Abstract
The rhizomes of the genus Atractylodes DC. consist of various bioactive components, including sesquiterpenes, which have attracted a great deal of research interest in recent years. In the present study, we reviewed the previously published literatures prior to November 2023 on the chemical structures, biosynthetic pathways, and pharmacological activities of the sesquiterpenoids from this genus via online databases such as Web of Science, Google Scholar, and ScienceDirect. Phytochemical studies have led to the identification of more than 160 sesquiterpenes, notably eudesmane-type sesquiterpenes. Many pharmacological activities have been demonstrated, particularly anticancer, anti-inflammatory, and antibacterial and antiviral activities. This review presents updated, comprehensive and categorized information on the phytochemistry and pharmacology of sesquiterpenes in Atractylodes DC., with the aim of offering guidance for the future exploitation and utilization of active ingredients in this genus.
1. Introduction
The genus Atractylodes DC. belongs to the family Asteraceae and mainly is distributed in Eastern Asia [1]. There are four accepted names for this genus according to World Flora Online: Atractylodes lancea DC. (A. lancea), Atractylodes macrocephala Koidz. (A. macrocephala), Atractylodes carlinoides (Hand.-Mazz.) Kitam. (A. carlinoides), and Atractylodes koreana (Nakai) Kitam. (A. koreana). Moreover, 60 synonyms of A. lancea are listed in this revision, such as Atractylodes japonica, Atractylodes chinensis, and Atractylodes ovata [2].
The rhizomes of the Atractylodes DC. genus are rich in essential oils, which have been traditionally used for the treatment of gastrointestinal, coronavirus, and rheumatic diseases in China, Korea, and Japan [3,4,5,6]. The rhizomes of A. lancea have been used as crude drugs in the Chinese and Japanese pharmacopoeia, which are referred to Cangzhu and Sojutsu, respectively [7,8]. In addition, A. lancea (known as Khod-Kha-Mao in Thailand) is also used for the treatment of fevers and colds in Thai traditional medicine [9]. A. macrocephala is not only used as functional food in China but has also been historically widely used in traditional Korean and Japanese medicine [10]. These traditional uses of Atractylodes DC. are closely related to its intrinsic chemical composition [11,12].
Sesquiterpenes are significant oily compositions with extensive dispersal in plants, currently gaining recognition due to their wide range of pharmacological effects, including antitumor, anti-inflammatory, antibacterial and antiviral, etc. [13,14,15]. According to the diverse skeletal structures of sesquiterpenes in Atractylodes DC., they can be divided into the following five categories: eudesmane-type (such as β-eudesmol, atractylon), guaiane-type (such as atractylmacrol A, atrchiterpene D), spirovetivane-type (such as hinesol, hinesolone), isopterocarpolone-type (such as 14-hydroxy-isopterocarpolone, Atractyloside I), and eremophilane-type [10,16,17,18,19,20,21]. β-eudesmol, atractylon, and hinesol are usually used as chemical markers for evaluating the quality of Atractylodes DC. in different regions [22,23,24,25]. Many studies have been conducted on Atractylodes DC. [26,27], yet there are still noticeable deficiencies in the literature. Various potential clinical uses and upcoming research paths have been suggested, offering a comprehensive collection of research discoveries on the sesquiterpenes of Atractylodes DC. Hence, the current work presents the chemical constituents, possible biosynthesis, and pharmacologic mechanisms of sesquiterpenoids from Atractylodes DC. genus rhizomes in order to encourage researchers to explore this genus in depth with the aim of discovering novel bioactive substances.
2. Methodology
This present review article considered the previously published literature prior to November 2023 concerning the chemical components, biosynthetic pathways, and pharmacological activities of sesquiterpenoids from the genus Atractylodes DC. The search was conducted using online databases such as Web of Science, Google Scholar, ScienceDirect, PubMed, CNKI, Baidu Scholar, and classic books on Dictionary of TCM. The key words searched included Atractylodes DC., Asteraceae, secondary metabolites, phytochemistry, sesquiterpenoids, biosynthetic, atractylenolides, biological activity, pharmacological, and the names of each species of the genus. The chemical structures were drawn using ChemDraw Professional 14.0 software.
3. Phytochemical Constituents
Our literature investigation revealed that essential oils are the main active ingredient in the genus Atractylodes, among which sesquiterpenoids are the characteristic components. Currently, 163 sesquiterpenoids have been isolated and identified from the genus Atractylodes DC., including 104 eudesmane-type, 32 guaiane-type, 14 spirovetivane-type, 11 isopterocarpolone-type, and 2 eremophilane-type sesquiterpenoids. Their specific chemical names, structures, sources, collection areas, and year of isolation are shown in Table 1, Table 2, Table 3, Table 4 and Table 5.
3.1. Eudesmane-Type Sesquiterpenes
This group of sesquiterpenoids possesses a 5,8α-dimethyl-3-(propan-2-yl)-decahydronaphthalene skeleton, which is abundant in the Atractylodes DC. genus [28,29,30]. Among them, compounds (1–5, 11–13, 28, 34, 39, 42, 61, 68, 77, 78, 82, 83, 85, 87, 89, 90–96, 98) have eudesmane lactone structures, compounds (21, 22, 36, 37, 41, 75, 76, 79, 80, 97) are N-containing eudesmanes, and compounds (18–20, 38, 40) possess epoxy ring groups. Atractylenolides (I–III) have lactone structures and possess antioxidant, anti-inflammatory, and anticancer properties [31]. Atractylenolide I (AT-I) (1), atractylenolide II (AT-II) (2), atractylenolide III (AT-III) (3), and atractylenolide IV (4) are widely present in A. lancea and A. macrocephala [32,33,34,35,36,37]. Atractylenolide V (5), atractylenolide VI (6), atractylenolide VII (7), and biatractylenolide II (8) have been reported in A. macrocephala [38,39,40,41]. A phytochemical investigation of A. macrocephala 95% ethanol extract identified five eudesmane-type sesquiterpenoids (9–13), and their structures were elucidated via NMR and high-resolution electrospray ionization mass spectroscopy (HRESIMS) analyses, X-ray diffraction analyses, and electronic circular dichroism (ECD) [18]. Four new sesquiterpenoids eudesm-4(15),7-diene-3α,9β,11-triol (14), and eudesm-4(15),7-diene-1β,3α,9β,11-tetraol (15), (7Z)-8β,13-diacetoxy-eudesma-4(15),7(11)-diene (16), 7-oxo-7,8-secoeudesma-4(15),11-dien-8-oic acid (17), were purified from the ethanol extract of A. macrocephala using column chromatography on silica gel, Sephadex LH-20, ODS, and high-performance liquid chromatography (HPLC) [42,43]. Zhang et al. [44] identified atramacronoids A–C (18–20) from the rhizomes of A. macrocephala using spectroscopic data analysis, chemical calculations, and X-ray diffraction, which were found to contain an unusual 6/6/5/5/6 skeleton furnished by an unexpected C-8–C-16 linkage. Subsequently, twenty undescribed eudesmane-type sesquiterpenes named atramacronoids D–W (21–40) were identified in the rhizomes of A. macrocephala using extensive spectroscopic data analysis, Snatzke’s rule, ECD calculations, and X-ray crystallographic analysis [45,46]. A chemical investigation of the ethanol extract of A. lancea resulted in the isolation of nine eudesmane-type sesquiterpenoids (41–49), and their structures were elucidated using spectroscopic techniques and HRESIMS [47]. Kamauchi et al. [48] gained two new eudesmane-type sesquiterpenoids, namely 3α-hydroxy pterocarpol (50) and (11R)-2,11,12-trihydroxy-b-selinene (51), along with three known sesquiterpenoids (52–54) in the fermented rhizomes of A. lancea using column chromatography. β-eudesmol (55) and atractylon (98) were widely distributed in genus species [26,49,50,51,52]. (1R,7R,10R)-1-hydroxylcarissone-11-O-β-d-glucopyranoside (56) was isolated from A. lancea via HPLC and elucidated through detailed spectroscopic methods [53]. A phytochemical investigation of the rhizomes of A. macrocephala led to the isolation of four new sesquiterpenes, atractylmacrols B–E (57–60), as well as known eudesmane sesquiterpenes (61) through the interpretation of their NMR spectroscopic data and HREIMS values [54]. Eight eudesmane-type sesquiterpenoids (62–69) were previously isolated from A. lancea with normal-phase and reverse-phase column chromatography and elucidated through detailed spectroscopic methods [55,56]. Xu et al. [57] identified (2S,7R,10S)-3-hydroxylcarissone-11-O-β-d-glucopyranoside (70) and (2R,7R,10S)-3-hydroxylcarissone-11-O-β-d-glucopyranoside (71) in the rhizomes of A. lancea using extensive spectroscopic analyses with experimental and ECD calculations. Eudesm-4(15)-ene-7α,11-diol (72), (5R,10S)-eudesm-4(15),7-diene-11-ol-9-one (73), and eudesm-4(15),7(11)-diene-9α,11-diol (74) were separated from A. lancea via silica gel column chromatography and preparative TLC [58]. Two new nitrogen-containing sesquiterpenoids, atractylenolactam A (75) and atractylenolactam B (76); two new sesquiterpene lactones, 8-methoxy-AT-V (77) and 15-acetoxyl AT-III (86); and four known analogs (78–82) were separated from A. macrocephala using column chromatography and preparative HPLC, and the absolute configurations were established using time-dependent density functional theory ECD (TDDFT-ECD) calculations [59,60]. Zhou et al. [61] isolated six eudesmane-type sesquiterpenoids (83–88) from A. lancea with repeated silica gel column chromatography, and their structures were determined using physiochemical and spectroscopic evidence. Nine atractylenolides (89–97) with lactone structures were isolated from A. macrocephala using silica gel, ODS column chromatography, and preparative HPLC [18,32,35,38,39,62]. Toda et al. [63] purified eudesma-4(14),7(11)-dien-8-one (100) from A. lancea using silica gel column chromatography and preparative TLC, identified using physiochemical and spectroscopic evidence. Three new eudesmane-type sesquiterpenoids, selina-4(14),7,11-trien-9-ol (101), selina-4(14),11-dien-7-ol (102), and atractin A (103), along with two known compounds, eudesm-4(15)-ene-7β,11-diol (99) and selina-4(14),7-dien-11-ol (104), were separated from A. macrocephala using silica gel column chromatography and preparative HPLC, combined with HRESIMS, extensive spectroscopic data, and ECD [28,64]. The eudesmane-type sesquiterpenoids from genus Atractylodes DC. are shown in Table 1.

Table 1.
Eudesmane-type sesquiterpenoids from genus Atractylodes DC.
Table 1.
Eudesmane-type sesquiterpenoids from genus Atractylodes DC.
| NO. | Compounds | Structure | Source | Collection Area | Year |
|---|---|---|---|---|---|
| 1 | Atractylenolide I | 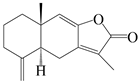 | A. macrocephala [32], A. lancea [33] | China (Yuqian town, Zhejiang province); China (Heilongjiang province) | 2017, 2010 |
| 2 | Atractylenolide II | 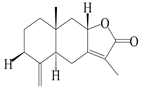 | A. macrocephala [32], A. lancea [34] | China (Yuqian town, Zhejiang province); Germany (Hospital for Traditional Chinese Medicine, Kötzting) | 2017, 1998 |
| 3 | Atractylenolide III/codonolactone | 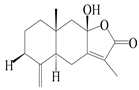 | A. macrocephala [32], A. lancea [35] | China (Yuqian town, Zhejiang province); Japan (Kampo Research Laboratories, Kracie Pharma, Ltd., Takaoka) | 2017, 2016 |
| 4 | Atractylenolide IV | 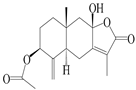 | A. macrocephala [39], A. lancea [36] | China (Pan’an county, Zhejiang province); China (Maoshan mountain of Jiangsu province) | 2014, 2008 |
| 5 | Atractylenolide V | 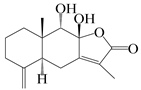 | A. macrocephala [38] | Korea (Ulsan-si Market) | 2016 |
| 6 | Atractylenolide VI | 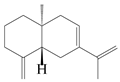 | A. macrocephala [40] | – | 2005 |
| 7 | Atractylenolide VII | 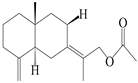 | A. macrocephala [40] | – | 2005 |
| 8 | Biatractylenolide II | 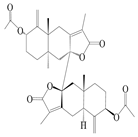 | A. macrocephala [41] | China (Qimen county, Anhui province) | 2017 |
| 9 | (1S,5R,10S)-atractylmacrene C | 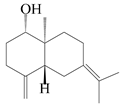 | A. macrocephala [18] | China (Bozhou Medicinal Materials Market) | 2021 |
| 10 | (1R,5S,10R)-atractylmacrene C | 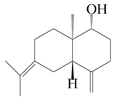 | A. macrocephala [18] | China (Bozhou Medicinal Materials Market) | 2021 |
| 11 | 4R,5R,8S,9S-diepoxyatractylenolide II | 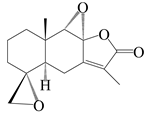 | A. macrocephala [18] | China (Bozhou Medicinal Materials Market) | 2021 |
| 12 | 4-oxo-8S,9S-epoxylatractylenolide II | 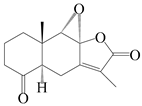 | A. macrocephala [18] | China (Bozhou Medicinal Materials Market) | 2021 |
| 13 | 8S,9S-epoxylatractylenolide II | 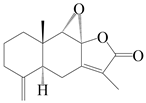 | A. macrocephala [18] | China (Bozhou Medicinal Materials Market) | 2021 |
| 14 | Eudesm-4(15),7-diene-3α,9β,11-triol | 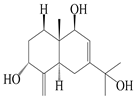 | A. macrocephala [42] | Vietnam (Quan ba city, Ha Giang province) | 2023 |
| 15 | Eudesm-4(15),7-diene-1β,3α,9β,11-tetraol | 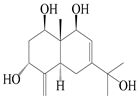 | A. macrocephala [42] | Vietnam (Quan ba city, Ha Giang province) | 2023 |
| 16 | (7Z)-8β,13-diacetoxy-eudesma-4(15),7(11)-diene | 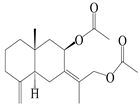 | A. macrocephala [43] | China (Jiaozuo city, Henan province) | 2022 |
| 17 | 7-oxo-7,8-secoeudesma-4(15),11-dien-8-oic acid | 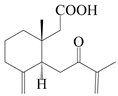 | A. macrocephala [43] | China (Jiaozuo city, Henan province) | 2022 |
| 18 | Atramacronoid A | 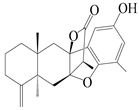 | A. macrocephala [44] | China (Bozhou Medicinal Materials Market) | 2023 |
| 19 | Atramacronoid B | 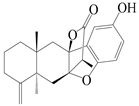 | A. macrocephala [44] | China (Bozhou Medicinal Materials Market) | 2023 |
| 20 | Atramacronoid C | 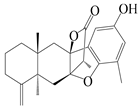 | A. macrocephala [44] | China (Bozhou Medicinal Materials Market) | 2023 |
| 21 | Atramacronoid D | 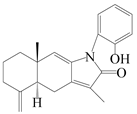 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 22 | Atramacronoid E | 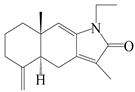 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 23 | Atramacronoid F | 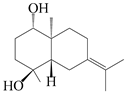 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 24 | Atramacronoid G | 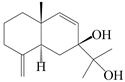 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 25 | Atramacronoid H | 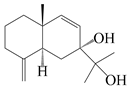 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 26 | Atramacronoid I | 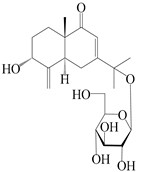 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 27 | Atramacronoid J | 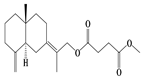 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 28 | Atramacronoid K | 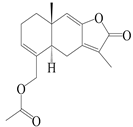 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 29 | Atramacronoid L | 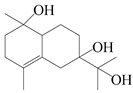 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 30 | Atramacronoid M | 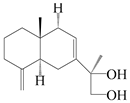 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 31 | Atramacronoid N | 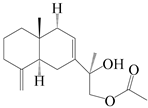 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 32 | Atramacronoid O | 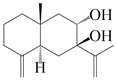 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 33 | Atramacronoid P | 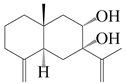 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 34 | Atramacronoid Q | 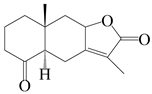 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 35 | Atramacronoid R | 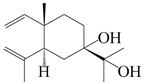 | A. macrocephala [45] | China (Bozhou Medicinal Materials Market) | 2023 |
| 36 | Atramacronoid S | 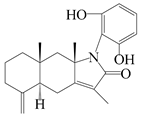 | A. macrocephala [46] | China (Bozhou Medicinal Materials Market) | 2023 |
| 37 | Atramacronoid T | 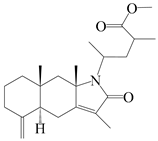 | A. macrocephala [46] | China (Bozhou Medicinal Materials Market) | 2023 |
| 38 | Atramacronoid U | 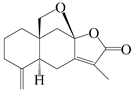 | A. macrocephala [46] | China (Bozhou Medicinal Materials Market) | 2023 |
| 39 | Atramacronoid V | 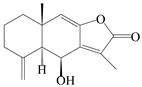 | A. macrocephala [46] | China (Bozhou Medicinal Materials Market) | 2023 |
| 40 | Atramacronoid W | 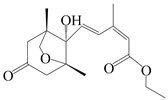 | A. macrocephala [46] | China (Bozhou Medicinal Materials Market) | 2023 |
| 41 | Atrchiterpene A | 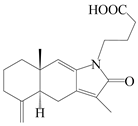 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 42 | Atrchiterpene B | 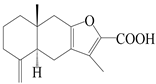 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 43 | Atrchiterpene C | 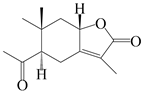 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 44 | 4(15)-eudesmene-1β,7,11-triol | 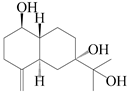 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 45 | 3-eudesmene-1β,7,11-triol | 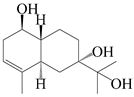 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 46 | Eudesmane-4α,11,15-triol | 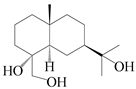 | A. lancea [39] | China (Heilongjiang province) | 2022 |
| 47 | (4α,7β,9α)-farfugane-4,9,11-triol | 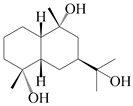 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 48 | (4α,7α,9α)-farfugane-4,9,11-triol | 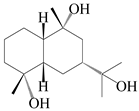 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 49 | (1β,4α,6β)-gorgonane-1β,4α,11-triol | 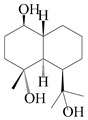 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 50 | 3α-hydroxy pterocarpol | 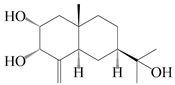 | A. lancea [48] | Japan (Tokyo city, Kinokuniyakanyakkyoku. Co., Ltd.) | 2015 |
| 51 | (11R)-2,11,12-trihydroxy-β-selinene | 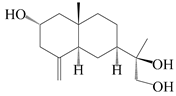 | A. lancea [48] | Japan (Tokyo city, Kinokuniyakanyakkyoku. Co., Ltd.) | 2015 |
| 52 | Pterocarpol | 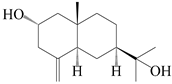 | A. lancea [48] | Japan (Tokyo city, Kinokuniyakanyakkyoku. Co., Ltd.) | 2015 |
| 53 | Kudtdiol | 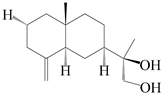 | A. lancea [48] | Japan (Tokyo city, Kinokuniyakanyakkyoku. Co., Ltd.) | 2015 |
| 54 | (11S)-2,11,13-trihydroxy-β-selinene | 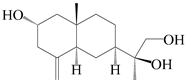 | A. lancea [48] | Japan (Tokyo city, Kinokuniyakanyakkyoku. Co., Ltd.) | 2015 |
| 55 | β-Eudesmol | 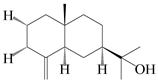 | A. macrocephala [50], A. lancea [49] | China (Qimen city); China (Anguo Chinese Herbs Market, Hebei province) | 2021, 2011 |
| 56 | (1R,7R,10R)-1-hydroxylcarissone-11-O-β-d-glucopyranoside | 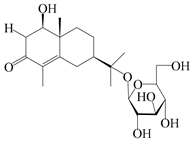 | A. lancea [53] | China (Huanggang city, Hubei province) | 2018 |
| 57 | Atractylmacrol B | 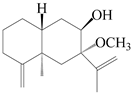 | A. macrocephala [54] | China (Juhuacun Chinese Traditional Medicine Market, Kunming city, Yunnan province) | 2018 |
| 58 | Atractylmacrol C | 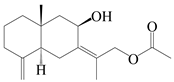 | A. macrocephala [54] | China (Juhuacun Chinese Traditional Medicine Market, Kunming city, Yunnan province) | 2018 |
| 59 | Atractylmacrol D | 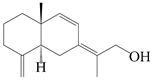 | A. macrocephala [54] | China (Juhuacun Chinese Traditional Medicine Market, Kunming city, Yunnan province) | 2018 |
| 60 | Atractylmacrol E | 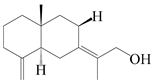 | A. macrocephala [54] | China (Juhuacun Chinese Traditional Medicine Market, Kunming city, Yunnan province) | 2018 |
| 61 | 8β-methoxy-atractylenolide I | 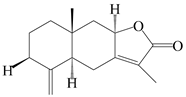 | A. macrocephala [54] | China (Juhuacun Chinese Traditional Medicine Market, Kunming city, Yunnan province) | 2018 |
| 62 | (3S)-3-hydroxyatractylenolide III 3-O-β-d-glucopyranoside | 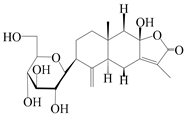 | A. lancea [55] | Japan (Tokyo city, Metropolitan Medical Plants Garden) | 2003 |
| 63 | Atractyloside C | 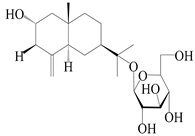 | A. lancea [56] | Japan (Tokyo city, Metropolitan Medical Plants Garden) | 1989 |
| 64 | Atractyloside D | 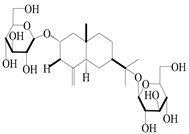 | A. lancea [56] | Japan (Tokyo city, Metropolitan Medical Plants Garden) | 1989 |
| 65 | Atractyloside E | 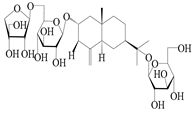 | A. lancea [56] | Japan (Tokyo city, Metropolitan Medical Plants Garden) | 1989 |
| 66 | Atractyloside F | 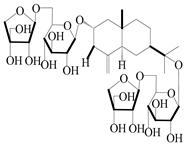 | A. lancea [56] | Japan (Tokyo city, Metropolitan Medical Plants Garden) | 1989 |
| 67 | Atractyloside G | 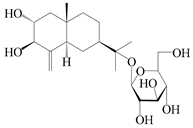 | A. lancea [56] | Japan (Tokyo city, Metropolitan Medical Plants Garden) | 1989 |
| 68 | Atractyloside H | 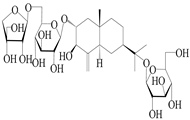 | A. lancea [56] | Japan (Tokyo city, Metropolitan Medical Plants Garden) | 1989 |
| 69 | Atractyloside G 2-O-β-d-glucopyranoside | 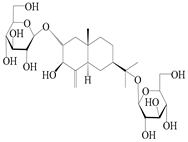 | A. lancea [56] | Japan (Tokyo city, Metropolitan Medical Plants Garden) | 1989 |
| 70 | (2S,7R,10S)-3-hydroxylcarissone-11-O-β-d-glucopyranoside | 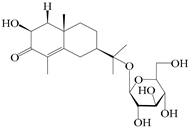 | A. lancea [57] | China (Huanggang city, Hubei province) | 2016 |
| 71 | (2R,7R,10S)-3-hydroxylcarissone-11-O-β-d-glucopyranoside | 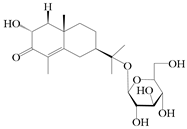 | A. lancea [57] | China (Huanggang city, Hubei province) | 2016 |
| 72 | Eudesm-4(15)-ene-7α,11-diol | 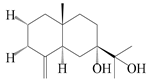 | A. lancea [58] | China (Lanzhou city, Gansu province) | 2008 |
| 73 | (5R,10S)-Eudesm-4(15),7-diene-11-ol-9-one | 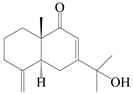 | A. lancea [58] | China (Lanzhou city, Gansu province) | 2008 |
| 74 | Eudesm-4(15),7(11)-diene-9α,11-diol | 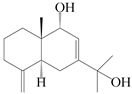 | A. macrocephala [37], A. lancea [58] | China (Hangzhou city, Zhejiang province); China (Lanzhou city, Gansu province) | 2011, 2008 |
| 75 | Atractylenolactam A | 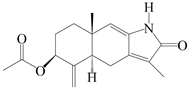 | A. macrocephala [59] | China (Jiaozuo city, Henan province) | 2022 |
| 76 | Atractylenolactam B | 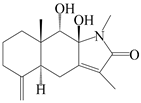 | A. macrocephala [59] | China (Jiaozuo city, Henan province) | 2022 |
| 77 | 8-methoxy-atractylenolide V | 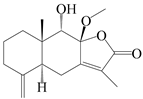 | A. macrocephala [59] | China (Jiaozuo city, Henan province) | 2022 |
| 78 | 15-acetoxyl atractylenolide III | 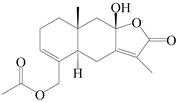 | A. macrocephala [59] | China (Jiaozuo city, Henan province) | 2022 |
| 79 | Taenialactam A | 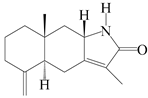 | A. macrocephala [59] | China (Jiaozuo city, Henan province) | 2022 |
| 80 | Taenialactam B | 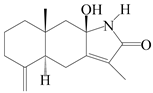 | A. macrocephala [59] | China (Jiaozuo city, Henan province) | 2022 |
| 81 | Eudesma-4(15),7(11)-dien-8-one | 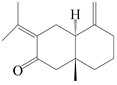 | A. macrocephala [60] | China (Zhejiang province) | 1987 |
| 82 | 8β-methoxyatractylenolide | 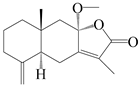 | A. macrocephala [60] | China (Zhejiang province) | 1987 |
| 83 | 4R,15-epoxyatractylenolide II | 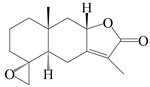 | A. macrocephala [52], A. lancea [61] | China (Pan’an county, Zhejiang province); China (Haerbin city, Heilongjiang province) | 2018, 2020 |
| 84 | Eudesma-7(11)-en-4-ol | 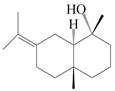 | A. macrocephala [54], A. lancea [61] | China (Juhuacun Chinese Traditional Medicine Market, Kunming city, Yunnan province); China (Haerbin city, Heilongjiang province) | 2018, 2020 |
| 85 | 8β,9α-dihydroxyatractylenolide II | 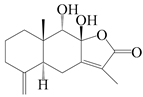 | A. macrocephala [52], A. lancea [61] | China (Pan’an county, Zhejiang province); China (Haerbin city, Heilongjiang province) | 2018, 2020 |
| 86 | Biepiasterolide | 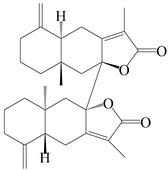 | A. lancea [61] | China (Haerbin city, Heilongjiang province) | 2020 |
| 87 | Atractylenother | 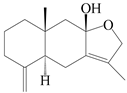 | A. macrocephala [39], A. lancea [61] | China (Pan’an county, Zhejiang province); China (Haerbin city, Heilongjiang province) | 2014, 2020 |
| 88 | Biatractylenolide | 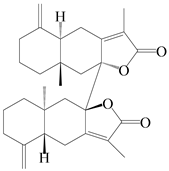 | A. lancea [61] | China (Haerbin city, Heilongjiang province) | 2020 |
| 89 | Isoatractylenolide I | 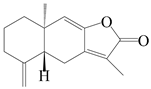 | A. macrocephala [32], A. lancea [61] | China (Yuqian town, Zhejiang province); China (Haerbin city, Heilongjiang province) | 2017, 2020 |
| 90 | 3β-acetoxyl atractylenolide I | 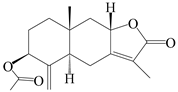 | A. macrocephala [32] | China (Yuqian town, Zhejiang province) | 2017 |
| 91 | 4R,15-epoxy-8β-hydroxyatractylenolide II | 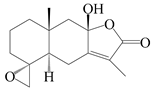 | A. macrocephala [39] | China (Pan’an county, Zhejiang Province) | 2014 |
| 92 | 8-epiatractylenolide III | 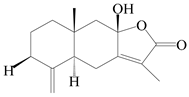 | A. macrocephala [39] | China (Pan’an county, Zhejiang Province) | 2014 |
| 93 | 8-epiasterolid | 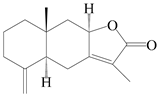 | A. macrocephala [18], A. lancea [35] | China (Bozhou Medicinal Materials Market); Kampo Research Laborato-ries, Kracie Pharma, Ltd., Ta-kaoka | 2021, 2016 |
| 94 | 3β-acetoxyl atractylon | 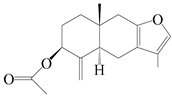 | A. macrocephala [62] | China (Qimen county, Anhui province) | 1997 |
| 95 | 4-ketone-atractylenolide III | 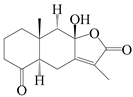 | A. macrocephala [38] | Korea (Ulsan-si) | 2016 |
| 96 | 13-hydroxyl-atractylenolide II | 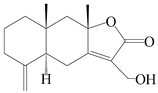 | A. macrocephala [38] | Korea (Ulsan-si) | 2016 |
| 97 | Atractylenolactam | 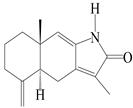 | A. macrocephala [38] | Korea (Ulsan-si) | 2016 |
| 98 | Atractylon | 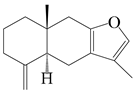 | A. macrocephala [52], A. lancea [35] | China (Pan’an county, Zhejiang province); Kampo Research Laboratories, Kracie Pharma, Ltd., Takaoka | 2018, 2016 |
| 99 | Eudesm-4(15)-ene-7β,11-diol | 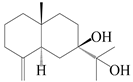 | A. macrocephala [38] | Korea (Ulsan-si) | 2016 |
| 100 | Eudesma-4(14),7(11)-dien-8-one | 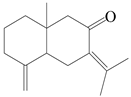 | A. lancea [63] | Japan (Koshiro Co., Ltd.) | 2017 |
| 101 | Selina-4(14),7,11-trien-9-ol | 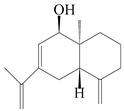 | A. macrocephala [28] | China (Jiaozuo city, Henan province) | 2022 |
| 102 | Selina-4(14),11-dien-7-ol | 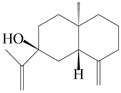 | A. macrocephala [28] | China (Jiaozuo city, Henan province) | 2022 |
| 103 | Atractin A | 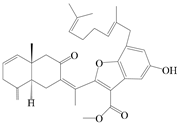 | A. macrocephala [64] | China (Jinan city, Shandong province) | 2022 |
| 104 | Selina-4(14),7-dien-11-ol | 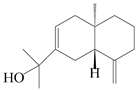 | A. macrocephala [28] | China (Jiaozuo city, Henan province) | 2022 |
‘–’ denotes no useful information found in the study.
3.2. Guaiane-Type Sesquiterpenes
The basic skeleton of guaiane sesquiterpenes contains a five-membered ring combined with a seven-membered ring, with methyl substitutions at C-1 and C-4 and an isopropyl substitution at C-7, which often forms a lactone structure. Si et al. [18] isolated two pairs of guaiane-type sesquiterpene enantiomers (105/106, and 107/108) from the rhizomes of A. macrocephala via chiral-phase HPLC resolution. Five guaiane-type sesquiterpenes containing an interesting epoxy unit (109–112) and a rare tricyclic carbon skeleton type (113) were isolated from rhizomes of A. lancea using silica gel column chromatography and preparative HPLC, and the structures and relative configurations were determined via NMR and MS spectroscopic data [58]. Liu et al. [65] elucidated a secoguaiane lactone glycoside featuring 6/7 cores, named secoatractylohexone A (114), and a 9,10-unsaturated guaiene-type glycoside, named dihydroxy-9-guaine-3-one-11-O-β-d-glucopyranoside (115), along with three known guaiane-type sesquiterpenes (116–118), from the rhizomes of A. lancea on the basis of extensive spectroscopic data and the application of the CD technique. 4,10,11-trihydroxyguaiane (119), atrchiterpene D (120), and macrochaetoside B (121) were elucidated from A. lancea using NMR spectra and HRESIMS [47]. The EtOAc fraction of the A. macrocephala rhizomes was subjected to silica gel, Sephadex LH-20 column chromatography, and semi-preparative HPLC to obtain atractylmacrol A (122) [54]. A new guaiane-type sesquiterpenoid glycoside, namely (3R,4S,7R,10R)-2-hydroxypancherione-11-O-β-d-glucopyranoside (123), was identified from A. lancea using NMR, MS, and ECD data [17]. The atractyloside A (124), 10-epi-atractyloside A (125) (1S,4S,5S,7R,10R)-10,11,14-trihydroxyguai-3-one-11-O-β-d-glucopyranoside (126), (1S,4S,5R,7R,10R)-11,14-dihydroxyguai-3-one 11-O-β-d-glucopyranoside (127), atractyloside B (128), and (1S,5R,7R,10R)-secoatractylolactone-11-O-β-d-glucopyranoside (129) have been isolated and identified in A. macrocephala and A. lancea [17,42,55,66]. Phytochemical investigations of the rhizomes of A. lancea identified two previously described guaiane-type sesquiterpenes, namely (1S,4S,5R,7R,10S)-4,11,14-trihydroxyguai-3-one-11-O-β-d-glucopyranoside (130) and (1S,4S,5R,7R)-4,11,14-trihydroxyguaia-9-en-3-one-11-O-β-d-glucopyranoside (131), and the structures of the isolated compounds were elucidated using NMR spectroscopic analyses [67,68]. Three guaiane lactone glycosides have been identified and isolated from A. lancea via HPLC and elucidated through detailed spectroscopic methods, namely (1R,7R,10S)-10,11-dihydroxy-4-guaien-3-one 11-O-β-d-glucopyranoside (132), atractyloside A 14-O-β-d-fructofuranoside (133), and 1β,5α,7α-H-3β,4α,11,14-tetrahydroxy-guaia-9-en-11-O-β-d-glucopyranoside (134) [53,69]. Guai-10(14)-en-11-ol (135) was isolated from A. macrocephala rhizomes via silica gel column chromatography; its chemical structure was determined by a combination of 1D and 2D NMR analysis and mass spectrometry [43]. A new guaiane-type sesquiterpene, named seco-guaione (136), was recently isolated from a 95% ethanol extraction of A. lancea using macroporous resin, silica gel, and semi-preparative HPLC, and the chemical structure was identified via physiochemical and spectroscopic evidence [29]. The guaiane-type sesquiterpenoids from genus Atractylodes DC. are shown in Table 2.

Table 2.
Guaiane-type sesquiterpenoids from genus Atractylodes DC.
Table 2.
Guaiane-type sesquiterpenoids from genus Atractylodes DC.
| NO. | Compounds | Structure | Source | Collection Area | Year |
|---|---|---|---|---|---|
| 105 | (4S,5S)-atractylmacrene A | 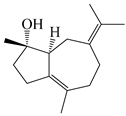 | A. macrocephala [18] | China (Bozhou Medicinal Materials Market) | 2021 |
| 106 | (4R,5R)-atractylmacrene A | 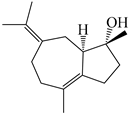 | A. macrocephala [18] | China (Bozhou Medicinal Materials Market) | 2021 |
| 107 | (1S,4S,5S)-atractylmacrene B | 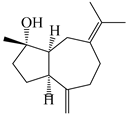 | A. macrocephala [18] | China (Bozhou Medicinal Materials Market) | 2021 |
| 108 | (1R,4R,5R)-atractylmacrene B | 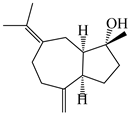 | A. macrocephala [18] | China (Bozhou Medicinal Materials Market) | 2021 |
| 109 | 4α,7α-epoxyguaiane-10α,11-diol | 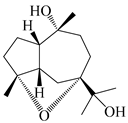 | A. lancea [58] | China (Lanzhou city, Gansu province) | 2008 |
| 110 | 7α,10α-epoxyguaiane-4α,11-diol | 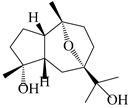 | A. lancea [58] | China (Lanzhou city, Gansu province) | 2008 |
| 111 | 10β,11β-epoxyguaiane-1α,4α-diol | 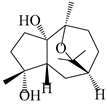 | A. lancea [58] | China (Lanzhou city, Gansu province) | 2008 |
| 112 | 10β,11β-epoxyguaiane-1α,4α,7α-triol | 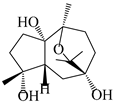 | A. lancea [58] | China (Lanzhou city, Gansu province) | 2008 |
| 113 | 1-Patchoulene-4α,7α-diol | 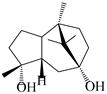 | A. lancea [58] | China (Lanzhou city, Gansu province) | 2008 |
| 114 | Secoatractylohexone A | 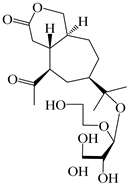 | A. lancea [65] | China (Maoshan mountain in Jiangsu province) | 2022 |
| 115 | Dihydroxy-9-guaine-3-one-11-O-β-d-glucopyranoside | 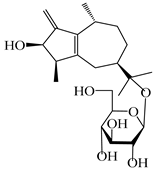 | A. lancea [65] | China (Maoshan mountain in Jiangsu province) | 2022 |
| 116 | (1S,4S,5S,7R,10S)-10,11,14-trihydroxyguai-3-one-11-O-β-d-glucopyranoside | 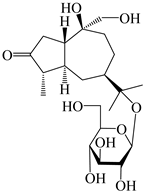 | A. lancea [65] | China (Maoshan mountain in Jiangsu province) | 2022 |
| 117 | (1S,4S,5R,7R,10R)-11,14-dihydroxyguai-3-one-11-O-β-d-glucopyranoside | 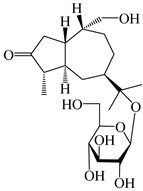 | A. lancea [65] | China (Maoshan mountain in Jiangsu province) | 2022 |
| 118 | (1S,5R,7R,10R)-secoatractylolactone | 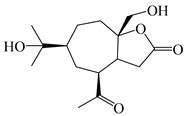 | A. macrocephala [42], A. lancea [65] | Vietnam (Quan ba city, Ha Giang province), China (Maoshan mountain in Jiangsu province) | 2023, 2022 |
| 119 | 4,10,11-trihydroxyguaiane | 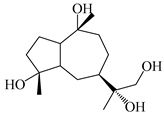 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 120 | Atrchiterpene D | 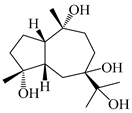 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 121 | Macrochaetoside B | 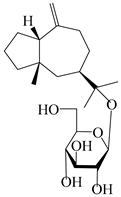 | A. lancea [47] | China (Heilongjiang province) | 2022 |
| 122 | Atractylmacrol A | 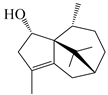 | A. macrocephala [54] | China (Juhuacun Chinese Traditional Medicine Market, Kunming city, Yunnan province) | 2018 |
| 123 | (3R,4R,7R,10R)-2-hydroxypancherione-11-O-β-d-glucopyranoside | 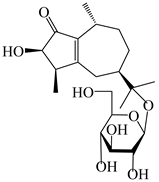 | A. lancea [17] | China (Huanggang city, Hubei province) | 2018 |
| 124 | Atractyloside A | 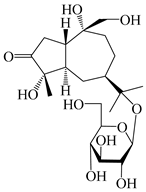 | A. lancea [55] | Japan (Tokyo Metropolitan Medical Plants Garden) | 2003 |
| 125 | 10-epi-atractyloside A | 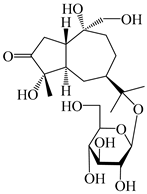 | A. lancea [55] | Japan (Tokyo Metropolitan Medical Plants Garden) | 2003 |
| 126 | (1S,4S,5S,7R,10R)-10,11,14-trihydroxyguai-3-one-11-O-β-d-glucopyranoside | 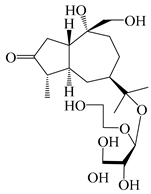 | A. lancea [66] | Japan (Tokyo Metropolitan Medical Plants Garden) | 2003 |
| 127 | (1S,4S,5R,7R,10R)-11,14-dihydroxyguai-3-one 11-O-β-d-glucopyranoside | 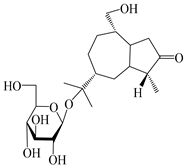 | A. lancea [66] | Japan (Tokyo Metropolitan Medical Plants Garden) | 2003 |
| 128 | Atractyloside B | 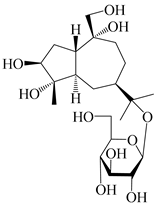 | A. lancea [55] | Japan (Tokyo Metropolitan Medical Plants Garden) | 2003 |
| 129 | (1S,5R,7R,10R)-secoatractylolactone-11-O-β-d-glucopyranoside | 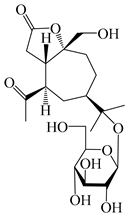 | A. macrocephala [42], A. lancea [55] | Vietnam (Quan ba city, Ha Giang province); Japan (Tokyo Metropolitan Medical Plants Garden) | 2023, 2003 |
| 130 | (1S,4S,5R,7R,10S)-4,11,14-trihydroxyguai-3-one-11-O-β-d-glucopyranoside | 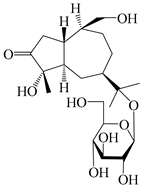 | A. lancea [67] | China (Maoshan mountain of Jiangsu province) | 2015 |
| 131 | (1S,4S,5R,7R)-4,11,14-trihydroxy-guaia-9-en-3-one-11-O-β-d-glucopyranoside | 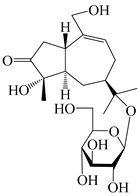 | A. lancea [68] | China (Nanjing city, Jiangsu province) | 2023 |
| 132 | (1R,7R,10S)-10,11-dihydroxy-4-guaien-3-one 11-O-β-d-glucopyranoside | 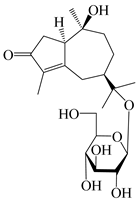 | A. lancea [53] | China (Huanggang city, Hubei province) | 2018 |
| 133 | Atractyloside A 14-O-β-d-fructofuranoside | 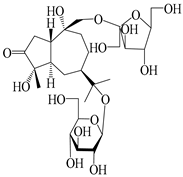 | A. lancea [66] | Japan (Tokyo Metropolitan Medical Plants Garden) | 2003 |
| 134 | 1β,5α,7α-H-3β,4α,11,14-tetrahydroxy-guaia-9-en-11-O-β-d-glucopyranoside | 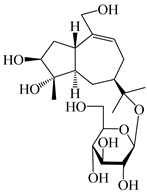 | A. lancea [69] | China (Maoshan mountain of Jiangsu province) | 2015 |
| 135 | Guai-10(14)-en-11-ol | 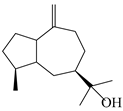 | A. macrocephala [43] | China (Jiaozuo city, Henan province) | 2022 |
| 136 | Seco-guaione | 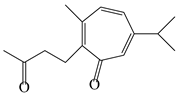 | A. lancea [29] | China (Bozhou city, Anhui province) | 2023 |
3.3. Spirovetivane-Type Sesquiterpenes
Spirovetivane-type sesquiterpenoids possess a five-membered ring and a six-membered ring connected by a spiro atom. Hinesol (137) was obtained from A. lancea using preparative silica gel column chromatography [70]. (4R,5S,7R)-hinesolone-11-O-β-d-glucopyranoside (138) and Hinesolone (139) were separated from the rhizomes of A. lancea using silica gel column chromatography [71,72]. Kamauchi et al. [48] obtained 2-oxo-hinesol (140), 2-oxo-12-hydroxy-hinesol (141), and 2-oxo-15-hydroxy-hinesol (142) from A. lancea fermented by marine fungus. Eight new spirovetivane-type sesquiterpenoids (143–150) were identified from the n-BuOH section of an aqueous EtOH extraction of A. lancea using NMR, MS, and ECD data [17]. The spirovetivane-type sesquiterpenoids from genus Atractylodes DC. are shown in Table 3.

Table 3.
Spirovetivane-type sesquiterpenoids from genus Atractylodes DC.
Table 3.
Spirovetivane-type sesquiterpenoids from genus Atractylodes DC.
| NO. | Compounds | Structure | Source | Collection Area | Year |
|---|---|---|---|---|---|
| 137 | Hinesol | 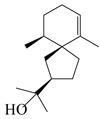 | A. lancea [70] | Japan (Uchida Wakanyaku Ltd., Lot No. 08M1145) | 2015 |
| 138 | (4R,5S,7R)-hinesolone-11-O-β-d-glucopyranoside | 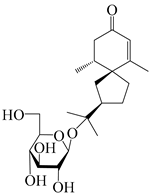 | A. lancea [71] | China (Jurong city, Jiangsu province) | 2020 |
| 139 | Hinesolone | 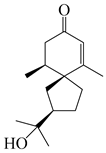 | A. lancea [72] | China (Chinese drug store, Taipei city, Taiwan province) | 2000 |
| 140 | 2-oxo-hinesol | 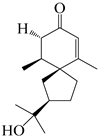 | A. lancea [48] | Japan (Kinokuniyakanyakkyoku. Co., Ltd.) | 2015 |
| 141 | 2-oxo-12-hydroxy-hinesol | 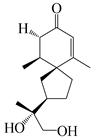 | A. lancea [48] | Japan (Kinokuniyakanyakkyoku. Co., Ltd.) | 2015 |
| 142 | 2-oxo-15-hydroxy-hinesol | 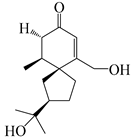 | A. lancea [48] | Japan (Kinokuniyakanyakkyoku. Co., Ltd.) | 2015 |
| 143 | (7R)-3,4-dehydrohinesolone-11-O-β-d-glucopyranoside | 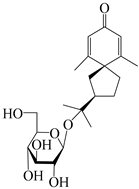 | A. lancea [17] | China (Huanggang city, Hubei province) | 2018 |
| 144 | (7R)-3,4-dehydrohinesolone-11-O-β-d-apiofuranosyl-(1→6)-β-Dglucopyranoside | 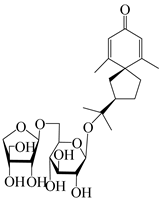 | A. lancea [17] | China (Huanggang city, Hubei province) | 2018 |
| 145 | (5R,7R)-14-hydroxy-3,4-dehydrohinesolone-11-O-β-d-glucopyranoside | 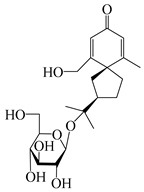 | A. lancea [17] | China (Huanggang city, Hubei province) | 2018 |
| 146 | (5R,7R)-14-hydroxy-3,4-dehydrohinesolone-11-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside | 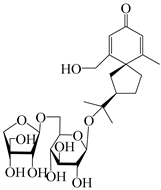 | A. lancea [17] | China (Huanggang city, Hubei province) | 2018 |
| 147 | (5R,7R)-14-hydroxy-3,4-dehydrohinesolone-14-O-β-d-xylopyranoside | 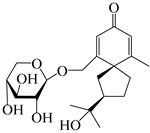 | A. lancea [17] | China (Huanggang city, Hubei province) | 2018 |
| 148 | (4R,5S,7R)-14-hydroxyhinesolone-14-O-β-d-xylopyranoside | 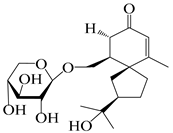 | A. lancea [17] | China (Huanggang city, Hubei province) | 2018 |
| 149 | (3S,4S,5S,7R)-3-hydroxyhinesolone-11-O-β-d-glucopyranoside | 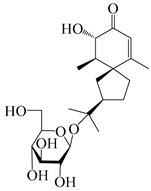 | A. lancea [17] | China (Huanggang city, Hubei province) | 2018 |
| 150 | (4S,5S,7R)-15-hydroxyhinesolone-15-O-β-d-xylopyranoside | 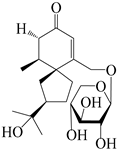 | A. lancea [17] | China (Huanggang city, Hubei province) | 2018 |
3.4. Isopterocarpolone-Type Sesquiterpenes
Isopterocarpolone-type sesquiterpenoids usually possess a 6-(2-hydroxypropan-2-yl)-4,8a-dimethyl-1,4α,5,6,7,8-hexahydronaphthalen-2-one skeleton. 14-hydroxy-isopterocarpolone (151) was identified in A. lancea via physiochemical and spectroscopic analyses [48]. Atractyloside I (152) was described in A. lancea [55]. Meanwhile, another Cis-isomerism (153) was also found in A. lancea [66]. Jiang et al. [53] reported that three isopterocarpolone-type sesquiterpenoids, (5R,7R,10S)-14-hydroxylisopterocarpolone-11-O-β-d-glueopyranoside (154), (5R,7R,10S)-3-O-β-d-glucopyranosylisopterocarpolone-11-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside (155), and (5R,7R,10S)-14-carboxylisopterocarpolone-11-O-β-d-glucopyranoside (156), were isolated from A. lancea using HPLC and elucidated through detailed spectroscopic methods. Five isopterocarpolone-type sesquiterpenoids (157–161) have also been identified in this species using extensive spectroscopic analyses with experimental and ECD calculations [57]. The isopterocarpolone-type sesquiterpenoids from genus Atractylodes DC. are shown in Table 4.

Table 4.
Isopterocarpolone-type sesquiterpenoids from genus Atractylodes DC.
Table 4.
Isopterocarpolone-type sesquiterpenoids from genus Atractylodes DC.
| NO. | Compounds | Structure | Source | Collection Area | Year |
|---|---|---|---|---|---|
| 151 | 14-hydroxy-isopterocarpolone | 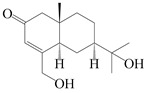 | A. lancea [48] | Japan (Kinokuniyakanyakkyoku. Co., Ltd.) | 2015 |
| 152 | Atractyloside I | 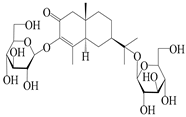 | A. lancea [55] | Japan (Tokyo Metropolitan Medical Plants Garden) | 2003 |
| 153 | Cis-atractyloside I | 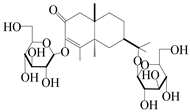 | A. lancea [66] | Japan (Tokyo Metropolitan Medical Plants Garden) | 2003 |
| 154 | (5R,7R,10S)-14-hydroxylisopterocarpolone-11-O-β-d-glueopyranoside | 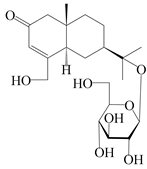 | A. lancea [53] | China (Huanggang city, Hubei province) | 2018 |
| 155 | (5R,7R,10S)-3-O-β-d-glucopyranosylisopterocarpolone-11-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside | 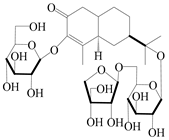 | A. lancea [53] | China (Huanggang city, Hubei province) | 2018 |
| 156 | (5R,7R,10S)-14-carboxylisopterocarpolone-11-O-β-d-glucopyranoside | 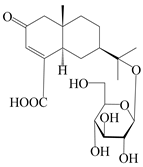 | A. lancea [53] | China (Huanggang city, Hubei province) | 2018 |
| 157 | (5R,7R,10S)-3-hydroxylisopterocarpolone-3-O-β-d-glucopyranoside | 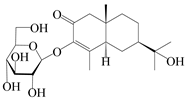 | A. lancea [57] | China (Huanggang city, Hubei province) | 2016 |
| 158 | (5R,7R,10S)-6″-O-acetylatractyloside I | 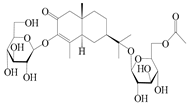 | A. lancea [57] | China (Huanggang city, Hubei province) | 2016 |
| 159 | (5R,7R,10S)-6′-O-acetylatractyloside I | 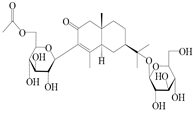 | A. lancea [57] | China (Huanggang city, Hubei province) | 2016 |
| 160 | (5R,7R,10S)-isopterocarpolone-11-O-β-d-apiofuranosyl-(1→6)-β-d-glucopyranoside | 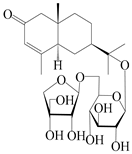 | A. lancea [57] | China (Huanggang city, Hubei province) | 2016 |
| 161 | (5R,7R,10S)-isopterocarpolone-11-O-β-d-glucopyranoside | 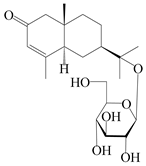 | A. lancea [66] | Japan (Tokyo Metropolitan Medical Plants Garden) | 2003 |
3.5. Eremophilane-Type Sesquiterpenes
Eremophilane-type sesquiterpenes are widely present in several genera (such as Ligularia, Senecio, and Cacalia) of Asteraceae [73,74]. However, this class of sesquiterpenoids shows few forms and narrow distribution in Atractylodes DC. species. Currently, only two eremophilane-type sesquiterpenoids, namely (3S,4R,5R,7R)-3,11-dihydroxy-11,12-dihydronootkatone-11-O-β-d-glucopyranoside (162) and (3S,4R,5S,7R)-3,4,11-trihydroxy-11,12-dihydronootkatone-11-O-β-d-glucopyranoside (163), have been isolated from A. lancea using RP-18, Sephadex LH-20 column chromatography, and semi-preparative HPLC and elucidated through NMR, HRESIMS, and ECD calculations [57]. The eremophilane-type sesquiterpenoids from genus Atractylodes DC. are shown in Table 5.

Table 5.
Eremophilane-type sesquiterpenoids from genus Atractylodes DC.
Table 5.
Eremophilane-type sesquiterpenoids from genus Atractylodes DC.
| NO. | Compounds | Structure | Source | Collection Area | Year |
|---|---|---|---|---|---|
| 162 | (3S,4R,5R,7R)-3,11-dihydroxy-11,12-dihydronootkatone-11-O-β-d-glucopyranoside | 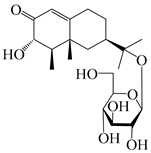 | A. lancea [57] | China (Huanggang city, Hubei province) | 2016 |
| 163 | (3S,4R,5S,7R)-3,4,11-trihydroxy-11,12-dihydronootkatone-11-O-β-d-glucopyranoside | 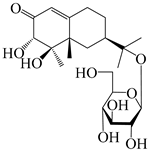 | A. lancea [57] | China (Huanggang city, Hubei province) | 2016 |
3.6. Biosynthesis of Sesquiterpenes
Farnesyl pyrophosphate (FPP) has been recognized as a sesquiterpenoid biosynthetic precursor and generates diverse sesquiterpene carbon skeletons via irregular coupling reactions [75,76]. FPP undergoes one cyclization or more to form germacryl cations, which lose a proton to produce the intermediate germacrenes A/B, followed by a series of protonation, structural rearrangements, and substitutions of various hydroxyls via oxidation reactions to produce eudesmane, guaiane, spirovetivane, isopterocarpolone, and eremophilane skeletons [77,78,79]. The possible biosynthetic pathways of various sesquiterpene types are shown in Figure 1.
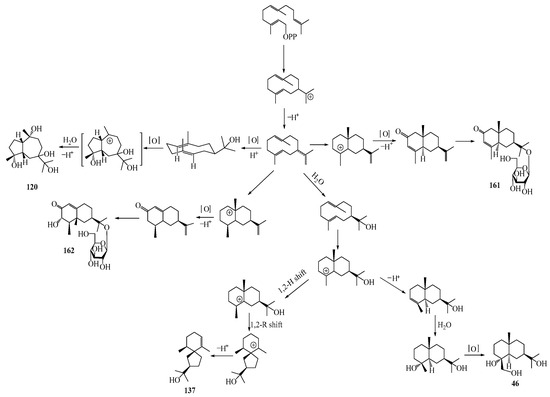
Figure 1.
The putative biosynthetic pathways of eudesmane-type (46), guaiane-type (120), spirovetivane-type (137), isopterocarpolone-type (161), and eremophilane-type (162) sesquiterpenes.
4. Pharmacological Activities
Pharmacological studies have shown that the majority of Atractylodes DC. species exhibit anticancer, anti-inflammatory, antibacterial and antiviral, antioxidant, neuroprotective, and gastrointestinal protection properties. The bioactivities and the corresponding pharmacological mechanisms of the crude extract and isolated sesquiterpenes are listed in Table 6. These findings support the traditional use of Atractylodes DC. in terms of pharmacological activity.

Table 6.
Pharmacological activities of sesquiterpenoids from genus Atractylodes DC.
4.1. Anticancer Activity
Mao et al. [80] determined that an appropriate concentration of atractylon can inhibit the proliferation and promote the apoptosis of intestinal cancer cells by suppressing the PI3K/AKT/mTOR signaling pathway. In addition, atractylon regulates the expression of thymopoietin antisense transcript 1 (TMPO-AS1) and coiled-coil domain-containing 183 antisense RNA 1 (CCDC183-AS1) and inhibits the invasion and migration of liver cancer cells [81]. β-eudesmol was found to have moderate activity against human cholangiocarcinoma (HuCCT-1) cell growth with an IC50 (concentration that inhibits cell growth by 50%) value of 16.80 ± 4.41 µg/mL through the Notch signaling pathway and its upstream/downstream molecules in the CCA cell line at the gene and protein expression levels [82]. Moreover, β-eudesmol treatment (2.5–5 mg/kg) significantly inhibited the growth of H22 and S180 mouse tumor in vivo, which indicated that it inhibited angiogenesis via suppressing CREB activation in growth factor signaling pathway [83]. Hinesol can induces the apoptosis of human leukemia-60 (HL-60) cells through the JNK signaling pathway in HL-60 cells [70]. Furthermore, hinesol reduced cell proliferation via the arresting cell cycle at the G1 phase and induced apoptosis. Further experiments revealed that hinesol inhibited the phosphorylation of MEK and extracellular signal-regulated kinase (ERK) and downregulated the expressions of NF-κB p65 and phosphor-p65 in nuclei [84]. Atramacronoid A induced SGC-7901 cells apoptosis through the promotion of the synthesis of neutrophil elastase [44]. AT-I, AT-II, and atractylon showed the most potent antitumor activity against B16 cells, and they could also induce cell differentiation and inhibit cell migration through inactivating Ras/ERK MAPK (for AT-I and AT-II) and PI3/AKT pathways [85]. AT-I can downregulate the expression of cyclin-dependent kinases (CDK1) in ovarian cancer SK-OV-3 and ovarian carcinoma (OVCAR)-3 cells through the PI3K/AKT pathway, which leads to cell cycle arrest in the G2/M phase, and plays an important role in the proliferation inhibition of tumor cells [86]. AT-I inhibited the self-renewal capacity of gastric stem-like cells (GCSLCs) via the suppression of their sphere formation capacity and cell viability. AT-I attenuated gastric cancer stem cell (GCSC) traits partly through inactivating Notch1, leading to a reduction in the expressions of its downstream targets Hes1, Hey1, and CD44 in vitro [87]. AT-I showed significant antitumor activity on A549 and HCC827 cells in vitro and in vivo, and the possible mechanism of action may be related to apoptosis induced by AT-I via a mitochondria-mediated apoptosis pathway [88]. Ye et al. [89] demonstrated that the G1-arresting and apoptotic effects of AT-II in B16 cells involve p38 activation as well as ERK and Akt inactivation, and the cytotoxic/apoptotic effects of AT-II are potentially p53-dependent. AT-II exerted significant antitumor effects on gastric carcinoma cells by modulating the Akt/ERK signaling pathway, which upregulated the expression level of Bax but downregulated the expression levels of B-cell lymphoma-2 (Bcl-2), p-Akt, and p-ERK compared to those of the control group [90]. Codonolactone, also named AT-III, which inhibited the programming of the epithelial–mesenchymal transition (EMT) in vitro and in vivo, inhibited the motility of metastatic breast cancer cells through the downregulation of transforming growth factor (TGF)-β signaling, and blocked the activation of Runx2 phosphorylation [91]. AT-III can induce the apoptosis of lung carcinoma cells via inhibiting cell growth, increasing lactate dehydrogenase release, and modulating the cell cycle in human lung carcinoma A549 cells. In addition, it also inhibited the proliferation and capillary tube formation of human umbilical vein endothelial cells [92].
4.2. Anti-Inflammatory Activity
Lipopolysaccharides (LPS) act as prototypical endotoxins, inducing inflammation, septic shock, and death, and are commonly used for in vitro models of inflammation [143]. Nitric oxide (NO) is one of the inflammatory mediators of many organs; inhibitors of NO production may have therapeutic potential in the treatment of inflammation accompanying the overproduction of NO [144]. It was determined that the existing cyclic ether on the skeleton of sesquiterpenes is responsible for protective activity against neuroinflammation in LPS-induced BV-2 microglia [45]. AT-I displayed a potent inhibitory effect on angiogenesis through the downregulation of NO, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, VEGF, and PlGF in chronic inflammation [93]. Jin et al. [94] reported that AT-I inhibited the LPS-induced phosphorylation of p38 and ERK mitogen-activated protein kinases (MAPKs) and showed anti-inflammatory activity in RAW264.7 cells. AT-I also inhibited the proliferation of vascular smooth muscle cells (VSMCs) induced by oxidized modified low-density lipoprotein (OXLDL). Migration contributes to antiatherosclerosis by responding to the expression of monocyte chemoattractant protein-1 (MCP-1) and by downregulating the expression of effective inflammatory mediators of the vascular inflammatory response [95]. AT-I extracted from A. macrocephala rhizomes effectively inhibited the increase in vascular permeability in mice caused by acetic acid and reduced cotton pellet granuloma tissue proliferation significantly, which proved that it was an active compound in acute and chronic inflammation models in mice [96]. AT-I was reported previously to act on white blood cell membranes and TLR4, and its anti-inflammatory activity is related to antagonizing the TLR4 pathway [97]. AT-I shows an anti-inflammatory effect by inhibiting TNF-α and IL-6 production. The anti-inflammatory molecular mechanism of AT-I may be associated with the inhibition of the NF-κB, ERK 1/2, and p38 signaling pathways [98]. Animal studies further demonstrated that AT-I and AT-III exert their anti-inflammatory effects by downregulating lipopolysaccharide (LPS)-induced TNF-α expression and inducible NOS (iNOS) expression. Meanwhile, AT-I showed more potent inhibition than AT-III in the production of TNF-α and NO in LPS-activated peritoneal macrophages [99]. Moreover, in vivo experiments revealed that AT-III could alleviate osteoarthritis by inhibiting chondrocyte senescence through reduced phosphorylation of IκB kinase (IKK) α/β, IκBα, and P65 in the NF-κB pathway [100]. Li et al. [101] discovered that atractylon significantly inhibited the ERK, JNK, and NF-κB expression induced by LPS in BV2 cells. It is suggested that atractylone is able to alleviate LPS-induced inflammatory responses through the downregulation of the ERK, JNK, and NF-κB pathways in BV2 cells. Atractylon significantly inhibited NO and prostaglandin E2 production, as well as inducible NO synthase and cyclooxygenase-2 expression in LPS-induced RAW 264.7 cells. Atractylon also significantly reduced the acetic acid-induced writhing response, carrageenan-induced pawedema, and hot-plate latent pain response [35]. Seo et al. [102] investigated the regulatory mechanism of β-eudesmol on mast cell-mediated inflammatory response; the results indicated that it inhibited the production and expression of IL-6 on phorbol 12-myristate 13-acetate and calcium ionophore A23187-stimulated human mast cells (HMCs) via suppressing the activation of p38 MAPKs and NF-κB in activated HMC-1 cells, as well as the activation of caspase-1 and expression of receptor-interacting protein-2.
4.3. Antimicrobial and Antiviral Activity
Previous studies have proven that the spatial arrangement of the terpenoid skeleton combined with an α-methylene-γ-lactone moiety exhibits obvious antiviral activity [145]. Atractyloside A not only possesses anti-influenza B virus infection effects in vivo and in vitro but also can regulate macrophage polarization to the M2-type, which can effectively attenuate the damage caused by influenza B virus infection [103]. Shi et al. [104] reports that atractylon has anti-influenza virus A H3N2, anti-influenza virus A H5N1 (avian influenza virus), and anti-influenza B virus effects at non-toxic concentrations. Cheng et al. [105] determined that atractylon significantly alleviated influenza A virus (IAV)-induced lung injury via regulating the Toll-like receptor 7 (TLR-7) signaling pathway and may warrant further evaluation as a possible agent for IAV treatment. The essential oil of A. lancea exhibited antibacterial activities against both Gram-positive and Gram-negative bacteria through the simultaneous disruption of the cell membrane [106]. The administration of A. macrocephala ethanol extracts (5–40 mg/mL) for 24 h remarkably inhibited the growth of Staphylococcus aureus, Escherichia coli, Bacillus subtilis, and Shigella felxneri bacteria. Meanwhile, the ethanol extracts from the above-ground portion of the plant showed greater antibacterial activity than extracts of rhizome tissues [107]. Li et al. [108] demonstrated that the essential oil of A. lancea had antimicrobial activity against clinical isolates of multidrug-resistant Escherichia coli. Wan et al. [109] discovered that atractylodes essential oil showed antifungal activity against Colletotrichum karstii, Colletotrichum gloeosporioides, Colletotrichum camelliae, Colletotrichum fioriniae, and Colletotrichum chongqingense with EC50 values of 0.089, 0.165, 0.108, 0.205, and 0.092 mg/mL, respectively, and had a significantly higher antifungal effect in the contact phase than that in the vapor phase (p < 0.05).
4.4. Insecticidal Activity
Sesquiterpenoids are well known as major constituents of essential oils and play important ecological roles in the plants’ interactions with pollinators and predators to adapt to the environment [146]. In previous reports, atractylon and β-eudesmol were toxic to fruit flies (LD50 = 1.63 and 2.65 μg/adult, respectively), while the crude oil of A. lancea had an LD50 value of 2.44 μg/adult [49]. β-eudesmol exhibited contact toxicity and ovicidal activity against Plutella xylostella diamondback moths [110]. Although hinesol and β-eudesmol expressed some repellent and contact toxicities against Tribolium castaneum adults (red flour beetles), they displayed a lower repellency level (p < 0.05) than those of N,N-Diethyl-3-methyl benzoyl amide (DEET), and their contact toxicity of them was unremarkable [111]. AT-III and atractylon were proven to possess contact and fumigant toxicities against Dermatophagoides farinae and Dermatophagoides pteronyssinus house dust mite adults using fabric-circle residual contact and vapor-phase toxicity bioassays. They were much more toxic toward house dust mite adults (D. farinae and D. pteronyssinus) than either DEET or dibutyl phthalate but slightly less active than benzyl benzoate [112]. He et al. [113] determined that the hexane-soluble phase of A. lancea has high lavicidal activity against Culex pipiens pallens Coquillett, wild Culex pipiens molestus Forskal, and Aedes albopictus Skuse, which have the potential to be developed as a novel insecticide.
4.5. Neuroprotective Activity
To date, sesquiterpene lactones from medicinal plants have been reported to exhibit a neuroprotective effect against glutamate-induced neurotoxicity in cultured neurons [147]. Biatractylenolide exerted a neuroprotective effect against glutamate-induced excitotoxicity via decreasing the formation of reactive oxygen species (ROS) and the activity of acetylcholinesterase (AChE) and increasing the expression of synapsin I and protein kinase C (PKC) in D-galactose-treated mice, which may have therapeutic potential in aging-related memory impairment [114]. In PC12 and SH-SY5Y cells, biatractylolide could modulate PI3K-Akt-GSK3β-dependent pathways to protect against glutamate-induced cell damage [115]. AT-III was shown to be able to protect phaeochromocytoma (PC) 12 cells from corticosterone-induced injury by inhibiting intracellular Ca2+ overloading and the mitochondrial apoptotic pathway, as well as modulating the MAPK/NF-κB inflammatory pathways, which may serve as a therapeutic agent in the treatment of depression [116]. Liu et al. [117] determined that AT-III exhibited a significant neuroprotective effect against glutamate-induced neuronal apoptosis via inhibiting the caspase signaling pathway, which markedly attenuated the caspases-3-like activity and may therefore have therapeutic potential in excitotoxicity-mediated neurological diseases. In a chronic unpredictable mild stress (CUMS) mouse model, AT-I (5–20 mg/kg) increased sucrose preference and shortened the immobility time in the forced swimming and tail suspension tests and reduced CUMS-induced decreases in serotonin and norepinephrine in the hippocampus [118]. Zhou et al. [119] found that AT-III produces antidepressant- and anxiolytic-like effects, which are related to the normalization of proinflammatory cytokine levels under chronic mild stress. AT-II may reduce the injury of neuronal HT22 cells by oxidative stress through phosphatidylinositol-3 kinase/protein kinase B [120]. In a Parkinson’s disease model, AT-I, AT-II, biepiasterolid, isoatractylenolide I, and AT-III showed a significant protective effect on MPP+-induced SH-SY5Y cells at 1–10 μM [121]. Lin et al. [122] determined that atractylon had a protective effect against sleep-disordered breathing (SDB)-induced nerve cell injury and cognitive dysfunction (CD) via decreasing chronic intermittent hypoxia (CIH)-induced CD and the expression of inflammatory factors in the hippocampal region by suppressing M1 microglial activation and the promotion of M2 microglial activation. Moreover, the downregulation of sirtuin 3 decreased the protective effect of atractylon against CIH-induced microglial cell injury.
4.6. Antioxidant Activity
The antioxidant activity of the sesquiterpene lactones has been proven by their DPPH and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonate) (ABTS) free radical scavenging activity, and most STLs have been reported to exert their antioxidant activity through the activation of the antioxidant response element (ARE) gene [148]. Selina-4(14),7,11-trien-9-ol and selina-4(14),7(11)-dien-8-one exhibited antioxidant activity by activating the Nrf2-ARE receptor in the Keap1-Nrf2-ARE signaling pathway. Furthermore, selina-4(14),7,11-trien-9-ol binds to Keap1 via hydrogen bonds at VAL-606, and selina-4(14),7(11)-dien-8-one binds to Keap1 via hydrogen bonds at VAL-463 and VAL-465 [19]. Atractylon was shown to inhibit carbon tetrachloride (CCl4)-induced cytotoxicity in primary cultured rat hepatocytes and CCl4-induced lipid peroxidation by rat liver microsomes [123]. Hwang et al. [124] further demonstrated that atractylon, at the concentrations of 0.01, 0.1, and 1.0 mg/mL, decreased the formation of malondialdehyde (MDA) and leakage of lactate dehydrogenase (LDH) and alanine aminotransferase (ALT) and activated the repair synthesis of DNA induced by a 30 min treatment of t-BHP (1.5 mM) in primary cultured rat hepatocytes. Xiao et al. [125] demonstrated that AT-II can markedly suppress ionizing radiation (IR) damage by promoting the expression of antioxidant factors heme oxygenase-1 (HO-1) and NAD(P)H dehydrogenase quinone oxido-reductase 1 (NQO-1), which are mediated by the nuclear factor-erythroid 2-like 2 (Nrf2) signaling pathway.
4.7. Activity in Gastrointestinal System
AT-I stimulates intestinal epithelial cell migration and proliferation via the polyamine-mediated Ca2+ signaling pathway, and it may be further developed as a promising therapeutic agent to treat diseases associated with gastrointestinal mucosal injury [126]. AT-III significantly and dose-dependently suppressed gastric ulcer formation via inhibiting matrix metalloproteinase (MMP)-2 and MMP-9 expression, decreasing the extracellular matrix (ECM) damage and preventing gastric ulcer formation [127]. Nogami et al. [128] demonstrated that β-eudesmol markedly inhibited ulcers in Shay rats, as well as histamine- and aspirin-induced gastric ulcers, and showed antisecretory activity on gastric acid secretion stimulated by histamine in a perfused rat stomach preparation. A remarkable antagonistic effect of β-eudesmol against the increased gastrointestinal movement induced by neostigmine was observed in vivo (p < 0.05). Improvements such as an increase in body weight and the normalization of gastrointestinal movement were observed after treatment with β-eudesmol in spleen-deficient mice [129]. Kimura et al. [130] further determined that an extract of A. lancea and β-eudesmol may stimulate gastric emptying or small intestinal motility by inhibiting the dopamine D2 receptor and 5-hydroxytryptamine 3 (HT3) receptor. AT-I could increase fecal water content, accelerate intestinal peristalsis, and thus improve the symptoms of constipation in rats via improving intestinal flora disturbance and increasing the content of acetic acid and propionic acid [131]. Atractyloside A improved gastrointestinal function by protecting the intestinal mucosal barrier via the inhibition of the p38 MAPK pathway [132]. Animal studies further demonstrated that the processing of A. lancea had more satisfactory effects than the crude in treatment of gastric ulcers. The antiulcer effects of A. lancea could be attributed to the anti-inflammatory properties via downregulating TNF-α, interleukin 6 (IL-6), IL-8, and prostaglandin E2 (PGE2) to the gastroprotective effects via upregulating epidermal growth factor (EGF) and trefoil factor2 (TFF2) [133]. Zhang et al. [134] investigated the effects of essential oils extracted from A. lancea on delayed gastric emptying, gastrointestinal hormone, and hypothalamic corticotropin-releasing factor (CRF) abnormalities induced by restraint stress in rats. The results suggested that the regulative effects of the essential oils on delayed gastric emptying are preformed mainly via inhibiting the release of central CRF and the activation of the vagal pathway, which are also involved in the release of gastrointestinal hormones such as motilin, gastrin, and somatostatin. Nakai et al. [135] discovered that an aqueous extract of A. lancea may improve both the delays in gastric emptying and ulcers.
4.8. Miscellaneous Activities
Yu et al. [136] discovered that AT-I could alleviate cerebral ischemia/reperfusion injury by reducing apoptosis and inflammatory responses through the inactivation of the nuclear factor-κB pathway. Additionally, AT-I mediated protective effects against acetaminophen-induced hepatotoxicity via the TLR4/MAPKs/NF-κB pathways, which attenuated the APAP-induced activation of TLR4, NF-κB, and MAPKs (including JNK and p38) [137]. Wang et al. [138] discovered that AT-III ameliorated bile duct ligation (BDL)-induced liver fibrosis by inhibiting the PI3K/AKT signaling pathway, as well as regulating the glutamine metabolic pathway. According to Chen et al. [139], AT-II and AT-III not only reduced agonist-induced platelet aggregation and ATP secretion, downregulated p-Akt and p-p38 MAPK levels, and inhibited platelet proliferation and clot contraction but also prolonged the time to first occlusion and prolonged bleeding. The administration of AT-I (1–300 μg/mL) or AT-III (1–300 μg/mL) to mesenchymal stem cells was found to significantly increase the expression of specific chondrogenic markers, including collagen gel aggrecan, Sox9, sonic hedgehog (Shh) and its target gene Gli-1. These effects indicate that atractylenolides may enhance chondrogenic differentiation by activating the Shh pathway [140]. The sesquiterpenoid extracted from A. lancea showed the inhibition of blood vessel development in zebra fish embryos, which became much more expressive with an increase in concentration. Vegfaa gene expression were downregulated by β-eudesmol at all concentrations. For zebra fish embryos, β-eudesmol and atractylodin were lethal, showing the antiangiogenic property of A. lancea extracts [141]. Tsuneki et al. [142] determined that β-eudesmol significantly inhibited angiogenesis in subcutaneously implanted Matrigel plugs in mice and in adjuvant-induced granuloma in mice through the blockade of the ERK signaling pathway.
5. Conclusions
The structural characteristics, biosynthetic pathways, and biological activities of sesquiterpenes from Atractylodes DC. species have been updated and summarized in the present review. Over 160 sesquiterpenes have been isolated and identified from the genus; among them, eudesmane-type sesquiterpenes were the main structures found in this genus, which accounted for more than 60% of the total sesquiterpenes. Meanwhile, the possible biosynthetic pathways of five categories of sesquiterpenes were also deduced in this review. In addition, improving pharmacological mechanisms support the traditional use of Atractylodes DC. Nevertheless, more research is needed in this field as current studies are still insufficient, and further exploration is required for future advancements. The primary focus of research on Atractylodes DC. species has been directed toward A. lancea and A. macrocephala, with little attention given to other members of the genus; however, it is worth noting that these overlooked species also possess significant value in terms of their active chemical components, making them a valuable addition to Atractylodes DC. resources. The mechanisms of their pharmacological activities, especially their antibacterial and antiviral activity, have not yet been clarified. Atractylon, at an appropriate concentration, can significantly inhibit the proliferation and promote the apoptosis of intestinal cancer cells via suppressing the PI3K/AKT/mTOR signaling pathway, which may be a potential candidate for the treatment of colorectal cancer and other related diseases. An additional investigation is warranted to delve into the therapeutic effectiveness, potential toxicity, and safety profiles of the active components, as well as to elucidate the correlation between chemical structure and biological activity, and to assess their practical use in clinical settings.
Author Contributions
Writing—original draft, Z.Q.; data curation, H.L.; supervision, Z.Z.; writing—review and editing, W.H.; literature collection, S.Z.; project administration, P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jilin Province Science and Technology Department (Grant No. 20220204071YY).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Editorial Committee of Chinese Botany, Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1987; p. 23. [Google Scholar]
- Atractylodes, D.C. The World Flora Online. Available online: http://www.Worldfloraonline.Org/taxon/wfo-4000003599 (accessed on 16 February 2024).
- Liu, J.; Yang, W.; Liu, Y.; Lu, C.; Ruan, L.; Zhao, C.; Huo, R.; Shen, X.; Miao, Q.; Lv, W.; et al. Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): A single-center, open-label, randomized controlled trial. Phytomedicine 2021, 91, 153671. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Zhang, B.X.; Cai, Q. Study on the pharmacodynamics and metabolomics of five medicinal species in Atractylodes DC. on rats with rheumatoid arthritis. Biomed. Pharmacother. 2020, 131, 110554. [Google Scholar] [CrossRef] [PubMed]
- Koonrungsesomboon, N.; Na-Bangchang, K.; Karbwang, J. Therapeutic potential and pharmacological activities of Atractylodes lancea (Thunb.) DC. Asian Pac. J. Tro. Med. 2014, 7, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, M. The state of Atractylodes lancea (Hosoba-okera) in Sado after the showa period. J. Stage 2023, 58, 10–17. [Google Scholar]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; p. 168. [Google Scholar]
- The Committee on the Japanese Pharmacopoeia. The Japanese Pharmacopoeia, 17th ed.; The Minister of Health, Labour and Welfare: Tokyo, Japan, 2016; p. 1791.
- Yang, L.; Yu, H.; Hou, A.; Man, W.J.; Wang, S.; Zhang, J.X.; Wang, X.J.; Zheng, S.W.; Jiang, H.; Kuang, H.X. A review of the ethnopharmacology, phytochemistry, pharmacology, application, quality control, processing, toxicology, and pharmacokinetics of the dried rhizome of atractylodes macrocephala. Front. Pharmacol. 2021, 12, 727154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Zhao, Z.Y.; Chang, L.K.; Cao, Y.; Wang, S.; Kang, C.Z.; Wang, H.Y.; Zhou, L.; Huang, L.Q.; Guo, L.P. Atractylodis Rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J. Ethnopharmacol. 2021, 266, 113415. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.L.; Hua, J.W.; Cheng, W.L.; Qin, L.P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Yao, J.; Song, X.; He, L.W.; Meng, X.C. Overview of chemical composition, pharmacological effects, and clinical application of medicinal plants of Atractylodes DC. Chin. Arch. Trad. Chin. Med. 2023, 41, 151–154. [Google Scholar]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2013, 30, 1226–1264. [Google Scholar] [CrossRef]
- Tian, X.H.; Hong, L.L.; Jiao, W.H.; Lin, H.W. Natural sesquiterpene quinone/quinols: Chemistry, biological activity, and synthesis. Nat. Prod. Rep. 2023, 40, 718–749. [Google Scholar] [CrossRef]
- Shulha, O.; Zidorn, C. Sesquiterpene lactones and their precursors as chemosystematic markers in the tribe Cichorieae of the Asteraceae revisited: An update (2008–2017). Phytochemistry 2019, 163, 149–177. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, S.; Mahomoodally, M.F.; Rengasamy, K.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef]
- Xu, K.; Jiang, J.S.; Feng, Z.M.; Yang, Y.N.; Li, L.; Zang, C.X.; Zhang, P.C. Bioactive sesquiterpenoid and polyacetylene glycosides from Atractylodes lancea. J. Nat. Prod. 2016, 79, 1567–1575. [Google Scholar] [CrossRef]
- Si, J.G.; Zhang, H.X.; Yu, M.; Li, L.Y.; Zhang, H.W.; Jia, H.M.; Ma, L.Y.; Qin, L.L.; Zhang, T.; Zou, Z.M. Sesquiterpenoids from the rhizomes of Atractylodes macrocephala and their protection against lipopolysaccharide-induced neuroinflammation in microglia BV-2 cells. J. Funct. Foods 2021, 83, 104541. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Li, Y.Z.; Huang, S.Q.; Zhang, H.W.; Deng, C.; Song, X.M.; Zhang, D.D.; Wang, W. Genus Chloranthus: A comprehensive review of its phytochemistry, pharmacology, and uses. Arab. J. Chem. 2022, 15, 104260. [Google Scholar] [CrossRef]
- Wang, L.Y.; Li, M.Y.; Jin, L.H.; Wei, Y.C.; Wang, J.M.; Pan, J.L.; Zhang, C.C.; Li, C.Y.; Jiang, F.S. Chemical characterization and antioxidant, anti-inflammatory, and anti-septic activities of the essential oil from the aerial parts of Atractylodes macrocephala Koidz. Arab. J. Chem. 2022, 15, 104215. [Google Scholar] [CrossRef]
- Xie, Z.Y.; Lin, M.Q.; He, X.L.; Dong, Y.J.; Chen, Y.G.; Li, B.; Chen, S.H.; Lv, G.Y. Chemical constitution, pharmacological effects and the underlying mechanism of atractylenolides: A Review. Molecules 2023, 28, 3987. [Google Scholar] [CrossRef]
- Song, B.H.; Wang, W.; Liu, R.P.; Cai, J.J.; Jiang, Y.Y.; Tang, X.M.; Wu, H.F.; Ao, H.; Chen, L. Geographic differentiation of essential oil from rhizome of cultivated Atractylodes lancea by using GC-MS and chemical pattern recognition analysis. Molecules 2023, 28, 2216. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, Y.; Song, B.; Tang, X.; Wu, H.; Jin, Z.; Chen, L. Discovery of quality markers in the rhizome of Atractylodes chinensis using GC–MS fingerprint and network pharmacology. Arab. J. Chem. 2023, 16, 105114. [Google Scholar] [CrossRef]
- Xu, R.; Lu, J.; Wu, J.; Yu, D.; Chu, S.; Guan, F.; Liu, W.; Hu, J.; Peng, H.; Zha, L. Comparative analysis in different organs and tissue-specific metabolite profiling of Atractylodes lancea from four regions by GC-MS and laser microdissection. J. Sep. Sci. 2022, 45, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.C.; Liu, Z.Q.; Yang, Z.J.; Zhang, G.Q.; Sun, L.L.; Wang, M.; Ren, X.L. Species differentiation and quality evaluation for Atractylodes medicinal plants by GC/MS coupled with chemometric analysis. Chem. Biodivers. 2023, 20, e202300793. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, Z.B.; Sun, Y.P.; Yang, B.Y.; Kuang, H.X. Research progress on chemical structure and biological activity of sesquiterpenes from Atractylodes. Chin. Tradit. Herbal. Drugs 2021, 52, 299–309. [Google Scholar]
- Kim, H.Y.; Kim, J.H. Sesquiterpenoids isolated from the rhizomes of genus Atractylodes. Chem. Biodivers 2022, 19, e202200703. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Gao, G.; Ma, G.L.; Xu, R.Z.; Guo, T.; Wu, L.M.; Liu, X.G.; Xie, Z.S.; Xu, J.Y.; Zhang, Z.Q.; et al. Two new sesquiterpenes from the rhizomes of Atractylodes macrocephala and their biological activities. Nat. Prod. Res. 2022, 36, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, H.J.; Hu, G.Q. Chemical constituents from Atractylodes chinensis and their angiogenesis inhibitory activities. Chin. Trad. Patent Med. 2023, 45, 3271–3276. [Google Scholar]
- Wu, Q.X.; Shi, Y.P.; Jia, Z.J. Eudesmane sesquiterpenoids from the Asteraceae family. Nat. Prod. Rep. 2006, 23, 699–734. [Google Scholar] [CrossRef]
- Bailly, C. Atractylenolides, essential components of Atractylodes-based traditional herbal medicines: Antioxidant, anti-inflammatory and anticancer properties. Eur. J. Pharmacol. 2021, 891, 173735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, C.; Sun, T.M.; Ran, X.K.; Kang, T.G.; Dou, D.Q. Two new compounds from Atractylodes macrocephala with neuroprotective activity. J. Asian Nat. Prod. Res. 2017, 19, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Li, G.; Dai, R.; Ma, Y.; Zhang, K.; Zhang, C.; Li, X.; Wang, J. Chemical constituents of Atractylodes chinensis (DC.) koidz. Biochem. Syst. Ecol. 2010, 38, 1220–1223. [Google Scholar] [CrossRef]
- Resch, M.; Steigel, A.; Chen, Z.L.; Bauer, R. 5-Lipoxygenase and cyclooxygenase-1 inhibitory active compounds from Atractylodes lancea. J. Nat. Prod. 1998, 61, 347–350. [Google Scholar] [CrossRef]
- Chen, L.G.; Jan, Y.S.; Tsai, P.W.; Norimoto, H.; Michihara, S.; Murayama, C.; Wang, C.C. Anti-inflammatory and antinociceptive constituents of Atractylodes japonica Koidzumi. J. Agric. Food Chem. 2016, 64, 2254–2262. [Google Scholar] [CrossRef]
- Duan, J.; Wang, Y.; Qian, S.H.; Su, S.L.; Tang, Y.P. A new cytotoxic prenylated dihydrobenzofuran derivative and other chemical constituents from the rhizomes of Atractylodes lancea DC. Arch. Pharm. Res. 2008, 31, 965–969. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, J.; Li, Y.Y. Screening for compounds with aromatase inhibiting activities from Atractylodes macrocephala Koidz. Molecules 2011, 16, 3146–3151. [Google Scholar] [CrossRef]
- Hoang, L.S.; Tran, M.H.; Lee, J.S.; Ngo, Q.M.T.; Woo, M.H.; Min, B.S. Inflammatory inhibitory activity of sesquiterpenoids from Atractylodes macrocephala rhizomes. Chem. Pharm. Bull. 2016, 64, 507–511. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.W. New eudesmane-type sesquiterpenoids from the processed rhizomes of Atractylodes macrocephala. J. Asian Nat. Prod. Res. 2014, 16, 123–128. [Google Scholar] [CrossRef]
- Ding, X.Y.; Liu, M.Y.; Zhang, W.L.; Lin, H.Y. New sesquiterpenoids from the rhizomes of Atractylodes Macrocephala. J. Chin. Pharm. Sci. 2005, 57, 37–42. [Google Scholar]
- Li, Y.Z.; Dai, M.; Peng, D.Y. New bisesquiterpenoid lactone from the wild rhizome of Atractylodes macrocephala Koidz grown in Qimen. Nat. Prod. Res. 2017, 31, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Hai, C.H.; Luyen, N.T.; Giang, D.H.; Minh, B.Q.; Trung, N.Q.; Chinh, P.T.; Hau, D.V.; Dat, N.T. Atractylodes macrocephala Koidz’s rhizomes contain anti-inflammatory sesquiterpenes. Chem. Pharm. Bull. 2023, 71, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.Z.; Zhao, X.; Du, Y.Y.; Xu, M.S.; Liu, X.G.; Xie, Z.S.; Gao, S.; Xu, J.Y.; Wang, P. New eudesmane sesquiterpenoids from Atractylodis macrocephalae rhizoma and their inhibitory activities against SREBPs. Chin. J. Chin. Mater. Med. 2022, 47, 428–432. [Google Scholar]
- Zhang, H.X.; Li, J.R.; Si, J.G.; Dong, C.Y.; Li, Q.; Yu, M.; Qin, L.L.; Li, L.Y.; Zhao, C.X.; Zhang, T.; et al. Atramacronoids A−C, three eudesmanolide sesquiterpene-phenol hybrids with an unprecedented C−C linkage from the rhizomes of Atractylodes macrocephala. Chinese Chem. Lett. 2023, 34, 107743. [Google Scholar] [CrossRef]
- Zhang, H.X.; Si, J.G.; Li, J.R.; Yu, M.; Qin, L.L.; Zhao, C.X.; Zhang, T.; Zou, Z.M. Eudesmane-type sesquiterpenes from the rhizomes of Atractylodes macrocephala and their bioactivities. Phytochemistry 2023, 206, 113545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Lin, C.Y.; Yin, L.Y.; Si, J.G.; Yu, M.; Li, J.R.; Li, L.Y.; Zhang, T.; Zou, Z.M. Bioactive constituents from the rhizomes of Atractylodes macrocephala. Fitoterapia 2023, 165, 105431. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.X.; Liu, Y.; Wang, S.Y.; Sun, Y.; Pan, J.; Guan, W.; Hao, Z.C.; Kuang, H.X.; Yang, B.Y. Cytotoxic sesquiterpenoids from Atractylodes chinensis (DC.) Koidz. Chem. Biodiversity 2022, 19, e202200812. [Google Scholar] [CrossRef] [PubMed]
- Kamauchi, H.; Kinoshita, K.; Takatori, K.; Sugita, T.; Takahashi, K.; Koyama, K. New sesquiterpenoids isolated from Atractylodes lancea fermented by marine fungus. Tetrahedron 2015, 71, 1909–1914. [Google Scholar] [CrossRef]
- Chu, S.S.; Jiang, G.H.; Liu, Z.L. Insecticidal compounds from the essential oil of Chinese medicinal herb Atractylodes chinensis. Pest Manag. Sci. 2011, 67, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.N.; Lin, M.; Zhuo, W.Y.; Li, Y.Z. Chemical constituents from the wild Atractylodes macrocephala Koidz and acetylcholinesterase inhibitory activity evaluation as well as molecular docking study. Molecules 2021, 26, 7299. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhao, Y.Y.; Zhao, Y.S.; Yan, Z.S.; Lu, L.T.; Zheng, Y.G.; Fang, H.Y. Analysis of essential oil from medicinal and non-medicinal parts of Atractylodes chinensis and antibacterial activity in vitro. China Pharm. 2022, 33, 2609–2614. [Google Scholar]
- Li, Y.; Yang, X.W. Chemical constituents of rhizomes of Atractylodes macrocephala. Mod. Chin. Med. 2018, 20, 382–386. [Google Scholar]
- Jiang, J.S.; Xu, K.; Feng, Z.M.; Yang, Y.N.; Zhang, P.C. Four new sesquiterpenes from Atractylodes lancea. Phytochem. Lett. 2018, 26, 88–92. [Google Scholar] [CrossRef]
- Wang, S.Y.; Ding, L.F.; Su, J.; Peng, L.Y.; Song, L.D.; Wu, X.D. Atractylmacrols A-E, sesquiterpenes from the rhizomes of Atractylodes macrocephala. Phytochem. Lett. 2018, 23, 127–131. [Google Scholar] [CrossRef]
- Kitajima, J.; Kamoshita, A.; Ishikawa, T.; Takano, A.; Fukuda, T.; Isoda, S.; Ida, Y. Glycosides of Atractylodes japonica. Chem. Pharm. Bull. 2003, 51, 152–157. [Google Scholar] [CrossRef]
- Shoji, Y.; Tomoko, H.; Kazue, I.; Marubayashi, N.; Ueda, I.; Kohda, H.; Goto, K.; Izumi, H.; Nuno, M.; Katsuki, S.; et al. Studies on the constituents of Atractylodes lancea. Chem. Pharm. Bull. 1989, 37, 2995–3000. [Google Scholar]
- Xu, K.; Feng, Z.M.; Yang, Y.N.; Jiang, J.S.; Zhang, P.C. Eight new eudesmane-and eremophilane-type sesquiterpenoids from Atractylodes lancea. Fitoterapia 2016, 114, 115–121. [Google Scholar] [CrossRef]
- Wang, H.X.; Liu, C.M.; Liu, Q.; Gao, K. Three types of sesquiterpenes from rhizomes of Atractylodes lancea. Phytochemistry 2008, 69, 2088–2094. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.N.; Xu, R.Z.; Zhang, X.W.; Sun, Y.R.; Feng, Q.M.; Li, Z.H.; Xu, J.Y.; Xie, Z.S.; Zhang, Z.Q.; et al. Sesquiterpene lactams and lactones with antioxidant potentials from Atractylodes macrocephala discovered by molecular networking. Front. Nutr. 2022, 9, 865257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L. The acetylenes from Atractylodes macrocephala. Planta. Med. 1987, 53, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Gao, H.R.; Wu, X.P.; Song, H.J.; Ma, D.N. Chemical constituents of petroleum ether extracted from Atractylodes Japonica. Acta. Chin. Med. Pharm. 2020, 48, 26–29. [Google Scholar]
- Chen, Z.L.; Cao, W.Y.; Zhou, G.X.; Wichtl, M. A sesquiterpene lactam from Artractylodes macrocephala. Phytochemistry 1997, 45, 765–767. [Google Scholar] [CrossRef]
- Toda, Y.; Shigemori, H.; Ueda, J.; Miyamoto, K. Isolation and identification of polar auxin transport inhibitors from Saussurea costus and Atractylodes japonica. Acta. Agrobotanica. 2017, 70, 1700. [Google Scholar] [CrossRef]
- Wen, S.S.; Wang, W.J.; Zheng, J.; Li, L.; Yang, S.J.; Zhang, L.Y.; Niu, C.; Xu, Y.W. Atractin A, a new sesquiterpenoid-geranylbenzofuran conjugate from Atractylodes macrocephala. Chem. Nat. Compd. 2022, 58, 1039–1041. [Google Scholar] [CrossRef]
- Liu, L.; Guan, F.; Chen, Y.; Wang, F.; Chen, P.; Yin, M.; Wang, B.; Li, L.; Wang, Q.; Gu, Y.; et al. Two novel sesquiterpenoid glycosides from the rhizomes of Atractylodes lancea. Molecules 2022, 27, 5753. [Google Scholar] [CrossRef]
- Kitajima, J.; Kamoshita, A.; Ishikawa, T.; Takano, A.; Fukuda, T.; Isoda, S.; Ida, Y. Glycosides of Atractylodes lancea. Chem. Pharm. Bull. 2003, 51, 673–678. [Google Scholar] [CrossRef]
- Yin, M.; Xiao, C.C.; Chen, Y.; Wang, M.; Guan, F.Q.; Wang, Q.Z.; Shan, Y.; Feng, X. A new sesquiterpenoid glycoside from rhizomes of Atractylodes lancea. Chin. Herb. Med. 2015, 7, 371–374. [Google Scholar] [CrossRef]
- Wang, M.D.; Chen, P.X.; Yin, M.; Xu, X.X.; Chen, Y.; Feng, X.; Guan, F.Q.; Liao, P.H.; Wang, Q.Z. Phytochemical and chemotaxonomic study on Atractylodes lancea. Biochem. Syst. Ecol. 2023, 111, 104734. [Google Scholar] [CrossRef]
- Yin, M.; Xiao, C.C.; Chen, Y.; Ming, W.; Guan, F.Q.; Dong, Y.F.; Feng, X. Two new sesquiterpenoid glycosides from rhizomes of Atractylodes lancea. Chem. Nat. Compd. 2015, 3, 495–499. [Google Scholar] [CrossRef]
- Masuda, Y.; Kadokura, T.; Ishii, M.; Takada, K.; Kitajima, J. Hinesol, a compound isolated from the essential oils of Atractylodes lancea rhizome, inhibits cell growth and induces apoptosis in human leukemia HL-60 cells. J. Natural. Med. 2015, 69, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Long, L.P.; Wang, L.S.; Qi, S.Z.; Yang, Y.R.; Gao, H.Y. New sesquiterpenoid glycoside from the rhizomes of Atractylodes lancea. Nat. Prod. Res. 2020, 34, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.Y.; Wu, Y.C.; Lin, H.C. Phytochemical and pharmacological studies on Chinese Changzhu. J. Chin. Chem. Soc. 2000, 47, 561–566. [Google Scholar] [CrossRef]
- Hou, C.; Kulka, M.; Zhang, J.; Li, Y.; Guo, F. Occurrence and biological activities of eremophilane-type sesquiterpenes. Mini-Rev. Med. Chem. 2014, 14, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Zhao, Y.; Liu, Y.Z.; Zhang, Z.Z. Two new eremophilane-type sesquiterpenoids from the rhizomes of Ligularia veitchiana (Hemsl.) Greenm. Helv. Chim. Acta 2008, 91, 1712–1716. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Wang, C.Y.; Han, X.; Dai, C.C. Early stem growth mutation alters metabolic flux changes enhance sesquiterpenoids biosynthesis in Atractylodes lancea (Thunb.) DC. Plant Cell Tiss. Org. 2022, 149, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Tissandié, L.; Viciana, S.; Brevard, H.; Meierhenrich, U.J.; Filippi, J.J. Towards a complete characterisation of guaiacwood oil. Phytochemistry 2018, 149, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zheng, W.; Qian, S.Y.; Yang, M.F.; Lu, Y.Z.; He, Z.J.; Kang, J.C. New guaiane-Type Sesquiterpenoids biscogniauxiaols A–G with anti-fungal and anti-inflammatory activities from the endophytic fungus Biscogniauxia Petrensis. J. Fungi 2023, 9, 393. [Google Scholar] [CrossRef]
- Ramirez, A.M.; Saillard, N.; Yang, T.; Franssen, M.C.; Bouwmeester, H.J.; Jongsma, M.A. Biosynthesis of sesquiterpene lactones in Pyrethrum (Tanacetum cinerariifolium). PLoS ONE 2013, 8, e65030. [Google Scholar] [CrossRef]
- Mao, J.J.; Wang, X.P.; Yu, M.H.; Sun, C.K. Effects of atractylon on proliferation and apoptosis of intestinal cancer cells through PI3K/AKT/mTOR signaling pathway. J. Modern Oncol. 2022, 68, 153–160. [Google Scholar]
- Cheng, Y.; Ping, J.; Chen, J.J.; Fu, Y.F.; Zhao, H.; Xue, J.H. Molecular mechanism of atractylon in the invasion and migration of hepatic cancer cells based on high-throughput sequencing. Mol. Med. Rep. 2022, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Vanaroj, P.; Chaijaroenkul, W.; Na-Bangchang, K. Atractylodin and β-eudesmol from Atractylodes lancea (Thunb.) DC. inhibit cholangiocarcinoma cell proliferation by downregulating the notch signaling pathway. Asian Pac. J. Cancer Prev. 2023, 24, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.L.; Li, Y.C.; Tsuneki, H.; Xiao, J.F.; Xia, M.Y.; Wang, M.W.; Kimura, I. Beta-eudesmol suppresses tumour growth through inhibition of tumour neovascularisation and tumour cell proliferation. J. Asian Nat. Prod. Res. 2008, 10, 159–167. [Google Scholar] [CrossRef]
- Guo, W.Q.; Liu, S.B.; Ju, X.; Du, J.H.; Xu, B.; Yuan, H.X.; Qin, F.J.; Li, L.Z. The antitumor effect of hinesol, extract from Atractylodes lancea (Thunb.) DC. by proliferation, inhibition, and apoptosis induction via MEK/ERK and NF-κB pathway in non–small cell lung cancer cell lines A549 and NCI-H1299. J. Cell Biochem. 2019, 120, 18600–18607. [Google Scholar] [CrossRef]
- Ye, Y.; Chou, G.X.; Wang, H.; Chu, J.H.; Fong, W.F.; Yu, Z.L. Effects of sesquiterpenes isolated from largehead atractylodes rhizome on growth, migration, and differentiation of B16 melanoma cells. Integr. Cancer Ther. 2011, 10, 92–100. [Google Scholar]
- Long, F.Y.; Jia, P.; Wang, H.F.; Qing, Y.; He, M.J.; Wang, X.L. Mechanisms and proliferation inhibitory effects of atractylenolideⅠon SK-OV-3 and OVCAR-3 ovarian cancer cell. J. Reg. Anat. Oper. Surg. 2017, 26, 89–93. [Google Scholar]
- Ma, L.; Mao, R.R.; Shen, K.; Zheng, Y.H.; Li, Y.Q.; Liu, J.W.; Ni, L. Atractylenolide I-mediated Notch pathway inhibition attenuates gastric cancer stem cell traits. Biochem. Biophys. Res. Commun. 2014, 450, 353–359. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Zhang, T.; Zhao, Z.; Zhao, Y.; Cheng, P.; Li, H.; Gao, H.; Su, X. Anti-tumor effects of Atractylenolide I isolated from Atractylodes macrocephala in human lung carcinoma cell lines. Molecules 2013, 18, 13357–13368. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, H.; Chu, J.H.; Chou, G.X.; Chen, S.B.; Mo, H.; Fong, W.F.; Yu, Z.L. Atractylenolide II induces g1 cell-cycle arrest and apoptosis in b16 melanoma cells. J. Ethnopharmacol. 2011, 136, 279–282. [Google Scholar] [CrossRef]
- Tian, S.; Yu, H.D. Atractylenolide II inhibits proliferation, motility and induces apoptosis in human gastric carcinoma cell lines HGC-27 and AGS. Molecules 2017, 22, 1886. [Google Scholar] [CrossRef]
- Fu, J.; Ke, X.; Tan, S.; Liu, T.; Wang, S.; Ma, J.; Lu, H. The natural compound codonolactone attenuates TGF-β1-mediated epithelial-to-mesenchymal transition and motility of breast cancer cells. Oncol. Rep. 2016, 35, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Bang, J.Y.; Kim, M.H.; Kang, I.C.; Kim, H.M.; Jeong, H.J. Atractylenolide III, a sesquiterpenoid, induces apoptosis in human lung carcinoma A549 cells via mitochondria-mediated death pathway. Food Chem. Toxicol. 2011, 49, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Duan, H.J.; He, L.C. Inhibitory effect of atractylenolide I on angiogenesis in chronic inflammation in vivo and in vitro. Eur. J. Pharmacol. 2009, 612, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Kim, K.W.; Li, J.; Lee, D.Y.; Yoon, D.; Jeong, J.T.; Kim, G.S.; Oh, H.; An, R.B.; Kim, Y.C. Anti-inflammatory components isolated from Atractylodes macrocephala in LPS-induced RAW264.7 macrophages and BV2 microglial cells. Appl. Biol. Chem. 2022, 65, 11. [Google Scholar] [CrossRef]
- Li, W.; Zhi, W.; Liu, F.; He, Z.; Wang, X.; Niu, X. Atractylenolide I restores HO-1 expression and inhibits Ox-LDL-induced VSMCs proliferation, migration and inflammatory responses in vitro. Exp. Cell Res. 2017, 353, 26–34. [Google Scholar] [CrossRef]
- Li, C.Q.; He, L.C.; Dong, H.Y.; Ju, Q.J. Screening for the anti-inflammatory activity of fractions and compounds from Atractylodes macrocephala koidz. J. Ethnopharmacol. 2007, 114, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; He, L.C. Establishment of the model of white blood cell membrane chromatography and screening of antagonizing TLR4 receptor component from Atractylodes macrocephala Koidz. Sci. China C. Life. Sci. 2006, 49, 182–189. [Google Scholar] [CrossRef]
- Ji, G.Q.; Chen, R.Q.; Zheng, J.X. Atractylenolide I inhibits lipopolysaccharide-induced inflammatory responses via mitogenactivated protein kinase pathways in RAW264.7 cells. Immunopharm. Immunot. 2014, 36, 420–425. [Google Scholar] [CrossRef]
- Li, C.Q.; He, L.C.; Jin, J.Q. Atractylenolide I and atractylenolide III inhibit lipopolysaccharide-induced TNF-alpha and NO production in macrophages. Phytother. Res. 2007, 21, 347–353. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Hu, X.F.; Cai, J.L.; Li, Y.L.; Zou, Y.; Wang, Y.H.; Xie, C.N.; Xu, S.Y.; Wang, Y.Q.; Zheng, Y.L.; et al. Atractylenolide-III alleviates osteoarthritis and chondrocyte senescence by targeting NF-κB signaling. Phytother. Res. 2023, 37, 4607–4620. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kim, Y.C.; Jin, H.G. Effects and mechanism of atractylone from rhizomes of Atractylodes macrocephala Koidzumi in LPS-induced neuro-inflammation in BV2 microglial cells. Nat. Prod. Res. Dev. 2020, 32, 826–830. [Google Scholar]
- Seo, M.-J.; Kim, S.-J.; Kang, T.-H.; Rim, H.-K.; Jeong, H.-J.; Um, J.-Y.; Hong, S.-H.; Kim, H.-M. The regulatory mechanism of β-eudesmol is through the suppression of caspase-1 activation in mast cell-mediated inflammatory response. Immunopharm. Immunot. 2011, 33, 178–185. [Google Scholar] [CrossRef]
- Han, J.H.; Zhu, X.Y.; Gao, Z.H.; Xiao, Y.; Zhang, J.X.; Wang, P.; Fang, J.B.; Li, Y.Q.; Zhu, Y.L.; Li, Y.; et al. Antiviral effects of atractyloside A on the influenza B virus (Victoria strain) infection. Front. Microbiol. 2023, 13, 1067725. [Google Scholar] [CrossRef]
- Shi, S.J.; Qin, Z.; Kong, S.Z.; Lai, X.P.; Su, Z.R.; Zeng, H.F. Effective component selection of atraotydin extract against influenza virus. Lishizhen Med. Materia. Med. Res. 2012, 23, 565–566. [Google Scholar]
- Cheng, Y.; Mai, J.Y.; Hou, T.L.; Ping, J.; Chen, J.J. Antiviral activities of atractylon from atractylodis rhizoma. Mol. Med. Rep. 2016, 14, 3704–3710. [Google Scholar] [CrossRef]
- Feng, H.; Wang, W.; Wu, M.C.; Fang, Y.P.; Wang, S.Z.; Yang, Y.; Ye, C.; Xiang, F. Antioxidant and antibacterial activities of essential oil from Atractylodes lancea rhizomes. Ind. Crop. Prod. 2020, 153, 112552. [Google Scholar]
- Peng, W.; Han, T.; Xin, W.-B.; Zhang, X.-G.; Zhang, Q.-Y.; Jia, M.; Qin, L.-P. Comparative research of chemical constituents and bioactivities between petroleum ether extracts of the aerial part and the rhizome of Atractylodes macrocephala. Med. Chem. Res. 2011, 20, 146–151. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, Y.J. Research on the antimicrobial activity of Atractylodes Lancea extract against clinical isolates of multidrug-resistant Escherichia coli. Guangming J. Chin. Med. 2023, 38, 1688–1691. [Google Scholar]
- Wan, Y.H.; Chen, Q.H.; Xu, W.; Chen, Y.J. Effect of Atractylodes essential oil on different Colletotrichum species causing tea brown blight disease. Sci. Hortic-Amsterdam. 2024, 324, 112611. [Google Scholar] [CrossRef]
- Dayrit, F.M.; Trono, C.M.; Morallo-Rejesus, B.; Maini, H. Anti-pest compounds from the volatile oil of Vitex negundo L. Philippine J. Sci. 1995, 124, 15–27. [Google Scholar]
- Guo, S.S.; Wang, Y.; Pang, X.; Geng, Z.F.; Cao, J.Q. Seven herbs against the stored product insect: Toxicity evidence and the active sesquiterpenes from Atractylodes lancea. Ecotox. Environ. Safe. 2019, 169, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Yun, Y.K.; Ahn, Y.J. Toxicity of atractylon and atractylenolide III identified in Atractylodes ovata rhizome to Dermatophagoides farinae and Dermatophagoides pteronyssinus. J. Agric. Food Chem. 2007, 55, 6027–6031. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhang, X.M.; Wang, Z.Q.; Ahn, Y.J.; Wang, M. Toxic effects of extract and active component from Atractylodes lancea on mosquito. J. Huazhong Agr. Univ. 2017, 36, 39–42. [Google Scholar]
- Ji, Z.H.; Liu, C.; Zhao, H.; Yu, X.Y. Neuroprotective effect of biatractylenolide against memory impairment in D-galactose-induced aging mice. J. Mol. Neurosci. 2015, 55, 678–683. [Google Scholar] [CrossRef]
- Zhu, L.; Ning, N.; Li, Y.; Zhang, Q.F.; Xie, Y.C.; Irshad, M.; Feng, X.; Tao, X.J. Biatractylolide modulates PI3K-Akt-GSK3β-Dependent pathways to protect against glutamate-induced cell damage in PC12 and SH-SY5Y Cells. Evid. Ba. Compl. Alt. Med. 2017, 2017, 1291458. [Google Scholar] [CrossRef]
- Gong, W.X.; Zhou, Y.Z.; Qin, X.M.; Du, G.H. Involvement of mitochondrial apoptotic pathway and MAPKs/NF-κB inflammatory pathway in the neuroprotective effect of atractylenolide III in corticosterone-induced PC12 cells. Chin. J. Nat. Med. 2019, 17, 264–274. [Google Scholar]
- Liu, C.; Zhao, H.; Ji, Z.H.; Xin, Y.Y. Neuroprotection of Atractylenolide III from Atractylodis macrocephalae against glutamate-induced neuronal apoptosis via inhibiting caspase signaling pathway. Neurochem. Res. 2014, 39, 1753–1758. [Google Scholar] [CrossRef]
- Gao, H.; Zhu, X.; Xi, Y.; Li, Q.; Shen, Z.; Yang, Y. Anti-depressant-like effect of atractylenolide I in a mouse model of depression induced by chronic unpredictable mild stress. Exp. Ther. Med. 2018, 15, 1574–1579. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, S.H.; Wu, F.L.; Zheng, Q.Y.; Zhang, F.S.; Luo, Y.X.; Jian, X.H. Atractylenolide III reduces depressive- and anxiogenic-like behaviors in rat depression models. Neurosci. Lett. 2021, 759, 136050. [Google Scholar] [CrossRef]
- Yu, X.W.; Qi, Y.W.; Li, Z. Atractylenolide II improves neuronal cell injury induced by oxidative stress through phosphatidylinositol-3 kinase/protein kinase B. Chin. J. Clin. Pharmacol. 2023, 39, 37–41. [Google Scholar]
- Tan, S.Y.; Yang, S.; Kang, H.M.; Zhou, K.; Wang, H.Q.; Zhang, Y.J.; Chen, S. Atractylenolide III ameliorated autophagy dysfunction via epidermal growth factor receptor-mammalian target of rapamycin signals and alleviated silicosis fibrosis in mice. Lab. Invest. 2023, 103, 100024. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, X.X.; Tan, D.; Jiang, Z.Y. Atractylon treatment prevents sleep-disordered breathing-induced cognitive dysfunction by suppression of chronic intermittent hypoxia-induced M1 microglial activation. Biosci. Rep. 2020, 40, BSR20192800. [Google Scholar] [CrossRef] [PubMed]
- Kiso, Y.; Tohkin, M.; Hikino, H. Mechanism of antihepatotoxic activity of atractylon, I: Effect on free radical generation and lipid peroxidation. Planta Med. 1985, 2, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.M.; Tseng, T.H.; Hsieh, Y.S.; Chou, F.P.; Wang, C.J.; Chu, C.Y. Inhibitory effect of atractylon on tert-butyl hydroperoxide induced DNA damage and hepatic toxicity in rat hepatocytes. Arch. Toxicol. 1996, 70, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Xu, C.; He, N.; Liu, Y.; Wang, Y.; Zhang, M.; Ji, K.; Du, L.; Wang, J.; Wang, Q.; et al. Atractylenolide II prevents radiation damage via MAPKp38/Nrf2 signaling pathway. Biochem. Pharmacol. 2020, 177, 114007. [Google Scholar] [CrossRef] [PubMed]
- Song, H.P.; Hou, X.Q.; Li, R.Y.; Yu, R.; Li, X.; Zhou, S.N.; Huang, H.Y.; Cai, X.; Zhou, C. Atractylenolide I stimulates intestinal epithelial repair through polyamine-mediated Ca2+ signaling pathway. Phytomedicine 2017, 28, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Chen, L.G.; Wu, C.H.; Chang, C.C.; Wang, C.C. Gastroprotective activity of atractylenolide III from Atractylodes ovata on ethanol-induced gastric ulcer in vitro and in vivo. J. Pharm. Pharmacol. 2010, 62, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Nogami, M.; Moriura, T.; Kubo, M.; Tani, T. Studies on the Origin, Processing and Quality of Crude Drugs. II.: Pharmacological Evaluation of the Chinese Crude Drug “Zhu” in Experimental Stomach Ulcer.(2).: Inhibitory Effect of Extract of Atractylodes lancea on Gastric Secretion. Chem. Pharm. Bull. 1986, 34, 3854–3860. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Xue, B.Y.; Liang, A.H.; Wang, L.; Fu, M.H.; Ye, Z.G. Effects of β-eudesmol, an active constituent from rhizoma Atractylodis on small intestine movement in rats. Chin. Pharm. J. 2002, 37, 266–268. [Google Scholar]
- Kimura, Y.; Sumiyoshi, M. Effects of an Atractylodes lancea rhizome extract and a volatile component β-eudesmol on gastrointestinal motility in mice. J. Ethnopharmacol. 2012, 141, 530–536. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhao, G.; Liu, P.L.; You, W.L. Effect of Atractylenolide I on intestinal flora in rats with slow transit constipation. J. Qingdao Univ. (Med. Sci.) 2023, 59, 216–220. [Google Scholar]
- Tu, J.; Xie, Y.; Xu, K.; Qu, L.; Lin, X.; Ke, C.; Yang, D.; Cao, G.; Zhou, Z.; Liu, Y. Treatment of spleen-deficiency syndrome with Atractyloside A from bran-processed Atractylodes lancea by protection of the intestinal mucosal barrier. Front. Pharmacol. 2020, 11, 583160. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, T.Z.; Cai, Q.; Jiang, N.; Ma, M.Y.; Min, D.Y.; Yuan, Y. Comparison of the anti-ulcer activity between the crude and bran-processed Atractylodes lancea in the rat model of gastric ulcer induced by acetic acid. J. Ethnopharmacol. 2014, 160, 211–218. [Google Scholar] [CrossRef]
- Zhang, H.; Han, T.; Sun, L.N.; Huang, B.K.; Chen, Y.F.; Zheng, H.C.; Qin, L.P. Regulative effects of essential oil from Atractylodes lancea on delayed gastric emptying in stress-induced rats. Phytomedicine 2008, 15, 602–611. [Google Scholar] [CrossRef]
- Nakai, Y.; Kido, T.; Hashimoto, K.; Kase, Y.; Sakakibara, I.; Higuchi, M.; Sasaki, H. Effect of the rhizomes of Atractylodes lancea and its constituents on the delay of gastric emptying. J. Ethnopharmacol. 2003, 84, 51–55. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Niu, P.; Su, Z.Y.; Ma, C.Y.; Wang, X.; Zhao, D.X.; Zhang, S. Role of atractylenolide I in cerebral ischemia reperfusion injury. Rev. Bras. Farmacogn. 2023, 33, 573–582. [Google Scholar] [CrossRef]
- Du, Z.Y.; Ma, Z.M.; Lai, S.L.; Ding, Q.C.; Hu, Z.Y.; Yang, W.W.; Qian, Q.Y.; Zhu, L.W.; Dou, X.B.; Li, S.T. Atractylenolide I ameliorates acetaminophen-induced acute liver injury via the TLR4/MAPKs/NF-κB signaling pathways. Front. Pharmacol. 2022, 13, 797499. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, K.; Tu, J.Y.; Ke, C.; Chen, N.P.; Wang, B.; Liu, Y.J.; Zhou, Z.S. Atractylenolide III ameliorates bile duct ligation-induced liver fibrosis by inhibiting the PI3K/AKT pathway and regulating glutamine metabolism. Molecules 2023, 28, 5504. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Guo, L.; Wu, X.; Zhang, T.; Liu, J.; Zhang, J. Atractylodes lactone compounds inhibit platelet activation. Platelets 2017, 28, 194–202. [Google Scholar] [CrossRef]
- Li, X.C.; Wei, G.; Wang, X.Z.; Liu, D.H.; Deng, R.D.; Li, H.; Zhou, J.H.; Li, Y.W.; Zeng, H.P.; Chen, D.F. Targeting of the Sonic Hedgehog pathway by atractylenolides promotes chondrogenic differentiation of mesenchymal stem cells. Biol. Pharm. Bull. 2012, 35, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Tshering, G.; Pimtong, W.; Plengsuriyakarn, T.; Na-Bangchang, K. Anti-angiogenic effects of beta-eudesmol and atractylodin in developing zebrafish embryos. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 243, 108980. [Google Scholar] [CrossRef]
- Tsuneki, H.; Ma, E.L.; Kobayashi, S.; Sekizaki, N.; Maekawa, K.; Sasaoka, T.; Wang, M.W.; Kimura, I. Antiangiogenic activity of β-eudesmol in vitro and in vivo. Eur. J. Pharmacol. 2005, 512, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Li, W.; Go, Y.; Oh, Y.C. Atractylodis rhizoma alba attenuates neuroinflammation in BV2 microglia upon LPS stimulation by inducing HO-1 activity and inhibiting NF-κB and MAPK. Int. J. Mol. Sci. 2019, 20, 4015. [Google Scholar] [CrossRef]
- Shanthini, N.; Pathangi, K.R. In vitro anti-inflammatory activity with ethanolic leaf extracts of Rivea hypocrateriformis LPS stimulated raw 264.7 macrophages. Curr. Bioact. Compd. 2023, 19, 51–59. [Google Scholar]
- Hwang, D.-R.; Wu, Y.-S.; Chang, C.-W.; Lien, T.-W.; Chen, W.-C.; Tan, U.-K.; Hsu, J.T.; Hsieh, H.-P. Synthesis and anti-viral activity of a series of sesquiterpene lactones and analogues in the subgenomic HCV replicon system. Bioorg. Med. Chem. 2006, 14, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Barreto, I.C.; de Almeida, A.S.; Sena Filho, J.G. Taxonomic insights and its type cyclization correlation of volatile sesquiterpenes in Vitex species and potential source insecticidal compounds: A review. Molecules 2021, 26, 6405. [Google Scholar] [CrossRef]
- Wu, Z.L.; Wang, Q.; Dong, H.Y.; Li, H.L.; Wang, S.M.; Zhang, W.D. Five rare dimeric sesquiterpenes exhibiting potential neuroprotection activity from Vladimiria souliei. Fitoterapia 2018, 128, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shah, I.; Ali, N.; Adhikari, A.; Tahir, M.N. Sesquiterpene lactone: A promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement. Altern. Med. 2017, 17, 27. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).