Abstract
Background: Anthralin is known for its efficacy in treating psoriasis and acne, possessing poor solubility. Addressing these limitations, the present study endeavors to develop a microemulgel formulation of anthralin aimed at enhancing solubility. Method: The solubility study was performed in various solvents. An o/w (oil-in-water) emulsion was formed using the water titration method, which was optimized by statistical experimental design half-run CCD. The final optimized batch was evaluated for physicochemical and in vitro properties Result: The final optimized batch showed a particle size (PS) of 417 nm, −25.2 mV zeta potential (ZP) and pH 5.8, which remained stable upon centrifugation, heating–cooling and freeze–thawing cycle. Furthermore, microemulsion with Carbopol 943 5% w/v was selected as the gel base for the formation of microemulgel characterized by PS, ZP, pH, and viscosity of 230 nm, −50.6 mV, 6.9 and 14,200 cps, respectively, that ensured it a high enough stability. In silico molecular docking between ligand and protein provides the binding energies validating the interaction. Hence, the in silico study was performed for psoriasis and P. acne proteins. An in vitro antibacterial activity study on Propionibacterium revealed a significant efficiency of the formulation and MTT assay using L929 cell line in the presence of the drug-loaded microemulgel indicated an inhibition of growth proving that formulation has anti-psoriatic activity. Conclusions: Combination therapy with Clindamycin might improve efficacy while reducing antibiotic resistance risks.
1. Introduction
The Global Psoriasis Atlas (www.globalpsoriasisatlas.org, accessed on 18 February 2025) and the World Health Organization’s (www.who.int, accessed on 18 February 2025) global report on psoriasis in addition to the National Psoriasis Foundation (www.psoriasis.org, accessed on 18 February 2025) reports that psoriasis is chronic condition affecting over 60 million people worldwide, with an age of onset of 20–30 and 50–60 years, and with a higher prevalence of 2.50% in Western Europe. In 2019, psoriasis accounted for over 3.5 million disability-adjusted life years (DALYs), with more than 40 million prevalent cases and 4.6 million new cases reported worldwide. Furthermore, it has been estimated that by 2030, age-standardized incidence rates per 1 lac were expected to decrease (53.67, 0.00 to 259.99), while incidence cases per 10,000 may increase (487.36, 423.62 to 551.10) [], and 81% of countries worldwide lack information on the epidemiology of psoriasis [].
Acne affects around 85% of adolescent and young adult due to the bacteria Cutibacterium (formerly Propionibacterium acnes a facultative anaerobic Gram-positive bacterium) and the fungi Malassezia colonization in the sebaceous sites of the skin []. According to a cross-sectional study of the antibiotic-resistant P. acnes, strains have been continuously increasing in Thai acne patients. The most common antibiotic resistance was erythromycin, followed by Clindamycin and tetracycline, respectively []. P. acne’s strains have specific genetic elements associated with their pathogenity. Hence identification and understanding of their role in health and disease by finding genetic differences is crucial for personalized medicines associated with targeted therapies in order to avoid or minimize antibiotic resistance [].
Anthralin inhibits leukotriene production and LT B4- omega oxidation by human neutrophils, and down-regulates epidermal growth factor receptors, potentially causing antipsoriatic effects. In vitro studies show it inhibits the monocyte secretion of IL-6, IL-8, and TNF-alpha, and even 10 microM anthralin can activate NF-B [,]. In acne treatment, several antibiotics are prescribed by oral or topical routes, such as tetracycline, oxytetracycline, doxycycline, minocycline, lymecycline, erythromycin, azithromycin, trimethoprim, and β-lactams ampicillin/amoxicillin/oxacillin; however, their resistance and adverse effects are cause for concern. On the other hand, benzoyl peroxide, azelaic acid, and salicylic acid were found to have anti-microbial properties []. Hence, new treatment options are necessary.
Previously, we have reported microemulsion (ME) and microemulgels for various applications [,,,]. In this study, we developed and characterized anthralin-loaded microemulsion-based gel (microemulgel) with the objective of dual treatment of psoriasis and acne suitable for topical applications. ME can increase the solubility of anthralin and enhance its penetrability through the skin after topical administration, while the gel base can effectively enhance the retention time on the skin. As microemulgels combine, the benefits of gels and microemulsions to treat skin disorders like acne and psoriasis increase. They have a solid gel matrix for controlled release and a small particle size (PS) that improves penetration through the stratum corneum with easy spreadability [,].
2. Results
2.1. Preparation of ME
2.1.1. Screening of Excipients
Microemulsions are essentially clear systems and determining the solubility of the drug in all the components is necessary. The drug solubility measured in oleic acid, Tween 80, and PEG 400 at room temperature (27 ± 1 °C) was found to be of 44.23 mg/mL, 56.017 mg/mL and 14.066 mg/mL, respectively. While Transcutol® exhibited the highest solubility for anthralin in preliminary screening (Figure 1), we opted to use PEG 400 in the final formulation. PEG is a common carrier for drugs with low molecular weights. It possesses low levels of toxicity, immunogenicity, antigenicity, and solubility in water-based solutions. After being administered to living things, it can be quickly eliminated even though it is not biodegradable. Protein or enzyme structure is unaffected by PEG’s presence in water-based solutions []. The study found that P-glycoprotein inhibition reduced the transdermal absorption of some drugs. Topical inhibitors can modify P-glycoprotein’s action, and the skin’s defensive role is diminished in aged individuals due to reduced dermis thickness [,]. PEG400 has been considered an inhibitor of P-gp []. PEG 400 is utilized as a cosurfactant because the surfactant taken solely, i.e., Tween 80, might fail to produce transient negative interfacial tension as well as a fluid interfacial film throughout ME development. Thus, a system of co-surfactants often comes into play to aid in the framework of ME at lower surfactant amounts by dropping the tension between the layers. Co-surfactants also enhance the mobility of surfactant hydrocarbon tails, enabling elevated oil diffusion. The inclusion of a cosurfactant also reduces the bending stress of the interface, giving rise to a more flexible interfacial layer that can accommodate the various curvatures needed to create ME across an array of compositions [,]
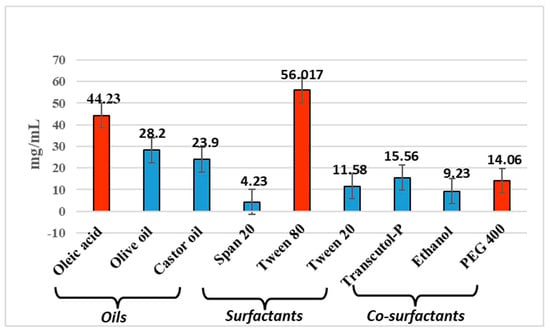
Figure 1.
Screening of excipients.
2.1.2. Construction of Pseudo-Ternary Phase Diagram
The aqueous phase titration method was used to construct a pseudo-ternary phase diagram to reach the required concentration of oil, surfactant, and co-surfactant. By adjusting the ratios of the oil and Smix components, a pseudo-ternary phase diagram was produced. Using aqueous phase titration, the concentration of Smix was reduced by 10% while the concentration of oil was increased by 90%. To create pseudo-ternary diagrams, Smix and oil (oleic acid) were used. Tween 80 to PEG 400 ratios of 2:1, 1:1, and 1:1.5 were also utilized. As shown in Figure 2, areas associated with Smix ratio 1:1, 1:1.5, and 2:1 batch were found to be 97,736.33 ± 496.42, 86,847 ± 977.42 and 102,191.66 ± 676.90. Based on this, 1:1.5 ratio was exempted owing to the least area and t-test revealed a significant difference in area between the 1:1 and 2:1 ratio (p = 0.025), indicating that the change in area is statistically non-significant. However, considering the toxicity of surfactants towards skin we selected 1:1 area for further analysis.
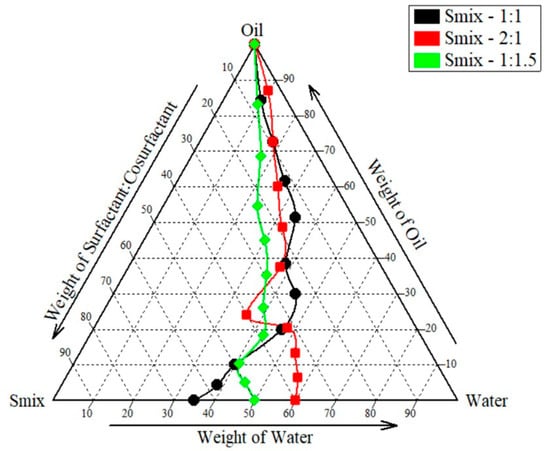
Figure 2.
Pseudo-ternary phase diagram.
2.2. Experimental Design for ME
Table 1 shows the dependent variables (DVs) and independent variables (IVs) of design with runs and Table 2 shows ANOVA and fit summary of design.

Table 1.
Selection of dependent variables (DVs), independent variables (IVs), and half-run central composite design (CCD) runs and results.

Table 2.
Summary of ANOVA for PS, ZP, and %T.
The model F-values of 31.74 for PS, 5.20 for ZP, and 9.35 for %T indicate that the model is significant. There is a 0.07%, 4.21%, and 1.20% chance that an F-value this large will occur owing to noise for PS, ZP, and %T, respectively. p-values less than 0.0500 suggest that model terms are significant, but values more than 0.1000 indicate that they are not. In case of PS, B, AB, AC, BC, A2, B2 are significant model terms, A2 is a significant model term for ZP and B, A2, B2 are significant model terms for %T. The lack of fit (LOF) F-value of 0.22 for PS, 0.0006 for ZP, and 0.12 for %T implies the Lack of Fit is not significant relative to the pure error, and there is a 66.14%, 99.14%, and 74.56% chance that a LOF F-value this large could occur due to noise, respectively.
The predicted R2 0.8773 of PS, 0.8542 of ZP, and 0.7402 of %T is in reasonable agreement with the adjusted R2 0.9518 for PS, 0.7296 for ZP, and 0.8430 for %T, respectively, i.e., the difference is less than 0.2. Adeq Precision measures the signal-to-noise ratio. A ratio greater than 4 is desirable. Hence, the ratio of 17.656 for PS, 6.203 of ZP, and 7.719 for %T indicates an adequate signal. This model can be used to navigate the design space.
Figure 3(a1) shows perturbation plot for PS, as oil (A) increases PS increases, Smix (B) slope shows change in PS as it moves away from the reference point and the water (C) line is flat, which has a negligible effect on PS. Figure 3(a2) shows a perturbation plot for ZP, as oil (A) increases ZP increases, while Smix (B) has a lesser effect on ZP and water (C) has a negligible effect on ZP. Figure 3(a3) shows a perturbation plot for %T, as oil (A) increases, %T decreases, Smix (B) has moderate effect on %T, and water (C) has negligible effect on %T. Considering this we may need to focus on the concentration of oil (A) and Smix (B) while optimizing the formulation. Also, Figure 3(b1–b3) shows a majority of points falling on the straight line, showing good prediction power.
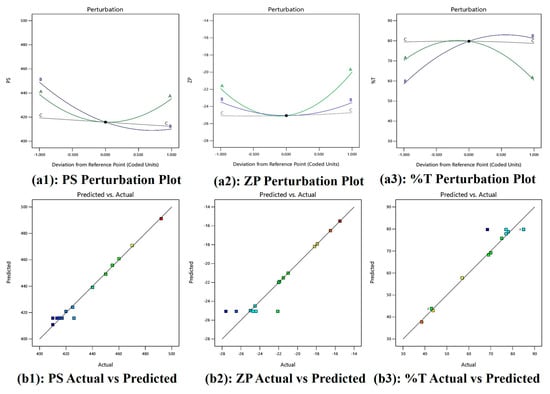
Figure 3.
(a1–a3) Perturbation plots and (b1–b3) actual vs. predicted plot of PS, ZP, and %T.
2.2.1. Analysis of PS
PS = 415.85 − 1.76 A − 19.44 B − 3.53 C + 10.71 AB − 26.19 AC − 21.01 BC + 21.25 A2 +13.75 B2 + 0.0092 C2
Equation (1) represents the effect of main effects, two factor interaction and quadratic effects of variables on PS. As per Equation (1), the main effects A, B, and C exhibit negative coefficients of −1.76, −19.44, and −3.53, respectively, indicating that each of these factors contributes to a reduction in particle size (PS). However, the higher negative coefficient of B (−19.44 coefficient) with (F-value of 48.80 and p-value of 0.0009) shows its significant effect on reducing PS. The non-ionic surfactant Tween 80 improves the stability of the emulsion. Because of steric hindrance, it creates a protective coating surrounding oil droplet, keeping them from coalescing and preserving their smaller particle size [,]. Positive AB interaction (F-value of 7.41, p-value of 0.0417) and its coefficient (+10.71) suggests this combination increases the PS. However, as can be seen in Figure 4(a1) and Figure 5(a1), PS becomes low at higher Smix and moderate oil content. At higher concentrations, these components exhibit an increase in particle size (PS). This may be attributed to the addition of oleic acid to Tween 80, which can lead to the formation of micellar aggregates due to oleic acid’s surfactant-like behavior, as reported in earlier studies []. Additionally, the AC (F-value of 44.28, p-value of 0.0012) negative coefficient (−26.19), as seen in Figure 4(a2) and Figure 5(a2), shows a steep slope indicating strong interaction between oil and water, as reported previously, with viscosity and particle size distribution being, most likely, two ways by which oleic acid (A) influences emulsion PS. Stable emulsions with smaller droplet sizes are produced when the oil–water interfacial region is better contacted due to increased viscosity. However, an excessive amount of oleic acid might cause droplets to enlarge, which will reduce the homogeneity of the emulsion [,]. Similarly, BC interaction (−21.01 coefficient) and (F-value of 28.50, p-value of 0.0031) decreases the PS and according to Figure 4(a3) and Figure 5(a3), PS becomes low at high Smix and moderate to high water content because more micelles are produced and active species could be easily dispersed, with a direct consequence in decreasing ME particle size. On the other hand, a lower emulsifier concentration leads to a larger particle size dispersion []. Finally, quadratic terms A2, B2, and C2 with positive coefficients of +21.25, +13.75, and +0.0092 imply an increase in PS with the increase in its concentration in the ME system.
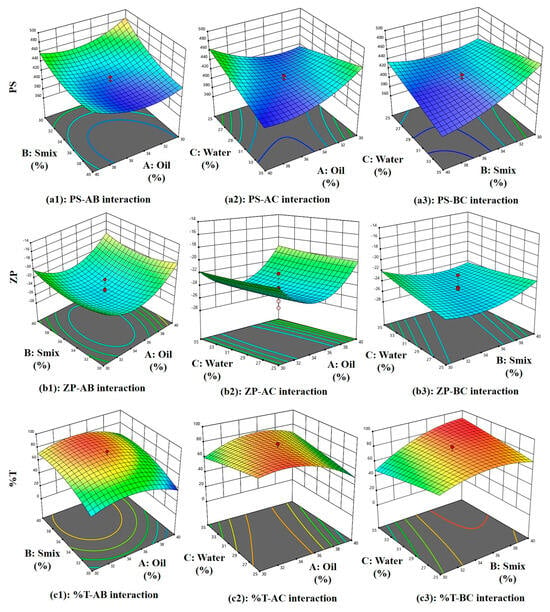
Figure 4.
Three-dimensional surface plots of the PS, ZP, and %T.
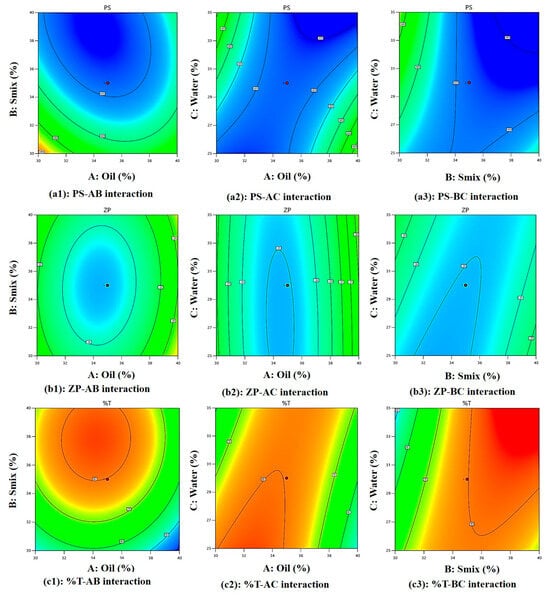
Figure 5.
Two-dimensional contour plots of the PS, ZP, and %T.
2.2.2. Analysis of ZP
ZP = −25.06 + 0.954 A − 0.0353 B + 0.176 C −0.298 AB + 0.189 AC −1.07 BC + 4.11 A2 + 1.56 B2 + 0.160 C2
Equation (2) represents the effect of main effects, two factor interaction, and quadratic effects of variables on ZP. As per Equation (2), main effect A(oil) has a positive effect (+0.954) and as reported previously, oleic acid ionizes in aqueous solutions, increasing oil droplet surface charge due to the formation of negatively charged carboxylate ions, enhancing the negative charge on oil droplets [], C(water) has a positive effect (+0.176), which increases ZP, and B(Smix) has a negative effect (−0.0353), which reduces the ZP since tween 80 and PEG-400 both being non-ionic in nature with no charge on their lipophilic portion, hence it reduces ZP and are used in pharmaceutical formulations, as reported previously []. However, these effects are insignificant, as suggested by their F-value of 1.02, p-value of 0.3587 for oil, F-value of 0.0014, p-value of 0.9716 for Smix, and F-value of 0.0350, p-value of 0.8589 for water. In Figure 4(b1) and Figure 5(b1) at higher Smix and oil content, ZP is low with clear optimal region at moderate oil and Smix. ZP reduces due to the negative effect of AB (−0.298) interaction (F-value of 0.0498, p-value of 0.8322) and BC (−1.07) interaction (F-value of 0.6418, p-value of 0.4594) owing to the non-ionic nature of tween 80 and PEG-400, stearic stabilization, and ionization of oleic acid in the presence of water. There is a positive effect (+0.189) of AC interaction (F-value of 0.0201, p-value of 0.8927), suggesting an increase in ZP due to oil and water interaction. Figure 4(b2) and Figure 5(b2) show at moderate oil and high water, higher ZP is observed. Figure 4(b3) and Figure 5(b3) show a steeper curve at both higher concentrations. Finally, quadratic terms A2, B2, and C2 with positive coefficients of +4.11, +1.56, and +0.160 imply an increase in ZP with the increase in its concentration in the ME system.
2.2.3. Analysis of %T
%T = 79.78 − 4.94 A + 11.31 B −0.353 C + 0.696 AB + 9.76 AC + 9.25 BC −14.50 A2 −10.00 B2 −0.755 C2
Equation (3) represents the effect of main effects, two factor interaction, and quadratic effects of variables on %T. As per Equation (3), the main effect of oil with a −4.94 coefficient (F-value of 2.20, p-value of 0.1981) and water with a −0.353 coefficient (F-value of 0.0112, p-value of 0.9197) does not significantly reduce the %T because oleic acid and water can decrease an %T by increasing ME turbidity. This is because oleic acid forms micelles or droplets that scatter light, reducing the amount of light that can pass through the emulsion. This increased turbidity is due to the formation of a new phase []. While Smix (+11.31) increases the %T significantly (F-value of 11.49, p-value of 0.0195). This is because the PS of a ME is influenced by the %T and the interaction of Tween 80 and PEG-400 (Smix). As reported previously, Tween 80 and PEG-400 adsorb the emulsion’s surface, decreasing the surface tension and increasing the kinetic stability as they prevent oil droplets from aggregating, but high concentrations can cause PS to increase. Surfactants and co-surfactants form micelles, increasing local osmotic pressure and causing droplets to move closer, causing aggregation. Larger particle sizes result in more non-uniform droplets []. All interaction effect has a positive influence on %T +0.696 for AB (F-value of 0.0218, p-value of 0.8884), which shows %T increases with an increase in Smix and a decrease in oil (moderately) (Figure 4(c1) and Figure 5(c1)), +9.76 for AC (F-value of 4.28, p-value of 0.0934), suggesting lower %T at high oil and water (Figure 4(c2) and Figure 5(c2)). These interaction effects can together be explained by the fact that oleic acid also acts as co-surfactant (HLB-1) [], with the hydrophilic head of oleic acid contains the carboxylic acid group (-COOH), while the hydrophobic tail contains the hydrocarbon group pubchem, which reduces surface tension between oil and water phases, resulting in smaller, uniform droplets. Tween 80 stabilizes the emulsion by forming a protective layer around oil droplets, preventing coalescence and increasing transmittance. PEG 400 further reduces interfacial tension and enhances oil phase solubility, resulting in a clearer emulsion with higher transmittance [] and +9.25 for BC (F-value of 3.84, p-value of 0.1073) producing highest %T at balanced water (around 30%) and Smix (above 33%), as seen in (Figure 4(c3) and Figure 5(c3)) because as reported previously, Tween-80 reduces transmittance and increases viscosity, while PEG decreases it. Both are surfactants, with Tween acting as a surfactant and PEG as an anionic cosurfactant. Combining them produces smaller, stable particle sizes and increases film flexibility, as surfactants alone cannot reduce surface tension []. Lastly, quadratic terms coefficient −14.50 for A2, −10.00 for B2 and −0.755 for C2 reduces %T at higher concentrations.
2.2.4. Optimization of ME
The optimized batch given by software is shown in Figure 6. The batch was prepared and the evaluated. The evaluation parameters were found to be in a good range of predicted values confirming the model’s validity. The evaluation of the optimized batch of ME is given in Section 2.3.
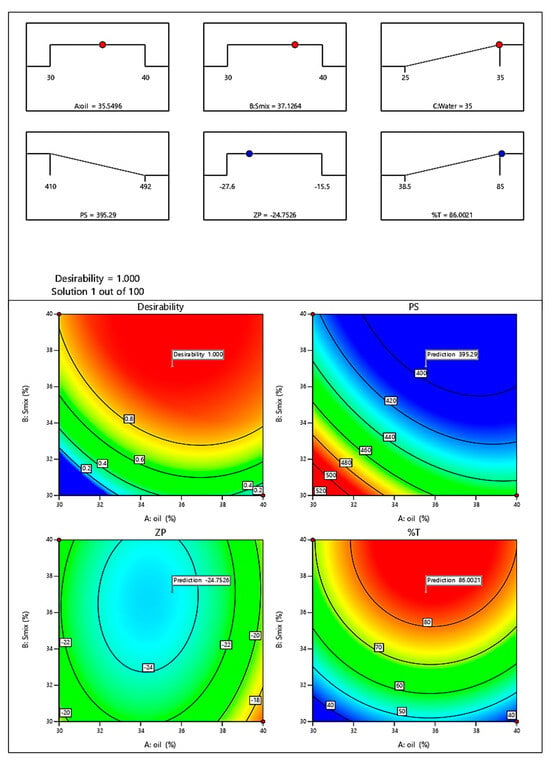
Figure 6.
Optimized batch of ME.
2.3. Evaluation of ME
2.3.1. Particle Size Analysis, PDI, and Zeta Potential
The microemulsion exhibits polydispersity index (PDI) 0.387, which with good interpretation is less than 0.5. The preparation’s particle size was determined by particle size analysis to be 417 nm. The final batch’s zeta potential was found to be −25.2 mV, demonstrating the emulsion’s good stability as shown in Figure 7.
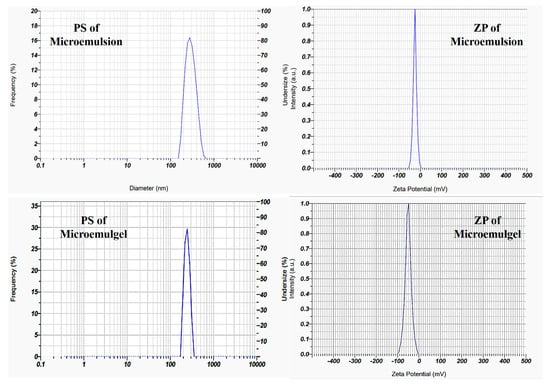
Figure 7.
Particle size and zeta potential of optimized batch of ME and microemulgel.
2.3.2. Dilution Test, %T, Viscosity, and Ph
Phase separation was not seen while diluting the microemulsion with water. This indicates that the o/w kind of microemulsion was used in the final batch. The microemulsion’s pH was found to be around 5.8, meaning that topical treatment is appropriate for it. The viscosity of microemulsion was found to be 3200 cps. Finally, the %T was found to be more than 90%, which indicates a lower particle size.
2.3.3. Thermodynamic Stability
Centrifugation of the microemulsion was performed using a BIO-LAB centrifuge (Mumbai, India) at 5000–10,000 rpm for 30–40 min at 30 °C. Microemulsion physical stability was proven by the absence of phase separation. Furthermore, the heating–cooling cycle and freeze–thaw cycle showed no visible phase separation of microemulsion indicating a stability of the system imparted by the use of optimal concentration of surfactant and co-surfactant.
2.4. Evaluation of Microemulsion-Based Gel
2.4.1. Physical Examination
Carbopol 934 was selected over Carbopol 940 and xanthan gum as Carbopol 934 has lower cross-linking density as compared to 940, resulting in required gel viscosity, making the gel matrix for drug to diffuse through, causing higher drug release. Also, xanthan gum is a natural polymer that can favor microbial growth. Carbopol 934 was selected over Carbopol 940 and xanthan gum based on preliminary formulation trials assessing stability, microbial resistance, spreadability, and extrudability. Xanthan gum, although commonly used as a natural gelling agent, showed visible microbial growth during stability studies, indicating poor preservative efficacy. Additionally, gel formulations prepared with Carbopol 934 demonstrated superior extrudability and spreadability compared to those with other gelling agents, as also reported previously []. Formulations ranging from 1% to 7% w/v of Carbopol 934 content exhibited a gradual transition in appearance, consistency, and texture. At lower percentage (1–2% w/v), the formulations remained transparent, homogeneous, and smooth, with a thin to gel-like consistency. As the concentration increased (3–7% w/v), the appearance changed to translucent while maintaining homogeneity. The consistency became progressively gel-like, with the 4% w/v formulation. At higher concentrations (5–7% w/v), the formulations retained a uniform gel-like texture, ensuring stability.
2.4.2. PS, ZP, and PDI
The microemulgel shows PDI 0.387, which is below 0.5 with good interpretation. Particle size analysis of the preparation showed the size as 230 nm, as shown in Figure 7. Particle size reduces after the incorporation in gel, as also previously reported by []. A higher zeta potential value denotes the presence of electrostatic repulsion between two droplets. Either +30 or −30 mV was chosen as the overall dividing point between stable and unstable emulgel. Particles with absolute values of zeta potential greater than or equal to 30 mV (>+30 mV or <−30 mV) are generally considered stable. The optimized batch’s zeta potential was found to be −50.6 mV, demonstrating the emulgel’s good stability.
2.4.3. pH and Viscosity
In individuals with psoriasis, studies have indicated that the skin pH can be lower than that of healthy skin. One study reported an average pH of 5.2 in psoriatic skin, compared to 5.6 in healthy skin []. The percentage ionization of the drug was calculated using the Henderson–Hasselbalch equation, employing a previously reported pKa value of 9.06 []. At pH 5.2, which corresponds to psoriatic skin conditions, the ionization was found to be only 0.01%, while at pH 5.6, representing healthy skin, it was slightly higher at 0.03%. These results indicate that the drug remains predominantly in its unionized form at both pH levels, which is favorable for topical absorption and permeability. This shows that drugs will be available for absorption through skin. Thus, anthralin’s absorption through skin varies with pH; lower pH increases absorption. The optimal pH range for topical application is 6–7 to balance efficacy and skin irritation []. The pH of the formed microemulgel was found around 6.9 which shows good absorption when applied on skin. Also, the viscosity of microemulgel was found to be 14,200 cps.
2.4.4. DSC of Microemulgel
The first endothermic peak at 101.6 °C suggests melting or dehydration, possibly indicating the presence of bound water or a polymorphic transition. The second peak at 134.8 °C suggests further thermal degradation or structural transformation of the sample. The absence of sharp exothermic peaks suggests no crystallization.
2.4.5. Texture Analysis
Understanding the arrangement and interactions between the formulation‘s component parts is aided by the examination of a semi-solid emulsion system‘s mechanical textural properties. Texture profile analysis (TPA) allows assessing particular mechanical characteristics, for example by subjecting a sample to an external compressional stress and evaluating the formulation‘s ability to produce both reversible and irreversible deformations. TPA can ascertain these mechanical properties. A product‘s hardness (expressed in N or gf), defined as its resistance to deformation, is determined by the initial maximum compression force. How long a gel stays in place after applying it to the skin is one indicator of its hardness, which in turn provides information on the gel’s ease of application. The resulting microemulsion-based gel exhibited 9.68 mm deformation at hardness. This result provides a clear indication of the material’s softness, while also highlighting potential limitations in wear resistance. These insights are crucial for determining the appropriate topical applications. It was discovered that the gel had a smooth and uniform texture, without any appearance variations or irregularities [].
The sample demonstrates a moderate hardness (64 gf) and significant deformation before reaching its maximum force (9.68 mm deformation), as shown in Figure 8. The material undergoes substantial deformation after reaching its peak force, suggesting soft or semi-solid properties. The absence of an abrupt force drop-off after peak load suggests the material maintains some structural integrity post-compression. A higher hardness value corresponds to a firmer gel with greater structural integrity, while a lower value suggests a softer, more spreadable formulation. The observed hardness ensures ease of application while maintaining an adequate viscosity and adhesion for an effective topical delivery.
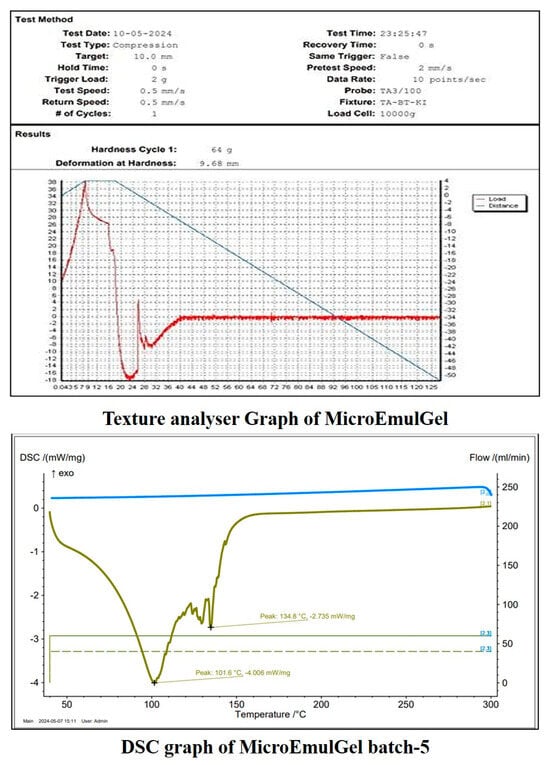
Figure 8.
Evaluation of microemulgel batch.
2.4.6. In Vitro Diffusion Study
Controlled-release formulations are designed to release the drug at a predetermined rate to maintain a consistent drug concentration over a specified period. For an 8 h study, the cumulative drug release should reflect a gradual release pattern rather than a burst release. Initial drug release (0–1 h) starts slowly to avoid a burst release, which is typical for controlled-release formulations. Early to mid-phase (1–4 h) drug release rate increases steadily, ensuring a consistent therapeutic effect. Late phase (4–8 h) release continues to increase gradually, aiming to reach around 90% and above cumulative release by the end of the 8 h. It was observed that the batch F5 showed controlled drug release from topical formulation as compared to reference product, as shown in Figure 9a. Hence batch F5 was selected as a final batch which contained 5% Carbopol 934 polymer.
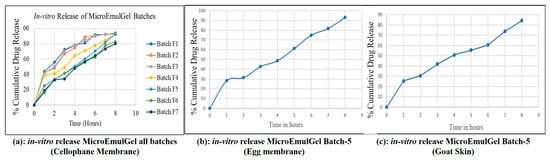
Figure 9.
In vitro release profiles of microemulgel batch.
2.4.7. In Vitro Drug Release: Egg Membrane and Goat Skin
It was determined how much medication will be released cumulatively during a time period of 8 h from the selected batch of microemulgel (Batch F5–5% w/v Carbopol 934 polymer containing optimized microemulsion formulation) was calculated Figure 9b,c. The release pattern appears to be gradual and sustained, suggesting a controlled release formulation. Initially, there is a rapid release phase (likely due to surface drug diffusion), followed by a steady increase over time. The total cumulative release is likely higher for the egg membrane compared to the goat skin, indicating that the membrane type significantly affects drug permeability.
2.4.8. Stability of Microemulgel
The three-month stability study of microemulgel showed an increase in PS to 253 nm, reduction in pH of formulation to 6.5 making it slightly acidic with no visible changes in physical stability.
2.5. In Vitro Activity
2.5.1. Antipsoriatic Activity
MTT assay for microemulgel was performed to determine the cytotoxic property of anthralin against L929 cell lines. Using samples with different drug loads, the minimum cell inhibition (47.90%) at 25 μg/mL concentration, maximum cell inhibition (60.34%)—to 100 μg/mL concentration of anthralin microemulgel and moderate cell inhibition (52.97%)—to 50 μg/mL. The cytotoxic activity of anthralin-loaded microemulgel was compared to microemulgel containing standard drug 5-fluouracil. The results demonstrated that anthralin microemulgel exhibits antipsoriatic potential.
As shown in Table 3 and Figure 10, both 5-FU and anthralin exhibited a dose-dependent cytotoxic effect, with an increasing % inhibition observed at higher concentrations. 5-FU demonstrated greater inhibition compared to anthralin across all tested concentrations. At 100 µg/mL, 5-FU exhibited 79.00% inhibition, whereas anthralin achieved 60.34% inhibition. The IC50 value was determined from the linear regression equation obtained from the % inhibition vs. concentration plot (Figure 10). The lower IC50 value of anthralin (36.18 µg/mL) indicates that it requires a lower concentration to achieve 50% inhibition compared to 5-FU (54.14 µg/mL), suggesting a relatively higher cytotoxic potency at lower doses. These results suggest that while 5-FU remains more cytotoxic overall, anthralin shows significant inhibition at lower concentrations, making it a potential candidate for further investigation in targeted cancer therapy.

Table 3.
Effects of active compounds against L929 (Mouse Connective Cell Line) by MTT assay.
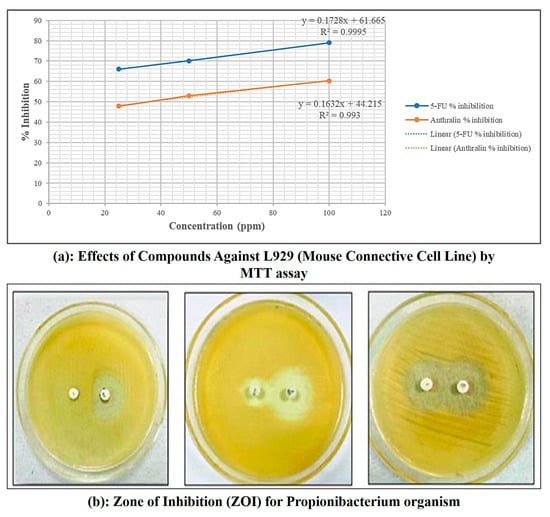
Figure 10.
MTT assay and zone of inhibition for Propionibacterium organism of microemulgel batch.
2.5.2. Antibacterial Activity
For this study propionic bacteria were used. After 24 h of incubation, the inhibitory effect of anthralin containing microemulgel was significant as compared to standard Clindamycin (Table 4). Zone of inhibition (ZoI) used as a measure for comparing bactericidal activity of anthralin microemulgel showed value of about 8.66, 10.66, 13.66 mm drug concentration of 25, 50 and 100 μg/mL, respectively, ZoI against the test organisms. The results demonstrated that anthralin microemulgel exhibits antibacterial potential. The increase in inhibition zone with concentration for both Clindamycin standard and antharalin microemulgel indicates a dose-dependent antibacterial effect.

Table 4.
Comparative data of antibacterial activity of Clindamycin (standard) and anthralin loaded in microemulgel.
2.6. In Silico Docking
Protein domains selected for docking were based on established ligand-binding or active sites, as reported in structural studies. The ligand used was anthralin, and docking was performed at biologically relevant sites previously identified as critical for therapeutic targeting for psoriasis [,,,,,,] and acne [,,,,,].
The docking study reveals that psoriasis-related proteins and Propionibacterium acnes proteins exhibit varying binding affinities with different ligands, highlighting their potential as drug targets as shown in (Table 5, Figure 11 and Figure 12). Among psoriasis proteins, NF-Kappa-B1 (PDB ID: 8TQD), Interleukin 12 receptor subunit beta−1 (PDB ID: 6WDP) and DYRK2 (PDB ID: 6HDR) exhibit highly negative binding energies, indicating strong and possibly irreversible ligand interactions. Overall, this analysis provides crucial insights into protein–ligand interactions, aiding the identification of potential drug candidates for psoriasis and acne treatment.

Table 5.
In silico docking study.
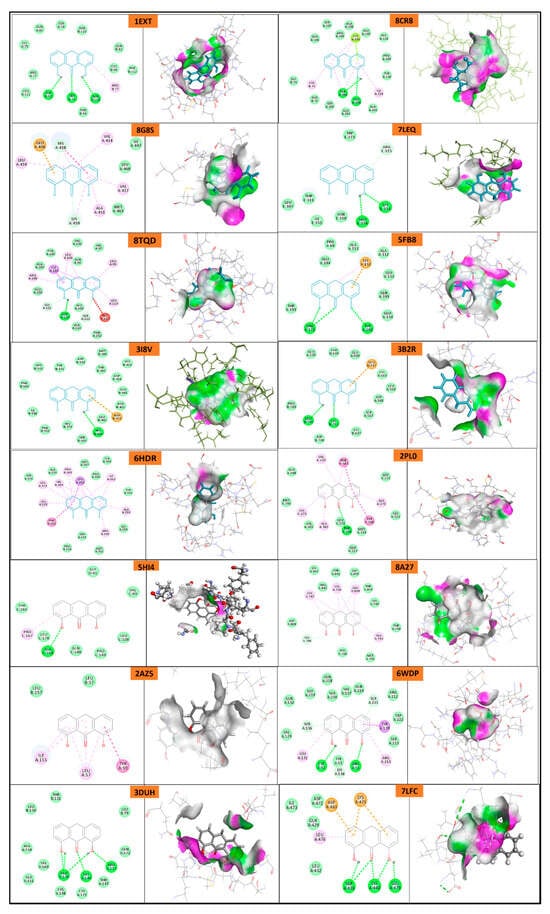
Figure 11.
Molecular docking of psoriasis proteins with anthralin.
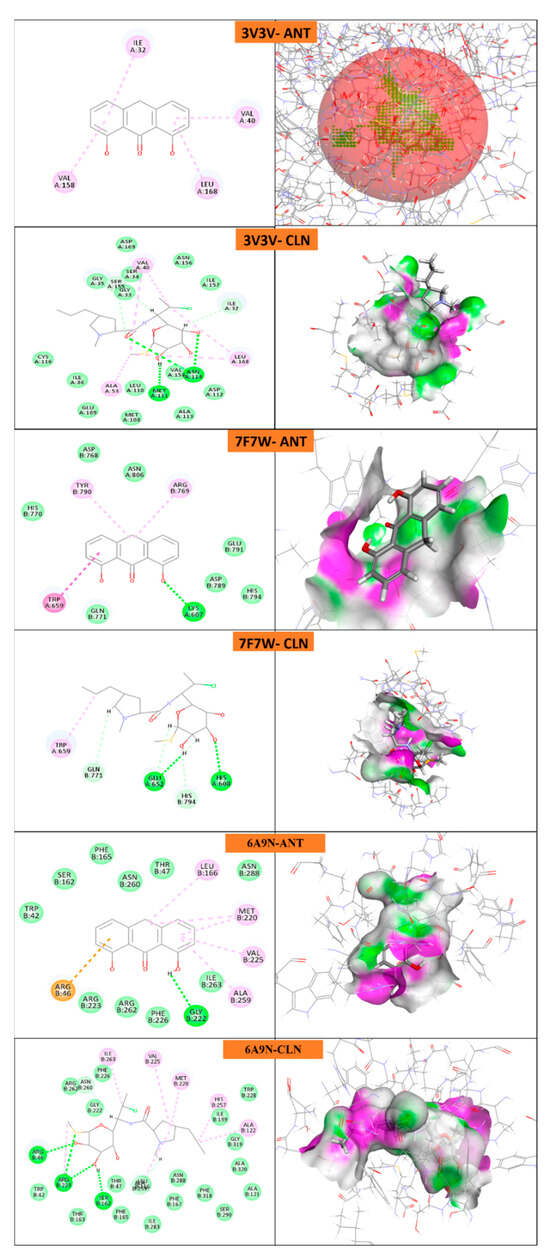
Figure 12.
Molecular docking of P. acne proteins with anthralin (ANT) and Clindamycin (CLN).
3. Discussion
The study successfully optimized an anthralin microemulgel for targeted delivery in psoriasis and acne treatment, addressing anthralin’s poor solubility and stability issues. The formulation demonstrated stable physicochemical properties with a particle size of 230 nm, a zeta potential of −50.6 mV, and a viscosity of 14,200 cps, ensuring effective retention and penetration on the skin. The in vitro antibacterial assay confirmed its efficacy against Propionibacterium acnes, while the MTT assay showed significant antipsoriatic activity. The formulation’s optimized composition, utilizing Carbopol 934 as a gel base, enhanced skin adherence and drug release. The study’s thermodynamic evaluation ensured long-term stability, confirming the microemulgel’s suitability for topical application. The drug release studies using egg membrane and goat skin confirmed sustained drug delivery over eight hours. The molecular docking results supported the formulation’s potential interaction with target proteins, suggesting its therapeutic viability. The formulation’s moderate hardness suggests it will provide good retention on the application site without excessive stiffness, ensuring optimal drug release and patient compliance. Anthralin requires a lower concentration (IC50 = 36.18 µg/mL) to reach 50% inhibition, indicating its superior effectiveness by comparison with 5-FU (IC50 = 54.14 μg/mL) at lower doses. Further optimization of anthralin microemulgel formulation is needed to enhance antibacterial efficacy. Combination therapy with Clindamycin might improve efficacy while reducing antibiotic resistance risks.
4. Materials and Methods
4.1. Materials
Anthralin was received as a gift sample from Yarrow chemicals, Mumbai, India. Oleic acid, olive oil, castor oil, Transcutol-P, methanol, PEG 400, PEG 600, tween 80, span 80, and tween 20 was received from SRL, Mumbai, India.
4.2. Pre-Formulation Study of Microemulsion
4.2.1. Screening of Co-Surfactant, Surfactant, and Oil
Solubility of anthralin in different components, a variety of oils, including oleic acid, olive oil, and castor oil, co-surfactants, including Transcutol-P, PEG 400, and PEG 600, and surfactants like tween 80, span 80, and tween 20, were examined. Excess drug was dissolved in 1 mL of the aforementioned oils, surfactant, and co-surfactant, and vortexed continuously for 48 h. The samples were centrifuged at 5000–7000 rpm for 15 min after which they were left to rest for 72 h. The supernatant liquid was then diluted with ethanol and used to measure the drug concentration via UV-spectroscopy by means of proper calibration curves (absorbance at 360 nm as a function of anthralin concentration) previously obtained.
4.2.2. Emulsification Study
The pseudo-ternary phase diagram comprising lipid, surfactant, cosurfactant and water were developed using the water titration method. This phase diagram was used to select the optimum ratio of Surfactant:Co-surfactant (S:CoS). Pseudo ternary phase diagrams were constructed for oleic acid, Tween 80, and PEG 400 using the water titration method []. Selected surfactant (Tween 80) was mixed with co-surfactant (PEG 400) at different weight ratios, i.e., 1:1, 2:1, and 1:1.5 w/w. The formation of microemulsion was confirmed by attaining a high transparency measured using an optical analyzer. The pseudo-ternary phase diagrams were mapped with CHEMIX software (version 13) and area values were calculated using ImageJ software (version 1.51p, National Institutes of Health).
4.2.3. Optimization by CCD
The half run Central Composite Design (CCD) model by Design Expert software (version 13.0.5, Stat-Ease, Minneapolis, MN, USA) was utilized in the study to examine the link between independent variables (IVs) and dependent variables (DVs). Table 2 lists the experimental levels, DVs, and IVs based on half CCD factorial models. With a minimum of 15 runs, the model looked at three variables at three different levels. The experimental data’s mean values were fitted using a polynomial equation, and each coefficient term’s significance was assessed using an ANOVA. Finding the regression coefficient (R2), predicted R2, and adjusted R2 allowed for the assessment of the experimental data and model. To evaluate the adequacy of the model, graphs displaying perturbation, actual versus predicted values, 3D contours, and 2D plots were created.
4.2.4. Preparation of Microemulsion
To prepare the microemulsion, the pseudo-ternary phase diagram was used with a drug dose of 1.2%. After the preparation of microemulsion, the microemulsion was evaluated for % transmittance, viscosity, pH, etc.
4.3. Evaluation of Microemulsion
4.3.1. Percent Transmittance Measurement:
With pure water, microemulsions were diluted up to 100 times. The formulation’s percent transmittance was measured at a wavelength of 660 nm using a UV-visible spectrophotometer with continuous phase (distilled water) serving as the reference.
4.3.2. PS, PDI, and ZP
A Malvern Zetasizer (Nano ZS 90, Malvern Instruments Ltd., Malvern, UK) was used to determine the Microemulsion’s PS, ZP, and PDI. The measurements of the samples were recorded using indisposable cuvettes. Cuvettes were cleaned with methanol and rinsed with a sample for analysis prior to each experiment [].
4.3.3. Dilution Test, Viscosity, and pH
This experiment was run to determine the type of microemulsion that developed. The prepared microemulsion was diluted with external/continuous phase water. A Brookfield viscometer (Brookfield instruments, AMETEK Brookfield, Mumbai, India) was used to measure the viscosity of the microemulsion. The microemulsion was put in the sample container and the appropriate spindle (No. 7) was placed perpendicularly into it. The spindle was allowed to spin continuously at its optimal speed. The viscosity of the formulation was tested at room temperature. The pH of the microemulsion was measured using the digital pH meter (Lab India PICO+, Mumbai, India) [].
4.3.4. Thermodynamic Stability
This characteristic was examined to evaluate the physical stability of the microemulsion. To evaluate the system’s capacity to cream or separate phases, the prepared microemulsion was spun for 10 min at 5000 rpm at room temperature. The produced ME system was subjected to a number of stress condition tests, including cycles of heating (40 °C) and cooling (4 °C), freezing (−21 °C), and thawing (+25 °C), while being stored for 48 h at a predetermined temperature. The Microemulsion’s appearance was visually examined [].
4.4. Preparation of Microemulgel
4.4.1. Selection of Polymer for Preparation of Gel Phase
Polymers such as xanthan gum, Carbopol 934, and Carbopol 940 were screened. Separately dispersed in warm water (70–75 °C) and allowed to soak for 24 h, the polymers were prepared for usage. By combining the polymers with distilled water, different gel concentrations were created.
4.4.2. Preparation of Micro-Emulsion-Based Gel
The Carbopol 934 polymer served as the gelling ingredient in the formation of the microemulsion-based gel. The polymer was submerged in water for 24 h. Polymer screening was conducted based on preliminary formulation trials evaluating stability, microbial resistance, spreadability, and extrudability, leading to the selection of Carbopol 934 over Carbopol 940 and xanthan gum. Then, to create a microemulsion-based gel, the prepared microemulsion was mixed with the gel phase. Finally, the pH was adjusted with trienthanolamine.
4.5. Evaluation of Micro-Emulsion-Based Gel
4.5.1. PS, ZP, and pH
Malvern Zetasizer (Nano ZS 90, Malvern Instruments Ltd., Malvern, UK) was used to determine the microemulsion’s PS and ZP using a disposable sizing cuvette at a temperature of 25°C []. The pH of microemulsion-based gels in 1% aqueous solutions was determined using a digital pH meter [].
4.5.2. Texture Analysis and Viscosity
The mechanical textural properties of formulations were analyzed at 25 °C using TexturePro CT V1.3 Build 15. A TA-BT-KT texture analyzer set to TPA mode (Stable Micro Systems, Surrey, UK) was used to examine the texture profiles. To analyze texture profiles, 50 mL falcon tubes containing 10 g gel were centrifuged at 4000 rpm for 10 min. The sample was positioned 10 mm above the analytical probe, which was then lowered 10 mm into the sample after being held at a constant speed of 1 mms¹ by the trigger force for 2 min. After that, it was brought back to the surface at a velocity of 0.5 mms1, and after 5 s, a second compression was initiated. The experimental procedure was carried out at 25 °C. The viscosity of the microemulgel was determined by using a Brookfield viscometer (Brookfield instruments, AMETEK Brookfield, Mumbai, India).
4.5.3. In Vitro Drug Release Study
In Vitro Diffusion Study: Cellophane Membrane
For the in vitro diffusion study, a modified Franz diffusion cell was employed. The permeation medium used was a cellophane membrane number 12, which has a molecular weight cut-off of 12,000 Dalton, an average pore size of 2.4 nm, and an approximate capacity of 1.61 mL/cm. Prior to use, the cellophane membrane was kept in phosphate buffer (pH 5.5) for a total of 12 h. The cell was divided into donor and receptor compartments, with one gram of gel in the donor compartment and 25 milliliters of phosphate buffer in the receptor compartment. The cell was stirred at 50 rpm and the temperature set at 37 °C. After 15 min for first 1 h then after one hour at intervals of one hour, two hours, three hours, four hours, five hours, six hours, seven hours, and eight hours, 1 mL aliquots were removed and replaced with fresh, warm phosphate buffer pH 5.5 each time. At 360 nm, the absorbance of the anthralin containing sample were measured [].
In Vitro Diffusion Study: Egg Membrane
Similarly to the in vitro diffusion study with cellophane membrane, another test was carried out employing egg membrane. A raw egg was obtained, and its contents were gently removed by carefully poking a small hole in it. The membrane was then collected after being immersed in 0.1 N HCl for an overnight period. A concentrated HCl solution can be applied to remove membrane effectively. Following a cleaning with distilled water and a pH 5.5 phosphate buffer, this fresh egg membrane was employed for investigation. Every single series of measurements was performed by using a newly detached egg membrane. The exact same experimental setup was used for the cellophane membrane investigation.
In Vitro Diffusion Study: Goat Skin
The goat’s fresh dorsal skin was donated by a butcher. Skin hair was extracted and preserved in tyrode solution. Cleaned goat skin piece was placed on the epidermis facing the donor compartment. The phosphate-buffer solution (pH 5.5) was then added to the receptor compartment, which was kept at 37 °C ± 0.5 °C using magnetic stirring. The membrane was coated with 1 gm of microemulgel, equivalent to (1.2% anthralin), and a drug release process was conducted like that used with cellophane membranes.
4.5.4. Stability of Microemulgel
After three months of storage at different temperatures (8 °C, 48 °C + 75% RH, and room temperature (RT), the stability of the microemulgel formulation was evaluated. Samples were examined for organoleptic changes, physical stability, pH, and PS [].
4.5.5. Antipsoriatic Activity
The L929 mouse connective tissue cell line was received from the National Center for Cell Sciences (NCCS), Pune, India and grown in DMEM media containing 10% fetal bovine serum. The cells were kept at 37 °C in a 5% CO2 incubator and seeded at a density of 1 × 10⁴ cells/mL in culture media for 24 h. To conduct the experiment, 70 μL of cell suspension (1 × 104 cells/well) were added to 100 μL of culture media on a 96-well tissue culture plate. Sample was added to wells at varying doses (25, 50, and 100 μg/mL), while control wells contained 0.2% DMSO in PBS with cell culture. All experiments were performed in triplicate to ensure reliability and reproducibility of the results. Control wells were utilized to evaluate cell viability and calculate the percentage of cells that survived after incubation. The cultures were incubated at 37 °C with 5% CO2 for 24 h in a CO2 incubator (Thermo Scientific BB150, Thermo Fisher Scientific Inc., Waltham, MA, USA). After incubation, the media were removed and 20 μL of MTT reagent (5 mg/mL in PBS) was added. The plates were incubated for a further 4 h at 37 °C to allow live cells to convert the MTT into formazan crystals, which were then viewed under a microscope. After removing the medium, 200 μL of DMSO was added and incubated at 37 °C for 10 min while protected from light with aluminum foil. Each sample’s absorbance was measured at 570 nm using an ELISA microplate reader (Benesphera E21, Avantor Performance Materials India Private Limited, Gurgoan, India), with triplicates tested for accuracy [].
4.5.6. Determination of Antibacterial Activity
The antimicrobial activity was performed to determine anti-acne activity of anthralin containing microemulgel by pour plate method. Nutrient Agar (NA-Himedia, HiMedia Laboratories Pvt. Ltd., Mumbai, India) was prepared by dissolving 28.0 g in 1000 mL distilled water, sterilized at 121 °C for 15 min, and poured into sterile Petri plates. Propionibacterium was used for microbial assays. A 24 h pure culture was prepared by suspending the microorganisms in saline solution, streaking onto culture slants, and incubating at 37 °C. Standard solutions, including Clindamycin (25 mg/mL), were prepared in sterile water and stored under refrigeration. Plates were prepared using the pour plate method by pouring melted agar into sterile plates and allowing its solidification before use.
A sterile, non-toxic cotton swab was dipped in the standardized inoculums (turbidity was adjusted to achieve confluent growth on the Petri plate), and the entire plate’s agar surface was streaked with the swab three times, turning the plate at a 60-degree angle between streaking. The streaked inoculums were then allowed to dry for 5–15 min with the lid on. Then, using sterile well punches, a bore was punched on the prepared plates (8 mm). With the assistance of a sterile micropipette, a 10 μL dosage of produced microemulsion and standard medication Clindamycin were injected into each bore in an aseptic environment. Plates were maintained at room temperature for 30 min before being incubated for 24 h at 37 °C. The diameter of the inhibitory zones was measured using a scale in mm [].
The antibacterial potential of test compounds was assessed using the mean diameter of the zone of inhibition around the disc in millimeters. The zones of inhibition of the studied microorganisms by the substances were determined on a millimeter scale.
4.5.7. In Silico Molecular Docking
In silico molecular docking was performed using BIOVIA Discovery studio (Discovery Studio 2021) on various proteins of psoriasis and P. acne bacteria. Briefly, the ligand was minimized and docked with minimized and prepared proteins. The docking score, 2D and 3D images are shown [].
5. Conclusions
The optimized anthralin microemulgel provides a promising solution for psoriasis and acne treatment, enhancing drug solubility, stability, and skin penetration. The formulation exhibited effective antibacterial and antipsoriatic properties. Its controlled-release mechanism ensures prolonged therapeutic action. Combination therapy strategies may further enhance treatment outcomes. These findings suggest microemulgels as an alternative for improving topical drug delivery in dermatological applications.
Author Contributions
Conceptualization, S.J.; Methodology, S.S., S.J. and A.C.; Software, S.S. and S.D.; Validation, S.U. and A.B.; Formal analysis, S.D., A.C. and H.A.M.; Investigation, A.C.; Resources, S.S. and S.D.; Data curation, S.D.; Writing – original draft, S.S.; Writing – review & editing, S.J., S.U. and A.B.; Visualization, A.B.; Supervision, S.J. and A.C.; Project administration, S.U. and H.A.M.; Funding acquisition, S.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R79), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available from corresponding author on request.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R79), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, K.; Zhao, Y.; Cao, X. Global Burden and Future Trends in Psoriasis Epidemiology: Insights from the Global Burden of Disease Study 2019 and Predictions to 2030. Arch. Dermatol. Res. 2024, 316, 114. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, Regional, and Worldwide Epidemiology of Psoriasis: Systematic Analysis and Modelling Study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Microbial Dysbiosis in the Skin Microbiome and Its Psychological Consequences. Microorganisms 2024, 12, 1908. [Google Scholar] [CrossRef]
- Laochunsuwan, A.; Taweechotipatr, M.; Udompataikul, M. In Vitro Study of Antibiotic Susceptibility of Propionibacterium Acnes Strains Isolated from Acne Vulgaris Patients. J. Med. Assoc. Thai. 2017, 100, S24–S31. [Google Scholar]
- Tomida, S.; Nguyen, L.; Chiu, B.H.; Liu, J.; Sodergren, E.; Weinstock, G.M.; Li, H. Pan-Genome and Comparative Genome Analyses of Propionibacterium Acnes Reveal Its Genomic Diversity in the Healthy and Diseased Human Skin Microbiome. mBio 2013, 4, e00003-13. [Google Scholar] [CrossRef]
- Brück, J.; Fehrenbacher, B.; Schaller, M.; Eberle, F.C.; Schittek, B.; Schäfer, I.; Carevic, M.; Müller-Hermelink, E.; Holstein, J.; Ghoreschi, K. Anthralin Modulates the Expression Pattern of Cytokeratins and Antimicrobial Peptides by Psoriatic Keratinocytes. J. Dermatol. Sci. 2017, 87, 236–245. [Google Scholar] [CrossRef]
- Pavithran, K. Dithranol: Recent Views on Its Mechanism of Action. Indian J. Dermatol. Venereol. Leprol. 2001, 67, 104–105. [Google Scholar] [PubMed]
- Blaskovich, M.A.T.; Elliott, A.G.; Kavanagh, A.M.; Ramu, S.; Cooper, M.A. In Vitro Antimicrobial Activity of Acne Drugs Against Skin-Associated Bacteria. Sci. Rep. 2019, 9, 14658. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Deore, G.; Chabukswar, A. Development of Microemulsion Based Nabumetone Transdermal Delivery for Treatment of Arthritis. Recent Pat. Drug. Deliv. Formul. 2018, 12, 130–149. [Google Scholar] [CrossRef]
- Jagdale, S.; Kothekar, P. Development of Emulgel Delivery of Mupirocin for Treatment of Skin Infection. Recent Pat. Antiinfect. Drug. Discov. 2020, 15, 137–156. [Google Scholar] [CrossRef]
- Jagdale, S.; Brahmane, S.; Chabukswar, A. Optimization of Microemulgel for Tizanidine Hydrochloride. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2019, 19, 158–179. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Pawar, S. Gellified Emulsion of Ofloxacin for Transdermal Drug Delivery System. Adv. Pharm. Bull. 2017, 7, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lotlikar, V.B.; Sharma, S.; Londhe, V.Y. Unlocking Relief: Formulation, Characterization, and in Vivo Assessment of Salicylic Acid–Loaded Microemulgel for Psoriasis Management. Naunyn. Schmiedebergs. Arch. Pharmacol. 2024, 398, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Ashara, K.C.; Paun, J.S.; Soniwala, M.M.; Chavda, J.R.; Mendapara, V.P.; Mori, N.M. Microemulgel: An Overwhelming Approach to Improve Therapeutic Action of Drug Moiety. Saudi Pharm. J. 2016, 24, 452–457. [Google Scholar] [CrossRef]
- Pandey, S.; Rahdar, A.; Gholami, A.; Amiri, Z.; Abdouss, H.; Pourmadadi, M.; Abdouss, M.; Behzadmehr, R. Formulation of Double Microemulsion Based on PH-ResponsivePEG/PVA/Zinc Oxide as a Potential Nano-Platform for Drug Delivery: Green Synthesis, and Physico-Chemical Characterization. J. Mol. Liq. 2024, 410, 125563. [Google Scholar] [CrossRef]
- Bajza, Á.; Kocsis, D.; Berezvai, O.; Laki, A.J.; Lukács, B.; Imre, T.; Iván, K.; Szabó, P.; Erdő, F. Verification of P-Glycoprotein Function at the Dermal Barrier in Diffusion Cells and Dynamic “Skin-on-a-Chip” Microfluidic Device. Pharmaceutics 2020, 12, 804. [Google Scholar] [CrossRef]
- Skazik, C.; Wenzel, J.; Marquardt, Y.; Kim, A.; Merk, H.F.; Bickers, D.R.; Baron, J.M. P-Glycoprotein (ABCB1) Expression in Human Skin Is Mainly Restricted to Dermal Components. Exp. Dermatol. 2011, 20, 450–452. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, M.; Yuan, M.; Yang, D.; Zhao, M.; Zhang, S.; Wang, P.; Zhang, R.; Gao, X. The Pharmaceutical Excipient PEG400 Affect the Absorption of Baicalein in Caco-2 Monolayer Model by Interacting with UDP-Glucuronosyltransferases and Efflux Transport Proteins. Pharmacol. Res. Perspect. 2022, 10, e00928. [Google Scholar] [CrossRef]
- Ding, Z.; Jiang, Y.; Liu, X. Nanoemulsions-Based Drug Delivery for Brain Tumors. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 327–358. ISBN 9780128122181. [Google Scholar]
- Nikumbh, K.V.; Sevankar, S.G.; Patil, M.P. Formulation Development, in Vitro and in Vivo Evaluation of Microemulsion-Based Gel Loaded with Ketoprofen. Drug. Deliv. 2015, 22, 509–515. [Google Scholar] [CrossRef]
- Koroleva, M.; Nagovitsina, T.; Yurtov, E. Nanoemulsions Stabilized by Non-Ionic Surfactants: Stability and Degradation Mechanisms. Phys. Chem. Chem. Phys. 2018, 20, 10369–10377. [Google Scholar] [CrossRef]
- Wu, S.; Wang, G.; Lu, Z.; Li, Y.; Zhou, X.; Chen, L.; Cao, J.; Zhang, L. Effects of Glycerol Monostearate and Tween 80 on the Physical Properties and Stability of Recombined Low-Fat Dairy Cream. Dairy Sci. Technol. 2016, 96, 377–390. [Google Scholar] [CrossRef]
- Novita, M.; Suyatma, N.E.; Sugiyono; Yuliani, S. Physical Properties of Beeswax-Oleic Acid Mixture Nanoemulsions as Affected by Lipid Ratio and Concentration of Emulsifier. Food Res. 2024, 8, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kawai, T.; Nonomura, Y. Effects of Fatty Acid Addition to Oil-in-Water Emulsions Stabilized with Sucrose Fatty Acid Ester. J. Oleo. Sci. 2018, 67, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, B.; Zhao, J. Dispersion Process and Effect of Oleic Acid on Properties of Cellulose Sulfate-Oleic Acid Composite Film. Materials 2015, 8, 2346–2360. [Google Scholar] [CrossRef]
- Taher, S.S.; Al-Kinani, K.K.; Hammoudi, Z.M.; Ghareeb, M. mohammed Co-Surfactant Effect of Polyethylene Glycol 400 on Microemulsion Using BCS Class II Model Drug. J. Adv. Pharm. Educ. Res. 2022, 12, 63–69. [Google Scholar] [CrossRef]
- Drzymała, J. An Estimation of the Surface Ionization Constant of Oleic Acid in Aqueous Sodium Chloride Solution. Colloid Polym. Sci. 1987, 265, 613–618. [Google Scholar] [CrossRef]
- Arifin, M.; Shafira, E.; Noviani, Y.; Desmiaty, Y.; Okta, F. Nanosuspension Formula of Curcuma Xanthorriza Rhizome Dry Extract: Impact of Tween 80-PEG 400 Ratio. Sci. Pharm. 2024, 3, 112–119. [Google Scholar] [CrossRef]
- Kaibara, K.; Iwata, E.; Eguchi, Y.; Suzuki, M.; Maeda, H. Dispersion Behavior of Oleic Acid in Aqueous Media: From Micelles to Emulsions. Colloid. Polym. Sci. 1997, 275, 777–783. [Google Scholar] [CrossRef]
- Miksusanti; Apriani, E.F.; Bihurinin, A.H.B. Optimization of Tween 80 and PEG-400 Concentration in Indonesian Virgin Coconut Oil Nanoemulsion as Antibacterial against Staphylococcus Aureus. Sains Malays. 2023, 52, 1259–1272. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, Y.; Ren, H.; Wei, D. Effect of Surfactant HLB Value on Settlement Stability of Magnetorheological Fluid. Open Access Libr. J. 2018, 5, 1. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Preparation of a Novel Curcumin Nanoemulsion by Ultrasonication and Its Comparative Effects in Wound Healing and the Treatment of Inflammation. RSC Adv. 2019, 9, 20192–20206. [Google Scholar] [CrossRef] [PubMed]
- Winarti, C.; Hamaisa, A.; Lesmayati, S.; Misgiyarta, M.; Gusmaini, G. Emulsifier Ratio and Shock Treatment Effect on the Characteristics of Eucalyptus–Peppermint Oil-Based Mouthwash Nanoemulsion. J. Appl. Pharm. Sci. 2024, 14, 83–89. [Google Scholar] [CrossRef]
- Alshetaili, A.S. Design, Statistical Optimization, and Characterization of Esculin-Loaded Transliposome Nanogel for Topical Delivery: In-Vitro, Ex Vivo Study and Dermatokinetic Evaluation. J. King Saud Univ. Sci. 2024, 36, 103166. [Google Scholar] [CrossRef]
- Vanti, G.; Grifoni, L.; Bergonzi, M.C.; Antiga, E.; Montefusco, F.; Caproni, M.; Bilia, A.R. Development and Optimisation of Biopharmaceutical Properties of a New Microemulgel of Cannabidiol for Locally-Acting Dermatological Delivery. Int. J. Pharm. 2021, 607, 121036. [Google Scholar] [CrossRef]
- Mijaljica, D.; Townley, J.P.; Klionsky, D.J.; Spada, F.; Lai, M. The Origin, Intricate Nature, and Role of the Skin Surface PH (PHSS) in Barrier Integrity, Eczema, and Psoriasis. Cosmetics 2025, 12, 24. [Google Scholar] [CrossRef]
- Upadrashta, S.M.; Wurster, D.E. Spectroscopic Analysis of the Ionization Behavior of Anthralin. Spectrosc. Lett. 1988, 21, 147–155. [Google Scholar] [CrossRef]
- Sa e Melo, T.; Dubertret, L.; Prognon, P. Physiocochemical Properties and Stability of Anthralin in Model Systems and Human Skin. J. Investig. Dermatol. 1983, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Çağlar, E.Ş.; Özdemir, Z.Ö.; Nath, E.Ö.; Barak, T.H.; Şentürk, T.B. In Vitro Evaluation of Skin Related Enzyme Inhibitory Effect and Emulgel Formulation Development Studies of Onobrychis Argyrea Subsp. Argyrea with Phytochemical Analysis. Chem. Biodivers. 2024, 21, e202400139. [Google Scholar] [CrossRef]
- Zhou, F.; Yao, H.; Ma, Z.; Hu, X. Investigating Small Molecule Compounds Targeting Psoriasis Based on CMAP Database and Molecular Dynamics Simulation. Ski. Res. Technol. 2023, 29, e13301. [Google Scholar] [CrossRef]
- Madan, S.; Akhtar, A.; Walia, R.; Hawwal, M.F.; Alam, P.; Tyagi, R.; Sahu, N.; Fantoukh, O.I.; Almarabi, I.; Saxena, S. Multi-Target Mechanism of Solanum Xanthocarpum for Treatment of Psoriasis Based on Network Pharmacology and Molecular Docking. Saudi Pharm. J. 2023, 31, 101788. [Google Scholar] [CrossRef]
- Chadha, H.; Chandra, P.; Meher, B.; Sachan, N. Molecular Docking Analysis of Psoriasis Specific Mediator IL-17 with Active Phytoconstituents from Cocos Nucifera, Carica Papaya, Ichnocarpus Frutescens. Lett. Appl. NanoBioScience 2025, 14, 18. [Google Scholar] [CrossRef]
- Ibezim, A.; Onah, E.; Dim, E.N.; Ntie-Kang, F. A Computational Multi-Targeting Approach for Drug Repositioning for Psoriasis Treatment. BMC Complement. Med. Ther. 2021, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Hari, G.; Kishore, A.; Karkala, S.R.P. Treatments for Psoriasis: A Journey from Classical to Advanced Therapies. How Far Have We Reached? Eur. J. Pharmacol. 2022, 929, 175147. [Google Scholar] [CrossRef]
- Yue, C.; Feng, J.; Gao, A. A Network Pharmacology and Molecular Docking Investigation on the Mechanisms of Shanyaotianhua Decoction (STT) as a Therapy for Psoriasis. Medicine 2023, 102, E34859. [Google Scholar] [CrossRef] [PubMed]
- Rapalli, V.K.; Waghule, T.; Gorantla, S.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Psoriasis: Pathological Mechanisms, Current Pharmacological Therapies, and Emerging Drug Delivery Systems. Drug Discov. Today 2020, 25, 2212–2226. [Google Scholar] [CrossRef]
- Darusman, F.; Fakih, T.M. Comprehensive In Silico Analysis of Christinin Molecular Behaviour from Ziziphus Spina-Christi Leaves on Propionibacterium Acnes. Pharm. Sci. Res. 2021, 8, 5. [Google Scholar]
- Cheon, D.; Kim, J.; Jeon, D.; Shin, H.C.; Kim, Y. Target Proteins of Phloretin for Its Anti-Inflammatory and Antibacterial Activities against Propionibacterium Acnes-Induced Skin Infection. Molecules 2019, 24, 1319. [Google Scholar] [CrossRef]
- Aloliqi, A.A. Towards Identification of Therapeutics against Multi-Infections and Cancers Causing Propionibacterium Acnes: Molecular Modeling and Dynamics Simulation Investigation. J. Mol. Liq. 2024, 415, 126373. [Google Scholar] [CrossRef]
- Lu, X.; Han, Y.; Zhang, Y.; Li, R.; Xu, J.; Yang, J.; Yao, J.; Lv, Z. An Integrated Network Pharmacology and Molecular Docking Approach to Reveal the Role of Arctigenin against Cutibacterium Acnes-Induced Skin Inflammation by Targeting the CYP19A1. Chem. Biol. Drug. Des. 2024, 104, e14598. [Google Scholar] [CrossRef]
- Grange, P.A.; Raingeaud, J.; Morelle, W.; Marcelin, A.G.; Calvez, V.; Dupin, N. Characterization of a Propionibacterium Acnes Surface Protein as a Fibrinogen-Binding Protein. Sci. Rep. 2017, 7, 6428. [Google Scholar] [CrossRef]
- Raphael, V.P.; Johnson, R.; Vidhya Thomas, K.; Kakkassery, J.T.; Ragi, K. In Vitro Antibacterial and in Silico Docking Studies of Two Schiff Bases on Staphylococcus Aureus and Its Target Proteins. Future J. Pharm. Sci. 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Alyami, M.H.; Alyami, H.S.; Alshehri, A.A.; Alsharif, W.K.; Shaikh, I.A.; Algahtani, T.S. Tamoxifen Citrate Containing Topical Nanoemulgel Prepared by Ultrasonication Technique: Formulation Design and In Vitro Evaluation. Gels 2022, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, R.; Khan, H.M.S.; Rasool, F.; Khan, K.U.R.; Umair, M.; Esatbeyoglu, T.; Korma, S.A. Development of Phytocosmeceutical Microemulgel Containing Flaxseed Extract and Its In Vitro and In Vivo Characterization. Pharmaceutics 2022, 14, 1656. [Google Scholar] [CrossRef]
- Ullah, N.; Amin, A.; Farid, A.; Selim, S.; Rashid, S.A.; Aziz, M.I.; Kamran, S.H.; Khan, M.A.; Rahim Khan, N.; Mashal, S.; et al. Development and Evaluation of Essential Oil-Based Nanoemulgel Formulation for the Treatment of Oral Bacterial Infections. Gels 2023, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Hemanth, K.M.; Sunil, K.J.; Spandana, V.; Sandeep, B.P. Anticancer Activity of Terpenoid Saponin Extract of Psidium Guajava on MCF-7 Cancer Cell Line Using DAPI and MTT Assays. Afr. J. Pharm. Pharmacol. 2021, 15, 206–211. [Google Scholar] [CrossRef]
- Poomanee, W.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Khunkitti, W.; Leelapornpisid, P. In-Vitro Investigation of Anti-Acne Properties of Mangifera Indica L. Kernel Extract and Its Mechanism of Action against Propionibacterium Acnes. Anaerobe 2018, 52, 64–74. [Google Scholar] [CrossRef]
- Al Jaouni, S.K.; Khan, H.M.S.; Yousuf, M.; Kamran, M.; Conte-Junior, C.A.; Selim, S.; Aadil, R.M.; Korma, S.A.; Umair, M.; Khan, K.U.R.; et al. Chemical Profiling, Formulation Development, In Vitro Evaluation and Molecular Docking of Piper Nigrum Seeds Extract Loaded Emulgel for Anti-Aging. Molecules 2022, 27, 5990. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).