Microwave-Assisted Extraction of Phenolic Compounds from Cocoa Pod Husk: Process Optimization and Impact of Drying Temperature on Bioactive Recovery
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Phenolic Compounds in Cocoa Pod Husk
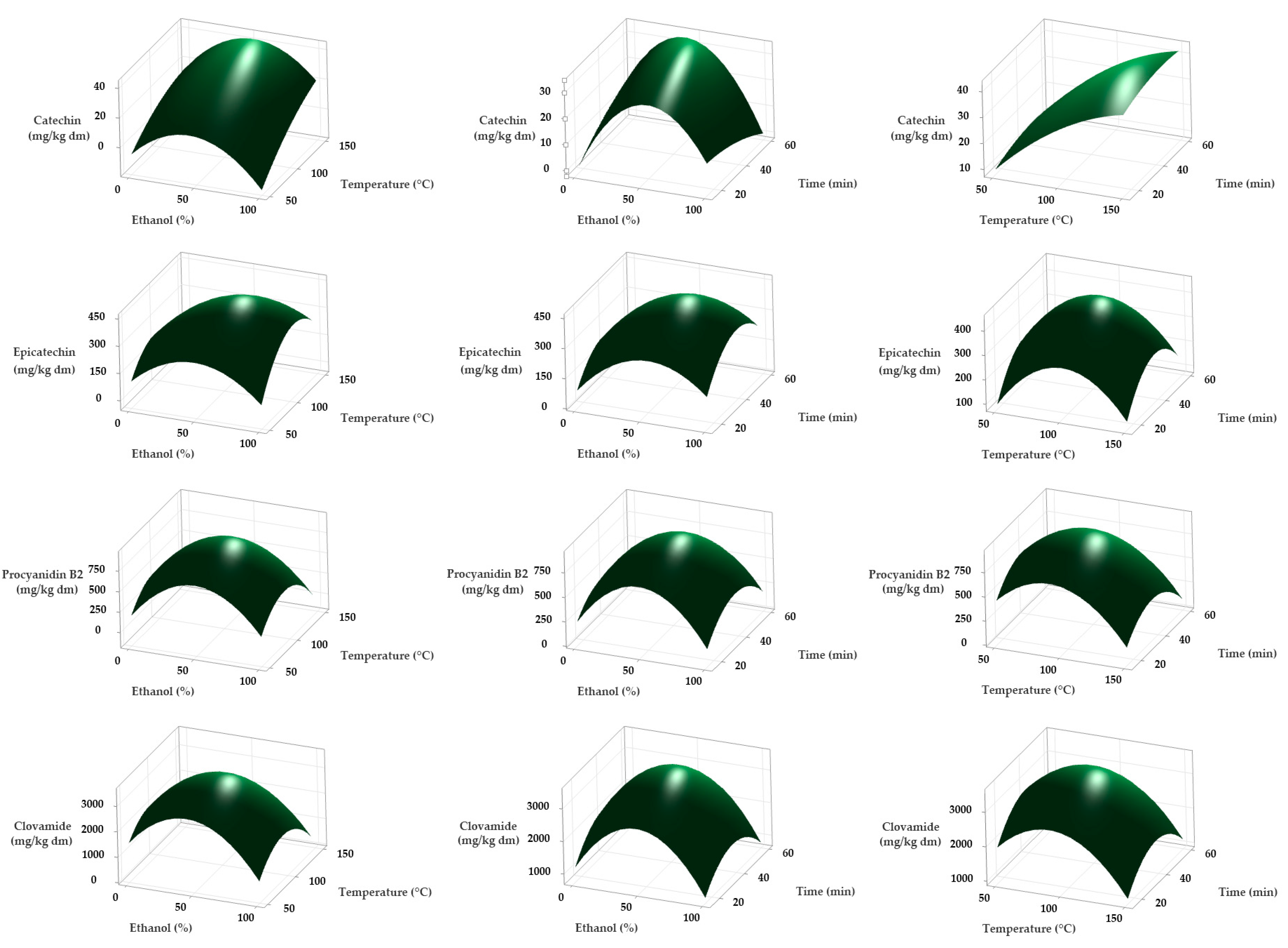
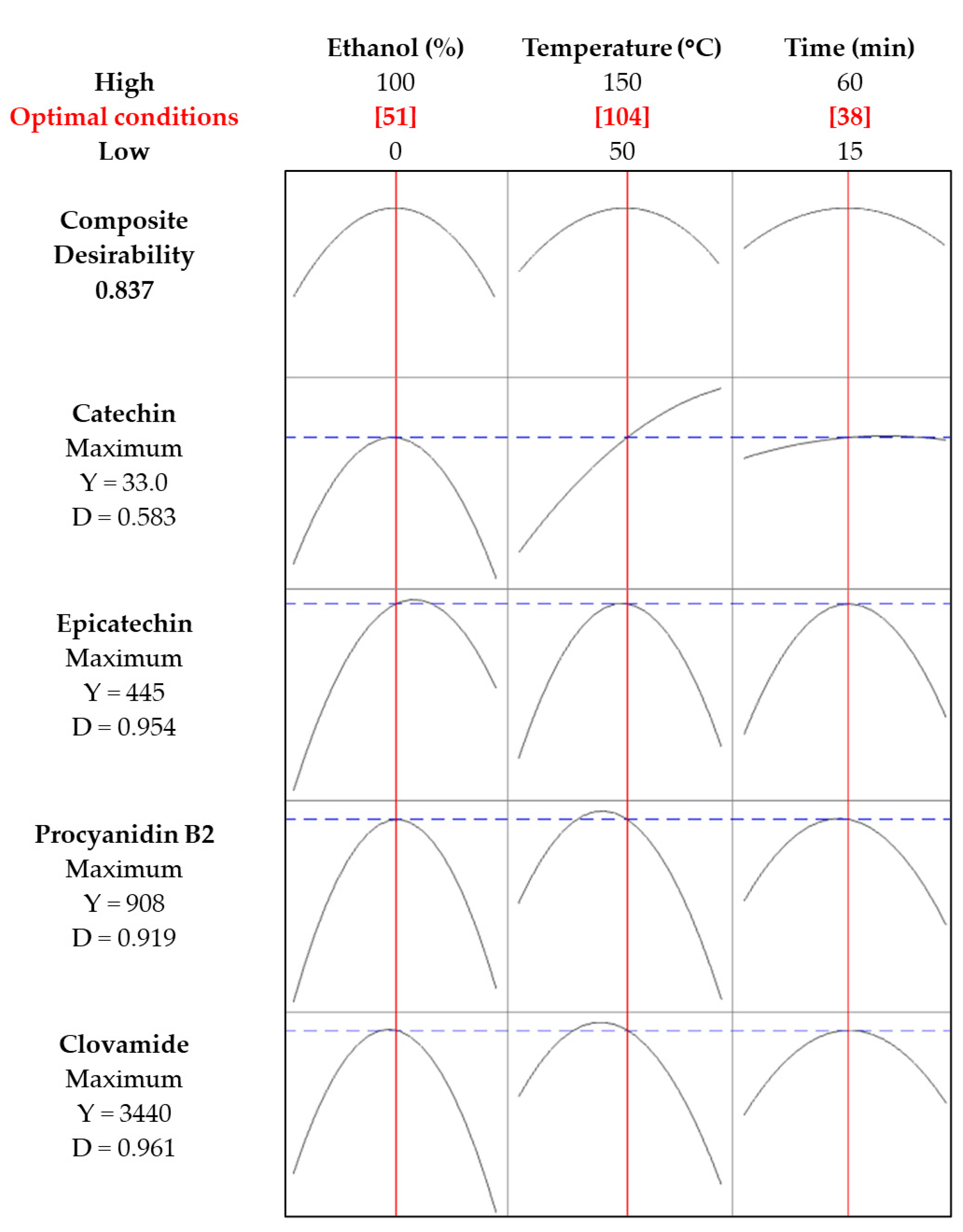
2.2. Optimization of Phenolic Compound Extraction by MAE
2.3. Effect of Drying Temperature on Phenolic Content
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Raw Material
3.3. Dehydration Process
3.4. Experimental Design
3.5. Microwave-Assisted Extraction
3.6. Quantification of Phenolic Compounds by HPLC-DAD-ESI-IT-MS/MS
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BBD | Box–Behnken Design |
| CPH | Cocoa pod husk |
| DAD | Diode-Array Detector |
| dm | Dry matter |
| ESI | Electrospray Ionization |
| HPLC | High-Performance Liquid Chromatography |
| IT | Ion Trap |
| LOQ | Limit of quantification |
| MAE | Microwave-assisted extraction |
| MS/MS | Tandem mass spectrometry |
| m/z | Mass-to-charge ratio |
| R2 | Coefficients of determination |
| RSM | Response Surface Methodology |
| RT | Retention time |
References
- Lima, G.V.S.; e Gonçalves, C.G.; Pinto, A.S.O.; da Silva, E.M.; de Souza, J.N.S.; Rogez, H. Impact of post-harvest processing and roasting conditions on the physicochemical properties, phenolic compounds, and antioxidant capacity of cocoa beans from the Brazilian Amazon. LWT 2024, 210, 116825. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organisation of the United Nations). FAOSTAT. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 2 May 2025).
- Cádiz-Gurrea, M.d.l.L.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Guerrero-Muñoz, N.; Villegas-Aguilar, M.d.C.; Pimentel-Moral, S.; Ramos-Escudero, F.; Segura-Carretero, A. LC-MS and spectrophotometric approaches for evaluation of bioactive compounds from Peru cocoa by-products for commercial applications. Molecules 2020, 25, 3177. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Tran, T.G.; Tran, N.L. Phytochemical compound yield and antioxidant activity of cocoa pod husk (Theobroma cacao L.) as influenced by different dehydration conditions. Dry Technol. 2021, 40, 2021–2033. [Google Scholar] [CrossRef]
- Mashuni; Hamid, F.H.; Muzuni; Kadidae, L.O.; Jahiding, M.; Ahmad, L.O.; Saputra, D. The determination of total phenolic content of cocoa pod husk based on microwave-assisted extraction method. AIP Conf. Proc. 2020, 2243, 030013. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Casimiro-Gonzales, S.; Cádiz-Gurrea, M.d.l.L.; Cancino Chávez, K.; Basilio-Atencio, J.; Ordoñez, E.S.; Muñoz, A.M.; Segura-Carretero, A. Optimizing vacuum drying process of polyphenols, flavanols and DPPH radical scavenging assay in pod husk and bean shell cocoa. Sci. Rep. 2023, 13, 13900. [Google Scholar] [CrossRef] [PubMed]
- Abdul Karim, A.; Azlan, A.; Ismail, A.; Hashim, P.; Abd Gani, S.S.; Zainudin, B.H.; Abdullah, N.A. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC Complement Altern. Med. 2014, 14, 381. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Rojas-García, A.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. High potential extracts from cocoa byproducts through sonotrode optimal extraction and a comprehensive characterization. Ultrason Sonochem. 2024, 106, 106887. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Plazola-Jacinto, C.P.; Hernández-Ortega, M.; Hernández-Navarro, M.D.; Villarreal, F.; Necoechea-Mondragón, H.; Ortiz-Moreno, A.; Ceballos-Reyes, G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal degradation of bioactive compounds during drying process of horticultural and agronomic products: A comprehensive overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Shi, M.; Nie, Y.; Zhao, Y.; Ye, J.-H.; Liang, Y.-R. Differential behaviors of tea catechins under thermal processing: Formation of non-enzymatic oligomers. Food Chem. 2016, 196, 347–354. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Biosci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Herrero, M.; Martín-Álvarez, P.J.; Senorans, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioproc. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- de Castro, M.L.; Castillo-Peinado, L. Microwave-assisted extraction of food components. In Innovative Food Processing Technologies Extraction, Separation, Component Modification and Process Intensification; Elsevier: Duxford, UK, 2016; pp. 57–110. [Google Scholar]
- Radoiu, M.; Kaur, H.; Bakowska-Barczak, A.; Splinter, S. Microwave-assisted industrial scale cannabis extraction. Technologies 2020, 8, 45. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Segovia, Á.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Extraction of phenolic compounds from cocoa shell: Modeling using response surface methodology and artificial neural networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Figueroa, J.G. Microwave-assisted extraction optimization and effect of drying temperature on catechins, procyanidins and theobromine in cocoa beans. Molecules 2023, 28, 3755. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, P.; Rosyidi, D.; Purwadi; Imam, T. Characteristics of catechin extracted from cocoa husks using microwave assisted extraction (MAE). Biodiversitas 2019, 20, 3626–3631. [Google Scholar]
- Van, T.N.; Thi, D.P.; Long, B.V.; Van, H.N.; Ngoc, L.T. Microwave-assisted extraction for maximizing the yield of phenolic compounds and antioxidant capacity from cacao pod husk (Theobroma cacao L.). Curr. Nutr. Food Sci. 2021, 17, 225–237. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2018, 105, 752–763. [Google Scholar] [CrossRef]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Factors affecting extraction and evolution of phenolic compounds during red wine maceration and the role of process modelling. Trends Food Sci. Technol. 2017, 69, 106–117. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. J. Industrial Crops 2017, 104, 210–220. [Google Scholar] [CrossRef]
- Dasiman, R.; Nor, N.M.; Eshak, Z.; Mutalip, S.S.M.; Suwandi, N.R.; Bidin, H. A review of procyanidin: Updates on current bioactivities and potential health benefits. Biointerface Res. Appl. Chem. 2022, 12, 5918–5940. [Google Scholar]
- Isidoro Haminiuk, C.W.; Vicente Plata-Oviedo, M.S.; de Mattos, G.; Carpes, S.T.; Branco, I.G. Fundamental and applied aspects of catechins from different sources: A review. Int. J. Food Sci. Technol. 2020, 55, 429–442. [Google Scholar]
- Locatelli, M.; Travaglia, F.; Giovannelli, L.; Coïsson, J.D.; Bordiga, M.; Pattarino, F.; Arlorio, M. Clovamide and phenolics from cocoa beans (Theobroma cacao L.) inhibit lipid peroxidation in liposomal systems. Food Res. Int. 2013, 50, 129–134. [Google Scholar] [CrossRef]
- Tranquilino-Rodríguez, E.; Martínez-Flores, H.E.; Rodiles-López, J.O.; Dios Figueroa-Cárdenas, J.D.; Pérez-Sánchez, R.E. Optimization in the extraction of polyphenolic compounds and antioxidant activity from Opuntia ficus-indica using response surface methodology. J. Food Process Preserv. 2020, 44, e14485. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Pan, H.; Fan, L. Optimisation of the microwave-assisted extraction process for six phenolic compounds in Agaricus blazei Murrill. Int. J. Food Sci. Technol. 2012, 47, 24–31. [Google Scholar] [CrossRef]
- Vo, T.P.; Pham, T.V.; Tran, T.N.H.; Vo, L.T.V.; Vu, T.T.; Pham, N.D.; Nguyen, D.Q. Ultrasonic-assisted and microwave-assisted extraction of phenolics and terpenoids from Abelmoschus sagittifolius (Kurz) Merr roots using natural deep eutectic solvents. ACS Omega 2023, 8, 29704–29716. [Google Scholar] [CrossRef]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef]
- Chen, J.; Thilakarathna, W.W.; Astatkie, T.; Rupasinghe, H.V. Optimization of catechin and proanthocyanidin recovery from grape seeds using microwave-assisted extraction. Biomolecules 2020, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Marsol-Vall, A.; Keskitalo, M.; Yang, B.; Suomela, J.-P. Effects of different drying temperatures on the content of phenolic compounds and carotenoids in quinoa seeds (Chenopodium quinoa) from Finland. J. Food Compost. Anal. 2018, 72, 75–82. [Google Scholar] [CrossRef]
- Yener, E.; Saroglu, O.; Sagdic, O.; Karadag, A. The effects of different drying methods on the in vitro bioaccessibility of phenolics, antioxidant capacity, and morphology of European plums (Prunes domestica L.). ACS Omega 2024, 9, 12711–12724. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Changes in the flavan-3-ols, anthocyanins, and flavanols composition of cocoa beans of different Theobroma cacao L. groups affected by roasting conditions. Eur. Food Res. Technol. 2015, 241, 663–681. [Google Scholar] [CrossRef]
- Mesquita, V.L.V.; Queiroz, C. Enzymatic browning. In Biochemistry of Foods; Eskin, N.A.M., Shahidi, F., Eds.; Academic Press: London, UK, 2013; Volume 3, pp. 387–418. [Google Scholar]
- De Taeye, C.; Kankolongo Cibaka, M.-L.; Jerkovic, V.; Collin, S. Degradation of (−)-epicatechin and procyanidin B2 in aqueous and lipidic model systems. First evidence of “chemical” flavan-3-ol oligomers in processed cocoa. J. Agric. Food Chem. 2014, 62, 9002–9016. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Jiang, X. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Latos-Brozio, M.; Masek, A. Natural polymeric compound based on high thermal stability catechin from green tea. Biomolecules 2020, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]

| Compound | RT (min) | m/z | MS/MS Fragments |
|---|---|---|---|
| Catechin | 20.2 | 289.2 | 165, 179, 245, 271 |
| Procyanidin B2 | 20.9 | 577.3 | 205, 289, 425, 559 |
| Epicatechin | 23.7 | 289.2 | 165, 179, 245, 271 |
| Clovamide | 28.4 | 358.2 | 178, 222, 314 |
| Run | Ethanol (%) | Temperature (°C) | Time (min) | Clovamide (mg/kg dm) | Procyanidin B2 (mg/kg dm) | Epicatechin (mg/kg dm) | Catechin (mg/kg dm) |
|---|---|---|---|---|---|---|---|
| 1 | 50 | 100 | 37.5 | 3471 | 929 | 413 | 31 |
| 2 | 100 | 100 | 60 | 613 | 130 | 199 | <LOQ |
| 3 | 0 | 100 | 60 | 1328 | 25 | 101 | 26 |
| 4 | 100 | 100 | 15 | 888 | 200 | 107 | <LOQ |
| 5 | 50 | 100 | 37.5 | 3397 | 845 | 466 | 40 |
| 6 | 0 | 50 | 37.5 | 816 | 36 | 12 | <LOQ |
| 7 | 0 | 100 | 15 | 1361 | 283 | 96 | <LOQ |
| 8 | 50 | 100 | 37.5 | 3570 | 988 | 454 | 27 |
| 9 | 100 | 50 | 37.5 | 305 | 54 | 54 | <LOQ |
| 10 | 0 | 150 | 37.5 | 808 | <LOQ | <LOQ | <LOQ |
| 11 | 50 | 50 | 15 | 2441 | 518 | 153 | <LOQ |
| 12 | 100 | 150 | 37.5 | 845 | 80 | 308 | 15 |
| 13 | 50 | 150 | 15 | 238 | <LOQ | 74 | 57 |
| 14 | 50 | 150 | 60 | 783 | <LOQ | 46 | 44 |
| 15 | 50 | 50 | 60 | 2672 | 565 | 160 | <LOQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, P.; Reyes, C.; Figueroa, J.G. Microwave-Assisted Extraction of Phenolic Compounds from Cocoa Pod Husk: Process Optimization and Impact of Drying Temperature on Bioactive Recovery. Molecules 2025, 30, 3497. https://doi.org/10.3390/molecules30173497
Gomez P, Reyes C, Figueroa JG. Microwave-Assisted Extraction of Phenolic Compounds from Cocoa Pod Husk: Process Optimization and Impact of Drying Temperature on Bioactive Recovery. Molecules. 2025; 30(17):3497. https://doi.org/10.3390/molecules30173497
Chicago/Turabian StyleGomez, Pablo, Cristhopher Reyes, and Jorge G. Figueroa. 2025. "Microwave-Assisted Extraction of Phenolic Compounds from Cocoa Pod Husk: Process Optimization and Impact of Drying Temperature on Bioactive Recovery" Molecules 30, no. 17: 3497. https://doi.org/10.3390/molecules30173497
APA StyleGomez, P., Reyes, C., & Figueroa, J. G. (2025). Microwave-Assisted Extraction of Phenolic Compounds from Cocoa Pod Husk: Process Optimization and Impact of Drying Temperature on Bioactive Recovery. Molecules, 30(17), 3497. https://doi.org/10.3390/molecules30173497






