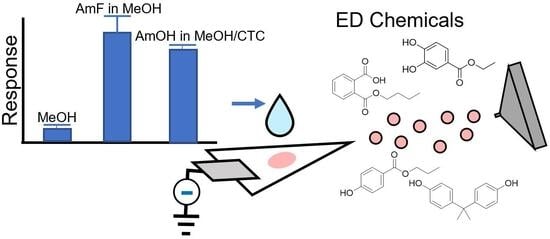
Negative Paper Spray Ionization Mass Spectrometry for the Determination of Endocrine-Disrupting Chemicals with Application to Paraben Analysis in Cosmetics
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Negative PSI for EDC-Related Model Analytes
2.1.1. Target Analytes
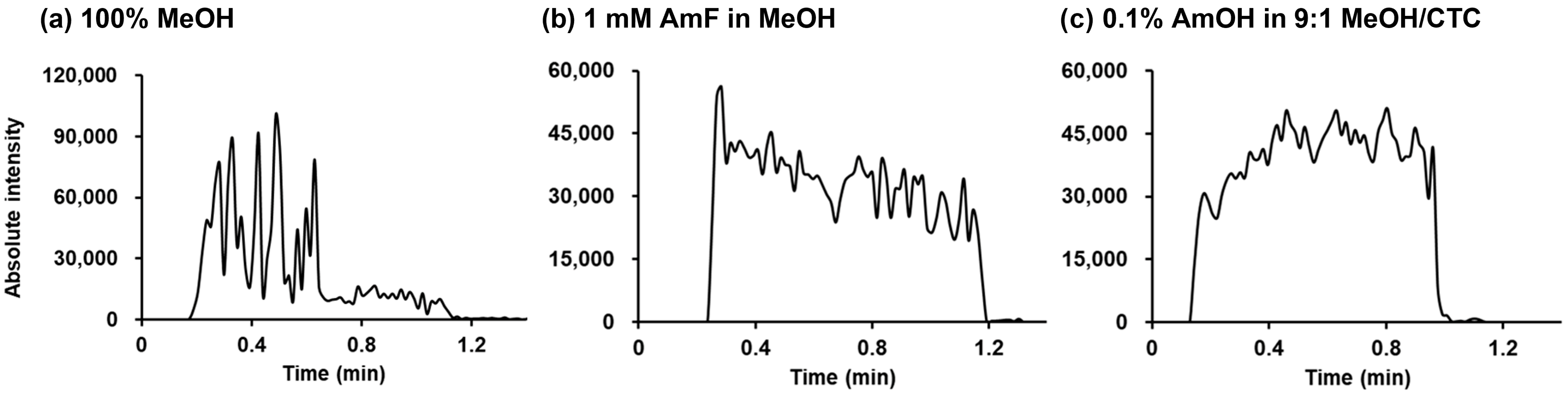
2.1.2. Effects of Spraying Solvent and Additive on the Ionization Yield of Model Analytes
2.1.3. Initial Quantitative Evaluation of Two PSI Spraying Solvent Systems: Linearity and LOQ
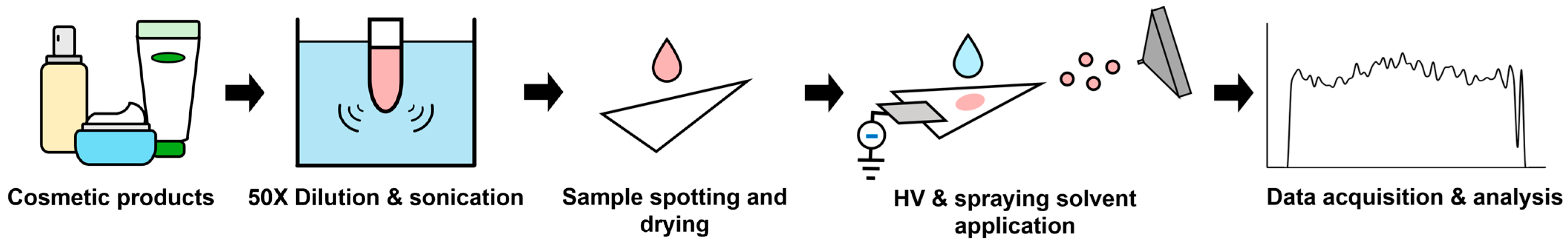
2.2. Application to Quantitative Analysis of Parabens in Cosmetics
3. Materials and Methods
3.1. Materials
3.2. Preparation of Standards for the Evaluation of Negative PSI-MS Performance
3.3. Standards and Sample Preparation for the Analysis of Parabens in Cosmetics
3.4. PSI-MS/MS
3.5. LC-MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| AmF | Ammonium fluoride |
| AmOH | Ammonium hydroxide |
| BP | Butylparaben |
| BPA | Bisphenol A |
| BPS | Bisphenol S |
| CE | Collision Energy |
| CTC | Carbon tetrachloride |
| EDC | Endocrine-disrupting chemicals |
| EIC | Extracted ion chronogram |
| EP | Ethylparaben |
| EPA | Ethyl protocatechuate |
| ESI | Electrospray ionization |
| 4-HBP | 4-Hydroxybenzophenone |
| IS | Internal standard |
| LC | Liquid chromatography |
| LOQ | Limit of quantitation |
| MBzP | Monobenzyl phthalate |
| MEHP | Mono-2-ethylhexyl phthalate |
| MeOH | Methanol |
| MnBP | Mono-n-butyl phthalate |
| MP | Methylparaben |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| 3-PBA | 3-Phenoxybenzoic acid |
| 4-PNP | 4-Nitrophenol |
| PP | Propylparaben |
| PSI | Paper spray ionization |
| PTFE-D | Hydrophobic polytetrafluoroethylene |
| TCP | 2,4,5-trichlorophenol |
References
- Chiang, S.; Zhang, W.; Ouyang, Z. Paper spray ionization mass spectrometry: Recent advances and clinical applications. Expert Rev. Proteom. 2018, 15, 781–789. [Google Scholar] [CrossRef]
- Espy, R.D.; Muliadi, A.R.; Ouyang, Z.; Cooks, R.G. Spray mechanism in paper spray ionization. Int. J. Mass Spectrom. 2012, 325–327, 167–171. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Song, W.-Y.; Kim, T.-Y. Characterization of Spray Modes and Factors Affecting the Ionization Efficiency of Paper Spray Ionization. Front. Chem. 2022, 10, 2022. [Google Scholar] [CrossRef]
- McBride, E.M.; Mach, P.M.; Dhummakupt, E.S.; Dowling, S.; Carmany, D.O.; Demond, P.S.; Rizzo, G.; Manicke, N.E.; Glaros, T. Paper spray ionization: Applications and perspectives. TrAC Trends Anal. Chem. 2019, 118, 722–730. [Google Scholar] [CrossRef]
- Cech, N.B.; Enke, C.G. Practical implications of some recent studies in electrospray ionization fundamentals. Mass Spectrom. Rev. 2001, 20, 362–387. [Google Scholar] [CrossRef]
- Cole, R.B.; Harrata, A.K. Solvent effect on analyte charge state, signal intensity, and stability in negative ion electrospray mass spectrometry; implications for the mechanism of negative ion formation. J. Am. Soc. Mass Spectrom. 1993, 4, 546–556. [Google Scholar] [CrossRef] [PubMed]
- McClory, P.J.; Håkansson, K. Corona Discharge Suppression in Negative Ion Mode Nanoelectrospray Ionization via Trifluoroethanol Addition. Anal. Chem. 2017, 89, 10188–10193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Pawliszyn, J. Coated Blade Spray with a Barrier: Improving Negative Electrospray Ionization and Sample Preparation. Anal. Chem. 2022, 94, 15879–15886. [Google Scholar] [CrossRef]
- Straub, R.F.; Voyksner, R.D. Negative ion formation in electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 1993, 4, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Han, S.M.; Lee, H.-J.; Kang, M.; Jeong, T.Y.; Son, J.; Min, H.; Cha, S.; Oh, H.B.; Oh, W.K.; et al. Influence of mobile phase composition on the analytical sensitivity of LC–ESI–MS/MS for the concurrent analysis of bisphenols, parabens, chlorophenols, benzophenones, and alkylphenols. Environ. Res. 2023, 221, 115305. [Google Scholar] [CrossRef]
- Lee, K.M.; Han, S.M.; Lee, S.; Jeong, T.Y.; Kim, H.J.; Min, H.; Kim, K.H.; Cha, S.; Oh, W.K.; Lee, J. Fluoride-assisted liquid chromatography-tandem mass spectrometry method for simultaneous analysis of propofol and its metabolites without derivatization in urine. J. Chromatogr. A 2021, 1652, 462360. [Google Scholar] [CrossRef]
- McFadden, J.R.; Ames, D.M. Assessment of ammonium fluoride as a mobile phase additive for sensitivity gains in electrospray ionization. Anal. Sci. Adv. 2023, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Jin, J.; Wang, L.; Zhao, X.; Guo, C.; Sun, X.; Dhanjai; Lu, X.; Chen, J. Ammonium hydroxide enhancing electrospray response and boosting sensitivity of bisphenol A and its analogs. Talanta 2018, 182, 590–594. [Google Scholar] [CrossRef]
- Monnin, C.; Ramrup, P.; Daigle-Young, C.; Vuckovic, D. Improving negative liquid chromatography/electrospray ionization mass spectrometry lipidomic analysis of human plasma using acetic acid as a mobile-phase additive. Rapid Commun. Mass Spectrom. 2018, 32, 201–211. [Google Scholar] [CrossRef]
- Kamel, A.M.; Brown, P.R.; Munson, B. Effects of Mobile-Phase Additives, Solution pH, Ionization Constant, and Analyte Concentration on the Sensitivities and Electrospray Ionization Mass Spectra of Nucleoside Antiviral Agents. Anal. Chem. 1999, 71, 5481–5492. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Tipple, C.A.; Yost, R.A. Application of paper spray ionization for explosives analysis. Rapid Commun. Mass Spectrom. 2017, 31, 1565–1572. [Google Scholar] [CrossRef]
- Zhu, J.; Cole, R.B. Formation and decompositions of chloride adduct ions, [M + Cl]−, in negative ion electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2000, 11, 932–941. [Google Scholar] [CrossRef]
- Li, C.; Attanayake, K.; Valentine, S.J.; Li, P. Facile Improvement of Negative Ion Mode Electrospray Ionization Using Capillary Vibrating Sharp-Edge Spray Ionization. Anal. Chem. 2020, 92, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Myridakis, A.; Balaska, E.; Gkaitatzi, C.; Kouvarakis, A.; Stephanou, E.G. Determination and separation of bisphenol A, phthalate metabolites and structural isomers of parabens in human urine with conventional high-pressure liquid chromatography combined with electrospray ionisation tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2509–2518. [Google Scholar] [CrossRef]
- Preindl, K.; Braun, D.; Aichinger, G.; Sieri, S.; Fang, M.; Marko, D.; Warth, B. A Generic Liquid Chromatography−Tandem Mass Spectrometry Exposome Method for the Determination of Xenoestrogens in Biological Matrices. Anal. Chem. 2019, 91, 11334–11342. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, P.; Xu, B.; Zhao, R.; Qiao, S.; Chen, X.; Tang, R.; Wu, D.; Song, L.; Wang, S.; et al. Determination of Nine Environmental Phenols in Urine by Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Anal. Toxicol. 2012, 36, 608–615. [Google Scholar] [CrossRef]
- Feng, Y.-L.; Liao, X.; Grenier, G.; Nguyen, N.; Chan, P. Determination of 18 phthalate metabolites in human urine using a liquid chromatography-tandem mass spectrometer equipped with a core–shell column for rapid separation. Anal. Methods 2015, 7, 8048–8059. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.M.; Han, S.M.; Lee, H.-J.; Sung, C.; Min, H.; Im, H.; Han, S.B.; Cha, S.; Lee, J. Comprehensive LC-MS/MS method combined with tandem hybrid hydrolysis for multiple exposure assessment of multiclass environmental pollutants. Environ. Res. 2022, 211, 113053. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liu, Z.; Zhang, L.; Xi, C.; Li, X.; Wang, G.; Yuan, R.; Mu, Z. Development of an HPLC–MS/MS method for the simultaneous analysis of six kinds of parabens in food. Anal. Methods 2013, 5, 1016–1023. [Google Scholar] [CrossRef]
- Bartella, L.; Di Donna, L.; Napoli, A.; Sindona, G.; Mazzotti, F. Paper spray tandem mass spectrometry: A rapid approach for the assay of parabens in cosmetics and drugs. J. Mass Spectrom. 2020, 55, e4526. [Google Scholar] [CrossRef]
- Ma, Q.; Bai, H.; Li, W.; Wang, C.; Li, X.; Cooks, R.G.; Ouyang, Z. Direct identification of prohibited substances in cosmetics and foodstuffs using ambient ionization on a miniature mass spectrometry system. Anal. Chim. Acta 2016, 912, 65–73. [Google Scholar] [CrossRef]
- Bernardo, R.A.; Sousa, J.C.P.; Gallimberti, M.; Junior, F.B.; Vaz, B.G.; Chaves, A.R. A fast and direct determination of bisphenol S in thermal paper samples using paper spray ionization mass spectrometry. Environ. Sci. Pollut. Res. 2021, 28, 57288–57296. [Google Scholar] [CrossRef]
- de Almeida Soares, D.; Pereira, I.; Sousa, J.C.P.; Bernardo, R.A.; Simas, R.C.; Vaz, B.G.; Chaves, A.R. Bisphenol determination in UHT milk and packaging by paper spray ionization mass spectrometry. Food Chem. 2023, 400, 134014. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Silva, M.J.; Kato, K.; Reidy, J.A.; Malek, N.A.; Hurtz, D.; Sadowski, M.; Needham, L.L.; Calafat, A.M. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ. Health Perspect. 2003, 111, 1148–1151. [Google Scholar] [CrossRef]
- Koch, H.M.; Calafat, A.M. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2063–2078. [Google Scholar] [CrossRef]
- Sangeetha, S.; Vimalkumar, K.; Loganathan, B.G. Environmental Contamination and Human Exposure to Select Endocrine-Disrupting Chemicals: A Review. Sustain. Chem. 2021, 2, 343–380. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Chen, Z.; Kim, S.; Sapra, K.J.; Bae, J.; Kannan, K. Urinary concentrations of benzophenone-type ultraviolet light filters and semen quality. Fertil. Steril. 2015, 104, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.; Hong, Y.C. Household insecticide use and urinary 3-phenoxybenzoic acid levels in an elder population: A repeated measures data. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Hryhorczuk, D.O.; Moomey, M.; Burton, A.; Runkle, K.; Chen, E.; Saxer, T.; Slightom, J.; Dimos, J.; McCann, K.; Barr, D. Urinary p-nitrophenol as a biomarker of household exposure to methyl parathion. Environ. Health Perspect. 2002, 110 (Suppl. S6), 1041–1046. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 1004/2014 of 18 September 2014 Amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products; Official Journal of the European Union: Brussels, Belgium, 2014.
- Ramutshatsha-Makhwedzha, D.; Munonde, T.S. Review of the Integrated Approaches for Monitoring and Treating Parabens in Water Matrices. Molecules 2024, 29, 5533. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pozo, L.; del Carmen Gómez-Regalado, M.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: A review. Talanta 2021, 234, 122642. [Google Scholar] [CrossRef]
- Frederiksen, H.; Jørgensen, N.; Andersson, A.-M. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography–tandem mass spectrometry (LC–MS/MS). J. Expo. Sci. Environ. Epidemiol. 2011, 21, 262–271. [Google Scholar] [CrossRef]
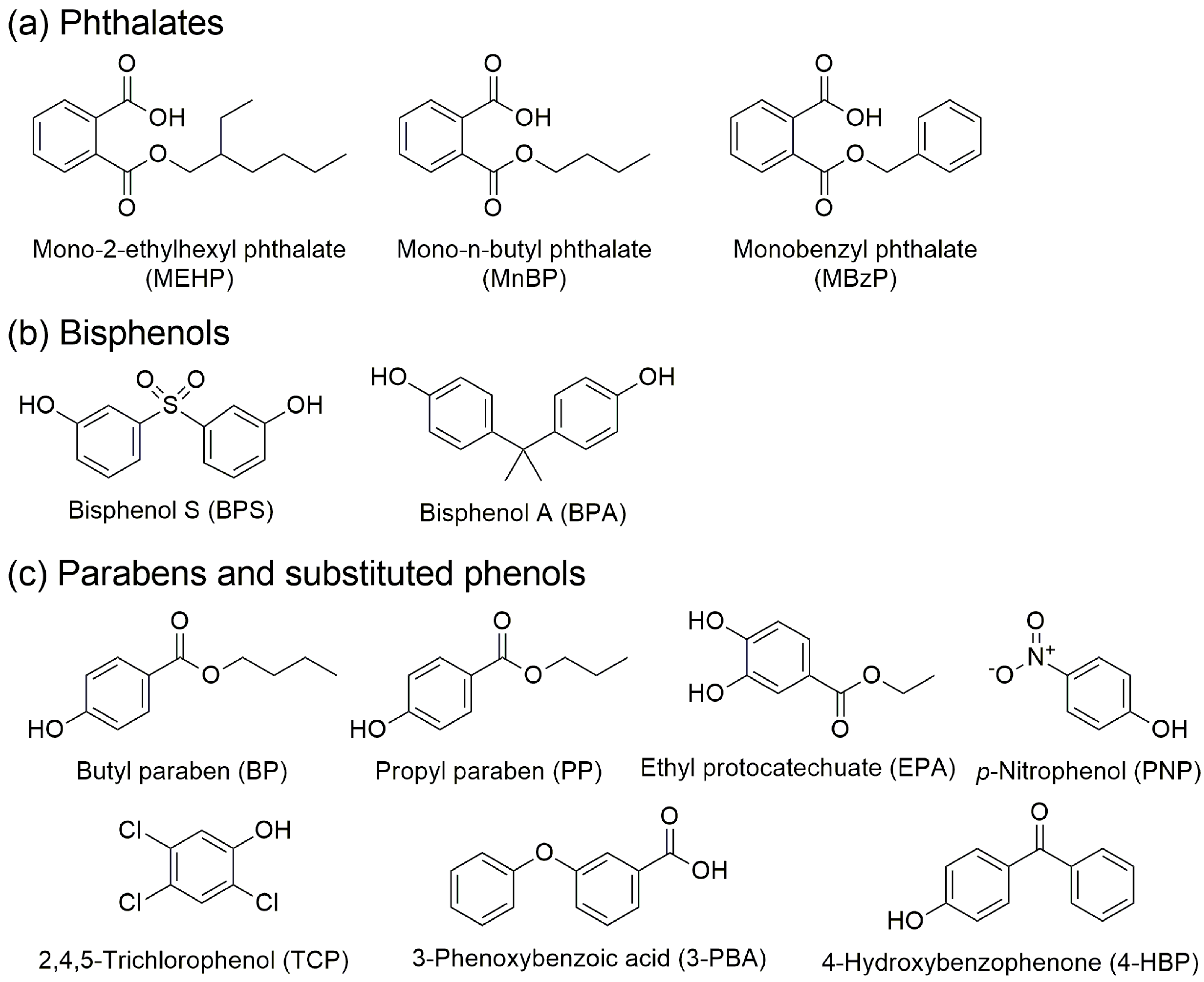
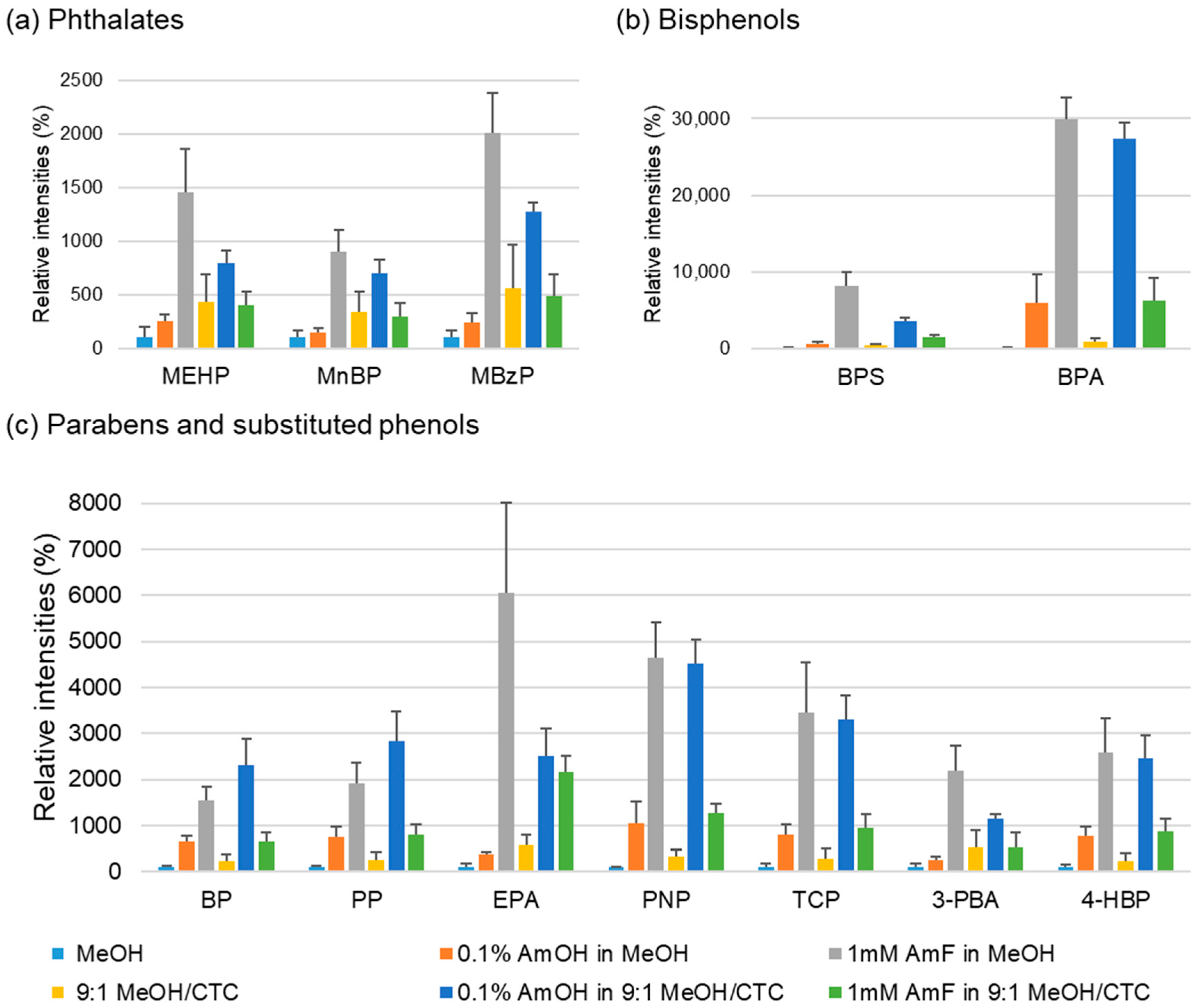
| Analytes | Spraying Solvents | |||||
|---|---|---|---|---|---|---|
| 1 mM AmF in MeOH | 0.1% AmOH in 9:1 MeOH/CTC | |||||
| Range (ppb) | R2 | LOQ (ppb) * | Range (ppb) | R2 | LOQ (ppb) * | |
| MEHP | 20–1000 | 0.9976 | 10.9 | 20–1000 | 0.9988 | 10.5 |
| MnBP | 20–1000 | 0.9980 | 14.7 | 20–1000 | 0.9986 | 11.3 |
| MBzP | 20–1000 | 0.9902 | 21.0 | 20–1000 | 0.9860 | 18.6 |
| BPS | 20–1000 | 0.9778 | 10.9 | 20–1000 | 0.9908 | 18.8 |
| BPA | 20–1000 | 0.9756 | 18.7 | 20–1000 | 0.9876 | 20.0 |
| BP | 10–1000 | 0.9997 | 6.6 | 10–1000 | 0.9975 | 6.7 |
| PP | 10–1000 | 0.9985 | 7.4 | 10–1000 | 0.9978 | 8.1 |
| EPA | 10–1000 | 0.9983 | 4.1 | 10–1000 | 0.9818 | 7.2 |
| PNP | 10–1000 | 0.9927 | 8.1 | 10–1000 | 0.9924 | 6.3 |
| TCP | 10–1000 | 0.9980 | 7.6 | 10–1000 | 0.9980 | 9.3 |
| 3-PBA | 10–1000 | 0.9638 | 4.0 | 20–1000 | 0.9976 | 16.3 |
| 4-HBP | 10–1000 | 0.9983 | 6.9 | 10–1000 | 0.9996 | 7.6 |
| Analytes | LOQ in Solution (ppb) 1 | LOQ in Sample (mg/kg) 2 | ||||
|---|---|---|---|---|---|---|
| PSI-MS/MS (AmF) | PSI-MS/MS (AmOH/CTC) | LC-MS/MS | PSI-MS/MS (AmF) | PSI-MS/MS (AmOH/CTC) | LC-MS/MS | |
| MP | 12.6 | 8.4 | 5.8 | 0.7 | 0.4 | 0.3 |
| EP | 8.0 | 5.8 | 11.7 | 0.4 | 0.3 | 0.6 |
| PP | 6.8 | 4.3 | 5.0 | 0.4 | 0.2 | 0.3 |
| BP | 11.4 | 5.8 | 8.5 | 0.6 | 0.3 | 0.4 |
| Analytes | Accuracy (%) | Intra-/Inter-day Precision (%RSD) | ||||
|---|---|---|---|---|---|---|
| PSI-MS/MS (AmF) | PSI-MS/MS (AmOH/CTC) | LC-MS/MS | PSI-MS/MS (AmF) | PSI-MS/MS (AmOH/CTC) | LC-MS/MS 1 | |
| MP | 93 | 100 | 77 | 4.2/6.0 | 3.7/5.1 | 4.0/- |
| EP | 109 | 110 | 90 | 2.5/5.4 | 2.8/4.4 | 7.0/- |
| PP | 109 | 106 | 110 | 3.4/3.9 | 2.6/4.2 | 7.0/- |
| BP | 104 | 102 | 94 | 4.0/6.6 | 1.1/3.7 | 5.9/- |
| Analytes and IS | Precursor Ion | Quantifying Ion | CE (eV) | Qualifying Ion | CE (eV) |
|---|---|---|---|---|---|
| MP | 151 | 92 | 26 | 136 | 17 |
| EP | 165 | 92 | 24 | 136 | 16 |
| BP | 193 | 92 | 25 | 136 | 18 |
| PP | 179 | 92 | 23 | 136 | 16 |
| EPA | 181 | 108 | 21 | 153 | 29 |
| PNP | 138 | 108 | 22 | 137 | 25 |
| TCP | 195 | 159 | 20 | 123 | 28 |
| 3-PBA | 213 | 93 | 23 | 169 | 13 |
| 4-HBP | 197 | 92 | 36 | 161 | 24 |
| MEHP | 277 | 134 | 18 | 127 | 18 |
| MnBP | 221 | 177 | 11 | 77 | 18 |
| MBzP | 255 | 77 | 21 | 183 | 13 |
| BPS | 249 | 108 | 29 | 92 | 42 |
| BPA | 227 | 212 | 18 | 133 | 33 |
| BP-d4 | 197 | 96 | 26 | ||
| MEHP-d4 | 281 | 138 | 19 | ||
| BPA-d16 | 241 | 223 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Amatya, S.S.; Bahng, H.; Lee, E.; Ko, Y.; Cha, S. Negative Paper Spray Ionization Mass Spectrometry for the Determination of Endocrine-Disrupting Chemicals with Application to Paraben Analysis in Cosmetics. Molecules 2025, 30, 4356. https://doi.org/10.3390/molecules30224356
Cho S, Amatya SS, Bahng H, Lee E, Ko Y, Cha S. Negative Paper Spray Ionization Mass Spectrometry for the Determination of Endocrine-Disrupting Chemicals with Application to Paraben Analysis in Cosmetics. Molecules. 2025; 30(22):4356. https://doi.org/10.3390/molecules30224356
Chicago/Turabian StyleCho, Seonyoung, Sarmila Shrestha Amatya, Hyerin Bahng, Eungyeong Lee, Yunsang Ko, and Sangwon Cha. 2025. "Negative Paper Spray Ionization Mass Spectrometry for the Determination of Endocrine-Disrupting Chemicals with Application to Paraben Analysis in Cosmetics" Molecules 30, no. 22: 4356. https://doi.org/10.3390/molecules30224356
APA StyleCho, S., Amatya, S. S., Bahng, H., Lee, E., Ko, Y., & Cha, S. (2025). Negative Paper Spray Ionization Mass Spectrometry for the Determination of Endocrine-Disrupting Chemicals with Application to Paraben Analysis in Cosmetics. Molecules, 30(22), 4356. https://doi.org/10.3390/molecules30224356