Functionalized Silica Fume for Efficient Cd2+ Removal from Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Materials
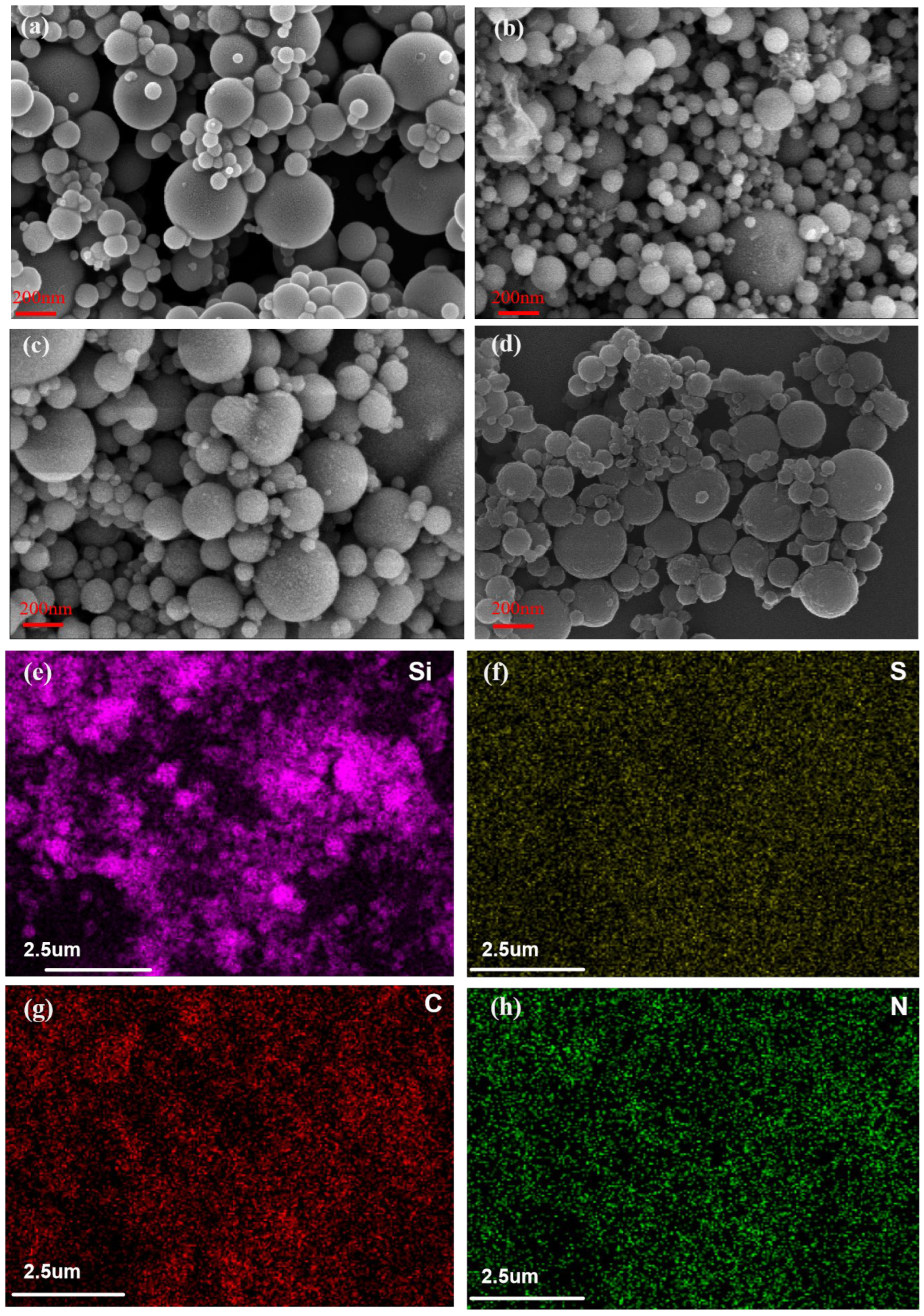
2.1.1. Scanning Electron Microscopy (SEM) Analysis
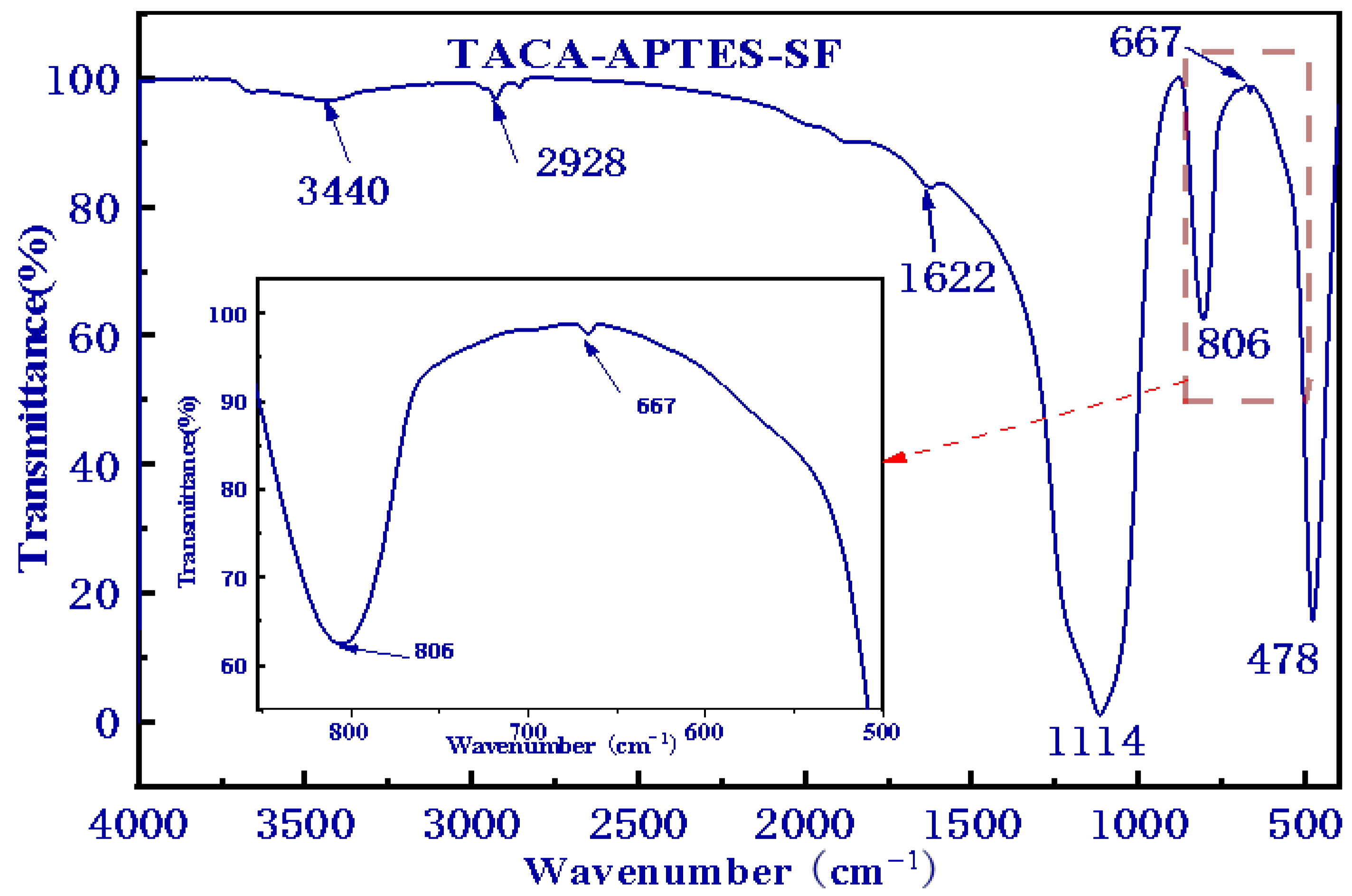
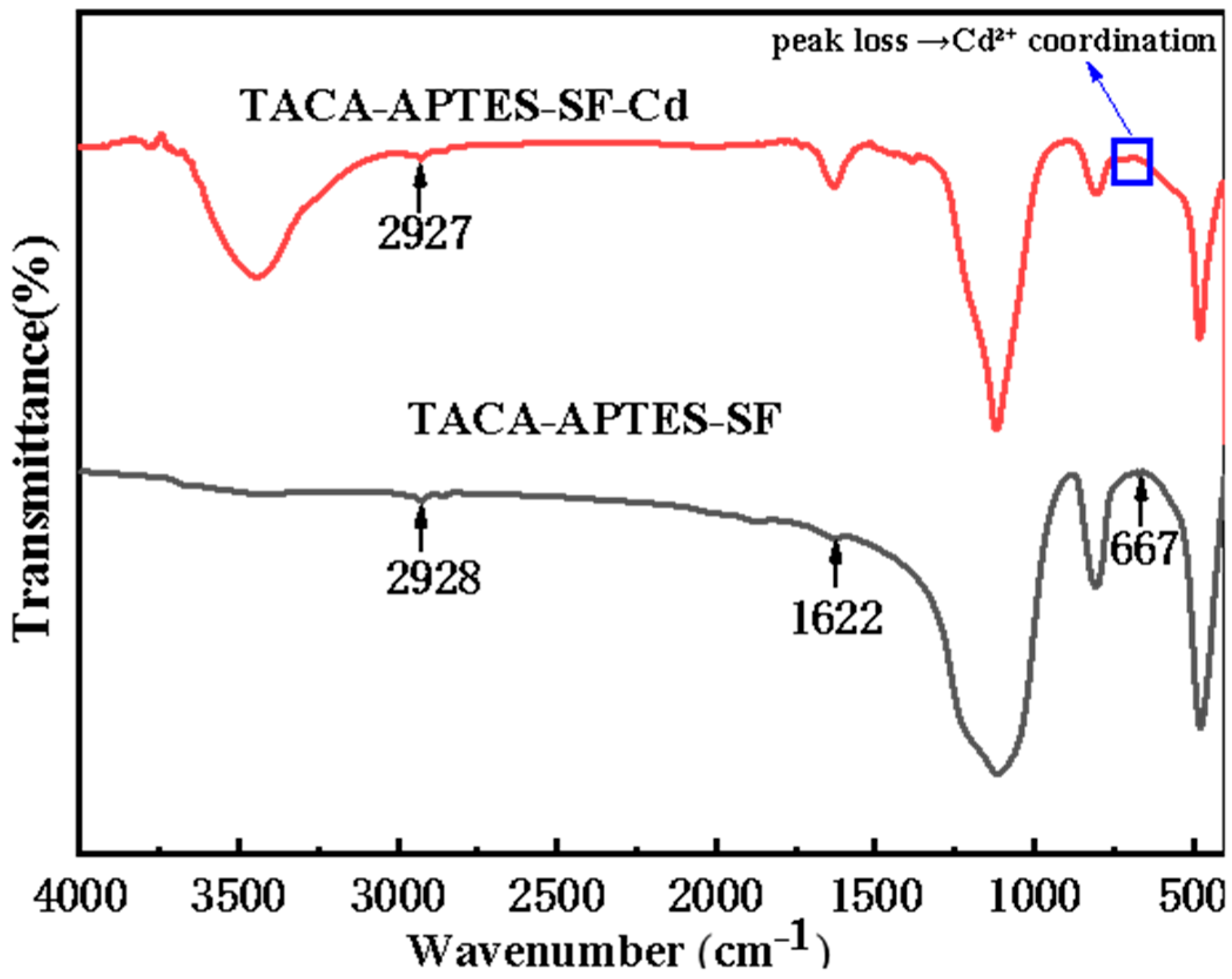
2.1.2. Fourier-Transform Infrared (FT-IR) Spectroscopy
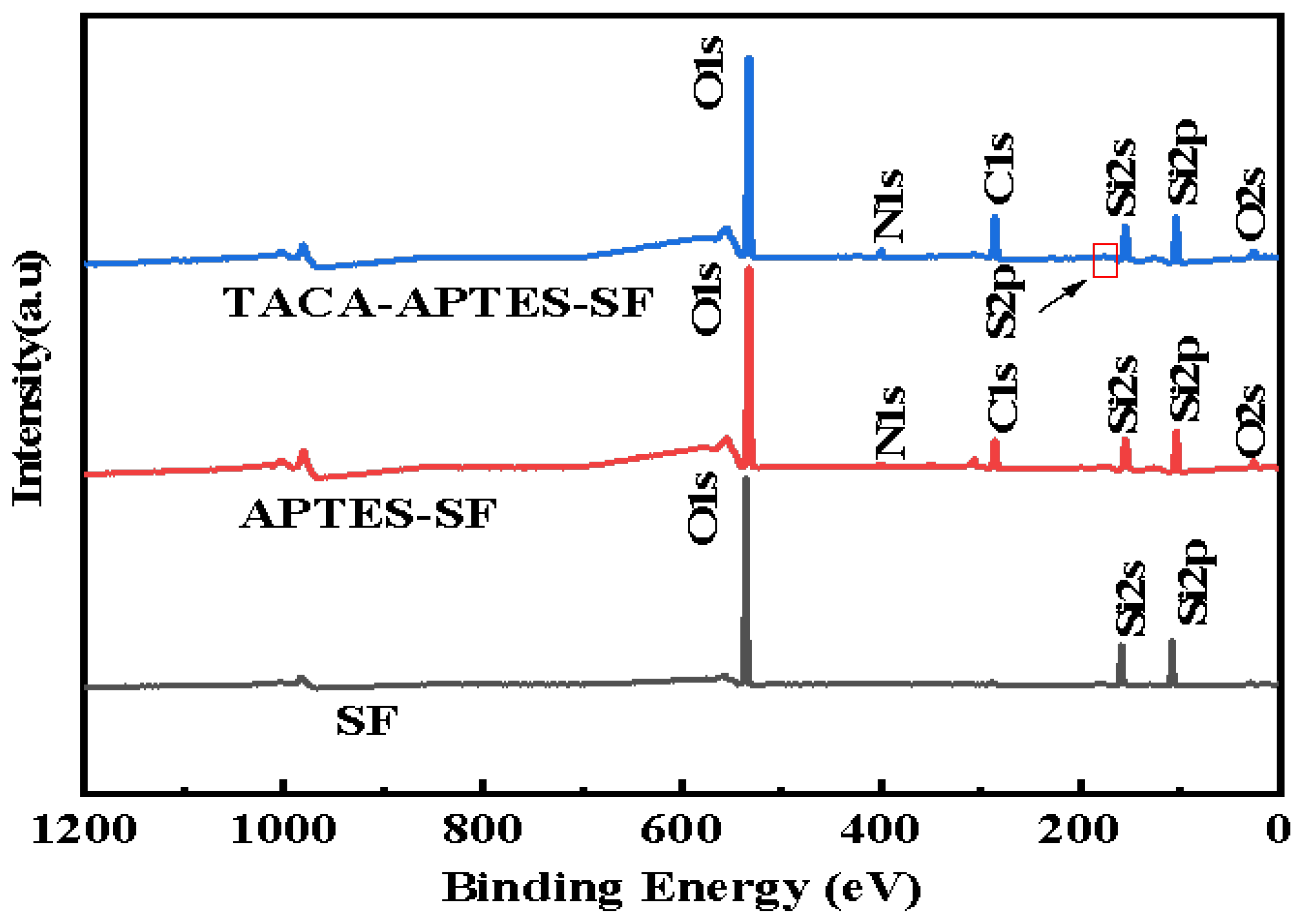
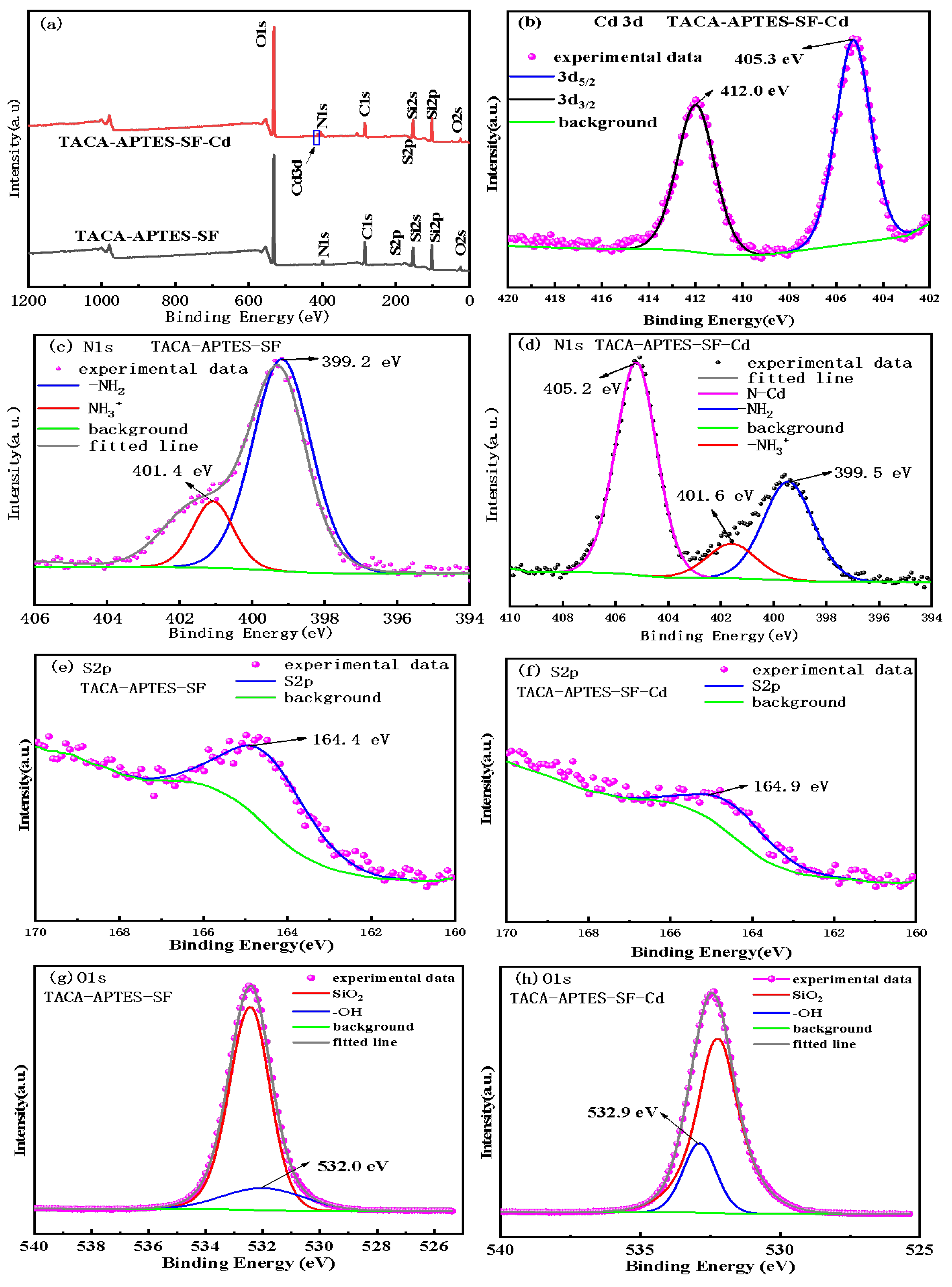
2.1.3. X-Ray Photoelectron Spectroscopy (XPS) Analysis
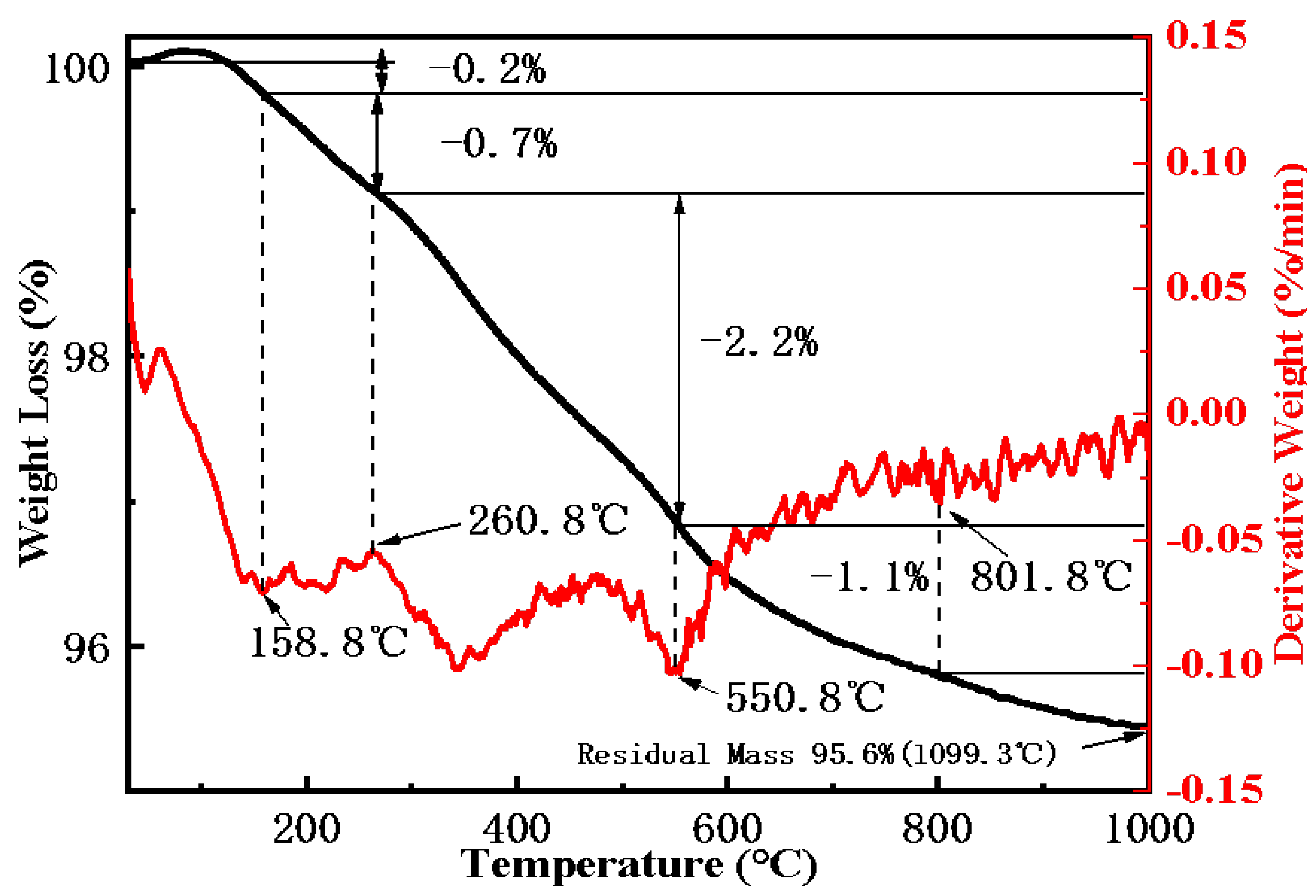
2.1.4. Thermogravimetric Analysis (TGA)
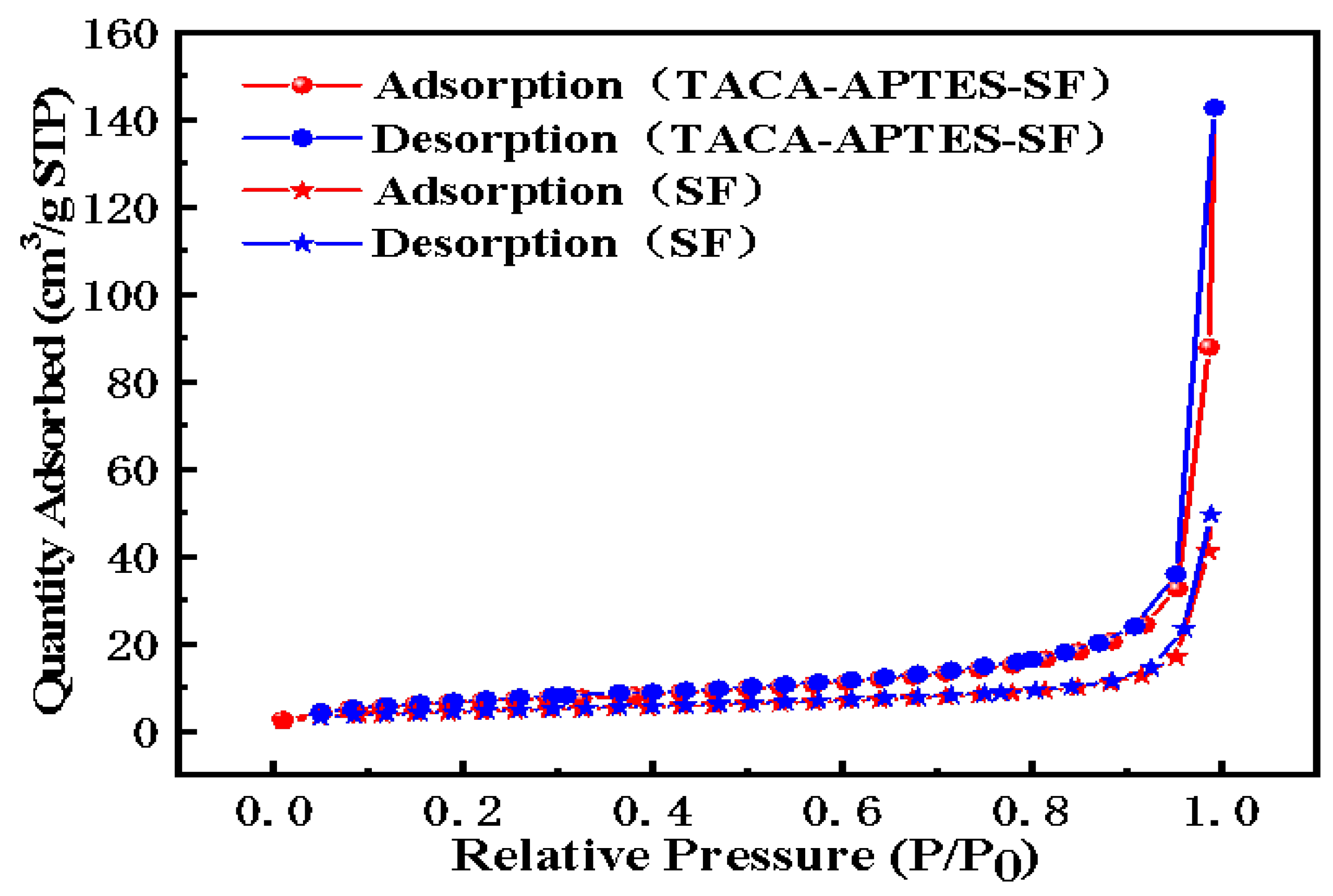
2.1.5. The N2 Adsorption–Desorption Isotherms
2.2. Adsorption Properties
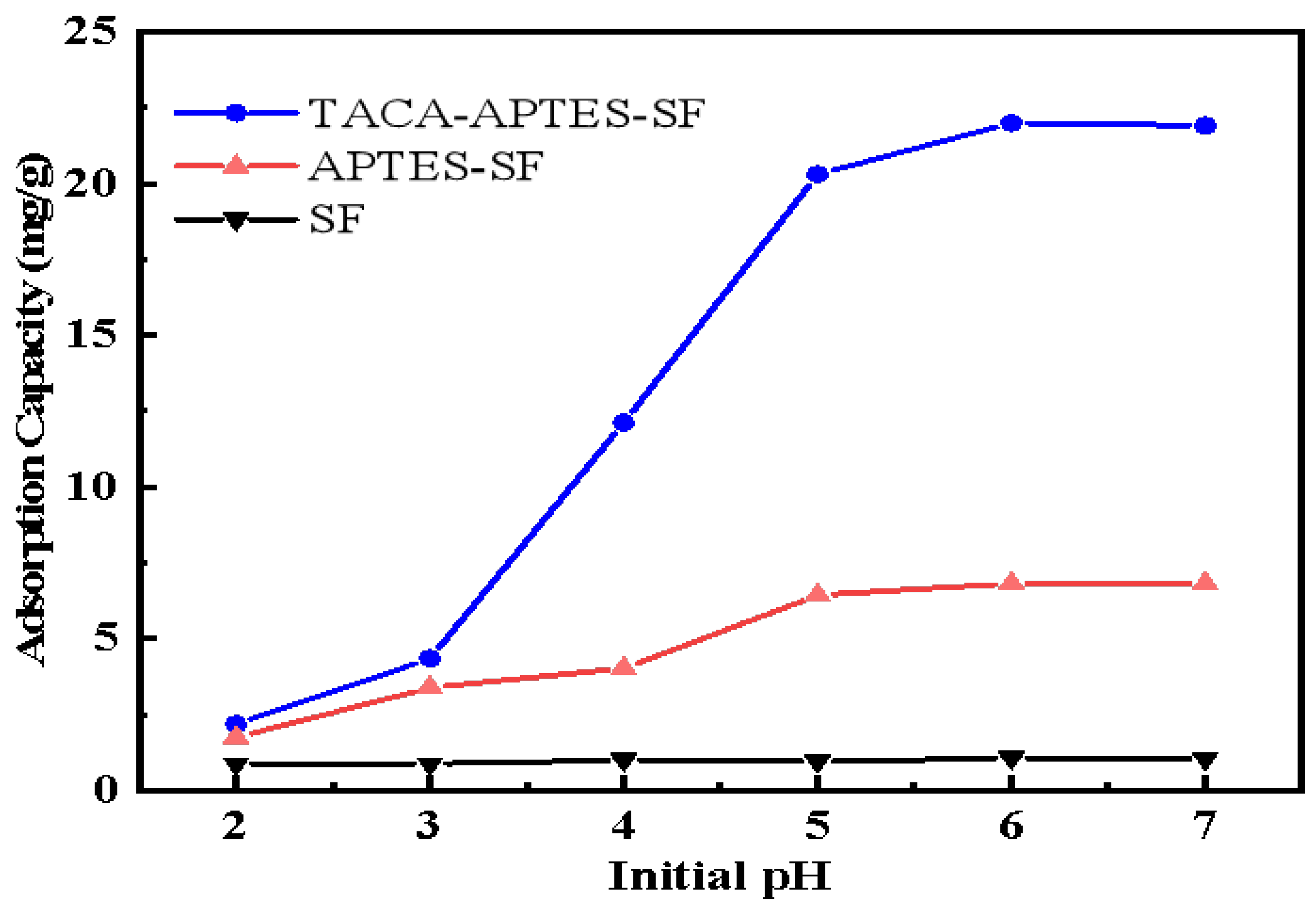
2.2.1. Effect of pH
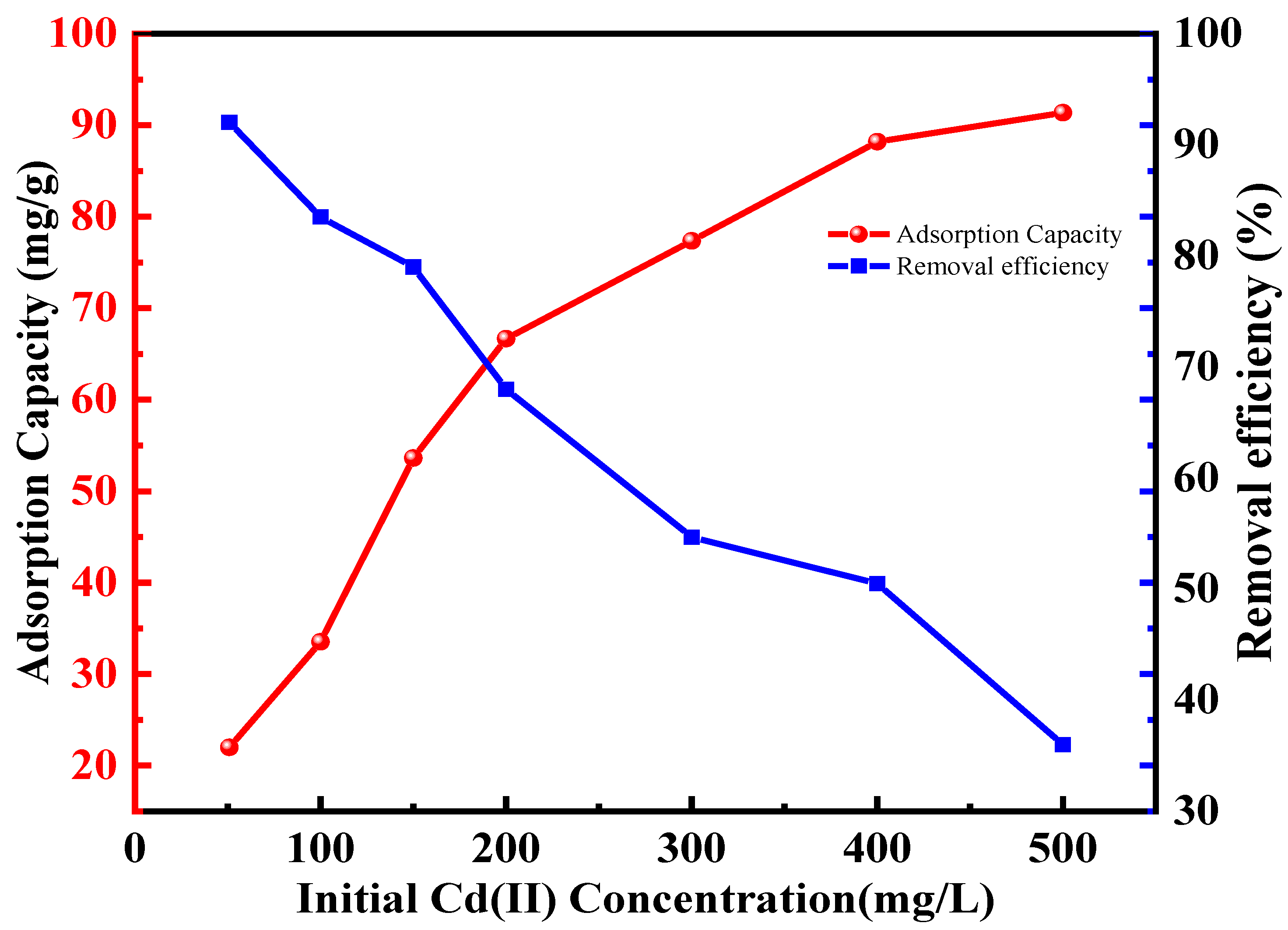
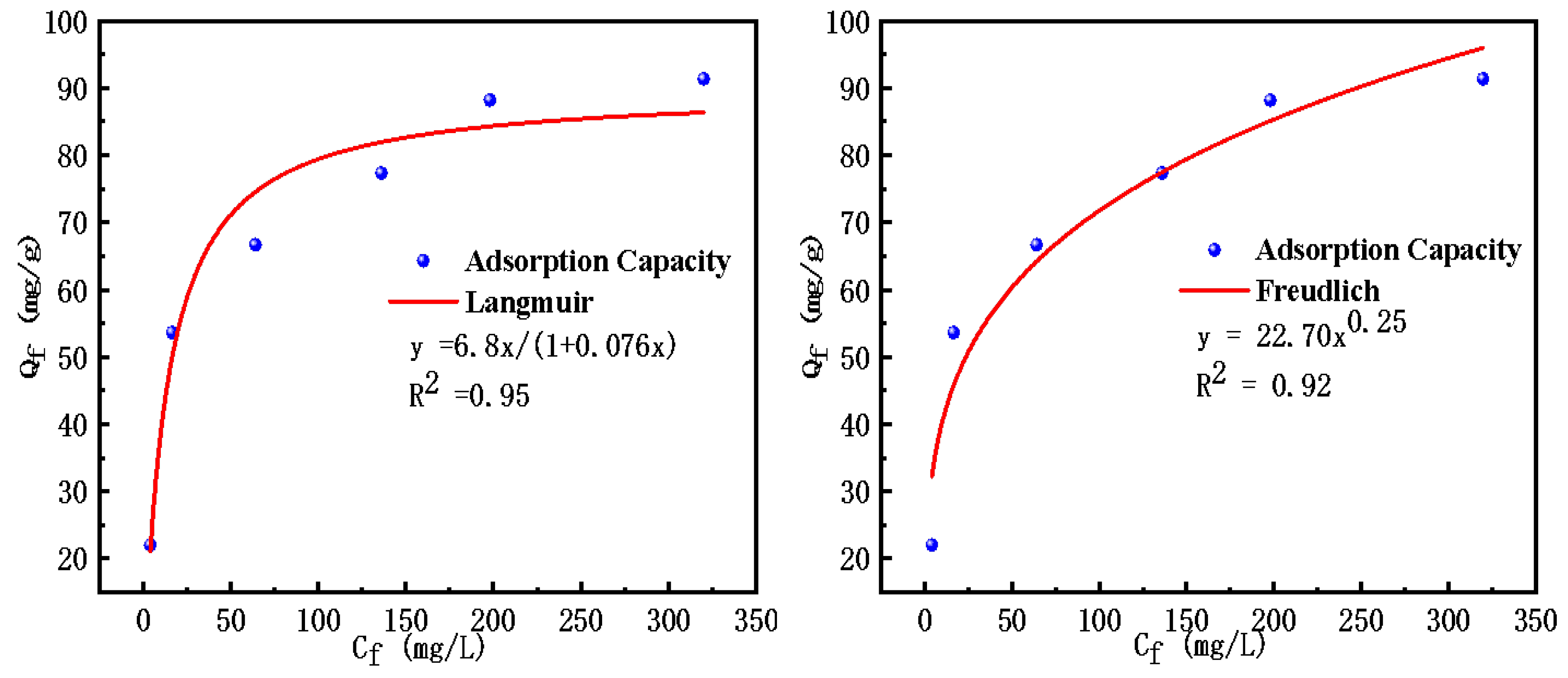
2.2.2. Adsorption Isotherms
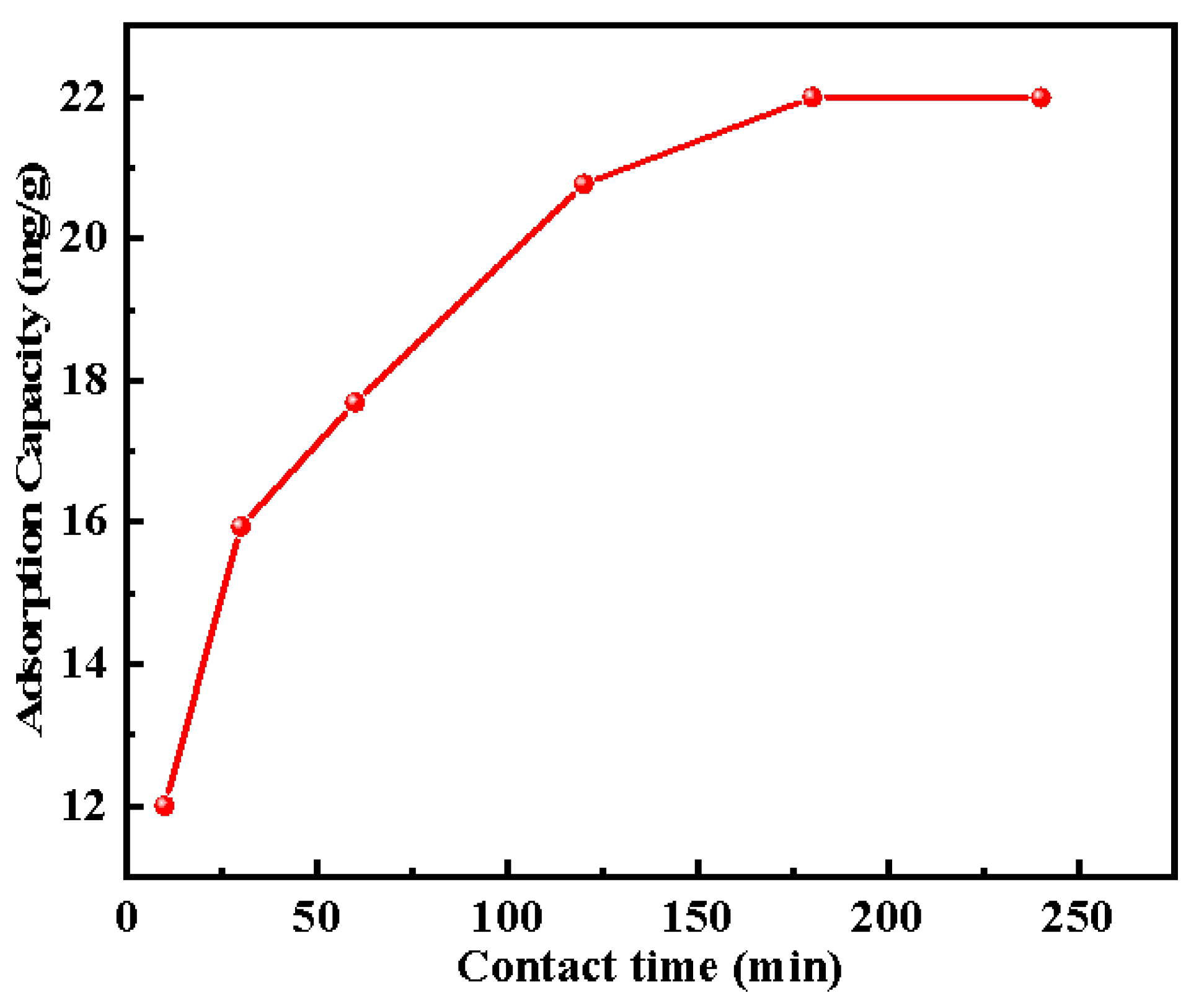
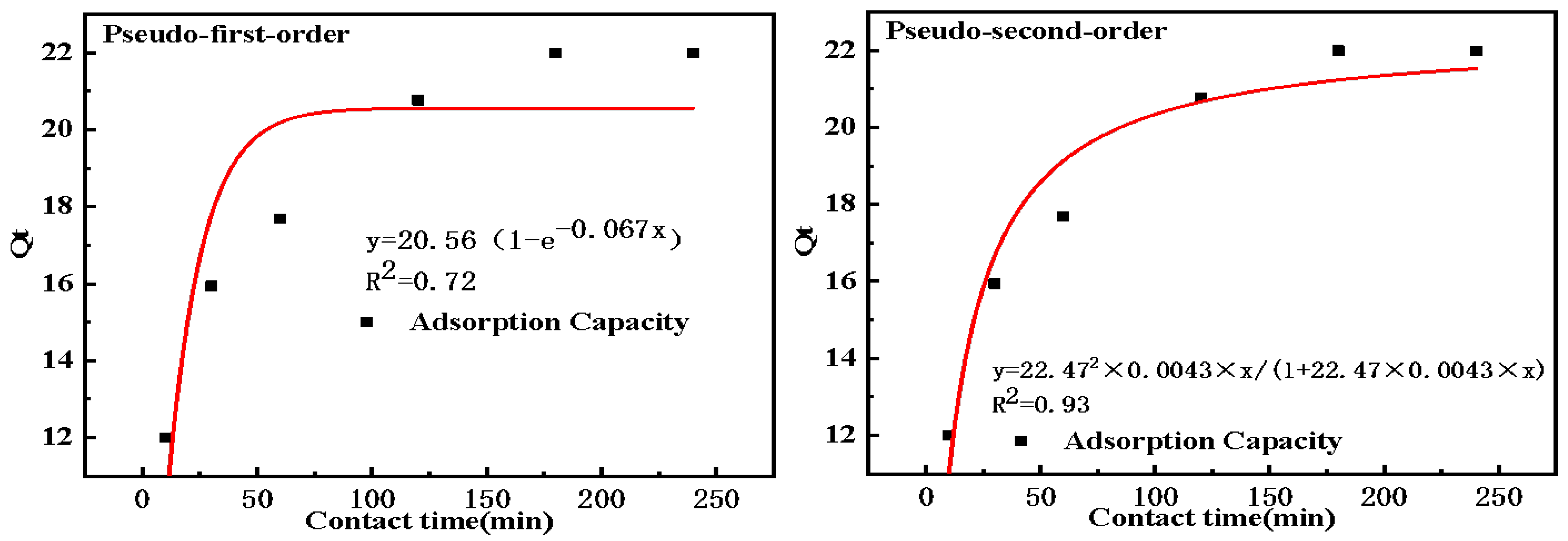
2.2.3. Adsorption Kinetics
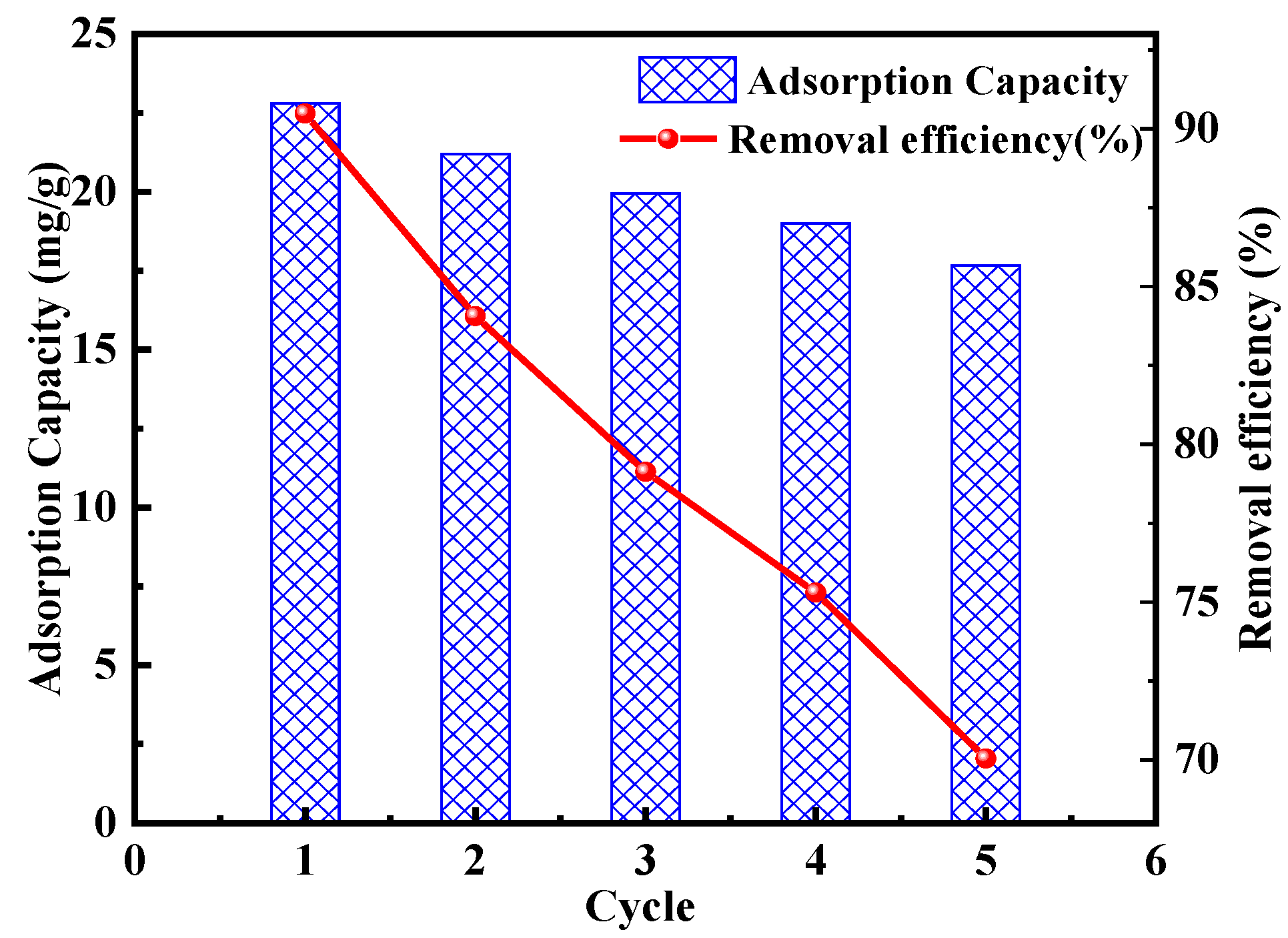
2.2.4. Reusability
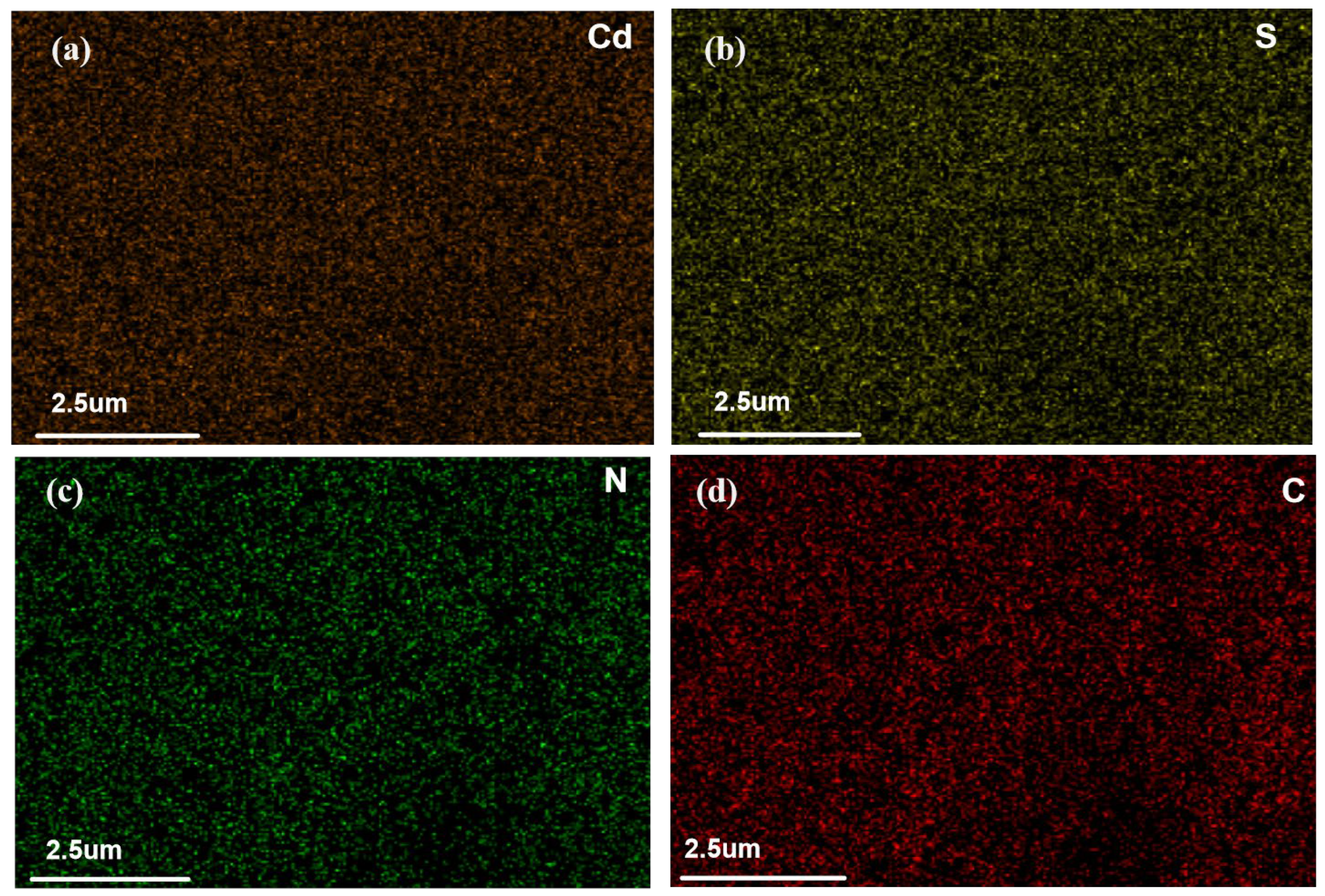
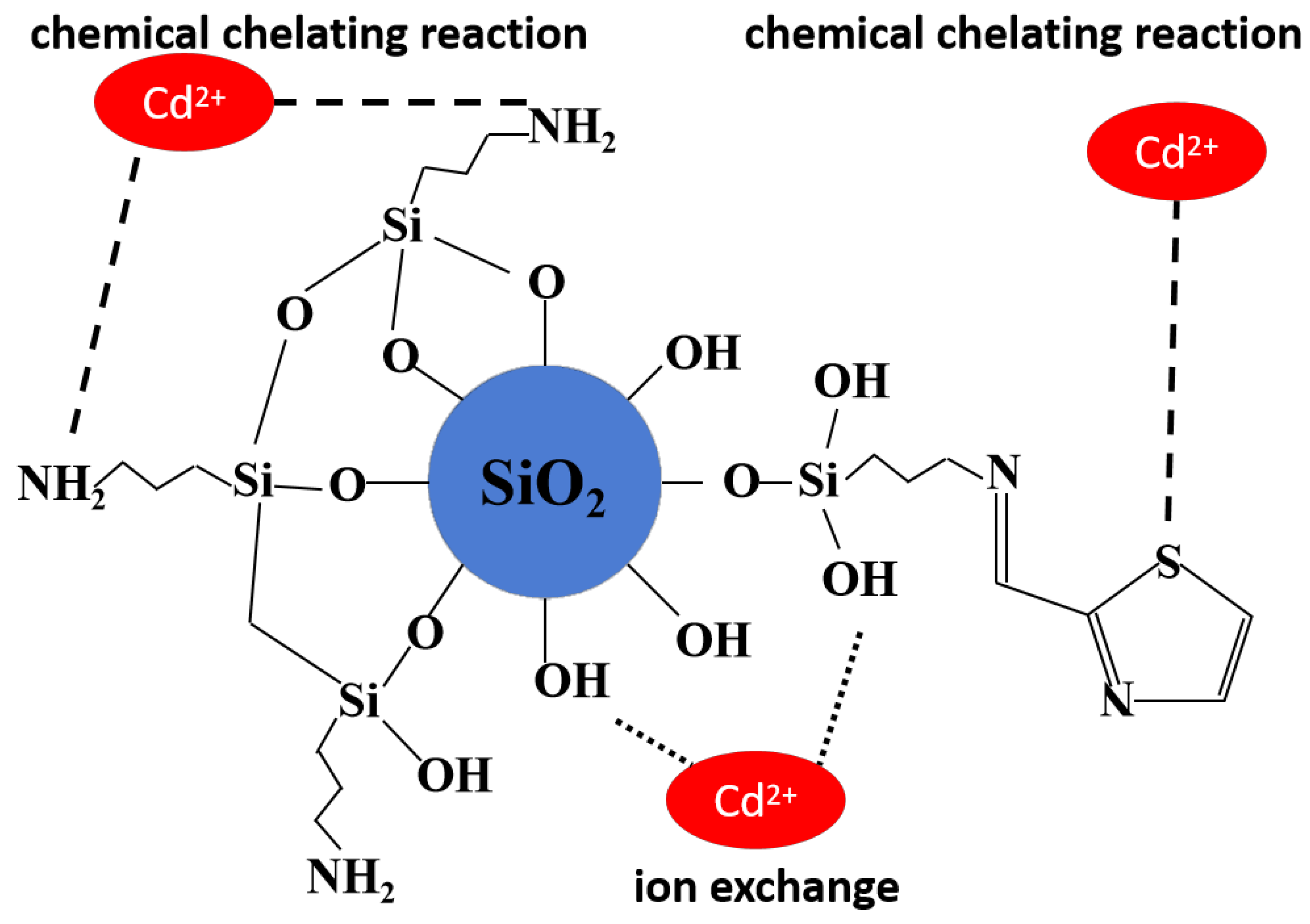
2.3. Adsorption Mechanism
3. Materials and Methods
3.1. Materials and Reagents
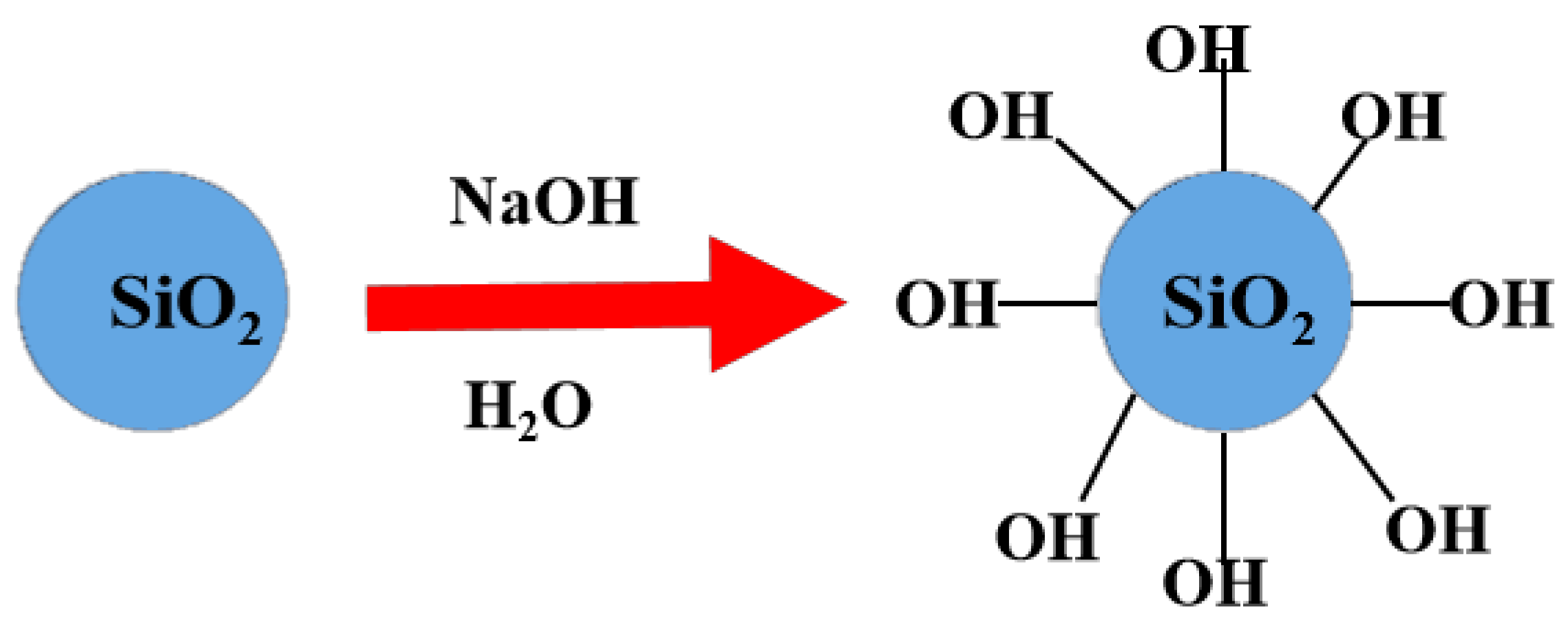
3.2. Pretreatment of SF
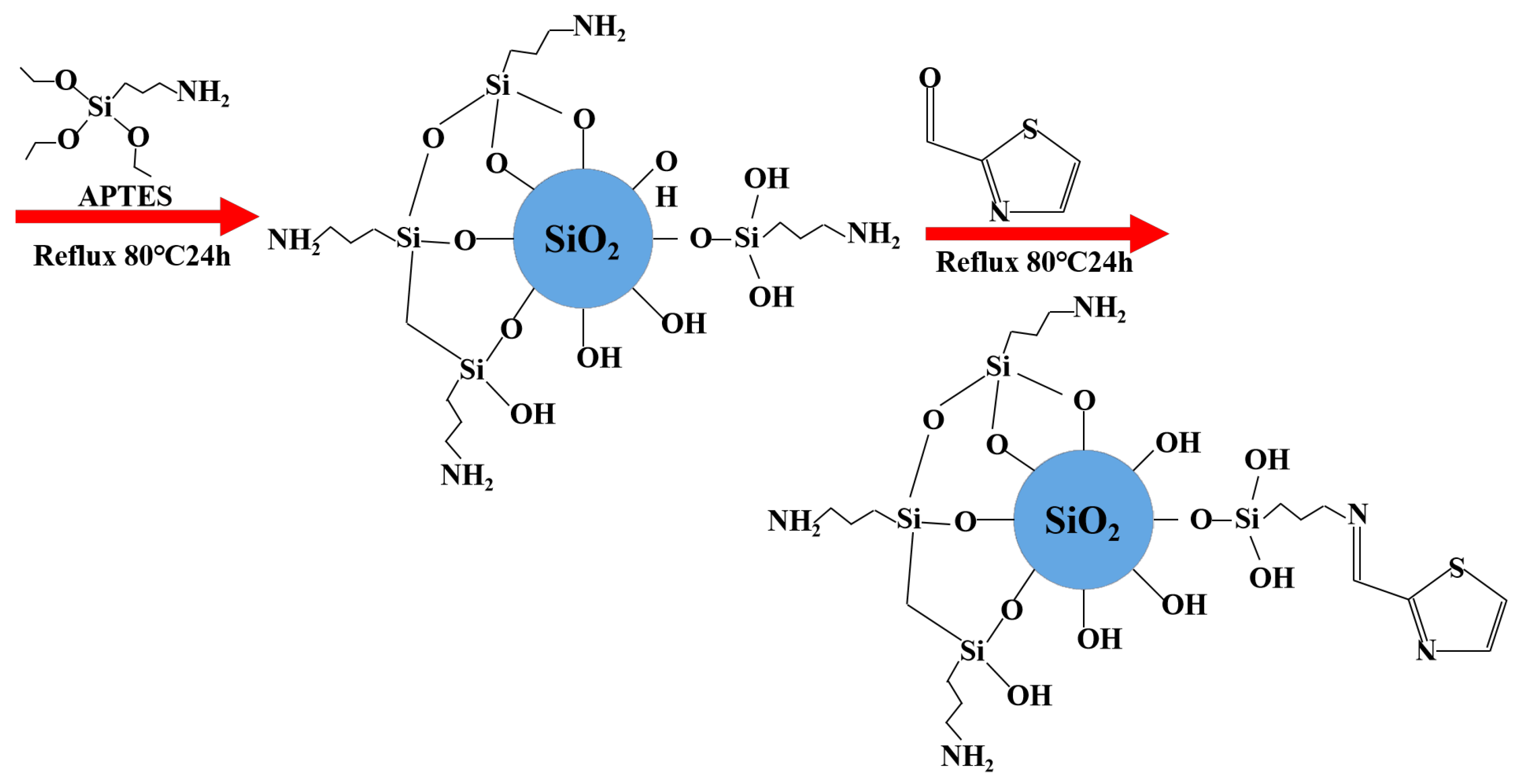
3.3. Synthesis of TACA-APTES-SF
3.4. Characterization
3.5. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Chen, T.; Wang, B.; Zhou, S.; Zhang, Z.; Li, Y.; Pan, X.; Wang, N. Enhanced removal of Cd2+ from water by AHP-pretreated biochar: Adsorption performance and mechanism. J. Hazard. Mater. 2022, 438, 129467. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Li, S.; Ma, W.; Li, Y.; He, Z.; Hu, H.; Wang, T. Effective removal of Cd(II) from aqueous solution based on multifunctional nanoporous silicon derived from solar kerf loss waste. J. Hazard. Mater. 2020, 385, 121522. [Google Scholar] [CrossRef]
- Jin, Z.; Xue, Z.; Li, B.; Ou, L.; Yan, L.; Yang, L.; Yin, K.; Jouha, J.; Shao, P.; Zeng, Z.; et al. High-performance spent coffee grounds-based 3D microporous biochar for the efficient capture of Cd2+ via a multi-pathway mechanism. Chem. Eng. J. 2024, 485, 149537. [Google Scholar] [CrossRef]
- Qin, X.; Cheng, S.; Xing, B.; Xiong, C.; Yi, G.; Shi, C.; Xia, H.; Zhang, C. Preparation of high-efficient MgCl2 modified biochar toward Cd(II) and tetracycline removal from wastewater. Sep. Purif. Technol. 2023, 325, 124625. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Sun, C.; Nie, Q.; Sun, S. Adsorption mechanism of shell powders on heavy metal ions Pb2+/Cd2+ and the purification efficiency for contaminated soils. Front. Earth Sci. 2023, 10, 1071228. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, J.; Yuan, Y.; Song, H.; Liu, Y.; Wang, S.; Tao, Y.; Zhao, Y.; Li, Z. Simultaneous scavenging of Cd(II) and Pb(II) from water by sulfide-modified magnetic pinecone-derived hydrochar. J. Clean. Prod. 2022, 341, 130758. [Google Scholar] [CrossRef]
- Liu, K.; Li, F.; Cui, J.; Yang, S.; Fang, L. Simultaneous removal of Cd(II) and As(III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: Synergistic effects and mechanisms. J. Hazard. Mater. 2020, 395, 122623. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shan, J.; Chen, Z.; Lichtfouse, E. Efficient recovery of phosphate from simulated urine by Mg/Fe bimetallic oxide modified biochar as a potential resource. Sci. Total Environ. 2021, 784, 147546. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, C.; Wang, Y.; Ding, M.; Xu, H.; Yan, X.; Gao, L. Water recovery from the high salinity brine: Effect of the interlayer structure in the polyamide nanofiltration membrane. J. Environ. Chem. Eng. 2024, 12, 111963. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Li, L.; Xu, F.; Jiang, H.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, R.; Chen, G.; Xing, B. Facile synthesis of multifunctional bone biochar composites decorated with Fe/Mn oxide micro-nanoparticles: Physicochemical properties, heavy metals sorption behavior and mechanism. J. Hazard. Mater. 2020, 399, 123067. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Xin, J.; Liu, G.; Wu, G.; Kong, Z.; Zhang, J. Clickable synthesis of 1,2,4-triazole modified lignin-based adsorbent for the selective removal of Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 4086–4093. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, S.; Guo, H.; Xing, B.; Liu, Y.; Zhang, C.; Ma, M. High-efficiency removal of lead/cadmium from wastewater by MgO modified biochar derived from crofton weed. Bioresour. Technol. 2022, 343, 126081. [Google Scholar] [CrossRef]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.-Q.; Zhang, Q.-P.; Liu, Q.-C.; Li, Y.-D.; Xiao, R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Ai, W.; Wei, C. A new method to prepare mesoporous silica from coal gasification fine slag and its application in methylene blue adsorption. J. Clean. Prod. 2019, 212, 1062–1071. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, D.; Li, X.; Yu, J.; Zhou, Y.; Luo, Y.; Ma, W. Synthesis of mesoporous silica materials (MCM-41) using silica fume as the silica source in a binary surfactant system assisted by post-hydrothermal treatment and its Pb2+ removal properties. Can. J. Chem. Eng. 2016, 95, 46–54. [Google Scholar] [CrossRef][Green Version]
- Zhu, W.; Li, X.T.; Wu, D.; Yu, J.; Zhou, Y.; Luo, Y.; Wei, K.; Ma, W. Synthesis of spherical mesoporous silica materials by pseudomorphic transformation of silica fume and its Pb2+ removal properties. Microporous Mesoporous Mater. 2016, 222, 192–201. [Google Scholar] [CrossRef]
- Wang, L.; Jin, M.M.; Guo, F.X.; Wang, Y.; Tang, S.W. Pore structural and fractal analysis of the influence of fly ash and silica fume on the mechanical property and abrasion resistance of concrete. Fractals-Complex Geom. Patterns Scaling Nat. Soc. 2021, 29, 2140003. [Google Scholar] [CrossRef]
- Barati, M.; Sarder, S.; McLean, A.; Roy, R. Recovery of silicon from silica fume. J. Non-Cryst. Solids 2011, 357, 18–23. [Google Scholar] [CrossRef]
- Zhu, W.J.; Zhou, Y.; Ma, W.H.; Li, M.M.; Yu, J.; Xie, K.Q. Using silica fume as silica source for synthesizing spherical ordered mesoporous silica. Mater. Lett. 2013, 92, 129–131. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.J.; He, P.Y.; Li, C.J. Cost-effective and facile one step synthesis of ZSM-5 from silica fume waste with the aid of metakaolin and its NOx removal performance. Powder Technol. 2020, 367, 558–567. [Google Scholar] [CrossRef]
- Cheng, S.; Meng, W.; Xing, B.; Shi, C.; Wang, Q.; Xia, D.; Nie, Y.; Yi, G.; Zhang, C.; Xia, H. Efficient removal of heavy metals from aqueous solutions by Mg/Fe bimetallic oxide-modified biochar: Experiments and DFT investigations. J. Clean. Prod. 2023, 403, 136821. [Google Scholar] [CrossRef]
- He, C.; Yang, Z.Q.; Ding, J.; Chen, Y.C.; Tong, X.W.; Li, Y. Effective removal of Cr(VI) from aqueous solution by 3-aminopropyltriethoxysilane-functionalized graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 448–458. [Google Scholar] [CrossRef]
- Fan, H.T.; Sun, T.; Xu, H.B.; Yang, Y.J.; Tang, Q.; Sun, Y. Removal of arsenic(V) from aqueous solutions using 3-[2-(2-aminoethylamino)ethylamino]propyl-trimethoxysilane functionalized silica gel adsorbent. Desalination 2011, 278, 238–243. [Google Scholar] [CrossRef]
- Kumar Yadav, V.; Suriyaprabha, R.; Heena Khan, S.; Singh, B.; Gnanamoorthy, G.; Choudhary, N.; Kumar Yadav, A.; Kalasariya, H. A novel and efficient method for the synthesis of amorphous nanosilica from fly ash tiles. Mater. Today Proc. 2020, 26, 701–705. [Google Scholar] [CrossRef]
- Yin, M.; Bai, X.; Wu, D.; Li, F.; Jiang, K.; Ma, N.; Chen, Z.; Zhang, X.; Fang, L. Sulfur-functional group tunning on biochar through sodium thiosulfate modified molten salt process for efficient heavy metal adsorption. Chem. Eng. J. 2022, 433, 134441. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Khalfaoui, M.; Knani, S.; Hachicha, M.A.; Lamine, A.B. New theoretical expressions for the five adsorption type isotherms classified by BET based on statistical physics treatment. J. Colloid Interface Sci. 2003, 263, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Wang, S.X.; Sun, W.T.; Li, Y. Selective adsorption of Pb(II) from aqueous solution using nanosilica functionalized with diethanolamine: Equilibrium, kinetic and thermodynamic. Microchem. J. 2019, 146, 270–278. [Google Scholar] [CrossRef]
- Kaewprachum, W.; Wongsakulphasatch, S.; Kiatkittipong, W.; Striolo, A.; Cheng, C.K.; Assabumrungrat, S. SDS modified mesoporous silica MCM-41 for the adsorption of Cu2+, Cd2+, Zn2+ from aqueous systems. J. Environ. Chem. Eng. 2020, 8, 102920. [Google Scholar] [CrossRef]
- Fan, H.-T.; Li, J.; Li, Z.-C.; Sun, T. An ion-imprinted amino-functionalized silica gel sorbent prepared by hydrothermal assisted surface imprinting technique for selective removal of cadmium (II) from aqueous solution. Appl. Surf. Sci. 2012, 258, 3815–3822. [Google Scholar] [CrossRef]
- Gourmand, C.; Bertagnolli, C.; Brandel, J.; Hubscher-Bruder, V.; Boos, A. Bioinspired Mesoporous Silica for Cd(II) Removal from Aqueous Solutions. Ind. Eng. Chem. Res. 2022, 61, 8188–8203. [Google Scholar] [CrossRef]
- Ianasi, C.; Piciorus, M.; Nicola, R.; Ciopec, M.; Negrea, A.; Niznansky, D.; Len, A.; Almásy, L.; Putz, A.M. Removal of cadmium from aqueous solutions using inorganic porous nanocomposites. Korean J. Chem. Eng. 2019, 36, 688–700. [Google Scholar] [CrossRef]
- Waseem, M.; Mustafa, S.; Naeem, A.; Shah, K.H.; Shah, I. Mechanism of Cd (II) sorption on silica synthesized by sol-gel method. Chem. Eng. J. 2011, 169, 78–83. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Othman Charles, S.; Al Hamouz, O.S.A. Removal of lead and arsenic ions by a new series of aniline based polyamines. Process Saf. Environ. Prot. 2017, 106, 180–190. [Google Scholar] [CrossRef]
- Kim, S.A.; Kamala-Kannan, S.; Lee, K.-J.; Park, Y.-J.; Shea, P.J.; Lee, W.-H.; Kim, H.-M.; Oh, B.-T. Removal of Pb(II) from aqueous solution by a zeolite-nanoscale zero-valent iron composite. Chem. Eng. J. 2013, 217, 54–60. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, S.X.; Zhang, L.B.; Li, Y.; Zhou, Y.; Peng, J.H. Preparation of 2-Aminothiazole-Functionalized Poly (glycidyl methacrylate) Microspheres and Their Excellent Gold Ion Adsorption Properties. Polymers 2018, 10, 159. [Google Scholar] [CrossRef]
- Guan, X.; Yuan, X.; Zhao, Y.; Bai, J.; Li, Y.; Cao, Y.; Chen, Y.; Xiong, T. Adsorption behaviors and mechanisms of Fe/Mg layered double hydroxide loaded on bentonite on Cd (II) and Pb (II) removal. J. Colloid Interface Sci. 2022, 612, 572–583. [Google Scholar] [CrossRef]
- Yamada, Y.; Kim, J.; Matsuo, S.; Sato, S. Nitrogen-containing graphene analyzed by X-ray photoelectron spectroscopy. Carbon 2014, 70, 59–74. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.N.; Wang, J.G.; Tang, Y.; Zhang, Z. Selective adsorption of Pb2+ and Cu2+ on amino-modified attapulgite: Kinetic, thermal dynamic and DFT studies. J. Hazard. Mater. 2021, 404, 124140. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xiong, C.; Zhao, M.H.; Wang, S.X.; Zhou, Y.; Dai, L.Q.; Zhang, L.B. Surface-functionalized pomelo peel-derived biochar with mercapto-1,2,4-triazloe for selective elimination of toxic Pb (II) in aqueous solutions. Adv. Powder Technol. 2021, 32, 1013–1022. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.; Li, S.; Ma, W.; Li, Y.; He, Z.; Hu, H. Effective removal of Cr(VI) from aqueous solution based on APTES modified nanoporous silicon prepared from kerf loss silicon waste. Environ. Sci. Pollut. Res. Int. 2020, 27, 10899–10909. [Google Scholar] [CrossRef]
- Ruiz-Canas, M.C.; Corredor, L.M.; Quintero, H.I.; Manrique, E.; Romero Bohorquez, A.R. Morphological and Structural Properties of Amino-Functionalized Fumed Nanosilica and Its Comparison with Nanoparticles Obtained by Modified Stober Method. Molecules 2020, 25, 2868. [Google Scholar] [CrossRef]
- Lin, O.H.; Md Akil, H.; Ishak, Z.A.M. Characterization and properties of activated nanosilica/polypropylene composites with coupling agents. Polym. Compos. 2009, 30, 1693–1700. [Google Scholar] [CrossRef]
| Adsorbent | Test Parameters | Adsorption Capacity (mg·g−1) | References | |
|---|---|---|---|---|
| Value of pH | Adsorption Equilibrium Duration (min) | |||
| MCM-41 | 7 | 180 | 8.56 | [31] |
| amino-functionalized silica | 6 | 20 | 59.9 | [32] |
| Bioinspired Mesoporous Silica | 6.6 | 120 | 116.896 | [33] |
| magnetic nanosilica | 6 | 120 | 4.11 | [34] |
| silica | 5 | 120 | 4.8332 | [35] |
| Functional SF | 6 | 120 | 91.37 | This study |
| Langmuir isotherm model | R2 | KL | Qm (mg·g−1) |
| 0.95 | 0.076 | 89.94 | |
| Freundlich isotherm model | R2 | KF | n |
| 0.92 | 22.70 | 4.00 |
| C0 (mg·L−1) | 50 | 100 | 150 | 200 | 300 | 400 | 500 |
| K1 | 0.9515 | 0.9091 | 0.8696 | 0.8333 | 0.7692 | 0.7143 | 0.6667 |
| Pseudo-first-order model | R2 | k1 (1·min−1) | Qf (mg·g−1) |
| 0.72 | 0.067 | 20.56 | |
| Pseudo-second-order model | R2 | k2 (g·mg−1·min−1) | Qf (mg·g−1) |
| 0.93 | 0.0043 | 22.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Wei, K. Functionalized Silica Fume for Efficient Cd2+ Removal from Aqueous Solutions. Molecules 2025, 30, 4141. https://doi.org/10.3390/molecules30204141
Zhu J, Wei K. Functionalized Silica Fume for Efficient Cd2+ Removal from Aqueous Solutions. Molecules. 2025; 30(20):4141. https://doi.org/10.3390/molecules30204141
Chicago/Turabian StyleZhu, Jianeng, and Kuixian Wei. 2025. "Functionalized Silica Fume for Efficient Cd2+ Removal from Aqueous Solutions" Molecules 30, no. 20: 4141. https://doi.org/10.3390/molecules30204141
APA StyleZhu, J., & Wei, K. (2025). Functionalized Silica Fume for Efficient Cd2+ Removal from Aqueous Solutions. Molecules, 30(20), 4141. https://doi.org/10.3390/molecules30204141





