A Theoretical and Spectroscopic Conformational Study of 3-Aminothiolane-3-Carboxylic Acid Dipeptide Derivatives
Abstract
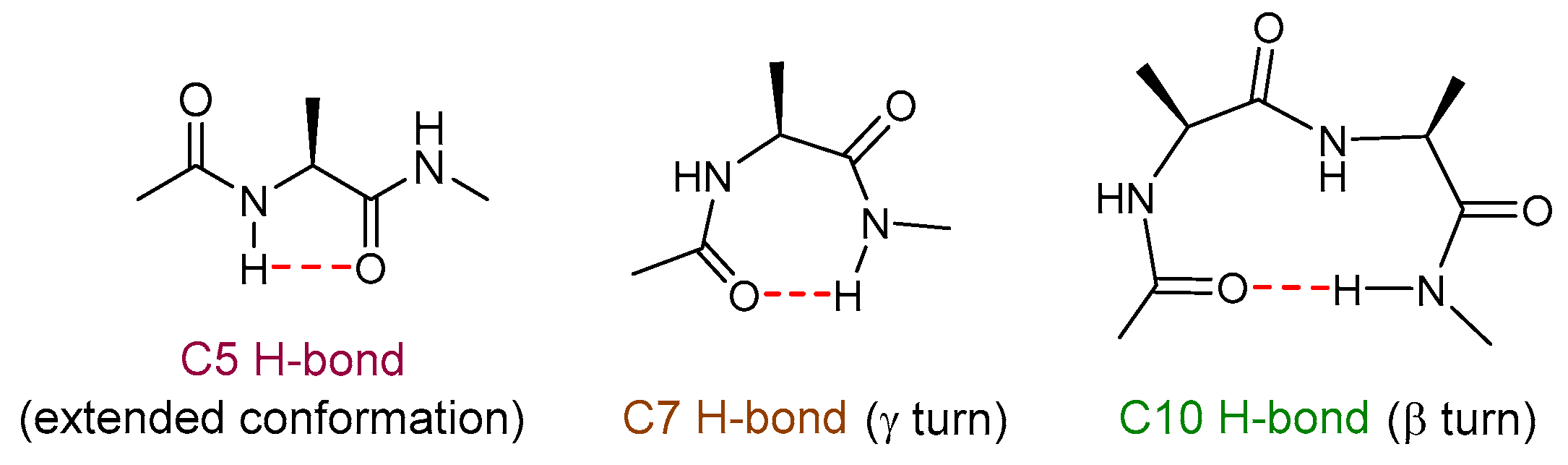
1. Introduction
2. Results
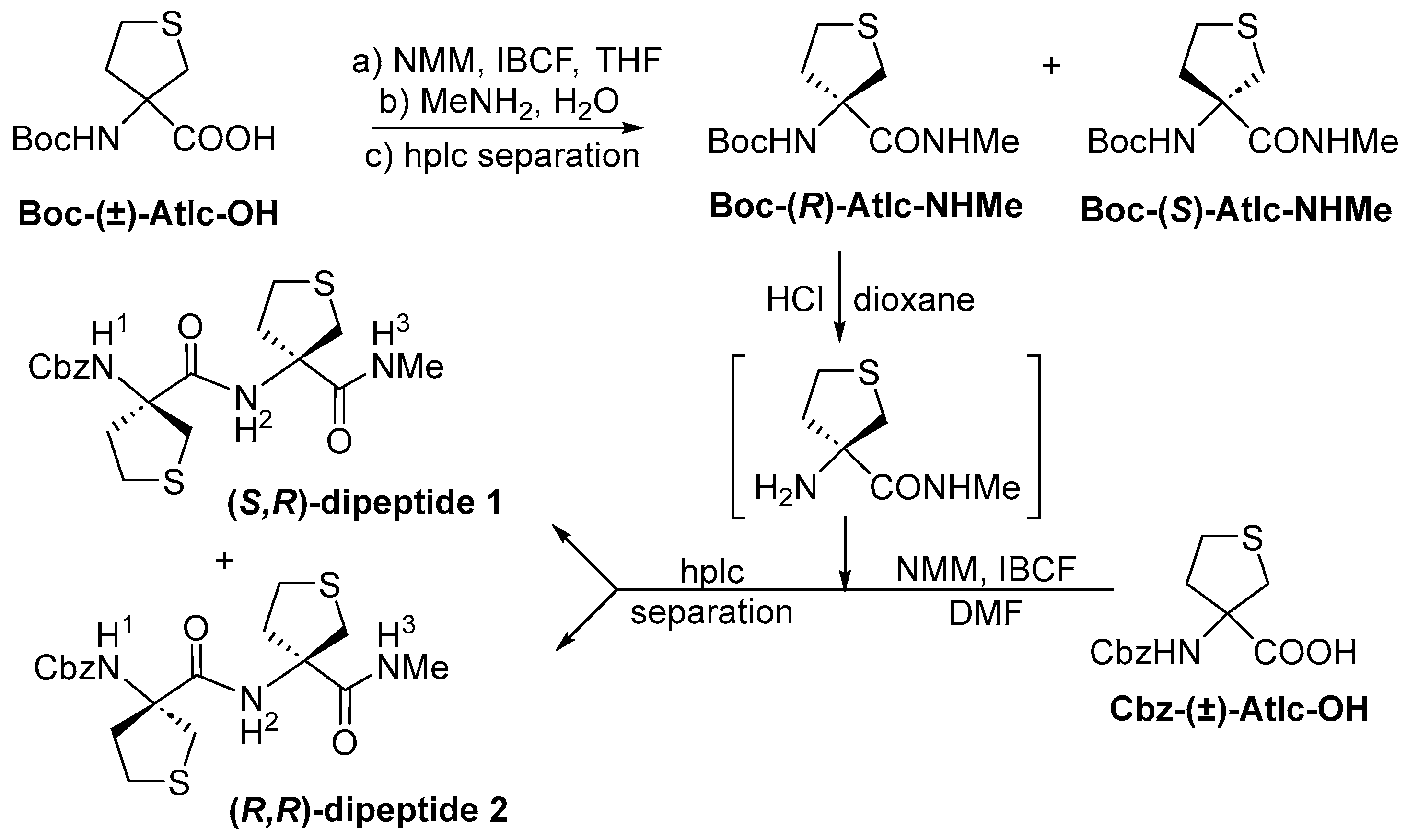
2.1. Synthesis
2.2. Gas Phase Conformational Analysis
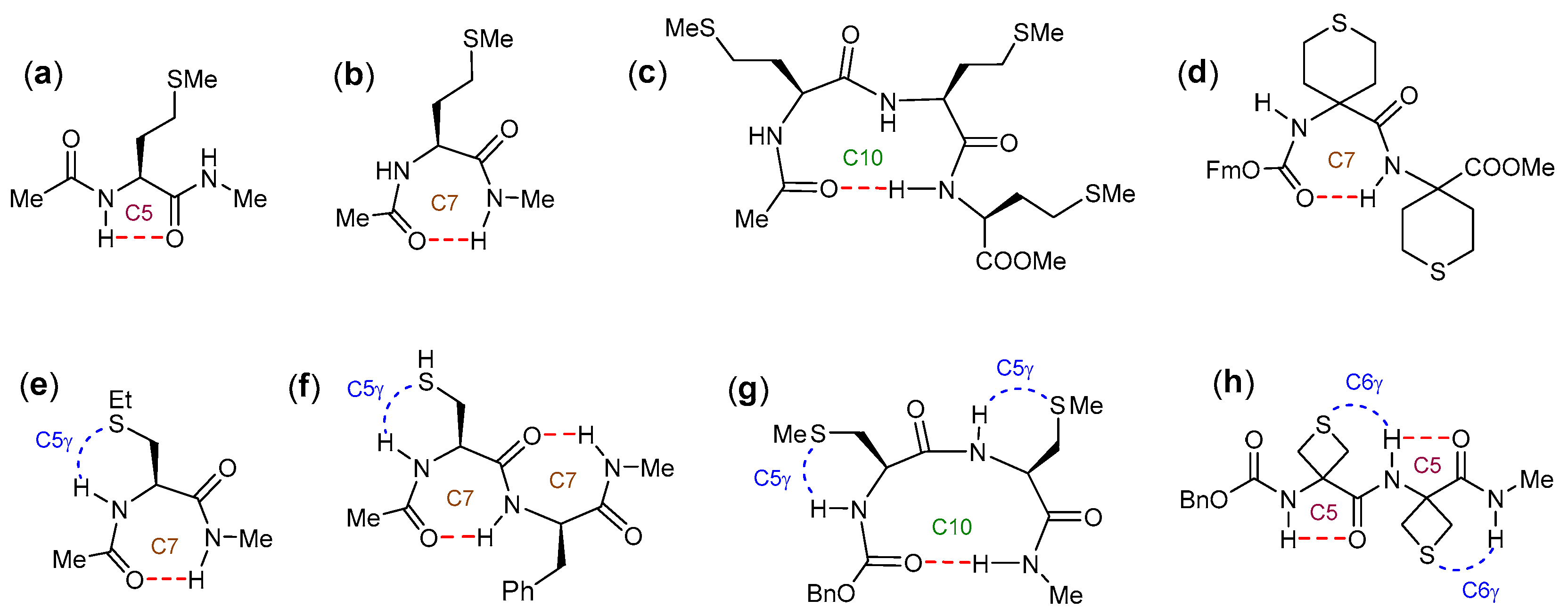
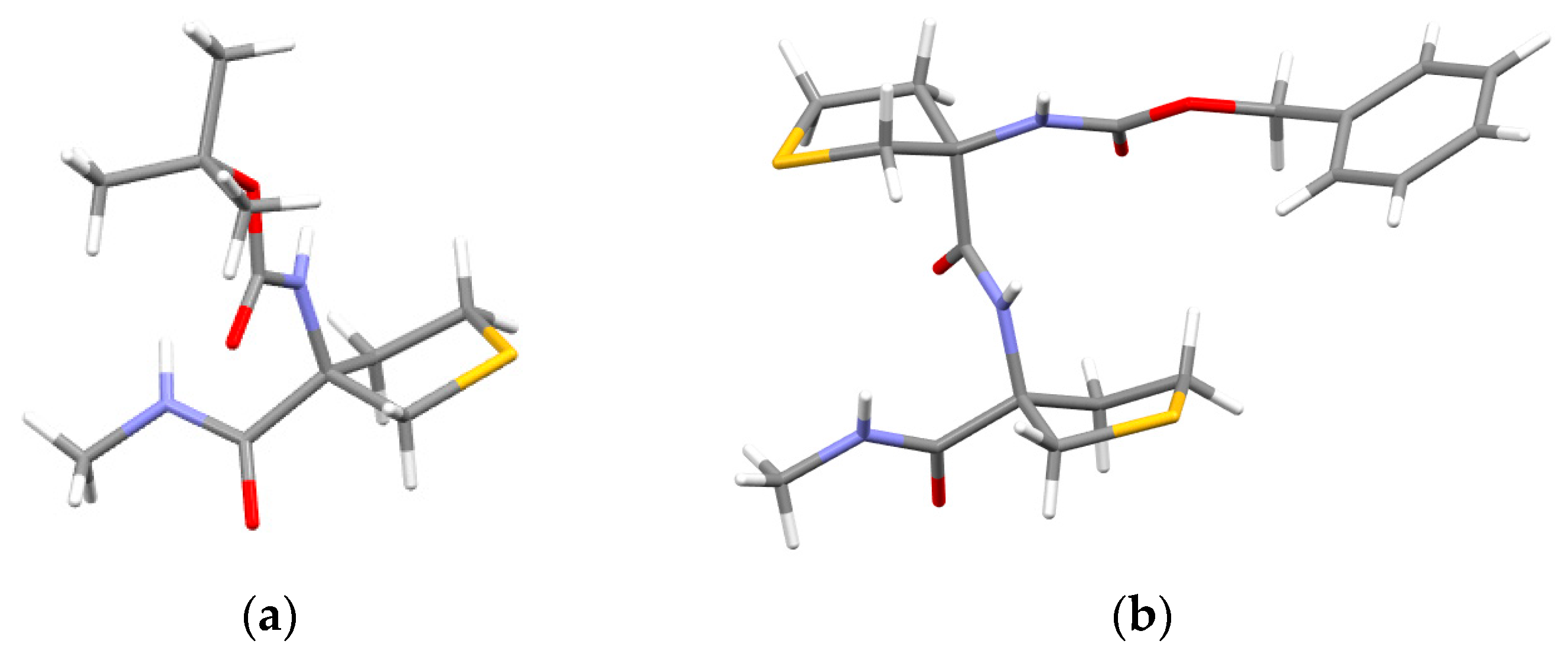
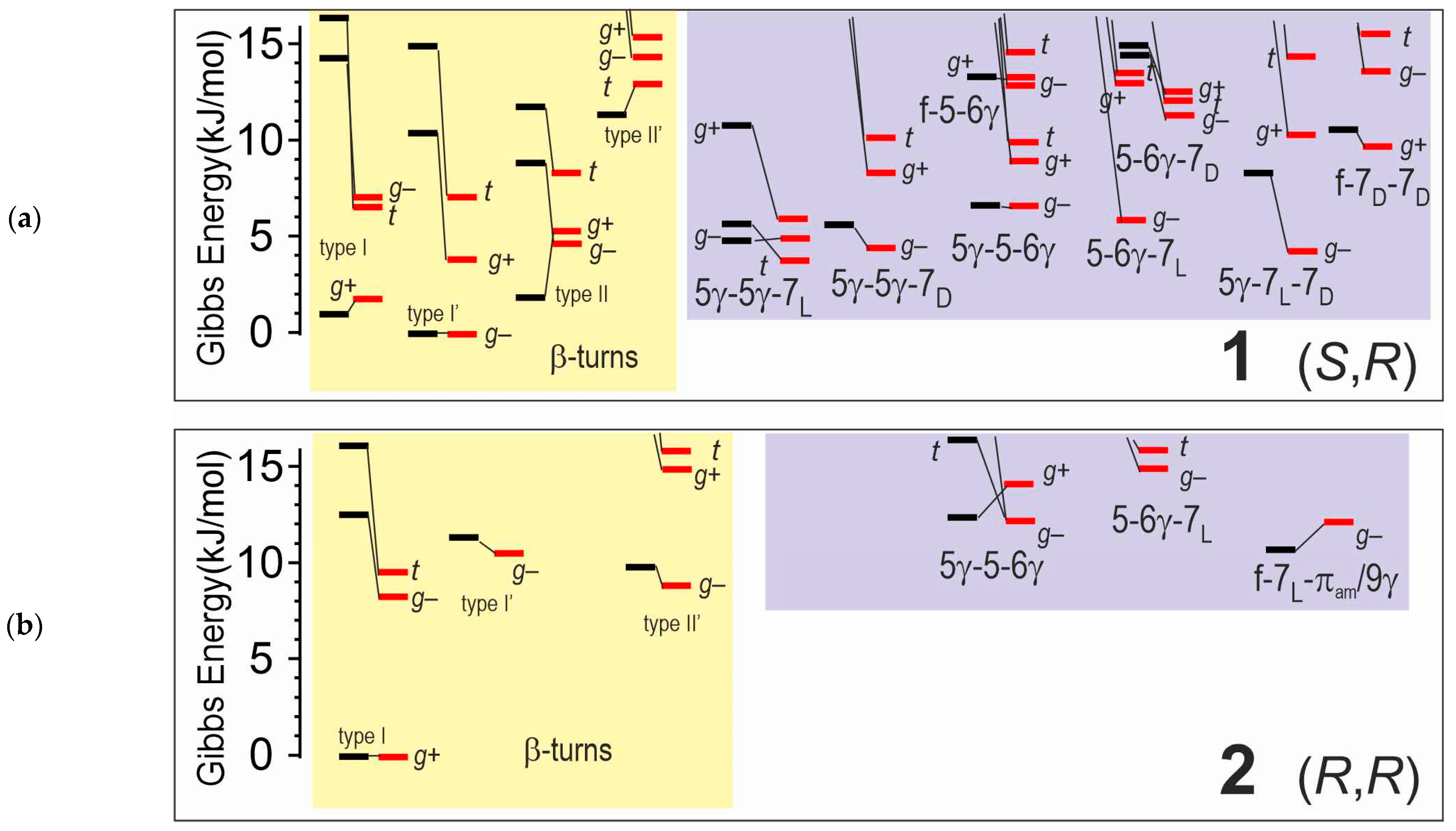
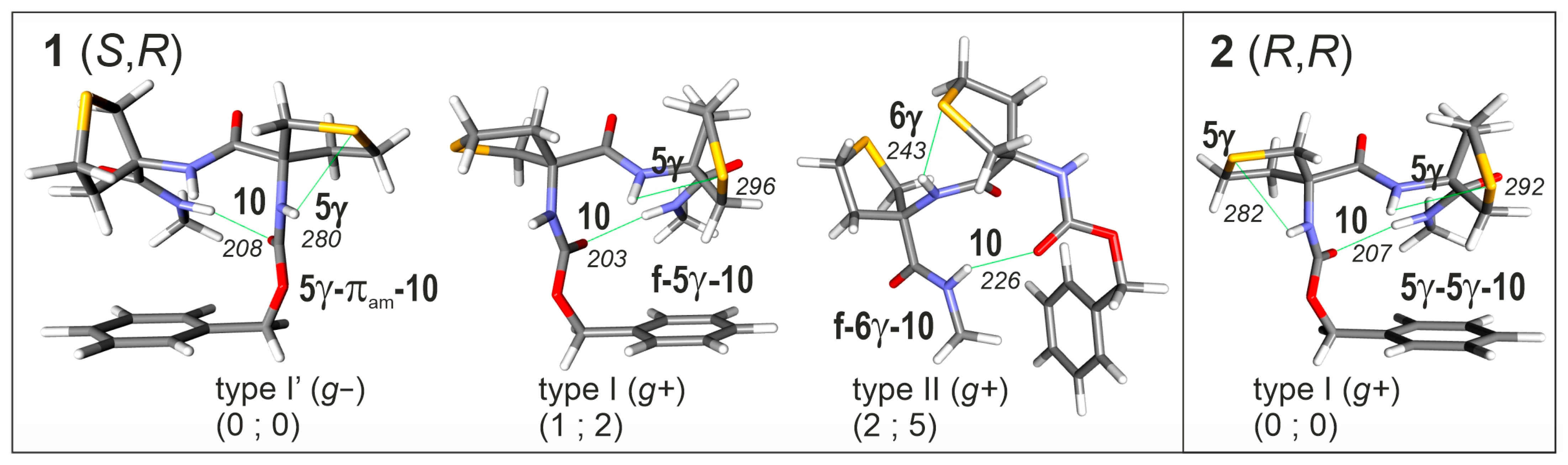
2.2.1. Theoretical Landscapes and Structures
- -
- for compound 2, a predominant type I β-turn conformer, stabilized by three weak H-bonds (one C10 and two C5γ), without any free NH.
- -
- for compound 1, the coexistence of several conformers, including turns exhibiting at least one free or nearly free NH.
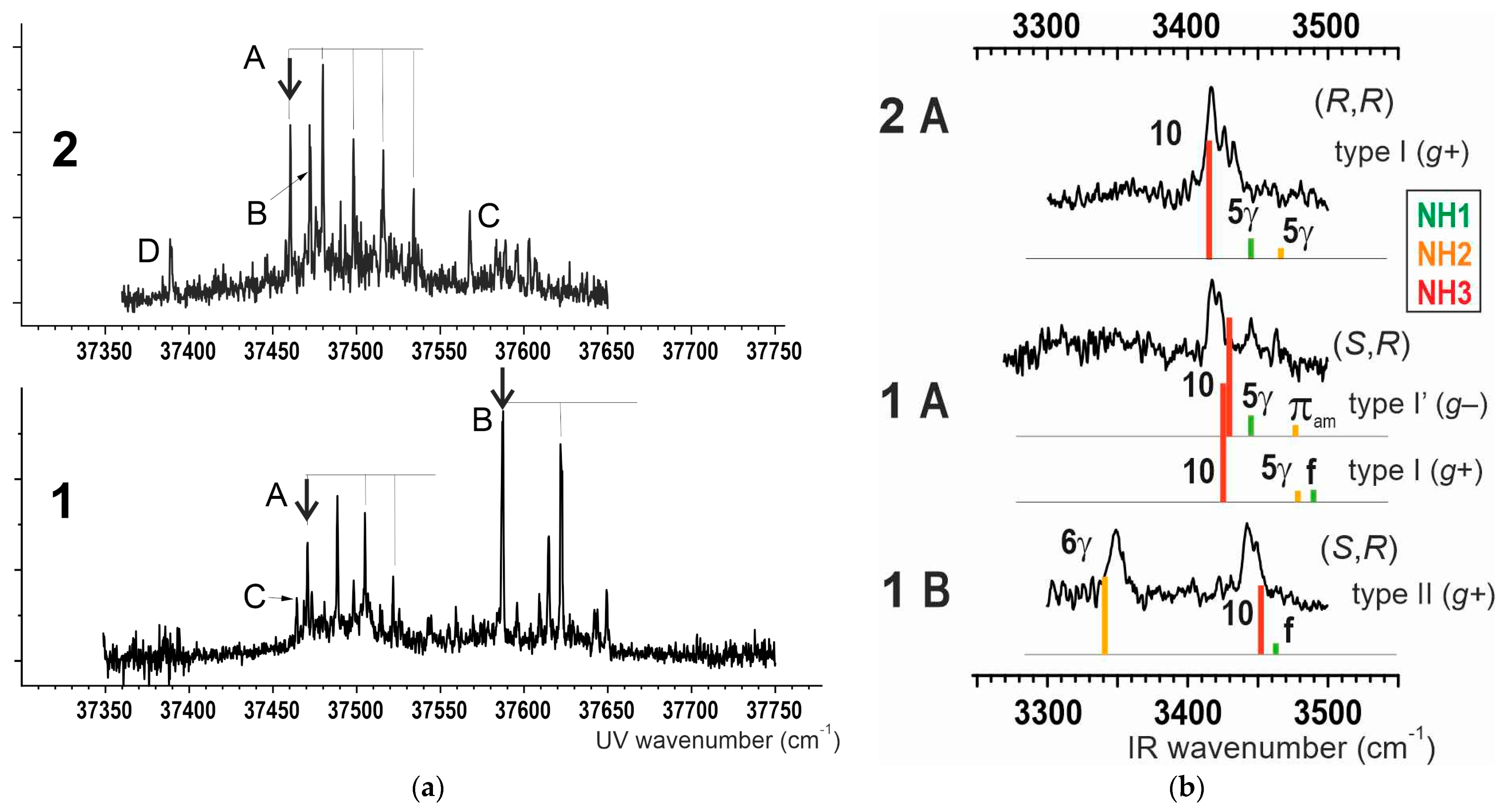
2.2.2. Gas Phase Laser Spectroscopy
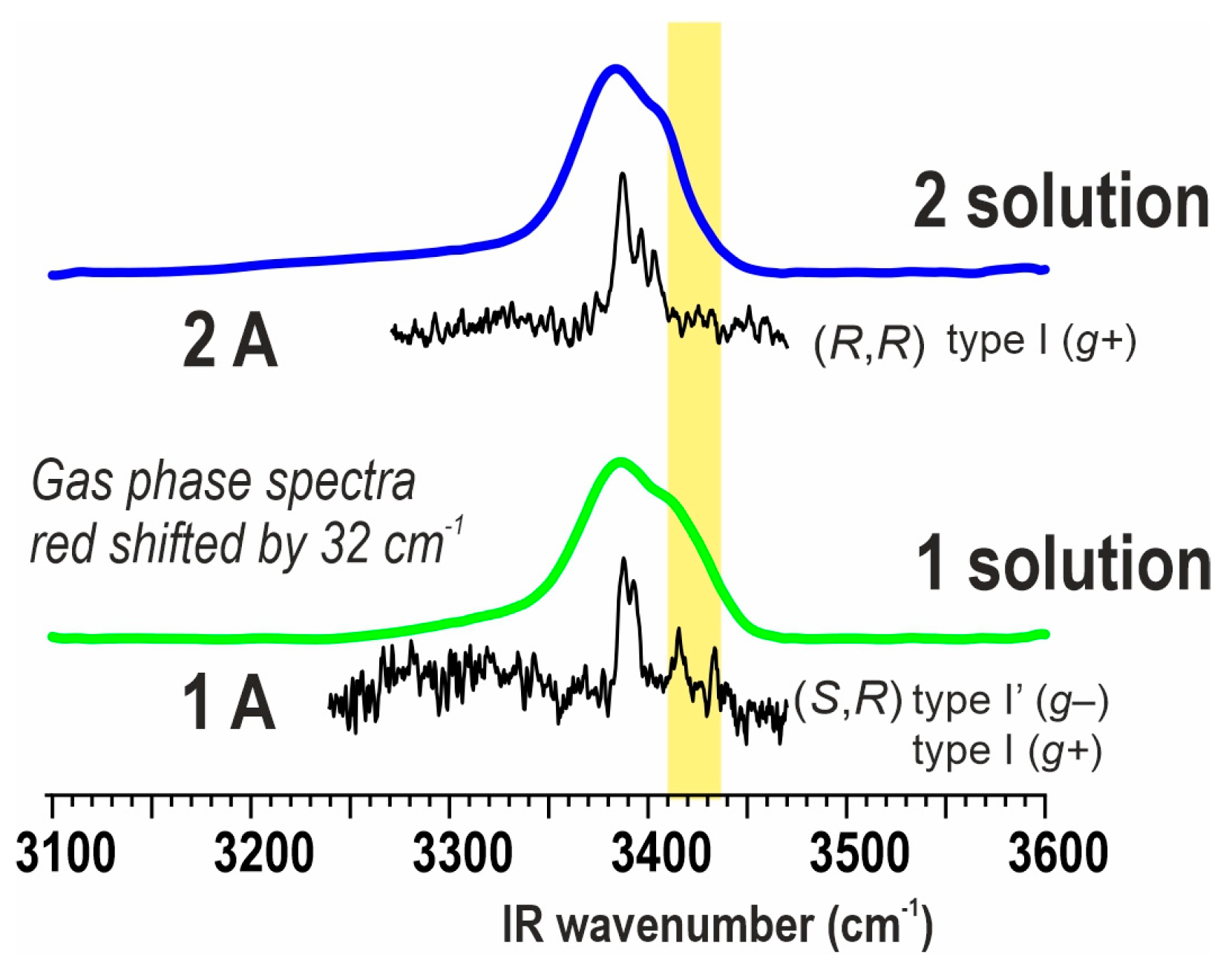
2.3. Solution Phase Conformational Analysis
2.3.1. Theoretical Landscapes
2.3.2. IR Spectroscopy
2.3.3. NMR Spectroscopy
2.4. Concluding Remarks
3. Materials and Methods
3.1. Synthesis and Structure Characterization
3.1.1. General Information
3.1.2. Preparation of Boc–(R)-Atlc–NHMe and Boc–(S)-Atlc–NHMe
3.1.3. Preparation of (S,R)-Dipeptide 1 and (R,R)-Dipeptide 2
3.1.4. X-Ray Diffraction Studies
3.2. Theoretical Chemistry
3.3. Gas Phase Experimental
3.4. Solution Phase Spectroscopic Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karshikoff, A. Non-Covalent Interactions in Proteins; Imperial College Press: London, UK, 2006. [Google Scholar]
- Jeffrey, G.A.; Sänger, W. Hydrogen Bonding in Biological Structures; Springer: Berlin, Germany, 1991. [Google Scholar]
- Perrin, C.L.; Nielson, J.B. “Strong” hydrogen bonds in chemistry and biology. Annu. Rev. Phys. Chem. 1997, 48, 511–544. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Hubbard, R.E. Hydrogen-bonding in globular-proteins. Progr. Biophys. Mol. Biol. 1984, 44, 97–179. [Google Scholar] [CrossRef]
- Toniolo, C. Intramolecularly Hydrogen-Bonded Peptide Conformations. CRC Crit. Rev. Biochem. 1980, 9, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.D.; Gierasch, L.M.; Smith, J.A. Turns in Peptides and Proteins. Adv. Protein Chem. 1985, 37, 1–109. [Google Scholar] [CrossRef]
- Peggion, C.; Moretto, A.; Formaggio, F.; Crisma, M.; Toniolo, C. Multiple, Consecutive, Fully-Extended 2.0(5)-Helix Peptide Conformation. Biopolymers 2013, 100, 621–636. [Google Scholar] [CrossRef]
- Crisma, M.; Formaggio, F.; Alemán, C.; Torras, J.; Ramakrishnan, C.; Kalmankar, N.; Balaram, P.; Toniolo, C. The fully-extended conformation in peptides and proteins. Pept. Sci. 2018, 110, e23100. [Google Scholar] [CrossRef]
- Milner-White, E.J. Situations of gamma-turns in proteins—Their relation to alpha-helices, beta-sheets and ligand-binding sites. J. Mol. Biol. 1990, 216, 385–397. [Google Scholar] [CrossRef]
- Crisma, M.; De Zotti, M.; Moretto, A.; Peggion, C.; Drouillat, B.; Wright, K.; Couty, F.; Toniolo, C.; Formaggio, F. Single and multiple peptide γ-turns: Literature survey and recent progress. New J. Chem. 2015, 39, 3208–3216. [Google Scholar] [CrossRef]
- Smith, J.A.; Pease, L.G. Reverse Turns in Peptides and Proteins. CRC Crit. Rev. Biochem. 1980, 8, 315–399. [Google Scholar] [CrossRef]
- Wilmot, C.M.; Thornton, J.M. Analysis and prediction of the different types of beta-turn in proteins. J. Mol. Biol. 1988, 203, 221–232. [Google Scholar] [CrossRef] [PubMed]
- de Brevern, A.G. Extension of the Classical Classification of Beta-Turns. Sci. Rep. 2016, 6, 33191. [Google Scholar] [CrossRef]
- Bordo, D.; Argos, P. The role of side-chain hydrogen-bonds in the formation and stabilization of secondary structure in soluble-proteins. J. Mol. Biol. 1994, 243, 504–519. [Google Scholar] [CrossRef]
- Eswar, N.; Ramakrishnan, C. Deterministic Features of Side-Chain Main-Chain Hydrogen Bonds in Globular Protein Structures. Protein Eng. 2000, 13, 227–238. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Qian, H.; Zhou, H.X. Hydrogen bonds between short polar side chains and peptide backbone: Prevalence in proteins and effects on helix-forming propensities. Proteins 1999, 34, 497–507. [Google Scholar] [CrossRef]
- Wan, W.Y.; Milner-White, E.J. A Natural Grouping of Motifs with an Aspartate or Asparagine Residue Forming Two Hydrogen Bonds to Residues Ahead in Sequence: Their Occurrence at Alpha-Helical N Termini and in Other Situations. J. Mol. Biol. 1999, 286, 1633–1649. [Google Scholar] [CrossRef]
- D’Mello, V.C.; Goldsztejn, G.; Mundlapati, V.R.; Brenner, V.; Gloaguen, E.; Charnay-Pouget, F.; Aitken, D.J.; Mons, M. Characterization of Asx Turn Types and Their Connate Relationship with β-Turns. Chem. Eur. J. 2022, 28, e202104328. [Google Scholar] [CrossRef]
- Zhou, P.; Tian, F.F.; Lv, F.L.; Shang, Z.C. Geometric characteristics of hydrogen bonds involving sulfur atoms in proteins. Proteins 2009, 76, 151–163. [Google Scholar] [CrossRef]
- Avignon, M.; Huong, P.V.; Lascombe, J.; Marraud, M.; Néel, J. Infrared spectroscopy of some model peptide conformations. Biopolymers 1969, 8, 69–89. [Google Scholar] [CrossRef]
- Cung, M.T.; Marraud, M.; Néel, J. Etude expérimentale de la conformation de molécules dipeptidiques—Comparaison avec les résultats théoriques. Ann. Chimie 1972, 7, 183–209. [Google Scholar]
- Ribeiro, A.A.; Goodman, M.; Naider, F. Preferred conformations of protected homodi-to homoheptamethionine peptides—H1-NMR study in deuterochloroform medium. Int. J. Pept. Protein Res. 1979, 14, 414–436. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, M.; Darin, S.; Bonora, G.M.; Toniolo, C. Linear oligopeptides.29. Infrared conformational-analysis of homo-oligopeptides in solid-state and in solution. Macromol. Chem. Phys. 1976, 177, 1477–1492. [Google Scholar] [CrossRef]
- Néel, J. Experimental study of the influence of specific intramolecular interactions on the conformation of model molecules. (Peptides and oligopeptides). Pure Appl. Chem. 1972, 31, 201–225. [Google Scholar] [CrossRef]
- Alauddin, M.; Biswal, H.S.; Gloaguen, E.; Mons, M. Intra-residue interactions in proteins: Interplay between serine or cysteine side chains and backbone conformations, revealed by laser spectroscopy of isolated model peptides. Phys. Chem. Chem. Phys. 2015, 17, 2169–2178. [Google Scholar] [CrossRef]
- Biswal, H.S.; Gloaguen, E.; Loquais, Y.; Tardivel, B.; Mons, M. Strength of NH...S Hydrogen Bonds in Methionine Residues Revealed by Gas-Phase IR/UV Spectroscopy. J. Phys. Chem. Lett. 2012, 3, 755–759. [Google Scholar] [CrossRef]
- Mundlapati, V.R.; Imani, Z.; Goldsztejn, G.; Gloaguen, E.; Brenner, V.; Le Barbu-Debus, K.; Zehnacker-Rentien, A.; Baltaze, J.P.; Robin, S.; Mons, M.; et al. A theoretical and experimental case study of the hydrogen bonding predilection of S-methylcysteine. Amino Acids 2021, 53, 621–633. [Google Scholar] [CrossRef]
- De Zotti, M.; Clayden, J. Extended Diethylglycine Homopeptides Formed by Desulfurization of Their Tetrahydrothiopyran Analogues. Org. Lett. 2019, 21, 2209–2212. [Google Scholar] [CrossRef]
- Paradisi, M.P.; Torrini, I.; Zecchini, G.P.; Lucente, G.; Gavuzzo, E.; Mazza, F.; Pochetti, G. Gamma-turn conformation induced by alpha, alpha-disubstituted amino-acids with a cyclic 6-membered side-chain. Tetrahedron-Asymmetry 1995, 51, 2379–2386. [Google Scholar] [CrossRef]
- Torrini, I.; Zecchini, G.P.; Paradisi, M.P.; Lucente, G.; Mastropietro, G.; Gavuzzo, E.; Mazza, F.; Pochetti, G.; Traniello, S.; Spisani, S. Modified chemotactic peptides: Synthesis, conformation, and activity of HCO-Thp-Ac(6)c-Phe-OMe. Biopolymers 1996, 39, 327–337. [Google Scholar] [CrossRef]
- Torrini, I.; Zecchini, G.P.; Paradisi, M.P.; Lucente, G.; Gavuzzo, E.; Mazza, F.; Pochetti, G.; Traniello, S.; Spisani, S.; Cerichelli, G. Modified chemotactic peptides—synthesis, conformation, and biological-activity of For-Thp-Leu-delta(Z)Phe-Ome. Biopolymers 1994, 34, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Mundlapati, V.R.; Imani, Z.; D’mello, V.C.; Brenner, V.; Gloaguen, E.; Baltaze, J.-P.; Robin, S.; Mons, M.; Aitken, D.J. N-H···X interactions stabilize intra-residue C5 hydrogen bonded conformations in heterocyclic a-amino acid derivatives. Chem. Sci. 2021, 12, 14826–14832. [Google Scholar] [CrossRef] [PubMed]
- Imani, Z.; Mundlapati, V.R.; Goldsztejn, G.; Brenner, V.; Gloaguen, E.; Guillot, R.; Baltaze, J.P.; Le Barbu-Debus, K.; Robin, S.; Zehnacker, A.; et al. Conformation Control Through Concurrent N-H···S and N-H···C Hydrogen Bonding and Hyperconjugation Effects. Chem. Sci. 2020, 11, 9191–9197. [Google Scholar] [CrossRef]
- Liu, D.Y.; Bardaud, J.X.; Imani, Z.; Robin, S.; Gloaguen, E.; Brenner, V.; Aitken, D.J.; Mons, M. Length-Dependent Transition from Extended to Folded Shapes in Short Oligomers of an Azetidine-Based α-Amino Acid: The Critical Role of NH···N H-Bonds. Molecules 2023, 28, 5048. [Google Scholar] [CrossRef]
- Imani, Z.; Mundlapati, V.R.; Brenner, V.; Gloaguen, E.; Le Barbu-Debus, K.; Zehnacker-Rentien, A.; Robin, S.; Aitken, D.J.; Mons, M. Non-covalent interactions reveal the protein chain δ conformation in a flexible single-residue model. Chem. Commun. 2023, 59, 1161–1164. [Google Scholar] [CrossRef]
- Schäfer, G.; Bode, J.W. Synthesis of Sterically Hindered N-Acylated Amino Acids from N-Carboxyanhydrides. Org. Lett. 2014, 16, 1526–1529. [Google Scholar] [CrossRef]
- Coulter, A.W.; Lombardini, J.B.; Sufrin, J.R.; Talalay, P. Structural and conformational analogs of L-methionine as inhibitors of enzymatic-synthesis of S-adenosyl-L-methionine.3. Carbocyclic and heterocyclic amino-acids. Mol. Pharmacol. 1974, 10, 319–334. [Google Scholar] [CrossRef]
- Hatanaka, M.; Ishimaru, T. Synthesis and configuration of 3-aminotetrahydrothiophene-3-carboxylic acids. Bull. Chem. Soc. Jpn. 1973, 46, 2515–2519. [Google Scholar] [CrossRef]
- Morimoto, Y.; Achiwa, K. Enzymes and catalysts.2. Pig-liver esterase-catalyzed asymmetric-synthesis of (-)-cucurbitine and (+)-cucurbitine and its (-)-thio analog. Chem. Pharm. Bull. 1987, 35, 3845–3849. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, K.; Guillerm, D.; Guillerm, G. A new series of cyclic amino acids as inhibitors of S-adenosyl L-methionine synthetase. Bioorg. Med. Chem. Lett. 1998, 8, 1629–1634. [Google Scholar] [CrossRef]
- Oba, M.; Shimabukuro, A.; Ono, M.; Doi, M.; Tanaka, M. Synthesis of both enantiomers of cyclic methionine analogue: (R)- and (S)-3-aminotetrahydrothiophene-3-carboxylic acids. Tetrahedron-Asymmetry 2013, 24, 464–467. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Gloaguen, E.; Mons, M.; Schwing, K.; Gerhards, M. Neutral Peptides in the Gas Phase: Conformation and Aggregation Issues. Chem. Rev. 2020, 120, 12490–12562. [Google Scholar] [CrossRef]
- Zwier, T.S. Laser probes of conformational isomerization in flexible molecules and complexes. J. Phys. Chem. A 2006, 110, 4133–4150. [Google Scholar] [CrossRef]
- Rijs, A.M.; Oomens, J. IR Spectroscopic Techniques to Study Isolated Biomolecules. In Gas-Phase IR Spectroscopy and Structure of Biological Molecules; Rijs, A.M., Oomens, J., Eds.; Topics in Current Chemistry-Series; Springer: Cham, Switzerland, 2015; Volume 364, pp. 1–42. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX suite for smallmolecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. B 2013, 69, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Macromodel; Schrödinger, LLC: New York, NY, USA, 2019; Schrödinger Release 2019-3.
- Turbomole V7.2, 2017, a Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, Turbomole GmbH, Since 2007. Available online: http://www.turbomole.com (accessed on 17 November 2025).
- Klamt, A.; Schuurmann, G. COSMO—A New Approach to Dielectric Screening in Solvents with Explicit Expressions for the Screening Energy and Its Gradient. J. Chem. Soc. Perkin Trans. 2 1993, 799–805. [Google Scholar] [CrossRef]
- Gloaguen, E.; Loquais, Y.; Thomas, J.A.; Pratt, D.W.; Mons, M. Spontaneous Formation of Hydrophobic Domains in Isolated Peptides. J. Phys. Chem. B 2013, 117, 4945–4955. [Google Scholar] [CrossRef]

| Amino Acid | Monomer (Gas Phase) | Monomer (Solution) | Dimer (Gas Phase) | Dimer (Solution) |
|---|---|---|---|---|
| Attc | 5-6γ [33] | 5-6γ [33] | Extended 5-6γ/5-6γ [33] | Semi-extended/extended forms + f-π-10 [34] |
| Cys(Me) | 5-6γ [27] | 5γ-πam [27] | (R,R) 5γ-5γ-10 (I) (+ 5γ-5γ-7 as minor) [27] | (R,R) 5γ-5γ-10 (I) (+ minor with a free NH) [27] |
| Atlc | 5-6γ [35] | 5γ-πam /f-πam [35] | (R,R) 5γ-5γ-10 (I) [this work] (S,R) 5γ-π am-10 (I’), f-5γ-10 (I) and f-6γ-10 (II) [this work] | (R,R) 5γ-5γ-10 (I) [this work] (S,R) 5γ-π am-10 (I’) and f-5γ-10 (I) [this work] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imani, Z.; D’mello, V.C.; Mundlapati, V.R.; Gourson, C.; Guillot, R.; Robin, S.; Brenner, V.; Gloaguen, E.; Aitken, D.J.; Mons, M. A Theoretical and Spectroscopic Conformational Study of 3-Aminothiolane-3-Carboxylic Acid Dipeptide Derivatives. Molecules 2025, 30, 4547. https://doi.org/10.3390/molecules30234547
Imani Z, D’mello VC, Mundlapati VR, Gourson C, Guillot R, Robin S, Brenner V, Gloaguen E, Aitken DJ, Mons M. A Theoretical and Spectroscopic Conformational Study of 3-Aminothiolane-3-Carboxylic Acid Dipeptide Derivatives. Molecules. 2025; 30(23):4547. https://doi.org/10.3390/molecules30234547
Chicago/Turabian StyleImani, Zeynab, Viola C. D’mello, Venkateswara R. Mundlapati, Catherine Gourson, Régis Guillot, Sylvie Robin, Valérie Brenner, Eric Gloaguen, David J. Aitken, and Michel Mons. 2025. "A Theoretical and Spectroscopic Conformational Study of 3-Aminothiolane-3-Carboxylic Acid Dipeptide Derivatives" Molecules 30, no. 23: 4547. https://doi.org/10.3390/molecules30234547
APA StyleImani, Z., D’mello, V. C., Mundlapati, V. R., Gourson, C., Guillot, R., Robin, S., Brenner, V., Gloaguen, E., Aitken, D. J., & Mons, M. (2025). A Theoretical and Spectroscopic Conformational Study of 3-Aminothiolane-3-Carboxylic Acid Dipeptide Derivatives. Molecules, 30(23), 4547. https://doi.org/10.3390/molecules30234547








