Abstract
Owing to the rapid advancements in optical and microsystem technologies, K9 glass is extensively utilized in the fabrication of high-precision optical components. Nevertheless, the intrinsic brittleness and elevated hardness of K9 glass, combined with the stringent demands of high-end optical systems for exceptional surface precision and minimal subsurface damage, present significant challenges for its chemical mechanical polishing (CMP) process. To overcome this challenge, we formulated a novel environmentally friendly and high-performance polishing slurry comprising cerium oxide (CeO2), aluminum oxide (Al2O3), guanidine carbonate (GC), and sodium laureth-6 carboxylate (SL-6C). The incorporation of a minor proportion of high-hardness Al2O3 abrasive particles significantly enhanced the mechanical friction within the polishing slurry, thereby markedly increasing the MRR. The judicious addition of GC facilitated the formation of a hydration layer on the glass substrate. The surfactant SL-6C modulated the surface charge of the abrasive particles through electrostatic and coordination interactions, which improved particle dispersion and mitigated agglomeration. This effect minimized the risk of surface scratching and enhanced interfacial lubrication, consequently reducing the energy required for the detachment of the reaction layer. CMP findings demonstrated that utilizing an optimized slurry formulation comprising 1 wt% CeO2, 0.05 wt% Al2O3, 0.2 wt% GC, and 0.2 wt% SL-6C yielded a surface roughness of K9 glass as low as 0.11 nm. Additionally, the MRR value reached 521.71 nm/min. Compared with the polishing slurry containing only CeO2, the MRR increased by 7 times. The observed synergistic interactions among Al2O3, GC, SL-6C, and CeO2 offered valuable insights for the advancement of high-performance CMP slurries.
1. Introduction
K9 glass is a high-quality borosilicate material characterized by several superior properties, including high optical transmittance, low refractive index, elevated hardness, a low thermal expansion coefficient, and excellent thermal stability [,]. These attributes facilitate effective control of light scattering, thereby significantly reducing aberrations within optical systems. Consequently, K9 glass is particularly well-suited for the fabrication of precision optical components. With the rapid advancement of optical and microsystem technologies, the cost-effectiveness and exceptional microfabrication capabilities of K9 glass have led to its widespread application in the production of microstructures and microdevices at micrometer and nanometer scales [,,]. Such applications are especially prominent in complex optical systems, encompassing lasers, microscopes, astronomical telescopes, microwave technologies, and industrial inspection instruments. High-end optical systems typically demand extremely high surface accuracy and minimal processing-induced damage layers []. Therefore, the implementation of ultra-smooth surface treatments on K9 glass is critically important for enhancing the functional performance of optical and microwave devices. The intrinsic brittleness and elevated hardness of K9 glass present considerable obstacles to ultra-precision machining []. Presently, chemical mechanical polishing (CMP) technology, which leverages the synergistic interaction between chemical reactions and mechanical abrasion to facilitate effective substrate polishing, offers a viable solution [,]. CMP not only efficiently removes surface defects from the substrate but also substantially minimizes internal damage. Owing to its capacity to optimize the balance between surface quality and processing efficiency, CMP has emerged as a critical technique for the production of high-quality, high-yield K9 glass components.
In the CMP process, the polishing slurry facilitates the removal of substrate material by inducing chemical reactions at the substrate surface while simultaneously exerting frictional forces []. The efficacy of the polishing slurry significantly influences both the surface roughness of the substrate and the material removal rate (MRR), thereby serving as a critical determinant of CMP efficiency. Typically, the polishing slurry comprises abrasive particles combined with chemical additives. Cerium oxide (CeO2), as an abrasive, is capable of engaging in specific chemical interactions with glass or silica surfaces, thereby enabling an enhanced MRR [,,]. Consequently, CeO2 abrasives are extensively employed in the polishing of silica-based materials. Nonetheless, polishing slurry composed exclusively of CeO2 abrasives exhibits limitations in terms of processing efficiency and surface quality. This has motivated efforts to optimize their performance through the incorporation of mixed abrasives and chemical additives []. Hence, a systematic investigation into the application of CeO2-based polishing slurry for the precision polishing of K9 glass, alongside the exploration of strategies to augment their effectiveness, holds significant theoretical importance and practical relevance.
Currently, the typical formulation of CeO2-based polishing slurries generally comprises the following categories of components: (i) abrasives (e.g., CeO2, La2O3, SiO2); (ii) oxidants/reductants (e.g., H2O2 and other peroxides); (iii) complexing/corrosion-inhibiting agents (e.g., citric acid, amino acids); (iv) dispersants/stabilizers (e.g., polyacrylic acid, PEG); (v) surfactants; and (vi) acids/bases (e.g., NaOH, NH4OH) for regulating the chemical environment [,,,,,,,]. The synergistic interactions among these components govern the interfacial reaction kinetics, particle dispersion stability, and abrasive–substrate contact behavior, thereby directly determining the MRR and surface quality. Previous studies have demonstrated that performance optimization of CeO2-based slurries can be achieved primarily via abrasive hybridization and the introduction of chemical additives. For example, Zhao et al. reported a CeO2-LaOF mixed abrasive system incorporating sodium N-lauroyl sarcosinate (SNLS) and sodium polyacrylate (PAAS) as dispersants []. Under alkaline conditions, this slurry exhibited significantly enhanced MRR and nanoscale surface roughness in quartz glass polishing, indicating that rare-earth oxyfluorides can effectively modulate the surface reactivity of CeO2 abrasives. Lv et al. formulated a polishing slurry consisting of potassium oleate (KOL), deionized water, CeO2, and molybdenum disulfide (MoS2) []. In this formulation, KOL functioned as both a surfactant and a pH regulator, thereby modulating the MRR of CeO2. Concurrently, MoS2 served as a solid lubricant, mitigating excessive mechanical damage to the substrate during the polishing process. Optimal concentrations of 0.2% KOL and 0.3% MoS2 in the polishing slurry yielded a quartz glass surface roughness of 0.48 nm and an MRR of 24.03 μm/h. He et al. investigated CeO2-SiO2 composite abrasives for K9 glass polishing and found that introducing 0.5 wt% SiO2 yielded the highest MRR (22.6 nm/min) and optimal surface roughness (1.3157 nm), highlighting the potential of composite abrasives for performance enhancement []. Nevertheless, the overall efficiency and surface precision remain insufficient for high-end applications. In addition to adding abrasives, appropriately formulated chemical additives also served a critical function. Polymer dispersants and small-molecule chelating agents (e.g., polyacrylates, carboxylates) have been shown to significantly improve the dispersion of CeO2 particles, modulate particle/substrate surface charge distribution, and reshape interfacial reaction pathways, thereby enhancing polishing uniformity and selectivity [,]. Moreover, the pH of the slurry significantly influenced the interaction between CeO2 and the substrate. It modulated the surface charge characteristics of both the particles and the substrate, thereby affecting their adsorption and reaction dynamics []. These factors subsequently influenced the formation and dissociation kinetics of transient Ce-O-Si bonds, thereby affecting both the efficiency of material removal and the extent of subsurface damage. Consequently, the combined modulation of pH and chemical additives has been extensively acknowledged as a critical approach for enhancing the performance of CeO2-based polishing slurries.
Despite prior research exploring the optimization of CeO2-based polishing slurries through the combination of mixed abrasives and chemical additives, challenges persist in achieving an optimal balance between MRR and surface quality in practical applications. Certain systems achieved relatively high removal efficiency but failed to adequately suppress surface damage and roughness, whereas others exhibited superior surface planarity at the expense of processing speed, limiting their suitability for large-scale, high-precision optical manufacturing. Therefore, striking an optimal balance between efficient MRR and high-quality surface finishing continues to be a central challenge for CeO2-based CMP processes. To address this issue, the present study proposed a novel slurry design strategy by incorporating Al2O3 into conventional CeO2 abrasives. It was expected to utilize the high chemical reactivity of CeO2 and the high hardness of Al2O3 in a synergistic manner to achieve an effective combination of chemical softening and mechanical wear, thereby achieving a balance between efficiency and precision. In terms of chemical additives, sodium laureth-6-carboxylate (SL-6C) and guanidine carbonate (GC) were introduced to improve particle dispersion and interfacial chemical reactivity. The SL-6C was capable of modifying the surface charge of abrasive particles, thereby improving the dispersion stability of the abrasive in the polishing slurry. Concurrently, the presence of GC reinforced the alkaline conditions, which facilitated the development of a softening layer on the glass surface. Herein, we aimed to develop a CeO2-Al2O3 mixed slurry capable of simultaneously achieving high MRR and superior surface quality, and to systematically evaluate its performance in the ultraprecision polishing of K9 glass. This work highlighted the synergistic mechanisms between mixed abrasives and the co-regulation of chemical additives, thereby offering not only a promising strategy for high-precision optical fabrication but also a new paradigm for the further optimization of CeO2-based CMP slurries.
2. Results and Discussion
2.1. Analysis of Abrasive Morphology and Evaluation of CMP Efficiency
Figure 1a illustrated the schematic representation of the synthesis process for the polishing slurry. Sodium laureth-6-carboxylate (SL-6C), guanidine carbonate (GC), cerium oxide (CeO2), and alumina (Al2O3) were sequentially introduced into deionized water and subjected to ultrasonic dispersion to ensure homogeneous distribution. The morphology of the abrasive particles was examined using transmission electron microscopy (TEM). As shown in Figure 1b–e, the CeO2 particles had a spherical morphology with an average size of 126 ± 17 nm. The Al2O3 particles had an irregular shape with distinct edges, which was beneficial for increasing the polishing rate. However, the size distribution of the Al2O3 particles was wide, with an average size of approximately 339 ± 130 nm. Therefore, it was not advisable to introduce too many Al2O3 particles into the polishing slurry to avoid affecting the uniformity of the abrasive distribution in the solution []. Upon dispersion of the two abrasives within the polishing slurry, nitrogen (N) and sodium (Na) elements were found to be uniformly distributed across the surfaces of both abrasives, indicating effective adherence of the additives to the abrasive surfaces (Figure 1f).
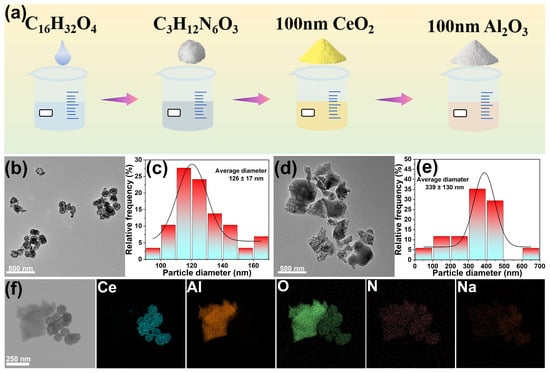
Figure 1.
(a) Diagrammatic representation of the synthesis process of the polishing slurry, (b) TEM images of CeO2, (c) Size distribution of CeO2, (d) TEM images of Al2O3, (e) Size distribution of Al2O3, (f) EDS images of CeO2-Al2O3 mixed abrasive.
To systematically evaluate the influence of individual components on polishing performance, a baseline slurry was prepared by dispersing CeO2 abrasives in deionized water and adjusting the pH to 10 using NaOH or acetic acid. A single-variable approach was then employed to determine the optimal concentrations of CeO2, Al2O3, GC, and SL-6C, using the material removal rate (MRR) and surface roughness (Ra) of K9 glass as the primary evaluation metrics. As shown in Figure 2a, increasing the CeO2 concentration from 0.5 wt% to 8 wt% resulted in a general rise in MRR, while Ra initially decreased slightly and then stabilized. Specifically, at 0.5 wt% CeO2, the MRR was only 46.70 nm/min with an Ra of 0.41 nm, whereas at 1 wt% CeO2 the MRR increased markedly to 68.33 nm/min and Ra decreased to 0.38 nm, indicating a favorable balance between MRR and surface finish. Further increasing to 2 wt% and 4 wt% CeO2 led to MRR values of 76.63 and 95.04 nm/min, respectively, with Ra remaining at approximately 0.39 nm. However, at 8 wt% CeO2, the MRR surged to 182.31 nm/min, but Ra increased to 0.46 nm, suggesting that excessive abrasive loading might promote particle agglomeration and induce micro-scratching, thereby degrading the surface quality. Taking into account the MRR, Ra values and cost issues, the optimal concentration of CeO2 abrasive was determined to be 1 wt%. Subsequently, we used a 1 wt% CeO2 solution as the base polishing slurry and conducted a screening of the amount of Al2O3. As shown in Figure 2b, increasing the Al2O3 concentration from 0.05 wt% to 0.5 wt% produced a general upward trend in MRR, while Ra initially decreased and then slightly rebounded. An excessive concentration of Al2O3 could lead to the aggregation of abrasive particles, which might induce localized micro-damage on the glass surface. This phenomenon adversely impacted the overall surface quality of the glass. Conversely, at an Al2O3 concentration of 0.05 wt%, the slurry demonstrated an optimal balance, achieving a high MRR while effectively reducing Ra. Consequently, this concentration was identified as the optimal Al2O3 content. Figure 2c,d illustrated the effects of GC and SL-6C concentrations on the MRR and Ra of K9 glass. GC was a strong base, which helped increase the alkalinity of the polishing slurry. This was beneficial for forming a softer and more elastic hydrated layer on the glass surface [,]. At an additive concentration of 0.2 wt% GC, the MRR reached its maximum value while concurrently maintaining the lowest Ra. However, increasing the concentration beyond this point led to a reduction in MRR and a marginal increase in Ra. This phenomenon might be attributed to excessive alkalinity, which potentially weakened the dispersion of abrasive particles, diminished chemical reactivity, or disrupted the synergistic interaction between chemical and mechanical processes at the interface of the abrasive particles and the substrate. SL-6C was an anionic surfactant characterized by the presence of carboxyl functional groups. It modulated the surface charge of abrasive particles via electrostatic and coordination interactions, thereby improving particle dispersion and mitigating agglomeration. This in turn minimized scratching risk while improving interfacial lubrication and lowering the detachment energy of the reaction layer. At an SL-6C concentration of 0.2 wt%, the polishing slurry attained its highest MRR and the lowest Ra. Increasing the concentration beyond this point might lead to excessive surfactant adsorption or over-lubrication, which could diminish the abrasive’s cutting effectiveness and consequently decrease the MRR. Integrating the findings from Figure 2a–d, the optimal polishing slurry was established as follows: 1 wt% CeO2 as the primary abrasive, 0.05 wt% Al2O3 as the secondary abrasive, 0.2 wt% GC and 0.2 wt% SL-6C as chemical additives, and pH was adjusted to 10. This formulation concurrently improved the dispersion stability of the particles, the interfacial lubricity, and the chemical reactivity of CeO2, resulting in a substantial increase in MRR and a marked reduction in Ra. The synergistic interaction between chemical corrosion and mechanical wear under these conditions underscored the critical importance of the coordinated selection of abrasives and chemical additives to optimize the performance of CeO2-based CMP slurries.
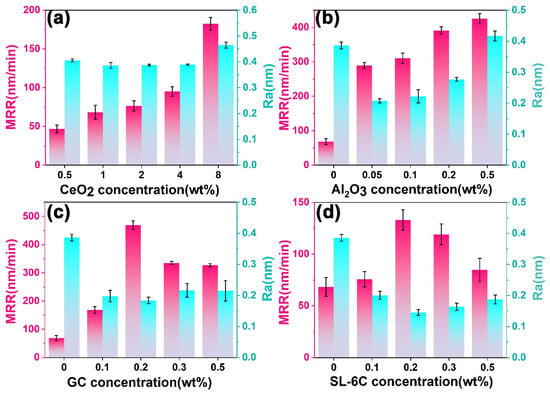
Figure 2.
Screening of optimal polishing slurry formulation incorporation (a) CeO2, (b) Al2O3, (c) GC and (d) SL-6C.
As illustrated in Figure 3a, the base polishing slurry employed consisted of a CeO2 suspension with a mass fraction of 1 wt% and a pH value of 10. A marked difference in performance was observed between this basic slurry and the optimized polishing slurry developed in the present study. Specifically, the MRR of the basic polishing slurry was relatively low, measured at approximately 68.33 nm/min, with an Ra of 0.38 nm. In contrast, the optimized slurry exhibited a substantially enhanced MRR of 521.71 nm/min, accompanied by a reduced Ra value of 0.11 nm. These results demonstrated that the optimized polishing slurry could simultaneously achieve high MRR and superior surface quality. Dynamic light scattering (DLS) analysis was utilized to evaluate the dispersion stability of the polishing slurry. This technique involves monitoring the intensity of scattered light and its temporal variations to derive kinetic information about the powder particles, which is subsequently used to calculate the particle size distribution. It is important to note that DLS measures the hydrated particle size within the solution rather than the actual physical size. For non-spherical particles, the hydrated size is estimated based on an equivalent volume diameter, which limits the accuracy of size determination for irregularly shaped nanomaterials. Thus, DLS primarily provides relative size ranges and trends when comparing similar materials. Consequently, electron microscopy is typically employed to ascertain the true particle size, while DLS offers insight into the overall particle size distribution within the sample. The DLS results revealed that the hydrated particle size of CeO2 in the pure CeO2 polishing slurry was 201.5 nm (Figure 3b). Over time, an increase in hydrated particle size was observed, indicating poor dispersion stability of the pure CeO2 slurry. Upon the addition of 0.05 wt% Al2O3 nanoparticles, the hydrated particle size of the mixed abrasive increased to 283.8 nm, suggesting that the incorporation of larger Al2O3 nanoparticles significantly enlarged the overall particle size (Figure 3c). Furthermore, the hydrated particle size continued to increase with storage time, implying that the polishing slurry containing only CeO2 and Al2O3 abrasives exhibited instability, with a tendency for particle agglomeration and sedimentation. In the optimized polishing slurry, DLS measurements primarily served to assess the overall dispersion state of the CeO2-Al2O3 mixed abrasives rather than to determine the precise size of individual particles. The measured hydrated particle size represented a weighted average reflecting the combined effects of both components. Complementary EDS analysis (Figure 1f) demonstrated that during slurry preparation, GC and SL-6C additives were uniformly adsorbed onto the abrasive surfaces, facilitating improved dispersion. As depicted in Figure 3d, the particle size of the mixed abrasive in the optimized polishing slurry was 210.1 nm, indicating that despite the large and irregular morphology of Al2O3 particles, the additives effectively enhanced abrasive dispersion. Over extended reaction times, only a slight increase in hydrated particle size was observed, signifying that the inclusion of GC and SL-6C markedly improved the dispersion stability of the polishing slurry. We further conducted Zeta potential and contact angle tests, as illustrated in Figure 3e,f. The optimized polishing slurry formulation resulted in an increase in the surface potential of the particles from −6.73 mV to −38.7 mV, accompanied by a decrease in the contact angle from 37.72 ± 0.71° to 29.11 ± 0.11°. These findings suggested that the incorporation of additives significantly enhanced both the wetting characteristics and the dispersion stability of the polishing slurry. The enhancement in surface wettability was anticipated to facilitate more efficient and uniform interactions between the particles and the substrate []. Furthermore, the real-time contact angle image depicted in Figure 3g demonstrated that the liquid film formed by the optimized polishing slurry on the glass surface exhibited a more homogeneous distribution, thereby providing direct evidence of the improved wetting properties.
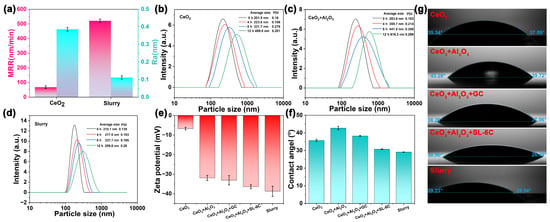
Figure 3.
Comparison for baseline slurry and optimized slurry in (a) MRR and Ra, DLS analysis of (b) baseline slurry, (c) Adding 0.05 wt% Al2O3 to the baseline slurry and (d) optimized slurry, (e) Zeta potential of different slurries, n = 3, (f) Contact angle of different slurries, n = 3, (g) Real-time contact angle images.
Previous studies have shown that by using abrasive mixtures and adding chemical additives, the performance of abrasive slurry based on CeO2 can be optimized. In the mixed abrasive polishing slurry, for the selection of the second type of abrasive, there were generally three approaches. The first was to choose a second type of abrasive with higher hardness, using the high hardness of the second abrasive to significantly increase the removal rate of cerium-based abrasive []. However, this situation might lead to poor surface quality of the wafer after polishing. The second was to add two-dimensional materials as solid lubricants []. The addition of solid lubricants could reduce the excessive friction between CeO2 and the workpiece, thereby improving the surface quality of the polished wafer. The third was to add lanthanide metal oxides []. During the polishing process, the lanthanide metal oxides might enhance the chemical activity of CeO2, thereby improving its polishing performance. For the selection of additives, generally, anionic-type surfactants were chosen. This type of surfactant not only improved the dispersion of CeO2 but also helped to enhance the chemical activity of CeO2, thereby improving the polishing performance of cerium-based abrasive. As summarized in Table 1, by comparing representative works with our own work, it could be seen that this polishing slurry formula led in both cases to the removal rate of K9 glass and the surface quality of K9 glass after polishing. These comparisons clearly demonstrated the superior polishing performance and practical potential of the designed slurry formulation.

Table 1.
Statistical data on the polishing effects of slurry with different abrasives and chemical additives.
Ultra-depth-of-field optical microscopy was employed to systematically examine the surface morphology of K9 glass before and after polishing (Figure 4a,d). Prior to polishing, the glass surface exhibited pronounced scratches, pits, and other irregular defects, indicative of severe processing-induced damage. After treatment with the optimized slurry, the K9 glass surface became markedly flatter, smoother, and more continuous, with virtually no discernible remnants of the original defects. This direct visual evidence highlighted the superior capability of the formulated slurry to remove surface damage and improve micro-scale flatness. Figure 4b,e further illustrated the subsurface damage state of the K9 glass substrates before and after polishing. Before polishing, a distinct damage layer with a thickness of approximately 44.06 nm was present, reflecting structural disruption introduced during prior machining. After polishing with the optimized slurry, the damage layer thickness was significantly reduced to 11.8 nm, suggesting that the slurry effectively removed surface defects. Figure 4c,f showed the three-dimensional (3D) surface topographies of K9 glass before and after polishing. Prior to polishing, the surface of the K9 glass exhibited pronounced undulations and a relatively high Ra value of 0.7117 nm, characterized by numerous protrusions and micro-cracks. In contrast, following treatment with the polishing slurry, the surface roughness significantly decreased to 0.1116 nm, and the surface morphology became notably smoother and more uniform.
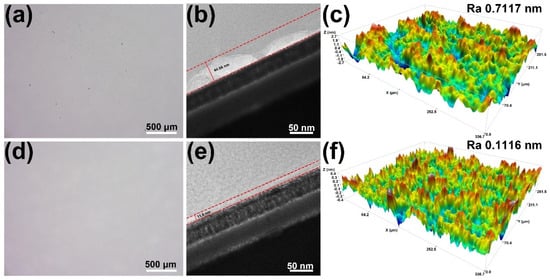
Figure 4.
Ultra-depth-of-field microscope images of (a) original surface of K9 glass and (d) polished surface using optimized slurry; TEM images of the surface damage layer of (b) the original glass and (e) polished surface using optimized slurry; 3D surface profile of (c) the original glass surface and (f) polished surface using optimized slurry.
2.2. Analysis of CMP Mechanism
XPS tests were conducted on pure CeO2 and the polishing slurry before and after the polishing process. The Ce 3d spectrum was decomposed into ten distinct peaks (Figure 5a–c). Specifically, the peaks labeled as v, v″, v‴, u, u″ and u‴ corresponded to the Ce4+ species, while v0, v’, u0 and u’ were classified as the Ce3+ species [,]. The semi-quantitative analysis based on peak area fitting was used to determine the concentration of Ce3+ ions. The concentration of Ce3+ ions on the surface of pure CeO2 was measured at 31.48%. Following the preparation of the polishing slurry, the Ce3+ ion concentration on the CeO2 surface increased markedly to 40.37%, suggesting that the incorporation of Al2O3, SL-6C, and GC substantially enhanced the chemical reactivity of CeO2. Subsequent to the polishing process using this slurry, the Ce3+ concentration on the CeO2 surface decreased to 35.88%. This reduction was likely attributable to the consumption of Ce3+ ions during the polishing process.
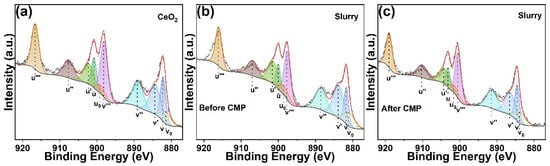
Figure 5.
Ce 3d XPS spectra of (a) CeO2, (b) Slurry before polishing and (c) Slurry after polishing.
We further explored the interactions between Al2O3, GC, SL-6C and CeO2 through theoretical calculations. According to the Frontier Molecular Orbital (FMO) calculations, the highest occupied molecular orbital (HOMO) exhibited a lower electron binding energy, indicating its donor characteristics, while the lowest unoccupied molecular orbital (LUMO) showed significant electron affinity, consistent with the receptor behavior [,,]. As shown in Figure 6, for pure CeO2, the HOMO (−8.151 eV) of a single repeating unit Ce(OH)4 was uniformly distributed throughout the molecule, while its LUMO (−6.049 eV) was located on the Ce atom, forming a HOMO-LUMO gap of 2.102 eV. When Al2O3 was introduced into the Ce(OH)4 structure, the gap between HOMO (−6.586 eV) and LUMO (−4.8651 eV) decreased to 1.735 eV, indicating that electron transfer occurred between CeO2 and Al2O3, and the chemical activity of the CeO2-Al2O3 mixed abrasive was higher []. In the CeO2 + GC system, the HOMO (−6.586 eV) was mainly distributed in GC, while the LUMO (−4.851 eV) was mainly distributed in CeO2, and the HUMO-LUMO gap (1.459 eV) was further reduced, indicating that the chemical activity between CeO2 + GC was also significantly enhanced. In the CeO2 + SL-6C system, its HUMO (−6.646 eV) was mainly distributed in SL-6C, while LUMO (−4.797 eV) was mainly distributed in CeO2, and the HUMO-LUMO gap (1.849 eV) was also lower than that of CeO2, indicating that the addition of SL-6C also significantly affected the chemical activity of CeO2. Theoretical calculations further supported the CMP experiment. We further calculated the changes in surface energy of each component after mixing with cerium oxide, as shown in Table 2. The surface energy of pure CeO2 was −36.9 kJ/A2. When GC, SL-6C, and Al2O3 were added, the surface energy gradually decreased to −37.9 kJ/A2, −40.7 kJ/A2, and −37.6 kJ/A2, indicating that the addition of all three materials could enhance the dispersion stability of CeO2 [,]. Particularly, the addition of SL-6C reduced its surface energy, suggesting that SL-6C, as a surfactant, had the best effect in improving the dispersion stability of CeO2.
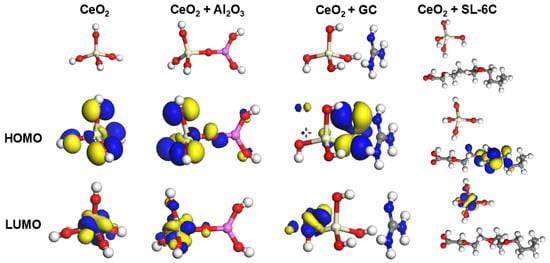
Figure 6.
HUMO and LUMO of CeO2, CeO2 + Al2O3, CeO2 + GC, and CeO2 + SL-6C.

Table 2.
HUMO, LUMO and surface energy of CeO2, CeO2 + Al2O3, CeO2 + GC, and CeO2 + SL-6C.
To elucidate the role of the optimized slurry formulation in surface chemical modification and material removal on K9 glass, XPS and FTIR analyses were performed on the substrates before polishing, after immersion in the optimized slurry for 72 h, and after polishing (Figure 7). The Si 2p spectra showed that the unpolished sample exhibited a dominant SiO2 peak at 103.15 eV and only a weak SiO32− signal at 102.33 eV (Figure 7a) [,]. After 72 h immersion, the SiO32− signal became markedly stronger, approaching but still slightly lower than that of SiO2, indicating that under alkaline conditions the slurry induced hydration/depolymerization of the glass surface, thereby generating a higher fraction of low-polymerized silicate species. After polishing, the SiO32− component further increased and surpassed the SiO2 peak, suggesting that chemical–mechanical coupling produced a depolymerized, silicate-rich layer that was more easily removed. Complementary evidence was provided by deconvolution of the O 1s spectra (Figure 7b). Before polishing, distinct peaks were observed at 533.71 eV (adsorbed H2O), 532.56 eV (SiO2), and 531.8 eV (SiO32−), while the metal oxide (M-O) contribution at 530.64 eV was minimal []. After 72 h immersion, the adsorbed water and SiO32− components increased while the SiO2 component slightly decreased, confirming that hydration facilitated partial cleavage of the Si-O-Si network and formation of hydroxylated surfaces. Critically, after polishing, SiO32− became the dominant O 1s component, and a new peak appeared at 531.14 eV attributable to a Si-O-Ce chemical state, implying that CeO2 interacted chemically with the glass surface through transient Ce-O-Si bonding [,]. This interaction lowered the binding strength of the reaction layer and enhanced its susceptibility to shear removal. In contrast, the M-O signal remained essentially unchanged across all three states, suggesting that bulk metal oxide content was not fundamentally altered or massively introduced. The N 1s spectra (Figure 7c) showed only background noise before polishing but a distinct peak at 400 eV after immersion and polishing, indicating adsorption or incorporation of N species (derived from GC) into the surface/reaction layer. These nitrogen-containing species might adjust surface charge and local chemistry through weak coordination or electrostatic interactions, thereby facilitating Ce-O-Si bond formation and accelerating interfacial reactions, consistent with the observed changes in Zeta potential and contact angle. FTIR spectra further corroborated these findings (Figure 7d). The unpolished sample displayed characteristic Si-O-Si stretching bands at 913 and 762 cm−1 [,,]. After 72 h of immersion, the intensities of these SiO2-related peaks decreased and exhibited slight shifts. After polishing, the peaks weakened and shifted further, revealing hydrolysis/cleavage and chemical modification of the Si-O-Si framework. This attenuation could be ascribed to partial Si-O bond breakage and new bonding with Ce or additives (e.g., Ce-O-Si), thereby diminishing the original Si-O-Si vibrational response.
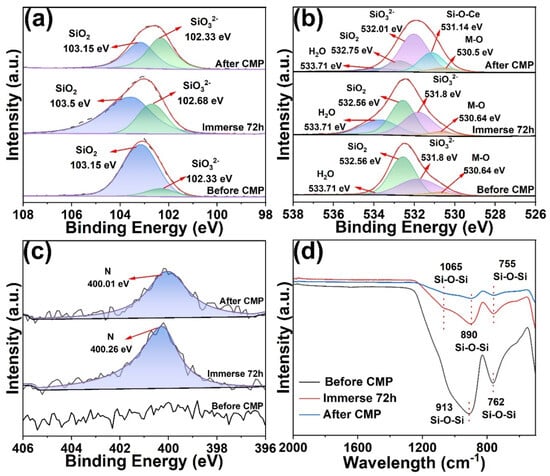
Figure 7.
XPS spectra of K9 glass surface before and after CMP, and after immersion in the optimized slurry for 72 h: (a) Si 2p spectra, (b) O 1s spectra, (c) N 1s spectra. (d) FTIR spectra of K9 glass surfaces before and after CMP, and after immersion in the optimized slurry for 72 h.
An additional experiment was conducted in which glass sheets were immersed in the polishing slurry for a duration of 24 h. A comparative analysis of Figure 7a and Figure 8a revealed that prolonging the immersion time resulted in an increased presence of the hydrated layer on the glass surface. Subsequently, the influence of specific additives within the polishing slurry on the formation of the hydrated layer during immersion was examined. Glass sheets were immersed for 24 h in slurries lacking GC, SL-6C, and Al2O3, respectively, followed by surface analysis using XPS. Analysis of the Si 2p spectra indicated that the absence of GC in the slurry corresponded with a markedly diminished SiO32− signal on the glass surface compared to samples containing GC, suggesting that the inclusion of GC facilitated the development of a softening layer on the glass surface (Figure 8a). Furthermore, the presence of the N 1s signal on the K9 glass surface was detected only after the addition of the GC additive to the polishing slurry (Figure 8b).
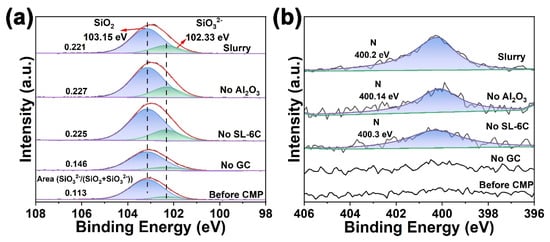
Figure 8.
XPS spectra of K9 glass surface after immersion in the different slurries for 24 h: (a) Si 2p spectra, (b) N 1s spectra.
Taken together, the systematic XPS and FTIR results demonstrated that, under the combined action of alkaline slurry and chemical additives, the K9 glass surface first underwent hydration/depolymerization to form a soft, SiO32−-rich reaction layer [,]. CeO2 then chemically interacted with this layer, producing transient Si-O-Ce bonds that weaken interfacial binding and render the layer more easily sheared away by abrasives (Figure 9). The intermediate state observed after immersion (enhanced H2O and SiO32− signals, emergence of N 1s) supported a two-step mechanism of chemical activation followed by mechanical removal. This mechanistic insight explains why the optimized formulation not only dramatically increases MRR but also minimizes subsurface damage and improves surface planarity, achieving high polishing efficiency and superior surface quality through synergistic chemical softening and mechanical removal.
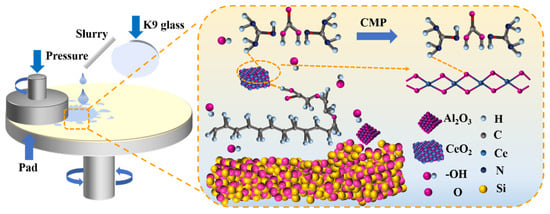
Figure 9.
Schematic diagram of the polishing mechanism of polishing slurry.
3. Materials and Methods
3.1. Materials
All the materials were purchased from Xilong Chemical and did not undergo any further purification.
3.2. Preparation of CeO2 Abrasive and Slurry
A homogeneous solution was prepared by dissolving 17.5 g cerium nitrate hexahydrate (Ce(NO3)3·6H2O), 3.95 g hexamethylenetetramine (HMT, C6H12N4), and 1 g tartaric acid (C4H6O6) in 5000 mL deionized water under ultrasonic dispersion for 10 min. The resulting solution was subsequently heated to 80 °C and maintained at this temperature for 8 h under continuous magnetic stirring (500 rpm). The precipitated product was isolated via centrifugation (10,000 rpm, 3 min) and subjected to sequential washing cycles using deionized water and anhydrous ethanol (3 times each) to remove residual ionic impurities. After vacuum drying at 60 °C for 24 h, the cerium-based precursor was transferred to a tubular furnace for calcination. The thermal treatment involved a controlled heating rate of 5 °C/min up to 800 °C, with a dwell time of 4 h under ambient atmosphere, yielding CeO2 abrasive.
Sodium laureth-6-carboxylate, guanidine carbonate, Al2O3, and CeO2 abrasives were combined in specific proportions with deionized water. Subsequently, the pH value of the above solution was tested using a pH meter. If the pH value was higher than 10, the pH of the solution was adjusted to 10 using acetic acid. If the pH value was lower than 10, the pH was adjustedto 10 using sodium hydroxide. Then, the prepared solution was subjected to ultrasonic treatment at ambient temperature for 1 h to produce a homogeneous suspension.
3.3. Characterization
High-resolution transmission electron microscopy (HR-TEM, JEM ARM-200F) was employed to characterize the morphology of the materials. The hydrodynamic particle size distribution and zeta potential were determined with a dynamic light scattering analyzer (ELSZ-2000ZS, Otsuka Electronics, Suzhou, China). The elemental composition and chemical states were examined by X-ray photoelectron spectroscopy (XPS, AXIS Supra, Shimadzu, Kyoto, Japan). During the XPS data processing procedure, the background subtraction method adopted was the Smart method, with the FWHM range set at 0.5–3.5 eV. The fitting constraints were fixed as 30% for the Lorentz/Gaussian mixture, 100% for the tail mixture, 0% for the tail height, and 0% for the tail index. The reference position was obtained by performing peak-fitting using the spectra of standard Ce4+ and Ce3+. The distances between the Ce4+ peaks and the distances between the Ce3+ peaks were calculated, and then peak-fitting was carried out using the bimodal peak-fitting mode to maintain the distances between the peaks as much as possible without changing them. The charge reference was calibrated using the C 1s at 284.8 eV, as the main purpose was to investigate the activity changes in the Ce element before and after polishing. The analysis of the total spectrum was not very significant for this.
3.4. CMP Test
K9 glass substrates (8-inch) were obtained from Qingdao Mingyu Technology Co., Ltd., (Qingdao, China). For comparative purposes, commercial Al2O3 abrasives with an average particle size of 100 nm were purchased from Zibo Xiyan Nanomaterials Co., Ltd., (Zibo, China). Polishing experiments were conducted on a polishing machine (Shenzhen Fangda Grinding Technology Co., Ltd., Shenzhen, China) equipped with a Suba 800 polishing pad (Dupont, Wilmington, DE, USA). The operating conditions were set as follows: platen rotation speed, 55 rpm; polishing head rotation speed, 55 rpm; applied weight, 80 kg. Then the load was 784 N, according to the formula P = F/S (where P represents pressure, F represents load, and S represents area), and it could be known that the pressure applied to the surface of the glass was 24.95 kPa; polishing duration, 2 min; and slurry feed rate, 50 mL/min.
After polishing, the K9 glass samples were repeatedly rinsed in deionized water and dried using an ultrasonic cleaner. The surface roughness was measured using a three-dimensional surface profilometer (Sneox 090, SENOFAR, Barcelona, Spain) over a scanning area of 336.71 × 281.51 µm2. The sample weight was recorded using a precision electronic balance with an accuracy of 0.1 mg. The polishing efficiency, expressed as the material removal rate (MRR, nm/min), was calculated according to Equation (1).
Here, M0 represented the quality of K9 glass before polishing, M represented the quality of K9 glass after polishing, ρ represented the density of K9 glass (2.53 g/cm3), S represented the area of K9 glass (314.2 cm2), and t represented the polishing time (2 min).
4. Conclusions
This study tackled the long-standing challenge of simultaneously achieving a high MRR and superior surface quality in CeO2-based polishing slurries by introducing a novel slurry design strategy that integrates CeO2-Al2O3 mixed abrasives with synergistic chemical additives. Leveraging the pronounced chemical reactivity of CeO2, robust transient Ce-O-Si bonds were established with the surface of K9 glass. Furthermore, owing to its elevated hardness and superior mechanical wear resistance, Al2O3 contributed to improving the MRR while preserving surface flatness. Through the co-integration of the two abrasives and precise pH adjustment to 10, the slurry established an effective synergy between chemical softening and mechanical stripping, thereby overcoming the intrinsic trade-off between MRR and Ra inherent to conventional single-abrasive CeO2 slurries. Performance evaluations demonstrated that the optimized composite-abrasive slurry achieves nearly a seven-fold increase in MRR and a 71% reduction in Ra compared with the baseline slurry, highlighting the pronounced benefits of coupling composite abrasives with tailored chemical additives. Complementary Zeta potential, contact angle, and DLS analyses confirmed that this formulation markedly enhanced particle surface potential, dispersion stability, and wettability, facilitating uniform adsorption and interfacial interactions of abrasives on the glass surface and thereby establishing favorable conditions for efficient CMP. XPS and FTIR analyses further elucidated the underlying chemo-mechanical synergy. Under alkaline conditions and in the presence of synergistic additives, the glass surface undergoes hydration and depolymerization to form a SiO32−-rich softened layer. Subsequent action of the CeO2-Al2O3 mixed abrasives enabled dual chemical–mechanical removal: CeO2 lowered interfacial binding energy by forming transient Si-O-Ce linkages, while Al2O3 enhanced shear and stripping capability, collectively promoting efficient detachment of the reaction layer. Attenuation and shifts in FTIR vibrational bands corroborated the disruption of the Si-O-Si framework and the formation of new chemical bonds such as Si-O-Ce. In summary, this work demonstrated and validated a new CMP slurry design concept based on the synergistic integration of CeO2-Al2O3 mixed abrasives, targeted chemical additives. This approach not only delivered a substantial increase in MRR but also ensured excellent surface integrity during ultra-precision polishing. By effectively coupling chemical softening with mechanical abrasion, the proposed slurry provided a robust and scalable pathway for high-efficiency, low-damage processing of hard and brittle optical materials. Beyond offering a viable paradigm for the precision manufacturing of optical components and semiconductor substrates, these findings also furnished new insights into mixed abrasive engineering and interfacial chemical synergy, guiding future optimization of CeO2-based CMP systems.
Author Contributions
S.L.: Writing—original draft, review & editing, Data curation. R.Y.: Investigation, Methodology. Z.Z.: Investigation, Methodology, Visualization. Z.H.: Investigation, Methodology. K.F.: Methodology, H.C.: editing, R.C.: Writing—original draft, Writing—review & editing. Y.L.: Writing—review & editing. Y.C.: Writing—review. All authors have read and agreed to the published version of the manuscript.
Funding
The Natural Science Foundation of Hubei Province (2024AFD140 and 2025AFD259) also provided support for this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the articl. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Authors Shaoping Li, Rui Ye, Zhemin Zou, Zhaobo He, Kai Feng, Huidong Cui, Yukun Chen and Yue Luo were employed by the company Hubei Sinophorus Electronic Materials Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Cheng, H.; Feng, Z.; Wang, Y.; Lei, S. Magnetic Bingham fluid-assisted deterministic polishing for super-smooth surfaces. Chin. Sci. Bull. 2005, 50, 172–178. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Geng, F.; Zhou, X.; Feng, S.; Ren, D.; Cheng, X.; Jiang, X.; Wu, W.; Zheng, W.; et al. Fluorescence and Raman spectra on surface of K9 glass by high fluence ultraviolet laser irradiation at 355nm. Opt. Commun. 2013, 308, 91–94. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Wang, X.; Zhang, F.-Y.; Wang, P.-P.; Zhang, Y.-L.; Sun, S.-F. Performance comparison of laser-etched microstructures on K9 glass and PMMA light guide plate. Optik 2021, 242, 167213. [Google Scholar] [CrossRef]
- Shang, X.; Shi, W.; Hai, W.; Su, J.; Dong, C. Optical characteristics of the undamaged and laser damaged K9 glass in terahertz band. Mater. Res. Express 2020, 7, 045202. [Google Scholar] [CrossRef]
- Qu, S.; Wang, Z.; Zhang, C.; Ma, Z.; Zhang, T.; Chen, H.; Wang, Z.; Yu, T.; Zhao, J. Material removal profile prediction and experimental validation for obliquely axial ultrasonic vibration-assisted polishing of K9 optical glass. Ceram. Int. 2021, 47, 33106–33119. [Google Scholar] [CrossRef]
- Tang, K.-E.; Yan, J.; Kwan, S.-Y.; Zhen, M.-Y.; Liu, C.-W. Using fast Fourier transform analysis for the rapid evaluation of the diffraction fringes of optical components produced through ultraprecision single-point diamond turning. Mech. Syst. Signal Process. 2025, 237, 113167. [Google Scholar] [CrossRef]
- Qu, S.; Zhang, C.; Liang, Y.; Ma, Z.; Meng, F.; Wang, Z.; Xu, P.; Yu, T.; Zhao, J. Experimental investigation of ultrasonic-vibration polishing of K9 optical glass based on ultrasonic atomization. Ceram. Int. 2022, 48, 9067–9074. [Google Scholar] [CrossRef]
- Ma, S.; Chen, Y.; Wang, C.; Li, Y.; Xu, Y.; Wu, Y. Application of surfactants in enhancing chemical mechanical polishing slurry performance for microelectronics: A review. Mater. Today Commun. 2025, 46, 112547. [Google Scholar] [CrossRef]
- Venkataswamy, R.; Trimble, L.; McDonald, A.; Nevers, D.; Bengochea, L.V.; Carswell, A.; Rossner, A.; Seo, J. Environmental impact assessment of chemical mechanical planarization consumables: Challenges, future needs, and perspectives. ACS Sustain. Chem. Eng. 2024, 12, 11841–11855. [Google Scholar] [CrossRef]
- Ma, J.; Xu, N.; Cheng, J.; Pu, Y. A review on the development of ceria for chemical mechanical polishing. Powder Technol. 2024, 444, 119989. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Cheng, J.; Xu, Y.; Wang, Y.; Qiu, J. Recent advances in CeO2 based abrasives for chemical mechanical polishing. Phys. Chem. Chem. Phys. 2025, 27, 13213–13233. [Google Scholar] [CrossRef]
- Gu, R.; Qi, W.; Wang, F.; Tan, B.; Shi, Y. Research progress on the application of cerium oxide and titanium oxide abrasives modification in chemical mechanical polishing. ECS J. Solid State Sci. Technol. 2025, 14, 094001. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Z.; Shi, C.; Feng, J.; Zhuang, X.; Li, L.; Meng, F.; Li, H.; Xue, Z.; Liu, D. Dispersion and polishing mechanism of a novel CeO2-LaOF-based chemical mechanical polishing slurry for quartz glass. Materials 2023, 16, 1148. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Z.; Shi, C.; Peng, Q.; Liu, X.; Liu, X.; Zhou, H.; Feng, J.; Wen, W. Atomic surface induced by novel green chemical mechanical polishing for aspheric thin-walled crucibles with large diameters. J. Manuf. Process. 2024, 117, 59–70. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Z.; Feng, J.; Yu, Z.; Meng, F.; Xu, G.; Wang, J.; Wen, W.; Liu, W. Atomic-level flatness on oxygen-free copper surface in lapping and chemical mechanical polishing. Nanoscale Adv. 2022, 4, 4263–4271. [Google Scholar] [CrossRef]
- Wei, K.-H.; Wang, Y.-S.; Liu, C.-P.; Chen, K.-W.; Wang, Y.-L.; Cheng, Y.-L. The influence of abrasive particle size in copper chemical mechanical planarization. Surf. Coatings Technol. 2013, 231, 543–545. [Google Scholar] [CrossRef]
- Matijević, E.; Babu, S. Colloid aspects of chemical–mechanical planarization. J. Colloid Interface Sci. 2008, 320, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Z.; Wu, B.; Hu, W.; Meng, F.; Li, Y. A review: Green chemical mechanical polishing for metals and brittle wafers. J. Phys. D Appl. Phys. 2021, 54, 373001. [Google Scholar] [CrossRef]
- Zhu, X.; Ding, J.; Mo, Z.; Jiang, X.; Sun, J.; Fu, H.; Gui, Y.; Ban, B.; Wang, L.; Chen, J. Evaluation of chemical mechanical polishing characteristics using mixed abrasive slurry: A study on polishing behavior and material removal mechanism. Appl. Surf. Sci. 2025, 679, 161157. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Shi, C.; Ren, Z.; Feng, J.; Zhou, H.; Liu, Z.; Meng, F.; Zhao, S. Novel green chemical mechanical polishing of fused silica through designing synergistic CeO2/h-BN abrasives with lubricity. Appl. Surf. Sci. 2023, 637, 157978. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Chen, L.; Yang, X.; Ju, B.; Liu, W.; Deng, X.; Wang, M.; Xu, Y. Atomic surface of fused silica with high material removal rate produced by novel chemical mechanical polishing using composite rare earth oxides. Colloids Surfaces A Physicochem. Eng. Asp. 2025, 712, 136420. [Google Scholar] [CrossRef]
- Lv, G.; Liu, S.; Cao, Y.; Zhang, Z.; Li, X.; Zhang, Y.; Liu, T.; Liu, B.; Wang, K. Effect of synergistic CeO2/MoS2 abrasives on surface roughness and material removal rate of quartz glass. Ceram. Int. 2024, 50, 48671–48679. [Google Scholar] [CrossRef]
- He, Q. Experimental study on polishing performance of CeO2 and nano-SiO2 mixed abrasive. Appl. Nanosci. 2018, 8, 163–171. [Google Scholar] [CrossRef]
- Cho, C.-W.; Kim, S.-K.; Paik, U.; Park, J.-G.; Sigmund, W.M. Atomic force microscopy study of the role of molecular weight of poly(acrylic acid) in chemical mechanical planarization for shallow trench isolation. J. Mater. Res. 2006, 21, 473–479. [Google Scholar] [CrossRef]
- Wortman-Otto, K.M.; Linhart, A.N.; Dudek, A.L.; Sherry, B.M.; Keleher, J.J. Role of molecular structure on modulating the interfacial dynamics for shallow trench isolation (STI) chemical mechanical planarization (CMP) applications. ECS J. Solid State Sci. Technol. 2021, 10, 024009. [Google Scholar] [CrossRef]
- Li, F.; Bai, Y.; Hu, H.; Qiao, G.; Li, L.; Zhang, F.; Zhang, X. pH-driven interfacial bond dynamics enable high-efficiency low-damage polishing of fused silica with CeO2 based slurries. J. Mater. Process. Technol. 2025, 341, 118896. [Google Scholar] [CrossRef]
- Ni, Z.; Fan, Q.; Chen, G.; Dai, M.; Chen, Z.; Bian, D.; Qian, S. Influence of cerium oxide abrasive particle morphologies on polishing performance. Cryst. Res. Technol. 2024, 59, 2300308. [Google Scholar] [CrossRef]
- Qu, S.; Cui, Z.; Chu, X.; Sun, X.; Dong, Z.; Yang, H.; Liu, Y.; Wang, Z.; Yu, T.; Zhao, J. Study on the polishing mechanism under the combined effect of K2CO3, KH550 and ultrasonic vibration for K9 optical glass. Appl. Surf. Sci. 2025, 693, 162746. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, N.; Li, X.; Mei, X.; Yang, L.; He, Y. The role of ammonium citrate and dodecyl pyridinium chloride on chemical mechanical polishing relevant to SiO2 dielectric layer. J. Manuf. Process. 2023, 107, 333–344. [Google Scholar] [CrossRef]
- Yang, H.; Jia, L.; Haraguchi, J.; Wang, Y.; Xu, B.; Zhang, Q.; Nan, Z.; Zhang, M.; Ohno, T. Nitrogen and sulfur co-doped CeO2 nanorods for efficient photocatalytic VOCs degradation. Catal. Sci. Technol. 2022, 12, 5203–5209. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Z.; Yu, S.; Chen, X.; Shi, C.; Zhou, H.; Meng, F.; Yu, J.; Wen, W. Unprecedented atomic surface of silicon induced by environmentally friendly chemical mechanical polishing. Nanoscale 2023, 15, 9304–9314. [Google Scholar] [CrossRef]
- Li, H.; Xia, M.; Wang, X.; Chong, B.; Ou, H.; Lin, B.; Yang, G. Efficient reduction of CO2 to C2 hydrocarbons by tandem nonthermal plasma and photocatalysis. Appl. Catal. B Environ. 2024, 342, 123423. [Google Scholar] [CrossRef]
- Shiu, Y.-J.; Cheng, Y.-C.; Tsai, W.-L.; Wu, C.-C.; Chao, C.-T.; Lu, C.-W.; Chi, Y.; Chen, Y.T.; Liu, S.-H.; Chou, P.-T. Pyridyl Pyrrolide boron complexes: The facile generation of thermally activated delayed fluorescence and preparation of organic light-emitting diodes. Angew. Chem. Int. Ed. 2016, 55, 3017–3021. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Shi, Y.; Wang, Y.; Qiu, J.; Xu, Y.; Wang, Y.; Niu, X.; Tan, B. Dual modification of Co doped CeO2 abrasives for enhanced chemical mechanical polishing performance. Ceram. Int. 2025, 51, 39227–39237. [Google Scholar] [CrossRef]
- Khan, M.A.; Ilyas, M.; Kalsoom, S.; Abbas, M.; Zohaib, H.M.; Ilyas, M.; Balouch, F.N.; Rasheed, M.; Iqbal, J. In-silico optimization of resveratrol interaction with nano-borophene: A DFT-guided study of supramolecular artistry. Comput. Biol. Chem. 2024, 112, 108179. [Google Scholar] [CrossRef]
- Sekkal, W.; Zaoui, A.; Benzerzour, M.; Abriak, N. Role of porosity on the stiffness and stability of (001) surface of the nanogranular C-S-H gel. Cem. Concr. Res. 2016, 87, 45–52. [Google Scholar] [CrossRef]
- Son, J.H.; Park, G.; Lee, D.-H.; Lee, Y.; Yun, Y.S.; Park, J.H.; Seo, J.-C.; Han, S.J. Selenium-promoted molten metal catalysts for methane pyrolysis: Modulating surface tension and catalytic activity. Appl. Catal. B Environ. 2025, 366, 125009. [Google Scholar] [CrossRef]
- Lv, G.; Cao, Y.; Liu, S.; Zhang, Z.; Li, X.; Wu, H.; Liu, T.; Liu, B.; Wang, K. Development and characterization of a novel alginate-based slurry and its fused silica CMP investigations. Ceram. Int. 2025, 51, 27146–27155. [Google Scholar] [CrossRef]
- Lv, G.; Hao, E.; Zhang, Z.; Liu, S.; Cao, Y.; Liu, T.; Li, X.; Wang, K. Effect of a novel alginate/5-aminovaleric acid slurry with CeO2/MoS2 abrasives on surface roughness and material removal rate of quartz glass. Appl. Surf. Sci. 2025, 698, 163096. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, J.; Jing, P.; Chi, K.; Yu, J.; Jia, X.; Xu, X.; Liu, B.; Bai, T.; Zhang, J. Promoting effect of LaOF on chemical mechanical polishing performance of cerium-based abrasives. J. Rare Earths 2025, 43, 2005–2015. [Google Scholar] [CrossRef]
- Kikugawa, M.; Goto, Y.; Yamazaki, K.; Manaka, Y.; Nanba, T.; Matsumoto, H.; Sato, A.; Aoki, M. Design of ammonia synthesis Ru catalysts with mesoporous structures through the Si-doping of cerium–lanthanum oxide. Appl. Catal. A Gen. 2025, 707, 120525. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y. Iron/silicon oxy-hydroxide co-precipitate trace-incorporated [-Fe-O]m-[Si-O-]n colloids: A facile strategy for silica xerogel strengthening and toughening. Constr. Build. Mater. 2025, 461, 139895. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Feng, J.; Yi, X.; Shi, C.; Gu, Y.; Zhao, F.; Liu, S.; Li, J. A novel atomic removal model for chemical mechanical polishing using developed mesoporous shell/core abrasives based on molecular dynamics. Nanoscale 2024, 16, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A. Chemical mechanical polishing of synthetic quartz using UV/ozone-treated CeO2 slurry. Mater. Lett. 2025, 382, 137856. [Google Scholar] [CrossRef]
- Gao, S.; Li, T.; Zhang, Y.; Yuan, S.; Kang, R. Insights into interfacial mechanism of CeO2/Silicon and atomic-scale removal process during chemo-mechanical grinding of silicon. Langmuir 2023, 39, 16606–16617. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).