Abstract
A series of novel fluorescent donor–acceptor–donor (D-A-D) dyes containing [1,2,5]oxadiazolo[3,4-d]pyridazine and its 1-oxide as electron-withdrawing groups has been synthesized and thoroughly investigated using X-ray diffraction and molecular spectroscopy methods. This study showed that the introduction of N-oxide into the 1,2,5-oxadiazole ring in the acceptor fragment leads to a significant decrease in the luminescence intensity and quantum yield of the dyes. A comprehensive comparison of the photophysical properties of the obtained compounds containing the 1,2,5-oxadiazole ring with the previously studied [1,2,5]thia- and 1,2,5-selenadiazolo[3,4-d]pyridazine analogs showed that the oxygen substitution in the acceptor fragment shifts the phosphorescence maximum from the NIR region of 980–1100 nm to the red region of 690–770 nm. In contrast, for oxygen- and sulfur-containing dyes, purely red fluorescence with a maximum in the spectral range of 620–900 nm is observed. The crystal structures of furoxan-containing 3d·½CHCl3 and furazan-containing 4d exhibit a non-planar [1,2,5]oxadiazolo[3,4-d]pyridazine fragment. We have found that short non-covalent interactions of the furoxan system with a lattice chloroform molecule in 3d lead to luminescence quenching. Meanwhile, in the 4d dye, the intermolecular π-π interactions of pyridazine nitrogen atoms with the N-carbazolyl group of the adjacent molecule should facilitate intermolecular charge transfer (ICT) emission. Thus, the luminescence maxima for these dyes can be tuned across a broad range of 700–1100 nm by varying the number of chalcogen atoms, highlighting the potential for tailoring optical properties in optoelectronic applications.
1. Introduction
Over the past two decades, donor–acceptor (D-A) molecules based on 1,2,5-chalcogenadiazoles have aroused significant scientific interest due to their potential applications in various optoelectronic devices. These molecules are of particular interest due to their unique physical properties, such as visible light absorption, extended π-conjugation that enhances molar extinction coefficients, and efficient charge carrier delocalization, which are essential for the performance of devices such as Grätzel cells. In addition, the applications include bulk heterojunction [1,2] dye-sensitized solar cells [3,4], organic light-emitting diodes [5,6], and n-type field-effect transistors [7,8].
Photovoltaic applications rely on light absorption, whereas electroluminescent devices require controlled emission within specific spectral ranges. However, the chemical design of D-A dyes with specified luminescence properties remains challenging, primarily due to the lack of systematic approaches for predicting absorption and emission spectra prior to their synthesis. Recent efforts have focused on developing criteria to guide the design of such dyes. For instance, symmetric donor–acceptor–donor (D-A-D) structures have exhibited enhanced intramolecular charge transfer (ICT) in comparison to more simplistic D-A systems [9]. Through the modification of the donor and acceptor moieties, the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) can be fine-tuned, thereby adjusting the absorption and emission maxima.
In our recent work, we demonstrated that D-A-D compounds incorporating 1,2,5-thiadiazolo[3,4-d]pyridazine as the central acceptor, with indoline and carbazole derivatives as donors, emit in the visible to near-infrared (NIR) range. These compounds were successfully employed as emitters in NIR OLEDs, achieving high external quantum efficiency [10]. Furthermore, the utilization of [1,2,5]selenadiazolo[3,4-d]pyridazine as the central acceptor resulted in dyes exhibiting dual emission spanning 700–900 nm and 900–1100 nm, thereby violating Kasha’s rule [11].
In our previous study, it was demonstrated that D-A-D-type molecules based on [1,2,5]oxadiazolo[3,4-d]pyridazine, with carbazole and cyclohexaindoline donor fragments, exhibited red emission [12]. However, the luminescent properties of [1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide derivatives remain unexplored, and a systematic comparison of the optical properties of chalcogen-based acceptors is still lacking.
This study represents the culmination of a comprehensive research cycle, with the objective of elucidating the influence of heavy atoms in the acceptor fragment of chalcogenadiazole-based D-A-D dyes on their luminescent properties. Specifically, we investigate how different acceptor fragments, combined with identical donor units, affect emission in the visible and NIR regions. We report the synthesis and characterization of a series of novel D-A-D compounds based on [1,2,5]oxadiazolo[3,4-d]pyridazine (furazan) and its 1-oxide (furoxan) paired with donors such as 1,2,3,3a,4,8b-hexahydrocyclopenta[b]indole, 1,2,3,3a,4,8b-hexahydrocyclohexa[b]indole, 1,2,3,4,4a,4a,9a-hexahydro-9λ2-1,4-methanocarbazole, and carbazole (Figure 1). A comparison of these results with data from previous studies [11,12] establishes a clear relationship between the chalcogen atom in the acceptor fragment and the emission spectral range, providing valuable insights for the design of optoelectronic materials.
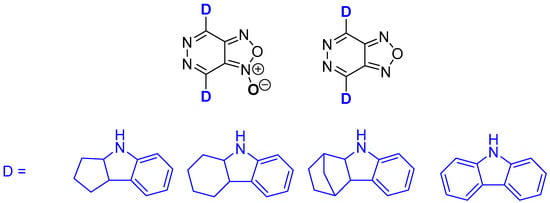
Figure 1.
Chemical structures of the synthesized dyes.
2. Results and Discussion
2.1. Synthesis
We have studied the substitution reactions of chlorine atoms in the pyridazine ring by amino groups. It was demonstrated that when the reaction between dichloride 1 and amines 2(a–c) (4 equiv.) was carried out in DMF at room temperature, the formation of bis-substitution products 3(a–c) occurred in high yields. The reduction of the furoxan ring to the furazan cycle was carried out under the action of triphenylphosphine in dichloromethane at room temperature for 3 h to obtain the target dyes 4(a–c), the yield of which varied within 65–72% (Scheme 1).
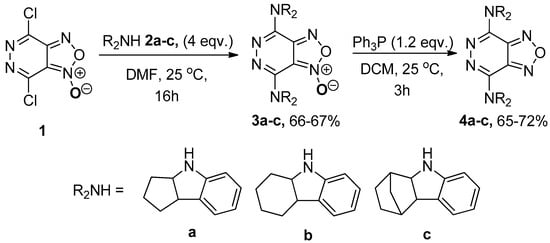
Scheme 1.
Synthesis of [1,2,5]oxadiazolo[3,4-d]pyridazines and [1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxides.
It has been demonstrated that carbazole reacts with 4,7-dichloro[1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide 1. It has been established that the use of the sodium salt of this amine in DMF for 24 h at 25 °C results in the formation of the bis-substitution product 3d in moderate yield (Scheme 2). The structure of the 3d compound was unambiguously confirmed via X-ray structural analysis (Figure 2).
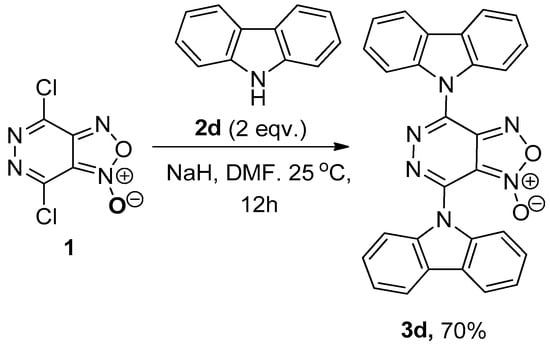
Scheme 2.
Synthesis of 4,7-di(9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide 3d.
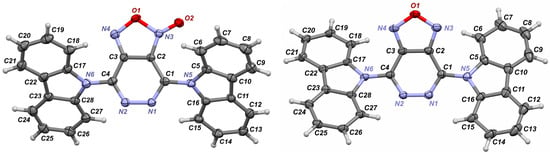
Figure 2.
Crystal structures of 3d ((left); only one crystallographicaly unique molecule is shown; the disorder of the furoxan cycle is omitted) and 4d (right). Herein and thereafter, anisotropic displacement parameters are set to a 50% probability level.
It was found that the 4d compound could be successfully synthesized from bis(hexahydrocarbazolyl) derivative 4b via dehydrogenation in toluene with 4,5-dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-dicarbonitrile (DDQ) to form the target product 4d with a high yield (Scheme 3) [13]. The structure of compound 4d was unambiguously confirmed using X-ray crystallography (Figure 2).
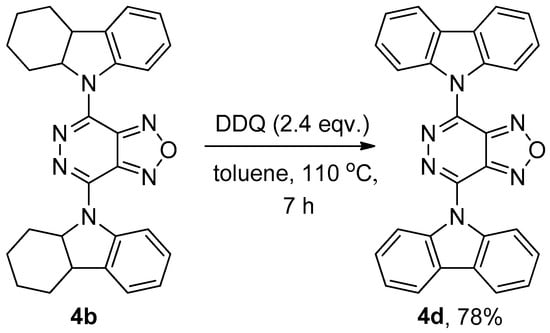
Scheme 3.
Synthesis of 4,7-di(9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine (4d).
2.2. X-Ray Analysis and DFT Calculations
The crystal structures of 3d·½CHCl3 and 4d have been studied using the single-crystal X-ray diffraction analysis. The asymmetric unit of 3d·½CHCl3 contains a disordered lattice chloroform molecule and two symmetrically non-equivalent molecules of 3d (Figure 2, left), whereby the furoxan fragment (C2N2O2) is disordered over two positions (see Supplementary Materials (ESI) for details). Both specimens crystalize in orthorhombic space groups (non-centrosymmetric Pca21, Z′ = 2 for 3d·½CHCl3 and centrosymmetric Pbca, Z′ = 1 for 4d) with Z = 8 in both cases. The unit cell parameters and packing plots are similar but differ slightly; the presence of the solvent molecule and the disorder of the furoxan fragment lead to a loss of symmetry elements (two 21 screw axes and a glide plane) in 3d·½CHCl3.
The selected bond distances for 3d and 4d are provided in Table 1. Expectedly, the furoxan fragments reveals a significant elongation of the O1-N3/O3-N10 bond by 0.10 Å and an elongation of the N3-C2/N10-C31 bond by 0.02 Å located at the N-oxide fragment in 3d, compared to those in the furazan fragment of 4d. In order to compare geometrical parameters, DFT calculations were performed at the PBE0/DEF2TZVP level of theory in the gas phase at 298K. Calculated bond distances are provided in Table 2. They are in good or in fairly good agreement with crystallographic data.

Table 1.
Selected bond distances for 3d and 4d (Å).

Table 2.
Calculated bond distances for 3d and 4d (Å).
Both molecules 3d and 4d demonstrate that the entire heterocycle N4C4O2 (3d)/N4C4O (4d) is not flat and demonstrates slight folding along two lines passing through the following atom pairs: C1/C4 and C2/C3. The folding angles (see ESI) are rather small (in the case of 3d, we have only evaluated its values due to high ESDs caused by the disorder of the furoxan cycle), not preventing a good conjugation within the π-system of the heterocycle. We suppose that the non-planarity [1,2,5]oxadiazolo[3,4-d]pyridazine heterocycle is not flat, likely due to intrinsic electronic effects rather than intra- and intermolecular van der Waals interactions (see below). Our idea was successfully confirmed by results of quantum calculations (see dihedral angles for optimized structures, Tables S9 and S10 in Supplementary Information).
On the contrary, nearly flat N-carbazolyl groups (C12H8N) are rotated by 29.38(6)° and 40.02(5)° with respect to the N2C4 plane of the central heterocycle in 4d, indicating that π-π conjugation between the C12H8N and N4C4O heterocycle systems is lost to a substantial extent. The presence of an additional oxygen atom in the furoxan ring makes the situation even more dramatic for 3d: the values of C12H8N-N2C4 dihedral rotation angles lie in the range of 41.6(1.5) to 46.3(1)° (see ESI). Such high values of dihedral angles are clearly the result of intramolecular van der Waals interactions within the molecule. Consequently, the N-carbazolyl group is unable to function as an effective antenna to enhance the luminescent properties of the investigated dyes. The optimized geometry of 3d perfectly reproduces C12H8N-N2C4 dihedral rotation angles. However, the optimized geometry of 4d indicates that both C12H8N-N2C4 dihedral angles should be of 47° in a gas phase, which contradicts with observations of the crystal structure, where one angle is much smaller. The latter is clearly induced by non-covalent interactions in a crystalline phase.
In 3d, pronounced π-π stacking is formed with an average distance of 3.35 Å between the π-systems (Figure 3). This phenomenon can be attributed to the minor deviation of the carbazolyl aromatic systems from each other within the crystal packing (Figure S3 in ESI). It should be noticed that the furoxan ring forms non-covalent contacts with a carbazolyl group of a neighboring molecule and with a lattice chloroform molecule. This observation potentially elucidates the comparatively inferior photophysical properties exhibited by 3d in comparison to 4d, as evidenced by phenomena such as luminescence quenching.
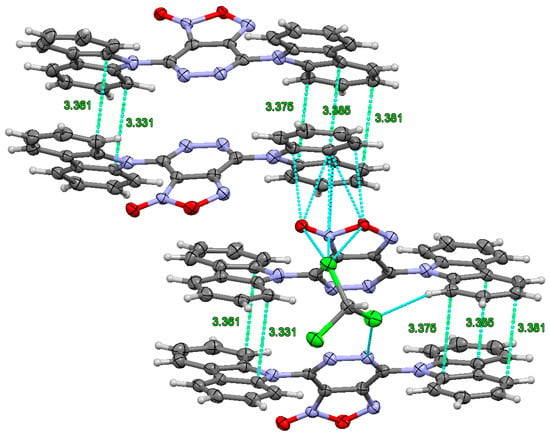
Figure 3.
Intermolecular contacts of carbazole rings in the crystal of 3d, forming π-π stacking, and short contacts of the furoxan ring.
In the crystal structure of 4d, the molecules are arranged in zigzag-shaped layers, supported by intermolecular π-π interactions of aromatic rings, including short contacts between nitrogen atoms (Figure 4); the angle in the zigzag is close to 90°. The distances between N-carbazolyl systems of neighboring molecules are in the range of 3.31 Å to 3.37 Å. More pronounced non-covalent interactions are between atoms N1 and N2 of one molecule and the N5, C1, C5, and C16 atoms of the N-carbazolyl substituent of another molecule (namely, the group that exhibits a smaller rotation angle): 2.959 Å for N1···N5, 3.061 Å for N1···C16, 3.241 Å for N1···C5, 3.024 Å for N2···C1, 3.080 Å for N2···N5, and 3.248 Å for N2···C16 (Figure 5). These particular interactions hinder the rotation of one of the two N-carbazolyl fragments to a greater angle, arrange molecules of 4d into 1D chains oriented along the b direction (see ESI), and should facilitate charge transfer from one molecule to another, enhancing its photophysical properties in the solid state, compared to those in 3d.
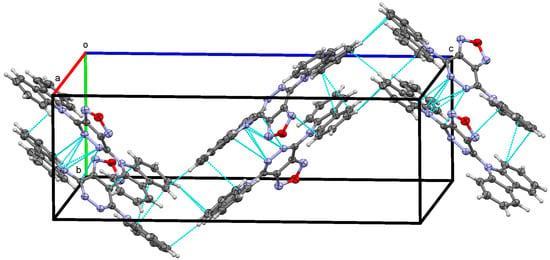
Figure 4.
Zigzag-shaped layers in the crystal packing of 4d. Only two layers are shown for clarity.
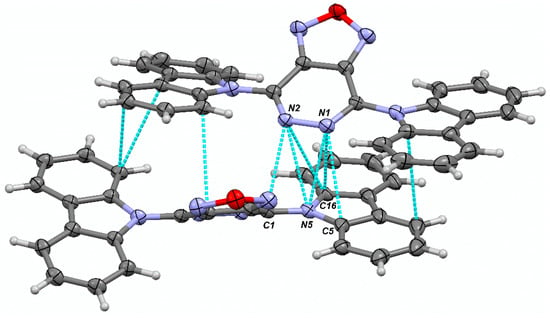
Figure 5.
Intermolecular non-valent contacts between two adjacent molecules of 4d.
Therefore, the crystal structure of the 3d compound reveals not only π-π stacking but also short intermolecular interactions between the furoxan rings that lead to luminescence quenching.
On the contrary, π-π stacking in the 4d compound containing the furazan system is observed primarily between two N-carbazolyl groups and between the pyridazine heterocycle and the N-carbazolyl group of adjacent molecules; the [1,2,5]oxadiazolo[3,4-d]pyridazine system might be considered as nearly unperturbed by intermolecular interactions. Consequently, the non-flat nature of the furazan system is likely caused by electronic effects.
2.3. Photophysical Properties
Optical absorption spectra measured for all investigated compounds dissolved in THF and toluene with a concentration of about 10−5 M/L reveal several bands (see Figure 6). Since the bands with the maxima at 280–295 nm depend on the nature of the solvent insignificantly, they are characterized by π-π* transitions. The long-wavelength band blue-shifts with the increase in solvent polarity for all dyes, so it is associated with the ICT state. Due to donor fragment variations from carbazole (3d and 4d dyes) to the b, a, and c moieties, the ICT peaks shift bathochromically by 25–50 nm. The photophysical parameters such as the absorption maximum wavelength (λabs), maximum molar extinction for the ICT band (εmax), photoluminescence (PL) maximum wavelength (λem), and Stokes shift (Δν) are listed in Table 3.
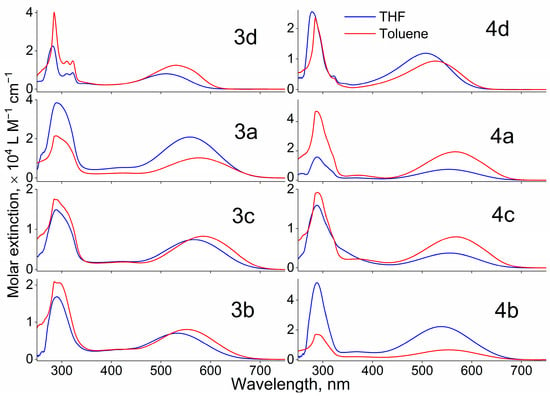
Figure 6.
UV-Vis spectra recorded for all investigated dyes dissolved in THF and toluene.

Table 3.
Photophysical parameters obtained for all dyes: absorption maximum wavelength (λabs), maximum molar extinction coefficient (ε), oscillator strength (f), wavelength of emission maximum (λem), full width at half-maximum (FWHM) for the emission spectrum, and Stokes shift (Δν).
Comparing compounds containing 1,2,5-oxadiazole (furazan) and 1,2,5-oxadiazole 2-oxide (furoxan) as acceptor fragments while using identical donor moieties, we observe qualitatively similar shapes in their absorption spectra. However, luminescence intensity strongly depends on the acceptor fragment. PL spectra were measured for all investigated dyes dissolved in toluene and THF upon excitation at λabs corresponding to the ICT state (see Figure 7 and Figure S10). The dyes emit their spectra in the orange-red spectral region. The λem of the 4d, 4a, 4c, and 4b compounds is red-shifted by 11–56 nm in comparison to analogs with 1,2,5-oxadiazole 2-oxide acceptor (3d, 3a, 3c, and 4b). The PL spectra for the 3a, 3c, and 3b compounds dissolved in toluene demonstrate a low signal-to-noise ratio that can be associated with more efficient non-radiative relaxation. The dominant non-radiative relaxation pathway can be explained by OH bonding of the oxygen at the N(1) position of the furoxan ring and hydrogen at the donor fragment. The emission spectra of compounds 3a and 3b in THF could not be accurately recorded due to their low luminescence intensity.
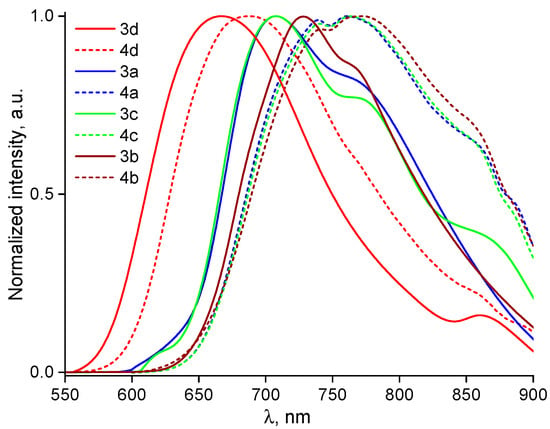
Figure 7.
Normalized PL spectra recorded for all dyes dissolved in toluene upon photoexcitation at the λabs of the ICT state.
Since PL decays for the 3d and 4d molecules demonstrate mono- and bi-exponential behaviors, respectively, with lifetimes relatively close to the lifetimes (τ) of the fast-time component, we have assumed that the dyes have different emission characteristics (see Figure 8, left). In the inset, one can see the biexponential PL decay of the toluene-dissolved 4d dye recorded within the time window of 250 μs, which demonstrates a weighted average lifetime of about 11 μs. In particular, the furoxan-based dye demonstrates pure fluorescence and later demonstrates both fluorescence and room temperature phosphorescence (RTP). To prove our assumption, we have measured time-resolved luminescence spectra with a delay of 5 μs at 300 K for all investigated dyes (see Figure 8, right). As a result, we observe RTP at the same spectral range as luminescence for the 4d, 4a, 4c, and 4d dyes (see Figure 8). Similar PL decays and relatively close lifetimes are observed for the 4a, 4c and 4d dyes. In addition, at 77 K, one can see the well-pronounced rise-time component for the 4d dye within the initial 30 ns in the PL decay associated with the population of the triplet state (see Figure S12). For furoxan-based dyes, we observe no RTP. Thus, the emission maxima of 4d, 4a, 4c, and 4d are red-shifted in comparison to furoxan analogs due to intensive RTP.
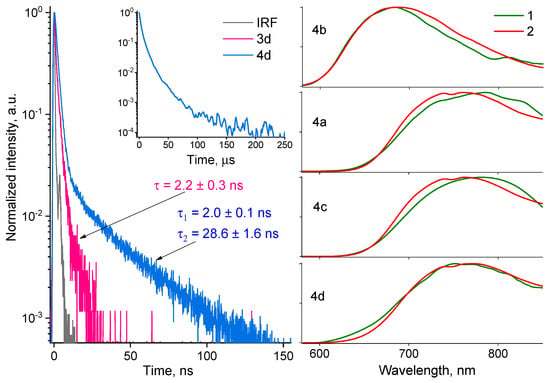
Figure 8.
(Left): PL decays for the 3d and 4d dyes recorded at 700 nm upon laser excitation at 450 nm at 300 K; (right): room temperature phosphorescence (1) recorded with a time delay of 5 us and steady-state luminescence (2) spectra for furazan-based dyes upon excitation at 450 nm. All the dyes were dissolved in toluene with a concentration of about 10−5 M.
The PL quantum yields (Φ) estimated for all the investigated dyes dissolved in toluene and THF using the absolute method are provided in Table 2. The relatively low polarity of toluene mitigates PL quenching and provides higher Φ values. The highest Φ values are achieved for 4a, 4c, and 4d (2.2%, 2.8%, and 2.0%, respectively). Since the UV-Vis spectra for the furazan- and furoxan-based compounds with the same donor fragments are quite similar, we conclude that the additional oxygen does not significantly affect the ICT process in the dyes. In our opinion, the strong decrease in luminescence efficiency is probably related to the formation of OH–hydrogen bonds between the dyes and solvents. However, the ICT absorption maxima slightly differ by ~10 nm due to ground state geometry rearrangement caused by steric repulsion between the donor fragment and furoxan’s oxygen. To summarize, both the hydrogen bond formation and repulsion of the furoxan oxygen atom from the electron density of the carbazole can result in fluorescence suppression.
To explore the impact of 3d·½CHCl3 on photophysical properties, we measured the PL spectra of the crystalline powders of the 3d and 4d dyes (see Figures S11 and S13). In contrast to the PL of the corresponding solutions, the red-shift in emission maximum is not observed (see Table 2). Moreover, Φ cannot be obtained for 3d even in the crystalline powder state. Since Φ for the 4d crystal is 1.1% which is four times higher than that for toluene-dissolved 4d, we consider that the presence of ½CHCl3 plays a crucial role in energy transfer, and as a result, it leads to emission quenching and a decrease in luminescence efficiency.
Within the framework of the research, we conducted a comprehensive study of the influence of the chalcogen in the acceptor fragment on the photophysical properties of D-A molecules (Table 3). We compared the results obtained for oxygen-containing molecules with sulfur and selenium-containing analogs investigated in our previous works [11,14]. The photophysical properties of furazan dyes are insignificantly different from molecules containing 1,2,5-thiadiazolo[3,4-d]pyridazine as an acceptor with the same donor fragments [14]. The emission maxima of the investigated dyes blue-shift by 15 nm in comparison to the ones for sulfur molecules. On the contrary, the luminescence spectra of molecules containing selenium in the acceptor differ greatly from the PL spectra of dyes under study. Firstly, the use of furoxan and furazan fragments as acceptors leads to the complete suppression of the phosphorescence band at room temperature in the near-infrared (NIR) spectral region [11]. Simultaneously, the luminescence band appears in the visible spectra region for molecules 4a and 4c (λem = 761–778 nm), which is not observed for similar selenium dyes with the same donors. In addition, the absorption spectra of the studied compounds are blue-shifted by 40–80 nm compared to selenium-containing analogs.
To summarize, the chalcogen variation in the acceptor moiety plays a crucial role in energy migration pathways. In particular, the substitution of selenium by oxygen in the acceptor moiety switches the relaxation process from dominant triplet phosphorescence to pure fluorescence. Moreover, the absorption and emission maxima positions are tuned by the variation of only one atom (O, S, and Se) in the acceptor of D-A-D molecules.
3. Materials and Methods
3.1. Materials and Reagents
Chemicals were purchased from commercial sources and used as received. 4,7-Dichloro[1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide 1 [15], 1,2,3,3a,4,8b-hexahydrocyclopenta[b]indole 2a [16], 2,3,4,4a,9,9a-hexahydro-1H-carbazole 2b [17], and 2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazole 2c [18] were prepared according to the previously described protocols. All synthetic operations were performed under a dry argon atmosphere. Solvents were purified via distillation over the appropriate drying agents.
3.2. Analytical Instruments
1H and 13C NMR spectra were taken with a Bruker AM-300 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) at frequencies of 300.1 and 75.5 MHz or a Bruker AV600 machine (Bruker AXS Handheld Inc., Kennewick, WA, USA) at frequencies of 600.1 and 150.9 MHz with TMS as the standard. MS spectra (EI, 70 eV) were obtained with a Finnigan MAT INCOS 50 instrument (Hazlet, NJ, USA). High-resolution MS spectra were measured on a Bruker MICROTOF II instrument (Bruker Daltonik Gmbh, Bremen, Germany) using electrospray ionization (ESI). The measurement was operated in a positive ion mode (interface capillary voltage –4500 V) or in a negative ion mode (3200 V); the mass range was from m/z 50 to 3000 Da; external or internal calibration was performed with the Electrospray Calibrant Solution (Fluka, Buchs, Switzerland). A syringe injection was used for solutions in acetonitrile, methanol, or water (a flow rate of 3 μL/min). Nitrogen was applied as a dry gas; the interface temperature was set at 180 °C. IR spectra were measured with a Bruker “Alpha-T” instrument in KBr pellets. The UV–Vis spectra of the investigated compounds dissolved in tetrahydrofuran (HPLC SuperGradient, Panreac, Spain) were recorded with a JASCO V-770 spectrophotometer (JASCO, Easton, MD, USA) operating within 200–2500 nm. The concentration of the solutions was about 10−5 M. The measurements were performed using quartz cells with a 1 cm path length. Excitation spectra and photoluminescence spectra were obtained using a Horiba Fluorolog QM spectrofluorometer (GMP, Fallanden, Switzerland) with a 75 W xenon arc lamp as the excitation source and a R13456 (Hamamatsu, Hamamatsu City, Japan) photomultiplier tube sensitive in the 200–980 nm emission range as the detector. The photoluminescence quantum yields were measured for solid samples via an absolute method using the same experimental setup equipped with a G8 integration sphere. The PL decays recorded within the time window on the order of hundreds of nanoseconds were obtained via TCSPC method, while the longer ones were measured using the single-shot transient digitizer (SSTD). The laser excitation wavelength is 500 nm.
X-ray diffraction data for 3d and 4d were collected at 100K on a four-circle Rigaku XtaLAB Synergy-S diffractometer equipped with an HyPix6000HE area detector (kappa geometry, shutterless ω-scan technique) using graphite-monochromatized Cu Kα radiation (λ = 1.54184 Å). The intensity data were integrated and corrected for absorption and decay using the CrysAlisPro program (version 1.171.42.89a, 2023) [19]. The structures were solved via dual methods using SHELXT-2014/5 [20] and refined via the full-matrix least-squares minimization method on F2 using SHELXL-2018/3 [21] in the OLEX2 program [22]. All non-hydrogen atoms were refined with individual anisotropic displacement parameters. All hydrogen atoms were placed in ideal calculated positions and refined as riding atoms with relative isotropic displacement parameters. The crystal data, data collection, and structure refinement details are summarized in Table S1 of ESI.
3.3. Experimental Details
3.3.1. General Procedure for the Preparation of Bis-Aminated Products 3(a–c)
Amine 2(a–c) (0.68 mmol) was added to a solution of 4,7-dichloro-[1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide 1 (35 mg, 0.17 mmol) in dry DMF (10 mL) at room temperature with stirring. The mixture was stirred at room temperature for 16 h. The mixture was poured into water (10 mL) and extracted with CH2Cl2 (3 × 35mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified using column chromatography (Silica gel Merck 60).
4,7-Bis(2,3,3a,8b-tetrahydrocyclopenta[b]indol-4(1H)-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide 3a
Violet solid, 51 mg (67%), and Rf = 0.4 (CH2Cl2). Mp = 196–198 °C. Eluent–CH2Cl2/hexane at a 1:1 (v/v) ratio. IR νmax (KBr, cm−1): 2952, 2863, 1613, 1508, 1484, 1451, 1402, 1264, 1219, 1158, 1099, 1025, 892, 749, 618, 569. 1H NMR (300 MHz, CDCl3): δ 8.63 (dd, J = 8.1, 4.8 Hz, 1H), 7.29–7.17 (m, 3H), 7.15–7.05 (m, 2H), 7.04–6.95 (m, 1H), 6.92 (t, J = 7.3 Hz, 1H), 5.73–5.63 (m, 1H), 5.47–5.37 (m, 1H), 4.09 (t, J = 7.5 Hz, 1H), 3.94–3.84 (m, 1H), 2.22–1.92 (m, 4H), 1.91–1.86 (m, 2H), 1.83–1.40 (m, 6H). 13C NMR (75 MHz, CDCl3): δ 145.1, 143.8, 143.6, 143.2, 137.4, 136.0, 135.3, 127.8, 127.3, 124.7, 124.7, 124.1, 122.1, 117.8, 111.8, 106.4, 68.8, 67.2, 46.2, 46.1, 36.7, 34.5, 34.1, 33.8, 24.4, 23.9. HRMS (ESI-TOF), m/z: calcd for C26H25N6O2 [M + H]+, 453.2034, found, 453.2031.
4,7-Bis(1,2,3,4,4a,9a-hexahydro-9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide 3b
Violet solid, 53 mg (66%), and Rf = 0.4 (CH2Cl2). Mp = 188–190 °C. Eluent–CH2Cl2/hexane at a 1:1 (v/v) ratio. IR νmax (KBr, cm−1): 2928, 2856, 1619, 1509, 1483, 1454, 1273, 1121, 1159, 1107, 1026, 752, 650. 1H NMR (300 MHz, CDCl3): δ 8.57 (dd, J = 7.9, 3.2 Hz, 1H), 7.31–7.25 (m, 2H), 7.21 (d, J = 7.3 Hz, 1H), 7.16–7.07 (m, 2H), 6.95 (t, J = 7.1 Hz, 1H), 6.89 (d, J = 7.9 Hz, 1H), 5.46–5.34 (m, 1H), 4.69–4.61 (m, 1H), 3.71–3.64 (m, 1H), 3.41–3.33 (m, 1H), 2.48–2.40 (m, 1H), 2.25–2.11 (m, 1H), 2.01–1.83 (m, 4H), 1.70–1.60 (m, 2H), 1.48–1.28 (m, 8H). 13C NMR (75 MHz, CDCl3): δ 145.9, 144.5, 143.5, 143.4, 138.4, 135.6, 134.5, 128.2, 127.7, 124.7, 124.0, 123.2, 122.6, 119.9, 112.7, 106.9, 65.3, 64.0, 41.3, 41.1, 30.4, 29.0, 28.0, 26.9, 23.3, 22.9, 22.3, 21.6. HRMS (ESI-TOF), m/z: calcd for C28H29N6O2 [M + H]+, 481.2347, found, 481.2332.
4,7-Bis(1,2,3,4,4a,9a-hexahydro-9H-1,4-methanocarbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide 3c
Violet solid, 57 mg (67%), and Rf = 0.4 (CH2Cl2). Mp = 196–198 °C. Eluent–CH2Cl2/hexane at a 1:1 (v/v) ratio. IR νmax (KBr, cm−1): 2954, 2873, 1610, 1507, 1482, 1447, 1405, 1349, 1254, 1176, 1112, 1026, 908, 750, 730, 619. 1H NMR (300 MHz, CDCl3): δ 8.64 (dd, J = 12.7, 8.2 Hz, 1H), 7.25–7.00 (m, 6H), 6.88 (t, J = 7.0 Hz, 1H), 5.13 (dd, J = 15.4, 7.9 Hz, 1H), 4.99–4.93 (m, 1H), 3.54 (d, J = 7.9 Hz, 1H), 3.34 (d, J = 8.1 Hz, 1H), 2.59–2.42 (m, 2H), 2.40–2.15 (m, 2H), 1.74–1.30 (m, 10H), 1.17–1.08 (m, 2H). 13C NMR (75 MHz, CDCl3): δ 146.5, 145.1, 144.1, 143.2, 138.0, 134.9, 133.8, 127.8, 127.4, 124.8, 124.2, 123.7, 121.8, 117.1, 112.3, 105.8, 69.8, 69.3, 51.0, 50.9, 44.0, 43.4, 43.1, 42.0, 32.3, 32.0, 28.6, 28.0, 25.5, 25.1. HRMS (ESI-TOF), m/z: calcd for C30H29N6O2 [M + H]+, 505.2347, found, 505.2344.
For 4,7-Di(9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide 3d, 60% sodium powered in mineral oil (12 mg, 0.48 mmol) was added to a solution of carbazole 2d (80 mg, 0.48 mmol) in dry DMF (10 mL) at room temperature with stirring. The reaction mixture was stirred for 30 min; then, 4,7-dichloro-[1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide 1 (50 mg, 0.24 mmol) was added. The mixture was stirred for 12 h at room temperature. The reaction mixture was treated in the same way as in the general procedure for the preparation of compounds 3a–c. The crude product was purified via column chromatography (Silica gel Merck 60, eluent–CH2Cl2/Hexane at a 1:1 (v/v) ratio) to afford 35 mg (70%) of target compound 3d as a dark red solid, with Rf = 0.3 (CH2Cl2/Hexane = 2:1(v/v)). Mp. = 280–283 °C. IR νmax (KBr, cm−1): 1629, 1609, 1492, 1458, 1334, 1297, 1243, 1218, 1150, 1008, 746, 719, 591. 1H NMR (300 MHz, CDCl3, ppm): 8.15–8.12 (m, 4H), 8.05 (d, J = 8.2 Hz, 2H), 7.66 (d, J = 8.2 Hz, 2H), 7.54–7.40 (m, 8H). 13C NMR (75 MHz, CDCl3, ppm): δ 144.9, 143.0, 139.2, 138.89, 138.86, 126.9, 126.7, 125.9, 125.3, 123.3, 123.2, 120.6, 120.3, 113.4, 111.6, 106.7. HRMS (ESI-TOF), m/z: found, 469.1395; calcd for C28H17N6O2 [M + H]+, 469.1408.
3.3.2. General Procedure for the Preparation of Di-Aminated Products 4
Bis-aminated products 3a–c (0.2 mmol) were added with stirring to a solution of Ph3P (62 mg, 0.24 mmol) in dry CH2Cl2 (10 mL). The mixture was stirred at room temperature for 3 h. Then, the mixture was poured into water (25 mL) and extracted with EtOAc (3 × 35mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified using column chromatography (Silica gel Merck 60).
4,7-Bis(2,3,3a,8b-tetrahydrocyclopenta[b]indol-4(1H)-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine 4a
Violet solid, 40 mg (72%), and Rf = 0.4 (CH2Cl2). Mp = 178–180 °C. Eluent–CH2Cl2/hexane at a 1:1 (v/v) ratio. IR νmax (KBr, cm−1): 2955, 2863, 1487, 1453, 1421, 1349, 1215, 894, 749, 666, 572. 1H NMR (300 MHz, CDCl3): 8.62 (d, J = 7.8 Hz, 2H), 7.29–7.19 (m, 4H), 7.02 (t, J = 6.9 Hz, 2H), 5.70–5.61 (m, 2H), 4.13–4.04 (m, 2H), 2.22–2.01 (m, 6H), 1.84–1.63 (m, 4H), 1.55–1.40 (m, 2H). 13C NMR (75 MHz, CDCl3): δ 144.2, 141.4, 141.3, 135.6, 127.7, 124.0, 123.1, 116.4, 67.4, 45.9, 36.3, 34.5, 23.9. HRMS (ESI-TOF), m/z: calcd for C26H25N6O [M + H]+, 437.2084, found, 437.2068.
4,7-Bis(1,2,3,4,4a,9a-hexahydro-9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine 4b
A yield of 144 mg (65%), violet solid, and Rf = 0.3 (a hexane–CH2Cl2 ratio of 2:1, v/v). Mp = 144–146 °C. IR νmax (KBr, cm−1): 2929, 2855, 1482, 1454, 1424, 1271, 1214, 1164, 1094, 1020, 971, 927, 886, 847, 752, 689, 576. 1H NMR (300 MHz, CDCl3): δ 8.55 (d, J = 8.0, 2H), 7.33 (t, J = 8.0, 2H), 7.27 (d, J = 7.3, 2H), 7.11 (t, J = 7.3, 2H), 5.42–5.35 (m, 2H), 3.72–3.66 (m, 2H), 2.43 (d, J = 14.5, 2H), 2.13–1.91 (m, 4H), 1.71–1.62 (m, 4H), 1.41–1.31 (m, 6H). 13C NMR (75 MHz, CDCl3): δ 143.0, 141.2, 141.0, 134.6, 127.5, 123.3, 122.5, 118.2, 63.7, 40.2, 27.7, 24.3, 22.6, 21.0. HRMS (ESI-TOF), m/z: calcd for C28H29N6O [M + H]+, 465.2397, found, 465.2395.
4,7-Bis(1,2,3,4,4a,9a-hexahydro-9H-1,4-methanocarbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine 4c
Violet solid, 40 mg (72%), and Rf = 0.4 (CH2Cl2). Mp = 178–180 °C. Eluent–CH2Cl2/hexane at a 1:1 (v/v) ratio. IR νmax (KBr, cm−1): 2953, 2870, 1482, 1451, 1350, 1254, 1173, 891, 748, 693, 648, 566. 1H NMR (300 MHz, CDCl3): 8.62 (d, J = 8.1 Hz, 2H), 7.28–7.17 (m, 4H), 7.00 (t, J = 7.3 Hz, 2H), 5.14 (d, J = 7.9 Hz, 2H), 3.53 (d, J = 7.9 Hz, 2H), 2.41 (d, J = 20.1 Hz, 4H), 1.74–1.44 (m, 10H), 1.13 (d, J = 10.4 Hz, 2H). 13C NMR (75 MHz, CDCl3): δ 145.4, 141.4, 141.4, 134.6, 127.8, 124.2, 122.9, 116.1, 69.3, 50.7, 43.5, 43.4, 32.0, 28.2, 25.4. HRMS (ESI-TOF), m/z: calcd for C30H29N6O [M + H]+, 489.2397, found, 489.2388.
4,7-Bis(1,2,3,4,4a,9a-hexahydro-9H-carbazol-9-yl)-[1,2,5]oxadiazolo[3,4-d]pyridazine 4d
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (74 mg, 0.32 mmol) was added to a solution of amine 2d (60 mg, 0.13 mmol) in toluene (12 mL). The mixture was refluxed for 7 h; diluted with EtOAc (30 mL); washed with aq. NaHSO3, Na2CO3, water, and brine; dried over MgSO4; and concentrated under reduced pressure. The crude product was purified using column chromatography (Silica gel Merck 60, eluent–CH2Cl2/hexane at a 2:1 (v/v) ratio) to afford 45 mg (78%) of target compound 4d as a dark red solid, with Rf = 0.3 (hexane–CH2Cl2 = 2:1 v/v). Mp. = 182–184 °C. IR νmax (KBr, cm−1): 1493, 1467, 1453, 1334, 1267, 1221, 1122, 744, 718, 675, 609. 1H NMR (300 MHz, CDCl3): δ 8.16 (d, J = 7.3, 4H), 8.07 (d, J = 8.1, 4H), 7.53 (td, J = 8.1, 1.7, 4H), 7.47 (t, J = 7.3, 4H). 13C NMR (75 MHz, CDCl3): δ 143.1, 142.6, 139.0, 126.8, 125.9, 123.4, 120.3, 113.2. HRMS (ESI-TOF), m/z: calcd for C28H17N6O [M + H]+, 453.1458, found, 453.1445.
3.4. DFT Quantum Calculations
DFT calculations were performed using the functional PBE1PBE(PBE0) [23] and the DEF2TZVP [24] basis with the GD3BJ empirical dispersion in the Gaussian 2016 program [25] (gas phase, 298K, see Supplementary Information).
4. Conclusions
We have successfully synthesized a series of novel D-A-D dyes based on [1,2,5]oxadiazolo[3,4-d]pyridazine and [1,2,5]oxadiazolo[3,4-d]pyridazine 1-oxide with various donor fragments. We have established that the emission of furazan-based dyes has the triplet-state phosphorescence nature as well as previously investigated [1,2,5]selenadiazolo[3,4-d]pyridazine derivatives. On the contrary, our studies reveal that the furoxan acceptor provides pure fluorescence in the red spectral region. Furazan derivatives demonstrate higher luminescence quantum yields compared to their furoxan analogs. The observed decrease in fluorescence quantum yields for furoxan derivatives can be attributed to two main factors: (1) a decrease in the overall conjugation of the π–electron system due to the steric repulsion between the furoxan’s external oxygen atom and the electron density of the carbazole system and (2) the formation of hydrogen bonds. The results obtained will subsequently help researchers design new high-performance donor–acceptor molecules with tailored luminescence properties. According to the results of X-ray diffraction studies of 3d·½CHCl3 and 4d, the [1,2,5]oxadiazolo[3,4-d]pyridazine heterocycle is not entirely flat, likely due to intrinsic electronic effects rather than intra- and intermolecular van der Waals interactions. Nearly flat N-carbazolyl groups are rotated with respect to the N2C4 plane of the central heterocycle by rather large angles due to intramolecular non-covalent interactions, indicating that π-π conjugation between the C12H8N and N4C4O/N4C4O2 heterocycle systems is lost to a substantial extent. Therefore, the N-carbazolyl group cannot effectively serve as an antenna to improve the photophysical properties of the dyes. Presumably, the short non-covalent interactions of the furoxan system with a lattice chloroform molecule in 3d lead to luminescence quenching, whereas intermolecular π-π interactions of the nitrogen atoms of the pyridazine ring with the N-carbazolyl group facilitate the intermolecular charge transfer in 4d.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30112374/s1. Characterization data including 1H and 13C NMR spectra for novel compounds and photoluminescence spectra. The crystal structures of 3d·½CHCl3 and 4d have been deposited at the Cambridge Crystallographic Data Center with the following reference CCDC numbers: 2401696 (3d·½CHCl3) and 2401695 (4d); they also contain the supplementary crystallographic data. These data can be obtained free of charge from the CCDC via https://www.ccdc.cam.ac.uk/structures/ (accessed on 3 March 2025). References [26,27,28] are cited in the supplementary materials.
Author Contributions
T.N.C.: investigation and formal analysis. A.V.T.: investigation and formal analysis. V.M.K.: investigation, formal analysis, data curation, writing—original draft, conceptualization, and writing—review and editing. E.D.K.: investigation. D.I.N.: investigation and formal analysis. M.E.M.: investigation. N.P.D.: investigation. M.N.E.: investigation. I.V.T.: supervision, writing—review and editing, conceptualization, and project administration. O.A.R.: supervision, conceptualization, writing—review and editing, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge financial support from the Russian Science Foundation (grant no. 24-73-00175).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bulavko, G.V.; Ishchenko, A.A. Organic Bulk Heterojunction Photovoltaic Structures: Design, Morphology and Properties. Russ. Chem. Rev. 2014, 83, 575–599. [Google Scholar] [CrossRef]
- Le, T.P.; Smith, B.H.; Lee, Y.; Litofsky, J.H.; Aplan, M.P.; Kuei, B.; Zhu, C.; Wang, C.; Hexemer, A.; Gomez, E.D. Enhancing Optoelectronic Properties of Conjugated Block Copolymers through Crystallization of Both Blocks. Macromolecules 2020, 53, 1967–1976. [Google Scholar] [CrossRef]
- Qin, C.; Numata, Y.; Zhang, S.; Yang, X.; Islam, A.; Zhang, K.; Chen, H.; Han, L. Novel Near-Infrared Squaraine Sensitizers for Stable and Efficient Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2014, 24, 3059–3066. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.; Yeh, C.; Zakeeruddin, S.M.; Gratzel, M. Porphyrin-Sensitized Solar Cells with Cobalt(II/III)-Based Redox Electrolyte Exceed 12 Percent E ciency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Zhang, J.; Tang, W.; Tang, A.; Peng, H.; Xu, Z.; Teng, F.; Wang, Y. Key issues and recent progress of high efficient organic light-emitting diodes. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 69–104. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Chmovzh, T.N.; Golovanov, I.S.; Knyazeva, E.A.; Mikhalchenko, L.V.; Saifutyarov, R.S.; Avetisov, I.C.; Woollins, J.D.; Taydakov, I.V.; Rakitin, O.A. Candle Light-Style OLEDs with Benzochalcogenadiazoles Cores. Dyes Pigment. 2021, 185, 108917. [Google Scholar] [CrossRef]
- Zhao, R.; Min, Y.; Dou, C.; Lin, B.; Ma, W.; Liu, J.; Wang, L. A Conjugated Polymer Containing a B ← N Unit for Unipolar N-Type Organic Field-Effect Transistors. ACS Appl. Polym. Mater. 2020, 2, 19–25. [Google Scholar] [CrossRef]
- Makala, M.; Barłóg, M.; Dremann, D.; Attar, S.S.; Fernández, E.G.; Al-Hashimi, M.; Jurchescu, O.D. High-performance n-type polymer field-effect transistors with exceptional stability. J. Mater. Chem. C 2024, 12, 17089–17098. [Google Scholar] [CrossRef]
- Biswas, S.; Pramanik, A.; Ahmed, T.; Sahoo, S.K.; Sarkar, P. Superiority of D–A–D over D–A Type of Organic Dyes for the Application in Dye-Sensitized Solar Cell. Chem. Phys. Lett. 2016, 649, 23–28. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Chmovzh, T.N.; Freidzon, A.Y.; Minyaev, M.E.; Barkanov, A.D.; Golovanov, I.S.; Mikhalchenko, L.V.; Avetisov, I.C.; Taydakov, I.V.; Rakitin, O.A. Small D-π-A-π-D Organic Dyes for near-Infrared Emitting OLEDs with Excellent External Quantum Efficiency. Dyes Pigment. 2023, 208, 110860. [Google Scholar] [CrossRef]
- Korshunov, V.M.; Chmovzh, T.N.; Tsorieva, A.V.; Gruzdev, G.A.; Rakhimkulov, D.M.; Taydakov, I.V.; Rakitin, O.A. Towards Deep NIR Emissive Simple D–A–D Dyes: A Novel Acceptor Block Providing Anti-Kasha’s Rule Emission. J. Mater. Chem. C 2024, 12, 19200–19211. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Kudryashev, T.A.; Gaisin, K.S.; Rakitin, O.A. 4,7-Di(9H-Carbazol-9-Yl)-[1,2,5]Oxadiazolo [3,4-d]Pyridazine. Molbank 2022, 2022, M1428. [Google Scholar] [CrossRef]
- Chmovzh, T.N.; Knyazeva, E.A.; Lyssenko, K.A.; Popov, V.V.; Rakitin, O.A. Safe Synthesis of 4,7-Dibromo[1,2,5]Thiadiazolo[3,4-d]Pyridazine and Its SNAr Reactions. Molecules 2018, 23, 2576. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, V.M.; Chmovzh, T.N.; Chkhetiani, G.R.; Taydakov, I.V.; Rakitin, O.A. New D–A–D Luminophores of the [1,2,5]Thiadiazolo[3,4-d]Pyridazine Series. Mendeleev Commun. 2022, 32, 371–373. [Google Scholar] [CrossRef]
- Chmovzh, T.; Knyazeva, E.; Popov, V.; Rakitin, O. 4,7-Dichloro[1,2,5]Oxadiazolo[3,4-d]Pyridazine 1-Oxide. Molbank 2018, 2018, M982. [Google Scholar] [CrossRef]
- Welmaker, G.S.; Sabalski, J.E. A Process for the Preparation of 1, 2, 3, 4, 8, 9, 10, 10a-Octahydro-7bH-Cyclopenta[b][1,4]Diazepino[6,7,1-Hi]Indole. Tetrahedron Lett. 2004, 45, 4851–4854. [Google Scholar] [CrossRef]
- Saito, K.; Shibata, Y.; Yamanaka, M.; Akiyama, T. Chiral Phosphoric Acid-Catalyzed Oxidative Kinetic Resolution of Indolines Based on Transfer Hydrogenation to Imines. J. Am. Chem. Soc. 2013, 135, 11740–11743. [Google Scholar] [CrossRef]
- Catellani, M.; Del Rio, A. Catalytic Arylation of Carbon-Carbon Double Bond Followed by N- or O-Cyclization. Russ. Chem. Bull. 1998, 47, 928–931. [Google Scholar] [CrossRef]
- CrysAlisPro. Rigaku Oxford Diffraction, version 1.171.42.89a; Rigaku Technologies: Cedar Park, TX, USA, 2023.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 229–341. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.P.G.A.; Petersson, G.A.; Nakatsuji, H.J.W.C.; et al. Gaussian 16 Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).