Antioxidant Properties, Bioactive Compounds Contents, and Chemical Characterization of Two Wild Edible Mushroom Species from Morocco: Paralepista flaccida (Sowerby) Vizzini and Lepista nuda (Bull.) Cooke
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield
2.2. Estimation of Bioactive Compounds
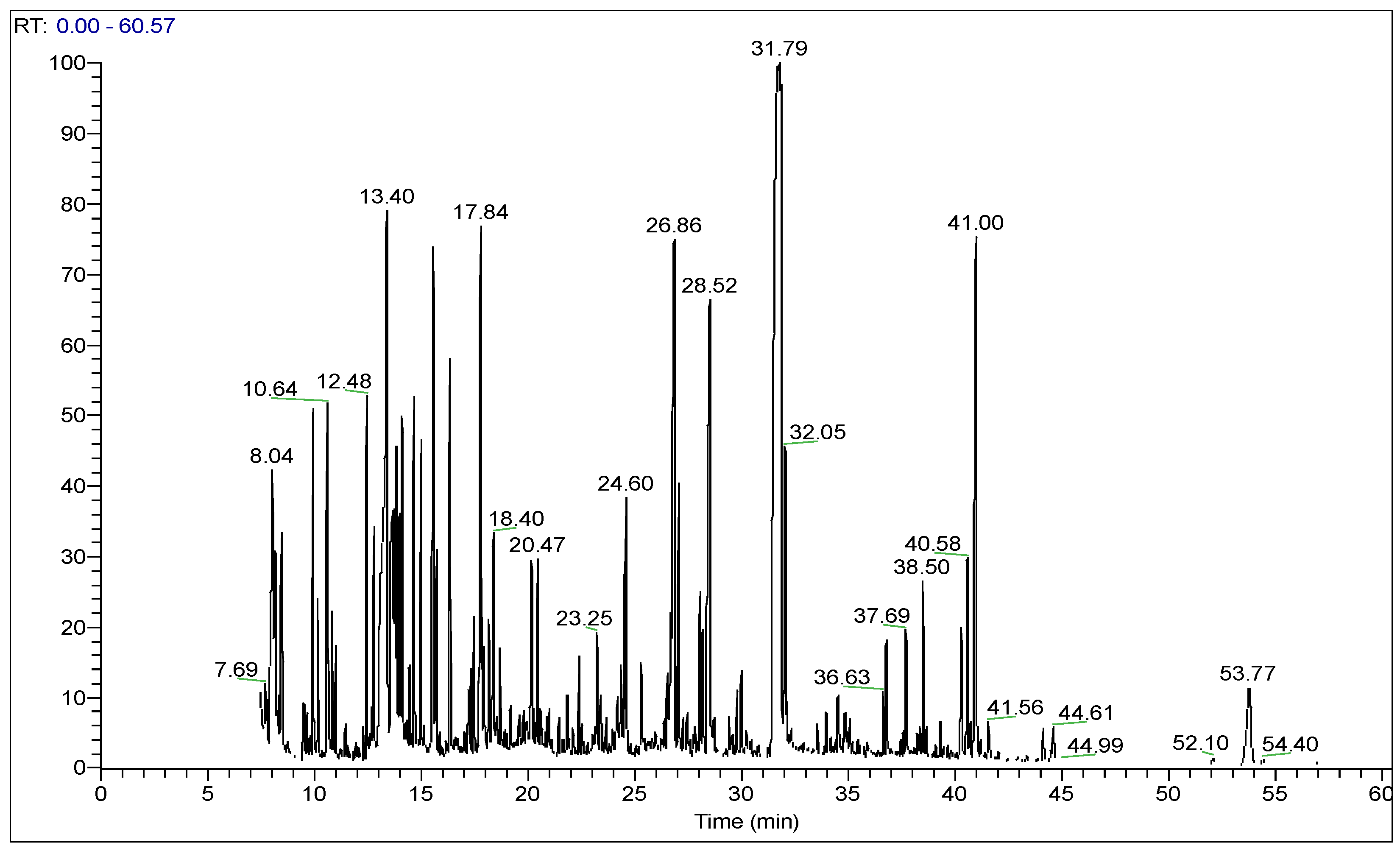
2.3. Phenolic Compounds by HPLC–MS Analysis
2.4. Biomolecules by GC–MS Analysis
2.5. Antioxidant Activity
3. Materials and Methods
3.1. Standards and Reagents
3.2. Mushroom Material
3.3. Preparation of Crude Methanolic Extracts
3.4. Estimation of Bioactive Compounds
3.5. Phenolic Compounds Analysis by HPLC–MS
3.6. Biomolecules Analysis by GC–MS
3.7. Evaluation of Antioxidant Activity
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Fernandes, Â.; Heleno, S.A. Chemical, Nutritional, and Bioactive Potential of Mushrooms. In Edible and Medicinal Mushrooms; Diego, C.Z., Pardo-Giménez, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 455–501. ISBN 978-1-119-14944-6. [Google Scholar]
- Benoutman, A.; Erbiai, E.H.; Edderdaki, F.Z.; Cherif, E.K.; Saidi, R.; Lamrani, Z.; Pintado, M.; Pinto, E.; Esteves da Silva, J.C.G.; Maouni, A. Phytochemical Composition, Antioxidant and Antifungal Activity of Thymus Capitatus, a Medicinal Plant Collected from Northern Morocco. Antibiotics 2022, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Pereira, E.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Biomolecule Profiles in Inedible Wild Mushrooms with Antioxidant Value. Molecules 2011, 16, 4328–4338. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Barros, L.; Abreu, R. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Bon, M.; Wilkinson, J.; Ovenden, D. The Mushrooms and Toadstools of Britain and North-Western Europe, 1st ed.; Hodder & Stoughton General Division: London, UK, 1987; ISBN 978-0-340-39953-8. [Google Scholar]
- Crevel, R. van Funga Nordica: Agaricoid, Boletoid and Cyphelloid Genera; CRC Press: Boca Raton, FL, USA, 1995; Volume 1, ISBN 978-90-5410-616-6. [Google Scholar]
- Vizzini, A.; Ercole, E. Paralepistopsis Gen. Nov. and Paralepista (Basidiomycota, Agaricales). Mycotaxon 2012, 120, 253–267. [Google Scholar] [CrossRef]
- Royal Botanic Gardens Kew Paralepista Flaccida—Species Fungorum. Available online: http://www.speciesfungorum.org/Names/NamesRecord.asp?RecordID=564347 (accessed on 21 October 2022).
- GBIF Secretariat Paralepista Flaccida (Sowerby) Vizzini. Available online: https://www.gbif.org/species/7978027 (accessed on 21 October 2022).
- Eyssartier, G.; Roux, P. Le guide des champignons. France et Europe 3e édition—Guillaume Eyssartier, Pierre Roux; Les Guides des fous de Nature; Belin: Paris, France, 2013; ISBN 978-2-7011-8289-6. [Google Scholar]
- Courtecuisse, R.; Duhem, B. Champignons de France et d’Europe - Régis Courtecuisse, Bernard Duhem; Guide Delachaux.; Delachaux et Niestlé: Paris, France, 2012; ISBN 978-2-603-02038-8. [Google Scholar]
- Bézivin, C.; Lohézic, F.; Sauleau, P.; Amoros, M.; Boustie, J. Cytotoxic Activity of Tricholomatales Determined with Murine and Human Cancer Cell Lines. Pharm. Biol. 2002, 40, 196–199. [Google Scholar] [CrossRef]
- Işıloğlu, M.; Yılmaz, F.; Merdivan, M. Concentrations of Trace Elements in Wild Edible Mushrooms. Food Chem. 2001, 73, 169–175. [Google Scholar] [CrossRef]
- El-Assfouri, A.; Ouazzani Touhami, A.; Zidane, L.; Fennane, M.; Douira, A. Inventaire Des Spécimens Fongiques de l’Herbier National de l’Institut Scientifique de Rabat. Bull. Inst. Sci. Rabat Maroc Sect. Sci. Vie 2003, 25, 1–23. [Google Scholar]
- Outcoumit, A.; Kholfy, S.E.; Touhami, A.O.; Douira, A. Bibliographic Inventory of Tangier Fungi: Catalogue of the Basidiomycetes Fungal Flora. IJPAES 2014, 4, 52. [Google Scholar]
- Kholfy, S.E.; El-Assfouri, A.; Ouazzani Touham, A.; Belahbib, N.; Douira, A. Bibliographic Catalog of Endemic or Rare Mushrooms of Morocco. Int. J. Plant Anim. Environ. Sci. 2014, 103–116. [Google Scholar]
- Haimed, M.; Nmichi, A.; Ouazzani Touhami, A.; Douira, A. Bibliographic Inventory of Moroccan Central Plateau Fungi. J. Anim. Plant Sci. 2013, 18, 2723–2749. [Google Scholar]
- Ouabbou, A.; El-Assfouri, A.; Ouazzani, A.; Benkirane, R.; Douira, A. Bibliographic Catalog of the Forest of Mamora (Morocco) Fungal Flora. J. Anim. Plant Sci. 2012, 15, 2200–2242. [Google Scholar]
- El kholfy, S.; Aït Aguil, F.; Ouazzani Touhami, A.; Benkirane, R.; Douira, A. Bibliographic Inventory of Moroccan Rif’s Fungi: Catalog of Rifain Fungal Flora. J. Anim. Plant Sci. 2011, 12, 1493–1526. [Google Scholar]
- Chen, M.-H.; Li, W.-S.; Lue, Y.-S.; Chu, C.-L.; Pan, I.-H.; Ko, C.-H.; Chen, D.-Y.; Lin, C.-H.; Lin, S.-H.; Chang, C.-P.; et al. Clitocybe Nuda Activates Dendritic Cells and Acts as a DNA Vaccine Adjuvant. Evid. Based Complement. Alternat. Med. 2013, 2013, e761454. [Google Scholar] [CrossRef]
- De, J.; Nandi, S.; Acharya, K. A Review on Blewit Mushrooms (Lepista Sp.) Transition from Farm to Pharm. J. Food Process. Preserv. 2022, 46, e17028. [Google Scholar] [CrossRef]
- Haimed, M.; Kholfy, S.E.; El-Assfouri, A.; Ouazzani-Touhami, A.; Benkirane, R.; Douira, A. Inventory of Basidiomycetes and Ascomycetes Harvested in the Moroccan Central Plateau. Int J Pure Appl Bio 2015, 3, 100–108. [Google Scholar]
- Alcántara, D.M.; Ferrezuelo, T.I.; Díaz, C.M.; Bouziane, H. Estudio de La Micobiota Del Norte de Marruecos II. Micobotánica-Jaén 2018, XIII, 1–44. [Google Scholar]
- Aliaño-González, M.J.; Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; López-Castillo, J.G.; Palma, M.; Barbero, G.F.; Carrera, C. Ultrasound-Assisted Extraction of Total Phenolic Compounds and Antioxidant Activity in Mushrooms. Agronomy 2022, 12, 1812. [Google Scholar] [CrossRef]
- Erbiai, E.H.; da Silva, L.P.; Saidi, R.; Lamrani, Z.; Esteves da Silva, J.C.G.; Maouni, A. Chemical Composition, Bioactive Compounds, and Antioxidant Activity of Two Wild Edible Mushrooms Armillaria Mellea and Macrolepiota Procera from Two Countries (Morocco and Portugal). Biomolecules 2021, 11, 575. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Bouchra, B.; da Silva, L.P.; Lamrani, Z.; Pinto, E.; da Silva, J.C.G.E.; Maouni, A. Chemical Composition and Antioxidant and Antimicrobial Activities of Lactarius Sanguifluus, a Wild Edible Mushroom from Northern Morocco. Euro-Mediterr. J. Environ. Integr. 2021, 6. [Google Scholar] [CrossRef]
- Marekov, I.; Momchilova, S.; Grung, B.; Nikolova-Damyanova, B. Fatty Acid Composition of Wild Mushroom Species of Order Agaricales—Examination by Gas Chromatography–Mass Spectrometry and Chemometrics. J. Chromatogr. B 2012, 910, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols Composition of Portuguese Wild Mushrooms with Antioxidant Capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef]
- Vaz, J.A.; Heleno, S.A.; Martins, A.; Almeida, G.M.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Wild Mushrooms Clitocybe Alexandri and Lepista Inversa: In Vitro Antioxidant Activity and Growth Inhibition of Human Tumour Cell Lines. Food Chem. Toxicol. 2010, 48, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Keleş, A.; Koca, I.; Gençcelep, H. Antioxidant Properties of Wild Edible Mushrooms. J. Food Process. Technol. 2011, 02. [Google Scholar] [CrossRef]
- Barros, L.; Venturini, B.A.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Chemical Composition and Biological Properties of Portuguese Wild Mushrooms: A Comprehensive Study. J. Agric. Food Chem. 2008, 56, 3856–3862. [Google Scholar] [CrossRef]
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of Antioxidant Activity and Antioxidant Compounds in Wild Edible Mushrooms. J. Food Compos. Anal. 2007, 20, 337–345. [Google Scholar] [CrossRef]
- Toledo, C.; Barroetaveña, C.; Fernandes, Â.; Barros, L.; Ferreira, I. Chemical and Antioxidant Properties of Wild Edible Mushrooms from Native Nothofagus Spp. Forest, Argentina. Molecules 2016, 21, 1201. [Google Scholar] [CrossRef]
- Sharma, S.K.; Gautam, N. Chemical, Bioactive, and Antioxidant Potential of Twenty Wild Culinary Mushroom Species. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Vaz, J.A.; Barros, L.; Martins, A.; Morais, J.S.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Phenolic Profile of Seventeen Portuguese Wild Mushrooms. LWT - Food Sci. Technol. 2011, 44, 343–346. [Google Scholar] [CrossRef]
- Pinto, S.; Barros, L.; Sousa, M.J.; Ferreira, I.C.F.R. Chemical Characterization and Antioxidant Properties of Lepista Nuda Fruiting Bodies and Mycelia Obtained by in Vitro Culture: Effects of Collection Habitat and Culture Media. Food Res. Int. 2013, 51, 496–502. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic Acids Determination by HPLC–DAD–ESI/MS in Sixteen Different Portuguese Wild Mushrooms Species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J. GC-MS Analysis of Biologically Active Compounds in Cosmopolitan Grasses. Acta Chromatogr. 2007, 279–282. [Google Scholar]
- André, P.; Villain, F. Free Radical Scavenging Properties of Mannitol and Its Role as a Constituent of Hyaluronic Acid Fillers: A Literature Review. Int. J. Cosmet. Sci. 2017, 39, 355–360. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Chan, P.T.; Kwan, K.Y.; Zhang, A. Reassessment of the Antioxidant Activity of Conjugated Linoleic Acids. J. Am. Oil Chem. Soc. 1997, 74, 749–753. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Study and Characterization of Selected Nutrients in Wild Mushrooms from Portugal by Gas Chromatography and High Performance Liquid Chromatography. Microchem. J. 2009, 93, 195–199. [Google Scholar] [CrossRef]
- Mizunoe, Y.; Kobayashi, M.; Sudo, Y.; Watanabe, S.; Yasukawa, H.; Natori, D.; Hoshino, A.; Negishi, A.; Okita, N.; Komatsu, M.; et al. Trehalose Protects against Oxidative Stress by Regulating the Keap1-Nrf2 and Autophagy Pathways. Redox Biol. 2018, 15, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Chuang, L.T.; Torun, H.; Colak, A.; Sesli˙, E.; Presley, J.; Smith, B.R.; Glew, R.H. Fatty Acid and Amino Acid Compositions of Selected Wild-Edible Mushrooms Consumed in Turkey. Int. J. Food Sci. Nutr. 2011, 62, 328–335. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized Analysis of Organic Acids in Edible Mushrooms from Portugal by Ultra Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a Novel Methodology for the Analysis of Ergosterol in Mushrooms. Food Anal. Methods 2014, 7, 217–223. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Fernandes, I.P.; Alves, M.J.; Barros, L.; González-Paramás, A.M.; Ferreira, I.C.F.R.; Barreiro, M.F. Phenolic Acids, Cinnamic Acid, and Ergosterol as Cosmeceutical Ingredients: Stabilization by Microencapsulation to Ensure Sustained Bioactivity. Microchem. J. 2019, 147, 469–477. [Google Scholar] [CrossRef]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The Importance of Antioxidants and Place in Today’s Scientific and Technological Studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-Radical Scavenging Capacity and Reducing Power of Wild Edible Mushrooms from Northeast Portugal: Individual Cap and Stipe Activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Malençon, G.; Bertault, R. Flore des champignons superieurs du Maroc: Tome I; Travaux de l’Institut Scientifique Chérifien et de la Faculté des Sciences de Rabat. Série Botanique et Biologie Végétale; Institut Scientifique Chérifien: Rabat, Morocco, 1970; Volume 1. [Google Scholar]
- Régis, C. LES CHAMPIGNONS DE FRANCE—Guide encyclopédique; Eclectis: Paris, France, 1994; Volume 1, ISBN 978-2-908975-19-2. [Google Scholar]
- Çayan, F.; Tel, G.; Duru, M.E.; Öztürk, M.; Türkoğlu, A.; Harmandar, M. Application of GC, GC-MSD, ICP-MS and Spectrophotometric Methods for the Determination of Chemical Composition and In Vitro Bioactivities of Chroogomphus Rutilus: The Edible Mushroom Species. Food Anal. Methods 2014, 7, 449–458. [Google Scholar] [CrossRef]
- Popova, M.; Silici, S.; Kaftanoglu, O.; Bankova, V. Antibacterial Activity of Turkish Propolis and Its Qualitative and Quantitative Chemical Composition. Phytomedicine 2005, 12, 221–228. [Google Scholar] [CrossRef] [PubMed]

| Bioactive Compounds | P. flaccida | L. nuda | One-Way ANOVA * |
|---|---|---|---|
| Extraction yield (%) | 30.32 ± 1.14 | 31.69 ± 2.04 | 0.4736 |
| Total phenolic (mg GAE/g dme) | 32.86 ± 0.52 a | 25.52 ± 0.56 b | <0.0001 |
| Total flavonoid (mg CE/g dme) | 10.34 ± 0.06 b | 19.02 ± 0.80 a | <0.0001 |
| Ascorbic acid (mg AAE/g dw) | 1.27 ± 0.06 | 1.31 ± 0.03 | 0.9048 |
| Tannin (mg CE/g dw) | 2.67 ± 0.04 | 2.26 ± 0.19 | >0.9999 |
| β-Carotene (µg/g dme) | 0.30 ± 0.02 | 0.64 ± 0.01 | 0.9982 |
| Lycopene (µg/g dme) | 0.23 ± 0.01 | 0.38 ± 0.01 | >0.9999 |
| N°. | Phenolic Compounds | P. flaccida (µg/g dw) | L. nuda (µg/g dw) | One-Way ANOVA * |
|---|---|---|---|---|
| 1 | Gallic acid | 132 ± 1.79 a | 131.7 ± 1.11 a | 0.9955 |
| 2 | Protocatechuic acid | 79.91 ± 2.02 b | 97.28 ± 1.10 a | <0.0001 |
| 3 | Chlorogenic acid | 136.3 ± 1.27 b | 327.6 ± 3.68 a | <0.0001 |
| 4 | Catechin | 102 ± 1.32 b | 400.2 ± 6.13 a | <0.0001 |
| 5 | p-Hydroxybenzoic acid | 138.5 ± 1.58 b | 587.9 ± 4.89 a | <0.0001 |
| 6 | Caffeic acid | 13.28 ± 0.60 b | 77.37 ± 0.66 a | <0.0001 |
| 7 | Vanillic acid | 26.59 ± 0.81 a | 23.53 ± 1.10 b | 0.0114 |
| 8 | Syringic acid | 11.25 ± 0.72 a | 8.57 ± 0.49 b | 0.001 |
| 9 | Rutin | nd | nd | - |
| 10 | Ellagic acid | 100.5 ± 3.62 b | 362.6 ± 2.80 a | <0.0001 |
| 11 | p-Coumaric acid | 35.9 ± 0.53 b | 124.2 ± 2.73 a | <0.0001 |
| 12 | Vanillin | nd | nd | - |
| 13 | Ferulic acid | 11.61 ± 0.32 b | 27.3 ± 0.53 a | <0.0001 |
| 14 | Rosmarinic acid | nd | nd | - |
| 15 | Salicylic acid | nd | nd | - |
| 16 | Methylparaben | 47.12 ± 1.04 b | 271.6 ± 3.21 a | <0.0001 |
| 17 | Quercetin | nd | nd | - |
| 18 | Cinnamic acid | 124.2 ± 0.44 b | 274.3 ± 1.00 a | <0.0001 |
| Compound Names | P. flaccida (%) | L. nuda (%) |
|---|---|---|
| Sugar compositions | 52.51 | 22.88 |
| Fatty acids | 11.71 | 29.72 |
| Amino acids | 16.03 | 18.29 |
| Organic acids | 10.53 | 11.11 |
| Other groups | 9.21 | 17.97 |
| Total | 99.99 | 99.97 |
| Assays | P. flaccida (mg/mL) | L. nuda (mg/mL) | Trolox (mg/mL) | One-Way ANOVA * |
|---|---|---|---|---|
| DPPH radical-scavenging activity | 1.18 ± 0.11 a | 0.98 ± 0.01 b | 0.020 ± 0.01 c | <0.0001 |
| β-carotene/linoleate assay | 0.22 ± 0.01 b | 0.39 ± 0.02 a | 0.006 ± 0.01 c | <0.0001 |
| Ferricyanide/Prussian blue assay | 0.63 ± 0.01 a | 0.48 ± 0.00 b | 0.080 ± 0.02 c | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erbiai, E.H.; Maouni, A.; Pinto da Silva, L.; Saidi, R.; Legssyer, M.; Lamrani, Z.; Esteves da Silva, J.C.G. Antioxidant Properties, Bioactive Compounds Contents, and Chemical Characterization of Two Wild Edible Mushroom Species from Morocco: Paralepista flaccida (Sowerby) Vizzini and Lepista nuda (Bull.) Cooke. Molecules 2023, 28, 1123. https://doi.org/10.3390/molecules28031123
Erbiai EH, Maouni A, Pinto da Silva L, Saidi R, Legssyer M, Lamrani Z, Esteves da Silva JCG. Antioxidant Properties, Bioactive Compounds Contents, and Chemical Characterization of Two Wild Edible Mushroom Species from Morocco: Paralepista flaccida (Sowerby) Vizzini and Lepista nuda (Bull.) Cooke. Molecules. 2023; 28(3):1123. https://doi.org/10.3390/molecules28031123
Chicago/Turabian StyleErbiai, El Hadi, Abdelfettah Maouni, Luís Pinto da Silva, Rabah Saidi, Mounir Legssyer, Zouhaire Lamrani, and Joaquim C. G. Esteves da Silva. 2023. "Antioxidant Properties, Bioactive Compounds Contents, and Chemical Characterization of Two Wild Edible Mushroom Species from Morocco: Paralepista flaccida (Sowerby) Vizzini and Lepista nuda (Bull.) Cooke" Molecules 28, no. 3: 1123. https://doi.org/10.3390/molecules28031123
APA StyleErbiai, E. H., Maouni, A., Pinto da Silva, L., Saidi, R., Legssyer, M., Lamrani, Z., & Esteves da Silva, J. C. G. (2023). Antioxidant Properties, Bioactive Compounds Contents, and Chemical Characterization of Two Wild Edible Mushroom Species from Morocco: Paralepista flaccida (Sowerby) Vizzini and Lepista nuda (Bull.) Cooke. Molecules, 28(3), 1123. https://doi.org/10.3390/molecules28031123











