Abstract
NADESs represent a modern class of extraction media that align with the principles of green chemistry. They are considered non-toxic and biodegradable, but relatively little is known about their biological activity. This study investigated the antioxidant, antibacterial, and antifungal properties of 40 NADESs. The systems were developed from primary (PRIM) based on choline chloride (ChCl), and specialized (HEVO) plant-derived metabolites, particularly based on thymol and menthol. Their antioxidant activity was evaluated using spectrophotometric tests. The antimicrobial activity was evaluated by the disk diffusion method. The data obtained were analyzed using principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA). NADESs based on PRIM exhibited negligible antioxidant activity and relatively low antimicrobial activity. By contrast, NADESs containing HEVO, particularly thymol-based systems, indicated significant antioxidant activity, with stronger activity observed at higher molar proportions of thymol. In the 1,8-cineole:thymol system, ABTS activity ranged from 167.37 ± 24.17 to 861.25 ± 33.03 mg Trolox equivalents/mL NADES (molar ratios 9:1 and 1:9, respectively). The 1,8-cineole:thymol system (1:9) also showed strong antimicrobial activity, with a maximum inhibition zone of 39.33 ± 2.52 mm against Staphylococcus aureus. In summary, NADESs based on HEVO exhibit significantly stronger biological activity than those containing only PRIM.
1. Introduction
Natural deep eutectic solvents (NADESs) have become the subject of numerous studies in recent years, focusing on their application in the extraction of plant compounds, chemical synthesis and the formulation of pharmaceutical products. These solvents are compositions of natural substances, both biosynthetically primary metabolites (PRIM) such as quarternary amines, organic acids, amino acids, fatty acids, sugars, and polyols, and highly evolutionary metabolites (HEVO) such as alkaloids, flavonoids, monoterpenes, and phenols [1,2,3]. Common NADES combinations include choline chloride:glycerol, choline chloride:lactic acid, thymol:menthol, and camphor:thymol (typically at a 1:1 molar ratio) [2,4].
These mixtures melt at a temperature significantly lower than their individual components, forming liquid systems at room temperature and often even much lower. It is assumed that the use of NADESs complies with the principles of green chemistry, as they are generally considered non-toxic, biodegradable, and biocompatible [5,6,7]. In addition, some NADESs possess desirable biological properties—such as antioxidant, antibacterial, and antifungal activities [8,9,10,11]—which represent significant advantages over conventional solvents. These properties make extracts obtained using NADESs more durable—by eliminating the risk of oxidation of the extracted compounds and enhancing microbiological stability. Moreover, they exhibit biological activity, resulting from the synergistic interaction between NADESs and the extracted compounds [8]. Furthermore, investigating the antimicrobial activity of NADESs seems particularly promising in the context of the increasing antibiotic resistance of pathogenic microorganisms [11].
Unfortunately, despite the well-known activities of many substances forming NADES systems, there are few reports on the activities of eutectic solvents designed on their basis. The unique supramolecular structure formed through hydrogen bonding between NADES components may exhibit distinct physicochemical and biological properties compared to the properties of the individual compounds [12]. An additional difficulty is the fact that the number of possible NADES combinations is almost unlimited, and extensive research is required to fully characterize their biological activities. This is particularly important for the pharmaceutical and food industries, because NADESs are increasingly used as drug solvents and extractants [1,8]. Several studies have demonstrated that NADES formulations significantly enhance the extraction efficiency of plant metabolites, such as alkaloids [7], lichen metabolites [3], triterpenes [13], anthocyanins [14], isoflavones [15,16], chlorogenic acid from various plant sources, and curcuminoids from Curcuma longa L., due to the higher solubility of these bioactive compounds in NADESs [4]. Furthermore, these solvents can be used in food cryoprotection [17], removal of heavy metals from food products [18,19], and as flavor enhancers [20] and food films [21]. This also prompts in-depth research into the biological activity of NADESs.
This study attempts a comprehensive assessment of the antioxidant and antimicrobial activity of 40 NADES compositions based on both PRIM and HEVO. Nineteen hydrophilic NADESs (11 PRIM-based and 8 HEVO-based compositions) and 21 lipophilic HEVO compositions forming volatile NADESs (VNADESs) were evaluated against both pathogenic fungi and bacteria (Gram-positive and Gram-negative). The aim of the study was to verify the hypothesis that NADES compositions containing HEVO have stronger antioxidant and antimicrobial properties than NADESs containing only PRIM, and that lipophilic NADESs exhibit more favorable biological properties than hydrophilic NADESs. Furthermore, we consider that the selection of NADESs with favorable biological activity will enable the rational design of extraction media, ensuring not only efficient extraction but also the stability of the obtained extracts, and additive pharmacological effects when crude NADES-based extracts are applied.
2. Results and Discussion
Liquids that are stable at room temperature were obtained by mixing the NADES components and heating them in a water bath at 60 °C for 30 min. After one week, only the NADESs based on choline chloride (ChCl) and arabinose had crystallized. The composition of NADESs tested is presented in Table 1.

Table 1.
Qualitative and quantitative composition of the obtained NADESs.
2.1. Antioxidant Activity of Testing NADESs
Screening tests for antioxidant activity revealed that HEVO-based NADESs exhibited significant activity, whereas PRIM-based liquids showed low activity (except for liquids containing malic and citric acids in the DPPH test) (see Figure S1). Spectrophotometric assay (DPPH, ABTS, FRAP, and CUPRAC) also revealed that the NADESs developed using HEVO exhibited significantly higher antioxidant activity than those based solely on PRIM (see Figure 1 and Table S1). The tested HEVO-based liquids, both hydrophilic and lipophilic, exhibited strong antioxidant activity against the ABTS radical and the ability to reduce Cu2+ ions to Cu+ (CUPRAC test). However, hydrophilic NADESs containing flavonoids and phenolic acids exhibited significantly higher antioxidant activity against the DPPH radical and the ability to reduce Fe3+ to Fe2+ (FRAP test), whereas lipophilic liquids (VNADESs) exhibited significantly lower activity in these tests (Figure 1). It is widely believed that the ABTS test is more sensitive than the DPPH test, and can be used to assess the antioxidant properties of both hydrophilic and lipophilic compounds [22]. Therefore, it appears to be suitable for a comprehensive assessment of the antioxidant properties of NADESs of varying polarity and composition. On the other hand, the CUPRAC test, performed in a neutral or slightly alkaline environment, is appropriate for assessing the activity of phenols (thymol), whose activity in the acidic environment of the FRAP test may be limited. Thus, as a rule, higher values are obtained for the same plant substances in the ABTS test compared to DPPH [23,24], and very often in the CUPRAC test compared to FRAP [25,26].
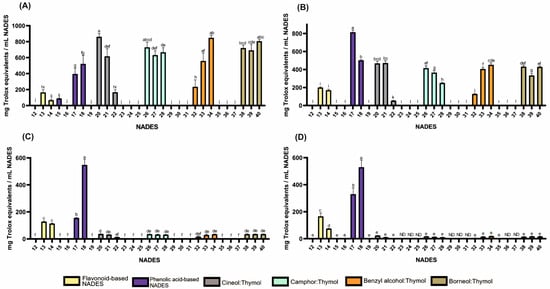
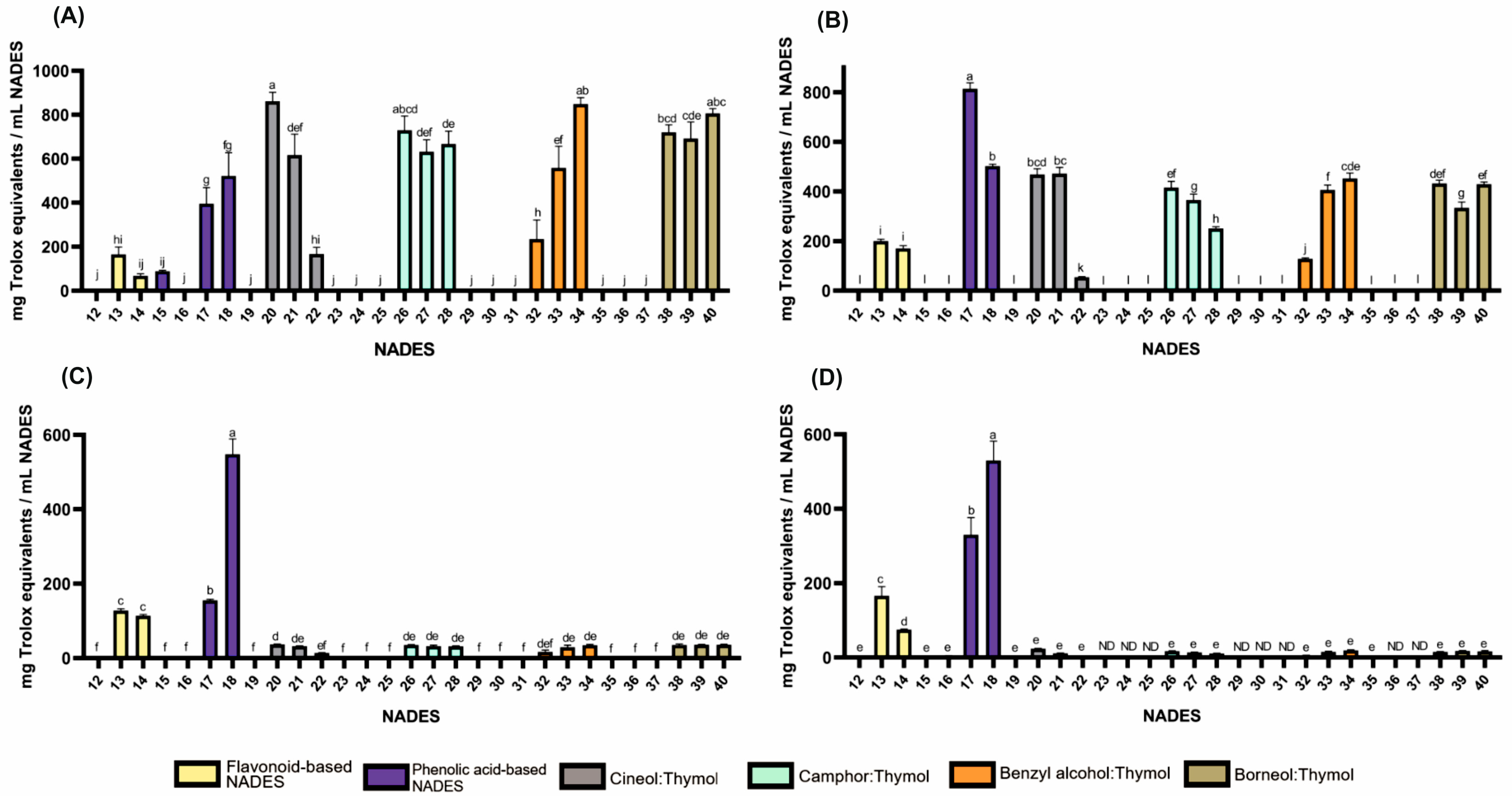
Figure 1.
Antioxidant activities of HEVO-based NADESs (12–40) determined by assays: (A)—ABTS, (B)—CUPRAC, (C)—DPPH, and (D)—FRAP. NADES numbering is in accordance with Table 1. Columns marked with different lowercase letters represent significant differences based on Welch’s ANOVA followed by the Games–Howell post hoc test (p < 0.05). Data are presented as means (n = 3) ± standard deviation (SD). ND—not detected.
As shown in Figure 1 and Table S1, antioxidant activity was observed in formulations containing flavonoids such as quercetin (165, 127, 200 and 166 mg Trolox equivalents per mL NADES (mg TE) for ABTS, DPPH, CUPRAC, and FRAP tests, respectively) and rutin (68, 114, 171 and 75 mg TE for ABTS, DPPH, CUPRAC, and FRAP tests, respectively) while the activity of the formulation containing naringin was negligible. Among formulations containing phenolic acids, those based on protocatechuic (395, 155, 813 and 330 mg TE for ABTS, DPPH, CUPRAC, and FRAP tests, respectively) and gentisic acids (522, 548, 502 and 530 mg ETx for ABTS, DPPH, CUPRAC, and FRAP tests, respectively) exhibited the strongest antioxidant properties. In contrast, compositions containing resorcylic and p-hydroxybenzoic acids generally exhibited no such activity, with the exception of α-resorcylic acid in the ABTS test.
Lipophilic NADESs exhibited significant antioxidant activity only in formulations containing thymol, with stronger activity observed at higher molar proportions of thymol. For example, for the 1,8-cineole:thymol system, the activity in the ABTS test ranged from 167 to 861 mg TE (for molar ratios of 9:1 and 1:9, respectively), while for the benzyl alcohol:thymol system, it ranged from 234 to 848 mg TE (for molar ratios of 9:1 and 4:6, respectively). The observed effect can be explained by the high antioxidant activity of thymol, which has been reported in numerous studies [27,28,29,30,31].
It should be noted that the volume of the reaction mixture in spectrophotometric tests was disproportionately large compared to the volume of NADESs added. Furthermore, some NADESs with significant activity had to be prediluted (e.g., NADESs containing gentisic acid). Thus, the mixtures subjected to spectrophotometric measurements constituted only solutions of NADES components, lacking the supermolecular structure that defines NADESs. This should be considered as a significant limitation of this study. However, preliminary bioautography tests, which involved using undiluted NADESs and evaporating free radical solvents by drying the chromatography plates, suggest that NADESs exhibit strong antioxidant activity even when their supermolecular structure is intact.
2.2. Antimicrobial Activity of Testing NADESs
To evaluate the antimicrobial and antifungal activity of different NADES formulations, we compared the broad spectrums of lipophilic and hydrophilic systems. Our findings highlighted a distinct advantage of lipophilic NADESs (HEVO-based) compared to hydrophilic ones. The superior performance of thymol-rich systems—exceeding even vancomycin (30 μg) in terms of the zone of inhibition (ZOI) against Gram-positive bacteria—highlights thymol’s potential as a natural antimicrobial agent when incorporated into NADES matrices (Figure 2; Table S2). Numerous studies have demonstrated the efficacy of pure thymol as an antimicrobial agent against Gram-positive bacteria (S. aureus, Staphylococcus epidermidis, Enterococcus faecalis, Bacillus cereus), Gram-negative bacteria (Escherichia coli, Salmonella Typhimurium, Pseudomonas aeruginosa), and yeast (Candida albicans) [32,33,34].
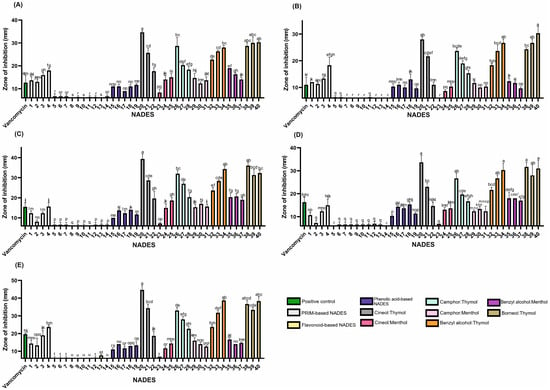
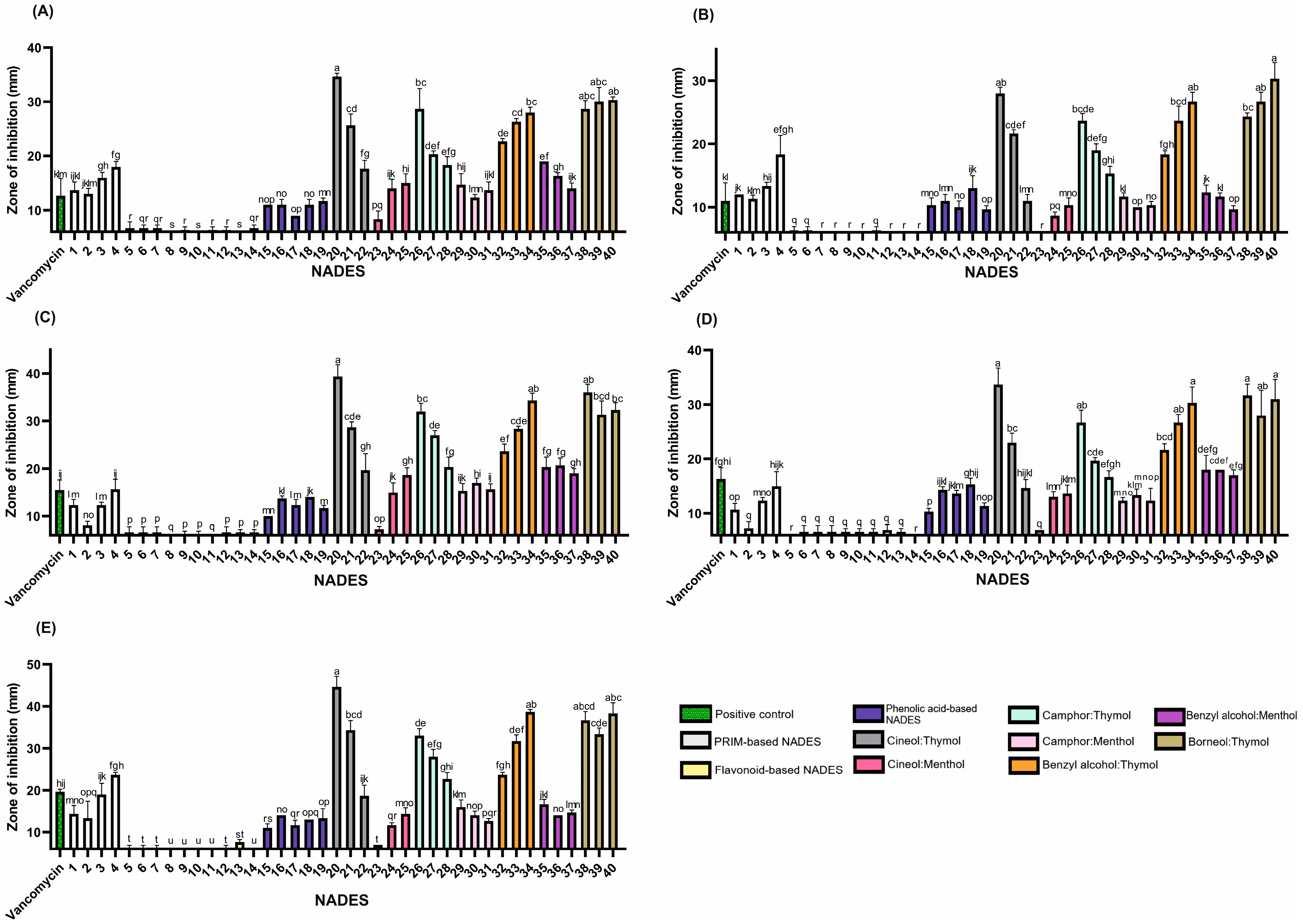
Figure 2.
The antibacterial activity of the tested NADESs against selected Gram-positive strains: (A)—B. cereus, (B)—E. faecalis, (C)—MRSA, (D)—MSSA, and (E)—S. epidermidis. NADES numbering is in accordance with Table 1. Columns marked with different lowercase letters represent significant differences based on Kruskal–Wallis ANOVA followed by the Conover–Inman post hoc test (p < 0.05). Data are presented as means (n = 3) ± standard deviation (SD).
Thymol’s antibacterial mechanisms are multifaceted, involving both membrane-level and intracellular actions. Its lipophilicity enables interaction with bacterial lipid membranes, disrupting membrane pumps and key enzymes. Furthermore, thymol can bind to large intracellular macromolecules, such as DNA, causing structural damage [32].
Thymol-based NADESs exhibited the most potent antimicrobial activity against all tested strains (Figure 2), except P. aeruginosa, which showed the highest sensitivity to the benzyl alcohol:menthol system. However, among all strains evaluated, P. aeruginosa was the least sensitive overall. In contrast, Gram-positive species were markedly more susceptible to thymol, emphasizing the influence of bacterial cell wall structure on antimicrobial sensitivity.
Lipophilic NADES systems demonstrated strong inhibitory effects against both methicillin-resistant S. aureus (MRSA) and methicillin-sensitive S. aureus strains (MSSA) (Figure 2; Table S2). Several mixtures nearly doubled the ZOI compared to the positive control (vancomycin, 30 µg). Effective combinations for MSSA included 1,8-cineole:thymol (1:9), benzyl alcohol:thymol (4:6), and borneol:thymol (1:2 and 3:7). For MRSA, camphor:thymol (3:7) and borneol:thymol (2:3) also produced enhanced activity, suggesting a heightened sensitivity of MRSA to these NADES formulations. These findings indicate the potential of such systems to combat antibiotic-resistant strains effectively.
The anti-MRSA activity of thymol was previously described by Yuan et al. [35], who demonstrated that co-treatment with thymol and vancomycin was more effective in eliminating MRSA biofilms in a mouse infection model than vancomycin monotherapy. The study showed that thymol inhibited biofilm formation and disrupted mature biofilms by reducing the production of polysaccharide intracellular adhesin and suppressing the release of extracellular DNA [35].
Interestingly, while thymol-based mixtures generally showed increased antimicrobial activity with higher thymol content, camphor:thymol NADESs displayed an inverse trend, suggesting complex interactions between components that influence overall efficacy. These findings contrast with studies on individual compounds, such as (±)-camphor and thymol, which reported minimum inhibitory concentrations (MICs) of 0.25 mg/mL for camphor and 0.007 mg/mL for thymol against B. cereus [34]. These values indicate that higher thymol content should enhance anti-B. cereus activity, which contrasts with the trend observed in our camphor:thymol systems.
This deviation suggests that the antimicrobial activity of thymol-based NADESs results from synergistic, additive, or antagonistic interactions between NADES components. Oxygenated terpenes such as camphor and 1,8-cineole have been shown to enhance the efficacy of phenolic compounds like eugenol in essential oil systems [36]. This suggests that thymol-based combinations may rely on a similar underlying mechanism.
In Gram-negative S. Typhimurium, thymol and benzyl alcohol mixtures exhibited similar inhibitory effects regardless of molar ratio, suggesting comparable antimicrobial potency of these two components against this pathogen (Figure 3; Table S2).
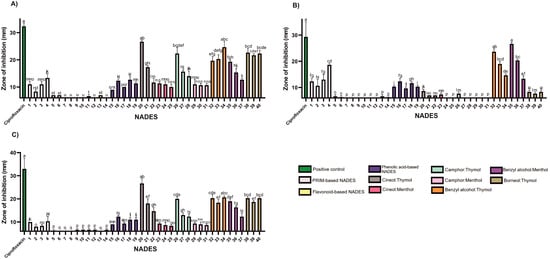
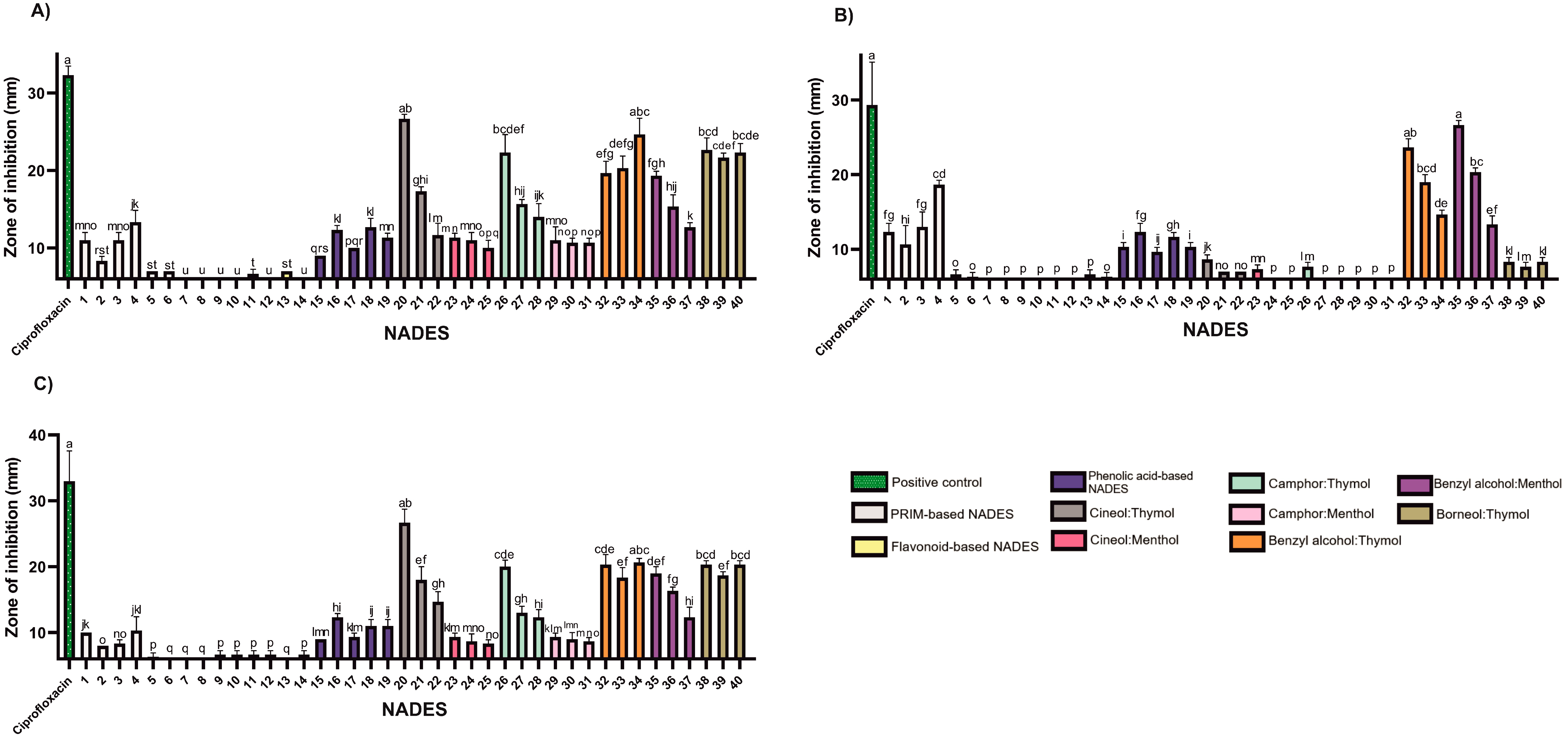
Figure 3.
The antibacterial activity of the tested NADESs against selected Gram-negative strains: (A)—E. coli, (B)—P. aeruginosa, (C)—S. Typhimurium. NADES numbering is in accordance with Table 1. Columns marked with different lowercase letters represent significant differences based on Kruskal–Wallis ANOVA followed by the Conover–Inman post hoc test (p < 0.05). Data are presented as means (n = 3) ± standard deviation (SD).
Certain findings from this study suggest that the antimicrobial effect may be attributed solely to thymol, such as in borneol-based systems against E. faecalis and P. aeruginosa. This assumption is based on previous studies reporting no antimicrobial activity of pure borneol against these strains [37]. In another study, S. Typhimurium showed susceptibility to (+)-borneol only at a very high MIC (800 mg/mL), while thymol demonstrated an MIC of 0.003 mg/mL, further supporting the prediction that the observed inhibition is solely based on the presence of thymol alone [34].
Previous research suggests that the reduced or absent activity of menthol may be related to its lack of a phenolic ring, which plays a role in electron destabilization and membrane disruption [38]. In menthol-based NADES formulations, increasing menthol content in 1,8-cineole:menthol systems correlated with increased antimicrobial activity. In contrast, camphor:menthol mixtures showed a decrease in activity with increasing menthol content. Based on trends observed in binary systems against S. epidermidis, camphor demonstrated the highest activity, followed by menthol and 1,8-cineole.
The tested NADES formulations exhibited limited activity against Gram-negative bacteria (Figure 3; Table S2), with inhibition zones smaller than those produced by the positive control, ciprofloxacin (5 μg). The outer membrane of Gram-negative bacteria, rich in lipopolysaccharides, provides substantial protection against hydrophobic agents [39], likely explaining their lower sensitivity to lipophilic NADESs.
Nevertheless, lipophilic NADESs (HEVO-based) consistently exhibited larger zones of inhibition than their hydrophilic counterparts (PRIM- and HEVO-based), suggesting greater efficacy. Benzyl alcohol emerged as particularly effective against P. aeruginosa. Mixtures such as benzyl alcohol:thymol and benzyl alcohol:menthol, especially at a 9:1 molar ratio, produced the strongest antimicrobial effects among NADESs tested—though still inferior to ciprofloxacin (Figure 3; Table S2).
Some researchers have demonstrated that the weak organic acid drug N-acetyl-L-cysteine (NAC) can effectively eradicate P. aeruginosa biofilms. They proposed that at pH < pKa, NAC is able to penetrate the matrix barrier, diffuse into bacterial cells, and achieve complete eradication of the biofilm [40].
While the mechanisms of our NADES formulations may differ, these findings highlight the potential role of acidity in overcoming P. aeruginosa resistance. In our study, acid-based hydrophilic NADESs (NADESs 1–4 and 15–19) showed notable antimicrobial activity against both Gram-positive and Gram-negative bacteria, including P. aeruginosa. This activity may stem from their acidic nature, which could disrupt bacterial enzymes or compromise membrane integrity, as also reported by Bedair and Samir [10].
In contrast, sugar-, polyalcohol-, and urea-based NADESs, (PRIM-based) and flavonoid-based NADESs consistently exhibited minimal or no antimicrobial activity, indicating their function primarily as inert solvents or stabilizers. This confirms that the antimicrobial effects of these formulations are primarily driven by the bioactivity of specific components, rather than by the NADES matrix as an inert system. Notably, dehydration effects often associated with carbohydrate-based NADESs were not observed in any tested pathogen, suggesting that this mechanism may not be relevant under current experimental conditions.
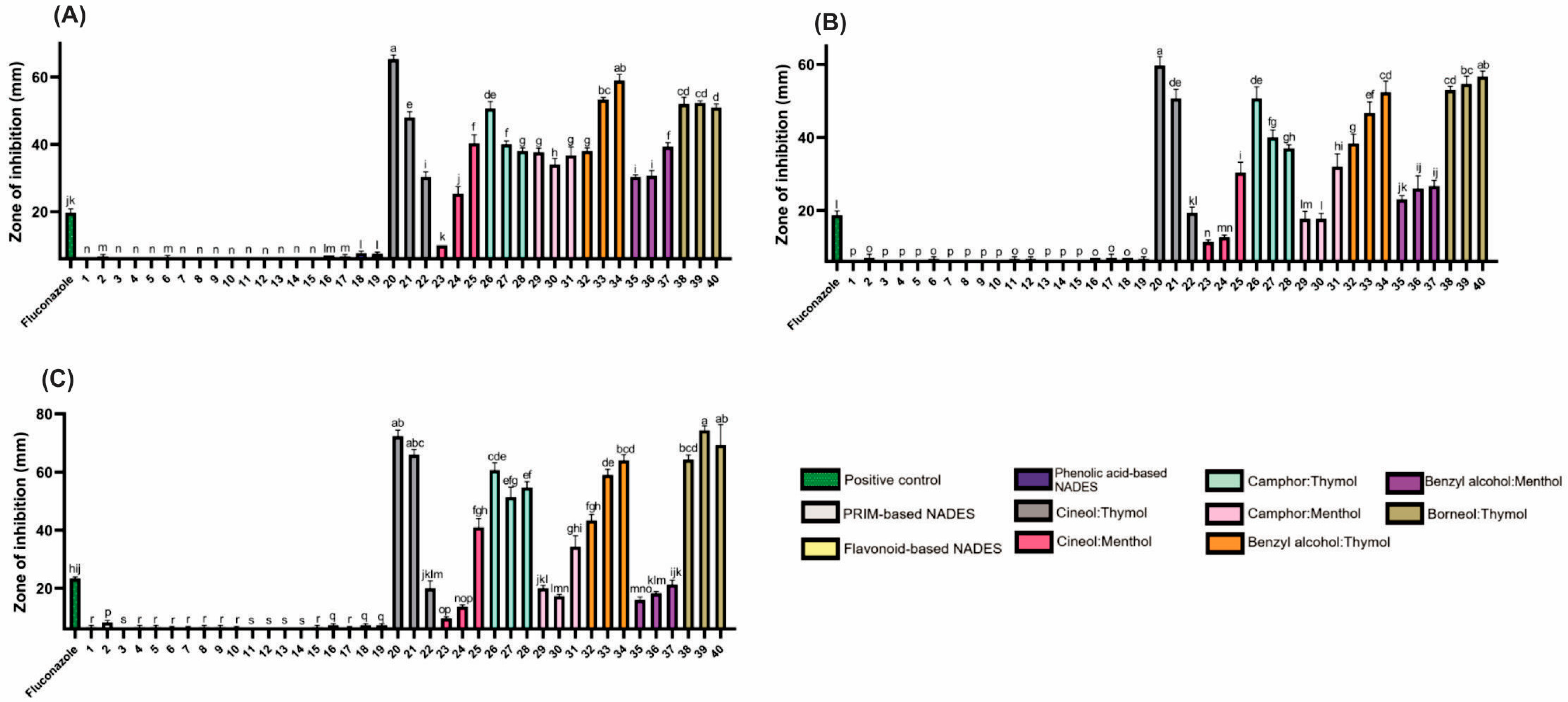
Hydrophilic NADESs (PRIM- and HEVO-based) exhibited negligible antifungal activity (Figure 4; Table S2). In contrast, lipophilic NADESs showed strong antifungal effects, suggesting potential as antifungal agents. Among all tested organisms, yeasts were the most sensitive, followed by Gram-positive, then Gram-negative bacteria. Thymol, similar to camphor, has demonstrated significant antifungal properties, particularly against Candida species [32].
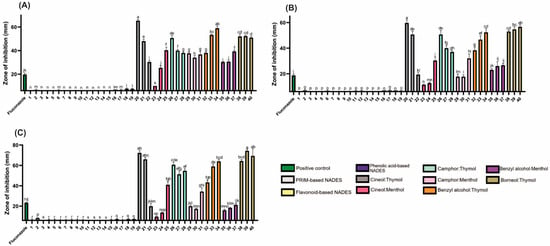
Figure 4.
The antifungal activity of the tested NADESs against selected Candida strains: (A)—C. albicans, (B)—C. glabrata, (C)—C. auris. NADES numbering is in accordance with Table 1. Columns marked with different lowercase letters represent significant differences based on Kruskal–Wallis ANOVA, followed by the Conover–Inman post hoc test (p < 0.05). Data are presented as means (n = 3) ± standard deviation (SD).
It is worth noting that the antibacterial and antifungal activity of the tested NADESs was inversely correlated with their antioxidant activity (Figure S2). As is well-known, there are compounds with both antioxidant and antimicrobial activity, and under certain conditions, positive correlations between their content and antibacterial and antifungal activity have been observed [41]. However, the negative effect of antioxidant activity on antibacterial and antifungal activity observed in this study may be the result of very high concentrations of NADESs in the microorganism growth environment.
2.3. Classification of NADESs Based on Antioxidant and Antimicrobial Profiles
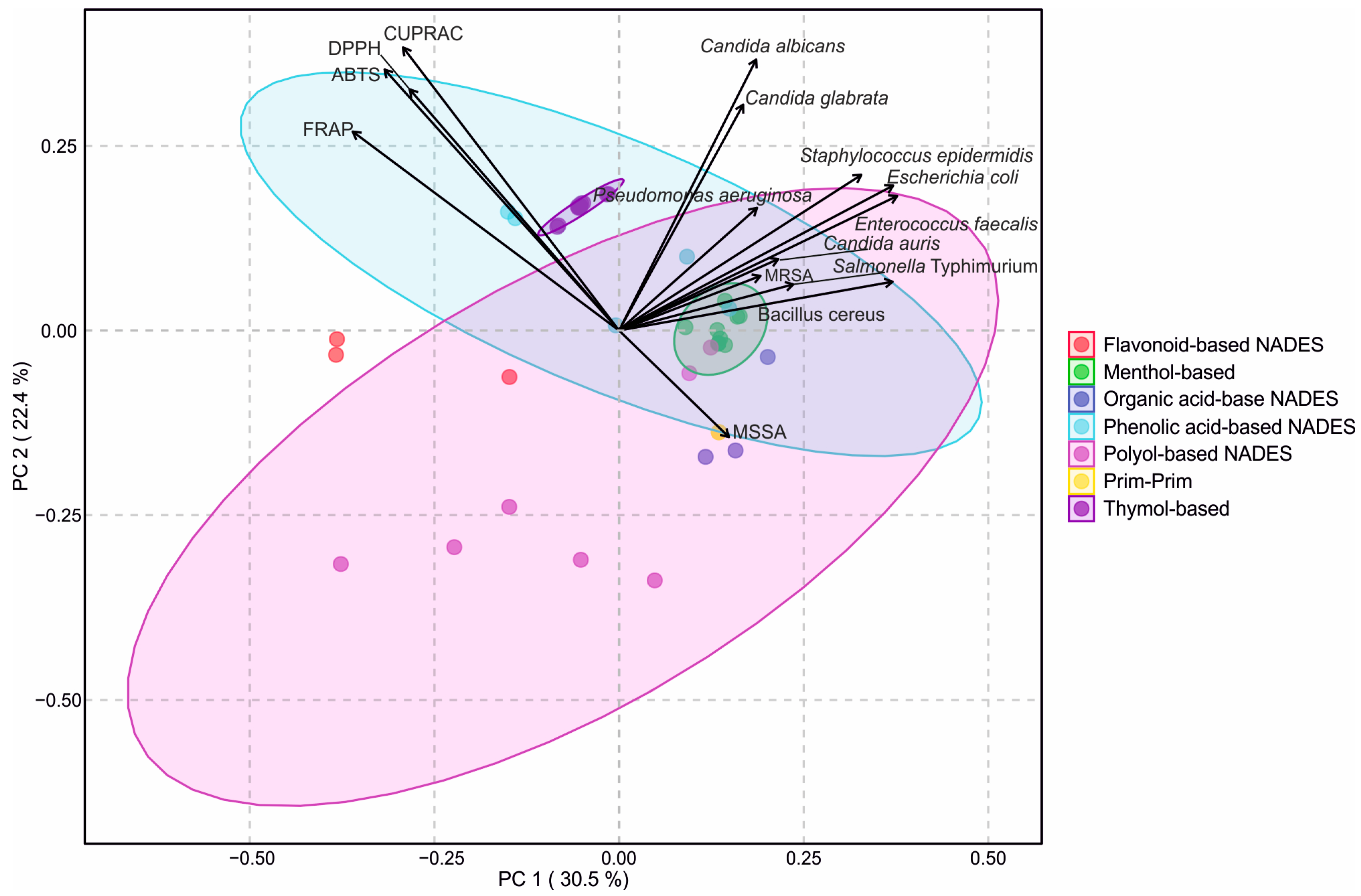
PCA was performed to visualize the relationships between antioxidant and antimicrobial activities of the tested NADESs (Figure 5). It was found that the first two principal components explained 47.1% of the total variance (PC1: 28.9%, PC2: 18.2%), allowing a clear two-dimensional separation of the solvent systems. The score plot revealed distinct clusters corresponding to the different groups of NADESs (flavonoid-based, menthol-based, organic acid-based, phenolic acid-based, polyol-based, PRIM–PRIM, and thymol-based). Colored ellipses representing 95% confidence intervals confirmed consistent grouping within each cluster.
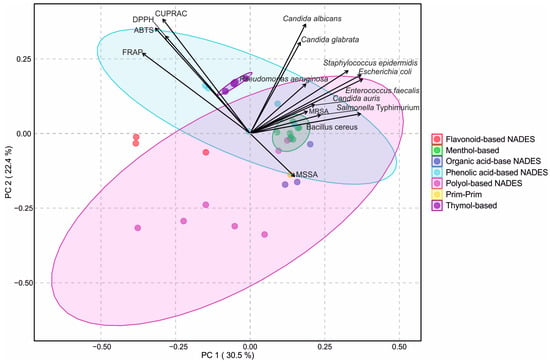
Figure 5.
Principal component analysis (PCA) biplot of antioxidant and antimicrobial activities of natural deep eutectic solvents (NADES). Seven different NADES groups are shown: flavonoid-based, menthol-based, organic acid-based, phenolic acid-based, polyol-based, prim–prim, and thymol-based. Vectors represent antioxidant assays (ABTS, CUPRAC, DPPH, FRAP) and microbial strains. Colored ellipses indicate 95% confidence intervals for sample clustering within each NADES group.
The antioxidant properties of the tested NADESs were strongly correlated with each other (Figure S2), and the loading vectors indicated that antioxidant assays were primarily negatively associated with PC1 and positively with PC2, which positioned phenolic acid- and flavonoid-based NADESs close to these variables, reflecting their strong antioxidant capacity. In contrast, antimicrobial activities were positively related to both PC1 and PC2, particularly the inhibitory responses against C. albicans and C. glabrata, which facilitated the separation of menthol- and thymol-based NADESs towards the antimicrobial space. Polyol- and organic acid-based NADESs were positioned more centrally, consistent with their moderate activity across both antioxidant and antimicrobial properties.
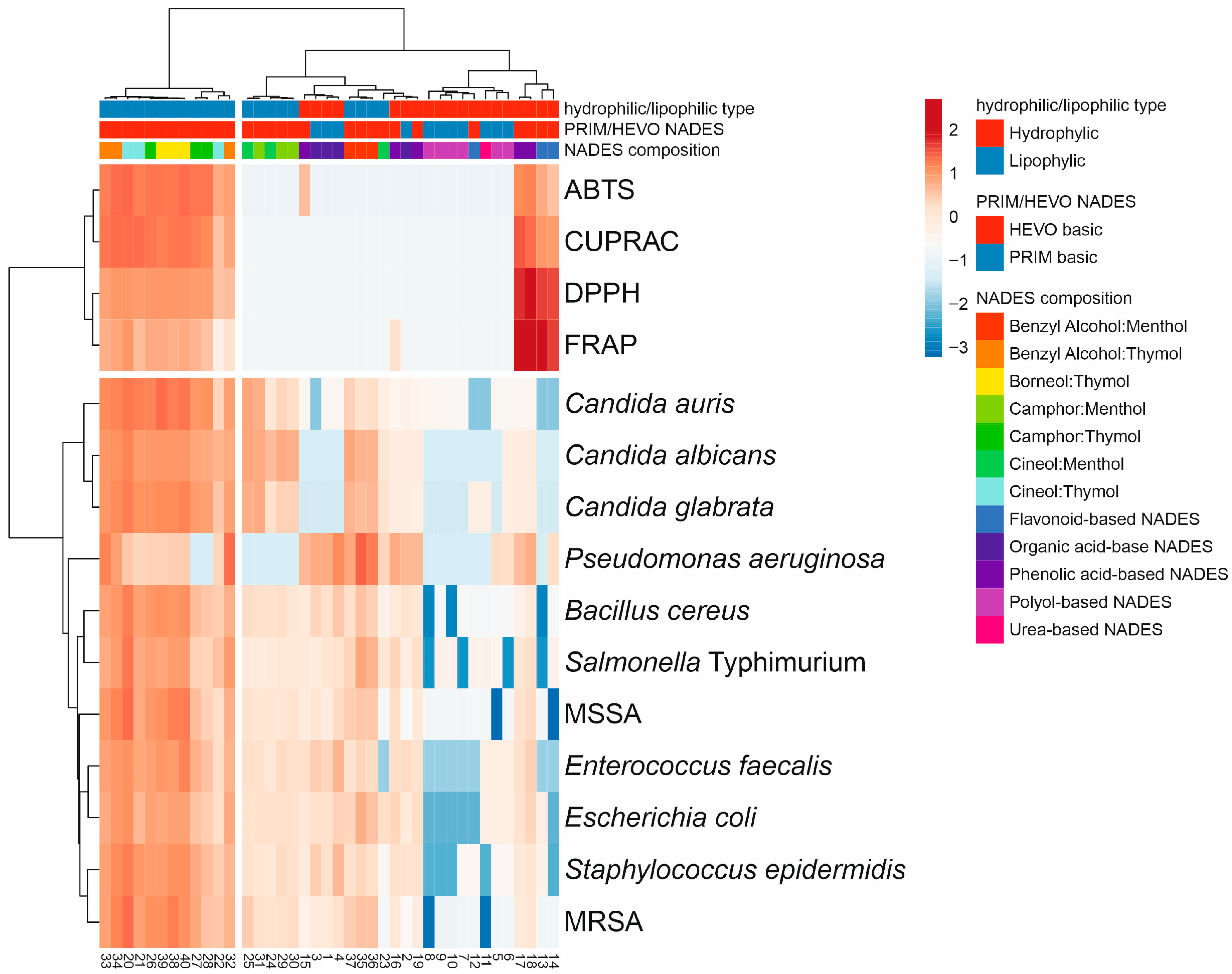
The hierarchical-clustering heatmap corroborated the PCA results and revealed clear grouping patterns (Figure 6). Figure 6 provides a comprehensive summary of the relationships between the type of NADESs and their mean antioxidant and antimicrobial activities, serving as an integrative overview of the biological performance of all systems. Comparison of standardized antioxidant and antimicrobial activities of the tested NADESs, according to their hydrophilic/lipophilic character and composition, showed that the first group of lipophilic NADESs, mainly HEVO-based and thymol-type, exhibited higher antimicrobial activity and generally stronger antioxidant capacity. In contrast, polyol- and organic acid-based NADESs were positioned in clusters with lower or moderate activity across both antioxidant and antimicrobial assays, together with some menthol-based NADESs. This group also contained phenolic- and flavonoid-based NADESs, which were characterized by relatively high antioxidant activity.
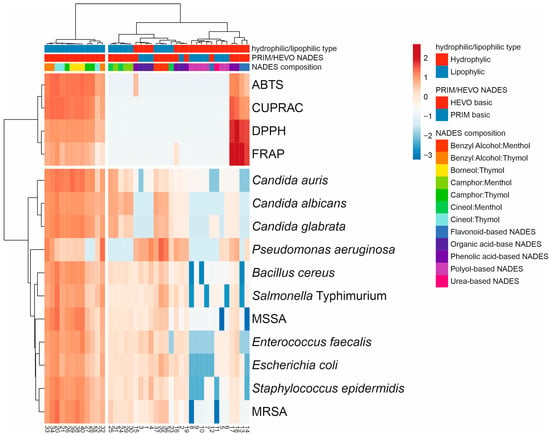
Figure 6.
Comprehensive summary of the relationships between the type of NADESs and their mean antioxidant and antimicrobial activities as a visualization of the heat map of NADES biological activity diversity (numbering according to Table 1). The colors range from dark blue (low activity) to dark red (high activity). The hierarchical cluster dendrograms were constructed based on lipophilicity, composition, and biological properties of the tested NADESs.
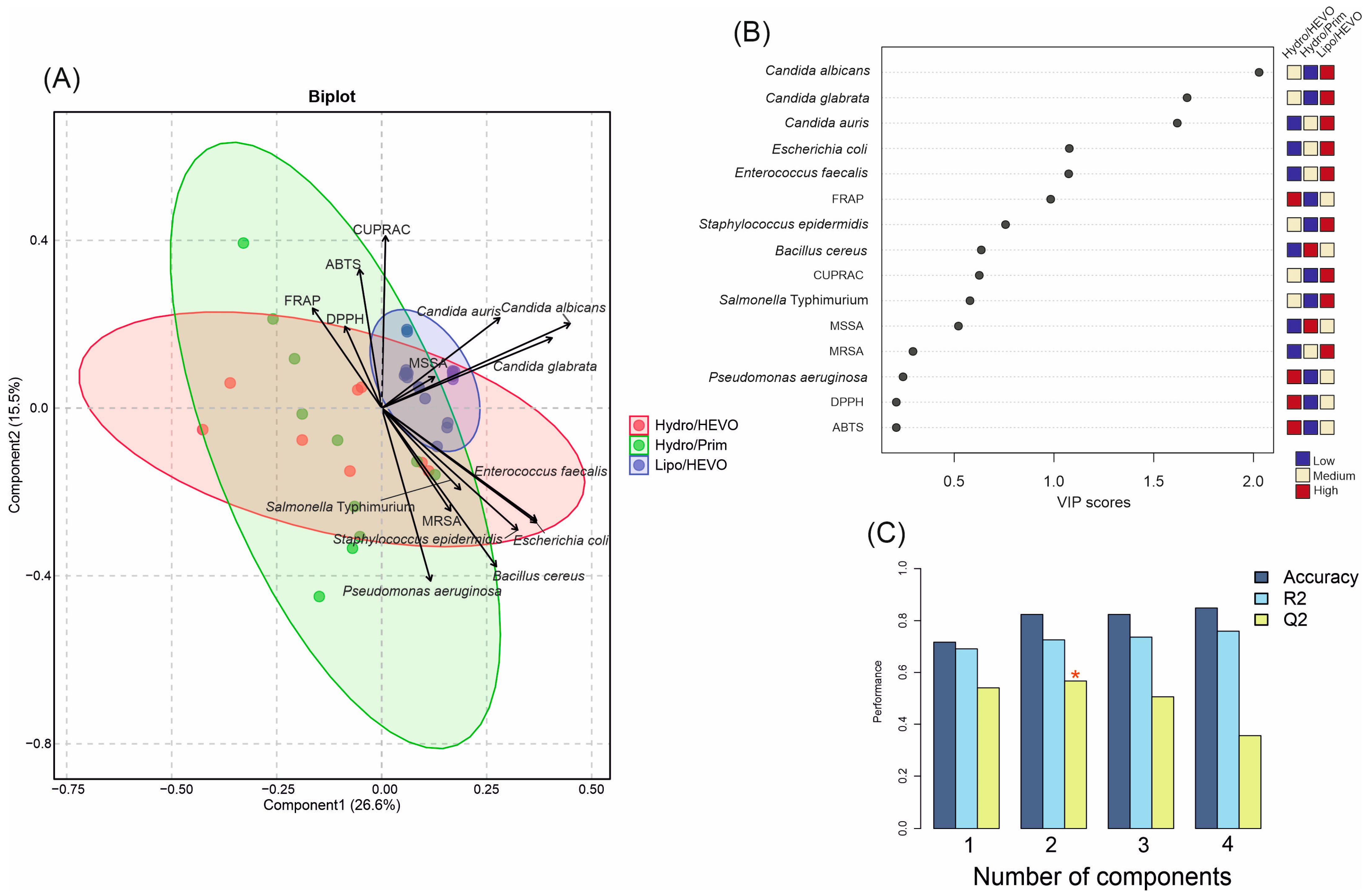
To further explore the discrimination power of the measured variables, a PLS-DA analysis was performed based on antioxidant and antimicrobial activities of the tested NADESs (Figure 7A). In contrast to the PCA, where seven groups were distinguished, the PLS-DA reduced the separation to three major groups according to their composition and polarity: Hydro/HEVO, Hydro/Prim, and Lipo/HEVO. The first two components explained together 42.1% of the variance (Component 1: 26.6%, Component 2: 15.5%), allowing for a clear visualization of the dataset. The cross-validation results demonstrated good model performance (Figure 7C). For one latent component, the model reached Accuracy = 0.71, R2 = 0.69, and Q2 = 0.54. With two components, classification markedly improved (Accuracy = 0.82, R2 = 0.72, Q2 = 0.56). For models with three and four components, although R2 slightly increased (0.74 and 0.76, respectively), Q2 values declined (0.51 and 0.36), suggesting a risk of overfitting. Thus, the model with two latent components was considered optimal, balancing high explanatory and predictive ability. The variable importance in projection (VIP) scores (Figure 7B) showed that among antimicrobial responses, inhibitory activities against Candida species played a dominant role in separating NADESs. Yeast inhibition was a key discriminator of Lipo/HEVO NADESs, which exhibited the highest activity against these microorganisms, as well as significant inhibitory effects against E. coli and E. faecalis. This observation was confirmed by the score plot (Figure 7A), which showed that Hydro/Prim NADESs and some Hydro/HEVO NADES clustered mainly along the negative values of Component 1, whereas the majority of Hydro/HEVO samples formed a distinct group on the positive side of Component 1. This component was largely influenced by inhibitory activity against Candida species, as well as activity against MSSA.
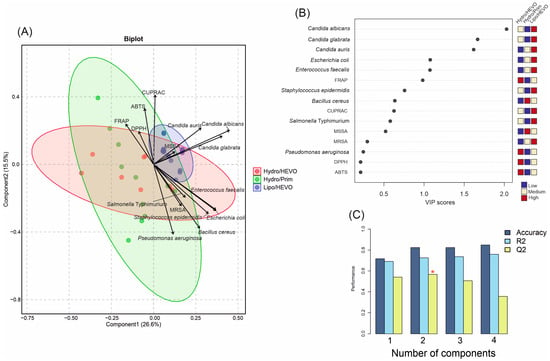
Figure 7.
(A) Partial least squares discriminant analysis (PLS-DA) biplot of antioxidant and antimicrobial activities of NADESs belonging to three major groups (Hydro/HEVO, Hydro/Prim, and Lipo/HEVO). Vectors indicate the contribution of variables (antioxidant assays and microbial strains). Colored ellipses represent 95% confidence intervals illustrating sample clustering within each group. (B) Variable importance in projection (VIP) scores of PLS-DA highlighting the most discriminant parameters. (C) Cross-validation results of the PLS-DA model. The red asterisk denotes the optimal number of components determined by cross-validation.
3. Materials and Methods
3.1. Materials and Chemicals
D-arabinose (≥99.0%), benzyl alcohol (≥99%), (-)-borneol (97%), (±)-camphor (≥95.5%), choline chloride (≥98%), 1,8-cineole (for synthesis), citric acid (≥99.5%), D-fructose (≥99%), gentisic acid (≥98%), D-(+)-glucose (≥99.5%), glycerol (≥99.0%), p-hydroxybenzoic acid (99%), DL-lactic acid (~90%), levulinic acid (98%), DL-malic acid (≥99%), DL-menthol (≥95%), naringin (≥95%), 1,3-propanediol (98%), protocatechuic acid (≥97%), quercetin (≥95%), α-resorcylic acid (≥97%), β-resorcylic acid (≥97%), rutin hydrate (≥94%), D-tagatose (≥98%), thymol (≥99%), urea (99.0-100.5%), DPPH, ABTS (98%), potassium persulfate (≥99%), 1,3,5-Tri(2-pyridyl)-2,4,6-triazine (TPTZ), iron (III) chloride (≥98%, FeCl3), sodium acetate (≥99%), copper(II) chloride (99%), neocuproine (≥98%), ammonium acetate (≥98%), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Acetic acid (99.5–99.9%) and hydrochloric acid (36%) were supplied by Avantor Performance Materials Poland S.A. (Gliwice, Poland). Water was deionized and purified using ULTRAPURE Milipore Direct-Q® 3UV–R (Merck, Darmstadt, Germany). HPTLC RP-18WF254s plates were purchased from Merck (Darmstadt, Germany). All NADESs were stored in sealed amber glass vials at 22 ± 2 °C and under relative humidity below 40% until analysis.
3.2. Antioxidant Panel
The antioxidant activity of NADESs was screened using bioautography on HPTLC plates. Two microlitres of each liquid were applied to the adsorbent surface, after which the plate surface was sprayed, separately, with DPPH and ABTS free radical solutions. The plates were then photographed.
Quantitative antioxidant activity was evaluated using four complementary assays—DPPH [42], ABTS [43], FRAP, and CUPRAC [26]—providing a comprehensive assessment encompassing free radical scavenging (ABTS; 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) and DPPH; 2,2-diphenyl-1-picrylhydrazyl) as well as Fe3+ (FRAP; Ferric Reducing Antioxidant Power) and Cu2+ (CUPRAC; Cupric ion Reducing Antioxidant Capacity). All measurements were performed using a UV–Vis Evolution 500 spectrophotometer (Thermo Electron Corporation, Waltham, MA, USA) and semi-micro cuvettes (Brand GMBH, Wertheim, Germany). HEVO-based NADESs, exhibiting particularly strong antioxidant activity, were diluted with methanol. For all tests, the NADES samples were incubated with the appropriate reagents for 30 min prior to measuring the absorbance. Values were expressed as the mg Trolox equivalent per mL NADES.
3.3. Antimicrobial Susceptibility Testing by Disk Diffusion Method (According to Kirby–Bauer)
3.3.1. Microorganisms
The antimicrobial activity of the tested NADESs were evaluated in accordance with EUCAST recommendations [44] against representative Gram-positive bacteria strains (S. aureus ATCC 29213, S. aureus ATCC BAA-1707, S. epidermidis ATCC 12228, B. cereus ATCC 10876, E. faecalis ATCC 51299), Gram-negative bacteria (S. Typhimurium ATCC 14028, E. coli ATCC 25922, P. aeruginosa ATCC 27853), and according to CLSI recommendations [45] for yeasts: C. albicans ATCC 10231, C. glabrata ATCC 90030, C. auris CDC B11903. The selected bacterial and yeast strains represent clinically relevant and well-characterized reference strains widely used for antimicrobial susceptibility testing and antimicrobial research [46,47,48,49,50,51].
The strains were obtained from certified microorganism collections of the American Type Culture Collection (ATCC, Manassas, VA, USA) and the Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA) and stored at 4 °C. Prior to testing, all strains were seeded on appropriate media to verify their viability and purity.
3.3.2. Procedure
- 1.
- Preparation of inoculum: Bacterial and yeast suspensions were prepared in sterile 0.85% NaCl and adjusted to a 0.5 McFarland standard (approximately 1–2 × 108 CFU/mL for bacteria, 1–5 × 106 CFU/mL for yeasts).
- 2.
- Plate inoculation: Microbiological media were poured aseptically into Petri dishes to a uniform height (approximately 4 mm) and left to dry at room temperature before inoculation. Mueller–Hinton agar (MHA; Sigma-Aldrich) for bacteria and Mueller–Hinton agar with 2% (w/v) glucose (MHA + 2% Glu) for yeast were inoculated evenly in three directions with the prepared microbial suspension, using a sterile swab to obtain uniform growth.
- 3.
- Preparation and application of test disks: 20 µL of each NADES was applied to sterile, blank 6 mm diameter paper disks (Oxoid™ Antimicrobial Susceptibility Test Discs, Thermo Fisher Scientific, Basingstoke, Hampshire, UK) and left to absorb under sterile conditions.
Commercial antibiotic disks were used as positive controls for test validation: ciprofloxacin (5 µg; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) for Gram-negative bacteria, vancomycin (30 µg; Oxoid™ Thermo Fisher Scientific, UK) for Gram-positive bacteria, and fluconazole (25 µg; BioMaxima, Lublin, Poland) for yeast.
The disks were placed on the surface of the inoculated agar using sterile tweezers. To ensure accurate and non-overlapping zones of inhibition, four disks were evenly spaced per plate for NADESs marked 1 to 19. For volatile mixtures (from 20 to 40), each disk was placed individually on a separate plate to prevent cross-interference.
Each test was performed in triplicate to ensure repeatability of results, and mean values were calculated.
- 4.
- Incubation: Plates were incubated at 35 ± 2 °C for 18 h for bacteria and 24 h for yeasts.
- 5.
- Measurement of inhibition zones: After incubation, the diameters of the microbial growth inhibition zones (including the diameter of the disk) were measured and the result was given in millimeters.
For the tested mixtures, relative activity was assessed based on the size of the inhibition zones compared to positive controls.
Aseptic Conditions
All procedures were performed under aseptic conditions to prevent contamination. All materials and instruments were sterilized prior to use. Disks were handled exclusively with sterile forceps, and culture plates were stored and used under aseptic conditions.
All experiments involving pathogenic microorganisms were conducted in accordance with institutional biosafety regulations, in a biosafety level 3 (BSL-3) laboratory at the Department of Pharmaceutical Microbiology, Medical University of Lublin, under a biosafety protocol approved by the Institutional Biosafety Committee.
3.4. Statistical and Multivariate Analysis
In order to detect differences between NADESs, statistical analyses were carried out using the Kruskal–Wallis ANOVA followed by the Conover–Inman post hoc test or Welch’s ANOVA followed by the Games–Howell post hoc test in PQStat Software (PQStat v.1.8.6.126, Poznań, Poland). Multivariate analyses were performed in MetaboAnalyst 5.0 [52]. PCA, PLS-DA, and heat mapping with hierarchical clustering (Euclidean distance, Ward’s linkage method) were applied to evaluate the relationships between antioxidant and antimicrobial activities of NADESs. Prior to multivariate analysis, data were normalized by sum and log transformation. For PLS-DA models, 5-fold cross-validation was applied to assess model performance.
4. Conclusions
In summary, these findings reinforce the potential of lipophilic NADES systems, particularly those based on thymol, exhibited potent antioxidant, and antimicrobial activity against a broad spectrum of pathogens. The variability in efficacy across different binary systems highlights the importance of component interactions, which may enhance or suppress antimicrobial effects. These findings highlight the potential of NADES preparations as versatile, natural antibacterial agents that can be used in pharmaceuticals and food preservation. However, further research is needed to clarify the mechanisms of their antibacterial action, optimize the preparations for maximum efficacy, and evaluate their safety profile through cytotoxicity studies. In addition, to fully exploit the potential of NADESs in combating microbial infections, it will be necessary to study a broader spectrum of microorganisms and perform quantitative antibacterial tests, such as minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC), as the disk diffusion method has limited precision in evaluating compounds with limited diffusion in agar (e.g., lipophilic NADESs) and does not provide data on MIC, MBC, and MFC values.
Furthermore, although trends suggest potential synergistic interactions, formal synergy tests would be necessary to confirm this.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30214219/s1, Figure S1: Photographs of bioautograms on HPTLC RP-18 plates after spraying with free radical solutions: (A)—ABTS, (B)—DPPH.; Table S1: Antioxidants’ activity of NADESs tested. Values are expressed as Trolox equivalent (mg Trolox/mL NADES); Table S2: Antimicrobial activity of tested NADESs. Values are expressed as diameters of the zones of inhibition (mm); Figure S2: Heatmap of Pearson correlation coefficients (red = positive, blue = negative) with hierarchical clustering using Euclidean distance and Ward’s method.; Table S3: Graphical representation of the results from the halo assay (disk diffusion test).
Author Contributions
Conceptualization, M.S.; methodology, M.S., M.K., A.G. and S.D.; software, M.K. and S.D.; validation, M.S., M.K., A.G. and S.D.; formal analysis, M.S., M.K., A.G., A.S.-K., K.D. and S.D.; investigation, M.S., M.K., A.G. and S.D.; resources, M.S., M.K., A.G. and S.D.; data curation, M.S., M.K., A.G. and S.D.; writing—original draft preparation, M.S., M.K., A.G., A.S.-K., K.D. and S.D.; writing—review and editing, M.S. and S.D.; visualization, M.K.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S., A.G., A.S.-K. and S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Lublin (DS 52).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chevé-Kools, E.; Choi, Y.H.; Roullier, C.; Ruprich-Robert, G.; Grougnet, R.; Chapeland-Leclerc, F.; Hollmann, F. Natural deep eutectic solvents (NaDES): Green solvents for pharmaceutical applications and beyond. Green Chem. 2025, 27, 8360–8385. [Google Scholar] [CrossRef]
- Kulinowska, M.; Dresler, S.; Skalska-Kamińska, A.; Hanaka, A.; Strzemski, M. Methodological aspects of green extraction of usnic acid using natural deep eutectic solvents. Molecules 2023, 28, 5321. [Google Scholar] [CrossRef]
- Dresler, S.; Baczewska, I.; Mykhailenko, O.; Zidorn, C.; Sowa, I.; Wójciak, M.; Feldo, M.; Wójciak, H.; Hanaka, A.; Strzemski, M. Extraction of lichen bioactive compounds using volatile natural deep eutectic solvents and comparative analytical approaches. Sci. Rep. 2025, 15, 22742. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural deep eutectic solvents (NADES): Phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.d.l.Á.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef]
- Strzemski, M.; Dresler, S.; Podkościelna, B.; Skic, K.; Sowa, I.; Załuski, D.; Verpoorte, R.; Zielińska, S.; Krawczyk, P.; Wójciak, M. Effectiveness of volatile natural deep eutectic solvents (VNADESs) for the green extraction of Chelidonium majus isoquinoline alkaloids. Molecules 2022, 27, 2815. [Google Scholar] [CrossRef]
- Jiménez-Ortega, L.A.; Kumar-Patra, J.; Kerry, R.G.; Das, G.; Mota-Morales, J.D.; Heredia, J.B. Synergistic antioxidant activity in deep eutectic solvents: Extracting and enhancing natural products. ACS Food Sci. Technol. 2024, 4, 2776–2798. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Khalid, R.M.; Hawash, D.; Al-Kaissi, E.; Al-Adham, I.S.I.; Al-Muhtaseb, N.; Jaber, N.; Al-Remawi, M.; Collier, P.J. Antimicrobial potential of natural deep eutectic solvents. Lett. Appl. Microbiol. 2022, 75, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Bedair, H.M.; Samir, T.M.; Mansour, F.R. Antibacterial and antifungal activities of natural deep eutectic solvents. Appl. Microbiol. Biotechnol. 2024, 108, 198. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Silva, E.; Reis, R.L.; Duarte, A.R.C. A closer look in the antimicrobial properties of deep eutectic solvents based on fatty acids. Sustain. Chem. Pharm. 2019, 14, 100192. [Google Scholar] [CrossRef]
- Marchel, M.; Cieśliński, H.; Boczkaj, G. Deep eutectic solvents microbial toxicity: Current state of art and critical evaluation of testing methods. J. Hazard. Mater. 2022, 425, 127963. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Morais, E.S.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Extraction of high value triterpenic acids from Eucalyptus globulus biomass using hydrophobic deep eutectic solvents. Molecules 2020, 25, 210. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Development of a method based on natural deep eutectic solvents for extraction of flavonoids from food samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef]
- Castro, V.I.B.; Craveiro, R.; Silva, J.M.; Reis, R.L.; Paiva, A.; Ana, A.R. Natural deep eutectic systems as alternative nontoxic cryoprotective agents. Cryobiology 2018, 83, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, F.; Chen, Z.G.; Wu, T.; Wang, Z.H. Green and efficient removal of cadmium from rice flour using natural deep eutectic solvents. Food Chem. 2018, 244, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zang, Y.Y.; Yang, S.; Chen, Z.G. Green and efficient removal of heavy metals from Porphyra haitanensis using natural deep eutectic solvents. J. Sci. Food Agric. 2021, 101, 2930–2939. [Google Scholar] [CrossRef]
- Kranz, M.; Hofmann, T. Food-grade synthesis of maillard-type taste enhancers using natural deep eutectic solvents (NADES). Molecules 2018, 23, 261. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidou, A.; Makris, D.P.; Lazaridou, A.; Biliaderis, C.G.; Mourtzinos, I. Physical properties of chitosan films containing pomegranate peel extracts obtained by deep eutectic solvents. Foods 2021, 10, 1262. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L. Essential oils as antioxidants: Their evaluation by DPPH, ABTS, FRAP, CUPRAC, and β-carotene bleaching methods. Monatshefte Fur. Chem. 2016, 147, 2083–2091. [Google Scholar] [CrossRef]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Szwerc, W.; Sawicki, J.; Kocjan, R.; Feldo, M.; Dresler, S. Carlina vulgaris L. as a source of phytochemicals with antioxidant activity. Oxid. Med. Cell. Longev. 2017, 2017, 1891849. [Google Scholar] [CrossRef]
- Stef, D.S.; Gergen, I.; Trașcă, T.I.; Riviş, A.; Ducu-Sandu, Ş.; Iosif, G.; Teodor-Ioan, T.; Adrian, R.; Lavinia, Ş.; Romeo, C.; et al. Assessing the influence of various factors on antioxidant activity of medicinal herbs. Rom. Biotech. Lett. 2017, 22, 12842. [Google Scholar]
- Gębalski, J.; Małkowska, M.; Kiełkowska, E.; Graczyk, F.; Wnorowska, S.; Hołyńska-Iwan, I.; Strzemski, M.; Wójciak, M.; Załuski, D. Chemical composition and in vitro biological activity of the polar and non-polar fractions obtained from the roots of Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Int. J. Mol. Sci. 2025, 26, 5619. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Aeschbach, R.; Löliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
- Chroho, M.; Rouphael, Y.; Petropoulos, S.A.; Bouissane, L. Carvacrol and thymol content affects the antioxidant and antibacterial activity of Origanum compactum and Thymus zygis essential oils. Antibiotics 2024, 13, 139. [Google Scholar] [CrossRef]
- Yildiz, S.; Turan, S.; Kiralan, M.; Ramadan, M.F. Antioxidant properties of thymol, carvacrol, and thymoquinone and its efficiencies on the stabilization of refined and stripped corn oils. J. Food Meas. Charact. 2021, 15, 621–632. [Google Scholar] [CrossRef]
- Modareskia, M.; Fattahi, M.; Mirjalili, M.H. Thymol screening, phenolic contents, antioxidant and antibacterial activities of Iranian populations of Trachyspermum ammi (L.) Sprague (Apiaceae). Sci. Rep. 2022, 12, 15645. [Google Scholar] [CrossRef]
- Abdollahi, A.; Fereydouni, N.; Moradi, H.; Karimivaselabadi, A.; Zarenezhad, E.; Osanloo, M. Nanoformulated herbal compounds: Enhanced antibacterial efficacy of camphor and thymol-loaded nanogels. BMC Complement. Med. Ther. 2024, 24, 138. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Dai, Y.; Ouyang, P.; Rehman, T.; Hussain, S.; Zhang, T.; Yin, Z.; Fu, H.; Lin, J.; He, C.; et al. Thymol inhibits biofilm formation, eliminates pre- existing biofilms, and enhances clearance of methicillin-resistant Staphylococcus aureus (MRSA) in a mouse peritoneal implant infection model. Microorganisms 2020, 8, 99. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Kundukad, B.; Schussman, M.; Yang, K.; Seviour, T.; Yang, L.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Mechanistic action of weak acid drugs on biofilms. Sci. Rep. 2017, 7, 4783. [Google Scholar] [CrossRef]
- Ispiryan, A.; Atkociuniene, V.; Makstutiene, N.; Sarkinas, A.; Salaseviciene, A.; Urbonaviciene, D.; Viskelis, J.; Pakeltiene, R.; Raudone, L. Correlation between antimicrobial activity values and total phenolic content/antioxidant activity in Rubus idaeus L. Plants 2024, 13, 504. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Sheehan, D.J. Interpretive breakpoints for fluconazole and Candida revisited: A blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 2006, 19, 435–447. [Google Scholar] [CrossRef]
- CLSI M100-ED29; 2021 Performance Standards for Antimicrobial Susceptibility Testing, 30th ed. CLSI: Pittsburgh, PA, USA, 2020; Volume 40, ISBN 9781684400324.
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Berkow, E.L.; Chow, N.; Welsh, R.M. Candida auris for the clinical microbiology laboratory: Not your grandfather’s Candida species. Clin. Microbiol. Newsl. 2017, 39, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, R.A.; Bäumler, A.J. Host adaptation and the emergence of infectious disease: The Salmonella paradigm. Mol. Microbiol. 2000, 36, 1006–1014. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).