Abstract
Murraya is a plant genus within the Rutaceae family comprising over 17 species, which are widely distributed in Asia, Australia, and the Pacific Islands. Furthermore, these species have been used in traditional medicine to treat fever, pain, and dysentery. Several reports have also extensively studied the leaves, seeds, stembark, and bark of Murraya from 1965 to 2023 to explore their natural product composition. Various phytochemical studies have revealed the isolation of 413 compounds recorded, comprising coumarins, terpenoids, flavonoids, and aromatics, as well as alkaloids, which constitute the largest proportion (46.9%). These isolated compounds have long been known to exhibit different bioactivities, such as cytotoxic and anti-inflammatory properties. Cytotoxic activity has been observed against HCT 116, HeLa, HepG2, and other cell lines. Previous studies have also reported the presence of antifungal, hepatoprotective, antihyperlipidemic, antidiarrheal, and antioxidant effects. Therefore, this review provides a comprehensive overview of Murraya species, highlighting their phytochemistry, biological activities, and potential as a source of active natural compounds.
1. Introduction
The Rutaceae family comprises over 150 genera that are distributed across the globe. Furthermore, one of these genera is Murraya, consisting of 17 species, which are spread across Asia, Australia, and the Pacific region. The ethnobotanical applications of the genus encompass a diverse range of uses, such as the landscaping of construction buildings, and some species can be grafted onto citrus rootstocks. Various plant parts of its members have also been used in traditional medicine to treat fever, pain, and dysentery [1].
Phytochemical studies on Marraya have been carried out since 1965, with a focus on identifying its potential biological activity. This genus has been investigated for various bioactivities, including cytotoxic [2], anti-inflammatory [3], antihyperlipidemic [4], antidiarrheal [5], and antioxidant [6] activities. In an initial study conducted by Chakraborty et al. [7] in 1965, a carbazole alkaloid-type compound named murrayanine (98) was isolated, showing significant anti-inflammatory potential [3]. Furthermore, other isolated alkaloid-type compounds included yuehchukene (186) and mahanine (26), which exhibited strong anti-implantation [8] and cytotoxic [2] activities, respectively.
The rapid growth of health problems has necessitated the need for urgent solutions, thereby making bioactive compounds from Murraya a starting point for drug development.
Over the course of 56 years, extensive studies have been conducted on the Marraya genus, leading to the identification of five classes of metabolites, with alkaloids being the main component. Based on the findings, there are no extensive reports on the phytochemistry and biological activities of the entire genus. Therefore, this review provides a comprehensive overview of Murraya species, highlighting their phytochemistry, biological activities, and potential as a source of active natural compounds. The results of this study are expected to serve as a foundation for future studies, which aim to identify chemical content from natural resources and discover new drugs.
2. Methodology
This study started with a literature search on Murraya species and all the synonyms were confirmed from the plant list (theplantlist.org, [accessed on 20 February 2023]), International Plant Names Index (ipni.org, [accessed on 20 February 2023]), Royal Botanical Gardens (kew.org), [accessed on 20 February 2023]), and tropicos (tropicos.org, [accessed on 20 February 2023]) databases. Furthermore, literature articles were collected from databases such as SciFinder, PubMed, Google Scholar, and Scopus. These articles were filtered based on their abstracts or keywords. A collection of relevant papers published between 1963 and 2023 was then obtained, focusing on the biological and phytochemical properties of Marraya. A systematic review was carried out using a flow diagram and meta-analysis studies were gathered from the database search. The identification of relevant papers was carried out with an approach involving title screening, gray literature exploration, review, excluding primary sources, and the removal of duplicate entries. The selected papers were then collected and subjected to further analysis, as shown in Figure 1 [9].
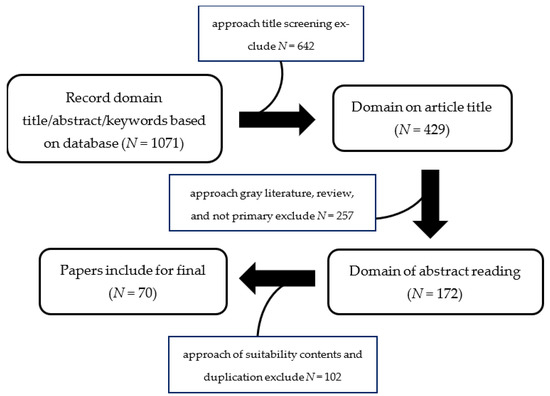
Figure 1.
The flow diagram for the systematic review.
3. Botany
The plants in the genus Murraya were often angiosperms widely distributed in tropical and subtropical regions, including East, Southern, and Southeast Asia, northern Australia, and several areas in South America. The plants were characterized by an average maximum height of 3.5 m, with alternate and odd-pinnate leaves, as well as terminal and/or axillary inflorescence. The seeds typically had a seed coat that could be membranous or fleshy, with straight embryos and elliptic cotyledons. Furthermore, the hypocotyl was partially enclosed between the cotyledons, with four or five petals [1]. One of the Murraya species, Murraya paniculata, is known as orange jasmine or kemuning in Indonesia and some other countries [10]. Additionally, the species Murraya koenigii is widely known as curry tree and the leaves are commonly used as a part of local cuisine in India [11,12,13] (Figure 2). The edibility of this variety of Murraya plants has been studied. Liaqat et al. [14], in the research on the toxicology of the Rutaceae family, including Murraya, stated that the oil content from Murraya is considered safe for internal use with caution.

Figure 2.
Murraya genus. (a) The flower and leaves of M. paniculata [15]. (b) The flowers and leaves of M. koenigii [16].
4. Phytochemistry
4.1. Overview of Isolated Compounds from Murraya Species
A total of 413 compounds were isolated based on the data obtained from the literature published between 1965 and 2023. The compounds isolated from the stem bark, bark, roots, leaves, and twigs of Murraya species included alkaloids, coumarin, flavonoids, steroids, terpenoids, and other components. Furthermore, previous reports suggested that alkaloids were the dominant metabolites, with a total of 193 compounds (46.9%), followed by coumarin and flavonoid with 121 (29.3%) and 48 (10.3%), respectively (Figure 3).
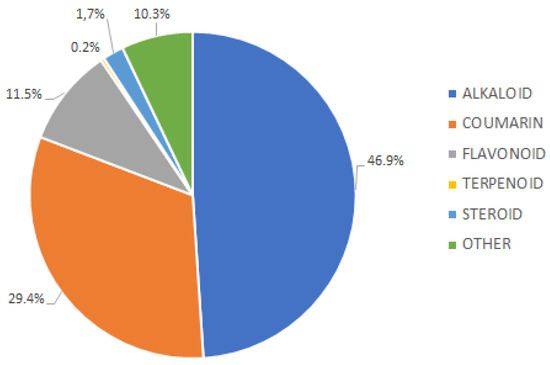
Figure 3.
Distribution of compound groups from the Murraya genus.
4.2. Alkaloid
At present, a total of 193 alkaloids have been identified in the form of carbazole, pyridine, pyrrole, N-substituted, indole, and dimerics, as shown in Table 1. The pyridinemonocarboxylate-type alkaloid, namely isomurralonginol nicotinate (1), was obtained from the leaves and stem of M. alata Drake [17]. Wu et al. [18] reported the presence of four new carbazole types, namely murrayamine F (2), murrayamines G (3), murrayamines H (4), and euchrestifoline (5), as well as four compounds (6–10) in M. euchrestifolia. Furthermore, other studies isolated new carbazole types from M. euchrestifolia, including murrayamine J (10), murrayamine K (11), murrayamine I (12), murrayamine M (13), murrayamine N (14), murrayamine D (15) [19], murrayamine O (19), and murrayamine P (20) [20], as well as three other alkaloids (16–18) [19].
The new binary carbazole type, namely bis-7-hydroxygirinimbine A (21) and bis-7-hydroxygirinimbine B (22), were isolated from the leaves of M. euchrestifolia [21]. A total of four carbazole-derivative-type alkaloids (23–26) were also isolated from M. euchrestifolia [19] along with a few other Murraya species [2]. McPhail et al. [22] reported the presence of a novel biscarbazole alkaloid, (+)-murrafoline (27), from the root bark of M. euchrestifolia. Furthermore, methyl-2-methyl-4-(N-2″b-methyl-1″,2″,3″,4″-tetrahydro-carbazol-1″a-ylindol-3′-yl)-butanoate (30) was found as a novel indole dimer from the root part of M. gleinei [23] (Figure 4).
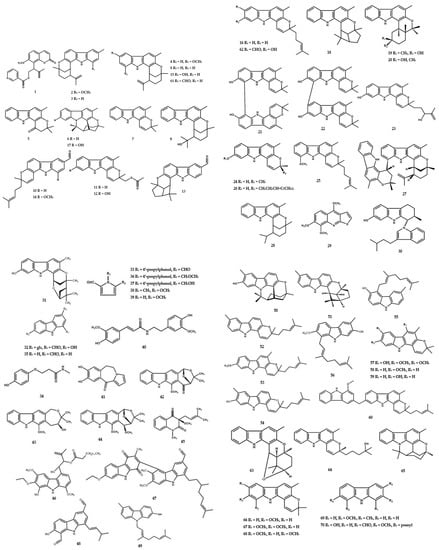
Figure 4.
Structures of alkaloids from the Murraya genus 1–70.

Table 1.
Alkaloids from the Murraya genus.
Table 1.
Alkaloids from the Murraya genus.
| Compounds | Part of Plant | Source | References |
|---|---|---|---|
| isomurralonginol nicotinate (1) | leaves and stems | M. alata Drake | [17] |
| murrayamines F (2) | leaves | M. euchrestifolia | [18] |
| murrayamines G (3) | leaves | M. euchrestifolia | [18] |
| murrayamines H (4) | leaves | M. euchrestifolia | [18] |
| euchrestifoline (5) | leaves | M. euchrestifolia | [18] |
| murrayazoline (6) | leaves | M. euchrestifolia | [18] |
| girinimbine (7) | leaves | M. euchrestifolia M. koenigii M. microphylla | [18,19,21,24,25] [26,27,28] [2,29] |
| murrayazolidine (8) | leaves | M. euchrestifolia M. koenigii | [18] [26,30] |
| murrayazolinine (9) | leaves | M. koenigii M. euchrestifolia M. koenigii | [4] [18] [26] |
| murrayamine-J (10) | leaves | M. euchrestifolia M. koenigii M. microphylla | [19] [26] [29] |
| murrayamine-K (11) | leaves | M. euchrestifolia | [19] |
| murrayamine-l (12) | leaves | M. euchrestifolia | [19] |
| murrayamine-M (13) | leaves | M. euchrestifolia | [19] |
| murrayamine-N (14) | leaves | M. euchrestifolia | [19] |
| murrayamine-D (15) | leaves | M. euchrestifolia | [19] |
| mahanimbine (16) | leaves | M. euchrestifolia M. koenigii M. microphylla | [19,24] [26,27,28,30,31,32] [2,29] |
| murrayamine-E (17) | leaves | M. euchrestifolia M. koenigii | [19] [31] |
| bicyclomahanimbine (18) | leaves | M. euchrestifolia M. koenigii | [19] [26,31] |
| murrayamine-O (19) | root bark | M. euchrestifolia | [20] |
| murrayamine-P (20) | root bark | M. euchrestifolia | [20] |
| bis-7-hydroxygirinimbine-A (21) | leaves | M. euchrestifolia | [21] |
| bis-7-hydroxygirinimbine-B (22) | leaves | M. euchrestifolia | [21] |
| murrayamine-C (23) | leaves | M. euchrestifolia M. koenigii | [24,25] [26] |
| murrayamine-A (24) | leaves | M. euchrestifolia M. koenigii M. microphylla | [19,24] [27] [2,29] |
| murrayamine-B (25) | leaves | M. euchrestifolia | [24] |
| mahanine (26) | leaves | M. euchrestifolia M. microphylla M. koenigii | [19,24] [2,29] [28,32,33] |
| (+)-murrafoline (27) | root bark | M. euchrestifolia | [22] |
| exozoline (28) | stem bark | M. exotica L. | [34] |
| skimianine (29) | leaves | M. gleinei | [35] |
| methyl 2-methyl-4-(N-2″b-methyl-1″,2″,3″,4″-tetrahydro-carbazol-1″a-ylindol-3′-yl)bustanoate (30) | root | M. gleinei | [23] |
| (1′R,3′R,4′R,6′S)-endocycliomurrayamine-A (31) | whole plant | M. koenigii | [4] |
| 3-formyle-7-hydroxy-9H-carbazole-1-O-β-D-glucopyranoside (32) | whole plant | M. koenigii | [4] |
| 4′-hydroxyphenyl-6-ethyl-1H-pyrrole-2-carboxalde-hyde (33) | whole plant | M. koenigii | [4] |
| 4-hydroxyphenoxy-N-methyl-propanamide (34) | whole plant | M. koenigii | [4] |
| 3-formylcarbazole (35) | whole plant | M. koenigii M. kwangsiensis | [4,28] [3] |
| pyrrolezanthine-6-methyl ether (36) | whole plant | M. koenigii | [4] |
| pyrolezanthine (37) | whole plant | M. koenigii | [4] |
| 5-hydroxymethyl-1-methylpyrrol-2-carbaldehyde (38) | whole plant | M. koenigii | [4] |
| 2-formyl-5-hydroxymethyl-pyrrole (39) | whole plant | M. koenigii | [4] |
| N-trans-feruloyl-3′-O-methyldopamine (40) | whole plant | M. koenigii | [4] |
| portulacatone (41) | whole plant | M. koenigii | [4] |
| claulansium A (42) | whole plant | M. koenigii | [4] |
| claulansium B (43) | whole plant | M. koenigii | [4] |
| 1′-omethylclaulamine B (44) | whole plant | M. koenigii | [4] |
| dunnine E (45) | whole plant | M. koenigii | [4] |
| mukoenigatin (46) | aerial part | M. koenigii | [36] |
| bikoeniquinonine (47) | aerial part | M. koenigii | [36] |
| murrayadinal (48) | aerial part | M. koenigii | [36] |
| karapinchamines A (49) | leaves | M. koenigii | [31] |
| karapinchamines B (50) | leaves | M. koenigii | [31] |
| bicyclomahanimbicine (51) | leaves | M. koenigii | [31] |
| mahanimbicine (52) | leaves | M. koenigii | [31] |
| methylmahanimbine (53) | leaves | M. koenigii | [31] |
| pyrayafoline D (54) | leaves | M. koenigii M. kwangsiensis | [31,32] [3] |
| eustifolin (55) | leaves | M. koenigii | [31] |
| euchrestine-B (56) | leaves | M. koenigii | [31,32] |
| kurryam (57) | seeds | M. koenigii | [5] |
| koenimbine (58) | seeds | M. koenigii M. microphylla | [5,26,27,32] [2,29] |
| koenine (59) | seeds | M. koenigii M. microphylla | [5,27,32] [2] |
| murrayakonine A (60) | stems and leaves | M. koenigii | [26] |
| murrayakonine B (61) | stems and leaves | M. koenigii | [26] |
| murrayakonine C (62) | stems and leaves | M. koenigii | [26] |
| murrayakonine D (63) | stems and leaves | M. koenigii | [26] |
| mahanimbinine (64) | stems and leaves | M. koenigii M. microphylla | [26] [29] |
| currayangine (65) | stems and leaves | M. koenigii | [26] |
| O-methylmurrayamine-A (66) | stems and leaves | M. koenigii M. microphylla | [26] [2] |
| koenigicine (67) | stems and leaves | M. koenigii | [26] |
| mukonicine (68) | stems and leaves | M. koenigii | [26] |
| 2-methoxy-3-methyl-9H-carbazole (69) | stems and leaves | M. koenigii | [26] |
| 1-hydroxy-7-methoxy-8-(3-methylbut-2-en-1-yl)-9H-carbazole-3-carbaldehyde (70) | stems and leaves | M. koenigii | [26] |
| 8,8″-biskoenigine (71) | stems and leaves | M. koenigii | [26,27] |
| clauraila A (72) | stems and leaves | M. koenigii | [26] |
| N-benzyl carbazole-A (73) | whole plant | M. koenigii | [27] |
| N-benzyl carbazole-B (74) | whole plant | M. koenigii | [27] |
| isokoenidine (75) | whole plant | M. koenigii M. microphylla | [27] [2] |
| iso-koenigine (76) | whole plant | M. koenigii | [27] |
| koenigine (77) | whole plant | M. koenigii M. microphylla | [27,32] [2,29] |
| koenidine (78) | whole plant | M. koenigii M. microphylla | [27,32] [2,29] |
| murrayakoeninol (79) | leaves | M. koenigii | [37] |
| koenoline (80) | whole plant | M. koenigii | [27,38] |
| N-methoxy-3-hydroxymethyl-9H-carbazole (81) | whole plant | M. koenigii | [27] |
| 3-hydroxymethyl-9-H-carbazole (82) | whole plant | M. koenigii | [27] |
| O-demethylmurrayanine (83) | whole plant | M. koenigii M. kwangsiensis | [27] [3] |
| murrastanine A (84) | bark and leaves | M. koenigii | [39] |
| murrastinine A (85) | bark and leaves | M. koenigii | [39] |
| murrastinine B (86) | bark and leaves | M. koenigii | [39] |
| murrastinine C (87) | bark and leaves | M. koenigii M. microphylla | [39] [2,29] |
| murrayatanine-A (88) | bark and leaves | M. koenigii | [39] |
| bismahanimboline (89) | bark and leaves | M. koenigii | [39] |
| murrafoline-I (90) | leaves | M. koenigii | [32] |
| mahabinine-A (91) | leaves | M. koenigii | [32] |
| bisgerayafoline D (92) | fruit | M. koenigii | [33] |
| bismahanimbinol (93) | fruit | M. koenigii | [33] |
| bispyrayafoline (94) | fruit | M. koenigii | [33] |
| O-methyl mahanine (95) | fruit | M. koenigii | [33] |
| O-methyl mukonal (96) | fruit | M. koenigii | [33] |
| 3,3′-[oxybis(methylene)]bis(9-methoxy-9H-carbazole) (97) | stem bark | M. koenigii | [28] |
| murrayanine (98) | stem bark | M. koenigii M. kwangsiensis M. microphylla | [7,26,27,28,38] [3] [29] |
| 3-formyl-9-methoxycarbazole (99) | stem bark | M. koenigii | [28] |
| carbazole-3-carboxylic acid (100) | stem bark | M. koenigii | [28] |
| koenigine-quinone A (101) | stem bark | M. koenigii | [40] |
| koenigine-quinone B (102) | stem bark | M. koenigii | [40] |
| bismurrayafoline D (103) | leaves | M. euchrestifolia | [41] |
| bismurrayafoline E (104) | leaves | M. koenigii | [42] |
| 9-carbethoxy-3-methylcarbazole (105) | roots | M. koenigii | [43] |
| 9-formyl-3-methylcarbazole (106) | roots | M. koenigii | [43] |
| 3-methyl-carbazole (107) | roots | M. koenigii | [43] |
| isomahanine (108) | fruits | M. koenigii M. euchrestifolia | [30] [19] |
| murrayanol (109) | fruits | M. koenigii | [30] |
| mukonal (110) | whole plant | M. koenigii | [44] |
| mukonicine (111) | leaves | M. koenigii | [45] |
| isomurrayazoline (112) | stem bark | M. koenigii | [46] |
| mukonine (113) | root | M. koenigii | [47] |
| (−)-bispyrayafoline C (114) | leaves and stems | M. kwangsiensis | [3] |
| (+)-bispyrayafoline C (115) | leaves and stems | M. kwangsiensis | [3] |
| (−) kwangsine A (116) | leaves and stems | M. kwangsiensis | [3] |
| (−) kwangsine A (117) | leaves and stems | M. kwangsiensis | [3] |
| (−) kwangsine B (118) | leaves and stems | M. kwangsiensis | [3] |
| (+) kwangsine B (119) | leaves and stems | M. kwangsiensis | [3] |
| (−) kwangsine C (120) | leaves and stems | M. kwangsiensis | [3] |
| (+) kwangsine C (121) | leaves and stems | M. kwangsiensis | [3] |
| kwangsine D (122) | leaves and stems | M. kwangsiensis | [3] |
| kwangsine E (123) | leaves and stems | M. kwangsiensis | [3] |
| kwangsine F (124) | leaves and stems | M. kwangsiensis | [3] |
| kwangsine G (125) | leaves and stems | M. kwangsiensis | [3] |
| kwangsine H (126) | leaves and stems | M. kwangsiensis | [3] |
| kwangsine I (127) | leaves and stems | M. kwangsiensis | [3] |
| kwangsine J (128) | leaves and stems | M. kwangsiensis | [3] |
| kwangsine K (129) | leaves and stems | M. kwangsiensis | [3] |
| kwangsine L (130) | leaves and stems | M. kwangsiensis | [3] |
| kwangsine M (131) | leaves and stems | M. kwangsiensis | [3] |
| pyrayaquinone B (132) | leaves and stems | M. kwangsiensis | [3] |
| pyrayafoline C (133) | leaves and stems | M. kwangsiensis M. microphylla | [3] [2] |
| euchrestine-A (134) | leaves and stems | M. kwangsiensis | [3] |
| euchrestine-C (135) | leaves and stems | M. kwangsiensis | [3] |
| 2-hydroxy-3-methylcarbazole (136) | leaves and stems | M. kwangsiensis M. microphylla | [3] [29] |
| 1-hydroxy-3-methyl-9H-carbazole (137) | leaves and stems | M. kwangsiensis | [3] |
| 3-hydro-xymethyl-9H-carbazole (138) | leaves and stems | M. kwangsiensis | [3] |
| 3-(methoxymethyl)carbazole (139) | leaves and stems | M. kwangsiensis | [3] |
| 1-methoxy-3-(methoxymethyl)carbazole (140) | leaves and stems | M. kwangsiensis | [3] |
| claulansine Q (141) | leaves and stems | M. kwangsiensis | [3] |
| claulansine R (142) | leaves and stems | M. kwangsiensis | [3] |
| 3-carboxylic acid carbazole (143) | leaves and stems | M. kwangsiensis | [3] |
| clausine E (144) | leaves and stems | M. kwangsiensis | [3] |
| 3-methyl-9H-carbazole (145) | leaves and stems | M. kwangsiensis | [3] |
| murrayafoline A (146) | leaves and stems | M. kwangsiensis | [3] |
| (+)-microphylines N (147) | leaves and stems | M. microphylla | [2] |
| (–)-microphylines N (148) | leaves and stems | M. microphylla | [2] |
| (+)-microphylines O (149) | leaves and stems | M. microphylla | [2] |
| (–)-microphylines O (150) | leaves and stems | M. microphylla | [2] |
| (+)-microphylines P (151) | leaves and stems | M. microphylla | [2] |
| (–)-microphylines P (152) | leaves and stems | M. microphylla | [2] |
| (+)-microphylines Q (153) | leaves and stems | M. microphylla | [2] |
| (–)-microphylines Q (154) | leaves and stems | M. microphylla | [2] |
| (+)-microphylines R (155) | leaves and stems | M. microphylla | [2] |
| (–)-microphylines R (156) | leaves and stems | M. microphylla | [2] |
| isogirinimbine (157) | leaves and stems | M. microphylla | [2] |
| heptazolidine (158) | leaves and stems | M. microphylla | [2] |
| (−)-mahanimbicine (159) | leaves and stems | M. microphylla | [2] |
| (−)-pyrayafoline D (160) | leaves and stems | M. microphylla | [2] |
| O-(−)-methylpyrayafoline D (161) | leaves and stems | M. microphylla | [2] |
| (−)-murrayamine-J (162) | leaves and stems | M. microphylla | [2] |
| (−)-murrayamine-B (163) | leaves and stems | M. microphylla | [2] |
| (−)-6-hydroxymahanimbine (164) | leaves and stems | M. microphylla | [2] |
| (2′S,3′R)-microphyline K (165) | leaves and stems | M. microphylla | [29] |
| (2′R,3′S)-microphyline K (166) | leaves and stems | M. microphylla | [29] |
| microphyline L (167) | leaves and stems | M. microphylla | [29] |
| microphyline M (168) | leaves and stems | M. microphylla | [29] |
| 6-hydroxygirinimbine (169) | leaves and stems | M. microphylla | [29] |
| 3-formyl-1-hydroxycarbazole (170) | leaves and stems | M. microphylla | [29] |
| clausine P (171) | leaves and stems | M. microphylla | [29] |
| 9H-1-hydroxy-7-methoxy-8-(3-methyl-2-buten-1-yl)-carbazole-3-carboxaldehyde (172) | leaves and stems | M. microphylla | [29] |
| clausine Q (173) | leaves and stems | M. microphylla | [29] |
| 1-hydroxy-3-methylcarbazole (174) | leaves and stems | M. microphylla | [29] |
| carbalexin B (175) | leaves and stems | M. microphylla | [29] |
| murrayacarine (176) | root bark | M. omphalocarpa | [48] |
| 3-formylindole (177) | stem bark | M. omphalocarpa | [49] |
| paniculidines D (178) | roots | M. paniculata | [50] |
| paniculidines E (179) | roots | M. paniculata | [50] |
| paniculidines F (180) | roots | M. paniculata | [50] |
| paniculidines A (181) | roots | M. paniculata | [50,51] |
| paniculidines B (182) | roots | M. paniculata | [50,51] |
| paniculidines C (183) | roots | M. paniculata | [50,51] |
| tanakine (184) | roots | M. paniculata | [50] |
| indol-3-carbaldehyde (185) | roots | M. paniculata | [50] |
| yuehchukene (186) | roots | M. paniculata | [8,50] |
| alanditrypinone (187) | leaves | M. paniculata | [52] |
| alantryphenone (188) | leaves | M. paniculata | [52] |
| alantrypinene (189) | leaves | M. paniculata | [52] |
| alantryleunone (190) | leaves | M. paniculata | [52] |
| murrayaculatine (191) | flower | M. paniculata | [53] |
| murradiate (192) | leaves and stems | M. tetramera | [54] |
| murradiol (193) | leaves and stems | M. tetramera | [54] |
A further investigation of M. koenigii by Wei et al. [4] identified three new alkaloid derivatives, including two carbazoles, namely (1′R,3′R,4′R,6′S)-endocycliomurrayamine-A (31), 3-formyle-7-hydroxy-9H-carbazole-1-O-β-D-glucopyranoside (32), and a pyrole-type 4′-hydroxyphenyl-6-ethyl-1H-pyrrole-2-carboxaldehyde (33). The aliphatic alkaloid-type (34,40), carbazole-type (35), and three substituted pyrole-type (36–39) compounds were also identified in the species [4].
A previous study reported the isolation of the lactam derivatives, portulacatone (41), along with sixteen other alkaloids, from M. koenigii [4]. A total of three oxepane-carbazole derivatives, namely claulansium A (42), claulansium B (43), and 1′-omethylclaulamine B (44), and one other compound (45) were also identified [4]. Furthermore, the aerial parts of M. koenigii contained the dimer alkaloid, bikoeniquinonine (47) [36], along with two dimer types, namely murrayakonine A (60) [26] and 8,8′-biskoenigine (71) [27] (Figure 5).
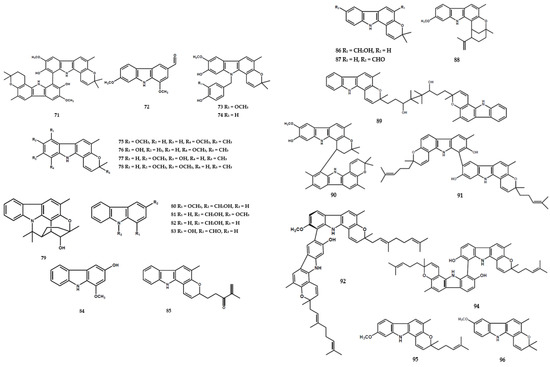
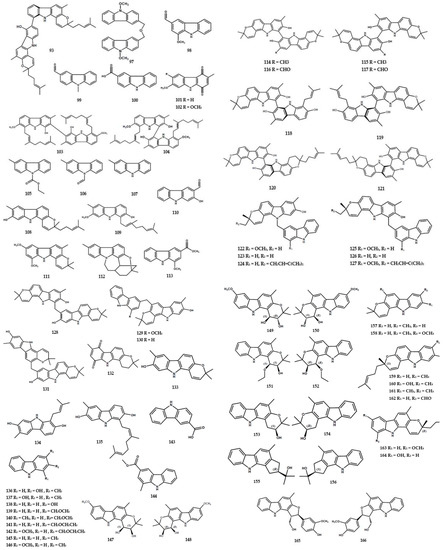
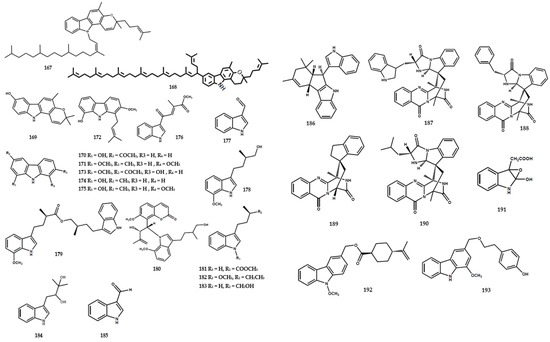
Figure 5.
Structures of alkaloids from the Murraya genus 71–193.
In further phytochemical studies, substituted carbazole and N-substituted carbazole structures were commonly found in the Murraya genus. Naz et al. [36] reported the isolation of mukoenigatin (46) and murrayadinal (48) from the aerial parts of M. koenigii. Karapinchamines A (49), karapinchamines B (50), eustifolin (55), and euchrestine B (56) were also obtained from its leaves [31]. An investigation by Nalli et al. [26] identified three N-substituted carbazoles, including 2-methoxy-3-methyl-9H-carbazole (69), 1-hydroxy-7-methoxy-8-(3-methylbut-2-en-1-yl)-9H-carbazole-3-carbaldehyde (70), and clauraila A (72).
The benzo[a]carbazole-type alkaloids were frequently found in the Murraya genus. Nakamura et al. [31] identified the presence of mahanimbicine (52) and two other compounds (53–54) in the leaves of M. koenigii. Another three compounds, namely kurryam (57), koenimbine (58), and koenine (59), were also obtained from its seeds [5]. Furthermore, O-methylmurrayamine-A (66), koenigicine (67), mukonicine (68 [26], N-benzyl carbazole-A (73), N-benzyl carbazole-B (74) [27], and murrastinine A-C (85–87) [39] were identified from M. koenigii and several other species.
Alkaloids were also identified in other forms, including substituted indole derivative types, encompassing 3-formylindole (177) from M. omphalocarpa [49], paniculidines D (178), paniculidines E (179), and seven compounds (180–186) from M. paniculata [50].
4.3. Coumarins
Several coumarins were identified in Murrya species, such as M. alata, M. gleinei, M. paniculata, and M. exotica. At present, a total of 121 compounds in this category have been reported, as shown in Table 2. Furthermore, these compounds were identified in the form of substituted simple coumarin, coumarin glycoside, alkoxycoumarin, 8-alkyl substituted, and furano type. Methoxy-substituted analog type, namely muralatin R (194), was obtained from M. alata [55]. Several coumarins have also been isolated from the same species, including meranzin (195), phebalosin (196), muralatin N (203), and meranzin hydrate (208). A previous study reported the presence of coumarin glycoside type, namely muralatin Q (213), in M. alata [17].
Coumarin-substituted cyclopropane was isolated from M. exotica in the form of an enantiomer, muratin A (214–215), and muratin B (216–217) [56]. Another study reported the isolation of a glycoside coumarin derivative, muratin F (221), from the same species [56] (Figure 6).
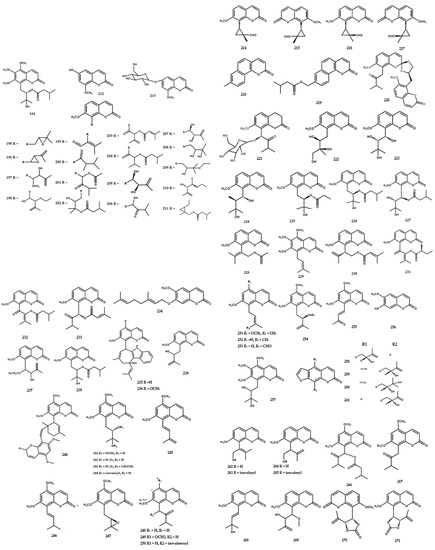
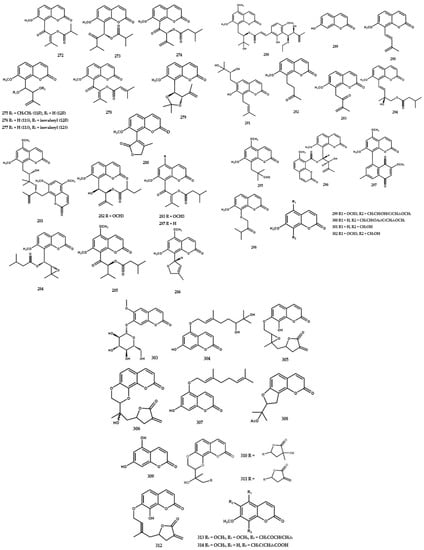
Figure 6.
Structures of coumarins from the Murraya genus 194–314.

Table 2.
Coumarins from the Murraya genus.
Table 2.
Coumarins from the Murraya genus.
| Compounds | Part of Plant | Source | References |
|---|---|---|---|
| muralatin R (194) | leaves | M. alata Drake | [55] |
| meranzin (195) | leaves and stems | M. alata Drake | [17] |
| phebalosin (196) | leaves and stems | M. alata Drake | [17] |
| murracarpin (197) | leaves and stems | M. alata Drake | [17] |
| 2′-O-ethylmurrangatin (198) | leaves and stems | M. alata Drake M. paniculata | [17] [57] |
| muralongin (199) | leaves and stems | M. alata Drake | [17] |
| muralatin L (200) | leaves and stems | M. alata Drake | [17] |
| hainanmurpanin (201) | leaves and stems | M. alata Drake M. gleinei | [17] [35] |
| muralatin M (202) | leaves and stems | M. alata Drake M. gleinei | [17] [35,58] |
| muralatin N (203) | leaves and stems | M. alata Drake | [17] |
| muralatin O (204) | leaves and stems | M. alata Drake M. paniculata | [17] [57,59] |
| murangatin (205) | leaves and stems | M. alata Drake M. gleinei M. paniculata M. elongata M. omphalocarpa | [17] [35,58] [51,57] [60] [61] |
| murpaniculol (206) | leaves and stems | M. alata Drake | [17] |
| minumicrolin (207) | leaves and stems | M. alata Drake M. gleinei M. omphalocarpa M. paniculata M. elongata M. exotica | [17] [35,58] [48,61] [57,59] [62] [63] |
| meranzin hydrate (208) | leaves and stems | M. alata Drake | [17] |
| yuehgesin-C (209) | leaves and stems | M. alata Drake M. omphalocarpa M. paniculata | [17] [61] [57,59] |
| muralatin K (210) | leaves and stems | M. alata Drake M. gleinei M. exotica | [17] [35] [63] |
| muralatin P (211) | leaves and stems | M. alata Drake | [17] |
| muralatin K (212) | leaves and stems | M. alata Drake M. paniculata | [17] [59] |
| muralatin Q (213) | leaves and stems | M. alata Drake | [17] |
| (−) murratin A (214) | leaves and twigs | M. exotica L. | [56] |
| (+) murratin A (215) | leaves and twigs | ||
| (−) murratin B (216) | leaves and twigs | M. exotica L. | [56] |
| (+) murratin B (217) | leaves and twigs | ||
| murratin C (218) | leaves and twigs | M. exotica L. | [56] |
| murratin D (219) | leaves and twigs | M. exotica L. | [56] |
| murratin E (220) | leaves and twigs | M. exotica L. | [56] |
| murratin F (221) | leaves and twigs | M. exotica L. | [56] |
| murratin G (222) | leaves and twigs | M. exotica L. | [56] |
| murratin H (223) | leaves and twigs | M. exotica L. | [56] |
| murratin I (224) | leaves and twigs | M. exotica L. | [56] |
| murratin J (225) | leaves and twigs | M. exotica L. | [56] |
| murratin K (226) | leaves and twigs | M. exotica L. | [56] |
| murratin L (227) | leaves and twigs | M. exotica L. | [56] |
| murratin M (228) | leaves and twigs | M. exotica L. | [56] |
| muralatin C (229) | leaves and twigs | M. exotica L. | [56] |
| 2-(7-methoxy-2-oxochromen-8-yl)-3-methylbut-2-enyl] 3-methylbut-2-enoate (230) | leaves and twigs | M. exotica L. | [56] |
| panitin C (231) | leaves and twigs | M. paniculata M. exotica L. | [57,59] [56] |
| exotimarin H (232) | leaves and twigs leaves and stems | M. exotica L. M. paniculata | [56] [59] |
| epimurpaniculol senecioate (233) | leaves and twigs | M. exotica L. | [56] |
| 7-geranyloxy-6-methoxycoumarin (234) | leaves and twigs | M. paniculata M. exotica L. | [57] [56] |
| exotines A (235) | roots | M. exotica L. | [64] |
| exotines B (236) | roots | M. exotica L. | [64] |
| murraxocin (237) | roots | M. exotica L. | [65] |
| murrayatin (238) | leaves | M. exotica L. M. paniculata | [66] [67] |
| auraptenol (239) | leaves | M. exotica L. | [63] |
| mexolide (240) | stem bark | M. exotica L. | [68] |
| murraglenin (241) | leaves | M. omphalocarpa | [48] |
| mexoticin (242) | roots | M. gleinei M. omphalocarpa M. paniculata | [35,58] [48,69] [57,70] |
| 5,7-dimethoxy-8-(2-hydroxyl-3-ethoxy-3-methylbutyl coumarin (243) | roots | M. paniculata | [57] |
| 5-methoxymurrayatin (244) | roots | M. paniculata | [57,59] |
| gleinadiene (245) | roots | M. gleinei | [58] |
| gleinene (246) | roots | M. gleinei | [58] |
| sibiricin (247) | roots | M. gleinei M. paniculata | [35] [57] |
| isomeranzin (248) | roots | M. paniculata | [57] |
| murrayone (249) | roots | M. paniculata | [57] |
| paniculatin (250) | roots | M. paniculata | [57,59] |
| coumurrayin (251) | roots | M. paniculata M. omphalocarpa | [57,70,71] [48,69] |
| osthol (252) | roots | M. paniculata | [51,57] |
| 7-methoxy-8-(3′-formylbut-2′-enyl)-coumarin (253) | roots | M. paniculata | [57] |
| omphamurin (254) | leaves | M. omphalocarpa M. paniculata | [48] [57] |
| toddalenone (255) | roots | M. gleinei M. omphalocarpa | [58] [61] |
| scopoletin (256) | leaves | M. gleinei | [35] |
| murragleinin (257) | leaves | M. gleinei M. paniculata | [35] [70] |
| gosferol (258) | seeds | M. koenigii | [72] |
| neobyakangelicol (259) | seeds | M. koenigii | [72] |
| byakangelicin (260) | seeds | M. koenigii | [72] |
| isogosferol (261) | seeds | M. koenigii | [72] |
| murralonginol (262) | roots | M. paniculata | [57] |
| murralonginol isovalerate (263) | roots | M. paniculata | [57] |
| isomurralonginol (264) | roots | M. paniculata | [57] |
| isomurralonginol isovalerate (265) | roots | M. paniculata M. omphalocarpa | [57] [61] |
| omphamurrayone (266) | leaves | M. omphalocarpa | [61] |
| 5,7-dimethoxy-8-(3′-methyl-2′-oxobutyl) coumarin (267) | root bark | M. omphalocarpa M. paniculata | [48,69] [70,73] |
| murraol (268) | root bark | M. omphalocarpa | [48] |
| (+)-murracarpin (269) | root bark | M. omphalocarpa M. paniculata | [48] [70] |
| (+)-murpanitin A (270) | leaves and stems | M. paniculata | [59] |
| (−)-murpanitin A (271) | leaves and stems | M. paniculata | [59] |
| murpanitins B (272) | leaves and stems | M. paniculata | [59] |
| murpanitins C (273) | leaves and stems | M. paniculata | [59] |
| murpanitins D (274) | leaves and stems | M. paniculata | [59] |
| murpanicin (275) | leaves and stems | M. paniculata | [57,59] |
| minumicrolin isovalerate (276) | leaves and stems | M. paniculata | [59] |
| murrangatin isovalerate (277) | leaves and stems | M. paniculata | [57,59] |
| kimcuongin (278) | leaves and stems | M. paniculata | [59] |
| minumicrolin acetonide (279) | leaves and stems | M. paniculata | [57,59] |
| microminutin (280) | leaves and stems | M. paniculata | [59] |
| panitin A (281) | roots | M. paniculata | [57] |
| panitin B (282) | roots | M. paniculata | [57] |
| panitin D (283) | roots | M. paniculata | [57] |
| panitin E (284) | roots | M. paniculata | [57] |
| panitin F (285) | roots | M. paniculata | [57] |
| panitin G (286) | roots | M. paniculata | [57] |
| exotimarin I (287) | roots | M. paniculata | [57] |
| 10′-ethoxyexotimarin F (288) | roots | M. paniculata | [57] |
| Umbelliferone (289) | roots | M. paniculata | [57] [74] |
| trans-dehydroosthol (290) | roots | M. paniculata | [57] |
| 6-(2′,3′-dihydroxy-3-methylbutyl)-8-prenylumbelliferone (291) | roots | M. paniculata | [57] |
| hassanon (292) | roots | M. paniculata | [57] |
| 5,7-dimethoxy-8-(3-methyl-2-keto-butyl)coumarin (293) | roots | M. paniculata | [57] |
| casegravol isovalerate (294) | roots | M. paniculata | [57] |
| seselinal (295) | roots | M. paniculata | [57] |
| cladimarin B (296) | roots | M. paniculata | [57] |
| toddacoumaquinone (297) | roots | M. paniculata | [57] |
| 8-(2′,-oxo-3′-methyl)butoxy-7-methoxycoumarin (298) | leaves | M. paniculata | [67] |
| omphalocarpin (299) | flowers | M. omphlocarpa M. paniculata | [48] [70] |
| (−)-murracarpin (300) | flowers | M. omphalocarpa M. paniculata | [48] [70] |
| murrayacarpin-A (301) | flowers | M. paniculata | [70] |
| murrayacarpin-B (302) | flowers | M. paniculata | [70] |
| scopolin (303) | flowers | M. paniculata | [70] |
| murrayacoumarin A (304) | leaves | M. siamensis | [74] |
| murrayacoumarin B (305) | leaves | M. siamensis | [74] |
| murrayacoumarin C (306) | leaves | M. siamensis | [74] |
| 5-geranyloxy-7-hydroxy-coumarin (307) | leaves | M. siamensis | [74] |
| columbianetin acetate (308) | leaves | M. siamensis | [74] |
| 5,7-dihydroxycoumarin (309) | leaves | M. siamensis | [74] |
| clauslactone B (310) | leaves | M. siamensis | [74] |
| clauslactone A (311) | leaves | M. siamensis | [74] |
| clauslactone E (312) | leaves | M. siamensis | [74] |
| murrayanone (313) | leaves | M. paniculata | [73] |
| murraculatin (314) | leaves | M. paniculata | [73] |
Several studies reported the isolation of various C-8-subtituted coumarins from the Murraya genus. Liang et al. [56] reported the presence of murratin G (222), murratin H (223), murratin I (224), murratin J (225), murratin K (226), murratin L (227), murratin M (228), muralatin C (229), 2-(7-methoxy-2-oxochromen-8-yl)-3-methylbut-2-enyl]-3-methylbut-2-enoate (230), and two other compounds (231 and 233) from M. exotica. Furthermore, exotimarin H (232) and 7-geranyloxy-6-methoxycoumarin (234) were identified in M. exotica [56] and M. paniculata [57].
A dimeric coumarin, mexolide (240), was identified from the stem bark of M. exotica [68]. A previous study reported the presence of a furanocoumarin type, consisting of gosferol (258), neobyakangelicol (259), byakangelicin (260), and isogosferol (261), in the seeds of M. koenigii [72] (Figure 6).
4.4. Flavonoid
A total of forty-eight flavonoids had been identified in the form of flavone, flavanone, flavanonol, and flavanoid glycoside, as shown in Table 3. A previous study reported the presence of 3,3′,4′,5,5′,7,8-heptamethoxyflavone (318) in M. exotica in 1970 [75] and further reports isolated 3,5,6,8,3′,4′,5′-heptamethoxyflavone (317) from the same species [63]. Furthermore, five flavone types were isolated from M. paniculata, encompassing 3′-hydroxy-5,6,7,4′,5′-pentamethoxyflavone (321), 5,3′-dihydroxy-6,7,4′,5′-tetramethoxy-flavone (322), 5,3′-dihydroxy-7,4′,5′-trimethoxyflavone (323), 5-hydroxy-7,8,3′,4′-tetramethoxyflavone (324), and 4′-hydroxy-5,6,7,3′,5′-pentamethoxyflavone (325) [76].
Six flavanone types were also identified in M. paniculata, including 5,7,3′,4′-tetramethoxyflavanone (326), 4′-hydroxy-5,7,3′-trimethoxyflavanone (327), 4′-hydroxy-5,7-dimethoxyflavanone (328), 5,6,7,3′,4′,5′-hexamethoxyflavanone (329), 6,7,8,3′,4′,5′-hexamethoxyflavanone (330), and 3-hydroxy-5,7,3′,4′-tetramethoxyflavanone (331) [76] (Figure 7).
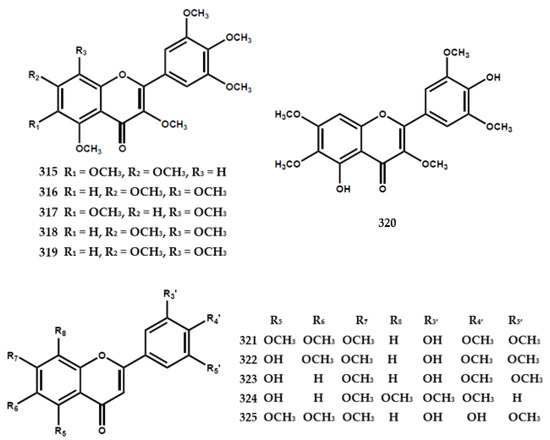
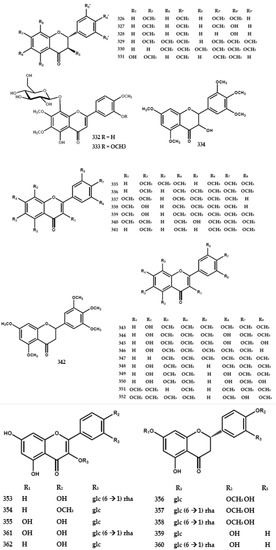
Figure 7.
Structures of flavonoids from the Murraya genus 315–362.
The flavanonol-type flavonoid was found in M. paniculata and identified as 5,7,3′,4′,5′-pentamethoxyflavanonol (334) [77]. Ferracin et al. [77] isolated six flavone types, including 5,6,7,3′,4′,5′-hexamethoxyflavone (335), 5,7,8,3′,4′,5′-hexamethoxy-flavone (336), 3,5,7,8,-3′,4′-hexamethoxyflavone (337), 5-hydroxy-3,7,8,3′,4′-penta-methoxy-flavone (338), 5-hydroxy-3,7,8,3′,4′,5′-hexamethoxyflavone (339), and 8-hydroxy-3,5,7,3′,4′,5′-hexa-methoxyflavone (340) (Figure 7).

Table 3.
Flavonoids from the Murraya genus.
Table 3.
Flavonoids from the Murraya genus.
| Compounds | Part of Plant | Source | References |
|---|---|---|---|
| 3,5,6,7,3′,4′,5′-heptamethoxyflavone (315) | leaves and stems | M. alata Drake | [17,63] |
| 3,5,7,8,3′,4′,5′-heptamethoxyflavone (316) | leaves and stems | M. alata Drake | [17] |
| 3,5,6,8,3′,4′,5′-heptamethoxyflavone (317) | leaves | M. exotica L. | [63] |
| 3,3′,4′,5,5′,7,8-Heptamethoxyflavone (318) | leaves | M. exotica L. | [75] |
| Exoticin (319) | leaves | M. gleinei | [35] |
| 5,4′-dihydroxy-3,6,7,3′,5′-pentamethoxyflavone (320) | fresh fruits | M. omphalocarpa | [78] |
| 3′-hydroxy-5,6,7,4′,5′-pentamethoxyflavone (321) | leaves and twigs | M. paniculata | [76] |
| 5,3′-dihydroxy-6,7,4′,5′-tetramethoxyflavone (322) | leaves and twigs | M. paniculata | [76] |
| 5,3′-dihydroxy-7,4′,5′-trimethoxyflavone (323) | leaves and twigs | M. paniculata | [76] |
| 5-hydroxy-7,8,3′,4′-tetramethoxyflavone (324) | leaves and twigs | M. paniculata | [76] |
| 4′-hydroxy-5,6,7,3′,5′-pentamethoxyflavone (325) | leaves and twigs | M. paniculata | [76] |
| 5,7,3′,4′-tetramethoxyflavanone (326) | leaves and twigs | M. paniculata | [76] |
| 4′-hydroxy-5,7,3′-trimethoxyflavanone (327) | leaves and twigs | M. paniculata | [76] |
| 4′-hydroxy-5, 7-dimethoxyflavanone (328) | leaves and twigs | M. paniculata | [76] |
| 5,6,7,3′,4′,5′-hexamethoxyflavanone (329) | leaves and twigs | M. paniculata | [76] |
| 6,7,8,3′,4′,5′-hexamethoxyflavanone (330) | leaves and twigs | M. paniculata | [76] |
| 3-hydroxy-5,7,3′,4′-tetramethoxyflavanone (331) | leaves and twigs | M. paniculata | [76] |
| 5,8,3′-trihydroxy-6,7,4′-trimethoxyflavone 8-O-β-glucopyranoside (332) | leaves and shoots | M. paniculata | [79] |
| 5,8-dihydroxy-6,7,3′,4′-tetramethoxyflavone 8-O-β-glucopyranoside (333) | leaves and shoots | M. paniculata | [79] |
| 5,7,3′,4′,5′-pentamethoxyflavanonol (334) | leaves and stems | M. paniculata | [77] |
| 5,6,7,3′,4′,5′-hexamethoxyflavone (335) | leaves and stems | M. paniculata | [77] |
| 5,7,8,3′,4′,5′-hexamethoxyflavone (336) | leaves and stems | M. paniculata | [77] |
| 3,5,7,8,3′,4′-hexamethoxyflavone (337) | leaves and stems | M. paniculata | [77] |
| 5- hydroxy-3,7,8,3′,4′-pentamethoxyflavone (338) | leaves and stems | M. paniculata | [77] |
| 5-hydroxy-3,7,8,3′,4′,5′-hexamethoxyflavone (339) | leaves and stems | M. paniculata | [77] |
| 8-hydroxy-3,5,7,3′,4′,5′-hexamethoxyflavone (340) | leaves and stems | M. paniculata | [77] |
| 5,7,3′,4′,5′-pentamethoxy-flavone (341) | leaves | M. paniculata | [80] |
| 5,7,3′,4′,5′-pentamethoxyflavanone (342) | leaves | M. paniculata | [80] |
| 5-hydroxy-6,7,8,3′,4′,5′- hexamethoxyflavone (343) | leaves | M. paniculata | [81] |
| 5,3′-dihydroxy-6,7,8,4′,5-pentamethoxyflavone (344) | leaves | M. paniculata | [81] |
| 6,7,8,4′-tetramethoxy- 5,3′,5′-trihydroxyflavone (345) | leaves and stems | M. paniculata | [81] |
| 5- hydroxy- 6,7,8,3′,4′-pentamethoxyflavone (346) | leaves and stems | M. paniculata | [81] |
| 6,7,8,3′,4′,5′-hexamethoxyflavone (347) | leaves and stems | M. paniculata | [81] |
| 5-hydroxy-6,7,3′,4′,5′-pentamethoxyflavone (348) | leaves | M. paniculata | [81] |
| 5,3′- dihydroxy-6,7,4′,5-tetramethoxyflavone (349) | leaves and stems | M. paniculata | [81] |
| 5,3′,5′-trihydroxy-6,7,4′-trimethoxyflavone (350) | leaves and stems | M. paniculata | [81] |
| 3,5,7,3′,4′,5′-hexamethoxytlavone (351) | flowers | M. paniculata | [53] |
| 4′-hydroxy-3,5,6,7,3′,5′-hexamethoxyflavone (352) | leaves | M. paniculata | [78] |
| kaempferol-3-O-rutinoside (353) | leaves and stem barks | M. tetramera | [82] |
| kaempferide-3-O-β-D-glucopyranoside (354) | leaves and stem barks | M. tetramera | [82] |
| kaempferol-3-O-β-D-glucopyranoside (355) | leaves and stem barks | M. tetramera | [82] |
| hesperitin-7-O-β-D-glucopyranoside (356) | leaves and stem barks | M. tetramera | [82] |
| neohesperidin (357) | leaves and stem barks | M. tetramera | [82] |
| hesperidin (358) | leaves and stem barks | M. tetramera | [82] |
| naringenin-7-O-β-D-glucopyranoside (359) | leaves and stem barks | M. tetramera | [82] |
| naringin (360) | leaves and stem barks | M. tetramera | [82] |
| rutin (361) | leaves and stem barks | M. tetramera | [82] |
| isoquercitrin (362) | leaves and stem barks | M. tetramera | [82] |
The flavonoid glycoside types, including 5,8,3′-trihydroxy-6,7,4′-trimethoxyflavone 8-O-β-glucopyranoside (332) and 5,8-dihydroxy-6,7,3′,4′-tetramethoxyflavone 8-O-β-glucopyranoside (333), were isolated from the leaves and shoots of M. paniculata [79]. Furthermore, Zhou et al. [82] found the presence of ten flavonoid glycosides, encompassing kaempferol-3-O-rutinoside (353), kaempferide-3-O-β-D-glucopyranoside (354), kaempferol-3-O-β-D-glucopyranoside (355), hesperitin-7-O-β-D-glucopyranoside (356), neohesperidin (357), hesperidin (358), naringenin-7-O-β-D-glucopyranoside (359), naringin (360), rutin (361), and isoquercitrin (362) in the leaves and stem bark of M. tetramera [82] (Figure 7).
4.5. Terpenoids and Steroids
Terpenoids and steroids were the smallest isolated secondary metabolite group from the Murraya genus (Figure 8). At present, one terpenoid, namely friedelin (363) had been identified from the leaves of M. euchrestifolia [19,24]. Furthermore, steroids were rarely isolated from the Murraya genus, with seven compounds being identified in this review. Wu et al. [19] identified sitosterol (364) from M. euchrestifolia, and other phytosterols were isolated from the leaves of M. exotica [83]. These phytosterols included (23S)-23-ethyl-24-methyl-cycloart-24(24′)-en-3β-ol (365), 3β-methoxy-(23S)-23-ethyl-24-methyl-cycloart-24(24′)-en-3β -ol (366), (23S)-23-ethyl-24-methyl-cycloart-24(24′)-3β-yl-acetate (367), (23ξ)-23-isopropyl-24-methyl-cycloart-25en-3β-ol (368), and (23ξ)-23-isopropyl-24-methyl-cycloart-25-en-3β-yl-acetate (369). A previous study reported the presence of stigmasterol (370) in the roots of M. gleinei [58] and the stem bark of M koenigii [28] (Figure 8).
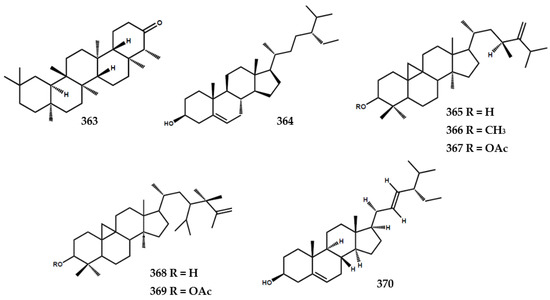
Figure 8.
Structures of terpenoids and steroids from the Murraya genus 363–370.
4.6. Other Compounds
A total of 43 compounds were identified and characterized as derivatives of alkylated and aromatic secondary metabolites (Table 4). A cyclic carotene, namely β-carotene (371), was isolated from the leaves of M. euchrestifolia along with ρ-hydroquinone (372) [24]. Furthermore, Barik et al. [65] discovered a new cinnamic acid derivate, namely marraxonin (373), from the leaves of M. exotica.

Table 4.
Other compounds from the Murraya genus.
A total of four new phenylpropanoid derivatives were obtained from M. koenigii and identified as (7′E,8S)-9-hydroxy-7′-propen-3′-5′-dimethoxyphenyl-3-methoxyphenyl-7,9-propane-diol-4-O-β-D-glucopyranoside (374), (7R)-2,6-dimethoxyphenyl-7,9-propane-diol-1-O-β-D-glucopyranoside (375), (2′R,4′R,7S)-2′,4-dihydroxy-3-methoxyphenyl-4′-hydromethyl-tetrahydro-1H-pyran-1-one (376), and (1R,10S)-1-hydroxy-7-(10-hydroxy-butyl)-2,3-dihydrobenzofuran-8(6H)-one (377) [84]. Furthermore, phenylpropanoid derivative types encompassing (1′R)-4-O-β-D-glucopyranoside-3,5-dimethoxyphenyl-1′-propanol (378), citrusin B (379), (7′R,8′R,8E)-4′,7′-dihydroxy-3′-methoxyphenyl-8′-hydroxymethyl-ethoxy-3,5-dimethoxyphenyl-8-propenoic acid methylester (380), (7S,8S)-1′-hydroxy-3′,5′-dimethoxyphenoxy-4-hydroxy-3-methoxyphenyl-7,9-propanediol (381), (7′E,7S,8S)-9′-hydroxy-7′-propen-3′-methoxy-phenyl-4-hydroxy-3-methoxyphenyl-7,9-propanediol (382), (1S,2R)-4,4′-hydroxy-3,3′-methoxyphenyl-1,3-propanediol (383), (7S, 8R)-4,4′-dihydroxy-3,3′-dimethoxyphenyl-7-ethoxy-9-propanol (384), (7S,8R)-4-hydroxy-3-methoxyphenyl-7,8,9-propanetriol (385), (7S,8R)-4-hydroxy-3,5-dimethoxy-phenyl-7,8,9-propanetriol (386), lariciresinol-4-O-β-D-glucopyranoside (387), and (7S,7′S, 8S,8′R)-4,4″,7′,9′-tetrahydroxy-3,3′,3″-trimethoxy-phenyl-7,9-propanediol (388) were also isolated from the species [84].
Ma et al. [6] reported the presence of new alkene types in M. koenigii, including (3S,4E,6E,10R)-2,10-dihydroxy-2-hydroxy-2-methylethyl-6,10-di-methyl-4,6,11-sencolaninic-3-β-D-glucopyranoside (389), (3R,5S,6E,8S,10E)-3,7,11-trimethyl-1,6,10-dodecatriene-3,5,8-triol (390), (5S,6R,7S,8R)-5-amino-(2Z,4Z)-1,2,3-trihydroxybuta-2,4-dienyl-oxy-pentane-6,7,8,9-tetraol (391), and (3E,6S,7E,9R,10S,11S,17R)-octadeca-3,7-diene-6,9, 10,11,17-penta-ol (392) (Figure 9).
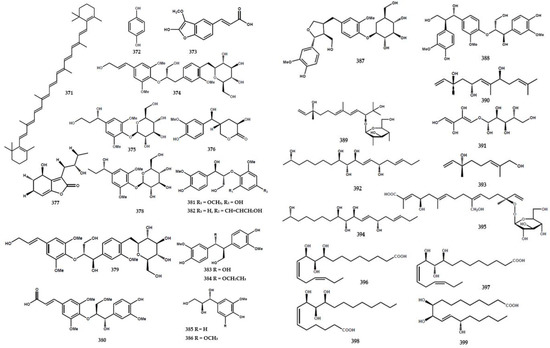
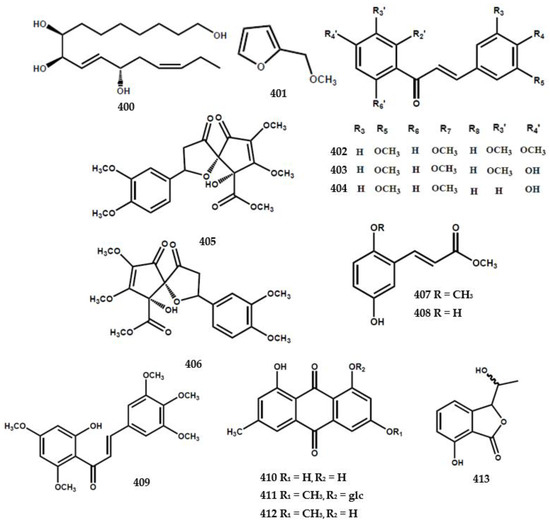
Figure 9.
Structures of other compounds from the Murraya genus 371–413.
5. Biological Activities
Murraya plant parts have long been used in several regions as traditional medicines to treat dysentery, fever, and dizziness. Several studies have also shown that the extracts and compounds obtained from the genus exhibited various bioactivities, including cytotoxicity, anti-inflammatory, antidiarrheal, antihyperlipidemic, and antioxidant properties (Table 5). The active compounds have potential for medicine purposes.
5.1. Cytotoxicity Properties
Ma et al. [2] reported that mahanine (26) showed significant cytotoxicity against four cell lines and PCK2 protein, with SPR (surface plasmon resonance) being identified as the possible mechanism. Furthermore, the potential binding sites were disclosed as Phe 525, Arg 436, Phe 530, Asn 533, and Gly 289. Changes in nuclear morphology, DNA breakage, activation-like activities, cleavage of poly(ADP-ribose) polymerase, release of cytochrome C into the cytoplasm, and stimulation of reactive oxygen species formation were observed to be signs of mahanine-induced cell death. Mahanine triggered the caspase-3, 6, 8, and 9 activities, but did not affect caspase-1-like activity [85]. Koenimbine (58) from M. koenigii showed the most potent inhibitory activity against B16 melanoma 4A5. Similar activity was also reported in Mahanimbine (64) and 2 other compounds (17 and 52) [31].
A previous study stated that three compounds from M. Koenigii, including pyrayafoline D, induced apoptotic cell death in HL-60 cell lines at a concentration of 30 µM. The apoptotic effect of these compounds was observed to be mediated by the loss of mitochondrial membrane potential and the subsequent activation of caspase-9/caspase-3 [32]. Furthermore, CHCl3 extract and koenoline (80) from M. koenigii exhibited cytotoxic activity with an ED50 range of 4.0 µg/mL to 26 µg/mL [38].
The primary screening results showed that compounds derived from M. siamensis had inhibitory activity. All test coumarins obtained through isolation showed a potent dose-dependent inhibitory effect on EBV-EA induction via TPA. Murrayacoumarin A (304) bearing an oxygenated geranyloxy side chain exhibited the most potent activity [74].
Ma et al. [27] reported several compounds from M. koenigii, including mahanine (26), mahanimbine (16), and 8,8′-biskoenigine (71), that showed significant PTP1B inhibitory activity with IC50 values of 1.773, 1.875, and 2.286 µM, respectively.

Table 5.
Biological activities from the Murraya genus.
Table 5.
Biological activities from the Murraya genus.
| Biological Activities | Cell Target/Process | Compounds or Extract [IC50/CD50] | Plant Species | References |
|---|---|---|---|---|
| Cytotoxic | Du145, HepG2, HeLa, and HCT-116 cell lines | murrayamine A (24) [0.3 ± 0.4 µM; 3.4 ± 0.3 µM; 0.4 ± 1.7 µM; 0.2 ± 0.4 µM]; mahanine (26) [2.2 ± 0.1 µM; 3.5 ± 0.9 µM; 0.02 ± 0.01 µM; 0.03 ± 0.08 µM]; | M. microphylla | [2] |
| HepG2, Du145, HeLa, and HCT116 cell | murrayamine A (24) [21.4 ± 3.1 µM; 19.7 ± 1.1 µM; 25.9 ± 3.7 µM; 20.0 ± 2.3 µM]; mahanine (26) [48.3 ± 3.4 µM; 46.9 ± 2.5 µM; 46.5 ± 0.2 µM; 44.8 ± 3.2 µM]; | M. microphylla | [29] | |
| inhibited melanogenesis B16 melanoma 4A5 | murrayamine-E (17) [2.9 µM]; mahanimbicine (52) [2.2 µM]; koenimbine (58) [1.2 µM]; mahanimbine (64) [1.4 µM]; | M. koenigii | [31] | |
| induced apoptosis in HL-60 cells through activation of the caspase-9/caspase-3 pathway | pyrayafoline D (54); murrafoline I (90), | M. koenigii | [32] | |
| HepG2 cells | (−)-bispyrayafoline C (114); (+)-bispyrayafoline C (115); kwangsine D (122); kwangsine E (123); kwangsine G (125); kwangsine H (126); kwangsine J (128); kwangsine K (129); kwangsine L (130); kwangsine M (131); euchrestine C (135); 1-hydroxy-3-methyl-9H-carbazole (137); murrayafoline A (146) [Range 9.9–44.3 µM] | M. kwangsiensis | [3] | |
| HL-60 and HeLa | murrastinine-C (87) [17 µg/mL and 1 µg/mL]; murrayatanine-A (88) [12 µg/mL and 5 µg/mL]; | M. koenigii | [39] | |
| KB cell culture | CHCl3 extract; koenoline (80); murrayanine (98) | M. koenigii | [38] | |
| bearing an oxygenated geranyloxy side-chain exhibited the most potent activity | murrayacoumarin A (304) | M. siamensis | [74] | |
| PTB1B inhibitory | mahanine (26), mahanimbine (16), and 8,8′-biskoenigine (71) | M. koenigii | [27] | |
| Anti-inflammatory | inhibitory activities against NO production | 3-formylcarbazole (35) [78.2 ± 2.6 µM]; O-demethylmurrayanine (83) [79.2 ± 2.1 µM]; murrayanine (98) [12.2 ± 0.2 µM]; 1-methoxy-3-(methoxymethyl)-carbazole (140) [65.1 ± 1.7 µM]; | M. kwangsiensis | [3] |
| potent inhibition against LPS-induced NO production in BV-2 microglial cells | panitin D (283) [19.6 ± 0.3 µM];; exotimarin I (287) [26.9 ± 0.8 µM]; trans-dehydroosthol (290) [12.4 ± 0.9 µM]; | M. paniculata | [57] | |
| inhibitory effects on LPS-induced NO production in BV-2 microglial cells | 2′-O-ethylmurrangatin (204) [53.2 ± 8.9 µM]; panitin C (231) [57.7 ± 5.8 µM]; exotimarin H (232) [53.2 ± 4.4 µM]; | M. paniculata | [59] | |
| inhibition of NO production | murratin D (219) [39.0 ± 4.3 µM]; muratin E (220) [36.8 ± 3.4 µM]; muralatin C (229) [32.7 ± 3.0 µM]; 2-(7-methoxy-2-ocochromen-8-yl)-3-methylbut-2-enyl)-3-methylbut-2-enoate (230) [38.1 ± 3.0 µM]; exotimarin H (232) [28.6 ± 0.9 µM]; | M. exotica | [56] | |
| inhibitions against LPS-induced NO production in RAW264.7 macrophages | (2′R,4′R,7S)-2′,4-dihydroxy-3-methoxyphenyl-4′-hydroxymethyl-tetrahydro-1H-pyran-1-one (376) [32.7 µM]; (1R,10S)-1-hydroxy-7-(10-hydroxybutyl)-2,3-dihydrobenzofuran-8(6H)-one (377) [7.9 µM]; (7′E,7S,8S)-9′-hydroxy-7′-propen-3′-methoxyphenyl-4-hydroxy-3-methoxy-phenyl-7,9-propanediol (382) [42.1 µM]; (7S,8R)-4,4′-dihydroxy-3,3′dimethoxy-phenyl-7-ethoxy-9-propanol (384) [58.9 µM]; lariciresinol-4-O-β-D-glucopyranoside (387) [62.4 µM]; | M. koenigii | [84] | |
| Hepatoprotective and Antihyperlipidemic | against D-galactosamine induced HL-7702 cells damage (hepatoprotective) the activations of PPARα and PPRγ (Antihyper-lidemic) | (1′R,3′R,4′R,6′S)-endocycliomurrayamine A (31); claulansiums A (42); 1′-O-methyl-claulamine B (44); dunnine E (45) 3-formyle-7-hydroxy-9H-carbazole-1-O-β -D-glucopyranoside (32); 4′-hydroxy-phenyl-6ethyl-1H-pyrrole-2-carbax-aldehyde (33); pyrolezanthine (37); portulacatone (41) | M. koenigii | [4] |
| inhibited nitric oxide production in BV-2 microglial cells stimulated with lipopolysaccharide | murradiate (192) and murradiol (193) | M. tetramera | [54] | |
| Antidiarrheal | inhibitory activity against castor-oil-induced diarrhea and PGE2-induced enteropooling in rats | kurryam (57); koenimbine (58) | M. koenigii | [5] |
| Antioxidant | antioxidative activities DPPH method | (3S,4E,6E,10R)-2,10-dihydroxy-2-hydroxy-2-methylethyl-6,10-di-methyl-4,6,11-sencolaninic-3-β-D-gluco-pyranoside (389) [38.4 µM]; (3R,5S,6E,8S,10E)-3,7,11-trimethyl-1,6,10-dodecatriene-3,5,8-triol (390) [23.5 µM]; (3E,6S,7E,9R,10S,11S,17R)-octadeca-3,7-diene-6,9,10,11,17-pentaol (392) [25.4 µM]; capsianoside V (395) [40.2 µM]; | M. koenigii | [6] |
5.2. Anti-Inflammatory Properties
Studies on the biological activities of plants from the Murraya genus identified the presence of anti-inflammatory activity. Furthermore, Murrayanine (98) from M. kwangsiensis showed significant inhibition of NO production in lipopolysaccharide-stimulated BV-2 microglial cells compared to a positive control [3]. Another study reported that three compounds from M. paniculata, including Panitin D (283), exotimarin I (287), and trans-dehydroosthol (290), showed moderate inhibitory effects on LPS-induced NO production in BV-2 microglial cells [57].
5.3. Hepatoprotective and Antihyperlipidemic Properties
Hepatoprotective properties refer to the ability of a substance to prevent damage to the liver. Previous studies reported that CHCL3 extract from M. koenigii exhibited potential hepatoprotective properties. Furthermore, four compounds, encompassing (1′R,3′R,4′R,6′S)-endocycliomurrayamine A (31), claulansiums A (42), 1′-O-methylclaulamine B (44), and dunnine E (45), showed moderate activity against D-galactosamine-induced toxicity in HL 7720. Antihyperlipidemic agents were substances known to promote the reduction of lipid and cholesterol levels. Several studies showed that compounds from Murraya exhibited moderate activity [4].
5.4. Antidiarrheal Properties
Mandal et al. [5] reported that kurryam (57) and koenimbine (58) exhibited significant inhibitory activity against castor-oil-induced diarrhea and PGE2-induced enteropooling in rats. Furthermore, a dose of 30 mg/kg had an equivalent effect to 5 mg/kg of the standard drug.
5.5. Antioxidant Properties
Previous studies on the bioactivity of the Murraya genus showed potent antioxidant activity. According to a previous report, (3S,4E,6E,10R)-2,10-dihydroxy-2-hydroxy-2-methylethyl-6,10-di-methyl-4,6,11-sencolaninic-3-β-D-glucopyranoside (389), (3R,5S,6E, 8S,10E)-3,7,11-tri-methyl-1,6,10-dodeca-triene-3,5,8-triol (390), (3E,6S,7E,9R,10S,11S,17R)-octadeca-3,7-diene-6,9,10,11,17-pentaol (392), and capsianoside V (395) from M. koenigii showed the potent inhibition of DPPH with an IC50 range of 21.4–49.5 µM [6].
6. Conclusions
In conclusion, Murraya species have been extensively studied, thereby contributing to the understanding of secondary metabolites and their biological activities in nature. Furthermore, alkaloids were observed to be the dominant compounds from Murraya, followed by coumarins and flavonoids. The literature reports showed that the genus exhibited various biological activities, such as cytotoxic and anti-inflammatory effects.
Author Contributions
Conceptualization, R.Y.; methodology, R.Y.; validation, R.Y., D.H. and U.S.; formal analysis, R.Y. and T.R.; resources, R.Y.; data curation, R.Y.; writing—original draft preparation, R.Y.; writing—review and editing, R.Y., U.S. and D.H.; visualization, R.Y. and D.H.; supervision, U.S., D.H. and S.F.; project administration, U.S. and D.H.; funding acquisition, U.S. and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Indonesian Ministry of Research, Technology and Higher Education for Grant of Penelitian Tesis Magister (PTM) 2022, 1318/UN6.3.1/PT.00/2022; 12 May 2022, for D.H.; and the Hibah Riset Unpad (HRU) 2023, for Article Review Grant for D.H., 2023, 1549/UN6.3.1/PT.00/2023. The APC was funded by Universitas Padjadjaran.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The study did not report any data.
Acknowledgments
The authors are grateful to the Indonesian Ministry of Research, Technology and Higher Education for Grant of Penelitian Tesis Magister (PTM), Indonesia, and to the Universitas Padjadjaran for Article Review Grant and supporting with study facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilson, A. Flora of Australia Volume 26 Meliaceae, Rutaceae, Zygophyllaceae; ABRS Canberra/CSIRO Publishing: Melbourne, Australia, 2013; pp. 501–503. [Google Scholar]
- Ma, X.L.; Zhu, S.S.; Liu, Y.; Chen, H.W.; Shi, Y.T.; Zeng, K.W.; Tu, P.F. Carbazole alkaloids with potential cytotoxic activities targeted on PCK2 protein from Murraya microphylla. Bioorganic Chem. 2021, 114, 105–113. [Google Scholar]
- Chen, Y.; Cao, N.; Lv, H.; Zeng, K.; Yuan, J.; Guo, X.; Zhao, M.; Tu, P.; Jiang, Y. Anti-inflammatory and cytotoxic carbazole alkaloids from Murraya kwangsiensis. Phytochemistry 2020, 170, 112186. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ma, Q.; Zhong, G.; Su, Y.; Yang, J.; Wang, A.; Ji, T.; Guo, H.; Wang, M.; Jiang, P.; et al. Structural characterization, hepatoprotective and antihyperlipidemic activities of alkaloid derivatives from Murraya koenigii. Phytochem. Lett. 2020, 35, 135–140. [Google Scholar] [CrossRef]
- Mandal, S.; Nayak, A.; Kar, M.; Banerjee, S.K.; Das, A.; Upadhyay, S.N.; Singh, R.K.; Banerji, A.; Banerji, J. Antidiarrhoeal activity of carbazole alkaloids from Murraya koenigii Spreng (Rutaceae) seeds. Fitoterapia 2010, 81, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.G.; Xu, K.; Sang, Z.P.; Wei, R.R.; Liu, W.M.; Su, Y.L.; Yang, J.B.; Wang, A.G.; Ji, T.F.; Li, L.J. Alkenes with antioxidative activities from Murraya koenigii (L.) Spreng. Bioorganic Med. Chem. Lett. 2016, 26, 799–803. [Google Scholar] [CrossRef]
- Chakraborty, D.P.; Barman, B.K.; Bose, P.K. On the constitution of murrayanine, A carbazole derivative isolated from Murraya koenigii Spreng. Tetrahedron 1965, 21, 681–685. [Google Scholar]
- Kong, Y.C.; Cheng, K.F.; Cambie, R.C.; Watermand, P.G. Yuehchukene: A Novel lndole Alkaloid with Anti-implantation Activity. J. Chem. Soc. Chem. Commun. 1985, 47–48. [Google Scholar] [CrossRef]
- Mengist, W.; Soromessa, T.; Legese, G. Method for conducting systematic literature review and meta-analysis for environmental science research. MethodsX 2020, 7, 100777. [Google Scholar] [CrossRef]
- Graf, A.B. Tropica, Color Cyclopedia of Exotic Plants and Trees, 3rd ed.; Roehrs Company: Farmingdale, NJ, USA, 1986. [Google Scholar]
- Shenoy, A.; Buttar, H.S.; Dicholkar, P.D.; Kaur, G.; Chintamaneni, M. Role of nutraceuticals functional foods, and spices in the management of metabolic syndrome and related disorders. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Isaza, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Integrated Taxonomy Information System-Report. Available online: itis.gov (accessed on 3 July 2023).
- Missouri Botanical Garden. Available online: missouribotanicalgarden.org/PlantFinder (accessed on 3 July 2023).
- Liaqat, I.; Riaz, N.; Saleem, Q.A.; Tahir, H.M.; Arshad, M.; Arshad, N. Toxicological Evaluation of Essential Oils from Some Plants of Rutaceae Family. eCAM 2018, 2018, 4394687. [Google Scholar]
- Photo by Nicole-George Nedelcu On Unsplash. Available online: unsplash.com/s/photos/murraya (accessed on 22 July 2023).
- Photo by Kavindu Maleesha on Unsplash. Available online: unsplash.com/s/photos/murraya (accessed on 22 July 2023).
- You, C.X.; Guo, S.S.; Geng, Z.F.; Zhang, W.J.; Liang, J.Y.; Zhang, Z.; Wang, C.F.; Du, S.S.; Deng, Z.W. Repellent activity of compounds from Murraya alata Drake against Tribolium castaneum. Ind. Crop. Prod. 2017, 95, 460–466. [Google Scholar] [CrossRef]
- Wu, T.S.; Wang, M.L.; Wu, P.L. Seasonal variations of carbazole alkaloids in Murraya euchrestifolia. Phytochemistry 1996, 43, 785–789. [Google Scholar]
- Wu, T.S.; Wang, M.L.; Wu, P.L.; Ito, C.; Furukawa, H. Carbazole alkaloids from the leaves of Murraya euchrestifolia. Phytochemistry 1996, 41, 1433–1435. [Google Scholar] [CrossRef]
- Wu, T.S.; Wang, M.L.; Wu, P.L. Murrayamine-O and -P, two cannabinol skeletal carbazole alkaloids from Murraya euchrestifolia. Tehtrahedron Lett. 1995, 30, 5385–5388. [Google Scholar]
- Wu, T.S.; Wang, M.L.; Lai, J.S.; Ito, C.; Furukawa, H. Binary carbazole alkaloids from Murraya euchrestifolia. Phytochemistry 1991, 30, 1052–1054. [Google Scholar]
- Mcphail, A.T.; Cross, P.M.; Wu, T.S.; Ohta, T.; Furukawa, H. Structure of murrafoline, a novel biscarbazole. Tetrahedron Lett. 1983, 24, 5377–5380. [Google Scholar] [CrossRef]
- Kumar, V.; Wickramaratne, D.B.M.; Jacobsson, U. Indole dimer from Murraya gleniei root. Tetrahedron Lett. 1990, 31, 5217–5218. [Google Scholar] [CrossRef]
- Wu, T.S. Murrayamine-A, -B, -C and (+)-mahanine, carbazole alkaloids from Murraya euchrestifolia. Phytochemistry 1991, 30, 1048–1051. [Google Scholar]
- Ito, C.; Kanbara, H.; Wu, T.S.; Furukawa, H. Murrayamine C from Murraya euchrestifolia. Phytochemistry 1992, 31, 1083–1084. [Google Scholar] [CrossRef]
- Nalli, Y.; Khajuria, V.; Gupta, S.; Arora, P.; Hassan, S.R.U.; Ahmed, Z.; Ali, A. Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities. Org. Biomol. Chem. 2016, 14, 3322–3332. [Google Scholar] [CrossRef]
- Ma, Q.; Tian, J.; Yang, J.; Wang, A.; Ji, T.; Wang, Y.; Su, Y. Bioactive carbazole alkaloids from Murraya koenigii (L.) Spreng. Fitoterapia 2013, 87, 1–6. [Google Scholar] [CrossRef]
- Rahman, M.M.; Gray, A.I. A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity. Phytochemistry 2005, 66, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cao, N.; Zhang, C.; Guo, X.; Zhao, M.; Tu, P.; Jiang, Y. Cytotoxic carbazole alkaloid derivatives from the leaves and stems of Murraya microphylla. Fitoterapia 2018, 127, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Reisch, J.; Goj, O.; Wickramasighe, A.; Herath, H.M.T.B.; Henkel, G. Carbazole alkaloids from seeds of Murraya koenigii. Phytochemistry 1992, 31, 2877–2879. [Google Scholar] [CrossRef]
- Nakamura, S.; Nakashima, S.; Oda, Y.; Yakota, N.; Fujimoto, K.; Matsumoto, T.; Ohta, T.; Ogawa, K.; Maeda, S.; Nishida, S.; et al. Alkaloids from Sri Lankan curry-leaf (Murraya koenigii) display melanogenesis inhibitory activity: Structures of karapinchamines A and B. Bioorganic Med. Chem. 2013, 21, 1043–1049. [Google Scholar] [CrossRef]
- Ito, C.; Itogawa, M.; Nakao, K.; Murata, T.; Tsuboi, M.; Kaneda, N.; Furukawa, H. Induction of apoptosis by carbazole alkaloids isolated from Murraya koenigii. Phytomedicine 2006, 13, 359–365. [Google Scholar] [CrossRef]
- Uvarani, C.; Jaivel, N.; Sankaran, M.; Chandraprakash, K.; Ata, A.; Mohan, P.S. Fitoterapia Axially chiral biscarbazoles and biological evaluation of the constituents from Murraya koenigii. Fitoterapia 2014, 94, 10–20. [Google Scholar] [CrossRef]
- Ganguly, S.N.; Sarkar, A. Exozoline, a new carbazole alkaloid from the leaves of Murraya exotica. Phytochemistry 1978, 17, 1816–1817. [Google Scholar] [CrossRef]
- Wickramaratne, D.B.M.; Kumar, V.; Balasubramaniam, S. Murragleinin, a coumarin frim Murraya gleinei leaves. Phytochemistry 1984, 23, 2964–2966. [Google Scholar] [CrossRef]
- Naz, S.; Saied, S.; Ahmed, A.; Muhammad, S. Three new carbazole alkaloids and biological activities of Murraya koenigii. J. Asian Nat. Prod. Res. 2015, 17, 7–13. [Google Scholar] [CrossRef]
- Chakraborty, M.; Saha, S.; Mukhapadhyay, S. Murrayakoeninol—A New Carbazole Alkaloid from Murraya koenigii (Linn) Spreng. Nat. Prod. Commun. 2009, 4, 355–358. [Google Scholar] [CrossRef]
- Fiebig, M.; Pezzuto, J.M.; Soejarto, D.D.; Kinghorn, A.D. Koenoline, a further cytotoxic carbazole alkaloid from Murraya koenigii. Phytochemistry 1985, 24, 3041–3043. [Google Scholar] [CrossRef]
- Tan, S.; Ali, A.M.; Nafiah, M.A.; Awang, K.; Ahmad, K. Isolation and cytotoxic investigation of new carbazole alkaloids from Murraya koenigii (Linn.) Spreng. Tetrahedron 2015, 71, 3946–3953. [Google Scholar] [CrossRef]
- Saha, C.; Chowdhury, B.K. Carbazoloquinones from Murraya koenigii. Phytochemistry 1998, 48, 363–366. [Google Scholar] [CrossRef]
- Ito, C.; Nakagawa, M.; Wu, T.S.; Furukawa, H. New Carbazole Alkaloids from Murraya euchrestifolia. Chem. Pharm. Bull. 1991, 39, 2525–2528. [Google Scholar] [CrossRef]
- Nutan, M.T.H.; Hasan, C.M.; Rashid, M.A.U. Bismurrayafoline E: A new dimeric carbazole alkaloid from Murraya koenigii. Fitoterapia 1999, 70, 130–133. [Google Scholar] [CrossRef]
- Chakrabarty, M.; Nath, A.C.; Khasnobis, S.; Chakrabarty, M.; Konda, Y.; Harigaya, Y.; Komiyama, K. Carbazole alkaloids from Murraya koenigi. Phytochemistry 1997, 46, 751–755. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Chakraborty, A. Mukonal, a probable biogenetic intermediate of pyranocarbazole alkaloids from murraya. Phytochemistry 1982, 23, 471–472. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mukherjee, S.; Shaw, A.K.; Ganguly, S.N. Mukonicine, a carbazole alkaloid from leaves of Murraya koenigii. Phytochemistry 1983, 22, 2328–2329. [Google Scholar] [CrossRef]
- Bhattacharya, L.; Roy, S.K.; Chakraborty, D.P. Structure of the carbazole alkaloid isomurrayazoline from Murraya koenigii. Phytochemistry 1982, 21, 2432–2433. [Google Scholar] [CrossRef]
- Biswas, A.K. Structure and synthesis of mukonine, a new carbazole alkaloid from Murraya koenigii. Phytochemistry 1978, 17, 834–835. [Google Scholar]
- Wu, T.S.; Liou, M.; Jong, T.T.; Chen, Y.J.; Lai, J.S. Indole alkaloids and coumarins from the root bark of Murraya paniculata var. omphalocarpa. Phytochemistry 1989, 28, 2873–2874. [Google Scholar]
- Chowdhury, B.K.; Chakraborty, D.P. 3-formylindole from Murraya exotica. Phytochemistry 1971, 10, 481–483. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, K.; Zhao, M.; Tu, P.; Li, J.; Jiang, Y. Three new indole alkaloid derivatives from the roots of Murraya paniculata. J. Asian Nat. Prod. Res. 2017, 20, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Tatara, S.; Ho, F.C.; Sankawa, U. 3-prenylidoles from Murraya paniculata and their biogenetic significance. Phytochemistry 1989, 28, 147–151. [Google Scholar] [CrossRef]
- Barros, F.A.P.; Filho, E.R. Four spiroquinazoline alkaloids from Eupenicillium sp. isolated as an endophytic fungus from leaves of Murraya paniculata (Rutaceae). Biochem. Syst. Ecol. 2005, 33, 257–268. [Google Scholar] [CrossRef]
- Wu, T.S.; Chan, Y.Y.; Leu, Y.L.; Huang, S.C. A flavonoid and indole alkaloid from flowers of Murraya paniculata. Phytochemistry 1994, 37, 287–288. [Google Scholar] [CrossRef]
- Lv, H.; Zhou, Y.; Wen, R.; Shi, M.; Zeng, K.; Xia, F. Murradiate and murradiol, two structurally unique heterodimers of carbazole-monoterpene and carbazole-phenylethanol from Murraya tetramera. Phytochem. Lett. 2016, 15, 113–115. [Google Scholar] [CrossRef]
- Lyu, H.; Wei, N.; Tu, P.; Wang, K.; Jiang, Y. A new coumarin from Murraya alata activates TRPV1 channel. Nat. Prod. Res. 2019, 34, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Shi, Y.; Zeng, K.; Zhao, M.; Tu, P.; Jiang, Y. Coumarin derivatives from the leaves and twigs of Murraya exotica L. and their antiinflammatory activities. Phytochemistry 2020, 177, 112416. [Google Scholar] [CrossRef]
- Wang, X.; Liang, H.; Zeng, K.; Zhao, M.; Tu, P.; Li, J.; Jiang, Y. Panitins A-G: Coumarin derivatives from Murraya paniculata from Guangxi Province, China show variable NO inhibitory activity. Phytochemistry 2019, 162, 224–231. [Google Scholar]
- Kumar, V.; Reisch, J.; Wicremaratne, D.B.M.; Hussain, R.A.; Adesina, K.S.; Balasubramaniam, S. Gleinene and gleinadiene, 5-7-dimethoxycoumarins from Murraya gleinei root. Phytochemistry 1987, 26, 511–514. [Google Scholar] [CrossRef]
- Liang, H.; Cao, N.; Zeng, K.; Zhao, M.; Tu, P.; Jiang, Y. Coumarin and spirocyclopentenone derivatives from the leaves and stems of Murraya paniculata (L.) Jack. Phytochemistry 2020, 172, 112258. [Google Scholar] [CrossRef]
- Talapatra, S.K.; Dutta, L.N.; Talapatra, B. Structure of murralongin, a novel monomeric coumarin from Murraya elongata. Tehtrahedron Lett. 1973, 50, 5005–5008. [Google Scholar] [CrossRef]
- Kinoshita, T.; Wu, J.B.; Ho, F.C. The isolation of a prenylcoumarin of chemotaxonomic significance from Murraya paniculata var. omphalocarpa. Phytochemistry 1996, 43, 125–128. [Google Scholar] [CrossRef]
- Talapatra, S.K.; Dutta, L.N.; Talapatra, B. The structure and stereochemistry of murrangatin. Tetrahedron 1973, 29, 28–31. [Google Scholar] [CrossRef]
- Barik, B.R.; Dey, A.K.; Das, P.C.; Chatterjee, A.; Shoolery, J.N. Coumarins of murraya exotica-absolute configuration of auraptenol. Phytochemistry 1983, 22, 792–794. [Google Scholar] [CrossRef]
- Liu, B.Y.; Zhang, C.; Zeng, K.W.; Li, J.; Guo, X.Y.; Zhao, M.B.; Tu, P.F.; Jiang, Y. Exotines A and B, Two Heterodimers of Isopentenyl-Substituted Indole and Coumarin Derivatives from murraya exotica. Org. Lett. 2015, 17, 4380–4383. [Google Scholar] [CrossRef] [PubMed]
- Barik, B.R.; Kundu, A.B. A cinnamic acid derivative and a coumarin from Murraya exotica. Phytochemistry 1987, 26, 3319–3321. [Google Scholar] [CrossRef]
- Barik, B.R.; Dey, A.K.; Chatterjee, A. Murrayatin, a coumarin from Murraya exotica. Phytochemistry 1983, 22, 2273–2275. [Google Scholar] [CrossRef]
- Rahman, A.; Shabbir, M.; Sultani, S.Z.; Jabbar, A.; Choudhary, M.I. Cinnamates and coumarins from the leaves of Murraya paniculata. Phytochemistry 1997, 44, 683–685. [Google Scholar] [CrossRef]
- Chakraborty, D.P.; Roy, S.; Chakraborty, A.; Mandal, A.K.; Chowdhury, B.K. Structure and synthesis of mexolide: A new antibiotic dicoumarin from Murraya exotica Linn. Tetrahedron 1980, 36, 3563–3564. [Google Scholar] [CrossRef]
- Wu, T.S.; Tien, H.J.; Arisawa, M.; Shimizu, M.; Morita, N. Flavonols and Coumarins from the fruit of Murraya omphalocarpa. Phytochemistry 1980, 19, 2227–2228. [Google Scholar] [CrossRef]
- Wu, T.S.; Liou, M.J.; Kuoh, C.S. Coumarins of the flowers of Murraya paniculata. Phytochemistry 1989, 28, 293–294. [Google Scholar] [CrossRef]
- Ramstad, E.; Lin, W.C.; Lin, T.; Koo, W. Coumurrayin, a new coumarin from Murraya paniculata (L.) jack. Tetrahedron Lett. 1968, 6, 811–813. [Google Scholar] [CrossRef]
- Adebajo, A.C. Reisch, J. Minor furocoumarins of Murraya koenigii. Fitoterapia 2000, 71, 334–337. [Google Scholar] [CrossRef]
- Wu, T.S. Coumarins from the leaves of Murraya paniculata. Phytochemistry 1988, 27, 2357–2358. [Google Scholar] [CrossRef]
- Ito, C.; Itogawa, M.; Onada, S.; Hosokawa, A.; Ruangrungsi, N.; Okuda, T.; Tokuda, H.; Nishino, H.; Furukawa, H. Chemical constituents of Murraya siamensis: Three coumarins and their anti-tumor promoting effect. Phytochemistry 2005, 66, 567–572. [Google Scholar] [CrossRef]
- Joshi, B.S.; Kamat, V.N. Isolation of 3,3’,4’,5,5’,7,8-heptamethoxyflavone from Murraya exotica. Phytochemistry 1970, 9, 889. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, M.; Tu, P.; Jiang, Y. Polymethoxylated flavonoids from Murraya paniculata (L.) Jack. Biochem. Syst. Ecol. 2020, 93, 104162. [Google Scholar] [CrossRef]
- Ferracin, R.J.; das, G.F. da Silva, M.F.; Fernandes, J.B.; Vieira, P.C. Flavonoids from the fruits of Murraya paniculata. Phytochemistry 1988, 41, 393–396. [Google Scholar]
- Silva, L.B.D.E.; de Silva, U.L.L.; Mahendran, M.; Jennings, R.C. 4′-hydroxy-3,5,6,7,3′,5′-hexamethoxyflavone from Murraya paniculata. Phytochemistry 1980, 19, 9422. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Shi, S.; Zan, K.; Tu, P. Glycosides of flavone methyl ethers from Murraya paniculata. Biochem. Syst. Ecol. 2012, 43, 10–13. [Google Scholar] [CrossRef]
- Kinoshita, T.; Firman, K. Myricetin 5,7,3′,4′,5′-pentamethyl ether and other methylated flavonoids from Murraya paniculata. Phytochemistry 1997, 45, 179–181. [Google Scholar] [CrossRef]
- Kinoshita, T.; Firman, K. Highly oxygenated flavonoids from murraya paniculata. Phytochemistry 1996, 42, 1207–1210. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, H.; Wang, W.; Tu, P.; Jiang, Y. Flavonoids and anthraquinones from Murraya tetramera. Biochem. Syst. Ecol. 2014, 57, 78–80. [Google Scholar] [CrossRef]
- Desoky, E.K. Phytosterols from Murraya exotica. Phytochemistry 1995, 40, 1769–1772. [Google Scholar] [CrossRef]
- Ma, Q.; Wei, R.; Yang, M.; Huang, X.; Zhong, G.; Sang, Z.; Dong, J.; Shu, J.; Liu, J.; Zhang, R.; et al. Structures and biological evaluation of phenylpropanoid derivatives from Murraya koenigii. Bioorganic Chem. 2019, 86, 159–165. [Google Scholar] [CrossRef]
- Roy, M.K.; Thalang, V.N.; Trakoontivakorn, G.; Nakahara, K. Mechanism of mahanine-induced apoptosis in human leukemia. Biochem. Pharmacol. 2004, 67, 41–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).