Abstract
This study investigates, for the first time, the relationship between carbon (δ13C) and nitrogen stable isotopic composition of Aspergillus niger mycelium, used as chitin and chitosan sources, and the fungus diet under controlled cultivation conditions. Four diets were tested, combining different carbon (C3- and C4-glucose) and nitrogen sources (KNO3 and NH4Cl). Results showed that carbon sources significantly influenced δ13C values of the mycelium: C4-glucose diets led to more negative Δ13C values (δ13CMYCELIUM-δ13CDIET) compared to C3-glucose diets. Nitrogen sources also affected isotopic fractionation, with KNO3 leading to negative Δ15N (δ15NMYCELIUM-δ15NDIET) and NH4Cl yielding positive Δ15N. Conversely, pH and temperature showed negligible effects on δ15N, while continuous aeration during growth significantly decreased δ15N, possibly due to partial assimilation of atmospheric nitrogen. These findings demonstrate that both nutrient and cultivation parameters can modulate the isotopic fractionation in A. niger, particularly for nitrogen. Although a direct correlation between diet composition and δ15N could not be established, this work provides the first experimental link between fungal metabolism and its isotopic fingerprint. The results offer a scientific foundation for applying stable isotope ratio analysis to authenticate and trace fungal-derived chitin and chitosan, with potential applications in food and winemaking industries.
1. Introduction
Chitosan is a natural biopolymer that, thanks to its chemical and physical properties, has recently found various applications in technological and industrial fields. In recent years, its use has also been proposed in winemaking, mainly as an antimicrobial, antioxidant, clarifying and chelating agent [1]. Commercially, this biopolymer is cost effectively obtained through the chemical deacetylation of chitin, which is the structural element of animal exoskeletons and fungal cell walls [2]. To date, the major source of industrial chitin comes from marine food production wastes, mainly crustacean shells, e.g., shrimp, crab or krill shells [3]. However, the International Organisation of Vine and Wine (OIV), through resolution OIV-Oeno 368-2009 [4], exclusively authorizes chitosan of fungal origin for winemaking purposes, which is normally obtained from citric acid production waste [5], while the animal-derived one is forbidden. The reason for this decision lies in the fact that the crustaceans can cause allergic reactions such as anaphylaxis [6], due to proteins like tropomyosin [7].
To verify the fungal origin of chitosan and prevent fraudulent substitution with animal-derived material, the OIV has outlined various analytical methods. Chitosan of fungal origin may simultaneously meet specific thresholds: residual glucan content > 2%, settled density ≥ 0.7 g/cm3, and viscosity < 15 cP in a 1% acetic acid solution. However, these parameters are not always concurrently satisfied and can potentially be manipulated to meet the specifications. Furthermore, these methods are labour-intensive, require over three hours per sample, and rely on instruments not always available in standard laboratories. Consequently, there is a growing demand for faster, more automated, and reliable approaches for chitosan authentication.
In response to these limitations, Perini et al. introduced a promising alternative based on stable isotope ratio (SIR) analysis of elements such as carbon and nitrogen, which enables the distinction between fungal- and animal-derived chitosan [8].
The method has been included in 2025 in the chitosan monograph (Resolution OIV-Oeno 368-2009) among the analytical techniques that can be used to guarantee its fungal origin with the OIV/OENO-SPECIF 23-728 resolution. More recently, Claverie et al. helped to define the isotopic ranges that characterize the two chitosan origins [9]. The author speculated that the isotopic differences they found could be due to the different sources of C, N, O and H used by either the fungus or the crustaceans, which are characterized by different isotopic compositions. For instance, the carbon absorbed by the fungus strain during its growth derives almost exclusively from either beet or cane sugar and could therefore likely reflect the typical carbon isotopic ratio (δ13C) of a C3 (from −29‰ to −25‰) or a C4 (between −14‰ and −12‰) plant [10]. As reported by Claverie et al., the δ13C of C3-fed fungal chitosan varied from −25.6‰ to −24.8‰, while the same parameter varied from −14.2‰ to −12.9‰ for C4-fed fungal chitosan [9]. Moreover, if the value of δ13C falls between −25.1‰ and −24.9‰, it would be necessary to proceed with the evaluation of the δ15N parameter, which must be above +2.7‰ to classify chitosan as from fungi. On the other hand, an average δ13C value of −21.1 ± 2.0‰ was reported for chitosan derived from crustaceans, which feed on marine phytoplankton having a typical δ13C of −20‰.
Both studies of Perini et al. [8] and Claverie et al. [9] were conducted exclusively on finished products (animal-derived chitosan from shrimp, crab and squid and fungal-derived chitosan from Aspergillus niger and Agaricus bisporus) provided by different producers. The isotopic relationship among the living organism (e.g., the fungus A. niger) fed on a specific diet, the diet composition and the chitin/chitosan produced by the organism itself has never been studied in detail, but it has only been inferred based on studies on terrestrial and aquatic organisms, such the one reported by DeNiro et al. [11]. Henn et al. [12] and Ruess et al. [13] in their respective studies on fungi (δ13C and δ15N variability), highlighted that the isotopic fractionation between mycelia and diet could vary depending on the chemoheterotrophic organism under study.
In this study, we focused on the initial stage of chitosan production, providing experimental evidence that links the isotopic composition of the A. niger diet to the stable isotopic signature (δ13C and δ15N) of the resulting fungal mycelium—the primary source of chitin. The effects on the subsequent stages, such as chitin extraction, purification, and deacetylation, were not considered here.
We investigated how different carbon and nitrogen sources, together with cultivation parameters such as temperature, pH and aeration, can influence the isotopic fractionation during fungal growth. This allowed us to identify the conditions that most strongly affect the isotopic composition of the final fungal biomass.
Specifically, the study aimed to quantify the extent of the isotopic fractionation under varying nutritional and environmental conditions and to determine which parameters have the greatest impact on δ13C and δ15N values. By establishing this relationship between the isotopic composition of the growth substrates and the resulting fungal signature, our work provides the first experimental evidence supporting the use of stable isotope ratios to authenticate the origin of chitosan.
2. Results and Discussion
2.1. Isotopic Analysis of Starting Spore Strain
To evaluate the possible correlation between the isotopic composition of the mycelium produced by A. niger and the starting spore of the fungus itself (see Section 3.1.2), the latter was isotopically analysed. Three replicates of the same starting spore sample were considered. The results of the δ13C and δ15N analysis are shown in Table 1.

Table 1.
Carbon (δ13C) and nitrogen (δ15N) values for three replicates of the starting spore sample.
The isotopic composition of the starting spore (average δ13C = −13.2‰ and δ15N = −1.3‰) doesn’t seem to be the major factor contributing to the δ13C and δ15N of the fungus mycelium.
In the case of diet A, the fungus was provided with a Glucose 1 (δ13C = −10.9‰) which resulted in a final mycelium having more negative δ13C (mean δ13C = −17.7‰, Table S1). In case the starting spore represented a relevant contribution to the δ13C of the final mycelium, a much less negative value would be expected. It can be therefore stated that the isotopic contribution of the starting spore is completely negligible and that the isotopic value of the final mycelium is solely correlated with the sources supplied to the fungus during its growth.
2.2. Estimation of the Inter-Days Repeatability
The replicates of both A and B diet sampled at different times (Table S1) were used to estimate the inter-days repeatability of the whole method (including the inoculum preparation, the mycelium growing and separation and finally in the stable isotope analysis). At 95% confidence level, the standard deviation of repeatability (sr) was estimated to be 0.3‰ for δ13C and 0.6‰ for δ15N. Correspondingly, the repeatability (r), calculated as 2.8 × sr, was 0.8‰ for δ13C and 1.8‰ for δ15N. Deviations among the isotopic values below these limits can therefore be considered as not significant.
2.3. Experiment 1 Test 1: Effect of Different Diets
The results of the carbon and nitrogen isotopic analyses of the mycelium samples obtained by growing of A. niger with four different diets (Diet A–D) are presented in Figure 1.
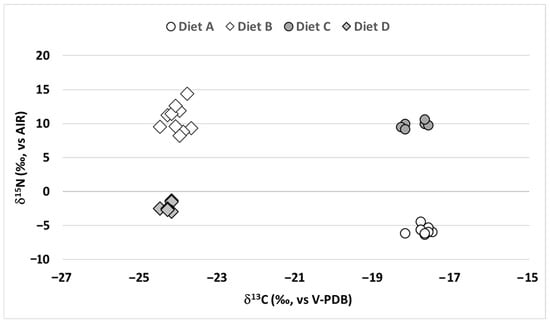
Figure 1.
Plot of δ13C vs. δ15N of the grown mycelium (chitin source) samples obtained from the fungus Aspergillus niger fed with four different diets.
2.3.1. Carbon Isotopic Ratio (δ13C) Shift Between the Diet and the Mycelium Produced by the Aspergillus niger Fungus
The mycelium obtained from A. niger fed on diets A and C (Figure 1 and Supplementary Table S1), both including a C4-carbon source (Glucose 1, δ13C = −10.9‰), exhibited a lower (more negative) δ13C than the diet. The Δ13C, defined as (δ13CMYCELIUM-δ13CDIET), was calculated as −6.8‰ and −7.1‰ for diets A and C, respectively. Conversely, mycelium from A. niger fed on diets B and D (Figure 1), both including a C3-carbon source (Glucose 2, δ13C = −23.7‰), showed smaller differences between the δ13C of diet and mycelium. The Δ13C values for diets B and D resulted in −0.4‰ and −0.6‰, respectively.
Earlier studies in fungi provided evidence of specifical biochemical pathways resulting in strong fractionation effects [14]. The enrichment in either the heavy or the light carbon isotope in fungal biomass compared to the bulk substrate (which is comparable to the just mentioned parameter Δ13C) of up to 7‰ was previously reported by Will et al. [15]. Moreover, deviations comparable to those found in this study were previously reported in other fungal species by Henn et al. [12]. For instance, when exposed to the same C3-based diet, the fungus Cryptoporus volvatus showed negligible enrichment with respect to its diet (+0.67‰), whereas Marasmius androsaceus and Suillus granulatus exhibited significant isotopic enrichments, having Δ13C (described in the study as isotopic discrimination and calculated as δ13CFUNGAL BIOMASS-δ13CBLANK MEDIUM) values of +4.87‰ and +4.91‰, respectively. On the other hand, in the same work and in disagreement with the results shown in this study, Henn et al., reported that the isotopic enrichment became negligible when the fungi were subjected to a C4-based diet [12].
According to Rossmann et al., glucose showed a pronounced isotopic asymmetry, which is more evident in sugars derived from C3 than from C4 plants [16]. The Δδ13C values, defined by the authors as deviations relative to the measured average value for the glucose molecule, were calculated and used to compare the average δ13C of the glucose molecule to the δ13C of every carbon atom in the structure (analysed after chemical degradation).
The calculation of the Δδ13C values revealed that for carbon 3 (Figure 2), C3 glucose was 13C-enriched compared to molecular δ13C, with a Δδ13C = 2.2‰, while C4 glucose had a Δδ13C = 1.2‰. For carbon 6 (Figure 2), C3 glucose was 13C-depleted compared to molecular δ13C, with a Δδ13C = −5.2‰, whereas C4 glucose had Δδ13C = −4.0‰. This asymmetry can explain the isotopic deviations observed in fungi fed on C3 vs. C4 diets, as suggested by Henn et al. [12]. In particular, the authors stated that carbon fractionation was related to the selective uptake of 13C-enriched C rather than from the selective loss of 12C from fungal biomass through depleted CO2. Once they stated that the focus to understand C fractionation was to be put on its uptake, they proposed a model called dual-uptake hypothesis. Briefly, they suggest two alternative routes in fungi C uptake, resulting in differential isotopic discrimination. In the first one, the hexose molecules are brought into the cell without catabolism, resulting in no C fractionation; in the second one, the hexose molecules are broken down extracellularly into triose fragments though two separate routes characterised by different rates, therefore resulting in C fractionation. Despite the requirement for confirmation of this hypothesis, it would support the results obtained in the present study. Moreover, in a following study of Henn, [17] the same authors assessed that the exact means by which 13C is selectively taken into the fungal cell from the medium is unclear. Therefore, to fully understand the metabolic pathway that the sugars undergo from source to fungus further studies are required.
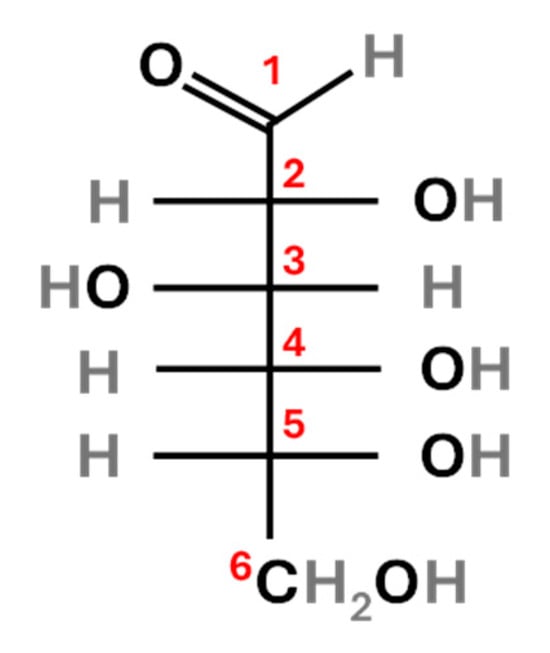
Figure 2.
Glucose structure: carbon atoms are labelled in red (1–6).
Henn et al. also highlighted significant isotopic effects associated with sucrose utilization by different species of basidiomycetes [12]. These effects are species-specific and vary consistently depending on whether the sucrose originates from a C3 or C4 carbon source. As such, it is crucial to study the isotopic behaviour of individual chemoheterotrophic organisms independently.
The shift toward more negative δ13C values during mycelium biosynthesis (from −10.9‰ to −17.7‰ in the C4 diet, and from −23.7‰ to −24.1‰ in the C3 one) was consistent with previously reported fractionation processes occurring during different biosynthetic pathways [18]. Furthermore, certain types of bacteria use carbon sources with specific δ13C signatures or possess metabolic pathways that yield specific δ13C values, which can support their identification [18].
The results demonstrate that under identical growth conditions (temperature and pH) the δ13C signatures of A. niger mycelium (chitin source) can be directly traced back to the isotopic composition of the diet with deviations depending on the type of carbon source (C3- or C4- derived glucose).
2.3.2. Nitrogen Isotopic Ratio (δ15N) Shift Between the Diet and the Final Mycelium Produced by the Aspergillus niger Fungus
As for δ15N, the mycelium obtained from A. niger fed on diets A and D (Figure 1), both including potassium nitrate as nitrogen source, showed lower δ15N values compared to the diet. The Δ15N, defined as (δ15NMYCELIUM-δ15NDIET), was −7.0‰ and −3.6‰ for diets A and D, respectively. In contrast, for diets B and C (Figure 1), both including ammonium chloride as nitrogen source, an unexpected positive difference between the δ15N of the mycelium and the diet was observed. The Δ15N values resulted +12.5‰ for diet B and +11.7‰ for diet C.
The enrichment in either the heavy or the light nitrogen isotope in fungal biomass compared to the bulk substrate (which is comparable to the just mentioned parameter Δ15N) of up to 4‰ was previously reported by Will et al. [15] and forest basidiomycetes were also found to become either enriched or depleted in 15N relative to atmospheric nitrogen.
A trend similar to that found in this study was reported by Emmerton et al. in a study on nitrogen assimilation and isotopic fractionation in the mycorrhizal fungi Paxillus involutus and Leccinum scabrum [19]. When supplied with ammonium sulphate, the δ15N of the nitrogen (N2) assimilated by P. involutus and L. scabrum displayed Δ15N values of +4‰ and +3‰, respectively. On the other hand, when provided with calcium nitrate the δ15N of the N2 assimilated by both fungi was significantly lower than the dietary one. In the same study, a different nitrogen isotopic fractionation was observed for an ericoid mycorrhizal fungus (ERM) [19]. When the ERM fungus was grown with calcium nitrate, no fractionation relative to the nitrogen source was observed, whereas a Δ15N of −15‰ was reported when including ammonium sulphate in the diet. Although these results are difficult to fully explain, Emmerton et al. [19] suggest that nitrogen fractionation during assimilation largely depends on the pathways preferentially used by the fungal species considered.
An analogous trend in δ15N values was also reported by Högberg et al. in their study on nitrogen isotopic fractionation in fungal species forming symbiotic associations (mycorrhizae) with Scots pine (Pinus sylvestris) [20]. Högberg et al. examined variations in δ15N values among three fungal species during nutrient transport from soil to plant, using potassium nitrate (δ15N = 0.6 ± 0.0‰) and ammonium chloride (δ15N = −0.5 ± 0.0‰) as nitrogen sources in solutions with pH ≈ 7.4 and 4, respectively. The authors reported δ15N values in some cases more negative and in others more positive than the nitrogen source, regardless of the diet. For example, Suillus bovinus exhibited more positive δ15N values than the nitrogen source with an ammonium chloride-bases diet, while with a potassium nitrate-based diet, δ15N values were more negative. This pattern supports the δ15N trends observed in the present study for A. niger grown with different nitrogen sources. The authors argued that the larger isotopic effect (which is related, in the present study, to the higher Δ15N) observed for the diets including the ammonium chloride rather than the potassium nitrate is to be expected, because of the larger relative difference in mass if 15N replaces 14N in the ammonium chloride rather than in the potassium nitrate.
It is worth making an additional observation on the difference in the Δ15N of diets including either the ammonium or the nitrate N source. The higher Δ15N in diets B and C (ammonium) than A and D (nitrate) could be related to the efficiency of the N source. A study on the removal of multiple nitrogenous wastes by the fungus A. niger showed that ammonium was metabolised rapidly prior to nitrite, since the latter was a less efficient source of nitrogen for cell growth compared to the former. It must be noticed that nitrite is not nitrate, therefore even though an exact comparison cannot be made, parallelism can still be noticed. The higher N uptake could be therefore related to a more consistent fractionation, as already stated in plants [20]. In support to this affirmation, Högberg et al. found out that plants given nitrate took up 12–33% of the N supplied and had less fractionation with respect to the source (0.0‰–1.7‰), whereas plants given ammonium took up 18–86% of the N supplied and had higher fractionation with respect to the source (0.9‰–5.8‰). Ruess et al. reported similar δ15N trends in fungi such as Laccaria laccata, Agrocybe gibberosa, and Chaetomium globosum [13]. They observed both positive (e.g., C. globosum Δ15N = 0.3‰, A. gibberosa Δ15N = 0.8‰) and negative (e.g., L. laccata Δ15N = −2.1‰) deviations in δ15N values between the fungus and the diet (e.g., potato dextrose or Pachlewska agar). Possible sources of variation in δ15N fractionation were attributed to differences in the type and quality of food, as well as to the metabolism and physiological state of the fungal species [13].
As Brauer et al. noted, factors such as pH, temperature, light exposure, and the nitrogen source included in the diet can influence mycelium biosynthesis in Aspergillus fungi [21]. In response to such stressors, the fungal cell wall, composed of mycelium, undergoes continuous remodelling to tolerate adverse extracellular conditions, ensuring growth and reproduction while avoiding cell death [22]. Furthermore, condition-specific alterations in the Aspergillus cell wall may occur, potentially leading to increased mycelium production and/or redistribution within the organism [23].
The results indicate that the nitrogen source in the substrate significantly influences both the direction and magnitude of nitrogen isotopic fractionation in the mycelium produced by the A. niger. However, the observed differences among treatments cannot be attributed solely to the chemical nature of the nitrogen source (nitrate or ammonium). Other factors, such as nitrogen assimilation efficiency, growth conditions, and internal metabolic processes, are likely to contribute to the observed isotopic variability.
Therefore, based on the available data, a direct and generalizable relationship between the diet and the δ15N values of chitin cannot be established. Instead, the nitrogen isotopic fractionation in A. niger appears to reflect the complex interaction between the type of nitrogen source and the fungus specific physiological responses to environmental and nutritional conditions.
2.4. Temporal and Condition-Dependent Fractionation of Carbon and Nitrogen Isotopes in Aspergillus niger Mycelium
Despite the hypotheses presented in Section 2.3.1 and Section 2.3.2, which tried to explain the pathways on which nitrogen and carbon fractionation in fungi are based, many authors agreed in affirming that the mechanism of C and N fractionation between the fungus and its source is still far from being understood (Henn & Chapela, [14], Henn et al., [17]). Therefore, to better understand the mechanisms underlying the variations in δ13C and δ15N between the diet and the mycelium produced by A. niger, fractionation tests described in Section 2.4.1 were designed and conducted. Since diets A and D (including potassium nitrate) were cultured in a pH ≈ 7 broth, while diets B and C (including ammonium chloride) were cultured in a pH ≈ 5 broth, an additional experiment (Section 2.4.2) was conducted to evaluate whether pH effects could explain the observed δ15N differences.
2.4.1. Experiment 1 Test 2: Monitoring over the Time
To better understand the overtime changes in the δ13C and δ15N values of mycelium during its production by the fungus A. niger, fractionation tests were designed and conducted using diets A and B as reported in Section 3.1.4. The results of the duplicate experiments on diets A and B are reported in Table 2 (missing data correspond to a missed mycelium sampling).

Table 2.
Results of the isotopic fractionation tests for carbon (δ13C) and nitrogen (δ15N) of the mycelium produced by the fungus Aspergillus niger fed with diets A and B. For each test, mycelium (chitin source) was sampled 4 times every other day (Sampling No. 1–4) from two fungi samples (Sample 1 and 2).
Considering the estimated repeatability (see Section 2.2), the results for the δ13C showed negligible variation (p > 0.05) over the collections, both for diet A and B. The δ13C of the mycelium produced by the fungi remained steady from the first sampling, which occurred 2–4 days after inoculation, to the last one.
A different trend was found for δ15N. Unlike δ13C, the δ15N values progressively increased for diet B, while decreased for diet A. The variation in the δ15N values indicates nitrogen fractionation processes during mycelium biosynthesis. As reported in paragraph 2.3.2, the variation in δ15N can be caused by the different conditions in which the A. niger fungus grows, particularly the nitrogen source administered in the diet (potassium nitrate vs. ammonium chloride).
2.4.2. Experiment 2: Effect of pH, Temperature and Airflow on the δ15N Values
To understand the possible effects induced by some fermentation parameters on the δ15N, two different experiments were set up. In the first one, the effect of temperature and pH were studied. The fungi fed on diet B and kept at 25 °C produced a mycelium having mean δ15N values of +10.6‰, +9.5‰ and +9.5‰ at pH 5.3, 7 and 8, respectively. The δ15N measured at different pH values were not statistically different, considering the repeatability estimated in Section 2.2.
On the other hand, temperature had a significant impact on the δ15N mycelium produced by A. niger fed on diet B and growing in a pH = 5.3 broth. The δ15N of mycelium produced at 15 °C (+6.4‰) was statistically different to the values measured at 25 °C (+13.6‰) and 40 °C (+14.5‰) (p < 0.05). The lower temperature of 15 °C contributed to slowing down the growth process and led to as high isotopic values as the other temperatures (Table 3).

Table 3.
Effect of temperature, pH and airflow on mycelium δ15N value (mean of three replicates each); diet B was considered to study the effect of temperature and pH, while diets B and C were considered to study the effect of the airflow; different letters indicate statistically different results (p < 0.05).
Finally, the effect of constant airflow applied during the fungi growth was considered. The pH was monitored and remained steady all the test long (pH = 5.3). For both diets, a statistical difference (p < 0.01 and Tukey test) was highlighted between the δ15N of mycelium samples produced under or without the airflow. Regardless of the diet, higher δ15N values were found for mycelium produced without the use of airflow (Table 3). This may be because, as hypothesized by some authors, Aspergillus species can fix the atmospheric nitrogen.
Pannington et al. reported that the ability of fungi to absorb atmospheric nitrogen has been an open question for years [24]. Lipman’s experiments seemed to demonstrate this ability for the Aspergillus species [25], but subsequent studies often led to opposite conclusions [26]. The atmospheric nitrogen δ15N is equal to 0‰ (standard AIR). Its assimilation by the fungus could justify a decrease in the δ15N value recorded in samples subjected to airflow, which improves atmospheric nitrogen dispersion in the broth. Further studies are required to better understand this phenomenon, eventually considering additional nitrogen and carbon sources, as well as fungal species other than Aspergillus.
3. Materials and Methods
3.1. Description of Samples and Processes
3.1.1. Ingredients
The A. niger ATCC6275 was purchased from the American Type Culture Collection (Manassas, VA, USA) in dried form, rehydrated according to the supplier’s instructions and allowed to grow in Potato Dextrose Broth (PDB, Oxoid, Basingstoke, UK) for 48 h at 25 °C. Then, the culture was supplemented with 20% v/v glycerol (Sigma Aldrich, Saint Louis, MO, USA) and stored at −80 °C until the tests. Water employed in the tests was twice demineralized by a Water Purifier (Stedim Arium 611DI, Sartorius AG, Göttingen, Germany) and sterilized at 121 °C for 15 min in VE-95 autoclave (Systec GmbH & Co. KG, Linden, Germany). Potassium nitrate (KNO3, δ15N = 1.3‰) and ammonium chloride (NH4Cl, δ15N = −1.8‰) (Merck, Darmstadt, Germany) were used as nitrogen sources, while two glucose samples (CARLO ERBA Reagents S.r.l., Milan, Italy) with different δ13C values were used as carbon sources (Glucose 1: δ13C = −10.9‰; Glucose 2: δ13C = −23.7‰). The two samples were chosen to mimic a C4 and C3 diet, respectively. The isotopic values were obtained by analysing the different ingredients in triplicate.
3.1.2. Preparation of the Starting Spore
The fungus culture (1 mL having a nominal concentration of 107 CFU/mL) was reactivated in 200 mL of PDB for 24 h at 25 °C, then multiplied for a variable interval between 3 and 30 days in growing volume and in the same conditions.
To analyse the isotopic composition of A. niger and evaluate their potential influence on subsequent mycelium analyses, 10 Petri dishes (9 cm diameter) were filled with potato dextrose agar (PDA, Oxoid, Basingstoke, UK) growth medium, onto which 1 mL of spore was spread. Dishes were incubated for 7 days at 25 °C, resulting in a complete covering of the surface by mould mycelium. After incubation, the mycelium was removed by a sterile stainless-steel spatula and divided into 3 Eppendorf tubes. The mycelium was then washed with deionized water and centrifuged (4500 rpm for 5 min with Thermo centrifuge KR4, Bremen, Germany) three times. The resulting samples were subsequently frozen and lyophilized.
3.1.3. Experiment 1 Test 1: Effect of Different Diets
The production of citric acid from A. niger results in approximately 80,000 tons/year of mycelial waste materials [27]. These fungal wastes represent a free natural source of chitin and chitosan. For this reason, in this study, the laboratory-synthesized mycelium samples were obtained from A. niger, which is considered as the commercially most used fungi for chitosan production. To evaluate the correlation between the isotopic composition of the diet provided to the growing fungus and of the mycelium used as chitin source finally produced by the fungus itself, four specific diets characterized by different sources of nitrogen and carbon were formulated.
For this purpose, 1 g of A. niger fungal mycelium was inoculated on the culture media (1 L for each test) consisting of water and specific carbon and nitrogen sources, in concentrations which aimed to simulate the diet provided to the fungus in common industrial processes. The complete description of the four diets (Diet A–D) is reported in Table 4.

Table 4.
Description of the nitrogen (potassium nitrate, ammonium chloride) and carbon (D-(+) glucose) sources supplied to the Aspergillus niger fungus in the different diets A, B, C and D.
After inoculation, the different media were incubated at 25 °C, and the fungal mycelium produced 10 days after inoculation was sampled.
For diets A and B, 5 samples of A. niger were considered for each one. The experiment was subsequently replicated, resulting in a total of 10 replicates for diets A (Table S1, samples 1–10) and B (Table S1, samples 11–20). For diets C and D, 3 samples of A.niger were considered for each diet. The experiment was duplicated, resulting in a total of 6 replicates for diets C (Table S1, samples 21–26) and D (Table S1, samples 27–32).
3.1.4. Experiment 1 Test 2: Monitoring over the Time
Diets A and B were finally selected to monitor the δ15N and δ13C variation in the mycelium produced by the A. niger fungus over time. For this purpose, the fungus inoculated in the culture medium was allowed to grow for 5–12 days, and the produced grown mycelium was subsequently sampled 4 times at two-day intervals. Since a small part of the fungal product needed to be sampled multiple times, it was necessary to use a larger amount of starting material (both A. niger and culture medium). Therefore, it was decided to simulate the industrial conditions applied to the bioreactors used for chitosan production, using a 3 L volume of culture medium inoculated with 5 mL of A. niger culture subject to a continuous air stream at 25 °C. In this part, two fungus samples were considered for each diet, repeating the experiment twice.
3.1.5. Experiment 2: Effect of the Variation in pH, Temperature and Presence or Absence of a Constant Airflow on δ15N Value
The effect of temperature and pH variation were evaluated separately in two different experiments. The fungi A. niger were given diet B and grown for 10 days. To evaluate the pH effect, the fungi were grown at a constant temperature of 25 °C in three different culture broths adjusted to pH 5.3, 7.0 and 8.0. Three replicates for each pH value were considered (see Table 3, Test 1). To evaluate the temperature effect, the fungi were grown for 10 days at a constant pH of 5.3 in three culture broths at constant temperatures of 15 °C, 25 °C and 40 °C. Three replicates for each temperature value were considered (see Table 3, Test 2). Therefore, the effect of the air stream applied during the growth was evaluated. The fungi were fed with diets B and C, both subject and not subject to a continuous air flow (1 mL/min). Three replicates for each combination (diet B–no flow; diet B–air flow; diet C–no flow; diet C–air flow) were considered (see Table 3, Test 3).
3.1.6. Mycelium Sample Isolation
The fungal mycelia produced by A. niger were washed with water using an ULTRA TURRAX® IKA® T25 digital and centrifuged three times (Thermo centrifuge at 4100 rpm for 5 min). The resulting mycelium samples were then freeze-dried with an ALPHA I-5 ChrisA 5Pascal lyophilized (Duration 24 h). Finally, they were homogenized using a mortar and pestle in liquid nitrogen.
3.2. Stable Isotope Ratio Analysis
For the determination of δ15N and δ13C values, samples were weighed 0.8 mg in tin capsules (4 mm diameter, 6 mm height) using a microbalance (XM1000P Sartorius Lab Instruments GmbH & Co. KG, Göttingen, Germany). Each capsule was closed with forceps and pressed to eliminate residual air. To simultaneously perform δ15N and δ13C analyses, tin capsules were inserted into an Elemental Analyzer (EA) (NC analyser FLASH EA 1112 SERIES, Thermo Scientific, Waltham, MA, USA) for total sample combustion. The gasses (CO2 and N2) resulting from the combustion were then transferred through a helium stream to an Isotope Ratio Mass Spectrometer (IRMS) (Finnigan DELTA XP, Thermo Scientific) for the isotopic ratio measurement. The δ15N and δ13C values were calculated using two internal reference standards (casein ST1: δ15N = 6.56‰ and δ13C = −23.51‰; casein ST2: δ15N = 7.38‰ and δ13C = −21.98‰), calibrated against international standard reference materials. For 13C/12C analysis, the reference standards used were fuel NBS-22 (δ13C = −30.03‰), sucrose IAEA-CH6 (δ13C = −10.45‰) (IAEA—International Atomic Energy Agency, Vienna, Austria), and L-glutamic acid USGS 40 (δ13C = −26.39‰ and δ15N = −4.52‰) (U.S. Geological Survey, Reston, VA, USA); for 15N/14N analysis, the standards used were glutamic acid USGS 40 (δ13C = −26.39‰ and δ15N = −4.52‰) (U.S. Geological Survey, Reston, VA, USA) and potassium nitrate IAEA-NO3 (δ15N = 4.7‰) (IAEA—International Atomic Energy Agency, Vienna, Austria).
The instrumental precision, expressed as reproducibility limit calculated as 2 * rad2 * SDreproducibility of the same sample, was 0.3 for both δ15N and δ13C.
The measured isotope ratios are reported in the delta (δ) notation corresponding to the relative deviations of the molar ratio (R) of the heavy elements (iE i.e., 13C) to light elements (jE i.e., 12C) isotopes in the samples from those in international standards V−PDB (Vienna−Pee Dee Belemnite) for δ13C and Air (atmospheric N2) for δ15N, as shown in the following equation:
where, ref is the international measurement standard, sample is the analysed sample, and iE/jE is the ratio of heavier to lighter isotopes.
The delta values are here multiplied by 1000 and expressed in the more common unit "per mil" (‰) rather than, as required by the International System of Units (SI), in milliurey units (mUr).
3.3. Statistics
The statistical analysis of the isotopic results was performed using the Statistica program version 14.0.1.25. Data normality was tested using the Kolmogorov–Smirnov and Lilliefors tests. To evaluate significant differences between groups, a one-way ANOVA was performed, with Tukey’s test as a post hoc test. The threshold p-value for significant differences was set at 0.05. Pearson’s correlation coefficient was used for assessing the linear correlation between two parameters.
4. Conclusions
This study investigated for the first time the correlation between the isotopic values of A. niger fungal mycelium used as a chitin source, a precursor of chitosan, and those of the diet supplied to the fungus for its growth.
Different combinations of carbon (glucose from C3 and C4 plants) and nitrogen sources (potassium nitrate and ammonium chloride) were considered. The use of glucose from C4 plants led to larger absolute Δ13C values (defined as δ13CMYCELIUM-δ13CDIET) than glucose from C3 plants and repeated experiments showed that this deviation remained steady over time.
The nitrogen source affected the δ15N values of the mycelium differently, with positive Δ15N (defined as δ15NMYCELIUM-δ15NDIET) values measured when the ammonium chloride was considered and negative Δ15N values when potassium nitrate was taken as the primary nitrogen source. δ15N did not seem to be affected by the broth pH or the temperature usually applied in industrial procedures (higher than 25 °C), but it was clearly affected by the application of an airflow during the growth.
Although the fractionation effects on the subsequent stages of chitosan production (i.e., chitin extraction, purification, and deacetylation to chitosan) have not been evaluated yet, this study represents the first step toward understanding how the isotopic composition of A. niger mycelium relates to its growth conditions and nutrient sources. Carbon isotopic ratios (δ13C) clearly reflected the isotopic composition of the carbon source, confirming the reliability of δ13C as an indicator of dietary carbon origin. Conversely, nitrogen isotopic ratios (δ15N) showed more complex fractionation behaviour: while the nitrogen source influenced the direction of isotopic enrichment (negative Δ15N for nitrate and positive Δ15N for ammonium), no simple or direct correlation could be established between the diet composition and the δ15N of the fungal mycelium.
These findings suggest that nitrogen isotopic fractionation in A. niger does not only depend on the chemical form of nitrogen, but also on the fungus metabolism and physiological responses to cultivation conditions such as aeration. Overall, this work provides the first experimental framework for interpreting isotopic variability in fungal chitin and its derived chitosan, offering a scientific basis for the development of isotopic tools for authentication and traceability of fungal-derived biopolymers. Although exploratory in nature, the study delivers fundamental insights that can guide future research aimed at expanding the dataset and strengthening the statistical robustness of isotopic models for chitosan origin determination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30204142/s1, Table S1.
Author Contributions
Conceptualization: M.P., S.P. and R.G.; Data curation: S.P. and F.V.; Formal analysis: S.P. and F.V.; Project administration: R.L.; Software: M.P.; Supervision: R.L. and M.P.; Validation: R.G.; Visualization: R.G.; Writing—original draft: M.P., R.G. and S.P.; Writing—review and editing: R.L. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be made available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation and Characterization of Chitin and Chitosan—A Review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Resolution OIV-Oeno 368-2009. Available online: https://www.oiv.int/public/medias/1094/oiv-oeno-368-2009-en.pdf (accessed on 5 July 2024).
- Cai, J.; Yang, J.; Du, Y.; Fan, L.; Qiu, Y.; Li, J.; Kennedy, J.F. Enzymatic Preparation of Chitosan from the Waste Aspergillus Niger Mycelium of Citric Acid Production Plant. Carbohydr. Polym. 2006, 64, 151–157. [Google Scholar] [CrossRef]
- Faber, M.A.; Pascal, M.; El Kharbouchi, O.; Sabato, V.; Hagendorens, M.M.; Decuyper, I.I.; Bridts, C.H.; Ebo, D.G. Shellfish Allergens: Tropomyosin and beyond. Allergy 2017, 72, 842–848. [Google Scholar] [CrossRef]
- Chagas, R.; Monteiro, S.; Boavida Ferreira, R. Assessment of Potential Effects of Common Fining Agents Used for White Wine Protein Stabilization. Am. J. Enol. Vitic. 2012, 63, 574–578. [Google Scholar] [CrossRef]
- Perini, M.; Nardin, T.; Venturelli, M.; Pianezze, S.; Larcher, R. Stable Isotope Ratio Analysis as a Fast and Simple Method for Identifying the Origin of Chitosan. Food Hydrocoll. 2020, 101, 105516. [Google Scholar] [CrossRef]
- Claverie, E.; Perini, M.; Onderwater, R.C.A.; Pianezze, S.; Larcher, R.; Roosa, S.; Yada, B.; Wattiez, R. Multiple Technology Approach Based on Stable Isotope Ratio Analysis, Fourier Transform Infrared Spectrometry and Thermogravimetric Analysis to Ensure the Fungal Origin of the Chitosan. Molecules 2023, 28, 4324. [Google Scholar] [CrossRef]
- O’Leary, M.H. Carbon Isotopes in Photosynthesis. Bioscience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of Diet on the Distribution of Carbon Isotopes in Animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Henn, M.R.; Chapela, I.H. Differential C Isotope Discrimination by Fungi during Decomposition of C(3)- and C(4)-Derived Sucrose. Appl. Environ. Microbiol. 2000, 66, 4180–4186. [Google Scholar] [CrossRef]
- Ruess, L.; Häggblom, M.M.; Langel, R.; Scheu, S. Nitrogen Isotope Ratios and Fatty Acid Composition as Indicators of Animal Diets in Belowground Systems. Oecologia 2004, 139, 336–346. [Google Scholar] [CrossRef]
- Henn, M.R.; Chapela, I.H. Ecophysiology of C and N Isotopic Fractionation in Forest Fungi and the Roots of the Saprotrophic-Mycorrhizal Divide. Oecologia 2001, 128, 480–487. [Google Scholar] [CrossRef]
- Will, O.H., III; Tieszen, L.L.; Gerlach, T.; Kellen, M. Alteration of Carbon Isotope Ratios by Eight Ustilago Species on Defined Media. Bot. Gaz. 1989, 150, 152–157. [Google Scholar] [CrossRef]
- Rossmann, A.; Butzenlechner, M.; Schmidt, H.L. Evidence for a Nonstatistical Carbon Isotope Distribution in Natural Glucose. Plant Physiol. 1991, 96, 609–614. [Google Scholar] [CrossRef]
- Henn, M.R. Biochemical Basis and Ecological Implications of Stable Carbon and Nitrogen Isotopic Fractionation by Basidiomycete Fungi; University of California, Berkeley: Berkeley, CA, USA, 2002; p. 318. [Google Scholar]
- Boschker, H.T.S.; Middelburg, J.J. Stable Isotopes and Biomarkers in Microbial Ecology. FEMS Microbiol. Ecol. 2002, 40, 85–95. [Google Scholar] [CrossRef]
- Emmerton, K.S.; Callaghan, T.V.; Jones, H.E.; Leake, J.R.; Michelsen, A.; Read, D.J. Assimilation and Isotopic Fractionation of Nitrogen by Mycorrhizal Fungi. New Phytol. 2001, 151, 503–511. [Google Scholar] [CrossRef]
- Högberg, P.; Högberg, M.N.; Quist, M.E.; Ekblad, A.; Näsholm, T. Nitrogen Isotope Fractionation during Nitrogen Uptake by Ectomycorrhizal and Non-mycorrhizal Pinus sylvestris. New Phytol. 1999, 142, 569–576. [Google Scholar] [CrossRef]
- Brauer, V.S.; Pessoni, A.M.; Freitas, M.S.; Cavalcanti-Neto, M.P.; Ries, L.N.A.; Almeida, F. Chitin Biosynthesis in Species. J. Fungi 2023, 9, 89. [Google Scholar] [CrossRef]
- Latgé, J.-P.; Beauvais, A.; Chamilos, G. The Cell Wall of the Human Fungal Pathogen Aspergillus Fumigatus: Biosynthesis, Organization, Immune Response, and Virulence. Annu. Rev. Microbiol. 2017, 71, 99–116. [Google Scholar] [CrossRef]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A.R. Chitin Synthesis and Fungal Pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef]
- Pennington, L.H. Upon Assimilation of Atmospheric Nitrogen by Fungi. Bull. Torrey Bot. Club 1911, 38, 135. [Google Scholar] [CrossRef]
- Lipman, C.B. Nitrogen Fixation by Yeasts and Other Fungi; University of California, Berkeley: Berkeley, CA, USA, 1911; p. 14. [Google Scholar]
- Jayasinghearachchi, H.S.; Seneviratne, G. Can Mushrooms Fix Atmospheric Nitrogen? J. Biosci. 2004, 29, 293–296. [Google Scholar] [CrossRef]
- Ali, S.; Ul-Haq, I.; Qadeer, M.A.; Iqbal, J. Production of Citric Acid by Aspergillus Niger Using Cane Molasses in a Stirred Fermentor. Electron. J. Biotechnol. 2002, 5, 19–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).