Development and Validation of an HPLC-DAD Method for the Quantitative Determination of Benzoyl Peroxide, Curcumin, Rosmarinic Acid, Resveratrol and Salicylic Acid in a Face Mask—In Vitro/Ex Vivo Permeability Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Chromatographic Method
2.2. Method Validation
2.2.1. System Suitability
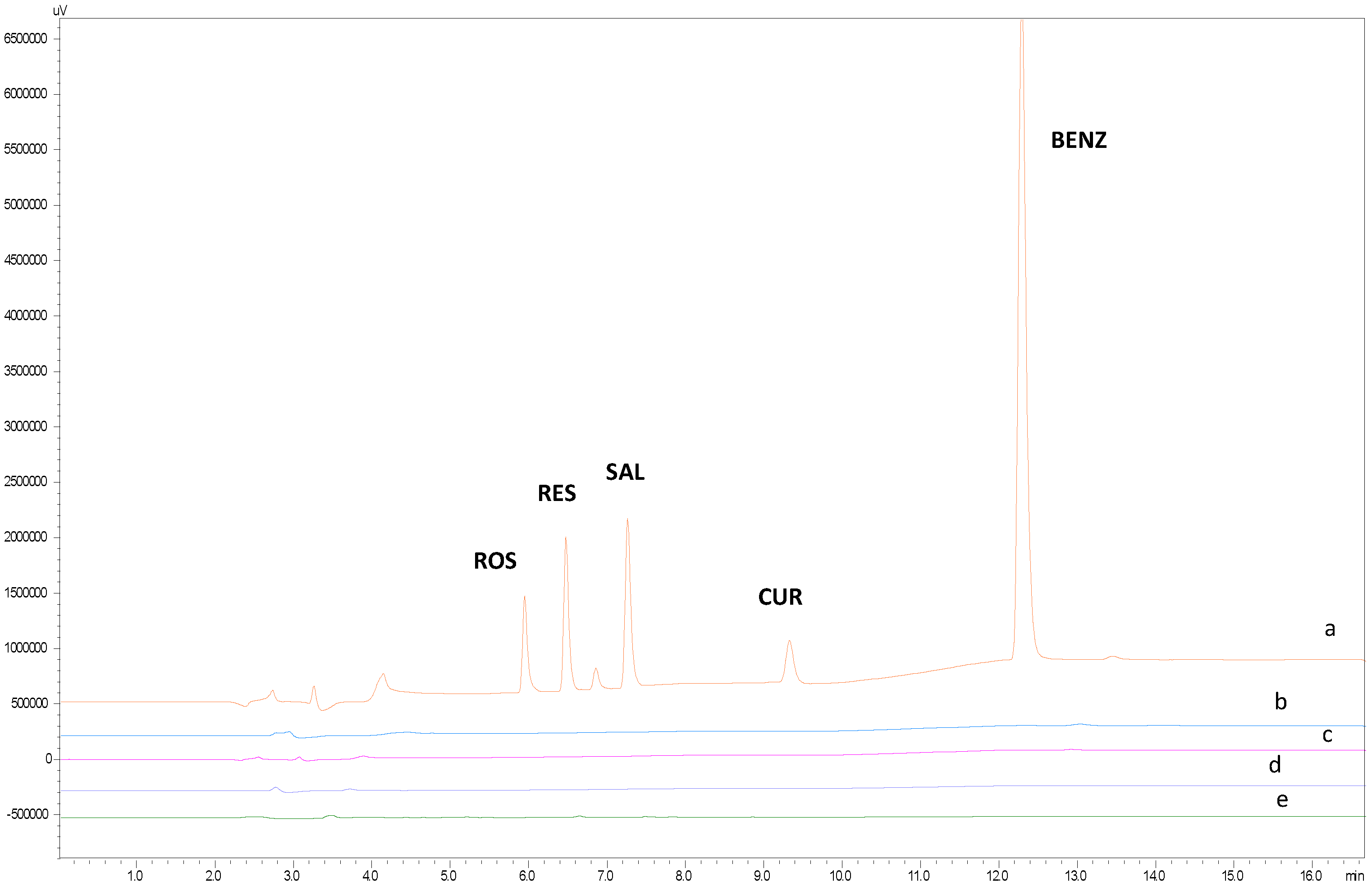
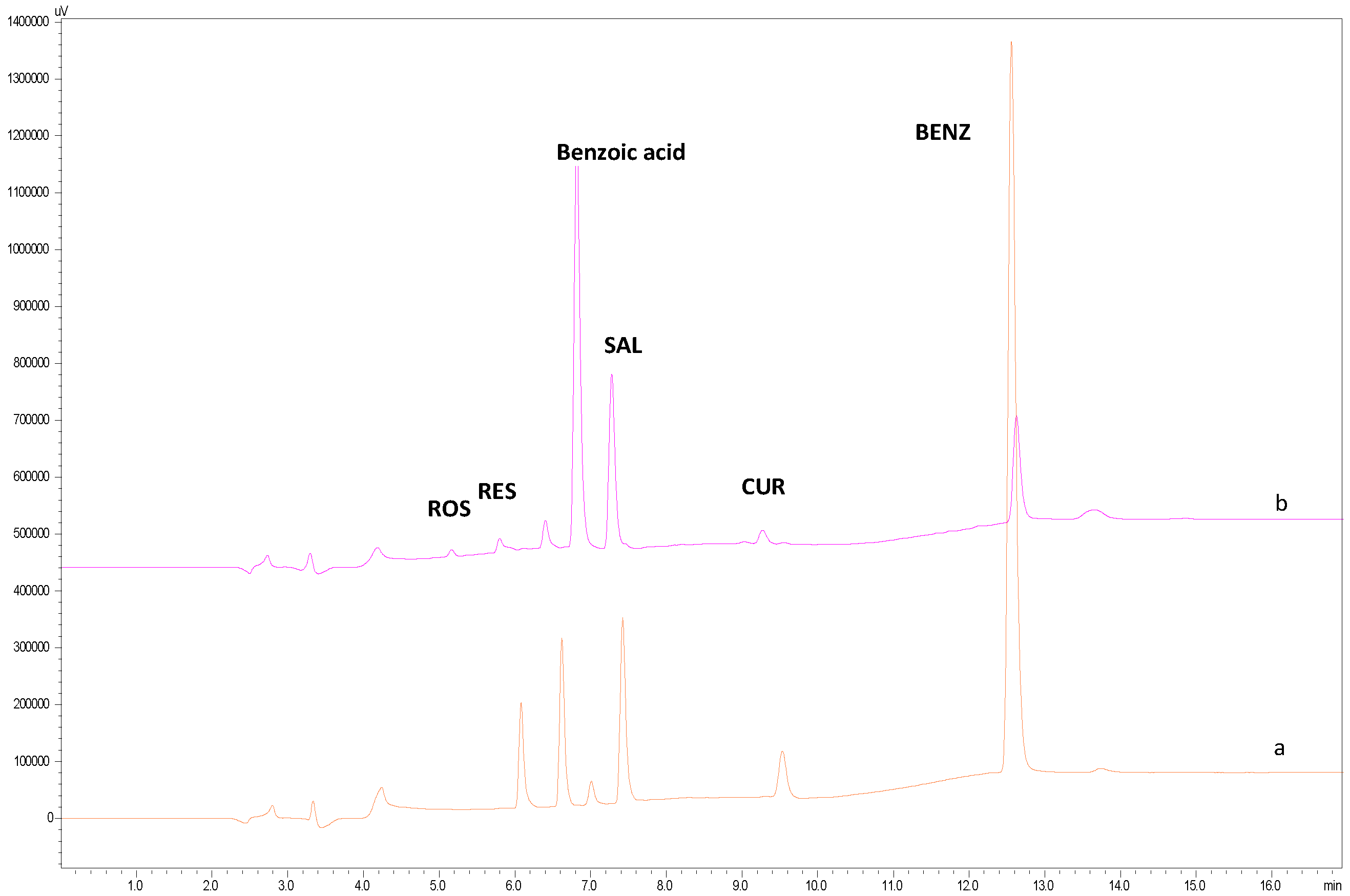
2.2.2. Selectivity
2.2.3. Linearity, LOD and LOQ
2.2.4. Precision
2.2.5. Accuracy
2.2.6. Robustness
2.3. Mask Preparation
2.4. Fourier-Transformed Infrared Spectroscopy (FTIR)
2.5. Samples Pretreatment in Mask
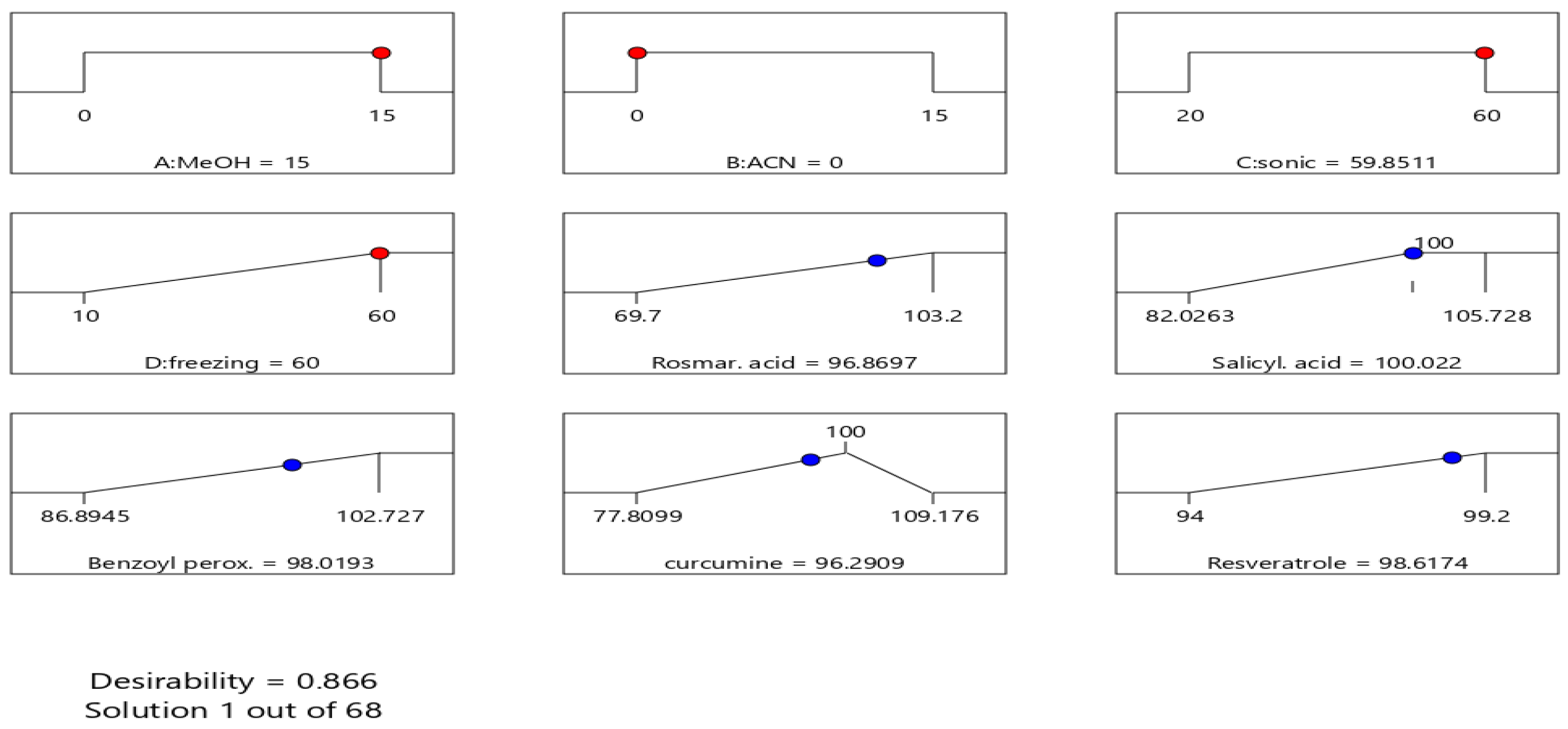
2.5.1. Experimental Design Methodology
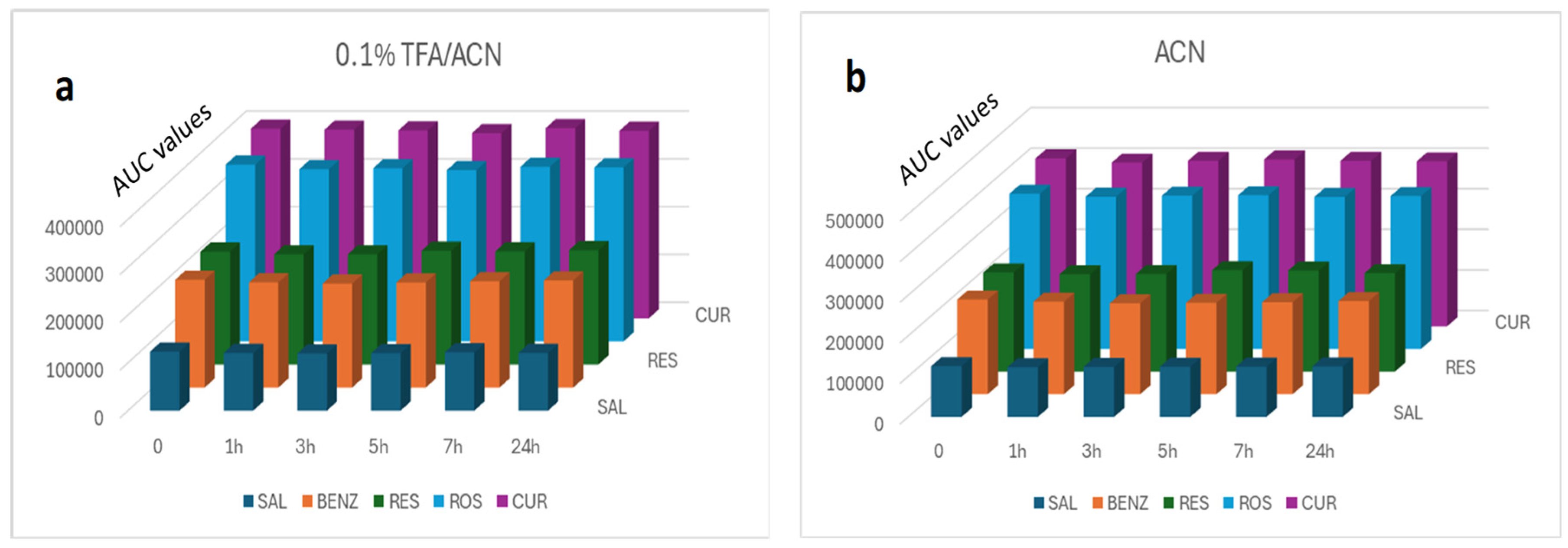
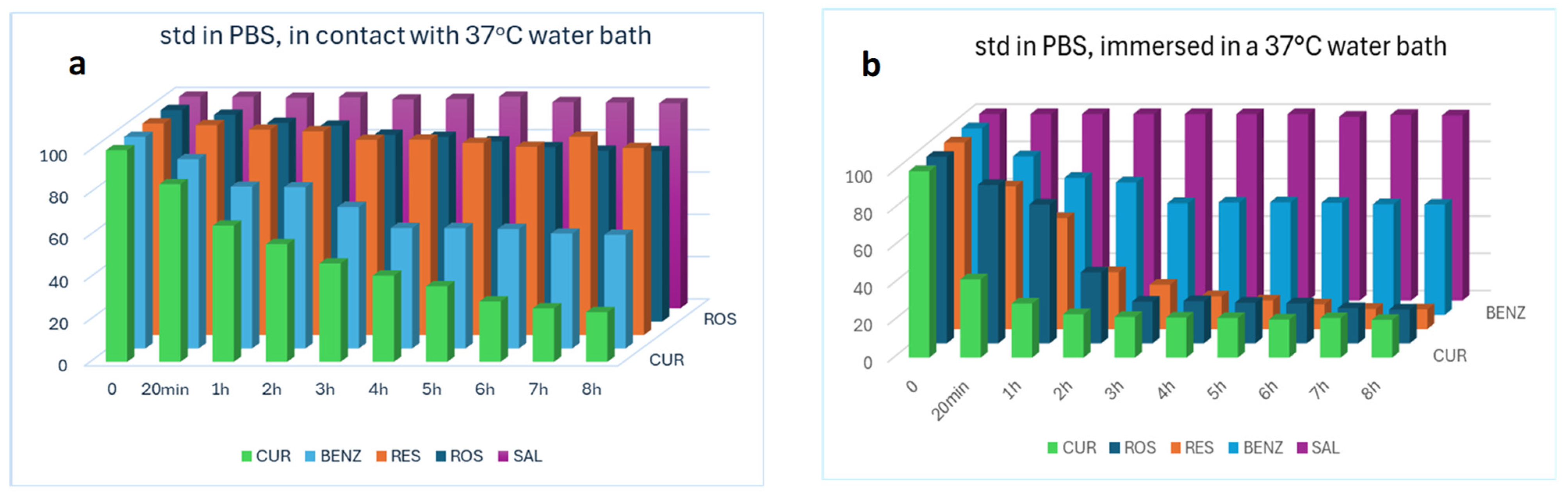
2.5.2. Validity of the Extraction
2.6. Transdermal Permeability
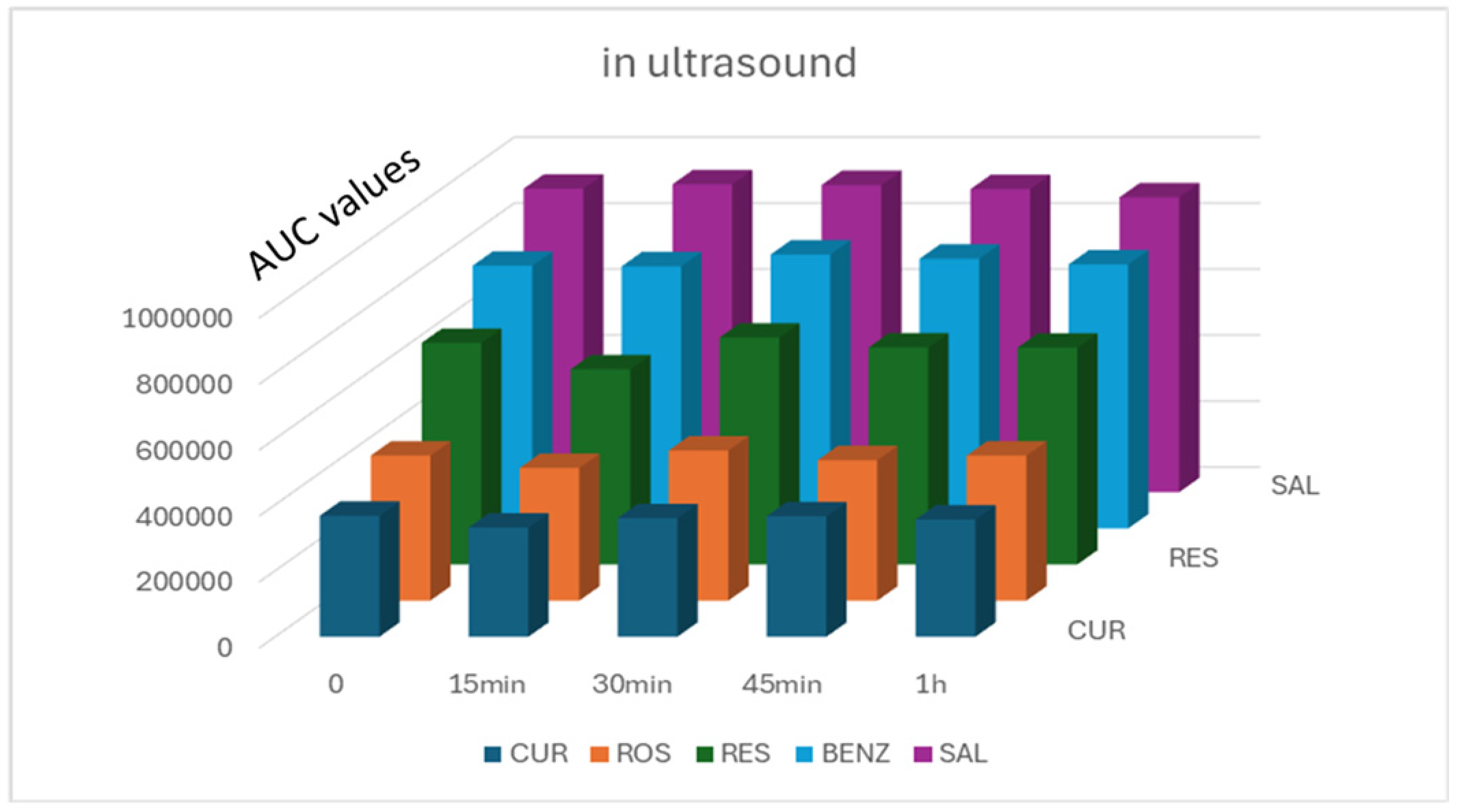
2.6.1. Preliminary Studies
2.6.2. Samples Pretreatment
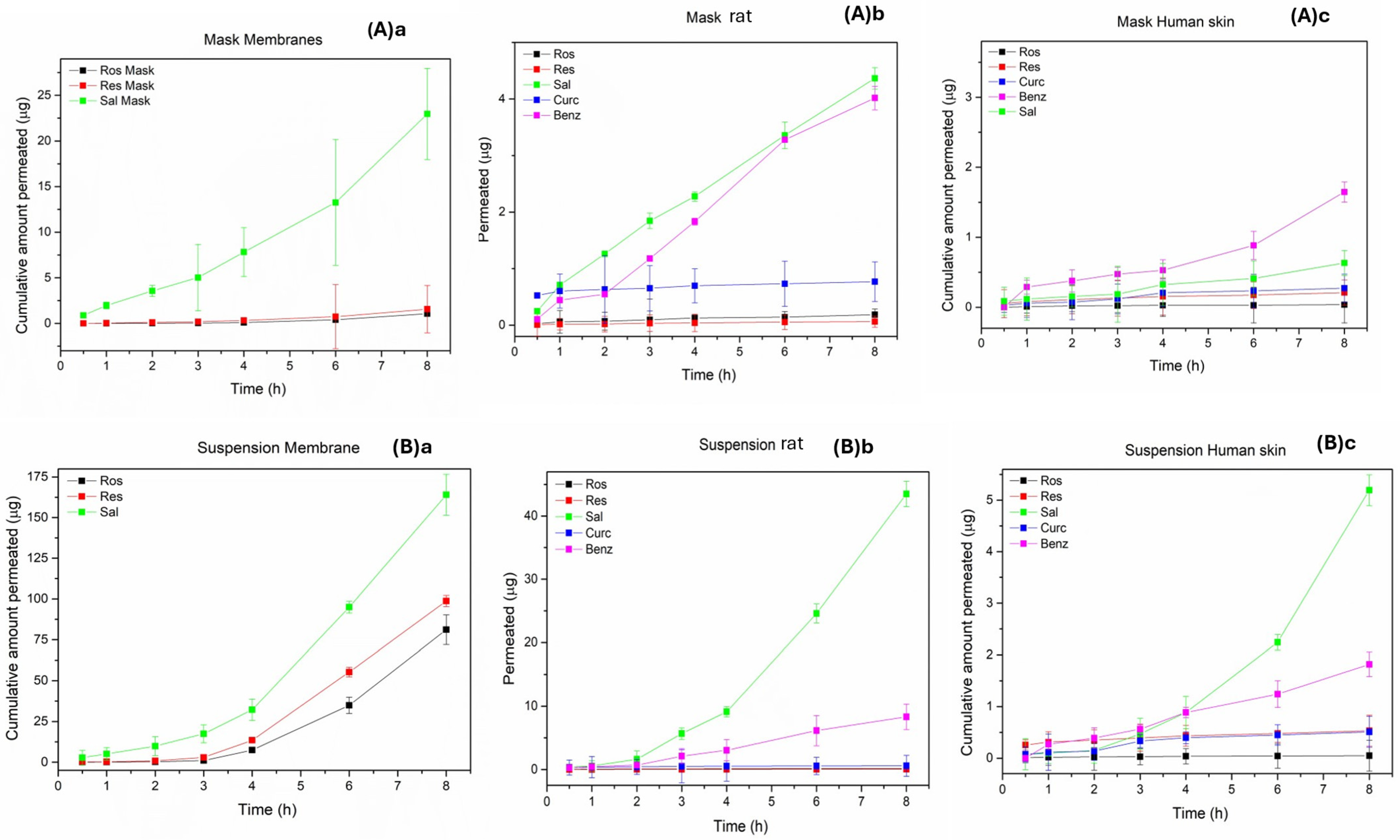
2.6.3. Evaluation of Permeability Results
3. Materials and Methods
3.1. Apparatus
3.1.1. Instrumentation
3.1.2. Permeability Studies: Franz Cells
3.2. Reagents and Solutions
3.3. Standard Solutions and Sample Pretreatment
3.3.1. Standard Solutions
3.3.2. Face Mask Extraction
3.3.3. Sample Processing in In Vitro/Ex Vivo Permeability Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Cong, T.-X.; Hao, D.; Wen, X.; Li, X.-H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef]
- Melnik, B.C.; Zouboulis, C.C. Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne. Exp. Dermatol. 2013, 22, 311–315. [Google Scholar] [CrossRef]
- Nascimento, L.D.; Guedes, A.; Magalha, G.; de Faria, F.; Guerra, R.; de C Almeida, F. Single-blind and comparative clinical study of the efficacy and safety of benzoyl peroxide 4% gel (BID) and adapalene 0.1% Gel (QD) in the treatment of acne vulgaris for 11 weeks. J. Dermatol. Treat. 2003, 14, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Mareledwane, N.G. A randomized, open-label, comparative study of oral doxycycline 100 mg vs. 5% topical benzoyl peroxide in the treatment of mild to moderate acne vulgaris. Int. J. Dermatol. 2006, 45, 1438–1439. [Google Scholar] [CrossRef] [PubMed]
- Sagransky, M.; Yentzer, B.A.; Feldman, S.R. Benzoyl peroxide: A review of its current use in the treatment of acne vulgaris. Expert. Opin. Pharmacother. 2009, 10, 2555–2562. [Google Scholar] [CrossRef]
- Truong, V.; Jun, M.; Jeong, W. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Wolter, F.; Stein, J. Biological activities of resveratrol and its analogs. Drugs Future 2002, 27, 0949. [Google Scholar] [CrossRef]
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 10, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Amoah, S.; Sandjo, L.; Kratz, J.; Biavatti, M. Rosmarinic Acid—Pharmaceutical and Clinical Aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef]
- Budhiraja, A.; Dhingra, G. Development and characterization of a novel antiacne niosomal gel of rosmarinic acid. Drug Deliv. 2015, 22, 723–730. [Google Scholar] [CrossRef]
- Budhiraja, A.; Dhingra, G. Evidence of curcumin and curcumin analogue effects in skin diseases: A narrative review. J. Cell Physiol. 2019, 234, 1165–1178. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Chang, K.-C.; Chen, L.-Y.; Hu, A. Low-dose blue light irradiation enhances the antimicrobial activities of curcumin against Propionibacterium acnes. J. Photochem. Photobiol. B 2018, 189, 21–28. [Google Scholar] [CrossRef]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Zarrintaj, P.; Salehi Moghaddam, A.; Mehrabi, T.; Alavi, S.; Mollapour Sisakht, M. Skin care and rejuvenation by cosmeceutical facial mask. J. Cosmet. Dermatol. 2018, 17, 693–702. [Google Scholar] [CrossRef]
- Sassi, A.B.; McCullough, K.D.; Cost, M.R.; Hillier, S.L.; Rohan, L.C. Permeability of tritiated water through human cervical and vaginal tissue. J. Pharm. Sci. 2004, 93, 2009–2016. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Robinson, J.R. Vaginal epithelial models. Pharm. Biotechnol. 1996, 8, 409–424. [Google Scholar] [PubMed]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Gaspar, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Studies and methodologies on vaginal drug permeation. Adv. Drug Deliv. Rev. 2015, 92, 14–26. [Google Scholar] [CrossRef]
- Neves, J.D.; Amaral, M.H.; Bahia, M.F. Performance of an in vitro mucoadhesion testing method for vaginal semisolids: Influence of different testing conditions and instrumental parameters. Eur. J. Pharm. Biopharm. 2008, 69, 622–632. [Google Scholar] [CrossRef]
- FDA-SUPAC-SS. Guidance for Industry. In SUPAC-SS Non-Sterile Semisolid Dosage Forms; Scale-Up and Post-Approval Changes: Chemistry, Manufacturing and Controls; In Vitro Release Testing and In Vivo Bioequivalence Documentation; U.S. Food and Drug Administration: Silver Spring, MD, USA, 1997; pp. 19–24. [Google Scholar]
- Twist, J.N.; Zatz, J.L. MembraneSolvent-Solute Interaction in a Model Permeation System. J. Pharm. Sci. 1988, 77, 536–540. [Google Scholar] [CrossRef]
- Franz, T.J. Percutaneous Absorption. On the Relevance of in Vitro Data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xu, X.; Kong, X.; Jiang, Y.; Mo, L.; Li, M.; Jin, Y.; Han, Y.; Li, X.-L.; Jin, T.; et al. Monitoring of salicylic acid content in human saliva and its relationship with plasma concentrations. J. Pharm. Biomed. Anal. 2022, 219, 11496. [Google Scholar] [CrossRef] [PubMed]
- Stephane, P. Stability of Cosmetic Formulations Containing UV Filters and Preservatives, Based on Physical and Chemical Parameters. MOJ Toxicol. 2015, 1, 12–21. [Google Scholar] [CrossRef]
- Pauwels, J.; D’aUtry, W.; Bossche, L.V.D.; Dewever, C.; Forier, M.; Vandenwaeyenberg, S.; Wolfs, K.; Hoogmartens, J.; Van Schepdael, A.; Adams, E. Optimization and validation of liquid chromatography and headspace-gas chromatography based methods for the quantitative determination of capsaicinoids, salicylic acid, glycol monosalicylate, methyl salicylate, ethyl salicylate, camphor and l-menthol in a topical formulation. J. Pharm. Biomed. Anal. 2012, 60, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Senta, R.D. Simultaneous Rp-HPLC determination of salicylamide, salicylic acid and deferasirox in the bulk API dosages forms. J. Taibah Univ. Sci. 2015, 9, 245–251. [Google Scholar] [CrossRef]
- Babu G, D.A.; Kumar, A. Development and validation of a hplc method for the quantification of benzoyl peroxide in flour. EPRA Int. J. Multidiscip. Res. (IJMR) 2023, 9, 16–21. [Google Scholar] [CrossRef]
- Usman, M.; Abbas, T. Development and validation of stability indicating HPLC–PDA method for the simultaneous determination of benzoyl peroxide and tretinoin in topical dosage form. Future J. Pharm. Sci. 2023, 9, 26. [Google Scholar] [CrossRef]
- Madhuri, A.B. Development and validation of HPLC method for simultaneous estimation of clindamycin phosphate and benzoyl peroxide in gel formulation. Int. J. Drug Res. Technol. 2016, 6, 34–42. [Google Scholar]
- Syed, H.K.; Liew, K.B.; Loh, G.O.K.; Peh, K.K. Stability indicating HPLC–UV method for detection of curcumin in Curcuma longa extract and emulsion formulation. Food Chem. 2015, 170, 321–326. [Google Scholar] [CrossRef]
- Pak, Y.; Patek, R.; Mayersohn, M. Sensitive and rapid isocratic liquid chromatography method for the quantitation of curcumin in plasma. J. Chromatogr. B 2003, 796, 339–346. [Google Scholar] [CrossRef]
- de Oliveira, K.B.; de Oliveira, B.H. HPLC/DAD determination of rosmarinic acid in Salvia officinalis: Sample preparation optimization by factorial design. J. Braz. Chem. Soc. 2013, 24, 85–91. [Google Scholar] [CrossRef]
- Wang, H. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation Study of Carnosic Acid, Carnosol, Rosmarinic Acid, and Rosemary Extract (Rosmarinus officinalis L.) Assessed Using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef]
- Chaowuttikul, C.; Palanuvej, C.; Ruangrungsi, N. Quantification of chlorogenic acid, rosmarinic acid, and caffeic acid contents in selected Thai medicinal plants using RP-HPLC-DAD. Braz. J. Pharm. Sci. 2020, 56, e17547. [Google Scholar] [CrossRef]
- Riccio, B.V.F.; Fonseca-Santos, B.; Ferrari, P.C.; Chorilli, M. Characteristics, Biological Properties and Analytical Methods of Trans-Resveratrol: A Review. Crit. Rev. Anal. Chem. 2020, 50, 339–358. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.F.; Guerra, C.C.; Czermainski, A.B.C.; Ferrari, L.; Bergold, A.M. Validation of a chromatographic method to routine analysis of trans-resveratrol and quercetin in red wines. Pesqui. Agropecu. Bras. 2017, 52, 335–343. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Shi, A.; Agyei, D.; Wang, Q. Formulation of water-in-oil-in-water (W/O/W) emulsions containing trans-resveratrol. RSC Adv. 2017, 7, 35917–35927. [Google Scholar] [CrossRef]
- Scalia, S.; Trotta, V.; Iannuccelli, V.; Bianchi, A. Enhancement of in vivo human skin penetration of resveratrol by chitosan-coated lipid microparticles. Colloids Surf. B Biointerfaces 2015, 135, 42–49. [Google Scholar] [CrossRef]
- Karavalasi, A.; Almpani, S.; Tserkezou, P.; Chachlioutaki, K.; Kamaris, G.; Markopoulou, C.K. Application of a Validated HPLC Method for the Determination of Resveratrol, Ferulic Acid, Quercetin, Retinol, and α-Tocopherol in a Cold Cream—Permeability Study. Appl. Sci. 2024, 14, 11843. [Google Scholar] [CrossRef]
- Agency, E.M. Temporary Interim Limits for NMBA, DIPNA and EIPNA Impurities in Sartan Blood Pressure Medicines. Eur. Med. Agency Amst. Neth. 2019, 31, 6–7. [Google Scholar]
- Harper, J.C. Benzoyl peroxide development, pharmacology, formulation and clinical uses in topical fixed-combinations. J. Drugs Dermatol. JDD 2010, 9, 482–487. [Google Scholar] [PubMed]
- Rahmasari, D.; Ermawati, D.; Nugraheni, R.W.; Putri, D.R.; Pratiwi, I.N. Design and Development of Peel-off Mask Gel Formulation of Citronella Oil for Acne Vulgaris. In Proceedings of the 2nd Health Science International Conference, SCITEPRESS—Science and Technology Publications, Malang, Indonesia, 5 October 2019; pp. 157–163. [Google Scholar] [CrossRef]
- Sarruf, F.D.; Contreras, V.J.P.; Martinez, R.M.; Velasco, M.V.R.; Baby, A.R. The Scenario of Clays and Clay Minerals Use in Cosmetics/Dermocosmetics. Cosmetics 2024, 11, 7. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I. Gelatin. In Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 92–115. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Honey in dermatology and skin care: A review. J. Cosmet. Dermatol. 2013, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Haftek, M.; Abdayem, R.; Guyonnet-Debersac, P. Skin Minerals: Key Roles of Inorganic Elements in Skin Physiological Functions. Int. J. Mol. Sci. 2022, 23, 6267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Dongye, C.; Xu, X.; Li, Z.; Yi, W.; Chen, X.; Wang, S. Co-pyrolysis behavior of spent bleaching clay and dealkaline lignin: An investigation into TG-FTIR, kinetics, and interaction mechanisms. Chem. Eng. J. 2025, 524, 169114. [Google Scholar] [CrossRef]
- Cebi, N.; Dogan, C.E.; Mese, A.E.; Ozdemir, D.; Arıcı, M.; Sagdic, O. A rapid ATR-FTIR spectroscopic method for classification of gelatin gummy candies in relation to the gelatin source. Food Chem. 2019, 277, 373–381. [Google Scholar] [CrossRef]
- Dimakopoulou-Papazoglou, D.; Serrano, S.; Rodríguez, I.; Ploskas, N.; Koutsoumanis, K.; Katsanidis, E. FTIR spectroscopy combined with machine learning for the classification of Mediterranean honey based on origin. J. Food Compos. Anal. 2025, 144, 107778. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, W. Study on thermal decomposition behavior of benzoyl peroxide under the influence of three impurities. Thermochim. Acta 2025, 750, 180037. [Google Scholar] [CrossRef]
- Telfah, A.; Thiabat, S.; Ababneh, A.M.; Ahmad, A.A.; Ababneh, Z.Q.; Al Bataineh, Q.M.; Tavares, C.J. Salicylic acid ionization dynamics in aqueous solution for pharmaceutical formulations. Colloids Surf. A Physicochem. Eng. Asp. 2025, 725, 137601. [Google Scholar] [CrossRef]
- Wu, S.-L.; Zhang, Y.; Wang, L.; Mei, Y.; Zeng, D.; Wang, Y.; Guan, J.; Liu, L. Resveratrol-modified polyethyleneimine as a high-efficiency and biocompatible gene delivery vector for enhanced transfection. J. Drug Deliv. Sci. Technol. 2025, 113, 107404. [Google Scholar] [CrossRef]
- Zhang, J.; Su, J.; Liu, X.; Chen, M.; Bai, B.; Yang, Y.; Fan, S.; Bo, T. Preparation and characterization of carboxymethyl cellulose-based edible thin films loaded with rosmarinic acid and citral nanoparticles. Carbohydr. Polym. Technol. Appl. 2025, 10, 100821. [Google Scholar] [CrossRef]
- Shen, Y.; Farajtabar, A.; Xu, J.; Wang, J.; Xia, Y.; Zhao, H.; Xu, R. Thermodynamic solubility modeling, solvent effect and preferential solvation of curcumin in aqueous co-solvent mixtures of ethanol, n-propanol, isopropanol and propylene glycol. J. Chem. Thermodyn. 2019, 131, 410–419. [Google Scholar] [CrossRef]
- Baldwin, H.; Elewski, B.; Hougeir, F.; Yamauchi, P.; Levy-Hacham, O.; Hamil, K.; Harper, J. Sixty Years of Benzoyl Peroxide Use in Dermatology. J. Drugs Dermatol. 2023, 22, 54–59. [Google Scholar] [CrossRef] [PubMed]
| Time (Min) | A% | B% | Flow (mL/Min) |
|---|---|---|---|
| 0 | 100 | 0 | 1.0 |
| 5 | 40 | 60 | 1.0 |
| 7 | 40 | 60 | 1.0 |
| 9 | 20 | 80 | 1.0 |
| 14 | 20 | 80 | 1.0 |
| 15 | 100 | 0 | 1.0 |
| 20 | run | stop | 1.0 |
| Name | Retention Time | Tailing Factor | K′ | Resolution | Theoretical Plates | HETP × 103 USP |
|---|---|---|---|---|---|---|
| ROS | 5.9 | 1.5 | 1.4 | -- | 25,068 | 9.97 |
| RES | 6.4 | 1.4 | 1.6 | 3.9 | 31,327 | 7.98 |
| SAL | 7.2 | 1.3 | 1.9 | 2.7 | 32,309 | 7.74 |
| CUR | 9.3 | 1.2 | 2.7 | 11.3 | 37,360 | 6.69 |
| BENZ | 12.2 | 1.3 | 3.9 | 16.8 | 65,485 | 3.82 |
| Substances | Concentration Range (μg/mL) | Calibration Curves | Correlation Coefficient (r2) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| ROS | 1.60–16.0 | y = 58,698x + 1713.6 | 0.9998 | 0.267 | 0.810 |
| RES | 0.37–15.10 | y = 160,221x + 1322.3 | 0.9999 | 0.047 | 0.142 |
| SAL | 2.04–20.40 | y = 14,367x + 5208.4 | 0.9994 | 0.636 | 1.925 |
| CUR | 0.92–9.20 | y = 88,301x − 7886.7 | 0.9994 | 0.296 | 0.897 |
| BENZ | 1.35–22.5 | y = 80,502x + 16,089 | 0.9991 | 0.083 | 0.251 |
| APIs | Intra-Day (n = 3) | Accuracy | Inter-Day Precision (n = 3) | |||||
|---|---|---|---|---|---|---|---|---|
| Concentration | %RSD | % Recovery | %RSD | %RSD | ||||
| (μg/mL) | 1nd Day | 2nd Day | 3rd Day | Total (n = 12) | ||||
| ROS | 1.6 | 0.32 | 99.4 | 0.39 | 0.38 | 0.31 | 0.07 | 0.25 |
| 8 | 0.15 | 101.1 | 0.01 | 0.20 | 0.14 | 0.12 | 0.20 | |
| 16 | 0.17 | 100.5 | 0.19 | 0.19 | 0.10 | 0.07 | 0.19 | |
| RES | 0.376 | 0.47 | 100.5 | 0.33 | 0.33 | 0.09 | 0.36 | 0.45 |
| 1.88 | 0.05 | 100.3 | 0.15 | 0.07 | 0.04 | 0.13 | 0.23 | |
| 15.1 | 0.17 | 100.0 | 0.01 | 0.19 | 0.10 | 0.08 | 0.14 | |
| SAL | 2.04 | 0.44 | 98.2 | 1.2 | 0.04 | 0.47 | 0.43 | 0.42 |
| 10.2 | 0.13 | 100.5 | 0.2 | 0.19 | 0.08 | 0.29 | 0.18 | |
| 20.4 | 0.15 | 100.2 | 0.23 | 0.22 | 0.02 | 0.10 | 0.14 | |
| CUR | 0.92 | 0.52 | 101.6 | 0.74 | 0.78 | 0.07 | 0.45 | 0.47 |
| 4.6 | 0.28 | 100.2 | 0.13 | 0.14 | 0.38 | 0.30 | 0.27 | |
| 9.22 | 0.23 | 101.3 | 0.03 | 0.03 | 0.32 | 0.05 | 0.27 | |
| BENZ | 1.35 | 1.12 | 103.1 | 0.33 | 1.36 | 0.63 | 0.82 | 0.98 |
| 4.5 | 0.41 | 99.1 | 0.97 | 0.48 | 0.15 | 0.16 | 0.43 | |
| 22.5 | 0.69 | 100.5 | 0.85 | 0.25 | 0.46 | 0.34 | 0.67 | |
| %RSD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | AUC ROS | Tf ROS | AUC RES | Tf RES | AUC SAL | Tf SAL | AUC CUR | Tf CUR | AUC BENZ | Tf BENZ |
| Mob.phase %B | 0.5 | 1.4 | 1.3 | 5.1 | 1.9 | 0 | 0.6 | 0.8 | 1.2 | 4.7 |
| (84, 85, 86) | ||||||||||
| Column (°C) | 1.3 | 0.5 | 0.2 | 1.3 | 0.2 | 1.7 | 2 | 1.7 | 0.7 | 2.5 |
| (39, 40, 41) | ||||||||||
| λ max | 2 | 0.5 | 3.3 | 0.5 | 2 | 0.5 | 2.9 | 0.9 | 1.1 | 0 |
| Inj. volume (19, 20, 21) | 4.6 | 2.1 | 4.9 | 3 | 4.5 | 0.5 | 4.8 | 0.8 | 1.4 | 0.8 |
| Study Type | Combined | Subtype | Randomized | |
| Design Type | D-optimal | Runs | 18 | |
| Design Model | Quadratic × Quadratic | Blocks | No Blocks | |
| Build Time (ms) | 104.00 | |||
| Mixture | Components | A (MeOH) + B (ACN) = 15 | ||
| Process | Factors | C Sonic (20–60 min) | ||
| D Freezing time (10–60 min) | ||||
| Parameters | ROS | SAL | RES |
|---|---|---|---|
| Std. Dev. | 4.81 | 2.67 | 0.67 |
| C.V. % | 5.35 | 2.79 | 0.69 |
| R2 | 0.705 | 0.85 | 0.85 |
| Adjusted R2 | 0.697 | 0.77 | 0.82 |
| Predicted R2 | 0.570 | 0.58 | 0.78 |
| Adeq Precision | 113.0 | 136.6 | 124.9 |
| % Recovery | |||||
|---|---|---|---|---|---|
| Samples | ROS | RES | SAL | CUR | BENZ |
| n1 | 98.1 | 102.1 | 99.9 | 101.2 | 100.6 |
| n2 | 97.6 | 100.3 | 98.7 | 99.7 | 99.5 |
| n3 | 99.9 | 101.4 | 98.9 | 101.1 | 99.8 |
| n4 | 97.3 | 96.3 | 101.9 | 95.4 | 96.5 |
| n5 | 98.0 | 98.1 | 100.0 | 99.8 | 99.0 |
| %RSD | 1.0 | 2.4 | 1.3 | 2.4 | 1.6 |
| ROS | RES | SAL | CUR | ΒΕΝΖ | |
|---|---|---|---|---|---|
| 105.4 | 106.9 | 105.3 | 103.0 | 106.2 | |
| 96.0 | 94.7 | 94.8 | 90.6 | 91.1 | |
| 102.0 | 102.5 | 98.5 | 100.2 | 94.6 | |
| 101.8 | 94.2 | 99.5 | 95.5 | 96.8 | |
| 104.4 | 99.7 | 96.6 | 98.0 | 99.9 | |
| Mean | 101.9 | 99.6 | 98.9 | 97.5 | 97.7 |
| %RSD | 3.2 | 4.8 | 3.6 | 4.3 | 5.2 |
| Cell | J (μg cm−2 min−1) Rat Skin | ||||
| ROS | RES | SAL | CUR | BENZ | |
| mask | 0.0035 ± 0.005 | 0.00135 ± 0.00005 | 0.10185 ± 0.00485 | 0.0049 ± 0.0006 | 0.104 ± 0.0041 |
| Suspension | 0.0031 ± 0.003 | 0.00125 ± 0.00005 | 1.3394 ± 0.0233 | 0.0029 ± 0.0008 | 0.2145 ± 0.0372 |
| Cell | Papp (cm min−1) Rat Skin | ||||
| ROS | RES | SAL | CUR | BENZ | |
| mask | 6.80 ± 0.97 | 2.78 ± 0.103 | 153.16 ± 7.293 | 9.51 ± 1.17 | 42.10 ± 167 |
| Suspension | 6.26 ± 0.60 | 2.52 ± 0.1 | 2626.27 ± 45.69 | 5.47 ± 1.50 | 86.84 ± 15.06 |
| Cell | J (μg cm−2 min−1) Membrane | ||||
| ROS | RES | SAL | CUR | BENZ | |
| mask | 0.03915 ± 0.002 | 0.0455 ± 0.0002 | 0.53335 ± 0.028 | ||
| Suspension | 3.062 ± 0.05 | 3.261 ± 0.128 | 5.05895 ± 0.368 | ||
| Cell | Papp (cm min−1) Membrane | ||||
| ROS | RES | SAL | CUR | BENZ | |
| mask | 76.02 ± 4.37 | 93.81 ± 0.41 | 802.03 ± 42.03 | ||
| Suspension | 6185.85 ± 101.01 | 6587.57 ± 257.67 | 9919.51 ± 722.06 | ||
| Cell | J (μg cm−2 min−1) Human | ||||
| ROS | RES | SAL | CUR | BENZ | |
| mask | 0.00285 ± 0.0022 | 0.00305 ± 0.00004 | 0.0135 ± 0.0002 | 0.00655 ± 0.00115 | 0.05225 ± 0.0008 |
| Suspension | 0.00075 ± 0.00005 | 0.00555 ± 0.00015 | 0.1777 ± 0.02 | 0.0117 ± 0.0004 | 0.04585 ± 0.0007 |
| Cell | Papp (cm min−1) Human | ||||
| ROS | RES | SAL | CUR | BENZ | |
| mask | 5.53 ± 4.17 | 6.29 ± 0.103 | 20.30 ± 0.30 | 12.72 ± 2.23 | 21.15 ± 0.30 |
| Suspension | 1.51 ± 0.10 | 11.21 ± 0.30 | 348.43 ± 40.20 | 22.08 ± 0.75 | 18.56 ± 0.26 |
| Rat Skin | Drugs Amounts in Suspension | Drugs Amounts in Mask | ||||||||
| Drug | Reference Sample | Donor | Acceptor | Membrane | Reference Sample | Donor | Acceptor | Membrane | ||
| Loaded (μg) | Founded (μg) | Loaded Sample (μg) | Final Founded (μg) | Final Founded (μg) | Loaded (μg) | Founded (μg) | Loaded Sample (μg) | Final Founded (μg) | Final Founded (μg) | |
| ROS | 495.0 | 510.0 | 510.0 | 0.12 | 228.6 | 515.0 | 512.0 | 512.0 | 0.13 | 162.7 |
| RES | 595.0 | 566.1 | 566.1 | 0.04 | 291.7 | 485.0 | 484.6 | 484.6 | 0.05 | 174.5 |
| SAL | 510.0 | 489.2 | 489.2 | 28.52 | 383.0 | 665.0 | 664.0 | 664.0 | 3.19 | 507.4 |
| CUR | 530.0 | 469.7 | 469.7 | 0.38 | 284.4 | 515.0 | 508.3 | 508.3 | 0.53 | 140.8 |
| BENZ * | 2470.0 | 2242.5 | 2242.5 | 6.82 | 1555.6 | 2470.0 | 2461.2 | 2461.2 | 3.27 | 1238.9 |
| Membrane | Drugs Amounts in Suspension | Drugs Amounts in Mask | ||||||||
| Drug | Reference Sample | Donor | Acceptor | Membrane | Reference Sample | Donor | Acceptor | Membrane | ||
| Loaded (μg) | Founded (μg) | Loaded Sample (μg) | Final Founded (μg) | Final Founded (μg) | Loaded (μg) | Founded (μg) | Loaded Sample (μg) | Final Founded (μg) | Final Founded (μg) | |
| ROS | 495.0 | 510.0 | 510.0 | 72.43 | 194.2 | 515.0 | 512.0 | 512.0 | 1.00 | 154.7 |
| RES | 595.0 | 566.1 | 566.1 | 88.74 | 158.5 | 485.0 | 484.6 | 484.6 | 1.35 | 117.2 |
| SAL | 510.0 | 489.2 | 489.2 | 145.46 | 261.7 | 665.0 | 664.0 | 664.0 | 19.16 | 554.9 |
| CUR | 530.0 | 469.7 | 469.72 | 0 | 239.1 | 515.0 | 508.3 | 508.3 | 0 | 153.6 |
| BENZ * | 2470.0 | 2242.5 | 2242.5 | 0 | 1456.1 | 2470.0 | 2461.2 | 2461.2 | 0 | 1044.2 |
| Human | Drugs Amounts in Suspension | Drugs Amounts in Mask | ||||||||
| Drug | Reference Sample | Donor | Acceptor | Membrane | Reference Sample | Donor | Acceptor | Membrane | ||
| Loaded (μg) | Founded (μg) | Loaded Sample (μg) | Final Founded (μg) | Final Founded (μg) | Loaded (μg) | Founded (μg) | Loaded Sample (μg) | Final Founded (μg) | Final Founded (μg) | |
| ROS | 495.0 | 510.0 | 510.0 | 0 | 297.2 | 515.0 | 512.0 | 512.0 | 0.00 | 171.7 |
| RES | 595.0 | 566.1 | 566.1 | 0.31 | 363.8 | 485.0 | 484.6 | 484.6 | 0.14 | 156.6 |
| SAL | 510.0 | 489.2 | 489.2 | 4.64 | 327.8 | 665.0 | 664.0 | 664.0 | 0.49 | 525.0 |
| CUR | 530.0 | 469.7 | 469.72 | 0.36 | 255.7 | 515.0 | 508.3 | 508.3 | 0.19 | 114.0 |
| BENZ * | 2470.0 | 2242.5 | 2242.5 | 1.52 | 1587.4 | 2470.0 | 2461.2 | 2461.2 | 1.35 | 1015.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almpani, S.; Mitsiou, M.; Monou, P.K.; Markopoulou, C.K. Development and Validation of an HPLC-DAD Method for the Quantitative Determination of Benzoyl Peroxide, Curcumin, Rosmarinic Acid, Resveratrol and Salicylic Acid in a Face Mask—In Vitro/Ex Vivo Permeability Study. Molecules 2025, 30, 4474. https://doi.org/10.3390/molecules30224474
Almpani S, Mitsiou M, Monou PK, Markopoulou CK. Development and Validation of an HPLC-DAD Method for the Quantitative Determination of Benzoyl Peroxide, Curcumin, Rosmarinic Acid, Resveratrol and Salicylic Acid in a Face Mask—In Vitro/Ex Vivo Permeability Study. Molecules. 2025; 30(22):4474. https://doi.org/10.3390/molecules30224474
Chicago/Turabian StyleAlmpani, Sofia, Maria Mitsiou, Paraskevi Kyriaki Monou, and Catherine K. Markopoulou. 2025. "Development and Validation of an HPLC-DAD Method for the Quantitative Determination of Benzoyl Peroxide, Curcumin, Rosmarinic Acid, Resveratrol and Salicylic Acid in a Face Mask—In Vitro/Ex Vivo Permeability Study" Molecules 30, no. 22: 4474. https://doi.org/10.3390/molecules30224474
APA StyleAlmpani, S., Mitsiou, M., Monou, P. K., & Markopoulou, C. K. (2025). Development and Validation of an HPLC-DAD Method for the Quantitative Determination of Benzoyl Peroxide, Curcumin, Rosmarinic Acid, Resveratrol and Salicylic Acid in a Face Mask—In Vitro/Ex Vivo Permeability Study. Molecules, 30(22), 4474. https://doi.org/10.3390/molecules30224474






