Abstract
In recent years, cadmium sulfide (CdS) has been widely investigated due to its excellent photocatalytic performance. However, its practical application in pollutant treatment is limited by its narrow photoresponse range and susceptibility to photocorrosion. Herein, we design a unique four-layer core–shell structure NaYF4:Yb3+,Er3+@NaYF4@CdS@Au (CSNPs@CdS@Au), with an inert NaYF4 shell coating on NaYF4:Yb3+,Er3+ (CNPs) to form NaYF4:Yb3+,Er3+@NaYF4 (CSNPs) and CdS depositing on CSNPs (CSNPs@CdS); Au nanoparticles are loaded on CdS (CSNPs@CdS@Au). Compared with CdS (9.81%), CSNPs (5.0%), CSNPs/CdS (6.9%), and CSNPs@CdS (81.0%), CSNPs@CdS@Au degrades 97.7% Rhodamine B (RhB) within 15 min, exhibiting superior photocatalytic performance, attributable to two key factors: (1) the NaYF4 inert shell encapsulation amplifies upconversion (UC) luminescence intensity by suppressing surface quenching; and (2) the electron transfer between Au nanoparticles and CdS effectively promotes spatial separation of photogenerated charge carriers and increases reactive active sites. Additionally, after five degradation cycles, CSNPs@CdS@Au still maintains a 93.25% degradation rate for RhB, confirming its excellent stability. This remarkable stability is attributed to the uniquely designed multilayer core–shell architecture, which significantly enhances structural integrity through physical isolation effects. This study establishes a material preparation strategy for efficient photocatalytic pollutant degradation.
1. Introduction
Environmental pollution has recently emerged as a pressing global challenge, as the survival of humankind and societal development hinge upon the availability of clean freshwater [1,2,3]. Rhodamine B (RhB), a widely used organic dye, is one of the common sources of water pollution [4,5,6,7]. Solar-driven semiconductor photocatalytic technology can effectively harness light to degrade pollutants and is considered an efficient and eco-friendly water purification method [8,9]. Cadmium sulfide (CdS), as a type of semiconductor, has a narrow bandgap of 2.42 eV [10] and excellent optical performance [11], which demonstrates outstanding performance in the fields of degrading organic pollutants [12] and photocatalytic hydrogen production [13,14], and so on. However, traditional CdS photocatalysts can only absorb light with wavelengths below 520 nm and cannot effectively utilize near-infrared (NIR) light [15], which accounts for a relatively large proportion in the solar spectrum. Therefore, achieving the efficient utilization of CdS for NIR light has become a pressing problem.
At present, combining upconversion (UC) materials is regarded as an effective strategy to expand the absorption of the solar spectrum for CdS. Rare-earth (RE) ions in UC nanoparticles (UCNPs) possess rich f-electron configurations. Under NIR light, they leverage transitions between 4f and 4f-5d energy levels to convert low-energy NIR light into high-energy ultraviolet (UV) or visible light [16], thereby expanding the light absorption range of semiconductor materials. It is reported that the Yb3+-Er3+ ion pair doped in the β-NaYF4 has the highest UC efficiency [17]. Balaji et al. synthesized NaYF4:Yb/Er UCNPs for NIR light-driven photocatalytic degradation of methyl orange dye [18]. Feng et al. fabricated a NaYF4:Yb3+,Er3+/Au/CdS UC photocatalyst for photocatalytic hydrogen production from ethanol [19]. However, the energy transfer loss in UCNPs and surface quenching lead to a decline in UC efficiency, thus preventing the full expansion of the absorption range of photocatalysts. The introduction of an inert shell or energy-capturing ions to construct a core–shell structure is an effective way to suppress energy migration and surface quenching. Constructing an inert shell (NaYF4) on the surface to form a core–shell structure isolates the luminescent ions from solvents and ligands, thereby reducing the occurrence of fluorescence quenching. Rinkel et al. proposed a method for synthesizing 10 nm β-NaYF4:Yb,Er/NaYF4 core–shell UC nanocrystals. A shell approximately 2.5 nm thick enhanced the UC efficiency by approximately 160-fold [20]. Huang et al. coated a layer of NaYF4 shell on the UCNPs, resulting in a significant enhancement of the fluorescence effect [21]. In addition, the particle size of UC materials is also an important factor affecting their fluorescence effect [22,23]. It is reported that the fluorescence intensity of micrometer-sized UC materials is higher than that of nanometer-sized UC materials [24,25]. Li et al. synthesized core–shell structured NaYF4:Yb,Tm@CdS microrods (~6 μm in length). Due to the fact that the surface has not been functionalized, the adhesion effect of CdS on the NaYF4 surface is not good [22]. Li et al. synthesized NaYF4:Yb,Tm@CdS microrods of length ~1.5 μm. TEM characterization revealed that CdS were not uniformly distributed on the surface of the microrods [26]. On the one hand, CdS is prone to fall off from the UC rod-shaped material, resulting in unstable catalytic performance [13]. On the other hand, the uniform distribution of CdS on rod-shaped UC materials is difficult to control. Consequently, effectively integrating micrometer-scale UC rods with CdS remains challenging. In contrast, nanoscale UCNPs, particularly after coating with an inert shell, are more suitable for integration with CdS photocatalysts.
Furthermore, the practical application of CdS remains severely limited due to photocorrosion issues [27]. Photogenerated holes (h+) in the valence band (VB) of CdS oxidize S2− within the material, following the reaction: CdS + 2h+→Cd2 + +S [28,29]. Theoretically, multilayered core–shell structures can enhance structural stability through physical isolation. Combining CdS with noble metals (e.g., Au) to form core–shell architectures is considered one of the most effective methods for improving catalyst stability [30,31,32]. It has been reported that Li et al. constructed a ternary CdS@Au/MXene composite. The incorporation of Au established dual Schottky barriers, resulting in a catalytic rate 26.6 times higher than that of pure CdS [33]. Dong et al. prepared a four-layer core–shell structure of Co3O4@TiO2@CdS@Au double-layer nanocages, which achieved a removal rate of 98.8% for pollutants within 10 min. After five circles, it still demonstrated excellent catalytic performance and stability. More importantly, the photocorrosion of CdS was significantly suppressed, which is a key drawback of CdS-based photocatalysts [34]. Therefore, loading Au onto the surface of CdS to form a core–shell structure is expected to simultaneously enhance catalytic performance and stability [27,35].
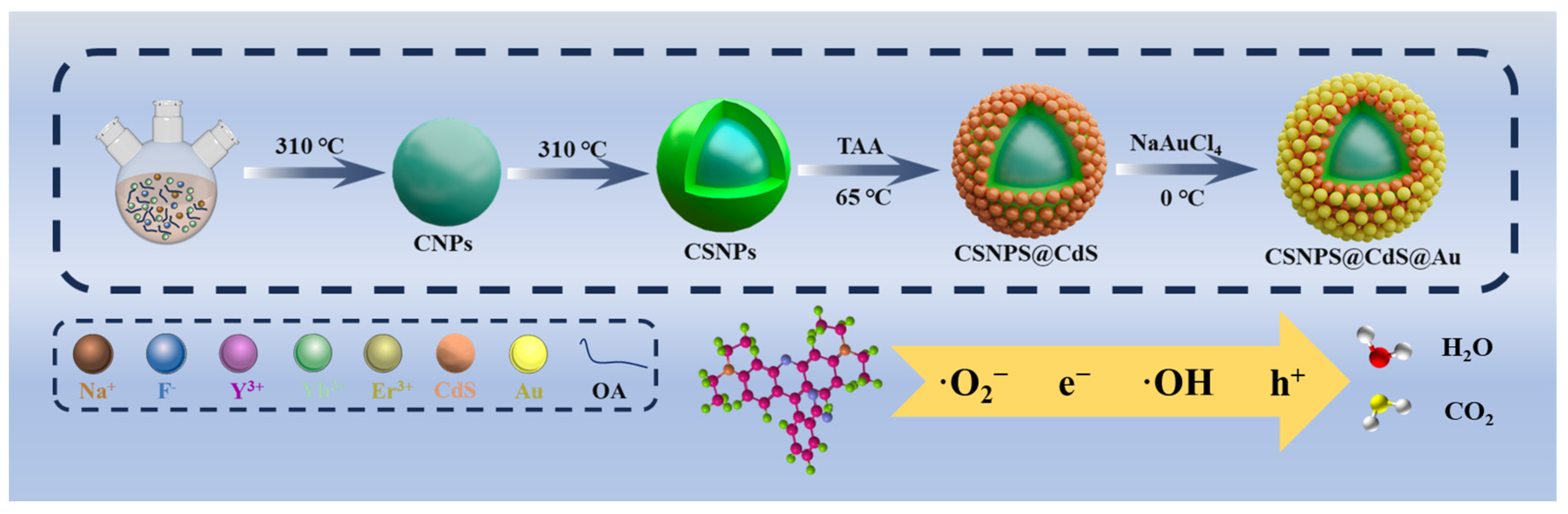
Inspired by the above studies, this research aims to design a novel photocatalyst capable of efficiently utilizing the solar spectrum, suppressing CdS photocorrosion, and enhancing reusability. Our design involves the following steps: (1) Coating NaYF4:Yb3+,Er3+ (CNPs) with an inert NaYF4 shell to prepare NaYF4:Yb3+,Er3+@NaYF4 (CSNPs), converting NIR/visible photons into UV emission. (2) Integrating CSNPs with CdS NPs to construct core–shell structured CSNPs@CdS, expanding CdS’s light absorption range. (3) Introducing Au NPs to form four-layer core–shell structured CSNPs@CdS@Au, enhancing CdS stability and catalytic efficiency. Under 300 W xenon lamp irradiation, CSNPs@CdS@Au demonstrated exceptional photocatalytic performance and stability in RhB degradation. This study contributes to advancing the NIR photocatalytic activity of CdS-based photocatalysts.
2. Materials and Methods
2.1. Chemicals and Materials
Yttrium chloride hexahydrate (99.9%), ytterbium chloride hexahydrate (99.9%), erbium chloride hexahydrate (99.9%), ammonium fluoride (AR), n-hexane (99%), sodium hydroxide (AR), sodium oleate (97.0%), oleic acid (OA, 90%), trisodium citrate dihydrate (98%), 3-aminopropyltriethoxysilane, and ammonia aqueous solution (25–28 wt%) were purchased from Aladdin Bio-Chemistry Shanghai China Technology Co., Ltd. (Shanghai, China), poly (vinylpyrrolidone) (PVP, MW = 55,000), Thioacetamide (TAA, 99.0%), cadmium chloride (99%) and 1-octadecene (ODE, 90% technical grade) were purchased from Beijing Innochem Technology Co., Ltd. (Beijing, China). Rh B and chloroauric acid (99.9%) were purchased from Anhui Zaisheng Technology Co., Ltd. (Hefei, China), and Cyclohexane (99.7%) was purchased from Hunan Huihong Reagent Co., Ltd. (Changsha, China). Deionized (DI) water was used during the entire experimental process. All chemical reagents were used without any purification.
2.2. Synthesis of CSNPs@CdS@Au NPs
As shown in Scheme 1, the synthesis of CSNPs@CdS@Au four-layer core–shell structure NPs is achieved using a four-step process. Firstly, lanthanide oleate complexes were used as precursors to synthesize NaYF4:Yb3+,Er3+ core NPs by thermal decomposition method. Then, NaYF4 inert shell layer was coated on the surface of NaYF4:Yb3+,Er3+ core to obtain CSNPs. Subsequently, CdS was encapsulated on the surface of CSNPs through ion deposition, and finally, gold was encapsulated on the surface of CdS from chloroauric acid through redox reaction.
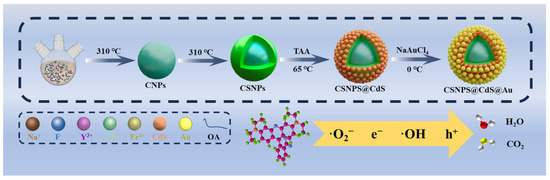
Scheme 1.
Schematic illustration of the preparation of CSNPs@CdS@Au NPs photocatalytic materials.
2.2.1. Synthesis of NaYF4:Yb3+,Er3+ Core NPs
RE fluoride NPs were prepared via thermal decomposition [36]. Into a three-necked flask containing a solvent mixture of DI water (6 mL), ethanol (7 mL), and n-hexane (14 mL), 0.4732 g (1.56 mmol) YCl3 6H2O, 0.1549 g (0.40 mmol) YbCl3 6H2O, 0.0158 g (0.04 mmol) ErCl3 6H2O, and 1.8570 g (6.1 mmol) sodium oleate were sequentially added. The molar ratio of Y:Yb:Er was set at 78:20:2. The solution was stirred at room temperature for 0.5 h and then transformed to a preheated water bath of 70 °C. The whole reaction system was maintained at 70 °C for 4 h with vigorous stirring. After the completion of the reaction, the upper oil phase containing Y0.78Yb0.20Er0.02-oleate complexes were washed three times with DI water (20 mL × 3) to remove the byproduct of NaCl. The above prepared Y0.78Yb0.20Er0.02-oleate complexes (2 mmol) were mixed with 12 mL OA and 30 mL ODE in a three-necked flask at room temperature with vigorous stirring. The solution was degassed and heated to 140 °C to form a transparent solution, and then cooled down to 50 °C. Subsequently, a methanol solution (20 mL) containing NaOH (0.2000 g, 5.0 mmol) and NH4F (0.2963 g, 8.0 mmol) was added to a flask and stirred continuously for 1.5 h. After that, methanol was removed by heating the solution at 100 °C. The reaction mixture was then heated to 310 °C and maintained for 1.5 h under a N2 atmosphere. After the completion of the reaction, the reaction system was cooled down to room temperature and the resultant solution was precipitated in 40 mL ethanol. The CNPs were collected by centrifugation (11,000 rpm) and washed five times with a mixture of cyclohexane and ethanol at a volume ratio of 1:3.
2.2.2. Synthesis of NaYF4:Yb3+, Er3+@NaYF4 Core-Inert Shell NPs
Into a flask containing a solvent mixture of DI water (6 mL), ethanol (7 mL) and n-hexane (14 mL), 0.6068 g (2 mmol) YCl3 6H2O and 1.8570 g (6.1 mmol) sodium oleate were sequentially added. The solution was stirred at 70 °C for 4 h. After the complement of the reaction naturally cooled to room temperature, the upper oil phase was washed three times with DI water (20 mL × 3) to remove the byproduct of NaCl. The supernatant was poured into a 100 mL three-necked flask and mixed with 12 mL OA and 30 mL ODE. The mixed solution was heated to 140 °C and continuously stirred to form a transparent solution, and then cooled down to 70 °C; subsequently, 4 mL of cyclohexane containing 0.3 g CNPs were added to the mixture solution and stirred for 5 min. After that, the cyclohexane was removed by maintaining the mixture solution at 70 °C under vacuum for 30 min, and then the solution was cooled down to 50 °C. Meanwhile, a methanol solution (20 mL) containing NaOH (0.2000 g, 5.0 mmol) and NH4F (0.2963 g, 8.0 mmol) was added to the reaction flask, and stirred continuously for 1.5 h. After that, methanol was removed by heating the solution at 100 °C. The reaction mixture was then heated to 310 °C and maintained for 1.5 h under N2 atmosphere. Then, the reaction system was cooled down to room temperature and the resultant solution was precipitated in 40 mL ethanol. The CSNPs were collected by centrifugation (11,000 rpm) and washed five times with a mixture of cyclohexane and ethanol at a volume ratio of 1:3.
2.2.3. Synthesis of CSNPs@CdS Three-Layer Core–Shell Structural Composite
Sandwich-structure NPs were prepared by the ion deposition method [26]. A quantity of 0.1200 g CSNPs was dispersed in 140 mL DI water by sonication treatment for 30 min. Trisodium citrate dihydrate (7 mL, 0.1 M) and CdCl2 (7 mL, 0.08 M) were sequentially added to the above solution under continuous stirring for 20 min and 40 min, respectively. After that, ammonia aqueous solution was slowly dropped into the mixture solution until the pH value reached 10.5. Subsequently, the mixture solution was slowly heated to 65 °C in a bath. Then, 10 mL TAA (0.063 M) was added into the above solution at a rate of 0.1 mL/min. Stirring was maintained at 65 °C for 1 h. The final product was washed with ethanol and DI water three times and dried at 60 °C for 12 h in an oven.
2.2.4. Synthesis of CSNPs@CdS@Au Four-Layer Core–Shell Structural Composites
Au NPs were loaded onto the surface of CSNPs@CdS particles [34]. CSNPs@CdS 30 mg was dispersed in 5 mL cyclohexane and sonicated for 30 min. PVP (30 mg) and 30 mL ethanol solution (v/v ratio of water/ethanol = 9:1) were then added to the above solution for 30 min. Then, 100 μL 0.6 mM HAuCl4·4H2O was added to the above solution, and the reaction was stirred for 60 min. NaBH4 (0.0057 g) was added under 0 °C for 1 h and freeze-dried to obtain the final product CSNPs@CdS@Au. x represents the volume of 0.6 mM HAuCl4·4H2O added in μL, the amount of Au added is denoted as Aux, and the final product is denoted as CSNPs@CdS@Aux. For example, Au100 indicates that 100 μL of 0.6 mM HAuCl4·4H2O solution was added during the synthesis of CSNPs@CdS@Au.
2.3. Characterization
X-ray powder diffractometer (XRD) was used to analyze the purity and phase structure of the prepared materials using a Bruker D8 Advance model from Germany in the 2θ range from 10° to 80° at a scan rate of 2°/min. A transmission electron microscope (TEM) was used to characterize the size and morphology of the prepared materials using a JEOL JEM-F200 model from Tokyo, Japan. X-ray photoelectron spectroscopy (XPS) was used to analyze the elemental composition and the chemical state of the surface of the prepared material samples, using the Thermo Scientific K-Alpha model from the Waltham, MA, USA. A PL fluorescence spectrometer was used (Edinburgh FLS1000, Manchester, UK). The absorbance curve of the solution was measured using a UV-Visible spectrophotometer (TU1810), with the wavelength range being 200–800 nm.
2.4. Photocatalytic Activity Measurement
The photocatalytic properties of the prepared composites (CSNPs@CdS@Au) were evaluated by degradation of RhB solution under irradiation of a 300 W xenon lamp. The visible band (λ > 420 nm) was obtained by using a filter. In each experiment, 25 mg of the photocatalyst was mixed with 50 mL of RhB (1.0 × 10−4 M). Prior to light irradiation, the suspension was magnetically stirred in the dark for 30 min to achieve adsorption–desorption equilibrium [37,38,39]. During light irradiation, aliquots of approximately 2.5 mL were drawn at quantitative time intervals, filtered through a 0.22 μm polyethersulfone (PES) membrane to remove the photocatalyst particles, and then the concentration of RhB remaining in the supernatant was detected by a UV-visible spectrophotometer (TU1810).
3. Results and Discussion
3.1. X-Ray Diffraction (XRD) Analysis
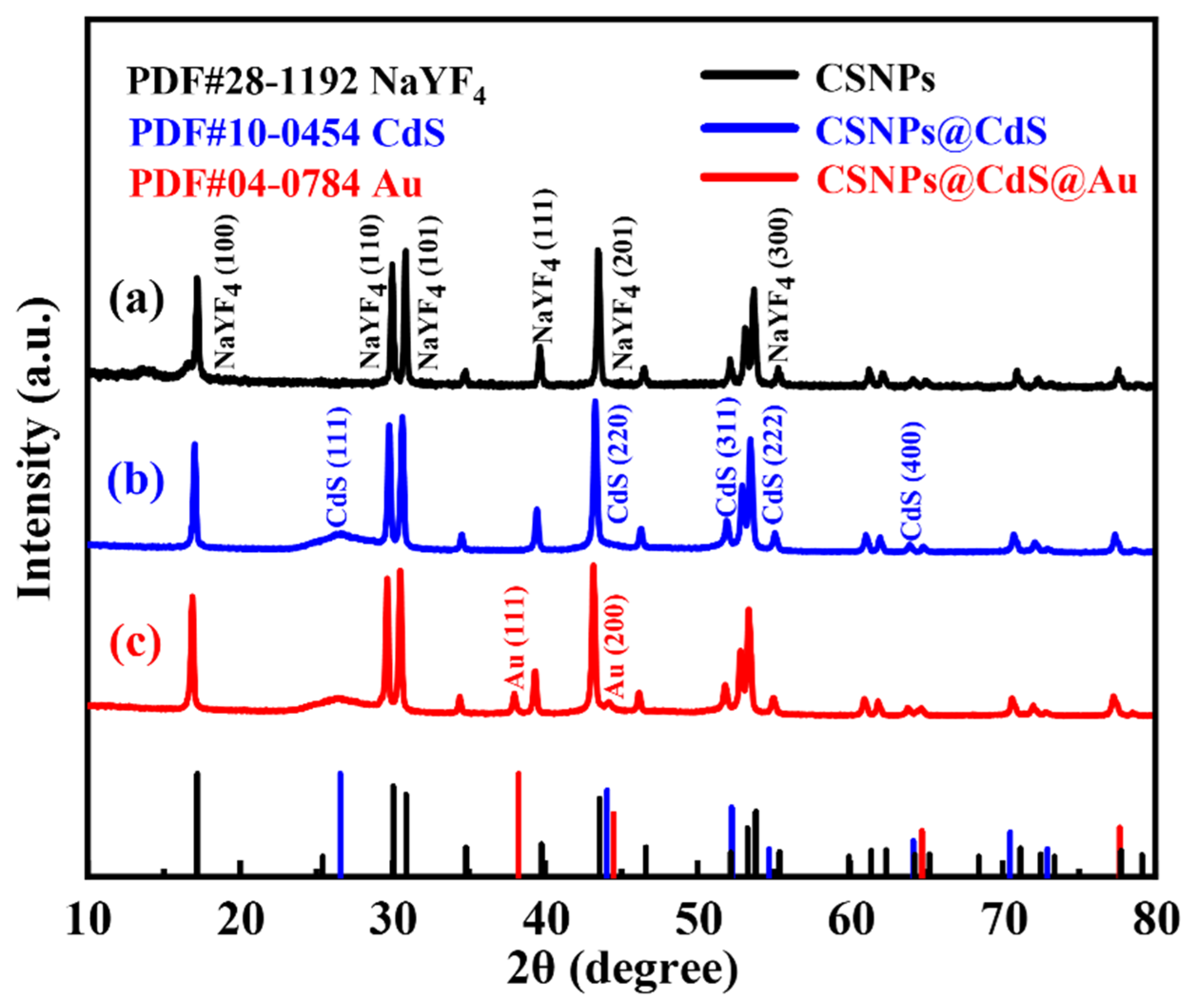
The XRD of CSNPs, CSNPs@CdS, and CSNPs@CdS@Au are shown in Figure 1. The black line in Figure 1a represents the XRD plot of CSNPs, the diffraction peaks appearing at diffraction angles of 17.20°, 30.06°, 30.78°, 39.67°, 43.49°, and 53.27° correspond to the diffraction peaks of NaYF4 (100), (110), (101), (111), (201), and (300), respectively, and by comparing with the standard diffractogram of hexagonal phase (JCPDS#28-1192) [40], it is possible to determine that the synthesized CSNPs is a hexagonal crystal phase [15]. Moreover, no other impurity peaks are observed in this figure, and the strong and sharp peaks indicate that the synthesized CSNPs nanomaterials under thermal decomposition conditions are highly crystalline. The blue line Figure 1b shows the XRD plots of CSNPs@CdS NPs, with the crystallographic planes corresponding to the diffraction peaks appearing at diffraction angles of 26.50°, 43.96°, 52.13°, 54.58°, and 64.02° as CdS (111), (220), (311), (222), and (400), respectively. The successful preparation of CdS particles can be determined by comparison with the CdS standard PDF card (JCPDS#10-0454). The red line in Figure 1c shows the XRD patterns of CSNPs@CdS@Au NPs, with diffraction peaks detected at diffraction angles of 38.18° and 44.39°, which correspond to the (111) and (200) planes of metallic Au (JCPDS#04-0784) [41], confirming the successful loading of Au NPs.
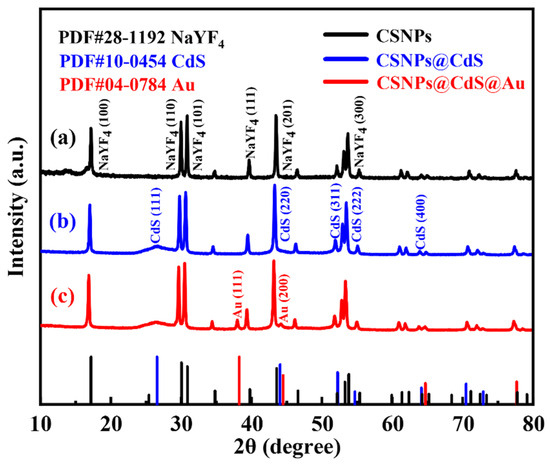
Figure 1.
XRD patterns of (a) CSNPs, (b) CSNPs@CdS, and (c) CSNPs@CdS@Au.
3.2. TEM Analysis
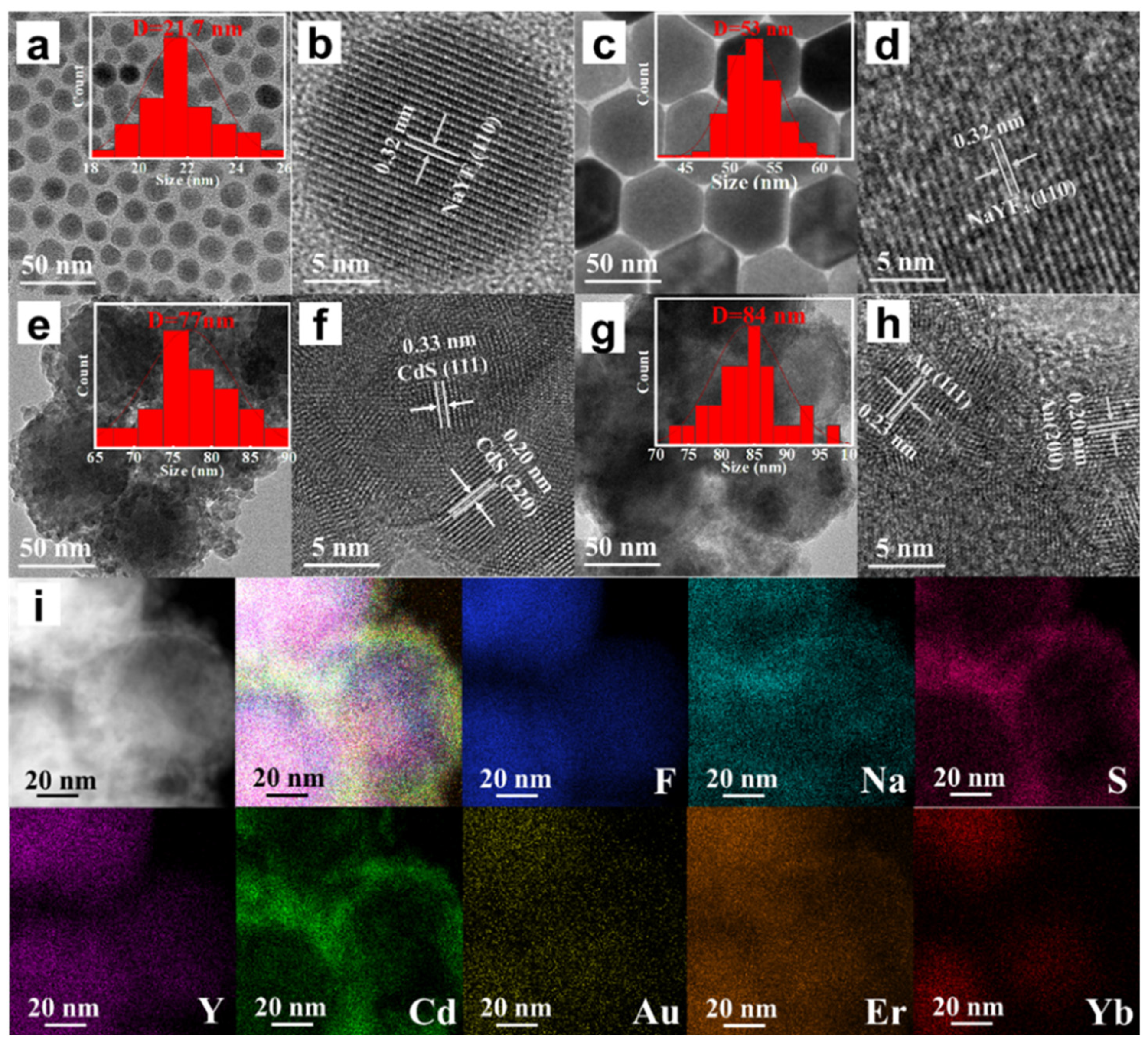
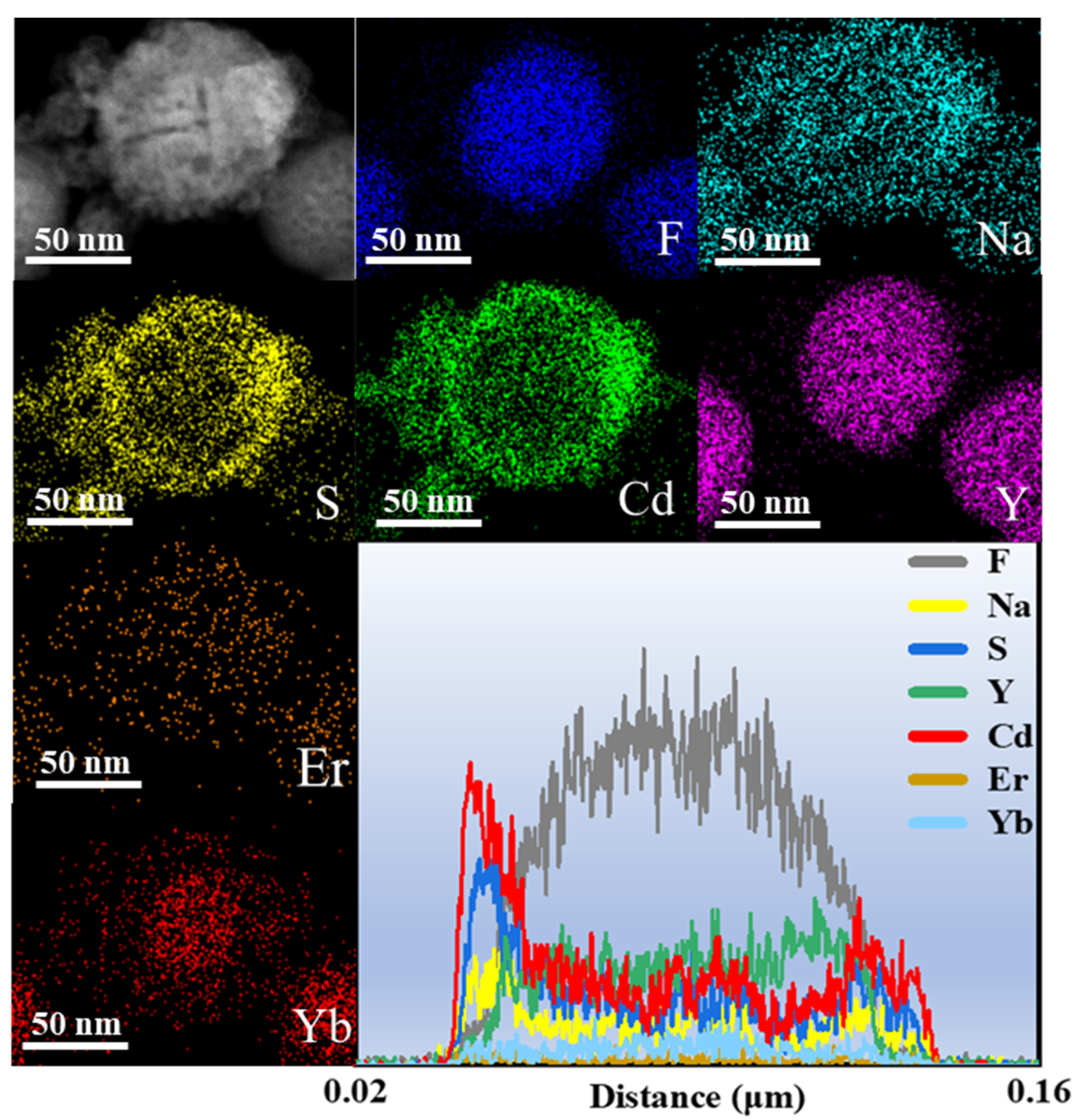
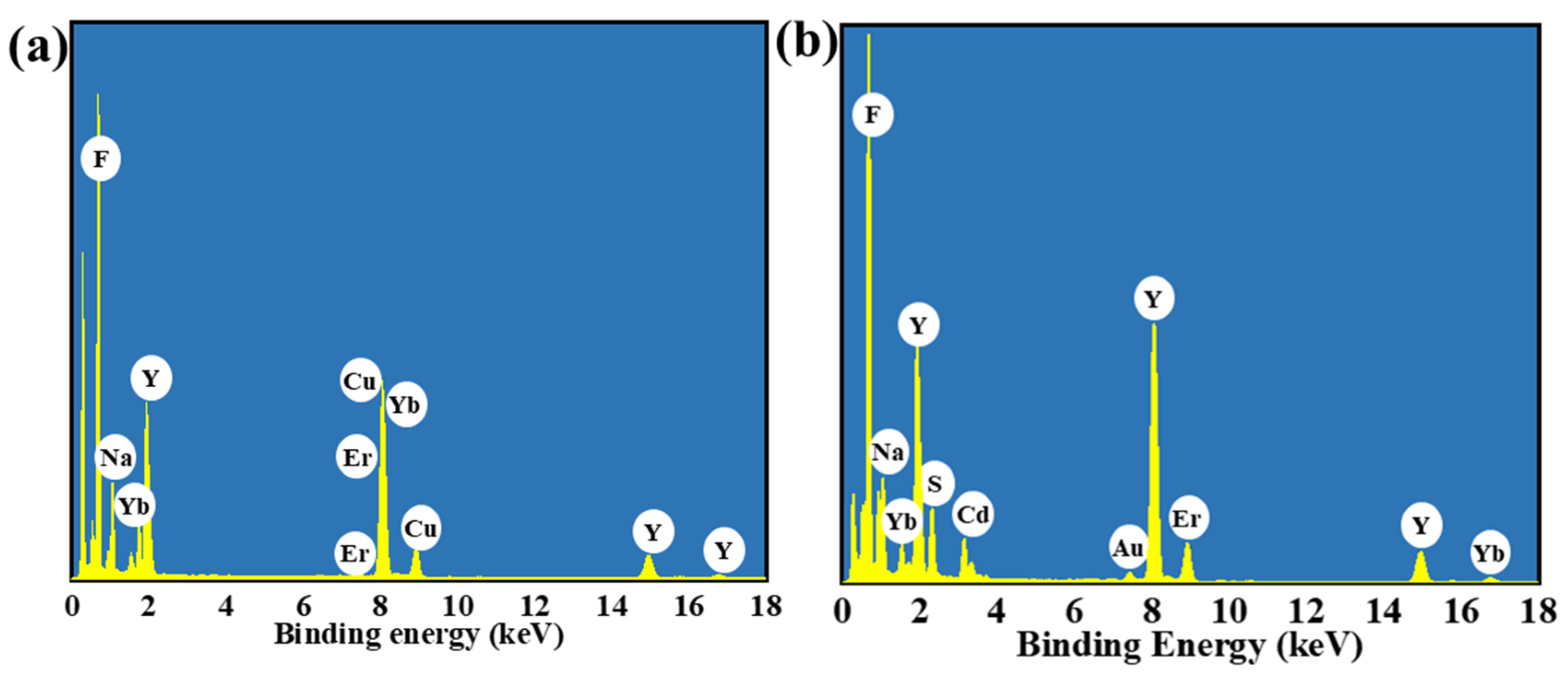
In order to investigate the microscopic morphological and structural changes in the composite nanomaterials, TEM tests were performed on a series of samples. Figure 2a shows the CNPs prepared by the thermal decomposition method. The CNPs have a uniform particle morphology with an average particle size of approximately 21.7 nm (inset of Figure 2a), and the lattice stripe spacing is 0.32 nm (Figure 2b), corresponding to the NaYF4 (110) crystal plane. In order to perform the fluorescence enhancement of the NPs, a layer of NaYF4 inert shell (not doped) was encapsulated on top of CNPs; the encapsulation of the inert shell layer reduces the surface defects of the Yb3+, Er3+-doped active nuclei, as shown in Figure 2c. The synthesized CSNPs NPs are uniform and well-dispersed, with a smooth hexagonal surface morphology. The average particle size is approximately 52.8 nm (inset of Figure 2c), and the lattice stripe spacing is 0.32 nm (Figure 2d), corresponding to the NaYF4 (110) crystal plane [42,43,44]. The core–shell CSNPs@CdS NPs were successfully synthesized via a redox method (Figure 2e). It can be seen from the image that the average particle size is approximately 77 nm (inset of Figure 2e). Furthermore, the elemental mapping of CSNPs@CdS (Figure 3) clearly shows that S and Cd are distributed in the shell layer, while the elements constituting the CSNPs core are concentrated in the center. Through the linear scanning plot of Figure 3, it can be observed that the S and Cd distributions show an elevated, then decreased, and then increased situation, which proves that we successfully prepared CSNPs@CdS with a core–shell structure. The CdS NPs were completely and uniformly coated on the surface of the CSNPs, enabling them to absorb the excitation light of the CSNPs while maintaining stability [45]. The measured spacings from the lattice fringes of CdS were found to be 0.33 and 0.20 nm by HR-TEM Figure 2f, which correspond to the (111) and (220) crystallographic planes of CdS, respectively. The TEM of the CSNPs@CdS@Au is shown in Figure 2g, and the morphology of the material is that of spherical particles of uniform size, The average particle size is about 84 nm (inset of Figure 2g). Comparing with Figure 2e, it can be clearly seen that the introduction of Au makes the surface of the coated CdS particles smoother. Figure 2h shows that the spacing of the two lattice fringes is 0.23 and 0.20 nm, respectively, corresponding to the (111) and (200) crystal planes of Au [46]. Figure 2i is the elemental mapping diagram of CSNPs@CdS@Au particles. It can be observed from the figure that elements such as F, Na, Y, Er, and Yb are distributed in the core center of the particle, S and Cd elements are distributed on the outer layer of the CSNPs, and Au element is evenly distributed on the outermost layer. The above results further indicate the successful preparation of the four core–shell composite material. Figure 4a confirms the presence of F, Na, Y, Yb, and Er elements in the CSNPs, with an Yb:Er molar ratio of 20:2, consistent with the expected composition. Figure 4b also confirms the presence of S, Cd, and Au elements, further confirming the successful preparation of the quad-core–shell composite material.
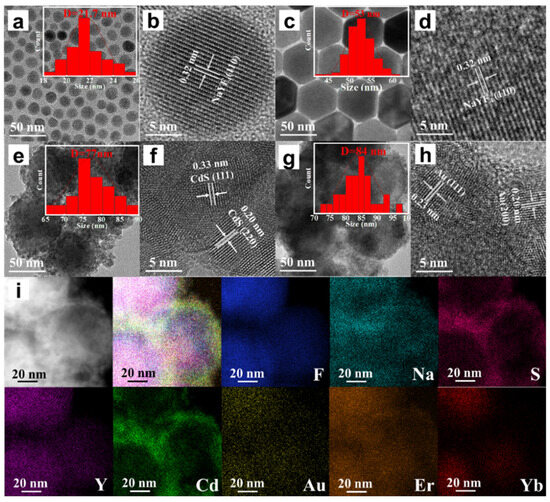
Figure 2.
(a) TEM image of CNPs; (b) HR-TEM image of CNPs; (c) TEM image of CSNPs; (d) HR-TEM image of CSNPs; (e) TEM image of CSNPs@CdS; (f) HR-TEM image of CSNPs@CdS; (g) TEM image of CSNPs@CdS@Au; (h) HR-TEM image of CSNPs@CdS@Au; (i) Elemental mapping images of CSNPs@CdS@Au NPs of the NPs.
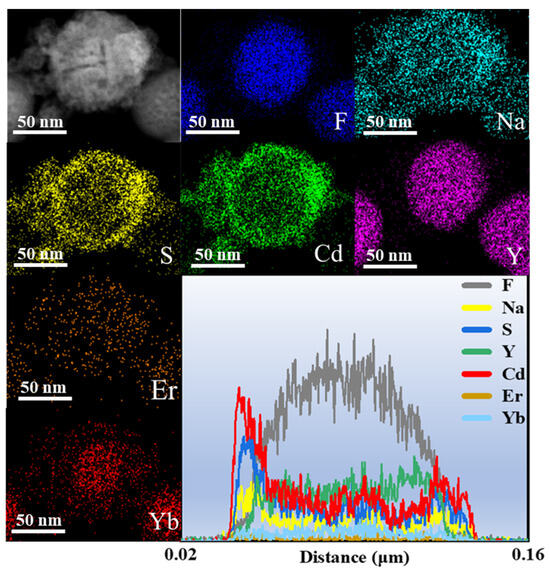
Figure 3.
The elemental mapping and line scan profile of CSNPs@CdS.
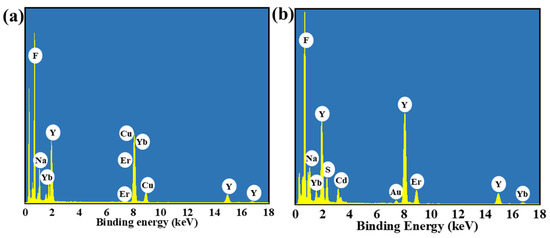
Figure 4.
(a) The EDX spectrum of CSNPs. (b) The EDX spectrum of CSNPs@CdS@Au.
3.3. XPS Analysis
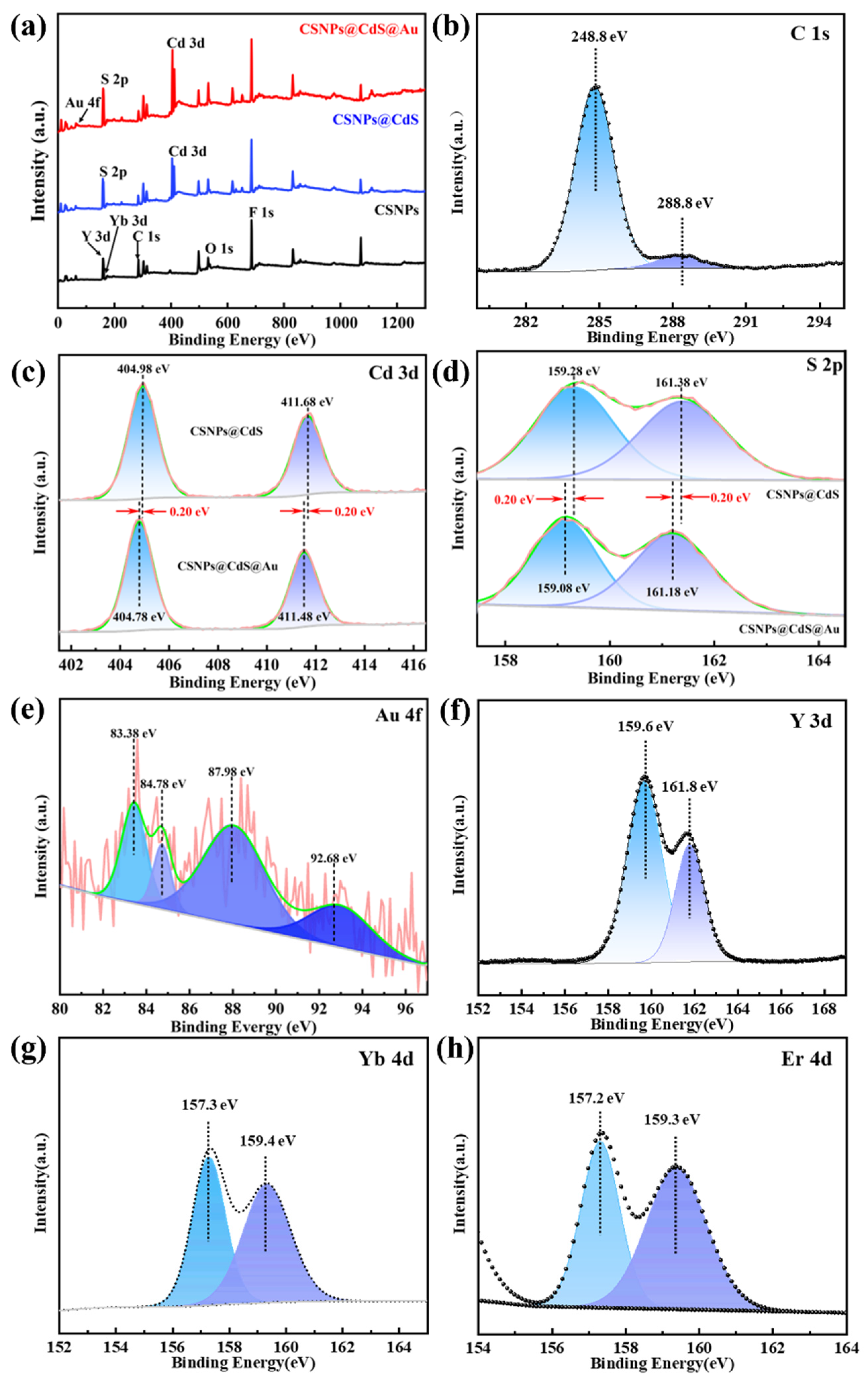
The chemical states of the involved elements of CSNPs@CdS@Au were investigated by XPS. The survey spectrum of CSNPs@CdS@Au reveals the co-presence of Cd, S, Na, Y, F, and Au elements (Figure 5a). Due to their relatively low doping concentrations (2% for Er3+ and 20% for Yb3+) and the shielding effect of the outer CdS and Au layers, the survey spectrum did not reveal the presence of Yb and Er. All XPS spectra were calibrated using the adventitious carbon C 1s (Figure 5b) band at 284.8 eV. The core energy spectrum of Cd exhibits two spin–orbit bands at approximately 411.48 eV and 404.78 eV, as shown in Figure 5c, which correspond to Cd 3d3/2 and 3d5/2, respectively. This confirms the oxidation state of CdS compounds as +2. And the high-resolution spectrum of S 2p (Figure 5d) exhibits two bands at 161.18 eV (S 2p1/2) and 159.08 eV (S 2p3/2) that can be assigned to S2−. The Cd and S element spectrum in CSNPs@CdS@Au material shows a shift compared with that in CSNPs@CdS. When the CdS shell wraps around Au NPs, the close contact between CdS and Au leads to a SPR effect [46], causing electrons to transfer from Au to CdS and inhibiting the recombination of electron–hole pairs [47]. The Au 4f spectra (Figure 5e) show the zero-oxidation state of Au 4f7/2 bands at 83.38 and 87.98 eV, and the peak of Au 4f5/2 bands at 84.78 and 92.68 eV, which may be due to the charge transfer induced by the Au NPs on the CdS carrier [48]. This indicates that the introduction of Au has caused an electron shift in CdS. In addition, the high-resolution spectra of Y 3d, Yb 4d, and Er 4d are obviously found in Figure 5f–h.
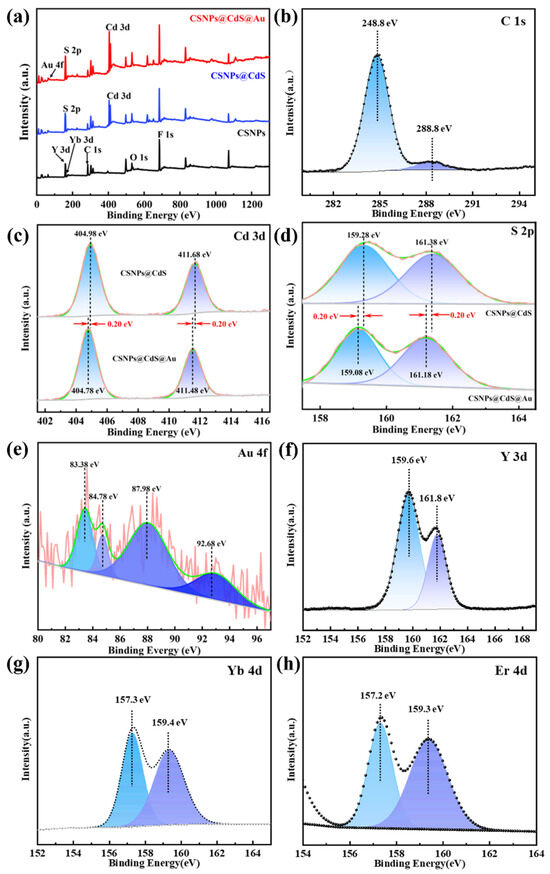
Figure 5.
(a) XPS survey spectra of CSNPs, CSNPs@CdS, and CSNPs@CdS@Au; (b) C1s; (c) Cd 3d (d) S 2p; (e) Au 4f; (f) Y 3d; (g) Yb 4d; (h) Er 4d.
3.4. Photoluminescence (PL) Spectra Analysis
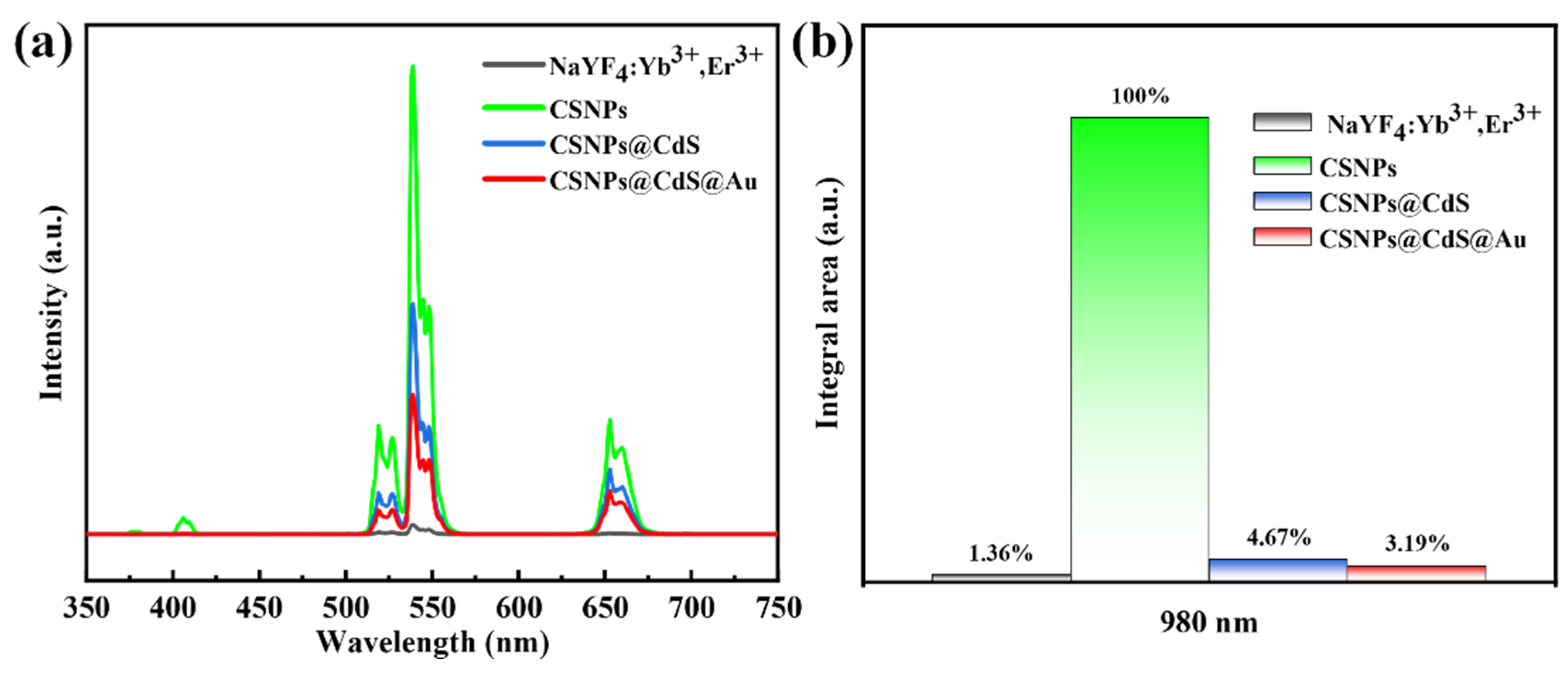
Figure 6a shows the UC PL spectra of CNPs, CSNPs, CSNPs@CdS, and CSNPs@CdS@Au, respectively, under excitation at 980 nm. It is well known that NaYF4 particles are an excellent host matrix for UC luminescence when co-doped with Yb3+ and Er3+ in NaYF4. Under 980 nm NIR light excitation, CNPs produces strong UC emission, with Yb3+ acting as a sensitizing ion for the sequential absorption of 980 nm NIR light and Er3+ acting as an activating ion for the emission of a series of spectra at wavelengths smaller than 980 nm [44]. There are three distinct bands at 520, 540, and 654 nm attributed to the 2H11/2→4I15/2, 4S3/2→4I15/2, and 4F9/2→4I15/2 transition, and a weak peak at 407 nm, which is usually attributed to the 2H9/2→4I15/2 leap of the Er3+ ion [42]. The higher emission intensity of the CSNPs core shell than that of the CNPs core is observed from the green line in the figure, which originates from the suppression of CNPs surface quenching. After coating the CSNPs, the fluorescence intensities of CSNPs@CdS and CSNPs@CdS@Au decreased significantly. By integrating the UV emission bands of the UC emission spectra of the four materials, as shown in Figure 6b, under the excited light of 980 nm, the UV emission intensity of CSNPs@CdS@Au is 3.19% of that of CSNPs. This shows that the CdS shell in CSNPs@CdS@Au effectively absorbs more than 96.81% of the UV emission. This shows that the unique four-layer core–shell structure of CSNPs@CdS@Au can greatly enhance the light absorption.
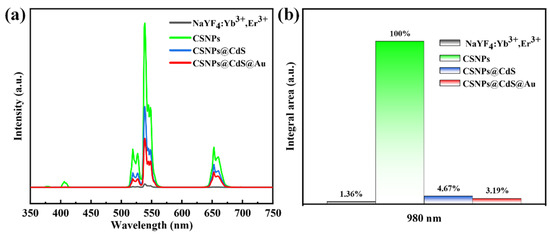
Figure 6.
(a) PL spectra of different materials. All the UC emission spectra were recorded upon 980 nm NIR excitation. (b) Integrated intensity of the UC emission in the 350–450 nm range for the samples shown in (a).
3.5. Photocatalytic Performance
To evaluate the photocatalytic performance of CSNPs@CdS@Au in the degradation of RhB, we compared it with CSNPs, CdS, CSNPs@CdS, and a physical mixture of CSNPs and CdS (CSNPs/CdS). A blank experiment without any catalyst was also conducted for reference. It is reported that RhB has a maximum absorption peak at a wavelength of 554 nm [49]. Figure 7a shows the time-dependent photocatalytic degradation of RhB by CSNPs@CdS@Au.
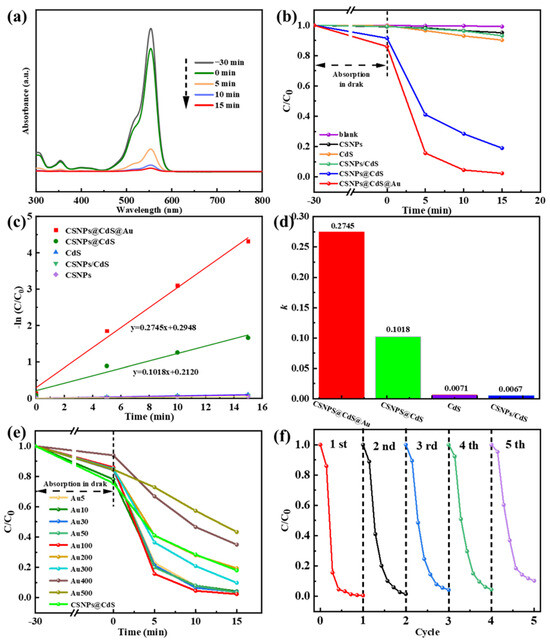
Figure 7.
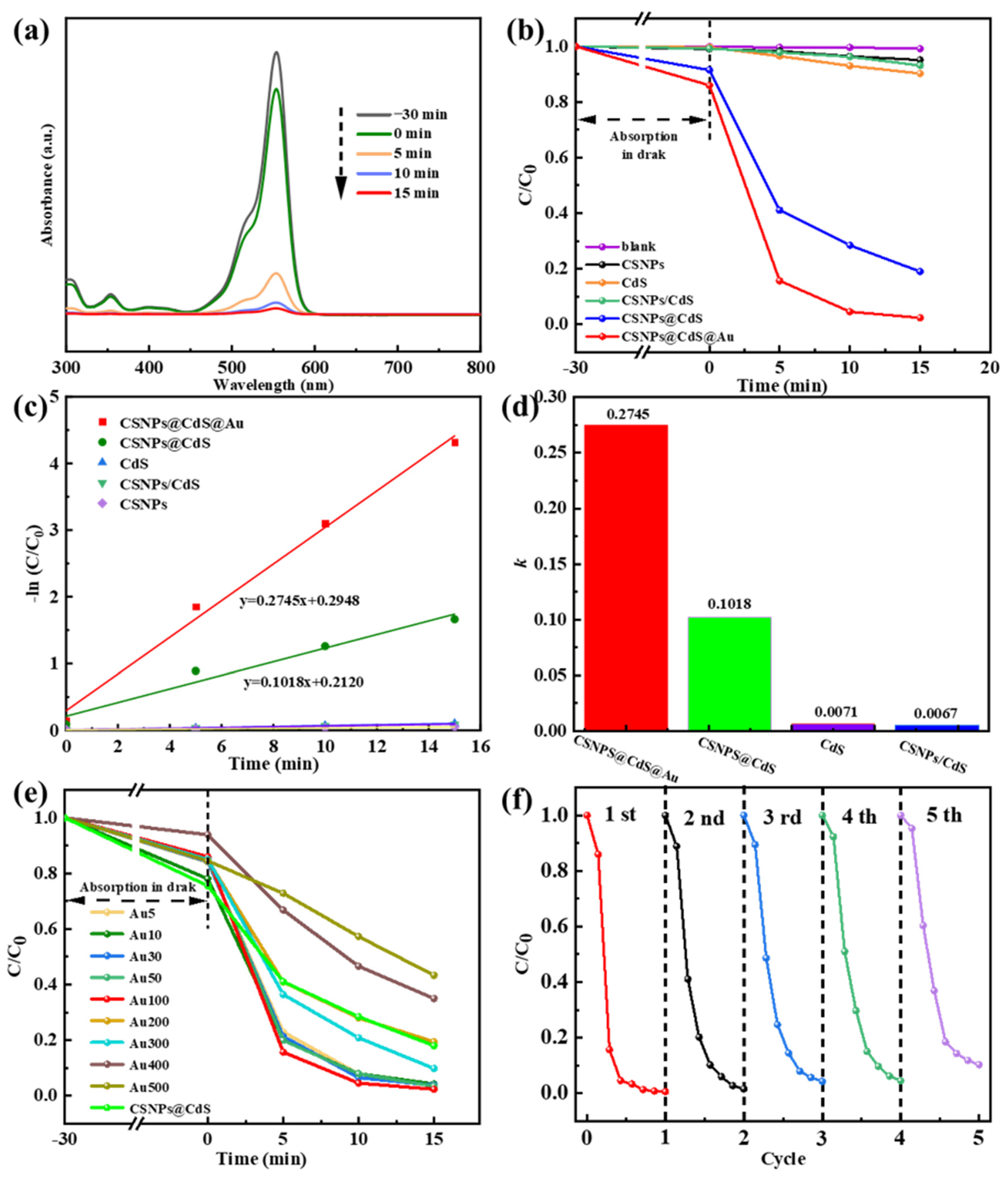
(a) UV-vis spectra depicting the photodegradation process of RhB solution by the CSNPs@CdS@Au composite material. (b) Degradation of RhB solution under simulated visible light. (c) Time-dependent pseudo-first-order kinetic curves. (d) Column graph of k. (e) Effect of Au content variation on the RhB degradation rate of the CSNPs@CdS@Au catalyst. (f) Cycling performance of the CSNPs@CdS@Au photocatalyst for RhB degradation.
As shown in Figure 7b, the catalytic experiments of different catalysts are compared, and it can be seen that the orange line represents the catalytic rate of bare CdS. After 15 min of catalysis by bare CdS, the degradation efficiency of RhB is approximately 9.8% [degradation efficiency = (1 − C/C0) × 100%, where C0 is the initial concentration and C is the post-irradiation concentration]. The black line represents CSNPs. The degradation efficiency of RhB within 15 min is approximately 5.0%, which is close to the adsorption effect, indicating that CSNPs primarily function as light converters to emit UV/vis light but lack inherent catalytic activity for RhB degradation by themselves. The green line represents the physical mixture catalyst composed of CdS and CSNPs. The degradation rate after 15 min is 6.9%, indicating that the physical mixture of the two does not enhance the catalytic efficiency. The blue line represents the catalytic rate of CSNPs@CdS. Within 15 min, it degraded 81% of RhB, indicating that the core–shell structure of CSNPs@CdS NPs has high catalytic performance. The CSNPs@CdS@Au catalyst (red line) demonstrated the highest activity, degrading 97.7% of RhB within 15 min. The incorporation of Au NPs significantly enhanced the performance compared to CSNPs@CdS. It can be seen that the four-layer core–shell structure of the CSNPs@CdS@Au catalyst, due to its structural specificity, has a superior performance compared with other catalysts, as well as catalysts of other materials reported previously [18,26,50,51].
Correspondingly, to further quantify the degradability of this series of samples, we performed the calculation of the first-order kinetic fitting constant k value using the equation below: −ln (C/C0) = kt. Where t represents irradiation time, k represents the pseudo-first-order kinetic constant, C0 represents the initial concentration of RhB, and C represents RhB concentration at a moment. From Figure 7c,d, The CSNPs@CdS@Au exhibit the highest k value (0.2745 min−1), which is significantly higher than CSNPs@Cds (0.1018 min−1), CSNPs/Cds (0.0067 min−1), and bare Cds (0.0071 min−1). As shown in Figure 7e, by controlling the content of Au element as the variable, the optimal ratio of Au loading onto CSNPs@Cds was obtained. When the content of added Au was lower than 100 μL, with the increase in Au content, the degradation efficiency in 15 min was 95.8% for the catalyst with the dosage of Au10, 96.2% for Au30, and 96.6% for Au50, which indicated that the higher the dosage of catalyst dosed, the higher the degradation rate of RhB. This may be due to an increase in the number of photocatalytic active sites (introduced by Au NPs), hence more reactive radicals being produced with higher Au loading (up to Au100). However, when the Au content exceeded 100 μL, the catalytic efficiency actually decreased. This might be due to the excessive increase in Au content, which occupied the active sites of CdS, resulting in a decrease in the catalytic rate. Correspondingly, cycling photocatalytic reactions were also performed on CSNPs@CdS@Au to verify the recycling ability of CSNPs@CdS@Au. From Figure 7f and Table 1, the CSNPs@CdS@Au demonstrates great recyclability because the photodegradation efficiency of RhB is still kept at 93.25% after five cycles. As a result of their excellent structural stability, CSNPs@CdS@Au possess high reliability. Table 2 presents the comparative results between the CdS-based catalyst and similar photocatalysts previously reported for RhB degradation.

Table 1.
Photocatalytic degradation rate after 15 min of reaction over 5 cycles.

Table 2.
Comparison of CdS-based catalysts for RhB catalysis in earlier reports.
3.6. Photocatalytic Mechanism
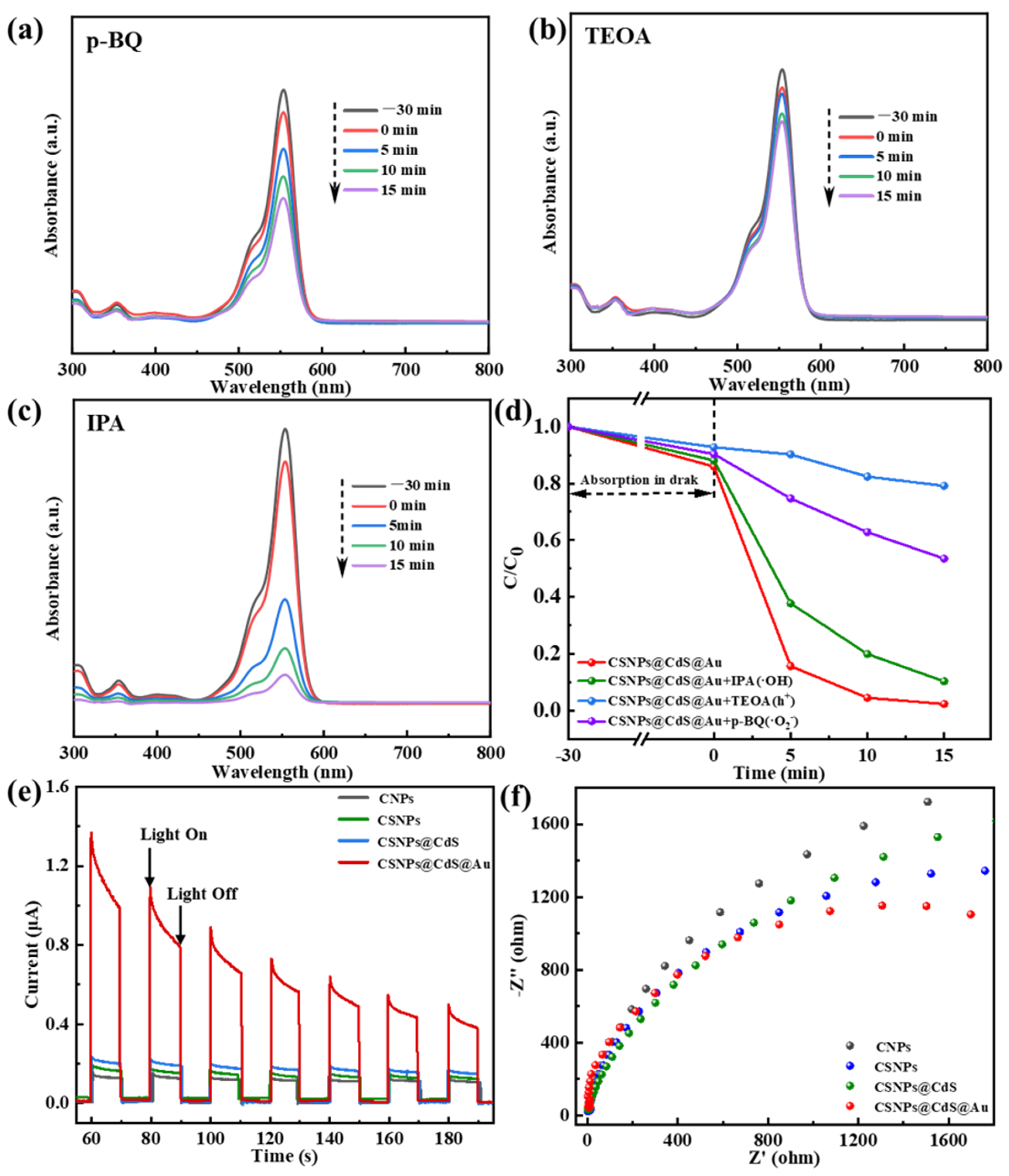
The active species of CSNPs@CdS@Au during the photocatalytic reaction were identified via trapping experiments. Various scavengers, including triethanolamine (TEOA), 1,4-Benzoquinone (p-BQ), and isopropyl alcohol (IPA), were introduced into the solution of RhB [34,53]. The h+, O2− and OH were trapped by TEOA, p-BQ, and IPA, respectively [54]. As shown in Figure 8a–d, the addition of p-BQ, TEOA, and IPA all resulted in a significant decrease in the catalytic rate. After 15 min of reaction, the degradation rates were 46.52% for p-BQ, 20.86% for TEOA, and 89.68% for IPA. The change in degradation rate was particularly pronounced when TEOA was introduced into the reaction system. It can therefore be inferred that the O2− serves as the dominant active species responsible for RhB degradation over this catalyst, while the e− acts as a secondary contributor. It is noted that RhB dye itself could act as a photosensitizer in the initial stage of the photocatalytic process. However, the active species trapping experiments and the significant performance difference between catalysts (e.g., bare CdS vs. CSNPs@CdS@Au) strongly suggest that the degradation is predominantly driven by the catalysts, where the UC process and the Au/CdS Schottky junction play decisive roles in generating active radicals (mainly O2−).
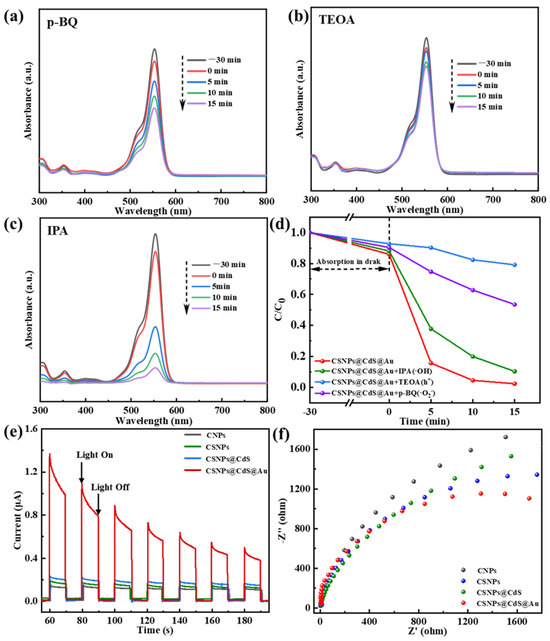
Figure 8.
Photocatalytic degradation of RhB by CSNPs@CdS@Au: trapping of active species, (a) p-BQ; (b) TEOA; (c) IPA; (d) Effects of scavengers on RhB degradation by CSNPs@CdS@Au; (e) transient photocurrent response curve, and (f) electrochemical impedance spectroscopy (EIS) of CSNPs@CdS@Au, CSNPs@CdS, CSNPs, and CNPs.
Meanwhile, Figure 8e displays the photocurrent response curves of various photocatalysts, which alternate with the on-off cycles of the lamp. Among them, the CSNPs@CdS@Au sample exhibits the highest transient photocurrent response, indicating superior photo-generated electron–hole transfer efficiency compared to other samples—typically signifying enhanced photocatalytic activity. The gradual increase in photocurrent intensity during the on-light intervals for the CSNPs@CdS, CSNPs, and CNPs samples may involve multiple factors: First, the slow rise in photocurrent in semiconductors (known as the relaxation phenomenon) reveals the semiconductor’s sensitivity to light intensity. The Au/CdS heterojunction formed by morphological differences alters carrier flow [33]. Second, it may be influenced by the synergistic effects between carrier flow direction and effective defects. Additionally, the decrease in photocurrent intensity in CSNPs@CdS@Au can be attributed to hole accumulation. To further validate the superiority of CSNPs@CdS@Au over other structured photocatalysts in terms of carrier mobility, electrochemical impedance spectroscopy (EIS) measurements were conducted (Figure 8f). Compared to other samples, CSNPs@CdS@Au exhibited a significantly smaller circular measurement radius. Furthermore, fitting the EIS curves yielded charge transfer resistance values of 8.59 Ω, 9.182 Ω, 12.76 Ω, and 13.33 Ω for CSNPs@CdS@Au, CSNPs@CdS, CSNPs, and CNPs, respectively. The results indicate that CSNPs@CdS@Au exhibits more efficient surface charge transfer capability, thereby achieving optimal photocatalytic activity, further confirming the superiority of the CSNPs@CdS@Au morphology.
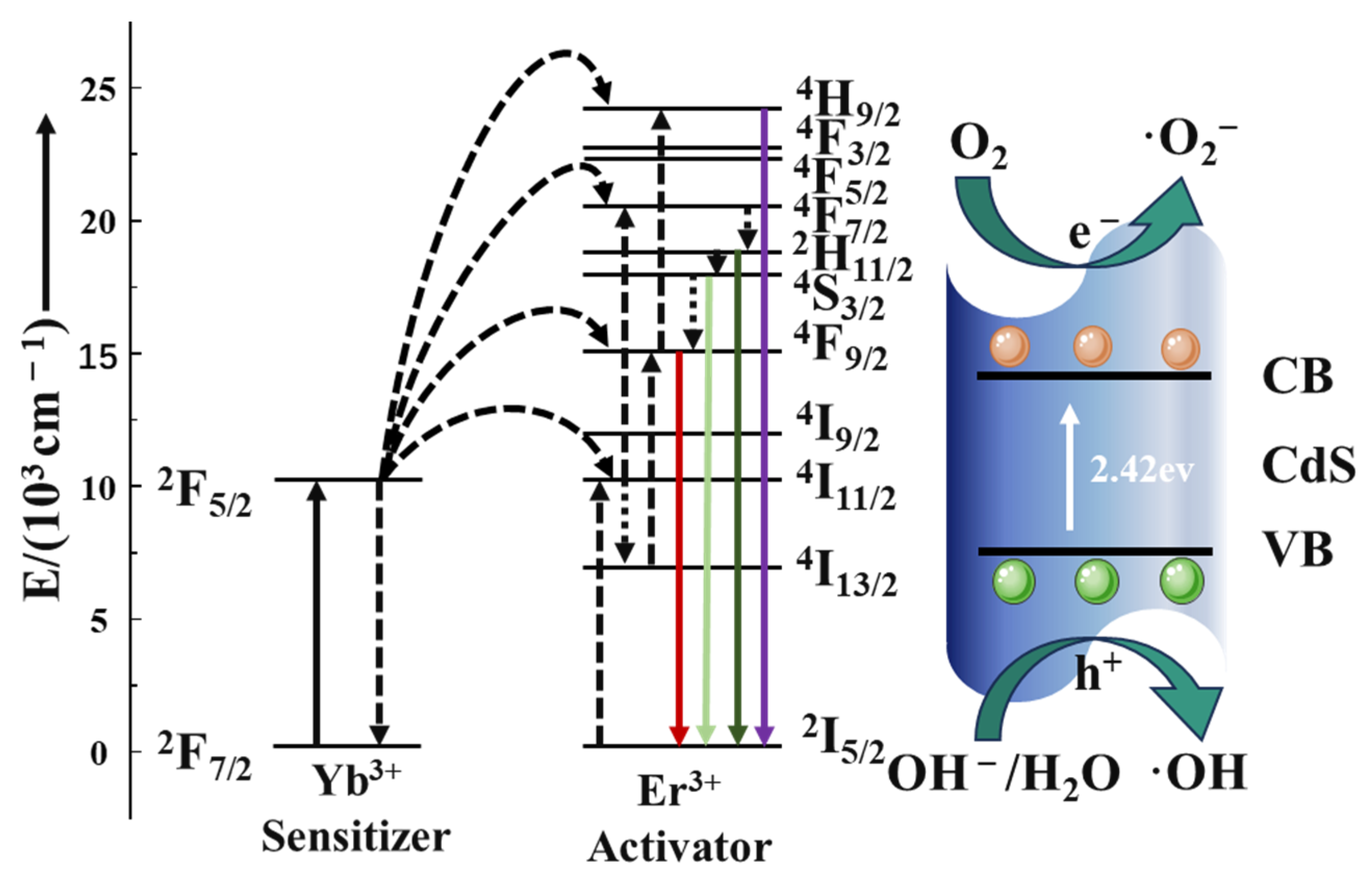
Under 980 nm laser irradiation, Yb3+ absorbs near-infrared photons and makes a 2F7/2→2F5/2 energy transition, which then transitions to the ground state 2F7/2 and transfers energy to nearby Er3+ [36]. As shown in Figure 9, after absorbing energy, the electrons of Er3+ can reach the excited state through the transition of 4I15/2→4I11/2, and the electrons in the excited state can transition to the excited state of a higher energy level through the transition of 4I11/2→4F7/2 or 4I13/2-4F9/2 due to energy matching [36]. Er3+-released energy through non-radiative relaxation can transition to 2H11/2 and 4S3/2 orbital energy levels [57], and when the electrons in these two orbitals transition back to the ground state, they radiate light at 522 and 541 nm, respectively. On the other hand, a 4F9/2→4I15/2 transition from a higher energy level to an electron in a 4F9/2 orbital through non-radiative relaxation or from an 4I13/2 orbital to a 4F9/2 orbital will radiate 654 nm of light. However, this emitted light wave energy can be absorbed by the catalyst and then produce electron–hole pairs to decompose RhB. These photogenerated carriers are the core of the photocatalytic reaction and have strong Redox ability, which can trigger a series of Redox reactions.
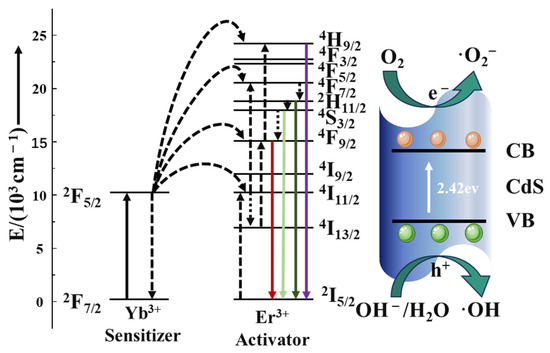
Figure 9.
Schematic illustration of the proposed UC-mediated photocatalytic mechanism.
4. Conclusions
In summary, we successfully developed a four-layer core–shell structured photocatalyst (CSNPs@CdS@Au). Experimental results demonstrate that the composite catalyst exhibits remarkable enhancement in both catalytic activity under visible light and cyclic stability compared to other catalysts. The performance enhancement mechanism can be attributed to three key factors: (1) the uniquely designed multilayer core–shell architecture significantly enhances structural stability through physical isolation effects; (2) the NaYF4 inert shell encapsulation amplifies UC luminescence intensity by suppressing surface quenching; and (3) the Schottky junction formed between Au NPs and CdS effectively promotes spatial separation of photogenerated charge carriers and increases reactive active sites. In addition, further analysis suggests that optimizing the quantum yield of UC materials (e.g., via optimizing lanthanide doping) or developing Z-scheme heterojunction configurations could unlock new frontiers in photocatalytic efficiency. This work not only provides crucial theoretical foundations and practical references for constructing efficient and stable photocatalytic systems but also opens new avenues for designing advanced catalysts for full-spectrum solar energy utilization.
Author Contributions
L.X.: Supervision, Funding acquisition, Writing–review and editing; Y.T. and P.Y.: Investigation, Methodology; T.D.: Investigation, Methodology; J.X.: Data curation; J.D.: Conceptualization, Methodology, Resources, Funding acquisition; R.Z.: Validation, Formal analysis, Funding acquisition; H.T.: Methodology, Validation; Y.L. and Z.T.: Conceptualization, Methodology, Writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
The National Natural Science Foundation of China (grant number 52474378 and 52374387), the Hunan Provincial Natural Science Foundation (2025JJ50334 and 2025JJ70008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Liu, S.; Li, Y.; Zhong, X.; Yang, K.; Li, X.; Jin, W.; Liu, H.; Xie, R. Metal Sulfide-Based Nanoarchitectures for Energetic and Environmental Applications. Small Struct. 2024, 5, 2300536. [Google Scholar] [CrossRef]
- Guo, H.; Deng, Y.; Yin, H.; Liu, J.; Zou, S. Fabricating BiOCl Nanoflake/FeOCl Nanospindle Heterostructures for Efficient Visible-Light Photocatalysis. Molecules 2023, 28, 6949. [Google Scholar] [CrossRef]
- Abdikarimova, U.; Bissenova, M.; Matsko, N.; Issadykov, A.; Khromushin, I.; Aksenova, T.; Munasbayeva, K.; Slyamzhanov, E.; Serik, A. Visible Light-Driven Photocatalysis of Al-Doped SrTiO3: Experimental and DFT Study. Molecules 2024, 29, 5326. [Google Scholar] [CrossRef]
- Khachane, M.; Bouddouch, A.; Bakiz, B.; Benlhachemi, A.; Kadmi, Y. High Photocatalytic Activity for the Degradation of Rhodamine B in Water. Int. J. Environ. Sci. Technol. 2022, 19, 8825–8834. [Google Scholar] [CrossRef]
- Abdelbar, N.M.; Ahmed, M.A.; Mohamed, A.A. A Novel Layered Double Hydroxide-Based Ternary Nanocomposite for the Effective Photocatalytic Degradation of Rhodamine B. RSC Adv. 2024, 14, 14523–14538. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of Rhodamine B in Water by Ultrasound-Assisted TiO2 Photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Alakhrasa, F.; Alhajria, E.; Haounatib, R.; Ouachtak, H.; Addi, A.; Saleh, T. Comparative Study of Photocatalytic Degradation of Rhodamine B Using Natural-Based Zeolite Composites. Surf. Interfaces 2020, 20, 100611. [Google Scholar] [CrossRef]
- Gaggero, E.; López-Muñoz, M.J.; Paganini, M.C.; Arencibia, A.; Bertinetti, S.; Fernández de Paz, N.; Calza, P. Mercury and Organic Pollutants Removal from Aqueous Solutions by Heterogeneous Photocatalysis with ZnO-Based Materials. Molecules 2023, 28, 2650. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, Y.; Sun, W. Exploring the Dynamics of Charge Transfer in Photocatalysis: Applications of Femtosecond Transient Absorption Spectroscopy. Molecules 2024, 29, 3995. [Google Scholar] [CrossRef] [PubMed]
- Dhruv, L.; Kori, D.K.K.; Das, A.K. Sodium alginate–CdS nanostructures: Reinforcing Chemoselectivity In nitro-organic Reduction and Dye Degradation Through Photoinduced Electron Transfer. ACS Appl. Nano Mater. 2024, 7, 6471–6486. [Google Scholar] [CrossRef]
- Li, J.; Shao, W.; Geng, M.; Wan, S.; Ou, M.; Chen, Y. Combined Schottky Junction and Doping Effect in CdxZn1−xS@Au/BiVO4 Z-Scheme Photocatalyst with Boosted Carriers Charge Separation for CO2 Reduction by H2O. J. Colloid Interf. Sci. 2022, 606, 1469–1476. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, H.; Wang, J.; Zhang, D.; Wang, M.; Jiang, Z. Enhancing Effect of NaYF4: Yb, Tm on the Photocatalytic Performance of BiVO4 Nnder NIR and Full Spectrum. J. Mater. Res. 2023, 38, 1894–1908. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, G.; Hong, X. Influence of Surface Defects and Palladium Deposition on the Activity of CdS Nanocrystals for Photocatalytic Hydrogen Production. Acta Phys.-Chim. Sin. 2019, 35, 215–222. [Google Scholar] [CrossRef]
- Zhu, L.-B.; Bao, N.; Zhang, Q.; Ding, S.-N. Synergistically Enhanced Photocatalytic Degradation by Coupling Slow-Photon Effect with Z-Scheme Charge Transfer in CdS QDs/IO-TiO2 Heterojunction. Molecules 2023, 28, 5437. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ahmed, S.B.; Shabir, M.; Imran, M.; Hassan, A.M.; Alatawi, N.S. Review on CdS-Derived Photocatalysts for Solar Photocatalytic Applications–Advances and Challenges. J. Ind. Eng. Chem. 2024, 130, 105–124. [Google Scholar] [CrossRef]
- Qamar, M.; Zhang, B.; Feng, Y. Enhanced Photon Harvesting in Dye-Sensitized Solar Cells by Doping TiO2 Photoanode with NaYF4:Yb3+,Tm3+ Microrods. Opt. Mater. 2019, 89, 368–374. [Google Scholar] [CrossRef]
- Schäfer, H.; Ptacek, P.; Eickmeier, H.; Haase, M. Synthesis of Hexagonal Yb3+,Er3+-Doped NaYF4 Nanocrystals at Low Temperature. Adv. Funct. Mater. 2009, 19, 3091–3097. [Google Scholar] [CrossRef]
- Balaji, R.; Kumar, S.; Reddy, K.L.; Sharma, V.; Bhattacharyyab, K.; Krishnana, V. Near-Infrared Driven Photocatalytic Performance of Lanthanide-Doped NaYF4@CdS Core-Shell Nanostructures with Enhanced Upconversion Properties. J. Alloys Compd. 2017, 724, 481–491. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, L.; Zhang, Y.; Yang, Y.; Fang, Z.; Wang, B.; Zhang, S.; Liu, P. Near-infrared-activated NaYF4:Yb3+, Er3+/Au/CdS for H2 production via photoreforming of bio-ethanol: Plasmonic Au as light nanoantenna, energy relay, electron sink and co-catalyst. J. Mater. Chem. A 2017, 5, 10311–10320. [Google Scholar] [CrossRef]
- Rinkel, T.; Raj, A.N.; Dîhnen, S.; Haase, M. Synthesis of 10 nm β-NaYF4:Yb,Er/NaYF4 Core/Shell Upconversion Nanocrystals with 5 nm Particle Cores. Angew. Chem. Int. Ed. 2015, 55, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, H.; Wang, Z.; Wang, P.; Zheng, Z.; Liu, Y.; Dai, Y.; Li, Y.; Huang, B. Efficient Near-Infrared Photocatalysts Based on NaYF4:Yb3+, Tm3+@NaYF4:Yb3+, Nd3+@ TiO2 Core@Shell Nanoparticles. Chem. Eng. J. 2019, 361, 1089–1097. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Zhu, J.; Yu, J.C. NaYF4:Yb,Tm/CdS Composite as a Novel Near-Infrared-Driven Photocatalyst. Appl. Catal. B Environ. 2010, 100, 433–439. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Kang, Y.; Niu, P.; Kang, X.; Yang, Z.; Ye, H.; Liu, G. Strong Interface Contact Detween NaYF4:Yb,Er and CdS Promoting Photocatalytic Hydrogen Evolution of NaYF4:Yb,Er/CdS Composites. J. Mater. Sci. Technol. 2022, 102, 1–7. [Google Scholar] [CrossRef]
- Dannareli, B.; Ruben, D.; Enric, P.; Emilio, J.; Juan, H.; Jacob, L.; Pablo, L.; Israel, R. Volumetric Temperature Mapping Using Light-Sheet Microscopy and Upconversion Fluorescence from Micro- and Nano-Rare Earth Composites. Micromachines 2023, 14, 2097. [Google Scholar] [CrossRef]
- Jones, C.; Panov, N.; Artiom, S.; Gibbons, J.; Hesse, F.; Bos, J.G.; Wang, X.; Vetrone, F.; Chen, G.; Hemmer, E.; et al. Effect of Light Scattering on Upconversion Photoluminescence Quantum Yield in Microscale-to-Nanoscale Materials. Opt. Express 2020, 28, 398353. [Google Scholar] [CrossRef]
- Feng, P.; Pan, Y.; Ye, H. Core–Shell Structured NaYF4:Yb,Tm@CdS Composite for Enhanced Photocatalytic Properties. RSC Adv. 2018, 8, 35306–35313. [Google Scholar] [CrossRef]
- Ling, S.; Cui, X.; Zhang, X.; Liu, B.; He, C.; Wang, J.; Qin, W.; Zhang, Y.; Gao, Y.; Bai, G. Glutathione-Protected Upconversion Nanocluster Decorated Cadmium Sulfide with Enhanced Photostability and Photocatalytic Activity. J. Colloid Interface Sci. 2018, 530, 120–126. [Google Scholar] [CrossRef]
- Farhadi, H.; Keramati, N. Investigation of Kinetics, Isotherms, Thermodynamics and Photocatalytic Regeneration of Exfoliated Graphitic Carbon Nitride/zeolite as Dye Adsorbent. Sci. Rep. 2023, 13, 14098. [Google Scholar] [CrossRef]
- Peana, M.; Pelucelli, A.; Chasapis, C.; Perlepes, S.; Bekiari, V.; Medici, S.; Zoroddu, M. Biological Effects of Human Exposure to Environmental Cadmium. Biomolecules 2023, 13, 36. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, L.; Zhang, L.; Zheng, G.; Zhang, X.; Zhen, Z. Activity and Stability Origin of Core–Shell Catalysts: Unignorable atomic Diffusion Behavior. Chem. Sci. 2025, 16, 3323. [Google Scholar] [CrossRef]
- Jiang, Y.; Chai, Y.; Jiang, Y.; Tian, S.; Wang, Z.; Cao, Y.; Lam, J.C.; Zhu, J.; Lin, R.; Zhu, W. Bacteria Photosensitized by CdS@Au@Polymeric Coatings for Sustainable Carbon Dioxide Fixation and Bioplastic Production. Chin. Chem. Lett. 2025, 111115. [Google Scholar] [CrossRef]
- Shao, L.; Lu, W.; Yu, R.; Han, X.; Zhao, J.; Chen, D.; Wang, Y.; Shi, X.; Su, X.; Teng, Z. Synthesis of Aminated Three-Layered Hollow Mesoporous Organosilica Spheres and in-situ Loading Gold Nanoparticles. Mater. Lett. 2023, 330, 133270. [Google Scholar] [CrossRef]
- Li, Z.; Huang, W.; Liu, J.; Lv, K.; Li, Q. Embedding CdS@Au into Ultrathin Ti3−xC2Ty to Build Dual Schottky Barriers for Photocatalytic H2 Production. ACS Catal. 2021, 11, 8510–8520. [Google Scholar] [CrossRef]
- Dong, Z.; Meng, C.; Li, Z.; Zeng, D.; Wang, Y.; Cheng, Z.; Cao, X.; Zhang, Z.; Liu, Y. Novel Co3O4@TiO2@CdS@Au Double-Shelled Nanocage for High-Efficient Photocatalysis Removal of U(VI): Roles of Spatial Charges Separation and Photothermal Effect. J. Hazard. Mater. 2023, 452, 131248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, G.; Wu, X.; Lv, K.; Chu, Y.; Qin, H.; Niu, J. Ultrathin Niobate Nanosheet Assembly with Au NPs and CdS QDs as a Highly Efficient Photocatalyst. Chem. Eur. J. 2022, 28, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, H.; Li, L. Power-Dependent Up-Conversion Emissions and Temperature Sensing Properties of NaYF4:Er/Yb@NaYF4 Phosphors. Phys. Scr. 2024, 99, 5. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Gusarov, S. Advances in Computational Methods for Modeling Photocatalytic Reactions: A Review of Recent Developments. Materials 2024, 17, 2119. [Google Scholar] [CrossRef]
- Khan, S.; Noor, T.; Iqbal, N.; Yaqoob, L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega 2024, 9, 21751–21767. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Wu, J.; Zhang, J.; Ling, H.; Zhao, Y.; Liu, W.; Di, M.; Shan, D.; Li, X.; Wang, B. Novel Simulated Sunlight-Driven NaYF4:Yb,Tm@MIL-53(Fe) Nanorods Photocatalysts for Degradation of Organic Pollutants and Enhanced Antibacterial Activity. Appl. Surf. Sci. 2023, 639, 158249. [Google Scholar] [CrossRef]
- Xu, Z.; Yue, W.; Li, C.; Wang, L.; Xu, Y.; Ye, Z.; Zhang, J. Rational Synthesis of Au–CdS Composite Photocatalysts for Broad-Spectrum Photocatalytic Hydrogen Evolution. ACS Nano 2023, 17, 11655–11664. [Google Scholar] [CrossRef]
- Gong, G.; Song, Y.; Tan, H.; Xie, S.; Zhang, C.; Xu, L.; Xu, J.; Zheng, J. Design of Core/active-Shell NaYF4:Ln3+@NaYF4:Yb3+ Nanophosphors with Enhanced Red-Green-Blue Upconversion Luminescence for Anti-Counterfeiting Printing. Compos. Part B 2019, 179, 107504. [Google Scholar] [CrossRef]
- Homann, C.; Krukewitt, L.; Frenzel, F.; Bettina, G.; Wurth, C.; Markus, H. NaYF4:Yb,Er/NaYF4 Core/shell Nanocrystals with High Upconversion Luminescence Quantum Yield. Angew. Chem. Int. Ed. 2018, 57, 8765–8769. [Google Scholar] [CrossRef]
- Xie, S.; Tong, C.; Tan, H.; Li, N.; Gong, L.; Xu, J.; Xu, L.; Zhang, C. Hydrothermal Synthesis and Inkjet Printing of Hexagonal-Phase NaYF4: Ln3+ Upconversion Hollow Microtubes for Smart Anti-Counterfeiting Encryption. Mater. Chem. Front. 2018, 2, 1997–2005. [Google Scholar] [CrossRef]
- Kuang, P.; Zheng, P.; Liu, Z.; Lei, J.; Wu, H.; Li, N.; Ma, T. Embedding Au Quantum Dots in Rimous Cadmium Sulfide Nanospheres for Enhanced Photocatalytic Hydrogen Evolution. Small 2016, 12, 6735–6744. [Google Scholar] [CrossRef]
- Tang, P.; Chen, M.; Li, H.; Zhong, T.; Liu, J.; Yang, M.; Deng, G. CdS@Au Bicro-Spheres as an Active and Durable Bifunctional Catalyst for the Urea-Assisted H2 Evolution Reaction. Mater. Lett. 2024, 354, 135353. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Han, J.; Li, R.; Huang, M. Stepwise Synthesis of Au@CdS-CdS Nanoflowers and Their Enhanced Photocatalytic Properties. Nanoscale Res. Lett. 2019, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Wang, H.; Yu, K.; Chang, Y.; Ma, X.; Dong, S. Photochemical Preparation of the Ternary Composite CdS/Au/g-C3N4with Enhanced Visible Light Photocatalytic Performance and its Microstructure. RSC Adv. 2016, 6, 77760–77767. [Google Scholar] [CrossRef]
- Jakiminska, A.; Pawlicki, M.; Macyk, W. Photocatalytic transformation of Rhodamine B to Rhodamine -110–The mechanism revisited. J. Photochem. Photobiol. A 2022, 433, 114176. [Google Scholar] [CrossRef]
- Gurugubelli, T.R.; Ravikumar, R.V.S.S.N.; Koutavarapu, R. Enhanced Photocatalytic Activity of ZnO–CdS Composite Nanostructures Towards the Degradation of Rhodamine B Under Solar Light. Catalysts 2022, 12, 84. [Google Scholar] [CrossRef]
- Abdelbar, N.M.; Ahmed, M.A.; Mohamed, A.A. Highly Efficient Photocatalytic Degradation of Rhodamine B by Immobilizing CdS Quantum Dots on ZnCr-Layered Double Hydroxide Nanosheets. Opt. Mater. 2024, 155, 115802. [Google Scholar] [CrossRef]
- Zhu, C.; Shen, M.; Qi, M.; Zhao, Y.; Xu, Z.; Li, P.; Ru, J.; Gao, W.; Zhang, X. Constructed CdS/Mn-MOF Heterostructure for Promoting Photocatalytic Degradation of Rhodamine B. Dyes Pigments 2023, 219, 111607. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuang, Y.; Dong, L.; Mu, H.; Li, D.; Wang, L.; Tian, S. Construction of CeO2/CdS Heterostructure and Study on Photocatalytic Mechanism of Rhodamine B Degradation. J. Inorg. Organomet. Polym. Mater. 2023, 34, 175–189. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Feng, W.; Zhong, X.; Li, J.; Liu, S.; Liu, H.; Ma, G.; Xie, R. Three-dimensional Zinc Oxide Decorated with Cadmium Sulfide Nanoparticles Heterogenous Nanoarchitectures with Expedited Charge Separation toward Efficient Photocatalytic Degradation of Organic Pollutants. Mater. Sci. Eng. B 2023, 292, 116459. [Google Scholar] [CrossRef]
- Wang, W.; Qin, X.; Wang, X.; Ma, K.; Wu, Z.; Si, H.; Zhang, J. Sulfur Vacancy-rich (α/β-CdS)/SiO2 Photocatalysts for Enhanced Visible-light-driven Photocatalytic Degradation of Rhodamine B. Environ. Pollut. 2024, 345, 123428. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, M.; Cao, R.; Wang, Y.; Ma, K.; Zhang, J. Facile Synthesis of a Novel CeCO3OH@(H/C–CdS) Catalyst with Synergistic Effect of Heterophase Junction and Heterojunction for Enhanced Visible-light Photocatalytic Degradation Efficiency at Room Temperature. J. Colloid. Interface Sci. 2025, 677, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, W.; Wu, Z.; Liang, Z.; Huang, Y.; Tan, Q.; Liu, T.; Han, D.; Niu, L. Near-Infrared Responsive Magnetic Photocatalyst Based on NaYF4:Yb3+/Er3+@Cu2O@MoS2@Fe3O4 for the Efficient Degradation of Organic Contaminants. New J. Chem. 2024, 48, 7688–7698. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).