Abstract
Significant enhancements of electrocatalytic activities for both half-reactions of water-electrolysis, i.e., oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), as well as pseudocapacitive charge-storage properties are demonstrated upon changing the structural order in a perovskite-type system. The structural change is prompted by the increase in the ionic radius of the A-site ion in A2Fe2O5. The structure of Sr2Fe2O5 consists of alternating layers of FeO6 octahedra and FeO4 tetrahedra, whereas Ba2Fe2O5 comprises seven different coordination geometries for Fe. We note that the catalytically active metal, i.e., iron, and the oxygen stoichiometry are the same for both materials. Nevertheless, the change in the structural order results in significantly greater electrocatalytic activity of Ba2Fe2O5, manifested in smaller overpotentials, smaller charge-transfer resistance, greater electrocatalytic current, and faster reaction kinetics. In addition, this material shows significantly enhanced pseudocapacitive properties, with greater specific capacitance and energy density compared to Sr2Fe2O5. These findings indicate the important role of structural order in directing the electrochemical properties.
1. Introduction
Efficient electrochemical energy conversion and storage devices require the development of functional materials with enhanced properties. Oxide materials have shown great promise for various applications, such as in water electrolyzers, solid oxide fuel cells (SOFCs), oxygen separation membranes, and oxygen sensors. Oxide systems derived from the perovskite structure are of particular interest, due to their interesting properties [1,2,3]. They exhibit a number of structures, which can be achieved by different methods, such as the partial removal of oxygen, which can be used to promote oxygen diffusivity and surface exchange kinetics [4,5].
Transition metal oxides adopting the perovskite structure have the general formula ABO3 (A = alkali, alkaline earth or lanthanide, B = usually transition metal). Oxygen-deficient perovskites (ODPs) can also be prepared, when some of the oxygen atoms are lost, giving the formula ABO3-δ, where δ is the number of oxygen vacancies per unit formula. Oxygen defects in such structures may be spread randomly or order systematically to yield several possible structures. ODPs having the oxygen deficiency of δ = 0.5, i.e., ABO2.5 or A2B2O5, often form the brownmillerite structure. Brownmillerites have been studied for a number of applications, such as oxygen carriers (Ca2Fe2O5) [6], photochemical oxygen production (Sr2Fe2O5) [7], oxygen ion and proton conduction (Ba2In2O5) [8], supercapacitors (Ca2FeCoO5) [9], and oxygen evolution (Ca2FeCoO5) [10].
Among these technologies, electrocatalytic water splitting is of great interest [11,12,13]. Water splitting has two half reactions, namely, hydrogen evolution reaction (HER) i.e., 2H2O + 2e− → H2 + 2OH−, and oxygen evolution reaction (OER) i.e., 4OH− → O2 + 2H2O + 4e−, in alkaline medium. However, both reactions have sluggish kinetics, leading to large overpotentials. The overpotentials can be significantly reduced by electrocatalysts. Traditional benchmark catalysts for HER and OER have been those based on precious metals. For example, Pt is a benchmark electrocatalyst for HER, which shows the best performance in both acidic and alkaline media [14]. Materials that have HER electrocatalytic activities comparable to Pt are scarce. Some of the traditional benchmark electrocatalysts for OER include RuO2 [15] and IrO2 [16]. While these catalysts show high activities for OER catalysis, the cost and scarcity of noble metals is a significant problem. Therefore, alternative materials have been explored to reduce the cost and make the water-splitting process more practical [17]. Perovskite oxide-based catalysts have emerged as promising alternatives, particularly for OER [18], Along with the use of earth abundant metals, it is highly desired to have stable catalysts that can operate under electrolysis conditions for extended periods of time. Perovskite oxides have shown high stability, especially in alkaline medium. These oxides can also have a variety of crystal structures. Many different metals from the periodic table can be used to modify the compositions and electrocatalytic properties to achieve efficient water splitting. Different approaches have been examined in an effort to enhance the electrocatalytic activity of perovskite oxides by metal substitutions in A or B-sites. For instance, we have previously explored the Ca substitution in the perovskite La1/3Sr2/3FeO3-δ to obtain a bilayer brownmillerite phase, La1/3Ca2/3FeO3-δ, with enhanced OER and HER activities [19]. Another example is the systematic substitution of Ca into the perovskite SrFe1/2Co1/2O3-δ, leading to the ordering of oxygen-vacancies in CaFe1/2Co1/2O3-δ, which has a brownmillerite structure and shows significantly lower OER and HER overpotentials [10]. Other examples, such as the changes in OER activity by varying the degree of Sr substitution in La1−xSrxNiO3, have also been investigated [20].
Another electrochemical property that is exhibited by oxide materials, particularly perovskite oxides, is pseudocapacitive charge storage. Pseudocapacitors behave somewhat similar to traditional capacitors but also include electron transfer reactions [21,22]. Their charge storage process involves both the formation of electric double-layer charge separation and reversible surface redox (faradic) reactions [22]. There are various types of pseudocapacitance, namely, redox pseudocapacitance, underpotential deposition, and intercalation pseudocapacitance [23]. In redox pseudocapacitance, both electrochemical adsorption of ions and faradaic charge-transfer take place on or near the surface of a material. Underpotential deposition is another mechanism, which occurs by the formation of a monolayer of metal ions above their redox potential on the surface of a different metal. Intercalation pseudocapacitance, which is the focus of this study, is another phenomenon that involves ion intercalation and a faradaic charge-transfer. This process has been observed in perovskite oxide materials. An example is the perovskite LaMnO3 [24], where a reversible intercalation of the oxide anion into the material takes place while Mn ions undergo a reversible redox reaction [24]. The mechanism involves several steps, initiated by the adsorption of hydroxide ion, which then loses a proton to leave behind an oxide ion that is intercalated into the material. As with many oxide properties, pseudocapacitance is also affected by structural changes. We have previously shown an example of the enhancement of pseudocapacitive properties by changing the structure from a disordered oxygen-deficient perovskite in SrCa2GaMn2O8 to an ordered brownmillerites system in Ca3GaMn2O8 [25]. Another example is the improvement of pseudocapacitive properties upon partial substitution of lanthanum by potassium in LaFeO3 to form La0.5K0.5FeO3 [26]. Similarly, the effects of Ca or Sr doping at the A-site of LaMnO3 on the pseudocapacitive properties have been investigated [27,28].
In the present work, we demonstrate considerable enhancements in electrochemical properties upon structural changes prompted by the change in the ionic radius of the A-site ion in A2Fe2O5 (B = Sr2+, Ba2+). Some properties of these materials, such as electrical conductivity and magnetism, have been studied before [29,30,31]. In this work, we investigate the electrochemical properties for water-electrolysis and pseudocapacitive energy storage. While both materials are synthesized under identical conditions and contain the same oxygen stoichiometry, the change in the structural order leads to a significant improvement of the electrochemical properties of Ba2Fe2O5 over Sr2Fe2O5. The former shows enhanced electrocatalytic activities for both half-reactions of water electrolysis, OER and HER, as well as significantly greater pseudocapacitive charge storage properties.
2. Results and Discussion
2.1. Crystal Structure
Crystal structures of both compounds were confirmed by Rietveld refinement analyses (Figure S1 and Tables S1 and S2) using powder X-ray diffraction data. The crystal structures of both materials were consistent with previous reports [30,31,32]. We note that both materials were synthesized under identical conditions in an argon atmosphere using the same iron precursor. Iodometric titrations were used to quantify the oxygen content, showing five oxygens per formula unit for both compounds. Therefore, the variation in their crystal structure is related to the change in the ionic radius of the A-site metal from 1.44 Å for Sr2+ to 1.61 Å for Ba2+ [33], leading to a significant change in the structural order. Sr2Fe2O5 has a brownmillerite structure [29,30], containing two distinct Fe positions, one with octahedral and another tetrahedral coordination environment. This leads to the formation of alternating layers of FeO6 octahedra and FeO4 tetrahedra in Sr2Fe2O5 (Figure 1a). The Sr2+ ions reside in spaces between these layers. On the other hand, the larger ionic radius of Ba2+ results in a significantly more complex structure for Ba2Fe2O5 [30,31]. This material contains seven different Fe positions, with several different coordination geometries, which may be described as tetrahedral, square pyramidal, and octahedral, although some polyhedra are significantly distorted, as shown in Figure 1b. The change in the structural arrangement between the two materials leads to major variations in electrochemical properties as described in the next sections.
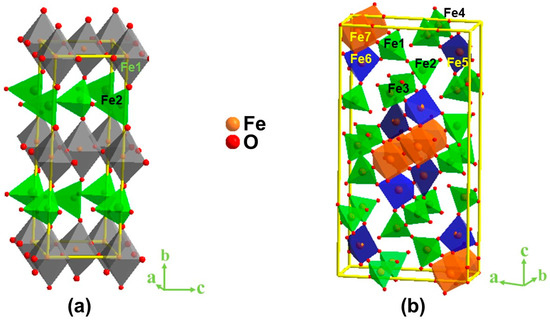
Figure 1.
Crystal structures of (a) Sr2Fe2O5 and (b) Ba2Fe2O5. The former has two crystallographically distinct Fe sites, while the latter has seven distinct Fe sites. The A-site atoms residing in spaces between the above polyhedra are omitted for clarity.
2.2. Electrocatalytic Properties for OER and HER
The electrocatalytic activities of both compounds toward both half-reactions of water-splitting, namely, oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) were studied in alkaline conditions.
Several parameters are often utilized to compare the activities of different electrocatalysts. One parameter is the onset potential, which refers to the start of the faradaic process, marked by a rise in the current density. Another parameter for gauging the catalytic performance is the overpotential beyond the ideal thermodynamic potential at a current density of 10 mA/cm2 (η10), which is associated with 10% solar-to-fuel conversion efficiency of a device in solar fuel synthesis [34]. Catalysts that enable the OER and HER at lower onset and overpotential are desired, as they lower the energy required for these processes to occur [35].
The electrocatalytic activities of the two materials for OER are represented by the polarization curves in Figure 2a. As observed in this plot, the two compounds show a similar onset potential at about 1.6 V. The overpotential for OER is calculated as η10 = ERHE − 1.23 V, where ERHE is the potential versus RHE at 10 mA/cm2, and 1.23 V is the thermodynamic potential for OER. The overpotential for Ba2Fe2O5 (0.50 V) is slightly lower than that of Sr2Fe2O5 (0.52 V). Importantly, the utilization of Ba2Fe2O5 as a catalyst leads to a current response, which is several-folds greater than that obtained using Sr2Fe2O5. This indicates the significantly higher electrocatalytic activity of Ba2Fe2O5 compared to Sr2Fe2O5. The OER activity of Ba2Fe2O5 is retained for many hours, as shown in the chronopotentiometry data in the inset of Figure 2a, indicating the stability of this catalyst. The kinetics of electrochemical reactions can be evaluated using the Tafel equation, η = a + b log j, where η is overpotential and j is current density. The linear fit of the plot of η versus log j, using the overpotential from the curved region of the polarization curve, will give the Tafel slope [25,36,37,38,39]. Faster reaction kinetics is associated with a smaller Tafel slope, indicating a more facile electron transfer. As shown in Figure 2b, the Tafel slope for Ba2Fe2O5 is smaller than that of Sr2Fe2O5, indicating a faster OER process, which is consistent with the higher electrocatalytic activity of Ba2Fe2O5.
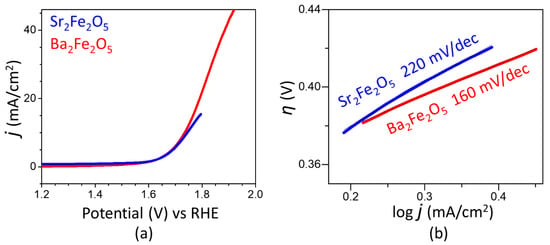
Figure 2.
(a) OER polarization curves in 1 M KOH. (b) Tafel plot showing Tafel slopes for both compounds.
The electrocatalytic activities of the two materials for HER are represented by the polarization curves in Figure 3a. As observed in this plot, the onset potential obtained using Ba2Fe2O5 (−0.35 V) is lower than that of Sr2Fe2O5 (−0.40 V). The overpotential for HER is calculated as η10 = ERHE − 0.0 V, where ERHE is the potential versus RHE at 10 mA/cm2, and 0.0 V is the thermodynamic potential for HER. The overpotential obtained for Ba2Fe2O5 (−0.47 V) is considerably lower than that of Sr2Fe2O5 (−0.56 V). We note that the overpotential for the precious metal benchmark catalyst Pt/C (20 wt. % Pt) has been reported to be close to −0.02 V vs. RHE [40]. However, the overpotential of Ba2Fe2O5 is lower than those of some other reported oxide catalysts, such as Sr3Mn2O6 (−0.59 V) [41] and SrLaCoO4-δ (−0.541 V) [36] and SrLaFeO4 (−0.691 V) [36]. The HER activity of Ba2Fe2O5 is retained for at least 15 h, as shown in the chronopotentiometry data in the inset of Figure 3a, indicating the stability of this catalyst. In addition, as shown in Figure 3b, Ba2Fe2O5 results in a smaller Tafel slope compared to Sr2Fe2O5, indicating the faster kinetics of HER process enabled by Ba2Fe2O5, consistent with its greater electrocatalytic activity. This is consistent with the electrochemical impedance spectroscopy data in the HER region (Figure 4a), which shows a smaller charge-transfer resistance for Ba2Fe2O5, indicating a more facile electron transfer compared to Sr2Fe2O5.
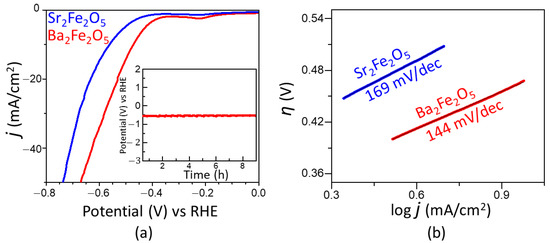
Figure 3.
(a) HER polarization curves in 1 M KOH. The inset shows chronopotentiometry data of Ba2Fe2O5 at a current density of −10 mA/cm2. (b) Tafel plot showing Tafel slopes for both compounds.
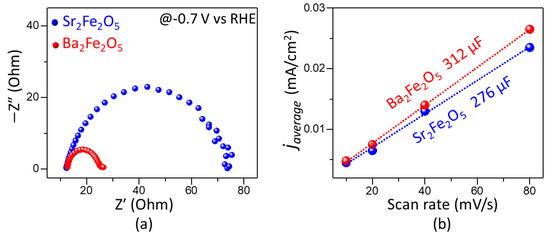
Figure 4.
(a) Nyquist plot for electrochemical impedance spectroscopy data obtained in the HER potential region of 0.7 V vs. RHE. (b) Plots of javerage vs. scan rate obtained from CVs in the non-faradaic region, giving the Cdl values as slope.
We have also evaluated the double-layer capacitance, Cdl, in the non-faradic region [42], where the current is generated mainly from electrical double layer charge and discharge, without contributions from electrode reactions [42,43]. The importance of Cdl is that it is directly related to the electrochemically active surface area [42,44,45,46]. The value of Cdl is determined from the equation Cdl = javerage/ν [47,48], where javerage is the average of the absolute values of the anodic and cathodic current-densities at the middle potential of the CVs in a non-faradic region (Figure S2). The slope of the plot of javerage vs. scan rate gives the Cdl. As shown in Figure 4b, Cdl was calculated from CVs at scan rates of 10, 20, 40, and 80 mV/s. Ba2Fe2O5 shows a greater Cdl value, which is consistent with its higher electrocatalytic activity compared to Sr2Fe2O5.
2.3. Pseudocapacitive Charge-Storage Properties
The important effect of structural changes on electrochemical properties is further demonstrated by investigation of the pseudocapacitive energy storage in the two materials. Pseudocapacitors store energy based on the faradaic processes that occur at or near the surface. Therefore, their properties lie in between those of traditional capacitors and batteries. Thus, in theory, they should be able to deliver both reasonable energy-density and power-density [49]. Pseudocapacitive properties in some oxides have been observed to occur by a reversible intercalation of the oxide anion [24,50,51]. As described by other researchers before [24,50], the process begins with the adsorption of the hydroxide ion on the electrode surface, followed by a loss of proton to another hydroxide ion to produce water and leave behind an oxide anion, which is intercalated into the electrode material [24,50].
The pseudocapacitive properties for the two materials were studied using the cyclic voltammetry data in a three-electrode cell configuration at scan rates of 5, 10, 25, 50, and 100 mV/s, as shown in Figure 5. The redox peaks are indicative of the faradaic reactions [24,52,53,54]. The redox peaks correspond to the Fe2+/3+ redox behavior for both compounds [52]. As observed in the CVs, as the scan rate increases, the oxidation peak shifts toward higher potentials and the reduction peak toward lower potentials. Such shifts occur due to the internal resistance of the electrode [55,56]. Moreover, the higher intensities of the redox peaks at higher scan rates are ascribed to the fast electronic and ionic transports [50,57]. Importantly, the redox peaks are not observed in a KNO3 solution (Figure 5), confirming that the faradaic processes arise from the oxide ion intercalation facilitated by OH- in an alkaline electrolyte [52].
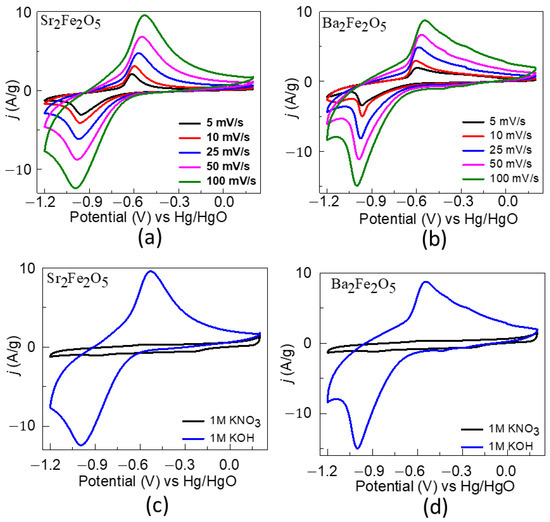
Figure 5.
(a,b) show CVs obtained using a three-electrode setup in 1 M KOH. (c,d) show comparisons of CVs obtained in 1 M KOH (blue) and 1 M KNO3 (black) electrolytes.
A symmetric two-electrode cell was constructed by loading the catalyst ink on two Ni foam electrodes with the area of 1 cm2. The galvanostatic charge–discharge (GCD) was then studied using this cell in the potential window of 0.0 to 1.3 V. The shape of the GCD cycle profiles at 0.5 A/g, shown in Figure 6a, is typical of pseudocapacitors [27,28]. The specific capacitance, Cs, of a two electrode cell is obtained using the equation [58,59]:
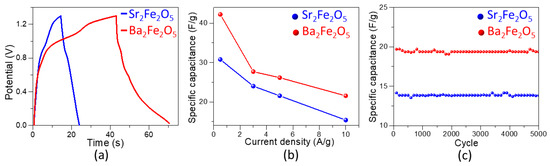
Figure 6.
(a) Galvanostatic charge–discharge (GCD) profiles at 0.5 A/g for symmetric cells of each material. (b) Secific capacitance from GCD of symmetric cells at current densities of 0.5, 3, 5, and 10 A/g. (c) Stability test over 5000 GCD cycles.
In this equation, I is the constant applied current, is the potential window, is the discharge time, and m is the total mass of the material loaded on both electrodes. The GCD experiments were done at various current densities, 0.5, 3, 5 and 10 A/g. The Cs values at each current density are shown in Figure 6b. As commonly observed in pseudocapacitors, the Cs decreases with the increase in current density [27,28]. At the current density of 0.5 A/g, the Cs values for Sr2Fe2O5 and Ba2Fe2O5 are ~31 F/g and ~42 F/g, respectively, indicating the significantly higher pseudocapacitive properties of the latter material. The specific capacitance from the symmetric cell of Ba2Fe2O5 is also superior to those of several previously reported oxide pseudocapacitors, such as La0.5Ca0.5MnO3 [27] and La0.85Sr0.15MnO3 [28], which show the respective specific capacitance values of ~6.5 F/g and less than ~8 F/g for symmetric cells at 0.5 A/g.
The energy density of the two-electrode cell is calculated by the following equation [60]:
In this equation, Cs is the specific capacitance from the two-electrode cell and V is the potential window in the GCD cycle. The constant 1/3.6 leads to the energy density in the unit of Wh/kg, considering that 1W = 1V 1A and 1F = 1. This energy density (E) value is further utilized to obtain the power density using the following equation [60]:
In this equation, is the discharge time in seconds and 3600 is a multiplier used to express the power density in W/kg. The two materials, Sr2Fe2O5 and Ba2Fe2O5, can deliver energy densities of ~7 Wh/kg and ~10 Wh/kg, respectively, at a power density of 1300 W/kg from a symmetric cell at a current density of 0.5 A/g. Therefore, the symmetric cell of Ba2Fe2O5 shows a significantly greater energy density, which outperforms some of the previously reported pseudocapacitors. An example is La0.85Sr0.15MnO3, where the energy density can be calculated as ~1.6 Wh/kg at 0.5 A/g, based on a specific capacitance of ~8 F/g from a symmetric cell with a reported potential window of 1.2 V [28]. Since the GCD discharge time at 0.5 A/g is not reported for the symmetric cell of La0.85Sr0.15MnO3, the power density cannot be estimated at 0.5 A/g. However, a higher energy density of 3.9 Wh/kg is reported for a low power density of 120 W/kg for the symmetric cell of La0.85Sr0.15MnO3 [28]. Similarly, the symmetric cell of Ba2Fe2O5 shows a better performance than that of La0.5Ca0.5MnO3 [27], where an energy density of 1.3 Wh/kg can be calculated based on the specific capacitance of ~6.5 F/g at 0.5 A/g for a symmetric cell with a reported potential window of 1.2 V [27]. For La0.5Ca0.5MnO3, a higher energy density of 7.6 Wh/kg is reported at a low power density of 160 W/kg [27].
Finally, stability studies for 5000 cycles for both materials were done using the two-electrode symmetric cell at a current density of 10 A/g. As shown in Figure 6c, both materials are stable and maintain a nearly constant specific capacitance even after 5000 cycles.
3. Experimental Methods
Both materials, Sr2Fe2O5 and Ba2Fe2O5, were synthesized by solid state synthesis method. The powders of the precursor compounds BaCO3, SrCO3, and Fe2O3 were ground and mixed thoroughly using agate mortar and pestle, pressed into pellets, and heated in argon at 1200 °C for 48 h (with an intermediate grinding and pelletizing). The phase purity and structures of the polycrystalline samples were confirmed by powder X-ray diffraction (XRD) at room temperature using Cu Kα1 radiation (λ = 1.54056 Å). The XRD data were analyzed by Rietveld refinement using the GSAS software [61] with EXPEGUI interface [62]. Iodometric titrations were performed by dissolving about 50 mg of the sample and excess KI (~2 g) in 100 mL of argon-purged 1 M HCl. Then, 5 mL of this solution was titrated against 0.025 M Na2S2O3, where 0.2 mL of a starch solution was added near the end point of the titration to act as the indicator. All iodometric titrations were performed under an argon atmosphere.
Electrocatalytic activities were measured using a three-electrode electrochemical workstation. A glassy carbon electrode coated with the catalyst ink, a commercial platinum electrode (for OER), or graphite rod (for HER) and Hg/HgO (in 1 M NaOH) were used as working, counter, and reference electrodes, respectively. The working electrode was prepared by drop-cast method for which the catalyst ink was prepared as described in our previous work [19,63], by mixing 35 mg of the catalytic material with 40 µL nafion, 7 mg carbon black, and 7 mL tetrahydrofuran (THF), followed by sonication for 15 min. The ink (40 μL) was loaded onto a glassy carbon electrode with a diameter of 5 mm and area of 0.196 cm2. Before starting each measurement, the 1 M KOH electrolyte (prepared in 18 MΩ nano pure water) was bubbled with argon gas for at least 30 min. Solution resistance values of ~10–22 Ω were recorded using electrochemical impedance spectroscopy in 0.1–100 kHz. All OER/HER potentials were iR-corrected. The potential can be converted to potential vs. the reversible hydrogen electrode (RHE) according to the Nernst equation [64], ERHE = EHg/HgO + 0.059 pH + E⁰Hg/HgO, where E⁰Hg/HgO = 0.098 V. In this work, the conversion to RHE potential was also verified by electrode calibration in the 1 M KOH. As shown in the Supporting Information, the Hg/HgO reference electrode was calibrated using Pt wires as the working and counter electrodes to run a cyclic voltammogram at a scan rate of 1 mV/s, and the average of the forward and return scans where the current crossed zero was taken as the thermodynamic potential [65]. This potential value (0.923 V) was nearly identical to that expected for 1 M KOH (pH = 14), i.e., 0.924 V. Therefore, this potential could be directly added to the experimental values to convert them into potentials vs. RHE [66]. Chronopotentiometry at 10 mA/cm2 for HER was performed by loading 40 µL of the ink on to glassy carbon electrode.
Pseudocapacitive properties were studied using both two- and three-electrode systems. For a two-electrode system, a symmetric cell was fabricated as described in the literature [67] to investigate the pseudocapacitive properties by galvanostatic charge–discharge (GCD) studies. The cell consisted of two Ni foams, separated by glass fiber filter paper, and sandwiched between two gold leads, connected to gold wires. In addition, 100 μL of the oxide ink was drop-casted on each electrode at 20 μL increments to obtain a total mass loading of ~1 mg/cm2. The electrodes were air-dried overnight. For three-electrode cells for pseudocapacitive experiments, the working electrode was prepared by drop-casting 10 µL of the oxide ink on a glass carbon electrode and overnight drying. CVs were run at scan rates of 5, 10, 25, 50, and 100 mV/s using a rotating disc electrode setup. All pseudocapacitive potentials are reported vs. Hg/HgO.
4. Conclusions
The change in the structural order has a profound impact on electrochemical properties. In the materials studied in this work, the active metal, i.e., Fe, and the oxygen stoichiometry are the same, but the A-site metal is varied. The A-site metal does not directly participate in the electrochemical processes. However, the variation in the ionic radius of the A-site metal leads to a structural change, which has a major impact on electrochemical properties, where Ba2Fe2O5 shows a significantly enhanced activity for both OER and HER, compared to Sr2Fe2O5. Structural changes also lead to the improvement of pseudocapacitive properties of Ba2Fe2O5, which shows a considerably higher specific capacitance and energy density compared to Sr2Fe2O5. This study highlights the importance of structural order in determining the functional properties of oxides that are derived from the perovskite structure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28165947/s1, Figure S1: Rietveld refinement profiles using powder X-ray diffraction data for (a) Sr2Fe2O5 and (b) Ba2Fe2O5. Black cross symbols, red solid curve, green vertical tick marks, and the pink curve correspond to the experimental data, calculated model, Bragg peak positions, and the difference plot, respectively; Figure S2: Cyclic voltammograms for both compounds in the non-faradic region to obtain double layer capacitance (Cdl) as shown in Figure 4b; Figure S3: Calibration of the Hg/HgO reference electrode in 1 M KOH, giving 0.923 V, which is nearly identical to that expected for 1 M KOH (pH = 14), i.e., 0.924 V. According to the Nernst equation, ERHE = EHg/HgO + 0.059 pH + E⁰Hg/HgO, where E⁰Hg/HgO = 0.098 V. Therefore, In 1 M KOH, ERHE = EHg/HgO + 0.924 V. See the Experimental section for more information on calibration; Table S1: The refined structural parameters of Sr2Fe2O5 using PXRD data. Space group: Ibm2, a = 5.6750(1) Å, b = 15.5870(2) Å, c = 5.53261(1), Rp = 0.024, wRp = 0.033, χ2 = 2.35%; Table S2: The refined structural parameters of Ba2Fe2O5 using PXRD data. Space group: P21/c, a = 6.9750(1) Å, b = 11.7342(2) Å, c = 23.4503(3) Å, β = 98.7555(7)° Rp = 0.02, wRp = 0.03, χ2 = 1.90%.
Author Contributions
Conceptualization, F.R.; methodology, S.B.K. and F.R.; validation, S.B.K.; formal analysis, S.B.K.; investigation, S.B.K.; resources, F.R.; writing—original draft preparation, S.B.K.; writing—review and editing, F.R.; visualization, S.B.K.; supervision, F.R.; project administration, F.R.; funding acquisition, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Science Foundation (NSF) under grant no. DMR-1943085.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available by corresponding author upon reasonable request.
Acknowledgments
The authors thank Narayan Acharya for help with chronopotentiometry measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adler, S.B. Factors Governing Oxygen Reduction in Solid Oxide Fuel Cell Cathodes. Chem. Rev. 2004, 104, 4791–4844. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.S.; Guo, R.; Roy, R. The perovskite structure—A review of its role in ceramic science and technology. Mater. Res. Innov. 2000, 4, 3–26. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Johnson, D.W.; Remeika, J.P.; Gallagher, P.K. Perovskite Oxides: Materials Science in Catalysis. Science 1977, 195, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Berenov, A.V.; Atkinson, A.; Kilner, J.A.; Bucher, E.; Sitte, W. Oxygen tracer diffusion and surface exchange kinetics in La0.6Sr0.4CoO3−δ. Solid State Ion. 2010, 181, 819–826. [Google Scholar] [CrossRef]
- Adler, S.B. Mechanism and kinetics of oxygen reduction on porous La1−xSrxCoO3−δ electrodes. Solid State Ion. 1998, 111, 125–134. [Google Scholar] [CrossRef]
- Ismail, M.; Liu, W.; Chan, M.S.C.; Dunstan, M.T.; Scott, S.A. Synthesis, Application, and Carbonation Behavior of Ca2Fe2O5 for Chemical Looping H2 Production. Energy Fuels 2016, 30, 6220–6232. [Google Scholar] [CrossRef]
- Saib, F.; Mekiri, M.; Bellal, B.; Chibane, M.; Trari, M. Photoelectrochemical properties of the brownmillerite Sr2Fe2O5: Application to electrochemical oxygen evolution. Russ. J. Phys. Chem. A 2017, 91, 1562–1570. [Google Scholar] [CrossRef]
- Fluri, A.; Gilardi, E.; Karlsson, M.; Roddatis, V.; Bettinelli, M.; Castelli, I.E.; Lippert, T.; Pergolesi, D. Anisotropic Proton and Oxygen Ion Conductivity in Epitaxial Ba2In2O5 Thin Films. J. Phys. Chem. C 2017, 121, 21797–21805. [Google Scholar] [CrossRef]
- Dhankhar, S.; Menon, S.S.; Gupta, B.; Baskar, K.; Singh, S. Electrochemical performance of brownmillerite calcium ferrite for application as supercapacitor. AIP Conf. Proc. 2017, 1832, 080050. [Google Scholar]
- Karki, S.B.; Hona, R.K.; Yu, M.; Ramezanipour, F. Enhancement of Electrocatalytic Activity as a Function of Structural Order in Perovskite Oxides. ACS Catal. 2022, 12, 10333–10337. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Xu, H.; Du, Y. Nanoboxes endow non-noble-metal-based electrocatalysts with high efficiency for overall water splitting. J. Mater. Chem. A 2021, 9, 857–874. [Google Scholar] [CrossRef]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Fang, S.; Zhu, X.; Liu, X.; Gu, J.; Liu, W.; Wang, D.; Zhang, W.; Lin, Y.; Lu, J.; Wei, S.; et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 2020, 11, 1029. [Google Scholar] [CrossRef]
- Das, D.; Das, A.; Reghunath, M.; Nanda, K.K. Phosphine-free avenue to Co2P nanoparticle encapsulated N,P co-doped CNTs: A novel non-enzymatic glucose sensor and an efficient electrocatalyst for oxygen evolution reaction. Green Chem. 2017, 19, 1327–1335. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Chen, Z.G.; Chen, Y.; Su, C.; Tadé, M.O.; Shao, Z. SrNb0.1Co0.7Fe0.2O3−δ perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution. Angew. Chem. Int. Ed. 2015, 54, 3897–3901. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Karki, S.B.; Andriotis, A.N.; Menon, M.; Ramezanipour, F. Bifunctional Water-Splitting Electrocatalysis Achieved by Defect-Order in LaA2Fe3O8 (A = Ca, Sr). ACS Appl. Energy Mater. 2021, 4, 12063–12066. [Google Scholar] [CrossRef]
- Sankannavar, R.; Sandeep, K.C.; Kamath, S.; Suresh, A.K.; Sarkar, A. Impact of Strontium-Substitution on Oxygen Evolution Reaction of Lanthanum Nickelates in Alkaline Solution. J. Electrochem. Soc. 2018, 165, J3236–J3245. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum: New York, NY, USA, 1999. [Google Scholar]
- Mefford, J.T.; Hardin, W.G.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 2014, 13, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.B.; Ramezanipour, F. Pseudocapacitive Energy Storage and Electrocatalytic Hydrogen-Evolution Activity of Defect-Ordered Perovskites SrxCa3–xGaMn2O8 (x = 0 and 1). ACS Appl. Energy Mater. 2020, 3, 10983–10992. [Google Scholar] [CrossRef]
- Mondal, R.; Mishra, N.K.; Maiyalagan, T.; Gupta, A.; Singh, P. La1–xKxFeO3−δ: An Anion Intercalative Pseudocapacitive Electrode for Supercapacitor Application. ACS Omega 2021, 6, 30488–30498. [Google Scholar] [CrossRef]
- Mo, H.; Nan, H.; Lang, X.; Liu, S.; Qiao, L.; Hu, X.; Tian, H. Influence of calcium doping on performance of LaMnO3 supercapacitors. Ceram. Int. 2018, 44, 9733–9741. [Google Scholar] [CrossRef]
- Lang, X.; Mo, H.; Hu, X.; Tian, H. Supercapacitor performance of perovskite La1−xSrxMnO3. Dalton Trans. 2017, 46, 13720–13730. [Google Scholar] [CrossRef]
- Greaves, C.; Jacobson, A.J.; Tofield, B.C.; Fender, B.E.F. A powder neutron diffraction investigation of the nuclear and magnetic structure of Sr2Fe2O5. Acta Crystallogr. Sect. B 1975, 31, 641–646. [Google Scholar] [CrossRef]
- Hona, R.K.; Ramezanipour, F. Variation in electrical conductivity of A2Fe2O5 (A = Sr, Ba): The role of structural order. Mater. Res. Express 2018, 5, 076307. [Google Scholar] [CrossRef]
- Zou, X.D.; Hovmoller, S.; Parras, M.; Gonzalez-Calbet, J.M.; Vallet-Regi, M.; Grenier, J.C. The complex perovskite-related superstructure Ba2Fe2O5 solved by HREM and CIP. Acta Crystallogr. Sect. A 1993, 49, 27–35. [Google Scholar] [CrossRef]
- Harder, M.; Müller-Buschbaum, H. Darstellung und Untersuchung von Sr2Fe2O5-Einkristallen Ein Beitrag zur Kristallchemie von M2Fe2O5-Verbindungen. Z. Anorg. Allg. Chem. 1980, 464, 169–175. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Zhong, X.; Zhang, J.; Luo, S.; Yi, W.; Zhang, L.; Hu, M.; Tang, J.; Zhou, X.; et al. Evaluation of A-Site Ba2+-Deficient Ba1-xCo0.4Fe0.4Zr0.1Y0.1O3-δ Oxides as Electrocatalysts for Efficient Hydrogen Evolution Reaction. Scanning 2018, 2018, 1341608. [Google Scholar] [CrossRef]
- Hona, R.K.; Karki, S.B.; Ramezanipour, F. Oxide Electrocatalysts Based on Earth-Abundant Metals for Both Hydrogen- and Oxygen-Evolution Reactions. ACS Sustain. Chem. Eng. 2020, 8, 11549–11557. [Google Scholar] [CrossRef]
- Alom, M.S.; Ramezanipour, F. Layered Oxides SrLaFe1-xCoxO4-δ (x=0–1) as Bifunctional Electrocatalysts for Water-Splitting. ChemCatChem 2021, 13, 3510–3516. [Google Scholar] [CrossRef]
- Allen, J.; Bard, L.R.F. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Hona, R.K.; Ramezanipour, F. Remarkable Oxygen-Evolution Activity of a Perovskite Oxide from the Ca2−xSrxFe2O6−δ Series. Angew. Chem. 2019, 58, 2060–2063. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zhou, W.; Zhu, Z.; Su, C.; Liu, M.; Shao, Z. A Perovskite Electrocatalyst for Efficient Hydrogen Evolution Reaction. Adv. Mater. 2016, 28, 6442–6448. [Google Scholar] [CrossRef]
- Karki, S.B.; Hona, R.K.; Ramezanipour, F. Sr3Mn2O6 and Sr3FeMnO6 for oxygen and hydrogen evolution electrocatalysis. J. Solid State Electrochem. 2022, 26, 1303–1311. [Google Scholar] [CrossRef]
- Jung, S.; McCrory, C.C.L.; Ferrer, I.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A 2016, 4, 3068–3076. [Google Scholar] [CrossRef]
- Lu, B.; Cao, D.; Wang, P.; Wang, G.; Gao, Y. Oxygen evolution reaction on Ni-substituted Co3O4 nanowire array electrodes. Int. J. Hydrogen Energy 2011, 36, 72–78. [Google Scholar] [CrossRef]
- Alom, M.S.; Ramezanipour, F. Vacancy effect on the electrocatalytic activity of LaMn1/2Co1/2O3−δ for hydrogen and oxygen evolution reactions. Chem. Commun. 2023, 59, 5870–5873. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, H.; Kwon, Y.; Kim, M.; Cho, E.; Kwon, H. Porous Co–P foam as an efficient bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. J. Mater.Chem. A 2016, 4, 18272–18277. [Google Scholar] [CrossRef]
- Hona, R.K.; Karki, S.B.; Cao, T.; Mishra, R.; Sterbinsky, G.E.; Ramezanipour, F. Sustainable Oxide Electrocatalyst for Hydrogen and Oxygen-Evolution Reactions. ACS Catal. 2021, 11, 14605–14614. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Sunarso, J.; Zhong, Y.; Shao, Z. Phosphorus-Doped Perovskite Oxide as Highly Efficient Water Oxidation Electrocatalyst in Alkaline Solution. Adv. Funct. Mater 2016, 26, 5862–5872. [Google Scholar] [CrossRef]
- Petrie, J.R.; Cooper, V.R.; Freeland, J.W.; Meyer, T.L.; Zhang, Z.; Lutterman, D.A.; Lee, H.N. Enhanced Bifunctional Oxygen Catalysis in Strained LaNiO3 Perovskites. J. Am. Chem. Soc. 2016, 138, 2488–2491. [Google Scholar] [CrossRef]
- Alom, M.S.; Ramezanipour, F. Pseudocapacitive charge storage in layered oxides SrLaFe1−xCoxO4−δ (x = 0–1). Mater. Lett. 2021, 295, 129859. [Google Scholar] [CrossRef]
- Che, W.; Wei, M.; Sang, Z.; Ou, Y.; Liu, Y.; Liu, J. Perovskite LaNiO3-δ oxide as an anion-intercalated pseudocapacitor electrode. J. Alloys Compd. 2018, 731, 381–388. [Google Scholar] [CrossRef]
- Kananke-Gamage, C.C.W.; Ramezanipour, F. Structure Effect on Pseudocapacitive Properties of A2LaMn2O7 (A = Ca, Sr). Energy Tech. 2023, 11, 2201249. [Google Scholar] [CrossRef]
- Alexander, C.T.; Mefford, J.T.; Saunders, J.; Forslund, R.P.; Johnston, K.P.; Stevenson, K.J. Anion-Based Pseudocapacitance of the Perovskite Library La1–xSrxBO3−δ (B = Fe, Mn, Co). ACS Appl. Mater. Interfaces 2019, 11, 5084–5094. [Google Scholar] [CrossRef]
- Alexander, C.T.; Forslund, R.P.; Johnston, K.P.; Stevenson, K.J. Tuning Redox Transitions via the Inductive Effect in LaNi1–xFexO3−δ Perovskites for High-Power Asymmetric and Symmetric Pseudocapacitors. ACS Appl. Energy Mater. 2019, 2, 6558–6568. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Su, C.; Zhou, W.; Liu, M.; Shao, Z. Perovskite SrCo0.9Nb0.1O3−δ as an Anion-Intercalated Electrode Material for Supercapacitors with Ultrahigh Volumetric Energy Density. Angew. Chem. Int. Ed. 2016, 55, 9576–9579. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Advanced Asymmetric Supercapacitors Based on Ni(OH)2/Graphene and Porous Graphene Electrodes with High Energy Density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Shi, P.; Li, Y.; Huang, L.; Mai, W.; Tan, S.; Cai, X. Growth of nickel (111) plane: The key role in nickel for further improving the electrochemical property of hexagonal nickel hydroxide-nickel & reduced graphene oxide composite. J. Power Sources 2014, 267, 356–365. [Google Scholar]
- Guo, D.; Zhang, H.; Yu, X.; Zhang, M.; Zhang, P.; Li, Q.; Wang, T. Facile synthesis and excellent electrochemical properties of CoMoO4 nanoplate arrays as supercapacitors. J. Mater. Chem. A 2013, 1, 7247–7254. [Google Scholar] [CrossRef]
- Vellacheri, R.; Al-Haddad, A.; Zhao, H.; Wang, W.; Wang, C.; Lei, Y. High performance supercapacitor for efficient energy storage under extreme environmental temperatures. Nano Energy 2014, 8, 231–237. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Nguyen, D.C.; Kim, N.H.; Lau, K.-t.; Lee, J.H. Ternary graphene-carbon nanofibers-carbon nanotubes structure for hybrid supercapacitor. Chem. Eng. J. 2020, 380, 122543. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994; pp. 86–748.
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Karki, S.B.; Andriotis, A.N.; Menon, M.; Ramezanipour, F. Enhancement of Electrocatalytic Activity for both Hydrogen and Oxygen Evolution Reactions of a Perovskite Oxide. J. Phys. Chem. C 2022, 126, 20011–20019. [Google Scholar] [CrossRef]
- Tsuji, E.; Motohashi, T.; Noda, H.; Kowalski, D.; Aoki, Y.; Tanida, H.; Niikura, J.; Koyama, Y.; Mori, M.; Arai, H.; et al. Brownmillerite-type Ca2FeCoO5 as a Practicable Oxygen Evolution Reaction Catalyst. ChemSusChem 2017, 10, 2864–2868. [Google Scholar] [CrossRef]
- Niu, S.; Li, S.; Du, Y.; Han, X.; Xu, P. How to Reliably Report the Overpotential of an Electrocatalyst. ACS Energy Lett. 2020, 5, 1083–1087. [Google Scholar] [CrossRef]
- Lee, J.G.; Hwang, J.; Hwang, H.J.; Jeon, O.S.; Jang, J.; Kwon, O.; Lee, Y.; Han, B.; Shul, Y.-G. A New Family of Perovskite Catalysts for Oxygen-Evolution Reaction in Alkaline Media: BaNiO3 and BaNi0.83O2.5. J. Am. Chem. Soc. 2016, 138, 3541–3547. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Chen, D.; Liu, J.; Zhang, Z.; Shao, Z.; Ciucci, F. Water Splitting with an Enhanced Bifunctional Double Perovskite. ACS Catal. 2018, 8, 364–371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).