Carbon-Rich Nanocomposites Based on Polyaniline/Titania Nanotubes Precursor: Synergistic Effect Between Surface Adsorption and Photocatalytic Activity
Abstract
1. Introduction
2. Results and Discussion
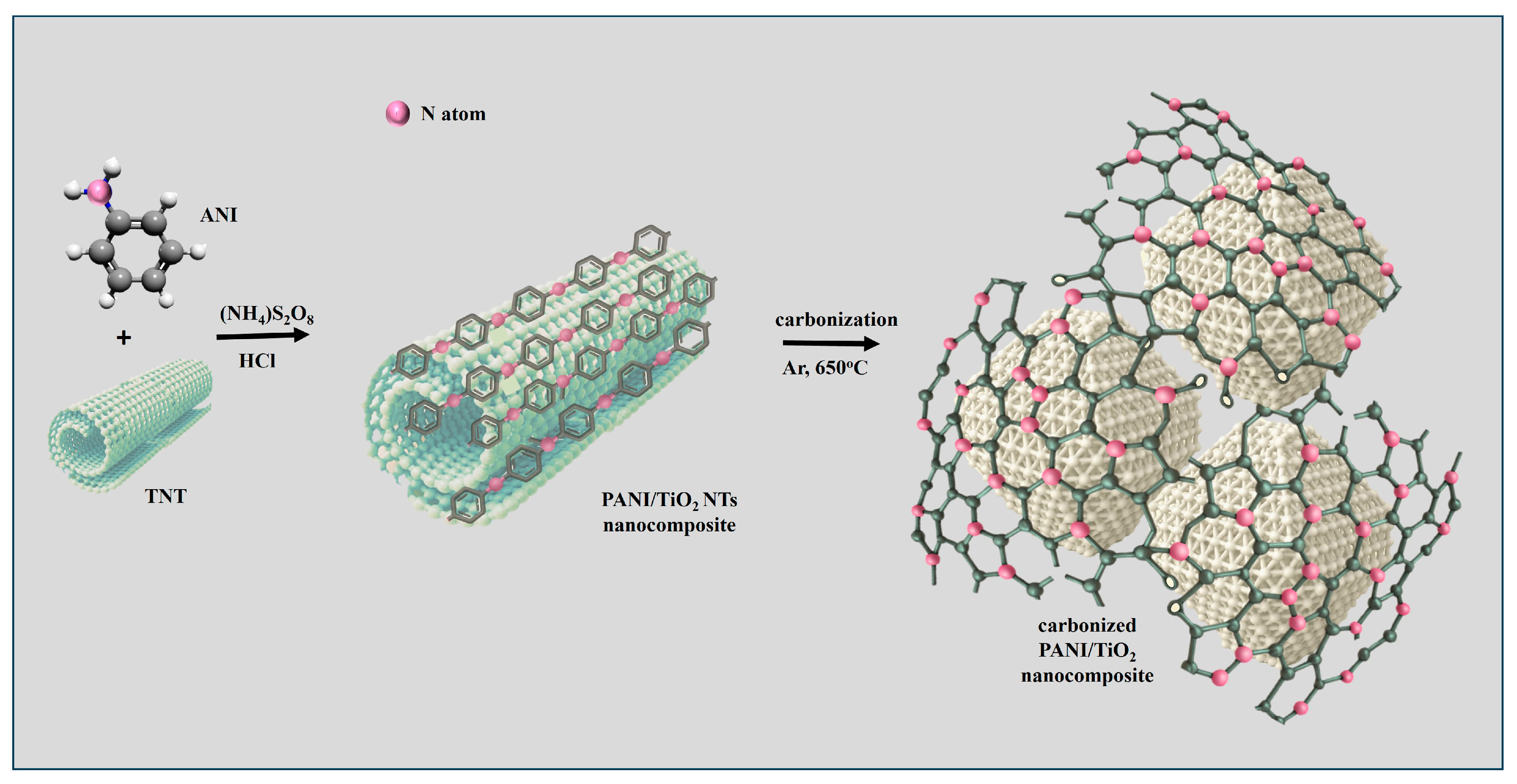
2.1. Morphological Properties of Synthesized Materials
2.2. Molecular Structure
2.3. Optical Properties and Band Structure Analysis
2.4. Crystallographic Analysis
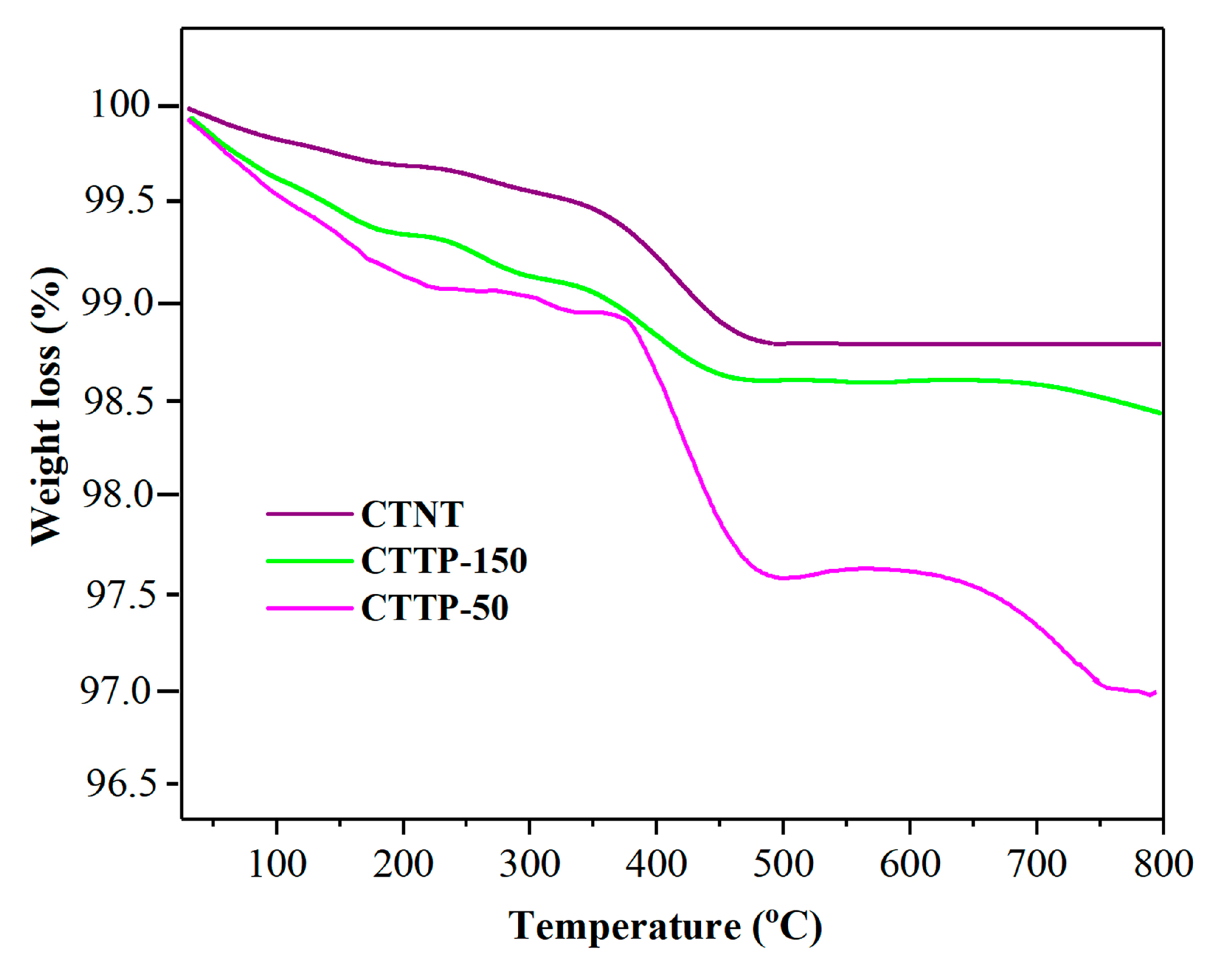
2.5. Thermal Analysis
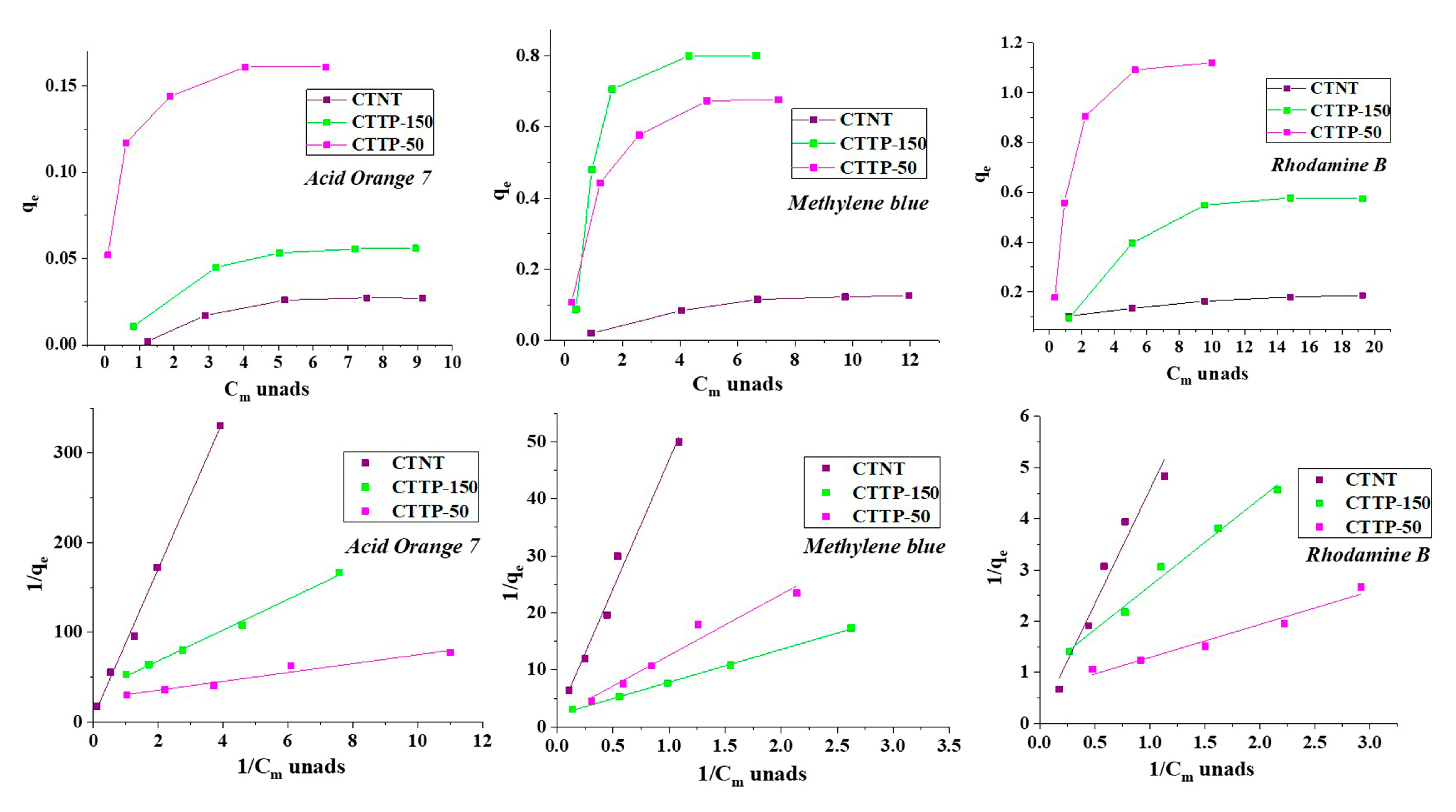
2.6. Adsorption Study
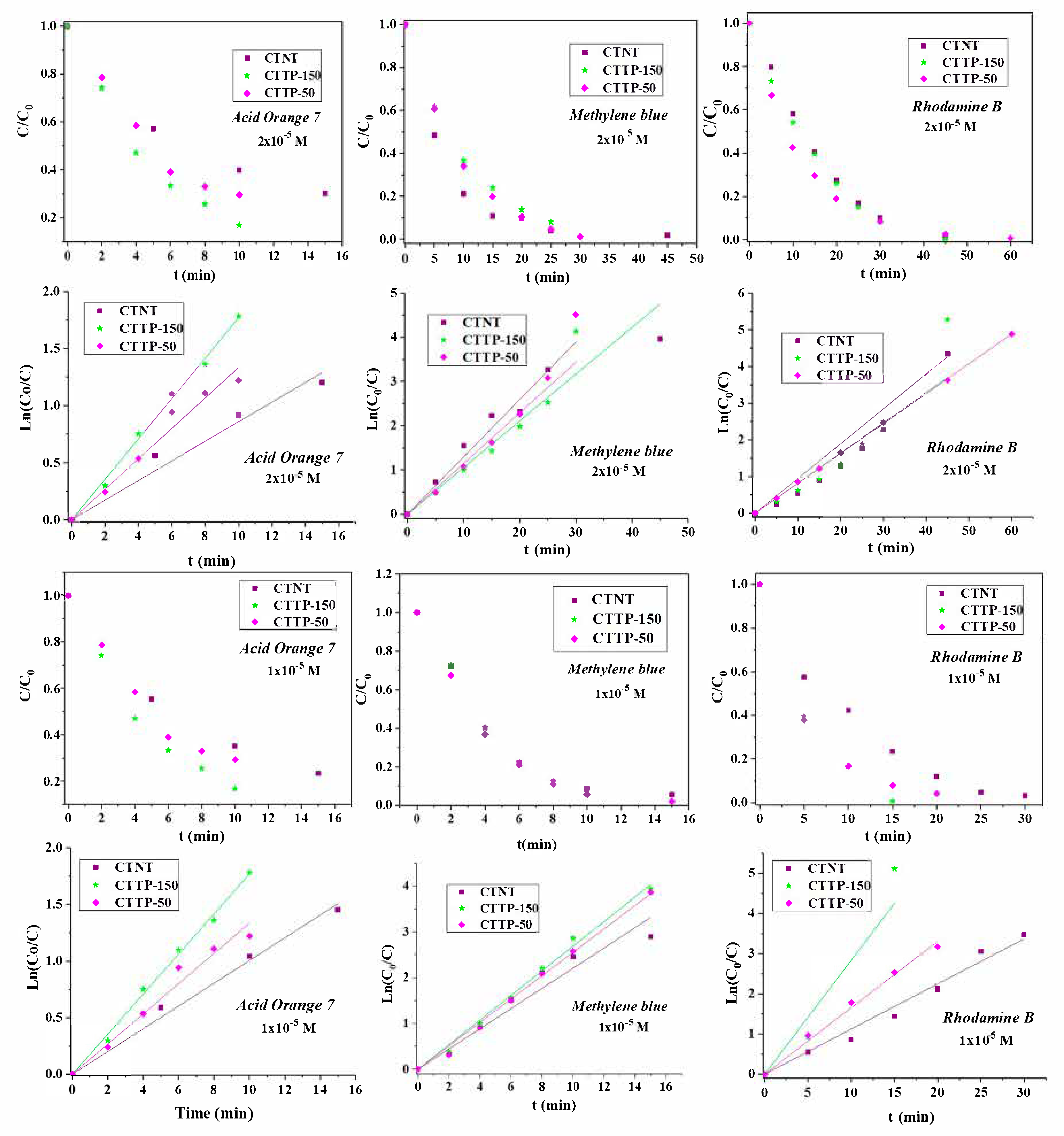
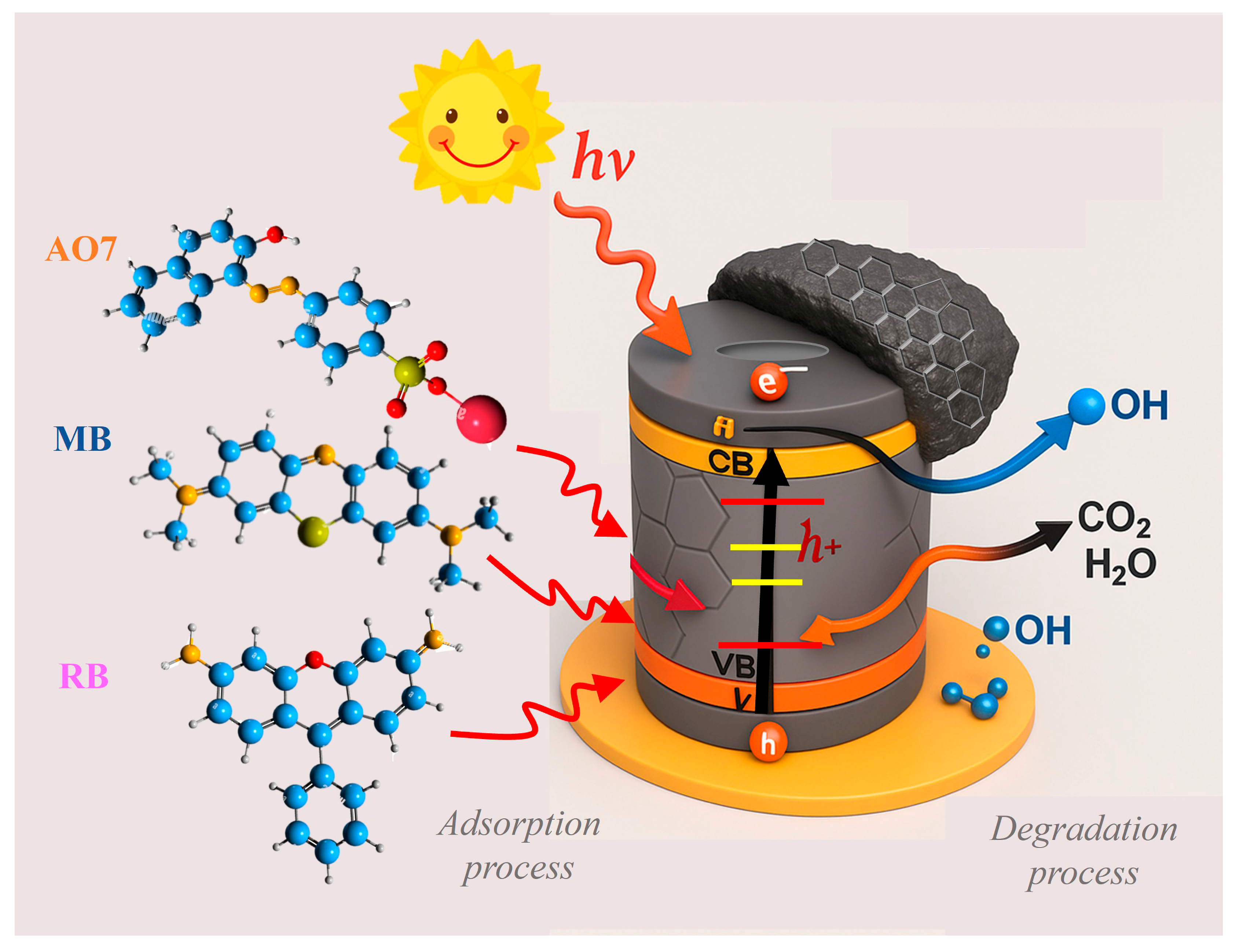
2.7. Photocatalytic Study
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Titania Nanotubes
3.3. Synthesis of Carbonized PANI/TiO2 NT Nanocomposites
3.4. Characterization
3.5. Adsorption Properties Study
3.6. Photocatalytic Ability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaharia, C.; Suteu, D. Textile Organic Dyes–Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents–A Critical Overview. In Organic Pollutants Ten Years After the Stockholm Convention: Environmental and Analytical Update; Intech Open: Rijeka, Croatia, 2012; p. 32373. [Google Scholar]
- Rahman, M.; Shaheen, S.; Ahmad, T. Photocatalytic Transformation of Organic Pollutants and Remediation Strategies of Carbon Emissions and Nitrogen Fixation in Inland Water. Mater. Today Catal. 2025, 9, 100103. [Google Scholar] [CrossRef]
- Sarkhosh, M. Utilizing Photocatalysis. In New Technologies for Energy Transition Based on Sustainable Development Goals; Kasinathan, K., Ladchumananandasivam, R., Mohamed, S.B., Eds.; Springer: Singapore, 2024; pp. 154–196. [Google Scholar]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Pichat, P. Are TiO2 Nanotubes Worth Using in Photocatalytic Purification of Air and Water? Molecules 2014, 19, 15075–15087. [Google Scholar] [CrossRef]
- Abdelfattah, I.; El-Shamy, A.M. A Comparative Study for Optimizing Photocatalytic Activity of TiO2-Based Composites with ZrO2, ZnO, Ta2O5, SnO, Fe2O3, and CuO Additives. Sci. Rep. 2024, 14, 27175. [Google Scholar] [CrossRef]
- Awofiranye, O.S.; Modise, S.J.; Naidoo, E.B. Overview of Polymer—TiO2 Catalyst for Aqueous Degradation of Pharmaceuticals in Heterogeneous Photocatalytic Process. J. Nanopart. Res. 2020, 22, 168. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.-Y.; Zang, X.; Fu, Y.-Q.; Jiang, S.-T.; Li, J.-B.; Wang, Q. A Review on Chitosan/Metal Oxide Nanocomposites for Applications in Environmental Remediation. Int. J. Biol. Macromol. 2024, 254, 127887. [Google Scholar] [CrossRef]
- Farooq, N.; Kallem, P.; Rehman, Z.U.; Khan, M.I.; Gupta, R.K.; Tahseen, T.; Mushtaq, Z.; Ejaz, N.; Shanableh, A. Recent Trends of Titania (TiO2) Based Materials: A Review on Synthetic Approaches and Potential Applications. J. King Saud Univ. Sci. 2024, 36, 103210. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present Applications of Titanium Dioxide for the Photocatalytic Removal of Pollutants from Water: A Review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An Overview on Limitations of TiO2-Based Particles for Photocatalytic Degradation of Organic Pollutants and the Corresponding Countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Radoičić, M.; Šaponjić, Z.; Janković, I.A.; Ćirić-Marjanović, G.; Ahrenkiel, S.P.; Čomor, M.I. Improvements to the Photocatalytic Efficiency of Polyaniline Modified TiO2 Nanoparticles. Appl. Catal. B Environ. 2013, 136–137, 133–139. [Google Scholar] [CrossRef]
- Radoičić, M.; Ćirić-Marjanović, G.; Spasojević, V.; Ahrenkiel, P.; Mitrić, M.; Novaković, T.; Šaponjić, Z. Superior Photocatalytic Properties of Carbonized PANI/TiO2 Nanocomposites. Appl. Catal. B Environ. 2017, 213, 155–166. [Google Scholar] [CrossRef]
- Jiang, D.; Otitoju, T.A.; Ouyang, Y.; Shoparwe, N.F.; Wang, S.; Zhang, A.; Li, S. A Review on Metal Ions Modified TiO2 for Photocatalytic Degradation of Organic Pollutants. Catalysts 2021, 11, 1039. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Tasisa, Y.E.; Sarma, T.K.; Krishnaraj, R.; Sarma, S. Band Gap Engineering of Titanium Dioxide (TiO2) Nanoparticles Prepared via Green Route and Its Visible Light Driven for Environmental Remediation. Results Chem. 2024, 11, 101850. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent Advances in Polyaniline Research: Polymerization Mechanisms, Structural Aspects, Properties and Applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, A.; Kumar, N.; Tiwari, A.; Lal, M.; Arya, S. A Review on Polyaniline and Its Composites: From Synthesis to Properties and Progressive Applications. J. Mater. Sci. 2024, 59, 6206–6244. [Google Scholar] [CrossRef]
- Gilja, V.; Novaković, K.; Travas-Sejdic, J.; Hrnjak-Murgić, Z.; Roković, M.K.; Žic, M. Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in Degradation of Azo Dye in Wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, C.; Yang, C.; Wang, X.; Wan, Z.; Zhao, E.; Xiao, Y.; Zhao, W.; Ma, M.; Chen, D.; et al. A Review on Conductive Polymers-Modified TiO2 Photocatalyst for Environmental Remediation. J. Environ. Chem. Eng. 2025, 13, 116518. [Google Scholar] [CrossRef]
- Radoičić, M.B.; Milošević, M.V.; Miličević, D.S.; Suljovrujić, E.H.; Ćirić-Marjanović, G.N.; Radetić, M.M.; Šaponjić, Z.V. Influence of TiO2 Nanoparticles on Formation Mechanism of PANI/TiO2 Nanocomposite Coating on PET Fabric and Its Structural and Electrical Properties. Surf. Coat. Technol. 2015, 278, 38–47. [Google Scholar] [CrossRef]
- Radoičić, M.; Ćirić-Marjanović, G.; Šaponjić, Z.V.; Mitrić, M.; Konstantinović, Z.; Stoiljković, M.; Nedeljković, J.M. Structural and Magnetic Properties of Nanocomposites Based on Nanostructured Polyaniline and Titania Nanotubes. J. Mater. Sci. 2013, 48, 5776–5787. [Google Scholar] [CrossRef]
- Radoičić, M.; Šaponjić, Z.; Ćirić-Marjanović, G.; Konstantinović, Z.; Mitrić, M.; Nedeljković, J. Ferromagnetic Polyaniline/TiO2 Nanocomposites. Polym. Compos. 2012, 33, 1482–1493. [Google Scholar] [CrossRef]
- Radoičić, M.; Šaponjić, Z.; Nedeljković, J.; Ćirić-Marjanović, G.; Stejskal, J. Self-Assembled Polyaniline Nanotubes and Nanoribbons/Titanium Dioxide Nanocomposites. Synth. Met. 2010, 160, 1325–1334. [Google Scholar] [CrossRef]
- Galloni, M.G.; Della Pina, C.; Bortolotto, V.; Nikonova, V.; Falletta, E.; Bianchi, C.L. Highly porous polyaniline (PANI): A novel green catalytic method for morphology control. J. Mater. Sci. 2025, 60, 5300–5325. [Google Scholar] [CrossRef]
- Mentus, S.; Ćirić-Marjanović, G.; Trchová, M.; Stejskal, J. Conducting Carbonized Polyaniline Nanotubes. Nanotechnology 2009, 20, 245601. [Google Scholar] [CrossRef]
- Janošević, A.; Pašti, I.; Gavrilov, N.; Mentus, S.; Ćirić-Marjanović, G.; Krstić, J.; Stejskal, J. Micro/mesoporous conducting carbonized polyaniline 5-sulfosalicylate nanorods/nanotubes: Synthesis, characterization and electrocatalysis. Synth. Met. 2011, 161, 2179–2184. [Google Scholar] [CrossRef]
- Gavrilov, N.; Pašti, I.A.; Vujković, M.; Travas-Sejdic, J.; Ćirić-Marjanović, G.; Mentus, S.V. High-Performance Charge Storage by N-Containing Nanostructured Carbon Derived from Polyaniline. Carbon 2012, 50, 3915–3927. [Google Scholar] [CrossRef]
- Janošević, A.; Pašti, I.; Gavrilov, N.; Mentus, S.; Krstić, J.; Mitrić, M.; Travas-Sejdic, J.; Ćirić-Marjanović, G. Microporous Conducting Carbonized Polyaniline Nanorods: Synthesis, Characterization and Electrocatalytic Properties. Microporous Mesoporous Mater. 2012, 152, 50–57. [Google Scholar] [CrossRef]
- Belhadj, H.; Moulefera, I.; Sabantina, L.; Benyoucef, A. Effects of Incorporating Titanium Dioxide with Titanium Carbide on Hybrid Materials Reinforced with Polyaniline: Synthesis, Characterization, Electrochemical and Supercapacitive Properties. Fibers 2022, 10, 46. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Wang, C.; Cui, B.; Wang, C. Multifunctional C/TiO2 from MXene/Polyaniline for Electromagnetic Protection and Supercapacitor. Energy Mater. Adv. 2024, 5, 0070. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Carbon Black, Titanium Dioxide, and Talc; World Health Organization: Lyon, France, 2010; Volume 93. [Google Scholar]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Swanepoel, R. Determination of the Thickness and Optical Constants of Amorphous Silicon. J. Phys. E Sci. Instrum. 1983, 16, 1214. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Stetsiv, Y.; Yatsyshyn, M.; Reshetnyak, O. Optical Parameters of Polyaniline Films on a Polyethylene Terephthalate Substrate. Proc. Shevchenko Sci. Soc. Ser. Chem. Sci. 2024, 75, 127–135. [Google Scholar] [CrossRef]
- Kolhar, P.; Sannakki, B.; Verma, M.; SV, P.; Alshehri, M.; Shah, N.A. Synthesis, Characterization and Investigation of Optical and Electrical Properties of Polyaniline/Nickel Ferrite Composites. Nanomaterials 2023, 13, 2223. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen Defect Dependent Variation of Band Gap, Urbach Energy and Luminescence Property of Anatase, Anatase–Rutile Mixed Phase and of Rutile Phases of TiO2 Nanoparticles. Phys. E 2014, 56, 364–371. [Google Scholar] [CrossRef]
- Kurban, M.; Polat, C.; Serpedin, E.; Kurban, H. Enhancing the Electronic Properties of TiO2 Nanoparticles through Carbon Doping: An Integrated DFTB and Computer Vision Approach. Comput. Mater. Sci. 2024, 244, 113248. [Google Scholar] [CrossRef]
- Yao, S.; Ma, Y.; Xu, T.; Wang, Z.; Lv, P.; Zheng, J.; Ma, C.; Yu, K.; Wei, W.; Ostrikov, K. Ti–C Bonds Reinforced TiO2@C Nanocomposite Na-Ion Battery Electrodes by Fluidized-Bed Plasma-Enhanced Chemical Vapor Deposition. Carbon 2021, 171, 524–531. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, S.P.; Wang, H.; Cui, Y.; Jiang, W.; Liu, S.; Wang, N.; Liu, C.; Chai, W.; Ding, W. Study on the Chemical Bond Structure and Chemical Stability of N Doped into TiO2 Film by N Ion Beam Implantation. Micro Nano Lett. 2016, 11, 758–761. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Kumar, R.; Choudhary, R.; Kolay, S.; Pandey, O.P.; Singh, K.; Bhargava, P. Carbon Coated Titanium Dioxide (CC-TiO2) as an Efficient Material for Photocatalytic Degradation. Energy Adv. 2022, 1, 926–934. [Google Scholar] [CrossRef]
- Altannyhi, K.A.; Elnaggar, E.M.; Elsayed, B.A.; Elsenety, M.M. Synthesis, characterization, and coating application of a highly conductive polyaniline-TiO2 nanocomposite. Egypt. J. Pet. 2024, 33, 14. [Google Scholar] [CrossRef]
- Rajakani, P.; Vedhi, C. Electrocatalytic Properties of Polyaniline–TiO2 Nanocomposites. Int. J. Ind. Chem. 2015, 6, 247–259. [Google Scholar] [CrossRef]
- Hashemi Monfared, A.; Jamshidi, M. Investigation of the corrosion protection performance of polyaniline-based nanocomposite coatings: A review. Prog. Org. Coat. 2019, 136, 105257. [Google Scholar] [CrossRef]
- Charles, H.; Anthony, P.D.; Ian, A.E. A general treatment and classification of the solute adsorption isotherm. Part II. Experimental interpretation. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar]
- Yao, Y.; Li, G.H.; Ciston, S.N.; Lueptow, R.M.; Gray, K.A. Photoreactive TiO2/carbon nanotube composites: Synthesis and reactivity. Environ. Sci. Technol. 2008, 42, 4952–4957. [Google Scholar] [CrossRef]
- Bourikas, K.; Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Adsorption of Acid Orange 7 on the surface of titanium dioxide. Langmuir 2005, 21, 9222–9230. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Manohar, P.; Luong, J.H.T.; Gedanken, A. Photocatalytic degradation of organic dyes and antimicrobial activities by polyaniline–nitrogen-doped carbon dot nanocomposite. Nanomaterials 2021, 11, 1128. [Google Scholar] [CrossRef]
- Wojtoniszak, M.; Dolat, D.; Morawski, A.; Mijowska, E. Carbon-modified TiO2 for photocatalysis. Nanoscale Res. Lett. 2012, 7, 235. [Google Scholar] [CrossRef]
- Police, A.K.R.; Vattikuti, S.P.; Baik, Y.J.; Chan, B. Eco-friendly, hydrogen fluoride-free, morphology-oriented synthesis of TiO2 with exposed (001) facets. Ceram. Int. 2019, 45, 2178–2184. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.; Wang, T.; Xu, M.; Yang, X.; Hou, J.; Cao, D.; Wang, Q. Construction of Z-scheme CdS/Ag/TiO2 NTs Photocatalysts for Photocatalytic Dye Degradation and Hydrogen Evolution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 276, 121215. [Google Scholar] [CrossRef]
- Wang, K.; Yang, N.; Xiao, B.; Shen, Y.; Zi, B.; Qiu, Z.; Zhou, T.; Hu, R.; Zhan, W.; Qiu, G.; et al. C,N-doped TiO2 nanoparticles with abundant surface Ti³⁺ and oxygen vacancies for efficient visible-light photocatalysis. ACS Appl. Nano Mater. 2024, 7, 923–935. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Mechanism of the OH Radical Generation in Photocatalysis with TiO2 of Different Crystalline Types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Rettenmaier, K.; Berger, T. Impact of Nanoparticle Consolidation on Charge Separation Efficiency in Anatase TiO2 Films. Front. Chem. 2021, 9, 772116. [Google Scholar] [CrossRef]
- Sieland, F.; Schneider, J.; Bahnemann, D.W. Photocatalytic activity and charge carrier dynamics of TiO2 powders with a binary particle size distribution. Phys. Chem. Chem. Phys. 2018, 20, 8119–8132. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Sienkiewicz, A.; Wanag, A.; Rokicka-Konieczna, P.; Morawski, A.W. The Role of Adsorption in the Photocatalytic Degradation of Organic Dyes by TiO2-Based Materials. Catalysts 2021, 11, 172. [Google Scholar] [CrossRef]
- Sha, M.S.; Anwar, H.; Musthafa, F.N.; Al-Lohedan, H.; Alfarwati, S.; Rajabathar, J.R.; Alahmad, J.K.; Cabibihan, J.-J.; Karnan, M.; Sadasivuni, K.K. Photocatalytic degradation of organic dyes using reduced graphene oxide (rGO). Sci. Rep. 2024, 14, 3608. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania Nanotubes Prepared by Chemical Processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Lin, X.; Li, M.; Li, Y.; Chen, W. Enhancement of the Catalytic Activity of Ordered Mesoporous TiO2 by Using Carbon Fiber Support and Appropriate Evaluation of Synergy between Surface Adsorption and Photocatalysis by Langmuir–Hinshelwood (L–H) Integration Equation. RSC Adv. 2015, 5, 105227–105238. [Google Scholar] [CrossRef]
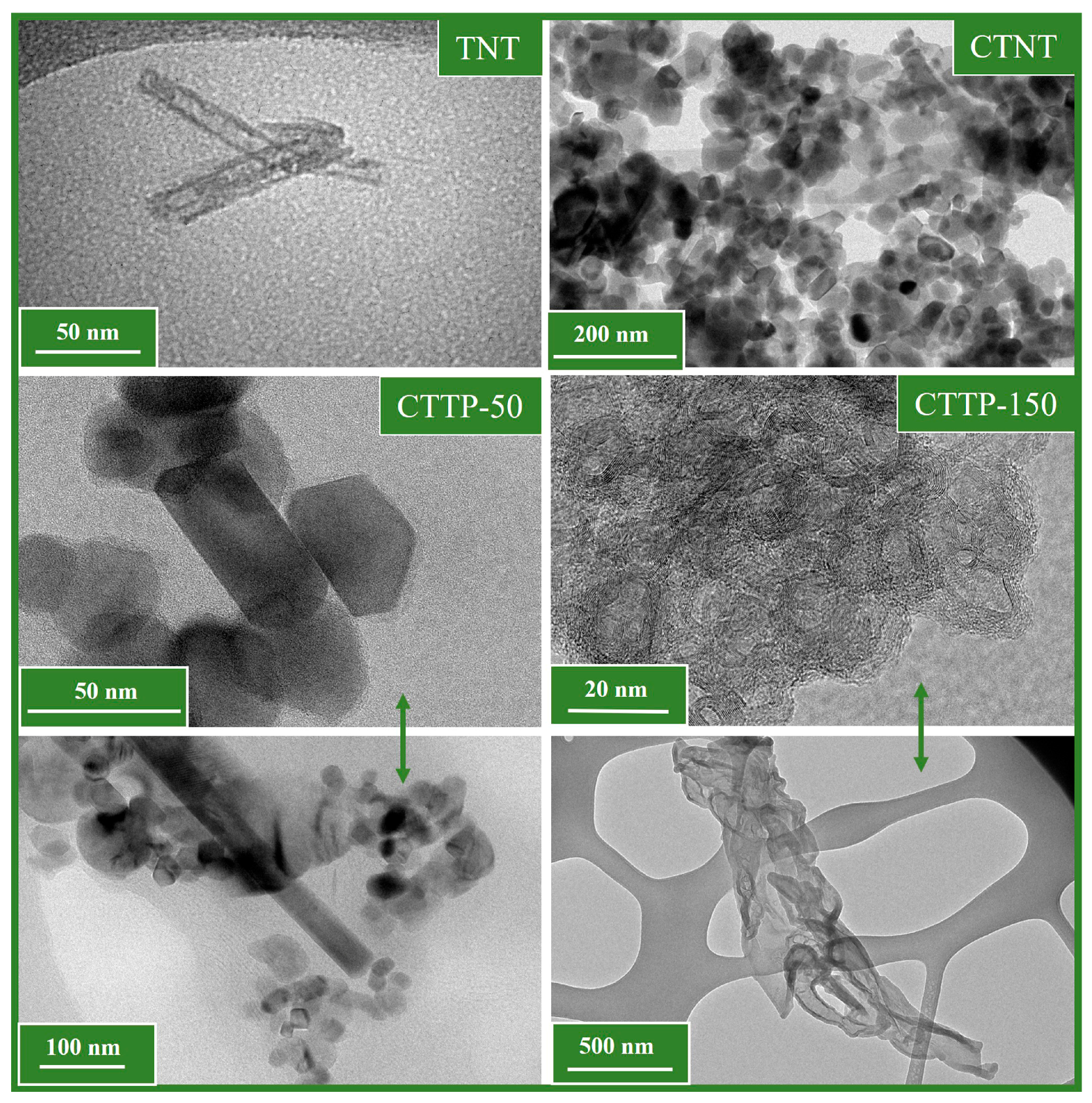
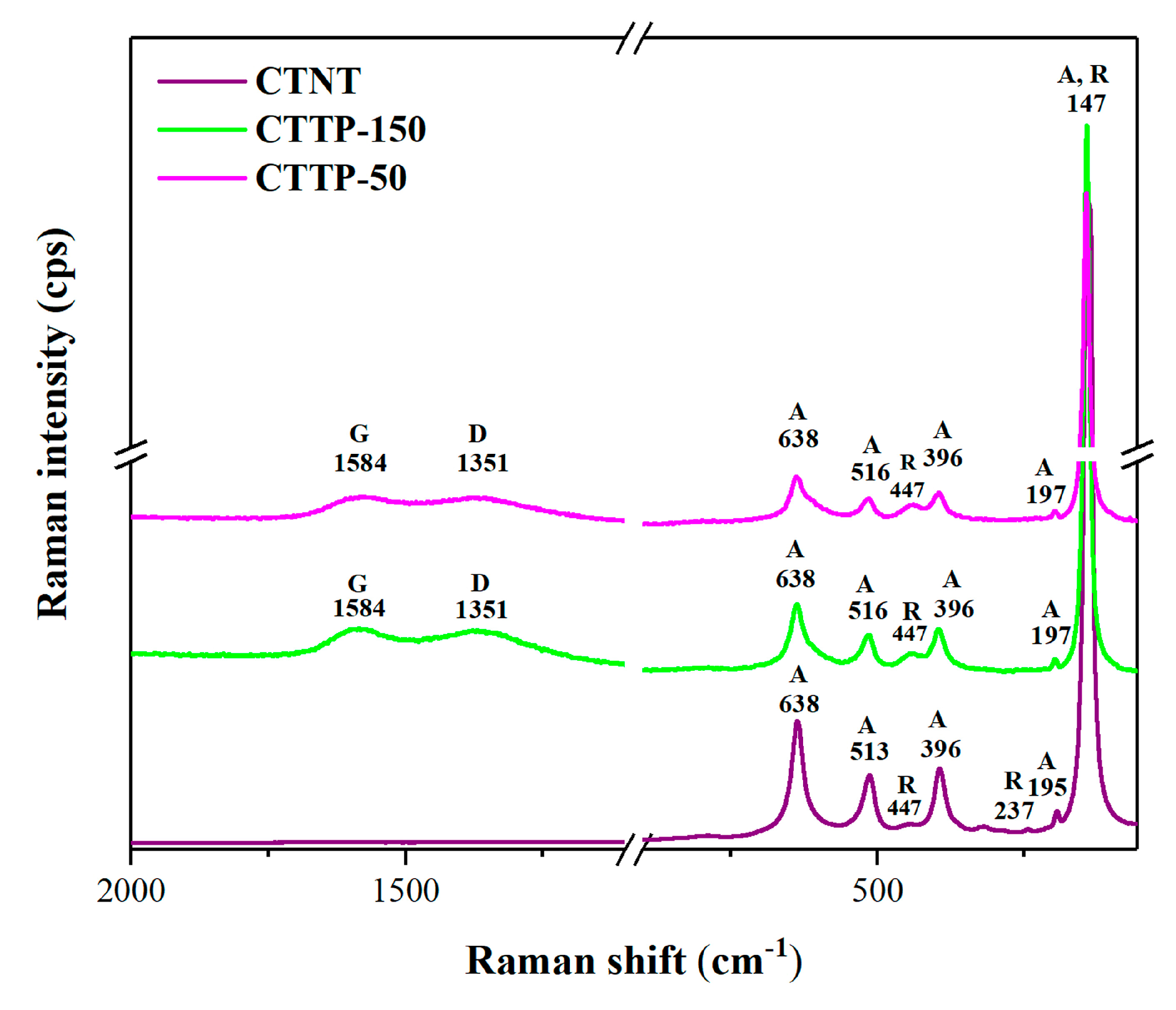
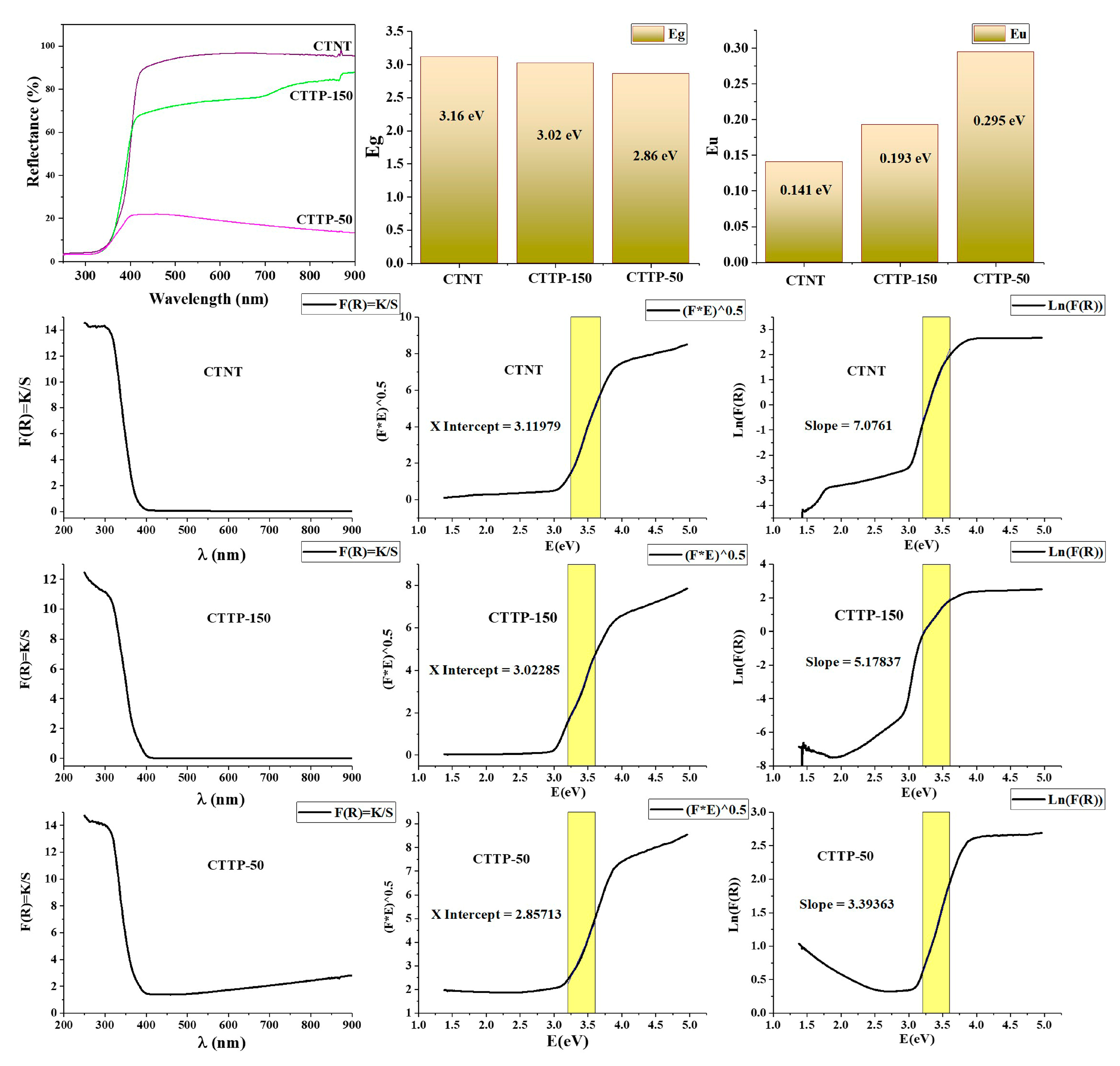
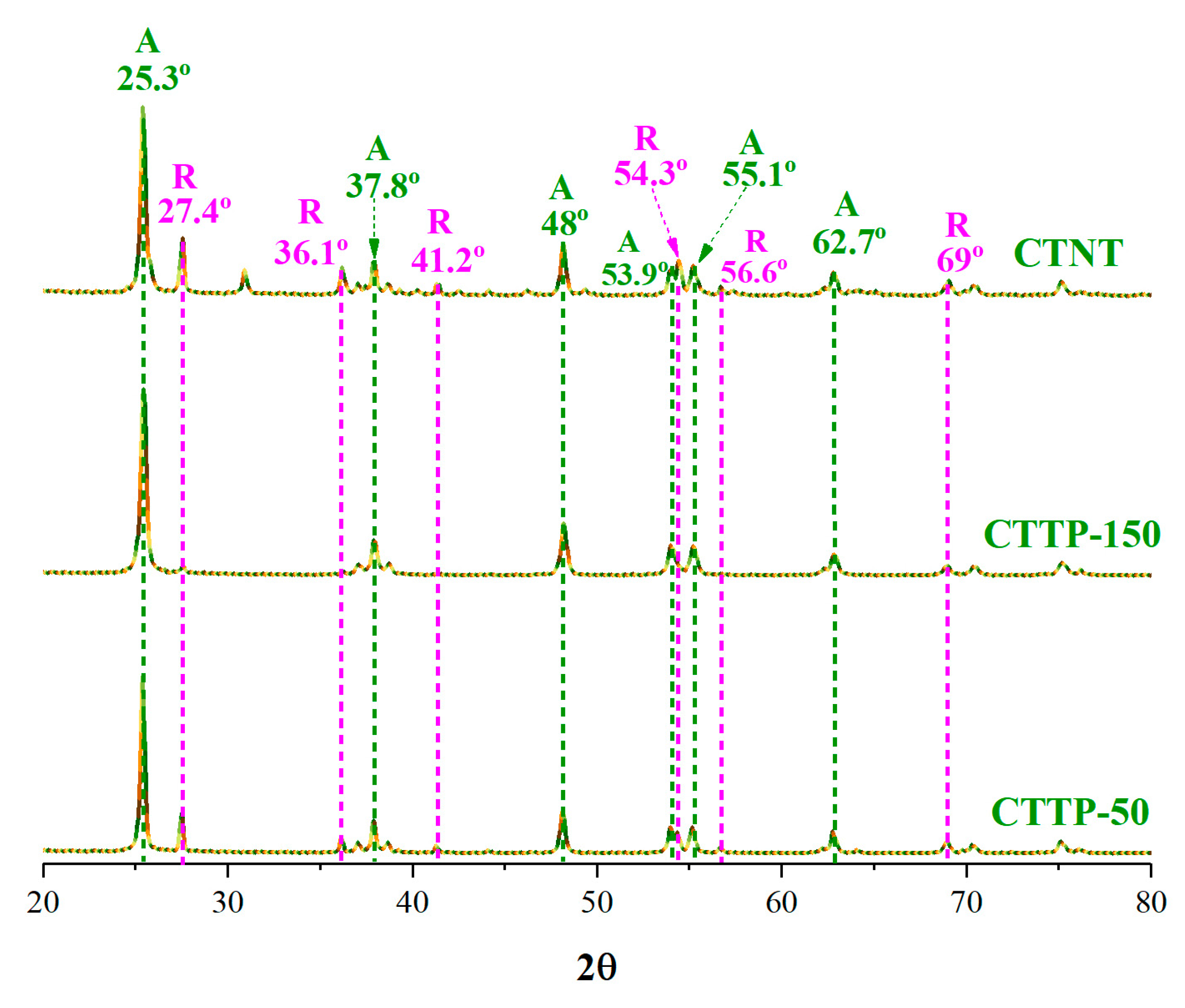
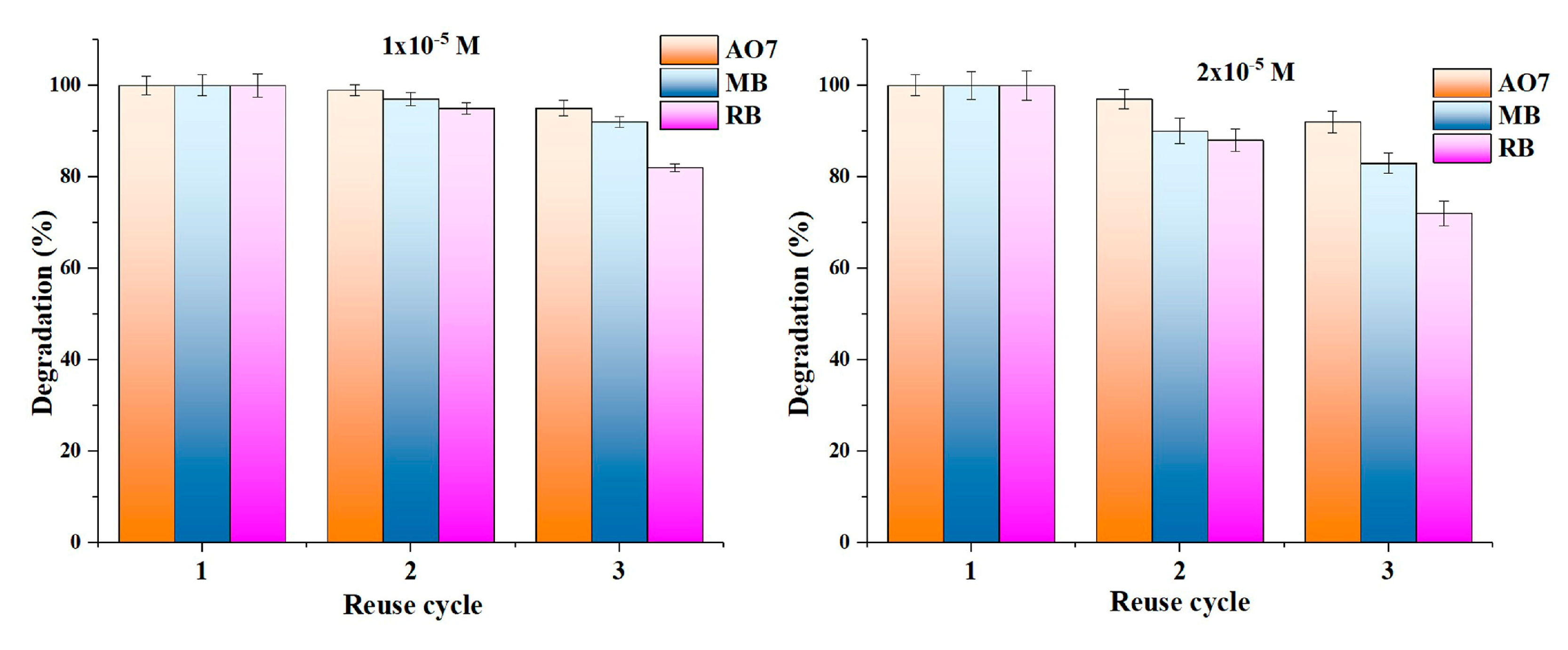
| Sample | Rutile (%) | Anatase (%) | FWHM (°) | Crystallite Size (nm) |
|---|---|---|---|---|
| CTNT | 16.8 | 83.92 | 0.442 | 18.4 |
| CTTP-150 | 10.03 | 89.97 | 0.457 | 17.8 |
| CTTP-50 | 22.65 | 77.35 | 0.302 | 27.0 |
| Sample | Acid Orange 7 (AO7) | Methylene Blue (MB) | Rhodamine B (RB) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax | Kads | kapp | qmax | Kads | kapp | qmax | Kads | kapp | ||||
| 1 × 10−5 M (3.50 mg/L) | 2 × 10−5 M (7.00 mg/L) | 1 × 10−5 M (3.74 mg/L) | 2 × 10−5 M (7.48 mg/L) | 1 × 10−5 M (4.79 mg/L) | 2 × 10−5 M (9.58 mg/L) | |||||||
| CTNT | 0.01839 | 0.06573 | 0.10157 | 0.07937 | 0.32484 | 0.03535 | 0.21876 | 0.10547 | 0.1957 | 0.02195 | 0.10938 | 0.09482 |
| CTTP-150 | 0.03938 | 5.14055 | 0.17188 | 0.17797 | 0.57774 | 0.36513 | 0.26564 | 0.11328 | 1.55003 | 1.00022 | 0.28126 | 0.11225 |
| CTTP-50 | 0.02974 | 1.95749 | 0.13282 | 0.13013 | 0.44623 | 0.17113 | 0.25001 | 0.12501 | 1.02208 | 0.57312 | 0.16407 | 0.08118 |
| Sample | Acid Orange 7 (AO7) | Methylene Blue (MB) | Rhodamine B (RB) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t (min) | % | t (min) | % | t (min) | % | t (min) | % | t (min) | % | t (min) | % | |
| 1 × 10−5 M (3.50 mg/L) | 2 × 10−5 M (7.00 mg/L) | 1 × 10−5 M (3.74 mg/L) | 2 × 10−5 M (7.48 mg/L) | 1 × 10−5 M (4.79 mg/L) | 2 × 10−5 M (9.58 mg/L) | |||||||
| CTNT | 10 | 64.80 | 10 | 60.10 | 15 | 94.50 | 30 | 97.00 | 15 | 76.50 | 45 | 98.70 |
| CTTP-150 | 10 | 83.20 | 10 | 83.20 | 15 | 97.90 | 30 | 98.40 | 15 | 99.40 | 45 | 99.49 |
| CTTP-50 | 10 | 70.50 | 10 | 70.50 | 15 | 98.05 | 30 | 98.90 | 15 | 92.10 | 45 | 99.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajić, B.; Milošević, M.; Kepić, D.; Ćirić-Marjanović, G.; Šaponjić, Z.; Radoičić, M. Carbon-Rich Nanocomposites Based on Polyaniline/Titania Nanotubes Precursor: Synergistic Effect Between Surface Adsorption and Photocatalytic Activity. Molecules 2025, 30, 2628. https://doi.org/10.3390/molecules30122628
Gajić B, Milošević M, Kepić D, Ćirić-Marjanović G, Šaponjić Z, Radoičić M. Carbon-Rich Nanocomposites Based on Polyaniline/Titania Nanotubes Precursor: Synergistic Effect Between Surface Adsorption and Photocatalytic Activity. Molecules. 2025; 30(12):2628. https://doi.org/10.3390/molecules30122628
Chicago/Turabian StyleGajić, Brankica, Milica Milošević, Dejan Kepić, Gordana Ćirić-Marjanović, Zoran Šaponjić, and Marija Radoičić. 2025. "Carbon-Rich Nanocomposites Based on Polyaniline/Titania Nanotubes Precursor: Synergistic Effect Between Surface Adsorption and Photocatalytic Activity" Molecules 30, no. 12: 2628. https://doi.org/10.3390/molecules30122628
APA StyleGajić, B., Milošević, M., Kepić, D., Ćirić-Marjanović, G., Šaponjić, Z., & Radoičić, M. (2025). Carbon-Rich Nanocomposites Based on Polyaniline/Titania Nanotubes Precursor: Synergistic Effect Between Surface Adsorption and Photocatalytic Activity. Molecules, 30(12), 2628. https://doi.org/10.3390/molecules30122628









