genus Windmill is unprecedented in the Americas. Compounds 2,7-dihydroxy-4,6-dimethoxyphenylene (48) and 2,6-dihydroxy-4,7-dimethoxy-9,10-dihydrophenanthrene (50) are novel discoveries in the Combretaceae family. These compounds were tested for in vitro cytotoxicity against five human cancer cell lines and for their free radical scavenging ability against 1,1-diphenyl-2-picrylhydrazine (DPPH). Compound 48 exhibited the most potent cytotoxicity against melanoma cells (IC50 of 2.59 ± 0.11 µM) and showed high selectivity compared to its effects on non-tumor mammalian cells (SI25.1).
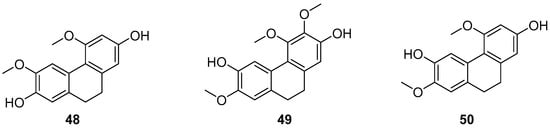
Figure 12.
The 9,10-dihydrophenanthrenes in Combretum laxum.
Pholidota cantonensis Rolfe, a perennial herbaceous plant in the Orchidaceae family with over 30 species, is native to regions in China such as Zhejiang, Jiangxi, Fujian, Taiwan, Hunan, Guangdong and Guangxi. Li et al. isolated two 9,10-dihydro phenanthrene compounds, phytol (51) and phocantone (52) (Figure 13), from the ethanol extract of the whole plant in Guangzhou. These compounds were evaluated for their cytotoxic activity against mouse leukemia P388D1 cancer cells, with IC50 values of 75.0 and 27.5 µM, respectively [48].
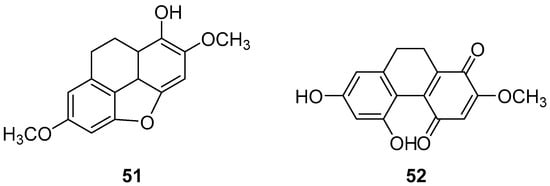
Figure 13.
The 9,10-dihydrophenanthrenes in Pholidota chinensis.
Cymbidium hybridum, an evergreen epiphytic herb from the Orchidaceae family, is predominantly found in East Asia, including China, Japan and South Korea, where it has been cultivated for centuries [49]. Shuang-shuang Lv extracted compounds from C. grandiflora and assessed their effects on human cancer cell lines using the MTT method. Shancidin (53) (Figure 14) was found to significantly impact all three cancer cell lines tested. The IC50 values for SMMC-7721, A549 and MGC80-3 cells were 12.57, 18.21 and 11.6 μM, respectively [50].
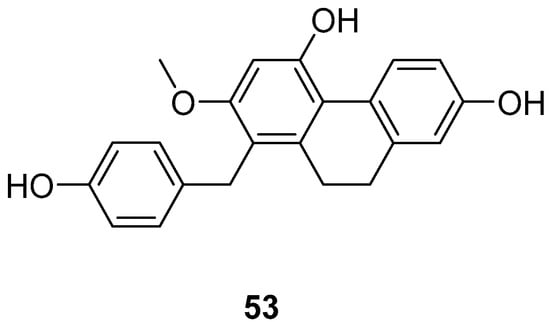
Figure 14.
The 9,10-dihydrophenanthrene in Cymbidium hybridum.
2.3. Antitumor 9,10-Dihydrophenanthrene Dimer Compounds
Panlong ginseng, a traditional Chinese medicine name, refers to the root or entire plant of Spiranthes australis Lindl. from the Orchidaceae family, distributed across various Chinese provinces and regions. Liu et al. extracted a 9,10-dihydrophenanthrene dimer compound (54) (Figure 15) and studied its activity, finding that the IC50 values for SGC-7901, HepG2 and B16-F10 cells were 63.8 ± 3.6, 78.4 ± 29.0 and 58.2 ± 2.6 µM, respectively [45].
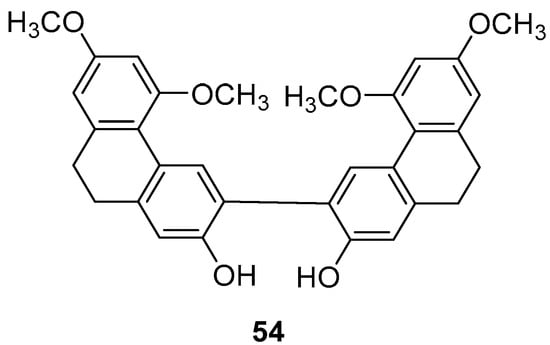
Figure 15.
The 9,10-dihydrophenanthrene dimer compound in Panlong ginseng.
The potential of natural compounds as anticancer agents has attracted researchers like Chiara Platella et al. to expand the library of natural compounds, particularly phenanthrenes, using biophysical and molecular docking techniques. They synthesized a dimer containing 9,10-dihydrophenanthrene (55–57) (Figure 16) and assessed its antiproliferative activity on HeLa adenocarcinoma, MCF7 breast cancer and A431 epidermoid carcinoma cells via the MTT method. The IC50 values for compound 56 on HeLa, MCF-7 and A431 cells were 25, 31 and 42 μM, respectively [51].
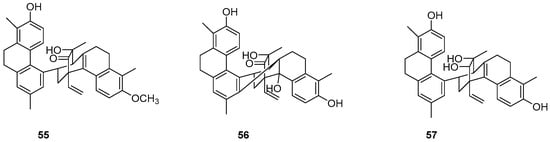
Figure 16.
The 9,10-dihydrophenanthrene dimer compounds.
The Dendrobium genus comprises perennial, primarily herbaceous plants with around 240 species distributed globally, predominantly in temperate and cold regions. These plants typically thrive along the water’s edge in grassy swamps and damp environments. In recent years, new structures of dimeric 9,10-dihydrophenanthrenes have been isolated from the Juncus plant. From 1997 to 2002, Dellagreca et al. identified only five types of dimeric phenanthrenes in Juncus acutus [52]. In 2003, six new dimeric 9,10-dihydrophenanthrene compounds (58–63) (Figure 17) were isolated from this species [53]. Diphenanthrenes are rare in this genus, with nine reported from Jasminum acuminatum and one from Wild jasmine [52,54,55]. In the process of bioactive natural products from traditional Chinese medicine, Wei Ma isolated four new phenanthreneoid dimers from the ethanol extract of Juncus effuses’ medulla in 2015, named effususins A–D (64–67) (Figure 18). These compounds’ effects on cell proliferation and survival were measured in SHSY-5Y, SMMC-7721, HepG-2 Hela and MCF-7 cells using the CCK-8 method, with paclitaxel as the positive control. Among the phenanthrene dimers, compound 64 showed moderate to strong cytotoxic activity against all five cancer cell lines, with IC50 values of 32.64, 13.60, 12.93, 25.09 and 12.49 µM, respectively, outperforming paclitaxel except against the SMMC-7721 cell line. The other three compounds exhibited no activity against these cell lines [56]. Csaba Bús et al. extracted a new dimeric phenanthrene, named compressin B (68) (Figure 19), from Juncus compressus Jacq. and tested its antiproliferative activity against three human tumor cell lines: HeLa and SiHa (cervical adenocarcinoma) and A2780 (ovarian cancer). The HeLa cell line was the most sensitive, with an IC50 value of 1.86 μM [57].
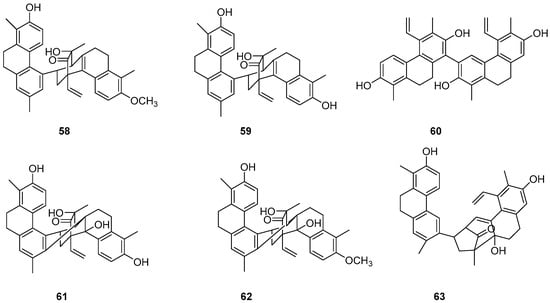
Figure 17.
The 9,10-dihydrophenanthrene dimer compounds in the Dendrobium genus.
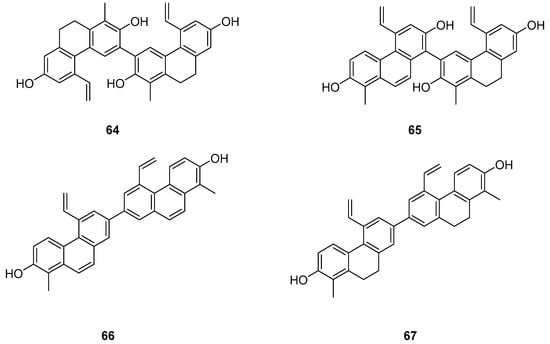
Figure 18.
The 9,10-dihydrophenanthrene dimer compounds in Juncus effuses.
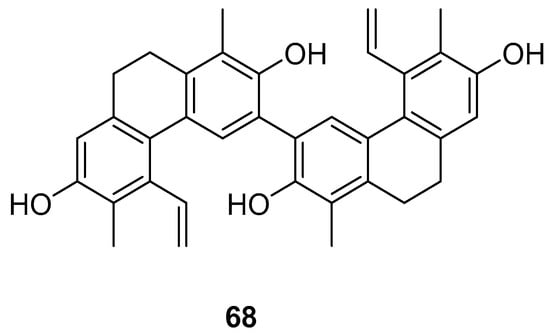
Figure 19.
The 9,10-dihydrophenanthrene dimer compound in J. compressus.
2.4. Other Derivatives of Antitumor Phenanthrenes
Dendrobium, one of the largest genera in the Dendrobium family, consists of over 1100 species, most of which are found in Asia, Europe and Australia [58]. In China, there are 74 species and two varieties, several of which are utilized in traditional or folk medicine. Phenanthrenes [59] and sesquiterpenes isolated from these plants exhibit significant antioxidant, anti-tumor, immunomodulatory and anti-inflammatory activities [60,61,62,63]. Zhao et al. isolated two new phenanthrenes and 9,10-dihydrophenanthrene derivatives (69–70) (Figure 20), along with six known homologues, from Dendrobium officinale stem extracts. Cell studies conducted on HI-60 and THP-1 cell lines revealed IC50 values of 35.32 ± 1.76, 20.78 ± 1.80, >50 and 45.32 ± 2.39 µM for compounds 69 and 70, respectively [13]. Denbinobin (71) (Figure 20), a phenanthrenequinone from the Dendrobium genus, can be extracted from D. candidum [64,65], D. nobile [18,59,66,67] and D. venustum [68]. Ephemeranthoquinone (72) (Figure 20) is also derived from D. hancockii, D. hongdie [69], D. longicornu [70,71], D. plicatile [72] and other plants. Zhai summarized the IC50 values of these compounds on MCF-7, HL-60, A549 and SW480 cells as 13.13 ± 0.47 and 3.63 ± 0.03, 3.08 ± 0.12 and 2.33 ± 0.12, 19.68 ± 1.12 and 14.79 ± 0.64 and 16.81 ± 0.13 and 6.66 ± 0.71 μM, respectively [73].
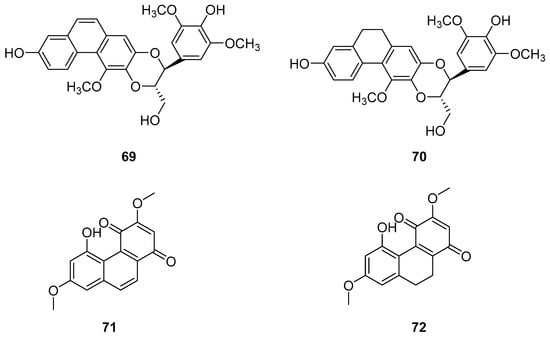
Figure 20.
The other derivatives of phenanthrenes in Dendrobium.
The earlier literature reports the isolation of significant quantities of stilbenes, steroids and triterpenes from a series of Indian orchids (Dendrobium rotundatum), which also contain a large amount of biphenyl, phenanthrene, phenanthropyran and pyran compounds, including 9,10-dihydrophenanthrene derivatives. Majumder continued to explore these phytochemicals and isolated a new 9,10-dihydrophenanthrene derivative from Dendrobium officinale, named rotundatin (73) (Figure 21) [74]. Over the past five years, numerous new phenanthrenes derivatives have been isolated from natural plants and their anticancer activities tested [75]. In 2016, a new biphenylphenanthrene compound 74 named 8-methoxy-12-(4-methoxybenzyl)-13,14-dihydro-12H-naphtho[2,1-a] xanthene-2,5,9,10-tetraol was isolated from Dendrobium officinale, displaying appropriate antiproliferative activity against MDA-231, HepG2 and HT-29 cancer cells, with IC50 values of 25.2, 51.3 and 30.4 μM [76]. Compound 75, a novel phenanthrene derivative isolated from Eria bambusifolia (Callostylis bambusifolia (Lindl.)) in 2016, showed effective antiproliferative activity against HL-60 cancer cells, with an IC50 of 14.5 μM [77]. That same year, a new phenanthrene derivative (76) (Figure 21) was isolated from Bai Ji, demonstrating potent inhibitory effects on MCF-7, HT-29, HUVEC and A549 tumor cells, with IC50 values of 12.6, 22.7, 33.5 and 22.6 μg/mL [78]. Further research indicated that compound 76 induces the stagnation of cancer cell division in the G0/G1 phase, inhibiting the transition from G1 to S phase. In 2017, six phenanthrenes were isolated from the stems of Dendrobium officinale, showing significant antiproliferative activity against HL-60 and THP-1 cancer cells [13]. Among them, compound 77 predominantly inhibits the growth of DU145 cells by arresting them in the G0/G1 phase of mitosis, with an IC50 of 1.5 μM [79]. In 2018, Li et al. extracted four new dihydrophenanthrofuran compounds, named bleochranols A–D (78–81) (Figure 22), from the roots of Bletilla striata. Of these, compound 80 exhibited the most potent activity. The IC50 values of bleochranol A against HL-60, SMMC-7721, A-549, MCF-7 and SW480 cells were 0.24 ± 0.03, 12.22 ± 0.26, 3.51 ± 0.09, 3.30 ± 0.99 and 12.97 ± 0.34 μM, respectively [80]. Juncunol (82) (Figure 22) was isolated from Juncus effusus L. and found to induce apoptosis in the human hepatoma cell line HepG2 [81]. Zhang et al. conducted cytotoxicity tests, revealing that the compounds AL-BII (83), AAI (84) (Figure 23) and AMH extracts exhibited notable cytotoxicity against HepG2 cells, with IC50 values of 0.2, 9.7 and 50.2 μM. AAI showed specific cytotoxicity to HepG2 cells, whereas AAD (85) (Figure 23) displayed no cytotoxicity. Among the AA derivatives, AL-BII demonstrated the strongest cytotoxicity and selectivity towards the NCI-H187 cell line but was non-toxic to A549 and MCF7 cells [82].
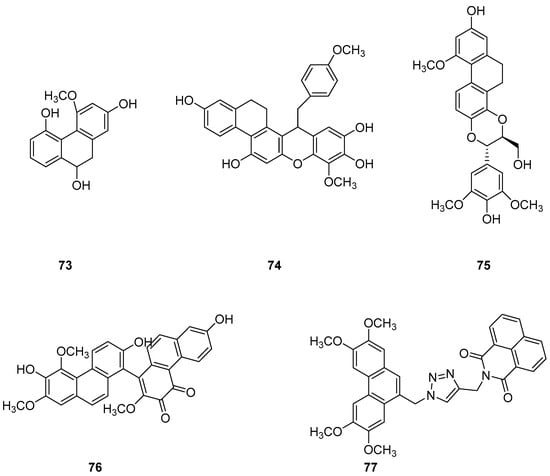
Figure 21.
The other derivatives of phenanthrenes in Indian orchids.
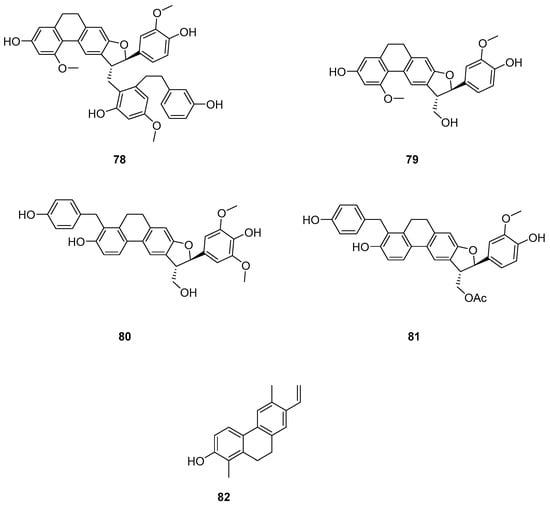
Figure 22.
The other derivatives of phenanthrenes in Bletilla striata.
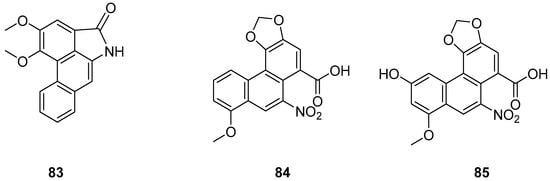
Figure 23.
The other derivatives of phenanthrenes in Aristolochia.
3. Toxicological Effects of Natural Phenanthrenes
Aristolochic acid (AA), a natural nitrophenanthrene carboxylic acid compound, consists of AAI and AAII in a ratio of approximately 1:1 [83]. These compounds are present in numerous plants, including Aristolochia, Asarum, Akebia, Clematis, Stephania, Menispermum, Dauricum and Asteraceae. AA has been utilized pharmacologically for a long time and was used to treat edema over 2500 years ago in ancient Greek and Egyptian practices [84]. In China, the medicinal plants containing AA are mainly from the Aristolochia and Asarum species. Several plants from the Aristolochiaceae family are used in traditional medicine globally, such as the fruit of Aristolochia, the canes of Caulis aristolochiae manshuriensis, the roots of Stephania tetrandra and Radix aucklandiae. Modern studies have shown that Aristolochia herbs possess diuretic, anti-infective, anti-inflammatory, anti-venom and anticancer properties [85,86,87,88]. It has also been documented that plants or plant-derived products containing AA are toxic, mutagenic and carcinogenic to humans [89,90], leading to a ban on their use in some countries.
3.1. Hepatotoxicity
AA is a key component in Chinese herbal medicines such as AA and asarum, commonly found in Southern Brazil and India. These herbs are used to treat various ailments including eczema, pneumonia, stroke, hepatitis, snakebite, arthritis, gout and coronary artery disease. Research published on 18 October 2017, by scientists from Singapore and Taiwan indicated that AA was responsible for a majority of hepatocellular carcinoma (HCC) cases in Taiwan [91]. In 2019, Ouyang monitored the metabolism of AAI and its residues in the liver and kidneys of rats fed with AAI, discovering that AAI metabolized in the kidney while AAT residues were found in both the liver and kidney [92]. In 2021, Wang et al. conducted toxicity tests on rats with varying doses of AA, finding that increased doses resulted in liver mitochondrial damage, reduction in organelles such as ER and ribosomes, liver inflammatory cell infiltration and a tendency toward fibrosis. These findings indicate that AA can cause hepatocyte necrosis and subsequently impair hepatocyte metabolic function. The prolonged and high-dose usage of AA leads to severe liver damage in a dose-dependent manner [93]. This aligns with Quan et al.’s 2020 findings that AA-containing herbs damaged mouse kidney mitochondria [94]. AA induces liver injury through oxidative stress and mitochondrial apoptosis pathways. The results showed that AST and ALT levels in serum were significantly elevated in all dose groups compared to the control, confirming dose-dependent toxicity [95]. In Wang’s study, the highest serum AST and ALT levels were observed in the 20 mg/kg-AA dose group, confirming the severe hepatic damage at this concentration [93]. Oxidative stress, primarily occurring in mitochondria, is a major pathological factor in many organisms and can lead to apoptosis [96]. The mitochondrial-mediated apoptosis pathway is one of the classic routes of apoptosis [97], which are critical factors in AA-induced hepatotoxicity (Figure 24).
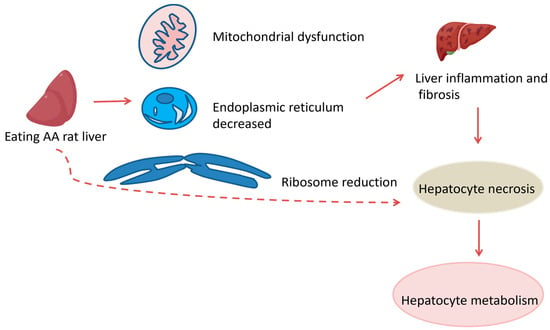
Figure 24.
Hepatotoxicity in rats. indicate the inhibitory effects.  indicate the promotional effects.
indicate the promotional effects.
3.2. Nephrotoxicity
In 1994, Cosyns et al. began to suspect that AA could cause kidney disease [98]. Debelle et al. linked the consumption of AA-containing herbs to Chinese herbal nephropathy (CHN)/AA nephropathy (AAN) in 2007, which is a progressive interstitial nephritis leading to end-stage renal disease and urothelial malignancies [99]. CHN was initially identified in Belgium as a new cause of chronic renal interstitial fibrosis associated with weight loss programs that included Chinese herbal medicine [100]. Subsequently, AA was confirmed as the pathogenic factor [101,102], and CHN was more accurately described as AAN [103,104]. In Southeastern Europe, a similar condition previously identified as Balkan endemic nephropathy (BEN) was later also recognized as AAN [105]. Following the recognition of AAN in the 1990s, numerous studies have elucidated the cellular and molecular mechanisms underlying the condition.
Ferroptosis, characterized by iron-dependent lipid peroxidation, requires sufficient free intracellular iron and polyunsaturated fatty acids (PUFAs) in the cell membrane. Acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) are involved in the synthesis and remodeling of PUFA-phosphatidylethanolamine (PE) in cell membranes. The inhibition of ACSL4, but not other ACSL family members, prevents ferroptosis. GPX4, a major endogenous antioxidant enzyme, prevents the accumulation of toxic lipid ROS during ferroptosis [106]. The depletion of glutathione (GSH) and inactivation of GPX4 lead to fatal iron-dependent accumulation of lipid ROS. The conditional knockout of GPX4 is linked with cancer, neurodegenerative diseases, acute kidney injury or liver injury, preventable or mitigated by inhibiting ferroptosis [106,107,108,109]. Acute AAN in mice has shown increased malondialdehyde (MDA) content, enhanced lipid peroxidation, decreased superoxide dismutase (SOD) activity, depleted GSH, and impaired antioxidant capacity. The expression of GPX4 was reduced, while that of ACSL4 was increased, suggesting the involvement of ferroptosis in AA-induced acute kidney injury. Ferroptosis inhibition by Fer-1 significantly improved renal function, reduced histopathological lesions, decreased lipid peroxidation and restored antioxidant capacity. In vitro, AA markedly reduced cell viability, induced ROS production, increased intracellular iron levels and decreased iron poisoning-related protein expression. Ferroptosis inhibition notably increased cell viability and mitigated AA-induced renal tubular epithelial cell injury [110]. As early as 2013, it was reported that AA could induce apoptosis in renal tubular epithelial cells (TECs) through the dephosphorylation of signal transducer and activator of transcription 3 (STAT3) and activation of the p53 signaling pathway (Figure 25) [111].
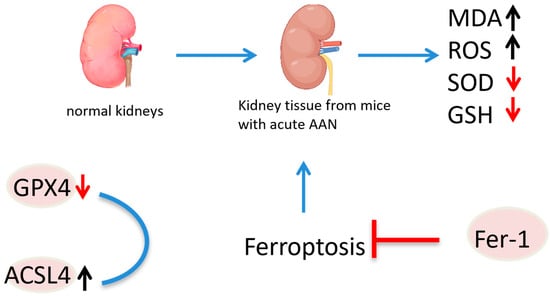
Figure 25.
Mechanism of aristolochic acid causing nephrotoxicity.  indicate the promotional effects,
indicate the promotional effects,  indicate the inhibitory effects,
indicate the inhibitory effects, 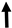 indicate the promotional effects,
indicate the promotional effects,  indicate the inhibitory effects in the figure.
indicate the inhibitory effects in the figure.
ROS or reactive nitrogen species (ROS/RNS) are products of normal cell metabolism, playing a crucial role in cell signal transduction and homeostasis. The excessive ROS/RNS production or depletion of endogenous antioxidant systems can lead to oxidative/nitrosative stress [112]. Exogenous substances like AA can also induce oxidative stress, resulting in cellular damage [113]. AA has been shown to damage DNA by stimulating ROS production. Yu used human cells to further explore the mechanism of AAI-induced DNA oxidative damage [114]. Romanov et al. noted that AA could induce apoptosis and cell arrest in the G2/M phase by generating ROS and activating the mitogen-activated protein kinase (MAP kinase), which in turn activates p38, leading to apoptosis (Figure 26) [48].
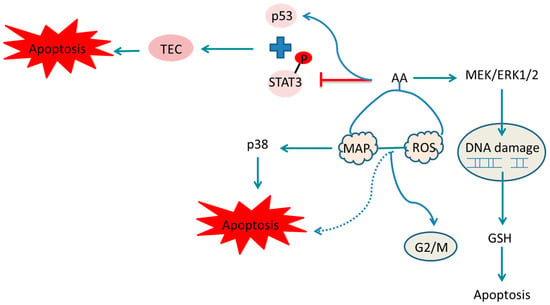
Figure 26.
Mechanism of aristolochic acid causing cellular damage and DNA damage.  indicate the promotional effects and
indicate the promotional effects and  indicate the inhibitory effects in the figure.
indicate the inhibitory effects in the figure.
Declèves’ experiments demonstrated inflammation-related oxidative stress in the AA-induced acute kidney injury mouse model [115]. Jin et al. found that AAI-induced oxidative stress in C57BL/6N mice was associated with the increased expression of NADPH oxidase 2 (NOX2), CYP2E1 and decreased catalase, SOD and glutathione synthetase levels, suggesting AA may interact with these enzymes to induce oxidative stress [116]. Research has indicated that mitochondria are a target of AA-induced nephropathy. Pozdzick’s data revealed AA toxicity primarily causes mitochondrial damage and impairs the activation of antioxidant enzymes. Additionally, Zhou et al. found that AA promoted mitochondrial DNA (mtDNA) damage, reduced the mtDNA copy number and decreased mitochondrial protein expression in cultured podocytes. AA treatment also lowered ATP content, the oxygen consumption rate and mitochondrial membrane potential in these cells and increased cellular ROS [117]. Furthermore, AA has been shown to induce apoptosis in renal tubular cells by inhibiting the PI3K/Akt signaling pathway [118,119], decreasing Bcl-2 levels and increasing Bcl-2 associated X protein (Bax) levels in human umbilical vein endothelial cells (HUVECs) [120]. Zhu and Hsin linked ER stress with AAI-induced apoptosis [121,122]. AAN is also associated with inflammation, as evidenced by the presence of mononuclear inflammatory cells in chronic renal scars in rats [123]. Honarpisheh’s data suggest that macrophages play a crucial role in AAN’s pathogenesis, related to excessive macrophage accumulation and activation [124]. AA-treated zebrafish embryos displayed inflammation, indicated by upregulated pro-inflammatory gene expression [125]. Similarly, Jadot observed increased renal mRNA expressions of pro-inflammatory cytokines in AA-treated mice [126]. Recent studies have also linked the NLRP3 inflammasome with AAN [127]. Kholia et al. and Wang et al. reported inflammation in AA rat models. These findings collectively demonstrate that AA can cause kidney disease, renal failure, and upper urinary tract cancer [128,129].
3.3. Carcinogenicity
Studies from the 1990s and early 21st century identified AA as a potent carcinogen and renal toxin, leading to its classification as a human carcinogen by the International Agency for Research on Cancer (IARC) in 2012. Upper urinary tract urothelial carcinoma (UC) is commonly found in patients with end-stage AA-related nephropathy, often occurring long after the initial onset of nephropathy. Both upper urinary tract and bladder UC are severe complications in renal transplant recipients with acid nephropathy, confirming the carcinogenic properties of AA [130]. Kwok’s research suggests that UC in traditional Chinese medicine is associated with AA exposure, potentially through skin contact or the inhalation of fine powder [131]. A recent comprehensive epidemiological study demonstrated that the use of herbal products containing AA is significantly linked with a higher dose-dependent risk of various cancers including liver, colorectal, kidney, bladder, prostate, pelvic and ureteral cancer. An increased risk of extrahepatic cholangiocarcinoma was observed particularly in women exposed to higher doses of AA, highlighting its genotoxicity [132]. AA is metabolized to form AA lactam (AL), which binds to DNA, preferentially to adenine and guanine. This binding induces errors in DNA synthesis, leading to substitutions of G:C to T:A and A:T to T:A. However, the AL–guanine adduct is recognized by global genomic nucleotide excision repair (GG-NER), making T > A substitutions a characteristic mutation of AA, typically found at the 5’-pyrimidine-A-purine-3’ site. This ultimately causes DNA damage and triggers liver cancer [133,134,135]. Additionally, AL-DNA adducts formed in the kidneys can cause renal tubular injury and renal interstitial fibrosis, persisting long-term, exacerbating renal damage and significantly increasing the risk of kidney disease and upper urinary tract epithelial cancer (Figure 27).
Figure 27.
Pathways for the metabolic activation of the aristolochic acids. The highlighted parts indicate the binding of aristolochic acid metabolites to DNA.
4. Conclusions and Prospect
With advancements in science and technology increasing health awareness, natural plant extracts have gained significant attention. As vital sources of natural medicines, these extracts have broad applications. In this paper, we summarized 84 natural phenanthrenes and their derivatives (Table 1). Phenanthrenes, primary active compounds found in plants like Dendrobium and Bletilla, have drawn considerable interest from scientists due to their anti-tumor potential and toxic effects.

Table 1.
Anticancer activity of phenanthrene natural products and their derivatives.
Historically, research on phenanthrenes has primarily focused on separation, extraction, pharmacological activities, mechanisms of action and chemical synthesis [136]. Currently, in China, natural plants containing phenanthrenes are well recognized medically among the public, yet knowledge of their chemical composition and active pharmacological substances remains limited. Phenanthrenes are significant chemical components of families like Caryophyllaceae and Orchidaceae, with more than 450 species compounds that have been isolated since the 1970s. It is important to note that almost all of the phenanthrene compounds have been studied for their potential biological activity, and several have shown multiple activities. During the screening process, some serve as promising resources for innovative drug lead compounds. Denbinobin, for instance, shows potential as a lead anticancer compound with a novel mechanism of action. AA, a nitrophenic acid compound, can cause various cancers such as urothelial, renal cell, hepatocellular, biliary tract and gastrointestinal cancers when ingested through Chinese herbal medicines, skin contact, or the inhalation of herbal powder [4]. In the future, we can transform its toxic effects into anticancer properties by modifying its structure. Therefore, we need new technologies to identify novel targets, such as PROTAC probe technology [137].
Although aristolochic acid compounds are highly toxic, we can engineer them through multiple pathways that may contribute to their ultimate pharmacological use in drug therapy. After that, we will work with other disciplines to study its mechanism of toxic effect conversion in multiple directions. In addition, we can perform structural modification, traditional Chinese medicine processing techniques or other biological approaches to achieve the toxicity-effect transformation of Aristolochic acid. The development of these new treatment strategies can achieve the effect of toxic effect conversion and provide new ideas for the treatment and development of phenanthrenes, so as to be better used in clinical practice in the future.
Author Contributions
Conceptualization, writing-original draft, Y.L., Z.D. and C.S. validation, Investigation, G.Z. and S.Y.; writing—review and editing, supervision, Z.Z.; conceptualization, writing—review and editing, supervision, funding acquisition, S.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hunan Science Fund for Distinguished Young Scholars (No. 2025JJ20098); the National Natural Science Foundation of China (No. 82204250); the China Postdoctoral Science Foundation (No. 2021M693961); the Young and Middle-Aged Talent Project of the Hubei Provincial Department of Education (No. Q20222808); and the High-level Talent Research Initiation Fund Project of Hunan University of Chinese Medicine (No. 0004010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kovács, A.; Vasas, A.; Hohmann, J. Natural Phenanthrenes and Their Biological Activity. Phytochemistry 2008, 69, 1084–1110. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, J.J.; Allen, R.W.; Gribble, G.W. A Simple Synthesis of Phenanthrene. Org. Prep. Proced. Int. 2020, 52, 166–169. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, J.; Carmella, S.G.; Hochalter, J.B.; Rauch, D.; Oliver, A.; Jensen, J.; Hatsukami, D.K.; Upadhyaya, P.; Zimmerman, C.; et al. Metabolism of [D10]Phenanthrene to Tetraols in Smokers for Potential Lung Cancer Susceptibility Assessment: Comparison of Oral and Inhalation Routes of Administration. J. Pharmacol. Exp. Ther. 2011, 338, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.A.; Perwaiz, S. Aristolochic Acids. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 298–301. [Google Scholar]
- Das, S.; Thakur, S.; Korenjak, M.; Sidorenko, V.S.; Chung, F.F.; Zavadil, J. Aristolochic acid-associated cancers: A public health risk in need of global action. Nat. Rev. Cancer 2022, 22, 576–591. [Google Scholar] [CrossRef]
- Thomas, P.C.; Charlie, X.; Kelvin, C.; Chun, G.L. Aristolochic Acids Detected in Some Raw Chinese Medicinal Herbs and Manufactured Herbal Products—A Consequence of Inappropriate Nomenclature and ImpreciseLabelling? Clin. Toxicol. 2006, 44, 371–378. [Google Scholar]
- Sborchia, M.; De Prez, E.G.; Antoine, M.H.; Bienfait, L.; Indra, R.; Valbuena, G.; Phillips, D.H.; Nortier, J.L.; Stiborová, M.; Keun, H.C.; et al. The Impact of P53 on Aristolochic Acid I-Induced Nephrotoxicity and DNA Damage In Vivo and In Vitro. Arch. Toxicol. 2019, 93, 3345–3366. [Google Scholar] [CrossRef]
- Sborchia, M.; Keun, H.C.; Phillips, D.H.; Arlt, V.M. The Impact of P53 on Aristolochic Acid I-Induced Gene Expression In Vivo. Int. J. Mol. Sci. 2019, 20, 6155. [Google Scholar] [CrossRef]
- Zhou, Q.; Pei, J.; Poon, J.; Lau, A.Y.; Zhang, L.; Wang, Y.; Liu, C.; Huang, L. Worldwide Research Trends on Aristolochic Acids (1957–2017): Suggestions for Researchers. PLoS ONE 2019, 14, e0216135. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhao, X.H.; Sun, Z.H.; Li, G.C.; Liu, G.C.; Sun, L.R.; Hou, J.Q.; Zhou, W. Recognition of the Toxicity of Aristolochic Acid. J. Clin. Pharm. Ther. 2019, 44, 157–162. [Google Scholar] [CrossRef]
- Moretti, C.; Rideau, M.; Chénieux, J.; Viel, C. Isolement de l’acide Aristolochique de Deux Aristoloches Malgaches. Détermination de Sa Cytotoxicité Sur Cellules Végétales. Comparaison Avec Les Cellules Animales. Planta Med. 1979, 35, 360–365. [Google Scholar] [CrossRef]
- Tóth, B.; Hohmann, J.; Vasas, A. Phenanthrenes: A Promising Group of Plant Secondary Metabolites. J. Nat. Prod. 2018, 81, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Y.; Deng, B.W.; Zhang, C.Y.; Cui, Y.D.; Bi, J.Y.; Zhang, G.G. New Phenanthrene and 9, 10-Dihydrophenanthrene Derivatives from the Stems of Dendrobium Officinale with Their Cytotoxic Activities. J. Nat. Med. 2018, 72, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Han, M.; Zhang, B.; Sun, L.; Jin, X.; Li, T. Chrysotoxene Induces Apoptosis of Human Hepatoblastoma HepG2 Cells in vitro and in vivo via Activation of the Mitochondria-Mediated Apoptotic Signaling Pathway. Oncol. Lett. 2018, 15, 4611–4618. [Google Scholar] [CrossRef] [PubMed]
- Nonpanya, N.; Prakhongcheep, O.; Petsri, K.; Jitjaicham, C.; Tungsukruthai, S.; Sritularak, B.; Chanvorachote, P. Ephemeranthol A Suppresses Epithelial to Mesenchymal Transition and FAK-Akt Signaling in Lung Cancer Cells. Anticancer Res. 2020, 40, 4989–4999. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Xin, W.; Liu, N.; Zhang, H. Nudol, a Phenanthrene Derivative from Dendrobium nobile, Induces Cell Cycle Arrest and Apoptosis and Inhibits Migration in Osteosarcoma Cells. Drug Des. Devel. Ther. 2019, 13, 2591–2601. [Google Scholar] [CrossRef]
- Yang, C.R.; Shih, K.S.; Liou, J.P.; Wu, Y.W.; Hsieh, I.N.; Lee, H.Y.; Lin, T.C.; Wang, J.H. Denbinobin Upregulates miR-146a Expression and Attenuates IL-1β-Induced Upregulation of ICAM-1 and VCAM-1 Expressions in Osteoarthritis Fibroblast-like Synoviocytes. J. Mol. Med. 2014, 92, 1147–1158. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, S.Y.; Han, S.-B.; Uddin, G.M.; Kim, C.Y.; Lee, J.K. Anti-Inflammatory Effects of Dendrobium nobile Derived Phenanthrenes in LPS-Stimulated Murine Macrophages. Arch. Pharm. Res. 2015, 38, 1117–1126. [Google Scholar] [CrossRef]
- Li, C.B.; Wang, C.; Fan, W.W.; Dong, F.W.; Xu, F.Q.; Wan, Q.L.; Luo, H.R.; Liu, Y.Q.; Hu, J.M.; Zhou, J. Chemical Components of Dendrobium crepidatum and Their Neurite Outgrowth Enhancing Activities. Nat. Prod. Bioprospect. 2013, 3, 70–73. [Google Scholar] [CrossRef]
- Réthy, B.; Kovács, A.; Zupkó, I.; Forgo, P.; Vasas, A.; Falkay, G.; Hohmann, J. Cytotoxic Phenanthrenes from the Rhizomes of Tamus Communis. Planta Med. 2006, 72, 767–770. [Google Scholar] [CrossRef]
- Fan, C.; Sun, X.; Wang, X.; Yu, H. Therapeutic Potential of the Chemical Composition of Dendrobium nobile Lindl. Front. Pharmacol. 2023, 14, 1163830. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.J.; Yang, L.; Yuan, M.Y.; Li, J.Y.; Hou, B.; Li, H.M.; Yang, X.Z.; Ding, C.C.; Hu, J.M. Sesquiterpene Amino Ether and Cytotoxic Phenols from Dendrobium Wardianum Warner. Fitoterapia 2017, 122, 76–79. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, C.; Zhuo, Y.; Gong, B.; Xu, W.; Zhang, G. 1,5,6-Trimethoxy-2,7-Dihydroxy-phenanthrene from Dendrobium Officinale Exhibited Antitumor Activities for HeLa Cells. Int. J. Mol. Sci. 2023, 24, 15375. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Isidori, M.; Lavorgna, M.; Monaco, P.; Previtera, L.; Zarrelli, A. Phenanthrenoids from the Wetland Juncus acutus. Phytochemistry 2002, 60, 633–638. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Y.; Ding, Y.Y.; Liu, F.; Li, N. Cytotoxic and Anti-Inflammatory Activities of Phenanthrenes from the Medullae of Juncus effusus L. Arch. Pharm. Res. 2016, 39, 154–160. [Google Scholar] [CrossRef]
- Ishiuchi, K.; Kosuge, Y.; Hamagami, H.; Ozaki, M.; Ishige, K.; Ito, Y.; Kitanaka, S. Chemical Constituents Isolated from Juncus effusus Induce Cytotoxicity in HT22 Cells. J. Nat. Med. 2015, 69, 421–426. [Google Scholar] [CrossRef]
- Miles, D.H.; Bhattacharyya, J.; Mody, N.V.; Atwood, J.L.; Black, S.; Hedin, P.A. The Structure of Juncusol. A Novel Cytotoxic Dihydrophenanthrene from the Estuarine Marsh Plant Juncus Roemerianus. J. Am. Chem. Soc. 1977, 99, 618–620. [Google Scholar] [CrossRef]
- Gainche, M.; Ripoche, I.; Senejoux, F.; Cholet, J.; Ogeron, C.; Decombat, C.; Danton, O.; Delort, L.; Vareille-Delarbre, M.; Berry, A.; et al. Anti-Inflammatory and Cytotoxic Potential of New Phenanthrenoids from Luzula sylvatica. Molecules 2020, 25, 2372. [Google Scholar] [CrossRef]
- Liu, W.; Meng, M.; Zhang, B.; Du, L.; Pan, Y.; Yang, P.; Gu, Z.; Zhou, Q.; Cao, Z. Dehydroeffusol Effectively Inhibits Human Gastric Cancer Cell-Mediated Vasculogenic Mimicry with Low Toxicity. Toxicol. Appl. Pharmacol. 2015, 287, 98–110. [Google Scholar] [CrossRef]
- Zhang, B.; Han, H.; Fu, S.; Yang, P.; Gu, Z.; Zhou, Q.; Cao, Z. Dehydroeffusol Inhibits Gastric Cancer Cell Growth and Tumorigenicity by Selectively Inducing Tumor-Suppressive Endoplasmic Reticulum Stress and a Moderate Apoptosis. Biochem. Pharmacol. 2016, 104, 8–18. [Google Scholar] [CrossRef]
- Yang, H.; Lee, P.; Jeong, E.J.; Kim, H.P.; Kim, Y.C. Selective Apoptosis in Hepatic Stellate Cells Mediates the Antifibrotic Effect of Phenanthrenes from Dendrobium nobile. Phytother. Res. 2012, 26, 974–980. [Google Scholar] [CrossRef]
- Nam, B.; Ryu, S.M.; Lee, D.; Jung, C.H.; Jin, C.H.; Kim, J.B.; Lee, I.S.; Han, A.R. Identification of Two New Phenanthrenes from Dendrobii Herba and Their Cytotoxicity towards Human Hypopharynx Squamous Carcinoma Cell (FaDu). Molecules 2019, 24, 2339. [Google Scholar] [CrossRef]
- Rabi, I.I.; Zacharias, J.R.; Millman, S.; Kusch, P. A New Method of Measuring Nuclear Magnetic Moment. Phys. Rev. 1938, 53, 318. [Google Scholar] [CrossRef]
- Longobardi, F.; Ventrella, A.; Napoli, C.; Humpfer, E.; Schütz, B.; Schäfer, H.; Kontominas, M.G.; Sacco, A. Classification of Olive Oils According to Geographical Origin by Using 1H NMR Fingerprinting Combined with Multivariate Analysis. Food Chem. 2012, 130, 177–183. [Google Scholar] [CrossRef]
- Maniara, G.; Rajamoorthi, K.; Rajan, S.; Stockton, G.W. Method Performance and Validation for Quantitative Analysis by 1H and 31P NMR Spectroscopy. Applications to Analytical Standards and Agricultural Chemicals. Anal. Chem. 1998, 70, 4921–4928. [Google Scholar] [CrossRef]
- Pauli, G.F. qNMR ? A Versatile Concept for the Validation of Natural Product Reference Compounds. Phytochem. Anal. 2001, 12, 28–42. [Google Scholar] [CrossRef]
- Wells, R.J.; Hook, J.M.; Al-Deen, T.S.; Hibbert, D.B. Quantitative Nuclear Magnetic Resonance (QNMR) Spectroscopy for Assessing the Purity of Technical Grade Agrochemicals: 2,4-Dichlorophenoxyacetic Acid (2,4-D) and Sodium 2,2-Dichloropropionate (Dalapon Sodium). J. Agric. Food Chem. 2002, 50, 3366–3374. [Google Scholar] [CrossRef]
- Deen, T.S.A.; Hibbert, D.B.; Hook, J.M.; Wells, R.J. Quantitative Nuclear Magnetic Resonance Spectrometry II. Purity of Phosphorus-Based Agrochemicals Glyphosate (N-(Phosphonomethyl)-Glycine) and Profenofos (O-(4-Bromo-2-Chlorophenyl) O-Ethyl S-Propyl Phosphorothioate) Measured by 1H and 31P QNMR Spectrometry. Anal. Chim. Acta 2002, 474, 125–135. [Google Scholar]
- Della Greca, M.; Fiorentino, A.; Molinaro, A.; Monaco, P.; Previtera, L. A Bioactive Dihydrodibenzoxepin from Juncus effusus. Phytochemistry 1993, 34, 1182–1184. [Google Scholar] [CrossRef]
- Chapatwala, K.D. Antimicrobial activity of Juncusol, a novel 9-10-dihydrophenanthrene from the marsh plant Juncus roemerianus. Life Sci. 1981, 29, 1997–2001. [Google Scholar] [CrossRef]
- Bús, C.; Kúsz, N.; Kincses, A.; Szemerédi, N.; Spengler, G.; Bakacsy, L.; Purger, D.; Berkecz, R.; Hohmann, J.; Hunyadi, A.; et al. Antiproliferative Phenanthrenes from Juncus Tenuis: Isolation and Diversity-Oriented Semisynthetic Modification. Molecules 2020, 25, 5983. [Google Scholar] [CrossRef]
- Su, X.H.; Yuan, Z.P.; Li, C.Y.; Zhong, Y.J.; Du, H.J.; Wen, Y.Y.; Li, Y.F.; Liang, B. Phenanthrenes from Juncus effusus. Planta Med. 2013, 79, 1447–1452. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Beak, N.; Kim, S.; Ahn, B. In Vitro and In Vivo Antitumoral Phenanthrenes from the Aerial Parts of Dendrobium nobile. Planta Med. 1995, 61, 178–180. [Google Scholar] [CrossRef]
- Kovács, A.; Forgo, P.; Zupkó, I.; Réthy, B.; Falkay, G.; Szabó, P.; Hohmann, J. Phenanthrenes and a Dihydrophenanthrene from Tamus Communis and Their Cytotoxic Activity. Phytochemistry 2007, 68, 687–691. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Q.M.; Yan, X.; Hu, C.; Wang, W.; Wang, R.K.; Luo, X.; Zhang, X.W. Bioactivity-Guided Isolation of Cytotoxic Phenanthrenes from Spiranthes sinensis. J. Agric. Food Chem. 2019, 67, 7274–7280. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, M.; Jiang, L.; Xie, Q.; Yuan, H.; Yang, Y.; Zafar, S.; Liu, Y.; Jian, Y.; Li, B.; et al. The medicinal uses of the genus Bletilla in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2021, 280, 63. [Google Scholar] [CrossRef]
- Bisoli, E.; Freire, T.V.; Yoshida, N.C.; Garcez, W.S.; Queiróz, L.M.M.; Matos, M.d.F.C.; Perdomo, R.T.; Garcez, F.R. Cytotoxic Phenanthrene, Dihydrophenanthrene, and Dihydrostilbene Derivatives and Other Aromatic Compounds from Combretum laxum. Molecules 2020, 25, 3154. [Google Scholar] [CrossRef]
- Li, B.; Ali, Z.; Chan, M.; Li, J.; Wang, M.; Abe, N.; Wu, C.R.; Khan, I.A.; Wang, W.; Li, S.X. Chemical Constituents of Pholidota cantonensis. Phytochemistry 2017, 137, 132–138. [Google Scholar] [CrossRef]
- Fei, Y.; Liu, Z.-X. Isolation and Characterization of the PISTILLATA Ortholog Gene from Cymbidium faberi Rolfe. Agronomy 2019, 9, 425. [Google Scholar] [CrossRef]
- Lv, S.; Fu, Y.; Chen, J.; Jiao, Y.; Chen, S. Six Phenanthrenes from the Roots of Cymbidium faberi Rolfe. and Their Biological Activities. Nat. Prod. Res. 2022, 36, 1170–1181. [Google Scholar] [CrossRef]
- Platella, C.; Criscuolo, A.; Riccardi, C.; Gaglione, R.; Arciello, A.; Musumeci, D.; DellaGreca, M.; Montesarchio, D. Exploring the Binding of Natural Compounds to Cancer-Related G-Quadruplex Structures: From 9,10-Dihydrophenanthrenes to Their Dimeric and Glucoside Derivatives. Int. J. Mol. Sci. 2023, 24, 7765. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Zarrelli, A. A New Dimeric 9,10-Dihydrophenanthrenoid from the rhizome of Juncus acutus. Tetrahedron Lett. 2002, 43, 2573–2575. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Temussi, F.; Zarrelli, A. New Dimeric Phenanthrenoids from the Rhizomes of Juncus acutus. Structure Determination and Antialgal Activity. Tetrahedron Lett. 2003, 59, 2317–2324. [Google Scholar] [CrossRef]
- Behery, F.A.A.; Naeem, Z.E.M.; Maatooq, G.T.; Amer, M.M.A.; Ahmed, A.F. A Novel Antioxidant Phenanthrenoid Dimer from Juncus acutus L. Nat. Prod. Res. 2013, 27, 155–163. [Google Scholar] [CrossRef]
- DellaGreca, M.; Previtera, L.; Zarrelli, A. Dimeric Phenanthrenoids from the Juncus acutus. Nat. Prod. Res. 2005, 19, 69–74. [Google Scholar] [CrossRef]
- Ma, W.; Liu, F.; Ding, Y.Y.; Zhang, Y.; Li, N. Four New Phenanthrenoid Dimers from Juncus effusus L. with Cytotoxic and Anti-Inflammatory Activities. Fitoterapia 2015, 105, 83–88. [Google Scholar] [CrossRef]
- Bús, C.; Kúsz, N.; Jakab, G.; Senobar Tahaei, S.; Zupkó, I.; Endrész, V.; Bogdanov, A.; Burián, K.; Csupor-Löffler, B.; Hohmann, J.; et al. Phenanthrenes from Juncus compressus Jacq. with Promising Antiproliferative and Anti-HSV-2 Activities. Molecules 2018, 23, 2085. [Google Scholar] [CrossRef]
- Luo, Q.; Tang, Z.; Zhang, X.; Zhong, Y.; Yao, S.; Wang, L.; Lin, C.; Luo, X. Chemical Properties and Antioxidant Activity of a Water-Soluble Polysaccharide from Dendrobium Officinale. Int. J. Biol. Macromol. 2016, 89, 219–227. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Zheng, C.J.; Gan, L.S.; Chen, G.Y.; Zhang, X.P.; Song, X.P.; Li, G.N.; Sun, C.G. Bioactive Phenanthrene and Bibenzyl Derivatives from the Stems of Dendrobium nobile. J. Nat. Prod. 2016, 79, 1791–1797. [Google Scholar] [CrossRef]
- Yang, D.; Liu, L.Y.; Cheng, Z.Q.; Xu, F.Q.; Fan, W.W.; Zi, C.T.; Dong, F.W.; Zhou, J.; Ding, Z.T.; Hu, J.M. Five New Phenolic Compounds from Dendrobium aphyllum. Fitoterapia 2015, 100, 11–18. [Google Scholar] [CrossRef]
- Zhao, W.; Ye, Q.; Tan, X.; Jiang, H.; Li, X.; Chen, K.; Kinghorn, A.D. Three New Sesquiterpene Glycosides from Dendrobium nobile with Immunomodulatory Activity. J. Nat. Prod. 2001, 64, 1196–1200. [Google Scholar] [CrossRef]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; He, T.; Chun, Z. In Vitro Antioxidant Activities of a Water-Soluble Polysaccharide Derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol. 2009, 45, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, C.; Zhao, X.; Wang, Y.; Feng, D.; Zhang, M.; Xie, H. (±)-Homocrepidine A, a Pair of Anti-Inflammatory Enantiomeric Octahydroindolizine Alkaloid Dimers from Dendrobium crepidatum. J. Nat. Prod. 2016, 79, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Wang, F.; Dong, H.; Guo, S.; Yang, J.; Xiao, P. Chemical Constituents of Dendrobium candidum. China J. Chin. Mater. Medica 2010, 35, 1715–1719. [Google Scholar]
- Li, R.; Yang, X.; He, P.; Gan, N. Studies on phenanthrene constituents from stems of Dendrobium candidum. J. Chin. Med. Mater. 2009, 32, 220–223. [Google Scholar]
- Cheng, L.; Guo, D.-L.; Zhang, M.-S.; Linghu, L.; Fu, S.-B.; Deng, Y.; He, Y.Q.; Xiao, S.J. Dihydrophenanthrofurans and Bisbibenzyl Derivatives from the Stems of Dendrobium nobile. Fitoterapia 2020, 143, 104586. [Google Scholar] [CrossRef]
- Ye, Q.; Zhao, W. New Alloaromadendrane, Cadinene and Cyclocopacamphane Type Sesquiterpene Derivatives and Bibenzyls from Dendrobium nobile. Planta Med. 2002, 68, 723–729. [Google Scholar] [CrossRef]
- Sukphan, P.; Sritularak, B.; Mekboonsonglarp, W.; Lipipun, V.; Likhitwitayawuid, K. Chemical Constituents of Dendrobium venustum and Their Antimalarial and Anti-Herpetic Properties. Nat. Prod. Commun. 2014, 9, 825–827. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Lian, X. Isolation of Stilbenoids and Lignans from Dendrobium Hongdie. Trop. J. Pharm. Res. 2015, 14, 2055. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.G.; Li, J.T.; Yin, B.L.; Liu, Y. Bibenzyls, 9,10-Dihydrophenanthrenes, and Phenanthraquinone from Dendrobium longicornu. Chem. Nat. Compd. 2010, 46, 790–791. [Google Scholar] [CrossRef]
- Hu, J.M.; Chen, J.J.; Yu, H.; Zhao, Y.X.; Zhou, J. Five New Compounds from Dendrobium longicornu. Planta Med. 2008, 74, 535–539. [Google Scholar] [CrossRef]
- Chen, D.-N.; Wang, Y.Y.; Liu, W.J.; Chen, Y.J.; Wu, Y.P.; Wang, J.X.; He, F.; Jiang, L. Stilbenoids from Aerial Parts of Dendrobium plicatile. Nat. Prod. Res. 2020, 34, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Lv, X.; Chen, J.; Peng, M.; Cai, J. Recent Research Progress on Natural Stilbenes in Dendrobium Species. Molecules 2022, 27, 7233. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.L.; Pal (Née Ray), S. Rotundatin, a New 9,10-Didydrophenanthrene Derivative from Dendrobium rotundatum. Phytochemistry 1992, 31, 3225–3228. [Google Scholar] [CrossRef]
- Li, J.; Feng, W.; Dai, R.; Li, B. Recent Progress on the Identification of Phenanthrene Derivatives in Traditional Chinese Medicine and Their Biological Activities. Pharmacol. Res. Mod. Chin. Med. 2022, 3, 100078. [Google Scholar] [CrossRef]
- Mittraphab, A.; Muangnoi, C.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. A New Bibenzyl-Phenanthrene Derivative from Dendrobium signatum and Its Cytotoxic Activity. Nat. Prod. Commun. 2016, 11, 657–659. [Google Scholar] [CrossRef]
- Zhan, R.; Wang, Z.C.; Yin, B.L.; Liu, Y.; Chen, Y.G. Novel 9, 10-Dihydrophenanthrene Derivatives from Eria bambusifolia with Cytotoxicity Aganist Human Cancer Cells In Vitro. Chin. J. Nat. Med. 2016, 14, 621–625. [Google Scholar] [CrossRef]
- Sun, A.; Liu, J.; Pang, S.; Lin, J.; Xu, R. Two Novel Phenanthraquinones with Anti-Cancer Activity Isolated from Bletilla striata. Bioorg. Med. Chem. Lett. 2016, 26, 2375–2379. [Google Scholar] [CrossRef]
- Kumar, N.P.; Nekkanti, S.; Sujana Kumari, S.; Sharma, P.; Shankaraiah, N. Design and Synthesis of 1,2,3-Triazolo-Phenanthrene Hybrids as Cytotoxic Agents. Bioorg. Med. Chem. Lett. 2017, 27, 2369–2376. [Google Scholar] [CrossRef]
- Li, J.Y.; Kuang, M.T.; Yang, L.; Kong, Q.H.; Hou, B.; Liu, Z.H.; Chi, X.Q.; Yuan, M.Y.; Hu, J.M.; Zhou, J. Stilbenes with Anti-Inflammatory and Cytotoxic Activity from the Rhizomes of Bletilla ochracea Schltr. Fitoterapia 2018, 127, 74–80. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Vizetto-Duarte, C.; Gangadhar, K.N.; Zengin, G.; Mollica, A.; Varela, J.; Barreira, L.; Custódio, L. In Vitro and In Silico Approaches to Unveil the Mechanisms Underlying the Cytotoxic Effect of Juncunol on Human Hepatocarcinoma Cells. Pharmacol. Rep. 2018, 70, 896–899. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, Y.; Yang, J.; Zhang, D.D.; Wang, Y.L.; Li, S.H.; Pan, Y.N.; Zhang, H.M.; Sun, Y. Comparative Analysis of Aristolochic Acids in Aristolochia Medicinal Herbs and Evaluation of Their Toxicities. Toxins 2022, 14, 879. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gong, S.; Wen, D.; Che, B.; Liao, Y.; Liu, H.; Feng, X.; Hu, S. Rapid Determination of Aristolochic Acid I and II in Aristolochia Plants from Different Regions by β-Cyclodextrin-Modified Capillary Zone Electrophoresis. J. Chromatogr. A 2004, 1049, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, J.; Fernandes, A. Ancient Medicinal Use of Aristolochia: Birthwort’s Tradition and Toxicity. Pharm. Hist. 2011, 53, 3–21. [Google Scholar] [PubMed]
- Dey, A.; Hazra, A.K.; Mukherjee, A.; Nandy, S.; Pandey, D.K. Chemotaxonomy of the Ethnic Antidote Aristolochia indica for Aristolochic Acid Content: Implications of Anti-Phospholipase Activity and Genotoxicity Study. J. Ethnopharmacol. 2021, 266, 113416. [Google Scholar] [CrossRef]
- Yang, H.Y.; Chen, P.C.; Wang, J.D. Chinese Herbs Containing Aristolochic Acid Associated with Renal Failure and Urothelial Carcinoma: A Review from Epidemiologic Observations to Causal Inference. BioMed Res. Int. 2014, 2014, 569325. [Google Scholar] [CrossRef]
- Dickman, K.G.; Sweet, D.H.; Bonala, R.; Ray, T.; Wu, A. Physiological and Molecular Characterization of Aristolochic Acid Transport by the Kidney. J. Pharmacol. Exp. Ther. 2011, 338, 588–597. [Google Scholar] [CrossRef]
- Han, J.; Xian, Z.; Zhang, Y.; Liu, J.; Liang, A. Systematic Overview of Aristolochic Acids: Nephrotoxicity, Carcinogenicity, and Underlying Mechanisms. Front. Pharmacol. 2019, 10, 648. [Google Scholar] [CrossRef]
- Arlt, V.M. Aristolochic Acid as a Probable Human Cancer Hazard in Herbal Remedies: A Review. Mutagenesis 2002, 17, 265–277. [Google Scholar] [CrossRef]
- Luciano, R.L.; Perazella, M.A. Aristolochic Acid Nephropathy: Epidemiology, Clinical Presentation, and Treatment. Drug Saf. 2015, 38, 55–64. [Google Scholar] [CrossRef]
- Ng, A.W.T.; Poon, S.L.; Huang, M.N.; Lim, J.Q.; Boot, A.; Yu, W.; Suzuki, Y.; Thangaraju, S.; Ng, C.C.Y.; Tan, P.; et al. Aristolochic Acids and Their Derivatives Are Widely Implicated in Liver Cancers in Taiwan and throughout Asia. Sci. Transl. Med. 2017, 9, eaan6446. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhang, Q.; Ma, G.; Zhu, L.; Wang, Y.; Chen, Z.; Wang, Y.; Zhao, L. New Dual-Spectroscopic Strategy for the Direct Detection of Aristolochic Acids in Blood and Tissue. Anal. Chem. 2019, 91, 8154–8161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, X.; Zhou, C.; Jia, Y.; Liu, S.; Xiong, Z.; Guo, X.; Fei, X.; Jiang, X.; Yu, W. Aristolochic Acid Induces Mitochondrial Apoptosis through Oxidative Stress in Rats, Leading to Liver Damage. Toxicol. Mech. Methods 2021, 31, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Jin, L.; Luo, K.; Jin, J.; Lim, S.W.; Shin, Y.J.; Ko, E.J.; Chung, B.H.; Yang, C.W. Assessment of Nephrotoxicity of Herbal Medicine Containing Aristolochic Acid in Mice. Korean J. Intern. Med. 2020, 35, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yuan, F.; Li, H.; Feng, Y.; Zhang, Y.; Zhang, C.; Zhang, J.; Song, Z.; Jia, L. The Ameliorations of Ganoderma applanatum Residue Polysaccharides against CCl4 Induced Liver Injury. Int. J. Biol. Macromol. 2019, 137, 1130–1140. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction-A Mini-Review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative Stress: The Mitochondria-Dependent and Mitochondria-Independent Pathways of Apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Cosyns, J.P.; Jadoul, M.; Squifflet, J.P.; De Plaen, J.F.; Ferluga, D.; Van Ypersele De Strihou, C. Chinese Herbs Nephropathy: A Clue to Balkan Endemic Nephropathy? Kidney Int. 1994, 45, 1680–1688. [Google Scholar] [CrossRef]
- Debelle, F.D.; Vanherweghem, J.-L.; Nortier, J.L. Aristolochic Acid Nephropathy: A Worldwide Problem. Kidney Int. 2008, 74, 158–169. [Google Scholar] [CrossRef]
- Depierreux, M.; Van Damme, B.; Vanden Houte, K.; Vanherweghem, J.L. Pathologic Aspects of a Newly Described Nephropathy Related to the Prolonged Use of Chinese Herbs. Am. J. Kidney Dis. 1994, 24, 172–180. [Google Scholar] [CrossRef]
- Vanhaelen, M.; Vanhaelen-Fastre, R.; But, P.; Vanherweghem, J.-L. Identification of Aristolochic Acid in Chinese Herbs. Lancet 1994, 343, 174. [Google Scholar] [CrossRef]
- Anandagoda, N.; Lord, G.M. Preventing Aristolochic Acid Nephropathy. Clin. J. Am. Soc. Nephrol. 2015, 10, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Cosyns, J.P. Aristolochic Acid and ‘Chinese Herbs Nephropathy’. Drug. Safety 2003, 26, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Pozdzik, A.A.; Salmon, I.J.; Debelle, F.D.; Decaestecker, C.; Van Den Branden, C.; Verbeelen, D.; Deschodt-Lanckman, M.M.; Vanherweghem, J.L.; Nortier, J.L. Aristolochic Acid Induces Proximal Tubule Apoptosis and Epithelial to Mesenchymal Transformation. Kidney Int. 2008, 73, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Jadot, I.; Declèves, A.E.; Nortier, J.; Caron, N. An Integrated View of Aristolochic Acid Nephropathy: Update of the Literature. Int. J. Mol. Sci. 2017, 18, 297. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Carlson, B.A.; Tobe, R.; Yefremova, E.; Tsuji, P.A.; Hoffmann, V.J.; Schweizer, U.; Gladyshev, V.N.; Hatfield, D.L.; Conrad, M. Glutathione Peroxidase 4 and Vitamin E Cooperatively Prevent Hepatocellular Degeneration. Redox Biol. 2016, 9, 22–31. [Google Scholar] [CrossRef]
- Seiler, A.; Schneider, M.; Förster, H.; Roth, S.; Wirth, E.K.; Culmsee, C.; Plesnila, N.; Kremmer, E.; Rådmark, O.; Wurst, W.; et al. Glutathione Peroxidase 4 Senses and Translates Oxidative Stress into 12/15-Lipoxygenase Dependent- and AIF-Mediated Cell Death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of Ferroptosis Regulator Glutathione Peroxidase 4 in Forebrain Neurons Promotes Cognitive Impairment and Neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Huang, X.; Liu, R.; Zhan, C.; Wu, H.; Fan, J.; Li, Z.; Yang, X. Aristolochic Acid Induces Acute Kidney Injury through Ferroptosis. Front. Pharmacol. 2024, 15, 1330376. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, P.; Huang, X.R.; Liu, F.; Lai, K.N.; Lan, H.Y. Activation of P53 Promotes Renal Injury in Acute Aristolochic Acid Nephropathy. J. Am. Soc. Nephrol. 2010, 21, 31–41. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-Y.; Wu, T.S.; Chen, T.W.; Liu, B.H. Aristolochic Acid I Induced Oxidative DNA Damage Associated with Glutathione Depletion and ERK1/2 Activation in Human Cells. Toxicol. Vitro 2011, 25, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Declèves, A.; Jadot, I.; Colombaro, V.; Martin, B.; Voisin, V.; Nortier, J.; Caron, N. Protective Effect of Nitric Oxide in Aristolochic Acid-induced Toxic Acute Kidney Injury: An Old Friend with New Assets. Exp. Physiol. 2016, 101, 193–206. [Google Scholar] [CrossRef]
- Kim, J.Y.; Leem, J.; Jeon, E.J. Protective Effects of Melatonin Against Aristolochic Acid-Induced Nephropathy in Mice. Biomolecules 2019, 10, 11. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, X.; Fang, L.; He, W.; Dai, C.; Yang, J. Aristolochic Acid Causes Albuminuria by Promoting Mitochondrial DNA Damage and Dysfunction in Podocyte. PLoS ONE 2013, 8, e83408. [Google Scholar] [CrossRef]
- Xie, X.C.; Zhao, N.; Xu, Q.H.; Yang, X.; Xia, W.K.; Chen, Q.; Wang, M.; Fei, X. Relaxin Attenuates Aristolochic Acid Induced Human Tubular Epithelial Cell Apoptosis In Vitro by Activation of the PI3K/Akt Signaling Pathway. Apoptosis 2017, 22, 769–776. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Wang, Y.; Shi, B.; Jin, Y.; Wang, Y.; Jiang, X.; Song, M.; Yu, W. Grape Seed Extract Proanthocyanidin Antagonizes Aristolochic Acid I-Induced Liver Injury in Rats by Activating PI3K-AKT Pathway. Toxicol. Mech. Methods 2023, 33, 131–140. [Google Scholar] [CrossRef]
- Yang, X.; Thorngren, D.; Chen, Q.; Wang, M.; Xie, X. Protective Role of Relaxin in a Mouse Model of Aristolochic Acid Nephropathy. Biomed. Pharmacother. 2019, 115, 108917. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Jin, J.; Guan, C.; Li, M.; Xi, C.; Ouyang, Z.; Chen, M.; Qiu, Y.; Huang, M.; et al. Endoplasmic Reticulum Stress Mediates Aristolochic Acid I-Induced Apoptosis in Human Renal Proximal Tubular Epithelial Cells. Toxicol. In Vitro 2012, 26, 663–671. [Google Scholar] [CrossRef]
- Hsin, Y.H.; Cheng, C.H.; Tzen, J.T.C.; Wu, M.J.; Shu, K.H.; Chen, H.C. Effect of Aristolochic Acid on Intracellular Calcium Concentration and Its Links with Apoptosis in Renal Tubular Cells. Apoptosis 2006, 11, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Debelle, F.A.A.A.A.D.; Nortier, J.D.L.; De Prez, E.G.; Garbar, C.H.; Vienne, A.R.; Salmon, I.J.; Deschodt-Lanckman, M.M.; Vanherweghem, J.-L. Aristolochic Acids Induce Chronic Renal Failure with Interstitial Fibrosis in Salt-Depleted Rats. J. Am. Soc. Nephrol. 2002, 13, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Honarpisheh, M.; Foresto-Neto, O.; Steiger, S.; Kraft, F.; Koehler, P.; Von Rauchhaupt, E.; Potempa, J.; Adamowicz, K.; Koziel, J.; Lech, M. Aristolochic Acid I Determine the Phenotype and Activation of Macrophages in Acute and Chronic Kidney Disease. Sci. Rep. 2018, 8, 12169. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.J.; Chen, Y.H. Developmental Nephrotoxicity of Aristolochic Acid in a Zebrafish Model. Toxicol. Appl. Pharmacol. 2012, 261, 59–65. [Google Scholar] [CrossRef]
- Jadot, I.; Colombaro, V.; Martin, B.; Habsch, I.; Botton, O.; Nortier, J.; Declèves, A.-E.; Caron, N. Restored Nitric Oxide Bioavailability Reduces the Severity of Acute-to-Chronic Transition in a Mouse Model of Aristolochic Acid Nephropathy. PLoS ONE 2017, 12, e0183604. [Google Scholar] [CrossRef]
- Wang, S.; Fan, J.; Mei, X.; Luan, J.; Li, Y.; Zhang, X.; Chen, W.; Wang, Y.; Meng, G.; Ju, D. Interleukin-22 Attenuated Renal Tubular Injury in Aristolochic Acid Nephropathy via Suppressing Activation of NLRP3 Inflammasome. Front. Immunol. 2019, 10, 2277. [Google Scholar] [CrossRef]
- Kholia, S.; Herrera Sanchez, M.B.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Brizzi, M.F.; Tetta, C.; Camussi, G. Human Liver Stem Cell-Derived Extracellular Vesicles Prevent Aristolochic Acid-Induced Kidney Fibrosis. Front. Immunol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Wang, L.; Liu, N.; Xue, X.; Zhou, S. The Effect of Overexpression of the Enhancer of Zeste Homolog 1 (EZH1) Gene on Aristolochic Acid-Induced Injury in HK-2 Human Kidney Proximal Tubule Cells In Vitro. Med. Sci. Monit. 2019, 25, 801–810. [Google Scholar] [CrossRef]
- Lemy, A.; Wissing, K.M.; Rorive, S.; Zlotta, A.; Roumeguere, T.; Muniz Martinez, M.-C.; Decaestecker, C.; Salmon, I.; Abramowicz, D.; Vanherweghem, J.-L.; et al. Late Onset of Bladder Urothelial Carcinoma After Kidney Transplantation for End-Stage Aristolochic Acid Nephropathy: A Case Series With 15-Year Follow-Up. Am. J. Kidney Dis. 2008, 51, 471–477. [Google Scholar] [CrossRef]
- Kwok, H.-C.; Chan, W. Aristolochic Acid Exposure via Dermal Contact or Inhalation of Herbal Powders: Evidence of Occupational Exposure in Herbalists with Urothelial Cancer. Chem. Res. Toxicol. 2024, 37, 873–877. [Google Scholar] [CrossRef]
- Chen, C.J.; Chiu, W.C.; Tseng, Y.H.; Lin, C.M.; Yang, H.Y.; Yang, Y.H.; Chen, P.C. Aristolochic Acid and the Risk of Cancers in Patients with Type 2 Diabetes: Nationwide Population-Based Cohort Study. Phytomedicine 2022, 99, 154023. [Google Scholar] [CrossRef]
- Au, C.K.; Chan, C.K.; Tung, K.K.; Zhang, J.; Chan, W. Quantitation of DNA Adducts of Aristolochic Acids in Repair-Deficient Cells: A Mechanistic Study of the DNA Repair Mechanism. Chem. Res. Toxicol. 2020, 33, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Bárta, F.; Dedíková, A.; Bebová, M.; Dušková, Š.; Mráz, J.; Schmeiser, H.H.; Arlt, V.M.; Hodek, P.; Stiborová, M. Co-Exposure to Aristolochic Acids I and II Increases DNA Adduct Formation Responsible for Aristolochic Acid I-Mediated Carcinogenicity in Rats. Int. J. Mol. Sci. 2021, 22, 10479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, J.; He, H.; Zhao, W.; Wong, Y.; Li, W.; Feng, S.; Liu, B.; Wang, J.; Luo, P. Hepatotoxic effects of aristolochic acid: Mechanisms and implications. Acta Mater. Medica 2024, 3, 349–362. [Google Scholar] [CrossRef]
- Sidorenko, V.S. Biotransformation and Toxicities of Aristolochic Acids. Adv. Exp. Med. Biol. 2020, 1241, 139–166. [Google Scholar]
- Yan, S.; Zhang, G.; Luo, W.; Xu, M.; Peng, R.; Du, Z.; Liu, Y.; Bai, Z.; Xiao, X.; Qin, S. PROTAC Technology: From Drug Development to Probe Technology for Target Deconvolution. Eur. J. Med. Chem. 2024, 276, 116725. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).