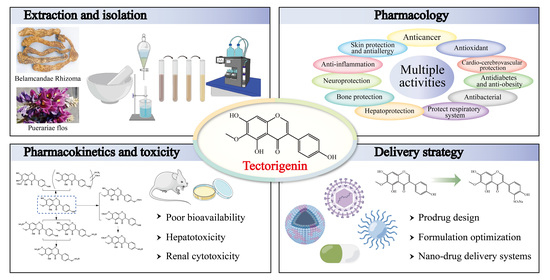
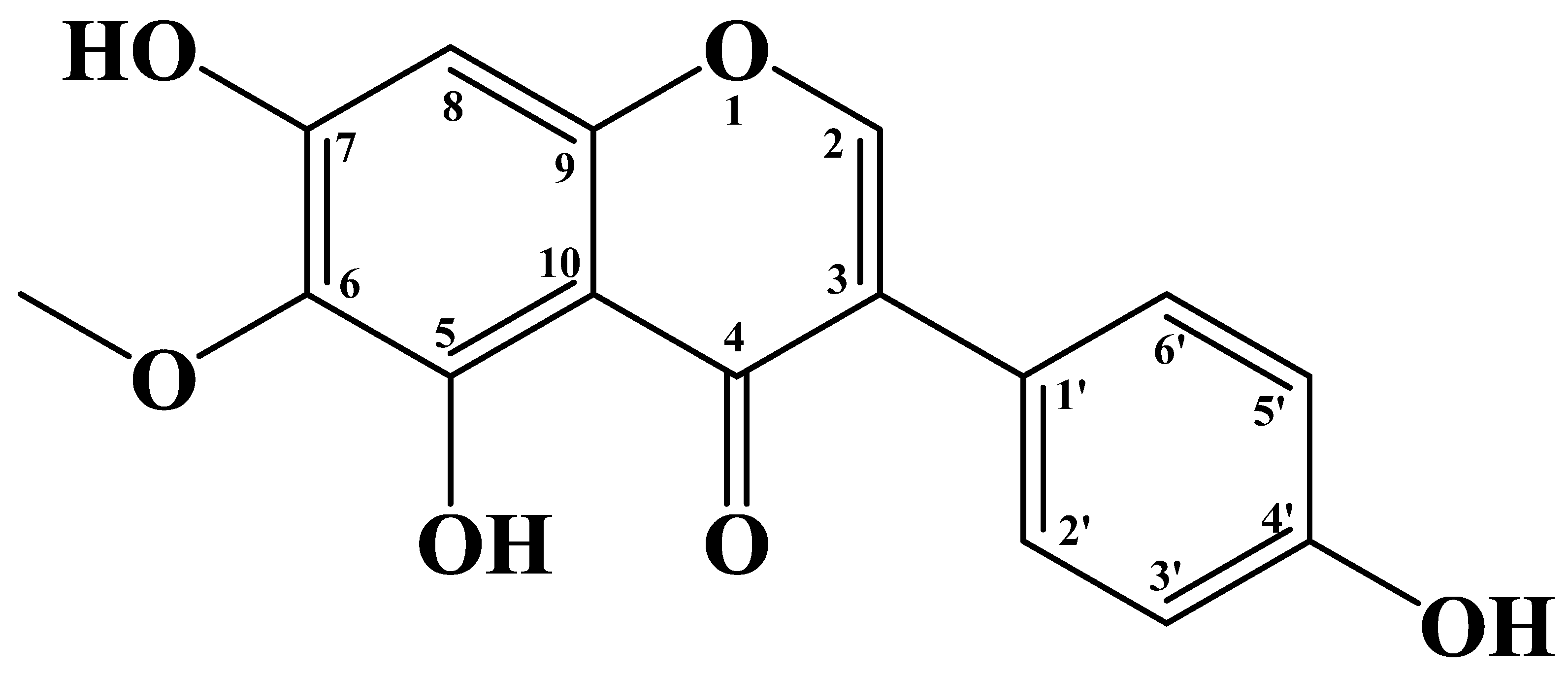
Tectorigenin: A Review of Its Sources, Pharmacology, Toxicity, and Pharmacokinetics
Abstract
1. Introduction
2. Sources of Tectorigenin
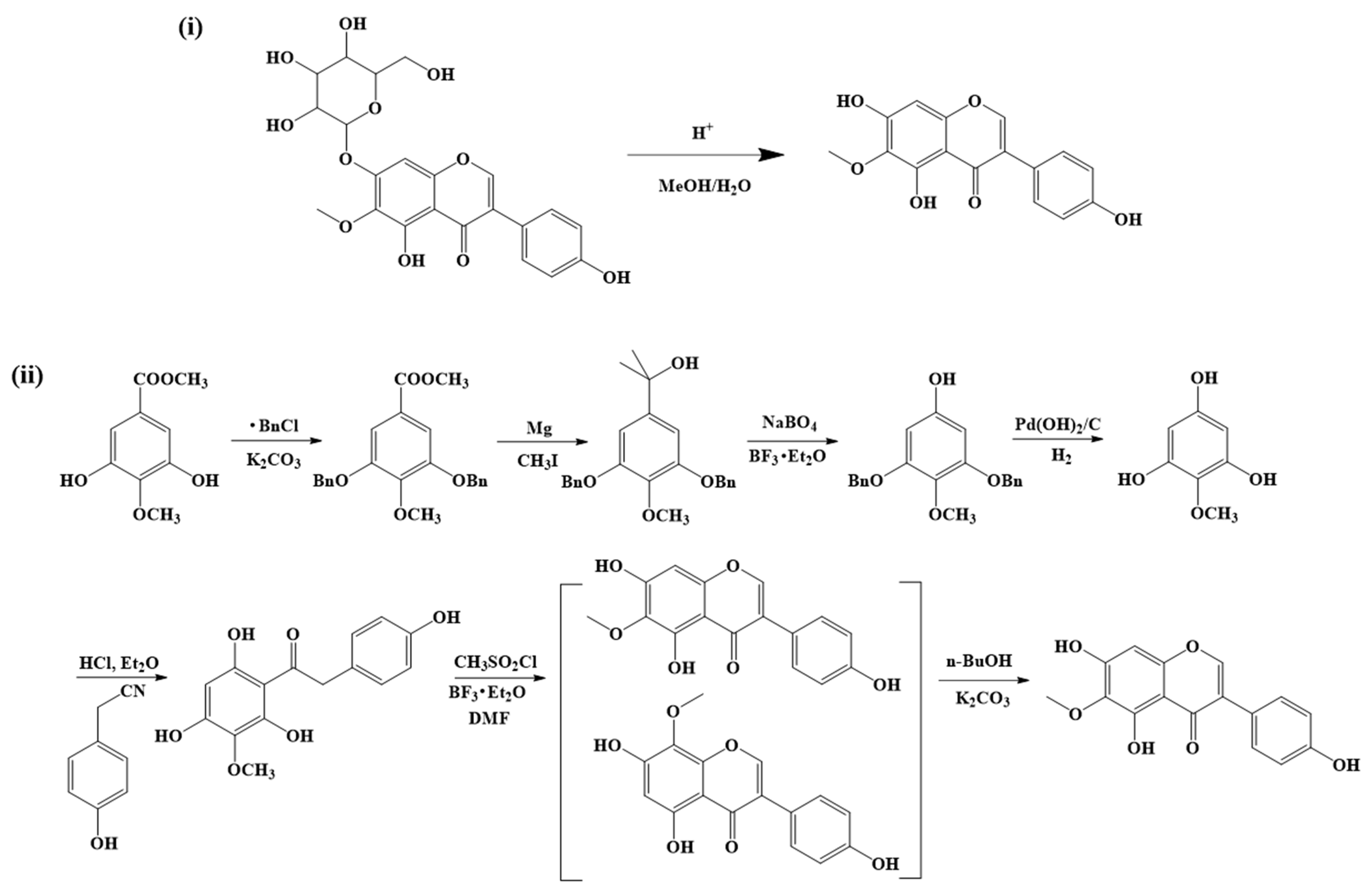
3. Extraction, Isolation, and Synthesis of Tectorigenin
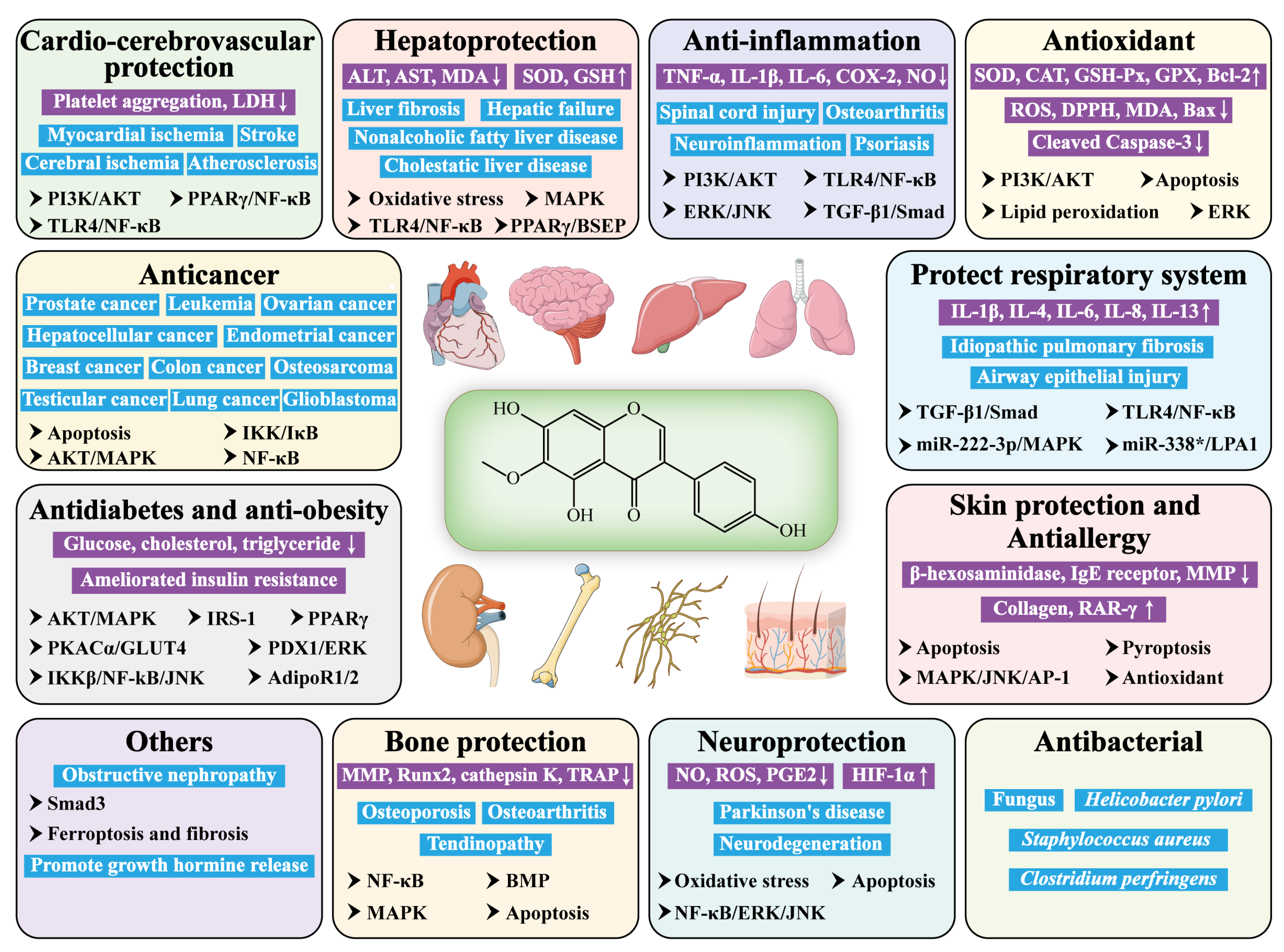
4. Pharmacological Insights of Tectorigenin
4.1. Anticancer Effects
4.1.1. Prostatic Cancer
4.1.2. Breast Cancer
4.1.3. Ovarian Cancer
4.1.4. Lung Cancer
4.1.5. Other Cancer
4.2. Antidiabetic and Anti-Obesity Effects
4.3. Hepatoprotective Effects
4.4. Anti-Inflammatory Effects
4.5. Antioxidant Effects
4.6. Antimicrobial Effects
4.7. Bone-Protective Effects
4.8. Anti-Skin-Damage and Antiallergic Effects
4.9. Cardioprotective and Cerebroprotective Effects
4.10. Protective Effects on the Respiratory System
4.11. Neuroprotective Effects
4.12. Other Effects
| Pharmacological Effects | Animal/Cell Line | Inducer | Route | Dosage | Duration | Mechanism of Action | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| Anticancer effects (Prostate cancer) | PC-3 and LNCaP cells | / | In vitro | 10, 50, 100 μM | 24 h | Up-regulated ERβ, decreased the tumor cell proliferation- related gene expression | ERβ, PDEF expression↑; PSA, prostate cancer-specific indicator gene DD3 (PCA3), hTERT, IGF-I receptor expression↓ | [69] |
| Anticancer effects (Prostate cancer) | LNCaP cells | / | In vitro | 100 μM | 24 h | Regulated the aberrant expression of genes relevant in proliferation, invasion, immortalization, and apoptosis | Prostate-derived Ets factor (PDEF), prostate -specific antigen (PSA), human telomerase reverse transcriptase (hTERT), insulin-like growth factor 1 (IGF-1) receptor expression↓; Telomerase activity↓; inhibited MMP and induced apoptosis in LNCaP cells | [73] |
| Anticancer effects (Prostate cancer) | RWPE-1, LNCaP and PC-3 cells | / | In vitro | 50 μM | 24, 72 h | Regulated cell cycle | Induced cycle arrest at G1 phase and p21 (WAF1) or p27kip1 protein expression | [75] |
| Anticancer effects (Prostate cancer) | RM-1, H22 and MFC cells | / | In vitro | / | 24, 48 h | / | Exhibited anti-prostate cancer activity with IC50 value of 0.08 μM | [53] |
| Anticancer effects (Breast cancer) | MCF-7 and T-47D cells | / | In vitro | / | 96 h | / | Stimulated MCF-7 and T-47D cell proliferation | [55] |
| Anticancer effects (Breast cancer) | MDA-MB-231, MCF-7 and hMSC cells | / | In vitro | 50, 100, 200 μM | 24, 48, 72, 96 h | Downregulated matrix metalloproteinases and AKT/MAPK signaling; upregulated caspase family | Induced apoptosis, G0/G1-phase arrest, migration and invasion; MMP-2, MMP-9, Bcl-2, p-AKT, and MAPK expression↓; Bax, cleaved poly [ADP-ribose] polymerase and cleaved caspase-3 expression↑ | [78] |
| Anticancer effects (Ovarian cancer) | MPSC1TR, A2780TR and SKOV3TR cells | / | In vitro | 25, 100 μM | 48 h | Inactivated AKT/IKK/IκB/NF-κB pathway | Activated caspases-3, -8 and -9; Nuclear translocation of NF-κB and FLICE inhibitory protein (FLIP), X-linked inhibitor of apoptosis protein (XIAP), Bcl-2, Bcl-xL and COX-2 expression↓; phosphorylation of IκB and IKK and the activation of AKT↓ | [82] |
| Anticancer effects (Ovarian cancer) | A2780 and IOSE80PC cells | / | In vitro | 1.56–100 μM | 48 h | / | IC50 against A2780 cells is 48.67 ± 0.31 μM | [41] |
| Anticancer effects (Lung cancer) | A549 cells | / | In vitro | 15, 30, 60, 120, 240 μg/mL | 24 h | / | Inhibited A549 cells growth with IC50 221.52 μg/mL | [48] |
| Anticancer effects (Lung cancer) | LLC, S180 and CPAE cells; C57BL/6 mice | Injection of LLCs (in vivo) | In vitro and in vivo | 1, 10, 100 μM (in vitro); 30 mg/kg (in vivo) | 3 days (in vitro); once a day for 10 days (in vivo) | / | Inhibited tumor growth | [83] |
| Anticancer effects (Lung cancer) | A549 and THP-1 cells | / | In vitro | 10, 25, 50 μM | 24 h | Suppressed lung cancer-induced pro-inflammatory response | TNF-α and IL-6 secretion↓; snail expression↓; E-cadherin↑ | [34] |
| Anticancer effects (Hepatocellular cancer) | HepG2 cells | / | In vitro | 2.5, 5, 10, 15, 20, 30, 40 μg/mL | 12, 24, 48 h | Induced apoptosis via mitochondrial-mediated pathway | HepG2 cell viability↓; induced the condensation of chromatin and fragmentation of nuclei; ROS, intracellular [Ca2+], caspase-9 and -3↑; mitochondrial membrane potential↓ | [84] |
| Anticancer effects (Osteosarcoma) | Saos2 and U2OS OS cells | / | In vitro | 100, 200, 400 µM | 24, 48, 72 h, | Inhibited the proliferation, migration and invasion | Inhibited migration and invasion; cleaved caspase3↑, MMP-1, MMP-2, and MMP-9↓ | [21] |
| Anticancer effects (Colon cancer) | Caco-2 cells | / | In vitro | 20 µM | 24 h | NF-κB pathway suppression | Arrested invasion; CXCL-10 expression, p-IκB, p-RelA↓; CXCL-10 promoter activity↓ | [85] |
| Anticancer effects (Leukemia) | HL-60, U-937, HepG2 and SNU-C5 cells | / | In vitro | 20, 50, 75 μM | 1–4 days | Induced differentiation and apoptosis | Inhibited HL-60, U-937, HepG2 and SNU-C cell growth with IC50 values of 22.3, 28, 84, and 62.7 μM, respectively; induced HL-60 cells differentiation, caused apoptotic changes of DNA | [86] |
| Anticancer effects (Testicular cancer) | TGCT, TCam-2 and NTera-2 cells | / | In vitro | 100, 250, 500 μM | 48, 72 h | Downregulated stem cell factors | Inhibited proliferation, the stem cell factors NANOG and POU5F1↓ | [87] |
| Anticancer effects (Endometrial cancer) | Ishikawa cells | / | In vitro | 0.1 μM | 72 h | Genomic aberrations | Induced array genes aberrated | [88] |
| Anticancer effects | COR-L23, C32, MCF-7, HepG2 | / | In vitro | 50, 100, 200, 400 µM | 48 h | Inhibited S phase and G2/M phase | IC50 against COR-L23, C32, MCF-7, HepG2 were 189, 207, 149, 105 µM, respectively; 400 µM increased significantly G2/M phase cells | [54] |
| Anticancer effects | P388, L1210, SNU C4, A549 and MA104 cells | / | In vitro | / | / | / | IC50 against P388, L1210, SNU C4, A549 and MA104 were 0.2, 0.04, 0.03, 0.29, >1 mM, respectively | [90] |
| Antidiabetic effects | Male SD rats | Streptozotocin | In vivo | 5, 10 mg/kg | A week | Antioxidant activity | Serum cholesterol, triglyceride, LDL- and VLDL-cholesterol↓; High-density lipoprotein (HDL)-cholesterol↑; DPPH radical, xanthine oxidase, superoxide anion radical, lipid peroxidation↓ | [42] |
| Antidiabetic effects | Male SD rats | Streptozotocin | In vivo | 10 mg/kg | Once per day for 3 days | / | Body weight↑; Serum glucose, cholesterol↓ | [90] |
| Antidiabetic effects | Male SD rats | Streptozotocin | In vivo | 100 mg/kg | 10 consecutive days | / | Exhibited high aldose reductase inhibitory potency with IC50 1.12 μM; inhibited the sorbitol accumulation in red blood cells, sciatic nerves, and lenses by 87.2%, 75.5%, and 50.5%, respectively | [91] |
| Antidiabetic effects | INS-1, RIN- m5F, and HEK293T cells; Male C57BL/6J mice | Glucose, palmitic acid (in vitro); high-fat +sucrose diet (in vivo) | In vivo and in vitro | 40 μg/mL (in vitro); 10, 20, 40 mg/kg (in vivo) | 3 h, 9 h, 24 h (in vitro); once every two days for one month (in vivo) | Enhanced PDX1 expression and protected pancreatic β-cells by activating ERK and reducing ER stress | Improved insulin secretion; weight gain, ROS↓; ameliorated hyperglycemia and glucose intolerance and lipotoxicity and apoptosis | [92] |
| Antidiabetic effects | Rat lenses | / | In vitro | / | / | / | Aldose reductase inhibition IC50 = 1.12 ± 0.08 μM | [47] |
| Antidiabetic effects | Rat lens | / | In vitro | / | / | / | Aldose reductase inhibition IC50 = 6.43 μM | [93] |
| Antidiabetic effects | HRGECs; Male type 2 diabetic (BKS.C g–m +/+ Lepr db/J, db/db) mice, a model homozygous for the diabetes spontaneous mutation | High-glucose (in vitro) | In vitro and in vivo | 5, 10 μM (in vitro); 75 mg/kg (in vivo) | 24 h (in vitro); 12 weeks (in vivo) | Restored the reduction of AdipoR1/2, pi-LKB1, pi-AMPKα, PPARα, decreased lipotoxicity, reduced macrophage infiltration and macrophage polarization | Attenuated metabolic disorders and exerted renoprotective effects; improved intrarenal lipid metabolism, endothelial functions, and renal insulin sensitivity | [96] |
| Antidiabetic effects | HUVECs | Palmitic acid | In vitro | 0.1, 1, 10 μM | 30 min | Inhibited IKKβ/NF-κB/JNK pathway | ROS, MMP, TNF-α, IL-6↓; protected endothelium-dependent relaxation | [97] |
| Antidiabetic effects | C2C12 myotubes; Male C57BL/6J mice | Palmitic acid (in vitro); high fat (in vivo) | In vitro and in vivo | 10 µg/mL (in vitro); 10, 20, 40 mg/kg or 50, 100, 200 mg/kg (in vivo) | 24 h (in vitro); every other day for 30 days (in vivo) | PKACα/AMPK/MEF2 pathway | Improved GLUT4, glucose uptake, and insulin sensitivity; ameliorated insulin resistance and hyperglycemia | [99] |
| Anti-obesity effects | 3T3-L1 cells; Male SD rats | Dexamethasone (in vitro); high-fat diet (in vivo) | In vitro and in vivo | 10, 25, 50, 75 μM (in vitro); 50, 100 mg/kg (in vivo) | 24 h (in vitro); once daily for 2 weeks (in vivo) | PPARγ and IKK/NF-κB pathway | Inhibited adipocyte differentiation; Triglyceride, glycerol-3-phosphate dehydrogenase, adipogenesis-related genes expression↓; IL-6, MCP-1↓; adiponectin secretion↑; increased glucose uptake and insulin sensitivity | [101] |
| Hepatoprotective effects | male ICR mice | CCl4 | In vivo | 50, 100 mg/kg | Once | Inhibited the β-glucuronidase activity | Inhibited the levels of serum ALT, AST and LDH by 22.4%, 44.4%, and 58.7%, respectively; MDA, Ca2+↓, GSH, GST↑ | [104] |
| Hepatoprotective effects | Male SD rats | CCl4, high-fat + cholesterol diet, alcohol | In vivo | 7.5, 15, 30 mg/kg | Once per day for 6 weeks | Antioxidant activity | Serum ALT, AST, hyaluronate, laminin, procollagen III N-terminal peptide↓; collagen in the livers↓, serum albumin concentration and ratio of albumin to globulin↑, liver lipid peroxidation↓, liver SOD and GPx↑ | [105] |
| Hepatoprotective effects | HepG2 cells; Male ICR mice | t-BHP (in vitro and in vivo) | In vitro and in vivo | 0.01, 0.1, 1, 10 μM (in vitro); 25, 50 mg/kg (in vivo) | 2 h (in vitro); once (in vivo) | Inhibited the hepatotoxicity | Inhibited the levels of plasma ALT and AST by 39% and 41%, respectively | [106] |
| Hepatoprotective effects | RAW 264.7 cells; Male C57BL/6J mice | LPS and D-GalN | In vitro and in vivo | 1, 10, 100 μM (in vitro); 12.5, 25, 50 mg/kg | 24 h (in vitro); once (in vivo) | TLR4/MAPK, TLR4/NF-κB, decreased inflammatory cytokine levels, promoted autophagy | Serum ALT, AST↓; Ameliorated the histological injury, apoptosis, and the mortality | [107] |
| Hepatoprotective effects | HSC-T6 cells | / | In vitro | 10, 20, 40, 60, 80, 100 μg/mL | 12, 24, 48 h | Inhibited proliferation and induced apoptosis | Cell viability↓; ROS, intracellular [Ca2+]↑; mitochondrial membrane potential↓; translocation of cytochrome c, caspase-3/9↑ | [109] |
| Hepatoprotective effects | Male C57BL/6N mice | High-fat diet | In vivo | 25, 50 mg/kg | Once per day for 6 weeks | LPS/TLR-4/NF-κB/TNF-α pathway, modulated gut microbiota | Ameliorated the obese characteristics; total cholesterol, total triglyceride, HDL-C, LDL-C, LPS, total bile acid, ALT, AST↓; fecal total bile acid↑; ameliorated the histological injury | [110] |
| Hepatoprotective effects | Primary mouse Kupffer cells; Male C57BL/6J mice | ANIT, DDC diet | In vitro and in vivo | 10 μM (in vitro); 75 mg/kg | 24 h (in vitro); once per day for 5 days (in vivo) | PPARγ/NF-κB, PPARγ/BSEP, inhibited macrophage activation | Serum AST, ALT, γ-glutamyltransferase, and AP↓; ameliorated the histological injury, cell apoptosis, inflammation, and bile metabolic dysfunction | [23] |
| Anti-inflammatory effects | Raw 264.7 cells | IFN-γ/LPS | In vitro | 50, 100, 200 μM | 24 h | Blocking of NF-κB activation | Nitric oxide synthase (iNOS)↓, NO↓, IL-1β↓, COX-2↓, Prostaglandin E2 (PGE2)↓ | [114] |
| Anti-inflammatory effects | PC12 cells | LPS | In vitro | 25, 50, 100, 200 μM | 24 h | Alleviated apoptosis, inflammation, and activation of NF-κB signaling in SCI cell models via inhibiting IGFBP6 | Cell viability↑, cell apoptosis↓, caspase-3/8/9, cleaved caspase-3/8/9; IL-1β, IL-6, TNF-α, IGFBP6, TLR4↓; inactivated IκBα and p65 | [116] |
| Anti-inflammatory effects | Female BALB/c mice | LPS | In vivo | 5, 10 mg/kg | 6 h | NF-κB P65 pathway | Inflammatory cell numbers↓, lung NF-κB p65 mRNA and protein level↓, myeloperoxidase↓, SOD↑, inhibited LPS-induced neutrophils in the lung | [117] |
| Anti-inflammatory effects | Swiss mice; Wistar rats | Acetic acid or carrageenan | In vivo | 50, 100 mg/kg; 10, 60 mg/kg | / | / | In mice, LD50 was 1.78 g/kg, had an analgesic effect; in inflammatory rats, reduced carrageenan-induced edema | [118] |
| Anti-inflammatory effects | HaCaT cells | M5 cytokines | In vitro | 2.5, 5, 10, 20, 40 μM | / | TLR4/NF-κB pathway; promoted autophagy | Promoted autophagy: LC3-II/LC3-I, beclin-1, LC3↑; P62↓; suppressed inflammation: IL-6, IL-1β, TNF-α↓; NOD-like receptor family pyrin domain containing 3 (NLRP3), apoptosis-associated speck-like protein (ASC) and caspase-1↓ | [119] |
| Antioxidant effects | Male SD rats | CCl4 | In vivo | 100 mg/kg | Once a day for 7 days | Prevented lipid peroxidation, antioxidation | IC50 of free radical scavenging potency was 275 μM; Liver MDA, SOD, CAT, GPx↓ by 75.6%, 63.8%, and 70.4%, respectively; serum AST, ALT↓ by 47.4% and 39.8%, respectively | [56] |
| Antioxidant effects | V79-4 cells | H2O2 | In vitro | 0.1, 1, 10 μg/mL | 25 h | Activated ERK pathway; enhanced antioxidative levels, apoptotic and cycle arrest | Prevented lipid peroxidation: intracellular ROS, DPPH↓; apoptotic cells, cell cycle arrest at G2/M↓; Cellular SOD, GPx, CAT↑; cell viability↑ | [127] |
| Antioxidant effects | Male SD rats | Bromobenzene | In vivo | 10 mg/kg | Once a day for 7 days | / | Inhibited the AFB1-induced mutagenicity by 90% and MNNG-induced one by 76%; prevented the MDA formation | [128] |
| Antioxidant effects | / | / | In vitro | 50, 100, 150, 200 μM | / | / | Exhibited reductive capability and DPPH scavenging activity, IC50 of OH−, O2•– scavenging activity and inhibition of lipid peroxidation were 87, 46.62, and 23 μg/mL, respectively | [60] |
| Anti-microbial effects (fungal) | 7 strains of fungi and 6 strains of bacteria | / | In vitro | / | 72 h | / | Inhibited dermatophytes of the genera Trichophyton (MIC: 3.12–6.25 μg/mL) and such yeast-like fungi as Can dida and Saccharomyces (25–50 μg/mL); inhibited the growth of P. aeruginosa, P. vulgαris, M. luteus and S. aureus (50–100 μg/mL) | [50] |
| Anti-microbial effects (HP) | HP ATCC43504, NCTC11637, NCTC11638, HP82516, HP82548 and HP4 | / | In vitro | / | 3 days | / | Inhibited HP growth (MIC: 50–100 μg/mL) | [130] |
| Anti-microbial effects (MRSA) | Methicillin-Resistant Staphylococcus aureus | / | In vitro | / | 18 h | Anti-MRSA action is related to cytoplasmic membrane permeability and ABC transporter | Inhibited all tested strains (MIC: 125 μg/mL) | [17] |
| Anti-microbial effects (Clostridium perfringens) | Caco-2 | / | In vitro | 4, 8, 16, 32 μg/mL | 96 h | Targeting type IV pilus (TFP) system | Inhibited gliding motility, biofilm formation, and adherence to Caco-2 cells; TFP-encoding genes↓ | [131] |
| Bone-protective effects | Bone marrow mononu clear (BMM) cells, RAW264.7 cells; Female C57BL/6 mice | RANKL (in vitro); Ovariectomized model (in vivo) | In vitro and in vivo | 10, 40, 80, 160 μM (in vitro); 1, 10 mg/kg (in vivo) | 7 days (in vitro); every 3 days for 6 weeks (in vivo) | NF-κB pathway | Reduced osteoclast differentiation, TRAP; NFATc1, cathepsin K, MMP-9↓; reduced the bone loss of trabecular bone; BMD, BV/TV, Tb.N↑, Tb.Sp↓; improved trabecular numbers, decreased osteoclasts numbers | [138] |
| Bone-protective effects | PDLCs and BMMs; Female ICR mice | Osteogenic medium (in vitro); RANKL, LPS (in vivo) | In vitro and in vivo | 10, 50, 100 μM (in vitro); 3 mg/kg (in vivo) | 14 days (in vitro); dosing on days 0 and 3 (in vivo) | BMP and MAPK pathways | ALP, OPN, OCN, Runx2, Osterix, BMP-2, BMP-4, Smad-4↑; TRAP, cathepsin-K, MMP-9↓; stimulated osteogenic differentiation, increased bone regeneration, inhibited osteoclast differentiation, and suppressed inflammatory bone loss | [120] |
| Bone-protective effects | Primary chondrocytes; Male SD rats | Medial collateral ligament transection and medial meniscal tear on the knee joints | In vitro and in vivo | 25, 50, 100, 200, 400 μM (in vitro); 0.75, 1.5 μg/kg (in vivo) | 24 h (in vitro); every 5 days for 8 weeks (in vivo) | Prevented articular cartilage degeneration and chondrocyte apoptosis via the NF-κB P65 and Bax/Bcl-2/caspase-3 pathway | Type X collagen, cyclooxigenase-2, MMP-3, and MMP-13 expression↓; Runx1, type II collagen, and aggrecan↑; inhibited apoptosis: p-Bad, caspase-3↑, Bax/Bcl-2 ratio↓, improved osteoarthritic injury | [139] |
| Bone-protective effects | Tendon-derived stem cells; Male SD rats | TNF-α (in vitro); Achilles tenotomy (in vivo) | In vitro and in vivo | 50, 100 µM (in vitro); 100 µM (in vivo) | 1 h (in vitro); once a week for 8 weeks (in vivo) | NF-κB and MAPK pathways | MMP-3, MMP-9, MMP-13, iNOS, COX-2, IL-6, IL-10, and collagen I, RUNX-2↓; inhibited apoptosis, senescence, and ossification, and alleviated calcification of tendon | [141] |
| Anti-skin-damage effects | HaCaT cells | Ultraviolet-B light | In vitro | 0.1, 1, 10 µM | 24 h | Antioxidant, apoptosis, and collagen degradation | Intracellular ROS↓, GSH level↑, GPx, CAT expression↑; caspase-3, Bcl-2/Bax ratio↓; attenuated collagen degradation | [22] |
| Anti-skin-damage effects | HaCaT, B16-F10, 293T cells; Kunming mice and New Zealand white rabbits | Ultraviolet | In vitro and in vivo | 10, 20, 40, 80, 160, 320 µM | 24, 48 h | Apoptosis and pyroptosis pathways; MAPK/JNK/AP-1 pathway | Targeting binding RAR-γ; inhibited UV-induced oxidative damage, inflammatory factor releases and MMP production, reversed the loss of collagen | [143] |
| Antiallergic effects | HMC-1 | / | In vitro | 10, 25, 50 µM | 72 h | Reduced generation of c-chain subunit | Inhibited the expression of IgE receptor | [145] |
| Antiallergic effects | RBL-2H3 and RAW 264.7 cells | / | In vitro | / | / | Inhibited release of b-hexosaminidase induced by IgE | Inhibited the passive cutaneous anaphylaxis reaction and inhibited the release of β-hexosaminidase from RBL-2H3 cells induced by IgE | [146] |
| Cardioprotective effects | Human blood | Arachidonic acid | In vitro | / | / | Competitive antagonism at thromboxane receptors | Inhibited whole blood platelet aggregation | [150] |
| Cardioprotective and cerebroprotective effects | HUVECs | H2O2 | In vitro | 0.1, 0.2, 0.5, 1, 10 μM | 24 h | PI3K/AKT | Cell viability↑, LDH↓, SOD↑, GSH-Px↑, MDA↓; Attenuated apoptosis | [39] |
| Cerebroprotective effects | HT-22 cells | OGD/R injury | In vitro | 1, 5, 10, 20 mM | 1 h | PI3K/AKT and PPARγ/NF-κB pathways | Cell viability↑; LDH, IL-1β, IL-6, TNF-α, ROS↓; alleviated apoptosis | [152] |
| Cerebroprotective effects | HT-22 cells; Male C57BL/6 mice | OGD/R injury (in vitro); Bilateral carotid artery stenosis (BCAS) (in vivo) | In vitro and in vivo | 1, 10, 50, 100, 200 μM (in vitro); 12.5, 25, 50 mg/kg (in vivo) | 24 h (in vitro); once daily for 15 days (in vivo) | Inhibited the TLR4/NF-κB pathway | Alleviated cognitive impairment, hippocampal tissue and myelin damage, and inflammation; Cell viability↑; alleviated apoptosis | [153] |
| Protective effects on the respiratory system | Male guinea pigs | Ovalbumin | In vivo | 10, 25 mg/kg | 14 days | TGF-β1/Smad and TLR4/NF-κB pathways | Inhibited pulmonary fibrosis and airway inflammation: the number of coughs, inflammatory cells, TGF-β1, p-Smad2/3/4, VEGFA, TNF-α, TLR4, MyD88, NF-κB, p-IKKβ↓; Smad7↑; inhibited pulmonary fibrosis | [24] |
| Protective effects on the respiratory system | 9HTE cells | Dexamethasone | In vitro | 0.1, 0.2,0.5, 1, 10 μM | 24 h | Enhanced miR-222-3p expression, and inhibited the MAPK pathway | Improved the cell viability, migration, and invasion, and alleviated apoptosis | [154] |
| Protective effects on the respiratory system | Pulmonary fibroblasts of male SD rats | Bleomycin | In vitro | 10, 100, 500 μM | 3 days | Enhanced miR-338* (miR-338-5p) expression, inhibited LPA1 expression | Cell viability↓ | [155] |
| Neuroprotective effects | Primary rat microglia | LPS | In vitro | / | / | / | Showed potency of inhibiting NO release from LPS-activated microglia with IC50 values of 9.3 µM | [40] |
| Neuroprotective effects | GBM-8401 and GBM-8901 cells | / | In vitro | 25, 50, 100, 200, 300 µM | 24 h | Cell cycle arrest | Glioblastoma cell viability↓; cell cycle arrest at G0/G1 phase; p-retinoblastoma protein, cyclin-dependent kinase 4 (CDK4)↓; p21 expression↑ | [160] |
| Neuroprotective effects | BV-2 cells; Male ICR mice (8 weeks) | LPS | In vitro and in vivo | 25, 50, 100 μM (in vitro); 5 or 10 mg/kg (in vivo) | 24 h (in vitro); once per day for 5 days (in vivo) | NF-κB/ERK/JNK pathway | Intracellular ROS, NO, PGE2, TNF-α, IL-6↓; Extracellular ERK, JNK, iNOS, COX-2↓; Hippocampus MDA, iNOS↓; Serum TNF-α, IL-6 levels↓; TLR4, MyD88 protein↓ | [115] |
| Neuroprotective effects | NT2/D1 cells | / | In vitro | 10, 20, 30 μM | 24 h | Upregulation of erythropoietin in neurons | Induced transcription of HIF-1α, reduced degradation of HIF-1α-OH | [164] |
| Neuroprotective effects | PC12 cells | β-Amyloid protein | In vitro | 25, 50, 100 μg/mL | 12, 24, 48 h | / | IC50 against MAO-B was 54.36 μg/mL; had a protective effect against Aβ(25–35)-induced cell damage | [57] |
| Neuroprotective effects | SH-SY5Y | MPP+ | In vitro | 0.1, 1, 10 μM | 24 h | Oxidative stress, apoptosis | Cell viability↑, caspase-3 activity and cytochrome c expression, Bax and Bcl-2 levels↓; ROS and NADPH oxidase↓; SOD, CAT, GPx↑ | [165] |
| Protective effects on obstructive nephropathy | Primary renal tubular epithelial cells; Male C57BL/6 mice | TGF-β1, erastin/RSL3 (in vitro); Unilateral ureteral obstruction (in vivo) | In vitro and in vivo | 20, 40, 60 μM (in vitro); 20 mg/kg (in vivo) | 24 h (in vitro); daily for 7 consecutive days (in vivo) | Inhibited Smad3-mediated ferroptosis and fibrosis | Blood urea nitrogen and creatinine, KIM-1↓, alleviated pathological damage and renal interstitial fibrosis | [166] |
| Effects on growth hormone release | Pituitary cells | / | In vitro | 2.5–20 μg/mL | 15 min | / | Promoted the release of growth hormone, twice as effective as in the control group | [167] |
5. Toxicity of Tectorigenin
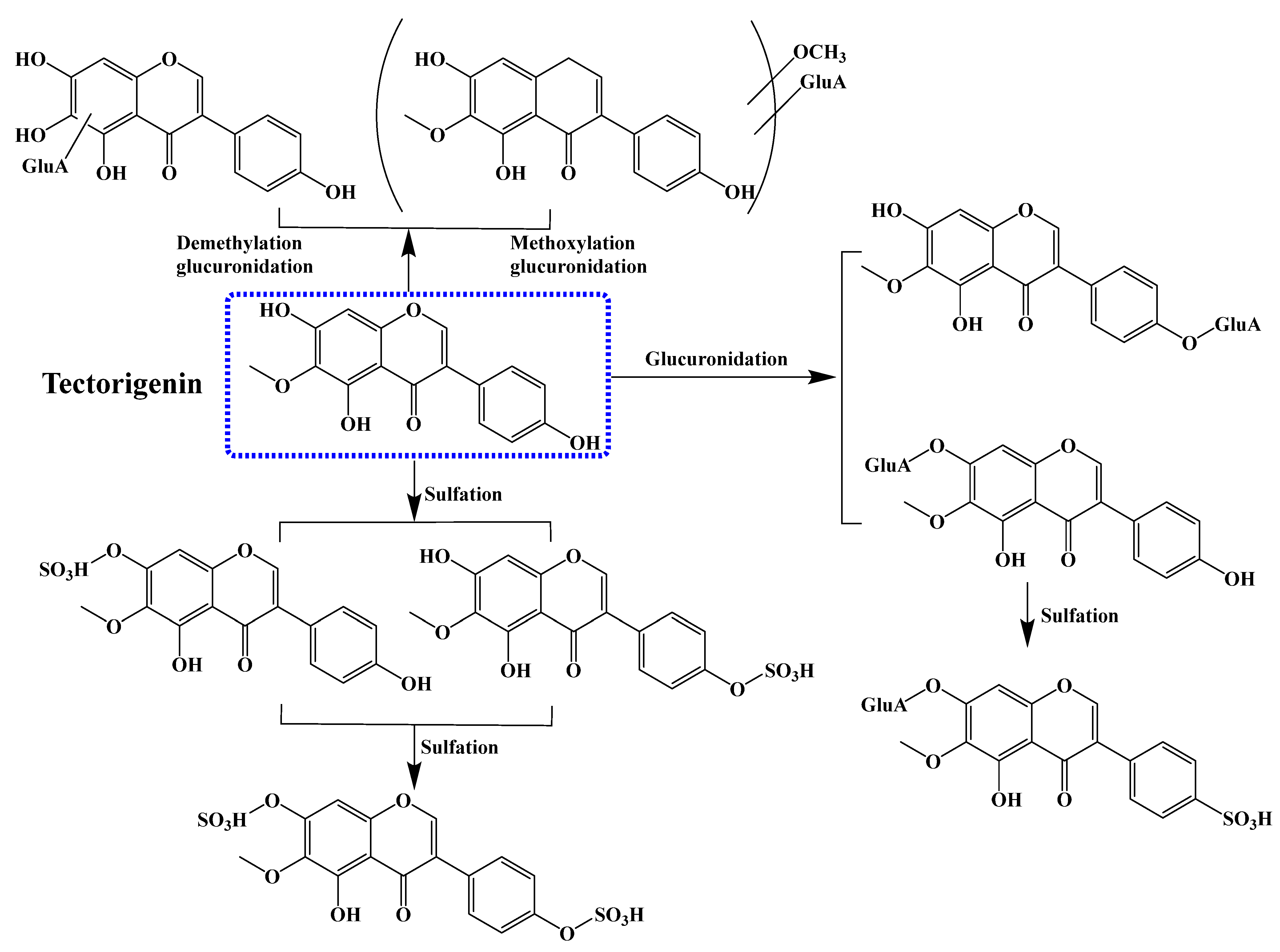
6. Pharmacokinetics of Tectorigenin
7. Delivery Strategy of Tectorigenin
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sin, V.J.; Anand, G.S.; Koh, H.L. Botanical medicine and natural products used for erectile dysfunction. Sex. Med. Rev. 2021, 9, 568–592. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Hernandez, I.; Alegre, L.; Van Breusegem, F.; Munne-Bosch, S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Proenca, C.; Ribeiro, D.; Freitas, M.; Carvalho, F.; Fernandes, E. A comprehensive review on the antidiabetic activity of flavonoids targeting PTP1B and DPP-4: A structure-activity relationship analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4095–4151. [Google Scholar] [CrossRef]
- Calderaro, A.; Patane, G.T.; Tellone, E.; Barreca, D.; Ficarra, S.; Misiti, F.; Lagana, G. The neuroprotective potentiality of flavonoids on Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 14835. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Q.; Zhou, X.; Wang, X.; Li, H.; Zhang, W.; Yuan, H.; Sun, C. Flavonoids regulate tumor-associated macrophages—From structure-activity relationship to clinical potential (Review). Pharmacol. Res. 2022, 184, 106419. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Terahara, N. Flavonoids in foods: A review. Nat. Prod. Commun. 2015, 10, 521–528. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Croft, K.D. Tea flavonoids and cardiovascular health. Mol. Aspects Med. 2010, 31, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Kafi, M.K.; Bolvari, N.E.; Pour, S.M.; Moghadam, S.K.; Shafaei, N.; Karimi, E.; Oskoueian, E. Encapsulated phenolic compounds from Ferula gummosa leaf: A potential phytobiotic against Campylobacter jejuni infection. J. Food Process. Pres. 2022, 46, e16802. [Google Scholar]
- Poorbagher, M.R.M.; Karimi, E.; Oskoueian, E. Hepatoprotective effect of nanoniosome loaded Myristica fragrans phenolic compounds in mice-induced hepatotoxicity. J. Cell. Mol. Med. 2022, 26, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Oskoueian, E.; Karimi, A.; Noura, R.; Ebrahimi, M. Borago officinalis L. flower: A comprehensive study on bioactive compounds and its health-promoting properties. J. Food Meas. Charact. 2018, 12, 826–838. [Google Scholar] [CrossRef]
- Oskoueian, E.; Karimi, E.; Noura, R.; Ebrahimi, M.; Shafaei, N.; Karimi, E. Nanoliposomes encapsulation of enriched phenolic fraction from pistachio hulls and its antioxidant, anti-inflammatory, and anti-melanogenic activities. J. Microencapsul. 2020, 37, 1–13. [Google Scholar] [CrossRef]
- Joung, D.K.; Mun, S.H.; Lee, K.S.; Kang, O.H.; Choi, J.G.; Kim, S.B.; Gong, R.; Chong, M.S.; Kim, Y.C.; Lee, D.S.; et al. The antibacterial assay of tectorigenin with detergents or ATPase inhibitors against methicillin-resistant Staphylococcus aureus. Evid. Based Complement. Alternat. Med. 2014, 2014, 716509. [Google Scholar] [CrossRef]
- Han, J.; Xu, K.; Yan, Q.; Sui, W.; Zhang, H.; Wang, S.; Zhang, Z.; Wei, Z.; Han, F. Qualitative and quantitative evaluation of Flos Puerariae by using chemical fingerprint in combination with chemometrics method. J. Pharm. Anal. 2022, 12, 489–499. [Google Scholar] [CrossRef]
- Wozniak, D.; Matkowski, A. Belamcandae chinensis rhizome—A review of phytochemistry and bioactivity. Fitoterapia 2015, 107, 1–14. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Kano, Y.; Yuan, D.; Qu, J. Oriental traditional herbal Medicine--Puerariae Flos: A systematic review. J. Ethnopharmacol. 2023, 306, 116089. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.H.; Cheng, Z.H.; Ou-Yang, H.N.; Luo, C.; Guo, Z.L. Tectorigenin inhibits osteosarcoma cell migration through downregulation of matrix metalloproteinases in vitro. Anticancer Drugs 2016, 27, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Noh, D.; Choi, J.G.; Huh, E.; Oh, M.S. Tectorigenin, a flavonoid-based compound of leopard lily rhizome, attenuates UV-B-induced apoptosis and collagen degradation by inhibiting oxidative stress in human keratinocytes. Nutrients 2018, 10, 1998. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, G.; Ma, C.; Wei, L.; Wu, H.; Zhang, W.; Tao, X.; Jiang, L.; Liang, Z.; Kang, L.; et al. Tectorigenin alleviates intrahepatic cholestasis by inhibiting hepatic inflammation and bile accumulation via activation of PPARγ. Br. J. Pharmacol. 2021, 178, 2443–2460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jing, W.; Qu, W.; Liu, Z.; Zhang, D.; Qi, X.; Liu, L. Tectorigenin inhibits inflammation and pulmonary fibrosis in allergic asthma model of ovalbumin-sensitized guinea pigs. J. Pharm. Pharmacol. 2020, 72, 956–968. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, Q.; Qi, L.W.; Qin, X.Y.; Qin, M.J. Characterization and determination of the major constituents in Belamcandae Rhizoma by HPLC-DAD-ESI-MS(n). J. Pharm. Biomed. Anal. 2011, 56, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Noh, D.; Choi, J.G.; Lee, Y.B.; Jang, Y.P.; Oh, M.S. Protective effects of Belamcandae Rhizoma against skin damage by ameliorating ultraviolet-B-induced apoptosis and collagen degradation in keratinocytes. Environ. Toxicol. 2019, 34, 1354–1362. [Google Scholar] [CrossRef]
- Farag, S.F.; Kimura, Y.; Ito, H.; Takayasu, J.; Tokuda, H.; Hatano, T. New isoflavone glycosides from Iris spuria L. (Calizona) cultivated in Egypt. J. Nat. Med. 2009, 63, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Wang, J. Ultrasound-assisted extraction of five isoflavones from Iris tectorum Maxim. Sep. Purif. Technol. 2011, 78, 49–54. [Google Scholar] [CrossRef]
- Yang, Q.; Zheng, X.; Wei, J.; Zhang, C. Determination of two isoflavones and their antitumor activity in Iris japonica Thunb. J. Sichuan Norm. Univ. (Nat. Sci.) 2020, 43, 5. [Google Scholar]
- Shi, G.R. Study on Active Substances and Functions of Leaf of Rehmannia glutinosa Libosch and Whole Plants of Iris japonica Thunb; Chinese Academy of Medical Sciences: Beijing, China, 2017. [Google Scholar]
- Di, Z.Z.; Zhang, Y.; Wang, G.H.; Zou, G.X.; Li, G.X. Research of quality control of Iris dichotoma based on medicinal material base of Belamcanda chinensis. Liaoning J. Tradit. Chin. Med. 2017, 44, 4. [Google Scholar]
- Zhao, Y.; Li, G.X. Determination of effective active ingredients of three plants in Iridaceae plants at different havest periods by HPLC. J. Liaoning Univ. TCM 2012, 14, 39–41. [Google Scholar]
- Mosihuzzman, M.; Naheed, S.; Hareem, S.; Talib, S.; Abbas, G.; Khan, S.N.; Choudhary, M.I.; Sener, B.; Tareen, R.B.; Israr, M. Studies on α-glucosidase inhibition and anti-glycation potential of Iris loczyi and Iris unguicularis. Life Sci. 2013, 92, 187–192. [Google Scholar] [CrossRef]
- Amin, A.; Mokhdomi, T.A.; Bukhari, S.; Wani, S.H.; Wafai, A.H.; Lone, G.N.; Qadri, A.; Qadri, R.A. Tectorigenin ablates the inflammation-induced epithelial-mesenchymal transition in a co-culture model of human lung carcinoma. Pharmacol. Rep. 2015, 67, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.A.; Mir, F.; Shawl, A.S.; Ganai, B.A.; Kamili, A.N.; Masood, A.; Tantry, M.A. Crocetenone, a new rotenoid with an unusual trans-fused ring system from Iris crocea. Nat. Prod. Commun. 2015, 10, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Amin, A.; Sofi, S.N.; Mokhdomi, T.A.; Bukhari, S.; Hassan, Q.P.; Qadri, R.A. RP- HPLC facilitated quantitative analysis of tectorigenin in the different species of iris plant and evaluation of its invitro anticancer potential. Int. J. Curr. Res. 2013, 5, 206–211. [Google Scholar]
- Okba, M.M.; Abdel Baki, P.M.; Abu-Elghait, M.; Shehabeldine, A.M.; El-Sherei, M.M.; Khaleel, A.E.; Salem, M.A. UPLC-ESI-MS/MS profiling of the underground parts of common Iris species in relation to their anti-virulence activities against Staphylococcus aureus. J. Ethnopharmacol. 2022, 282, 114658. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Kovalyov, V.; Kovalyov, S.; Krechun, A. Isoflavonoids from the rhizomes of Iris hungarica and antibacterial activity of the dry rhizomes extract. Ars Pharm. 2018, 58, 39–45. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Sun, L.; Lian, Y. Tectorigenin protect HUVECs from H2O2 -induced oxidative stress injury by regulating PI3K/Akt pathway. Tissue. Cell. 2021, 68, 101475. [Google Scholar] [CrossRef]
- Yuan, D.; Xie, Y.Y.; Bai, X.; Wu, X.; Yang, J.Y.; Wu, C.F. Inhibitory activity of isoflavones of Pueraria flowers on nitric oxide production from lipopolysaccharide-activated primary rat microglia. J. Asian Nat. Prod. Res. 2009, 11, 471–481. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Son, S.R.; Kim, J.Y.; Choi, J.H.; Jang, D.S. Chemical constituents of the flowers of Pueraria lobata and their cytotoxic properties. Plants 2022, 11, 1651. [Google Scholar] [CrossRef]
- Lee, K.T.; Sohn, I.C.; Kim, D.H.; Choi, J.W.; Kwon, S.H.; Park, H.J. Hypoglycemic and hypolipidemic effects of tectorigenin and kaikasaponin III in the streptozotocin-induced diabetic rat and their antioxidant activity in vitro. Arch. Pharm. Res. 2000, 23, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.Z.; Luo, M.; Wang, W.; Huang, Y.Y.; Efferth, T.; Wang, H.M.; Fu, Y.J. Efficient extraction and preparative separation of four main isoflavonoids from Dalbergia odorifera T. Chen leaves by deep eutectic solvents-based negative pressure cavitation extraction followed by macroporous resin column chromatography. J. Chromatogr. B 2016, 1033–1034, 40–48. [Google Scholar] [CrossRef]
- Umehara, K.; Nemoto, K.; Matsushita, A.; Terada, E.; Monthakantirat, O.; De-Eknamkul, W.; Miyase, T.; Warashina, T.; Degawa, M.; Noguchi, H. Flavonoids from the heartwood of the thai medicinal plant Dalbergia parviflora and their effects on estrogenic-responsive human breast cancer cells. J. Nat. Prod. 2009, 72, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.L.; Wu, C.C.; Chang, F.R.; Wang, W.Y.; Khalil, A.T.; Lee, K.H.; Wu, Y.C. Antiplatelet and anti-HIV constituents from Euchresta formosana. Nat. Prod. Res. 2003, 17, 91–97. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.I.; Jung, J.C.; Lee, J. Aldose reductase inhibitory effect by tectorigenin derivatives from Viola hondoensis. Bioorg. Med. Chem. 2006, 14, 7592–7594. [Google Scholar] [CrossRef]
- Zhan, G.; Pan, L.; Tu, K.; Jiao, S. Antitumor, antioxidant, and nitrite scavenging effects of Chinese water chestnut (Eleocharis dulcis) peel flavonoids. J. Food Sci. 2016, 81, 2578–2586. [Google Scholar] [CrossRef]
- Shin, K.H.; Kim, Y.P.; Lim, S.S.; Lee, S.; Ryu, N.; Yamada, M.; Ohuchi, K. Inhibition of prostaglandin E2 production by the isoflavones tectorigenin and tectoridin isolated from the rhizomes of Belamcanda chinensis. Planta Med. 1999, 65, 776–777. [Google Scholar] [CrossRef]
- Oh, K.B.; Kang, H.; Matsuoka, H. Detection of antifungal activity in Belamcanda chinensis by a single-cell bioassay method and isolation of its active compound, tectorigenin. Biosci. Biotechnol. Biochem. 2001, 65, 939–942. [Google Scholar] [CrossRef]
- Wozniak, D.; Janda, B.; Kapusta, I.; Oleszek, W.; Matkowski, A. Antimutagenic and anti-oxidant activities of isoflavonoids from Belamcanda chinensis (L.) DC. Mutat. Res. 2010, 696, 148–153. [Google Scholar] [CrossRef]
- Miyazawa, M.; Sakano, K.; Nakamura, S.; Kosaka, H. Antimutagenic activity of isoflavone from Pueraria lobata. J. Agric. Food Chem. 2001, 49, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, X.L.; Li, H.; Qin, X.Y.; Ge, C.Y.; Liu, R.; Qi, L.W.; Qin, M.J. Application of an efficient strategy for discovery and purification of bioactive compounds from chinese herbal medicines, a case study on the Puerariae thomsonii flos. J. Pharm. Biomed. Anal. 2013, 75, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Houghton, P.J.; Hylands, P.J. Cytotoxic effects of compounds from Iris tectorum on human cancer cell lines. J. Ethnopharmacol. 2008, 118, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Monthakantirat, O.; De-Eknamkul, W.; Umehara, K.; Yoshinaga, Y.; Miyase, T.; Warashina, T.; Noguchi, H. Phenolic constituents of the rhizomes of the thai medicinal plant Belamcanda chinensis with proliferative activity for two breast cancer cell lines. J. Nat. Prod. 2005, 68, 361–364. [Google Scholar] [CrossRef]
- Jung, S.H.; Lee, Y.S.; Lim, S.S.; Lee, S.; Shin, K.H.; Kim, Y.S. Antioxidant activities of isoflavones from the rhizomes of Belamcanda chinensis on carbon tetrachloride-induced hepatic injury in rats. Arch. Pharm. Res. 2004, 27, 184–188. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Wang, Y.; Li, R.; Niu, H.; Liu, C.; Zhang, Y. Ionic-liquid-based ultrasound-assisted extraction combined with countercurrent chromatography and semipreparative LC for the preparation of monoamine oxidase B inhibitors from Pueraria thomsonii. J. Sep. Sci. 2022, 45, 1116–1127. [Google Scholar] [CrossRef]
- Guo, C.L. Extraction, Purification and Microbial Biotransformation of Tectorigenin Isolated from both Belamcanda Chinensis and the Flowers of Pueraria thunbergiana; Agricultural University of Hebei: Baoding, China, 2013. [Google Scholar]
- Kim, M.Y.; Yoo, Y.M.; Chung, I.M.; Nam, J.H.; Park, H.J. Quantitative analysis of the isoflavone content in the flower and the root of Pueraria thunbergiana before and after acid hydrolysis. Nat. Prod. Sci. 2008, 14, 187–191. [Google Scholar]
- Han, T.; Cheng, G.; Liu, Y.; Yang, H.; Hu, Y.T.; Huang, W. In vitro evaluation of tectoridin, tectorigenin and tectorigenin sodium sulfonate on antioxidant properties. Food Chem. Toxicol. 2012, 50, 409–414. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; He, J.; Yan, J.; Li, H. Fluorescence spectroscopy and docking study in two flavonoids, isolated tectoridin and its aglycone tectorigenin, interacting with human serum albumin: A comparison study. Luminescence 2016, 31, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kagal, S.; Karmarkar, S.; Venkataraman, K. Synthetical experiments in the chromone group: Part XXXII. A synthesis of tectorigenin. In Proceedings of the Indian Academy of Sciences-Section A; Springer: Berlin/Heidelberg, Germany, 1956. [Google Scholar]
- Baker, W.; Downing, D.F.; Floyd, A.J.; Gilbert, B.; Ollis, W.D. Synthesis of isoflavones. V. Irigenin and tectorigenin. J. Chem. Soc. Perkin. 1 1970, 9, 1219–1223. [Google Scholar] [CrossRef]
- Baker, W.; Downing, D.F.; Floyd, A.J.; Gilbert, B.; Ollis, W.D.; Russell, R.C. Synthesis of irigenin and of tectorigenin, derivatives of 5,7-dihydroxy-6-methoxyisoflavone. Tetrahedron Lett. 1960, 1, 6–10. [Google Scholar] [CrossRef]
- Varady, J. A new method for the ring isomerization of isoflavones. Direct synthesis of tectorigenin, 4′-methyl-tectorigenin, caviunin and other isoflavones. Tetrahedron Lett. 1965, 6, 4273–4275. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Fang, R.Q.; Shi, L.; Ding, H.; Zhu, H.L. Synthesis, crystal structure, and growth inhibition of human hepatoma cell (HepG2) of polyphenolic compounds based on gallates. Canad. J. Chem. 2011, 85, 951–957. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Li, H.Q.; Xue, J.Y.; Shi, L.; Zhu, H.L. Efficient method for the synthesis of tectorigenin. Synth. Commun. 2008, 38, 525–529. [Google Scholar] [CrossRef]
- Strom, S.S.; Yamamura, Y.; Duphorne, C.M.; Spitz, M.R.; Babaian, R.J.; Pillow, P.C.; Hursting, S.D. Phytoestrogen intake and prostate cancer: A case-control study using a new database. Nutr. Cancer 1999, 33, 20–25. [Google Scholar] [CrossRef]
- Stettner, M.; Kaulfuss, S.; Burfeind, P.; Schweyer, S.; Strauss, A.; Ringert, R.H.; Thelen, P. The relevance of estrogen receptor-beta expression to the antiproliferative effects observed with histone deacetylase inhibitors and phytoestrogens in prostate cancer treatment. Mol. Cancer Ther. 2007, 6, 2626–2633. [Google Scholar] [CrossRef][Green Version]
- Branca, F. Dietary phyto-oestrogens and bone health. Proc. Nutr. Soc. 2003, 62, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.; Esch, H.L.; Wagner, J.; Rohnstock, L.; Metzler, M. Estrogenic and genotoxic potential of equol and two hydroxylated metabolites of daidzein in cultured human ishikawa cells. Toxicol. Lett. 2005, 158, 72–86. [Google Scholar] [CrossRef]
- Beck, V.; Rohr, U.; Jungbauer, A. Phytoestrogens derived from red clover: An alternative to estrogen replacement therapy? J. Steroid Biochem. Mol. Biol. 2005, 94, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Thelen, P.; Scharf, J.G.; Burfeind, P.; Hemmerlein, B.; Wuttke, W.; Spengler, B.; Christoffel, V.; Ringert, R.H.; Seidlova-Wuttke, D. Tectorigenin and other phytochemicals extracted from leopard lily Belamcanda chinensis affect new and established targets for therapies in prostate cancer. Carcinogenesis 2005, 26, 1360–1367. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Hesse, O.; Jarry, H.; Rimoldi, G.; Thelen, P.; Christoffel, V.; Wuttke, W. Belamcanda chinensis and the thereof purified tectorigenin have selective estrogen receptor modulator activities. Phytomedicine 2004, 11, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, C.; Bektic, J.; Spengler, B.; Galvin, D.; Christoffel, V.; Klocker, H.; Fitzpatrick, J.M.; Watson, R.W. Phytoestrogens derived from Belamcanda chinensis have an antiproliferative effect on prostate cancer cells in vitro. J. Urol. 2004, 172, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Leclercq, G. Relevance of breast cancer cell lines as models for breast tumours: An update. Breast Cancer Res. Treat 2004, 83, 249–289. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yuan, S.; Shen, J.; Wu, M.; Pan, L.; Kong, X. Suppression of human breast cancer cells by tectorigenin through downregulation of matrix metalloproteinases and MAPK signaling in vitro. Mol. Med. Rep. 2018, 17, 3935–3943. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Pistollato, F.; Calderon Iglesias, R.; Ruiz, R.; Aparicio, S.; Crespo, J.; Dzul Lopez, L.; Giampieri, F.; Battino, M. The use of natural compounds for the targeting and chemoprevention of ovarian cancer. Cancer Lett. 2017, 411, 191–200. [Google Scholar] [CrossRef]
- Yang, Y.I.; Lee, K.T.; Park, H.J.; Kim, T.J.; Choi, Y.S.; Shih Ie, M.; Choi, J.H. Tectorigenin sensitizes paclitaxel-resistant human ovarian cancer cells through downregulation of the Akt and NFκB pathway. Carcinogenesis 2012, 33, 2488–2498. [Google Scholar] [CrossRef]
- Jung, S.H.; Lee, Y.S.; Lee, S.; Lim, S.S.; Kim, Y.S.; Ohuchi, K.; Shin, K.H. Anti-angiogenic and anti-tumor activities of isoflavonoids from the rhizomes of Belamcanda chinensis. Planta Med. 2003, 69, 617–622. [Google Scholar]
- Jiang, C.P.; Ding, H.; Shi, D.H.; Wang, Y.R.; Li, E.G.; Wu, J.H. Pro-apoptotic effects of tectorigenin on human hepatocellular carcinoma HepG2 cells. World. J. Gastroenterol. 2012, 18, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Yu, Y.M.; Kong, Z.F.; Cao, Y.S.; Zhang, S. Tectorigenin inhibits Caco-2 human colon cells via NF-κB pathway suppression. Bangl. J. Pharmacol. 2015, 10, 948. [Google Scholar] [CrossRef]
- Lee, K.T.; Sohn, I.C.; Kim, Y.K.; Choi, J.H.; Choi, J.W.; Park, H.J.; Itoh, Y.; Miyamoto, K. Tectorigenin, an isoflavone of Pueraria thunbergiana Benth., induces differentiation and apoptosis in human promyelocytic leukemia HL-60 cells. Biol. Pharm. Bull. 2001, 24, 1117–1121. [Google Scholar] [CrossRef]
- Hasibeder, A.; Venkataramani, V.; Thelen, P.; Radzun, H.J.; Schweyer, S. Phytoestrogens regulate the proliferation and expression of stem cell factors in cell lines of malignant testicular germ cell tumors. Int. J. Oncol. 2013, 43, 1385–1394. [Google Scholar] [CrossRef]
- O’Toole, S.A.; Sheppard, B.L.; Sheils, O.; O’Leary, J.J.; Spengler, B.; Christoffel, V. Analysis of DNA in endometrial cancer cells treated with phyto-estrogenic compounds using comparative genomic hybridisation microarrays. Planta Med. 2005, 71, 435–439. [Google Scholar] [CrossRef]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New insights into the mechanisms of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef]
- Bae, E.A.; Han, M.J.; Lee, K.T.; Choi, J.W.; Park, H.J.; Kim, D.H. Metabolism of 6″-O-xylosyltectoridin and tectoridin by human intestinal bacteria and their hypoglycemic and in vitro cytotoxic activities. Biol. Pharm. Bull. 1999, 22, 1314–1318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, S.H.; Lee, Y.S.; Lee, S.; Lim, S.S.; Kim, Y.S.; Shin, K.H. Isoflavonoids from the rhizomes of Belamcanda chinensis and their effects on aldose reductase and sorbitol accumulation in streptozotocin induced diabetic rat tissues. Arch. Pharm. Res. 2002, 25, 306–312. [Google Scholar] [CrossRef]
- Yao, X.; Li, K.; Liang, C.; Zhou, Z.; Wang, J.; Wang, S.; Liu, L.; Yu, C.L.; Song, Z.B.; Bao, Y.L.; et al. Tectorigenin enhances PDX1 expression and protects pancreatic β-cells by activating ERK and reducing ER stress. J. Biol. Chem. 2020, 295, 12975–12992. [Google Scholar] [CrossRef]
- Qu, J.; Wu, Z.; Gao, J.; Wen, H.; Wang, T.; Yuan, D. Excretion of tectoridin metabolites in rat urine and bile orally administrated at different dosages and their inhibitory activity against aldose reductase. Fitoterapia 2014, 99, 99–108. [Google Scholar] [CrossRef]
- Munoz, M.; Lopez-Oliva, M.E.; Rodriguez, C.; Martínez, M.P.; Saenz-Medina, J.; Sanchez, A.; Climent, B.; Benedito, S.; Garcia-Sacristan, A.; Rivera, L.; et al. Differential contribution of Nox1, Nox2 and Nox4 to kidney vascular oxidative stress and endothelial dysfunction in obesity. Redox Biol. 2020, 28, 101330. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ma, C.; Wu, H.; Zhang, H.; Yuan, F.; Yang, G.; Yang, Q.; Jia, L.; Liang, Z.; Kang, L. Tectorigenin attenuates diabetic nephropathy by improving vascular endothelium dysfunction through activating AdipoR1/2 pathway. Pharmacol. Res. 2020, 153, 104678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, X.L.; Zhang, D.Y.; Gao, X.J.; Zhou, L.; Qin, X.Y.; Xie, G.Y.; Liu, K.; Qin, Y.; Liu, B.L.; et al. Tectorigenin attenuates palmitate-induced endothelial insulin resistance via targeting ROS-associated inflammation and IRS-1 pathway. PLoS ONE 2013, 8, e66417. [Google Scholar] [CrossRef]
- Zanquetta, M.M.; Alves-Wagner, A.B.; Mori, R.C.; Campello, R.S.; Machado, U.F. Recovery of insulin sensitivity and Slc2a4 mRNA expression depend on T3 hormone during refeeding. Metabolism 2014, 63, 328–334. [Google Scholar] [CrossRef]
- Yao, X.; Liu, L.; Shao, W.; Bai, M.; Ding, X.; Wang, G.; Wang, S.; Zheng, L.; Sun, Y.; Wang, G.; et al. Tectorigenin targets PKACα to promote GLUT4 expression in skeletal muscle and improve insulin resistance in vitro and in vivo. Int. J. Biol. Sci. 2023, 19, 1579–1596. [Google Scholar] [CrossRef]
- Pappachan, J.M.; Fernandez, C.J.; Chacko, E.C. Diabesity and antidiabetic drugs. Mol. Aspects Med. 2019, 66, 3–12. [Google Scholar] [CrossRef]
- Li, Q.Y.; Chen, L.; Yan, M.M.; Shi, X.J.; Zhong, M.K. Tectorigenin regulates adipogenic differentiation and adipocytokines secretion via PPARγ and IKK/NF-κB signaling. Pharm. Biol. 2015, 53, 1567–1575. [Google Scholar] [CrossRef]
- Zhou, M.; Deng, Y.; Liu, M.; Liao, L.; Dai, X.; Guo, C.; Zhao, X.; He, L.; Peng, C.; Li, Y. The pharmacological activity of berberine, a review for liver protection. Eur. J. Pharmacol. 2021, 890, 173655. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Habtemariam, S. Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomed. Pharmacother. 2019, 112, 108600. [Google Scholar] [CrossRef]
- Lee, H.W.; Choo, M.K.; Bae, E.A.; Kim, D.H. Beta-glucuronidase inhibitor tectorigenin isolated from the flower of Pueraria thunbergiana protects carbon tetrachloride-induced liver injury. Liver Int. 2003, 23, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.X.; Shi, D.H.; Chen, Y.X.; Cui, J.T.; Wang, Y.R.; Jiang, C.P.; Wu, J.H. The therapeutic effects of tectorigenin on chemically induced liver fibrosis in rats and an associated metabonomic investigation. Arch. Pharm. Res. 2012, 35, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Bae, E.A.; Kim, D.H. Hepatoprotective effect of tectoridin and tectorigenin on tert-butyl hyperoxide-induced liver injury. J. Pharmacol. Sci. 2005, 97, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Y.; Fan, L.; Xu, K.; Ji, F.; Xie, Z.; Ouyang, X.; Wu, D.; Li, L. Tectorigenin protects against experimental fulminant hepatic failure by regulating the TLR4/mitogen-activated protein kinase and TLR4/nuclear factor-κB pathways and autophagy. Phytother. Res. 2019, 33, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, Y.R.; Huang, W.Y.; Tan, R.X. Anti-proliferative and pro-apoptotic effects of tectorigenin on hepatic stellate cells. World J. Gastroenterol. 2010, 16, 3911–3918. [Google Scholar] [CrossRef]
- Duan, R.; Huang, K.; Guan, X.; Li, S.; Xia, J.; Shen, M.; Sun, Z.; Yu, Z. Tectorigenin ameliorated high-fat diet-induced nonalcoholic fatty liver disease through anti-inflammation and modulating gut microbiota in mice. Food Chem. Toxicol. 2022, 164, 112948. [Google Scholar] [CrossRef]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar] [CrossRef]
- Kim, Y.P.; Yamada, M.; Lim, S.S.; Lee, S.H.; Ryu, N.; Shin, K.H.; Ohuchi, K. Inhibition by tectorigenin and tectoridin of prostaglandin E2 production and cyclooxygenase-2 induction in rat peritoneal macrophages. Biochim. Biophys. Acta 1999, 1438, 399–407. [Google Scholar] [CrossRef]
- Hong, J.; Shin, K.H.; Lim, S.S.; Kwak, J.H.; Zee, O.; Ishihara, K.; Hirasawa, N.; Seyama, T.; Ohuchi, K. Lead compounds for anti-inflammatory drugs isolated from the plants of the traditional oriental medicine in Korea. Inflamm. Allergy Drug Targets 2008, 7, 195–202. [Google Scholar] [CrossRef]
- Pan, C.H.; Kim, E.S.; Jung, S.H.; Nho, C.W.; Lee, J.K. Tectorigenin inhibits IFN-gamma/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells. Arch. Pharm. Res. 2008, 31, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Kim, Y.J.; Kim, B.Y.; Park, G.; Jeong, S.J. The anti-neuroinflammatory activity of tectorigenin pretreatment via downregulated NF-κB and ERK/JNK pathways in BV-2 microglial and microglia inactivation in mice with lipopolysaccharide. Front. Pharmacol. 2018, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yan, K.; Xing, S.; Cheng, J. Tectorigenin alleviates the apoptosis and inflammation in spinal cord injury cell model through inhibiting insulin-like growth factor-binding protein 6. Open Med. 2023, 18, 20230680. [Google Scholar] [CrossRef]
- Ma, C.H.; Liu, J.P.; Qu, R.; Ma, S.P. Tectorigenin inhibits the inflammation of LPS-induced acute lung injury in mice. Chin. J. Nat. Med. 2014, 12, 841–846. [Google Scholar] [CrossRef]
- Ha, L.M.; Que, D.T.N.; Huyen, D.T.T.; Long, P.Q.; Dat, N.T. Toxicity, analgesic and anti-inflammatory activities of tectorigenin. Immunopharmacol. Immunotoxicol. 2013, 35, 336–340. [Google Scholar] [CrossRef]
- Li, J.; Yan, W.; Ren, F.; Sang, H. Tectorigenin inhibits inflammation in keratinocytes by inhibition of NLRP3 inflammasome regulated by the TLR4/NF-κB pathway. Allergol. Immunopathol. 2023, 51, 82–89. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, G.T.; Yun, H.M.; Kim, Y.C.; Kwon, I.K.; Kim, E.C. Tectorigenin promotes osteoblast differentiation and in vivo bone healing, but suppresses osteoclast differentiation and in vivo bone resorption. Mol. Cells 2018, 41, 476–485. [Google Scholar]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Tayab, M.A.; Chowdhury, K.A.A.; Jabed, M.; Mohammed Tareq, S.; Kamal, A.; Islam, M.N.; Uddin, A.M.K.; Hossain, M.A.; Emran, T.B.; Simal-Gandara, J. Antioxidant-rich woodfordia fruticosa leaf extract alleviates depressive-like behaviors and impede hyperglycemia. Plants 2021, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Arman, M.S.I.; Tayab, M.A.; Islam, M.N.; Xiao, J. Recent advances in the biosynthesis, bioavailability, toxicology, pharmacology, and controlled release of citrus neohesperidin. Crit. Rev. Food Sci. Nutr. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Chen, W.; Zhuang, J.; Li, Y.; Shen, Y.; Zheng, X. Myricitrin protects against peroxynitrite-mediated DNA damage and cytotoxicity in astrocytes. Food Chem. 2013, 141, 927–933. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Arct, J.; Pytkowska, K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Kim, S.Y.; Kim, H.S.; Kim, D.H.; Hyun, J.W. Cytoprotective effect of tectorigenin, a metabolite formed by transformation of tectoridin by intestinal microflora, on oxidative stress induced by hydrogen peroxide. Eur. J. Pharmacol. 2005, 519, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Jung, G.O.; Choi, J.; Lee, K.T.; Park, H.J. Potent antimutagenic and their anti-lipid peroxidative effect of kaikasaponin III and tectorigenin from the flower of Pueraria thunbergiana. Arch. Pharm. Res. 2002, 25, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Bae, E.A.; Han, M.J.; Kim, D.H. In vitro anti-Helicobacter pylori activity of irisolidone isolated from the flowers and rhizomes of Pueraria thunbergiana. Planta Med. 2001, 67, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, J.; Zhang, J.; Wang, T.; Zhou, Y.; Lv, Q.; Hu, N.; Shen, X.; Deng, X. Tectorigenin reduces type IV pilus-dependent cell adherence in Clostridium perfringens. FEMS Microbiol. Lett. 2019, 366, 112. [Google Scholar] [CrossRef]
- Guan, Z.; Luo, L.; Liu, S.; Guan, Z.; Zhang, Q.; Li, X.; Tao, K. The role of depletion of gut microbiota in osteoporosis and osteoarthritis: A narrative review. Front. Endocrinol. 2022, 13, 847401. [Google Scholar] [CrossRef]
- Shan, S.K.; Lin, X.; Li, F.; Xu, F.; Zhong, J.Y.; Guo, B.; Wang, Y.; Zheng, M.H.; Wu, F.; Yuan, L.Q. Exosomes and bone disease. Curr. Pharm. Des. 2019, 25, 4536–4549. [Google Scholar] [CrossRef]
- Wang, S.; Deng, Z.; Ma, Y.; Jin, J.; Qi, F.; Li, S.; Liu, C.; Lyu, F.J.; Zheng, Q. The role of autophagy and mitophagy in bone metabolic disorders. Int. J. Biol. Sci. 2020, 16, 2675–2691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, Y.L.; Wang, R.; Wang, X.Q.; Zhang, H. Exercise for osteoporosis: A literature review of pathology and mechanism. Front. Immunol. 2022, 13, 1005665. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Hartmann, L.C. Selective estrogen-receptor modulators—Mechanisms of action and application to clinical practice. N. Engl. J. Med. 2003, 348, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Kim, M.H.; Kim, H.; Kim, H.Y.; Nam, S.Y.; Han, N.R.; Lee, B.; Cho, H.; Moon, P.D.; Kim, H.M. PCE17 and its active compounds exert an anti-osteoporotic effect through the regulation of receptor activator of nuclear factor-κB ligand in ovariectomized mice. J. Food Biochem. 2018, 42, e12561. [Google Scholar] [CrossRef]
- Ma, C.; Xu, K.; Meng, J.; Ran, J.; Adel Abdo Moqbel, S.; Liu, A.; Yan, S.; Wu, L. Tectorigenin inhibits RANKL-induced osteoclastogenesis via suppression of NF-κB signalling and decreases bone loss in ovariectomized C57BL/6. J. Cell. Mol. Med. 2018, 22, 5121–5131. [Google Scholar] [CrossRef]
- Wang, C.L.; Li, D.; Wang, C.D.; Xiao, F.; Zhu, J.F.; Shen, C.; Zuo, B.; Cui, Y.M.; Wang, H.; Gao, Y.; et al. Anti-inflammatory and anti-osteoarthritis effects of tectorigenin. Biol. Open 2017, 6, 1130–1136. [Google Scholar] [CrossRef]
- Aicale, R.; Oliviero, A.; Maffulli, N. Management of achilles and patellar tendinopathy: What we know, what we can do. J. Foot Ankle. Res. 2020, 13, 59. [Google Scholar] [CrossRef]
- Moqbel, S.A.A.; Xu, K.; Chen, Z.; Xu, L.; He, Y.; Wu, Z.; Ma, C.; Ran, J.; Wu, L.; Xiong, Y. Tectorigenin alleviates inflammation, apoptosis, and ossification in rat tendon-derived stem cells via modulating NF-κB and MAPK pathways. Front. Cell Dev. Biol. 2020, 8, 568894. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Dai, X.; Jin, J.; Jia, Y.; Yang, K.; Han, J.; Zhang, Z.; Ding, X.; Yao, C.; Sun, T.; Zhu, C.; et al. A non-retinol retinoic acid receptor-γ (RAR-γ/NR1B3) selective agonist, tectorigenin, can effectively inhibit the ultraviolet a-induced skin damage. Br. J. Pharmacol. 2022, 179, 4722–4737. [Google Scholar] [CrossRef]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J. Dermatolog. Treat 2017, 28, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Yoshihira, K.; Tokumaru, M.; Zisheng, X.; Murakami, N. Inhibitors for expression of IgE receptor on human mast cell from Puerariae Flos. Bioorg. Med. Chem. Lett. 2010, 20, 3872–3875. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Shin, Y.W.; Lee, H.U.; Lee, C.S.; Kim, D.H. Passive cutaneous anaphylaxis-inhibitory action of tectorigenin, a metabolite of tectoridin by intestinal microflora. Biol. Pharm. Bull. 2004, 27, 1099–1102. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, W.; Jiang, X.; Liu, Y. Mesenchymal stem cell-derived exosomes in cardiovascular and cerebrovascular diseases: From mechanisms to therapy. Biomed. Pharmacother. 2023, 163, 114817. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Ministrini, S.; Carbone, F.; Camici, G.G.; Montecucco, F. Cytokines as therapeutic targets for cardio- and cerebrovascular diseases. Basic. Res. Cardiol. 2021, 116, 23. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Yadav, J.S. The role of antiplatelet therapy in carotid stenting for ischemic stroke prevention. Stroke 2006, 37, 1572–1577. [Google Scholar] [CrossRef]
- Applova, L.; Karlíckova, J.; Ríha, M.; Filipsky, T.; Macakova, K.; Spilkova, J.; Mladenka, P. The isoflavonoid tectorigenin has better antiplatelet potential than acetylsalicylic acid. Phytomedicine 2017, 35, 11–17. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Li, S.; Yang, X.; Qin, Y.; Zhang, Y.; Liu, C. Screening and isolation of potential lactate dehydrogenase inhibitors from five chinese medicinal herbs: Soybean, Radix pueraria, Flos pueraria, Rhizoma belamcandae, and Radix astragali. J. Sep. Sci. 2016, 39, 2043–2049. [Google Scholar] [CrossRef]
- Yao, L.; Yang, M.; Zhang, J.; Wang, F.; Liu, Q.; Xie, X.; Liu, Z.; Guo, Q.; Su, H.; Zhai, J.; et al. Tectorigenin attenuates the OGD/R-induced HT-22 cell damage through regulation of the PI3K/AKT and the PPARγ/NF-κB pathways. Hum. Exp. Toxicol. 2021, 40, 1320–1331. [Google Scholar] [CrossRef]
- Feng, W. Tectorigenin attenuates cognitive impairments in mice with chronic cerebral ischemia by inhibiting the TLR4/NF-κB signaling pathway. Biosci. Biotechnol. Biochem. 2021, 85, 1665–1674. [Google Scholar] [CrossRef]
- Qian, X.; Xiao, Q.; Li, Z. Tectorigenin regulates migration, invasion, and apoptosis in dexamethasone-induced human airway epithelial cells through up-regulating miR-222-3p. Drug Dev. Res. 2021, 82, 959–968. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Chen, S.; Wu, J.; Ye, X.; Xu, L.; Chen, H.; Zhang, D.; Tan, R.; Wang, Y. Tectorigenin inhibits the in vitro proliferation and enhances miR-338* expression of pulmonary fibroblasts in rats with idiopathic pulmonary fibrosis. J. Ethnopharmacol. 2010, 131, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J. Disease modification and Neuroprotection in neurodegenerative disorders. Transl. Neurodegener. 2017, 6, 25. [Google Scholar] [CrossRef]
- Crews, F.T.; Bechara, R.; Brown, L.A.; Guidot, D.M.; Mandrekar, P.; Oak, S.; Qin, L.; Szabo, G.; Wheeler, M.; Zou, J. Cytokines and alcohol. Alcohol. Clin. Exp. Res. 2006, 30, 720–730. [Google Scholar] [CrossRef]
- Yeh, L.T.; Hsu, L.S.; Chung, Y.H.; Chen, C.J. Tectorigenin inhibits glioblastoma proliferation by G0/G1 cell cycle arrest. Medicina 2020, 56, 681. [Google Scholar] [CrossRef]
- Marti, H.H.; Wenger, R.H.; Rivas, L.A.; Straumann, U.; Digicaylioglu, M.; Henn, V.; Yonekawa, Y.; Bauer, C.; Gassmann, M. Erythropoietin gene expression in human, monkey and murine brain. Eur. J. Neurosci. 1996, 8, 666–676. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Lin, S.H.; Lin, H.C.; Hung, C.C.; Wang, C.Y.; Lin, Y.C.; Hung, K.S.; Lien, C.C.; Kuan, C.Y.; Lee, Y.H. Cell type-specific dependency on the PI3K/Akt signaling pathway for the endogenous Epo and VEGF induction by baicalein in neurons versus astrocytes. PLoS ONE 2013, 8, e69019. [Google Scholar] [CrossRef][Green Version]
- John, M.J.; Jaison, V.; Jain, K.; Kakkar, N.; Jacob, J.J. Erythropoietin use and abuse. Indian J. Endocrinol. Metab. 2012, 16, 220–227. [Google Scholar]
- Liu, E.Y.; Zheng, Z.X.; Zheng, B.Z.; Xia, Y.; Guo, M.S.; Dong, T.T.; Tsim, K.W.K. Tectorigenin, an isoflavone aglycone from the rhizome of Belamcanda chinensis, induces neuronal expression of erythropoietin via accumulation of hypoxia-inducible factor-1α. Phytother. Res. 2020, 34, 1329–1337. [Google Scholar] [CrossRef]
- Gong, P.; Deng, F.; Zhang, W.; Ji, J.; Liu, J.; Sun, Y.; Hu, J. Tectorigenin attenuates the MPP(+)-induced SH-SY5Y cell damage, indicating a potential beneficial role in Parkinson’s disease by oxidative stress inhibition. Exp. Ther. Med. 2017, 14, 4431–4437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Yang, J.; Zhu, B.; Fan, J.; Hu, Q.; Wang, L. Tectorigenin protects against unilateral ureteral obstruction by inhibiting Smad3-mediated ferroptosis and fibrosis. Phytother. Res. 2022, 36, 475–487. [Google Scholar] [CrossRef]
- Jung, D.Y.; Ha, H.; Kim, C. Induction of growth hormone release by Pueraria thunbergiana BENTH. Horm. Metab. Res. 2004, 36, 86–91. [Google Scholar] [PubMed]
- Tomonaga, T.; Mine, T.; Kojima, I.; Taira, M.; Hayashi, H.; Isono, K. Isoflavonoids, genistein, psi-tectorigenin, and orobol, increase cytoplasmic free calcium in isolated rat hepatocytes. Biochem. Biophys. Res. Commun. 1992, 182, 894–899. [Google Scholar] [CrossRef]
- Cheng, H.H.; Liang, W.Z.; Liao, W.C.; Kuo, C.C.; Hao, L.J.; Chou, C.T.; Jan, C.R. Investigation of effect of tectorigenin (O-methylated isoflavone) on Ca2+ signal transduction and cytotoxic responses in canine renal tubular cells. Chin. J. Physiol. 2020, 63, 60–67. [Google Scholar]
- Madden, J.C.; Thompson, C.V. Pharmacokinetic tools and applications. Methods Mol. Biol. 2022, 2425, 57–83. [Google Scholar] [PubMed]
- Wang, S.; Gong, T.; Lu, J.; Kano, Y.; Yuan, D. Simultaneous determination of tectorigenin and its metabolites in rat plasma by ultra performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. B 2013, 933, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Z.; Gu, J. UGT1A1 and UGT1A9 are responsible for phase ii metabolism of tectorigenin and irigenin in vitro. Molecules 2022, 27, 4104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Qi, L.W.; Yang, X.L.; Huang, W.Z.; Li, P.; Yang, Z.L. Identification of the major metabolites of tectorigenin in rat bile by liquid chromatography combined with time-of-flight and ion trap tandem mass spectrometry. Rapid. Commun. Mass. Spectrom. 2008, 22, 2677–2684. [Google Scholar] [CrossRef]
- Zhang, W.D.; Yang, X.L.; Cao, J.; Li, P.; Yang, Z.L. Identification of key metabolites of tectorigenin in rat urine by HPLC-MS(n). Biomed. Chromatogr. 2009, 23, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, G.; Zhao, L.; Wang, S.; Kano, Y.; Yuan, D. Excretion of tectorigenin in rat urine orally administrated at different dosages by ultra-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Eur. J. Drug. Metab. Pharmacokinet 2015, 40, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Qi, L.W.; Yang, X.L.; Lu, Y.W.; Li, P.; Yang, Z.L. Determination of tectorigenin in rat plasma: Application to a pharmacokinetic study after oral administration of tectorigenin or its prodrug tectoridin. Chromatographia 2008, 68, 1021. [Google Scholar] [CrossRef]
- Li, J.; Yao, Y.; Zhou, M.; Yu, Z.; Wang, X. Pharmacokinetics of tectorigenin, tectoridi, irigenin, and iridin in mouse blood after intravenous administration by UPLC-MS/MS. Acta Chromatogr. 2021, 34, 246–252. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Bu, F.; Chen, N.; Zhang, H.; Gu, J. Simultaneous determination of eight constituents in rat plasma by HPLC-MS/MS and its application to a pharmacokinetic study after oral administration of Shejin-liyan Granule. Biomed. Chromatogr. 2019, 33, e4648. [Google Scholar] [CrossRef]
- Zhang, W.D.; Yang, W.J.; Wang, X.J.; Gu, Y.; Wang, R. Simultaneous determination of tectorigenin, irigenin and irisflorentin in rat plasma and urine by UHPLC-MS/MS: Application to pharmacokinetics. J. Chromatogr. B 2011, 879, 3735–3741. [Google Scholar] [CrossRef]
- Yang, M.; Yang, X.; An, J.; Xiao, W.; Wang, Z.; Huang, W.; Yang, Z.; Li, F. Comparative pharmacokinetic profiles of tectorigenin in rat plasma by UPLC-MS/MS after oral administration of Iris tectorum Maxim extract and pure tectoridin. J. Pharm. Biomed. Anal. 2015, 114, 34–41. [Google Scholar] [CrossRef]
- Bai, X.; Xie, Y.; Liu, J.; Qu, J.; Kano, Y.; Yuan, D. Isolation and identification of urinary metabolites of kakkalide in rats. Drug Metab. Dispos. 2010, 38, 281–286. [Google Scholar] [CrossRef]
- Zhang, G.; Qi, W.; Xu, L.; Kano, Y.; Yuan, D. Pharmacokinetics of irisolidone and its main metabolites in rat plasma determined by ultra performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. B 2015, 1005, 23–29. [Google Scholar] [CrossRef]
- Qu, J.; Gao, J.; Sun, J.; Zhang, L.; Makino, T.; Yuan, D. Pharmacokinetics of conjugated metabolites in rat plasma after oral administration of tectoridin. J. Chromatogr. B 2012, 902, 61–69. [Google Scholar] [CrossRef]
- Chen, Y.; Song, W.; Peng, Z.H.; Ge, B.Y.; Han, F.M. Identification of metabolites of tectoridin in-vivo and in-vitro by liquid chromatography-tandem mass spectrometry. J. Pharm. Pharmacol. 2008, 60, 709–716. [Google Scholar] [CrossRef]
- Thelen, P.; Seseke, F.; Ringert, R.H.; Wuttke, W.; Seidlova-Wuttke, D. Pharmacological potential of phytoestrogens in the treatment of prostate cancer. Urologe. A 2006, 45, 195–201. [Google Scholar] [CrossRef]
- Shin, J.E.; Bae, E.A.; Lee, Y.C.; Ma, J.Y.; Kim, D.H. Estrogenic effect of main components kakkalide and tectoridin of Puerariae Flos and their metabolites. Biol. Pharm. Bull. 2006, 29, 1202–1206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, Y.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Chen, Z.; Wu, L. Metabolism and pharmacological activities of the natural health-benefiting compound diosmin. Food Funct. 2020, 11, 8472–8492. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ravetti, S.; Garro, A.G.; Gaitan, A.; Murature, M.; Galiano, M.; Brignone, S.G.; Palma, S.D. Naringin: Nanotechnological strategies for potential pharmaceutical applications. Pharmaceutics 2023, 15, 863. [Google Scholar] [CrossRef]
- Biasutto, L.; Zoratti, M. Prodrugs of quercetin and resveratrol: A strategy under development. Curr. Drug Metab. 2014, 15, 77–95. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, H.; Li, S.; Zhang, H.; Zhang, D.; Wu, A.; Zhang, C.; Li, C.; Fu, X.; Chen, S.; et al. A double-layered gastric floating tablet for zero-order controlled release of dihydromyricetin: Design, development, and in vitro/in vivo evaluation. Int. J. Pharm. 2023, 638, 122929. [Google Scholar] [CrossRef]
- Taghavinia, F.; Teymouri, F.; Farokhrouz, F.; Bagherabad, E.H.; Farjami, S.; Karimi, E.; Oskoueian, E.; Le, H.H.; Shakeri, M. Nanoliposome-loaded phenolics from Nasturtium officinale improves health parameters in a colorectal cancer mouse model. Animals 2022, 12, 3492. [Google Scholar] [CrossRef]
- Beyrami, M.; Karimi, E.; Oskoueian, E. Synthesized chrysin-loaded nanoliposomes improves cadmium-induced toxicity in mice. Environ. Sci. Pollut. Res. Int. 2020, 27, 40643–40651. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Karimi, E.; Oskoueian, E.; Es-Haghi, A.; Yazdi, M.E.T. Comparative study on the biological effects of sodium citrate-based and apigenin-based synthesized silver nanoparticles. Nutr. Cancer 2021, 73, 1511–1519. [Google Scholar] [CrossRef]
- Tabatabaeain, S.F.; Karimi, E.; Hashemi, M. Satureja khuzistanica essential oil-loaded solid lipid nanoparticles modified with chitosan-folate: Evaluation of encapsulation efficiency, cytotoxic and pro-apoptotic properties. Front. Chem. 2022, 10, 904973. [Google Scholar] [CrossRef] [PubMed]
- Moeini, S.; Karimi, E.; Oskoueian, E. Antiproliferation effects of nanophytosome-loaded phenolic compounds from fruit of Juniperus polycarpos against breast cancer in mice model: Synthesis, characterization and therapeutic effects. Cancer Nanotechnol. 2022, 13, 20. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef] [PubMed]
- Javia, A.; Vanza, J.; Bardoliwala, D.; Ghosh, S.; Misra, L.A.; Patel, M.; Thakkar, H. Polymer-drug conjugates: Design principles, emerging synthetic strategies and clinical overview. Int. J. Pharm. 2022, 623, 121863. [Google Scholar]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(lactic acid)-based microparticles for drug delivery applications: An overview of recent advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Dhote, V.; Mahajan, S.C.; Mishra, P.K.; Mishra, D.K. Solid dispersion in pharmaceutical drug development: From basics to clinical applications. Curr. Drug Deliv. 2014, 11, 155–171. [Google Scholar] [CrossRef]
- Shuai, S.; Yue, S.; Huang, Q.; Wang, W.; Yang, J.; Lan, K.; Ye, L. Preparation, characterization and in vitro/vivo evaluation of tectorigenin solid dispersion with improved dissolution and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 413–422. [Google Scholar] [CrossRef]
- Zhang, Y.; He, L.; Yue, S.; Huang, Q.; Zhang, Y.; Yang, J. Characterization and evaluation of a self-microemulsifying drug delivery system containing tectorigenin, an isoflavone with low aqueous solubility and poor permeability. Drug Deliv. 2017, 24, 632–640. [Google Scholar] [CrossRef]
- Kim, Y.H.; Tabata, Y. Dual-controlled release system of drugs for bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Wang, F.; Zhao, N.; Yang, C.Y.; Wang, G.Y.; Wei, Y.; Zhang, W.Y. Preparation and release mechanism of tectorigenin intragastric floating sustained-release tablets. Chin. J. Chin. Mater. Med. 2017, 42, 5. [Google Scholar]
| No. | Plant | Family | Part | Ref. |
|---|---|---|---|---|
| 1 | Belamcanda chinensis (L.) DC. | Iridaceae | Rhizomes | [25,26] |
| 2 | Iris spuria L. (Calizona) | Iridaceae | Rhizomes | [27] |
| 3 | Iris tectorum Maxim | Iridaceae | Roots and rhizomes | [28] |
| 4 | Iris japonica Thunb. | Iridaceae | Whole plant | [29,30] |
| 5 | Iris dichotoma Pall. | Iridaceae | Rhizomes | [31] |
| 6 | Iris germanica L. | Iridaceae | Rhizomes | [32] |
| 7 | Iris unguicularis Poiret | Iridaceae | Rhizomes | [33] |
| 8 | Iris loczyi Kan. | Iridaceae | Whole plant | [33] |
| 9 | Iris kashmiriana Baker | Iridaceae | Rhizomes | [34] |
| 10 | Iris crocea Jacq. ex R. C. Foster | Iridaceae | Roots and rhizomes | [35,36] |
| 11 | Iris ensata Thunb. | Iridaceae | Rhizomes | [36] |
| 12 | Iris germanica L. | Iridaceae | Underground parts | [37] |
| 13 | Iris hungarica Waldst. et Kit. | Iridaceae | Rhizomes | [38] |
| 14 | Iris confusa Sealy | Iridaceae | Underground parts | [37] |
| 15 | Iris pseudacorus L. | Iridaceae | Underground parts | [37] |
| 16 | Pueraria lobata (Willd.) Ohwi | Leguminosae | Flowers | [40,41] |
| 17 | Pueraria thomsonii Benth. | Leguminosae | Flowers | [18,40] |
| 18 | Pueraria thunbergiana Benth. | Leguminosae | Flowers | [42] |
| 19 | Dalbergia odorifera T. Chen | Leguminosae | Leaves | [43] |
| 20 | Dalbergia parviflora Roxb. | Leguminosae | Heartwood | [44] |
| 21 | Euchresta formosana (Hayata) Ohwi | Leguminosae | Roots | [45] |
| 22 | Codonopsis pilosula (Franch.) Nannf. | Campanulaceae | Roots | [46] |
| 23 | Morus alba L. | Moraceae | Velamen and leaves | [46] |
| 24 | Viola hondoensis W. Becker et H. Boissieu. | Violaceae | Aerial parts | [47] |
| 25 | Eleocharis dulcis (Burm. f.) Trin. ex Hensch. | Cyperaceae | Peel | [48] |
| Inclusion of Drug Components | Dosage and Route | Animal Model | Pharmacokinetic Parameters | Ref. |
|---|---|---|---|---|
| Tectorigenin | 130 mg/kg, oral administration | Male Sprague-Dawley rats | Cmax = 12.0 ± 0.63 μmol/L, Tmax = 0.23 ± 0.15 h, t1/2 = 11.7 ± 5.74 h, AUC(0–t) = 84.2 ± 8.15 μmol/L × h | [171] |
| Tectorigenin | 80 mg/kg, oral administration | Sprague-Dawley rats | Cmax = 1.46 ± 0.30 μg/mL, Tmax = 1.20 ± 0.30 h, AUC(0–t) = 7.31 ± 1.20 μg/mL × h | [176] |
| Tectoridin | 130 mg/kg, oral administration | Sprague-Dawley rats | Cmax = 1.08 ± 0.25 μg/mL, Tmax = 7.11 ± 1.10 h, AUC(0–t) = 8.02 ± 1.10 μg/mL × h | [176] |
| Tectorigenin | 5 mg/kg, sublingual intravenous administration | Male mice | Cmax = 349.0 ± 172.4 ng/mL, t1/2 = 2.4 ± 1.5 h, AUC(0–t) = 219 ± 94 ng/mL × h | [177] |
| Shejin-liyan Granule | 2.0 g/kg, oral administration | Sprague-Dawley rats | Cmax 1 = 112.72 ± 60.04 ng/mL, Cmax 2 = 463.67 ± 170.46 ng/mL, Tmax 1 = 0.75 ± 0.35 h, Tmax 2 = 6.00 ± 2.19 h, t1/2 = 12.22 ± 2.42 h, AUC(0–t) = 5340.68 ± 1223.89 ng/mL × h | [178] |
| Belamcandae Rhizoma extract | 50 mg/kg, oral administration | Male Sprague-Dawley rats | Cmax = 1473.2 ± 156.9 ng/mL, Tmax = 0.5 ± 0.0 h, t1/2 = 3.55 ± 0.34 h, AUC(0–t) = 5800.28 ± 658.0 ng/mL × h | [179] |
| Extract of rhizome of Iris tectorum | 46 mg/kg, oral administration | Male Sprague-Dawley rats | Cmax = 740.3 ± 96.3 ng/mL, Tmax = 0.2 ± 0.1 h, t1/2 = 6.6 ± 2.6 h, AUC(0–t) = 4189.5 ± 60.1 ng/mL × h | [180] |
| Tectoridin | 32 mg/kg, oral administration | Male Sprague-Dawley rats | Cmax = 476.0 ± 57.8 ng/mL, Tmax = 0.25 ± 0.2 h, t1/2 = 3.4 ± 1.4 h, AUC(0–t) = 1760.9 ± 64.2 ng/mL × h | [180] |
| Irisolidone | 100 mg/kg, oral administration | Male Sprague-Dawley rats | Cmax = 0.918 ± 0.400 μmol/L, Tmax = 11.3 ± 1.03 h, t1/2 = 12.8 ± 8.28 h, AUC(0–t) = 11.8 ± 5.70 μmol/L × h | [182] |
| Tectoridin | 200 mg/kg, oral administration | Male Sprague-Dawley rats | Cmax = 8.67 ± 3.07 μmol/L, Tmax = 4.92 ± 2.87 h, AUC(0–t) = 72.0 ± 22.0 μmol/L × h | [183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, J.; Fu, F.; Han, C.; Wu, Y.; Xia, Q.; Du, D. Tectorigenin: A Review of Its Sources, Pharmacology, Toxicity, and Pharmacokinetics. Molecules 2023, 28, 5904. https://doi.org/10.3390/molecules28155904
Rong J, Fu F, Han C, Wu Y, Xia Q, Du D. Tectorigenin: A Review of Its Sources, Pharmacology, Toxicity, and Pharmacokinetics. Molecules. 2023; 28(15):5904. https://doi.org/10.3390/molecules28155904
Chicago/Turabian StyleRong, Juan, Fei Fu, Chenxia Han, Yaling Wu, Qing Xia, and Dan Du. 2023. "Tectorigenin: A Review of Its Sources, Pharmacology, Toxicity, and Pharmacokinetics" Molecules 28, no. 15: 5904. https://doi.org/10.3390/molecules28155904
APA StyleRong, J., Fu, F., Han, C., Wu, Y., Xia, Q., & Du, D. (2023). Tectorigenin: A Review of Its Sources, Pharmacology, Toxicity, and Pharmacokinetics. Molecules, 28(15), 5904. https://doi.org/10.3390/molecules28155904