Tailored Lignin Fractions via Ionic Liquid Pretreatment for Sustainable Polymer Systems
Abstract
1. Introduction
2. Results and Discussion
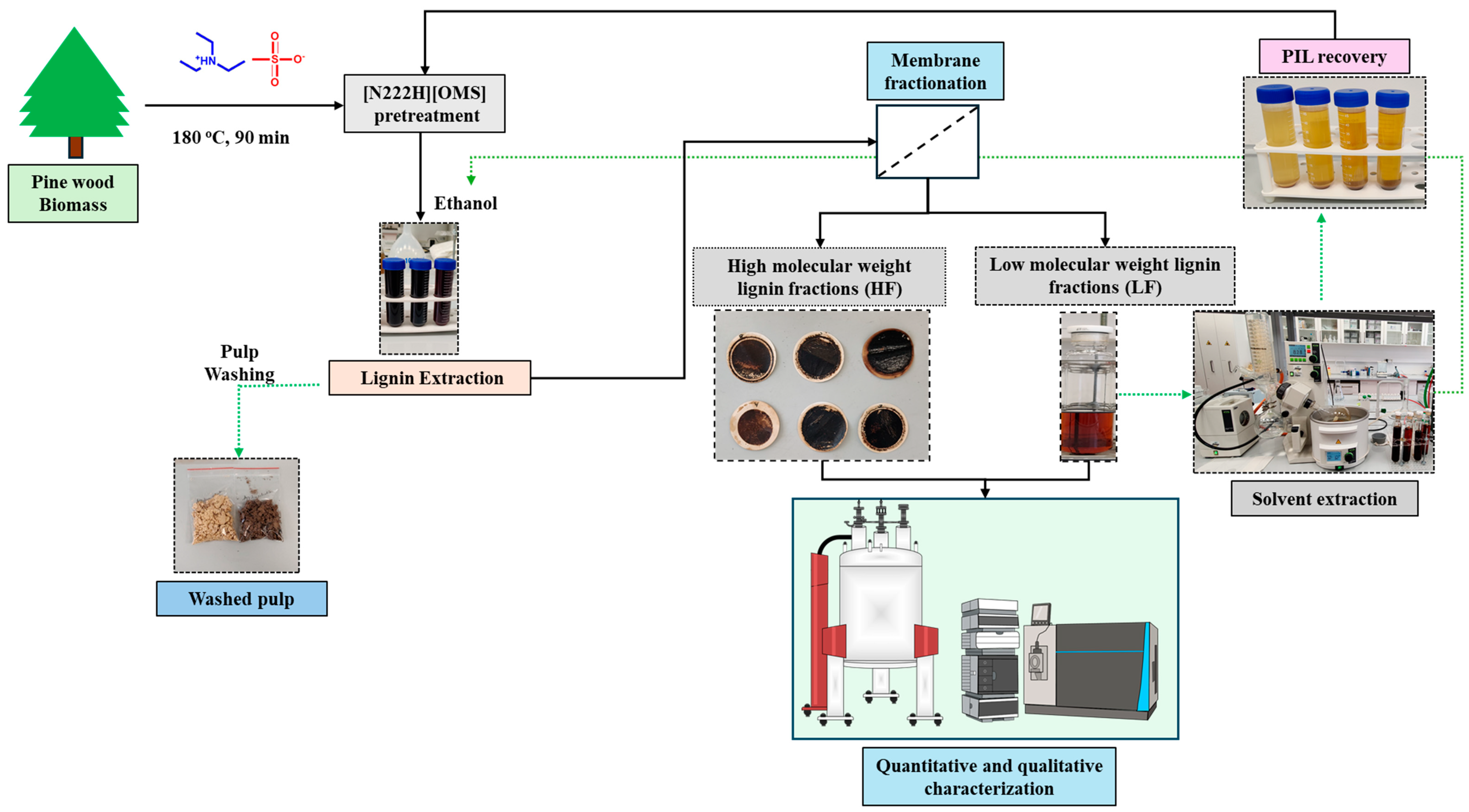
2.1. Ionic Liquid Fractionation of Pine Wood Biomass
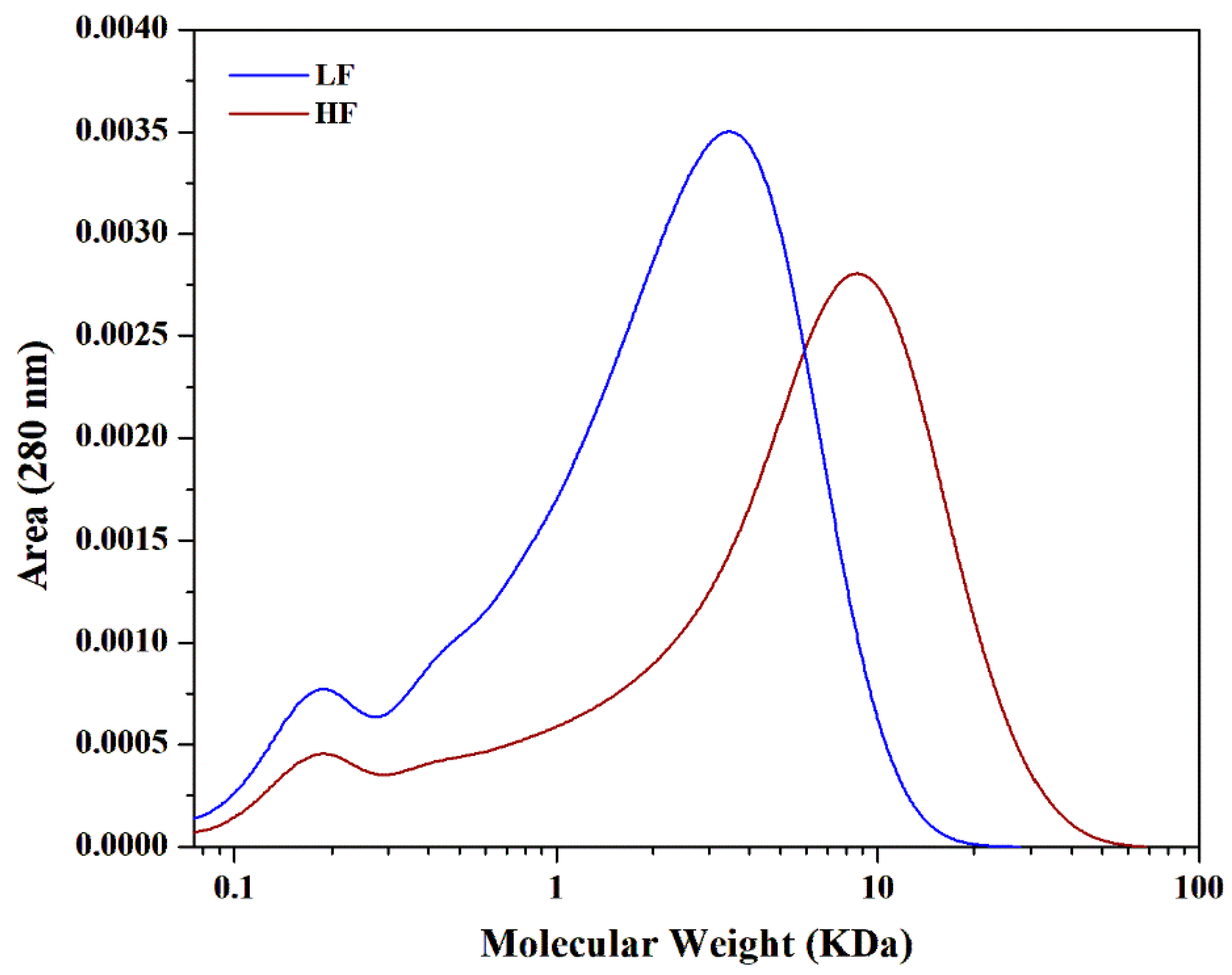
2.2. Differences in the Molecular Weights of HF and LF Fractions
2.3. Intensities of Different Functional Groups in HF and LF Compared with the Pretreated Pulp
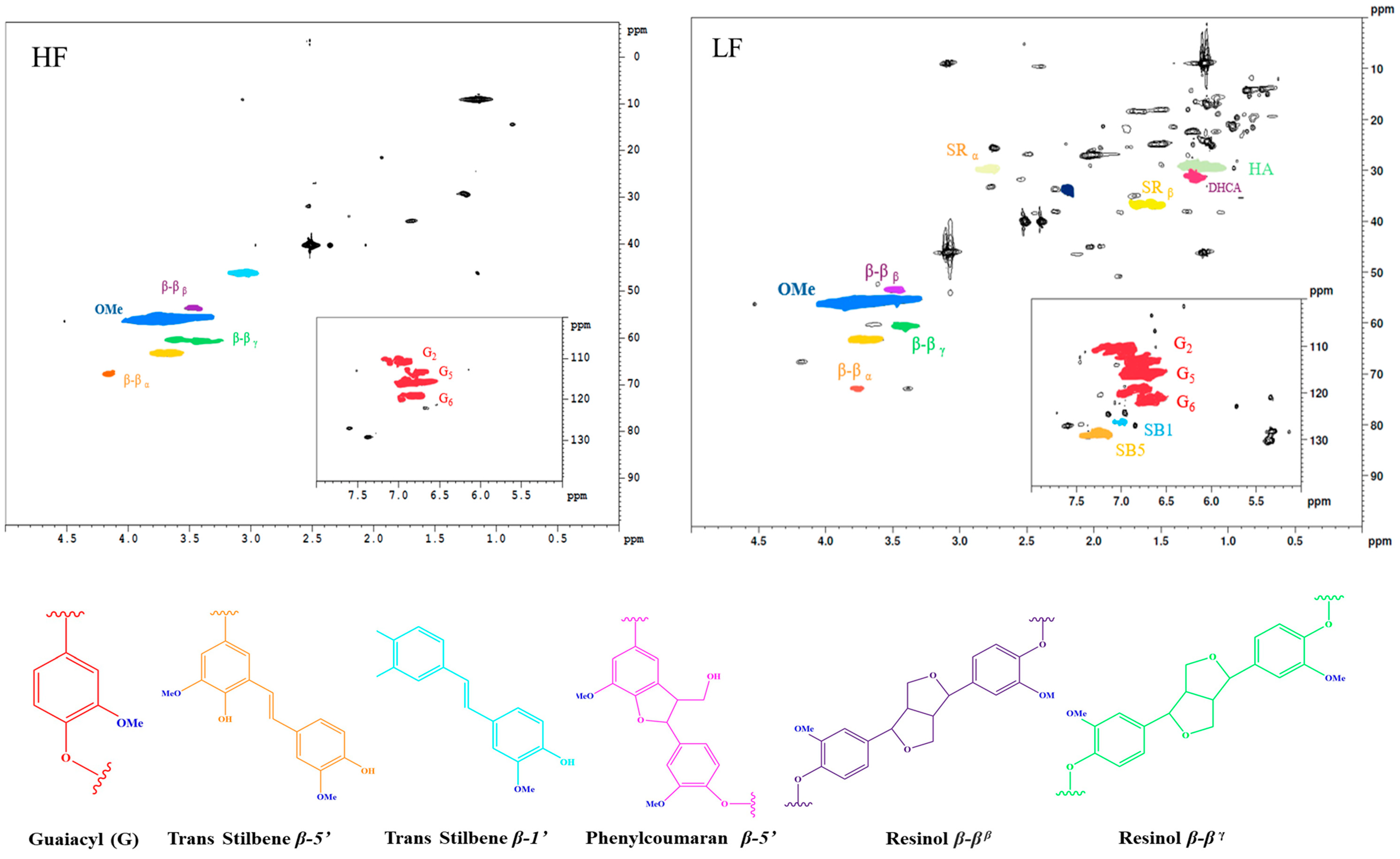
2.4. Understanding the Peculiarities in HF and LF Using 2D HSQC NMR
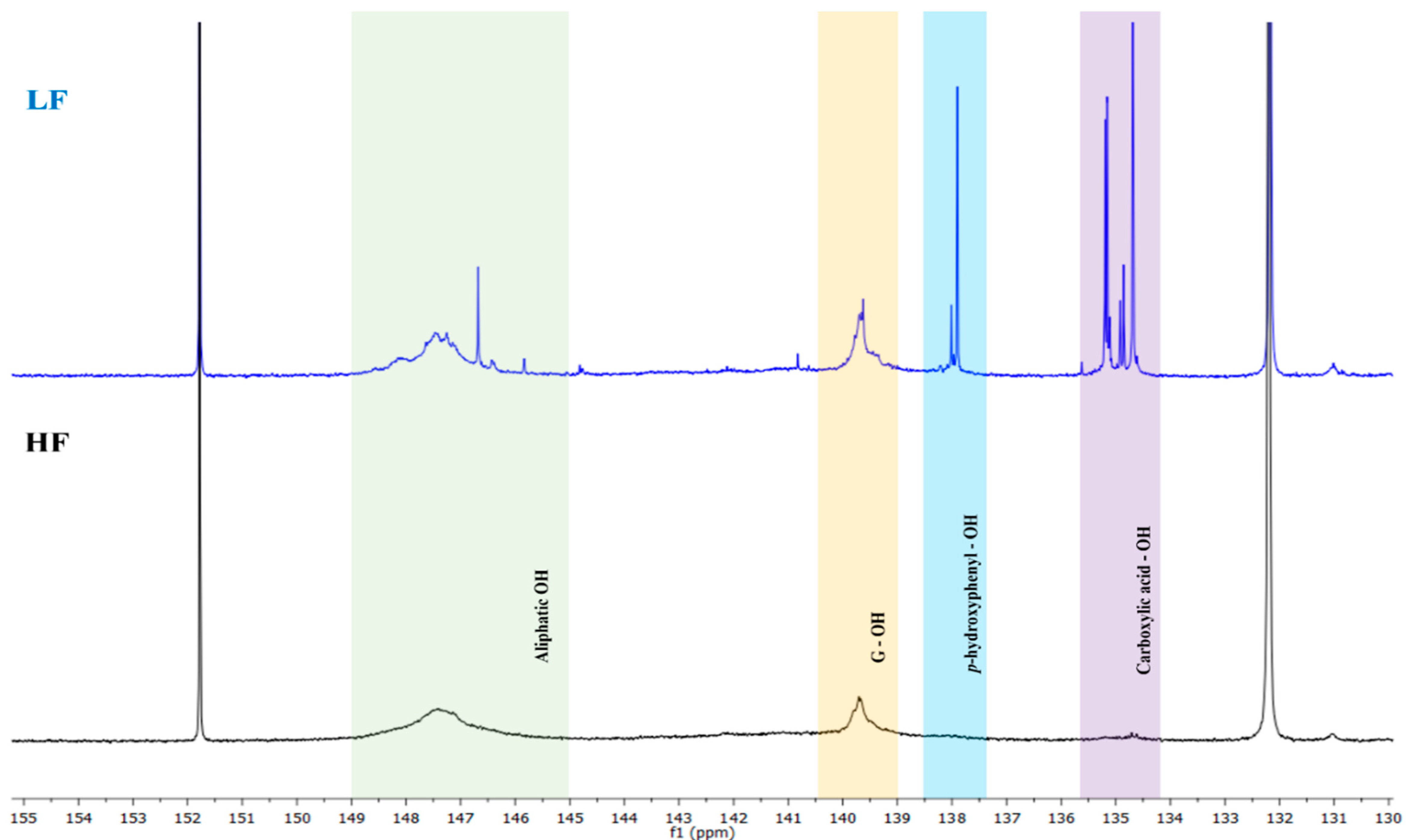
2.5. 31P NMR of Different Lignin Fractions
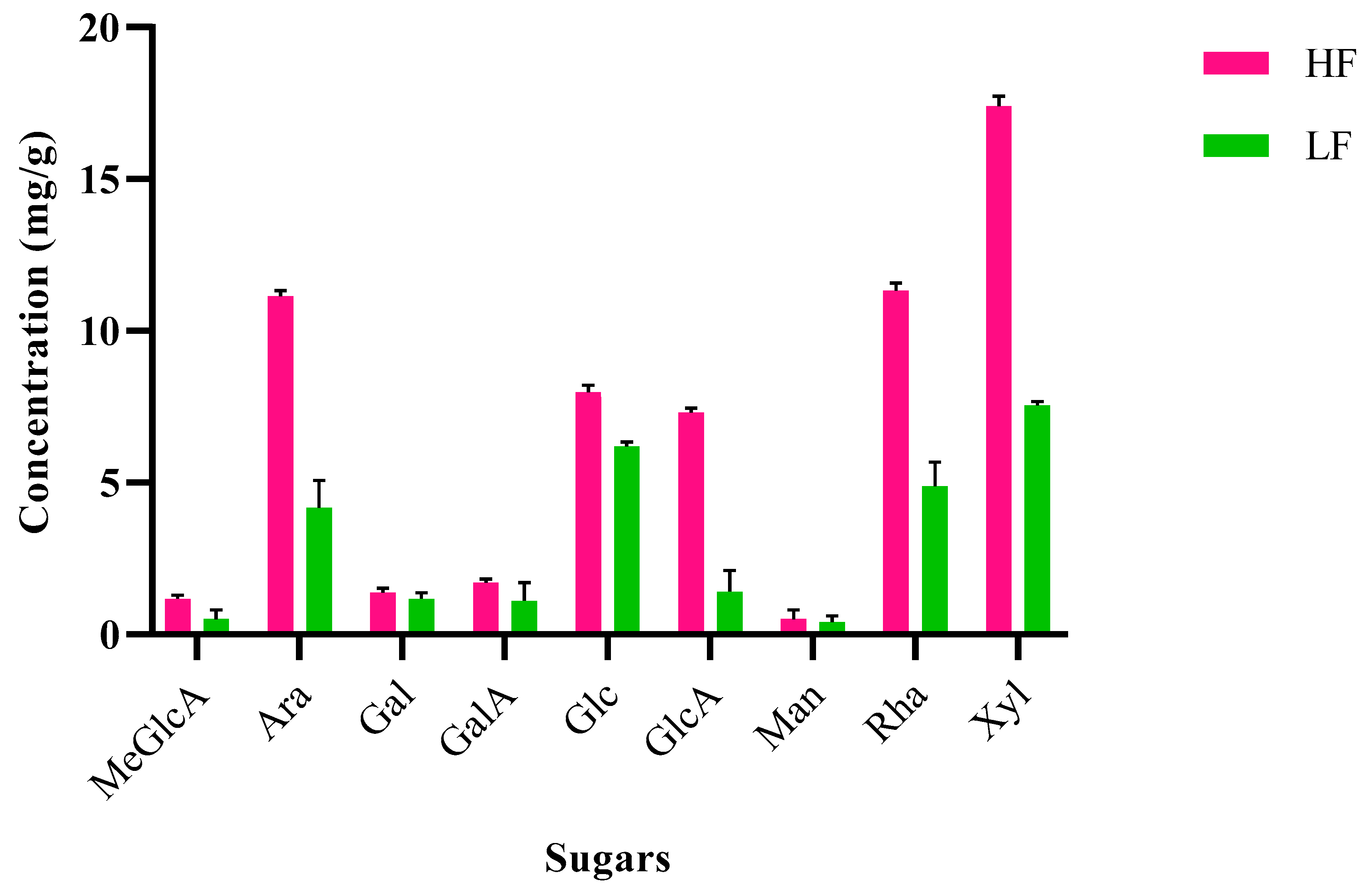
2.6. Different Sugars Concentration in Lignins
2.7. Py-GC/MS of HF and LF
3. Materials and Methods
3.1. Reagents and Biomass Preparation
3.2. Protic Ionic Liquid (PIL) Preparation and Its Characterization
3.3. Pine Wood Pretreatment
3.4. Lignin Fractionation
3.5. Delignification Quantification
3.6. Lignin Characterization
3.6.1. Molecular Weight Analysis
3.6.2. Nuclear Magnetic Resonance (2D HSQC and 31P NMR) Spectroscopy
3.6.3. ATR-FTIR (Attenuated Total Reflectance/Fourier Transform Infrared) Analysis of Lignin Fractions
3.6.4. Carbohydrate Quantification of Lignin Fractions
3.6.5. Pyrolysis-GC/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, Y.; Koelewijn, S.F.; van den Bossche, G.; van Aelst, J.; van den Bosch, S.; Renders, T.; Navare, K.; Nicolaï, T.; van Aelst, K.; Maesen, M.; et al. A Sustainable Wood Biorefinery for Low-Carbon Footprint Chemicals Production. Science 2020, 367, 1385–1390. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.Y.; Capanema, E.A.; Sulaeva, I.; Schlee, P.; Huang, Z.; Feng, M.; Borghei, M.; Rojas, O.J.; Potthast, A.; Rosenau, T. New Opportunities in the Valorization of Technical Lignins. ChemSusChem 2021, 14, 1016–1036. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; De Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Momayez, F.; Hedenström, M.; Stagge, S.; Jönsson, L.J.; Martín, C. Valorization of Hydrolysis Lignin from a Spruce-Based Biorefinery by Applying γ-Valerolactone Treatment. Bioresour. Technol. 2022, 359, 127466. [Google Scholar] [CrossRef] [PubMed]
- Svensson, I.; Roncal, T.; De Winter, K.; Van Canneyt, A.; Tamminen, T.; Mikkelson, A.; Barrio, A. Valorisation of Hydrolysis Lignin Rest From Bioethanol Pilot Plant: Process Development and Upscaling. Ind. Crops Prod. 2020, 156, 112869. [Google Scholar] [CrossRef]
- Borrega, M.; Pihlajaniemi, V.; Liitiä, T.; Wikström, L.; Tamminen, T. Evaluation of Chemical Additives in Hydrothermal Pre-Treatment of Wood for the Integrated Production of Monosugars and Hydrolysis Lignins for PLA-Based Biocomposites. Biomass Convers. Biorefinery 2023, 13, 7491–7503. [Google Scholar] [CrossRef]
- Tofani, G.; Jasiukaitytė-Grojzdek, E.; Grilc, M.; Likozar, B. Organosolv Biorefinery: Resource-Based Process Optimisation, Pilot Technology Scale-up and Economics. Green Chem. 2023, 26, 186–201. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Yang, M.; Singh, S.; Cheng, G. Transforming Lignocellulosic Biomass into Biofuels Enabled by Ionic Liquid Pretreatment. Bioresour. Technol. 2021, 322, 124522. [Google Scholar] [CrossRef]
- Nakasu, P.Y.S.; Pin, T.C.; Hallett, J.P.; Rabelo, S.C.; Costa, A.C. In-Depth Process Parameter Investigation into a Protic Ionic Liquid Pretreatment for 2G Ethanol Production. Renew. Energy 2021, 172, 816–828. [Google Scholar] [CrossRef]
- Gschwend, F.J.V.; Malaret, F.; Shinde, S.; Brandt-Talbot, A.; Hallett, J.P. Rapid Pretreatment of: Miscanthus Using the Low-Cost Ionic Liquid Triethylammonium Hydrogen Sulfate at Elevated Temperatures. Green Chem. 2018, 20, 3486–3498. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An Economically Viable Ionic Liquid for the Fractionation of Lignocellulosic Biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Lee, J.M. Recyclability of an Ionic Liquid for Biomass Pretreatment. Bioresour. Technol. 2014, 169, 336–343. [Google Scholar] [CrossRef]
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.S.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of Low-Cost Ionic Liquids for Lignocellulosic Biomass Pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef]
- Wang, F.L.; Li, S.; Sun, Y.X.; Han, H.Y.; Zhang, B.X.; Hu, B.Z.; Gao, Y.F.; Hu, X.M. Ionic Liquids as Efficient Pretreatment Solvents for Lignocellulosic Biomass. RSC Adv. 2017, 7, 47990–47998. [Google Scholar] [CrossRef]
- Chambon, C.L.; Verdía, P.; Fennell, P.S.; Hallett, J.P. Process Intensification of the IonoSolv Pretreatment: Effects of Biomass Loading, Particle Size and Scale-up from 10 ML to 1 L. Sci. Rep. 2021, 11, 15383. [Google Scholar] [CrossRef]
- Sun, L.L.; Sun, S.N.; Cao, X.F.; Yao, S.Q. An Integrated Biorefinery Strategy for Eucalyptus Fractionation and Co-Producing Glucose, Furfural, and Lignin Based on Deep Eutectic Solvent/Cyclopentyl Methyl Ether System. Carbohydr. Polym. 2024, 343, 122420. [Google Scholar] [CrossRef]
- Chen, W.; Dong, T.; Bai, F.; Wang, J.; Li, X. Lignin–Carbohydrate Complexes, Their Fractionation, and Application to Healthcare Materials: A Review. Int. J. Biol. Macromol. 2022, 203, 29–39. [Google Scholar] [CrossRef]
- Argyropoulos, D.D.S.; Crestini, C.; Dahlstrand, C.; Furusjö, E.; Gioia, C.; Jedvert, K.; Henriksson, G.; Hulteberg, C.; Lawoko, M.; Pierrou, C.; et al. Kraft Lignin: A Valuable, Sustainable Resource, Opportunities and Challenges. ChemSusChem 2023, 16, 202300492. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.; Capanema, E.A.; Zhu, X.; Sulaeva, I.; Potthast, A.; Rosenau, T.; Rojas, O.J. Spruce Milled Wood Lignin: Linear, Branched or Cross-Linked? Green Chem. 2020, 22, 3985–4001. [Google Scholar] [CrossRef]
- Tarasov, D.; Schlee, P.; Pranovich, A.; Moreno, A.; Wang, L.; Rigo, D.; Sipponen, M.H.; Xu, C.; Balakshin, M. AqSO Biorefinery: A Green and Parameter-Controlled Process for the Production of Lignin-Carbohydrate Hybrid Materials. Green Chem. 2022, 24, 6639–6656. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; Lima, V.; Araújo, L.C.P.; Botaro, V.R. Lignin Fractionation Methods: Can Lignin Fractions Be Separated in a True Industrial Process? Ind. Eng. Chem. Res. 2021, 60, 10863–10881. [Google Scholar] [CrossRef]
- Liu, R.; Smeds, A.; Tirri, T.; Zhang, H.; Willför, S.; Xu, C. Influence of Carbohydrates Covalently Bonded with Lignin on Solvent Fractionation, Thermal Properties, and Nanoparticle Formation of Lignin. ACS Sustain. Chem. Eng. 2022, 10, 14588–14599. [Google Scholar] [CrossRef]
- van Leuken, S.H.M.; van Osch, D.J.G.P.; Kouris, P.D.; Yao, Y.; Jedrzejczyk, M.A.; Cremers, G.J.W.; Bernaerts, K.V.; van Benthem, R.A.T.M.; Tuinier, R.; Boot, M.D.; et al. Quantitative Prediction of the Solvent Fractionation of Lignin. Green Chem. 2023, 25, 7534–7540. [Google Scholar] [CrossRef]
- Duval, A.; Vilaplana, F.; Crestini, C.; Lawoko, M. Solvent Screening for the Fractionation of Industrial Kraft Lignin. Holzforschung 2016, 70, 11–20. [Google Scholar] [CrossRef]
- Schuerch, C. The Solvent Properties of Liquids and Their Relation to the Solubility, Swelling, Isolation and Fractionation of Lignin. J. Am. Chem. Soc. 1952, 74, 5061–5067. [Google Scholar] [CrossRef]
- Liu, R.; Smeds, A.; Wang, L.; Pranovich, A.; Hemming, J.; Willför, S.; Zhang, H.; Xu, C. Fractionation of Lignin with Decreased Heterogeneity: Based on a Detailed Characteristics Study of Sequentially Extracted Softwood Kraft Lignin. ACS Sustain. Chem. Eng. 2021, 9, 13862–13873. [Google Scholar] [CrossRef]
- Zhu, W.; Westman, G.; Theliander, H. Investigation and Characterization of Lignin Precipitation in the Lignoboost Process. J. Wood Chem. Technol. 2014, 34, 77–97. [Google Scholar] [CrossRef]
- Hämäläinen, V.; Grönroos, T.; Suonpää, A.; Heikkilä, M.W.; Romein, B.; Ihalainen, P.; Malandra, S.; Birikh, K.R. Enzymatic Processes to Unlock the Lignin Value. Front. Bioeng. Biotechnol. 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Puss, K.K.; Lukk, T.; Loog, M.; Kikas, T.; Salmar, S. Enzymatic Conversion of Hydrolysis Lignin—A Potential Biorefinery Approach. Energies 2023, 16, 370. [Google Scholar] [CrossRef]
- Gschwend, F.J.V.; Chambon, C.L.; Biedka, M.; Brandt-Talbot, A.; Fennell, P.S.; Hallett, J.P. Quantitative Glucose Release from Softwood after Pretreatment with Low-Cost Ionic Liquids. Green Chem. 2019, 21, 692–703. [Google Scholar] [CrossRef]
- Lawoko, M.; Henriksson, G.; Gellerstedt, G. Structural Differences between the Lignin-Carbohydrate Complexes Present in Wood and in Chemical Pulps. Biomacromolecules 2005, 6, 3467–3473. [Google Scholar] [CrossRef]
- Bhatia, R.; Lad, J.B.; Bosch, M.; Bryant, D.N.; Leak, D.; Hallett, J.P.; Franco, T.T.; Gallagher, J.A. Production of Oligosaccharides and Biofuels from Miscanthus Using Combinatorial Steam Explosion and Ionic Liquid Pretreatment. Bioresour. Technol. 2021, 323, 124625. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.N.M.; Iqbal, A.; Shen, C.C.; Bhawani, S.A.; Adam, F. Synthesis of Lignin Based Composites of TiO2 for Potential Application as Radical Scavengers in Sunscreen Formulation. BMC Chem. 2019, 13, 17. [Google Scholar] [CrossRef]
- Mohamad Haafiz, M.K.; Hassan, A.; Zakaria, Z.; Inuwa, I.M.; Islam, M.S. Physicochemical Characterization of Cellulose Nanowhiskers Extracted from Oil Palm Biomass Microcrystalline Cellulose. Mater. Lett. 2013, 113, 87–89. [Google Scholar] [CrossRef]
- Jiang, B.; Shen, F.; Jiang, Y.; Huang, M.; Zhao, L.; Lei, Y.; Hu, J.; Tian, D.; Shen, F. Extraction of Super High-Yield Lignin-Carbohydrate Complexes from Rice Straw without Compromising Cellulose Hydrolysis. Carbohydr. Polym. 2024, 323, 121452. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Love, R.; Whiteside, W.S.; Doh, H.; Darby, D.O.; Bridges, W.S. Isolation and Characterisation of Cellulose Nanocrystals from Kudzu (Pueraria Montana Var. Lobata): A Sustainable Packaging Material. Int. J. Food Sci. Technol. 2023, 58, 4752–4760. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Shanmugam, S.; Kikas, T. Synergistic Effects of Torrefaction and Alkaline Pretreatment on Sugar and Bioethanol Production from Wood Waste. Energies 2023, 16, 7606. [Google Scholar] [CrossRef]
- You, T.T.; Zhang, L.M.; Zhou, S.K.; Xu, F. Structural Elucidation of Lignin-Carbohydrate Complex (LCC) Preparations and Lignin from Arundo Donax Linn. Ind. Crops Prod. 2015, 71, 65–74. [Google Scholar] [CrossRef]
- Giummarella, N.; Zhang, L.; Henriksson, G.; Lawoko, M. Structural Features of Mildly Fractionated Lignin Carbohydrate Complexes (LCC) from Spruce. RSC Adv. 2016, 6, 42120–42131. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A. Comprehensive Structural Analysis of Biorefinery Lignins with a Quantitative 13C NMR Approach. RSC Adv. 2015, 5, 87187–87199. [Google Scholar] [CrossRef]
- Diment, D.; Tkachenko, O.; Schlee, P.; Kohlhuber, N.; Potthast, A.; Budnyak, T.M.; Rigo, D.; Balakshin, M. Study toward a More Reliable Approach to Elucidate the Lignin Structure—Property—Performance Correlation. Biomacromolecules 2023, 25, 200–212. [Google Scholar] [CrossRef]
- Sapouna, I.; Lawoko, M. Deciphering Lignin Heterogeneity in Ball Milled Softwood: Unravelling the Synergy between the Supramolecular Cell Wall Structure and Molecular Events. Green Chem. 2021, 23, 3348–3364. [Google Scholar] [CrossRef]
- Karlsson, M.; Romson, J.; Elder, T.; Emmer, Å.; Lawoko, M. Lignin Structure and Reactivity in the Organosolv Process Studied by NMR Spectroscopy, Mass Spectrometry, and Density Functional Theory. Biomacromolecules 2023, 24, 2314–2326. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, C.; Li, X.; Li, H.; Zhang, Y.; Huang, Y.; Chen, S.; Hou, Q. Microwave-Assisted DES Fabrication of Lignin-Containing Cellulose Nanofibrils and Its Derived Composite Conductive Hydrogel. Carbohydr. Polym. 2024, 328, 121741. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.; Capanema, E.; Berlin, A. Isolation and Analysis of Lignin–Carbohydrate Complexes Preparations with Traditional and Advanced Methods, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 42, ISBN 9780444632814. [Google Scholar]
- Zhao, Y.; Shakeel, U.; Saif Ur Rehman, M.; Li, H.; Xu, X.; Xu, J. Lignin-Carbohydrate Complexes (LCCs) and Its Role in Biorefinery. J. Clean. Prod. 2020, 253, 120076. [Google Scholar] [CrossRef]
- Carvalho, D.M.D.; Lahtinen, M.H.; Lawoko, M.; Mikkonen, K.S. Enrichment and Identification of Lignin-Carbohydrate Complexes in Softwood Extract. ACS Sustain. Chem. Eng. 2020, 8, 11795–11804. [Google Scholar] [CrossRef]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin-Carbohydrate Complexes: Properties, Applications, Analyses, and Methods of Extraction: A Review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef]
- Peil, S.; Mouhoubi, R.; Streekstra, R.; Ridard, H.; Veith, L.; Ali, W.; Mayer-Gall, T.; Duvigneau, J.; Wurm, F.R. Encapsulation of Ammonium Polyphosphate in Lignin Nanocontainers Enhances Dispersion and Flame Retardancy in Polylactic Acid Foams. ACS Appl. Polym. Mater. 2024, 6, 6096–6107. [Google Scholar] [CrossRef]
- Watt, T.R.; Peil, S.; Jonkers, W.A.; Regenspurg, J.A.; Wurm, F.R.; de Vos, W.M. All-Lignin Polyelectrolyte Multilayers as Renewable and Biodegradable Nanofiltration Membranes. ACS Appl. Polym. Mater. 2023, 5, 8547–8558. [Google Scholar] [CrossRef]
- Dong, H.; Zheng, L.; Yu, P.; Jiang, Q.; Wu, Y.; Huang, C.; Yin, B. Characterization and Application of Lignin-Carbohydrate Complexes from Lignocellulosic Materials as Antioxidants for Scavenging in Vitro and in Vivo Reactive Oxygen Species. ACS Sustain. Chem. Eng. 2020, 8, 256–266. [Google Scholar] [CrossRef]
- Wang, X.; Han, J.; Pang, S.; Li, J.; Zhao, J.; Qin, C.; Yao, S.; Liu, Y.; Liang, C. Structural Enrichment and Identification of Lignin-Carbohydrate Complex in Alkaline Stabilized System. Carbohydr. Polym. 2022, 296, 119873. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zeng, X.; Hu, S.; Zhou, Y.; Xie, M.; Yue, F. Highly Uncondensed Lignin Extraction in the Novel Green L-Cysteine/Lactic Acid Cosolvent System. Chem. Eng. J. 2024, 497, 154340. [Google Scholar] [CrossRef]
- Domínguez-robles, J.; Tamminen, T.; Liitiä, T.; Soledad, M.; Rodríguez, A.; Jääskeläinen, A. International Journal of Biological Macromolecules Aqueous Acetone Fractionation of Kraft, Organosolv and Soda Lignins. Int. J. Biol. Macromol. 2018, 106, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, J.; Tarasov, D.; Koitto, T.; Rinke, P.; Balakshin, M.; Todorović, M. Machine Learning Optimization of Lignin Properties in Green Biorefineries. ACS Sustain. Chem. Eng. 2022, 10, 9469–9479. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Janus, R.; Lewandowski, M.; Magdziarz, A. On Mechanism of Lignin Decomposition—Investigation Using Microscale Techniques: Py-GC-MS, Py-FT-IR and TGA. Renew. Energy 2021, 177, 942–952. [Google Scholar] [CrossRef]
- Dier, T.K.F.; Rauber, D.; Durneata, D.; Hempelmann, R.; Volmer, D.A. Sustainable Electrochemical Depolymerization of Lignin in Reusable Ionic Liquids. Sci. Rep. 2017, 7, 5041. [Google Scholar] [CrossRef]
- Khan, S.; Rauber, D.; Shanmugam, S.; Kay, C.W.M.; Konist, A.; Kikas, T. Efficient Lignin Fractionation from Scots Pine (Pinus Sylvestris) Using Ammonium-Based Protic Ionic Liquid: Process Optimization and Characterization of Recovered Lignin. Polymers 2022, 14, 4637. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. NMR Characterization of Pyrolysis Oils from Kraft Lignin. Energy Fuels 2011, 25, 2322–2332. [Google Scholar] [CrossRef]
- Zhao, C.; Li, S.; Zhang, H.; Yue, F.; Lu, F. Structural Insights into the Alkali Lignins Involving the Formation and Transformation of Arylglycerols and Enol Ethers. Int. J. Biol. Macromol. 2020, 152, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Eugenio, M.E.; Martín-Sampedro, R.; Santos, J.I.; Wicklein, B.; Martín, J.A.; Ibarra, D. Properties versus Application Requirements of Solubilized Lignins from an Elm Clone during Different Pre-Treatments. Int. J. Biol. Macromol. 2021, 181, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Characterization of Lignin Structures and Lignin-Carbohydrate Complex (LCC) Linkages by Quantitative 13C and 2D HSQC NMR Spectroscopy. J. Agric. Food Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef]
- Xu, C.; Liu, R.; Smeds, A.; Willf, S. Industrial Crops & Products Structural Properties of Softwood Lignin Fractions: Revealed by NMR and Py-GC/MS. Ind. Crop. Prod. 2024, 209, 118055. [Google Scholar] [CrossRef]


| Lignin | Mn | Mw | PDI |
|---|---|---|---|
| HF | 1452 | 9535 | 6.56 |
| LF | 841 | 3342 | 3.97 |
| Aliphatic-OH a | Phenolic-OH from Lignin | Total Phenolic-OH | Carboxylic Acid OH e | Total OH | |||
|---|---|---|---|---|---|---|---|
| C5-Substituted OH b | Guaiacyl-OH c | p-Hydroxyphenyl-OH d | |||||
| HF | 1.97 | 1.09 | 0.93 | 0.21 | 2.23 | 0.23 | 4.43 |
| LF | 1.69 | 0.64 | 0.89 | 0.42 | 1.95 | 0.90 | 4.54 |
| Retention Time (min) | Compound | Chemical Formula | Py–GC/MS Peak Area (%) |
|---|---|---|---|
| Phenol-type (H) | |||
| 6.58 | Phenol | C6H6O | 0.45 |
| 7.91 | p-Creosol | CH3C6H4OH | 0.57 |
| Guaiacyl-type (G) | |||
| 8.52 | Guaiacol | C7H8O2 | 5.79 |
| 12.48 | 4-Vinylguaiacol | C9H10O | 10.32 |
| 11.95 | 4-Ethylguaiacol | C9H12O2 | 3.20 |
| 13.53 | 4-Propylguaiacol | C10H14O2 | 0.86 |
| 9.68 | 2,4-Dimethylphenol | C8H10O | 0.93 |
| 10.46 | Methyl guaiacol | C8H10O2 | 19.36 |
| 13.46 | Eugenol | C10H12O2 | 2.21 |
| 14.92 | Cis-Isoeugenol | C10H12O2 | 9.54 |
| 15.23 | Homo vanillin | C9H10O3 | 0.85 |
| 15.54 | Acetovanillone | C10H10O4 | 2.91 |
| 16.12 | Guaiacyl acetone | C10H12O3 | 1.83 |
| 16.89 | Guaiacyl propenol | G-C3H6O | 1.28 |
| 16.93 | 1-guaiacyl-2-propen-1-ol | G-C3H6O | 1.18 |
| 17.98 | Dihydro coniferyl alcohol | C10H14O3 | 1.66 |
| Catechol-type (Ca) | |||
| 10.92 | Catechol | C6H6O2 | 0.84 |
| 11.88 | 4-Methylcathecol | C7H8O2 | 1.93 |
| Other aromatic compounds | |||
| 12.53 | Veratrole | C6H4(OCH3)2 | 0.46 |
| 23.18 | 4-(1-propenyl) veratrole | C6H4(OCH3)2-CH=CH-CH3 | 0.3 |
| Fatty acids | |||
| 19.78 | Hexadecenoic acid | C16H30O2 | 0.39 |
| 23.93 | cis-13-octadecenoic acid | C18H34O2 | 3.97 |
| Other identified compounds | |||
| 1.12 | Carbon dioxide | CO2 | 11.85 |
| 2.38 | Triethylamine | N(CH2CH3)3 | 2.07 |
| 3.06 | Methylbenzene | C6H5CH3 | 1.53 |
| 2.08 | Benzene | C6H6 | 0.29 |
| 8.92 | 2,3-Dimethylanisole | C9H12O | 0.49 |
| 1.82 | 2-Methyl-furan | C5H6O | 1.5 |
| Unidentified compounds | |||
| 1.36 | Unknown | - | 3.05 |
| 2.24 | Unknown | - | 4.54 |
| 19.22 | Unknown | - | 0.79 |
| 19.57 | Unknown | - | 0.38 |
| 23.35 | Unknown | - | 0.72 |
| 25.92 | Unknown | - | 1.04 |
| Retention Time (min) | Compound | Chemical Formula | Py–GC/MS Peak Area (%) |
|---|---|---|---|
| Phenol-type (H) | |||
| 6.58 | Phenol | C6H6O | 0.41 |
| 7.92 | p-Creosol | CH3C6H4OH | 0.64 |
| Guaiacyl-type (G) | |||
| 8.52 | Guaiacol | C7H8O2 | 4.23 |
| 10.43 | 3-Methylguaiacol | C8H10O2 | 0.31 |
| 12.62 | 4-Vinylguaiacol | C9H10O | 7.88 |
| 11.92 | 4-Ethylguaiacol | C9H12O2 | 2.33 |
| 13.43 | 4-Propylguaiacol | C10H14O2 | 0.66 |
| 9.61 | 2,4-Dimethylphenol | C8H10O | 1.01 |
| 10.43 | Methyl guaiacol | C8H10O2 | 16.31 |
| 13.24 | Eugenol | C10H12O2 | 2.23 |
| 14.76 | Trans-Isoeugenol | C10H12O2 | 9.29 |
| 15.13 | Hom vanillin | C9H10O3 | 1.29 |
| 15.52 | Acetovanillone | C10H10O4 | 0.97 |
| 16.13 | Guaiacyl acetone | C10H12O3 | 1.54 |
| 16.83 | Guaiacyl propenol | Guaiacyl propenol | 1.53 |
| 16.92 | 1-guaiacyl-2-propen-1-ol | G-C3H6O | 1.06 |
| 17.92 | Dihydro coniferyl alcohol | C10H14O3 | 1.01 |
| Other aromatic compounds | |||
| 13.89 | Veratrole | C6H4(OCH3)2 | 0.33 |
| 13.97 | 4-(1-propenyl) veratrole | C6H4(OCH3)2-CH=CH-CH3 | 0.23 |
| Carbohydrate units | |||
| 15.71 | 1,6-anhydro-β-d-glucopyranose | C6H10O5 | 2.42 |
| Other identified compounds | |||
| 1.15 | Carbon dioxide | CO2 | 17.37 |
| 2.23 | Triethylamine | N(CH2CH3)3 | 10.28 |
| 3.06 | benzene, methyl- (cas) | C6H5CH3 | 1.18 |
| 3.02 | 1-methoxy-3-methylbenzene | C6H5CH3 | 0.16 |
| 8.88 | 2,3-dimethylanisole | C9H12O | 0.33 |
| 13.25 | 2,6-dimethyl-3(2h)-benzofuranone, | C10H10O2 | 0.14 |
| 28.09 | Bis(ethylhexyl)phthalate | C6H4(COOC8H17)2 | 6.69 |
| Unidentified compounds | |||
| 1.35 | Unknown | - | 4.47 |
| 15.27 | Unknown | - | 0.38 |
| 15.38 | Unknown | - | 0.58 |
| 15.74 | Unknown | - | 0.57 |
| 16.97 | Unknown | - | 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Rauber, D.; Veerabagu, U.; Wu, R.; Kay, C.W.M.; Xu, C.; Shanmugam, S.; Kikas, T. Tailored Lignin Fractions via Ionic Liquid Pretreatment for Sustainable Polymer Systems. Molecules 2025, 30, 2630. https://doi.org/10.3390/molecules30122630
Khan S, Rauber D, Veerabagu U, Wu R, Kay CWM, Xu C, Shanmugam S, Kikas T. Tailored Lignin Fractions via Ionic Liquid Pretreatment for Sustainable Polymer Systems. Molecules. 2025; 30(12):2630. https://doi.org/10.3390/molecules30122630
Chicago/Turabian StyleKhan, Sharib, Daniel Rauber, Udayakumar Veerabagu, Ruijie Wu, Christopher W. M. Kay, Chunlin Xu, Sabarathinam Shanmugam, and Timo Kikas. 2025. "Tailored Lignin Fractions via Ionic Liquid Pretreatment for Sustainable Polymer Systems" Molecules 30, no. 12: 2630. https://doi.org/10.3390/molecules30122630
APA StyleKhan, S., Rauber, D., Veerabagu, U., Wu, R., Kay, C. W. M., Xu, C., Shanmugam, S., & Kikas, T. (2025). Tailored Lignin Fractions via Ionic Liquid Pretreatment for Sustainable Polymer Systems. Molecules, 30(12), 2630. https://doi.org/10.3390/molecules30122630








