A Competing Hydrogen Bond Network Offers Access to a New Conformation in 24-Atom Triazine Macrocycles
Abstract
1. Introduction
2. Results
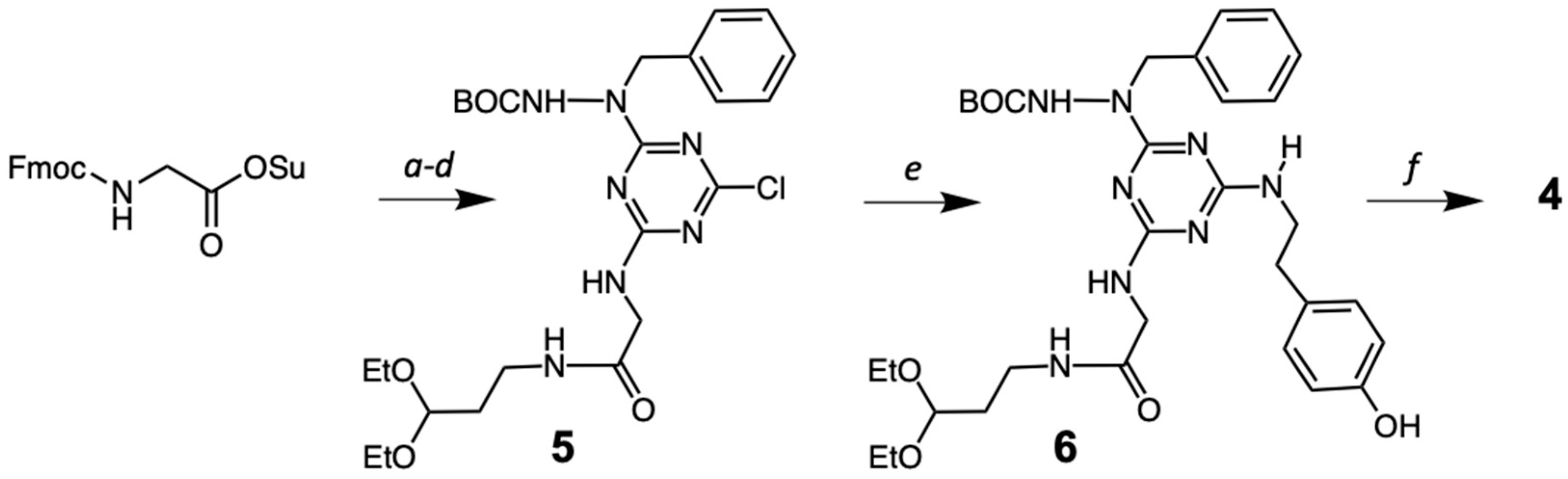
2.1. Synthesis
2.2. Crystallography
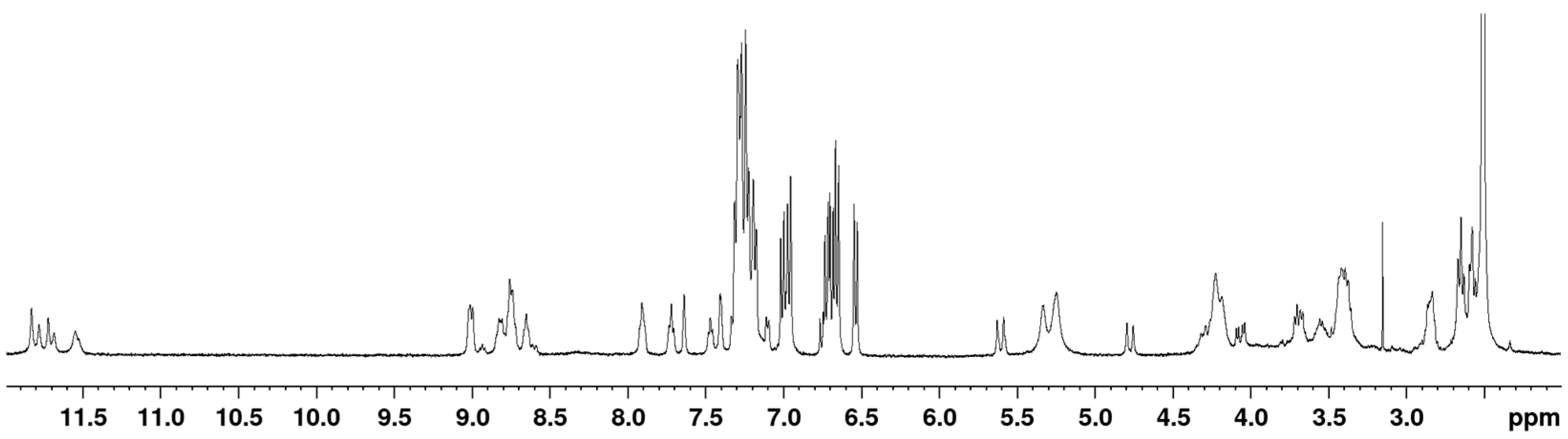
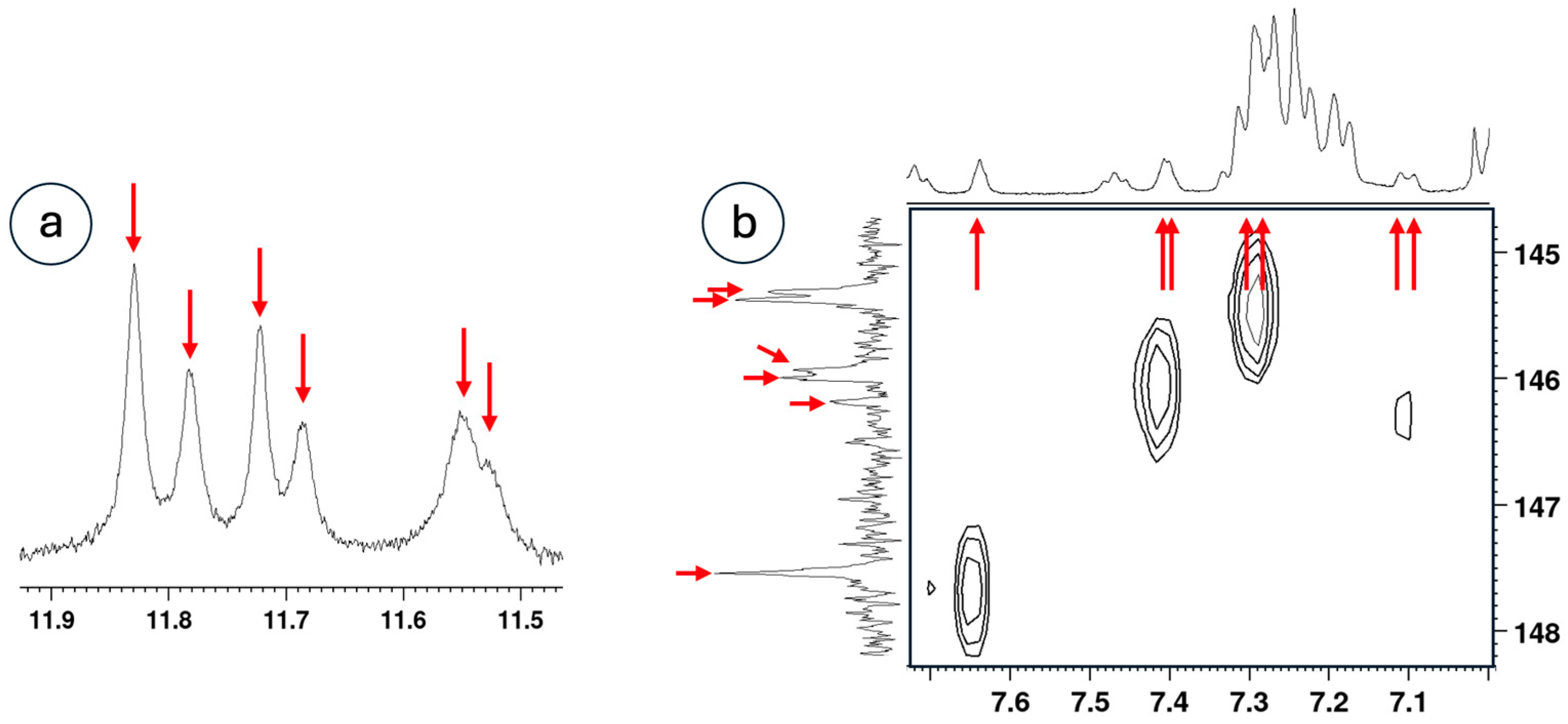
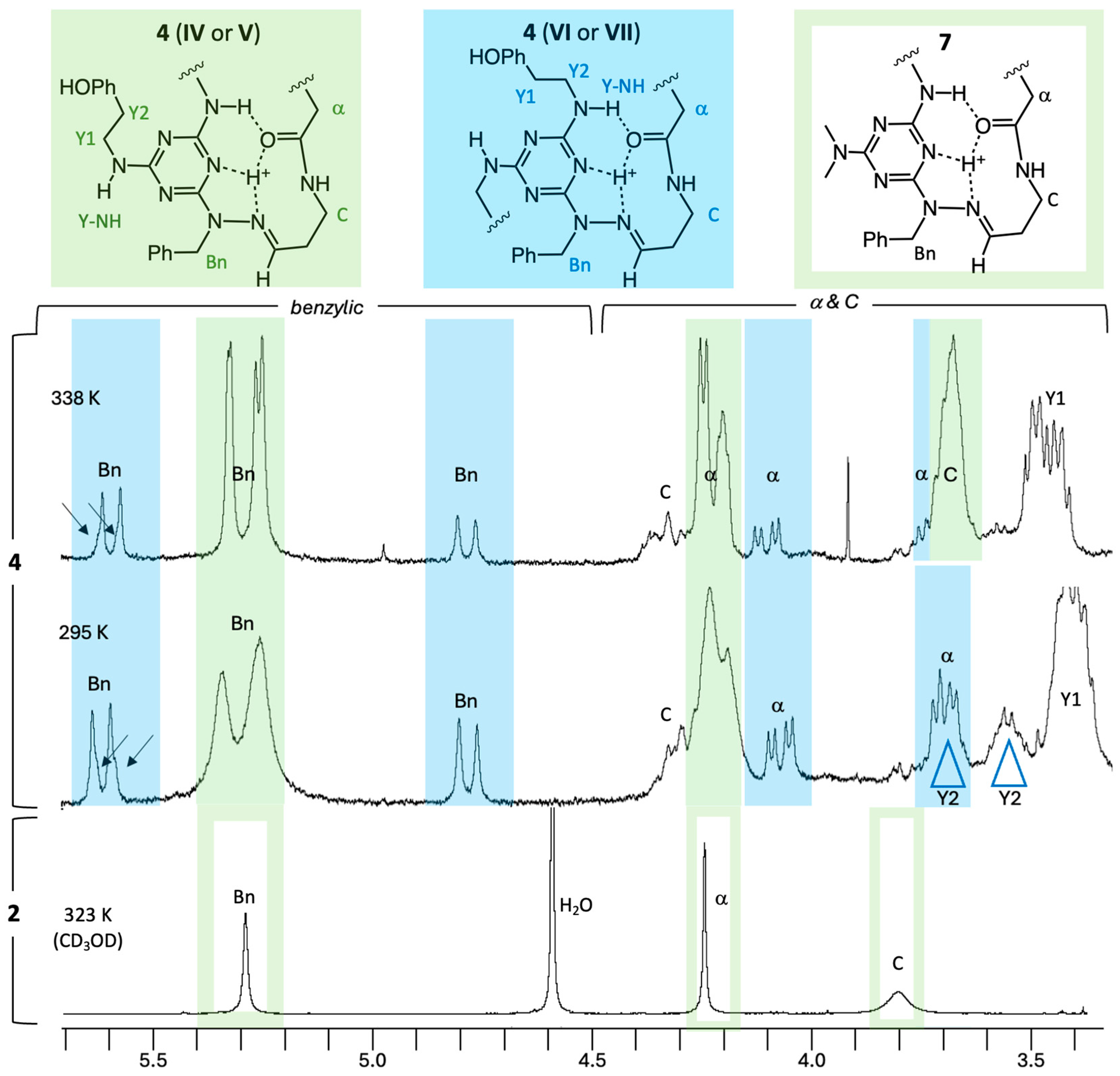
2.3. NMR Analysis
2.4. QTAIM Characterization of the VI–VI Motif
3. Discussion
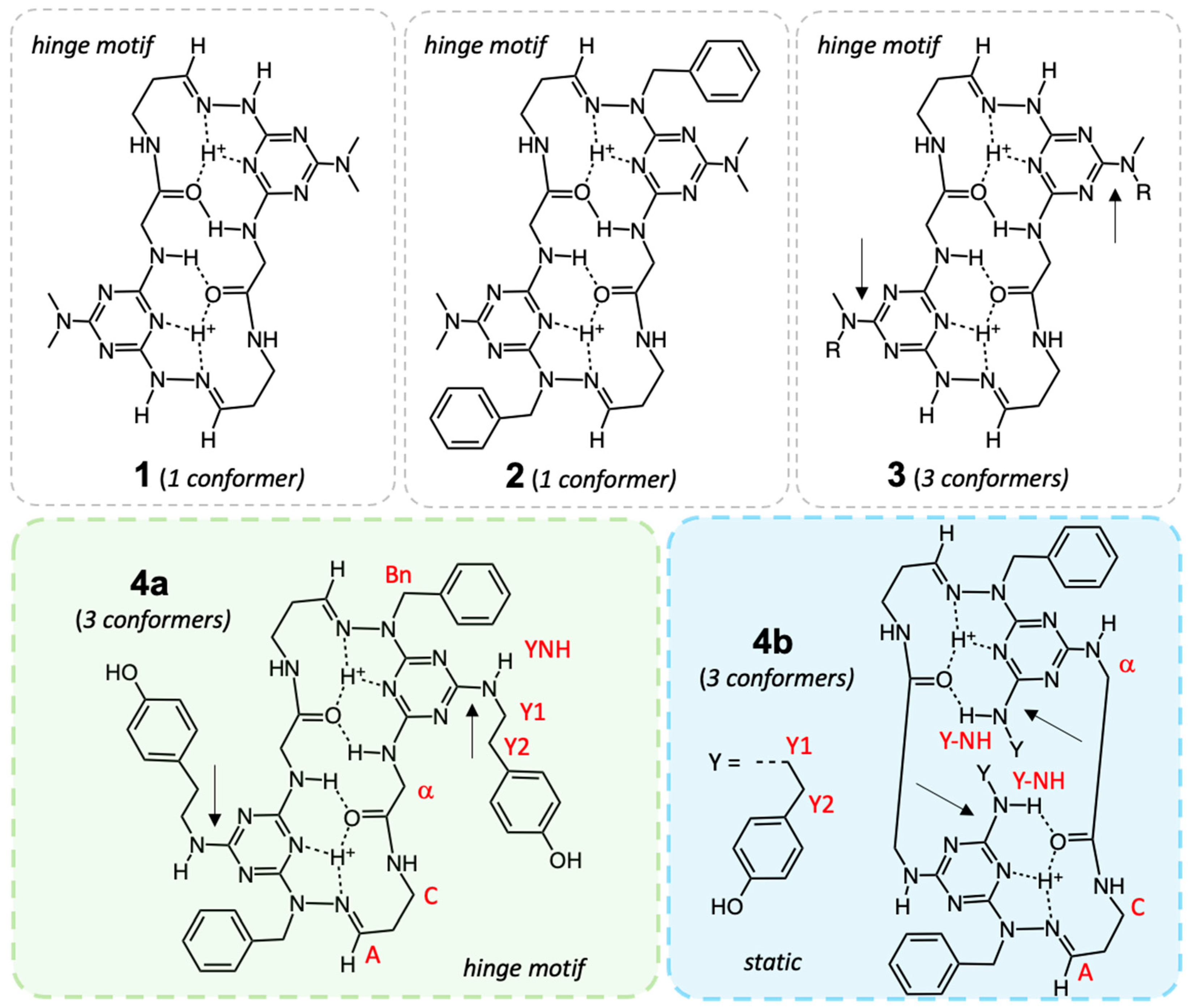
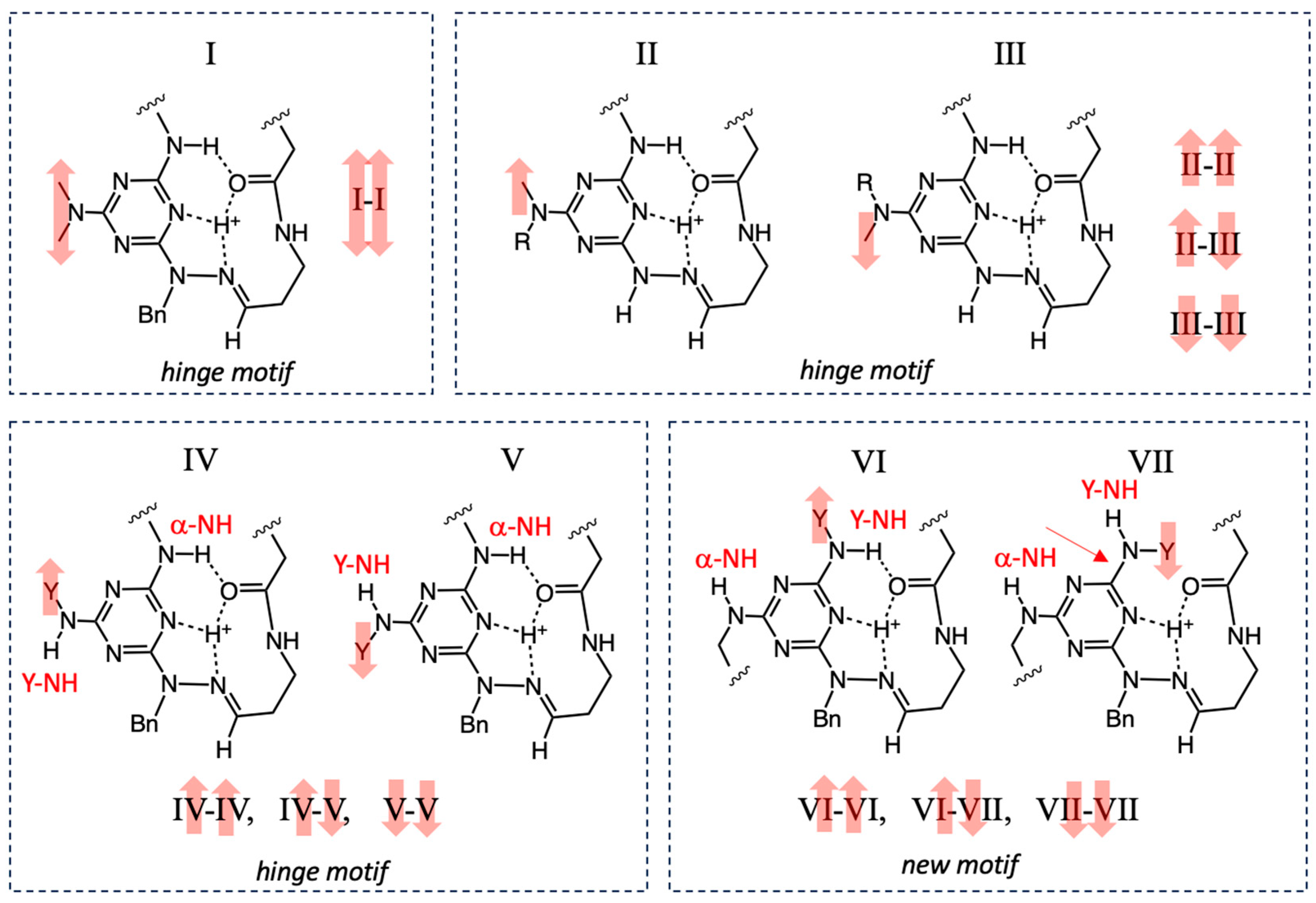
3.1. The Introduction of Isomers
3.2. An Existence Theorem—Evidence from X-Ray Crystallography
3.3. Counting Isomers Theoretically
3.4. Counting Isomers Using 1H NMR Spectroscopy
3.5. Evidence for Different Dynamic Behaviors Differentiates Hinge and New Motifs
4. Materials and Methods
4.1. General Methods
4.2. Synthesis of 5
4.3. Synthesis of 6
4.4. Synthesis of the Macrocycle
4.5. QTAIM Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BOC | tert-butyloxycarbonyl |
| CP | Critical point |
| DIPEA | Diisopropylethylamine |
| Fmoc | 9-fluorenylmethoxycarbonyl |
| Gly-OSu | Glycine succinimide ester |
| IMHB | Intramolecular Hydrogen Bond |
| QTAIM | Quantum Theory of Atoms in Molecules |
| TLC | Thin-layer chromatography |
| THF | Tetrahydrofuran |
References
- Wang, H.; Wang, D.; Wu, Y.; Zhao, Y. Macrocycle-Based Hierarchically Porous Hydrogen-Bonded Organic Frameworks. Chem. Eur. J. 2024, 30, e202303618. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Li, C.; Guo, D.-S. Macrocycle-containing metal–organic frameworks in adsorption and related applications. J. Mater. Chem. A 2025, 13, 23354–23376. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Zhang, S.; Wang, Y.; Yang, Y.-W. Smart organic materials based on macrocycle hosts. Chem. Soc. Rev. 2023, 52, 6644–6663. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, P.; Feng, H.; Zeng, R.; Li, S.; Zhang, Q. Macrocycle-Based Supramolecular Drug Delivery Systems: A Concise Review. Molecules 2024, 29, 3828. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Fang, R.; Rao, Q. An Insight into the Medicinal Chemistry Perspective of Macrocyclic Derivatives with Antitumor Activity: A Systematic Review. Molecules 2022, 27, 2837. [Google Scholar] [CrossRef]
- Viarengo-Baker, L.A.; Whitty, A. Macrocycles for Conventionally Druggable Targets: Lessons from Macrocyclic Kinase Inhibitors. J. Med. Chem. 2025, 68, 15260–15284. [Google Scholar] [CrossRef]
- Kim, T.; Baek, E.; Kim, J. Exploring Macrocyclic Chemical Space: Strategies and Technologies for Drug Discovery. Pharmaceuticals 2025, 18, 617. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Yang, Y.-W. Macrocycle-Based Antibacterial Materials. Chem. Mater. 2024, 36, 2177–2193. [Google Scholar] [CrossRef]
- Brudy, C.; Walz, C.; Spiske, M.; Dreizler, J.K.; Hausch, F. A Roadmap to Macrocyclization in Drug Discovery. J. Med. Chem. 2024, 67, 14768–14785. [Google Scholar] [CrossRef]
- Lisowsko, J. Imine- and Amine-Type Macrocycles Derived from Chiral Diamines and Aromatic Dialdehydes. Molecules 2022, 27, 4097. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, L.; Sun, B.; Wang, M.; Li, H.; Stoddart, J.F.; Huang, F. Applications of macrocycle-based solid-state host–guest chemistry. Nat. Rev. Chem. 2023, 7, 768–782. [Google Scholar] [CrossRef]
- Esteve, F.; Yang, Z.; Lehn, J.-M. Emerging Complex Behavior Driven by Self-Organization: Dynamic Covalent Libraries of Acylhydrazones in Water. J. Am. Chem. Soc. 2025, 147, 28408–28418. [Google Scholar] [CrossRef]
- Yang, Z.; Esteve, F.; Antheaume, C.; Lehn, J.-M. Triply Adaptive Libraries of Dynamic Covalent Macrocycles: Switching between Sorted and Unsorted States. J. Am. Chem. Soc. 2024, 146, 15438–15445. [Google Scholar] [CrossRef]
- Yang, Z.; Esteve, F.; Antheaume, C.; Lehn, J.-M. Dynamic covalent self-assembly and self-sorting processes in the formation of imine-based macrocycles and macrobicyclic cages. Chem. Sci. 2023, 14, 6631–6642. [Google Scholar] [CrossRef]
- Yepremyan, A.; Mehmood, A.; Asgari, P.; Janesko, B.G.; Simanek, E.E. Synthesis of Macrocycles Derived from Substituted Triazines. Chembiochem 2019, 20, 241–246. [Google Scholar] [CrossRef]
- Sharma, V.R.; Mehmood, A.; Janesko, B.G.; Simanek, E.E. Efficient syntheses of macrocycles ranging from 22–28 atoms through spontaneous dimerization to yield bis-hydrazones. RSC Adv. 2020, 10, 3217–3220. [Google Scholar] [CrossRef]
- Patterson-Gardner, C.J.; Pan, H.; Janesko, B.G.; Simanek, E.E. Conservation of Structure and Dynamic Behavior in Triazine Macrocycles with Opportunities for Subtle Control of Hinge Motion. Org. Biomol. Chem. 2025, 23, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.J.; Gloor, C.J.; Claton, L.E.; Mekhail, M.A.; Pan, H.; Stewart, M.D.; Green, K.N.; Pavan, G.M.; Capelli, R.; Simanek, E.E. A Model for the Rapid Assessment of Solution-Structures for 24-Atom Macrocycles: The Impact of b-Branched Amino Acids on Conformation. J. Org. Chem. 2023, 88, 2692–2702. [Google Scholar] [CrossRef] [PubMed]
- Stokes, G.A.; Patterson-Gardner, C.J.; Engstrom, A.M.; Menke, A.J.; Reddy, K.H.V.; Lokey, R.S.; Simanek, E.E. Triazine Macrocycle Libraries: Synthesis, logD Prediction, and a Surprisingly Hydrophobic, Membrane-Permeable Diamine. ACS Med. Chem. Lett. 2025, 16, 1017–1023. [Google Scholar] [CrossRef]
- Capelli, R.; Menke, A.J.; Pan, H.; Janesko, B.G.; Simanek, E.E.; Pavan, G.M. Well-Tempered Metadynamics Simulations Predict the Structural and Dynamic Properties of a Chiral 24-Atom Macrocycle in Solution. ACS Omega 2022, 7, 30291–30296. [Google Scholar] [CrossRef]
- Menke, A.J.; Reibenspies, J.H.; Patterson-Gardner, C.J.; Engstrom, A.; Lokey, R.S.; Simanek, E.E. Choreoisosteres: Pseudoatom Variation in Macrocyclic Hinges Conserves Structure and Dynamics. ACS Phys. Chem. Au 2025, 5, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.J.; Mellberg, J.M.; Pan, H.; Reibenspies, J.H.; Simanek, E.E. Controlling Swing Rates in Mortise Molecular Hinges. Chem. Eur. J. 2023, 29, e202300987. [Google Scholar] [CrossRef]
- Menke, A.J.; Henderson, N.C.; Kouretas, L.; Estenson, A.; Janesko, B.G.; Simanek, E.E. Computational and Experimental Evidence for Templated Macrocyclization: The Role of a Hydrogen Bond Network in the Quantitative Dimerization to 24-Atom Macrocycles. Molecules 2023, 28, 1144. [Google Scholar] [CrossRef] [PubMed]
- Claton, L.E.; Stokes, G.A.; Downum, A.L.; Simanek, E.E. Designing macrocycles to adopt persistent, isoenergetic conformations. J. Org. Chem. 2025, 90, 15215–15221. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.F.; Hernández-Trujillo, J.; Tang, T.-H.; Bader, R.F.W. Hydrogen–Hydrogen Bonding: A Stabilizing Interaction in Molecules and Crystals. Chem. Eur. J. 2003, 9, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- APEX3; Bruker-AXS: Madison, WI, USA, 2016.
- Software Packages SMART and SAINT, Version 4.0; Siemens Analytical X-ray Instrument Inc.: Madison, WI, USA, 1996.
- SADABS: Area-Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 1996.
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Casanova, B.B.; Muniz, M.N.; de Oliveira, T.; de Oliveira, L.F.; Machado, M.M.; Fuentefria, A.M.; Gosmann, G.; Gnoatto, S.C.B. BnHNNHBOC was prepared according to the procedure reported in: Synthesis and Biological Evaluation of Hydrazone Derivatives as Antifungal Agents. Molecules 2004, 20, 9229–9241. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]

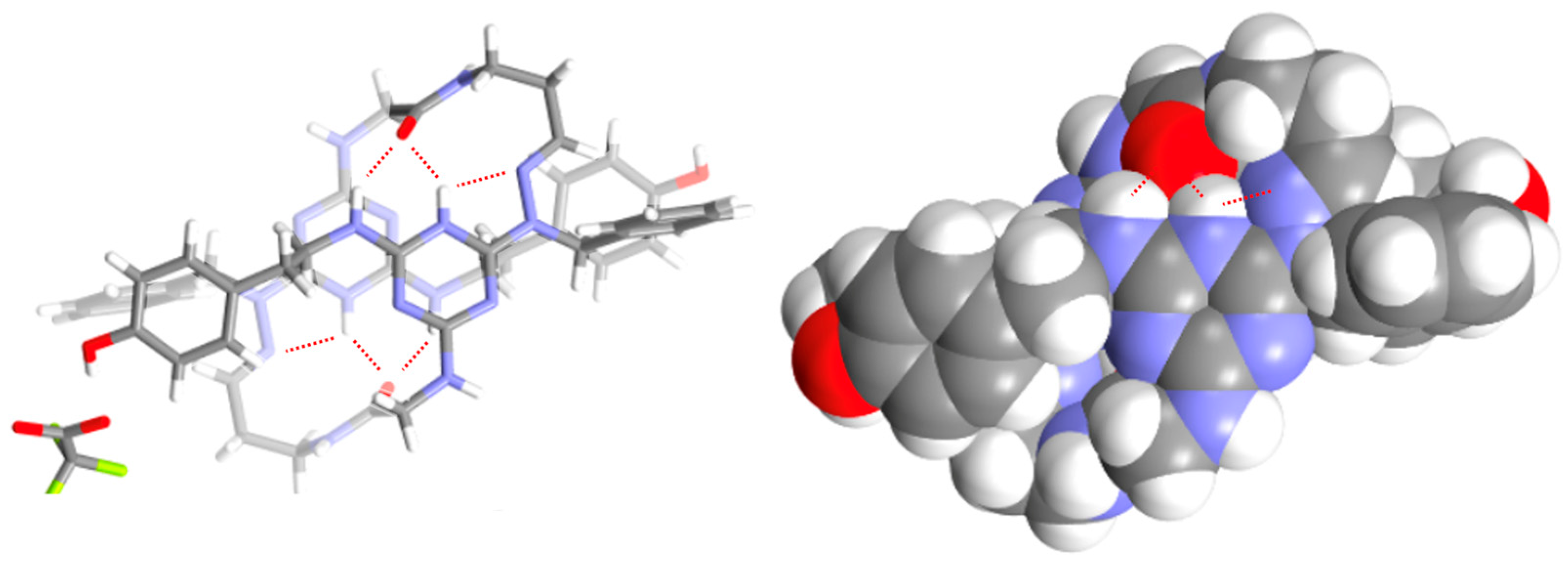

| Property | CP1 | CP2 | CP1′ | CP2′ |
|---|---|---|---|---|
| Interaction | NY–H⋯O | NT–H+⋯O | N′Y–H′⋯O′ | N′T–H′+⋯O′ |
| DA-H (Å) a | 2.072 | 1.882 | 2.073 | 1.882 |
| DD-H (Å) b | 1.019 | 1.031 | 1.019 | 1.031 |
| DA-CP (Å) c | 1.298 | 1.216 | 1.298 | 1.216 |
| DH-CP (Å) d | 0.779 | 0.667 | 0.779 | 0.667 |
| ρCP (e·Å−3) e | 0.123 | 0.195 | 0.123 | 0.195 |
| ∇2ρCP (e·Å−5) f | 1.79 | 2.39 | 1.79 | 2.39 |
| ε g | 0.040 | 0.088 | 0.040 | 0.088 |
| GCP (kJ·mol−1) h | 41.8 | 64.5 | 41.8 | 64.5 |
| VCP (kJ·mol−1) i | −35.1 | −63.8 | −35.1 | −63.9 |
| EHB (kJ·mol−1) j | −17.6 | −31.9 | −17.6 | −31.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, K.H.V.; Mehmood, A.; Yepremyan, A.; Simanek, E.E. A Competing Hydrogen Bond Network Offers Access to a New Conformation in 24-Atom Triazine Macrocycles. Molecules 2025, 30, 4475. https://doi.org/10.3390/molecules30224475
Reddy KHV, Mehmood A, Yepremyan A, Simanek EE. A Competing Hydrogen Bond Network Offers Access to a New Conformation in 24-Atom Triazine Macrocycles. Molecules. 2025; 30(22):4475. https://doi.org/10.3390/molecules30224475
Chicago/Turabian StyleReddy, K. Harsha Vardan, Arshad Mehmood, Akop Yepremyan, and Eric E. Simanek. 2025. "A Competing Hydrogen Bond Network Offers Access to a New Conformation in 24-Atom Triazine Macrocycles" Molecules 30, no. 22: 4475. https://doi.org/10.3390/molecules30224475
APA StyleReddy, K. H. V., Mehmood, A., Yepremyan, A., & Simanek, E. E. (2025). A Competing Hydrogen Bond Network Offers Access to a New Conformation in 24-Atom Triazine Macrocycles. Molecules, 30(22), 4475. https://doi.org/10.3390/molecules30224475








