Abstract
The western hemlock looper, Lambdina fiscellaria lugubrosa (Hulst) is a destructive defoliator of coniferous forests and a major cause of economic losses in forestry. A novel and efficient stereoselective synthesis of the sex pheromone of the western hemlock looper (1, 2 and 3) has been successfully achieved. The synthetic strategy integrates several key transformations, including Evans’ chiral auxiliaries, Grignard cross-coupling, hydroboration–oxidation, sulfone alkylation, and hydrogenation, providing an efficient and scalable approach for sex pheromone production. The three synthesized pheromone components were subsequently tested for their ability to attract Semiothisa cinerearia (Bremer & Grey) using both Y-tube and cage bioassays. Notably, compound 1 exhibited a cross-species attractive effect on S. cinerearia, a species that had not previously been documented to respond to the pheromone of L. fiscellaria lugubrosa. This discovery underscores the potential for cross-species attraction, broadens our understanding of pheromone specificity, and emphasizes the value of stereoselectively synthesized pheromones as molecular tools for cross-species pest monitoring and integrated pest management.
1. Introduction
The western hemlock looper, L. fiscellaria lugubrosa, is a member of the Geometridae family and a significant defoliator of its primary host, the western hemlock (Tsuga heterophylla), as well as associated conifer species in North America and Canada [1,2,3,4]. Its larvae feed on various conifer species associated with hemlock, including Sitka spruce (Picea sitchensis), subalpine fir (Abies lasiocarpa), grand fir (Abies grandis), Pacific silver fir (Abies amabilis), western white pine (Pinus monticola), and western redcedar (Thuja plicata), among others [5,6]. These inchworm-like caterpillars consume foliage of all ages, and severe defoliation during outbreaks can lead to tree mortality after just one year of feeding [3,7]. Therefore, monitoring and controlling this pest to prevent its outbreak is especially important.
Pheromone-based pest management is integral to sustainable agriculture and forestry, offering targeted, eco-friendly alternatives to chemical pesticides [8]. Strategies such as mating disruption, mass trapping, and monitoring exploit insect communication pathways to suppress pest populations while minimizing non-target impacts and environmental contamination. Additionally, these methods mitigate the risk of pesticide resistance, enhancing long-term control efficacy [9]. In, 1993, Slessor and coworkers first recognized (5R,11S)-5,11-dimethylheptadecane (1), (S)-7-methylheptadecane (2) and (S)-2,5-dimethylheptadecane (3) as the sex pheromone of the western hemlock looper by means of field trapping experiments (Figure 1) [10,11,12]. Given its significant role in the monitoring and control of this pest, there have been several reports on syntheses of this pheromone components based on chiral pool strategy. Slessor’s group firstly reported the synthesis of the sex pheromone of the western hemlock looper from methoxybornyl sulfoxides as a chiral precursor and Bakers’ yeast reduction [10,11]. In 1996, Mori’s group realized the synthesis of (5R,11S)-5,11-dimethylheptadecane (1) in 13 steps from the enantiomers of methyl3-hydroxy-2-methylpropanoate, and 2,5-dimethylheptadecane (3) with a total yield of 29% in 5 steps from (S)-citronellol [13]. In 2023, Zhong’s group achieved the synthesis of (5R,11S)-5,11-dimethylheptadecane (1) and (S)-7-methylheptadecane (2) using the enantiomers of 2-methyloxiraneoxide as chiral sources [14]. While the previously reported routes were efficient, our study focuses on developing a more diverse and convergent synthetic strategy, enabling flexible assembly of structurally related pheromone analogues from shared chiral building blocks. To facilitate the utilization of the sex pheromone, the development of a novel and efficient synthetic route based on a chiral auxiliary strategy is of great significance.
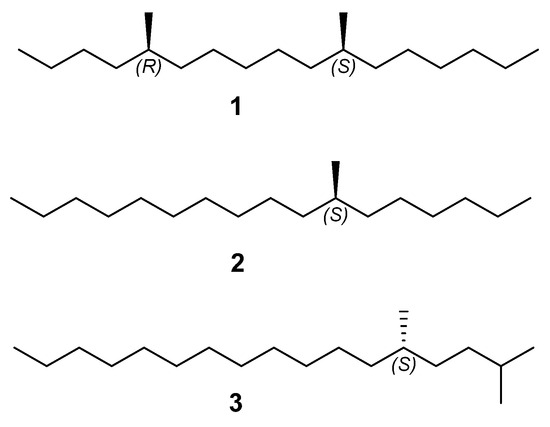
Figure 1.
The sex pheromone components of L. fiscellaria lugubrosa.
While pheromones are typically considered species-specific, increasing evidence suggests that pheromone components may also elicit responses in non-target species. Such cross-attraction phenomena are of particular interest in chemical ecology, as they can reveal evolutionary relationships in pheromone communication systems and offer potential for developing broad-spectrum monitoring tools [15,16,17]. In this study, we report the stereoselective synthesis of three sex pheromone components of L. fiscellaria lugubrosa and evaluate their behavioral activity in S. cinerearia, another geometrid species of ecological relevance. Laboratory-based Y-tube olfactometer and cage bioassays were employed to assess the potential of these compounds to act as attractants. Interestingly, component 1 was found to elicit cross-attractive effect on S. cinerearia, representing the first evidence of interspecies pheromone communication involving L. fiscellaria lugubrosa. Our work presents two complementary advances that have not been simultaneously achieved in previous studies. First, we developed a modular and stereoselective synthetic strategy based on Evans’ chiral auxiliaries, which offers a shorter, more convergent, and scalable route compared with previously reported methods employing chiral building blocks or long linear sequences. Second, we demonstrate a novel biological phenomenon in which one of the synthesized pheromone components functions as a cross-attractant in S. cinerearia, as supported by both Y-tube olfactometer and cage bioassays. These results not only expand current understanding of pheromone-mediated communication in geometrid moths but also provide a new chemical–ecological basis for developing sustainable pest management strategies that harness cross-attraction mechanisms.
2. Results
2.1. Synthesis of Sex Pheromone of L. fiscellaria lugubrosa 1 and 2
The synthesis of sex pheromone of L. fiscellaria lugubrosa 1 and 2 is summarized in Scheme 1. Treatment of hex-5-enoic acid (4) with oxalyl chloride produced the corresponding acyl chloride, which was then acylated with (R)-4-isopropyloxazolidin-2-one (5) to yield (R)-3-(hex-5-enoyl)-4-isopropyloxazolidin-2-one (6) in 90% yield [18]. Subsequent diastereoselective methylation and reductive cleavage resulted in allylic alcohol 8, exhibiting an enantiomeric excess (ee) of 99% as estimated by 1H NMR analysis of its Mosher ester [19]. Its specific rotation {[α]D25 = +7.17 (c 0.61, CHCl3)} was consistent with the literature value {[α]D20 = +9.1 (c 3.03, CHCl3)} for (R)-2-methylhex-5-en-1-ol (8), confirming that compound 8 possesses the R configuration [20,21]. The tosylate of 8 was then coupled with pentylmagnesium bromide (9) under the catalysis of lithium tetrachlorocuprate (II) (Li2CuCl4), affording (S)-5-methylundec-1-ene (10) in 83% yield [22,23]. Hydroboration–oxidation of 10 using 9-borabicyclo [3.3.1]nonane (9-BBN) and hydrogen peroxide (H2O2) resulted in (S)-5-methylundecan-1-ol (11) with an 80% yield [24]. Subsequent iodination with I2/PPh3/imidazole in THF furnished chiral alkyl iodide 12 in 88% yield [25]. Parallel iodination of (R)-2-methylhex-5-en-1-ol (8) afforded(R)-6-iodo-5-methylhex-1-ene (13) in 80% yield [25], which was transformed into sulfone 14 in 70% yield [26]. Sulfone 14 was then alkylated with chiral alkyl iodide 12 to give 15 with a 5,11-dimethyl skeleton by using n-butyllithium and hexamethylphosphoric triamide in 75% yield [26,27]. Reductive desulfonylation of 15 with magnesium in methanol, followed by Pd/C-catalyzed hydrogenation, delivered (5R,11S)-5,11-dimethylheptadecane (1) in 68% yield [26,28]. The specific rotation of 1 was −0.93 (c 0.63, CHCl3), aligning closely with the reported value in the literature of −0.90 (c 1.99, Hexane). Additionally, the structures of 1 was confirmed using 1H NMR, 13C NMR and HRMS spectra, consistent with the literature reports [13].
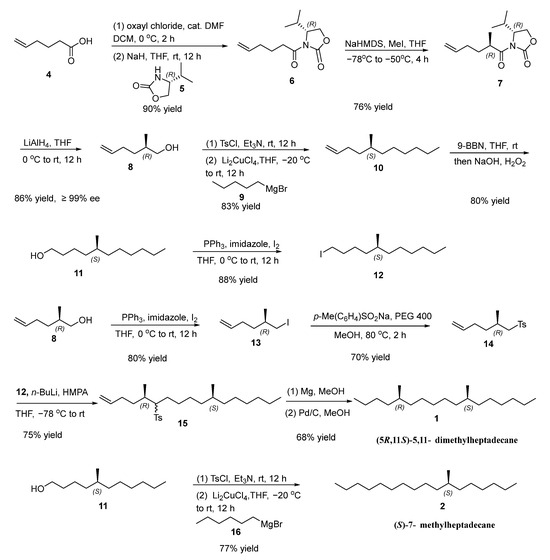
Scheme 1.
Synthesis of (5R,11S)-5,11-dimethylheptadecane (1) and (S)-7-methylheptadecane (2).
In parallel, alcohol 11 was converted to its tosylate, which coupled with hexylmagnesium bromide under Li2CuCl4 catalysis to furnish (S)-7-methylheptadecane (2) in 77% yield [22]. The sex pheromone of L. fiscellaria lugubrosa 2 was characterized with 1H NMR, 13C NMR (Supplementary Materials) and HRMS spectra. The specific rotation of 2 was −1.76 (c 0.87, CHCl3), which aligns closely with the published literature value of −1.97 (c 3.04, CHCl3) [14].
2.2. Synthesis of Sex Pheromone of L. fiscellaria lugubrosa 3
The sex pheromone of L. fiscellaria lugubrosa 3 was prepared according to the similar strategy of constructing 1, as shown in Scheme 2. Starting from tetradecanoic acid, we synthesized (S)-4-isopropyl-3-tetradecanoyloxazolidin-2-one (19) using oxalyl chloride and (S)-4-isopropyloxazolidin-2-one in 85% yield [18]. Subsequent diastereoselective methylation with iodomethane (MeI) in the presence of sodium bis(trimethylsilyl)amide (NaHMDS) produced (S)-4-isopropyl-3-((S)-2-methyltetradecanoyl)oxazolidin-2-one (20) in 78% yield [19]. Reductive cleavage of the chiral auxiliary with Lithium aluminum hydride (LiAlH4) gave (S)-2-methyltetradecan-1-ol (21) in 81% yield and 99% enantiomeric excess (ee), as estimated by 1H NMR spectra of its Mosher ester. The specific rotation of 21 {[α]D20 = −10.60 (c 1.10, CHCl3)} closely matched the literature value {[α]D20 =−11.36 (c 0.88, CHCl3)}, allowing for the assignment of its absolute configuration as S [29,30]. The final transformation into the target sex pheromone 3 was achieved through the tosylation of compound 21 with TsCl, followed by coupling reaction with iso-butylmagnesium bromide in a yield of 72% [22,23]. The structure of 3 was characterized by 1H NMR, 13C NMR, HRMS and specific rotation, all of which were consistent with the literature reference [13].
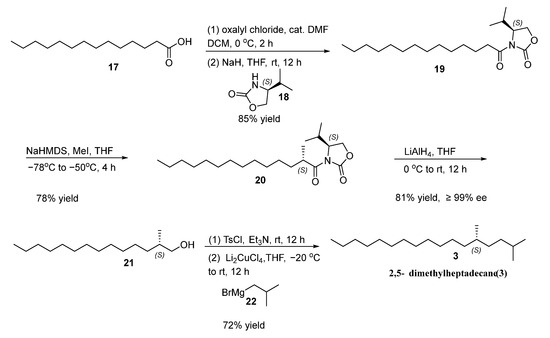
Scheme 2.
Synthesis of (S)-2,5-dimethylheptadecane (3).
2.3. Evaluation of the Cross-Species Attractive Effect on Male S. cinerearia
Building upon our efficient asymmetric syntheses and stereochemical assignments of the three L. fiscellaria lugubrosa pheromone components (1–3), we next examined their ecological activity in a non-conspecific geometrid. Given the potential conservation of pheromone biosynthesis and recognition within Geometridae, we selected S. cinerearia as a model to evaluate cross-species attraction [31]. S. cinerearia similar to L. fiscellaria lugubrosa, belongs to Geometridae, which is one of the most species-rich families of lepidopteran insects. It also ranks among the most economically significant pests affecting the Chinese scholar tree [32]. We conducted Y-tube olfactometer and cage bioassay to determine the activity and dose–response profiles of compounds 1–3 (Figure 2) [33,34,35], thereby testing the hypothesis of cross-species recognition and informing broader pheromone-based applications.
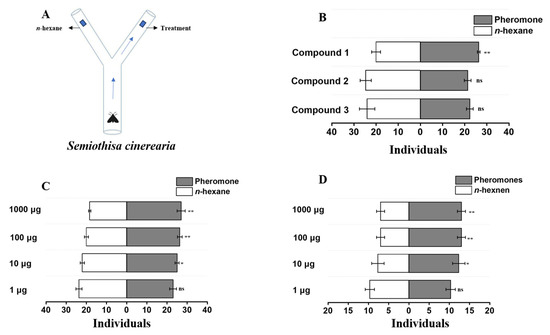
Figure 2.
Evaluation of the cross-species attractive effect. (A) Schematic of the Y-tube olfactometer for male S. cinerearia behavioral bioassays. (B) Y-Tube olfactometer assay of male S. cinerearia behavioral responses to 0.1 mg of compound 1,2 and 3 (n = 3 replicates per compound). (C) Y-Tube olfactometer assay of male S. cinerearia behavioral responses to different doses of compound 1 (n = 3 replicates per dose). (D) Cage bioassay for evaluating male S. cinerearia behavioral responses to different doses of compound 1 (n = 3 replicates per dose). Statistically significant differences were evaluated using Student’s t-test (*, p < 0.05; **, p < 0.01; ns, no significant difference).
We first employed a Y-tube olfactometer (Figure 2A) to evaluate the behavioral responses of male S. cinerearia to the three pheromone components (1, 2, and 3) of L. fiscellaria lugubrosa, using 0.1 mg as the test dose. As shown in Figure 2B, compound 1 elicited both attraction and retention effects in males, whereas compounds 2 and 3 did not induce behavioral responses. To further examine the dose-dependent preference, we tested compound 1 at four concentrations (1 μg, 10 μg, 100 μg, and 1000 μg). The results (Figure 2C) demonstrated that compound 1 began to exhibit detectable attractiveness to males at doses above 10 μg. To validate these findings under more realistic conditions, we conducted cage bioassays with the same four doses of compound 1. The results were consistent with those from the Y-tube assays and showed an obvious dose-dependent effect; attraction was observed at ≥10 μg, while a good cross-attractive effect was recorded at doses ≥100 μg (Figure 2D). These results reveal that sex pheromones may retain conserved recognition mechanisms across species. Compound 1 plays a pivotal role not only in intraspecific communication of L. fiscellaria lugubrosa but also triggers cross-attraction in S. cinerearia. This expands our understanding of pheromone specificity and evolutionary commonality, while providing a novel molecular tool for cross-species pest monitoring.
3. Discussion
In this study, we successfully developed a novel and efficient stereoselective synthesis of the three sex pheromone components of the western hemlock looper, L. fiscellaria lugubrosa. Starting from acid 4, (5R,11S)-5,11-dimethylheptadecane (1) and (S)-7-methylheptadecane (2) were synthesized in a total of 8 and 6 steps, respectively, with overall yields of 18% and 30%. The overall yield of (S)-2,5-dimethylheptadecane (3) was 39%, achieved from acid 17 in 4 steps. All products exhibited optical purities greater than 99%.
Compared to reported synthetic methods, our approach offers advantages such as inexpensive starting materials, a shorter synthetic route, good overall yields, and excellent optical purity of the target compounds. Slessor’s biocatalytic asymmetric synthesis of (S)-2,5-dimethylheptadecane (3) involved eight steps with an overall yield of only 13%, making this approach impractical for large-scale production [10,11]. Mori’s classical synthesis of (5R,11S)-5,11-dimethylheptadecane (1) required 19 consecutive steps, which severely limits its scalability [13]. Zhong’s route employed chiral 2-methyloxirane as the chiral source to synthesize (5R,11S)-5,11-dimethylheptadecane (1) in 13 steps with an overall yield of 17%. Although the chiral starting materials used in Zhong’s strategy are inexpensive and readily available, the reaction conditions are relatively demanding, often requiring elevated temperatures, and the optical purity of the final product reached only 96%, which makes it difficult to scale up for practical application [14]. In contrast, our synthetic strategy was deliberately designed with scalability, efficiency, and practicality in mind. The use of Evans’ chiral auxiliaries provides a robust and easily removable stereochemical control element, while the resulting intermediates are stable and suitable for multigram-scale synthesis. By employing inexpensive starting carboxylic acids and well-established transformations—such as hydroboration–oxidation, sulfone alkylation, and Grignard cross-coupling—the present route circumvents the need for multiple protecting-group manipulations in previously reported methods. Importantly, the integration of Evans’ auxiliary chemistry with a convergent Grignard cross-coupling and sulfone alkylation strategy for the assembly of methyl-branched long-chain alkanes—the characteristic structural motif of geometrid pheromones—has not, to our knowledge, been previously reported in a concise and high-optical-purity synthetic route. Our approach offers several distinct advantages: (i) exceptional stereochemical control with optical purities exceeding 99%, (ii) a reduced number of protecting-group steps and shorter linear sequences for key intermediates, and (iii) modularity that facilitates straightforward analogue synthesis with varied chain lengths or branching patterns without the need to redesign the entire route. Overall, these methodological advances enhance the practical accessibility of pheromone components and provide a versatile platform for structure–activity relationship exploration and scalable production, thereby supporting future applications in integrated pest management (IPM).
Beyond the achievement in asymmetric synthesis, the behavioral assays revealed a biologically important and unexpected finding. Among the three synthesized pheromone components, compound 1 exhibited cross-attractive effect on male S. cinerearia, a geometrid moth not previously reported to respond to L. fiscellaria lugubrosa sex pheromone. Y-tube olfactometer tests demonstrated that compound 1 elicited a dose-dependent behavioral response, with attraction detectable at 10 μg and increasing significantly at ≥100 μg. These results were further validated under more naturalistic conditions using cage bioassays, which confirmed that compound 1 consistently attracted male S. cinerearia at higher doses. While S. cinerearia and L. fiscellaria lugubrosa belong to different genera, both are Geometrid moths, which may explain partial overlap in pheromone communication systems. In the future, we will continue to investigate the interaction mechanism between compound 1 and the olfactory receptors of S. cinerearia to further elucidate the biological basis of this phenomenon. Our finding suggests that structurally similar pheromone components may act as interspecific cues, potentially influencing mating behaviors or serving as ecological signals across species boundaries.
4. Materials and Methods
4.1. General Information for Synthesizing Sex Pheromone of L. fiscellaria lugubrosa
All reactions were performed under an inert atmosphere of argon, utilizing a Schlenk line system. Reagents were sourced commercially and used without further purification, while solvents underwent distillation before application, following standard protocols. Proton NMR (1H NMR) spectra were obtained at 500 MHz using TMS at δ 0.00 ppm or CDCl3 at δ 7.26 ppm, and carbon NMR (13C NMR) spectra were recorded at 126 MHz, with CDCl3 set at δ 77.16 ppm as the internal standard, employing a Bruker DP-X500 spectrometer (Bruker Corporation, Beijing, China). High-resolution mass spectrometry (HRMS) data were collected using a Waters LCT Premier™ system (Waters Corporation, Beijing, China) equipped with an electrospray ionization (ESI) source. Optical rotation measurements were taken with a Rudolph Research Analytical AUTOPOL-IV polarimeter (Rudolph Research Analytical, Beijing, China).
Synthesis of (R)-3-(hex-5-enoyl)-4-isopropyloxazolidin-2-one (6)
Hex-5-enoic acid (4) (1.72 g, 15 mmol) was dissolved in dry dichloromethane (DCM) (20 mL) at 0 °C under an argon atmosphere. Oxalyl chloride (2.86 g, 22.5 mmol) and a catalytic amount of DMF were then added slowly; the reaction mixture was stirred for 2 h at 0 °C. The solvent was removed under reduced pressure to give the crude hex-5-enoyl chloride as a slight yellow oil.
NaH (0.9 g, 60% in mineral oil, 22.5 mmol) was dissolved in dry tetrahydrofuran (20 mL) at 0 °C under an argon atmosphere. (R)-4-isopropyloxazolidin-2-one (5) (3.46 g, 19.5 mmol) in tetrahydrofuran (20 mL) was added slowly. The resulting mixture was warmed to 25 °C and stirred for 2 h. Then, the crude hex-5-enoyl chloride in dry tetrahydrofuran (10 mL) was added slowly at 0 °C, the resulting mixture was allowed to rise to room temperature and stirred for another 4 h. The reaction was quenched with saturated aqueous NH4Cl solution (20 mL). The layers were separated, and the aqueous phase was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were washed with brine (50 mL) and dried over anhydrous Na2SO4, concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether = 1:5) to provide (R)-3-(hex-5-enoyl)-4-isopropyloxazolidin-2-one (6) as a light yellow oil (3.04 g, 90% yield). [α]D25 = −67.66 (c 1.80, CHCl3). 1H NMR (400 MHz, Chloroform-d) δ 5.85–5.75 (m, 1H), 5.12–4.94 (m, 2H), 4.45–4.41 (m 1H), 4.30–4.17 (m, 2H), 3.03–2.84 (m, 2H), 2.41–2.33 (m, 1H), 2.13 (q, J = 7.2 Hz, 2H), 1.83–1.71 (m, 2H), 0.91 (d, J = 7.0 Hz, 3H), 0.87 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 173.20, 154.14, 137.91, 115.36, 63.42, 58.45, 34.90, 33.11, 28.46, 23.63, 18.04, 14.73. HRMS (ESI, m/z): calculated for [M + Na]+ C12H19NO3Na 248.1257, found: 248.1240.
Synthesis of (R)-4-isopropyl-3-((R)-2-methylhex-5-enoyl)oxazolidin-2-one (7)
(R)-3-(hex-5-enoyl)-4-isopropyloxazolidin-2-one (6) (3.38 g, 15 mmol) was dissolved in dry tetrahydrofuran (40 mL) at −78 °C under an argon atmosphere. Sodium hexamethyldisilazide (NaHMDS, 11.25 mL, 2.0 M in THF, 22.5 mmol) was added slowly and the resulting mixture was stirred for 1 h, MeI (4.67 mL, 75 mmol) was then added dropwise. The reaction solution was stirred for 2 h at −78 °C and warmed up to −60 °C to react for 3 h. The reaction was quenched with saturated aqueous NH4Cl solution (30 mL). The layers were separated, and the aqueous phase was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were washed with brine (50 mL) and dried over anhydrous Na2SO4, concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether = 1:8) to provide (R)-4-isopropyl-3-((R)-2-methylhex-5-enoyl)oxazolidin-2-one (7) as a colorless oil (2.73 g, 76% yield). [α]D25 = −91.08 (c 0.55, CHCl3). 1H NMR (400 MHz, Chloroform-d) δ 5.83– 5.73 (m, 1H), 5.03–4.89 (m, 2H), 4.46–4.42 (m, 1H), 4.28–4.18 (m, 2H), 3.80–3.71 (m, 1H), 2.38–2.31 (m, 1H), 2.06 (q, J = 7.1 Hz, 2H), 1.90–1.81 (m, 1H), 1.52–1.45 (m, 1H), 1.21 (d, J = 6.9 Hz, 3H), 0.91 (d, J = 7.0 Hz, 3H), 0.87 (d, J = 6.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 177.17, 153.78, 138.30, 114.99, 63.37, 58.57, 37.34, 32.30, 31.68, 28.60, 18.08, 18.06, 14.85. HRMS (ESI, m/z): calculated for [M + Na]+ C13H21NO3Na 262.1414, found: 262.1417.
Synthesis of (R)-2-methylhex-5-en-1-ol (8)
LiAlH4 (1.50 g, 38.75 mmol) was dissolved in dry tetrahydrofuran (15 mL) at 0 °C under an argon atmosphere, the solution of (R)-4-isopropyl-3-((R)-2-methylhex-5-enoyl)oxazolidin-2-one (7) (2.65 g, 11.07 mmol) in dry tetrahydrofuran (10 mL) was then added dropwise over 20 min at 0 °C. The reaction mixture was warmed to 25 °C and stirred for 12 h, followed by the slow addition of saturated aqueous ammonium chloride (20 mL) and diluted with ethyl acetate. The precipitate was filtered off, and the filtrate was then dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether 1:5) to provide (R)-2-methylhex-5-en-1-ol (8) as a colorless oil (1.09 g, 86% yield, ≥99% ee, determined by 1H NMR analysis of the ester derived from (S)-MTPACl). [α]D25 = +7.17 (c 0.61, CHCl3). 1H NMR (400 MHz, Chloroform-d) δ 5.86–5.76 (m, 1H), 4.98 (dd, J = 27.7, 13.6 Hz, 2H), 3.53–3.41 (m, 2H), 2.18–2.00 (m, 2H), 1.70–1.59 (m, 1H), 1.56–1.47 (m, 1H), 1.41 (s, 1H), 1.25–1.16 (m, 1H), 0.93 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 139.03, 114.54, 68.31, 35.35, 32.43, 31.31, 16.57. HRMS (ESI, m/z): calculated for [M + K]+ C7H14OK 153.0676, found: 153.0662.
Synthesis of (S)-5-methylundec-1-ene (10)
Tosyl chloride (1.90 g, 10.0 mmol) in dry dichloromethane (20 mL) was added slowly to a solution of (R)-2-methylhex-5-en-1-ol (8) (0.57 g, 5.0 mmol) and triethylamine (1.24 mL, 8.75 mmol) in dichloromethane (10 mL) under an argon atmosphere. The mixture was stirred for 18 h at room temperature. Afterward, the reaction mixture was quenched with saturated aqueous sodium bicarbonate (20 mL), extracted with dichloromethane (3 × 30 mL), the combined organic layers were washed with brine (30 mL) and dried over anhydrous Na2SO4, concentrated under reduced pressure. The residue was purified by column chromatography on silica gel with ethyl acetate/petroleum ether (1:5) to yield the desired tosylate product as a colorless oil.
0.1 M solution of Li2CuCl4 in THF (5.0 mL, 0.5 mmol) and 1.0 M solution of pentylmagnesium bromide (9) (15.0 mL, 15.0 mmol) were added to a solution of the previously obtained tosylate (5.0 mmol, 1.0 equiv.) in dry tetrahydrofuran (10 mL) at –20 °C under an argon atmosphere. The mixture was allowed to warm up to room temperature and stirred overnight. The reaction was quenched with saturated aqueous NH4Cl solution (30 mL). The layers were separated, and the aqueous phase was extracted with ethyl acetate (3 × 40 mL). The combined organic layers were washed with brine (40 mL) and dried over anhydrous Na2SO4. The residue was purified by silica gel column chromatography (petroleum ether) to provide (S)-5-methylundec-1-ene (10) (0.70 g, 83% yield) as a colorless oil. [α]D25 = −1.22 (c 0.65, CHCl3). 1H NMR (400 MHz, Chloroform-d) δ 5.87–5.77 (m, 1H), 5.05–4.90 (m, 2H), 2.13–1.98 (m, 2H), 1.44–1.36 (m, 2H), 1.31–1.24 (m, 9H), 1.21–1.07 (m, 2H), 0.90–0.85 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 139.65, 114.06, 37.11, 36.40, 32.44, 32.10, 31.55, 29.83, 27.13, 22.85, 19.68, 14.28. HRMS (ESI, m/z): calculated for [M + Na]+ C12H24Na 191.1770, found: 191.1786.
Synthesis of (S)-5-methylundecan-1-ol (11)
9-Borabicyclo [3.3.1] nonane (9-BBN) (22.8 mL, 0.5 M in THF, 11.4 mmol) was added to a solution of (S)-5-methylundec-1-ene (10) (0.64 g, 3.8 mmol) in dry tetrahydrofuran (10 mL) slowly at room temperature under an argon atmosphere. The reaction was stirred for 12 h, followed by the addition of sodium hydroxide solution (7.6 mL, 3 M, 22.8 mmo) at 0 °C. After stirring for 30 min, the temperature was lowered to –20 °C, and hydrogen peroxide solution (7.6 mL, 30%) was added slowly. The reaction was stirred for 3 h at room temperature and was quenched with saturated aqueous ammonium chloride (15 mL). The layers were separated, and the aqueous phase was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were washed with brine (50 mL) and dried over anhydrous Na2SO4. The residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether = 1:10) to provide (S)-5-methylundecan-1-ol (11) (0.57 g, 80% yield) as a colorless oil. [α]D25 = −1.41 (c 0.85, CHCl3). 1H NMR (400 MHz, Chloroform-d) δ 3.64 (t, J = 6.6 Hz, 2H), 1.62–1.48 (m, 2H), 1.43–1.19 (m, 14H), 1.16–1.04 (m, 2H), 0.94–0.79 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 63.25, 37.16, 36.98, 33.30, 32.89, 32.09, 29.82, 27.17, 23.36, 22.84, 19.77, 14.26. HRMS (ESI, m/z): calculated for [M + Na]+ C12H26ONa 209.1876, found: 209.1882.
Synthesis of (S)-1-iodo-5-methylundecane (12)
To a stirred mixture of (S)-5-methylundecan-1-ol (11) (0.37 g, 2.0 mmol), triphenylphosphine (0.63 g, 2.4 mmol) and imidazole (0.30g, 4.4 mmol) in dry tetrahydrofuran (5 mL) were added to iodine (0.61 g, 2.4 mmol) in dry tetrahydrofuran (5 mL) at room temperature under an argon atmosphere. After 3 h, the reaction was quenched with 5% Na2S2O3 aqueous solution, and the resulting mixture was extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with 5% Na2S2O3 aqueous solution, water and brine, dried over anhydrous Na2SO4. The residue was purified by silica gel column chromatography (petroleum ether) to provide (S)-1-iodo-5-methylundecane (12) (0.52 g, 88% yield) as a light-yellow oil. [α]D25 = +0.88 (c 0.90, CHCl3). 1H NMR (500 MHz, Chloroform-d) δ 3.19 (t, J = 7.0 Hz, 2H), 1.85–1.76 (m, 2H), 1.45–1.34 (m, 3H), 1.32–1.24 (m, 10H), 1.15–1.07 (m, 2H), 0.88 (t, J = 6.9 Hz, 3H), 0.85 (d, J = 6.6 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 37.11, 36.01, 34.02, 32.74, 32.09, 29.81, 28.16, 27.15, 22.84, 19.77, 14.27, 7.52. HRMS (ESI, m/z): calculated for [M + H]+ C12H26I 297.1074, found: 297.1063.
Synthesis of (R)-6-iodo-5-methylhex-1-ene (13)
Following the similar procedure for the synthesis of (S)-1-iodo-5-methylundecane (12). To a stirred mixture of (R)-2-methylhex-5-en-1-ol (8) (0.29 g, 2.5 mmol), triphenylphosphine (0.79 g, 3.0 mmol) and imidazole (0.37g, 5.5 mmol) in dry tetrahydrofuran (8 mL) was added iodine (0.76 g, 3.0 mmol) in dry tetrahydrofuran (8 mL) at room temperature under an argon atmosphere. After 3 h, the reaction was quenched with 5% Na2S2O3 aqueous solution, and the resulting mixture was extracted with ethyl acetate (3 × 40 mL). The combined organic layers were washed with 5% Na2S2O3 aqueous solution, water and brine, dried over anhydrous Na2SO4. The residue was purified by silica gel column chromatography (petroleum ether) to provide (R)-6-iodo-5-methylhex-1-ene (13) (0.45 g, 80% yield) as a light-yellow oil. [α]D25 = −2.90 (c 0.83, CHCl3). 1H NMR (500 MHz, Chloroform-d) δ 5.83–5.75 (m, 1H), 5.05–5.01 (m, 1H), 4.99–4.95 (m, 1H), 3.24 (dd, J = 9.6, 4.4 Hz, 1H), 3.17 (dd, J = 9.7, 5.7 Hz, 1H), 2.09–2.04 (m, 2H), 1.52–1.45 (m, 2H), 1.35–1.27 (m, 1H), 0.99 (d, J = 6.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 138.40, 114.97, 35.70, 34.17, 31.24, 20.60, 17.75. HRMS (ESI, m/z): calculated for [M + H]+ C17H14I 225.0135, found: 225.0145.
Synthesis of (R)-1-methyl-4-((2-methylhex-5-en-1-yl)sulfonyl)benzene (14)
To a stirred mixture of sodium p-toluenesulfinate (0.25 g, 1.4 mmol), PEG-400 (3 mL), and DMSO (1.5 mL) was added (R)-6-iodo-5-methylhex-1-ene (13) (0.22 g, 1.0 mmol) at room temperature under an argon atmosphere. The mixture was warmed to 80 °C and stirred for 2 h. The reaction was quenched with a 10% NaCl solution (10 mL) after cooling to room temperature. The crude product was extracted with ethyl acetate (3 × 40 mL). The combined organic layers were washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether = 1:8) to provide (R)-1-methyl-4-((2-methylhex-5-en-1-yl)sulfonyl)benzene (14) (0.18 g, 70% yield) as a colorless oil. [α]D25 = −3.06 (c 0.95, CHCl3). 1H NMR (500 MHz, Chloroform-d) δ 7.78 (d, J = 8.2 Hz, 2H), 7.35 (d, J = 8.0 Hz, 2H), 5.75–5.66 (m, 1H), 4.99–4.89 (m, 2H), 3.07 (dd, J = 14.2, 4.7 Hz, 1H), 2.91 (dd, J = 14.2, 7.7 Hz, 1H), 2.45 (s, 3H), 2.15–2.05 (m, 1H), 2.04–1.92 (m, 2H), 1.56–1.49 (m, 1H), 1.37–1.29 (m, 1H), 1.07 (d, J = 6.7 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 144.63, 137.95, 137.36, 130.02, 128.04, 115.15, 62.75, 35.93, 30.76, 28.29, 21.76, 19.87. HRMS (ESI, m/z): calculated for [M + H]+ C14H21O2S 253.1257, found: 253.1259.
Synthesis of 1-(((5R,11S)-5,11-dimethylheptadec-1-en-6-yl)sulfonyl)-4-methylbenzene (15)
To a stirred solution of (R)-1-methyl-4-((2-methylhex-5-en-1-yl)sulfonyl)benzene (14) (0.15 g, 0.6 mmol) in dry tetrahydrofuran (10 mL) was added n-BuLi (2.4 M, 0.38 mL, 0.9 mmol) slowly at –78 °C under an argon atmosphere. The reaction mixture was stirred at –78 °C for 30 min and then at –35 °C for 30 min. After cooling to –78 °C, (S)-1-iodo-5-methylundecane (12) (0.21 g, 0.72 mmol) dissolved in HMPA (0.42 mL) and dry tetrahydrofuran (1 mL) was added dropwise. The reaction was then allowed to warm gradually to room temperature and stirred for overnight. The resulting mixture was poured into 1 M HCl (6 mL) and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with a saturated NaHCO3 solution and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether = 1:10) to provide 1-(((5R,11S)-5,11-dimethylheptadec-1-en-6-yl)sulfonyl)-4-methylbenzene (15) (0.19 g, 75% yield) as a colorless oil. [α]D25 = −0.38 (c 1.04, CHCl3). 1H NMR (500 MHz, Chloroform-d) δ 7.75 (d, J = 8.1 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 5.80–5.62 (m, 1H), 5.01–4.89 (m, 2H), 2.91–2.85 (m, 1H), 2.45 (s, 3H), 2.23–2.10 (m, 1H), 2.04–1.81 (m, 3H), 1.66–1.61 (m, 1H), 1.40–1.35 (m, 1H), 1.33–1.11 (m, 17H), 1.04–1.00 (m, 3H), 0.88 (t, J = 6.9 Hz, 3H), 0.79 (d, J = 6.5 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 144.39, 137.91 (138.50), 136.54 (136.86), 129.86 (129.83), 128.73 (128.70), 115.18 (114.97), 68.30 (69.70), 37.16 (37.13), 36.69 (36.72), 35.18, 32.73 (32.78), 32.27, 32.09 (31.99), 31.59, 31.48 (31.06), 29.81, 29.49 (29.19), 27.16 (27.04), 25.14, 22.84 (23.92), 21.74, 19.74 (18.09), 14.85, 14.27. HRMS (ESI, m/z): calculated for [M + H]+ C26H45O2S 421.3135, found: 421.3117.
Synthesis of (5R,11S)-5,11-dimethylheptadecane (1)
To a stirred solution of magnesium powder (0.13 g, 5.4 mmol) in dry tetrahydrofuran (1.0 mL), a catalytic amount of MeMgBr (3.0 M, two drops) was added to activate the metal under an argon atmosphere. After stirring for 15 min at room temperature, a solution of 1-(((5R,11S)-5,11-dimethylheptadec-1-en-6-yl)sulfonyl)-4-methylbenzene (15) (0.15 g, 0.35 mmol) in dry MeOH (5.0 mL) was added, the mixture was then stirred at 50 °C for 4 h. Next, the mixture was evaporated to remove MeOH and an ice-cooled 1 M HCl solution (2 mL) was added to dissolve the residues. The crude products were extracted with Et2O (3 × 10 mL), washed with saturated NaHCO3 solution and brine, dried over Na2SO4, and concentrated in vacuo. The residue was filtered through a silica gel pad and concentrated in vacuo to afford the crude product. The crude product was dissolved in 3 mL MeOH and added to a suspension of palladium/carbon (30 mg, 10% wt.) in MeOH (2 mL) at room temperature under a hydrogen atmosphere. The reaction mixture was stirred for 12 h and concentrated in vacuo. The residue was purified by silica gel column chromatography (petroleum ether) to provide (5R,11S)-5,11-dimethylheptadecane (1) (63.9 mg, 68% yield) as a colorless oil. [α]D25 = −0.93 (c 0.63, CHCl3). 1H NMR (500 MHz, Chloroform-d) δ 1.41–1.33 (m, 2H), 1.31–1.21 (m, 22H), 1.14–1.03 (m, 4H), 0.91–0.87 (m, 6H), 0.86–0.81 (m, 6H). 13C NMR (126 MHz, CDCl3) δ 37.27, 36.94, 32.92, 32.90, 32.13, 30.54, 30.20, 29.90, 29.86, 29.51, 27.28, 27.21, 23.21, 22.86, 19.89, 14.33, 14.28. HRMS (ESI, m/z): calculated for [M + K]+ C19H40K 307.2762, found:307.2751.
Synthesis of (S)-7-methylheptadecane (2)
Following the similar procedure for the synthesis of (S)-5-methylundec-1-ene (10). Tosyl chloride (0.19 g, 1.0 mmol) in dry dichloromethane (2 mL) was added slowly to a solution of (S)-5-methylundecan-1-ol (11) (0.57 g, 0.5 mmol) and triethylamine (0.12 mL, 0.88 mmol) in dichloromethane (2 mL) under an argon atmosphere. The mixture was stirred for 18 h at room temperature. Afterward, the reaction mixture was quenched with saturated aqueous sodium bicarbonate (4 mL), extracted with dichloromethane (3 × 15 mL), the combined organic layers were washed with brine (20 L) and dried over anhydrous Na2SO4, concentrated under reduced pressure. The residue was purified by column chromatography on silica gel with ethyl acetate/petroleum ether (1:5) to yield the desired tosylate product as a colorless oil.
0.1 M solution of Li2CuCl4 in THF (0.5 mL, 0.05 mmol) and 1.0 M solution of hexylmagnesium bromide (16) (1.5 mL, 1.5 mmol) were added to a solution of the previously obtained tosylate (0.5 mol, 1.0 equiv.) in dry tetrahydrofuran (2 mL) at –20 °C under an argon atmosphere. The mixture was allowed to warm up to room temperature and stirred overnight. The reaction was quenched with saturated aqueous NH4Cl solution (5 mL). The layers were separated, and the aqueous phase was extracted with ethyl acetate (3 × 30 mL). The combined organic layers were washed with brine (30 mL) and dried over anhydrous Na2SO4. The residue was purified by silica gel column chromatography (petroleum ether) to provide (S)-7-methylheptadecane (2) (98 mg, 77% yield) as a colorless oil. [α]D25 = −1.76 (c 0.87, CHCl3). 1H NMR (400 MHz, Chloroform-d) δ 1.38–1.03 (m, 28H), 1.09–1.04 (m, 1H), 0.90–0.82 (m, 9H). 13C NMR (101 MHz, CDCl3) δ 37.27, 32.92, 32.13, 32.10, 30.21, 29.90, 29.87, 29.83, 29.53, 27.25, 27.22, 22.86, 19.88, 14.28. HRMS (ESI, m/z): calculated for [M + K]+ C18H38K 293.2605, found: 293.2601.
Synthesis of (S)-4-isopropyl-3-tetradecanoyloxazolidin-2-one (19)
Following the similar procedure for the synthesis of (R)-3-(hex-5-enoyl)-4-isopropyloxazolidin-2-one (6), tetradecanoic acid (17) (0.91 g, 4 mmol) and (S)-4-isopropyloxazolidin-2-one (0.67 g, 5.2 mmol) were reacted to afford (S)-4-isopropyl-3-tetradecanoyloxazolidin-2-one (19) (1.15 g, 85% yield) as a white solid. The melting point was 56–57 °C; [α]D25 = +56.03 (c 3.02, CHCl3). 1H NMR (500 MHz, Chloroform-d) δ 4.44–4.41 (m, 1H), 4.25 (t, J = 8.7 Hz, 1H), 4.19 (dd, J = 9.1, 3.0 Hz, 1H), 3.00–2.94 (m, 1H), 2.87–2.81 (m, 1H), 2.41–2.32 (m, 1H), 1.69–1.59 (m, 2H), 1.35–1.24 (m, 20H), 0.90 (d, J = 7.1 Hz, 3H), 0.88–0.86 (m, 6H). 13C NMR (126 MHz, CDCl3) δ 173.56, 154.20, 63.42, 58.50, 35.65, 32.04, 29.80, 29.77, 29.73, 29.61, 29.50, 29.48, 29.26, 28.51, 24.60, 22.81, 18.10, 14.78, 14.24. HRMS (ESI, m/z): calculated for [M + K]+ C20H37O3NK 378.2405, found: 378.2405.
Synthesis of (S)-4-isopropyl-3-((S)-2-methyltetradecanoyl)oxazolidin-2-one (20)
Following the similar procedure for the synthesis of (R)-4-isopropyl-3-((R)-2-methylhex-5-enoyl)oxazolidin-2-one (7), (S)-4-isopropyl-3-tetradecanoyloxazolidin-2-one (19) (1.02 g, 3 mmol) and methyl iodide (0.93 mL, 15 mmol) were reacted to afford (S)-4-isopropyl-3-((S)-2-methyltetradecanoyl)oxazolidin-2-one (20) (0.83 g, 78% yield) as a colorless oil. [α]D25 = +60.61 (c 1.96 CHCl3). 1H NMR (500 MHz, Chloroform-d) δ 4.46–4.43 (m, 1H), 4.25 (t, J = 8.7 Hz, 1H), 4.20 (dd, J = 9.1, 2.9 Hz, 1H), 3.75–3.68 (m, 1H), 2.38–2.32 (m, 1H), 1.74–1.66 (m, 1H), 1.38–1.32 (m, 1H), 1.30– 1.23 (m, 20H), 1.19 (d, J = 6.9 Hz, 3H), 0.91 (d, J = 7.0 Hz, 3H), 0.89–0.86 (m, 6H). 13C NMR (126 MHz, CDCl3) δ 177.46, 153.80, 63.32, 58.57, 37.86, 33.26, 32.06, 29.81, 29.81, 29.78, 29.73, 29.65, 29.50, 28.57, 27.45, 22.82, 18.08, 17.99, 14.82, 14.25. HRMS (ESI, m/z): calculated for [M + Na]+ C21H39O3NNa 378.2822, found: 378.2813.
Synthesis of (S)-2-methyltetradecan-1-ol (21)
Following the similar procedure for the synthesis of (R)-2-methylhex-5-en-1-ol (8), (S)-4-isopropyl-3-((S)-2-methyltetradecanoyl)oxazolidin-2-one (20) (0.64 g, 1.8 mmol) and LiAlH4 (0.24 g, 6.3 mmol) were reacted to afford (S)-2-methyltetradecan-1-ol (21) (0.35 g, 81% yield, ≥99% ee, determined by 1H NMR analysis of the ester derived from (S)-MTPACl) as a colorless oil. [α]D25= −10.60 (c 1.10, CHCl3). 1H NMR (500 MHz, Chloroform-d) δ 3.51 (dd, J = 10.5, 5.8 Hz, 1H), 3.42 (dd, J = 10.5, 6.6 Hz, 1H), 1.64–1.57 (m, 1H), 1.39–1.35 (m, 2H), 1.31–1.26 (s, 20H), 1.13–1.07 (m, 1H), 0.91 (d, J = 6.7 Hz, 3H), 0.88 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 68.58, 35.92, 33.30, 32.07, 30.10, 29.83, 29.81, 29.80, 29.51, 27.13, 22.84, 16.73, 14.26. HRMS (ESI, m/z): calculated for [M + Na]+ C15H32ONa 251.2345, found: 251.2364.
Synthesis of (S)-2,5-dimethylheptadecane (3)
Following the similar procedure for the synthesis of (S)-5-methylundec-1-ene (10), (S)-2-methyltetradecan-1-ol (21) (0.18 g, 0.8 mmol) and isobutylmagnesium bromide (22) (4.8 mL, 0.5 M in THF, 2.4 mmol) were reacted to afford (S)-2,5-dimethylheptadecane (3) (0.15 g, 72% yield) as a colorless oil. [α]D25 = −0.77 (c 1.04, CHCl3). literature value: [α]D18 = −0.37 (c 2.98, Hexane). 1H NMR (400 MHz, Chloroform-d) δ 1.51–1.43 (m, 1H), 1.34–1.23 (m, 23H), 1.18–1.05 (m, 4H), 0.90–0.83 (m, 12H). 13C NMR (101 MHz, CDCl3) δ 37.27, 36.55, 34.93, 33.18, 32.10, 30.21, 29.90, 29.87, 29.83, 29.53, 28.50, 27.26, 22.99, 22.86, 22.76, 19.92, 14.28. HRMS (ESI, m/z): calculated for [M + K]+ C19H32NO3 307.2762, found: 307.2758.
4.2. Behavioral Assays: Y-Tube Olfactometer and Cage Tests
4.2.1. Insects
Pupae of male S. cinerearia were collected from Sishui, Shandong Province, China, and reared to adult emergence at the Institute of Industrial Crops, Shandong Academy of Agricultural Sciences. Insects were maintained under controlled environmental conditions at a temperature of 25 ± 1 °C, with a relative humidity of 70–80%, and a photoperiod of 16 h light/8 h dark. Only healthy, active and unmated males, 2 days post-eclosion with an average body length of approximately 12–14 mm and a body weight of 15–20 mg were selected for behavioral assays.
4.2.2. Preparation of Pheromone Component Solutions
Each pheromone component (compounds 1–3) was synthesized as described above and stored at –20 °C until use. Stock solutions were prepared in high-purity hexane (HPLC grade) at a concentration of 1 mg/mL. Working solutions of the desired doses (1 µg, 10 µg, 100 µg, and 1000 µg) were obtained by serial dilution with the same solvent. For behavioral assays, aliquots (10 µL) of each working solution were pipetted onto a 1 × 0.5 cm piece of filter paper, which was allowed to air dry for 2 min before being placed in the Y-tube olfactometer or attached inside the cage bioassay setup. Filter papers treated with hexane alone served as solvent control.
4.2.3. Y-Tube Olfactometer Assay
Behavioral choices were measured in a glass Y-tube (common arm: 15 cm; side arms: 17 cm; I.D. 2.5 cm; 60° bifurcation). Charcoal-filtered, humidified air was pulled through each arm at 200 mL min−1 (verified with a flowmeter). One arm received the treatment lure; the other received a solvent control. A single male S. cinerearia was released at the base of the common arm; a choice was scored when the moth moved ≥5 cm into an arm and remained for ≥30 s within a 3 min limit. Non-responders were recorded and excluded from choice analyses but reported as no choice. Between replicates, glassware was rinsed with hexane and baked (120 °C, ≥1 h). For each stimulus (or dose), 50 males were tested (3 biological replicates).
4.2.4. Cage Bioassay
Semi-field attraction was evaluated in mesh cages (60 × 60 × 50 cm). A treatment lure (Compound 1 at 1, 10, 100, or 1000 μg) and a solvent control were put at opposite corners, 50–60 cm apart. Groups of 20 males S. cinerearia were released from the cage center. After 10, 20, 30 min, the number of males within a 10 cm radius of each lure was recorded; the final read at 30 min was used for analysis (3 biological replicates).
4.2.5. Data Analysis
For the Y-tube and cage bioassays, treatment versus control choices were analyzed using SPSS software (version 23.0; IBM Corporation, Chicago, IL, USA). Comparisons between treatment and control groups were performed using Student’s t-test, with statistical significance defined as p < 0.05 (*) and p < 0.01 (**).
5. Conclusions
In summary, our work demonstrates both a methodological advance in asymmetric synthesis and a novel ecological finding in insect chemical communication. A concise and efficient stereoselective synthetic strategy was established for three sex pheromone components of L. fiscellaria lugubrosa, achieving excellent enantiomeric purity and improved overall yields. Importantly, behavioral assays revealed that compound 1 exhibits attractive effects on male S. cinerearia, with dose-dependent responses validated in both Y-tube and cage experiments. These results not only expand our understanding of pheromone-mediated communication within the Geometridae but also suggest new avenues for pest management. Collectively, this study bridges advances in synthetic chemistry with insect chemical ecology, highlighting the potential of stereoselectively synthesized pheromone components to uncover novel biological phenomena and support practical applications in integrated pest control.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30214216/s1, the detailed synthetic procedure for compound 1,2 and 3; 1H NMR and 13C NMR spectra for all the synthetic compounds and synthetic procedure of mosher esters of chiral primary alcohols 8 and 21; Experimental data of compounds 1, 2, and 3 on male S. cinerearia in Y-tube and cage assays.
Author Contributions
Conceptualization, J.Z. (Jiangchun Zhong) and C.S.; methodology performing the experiments and writing—original draft preparation, Y.Z. (Yun Zhou); Investigation, Y.G. and J.Z. (Jianhua Zhang); data curation, J.W. (Jionglin Wang), J.W. (Jianan Wang), X.L. and J.Z. (Jianhua Zhang); participating in the chemical synthesis and resources, Y.Z. (Yueru Zhang), X.F., X.W. and J.H.; writing—review and editing, J.Z. (Jiangchun Zhong) and C.S.; funding acquisition, Y.Z. (Yun Zhou) and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Shandong Province (ZR2023QB024), the Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2024F03), the Technology Innovation Guidance Plan of Shandong Province (2024LYXZ005), the Shandong Province Modern Agricultural Technology System Chinese Herbal Medicine Industrial Innovation Team (SDAIT-20) and Shandong Province Science and Technology based Small and Medium sized Enterprises Innovation Capability Enhancement Project (2024TSGC0698).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this article are available in the Supplementary Materials.
Acknowledgments
We thank the Innovative Institute of Chinese Herbal Medicines, Shandong Academy of Agricultural Sciences, for the support in this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thomson, M.G. Appraisal of western hemlock looper infestations. For. Chron. 1957, 33, 141–147. [Google Scholar] [CrossRef][Green Version]
- McCloskey, S.P.J.; Daniels, L.D.; McLean, J.A. Potential Impacts of Climate Change on Western Hemlock Looper Outbreaks. Northwest Sci. 2009, 83, 225–238. [Google Scholar] [CrossRef]
- Alfaro, R.I.; Taylor, S.; Brown, G.; Wegwitz, E. Tree mortality caused by the western hemlock looper in landscapes of central British Columbia. For. Ecol. Manag. 1999, 124, 285–291. [Google Scholar] [CrossRef]
- Evenden, M.; Borden, J.; Van Sickle, G.; Gries, G. Development of a pheromone-based monitoring system for western hemlock looper (Lepidoptera: Geometridae): Effect of pheromone dose, lure age, and trap type. Environ. Entomol. 1995, 24, 923–932. [Google Scholar] [CrossRef]
- Kinghorn, J. The influence of stand composition on the mortality of various conifers, caused by defoliation by the western hemlock looper on Vancouver Island, British Columbia. For. Chron. 1954, 30, 380–400. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Carroll, A.L. Insights into herbivore distribution and abundance: Oviposition preferences of western hemlock and phantom hemlock loopers. Can. Entomol. 2011, 143, 72–81. [Google Scholar] [CrossRef]
- Magnussen, S.; Alfaro, R. Linking aerial survey data of forest insect defoliation and tree ring data to estimate forest level growth losses. Dendrochronologia 2012, 30, 287–294. [Google Scholar] [CrossRef]
- Xia, Y.H.; Ding, B.J.; Dong, S.L.; Wang, H.L.; Hofvander, P.; Löfstedt, C. Release of moth pheromone compounds from Nicotiana benthamiana upon transient expression of heterologous biosynthetic genes. BMC Biol. 2022, 20, 80. [Google Scholar] [CrossRef]
- Mori, K. Significance of chirality in pheromone science. Bioorg. Med. Chem. 2007, 15, 7505–7523. [Google Scholar] [CrossRef]
- Li, J.; Gries, G.; Gries, R.; Bikic, J.; Slessor, K.N. Chirality of synergistic sex pheromone components of the western hemlock looper Lambdina fiscellaria lugubrosa (Hulst) (Lepidoptera: Geometridae). J. Chem. Ecol. 1993, 19, 2547. [Google Scholar] [CrossRef]
- Li, J.; Gries, R.; Gries, G.; Slessor, K.N.; King, G.G.S.; Bowers, W.W.; West, R.J. Chirality of 5,11-dimethylheptadecane, the major sex pheromone component of the hemlock looper, Lambdina fiscellaria (Lepidoptera: Geometridae). J. Chem. Ecol. 1993, 19, 1057. [Google Scholar] [CrossRef]
- Gries, G.; Gries, R.; Krannitz, S.H.; Li, J.; King, G.; Slessor, K.N.; Borden, J.H.; Bowers, W.W.; West, R.J.; Underhill, E.W. Sex pheromone of the western hemlock looper, Lambdina fiscellaria lugubrosa (Hulst) (Lepidoptera: Geometridae). J. Chem. Ecol. 1993, 19, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Horikiri, H. Pheromone synthesis. Part CLXXIV. Synthesis of (5R,11S)-5,11-dimethylheptadecane and (S)-2,5-dimethylheptadecane, the major and the minor components of the sex pheromone of the geometrid moth, Lambdina fiscellaria lugubrosa. Liebigs Ann. 1996, 1996, 501. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; An, B.; Wu, J.; Li, Y.; Bian, Q.; Wang, M.; Zhong, J. Asymmetric synthesis of sex pheromone of the western hemlock looper, Lambdina fiscellaria lugubrosa (Hulst). Tetrahedron Lett. 2023, 118, 154401. [Google Scholar] [CrossRef]
- Ganyard, M.C.; Brady, U.E. Inhibition of Attraction and Cross-attraction by Interspecific Sex Pheromone Communication in Lepidoptera. Nature 1971, 234, 415–416. [Google Scholar] [CrossRef]
- Gemeno, C.; Lutfallah, A.F.; Haynes, K.F. Pheromone Blend Variation and Cross-Attraction Among Populations of the Black Cutworm Moth (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2000, 93, 1322–1328. [Google Scholar] [CrossRef]
- Silva, W.D.; Millar, J.G.; Hanks, L.M.; Costa, C.M.; Leite, M.O.G.; Tonelli, M.; Bento, J.M.S. Interspecific Cross-Attraction between the South American Cerambycid Beetles Cotyclytus curvatus and Megacyllene acuta is Averted by Minor Pheromone Components. J. Chem. Ecol. 2018, 44, 268–275. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Akasaka, K.; Masuda, Y.; Konishi, S.; Yang, C.Y.; Takikawa, H.; Mori, K. Pheromone synthesis. Part 265: Synthesis and stereochemical composition of two pheromonal compounds of the female Korean apricot wasp, Eurytoma maslovskii. Tetrahedron 2020, 76, 131410. [Google Scholar] [CrossRef]
- Yu, S.; Yuan, G.; Liu, J.; Bian, Q.; Wang, M.; Zhong, J. Asymmetric synthesis of the sex pheromone of the apple leafminer, Lyonetia prunifoliella. Chirality 2023, 35, 118–128. [Google Scholar] [CrossRef]
- Evans, D.; Ennis, M.; Mathre, D.J. Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of .alpha.-substituted carboxylic acid derivatives. J. Am. Chem. Soc. 1982, 104, 1737–1739. [Google Scholar]
- Boeckman, R.K., Jr.; Charette, A.B.; Asberom, T.; Johnston, B.H. The chemistry of cyclic vinyl ethers. 6. Total synthesis of polyether ionophore antibiotics of the calcimycin (A-23187) class. J. Am. Chem. Soc. 1991, 113, 5337–5353. [Google Scholar] [CrossRef]
- Chow, S.; Fletcher, M.T.; Lambert, L.K.; Gallagher, O.P.; Moore, C.J.; Cribb, B.W.; Allsopp, P.G.; Kitching, W. Novel Cuticular Hydrocarbons from the Cane Beetle Antitrogus parvulus 4, 6, 8, 10, 16-Penta-and 4, 6, 8, 10, 16, 18-Hexamethyldocosanes Unprecedented anti-anti-anti-Stereochemistry in the 4, 6, 8, 10-Methyltetrad. J. Org. Chem. 2005, 70, 1808–1827. [Google Scholar] [CrossRef]
- Zhu, G.; Liang, B.; Negishi, E.I. Efficient and selective synthesis of (S, R, R, S, R, S)-4, 6, 8, 10, 16, 18-hexamethyl-docosane via Zr-catalyzed asymmetric carboalumination of alkenes (ZACA reaction). Org. Lett. 2008, 10, 1099–1101. [Google Scholar] [CrossRef]
- Wang, G.-J.N.; Molina-Lopez, F.; Zhang, H.; Xu, J.; Wu, H.-C.; Lopez, J.; Shaw, L.; Mun, J.; Zhang, Q.; Wang, S.; et al. Nonhalogenated Solvent Processable and Printable High-Performance Polymer Semiconductor Enabled by Isomeric Nonconjugated Flexible Linkers. Macromolecules 2018, 51, 4976–4985. [Google Scholar] [CrossRef]
- Kuwahara, S.; Liang, T.; Leal, W.S.; Ishikawa, J.; Kodama, O. Synthesis of all four possible stereoisomers of 5, 9-dimethylpentadecane, the major sex pheromone component of the coffee leaf miner moth, Perileucoptera coffeella. Biosci. Biotechnol. Biochem. 2000, 64, 2723–2726. [Google Scholar] [CrossRef] [PubMed]
- Taguri, T.; Yamakawa, R.; Fujii, T.; Muraki, Y.; Ando, T. Stereospecific inversion of secondary tosylates to yield chiral methyl-branched building blocks, applied to the asymmetric synthesis of leafminer sex pheromones. Tetrahedron Asymmetry 2012, 23, 852–858. [Google Scholar] [CrossRef]
- LeBel, N.A.; Balasubramanian, N. Stereospecific synthesis of 2,3,6-trisubstituted piperidines: An efficient total synthesis of (±)-pumiliotoxin C. J. Am. Chem. Soc. 1989, 111, 3363–3368. [Google Scholar] [CrossRef]
- Brown, A.C.; Carpino, L.A. Magnesium in methanol: Substitute for sodium amalgam in desulfonylation reactions. J. Org. Chem. 1985, 50, 1749–1750. [Google Scholar] [CrossRef]
- Yuan, G.; Yang, Y.; Liu, J.; Bian, Q.; Wang, M.; Zhong, J.C. Synthesis of the enantiomers of 13-methylheptacosane, the sex pheromone of pear psylla, Cacopsylla pyricola. Chirality 2021, 33, 274–280. [Google Scholar] [CrossRef]
- Bello, J.E.; McElfresh, J.S.; Millar, J.G. Isolation and determination of absolute configurations of insect-produced methyl-branched hydrocarbons. Proc. Natl. Acad. Sci. USA 2015, 112, 1077–1082. [Google Scholar] [CrossRef]
- Chi, S.; Wang, Y.; Wang, Z.; Li, H.; Gu, S.; Ren, Y. A chromosome-level genome of Semiothisa cinerearia provides insights into its genome evolution and control. BMC Genom. 2022, 23, 718. [Google Scholar] [CrossRef]
- Li, Z.-M.; Yao, E.-Y.; Liu, T.-L.; Liu, Z.-P.; Wang, S.-H.; Zhu, H.-Q.; Zhao, G.; Ren, Z.-L. Structural elucidation of sex pheromone components of the Geometridae Semiothisa cinerearia (Bremer et Grey) in China. Chin. J. Chem. 1993, 11, 251–256. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Yin, X.; Gong, Z.; Xing, H.; Miao, J.; Wang, S.; Liu, J.; Na, R.; Li, Q.X. Modular synthesis of the pheromone (2S,7S)-2,7-nonanediyl dibutyrate and its racemate and their field efficacy to control orange wheat blossom midge, Sitodiplosis mosellana (Géhin) (Diptera: Cecidomyiidae). Pest Manag. Sci. 2023, 79, 97–104. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Qu, C.; Zhang, Y.; Huang, J.; Pan, S.; Yang, Z.; Shi, Z.; Huang, Y.; Qin, Y.; et al. Editing Plant Volatile Isoeugenol for Integrated Aphid Management: Discovering Ecofriendly Insect Behavioral Regulators with Push–Pull Activity Targeting Odorant-Binding Proteins. J. Agric. Food Chem. 2025, 73, 20719–20730. [Google Scholar] [CrossRef]
- Miller, J.R.; McGhee, P.S.; Siegert, P.Y.; Adams, C.G.; Huang, J.; Grieshop, M.J.; Gut, L.J. General principles of attraction and competitive attraction as revealed by large-cage studies of moths responding to sex pheromone. Proc. Natl. Acad. Sci. USA 2010, 107, 22–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).