A Nano-Based Approach to Deliver Satureja thymbra Essential Oil to the Skin: Formulation and Characterization
Abstract
1. Introduction
2. Results
2.1. Quali-Quantitative Determination of Volatile Compounds in S. thymbra Essential Oil
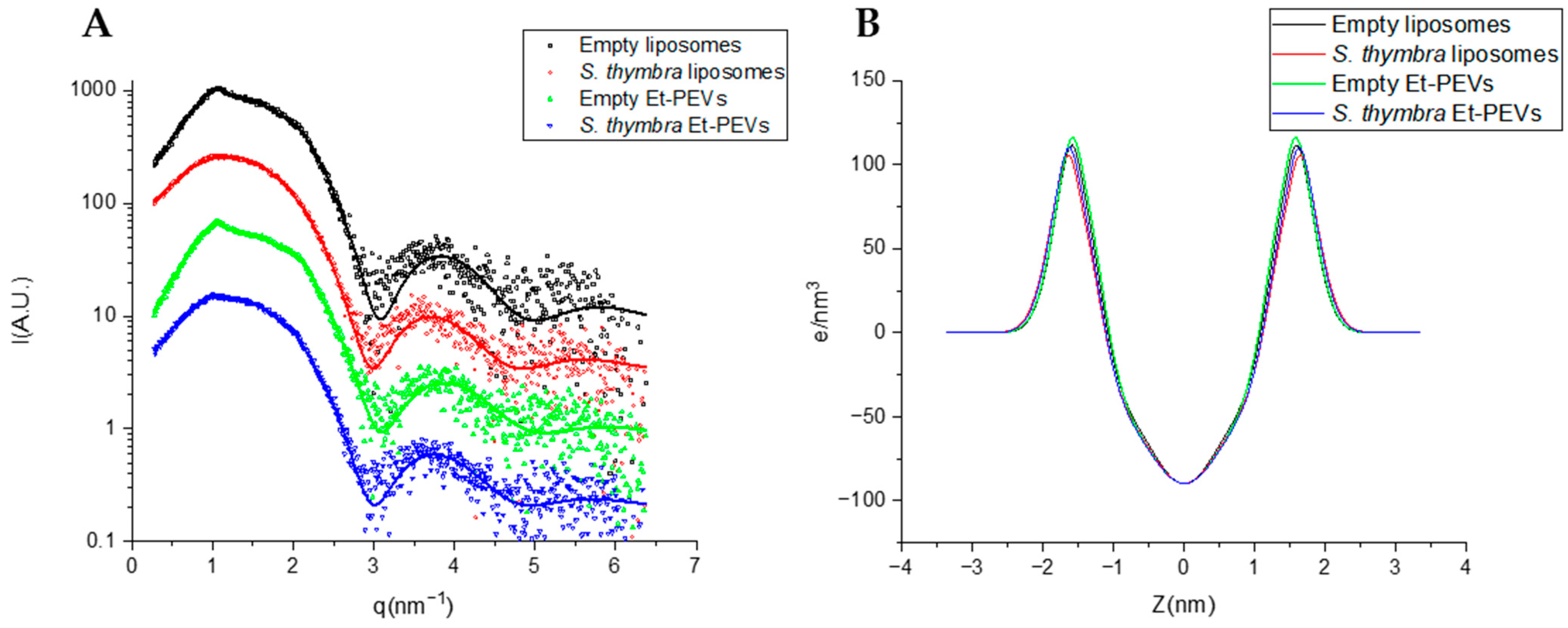
2.2. Characterization of Liposomes and Et-PEVs
2.3. Antioxidant Activity
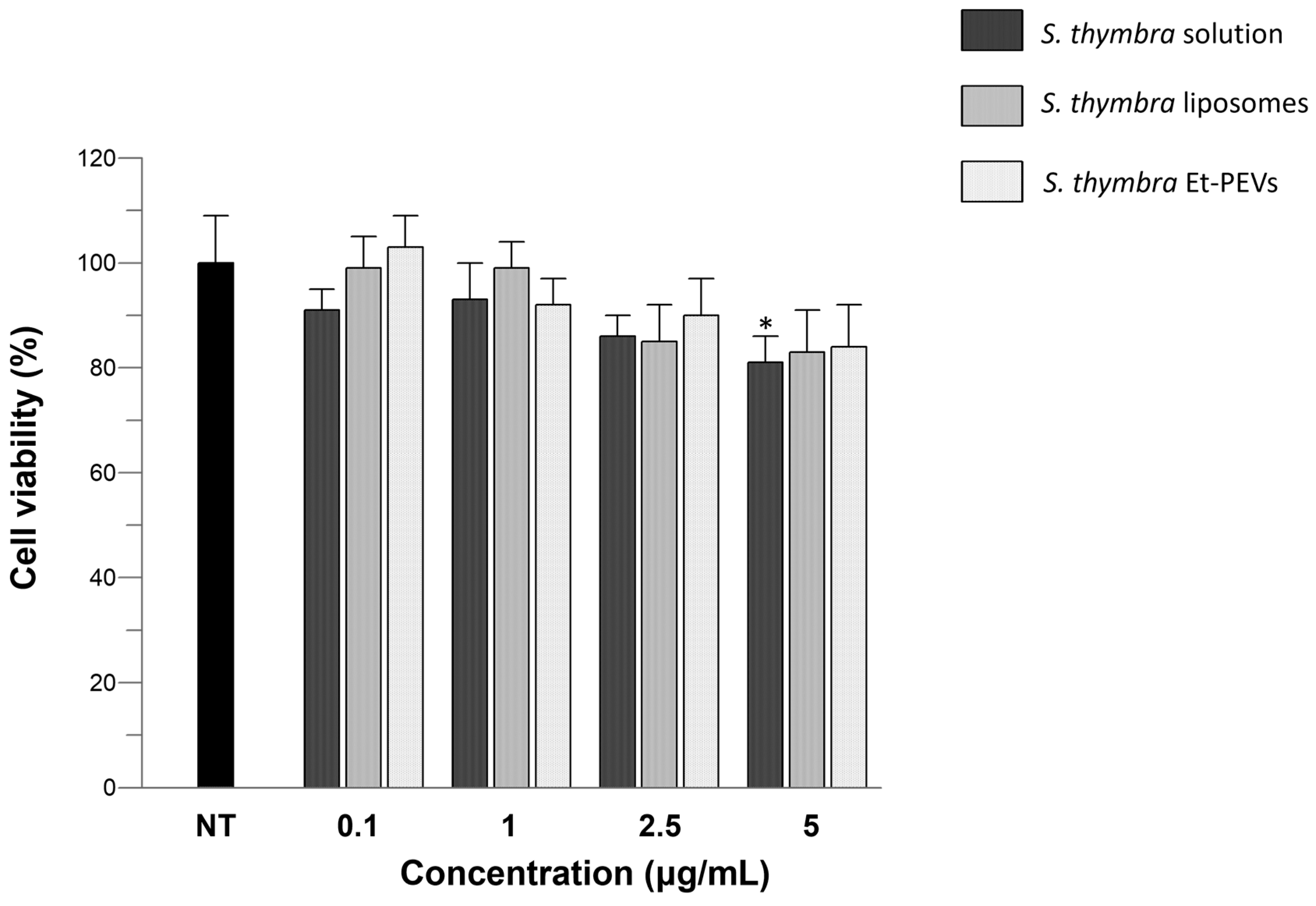
2.4. Viability of Skin Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Material and Preparation of the Essential Oil
4.3. GC–MS Analysis
4.4. HPLC–DAD Analysis
4.5. Preparation of the Phospholipid Vesicles
4.6. Characterization of the Phospholipid Vesicles
4.7. Small-Angle X-ray Scattering Analysis
4.8. Antioxidant Activity: DPPH• and FRAP Assays
4.9. Viability of Skin Cells
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azaz, A.D.; Kürkcüoglu, M.; Satil, F.; Baser, K.H.C.; Tümen, G. In vitro antimicrobial activity and chemical composition of some Satureja essential oils. Flavour Fragr. J. 2005, 20, 587–591. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M.; Sohraby, M. Summer Savory: From the Selection of Traditional Applications to the Novel Effect in Relief, Prevention, and Treatment of a Number of Serious Illnesses such as Diabetes, Cardiovascular Disease, Alzheimer’s Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 140–144. [Google Scholar] [CrossRef]
- Tepe, B.; Cilkiz, M. A pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2016, 54, 375–412. [Google Scholar] [CrossRef]
- Nikolić, M.; Jovanović, K.K.; Marković, T.; Marković, D.; Gligorijević, N.; Radulović, S.; Soković, M. Chemical composition, antimicrobial, and cytotoxic properties of five Lamiaceae essential oils. Ind. Crops Prod. 2014, 61, 225–232. [Google Scholar] [CrossRef]
- Capone, W.; Mascia, C.; Spanedda, L.; Chiappini, M. Chemical composition and antibacterial activity of the essential oil from Sardinian Satureja thymbra. Fitoterapia 1989, 60, 90–92. [Google Scholar]
- Atzei, A.D. Le Piante Nella Tradizione Popolare della Sardegna; Carlo Delfino Editore: Sassari, Italy, 2003. [Google Scholar]
- Dell’agli, M.; Sanna, C.; Rubiolo, P.; Basilico, N.; Colombo, E.; Scaltrito, M.M.; Ndiath, M.O.; Maccarone, L.; Taramelli, D.; Bicchi, C.; et al. Anti-plasmodial and insecticidal activities of the essential oils of aromatic plants growing in the Mediterranean area. Malar. J. 2012, 11, 219. [Google Scholar] [CrossRef]
- Ravid, U.; Putievsky, E. Composition of essential oils of Thymbra spicata and Satureja thymbra chemotypes. Planta Med. 1985, 51, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Capone, W.; Mascia, C.; Melis, M.; Spanedda, L. Determination of terpenic compounds in the essential oil from Satureja thymbra L. growing in Sardinia. J. Chromatogr. A 1988, 457, 427–430. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Evergetis, E.; Mallouchos, A.; Kalpoutzakis, E.; Nychas, G.-J.; Haroutounian, S.A. Characterization of the Essential Oil Volatiles of Satureja thymbra and Satureja parnassica: Influence of Harvesting Time and Antimicrobial Activity. J. Agric. Food Chem. 2006, 54, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Cocco, V.; Falconieri, D.; Porcedda, S.; Marongiu, B.; Maxia, A.; Frau, M.A.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Isolation of the Volatile Oil from Satureja thymbra by Supercritical Carbon Dioxide Extraction: Chemical Composition and Biological Activity. Nat. Prod. Commun. 2011, 6, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential oils encapsulated in liposomes: A review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef]
- Glamoclija, J.; Soković, M.; Vukojevic, J.; Milenković, I.; Van Griensven, L. Chemical composition and antifungal activities of essential oils of Satureja thymbra L. and Salvia pomifera ssp. Calycina (sm.) hayek. J. Essent. Oil Res. 2006, 18, 115–117. [Google Scholar] [CrossRef]
- Fleisher, Z.; Fleisher, A. Extract Analyses of Satureja thymbra L. And Thymbra spicata L. Aromatic Plants of the Holy Land and the Sinai. Part XVII. J. Essent. Oil Res. 2005, 17, 32–35. [Google Scholar] [CrossRef]
- Economou, G.; Panagopoulos, G.; Tarantilis, P.; Kalivas, D.; Kotoulas, V.; Travlos, I.; Polysiou, M.; Karamanos, A. Variability in essential oil content and composition of Origanum hirtum L., Origanum onites L., Coridothymus capitatus (L.) and Satureja thymbra L. populations from the Greek island Ikaria. Ind. Crops Prod. 2011, 33, 236–241. [Google Scholar] [CrossRef]
- Katar, D.; Kaçar, O.; Kara, N.; Aytac, Z.; Göksu, E.; Kara, S.; Katar, N.; Erbaş, S.; Telci, I.; Elmastaş, M. Ecological variation of yield and aroma components of summer savory (Satureja hortensis L.). J. Appl. Res. Med. Aromat. Plants 2017, 7, 131–135. [Google Scholar] [CrossRef]
- Khalil, N.; El-Jalel, L.; Yousif, M.; Gonaid, M. Altitude impact on the chemical profile and biological activities of Satureja thymbra L. essential oil. BMC Complement. Med. Ther. 2020, 20, 186. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2015, 95, 471–479. [Google Scholar] [CrossRef]
- Fitsiou, E.; Anestopoulos, I.; Chlichlia, K.; Galanis, A.; Kourkoutas, I.; Panayiotidis, M.I.; Pappa, A. Antioxidant and Antiproliferative Properties of the Essential Oils of Satureja thymbra and Satureja parnassica and their Major Constituents. Anticancer. Res. 2016, 36, 5757–5764. [Google Scholar] [CrossRef]
- Giweli, A.; Džamić, A.M.; Soković, M.; Ristić, M.S.; Marin, P.D. Antimicrobial and Antioxidant Activities of Essential Oils of Satureja thymbra Growing Wild in Libya. Molecules 2012, 17, 4836–4850. [Google Scholar] [CrossRef]
- Öztürk, M. Anticholinesterase and antioxidant activities of Savoury (Satureja thymbra L.) with identified major terpenes of the essential oil. Food Chem. 2012, 134, 48–54. [Google Scholar] [CrossRef]
- Vanti, G.; Tomou, E.-M.; Stojković, D.; Ćirić, A.; Bilia, A.R.; Skaltsa, H. Nanovesicles Loaded with Origanum onites and Satureja thymbra Essential Oils and Their Activity against Food-Borne Pathogens and Spoilage Microorganisms. Molecules 2021, 26, 2124. [Google Scholar] [CrossRef] [PubMed]
- NIST National Institute of Standards and Technology. Available online: https://webbook.nist.gov/chemistry (accessed on 13 January 2023).
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 16, pp. 65–120. [Google Scholar]
- Gil, K.A.; Jerković, I.; Marijanović, Z.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.G. Evaluation of an innovative sheep cheese with antioxidant activity enriched with different thyme essential oil lecithin liposomes. LWT 2021, 154, 112808. [Google Scholar] [CrossRef]
- De Luca, M.; Tuberoso, C.I.G.; Pons, R.; García, M.T.; Morán, M.D.C.; Ferino, G.; Vassallo, A.; Martelli, G.; Caddeo, C. Phenolic fingerprint, bioactivity and nanoformulation of Prunus Spinosa L. fruit extract for skin delivery. Pharmaceutics 2023, 15, 1063. [Google Scholar] [CrossRef]
- Caddeo, C.; Tuberoso, C.I.G.; Floris, S.; Masala, V.; Sanna, C.; Pintus, F. A Nanotechnological Approach to Exploit and Enhance the Bioactivity of an Extract from Onopordum illyricum L. Leaves. Plants 2023, 12, 1453. [Google Scholar] [CrossRef]
- Caddeo, C.; Pons, R.; Carbone, C.; Fernàndez-Busquets, X.; Cardia, M.C.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Physico-chemical characterization of succinyl chitosan-stabilized liposomes for the oral co-delivery of quercetin and resveratrol. Carbohydr. Polym. 2017, 157, 1853–1861. [Google Scholar] [CrossRef]



| Peak No. | tr (min) | RI | Compound | % ± SD |
|---|---|---|---|---|
| 1 | 6.17 | 923 | α-Thujene | 3.41 ± 0.15 |
| 2 | 6.40 | 930 | α-Pinene | 4.38 ± 0.19 |
| 3 | 6.95 | 946 | Camphene | 1.30 ± 0.06 |
| 4 | 7.97 | 974 | Sabinene | 0.61 ± 0.02 |
| 5 | 8.06 | 976 | β-Pinene | 2.46 ± 0.09 |
| 6 | 8.24 | 980 | 1-Octen-3-ol | 0.58 ± 0.02 |
| 7 | 8.55 | 988 | 3-Octanone | 0.09 ± 0.00 |
| 8 | 8.74 | 992 | β-Myrcene | 2.84 ± 0.01 |
| 9 | 8.92 | 996 | 3-Octanol | 0.24 ± 0.01 |
| 10 | 9.23 | 1003 | α-Phellandrene | 0.59 ± 0.03 |
| 11 | 9.47 | 1010 | 3-Carene | 0.12 ± 0.01 |
| 12 | 9.76 | 1017 | α-Terpinene | 3.76 ± 0.16 |
| 13 | 10.12 | 1026 | p-Cymene | 7.27 ± 0.03 |
| 14 | 10.28 | 1030 | Limonene | 1.09 ± 0.04 |
| 15 | 10.76 | 1041 | (Z)-β-Ocimene | 1.54 ± 0.07 |
| 16 | 11.09 | 1049 | o-Cymene | 1.45 ± 0.06 |
| 17 | 11.21 | 1051 | (E)-β-Ocimene | 1.50 ± 0.07 |
| 18 | 11.68 | 1061 | γ-Terpinene | 20.38 ± 1.05 |
| 19 | 11.95 | 1067 | cis-Sabinene hydrato | 0.21 ± 0.01 |
| 20 | 12.92 | 1086 | Terpinolene | 0.12 ± 0.01 |
| 21 | 12.96 | 1087 | p-Cimenene | 0.04 ± 0.00 |
| 22 | 13.33 | 1094 | trans-Sabinene hydrate | 0.07 ± 0.00 |
| 23 | 13.49 | 1097 | Linalool | 0.13 ± 0.01 |
| 24 | 16.37 | 1164 | Borneol | 3.71 ± 0.17 |
| 25 | 16.93 | 1176 | Terpinen-4-ol | 0.40 ± 0.01 |
| 26 | 17.57 | 1190 | Terpineol | 0.09 ± 0.00 |
| 27 | 20.03 | 1246 | Thymol methyl ether | 6.07 ± 0.26 |
| 28 | 20.27 | 1251 | α-Thymoquinone | 0.20 ± 0.01 |
| 29 | 22.35 | 1296 | Thymol | 23.88 ± 1.22 |
| 30 | 22.64 | 1302 | Carvacrol | 7.08 ± 0.31 |
| 31 | 24.88 | 1361 | Thymol acetate | 0.45 ± 0.02 |
| 32 | 25.72 | 1381 | Copaene | 0.02 ± 0.00 |
| 33 | 26.08 | 1390 | β-Bourbonene | 0.02 ± 0.00 |
| 34 | 27.53 | 1424 | β-Caryophyllene | 2.59 ± 0.12 |
| 35 | 28.91 | 1456 | α-Humulene | 0.35 ± 0.01 |
| 36 | 29.90 | 1478 | γ-Muurolene | 0.04 ± 0.00 |
| 37 | 30.04 | 1481 | Germacrene D | 0.26 ± 0.01 |
| 38 | 31.78 | 1523 | δ-Cadinene | 0.05 ± 0.00 |
| 39 | 34.00 | 1580 | Caryophyllene oxide | 0.32 ± 0.01 |
| Total identified | 99.73 ± 4.63 | |||
| Not identified | 0.27 ± 0.01 |
| MD (nm ± SD) | PI ± SD | ZP (mV ± SD) | EE (% ± SD) | |
|---|---|---|---|---|
| Empty liposomes | 96 ± 3.8 | 0.33 ± 0.04 | −12 ± 1.9 | -- |
| S. thymbra liposomes | *** 86 ± 0.2 | *** 0.20 ± 0.04 | *** −18 ± 3.0 | 87 ± 9.2 carvacrol 90 ± 8.7 thymol |
| Empty Et-PEVs | *** 107± 6.9 | 0.37 ± 0.06 | −12 ± 1.8 | -- |
| S. thymbra Et-PEVs | °°°§§§ 79 ± 2.7 | °°°§§ 0.24 ± 0.03 | °°° −17 ± 2.7 | 95 ± 6.8 carvacrol 97 ± 5.0 thymol |
| SAXS Parameters | Empty Liposomes | S. thymbra Liposomes | Empty Et-PEVs | S. thymbra Et-PEVs |
|---|---|---|---|---|
| χ2 | 1.7 | 1.8 | 1.6 | 1.3 |
| d (Å) | 58.0 ± 0.4 | - | 58.3 ± 0.4 | 61.1 ± 0.9 |
| η1 | 0.18 ± 0.04 | - | 0.09 ± 0.03 | 0.13 ± 0.08 |
| Nc | 3.5 ± 0.4 | - | 3.7 ± 0.3 | 3.2 ± 0.8 |
| % correlated bilayers | 14 ± 3 | - | 12 ± 1 | 6 ± 1 |
| σH (Å) | 2.94 ± 0.14 | 3.27 ± 0.2 | 3.14 ± 0.16 | 3.10 ± 0.16 |
| ρH (e/nm3) | 123 ± 6 | 119 ± 6 | 130 ± 7 | 122 ± 6 |
| ZH (Å) | 15.1 ± 0.2 | 15.3 ± 0.2 | 14.8 ± 0.1 | 15.3 ± 0.2 |
| σC (Å) | 4.6 ± 1.0 | 6.0 ± 1.0 | 6.0 ± 0.9 | 5.5 ± 0.9 |
| DPPH• Assay | FRAP Assay | ||
|---|---|---|---|
| AA (%) | TE (µg Trolox Equivalents/mL) | FE (mg Fe2+ Equivalents/mL) | |
| S. thymbra solution | 65 ± 1.9 | 124 ± 6.1 | 12 ± 0.22 |
| S. thymbra liposomes | ** 84 ± 2.3 | ** 164 ± 4.2 | 12 ± 1.12 |
| S. thymbra Et-PEVs | * 85 ± 5.0 | * 171 ± 11.0 | 12 ± 1.46 |
| Empty liposomes | 60 ± 3.5 | 116 ± 2.0 | 1.2 ± 0.22 |
| Empty Et-PEVs | 69 ± 9.8 | 142 ± 9.5 | 1.2 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pani, S.; Caddeo, C.; Sanna, C.; Pintus, F.; Floris, S.; Pons, R.; Dupont, A.; Tuberoso, C.I.G. A Nano-Based Approach to Deliver Satureja thymbra Essential Oil to the Skin: Formulation and Characterization. Molecules 2024, 29, 1041. https://doi.org/10.3390/molecules29051041
Pani S, Caddeo C, Sanna C, Pintus F, Floris S, Pons R, Dupont A, Tuberoso CIG. A Nano-Based Approach to Deliver Satureja thymbra Essential Oil to the Skin: Formulation and Characterization. Molecules. 2024; 29(5):1041. https://doi.org/10.3390/molecules29051041
Chicago/Turabian StylePani, Simone, Carla Caddeo, Cinzia Sanna, Francesca Pintus, Sonia Floris, Ramon Pons, Aurélien Dupont, and Carlo Ignazio Giovanni Tuberoso. 2024. "A Nano-Based Approach to Deliver Satureja thymbra Essential Oil to the Skin: Formulation and Characterization" Molecules 29, no. 5: 1041. https://doi.org/10.3390/molecules29051041
APA StylePani, S., Caddeo, C., Sanna, C., Pintus, F., Floris, S., Pons, R., Dupont, A., & Tuberoso, C. I. G. (2024). A Nano-Based Approach to Deliver Satureja thymbra Essential Oil to the Skin: Formulation and Characterization. Molecules, 29(5), 1041. https://doi.org/10.3390/molecules29051041









