Molecular Diversity of Lupane Hybrids in Drug Design and Materials Science
Abstract
1. Introduction
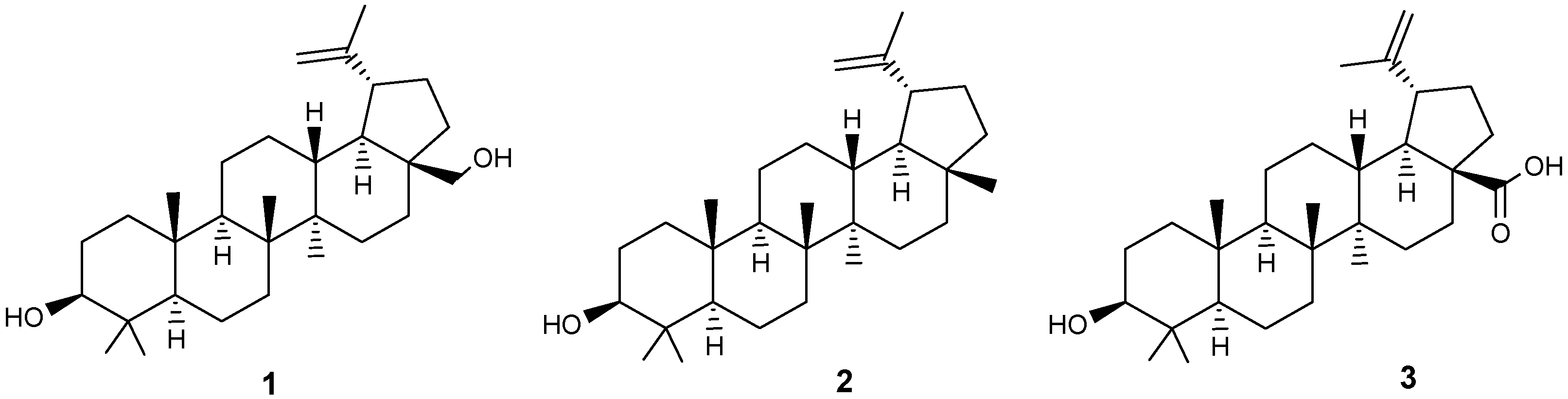
2. Antitumor Properties of Lupanoid Derivatives
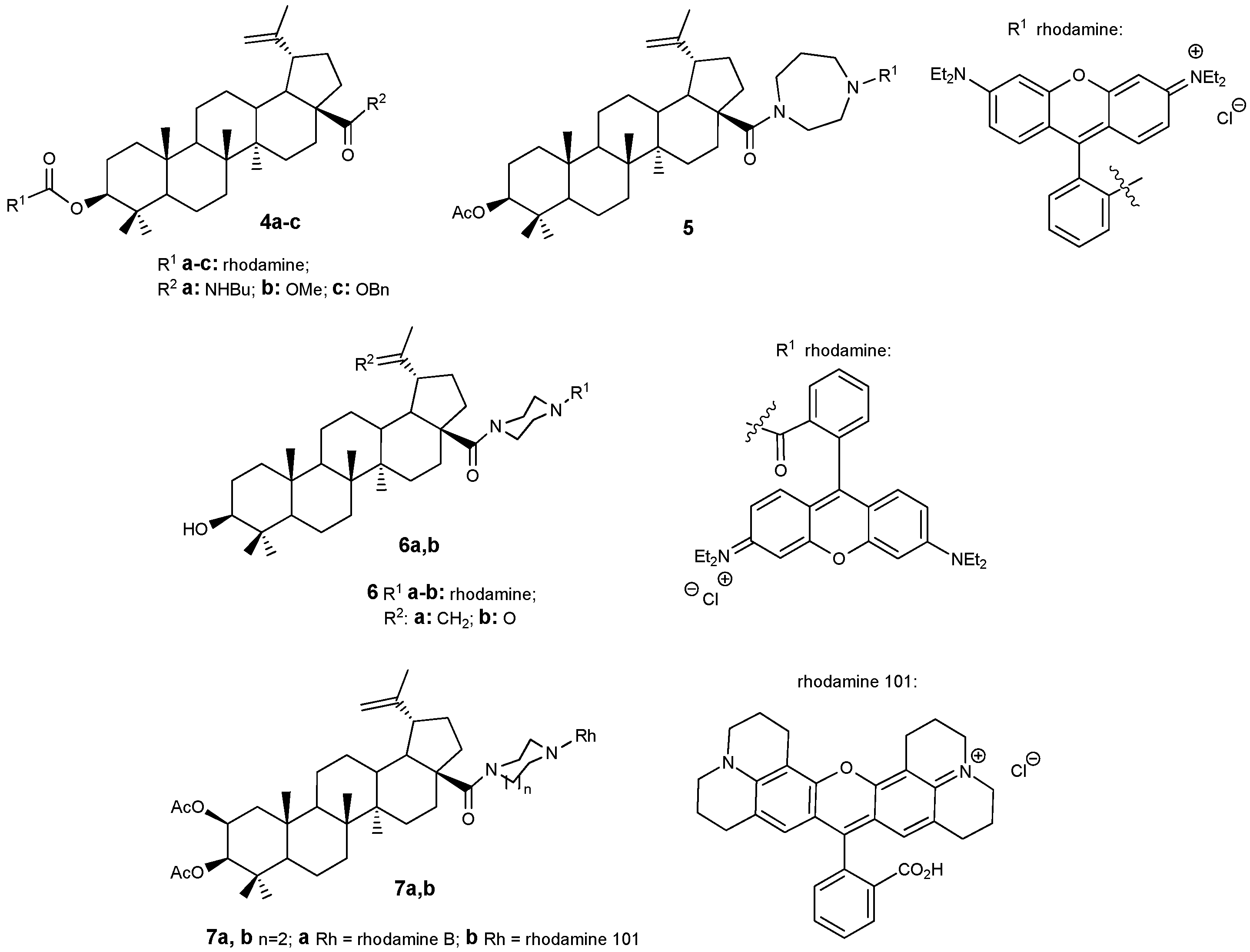
2.1. BA-rhodamine and BA-triphenylphosphonium Conjugates as Mitochondrial Disruptors
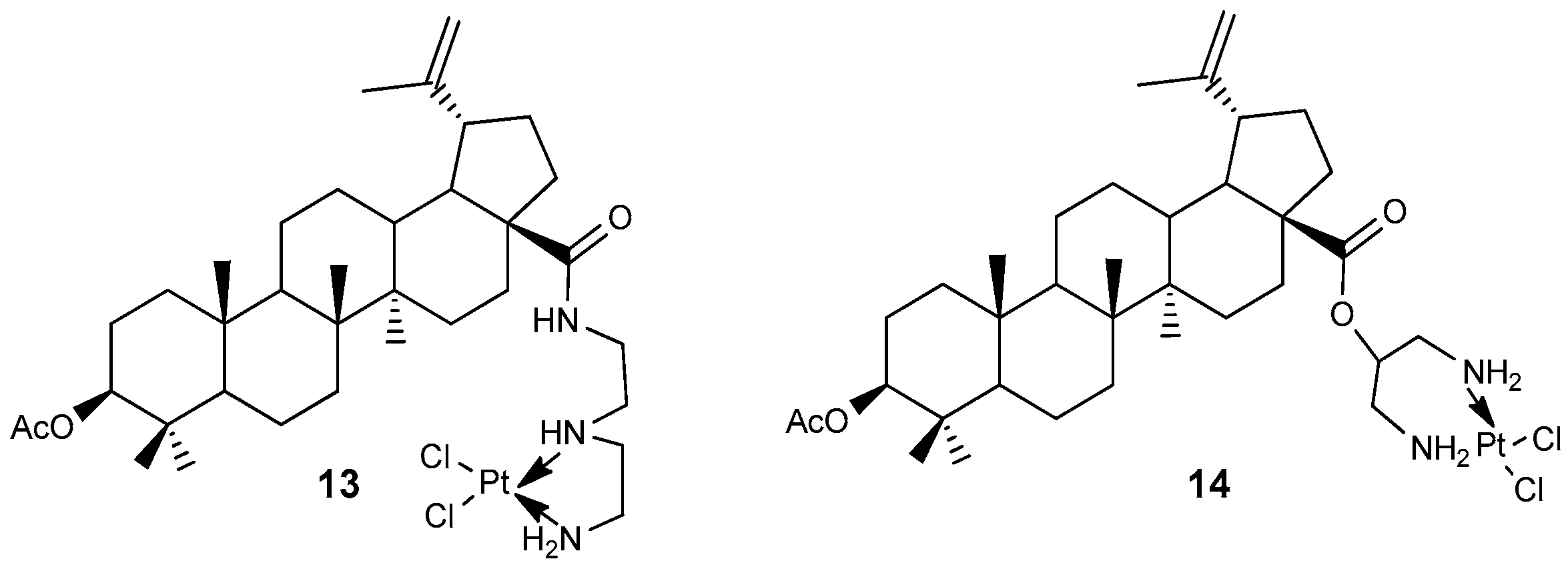
2.2. BA-cisplatin Complexes
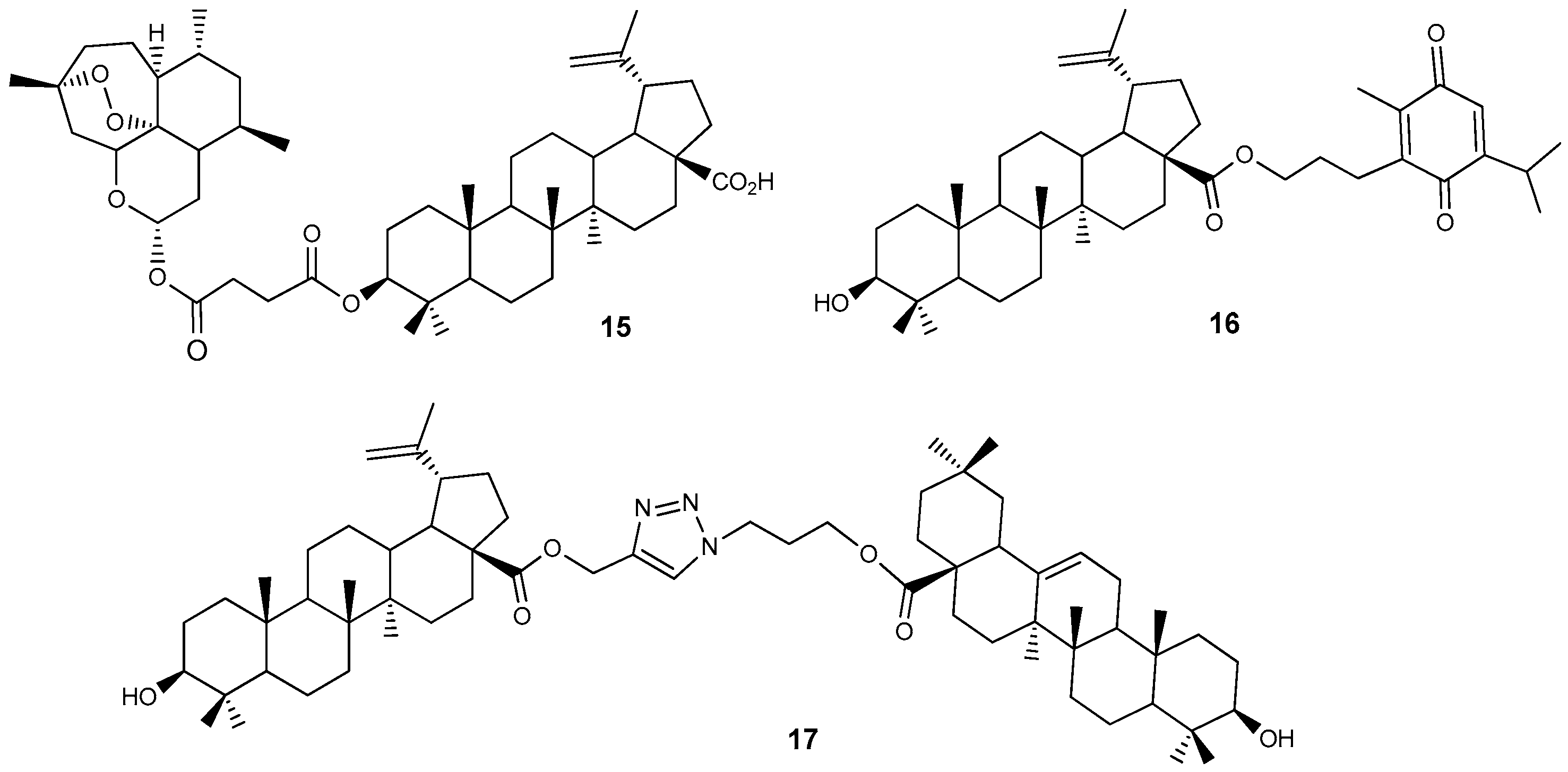
2.3. Lupanoid Conjugates with Natural Products and Their Antitumor Activity
2.4. BA Analogues as Topoisomerase I and IIA Inhibitors
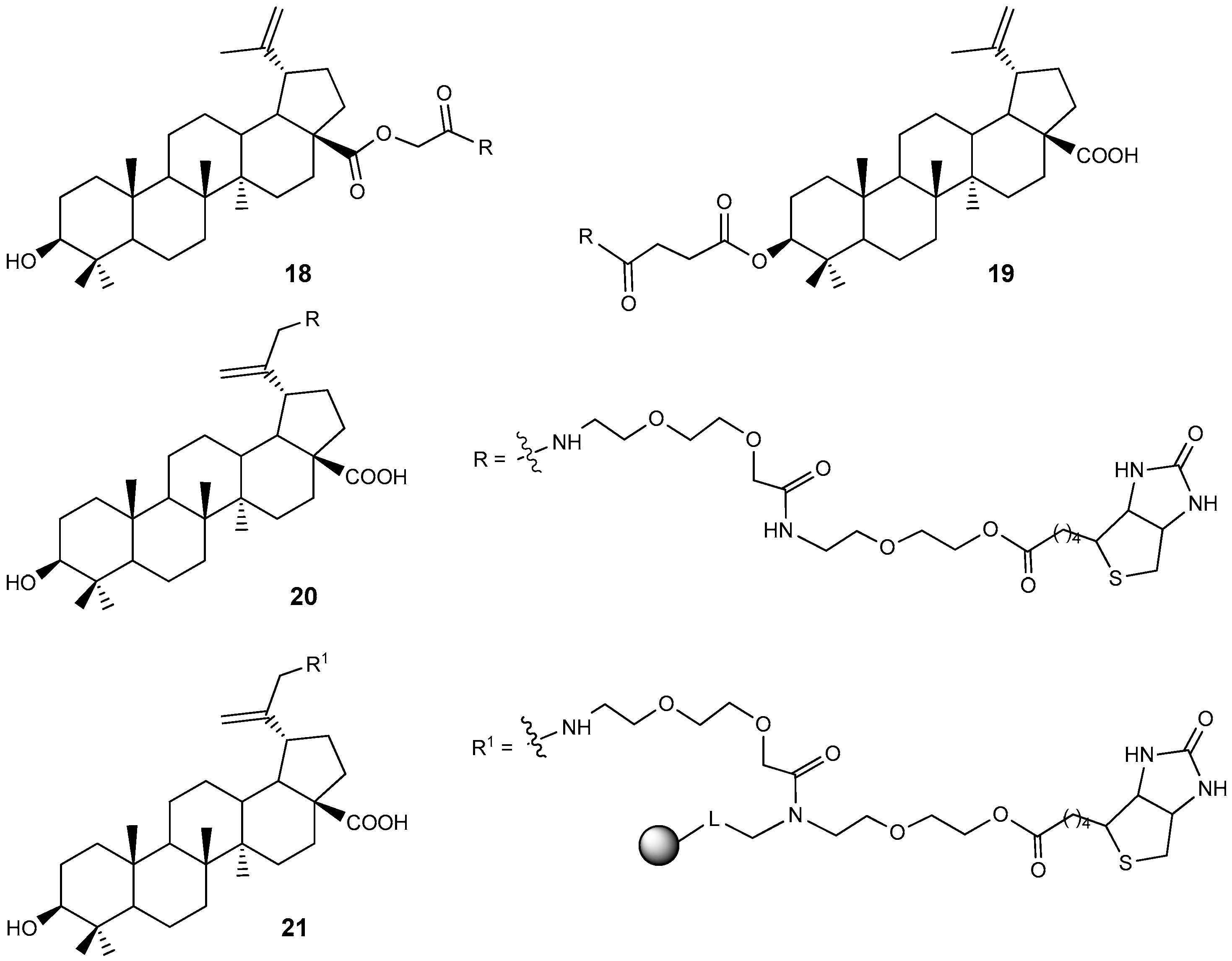
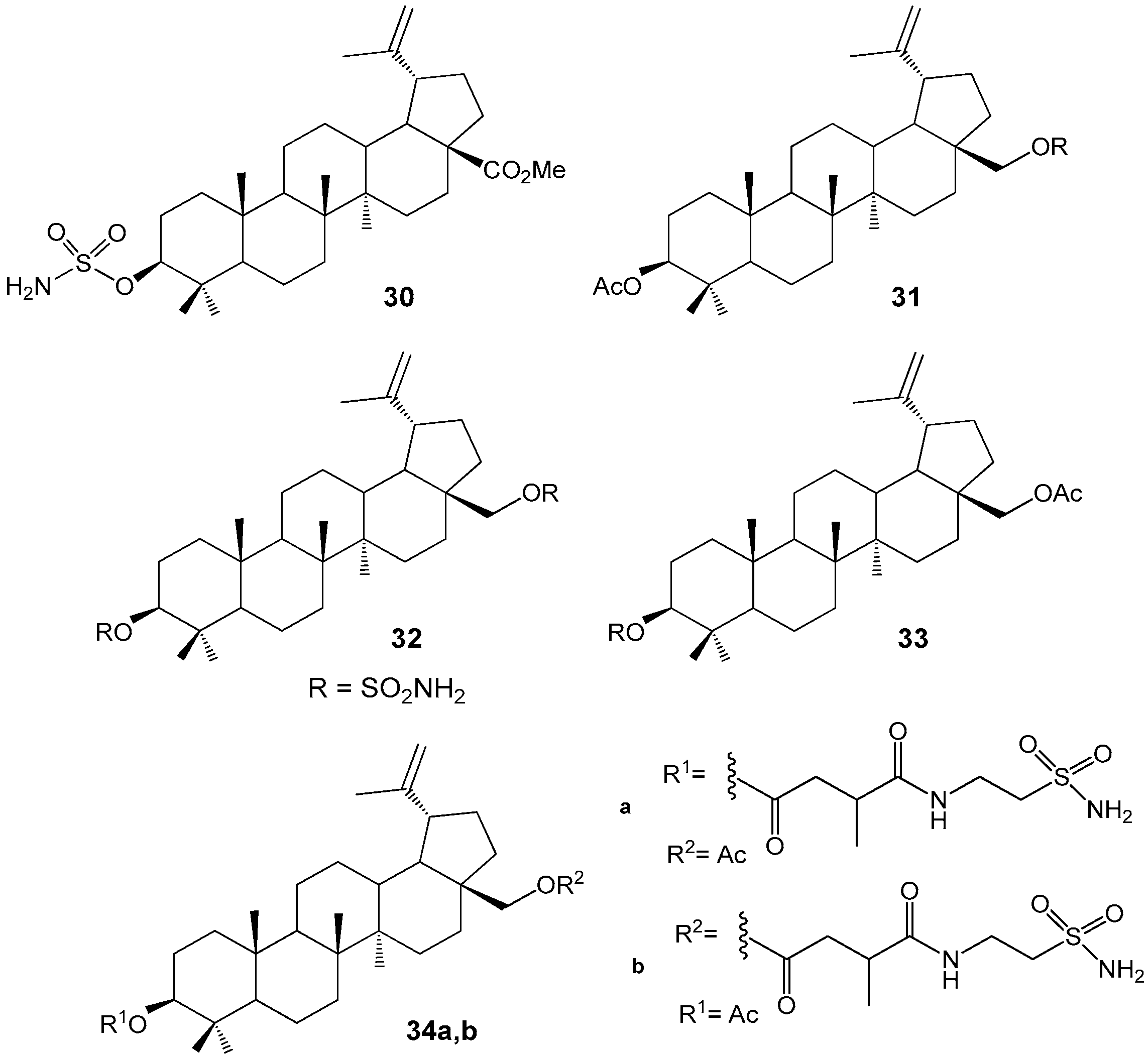
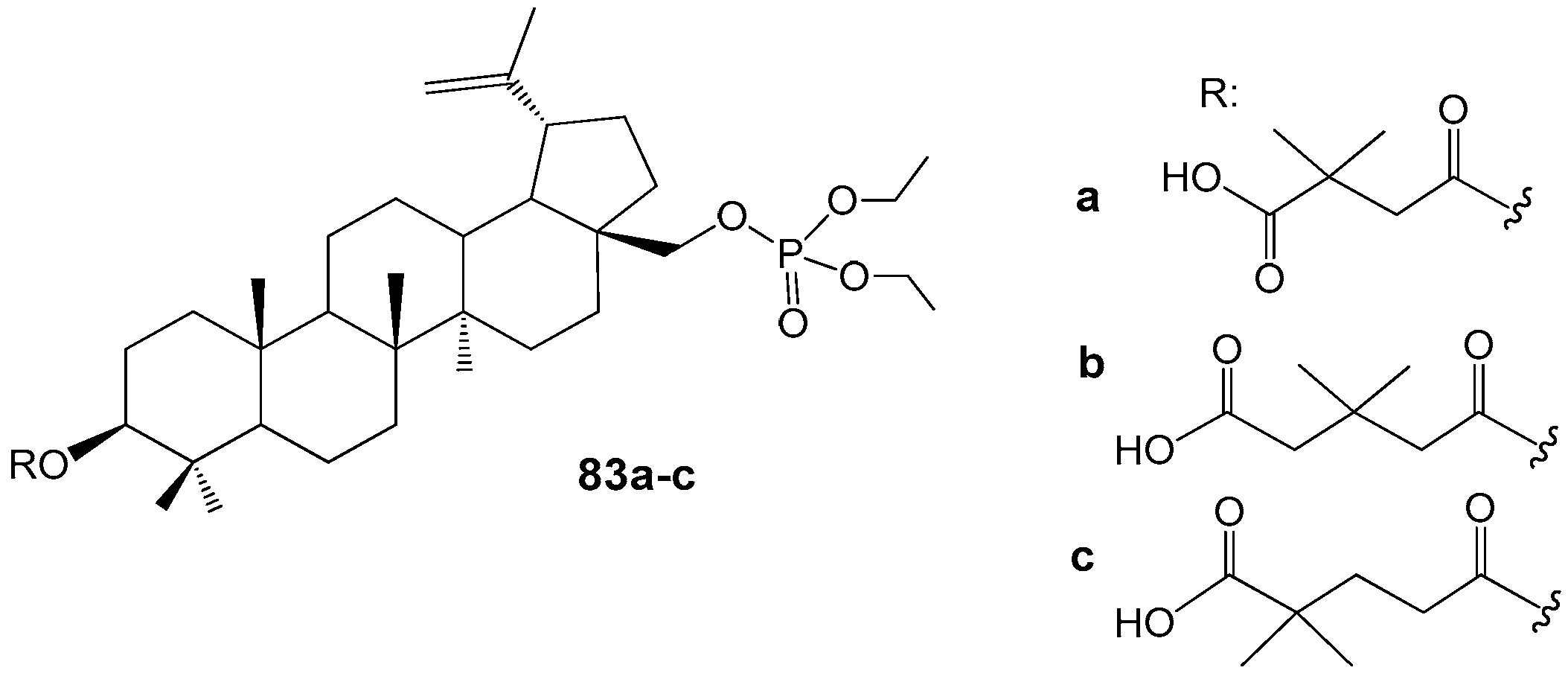
2.5. BA and BE Sulfomate and Sulfonamides with Antitumor and Carbonic Anhydrase Inhibition Activity
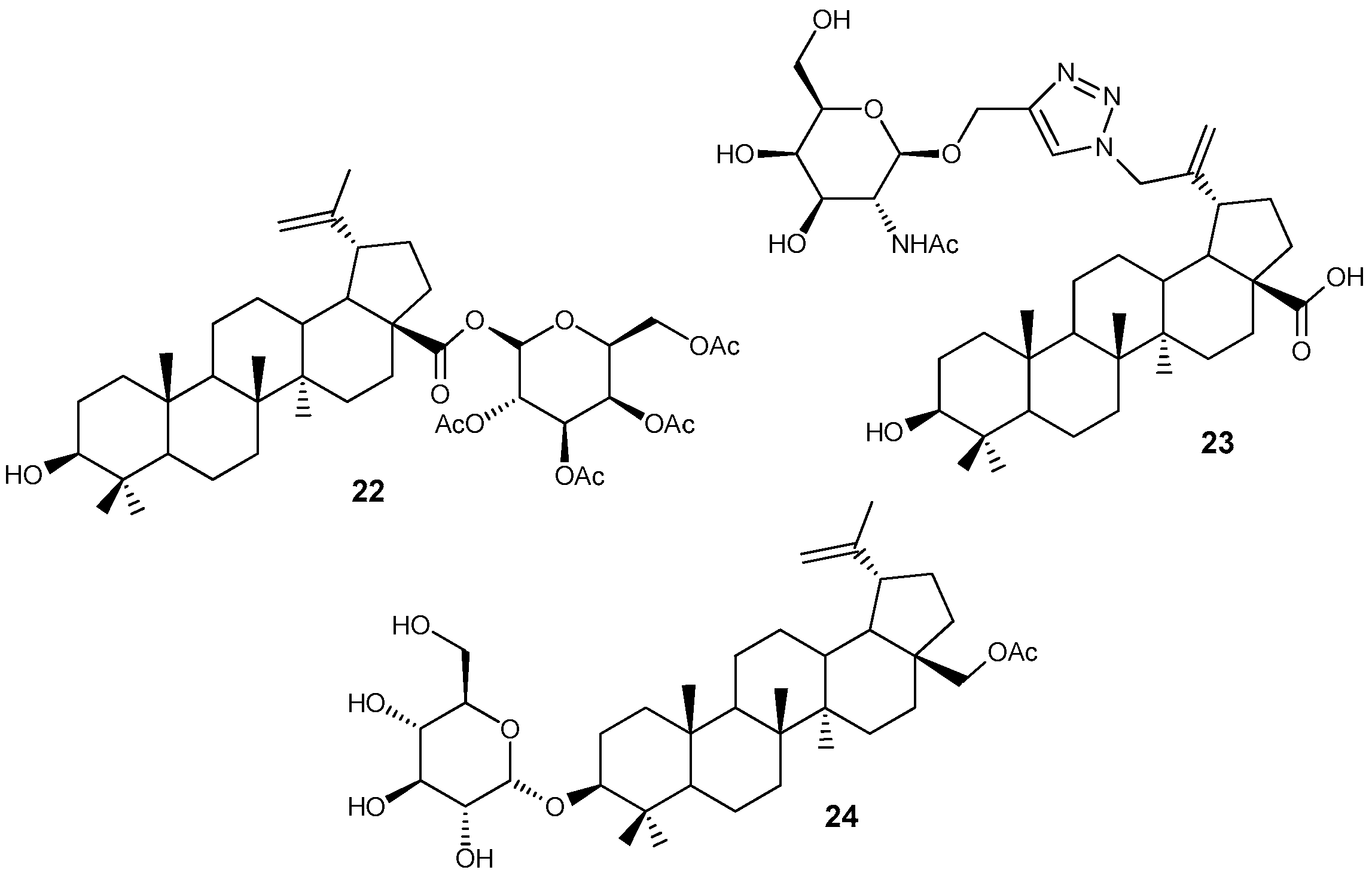
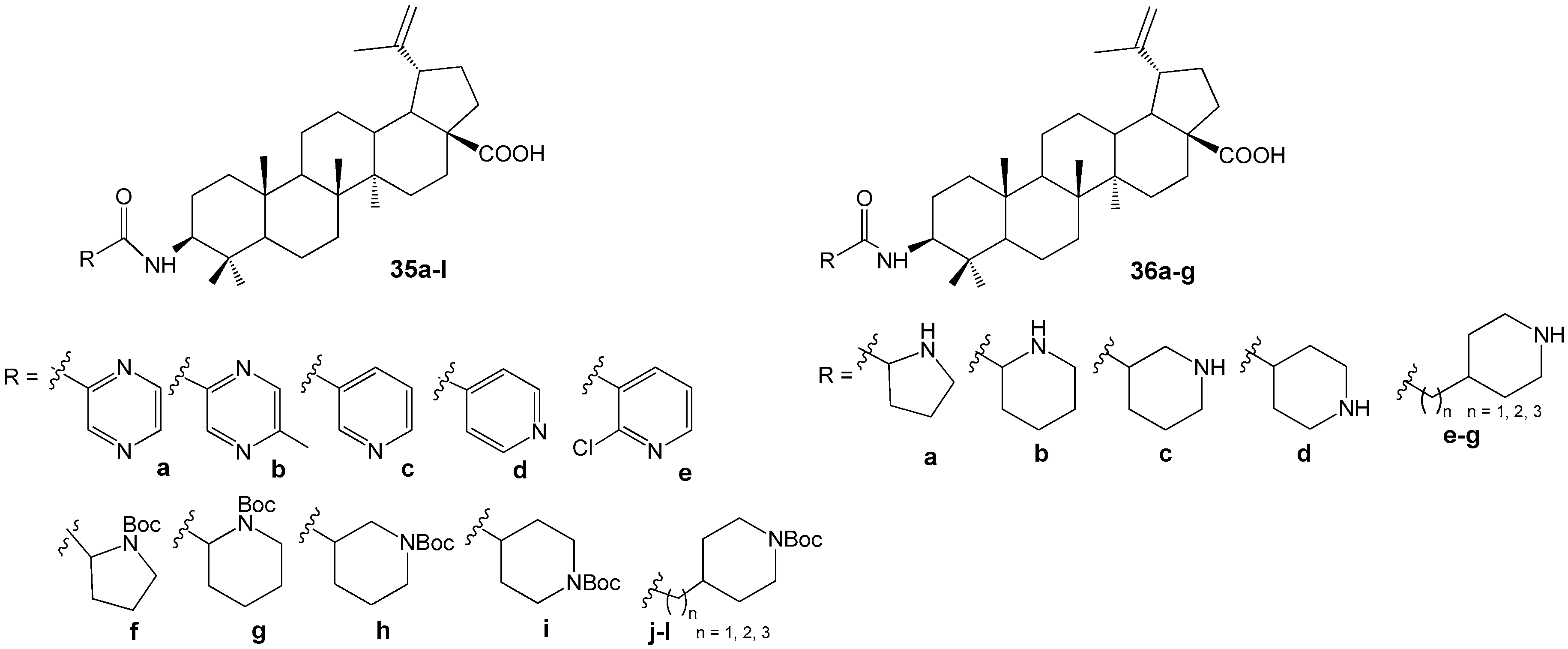
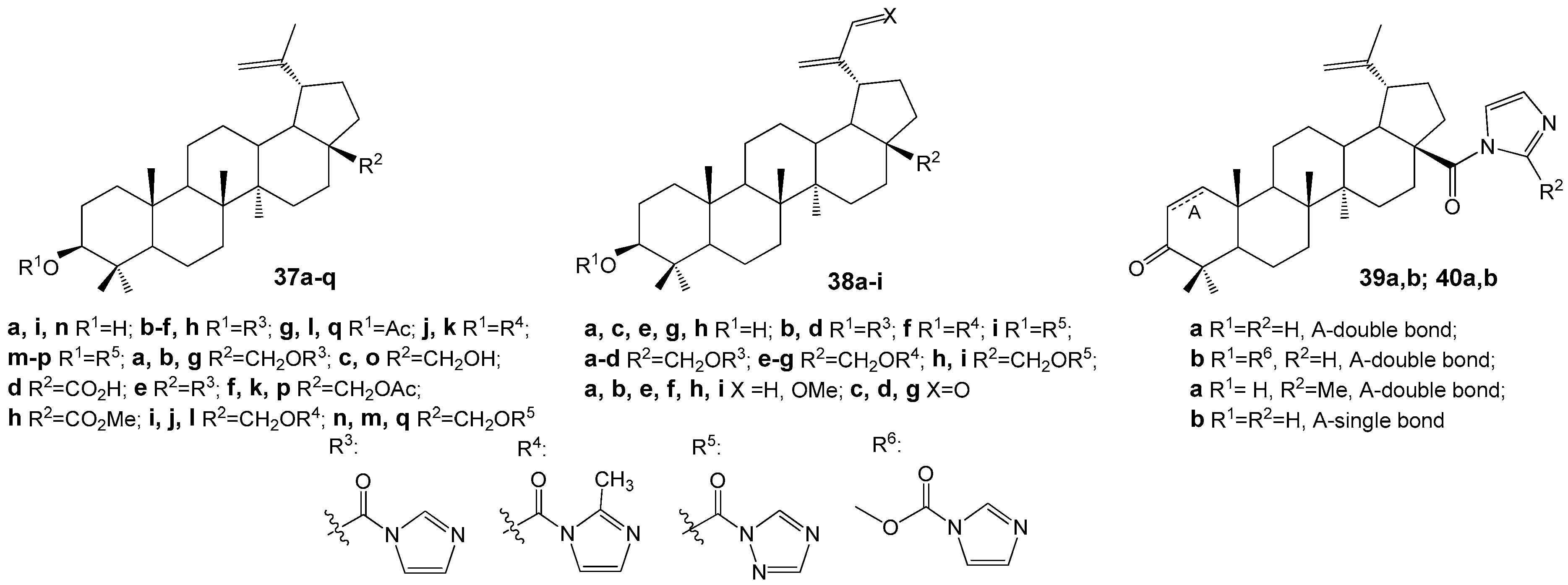
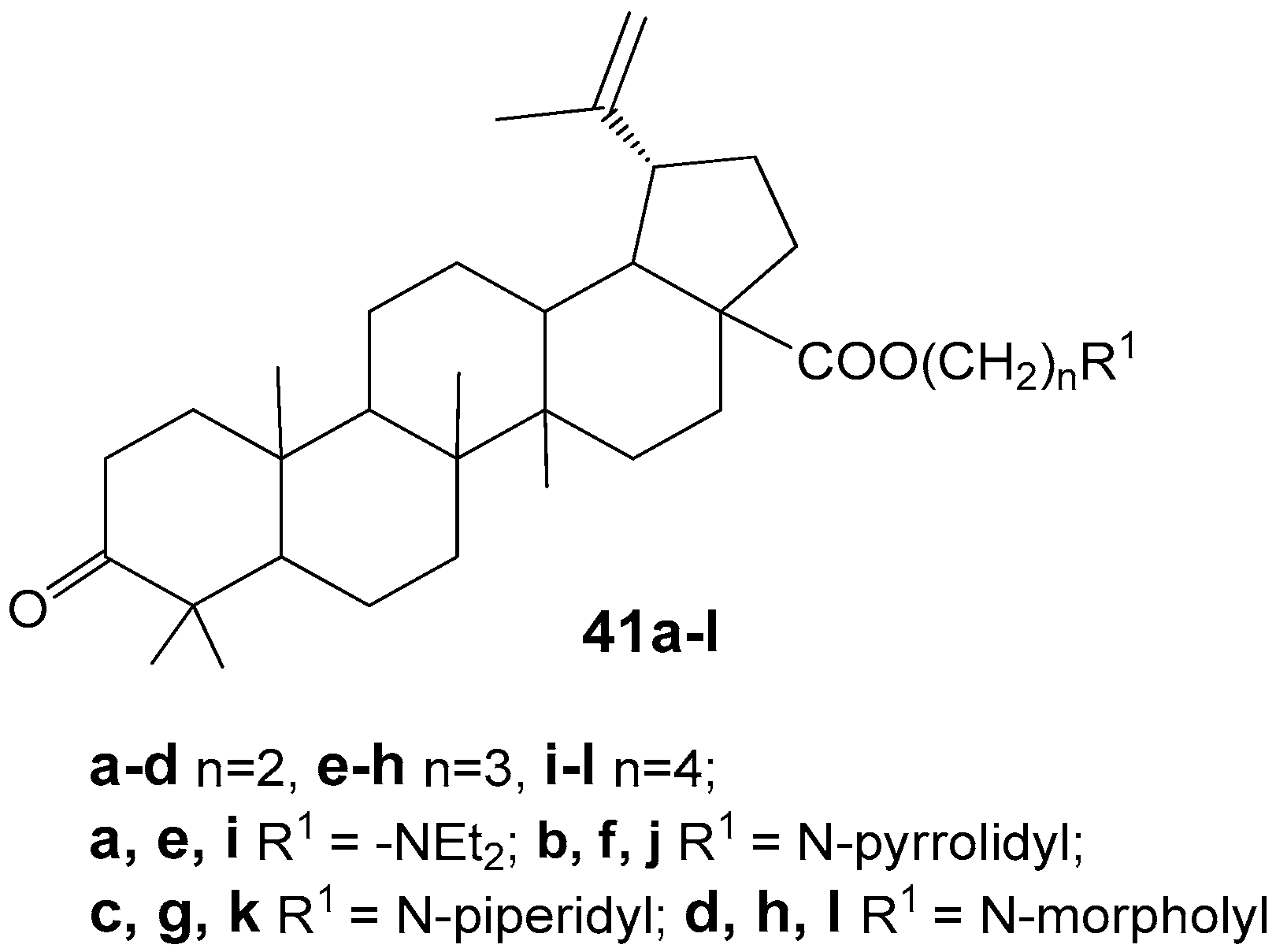
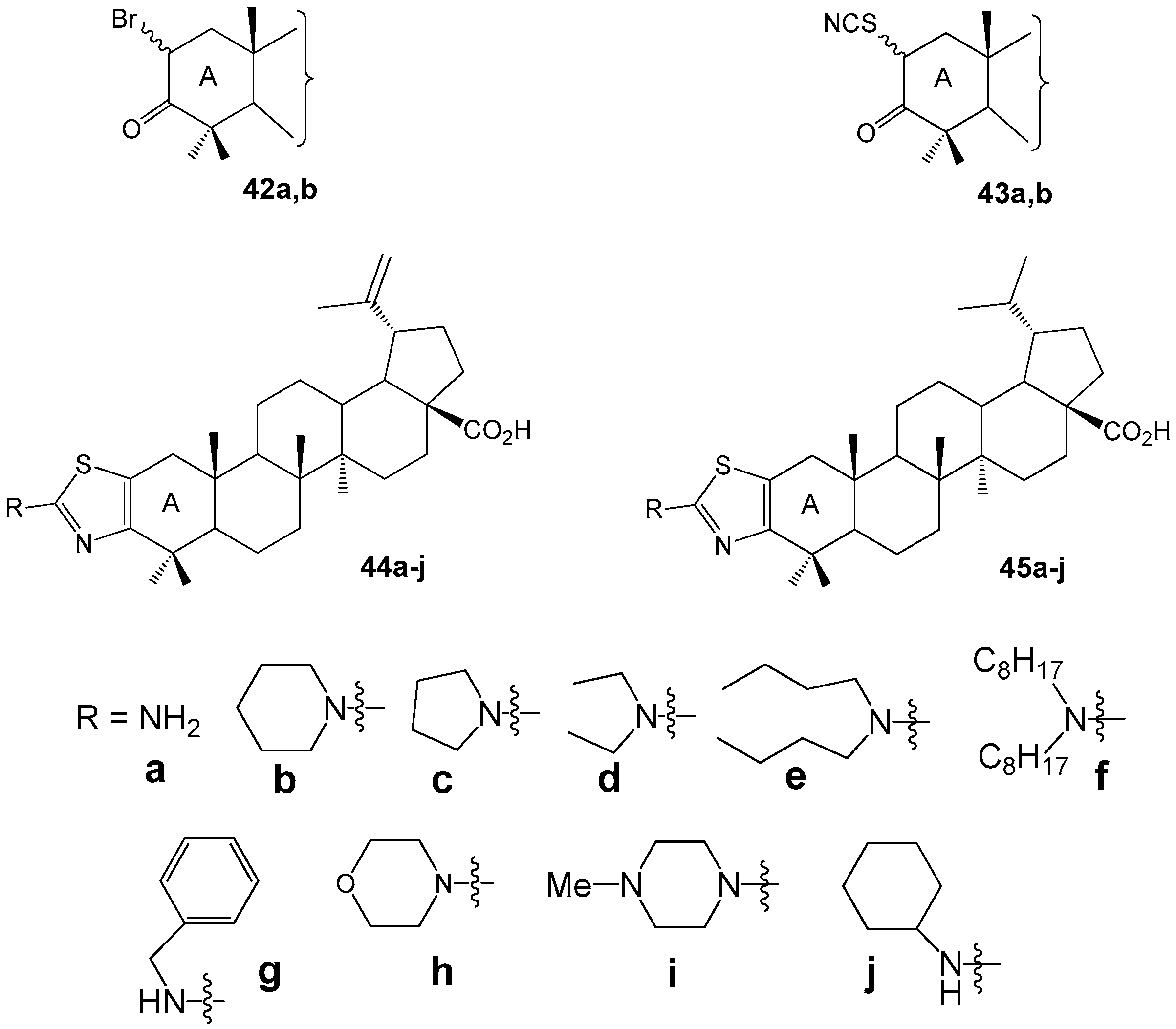
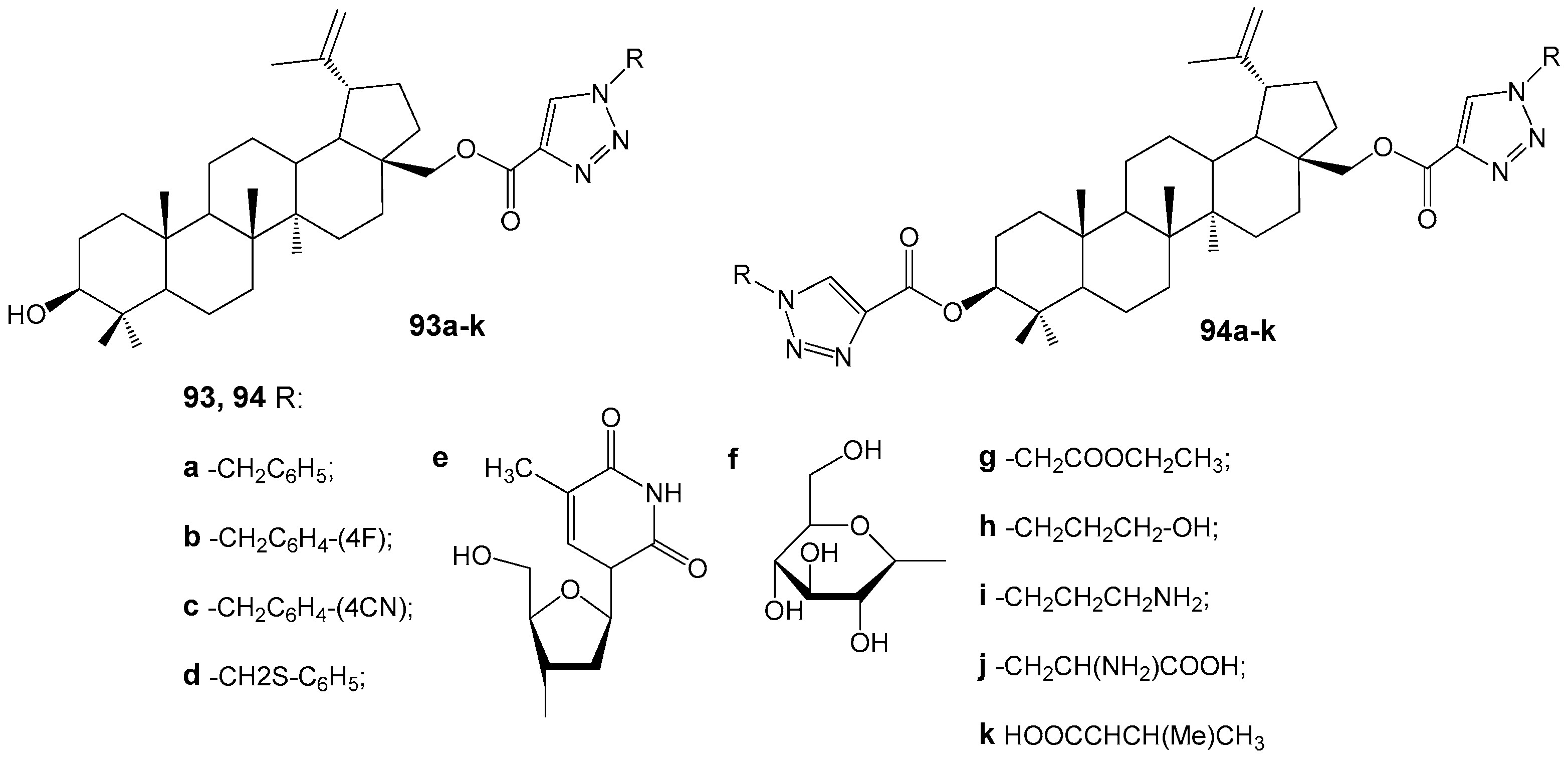
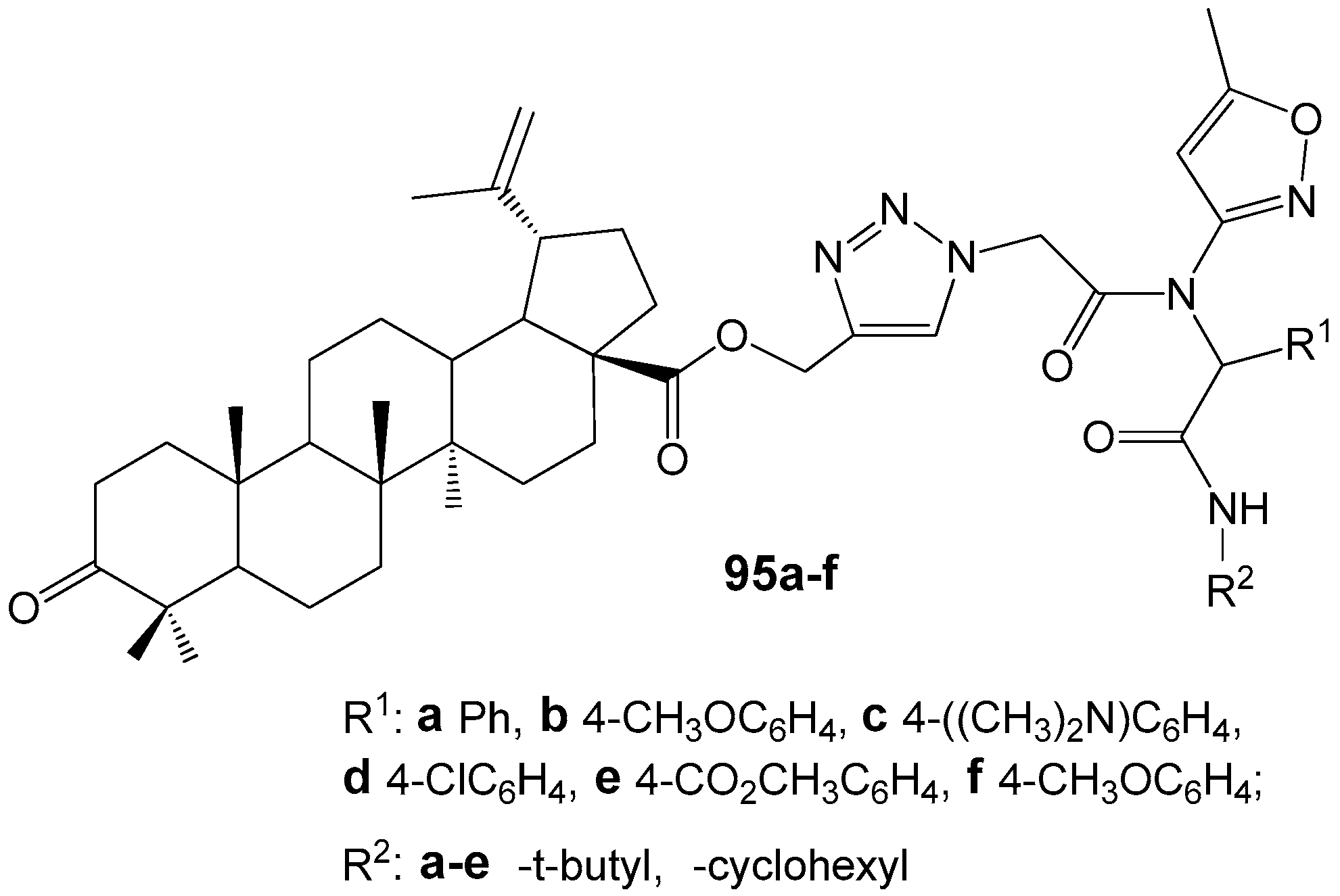
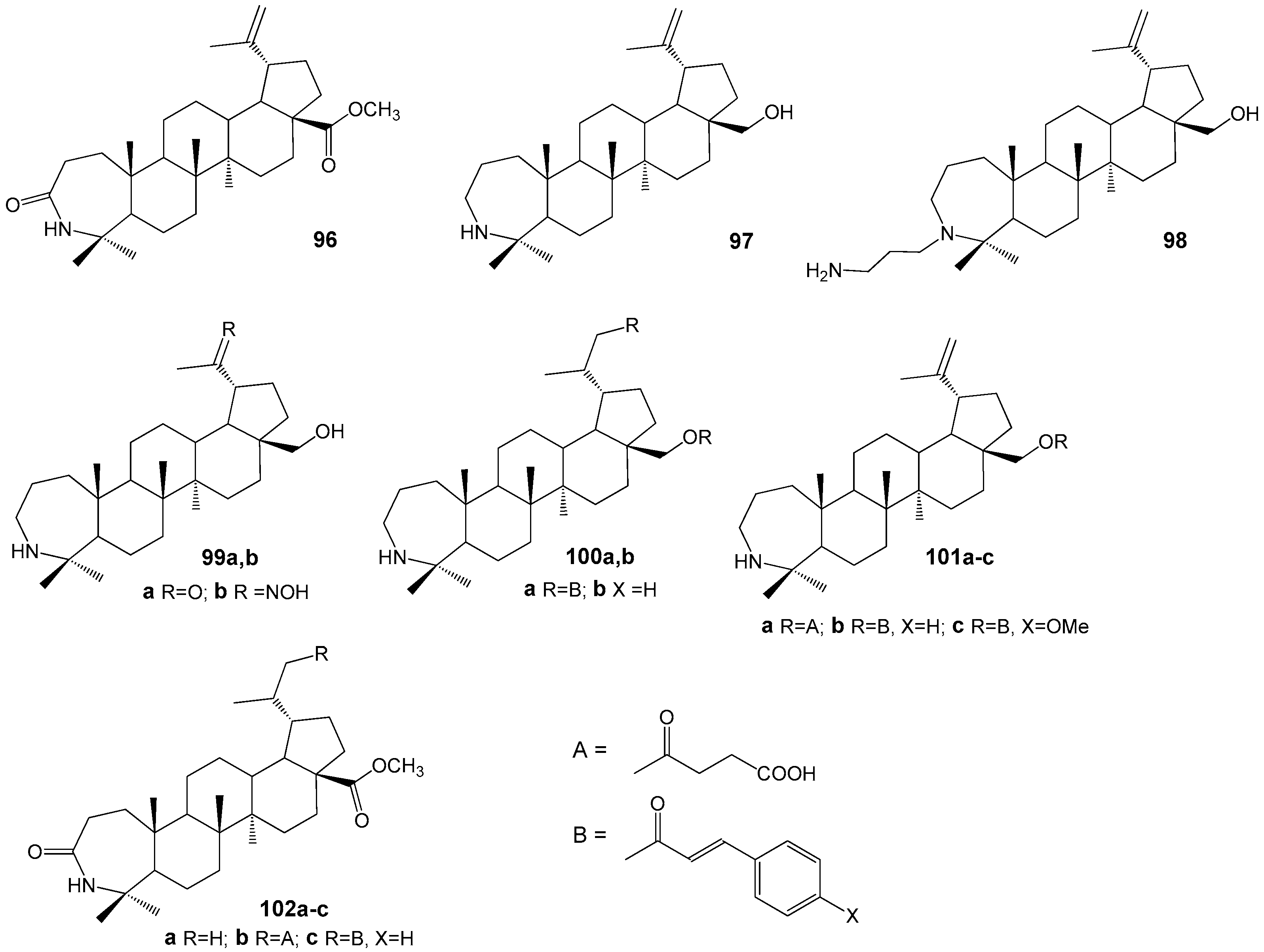
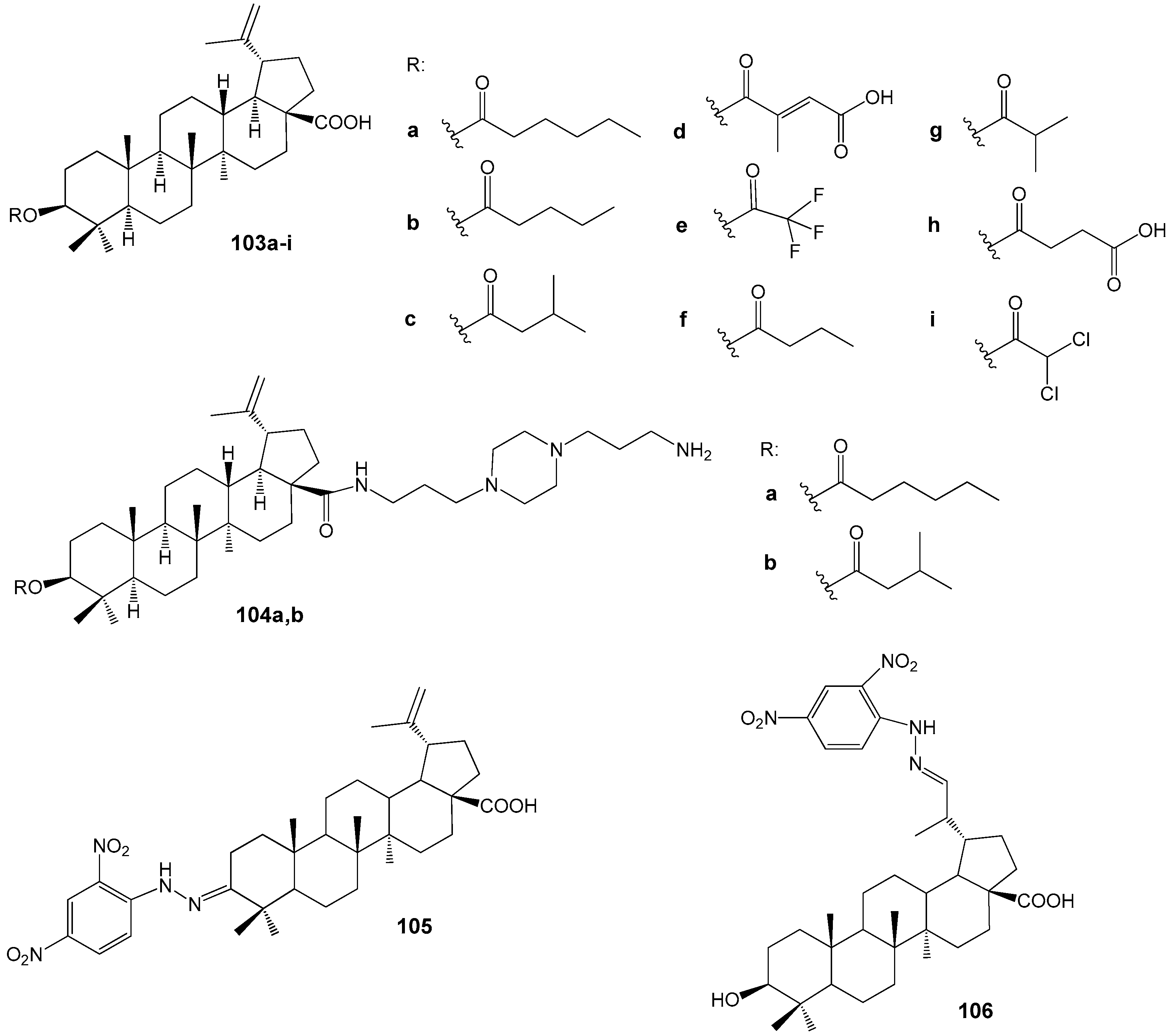
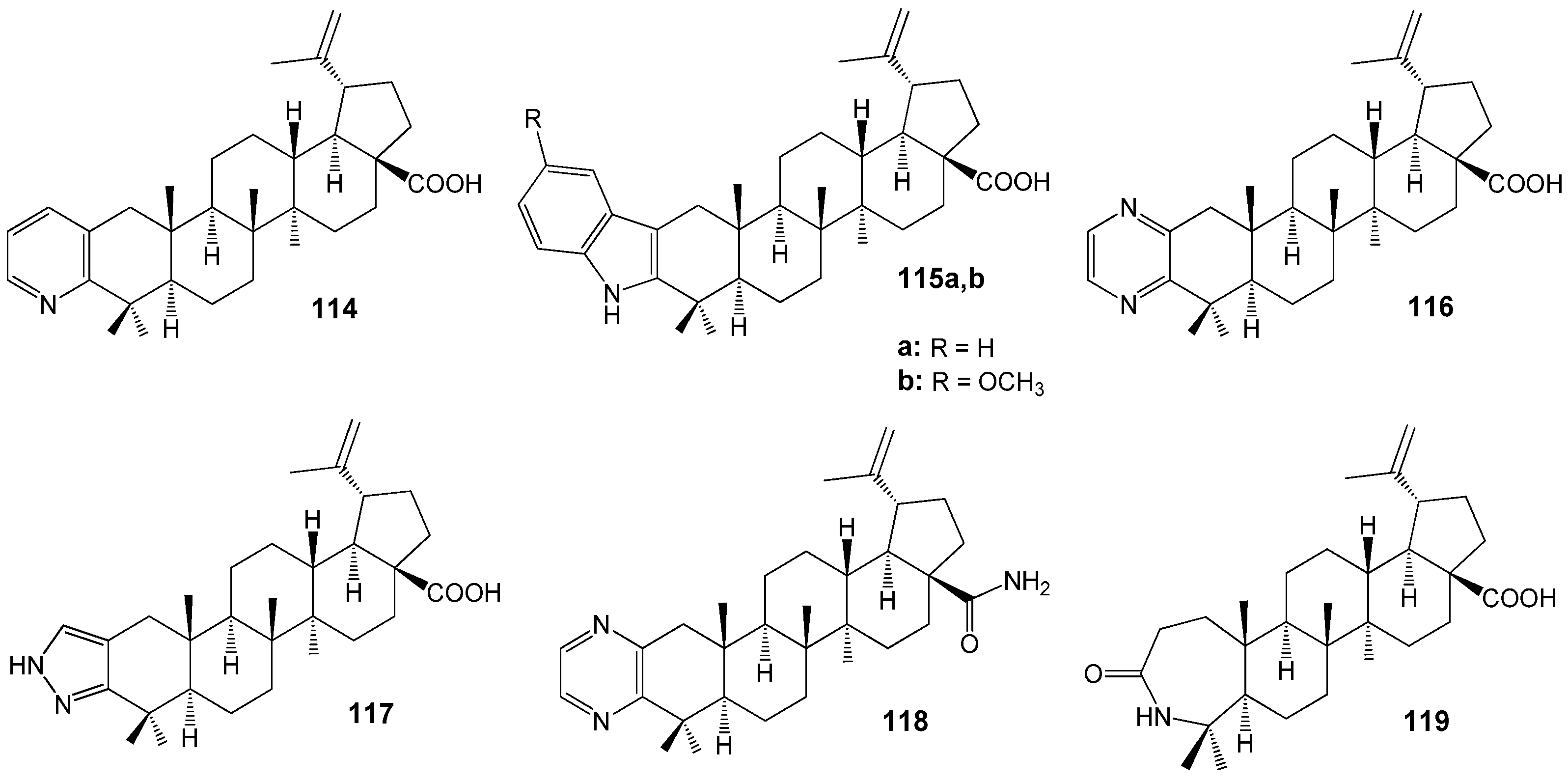
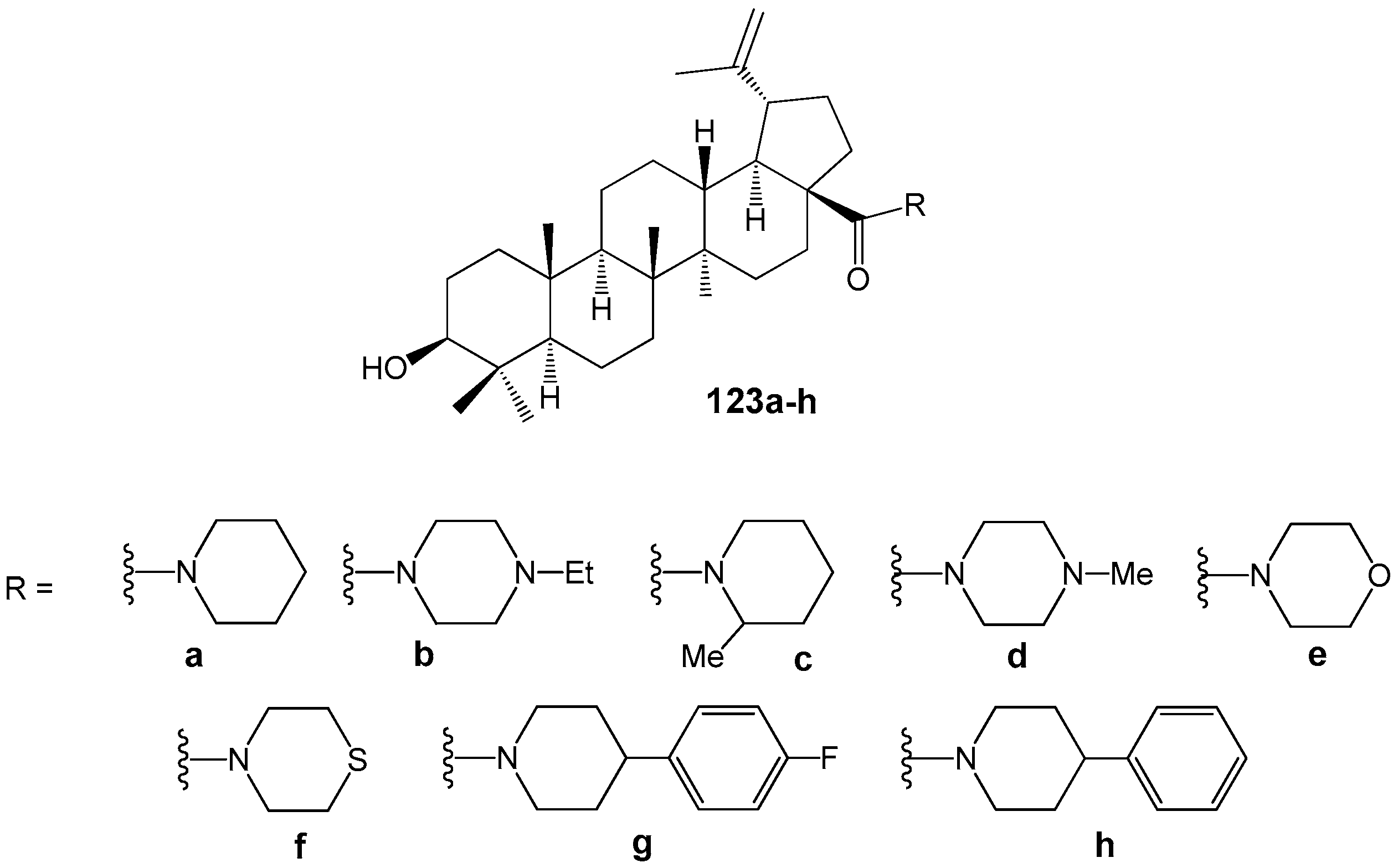
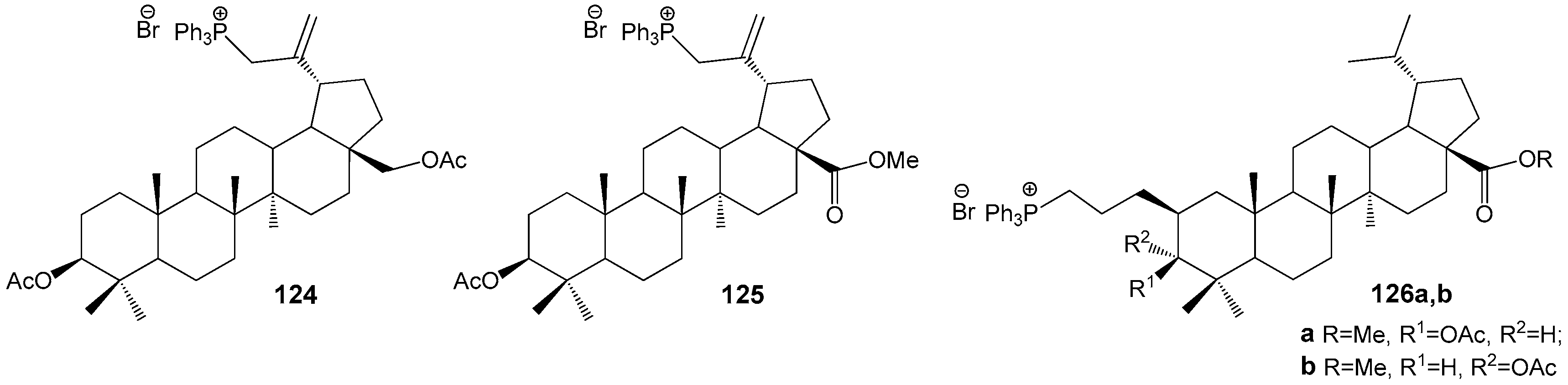
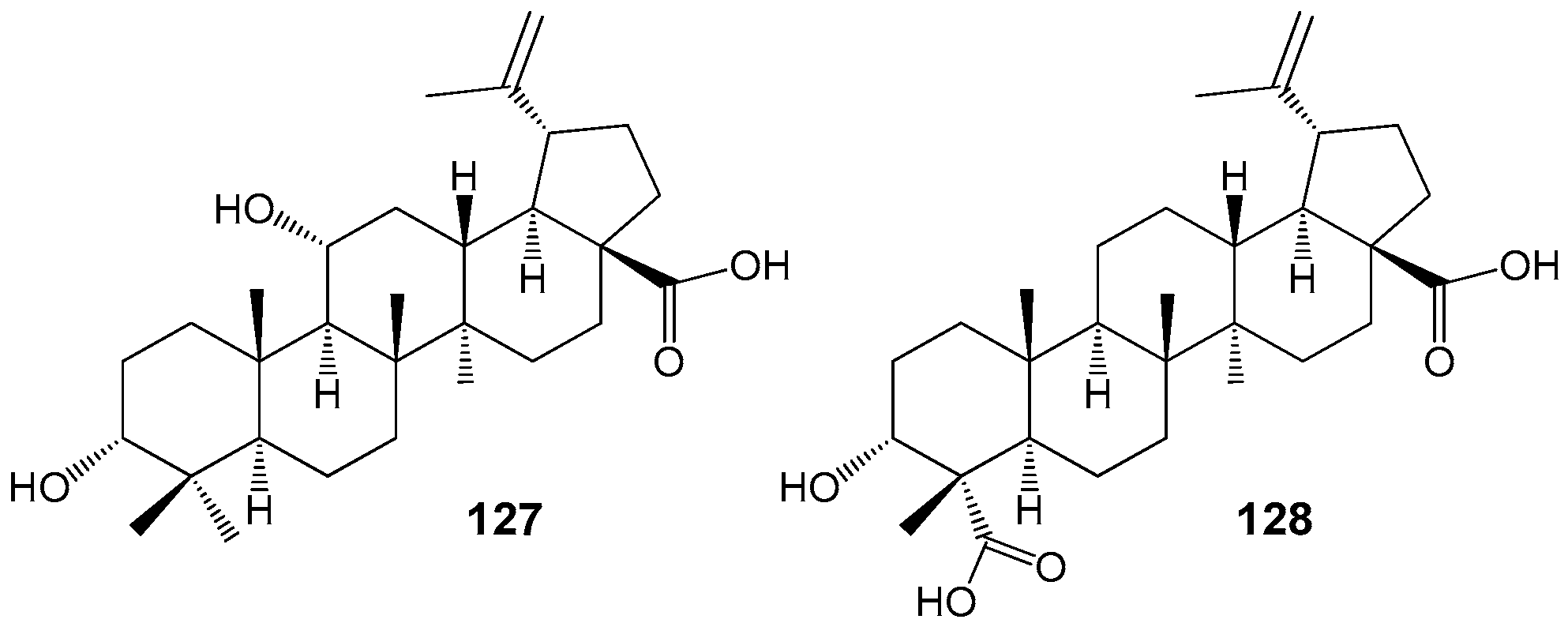
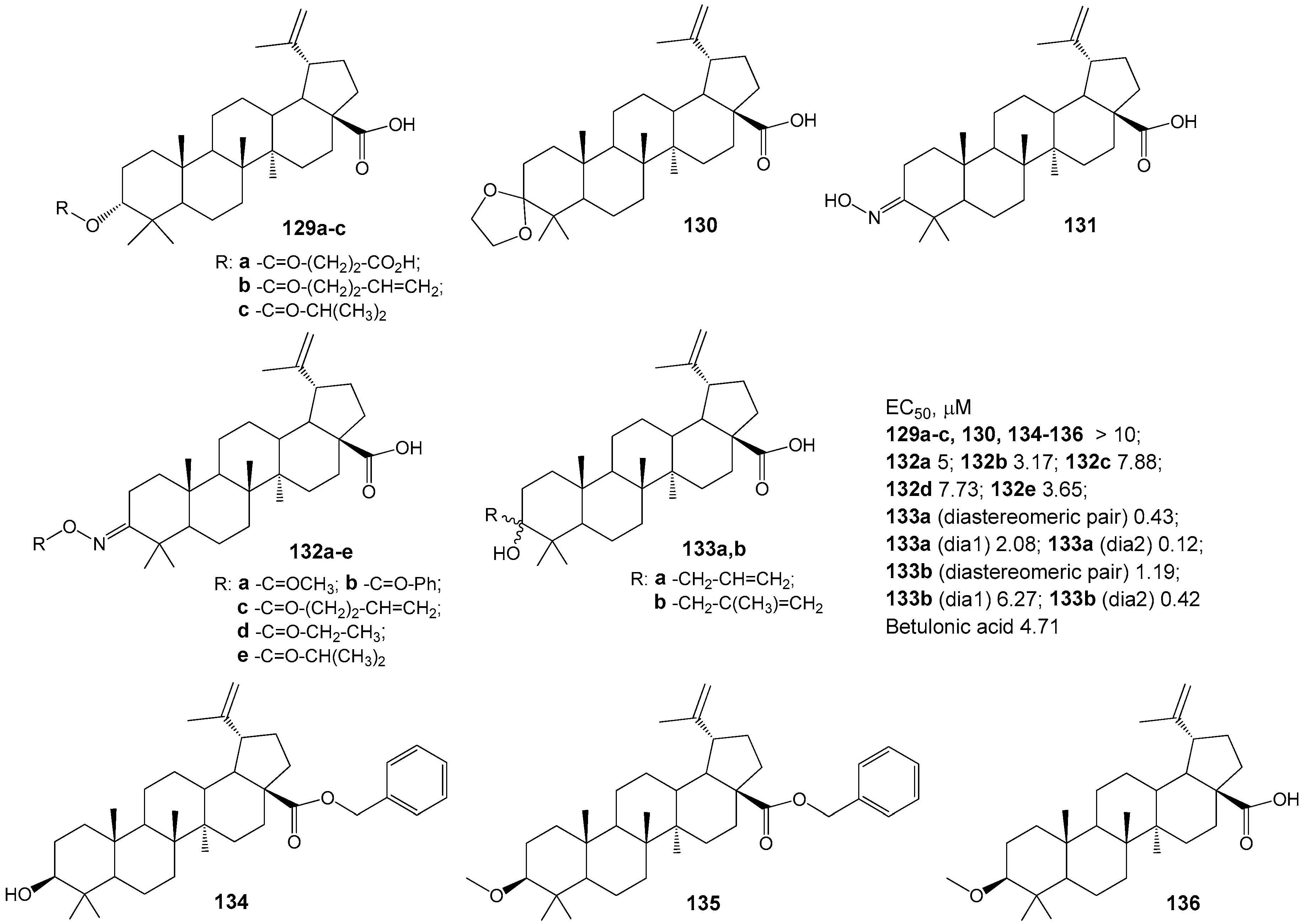
2.6. BA, BE and Lupeol Conjugates with Heterocycles
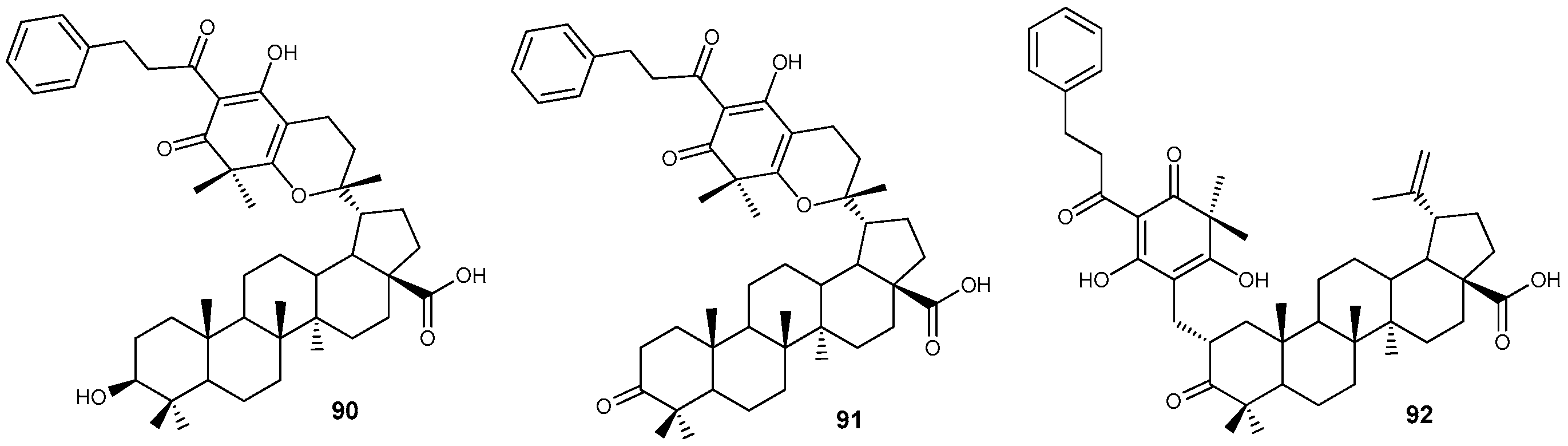
3. Anti-Inflammatory Activity of Lupanoids
4. Antiviral Activity of Lupanoids
4.1. BA and BE Hybrids with Anti-HIV Activity
4.2. BA and BE Hybrids with Anti-Influenza Virus Activity
4.3. Anti-SARS Activity of Lupanoids
4.4. Activity of Lupanoids Against HBV and HSV
5. Antibacterial, Antifungal and Antiparasitic Properties of Lupanoids
5.1. Activity of Lupanoids Against ESKAPE Group Bacteria
5.2. Lupanoids with Anti-Tuberculosis and Anti-Fungal Activity
5.3. Antiplasmodial, Antileishmanial, Antitrypanosomal and Antischistosomiasis Properties of Lupanoids
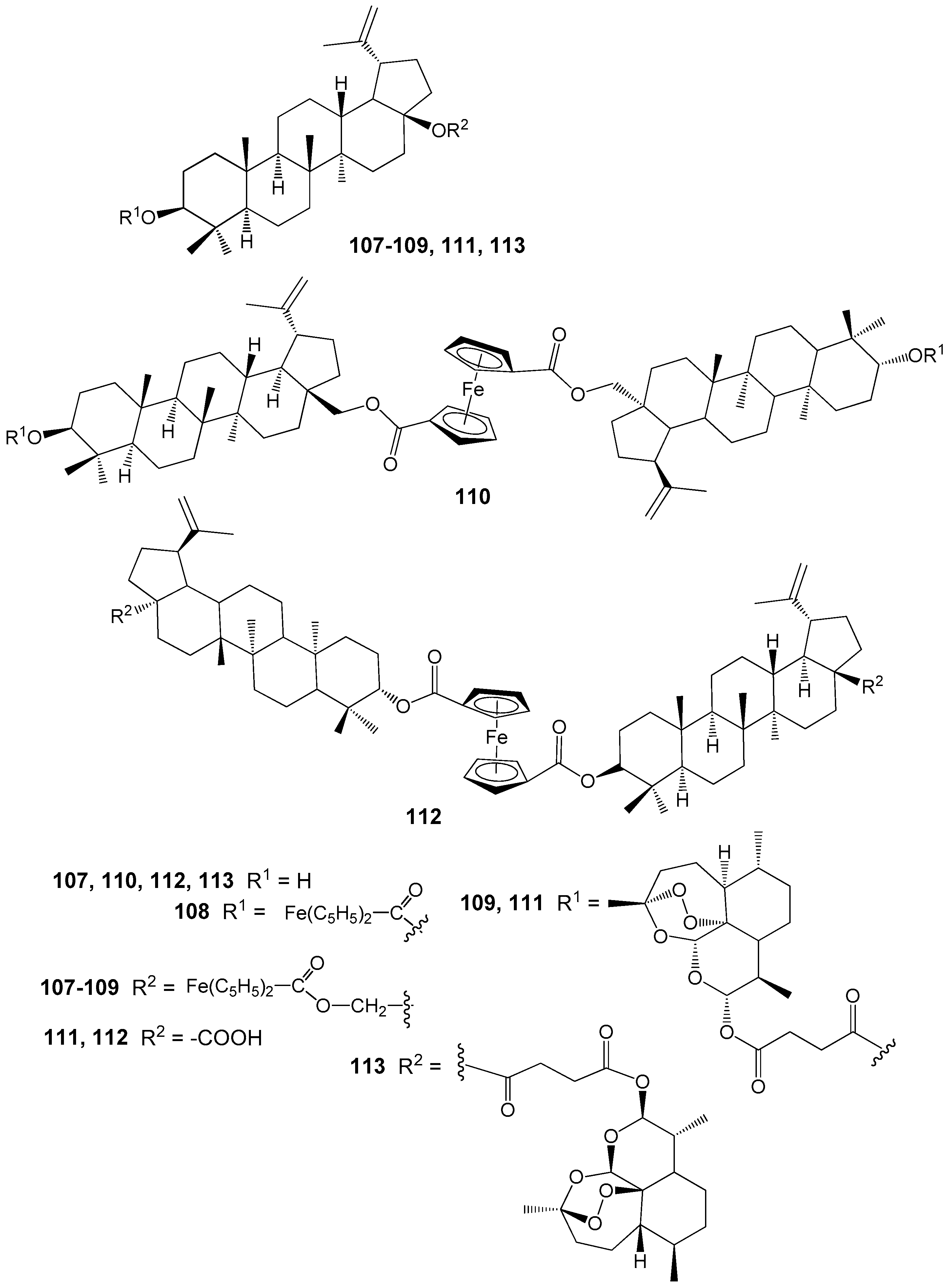
6. Compounds from Lupane Series as Potential Agents for Treatment Metabolic Disorders
7. Triterpenoid Derivatives from Lupane Series in Materials Science
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Hu, J.; Chen, Y. Betulinic acid and the pharmacological effects of tumor suppression (review). Mol. Med. Rep. 2016, 14, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent developments in the functionalization of betulinic acid and its natural analogues: A route to new bioactive compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Y.-m. Recent updates on anticancer activity of betulin and betulinic acid hybrids (A review). Russ. J. Gen. Chem. 2023, 93, 610–627. [Google Scholar] [CrossRef]
- Hodon, J.; Borkova, L.; Pokorny, J.; Kazakova, A.; Urban, M. Design and synthesis of pentacyclic triterpene conjugates and their use in medicinal research. Eur. J. Med. Chem. 2019, 182, 111653–111658. [Google Scholar] [CrossRef] [PubMed]
- Grymel, M.; Zawojak, M.; Adamek, J. Triphenylphosphonium analogues of betulin and betulinic acid with biological activity: A comprehensive review. J. Nat. Prod. 2019, 82, 1719–1730. [Google Scholar] [CrossRef]
- Baltina, L.A.; Komissarova, N.G. Transformations of pentacyclic triterpenoids as a route to the future medicines. Stud. Nat. Prod. Chem. 2023, 76, 331–407. [Google Scholar] [CrossRef]
- Allouche, Y.; Jiménez, A.; Uceda, M.; Aguilera, M.P.; Gaforio, J.J.; Beltrán, G. Triterpenic content and chemometric analysis of virgin olive oils from forty olive cultivars. J. Agric. Food Chem. 2009, 57, 3604–3610. [Google Scholar] [CrossRef]
- Razboršek, M.I.; Vončina, D.B.; Doleček, V.; Vončina, E. Determination of oleanolic, betulinic and ursolic acid in lamiaceae and mass spectral fragmentation of their trimethylsilylated derivatives. Chromatographia 2008, 67, 433–440. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Dubey, K.K.; Goel, N. Evaluation and optimization of downstream process parameters for extraction of betulinic acid from the bark of Ziziphus jujubae L. Sci. World J. 2013, 2013, 469674. [Google Scholar] [CrossRef]
- Pezzuto, J.M.; Kim, D.S.H.L. Methods of Manufacturing Betulinic Acid. U.S. Patent 5,804,575, 8 September 1998. [Google Scholar]
- Czarnotta, E.; Dianat, M.; Korf, M.; Granica, F.; Merz, J.; Maury, J.; Baallal Jacobsen, S.A.; Förster, J.; Ebert, B.E.; Blank, L.M. Fermentation and purification strategies for the production of betulinic acid and its lupane-type precursors in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Decker, M. Hybrid molecules incorporating natural products: Applications in cancer therapy, neurodegenerative disorders and beyond. Curr. Med. Chem. 2011, 18, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Dehaen, W.; Mashentseva, A.A.; Seitembetov, T.S. Allobetulin and its derivatives: Synthesis and biological activity. Molecules 2011, 16, 2443–2466. [Google Scholar] [CrossRef]
- Kommera, H.; Kaluđerović, G.N.; Kalbitz, J.; Dräger, B.; Paschke, R. Small structural changes of pentacyclic lupine-type triterpenoid derivatives lead to significant differences in their anticancer properties. Eur. J. Med. Chem. 2010, 45, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Stark, S.; Nitsche, C.; Barthel, A.; Siewert, B. Alkylidene branched lupane derivatives: Synthesis and antitumor activity. Eur. J. Med. Chem. 2012, 53, 337–345. [Google Scholar] [CrossRef]
- Baratto, L.C.; Porsani, M.V.; Pimentel, I.C.; Pereira Netto, A.B.; Paschke, R.; Oliveira, B.H. Preparation of betulinic acid derivatives by chemical and biotransformation methods and determination of cytotoxicity against selected cancer cell lines. Eur. J. Med. Chem. 2013, 68, 121–131. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, H.; Wang, L.; Li, Y.; Sun, P.; Wu, X.; Wang, G.; Chen, W.; Ye, W. Betulinic acid and its derivatives as potential antitumor agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.; Moreno-Jiménez, M.; González-Laredo, R.; Gallegos-Infante, J.; Rocha-Guzmán, N. Lupane-type triterpenes and their anti-cancer activities against most common malignant tumors: A review. EXCLI J. 2016, 15, 1611–2156. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Gheorgheosu, D.; Jung, M.; Ören, B.; Schmid, T.; Dehelean, C.; Muntean, D.; Brüne, B. Betulinic acid suppresses NGAL induced epithelial to mesenchymal transition in melanoma. Biol. Chem. 2013, 394, 773–781. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Q.; Wang, Z.; Liu, X.-Y. Activated STAT3 correlates with prognosis of non-small cell lung cancer and indicates anticancer strategies. Cancer Chemother. Pharmacol. 2015, 75, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; He, J.; Yi, S.; Wen, L.; Zhao, J.; Zhang, B.; Cui, G. Betulinic acid inhibits autophagic flux and induces apoptosis in human multiple myeloma cells in vitro. Acta Pharmacol. Sin. 2012, 33, 1542–1548. [Google Scholar] [CrossRef]
- Ehrhardt, H.; Fulda, S.; Führer, M.; Debatin, K.M.; Jeremias, I. Betulinic acid-induced apoptosis in leukemia cells. Leukemia 2004, 18, 1406–1412. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Kerzig, C.; Kramell, A.E.; Csuk, R. Rhodamine B conjugates of triterpenoic acids are cytotoxic mitocans even at nanomolar concentrations. Eur. J. Med. Chem. 2017, 127, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Denner, T.C.; Heise, N.V.; Hoenke, S.; Csuk, R. Synthesis of rhodamine-conjugated lupane type triterpenes of enhanced cytotoxicity. Molecules 2024, 29, 2346. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Nedopekina, D.A.; Shakurova, E.R.; Khalitova, R.R.; Gubaidullin, R.R.; Odinokov, V.N.; Dzhemilev, U.M.; Bel’skii, Y.P.; Bel’skaya, N.V.; Stankevich, S.A.; et al. Synthesis of lupane triterpenoids with triphenylphosphonium substituents and studies of their antitumor activity. Russ. Chem. Bull. 2013, 62, 188–198. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Nedopekina, D.A.; Khalitova, R.R.; Gubaidullin, R.R.; Odinokov, V.N.; Bel’skii, Y.P.; Bel’skaya, N.V.; Khazanov, V.A. Triphenylphosphonium cations of betulinic acid derivatives: Synthesis and antitumor activity. Med. Chem. Res. 2017, 26, 518–531. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Nemtarev, A.V.; Abdullin, T.I.; Grigor’eva, L.R.; Kuznetsova, E.V.; Akhmadishina, R.A.; Ziganshina, L.E.; Cong, H.H.; Mironov, V.F. Design, synthesis, and cancer cell growth inhibitory activity of triphenylphosphonium derivatives of the triterpenoid betulin. J. Nat. Prod. 2017, 80, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, D.; Vanchanagiri, K.; Baratto, L.C.; Schmidt, H.; Paschke, R. Synthesis and studies of anticancer properties of lupane-type triterpenoid derivatives containing a cisplatin fragment. Eur. J. Med. Chem. 2014, 75, 460–466. [Google Scholar] [CrossRef]
- Ackermann, A.; Karagöz, A.Ç.; Ghoochani, A.; Buchfelder, M.; Eyüpoglu, I.; Tsogoeva, S.B.; Savaskan, N. Cytotoxic profiling of artesunic and betulinic acids and their synthetic hybrid compound on neurons and gliomas. Oncotarget 2017, 8, 61457–61474. [Google Scholar] [CrossRef]
- Pattnaik, B.; Lakshmi, J.K.; Kavitha, R.; Jagadeesh, B.; Bhattacharjee, D.; Jain, N.; Mallavadhani, U.V. Synthesis, structural studies, and cytotoxic evaluation of novel ursolic acid hybrids with capabilities to arrest breast cancer cells in mitosis. J. Asian Nat. Prod. Res. 2017, 19, 260–271. [Google Scholar] [CrossRef]
- Soural, M.; Hodon, J.; Dickinson, N.J.; Sidova, V.; Gurska, S.; Dzubak, P.; Hajduch, M.; Sarek, J.; Urban, M. Preparation of conjugates of cytotoxic lupane triterpenes with biotin. Bioconjugate Chem. 2015, 26, 2563–2570. [Google Scholar] [CrossRef]
- Eignerova, B.; Tichy, M.; Krasulova, J.; Kvasnica, M.; Rarova, L.; Christova, R.; Urban, M.; Bednarczyk-Cwynar, B.; Hajduch, M.; Sarek, J. Synthesis and antiproliferative properties of new hydrophilic esters of triterpenic acids. Eur. J. Med. Chem. 2017, 140, 403–420. [Google Scholar] [CrossRef]
- Bache, M.; Bernhardt, S.; Passin, S.; Wichmann, H.; Hein, A.; Zschornak, M.; Kappler, M.; Taubert, H.; Paschke, R.; Vordermark, D. Betulinic acid derivatives NVX-207 and B10 for treatment of glioblastoma—An in vitro study of cytotoxicity and radiosensitization. Int. J. Mol. Sci. 2014, 15, 19777–19790. [Google Scholar] [CrossRef] [PubMed]
- Yamansarov, E.Y.; Skvortsov, D.A.; Lopukhov, A.V.; Kovalev, S.V.; Evteev, S.A.; Petrov, R.A.; Klyachko, N.L.; Ivanenkov, Y.A.; Majouga, A.G. New ASGPR-targeted ligands based on glycoconjugated natural triterpenoids. Russ. Chem. Bull. 2019, 68, 2331–2338. [Google Scholar] [CrossRef]
- Bar, F.M.A.; Khanfar, M.A.; Elnagar, A.Y.; Liu, H.; Zaghloul, A.M.; Badria, F.A.; Sylvester, P.W.; Ahmad, K.F.; Raisch, K.P.; El Sayed, K.A. Rational design and semisynthesis of betulinic acid analogues as potent topoisomerase inhibitors. J. Nat. Prod. 2009, 72, 1643–1650. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Csuk, R. Synthesis and cytotoxic activity of pentacyclic triterpenoid sulfamates. Arch. Der Pharm. 2015, 348, 46–54. [Google Scholar] [CrossRef]
- Vanchanagiri, K.; Emmerich, D.; Bruschke, M.; Bache, M.; Seifert, F.; Csuk, R.; Vordermark, D.; Paschke, R. Synthesis and biological investigation of new carbonic anhydrase IX (CAIX) inhibitors. Chem. Biol. Interact. 2018, 284, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Güttler, A.; Eiselt, Y.; Funtan, A.; Thiel, A.; Petrenko, M.; Keßler, J.; Thondorf, I.; Paschke, R.; Vordermark, D.; Bache, M. Betulin sulfonamides as carbonic anhydrase inhibitors and anticancer agents in breast cancer cells. Int. J. Mol. Sci. 2021, 22, 8808. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-W.; He, Y.; Wang, J.; Gao, W.; Liu, T.; Qin, M.; Wang, X.; Gao, C.; Wang, Y.; Liu, M.-Y.; et al. Synthesis of heterocycle-modified betulinic acid derivatives as antitumor agents. Eur. J. Med. Chem. 2015, 95, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.C.; Salvador, J.A.R.; Marín, S.; Cascante, M.; Moreira, J.N.; Dinis, T.C.P. Synthesis and structure-activity relationship study of novel cytotoxic carbamate and N-acylheterocyclic bearing derivatives of betulin and betulinic acid. Bioorg. Med. Chem. 2010, 18, 4385–4396. [Google Scholar] [CrossRef]
- Yang, S.-J.; Liu, M.-C.; Zhao, Q.; Hu, D.-Y.; Xue, W.; Yang, S. Synthesis and biological evaluation of betulonic acid derivatives as antitumor agents. Eur. J. Med. Chem. 2015, 96, 58–65. [Google Scholar] [CrossRef]
- Borková, L.; Frydrych, I.; Jakubcová, N.; Adámek, R.; Lišková, B.; Gurská, S.; Medvedíková, M.; Hajdúch, M.; Urban, M. Synthesis and biological evaluation of triterpenoid thiazoles derived from betulonic acid, dihydrobetulonic acid, and ursonic acid. Eur. J. Med. Chem. 2020, 185, 111806. [Google Scholar] [CrossRef]
- Grishko, V.V.; Tolmacheva, I.A.; Nebogatikov, V.O.; Galaiko, N.V.; Nazarov, A.V.; Dmitriev, M.V.; Ivshina, I.B. Preparation of novel ring-A fused azole derivatives of betulin and evaluation of their cytotoxicity. Eur. J. Med. Chem. 2017, 125, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhao, Y.; Deng, S.; Hou, L.; Song, J.; Wang, M.; Bu, M. Lupeol-3-carbamate derivatives: Synthesis and biological evaluation as potential antitumor agents. Molecules 2024, 29, 3990–4007. [Google Scholar] [CrossRef]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. Thiazolidine-2,4-diones as multi-targeted scaffold in medicinal chemistry: Potential anticancer agents. Eur. J. Med. Chem. 2014, 87, 814–833. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhao, Y.; Guo, X.; Hong, X.; Li, G.; Wang, Y.; Li, Q.; Bu, M.; Wang, M. ThiazolidinedioneConjugated Lupeol Derivatives as Potent Anticancer Agents Through a Mitochondria-Mediated Apoptotic Pathway. Molecules 2024, 29, 4957–4982. [Google Scholar] [CrossRef]
- Lomkova, E.A.; Chytil, P.; Janoušková, O.; Mueller, T.; Lucas, H.; Filippov, S.K.; Trhlíková, O.; Aleshunin, P.A.; Skorik, Y.A.; Ulbrich, K.; et al. Biodegradable Micellar HPMA-Based polymer–drug conjugates with betulinic acid for passive tumor targeting. Biomacromolecules. Biomacromolecules 2016, 17, 3493–3507. [Google Scholar] [CrossRef]
- Dai, L.; Li, D.; Cheng, J.; Liu, J.; Deng, L.-H.; Wang, L.-Y.; Lei, J.-D.; He, J. Water soluble multiarm-polyethylene glycol–betulinic acid prodrugs: Design, synthesis, and in vivo effectiveness. Polym. Chem. 2014, 5, 5775–5783. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Zhou, S.-F. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica 2008, 38, 802–832. [Google Scholar] [CrossRef]
- Darby, R.A.J.; Callaghan, R.; McMahon, R.M. P-glycoprotein inhibition: The past, the present and the future. Curr. Drug Metab. 2011, 12, 722–731. [Google Scholar] [CrossRef]
- Delou, J.M.; Capella, M.A.M.; Gattass, C.R. Betulinic acid does not modulate the activity of P-gp/ABCB1 or MRP1/ABCC1 in a non-tumoral renal cell line: Possible utility in multidrug resistance cancer chemotherapy. Mol. Med. Rep. 2009, 2, 271–275. [Google Scholar] [CrossRef]
- Fernandes, J.; Castilho, R.O.; Da Costa, M.R.; Wagner-Souza, K.; Coelho Kaplan, M.A.; Gattass, C.R. Pentacyclic triterpenes from Chrysobalanaceae species: Cytotoxicity on multidrug resistant and sensitive leukemia cell lines. Cancer Lett. 2003, 190, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Chen, K.; Zhao, W.; Zhao, X.; Luo, J.; Wang, Q.; Gao, C.; Li, X.; Wang, C. Methotrexate combined with 4-hydroperoxycyclophosphamide downregulates multidrug-resistance P-glycoprotein expression induced by methotrexate in rheumatoid arthritis fibroblast-like synoviocytes via the JAK2/STAT3 pathway. J. Immunol. Res. 2018, 2018, 3619320. [Google Scholar] [CrossRef]
- Semenenko, O.M.; Lipson, V.V.; Sadchenko, A.O.; Vashchenko, O.V.; Kasian, N.A.; Sviechnikova, L.V.; Lisetski, L.M.; Babak, M.L.; Vakula, V.M.; Borysov, O.V.; et al. Synthesis of methotrexate-betulonic acid hybrids and evaluation of their effect on artificial and Caco-2 cell membranes. Steroids 2024, 201, 109332. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Saeed Saify, Z. Naturally occurring and synthetic agents as potential anti-inflammatory and immunomodulants. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.E.; Coutinho, A.E.; Zhang, Z.; Kipari, T.; Savill, J.S.; Seckl, J.R. Changing glucocorticoid action: 11β-hydroxysteroid dehydrogenase type 1 in acute and chronic inflammation. J. Steroid Biochem. Mol. Biol. 2013, 137, 82–92. [Google Scholar] [CrossRef]
- Otsuka, H.; Fujioka, S.; Komiya, T.; Goto, M.; Hiramatsu, Y.; Fujimura, H. Studies on anti-inflammatory agents. V. A new anti-inflammatory constituent of Pyracantha crenulata Roem. Chem. Pharm. Bull. 1981, 29, 3099–3104. [Google Scholar] [CrossRef]
- Del Carmen Recio, M.; Giner, R.; Máñez, S.; Gueho, J.; Julien, H.; Hostettmann, K.; Ríos, J. Investigations on the steroidal anti-inflammatory activity of triterpenoids from Diospyros Leucomelas. Planta Med. 1995, 61, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Nader, M.A.; Baraka, H.N. Effect of betulinic acid on neutrophil recruitment and inflammatory mediator expression in lipopolysaccharide-induced lung inflammation in rats. Eur. J. Pharm. Sci. 2012, 46, 106–113. [Google Scholar] [CrossRef]
- Oliveira-Costa, J.F.; Barbosa-Filho, J.M.; De Azevedo Maia, G.L.; Guimarães, E.T.; Meira, C.S.; Ribeiro-dos-Santos, R.; Pontes De Carvalho, L.C.; Soares, M.B.P. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474. [Google Scholar] [CrossRef]
- Meira, C.S.; Espírito Santo, R.F.D.; Santos, T.B.D.; Orge, I.D.; Silva, D.K.C.; Guimarães, E.T.; Aragão França, L.S.D.; Barbosa-Filho, J.M.; Moreira, D.R.M.; Soares, M.B.P. Betulinic acid derivative BA5, a dual NF-kB/calcineurin inhibitor, alleviates experimental shock and delayed hypersensitivity. Eur. J. Pharmacol. 2017, 815, 156–165. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Nigmatullina, L.R.; Karachurina, L.T.; Baltina, L.A.; Zarudii, F.S.; Davydova, V.A.; Galin, F.Z.; Tolstikov, G.A. The synthesis and the anti-inflammatory and antiulcer activities of a number of 2-substituted derivatives of betulonic acid, methylbetulone, and lupenone. Pharm. Chem. J. 2000, 34, 588–591. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Khalitova, R.R.; Bel’skii, Y.P.; Ivanova, A.N.; Shakurova, E.R.; Bel’skaya, N.V.; Odinokov, V.N.; Danilets, M.G.; Ligacheva, A.A. Synthesis of conjugates of lupane triterpenoids with chromane antioxidants and in vitro study of their influence on the production of nitrogen monoxide and on the arginase activity in activated macrophages. Russ. Chem. Bull. 2010, 59, 2219–2229. [Google Scholar] [CrossRef]
- Meira, C.S.; Santos, E.D.S.; Santo, R.F.D.E.; Vasconcelos, J.F.; Orge, I.D.; Nonaka, C.K.V.; Barreto, B.C.; Caria, A.C.I.; Silva, D.N.; Barbosa-Filho, J.M.; et al. Betulinic acid derivative BA5, attenuates inflammation and fibrosis in experimental chronic Chagas disease cardiomyopathy by inducing IL-10 and M2 polarization. Front. Immunol. 2019, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Z.; Luo, J.; Yang, F.; Liu, T.; Liu, M.; Qiu, W.-W.; Tang, J. Synthesis and biological evaluation of heterocyclic ring-fused betulinic acid derivatives as novel inhibitors of osteoclast differentiation and bone resorption. J. Med. Chem. 2012, 55, 3122–3134. [Google Scholar] [CrossRef]
- Chen, S.; Bai, Y.; Li, Z.; Jia, K.; Jin, Y.; He, B.; Qiu, W.-W.; Du, C.; Siwko, S.; Chen, H.; et al. A betulinic acid derivative SH479 inhibits collagen-induced arthritis by modulating T cell differentiation and cytokine balance. Biochem. Pharmacol. 2017, 126, 69–78. [Google Scholar] [CrossRef]
- Govdi, A.I.; Sokolova, N.V.; Sorokina, I.V.; Baev, D.S.; Tolstikova, T.G.; Mamatyuk, V.I.; Fadeev, D.S.; Vasilevsky, S.F.; Nenajdenko, V.G. Synthesis of new betulinic acid-peptide conjugates and in vivo and in silico studies of the influence of peptide moieties on the triterpenoid core activity. Med. Chem. Commun. 2015, 6, 230–238. [Google Scholar] [CrossRef]
- World Health Organization. Rabies. Available online: https://www.who.int/news-room/fact-sheets/detail/rabies (accessed on 5 June 2024).
- World Health Organization. HIV and AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 15 July 2025).
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 28 February 2025).
- World Health Organization. Ebola Virus Disease. Available online: https://www.who.int/health-topics/ebola#tab=tab_1 (accessed on 24 April 2025).
- Happi, A.N.; Happi, C.T.; Schoepp, R.J. Lassa fever diagnostics: Past, present, and future. COVIRO 2019, 37, 132–138. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.-S.; Lee, K.-H. Anti-AIDS agents. 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef]
- Li, F.; Goila-Gaur, R.; Salzwedel, K.; Kilgore, N.R.; Reddick, M.; Matallana, C.; Castillo, A.; Zoumplis, D.; Martin, D.E.; Orenstein, J.M.; et al. Pa-457: A potent HIV inhibitor that disrupts core condensation by targeting a late step in gag processing. Proc. Natl. Acad. Sci. USA 2003, 100, 13555–13560. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Hashimoto, F.; Cosentino, L.M.; Chen, C.-H.; Garrett, P.E.; Lee, K.-H. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J. Med. Chem. 1996, 39, 1016–1017. [Google Scholar] [CrossRef]
- Smith, P.F.; Ogundele, A.; Forrest, A.; Wilton, J.; Salzwedel, K.; Doto, J.; Allaway, G.P.; Martin, D.E. Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-O-(3′,3′-dimethylsuc-cinyl)betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob. Agents Chemother. 2007, 51, 3574–3581. [Google Scholar] [CrossRef]
- Dang, Z.; Ho, P.; Zhu, L.; Qian, K.; Lee, K.-H.; Huang, L.; Chen, C.-H. New betulinic acid derivatives for bevirimat-resistant human immunodeficiency virus type-1. J. Med. Chem. 2013, 56, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Gulick, R.M. Choosing initial antiretroviral therapy: Current recommendations for initial therapy and newer or investigational agents. Top. Antivir. Med. 2015, 23, 128–131. [Google Scholar] [PubMed] [PubMed Central]
- Nowicka-Sans, B.; Protack, T.; Lin, Z.; Li, Z.; Zhang, S.; Sun, Y.; Samanta, H.; Terry, B.; Liu, Z.; Chen, Y.; et al. Identification and characterization of BMS-955176, a second-generation HIV-1 maturation inhibitor with improved potency, antiviral spectrum, and gag polymorphic coverage Antimicrob. Agents Chemother. 2016, 60, 3956–3969. [Google Scholar] [CrossRef] [PubMed]
- Olender, S.A.; Taylor, B.S.; Wong, M.; Wilkin, T.J. CROI 2015: Advances in antiretroviral therapy. Top. Antivir. Med. 2015, 23, 28–45. [Google Scholar] [PubMed] [PubMed Central]
- Regueiro-Ren, A.; Liu, Z.; Chen, Y.; Sin, N.; Sit, S.-Y.; Swidorski, J.J.; Chen, J.; Venables, B.L.; Zhu, J.; Nowicka-Sans, B.; et al. Discovery of BMS-955176, a second generation HIV-1 maturation inhibitor with broad spectrum antiviral activity. ACS Med. Chem. Lett. 2016, 7, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Kashiwada, Y.; Chen, C.-H.; Qian, K.; Morris-Natschke, S.L.; Lee, K.-H.; Takaishi, Y. Conjugates of betulin derivatives with AZT as potent anti-HIV agents. Bioorg. Med. Chem. 2010, 18, 6451–6469. [Google Scholar] [CrossRef]
- Bori, I.D.; Hung, H.-Y.; Qian, K.; Chen, C.-H.; Morris-Natschke, S.L.; Lee, K.-H. Anti-AIDS agents 88. Anti-HIV conjugates of betulin and betulinic acid with AZT prepared via click chemistry. Tetrahedron Lett. 2012, 53, 1987–1989. [Google Scholar] [CrossRef]
- Mayaux, J.F.; Bousseau, A.; Pauwels, R.; Huet, T.; Henin, Y.; Dereu, N.; Evers, M.; Soler, F.; Poujade, C.; De Clercq, E. Triterpene derivatives that block entry of human immunodeficiency virus type 1 into cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3564–3568. [Google Scholar] [CrossRef]
- Dang, Z.; Qian, K.; Ho, P.; Zhu, L.; Lee, K.-H.; Huang, L.; Chen, C.-H. Synthesis of betulinic acid derivatives as entry inhibitors against HIV-1 and bevirimat resistant HIV-1 variants. Bioorg. Med. Chem. Lett. 2012, 22, 5190–5194. [Google Scholar] [CrossRef]
- Liu, Y.; Ke, Z.; Wu, K.Y.; Liu, S.; Chen, W.; Jiang, S.; Jiang, Z. An amphiphilic conjugate approach toward the design and synthesis of betulinic acidepolyphenol conjugates as inhibitors of the HIV-1 gp41 fusion core formation. ChemMedChem 2011, 6, 1654–1664. [Google Scholar] [CrossRef]
- Marciniec, K.; Chrobak, E.; Dąbrowska, A.; Bębenek, E.; Kadela-Tomanek, M.; Pęcak, P.; Boryczka, S. Phosphate derivatives of 3-carboxyacylbetulin: Synthesis, in vitro anti-HIV and molecular docking study. Biomolecules 2020, 10, 1148–1166. [Google Scholar] [CrossRef] [PubMed]
- Grishko, V.V.; Galaiko, N.V.; Tolmacheva, I.A.; Kucherov, I.I.; Eremin, V.F.; Boreko, E.I.; Savinova, O.V.; Slepukhin, P.A. Functionalization, cyclization and antiviral activity of A-secotriterpenoids. Eur. J. Med. Chem. 2014, 83, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.-H.; Song, J.H.; Kang, K.B.; Sung, S.H.; Ko, H.-J.; Yang, H. Anti-influenza activity of betulinic acid from Zizyphus jujuba on influenza A/PR/8 virus. Biomol. Ther. 2015, 23, 345–349. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, R.; Ooi, L.S.M.; But, P.P.H.; Ooi, V.E.C. Antiviral triterpenoids from the medicinal plant Schefflera heptaphylla. Phytother. Res. 2007, 21, 466–470. [Google Scholar] [CrossRef]
- Tung, N.H.; Kwon, H.-J.; Kim, J.-H.; Ra, J.C.; Kim, J.A.; Kim, Y.H. An anti-influenza component of the bark of Alnus Japonica. Arch. Pharm. Res. 2010, 33, 363–367. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Medvedeva, N.I.; Baikova, I.P.; Tolstikov, G.A.; Lopatina, T.V.; Yunusov, M.S.; Zaprutko, L. Synthesis of triterpenoid acylates—An effective reproduction inhibitors of influenza A (H1N1) and papilloma viruses. Russ. J. Bioorg. Chem. 2010, 36, 771–778. [Google Scholar] [CrossRef]
- Han, X.; Shi, Y.; Si, L.; Fan, Z.; Wang, H.; Xu, R.; Jiao, P.; Meng, K.; Tian, Z.; Zhou, X.; et al. Design, synthesis and biological activity evaluation of novel conjugated sialic acid and pentacyclic triterpene derivatives as anti-influenza entry inhibitors. Med. Chem. Commun. 2016, 7, 1932–1945. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Shi, Y.; Si, L.; Jiao, P.; Fan, Z.; Han, X.; Wu, X.; Zhou, X.; Yu, F.; et al. Design, synthesis and biological evaluation of novel l-ascorbic acid-conjugated pentacyclic triterpene derivatives as potential influenza virus entry inhibitors. Eur. J. Med. Chem. 2016, 110, 376–388. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Xu, R.; Wang, S.; Zhang, Y.; Zhang, L.; Zhou, D.; Xiao, S. Synthesis, structure activity relationship and in vitro anti-influenza virus activity of novel polyphenol-pentacyclic triterpene conjugates. Eur. J. Med. Chem. 2019, 163, 560–568. [Google Scholar] [CrossRef]
- Wen, C.-C.; Kuo, Y.-H.; Jan, J.-T.; Liang, P.-H.; Wang, S.-Y.; Liu, H.-G.; Lee, C.-K.; Chang, S.-T.; Kuo, C.-J.; Lee, S.-S.; et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef]
- Ye, W.C.; Ji, N.N.; Zhao, S.X.; Liu, J.H.; Ye, T.; McKervey, M.A.; Stevenson, P. Triterpenoids from Pulsatilla chinensis. Phytochemistry 1996, 42, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Li, H.; Gou, Y.; Zhang, H.; Vlessidis, A.G.; Zhou, H.; Evmiridis, N.P.; Liu, Z. Betulinic acid-mediated inhibitory effect on hepatitis B virus by suppression of manganese superoxide dismutase expression. FEBS J. 2009, 276, 2599–2614. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Herpes Simplex Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 30 May 2025).
- Gong, Y.; Raj, K.; Luscombe, C.; Gadawski, I.; Tam, T.; Chu, J.; Gibson, D.; Carlson, R.; Sacks, S. The synergistic effects of betulin with acyclovir against herpes simplex viruses. Antiviral Res. 2004, 64, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Heidary Navid, M.; Laszczyk-Lauer, M.N.; Reichling, J.; Schnitzler, P. Pentacyclic triterpenes in birch bark extract inhibit early step of herpes simplex virus type 1 replication. Phytomedicine 2014, 21, 1273–1280. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, F.; Jin, Q.; Li, X.; Zhan, Q.; Chen, M.; Wang, S.; Wu, Z.; Ye, W.; Wang, L. Discovery of unusual phloroglucinol–triterpenoid adducts from Leptospermum scoparium and Xanthostemon chrysanthus by building blocks-based molecular networking. Chin. Chem. Lett. 2024, 35, 108881. [Google Scholar] [CrossRef]
- Song, B.-H.; Yun, S.-I.; Woolley, M.; Lee, Y.-M. Zika virus: History, epidemiology, transmission, and clinical presentation. J. Neuroimmunol. 2017, 308, 50–64. [Google Scholar] [CrossRef]
- Do Rosário, M.S.; De Jesus, P.A.P.; Vasilakis, N.; Farias, D.S.; Novaes, M.A.C.; Rodrigues, S.G.; Martins, L.C.; Vasconcelos, P.F.D.C.; Ko, A.I.; Alcântara, L.C.J.; et al. Guillain–Barre’syndrome after Zika virus infection in Brazil. Am. J. Trop. Med. Hyg. 2016, 95, 1157–1160. [Google Scholar] [CrossRef]
- Li, H.; Saucedo-Cuevas, L.; Regla-Nava, J.A.; Chai, G.; Sheets, N.; Tang, W.; Terskikh, A.V.; Shresta, S.; Gleeson, J.G. Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell 2016, 19, 593–598. [Google Scholar] [CrossRef]
- Navabi, S.P.; Sarkaki, A.; Mansouri, E.; Badavi, M.; Ghadiri, A.; Farbood, Y. The effects of betulinic acid on neurobehavioral activity, electrophysiology and histological changes in an animal model of the Alzheimer’s disease. Behav. Brain Res. 2018, 337, 99–106. [Google Scholar] [CrossRef]
- Cavalcante, B.R.R.; Aragão-França, L.S.; Sampaio, G.L.A.; Nonaka, C.K.V.; Oliveira, M.S.; Campos, G.S.; Sardi, S.I.; Dias, B.R.S.; Menezes, J.P.B.; Rocha, V.P.C.; et al. Betulinic acid exerts cytoprotective activity on Zika virus-infected neural progenitor cells. Front. Cell. Infect. Microbiol. 2020, 10, 558324. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 19 September 2024).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Sahin, O.; Grover, M.; Zhang, Q. New and alternative strategies for the prevention, control, and treatment of antibiotic resistant. Transl. Res. 2020, 223, 76–88. [Google Scholar] [CrossRef]
- Penesyan, A.; Nagy, S.S.; Kjelleberg, S.; Gillings, M.R.; Paulsen, I.T. Rapid microevolution of biofilm cells in response to antibiotics. NPJ Biofilms Microbiomes 2019, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.; Chung, L.Y.; Navaratnam, P. Potential targets by pentacyclic triterpe-noids from Callicarpa farinosa against methicillin-resistant and sensitive Staphylococcus aureus. Fitoterapia 2014, 94, 48–54. [Google Scholar] [CrossRef]
- Bębenek, E.; Jastrzębska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel triazole hybrids of betulin: Synthesis and biological activity profile. Molecules 2017, 22, 1876. [Google Scholar] [CrossRef]
- Murlykina, M.; Pavlovska, T.; Semenenko, O.; Kolomiets, O.; Sanin, E.; Morozova, A.; Kornet, M.; Musatov, V.; Kulyk, K.; Mazepa, A.; et al. Effective and versatile construction of hybrid-molecules combining natural betulonic acid core and synthetic heterocycle-containing peptidomimetic fragments and their microbiological properties. ChemistrySelect 2023, 8, e202301250. [Google Scholar] [CrossRef]
- World Health Organization. Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 15 March 2025).
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-Q.; Wang, Y.; Franzblau, S.G.; Montenegro, G.; Yang, D.; Timmermann, B.N. Antitubercular constituents of Valeriana laxiflora. Planta Med. 2004, 70, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, S.; Panseeta, P.; Kunchanawatta, S.; Distaporn, T.; Ruktasing, S.; Suksamrarn, A. Ceanothane- and lupane-type triterpenes with antiplasmodial and antimycobacterial activities with antiplasmodial and from Ziziphus cambodiana. Chem. Pharm. Bull. 2006, 54, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Tanachatchairatana, T.; Bremner, J.B.; Chokchaisiri, R.; Suksamrarn, A. Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic and ursolic acids. Chem. Pharm. Bull. 2008, 56, 194–198. [Google Scholar] [CrossRef]
- Jiménez-Arellanes, A.; Meckes, M.; Torres, J.; Luna-Herrera, J. Antimycobacterial triterpenoids from Lantana hispida (Verbenaceae). J. Ethnopharmacol. 2007, 111, 202–205. [Google Scholar] [CrossRef]
- Akihisa, T.; Franzblau, S.G.; Ukiya, M.; Okuda, H.; Zhang, F.; Yasukawa, K.; Suzuki, T.; Kimura, Y. Antitubercular activity of triterpenoids from Asteraceae flowers. Biol. Pharm. Bull. 2005, 28, 158–160. [Google Scholar] [CrossRef]
- Medvedeva, N.I.; Kazakova, O.B.; Lopatina, T.V.; Smirnova, I.E.; Giniyatullina, G.V.; Baikova, I.P.; Kataev, V.E. Synthesis and antimycobacterial activity of triterpenic A-ring azepanes. Eur. J. Med. Chem. 2018, 143, 464–472. [Google Scholar] [CrossRef]
- Innocente, A.; Casanova, B.B.; Klein, F.; Lana, A.D.; Pereira, D.; Muniz, M.N.; Sonnet, P.; Gosmann, G.; Fuentefria, A.M.; Gnoatto, S.C.B. Synthesis of isosteric triterpenoid derivatives and antifungal activity. Chem. Biol. Drug Des. 2014, 83, 344–349. [Google Scholar] [CrossRef]
- Saccoliti, F.; Di Santo, R.; Costi, R. Recent advancement in the search of innovative antiprotozoal agents targeting trypanothione metabolism. ChemMedChem 2020, 15, 2420–2435. [Google Scholar] [CrossRef]
- Sarma, N.; Patouillard, E.; Cibulskis, R.E.; Arcand, J.-L. The economic burden of malaria: Revisiting the evidence. Am. J. Trop. Med. Hyg. 2019, 101, 1405–1415. [Google Scholar] [CrossRef]
- World Health Organization. Malaria. Available online: https://www.who.int/health-topics/malaria#tab=tab_1 (accessed on 11 December 2024).
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 12 January 2023).
- World Health Organization. Human African Trypanosomiasis (Sleeping Sickness). Available online: https://www.who.int/health-topics/human-african-trypanosomiasis#tab=tab_1. (accessed on 2 May 2023).
- Cunha, A.B.; Batista, R.; Castro, M.Á.; David, J.M. Chemical strategies towards the synthesis of betulinic acid and its more potent antiprotozoal analogues. Molecules 2021, 26, 1081. [Google Scholar] [CrossRef]
- Ziegler, H.L.; Franzyk, H.; Sairafianpour, M.; Tabatabai, M.; Tehrani, M.D.; Bagherzadeh, K.; Hägerstrand, H.; Stærk, D.; Jaroszewski, J.W. Erythrocyte membrane modifying agents and the inhibition of Plasmodium falciparum growth: Structure-activity relationships for betulinic acid analogues. Bioorg. Med. Chem. 2004, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.N.; Maria, N.R.; Schuck, D.C.; Cruz, L.N.; De Moraes, M.S.; Nakabashi, M.; Graebin, C.; Gosmann, G.; Garcia, C.R.; Gnoatto, S.C. Two series of new semisynthetic triterpene derivatives: Differences in anti-malarial activity, cytotoxicity and mechanism of action. Malar. J. 2013, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Cargnin, S.T.; Staudt, A.F.; Medeiros, P.; De Medeiros Sol Sol, D.; De Azevedo Dos Santos, A.P.; Zanchi, F.B.; Gosmann, G.; Puyet, A.; Garcia Teles, C.B.; Gnoatto, S.B. Semisynthesis, cytotoxicity, antimalarial evaluation and structure-activity relationship of two series of triterpene derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Innocente, A.M.; Silva, G.N.S.; Cruz, L.N.; Moraes, M.S.; Nakabashi, M.; Sonnet, P.; Gosmann, G.; Garcia, C.R.S.; Gnoatto, S.C.B. Synthesis and antiplasmodial activity of betulinic acid and ursolic acid analogues. Molecules 2012, 17, 12003–12014. [Google Scholar] [CrossRef] [PubMed]
- De Silva, G.N.S.; Schuck, D.C.; Cruz, L.N.; Moraes, M.S.; Nakabashi, M.; Gosmann, G.; Garcia, C.R.S.; Gnoatto, S.C.B. Investigation of antimalarial activity, cytotoxicity and action mechanism of piperazine derivatives of betulinic acid. Trop. Med. Int. Health 2015, 20, 29–39. [Google Scholar] [CrossRef]
- Ullah, A.; Baratto, L.C.; Paula, R.C.; Silva, L.H.V.; Soares, M.J.; Oliveira, B.H. Preparation of derivatives of betulinic acid, steviol and isosteviol and evaluation of antitrypanosomal and antimalarial activities. J. Braz. Chem. Soc. 2016, 27, 1245–1253. [Google Scholar] [CrossRef]
- Karagöz, A.Ç.; Leidenberger, M.; Hahn, F.; Hampel, F.; Friedrich, O.; Marschall, M.; Kappes, B.; Tsogoeva, S.B. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorg. Med. Chem. 2019, 27, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Carmona, D.B.; Escalante-Erosa, F.; García-Sosa, K.; Ruiz-Pinell, G.; Gutierrez-Yapu, D.; Chan-Bacab, M.J.; Giménez-Turba, A.; Peña-Rodríguez, L.M. Antiprotozoal activity of betulinic acid derivatives. Phytomedicine 2010, 17, 379–382. [Google Scholar] [CrossRef]
- Haavikko, R.; Nasereddin, A.; Sacerdoti-Sierra, N.; Kopelyanskiy, D.; Alakurtti, S.; Tikka, M.; Jaffe, C.L.; Yli-Kauhaluoma, J. Heterocycle-fused lupane triterpenoids inhibit Leishmania donovani amastigotes. Med. Chem. Commun. 2014, 5, 445–451. [Google Scholar] [CrossRef]
- Sousa, M.C.; Varandas, R.; Santos, R.C.; Santos-Rosa, M.; Alves, V.; Salvador, J.A.R. Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: Synergistic effects with miltefosine. PLoS ONE 2014, 9, e89939. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.P.A.; Staudt, A.F.; Teles, C.B.G.; Gosmann, G.; Gnoatto, S.B.; Cargnin, S.T. Effective approach to semi-synthesis of lupane and ursane brominated derivatives and its effects on viability of Leishmania amazonensis. Ann. Med. Chem. Res. 2017, 3, 1020. [Google Scholar]
- Hoet, S.; Pieters, L.; Muccioli, G.G.; Habib-Jiwan, J.-L.; Opperdoes, F.R.; Quetin-Leclercq, J. Antitrypanosomal activity of triterpenoids and sterols from the leaves of Strychnos spinosa and related compounds. J. Nat. Prod. 2007, 70, 1360–1363. [Google Scholar] [CrossRef]
- Meira, C.S.; Barbosa-Filho, J.M.; Lanfredi-Rangel, A.; Guimarães, E.T.; Moreira, D.R.M.; Soares, M.B.P. Antiparasitic evaluation of betulinic acid derivatives reveals effective and selective anti-Trypanosoma cruzi inhibitors. Exp. Parasitol. 2016, 166, 108–115. [Google Scholar] [CrossRef]
- World Health Organization. Schistosomiasis. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 1 February 2023).
- Spivak, A.Y.; Keiser, J.; Vargas, M.; Gubaidullin, R.R.; Nedopekina, D.A.; Shakurova, E.R.; Khalitova, R.R.; Odinokov, V.N. Synthesis and activity of new triphenylphosphonium derivatives of betulin and betulinic acid against Schistosoma manson in vitro and in vivo. Bioorg. Med. Chem. 2014, 22, 6297–6304. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [CrossRef]
- Wardecki, T.; Werner, P.; Thomas, M.; Templin, M.F.; Schmidt, G.; Brandner, J.M.; Merfort, I. Influence of birch bark triterpenes on keratinocytes and fibroblasts from diabetic and nondiabetic donors. J. Nat. Prod. 2016, 79, 1112–1123. [Google Scholar] [CrossRef]
- De Melo, C.L.; Queiroz, M.G.R.; Arruda Filho, A.C.V.; Rodrigues, A.M.; De Sousa, D.F.; Almeida, J.G.L.; Pessoa, O.D.L.; Silveira, E.R.; Menezes, D.B.; Melo, T.S.; et al. Betulinic acid, a natural pentacyclic triterpenoid, prevents abdominal fat accumulation in mice fed a high-fat diet. J. Agric. Food Chem. 2009, 57, 8776–8781. [Google Scholar] [CrossRef]
- Yoon, J.J.; Lee, Y.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Betulinic acid inhibits high glucose-induced vascular smooth muscle cells proliferation and migration. J. Cell. Biochem. 2010, 111, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp-Fenske, K.; Bollinger, L.; Xu, H.; Yao, Y.; Horke, S.; Förstermann, U.; Li, H. Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. J. Pharmacol. Exp. Ther. 2007, 322, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Vergoten, G.; Bailly, C. In silico analysis of the antidiabetic terpenoid acankoreagenin binding to PPARγ. Silico Pharmacol. 2021, 9, 32–39. [Google Scholar] [CrossRef]
- Al-Mutary Mohsen, G.; Abu-Taweel, G.M.; Rajagopal, R.; Sun-Ju, K.; Kim, H.-J.; Kim, Y.O.; Mothana, R.A.; Kadaikunnan, S.; Khaled, J.M.; Siddiqui, N.A.; et al. Betulinic acid lowers lipid accumulation in adipocytes through enhanced NCoA1–PPAR interaction. J. Infect. Public Health 2019, 12, 726–732. [Google Scholar] [CrossRef]
- Ríos, J.; Máñez, S. New pharmacological opportunities for betulinic acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef]
- Lu, M.-X.; Yang, Y.; Zou, Q.-P.; Luo, J.; Zhang, B.-B.; Liu, X.-Q.; Hwang, E.-H. Anti-diabetic efects of acankoreagenin from the leaves of Acanthopanax Gracilistylus herb in RIN-m5F cells via suppression of NF-kB activation. Molecules 2018, 23, 958. [Google Scholar] [CrossRef]
- Tang, J.-J.; Li, J.-G.; Qi, W.; Qiu, W.-W.; Li, P.-S.; Li, B.-L.; Song, B.-L. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 2011, 13, 44–56. [Google Scholar] [CrossRef]
- Szuster-Ciesielska, A.; Plewka, K.; Daniluk, J.; Kandefer-Szerszeń, M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF alpha, TGF-beta) production and by influencing intracellular signaling. Toxicology 2011, 280, 152–163. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Sharma, M.C.; Dobhal, M.P.; Gupta, R.S. Evaluation of antidiabetic and antioxidant potential of lupeol in experimental hyperglycaemia. Nat. Prod. Res. 2012, 26, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Genet, C.; Strehle, A.; Schmidt, C.; Boudjelal, G.; Lobstein, A.; Schoonjans, K.; Souchet, M.; Auwerx, J.; Saladin, R.; Wagner, A. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: Potential impact in diabetes. J. Med. Chem. 2010, 53, 178–190. [Google Scholar] [CrossRef]
- Sato, H.; Macchiarulo, A.; Thomas, C.; Gioiello, A.; Une, M.; Hofmann, A.F.; Saladin, R.; Schoonjans, K.; Pellicciari, R.; Auwerx, J. Novel potent and selective bile acid derivatives as TGR5 agonists: Biological screening, structure-activity relationships, and molecular modeling studies. J. Med. Chem. 2008, 51, 1831–1841. [Google Scholar] [CrossRef]
- Denner, T.-C.; Heise, N.V.; Zacharias, J.; Csuk, R. Lupane acetates in small molecule drug hybrids: Probing their inhibitory activity for carbonic anhydrase II. Eur. J Med. Chem. Rep. 2024, 10, 100139. [Google Scholar] [CrossRef]
- Yang, D.-K.; Huang, X.-Y.; Zhu, Y.-M. Bistable cholesteric reflective displays: Materials and drive schemes. Annu. Rev. Mater. Sci. 1997, 27, 117–146. [Google Scholar] [CrossRef]
- Bauer, M.; Boeffel, C.; Kuschel, F.; Zaschke, H. Evaluation of chiral dopants for LCD applications. J. Soc. Inf. Disp. 2006, 14, 805–812. [Google Scholar] [CrossRef]
- Eelkema, R.; Feringa, B.L. Amplification of chirality in liquid crystals. Org. Biomol. Chem. 2006, 4, 3729–3745. [Google Scholar] [CrossRef] [PubMed]
- Pieraccini, S.; Masiero, S.; Ferrarini, A.; Spada, G.P. Chirality transfer across length-scales in nematic liquid crystals: Fundamentals and applications. Chem. Soc. Rev. 2011, 40, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.; Akagi, K. Powerful helicity inducers: Axially chiral binaphthyl derivatives. Liq. Cryst. 2008, 35, 953–965. [Google Scholar] [CrossRef]
- Yokokoji, O.; Oiwa, M.; Koike, T.; Inoue, S. Synthesis of new chiral compounds for cholesteric liquid crystal display. Liq. Cryst. 2008, 35, 995–1003. [Google Scholar] [CrossRef]
- Babak, N.L.; Gella, I.M.; Semenenko, A.N.; Shishkina, S.V.; Shishkin, O.V.; Musatov, V.I.; Lipson, V.V. α,β-Unsaturated ketones based on allobetulon. Russ. J. Org. Chem. 2014, 50, 1048–1055. [Google Scholar] [CrossRef]
- Babak, N.L.; Semenenko, A.N.; Gella, I.M.; Musatov, V.I.; Shishkina, S.V.; Novikova, N.B.; Sofronov, D.S.; Morina, D.A.; Lipson, V.V. Synthesis of pyrrol-2-yl- and pyrazol-4-ylmethylidene derivatives of betulin and allobetuline. Russ. J. Org. Chem. 2015, 51, 715–726. [Google Scholar] [CrossRef]
- Semenenko, A.N.; Babak, N.L.; Gella, I.M.; Musatov, V.I.; Shishkina, S.V.; Sofronov, D.S.; Lipson, V.V. Cyclopropanation and epoxidation of 2-ylidene derivatives of lupane series. Russ. J. Org. Chem. 2017, 53, 282–289. [Google Scholar] [CrossRef]
- Semenenko, A.N.; Babak, N.L.; Shishkina, S.V.; Musatov, V.I.; Mazepa, A.V.; Lipson, V.V. Synthesis, molecular and crystal structure of spirocyclopropyl derivatives of lupane series and their ability to induce cholesteric mesophase in nematic solvents. J. Mol. Struct. 2018, 1171, 605–613. [Google Scholar] [CrossRef]
- Babak, N.L.; Shishkin, O.V.; Shishkina, S.V.; Gella, I.M.; Musatov, V.I.; Novikova, N.B.; Lipson, V.V. Synthesis and spatial structure of new chiral dopants from allobetulin series for cholesteric liquid-crystal compositions. Struct. Chem. 2016, 27, 295–303. [Google Scholar] [CrossRef]
- Ramírez-López, P.; Torre, M.C.D.L.; Asenjo, M.; Ramírez-Castellanos, J.; González-Calbet, J.M.; Rodríguez-Gimeno, A.; Arellano, C.R.D.; Sierra, M.A.A. A new family of clicked estradiol-based low-molecular-weight gelators having highly symmetry-dependent gelation ability. Chem. Commun. 2011, 47, 10281–10283. [Google Scholar] [CrossRef] [PubMed]
- Panja, A.; Ghosh, S.; Ghosh, K. A sulfonyl hydrazone cholesterol conjugate: Gelation, anion interaction and its application in dye adsorption. New J. Chem. 2019, 43, 10270–10277. [Google Scholar] [CrossRef]
- Ghosh, K.; Panja, A.; Panja, S. Cholesterol appended bis-1,2,3-triazoles as simple supramolecular gelators for the naked eye detection of Ag+, Cu2+ and Hg2+ ions. New J. Chem. 2016, 40, 3476–3483. [Google Scholar] [CrossRef]
- Bag, B.G.; Maity, G.C.; Pramanik, S.R. A terpenoid-based gelators: The first arjunolic acid derived organogelator for alcohols and mixed solvents. Supramol. Chem. 2005, 17, 383–385. [Google Scholar] [CrossRef]
- Bag, B.G.; Maity, G.C.; Dinda, S.K. Donor-acceptor interaction promoted gelation: Visual observation of color change. Org. Lett. 2006, 8, 5457–5461. [Google Scholar] [CrossRef]
- Bag, B.G.; Dinda, S.K.; Dey, P.P.; Mallia, V.A.; Weiss, R.G. Self-Assembly of esters of arjunolic acid into fibrous networks and the properties of their organogels. Langmuir 2009, 25, 8663–8671. [Google Scholar] [CrossRef]
- Lu, J.; Hu, J.; Song, Y.; Ju, Y. A new dual-responsive organogel based on uracil-appended glycyrrhetinic acid. Org. Lett. 2011, 13, 3372–3375. [Google Scholar] [CrossRef]
- Gao, A.; Li, Y.; Lv, H.; Liu, D.; Zhao, N.; Ding, Q.; Cao, X. Melamine tunable effect in a lenalidomide-based supramolecular self-assembly system via hydrogen bonding. New J. Chem. 2017, 41, 7924–7931. [Google Scholar] [CrossRef]
- Zhikol, O.A.; Shishkina, S.V.; Lipson, V.V.; Semenenko, A.N.; Mazepa, A.V.; Borisov, A.V.; Mateychenko, P.V. Low molecular weight supramolecular dehydroepiandrosterone-based gelators: Synthesis and molecular modeling study. New J. Chem. 2019, 43, 13112–13121. [Google Scholar] [CrossRef]
- Lipson, V.; Zhikol, O.; Shishkina, S.; Semenenko, A.; Kulyk, K.; Mateychenko, P.; Musatov, V.; Mazepa, A.; Vakula, V.; Borisov, A.; et al. Low molecular weight supramolecular allobetuline-, cyclohexanol-, or undecanol-appended 1,2,3-triazole-based gelators: Synthesis and molecular dynamics simulation study. SynOpen 2023, 07, 694–702. [Google Scholar] [CrossRef]




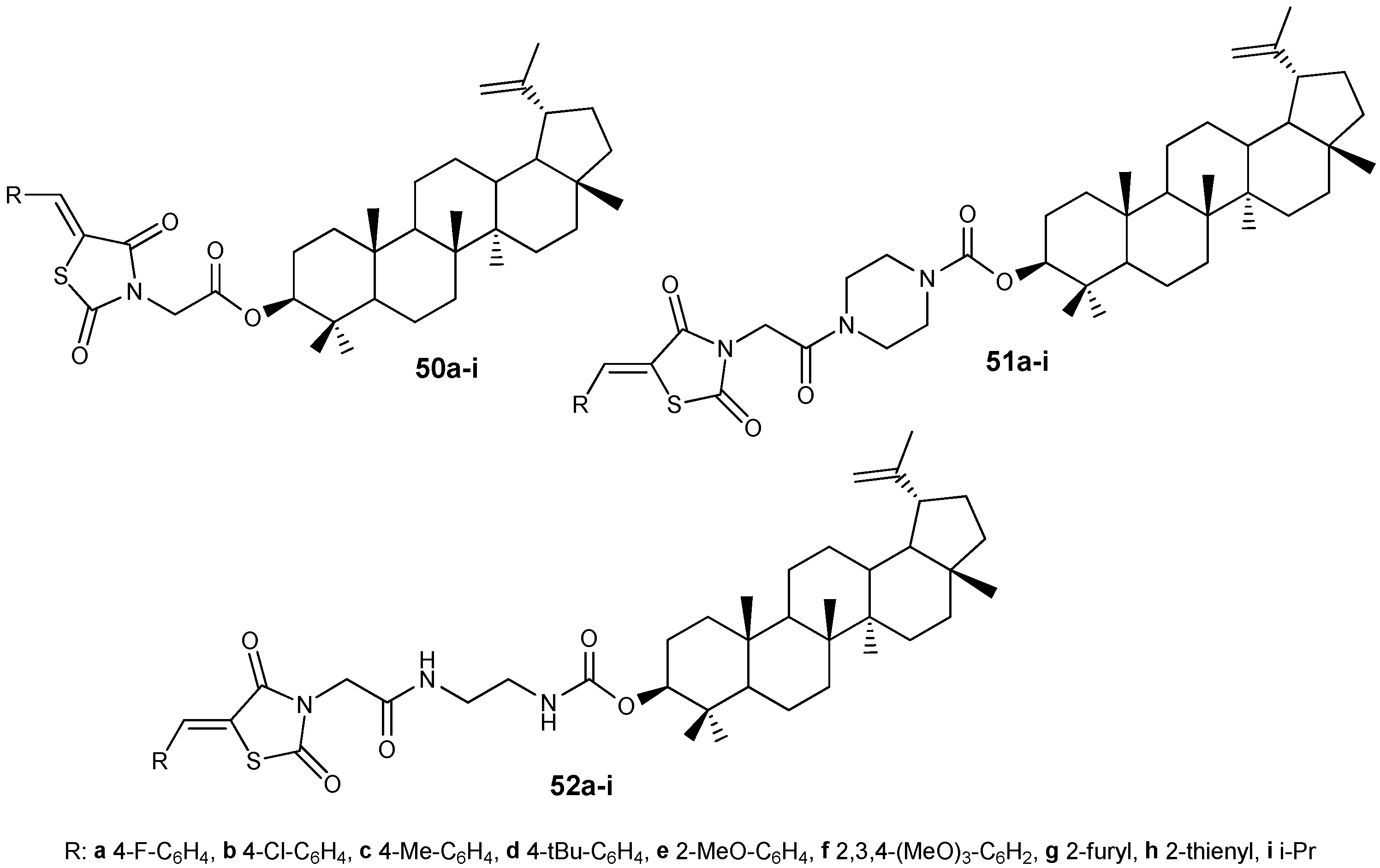
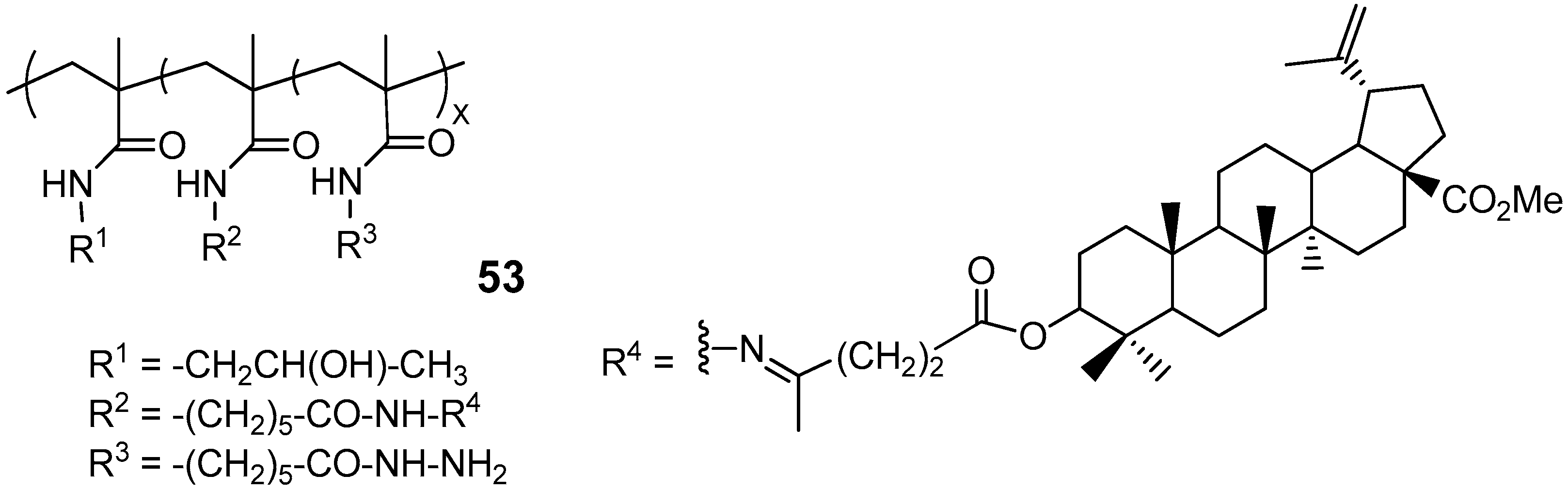

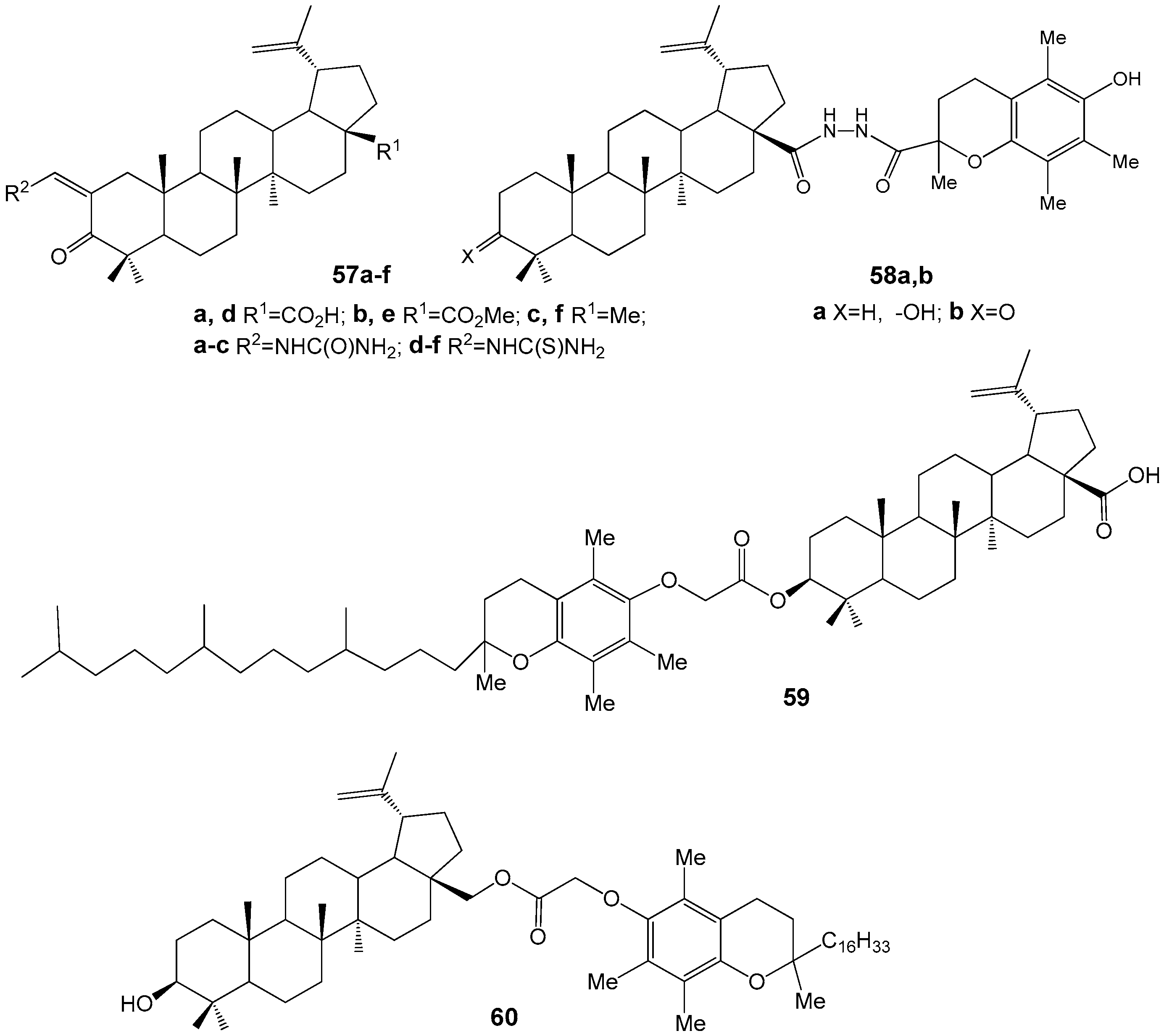
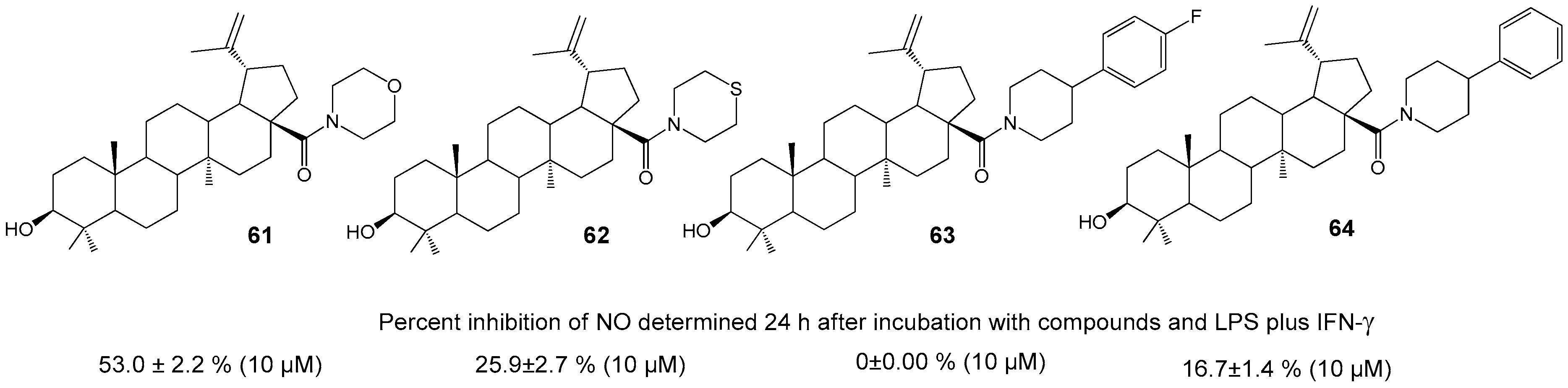

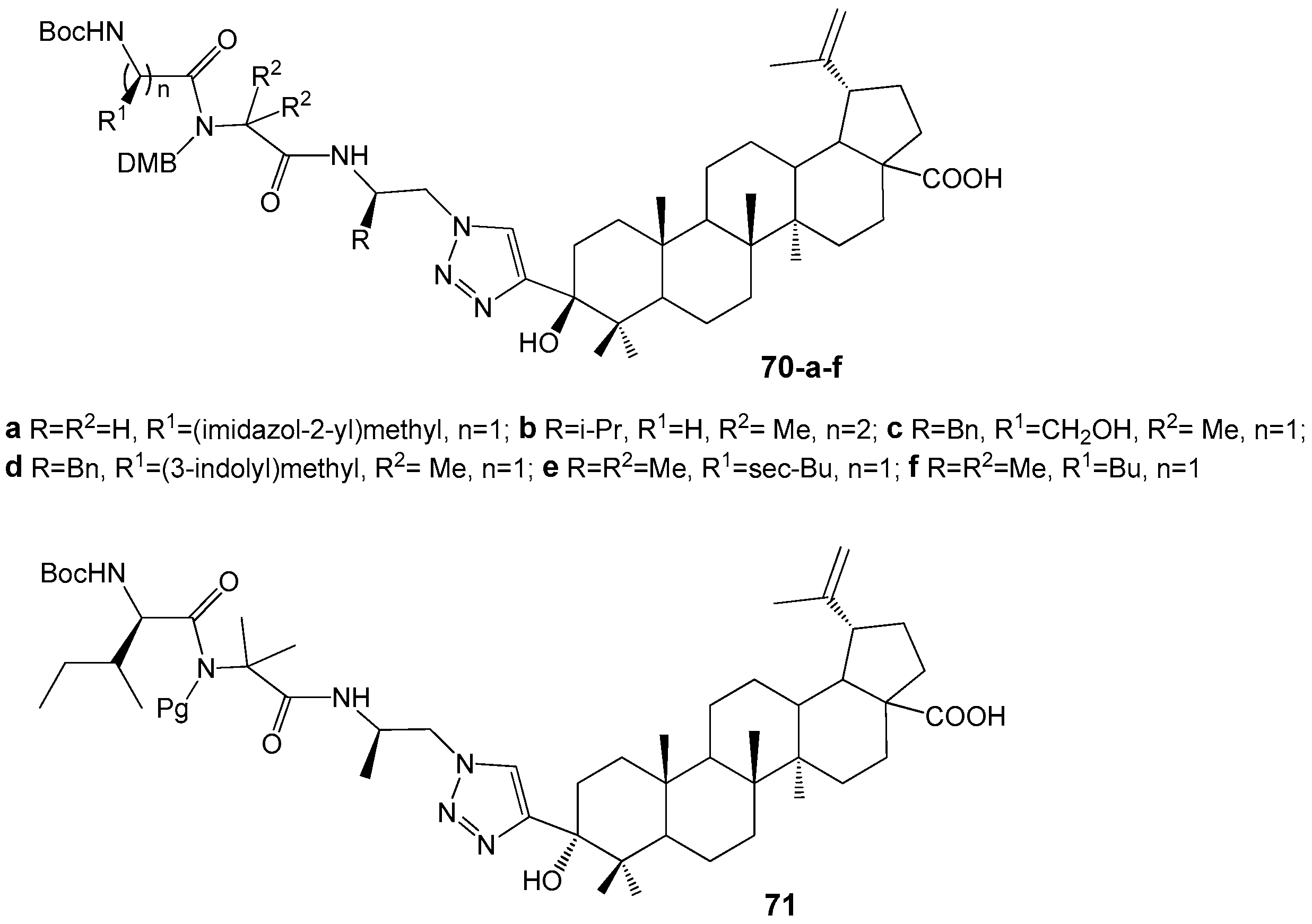
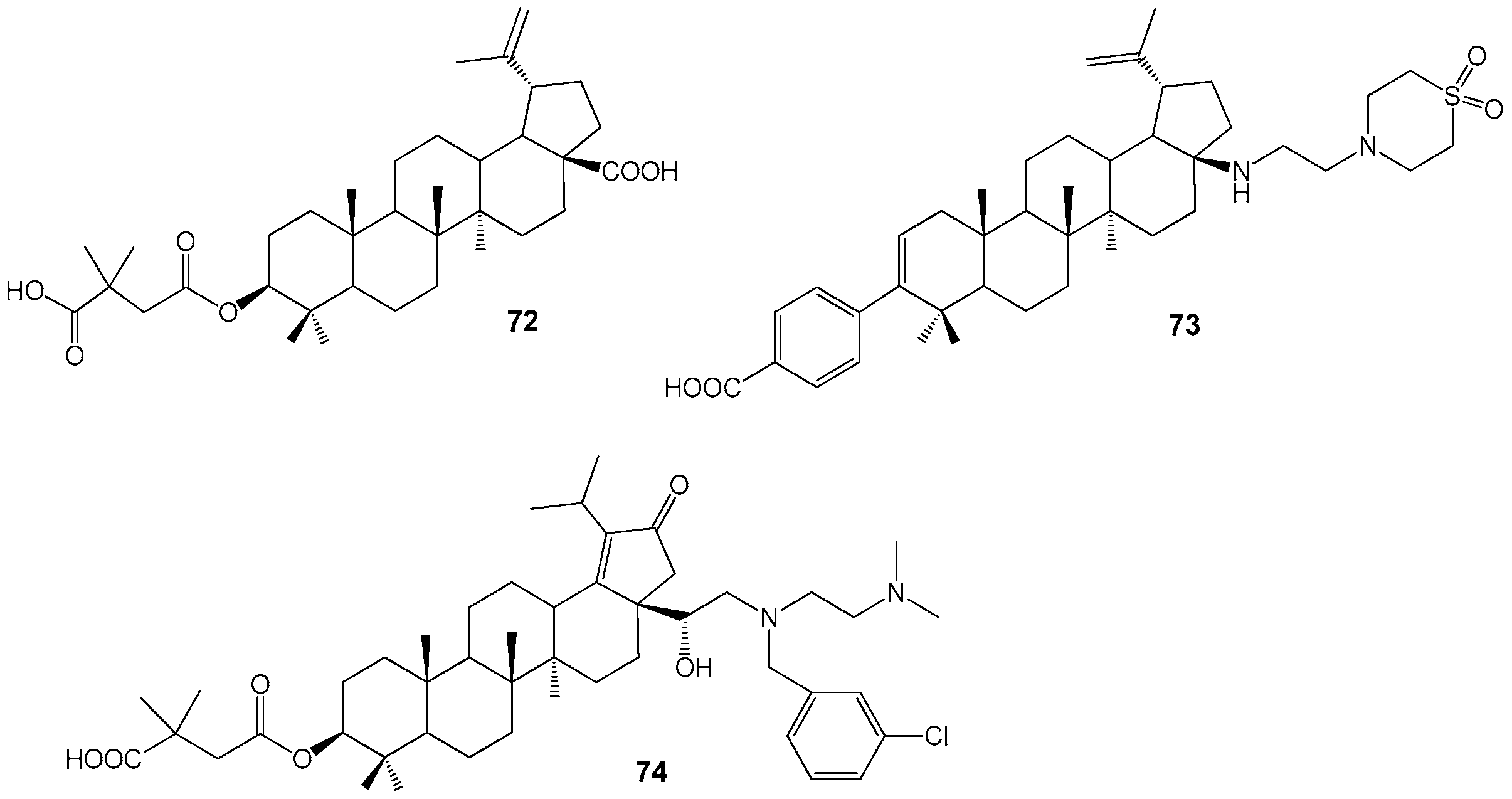
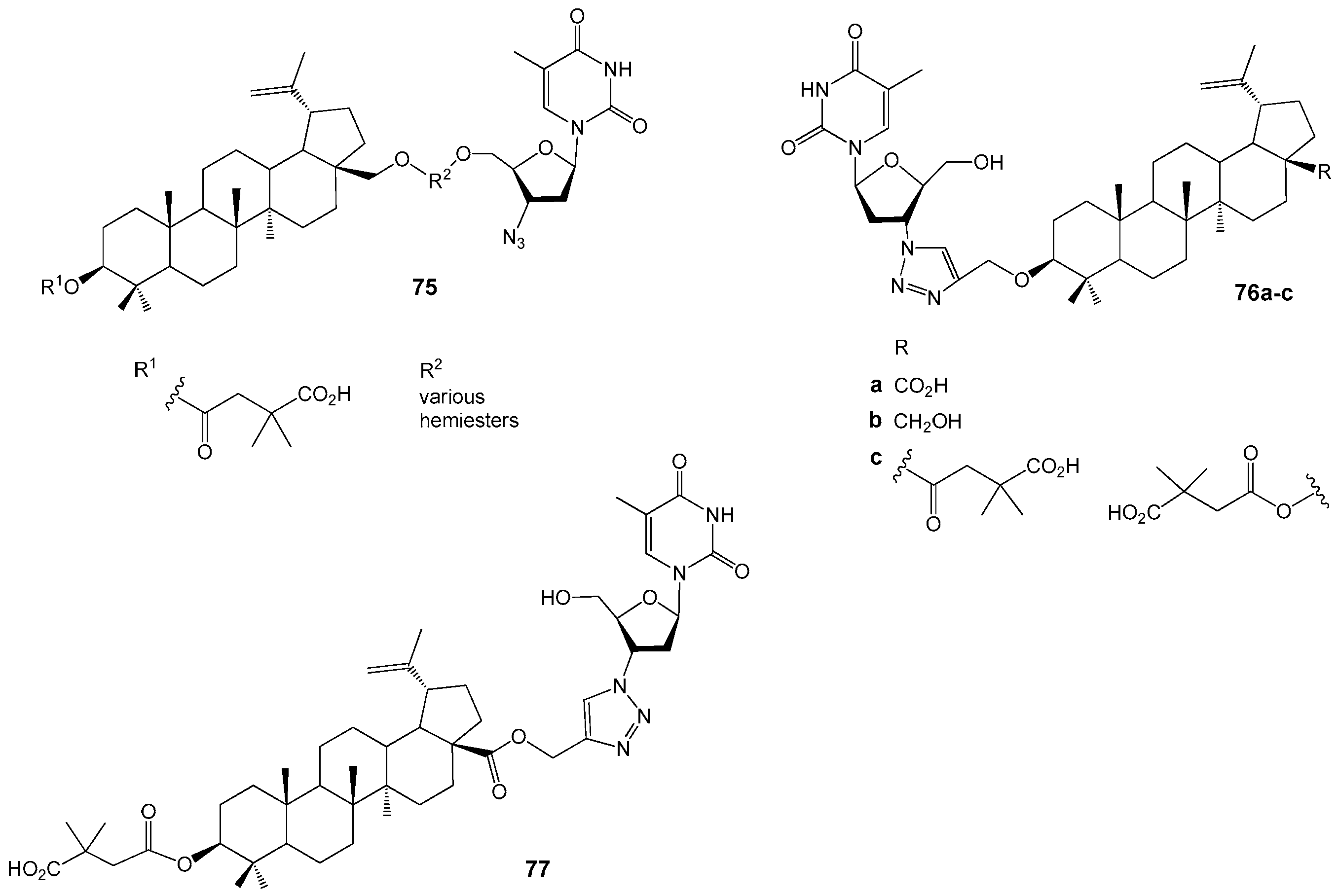
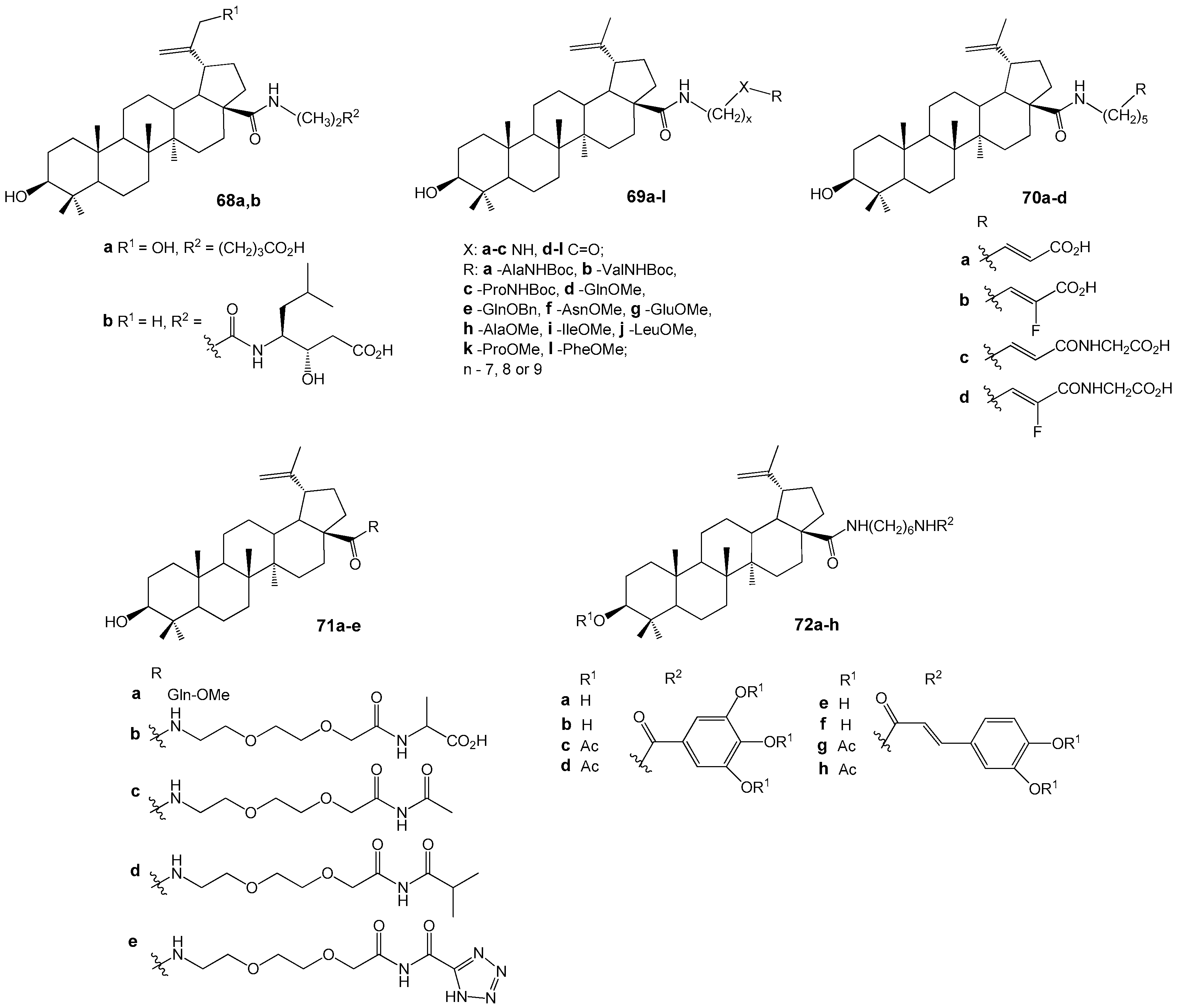



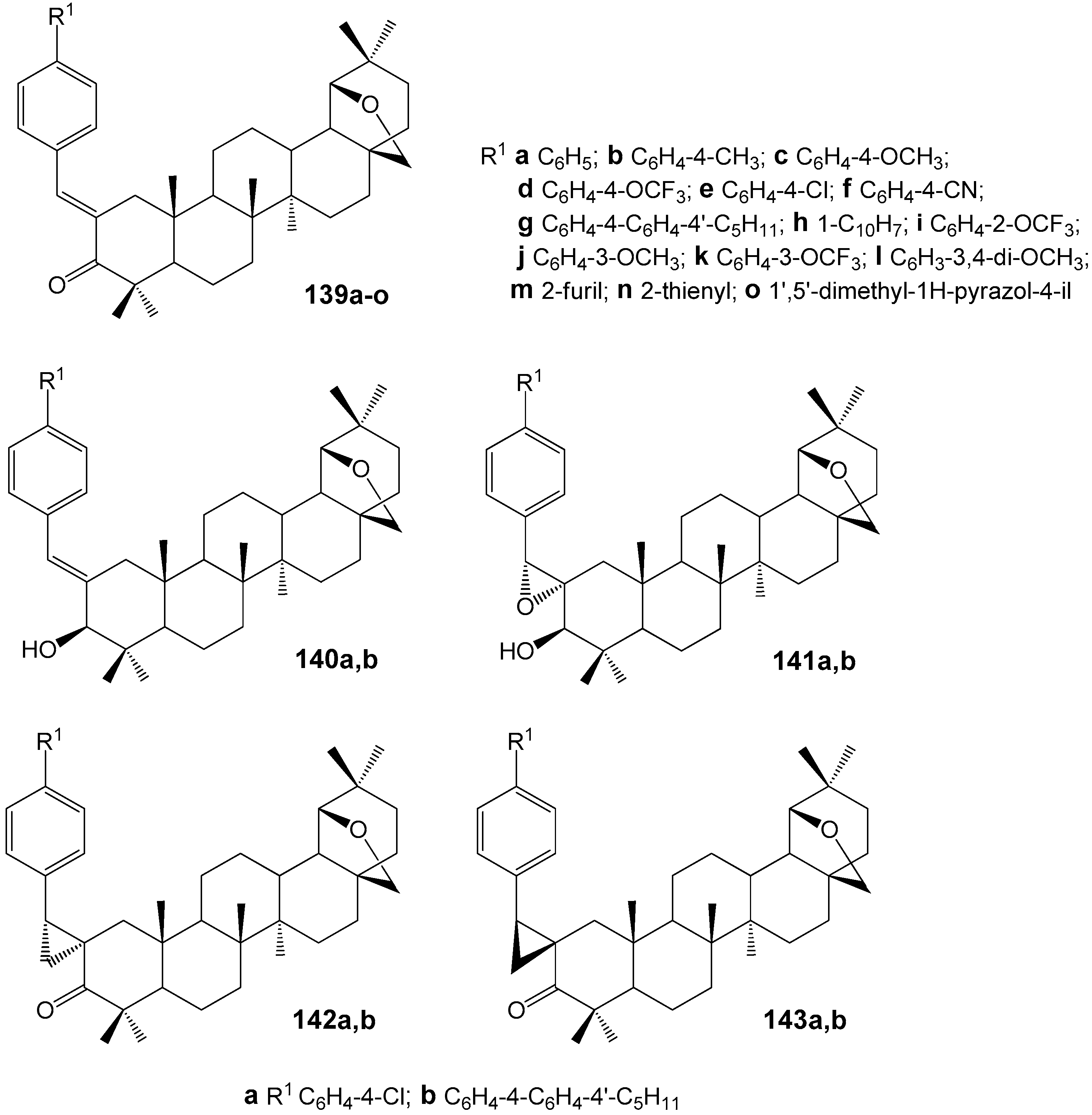
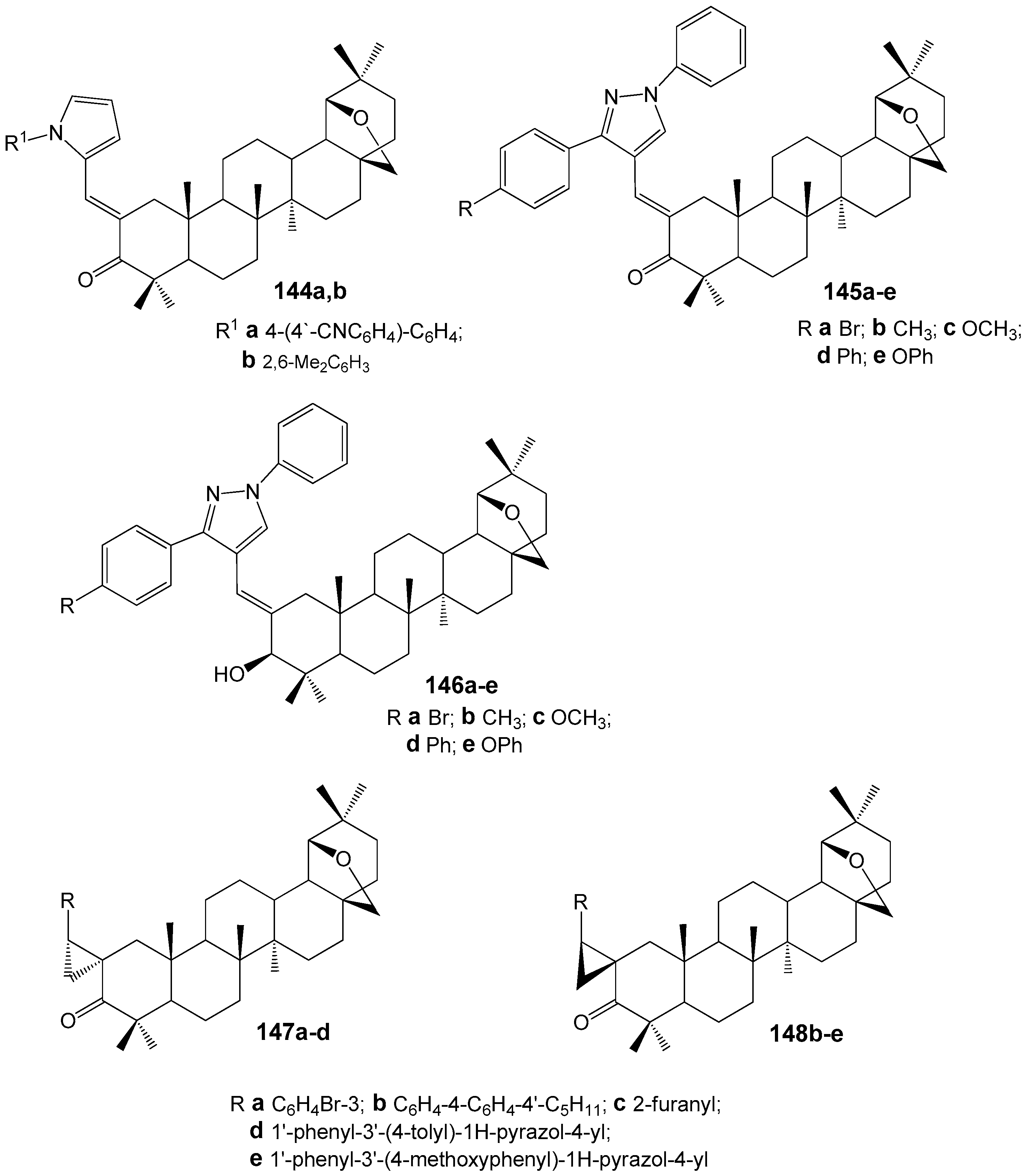
| Compound | DLD-1 | HT-29 | HeLa |
|---|---|---|---|
| BA | 70.75 ± 0.01 | 87.05 ± 3.89 | 62.65 ± 12.17 |
| BA-levulinate | 15.42 ± 1.58 | 33.13 ± 13.14 | 37.90 ± 0.01 |
| methylated BA-levulinate | 105.19 ± 10.05 | 68.70 ± 3.54 | 63.61 ± 1.55 |
| 53 | 64.20 ± 5.28 | 27.72 ± 9.31 | 40.90 ± 11.98 |
| Compound | 139e | 140a | 141a | 142a | 143a |
|---|---|---|---|---|---|
| |β|, (µm−1 mol. pats−1) | 29.7 ± 1.4 | 71.4 ± 3.4 | 84.3 ± 3.7 | 57.8 ± 2.6 | 3.4 ± 0.2 |
| Compound | 139g | 140b | 141b | 142b | 143b |
| |β|, (µm−1 mol. pats−1) | 10.8 ± 0.5 | 144.5 ± 3.1 | 174.9 ± 3.9 | 174.3 ± 0.9 | 122.5 ± 3.0 |
| Compound | 144a | 144b | 145a | 145b | 145c | 145d |
|---|---|---|---|---|---|---|
| |β|, (µm−1 mol. pats−1) | 89.7 ± 2.5 | 6.8 ± 1.1 | 108.9 ± 4.3 | 129.9 ± 2.8 | 145.0 ± 1.3 | 158.6 ± 8.7 |
| Compound | 145e | 146a | 146b | 146c | 146d | 146e |
| |β|, (µm−1 mol. pats−1) | 106.4 ± 2.4 | 51.9 ± 1.6 | 52.7 ± 2.1 | 63.8 ± 0.3 | 61.7 ± 1.3 | 49.2 ± 2.0 |
| Compound | 147a | 147b | 147c | 147d | 148b | 148c |
| |β|, (µm−1 mol. pats−1) | 5.2 ± 0.4 | 174.7 ± 0.9 | 25.66 ± 0.5 | 107.5 ± 3.3 | 123.5 ± 3.0 | 61.8 ± 0.4 |
| Compound | 148d | 148e | ||||
| |β|, (µm−1 mol. pats−1) | 213.8 ± 1.1 | 230.5 ± 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipson, V.V.; Shirobokova, M.G.; Gümüş, M.K.; Ozturkcan, A.; Chebanov, V.A. Molecular Diversity of Lupane Hybrids in Drug Design and Materials Science. Molecules 2025, 30, 4108. https://doi.org/10.3390/molecules30204108
Lipson VV, Shirobokova MG, Gümüş MK, Ozturkcan A, Chebanov VA. Molecular Diversity of Lupane Hybrids in Drug Design and Materials Science. Molecules. 2025; 30(20):4108. https://doi.org/10.3390/molecules30204108
Chicago/Turabian StyleLipson, Victoria V., Maria G. Shirobokova, Mustafa Kemal Gümüş, Arda Ozturkcan, and Valentyn A. Chebanov. 2025. "Molecular Diversity of Lupane Hybrids in Drug Design and Materials Science" Molecules 30, no. 20: 4108. https://doi.org/10.3390/molecules30204108
APA StyleLipson, V. V., Shirobokova, M. G., Gümüş, M. K., Ozturkcan, A., & Chebanov, V. A. (2025). Molecular Diversity of Lupane Hybrids in Drug Design and Materials Science. Molecules, 30(20), 4108. https://doi.org/10.3390/molecules30204108








