Abstract
A series of benzocycloalkanone derivatives have been prepared and evaluated as antimalarial and antitrypanosomal agents. The compounds were obtained by direct coupling of preformed 4-substituted benzaldehyde and indanone or tetralone substitutes through aldol condensation of Claisen-Schmidt using sodium hydroxide as a catalyst in ethanol at room temperature. Although designed to inhibit the formation of β-hematin in vitro, only three compounds, 10, 11, and 12, showed activities greater than 50% (75.16%, 63.02%, and 56.17%, respectively). The results of the in vivo antimalarial evaluation show that 10, 11, and 12 reduced parasitemia marginally, and an insignificant increase in the days of survival of the mice was observed. As trypanocidals, all compounds showed marginal activity as inhibitors of the proliferation of T. cruzi epimastigotes, except compound 33, with an activity of 51.08 ± 3.4% compared to the activity shown by the reference compound benznidazole 59.99 ± 2.9%. The compounds appear to have little cytotoxic effect against VERO cells in vitro; this new class of Michael acceptor agents clearly warrants further investigation.
1. Introduction
A parasite is an organism that lives on or in a host organism and obtains its food from or at the expense of its host. There are three main classes of parasites that can cause disease in humans and animals: protozoa, helminths, and ectoparasites. Protozoa, together with helminths, represent the main cause of parasitic disease in humans and animals [1]. Despite great advances in modern medicine, parasitic infections continue to affect a large number of humans as well as the economies of the affected countries, either directly or indirectly. The prevalence of major human protozoan parasitic diseases (PPDs) is estimated at approximately 790 million individual cases, with an annual death toll of 810,000. Most PPDs are widely regarded as poverty-related and neglected tropical diseases that have been largely ignored for many years [2].
Chagas disease (CD), also known as American trypanosomiasis, caused by the protozoan Trypanosoma cruzi, is a neglected tropical disease (NTD) with high prevalence and significant morbidity and mortality. It is endemic in 21 countries; the World Health Organization (WHO) estimates the prevalence of CD in about 7 million cases, resulting in more than 8000 deaths per year. It is mostly transmitted when humans come into contact with feces and/or urine of infected blood-sucking triatomine bugs (vector-borne transmission). Other routes of transmission have been identified and include blood transfusion and congenital infections. There are even reported cases of oral infection through ingestion of contaminated foods [3,4]. Due to immigration from South and Central America, hundreds of thousands of people in countries such as Canada and the United States of America, as well as in many European and some African, Eastern Mediterranean, and Western Pacific countries, can also carry the disease [5].
CD has two clinical phases: acute and chronic. The acute phase is relatively rare, with no specific symptoms detected, but it can be fatal in children. During the chronic phase that succeeds the acute phase, up to 30% of patients suffer from cardiac disorders and up to 10% experience digestive, neurological, or mixed disorders. To date, there is no perspective on an efficacious vaccine against trypanosomiasis, and the alternative is the development of safe and efficient chemotherapies to treat this disease. CD can be treated with two antiparasitic medicines: benznidazole and nifurtimox (Figure 1). Both medicines are nearly 100% effective in curing the disease if given soon after infection, including cases of congenital transmission. However, the efficacy of both diminishes the longer a person has been infected and the adverse reactions are more frequent at older age [4].

Figure 1.
Structures of benznidazole and nifurtimox.
Malaria, although not included in the WHO list of NTDs, is a life-threatening disease caused by parasites that are transmitted to people through the bites of infected female Anopheles mosquitoes. Six parasite species cause malaria in humans, and two of these species, P. falciparum and P. vivax, pose the greatest threat. According to the world malaria report, there were an estimated 247 million malaria cases in 2021, and 619,000 estimated deaths [6].
Among the factors that have precipitated the resurgence of malaria, climate change and environmental problems are mentioned, which have degenerated into changes in the life cycle, the duration of activity, and the proliferation of Anopheles [7]. The other two factors to take into consideration in the resurgence of malaria are an increase in the resistance of Anopheles strains to residual insecticides and the resistance to standard drugs, such as chloroquine (CQ), pyrimethamine, and artemisinin, and its derivatives (Figure 2) [8,9]. However, new scientific advancements are providing new tools for malaria control, including the first effective malaria vaccine, RTS,S/AS01 (RTS,S), which was approved by the WHO in October 2022 [10], and a new chlorfenapyr long-lasting insecticidal net that could mitigate the effects of insecticide resistance among mosquitoes has been tested in a clinical trial (Figure 3) [11].
Figure 2.
Structures of chloroquine, pyrimethamine, and artemisinin.
Figure 3.
Structures of chlorfenapyr and licochalcone A.
Despite the difference in epidemiology and visibility, these diseases share a similar history of strategies for their treatment and control. Various aspects of the discovery and development of drugs against these diseases have been reviewed previously [12,13,14,15]. In addition, it has been described how the existence of strains resistant to drugs in clinical use for both pathologies does not facilitate their eradication, which emphasizes the need for new drug options for their treatment.
Since the antimalarial activity of licochalcone A (Figure 3), a natural product isolated from Chinese licorice roots, has been reported, several research groups around the world have isolated from natural sources and synthesized a wide variety of chalcone derivatives to study their chemotherapeutic activities and modify their pharmacokinetic properties [16,17,18,19,20,21,22,23,24,25,26,27]. Chalcone compounds have a common chemical scaffold of 1,3-diaryl-2-propen-1-one, which exists as trans and cis isomers, with the trans isomer being thermodynamically more stable (Figure 4); it can act as a Michael acceptor and inhibit the active site due to its ability to form covalent bonds in some receptors containing a thiol group, such as cysteine proteases or trypanothione reductase.
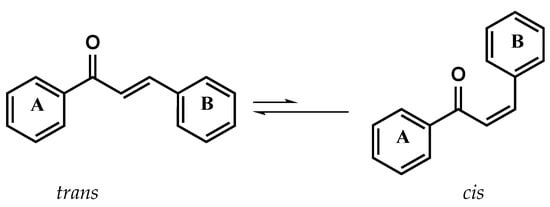
Figure 4.
Structures of chalcone trans and cis isomers.
Reports indicate that the chalcones can exert parasiticide activity through their action on several targets, namely: by inhibition of falcipain-2 [28,29,30], sorbitol-induced hemolysis [31], protein kinases (Pfmrk and PfPK5) [32], topoisomerase-II [33], β-hematin [21,22,34], plasmepsin-II, [35] cruzaine, [36] TcGAPDH, [37,38], and Trypanothione reductase [36,37,38]. As part of our program focused on the discovery of new molecules with potential antiparasitic activity, we have recently designed and synthesized a series of quinolinylbenzocycloalkanones, 4-benzylsulfanyl, and 4-benzylsulfonyl chalcones (Figure 5), where some of them were excellent candidates as antimalarials [21,22,39].
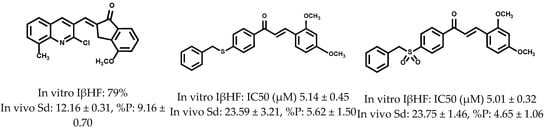
Figure 5.
Structures of quino-linylbenzocycloalkanones, 4-benzylsulfanyl, and 4-benzylsulfonyl chalcones actives in vitro and in vivo as antimalarials.
Based on these results, we infer that the rigidization of the carbonyl group in these structures favors a cooperative effect necessary to improve the activity during the inhibition of the formation of β-hematin, as well as in the decrease in the parasitemia of infected mice in the rodent malaria model. In addition, disubstitution with methoxyl groups in the indanone ring appears to play an important role in both antiparasitic activity and selectivity. Inspired by these encouraging results, and because of their simple synthetic procedure, we report the synthesis of 4-benzylsulfanyl and 4-benzyloxybenzocycloalkanone derivatives, their antimalarial evaluation in vitro as inhibitors of β-hematin formation and in vivo against a CQ sensitive strain of P. berghei, and trypanocidal activity in vitro against epimastigotes of T. cruzi.
2. Results and Discussion
2.1. Synthesis of 4-Benzylsulfanyl and 4-Benzyloxybenzocycloalcanone Derivatives 7–39
The synthesis of derivatives 7–39 is mentioned in (Scheme 1) through a facile synthesis in one and two steps. Compound 3 was prepared according to the published procedures [39]. The most important change in the design of these derivatives is the addition of benzyl mercaptan or benzyloxy moieties to position 4 of benzaldehyde. We designed this inclusion because we wanted to evaluate the antimalarial and trypanocidal activities of these compounds with sulfur or oxygen atoms. The final compounds 7–39 were synthesized through aldol condensation of Claisen-Schmidt between 3 or 4 and several substituted 1-indanones 5a–m and 1-tetralones 6a–d, using sodium hydroxide as a catalyst in ethanol at room temperature (rt). These conditions were found to be satisfactory for the synthesis in good yields. Only (E) isomers were obtained, which were confirmed by singlets (s) or triplets (t) in the 1H-NMR spectra for indanone core between 7.37 and 7.69 or between 7.76 and 7.90 ppm for tetralone core with coupling constants (J) around 1.5–1.7 Hz, and confirmed by the COSY experiment. Singlets or doublets (d) were around 3.80 and 4.06 ppm J = 1.5 Hz for H3 at the indanone core and t around 2.90 and 3.12 ppm J = 6.5 Hz for H3 at the tetralone core, whereas protons of the –SCH2– or –OCH2– groups appear as an s between 4.12 and 5.19 ppm in each compound, respectively. Protons of the –OCH3 group appear as a s between 3.83 and 3.91 ppm in each compound; the remaining aromatic protons are reported according to the substitution pattern. The 13C NMR spectrum of the same compounds exhibits signals between 135–140 ppm for Cβ and 186–194 ppm for C=O, which were also confirmed by DEPT 135° and 2D NMR experiments as HETCOR, HMQC, or HMBC (Supplementary Materials). The infrared (IR) spectra of the compounds show one characteristic intense stretching band between 1698 and 1679 cm−1 for carbonyl present in the 1-indanone ring and between 1648 and 1667 cm−1 for carbonyl in 1-tetralone, confirming the presence of α,β unsaturated C=O. For compounds with a sulfanyl group, a characteristic signal is observed for these functional groups between 1310 and 1460 cm−1. The analytical data for all compounds are summarized in the experimental section.
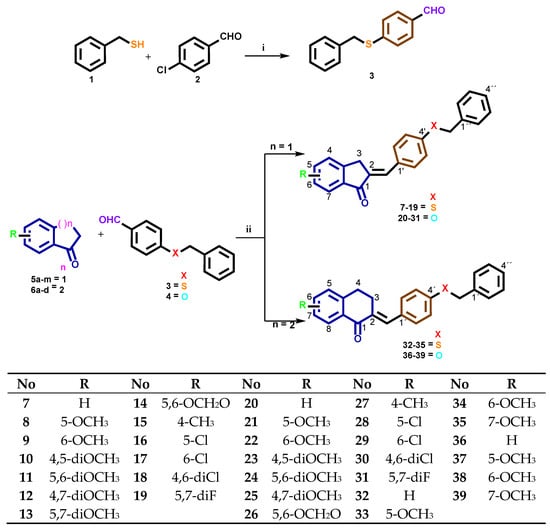
Scheme 1.
Benzylsulfanyl and 4-benzyloxybenzocycloalcanone derivatives 7–39. i: Ethanol, KOH, Δ, 24 h; ii: Ethanol, NaOH, rt, 24 h.
2.2. Antiprotozoal Activity
The novel synthesized compounds 7–39 were tested in vitro as inhibitors of β-hematin formation, and in vivo in a murine model (see Table 1) [39,40]. Most of the compounds evaluated (7–9 and 13–39) exhibited inhibition percentages of less than 50%, with a concentration of 10 μM. There were three compounds 10, 11, and 12 that reduced heme crystallization to 75.16%, 63.02%, and 56.17%, respectively, with an IC50 33.1 ± 2.5, 0.82 ± 0.04, and 0.66 ± 0.12 µM. The values are comparable to CQ 95.34% with an IC50 value of 0.18 ± 0.03 µM. Compounds with a percentage greater than 50% inhibition of β-hematin formation in vitro were tested in vivo in mice infected with P. berghei ANKA, a chloroquine-susceptible strain of murine malaria. The antimalarial potential of these compounds was assessed by their ability to reduce parasitemia and increase survival on the fourth day post-infection compared to the untreated control group.

Table 1.
The half maximal inhibitory concentrations (IC50 values) of compounds 7–39 to inhibit the formation of β-hematin (βHF), effects on P. berghei-infected mice (20 mg kg−1).
Mice were treated ip once daily with the test compounds (20 mg kg−1) or CQ (20 mg kg−1) for consecutive days (days 1–4 post-infection). Figure 6 shows the survival times and percentage of parasitemia on day four compared with those of control mice receiving only saline solution [41]. The Institute of Immunology Bioethical Committee approved the study according to universal guidelines of the National Research Council’s Institute for Laboratory Animal Research (ILAR) and the ethical principles for medical research by the World Medical Association Declaration of Helsinki. Structures 10, 11, and 12 used as a single therapy extended the average survival time of infected mice to 18.8 ± 4.91, 14.2 ± 2.16, and 12.3 ± 2.40 days, respectively; however, they were not able to decrease or delay the evolution of malaria (9.1 ± 2.44, 7.6 ± 2.3, and 12.5 ± 3.1%), respectively. CQ prolonged mouse survival time to 28 ± 1.34 days and decreased the development of malaria to 1.4 ± 0.54%.
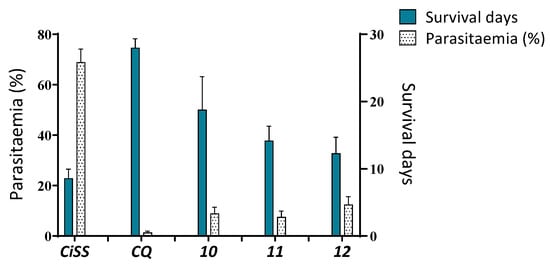
Figure 6.
Effects of compounds 10, 11, and 12 on parasitemia and survival in male Balb-C mice experimentally infected with P. berghei. Right Y axis: % of parasitemia on the fourth-day post-infection. Left Y axis: Survival time post-treatment (days). CiSS = Control infected and treated with saline solution. CQ = Chloroquine.
As can be seen in Table 1 and Figure 7, the benzylthio compounds 10, 11, and 12 had the best in vitro and in vivo activity as potential antimalarial agents, while the benzyloxy derivatives showed weak activity in both tests. As an antimalarial, the incorporation of a 1-tetralone group decreased activity when compared with 1-indanone. The presence of a withdrawing electron substituent group -Cl, -F, or the mono substitution with -CH3, -OCH3 at 1-indanone core, also resulted in decreased activity. When these results are compared with those previously reported by our group, no significant changes in antimalarial activity are observed [21,22,39].
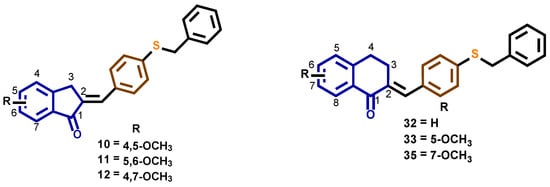
Figure 7.
Structures of compounds 10, 11, 12, 32, 33, and 35.
In the other assay, compounds 7–39 were tested in vitro against epimastigotes of T. cruzi (Y strain). Anti-T. cruzi properties were expressed in terms of % inhibition of the proliferation of T. cruzi epimastigotes for each compound at concentrations of 50 µM after 96 h of parasite exposition. Six compounds, 10, 11, 12, 32, 33, and 35, showed the best results (Table 2). The results described above represent the first report that benzocycloalcanone derivatives had in vitro activity against the parasitic protozoan T. cruzi; however, none of the new analogs showed improved activity compared to benznidazole used as a reference drug, except compound (E)-2-[4-(benzylthio)benzylidene]-3,4-dihydro-5-methoxynaphthalen-1(2H)-one 33, which showed a % of inhibition of proliferation of 51.08 ± 3.4, compared with benznidazole 59.99 ± 2.9%. A low cytotoxicity against VERO cells was observed for 10, 11, 19, 32, 33, and 35 (IC50 > 150 μM), while benznidazole presents a IC50 > 200 μM. In general terms, compounds with sulfur in position 4 of the benzylidene ring, accompanied by a fragment of tetralone, are better candidates as inhibitors of protozoan T. cruzi proliferation.

Table 2.
Effect of compounds 5–37 on the proliferation of epimastigotes of Trypanosoma cruzi, and VERO cells.
3. Materials and Methods
Melting points were determined on a Thomas micro hot stage apparatus and are uncorrected. Thin-layer chromatography (TLC) was carried out on MerckTM silica F254 0.255-mm plates, and spots were visualized by UV fluorescence at 254 nm. IR spectra were determined by a Perkin-ElmerTM Spectrum two spectrophotometer and are expressed in cm−1. The 1H and 13C NMR spectra were performed using a spectrometer JEOL EclipseTM 270 (at 270 MHz for 1H and 67.9 MHz for 13C spectra) or on a Bruker TM DRX-500 Avance spectrometer (at 500 MHz for 1H and 125 MHz for 13C spectra), using CDCl3 as the solvent, and are reported in ppm downfield from the residual CHCl3 (δ 7.25 for 1H NMR and 77.0 for 13C NMR, respectively). Elemental analyses were achieved using a Perkin ElmerTM 2400 CHN elemental analyzer, and the results were within ± 0.4% of the predicted values. Chemical reagents were obtained from Aldrich Chemical CoTM, St. Louis, MO, USA. All solvents were distilled and dried in the usual manner. The 4-(Benzyloxy)benzaldehyde 4 was purchased from MerckTM.
3.1. Synthesis of 4-(Benzylthio)benzaldehyde 3
A mixture of benzylmercaptan 1 (2.73 g, 22 mmol) and potassium hydroxide (1.23 g, 22 mmol) in 95% ethanol (10 mL) was heated to reflux until KOH had completely dissolved and was cooled to room temperature. 4-chlorobenzaldehyde 2 (2.81 g, 20 mmol) dissolved in 95% ethanol was then added dropwise. The solution was heated to reflux for 24 h. When cooled, a yellow solid precipitate formed, which was filtered and washed with ethanol and water. The solid was dissolved in ether, washed with water and 1N NaOH aqueous solution, dried with MgSO4, and the solvent removed to give a white solid. Yield: 76%; Mp: 62–64 °C; IR (ZnSe) cm−1: 2800, 1683, 1574; 1H NMR (CDCl3, 270 MHz) δ ppm: 4.23 (s, 2H, CH2), 7.24–7.38 (m, 7H, Ar), 7.73(d, 2H, H2,6, J = 8.7 Hz), 9.90 (s, 1H, CHO); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 37.0, 126.9, 127.7, 128.8, 130.1, 133.6, 136.0, 146.4, 191.3. Anal. Calcd for C14H12OS: C, 73.65; H, 5.30; S, 14.04. Found: C, 73.66; H, 5.29; S, 14.10.
3.2. General Procedure for the Synthesis of (E)-2-[4-(Benzylthio)benzylidene or (E)-2-[4-(Benzyloxy)benzylidene]-Benzocycloalkanone Derivatives 7–39
A mixture of 4-(benzylthio)benzaldehyde 3 or 4-(benzyloxy)benzaldehyde 4 (0.43 mmol), 1-indanone or 1-tetralone respective (0.43 mmol), and sodium hydroxide one pellet in ethanol 95% (5 mL) was stirred at room temperature for 24 h. The resulting precipitate was collected by filtration, washed with cold water, sodium bisulfite 10% aqueous solution, diethyl ether, and recrystallized from ethanol-water (9:1).
3.2.1. (E)-2-[4-(Benzylthio)benzylidene]-2,3-dihydroinden-1-one 7
Yield 82%; m.p. 155–157 °C; IR (ZnSe) cm−1: 1682, 1629, 1571, 1495, 1327, 1273, 1229; 1H NMR (CDCl3, 270 MHz) δ ppm: 4.00 (d, 2H, H3, J = 1.7 Hz), 4.19 (s, 2H, PhCH2), 7.26–7.44 (m, 7H, Art), 7.52–7.64 (m, 6H, are, Hv), 7.88 (d, 1H, H7, J = 7.7 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 32.6, 37.9, 124.5, 126.2, 127.5, 127.8, 128.4, 128.7, 128.8, 131.2, 132.9, 133.4, 134.3, 134.7, 136.7, 138.1, 139.7, 149.5, 194.4. Anal. Calca for C23H18OS: C, 80.67; H, 5.30; S, 9.36. Found: C, 80.89; H, 5.28; S, 9.30.
3.2.2. (E)-2-[4-(Benzylthio)benzylidene]-2,3-dihydro-5-methoxyinden-1-one 8
Yield 79%; m.p. 166–168 °C; IR (ZnSe) cm−1: 1682, 1622, 1602, 1580, 1487, 1337, 1254; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.89 (s, 3H, OCH3), 3.94 (d, 2H, H3, J = 1.5 Hz), 4.18 (s, 2H, PhCH2), 6.93 (dd, 1H, H6, J = 8.3, 2.2 Hz), 7.26–7.34 (m, 8H, Ar), 7.51 (d, 2H, H2′,6′, J = 8.2 Hz), 7.53 (s, 1H, Hv), 7.82 (d, 1H, H7, J = 8.4 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 32.6, 38.0, 55.8, 109.8, 115.3, 126.2, 127.7, 128.4, 128.7, 128.9, 131.0, 131.5, 132.1, 133.1, 134.9, 136.8, 139.3, 152.4, 165.3, 192.8. Anal. Calce for C24H20O2S: C, 77.39; H, 5.41; S, 8.61. Found: C, 77.43; H, 5.45; S, 8.72.
3.2.3. (E)-2-[4-(Benzylthio)benzylidene]-2,3-dihydro-6-methoxyinden-1-one 9
Yield 72%; m.p. 175–177 °C; IR (ZnSe) cm−1: 1684, 1617, 1584, 1489, 1282, 1257; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.85 (s, 3H, OCH3), 3.92 (d, 2H, H3, J = 1.5 Hz), 4.19 (s, 2H, PhCH2), 7.18 (dd, 1H, H5, J = 8.4, 2.5 Hz), 7.26–7.32 (m, 8H, Ar), 7.42 (d, 1H, H4, J = 8.4 Hz), 7.53 (d, 2H, H2′, 6′, J = 8.5 Hz), 7.57 (t, 1H, Hv, J = 1.5 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 31.9, 37.9, 55.7, 105.8, 124.0, 126.9, 127.5, 128.3, 128.8, 128.9, 131.1, 132.9, 133.3, 135.2, 136.7, 139.4, 139.7, 142.4, 159.7, 194.3. Anal. Calca for C24H20O2S: C, 77.39; H, 5.41; S, 8.61. Found: C, 77.41; H, 5.42; S, 8.47.
3.2.4. (E)-2-[4-(Benzylthio)benzylidene]-2,3-dihydro-4,5-dimethoxyinden-1-one 10
Yield 79%; m.p. 173–175 °C; IR (ZnSe) cm−1: 1685, 1617, 1584, 1493, 1340, 1280, 1269; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.96 (s, 5H, OCH3, H3), 3.97 (s, 3H, OCH3), 4.19 (s, 2H, PhCH2), 7.01 (d, 1H, H6, J = 8.4 Hz), 7.26–7.37 (m, 7H, Ar), 7.54 (s, 1H, Hv), 7.56 (d, 2H, H2′,6′, J = 8.6 HZ), 7.67 (d, 1H, H7, J = 8.4 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 29.5, 37.9, 56.3, 60.7, 112.6, 121.2, 127.5, 128.4, 128.7, 128.8, 131.1, 132.2, 132.7, 132.9, 134.7, 136.8, 139.5, 142.4, 145.2, 157.7, 192.9. Anal. Calce for C25H22O3S: C, 74.60; H, 5.51; S, 7.97. Found: C, 74.65; H, 5.53; S, 8.05.
3.2.5. (E)-2-[4-(Benzylthio)benzylidene]-2,3-dihydro-5,6-dimethoxyinden-1-one 11
Yield 81%; m.p. 160–164 °C; IR (ZnSe) cm−1: 1681, 1625, 1585, 1496, 1303, 1275, 1224; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.91 (d, 2H, H3, J = 1.7 Hz), 3.93 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.18 (s, 2H, PhCH2), 6.96 (s, 1H, H4), 7.25–7.33 (m, 7H, Ar), 7.50–754 (m, 4H, Ar, Hv); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 32.3, 37.9, 56.3, 56.4, 105.1, 107.2, 127.5, 128.5, 128.7, 128.9, 130.9, 131.2, 131.9, 133.1, 135.1, 136.8, 139.2, 144.8, 149.8, 155.5, 193.1. Anal. Calca for C25H22O3S: C, 74.60; H, 5.51; S, 7.97. Found: C, 74.61; H, 5.56; S, 8.12.
3.2.6. (E)-2-[4-(Benzylthio)benzylidene]-2,3-dihydro-4,7-dimethoxyinden-1-one 12
Yield 68%; m.p. 205–207 °C; IR (ZnSe) cm−1: 1694, 1628, 1587, 1492, 1283, 1265; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.83 (d, 2H, H3, J = 1.3 Hz), 3.87 (s, 3H, OCH3), 3.92 (s, 1H, OCH3), 4.17 (s, 2H, PhCH2), 6.76 (d, 1H, H5, J = 8.9 Hz), 7.01 (d, 1H, H6, J = 8.9 Hz), 7.26–7.33 (m, 7H, Ar), 7.52 (s, 1H, Hv), 7.54 (d, 2H, H2′,6′, J = 8.4 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 29.3, 37.9, 55.9, 56.2, 110.1, 116.7, 127.3, 127.5, 128.5, 128.7, 128.9, 131.1, 132.5, 133.0, 134.4, 136.8, 139.2, 139.8, 150.0, 152.5, 192.4. Anal. Calce for C25H22O3S: C, 74.60; H, 5.51; S, 7.97. Found: C, 74.71; H, 5.51; S, 7.85.
3.2.7. (E)-2-[4-(Benzylthio)benzylidene]-2,3-dihydro-5,7-dimethoxyinden-1-one 13
Yield 87%; m.p.176–178 °C; IR (ZnSe) cm−1: 1683, 1629, 1585, 1487, 1324, 1275, 1229; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.87 (s, 5H, OCH3, H3), 3.92 (s, 3H, OCH3), 4.17 (s, 2H, PhCH2), 6.31 (s, 1H, H6), 6.53 (s, 1H, H4), 7.23–7.34 (m, 7H, Ar), 7.44 (t, 1H, Hv, J = 1.7 Hz), 7.47 (d, 2H, H2′,6′, J = 8.4 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 32.8, 38.1, 55.9, 56.0, 97.7, 101.6, 120.7, 127.5, 128.6, 128.7, 128.9, 130.8, 133.4, 135.2, 136.9, 138.7, 154.4, 160.2, 167.0, 190.7. Anal. Calca for C25H22O3S: C, 74.60; H, 5.51; S, 7.97. Found: C, 74.60; H, 5.54; S, 8.09.
3.2.8. (E)-2-[4-(Benzylthio)benzylidene]-2,3-dihydro-5,6-methylendioxyinden-1-one 14
Yield 80%; m.p.194–196 °C; IR (ZnSe) cm−1: 1687, 1631, 1593, 1513, 1465, 1294, 1041; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.86 (s, 2H, H3), 4.17 (s, 2H, PhCH2), 6.06 (s, 2H, OCH2O), 6.89 (s, 1H, H4), 7.23–7.35 (m, 8H, Ar), 7.49 (m, 3H, Ar, Hv); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 32.5, 38.0, 102.3, 103.3, 103.4, 105.5, 127.5, 128.5, 128.7, 128.8, 131.0, 132.0, 133.0, 134.9, 136.8, 139.4, 146.9, 147.2, 154.1, 192.5. Anal. Calce for C24H18O3S: C, 74.59; H, 4.69; S, 8.30. Found: C, 74.62; H, 4.73; S, 8.43.
3.2.9. (E)-2-[4-(Benzylthio)benzylidene]-2,3-dihydro-4-methylinden-1-one 15
Yield 87; m.p. 167–169 °C; IR (ZnSe) cm−1: 1670, 1637, 1576, 1478, 1247, 830; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.42 (s, 3H, CH3), 3.84 (s, 2H, H3), 4.19 (s, 2H, PhCH2), 7.25–7.42 (m, 9H, Ar), 7.55 (d, 2H, H2′,6′, J = 8.4 Hz), 7.59 (t, 1H, Hv, J = 1.5 Hz), 7.73 (d, 1H, H7, J = 7.5 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 18.1, 31.3, 37.9, 121.9, 127.5, 127.9, 128.4, 128.7, 128.8, 131.1, 132.9, 133.3, 134.4, 135.3, 135.4, 136.7, 137.9, 139.7, 148.5, 194.7. Anal. Calca for C24H20OS: C, 80.86; H, 5.65; S, 8.99. Found: C, 80.90; H, 5.67; S, 8.95.
3.2.10. (E)-2-[4-(Benzylthio)benzylidene]-5-chloro-2,3-dihydroinden-1-one 16
Yield 80%; m.p. 140–142 °C; IR (ZnSe) cm−1: 1692, 1627, 1600, 1469, 1320, 1262, 1068, 810; 1H NMR (CDCl3, 500 MHz) δ ppm: 4.06 (d, 2H, H3, J = 1.8 Hz), 4.26 (s, 2H, PhCH2), 7.05 (s, 1H, H4), 7.14–7.34 (m, 7H, Ar), 7.56 (dd, 1H, H6, J = 8.0, 2.1 Hz), 7.68 (d, 2H, H2′,6′, J = 8.2 Hz), 7.69 (s, 1H, Hv), 7.89 (d, 1H, H7, J = 8.0 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 33.2, 38.1, 124.7, 126.6, 126.8, 127.8, 128.6, 128.7, 128.8, 130.6, 131.9, 137.0, 137.1, 138.4, 139.2, 147.8, 192.4. Anal. Calce for C23H17ClOS: C, 73.29; H, 4.55; S, 8.51. Found: C, 73.15; H, 4.58; S, 8.33.
3.2.11. (E)-2-[4-(Benzylthio)benzylidene]-6-chloro-2,3-dihydroinden-1-one 17
Yield 67%; m.p. 204 °C; IR (ZnSe) cm−1: 1687, 1620, 1586, 1494, 1257, 1092, 812; 1H NMR (CDCl3, 500 MHz) δ ppm: 4.02 (d, 2H, H3, J = 1.5 Hz), 4.27 (s, 2H, PhCH2), 7.22–7.25 (m, 2H, H4,4″), 7.33–7.44 (m, 7H, Ar), 7.56–7.67 (m, 3H, Hv, Ar), 7.91 (d, 1H, H7, J = 2.4 Hz); 13C NMR (CDCl3, 125.7 MHz) δ: 31.9, 38.1, 124.9, 127.8, 128.1, 128.6, 128.7, 128.9, 130.7, 131.3, 132.0, 132.9, 137.0, 136.8, 138.1, 138.2, 139.7, 145.4, 196.3; Anal. Calca for C23H17ClOS: C, 73.29; H, 4.55; S, 8.51. Found: C, 73.38; H, 4.52; S, 8.60.
3.2.12. (E)-2-[4-(Benzylthio)benzylidene]-4,6-dichloro-2,3-dihydroinden-1-one 18
Yield 73%; m.p. 181–183 °C; IR (ZnSe) cm−1: 1695, 1614, 1584, 1495, 1312, 1269, 1093; 1H NMR (CDCl3, 500 MHz) δ ppm: 3.99 (d, 2H, H3, J = 2.0 Hz), 4.28 (s, 2H, PhCH2), 7.15 (m, 1H, H4″), 7.32–7.46 (m, 7H, Ar), 7.58–7.67 (m, 2H, Ar), 7.68 (s, 1H, Hv), 7.66 (d, 1H, H7, J = 2.0 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 32.4, 38.7, 124.7, 127.8, 128.6, 128.7, 128.8, 130.1, 131.2, 132.3, 132.5, 132.8, 133.1, 137.1, 137.6, 138.3, 138.9, 143.0, 193.4; Anal. Calce for C23H16Cl2OS: C, 67.16; H, 3.92; S, 7.80. Found: C, 66.97; H, 4.11; S, 8.03.
3.2.13. (E)-2-[4-(Benzylthio)benzylidene]-5,7-difluoro-2,3-dihydroinden-1-one 19
Yield 77%; m.p. 170–172 °C; IR (ZnSe) cm−1: 1693, 1618, 1592, 1431, 1327, 1256, 847; 1H NMR (CDCl3, 270 MHz) δ ppm: 3.99 (d, 2H, H3, J = 1.5 Hz), 4.20 (s, 2H, PhCH2), 6.79 (t, 1H, H6, J = 7.9 Hz), 7.01 (d, 1H, H4, J = 7.9 Hz), 7.27–7.36 (m, 7H, Ar), 7.50 (d, 2H, H2′,6′, J = 8.4 Hz), 7.57 (t, 1H, Hv, J = 1.5 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 32.8, 37.8, 104.0 (q, J: 24 Hz), 109.3 (d, J: 25 Hz), 127.5, 128.3, 128.8, 128.9, 131.1, 132.3, 133.1, 133.8, 136.6, 140.3, 153.3 (d, J: 12 Hz), 189.3. Anal. Calca for C23H16F2OS: C, 73.00; H, 4.26; S, 8.47. Found: C, 72.97; H, 4.23; S, 8.24.
3.2.14. (E)-2-[4-(Benzyloxy)benzylidene]-2,3-dihydroinden-1-one 20
Yield 79%; m.p. 170 °C; IR (ZnSe) cm−1: 1679, 1628, 1596, 1509, 1424, 1251, 955, 821; 1H NMR (CDCl3, 500 MHz) δ ppm: 3.97 (s, 2H, H3), 5.11 (s, 2H, PhCH2), 7.04 (d, 2H, H2′,6′, J = 8.5 Hz), 7.34 (t, 1H, H4″, J = 5.0 Hz), 7.39–7.45 (m, 5H, Ar), 7.53 (d, 1H, H4, J = 7.5 Hz), 7.59 (t, 1H, H5, J = 7.5 Hz), 7.62 (d, 2H, H3′,5′, J = 8.5 Hz), 7.37 (t, 1H, Hv, J = 1.7 Hz), 7.88 (d, 1H, H7, J = 7.5 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 32.5, 70.2, 115.4, 124.3, 126.1, 127.4, 127.6, 128.2, 128.5, 128.7, 132.5, 132.6, 133.7, 134.3, 136.5, 138.3, 160.1, 194.3. Anal. Calcd for C23H18O2: C, 84.64; H, 5.56; O, 9.80. Found: C, 84.69; H, 5.59; O, 9.57.
3.2.15. (E)-2-[4-(Benzyloxy)benzylidene]-2,3-dihydro-5-methoxyinden-1-one 21
Yield 91%; m.p. 141–142 °C; IR (ZnSe) cm−1: 1682, 1627, 1598, 1509, 1291, 1247, 821, 1H NMR (CDCl3, 500 MHz) δ ppm: 4.05 (s, 5H, OCH3, H3), 5.10 (s, 2H, PhCH2), 7.05 (s, 1H, H4), 7.26–7.34 (m, 8H, Ar), 7.53 (d, 2H, H2′,6′, J = 8.2 Hz), 7.75 (s, 1H, Hv), 7.84 (d, 1H, H7, J = 8.4 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 32.6, 37.3, 69.9, 107.4, 115.2, 122.4, 126.7, 127.1, 128.3, 128.7, 129.1, 131.9, 133.7, 133.9, 136.3, 140.3, 142.4, 159.0, 161.1, 194.2. Anal. Calcd for C24H20O3: C, 80.88; H, 5.66; O, 13.47. Found: C, 81.01; H, 5.65; O, 13.53.
3.2.16. (E)-2-[4-(Benzyloxy)benzylidene]-2,3-dihydro-6-methoxyinden-1-one 22
Yield 87%; m.p. 178–179 °C; IR (ZnSe) cm−1: 3078, 1680, 1620, 1598, 1566, 1508, 1489, 1277, 819; 1H NMR (CDCl3, 500 MHz) δ ppm: 3.85 (s, 3H, OCH3), 3.90 (s, 2H, H3), 5.12 (s, 2H, PhCH2), 7.03 (d, 2H, H2′,6′, J = 8.0 Hz), 7.17 (d, 1H, H4, J = 8.0 Hz), 7.34–7.45 (m, 8H, Ar), 7.60–7.62 (m, 2H, Hv,7); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 31.8, 35.6, 70.1, 105.8, 115.4, 123.6, 126.8, 127.5, 128.2, 128.5, 128.7, 132.5, 133.5, 133.6, 136.5, 139.5, 142.3, 159.6, 160.0, 194.3. Anal. Calcd for C24H20O3: C, 80.88; H, 5.66; O, 13.47. Found: C, 80.87; H, 5.67; O, 13.51.
3.2.17. (E)-2-[4-(Benzyloxy)benzylidene]-2,3-dihydro-4,5-dimethoxyinden-1-one 23
Yield 89%; m.p. 167–168 °C; IR (ZnSe) cm−1: 1688, 1628, 1598, 1508, 1494, 1338, 1275, 1046; 1H NMR (CDCl3, 500 MHz) δ ppm: 3.93 (d, 2H, CH2, J = 1.5 Hz), 3.95 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.10 (s, 2H, PhCH2), 6.99 (d, 1H, H6, J = 8.5 Hz), 7.04 (d, 2H, H2′,6′, J = 8.5 Hz), 7.33 (t, 1H, H4″, J = 7.5 Hz), 7.38–7.44 (m, 4H, Ar), 7.56 (t, 1H, Hv, J = 1.5 Hz), 7.62 (d, 2H, H3′,5′, J = 8.5 Hz), 7.65 (d, 1H, H7, J = 8.5 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 29.4, 56.2, 60,5, 70.1, 112.5, 115.4, 120.9, 127.5, 128.2, 128.5, 128.7, 132.4, 132.5, 132.9, 133.0, 136.5, 142.4, 145.2, 157.4, 159.9, 192.9. Anal. Calcd for C25H22O4: C, 77.70; H, 5.74; O, 16.56. Found: C, 77.68; H, 5.73; O, 16.73.
3.2.18. (E)-2-[4-(Benzyloxy)benzylidene]-2,3-dihydro-5,6-dimethoxyinden-1-one 24
Yield 73%; m.p. 207–208 °C; IR (ZnSe) cm−1: 2941, 1688, 1628, 1598, 1508, 1484, 1275, 1242, 813; 1H NMR (CDCl3, 500 MHz) δ ppm: 3.82 (s, 2H, H3,), 3.89 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 5.07 (s, 2H, PhCH2), 6.91 (d, 1H, H4), 6.99 (d, 2H, H2′,6′, J = 8.0 Hz), 7.32 (t, 1H, H4″, J = 7.0 Hz), 7.36–7.42 (m, 4H, Ar), 7.49 (s, 1H, Hv), 7.53 (m, 3H, H3′,5′,7′); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 32.2, 56.1, 56.2, 70.1, 105.1, 107.2, 115.3, 127.5, 128.2, 128.6, 128.7, 131.3, 132.2, 132.3, 133.3, 136.5, 144.7, 149.6, 155.3, 159.8, 193.2. Anal. Calcd for C25H22O4: C, 77.70; H, 5.74; O, 16.56. Found: C, 77.74; H, 5.79; O, 16.69.
3.2.19. (E)-2-[4-(Benzyloxy)benzylidene]-2,3-dihydro-4,7-dimethoxyinden-1-one 25
Yield 83%; m.p. 209–210 °C; IR (ZnSe) cm−1: 1694, 1633, 1598, 1494, 1241, 1061, 1006, 981; 1H NMR (CDCl3, 500 MHz) δ ppm: 3.80 (d, 2H, H3, J = 1.5 Hz), 3.86 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 5.09 (s, 2H, PhCH2), 6.75 (d, 1H, H5, J = 8.5 Hz), 6.97 (d, 1H, H6, J = 8.5 Hz), 7.01 (d, 2H, H2′,6′, J = 8.5 Hz), 7.33 (t, 1H, H4″, J = 7.0 Hz), 7.37–7.43 (m, 4H, Ar), 7.54 (t, 1H, Hv, J = 1.5 Hz), 7.61 (d, 2H, H3′,5′); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 29.2, 55.9, 56.1, 70.1, 110.1, 115.3, 116.5, 127.5, 128.1, 128.6, 128.7, 132.4, 132.7, 132.8, 136.5, 139.8, 150.1, 152.4, 159.8, 192.4. Anal. Calcd for C25H22O4: C, 77.70; H, 5.74; O, 16.56. Found: C, 77.81; H, 5.73; O, 16.80.
3.2.20. (E)-2-[4-(Benzyloxy)benzylidene]-2,3-dihydro-5,6-methylendioxyinden-1-one 26
Yield 79%; m.p. 184–185 °C; IR (ZnSe) cm−1: 3103, 1681, 1629, 1597, 1509, 1465, 1294, 1249, 825; 1H NMR (CDCl3, 500 MHz) δ ppm: 3.92 (d, 2H, H3, J = 1.2 Hz), 5.18 (s, 2H, PhCH2), 6.13 (s, 2H, OCH2O), 6.97 (s, 1H, H4), 7.02 (d, 2H, H3′,5′, J = 8.5 Hz), 7.32–7.53 (m, 7H, Ar), 7.61 (s, 1H, H7), 7.69 (s, 1H, Hv); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 35.2, 69.8, 102.4, 106.1, 106.5, 115.1, 127.7, 127.9, 128.2, 128.3, 132.3, 132.4, 136.1, 137.0, 139.4, 141.1, 147.9, 150.2, 160.3, 193.0. Anal. Calcd for C25H22O4: C, 77.82; H, 4.90; O, 17.28. Found: C, 77.73; H, 5.07; O, 17.04.
3.2.21. (E)-2-[4-(Benzyloxy)benzylidene]-2,3-dihydro-4-methylinden-1-one 27
Yield 81%; m.p. 127–129 °C; IR (ZnSe) cm−1: 1688, 1630, 1598, 1508, 1278, 1249, 830, 744; 1H NMR (CDCl3, 500 MHz) δ ppm: 2.43 (s, 3H, CH3), 3.82 (s, 2H, H3), 5.12 (s, 2H, PhCH2), 7.05 (d, 2H, H2′,6′, J = 9.0 Hz), 7.30–7.36 (m, 3H, H4″,5,6), 7.39–7.35 (m, 4H, Ar), 7.62–7.64 (m, 3H, Hv,3′,5′), 7.73 (d, 1H, H7, J = 7.5 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 17.9, 31.2, 70.1, 115.4, 121.7, 127.5, 127.8, 128.2, 128.5, 128.7, 132.5, 132.7, 133.6, 134.9, 135.2, 136.5, 138.0, 148.5, 160.0, 194.7. Anal. Calcd for C24H20O2: C, 84.68; H, 5.92; O, 9.40. Found: C, 84.69; H, 5.97; O, 9.53.
3.2.22. (E)-2-[4-(Benzyloxy)benzylidene]-5-chloro-2,3-dihydroinden-1-one 28
Yield 85%; m.p. 189–190 °C; IR (ZnSe) cm−1: 1693, 1633, 1599, 1494, 1321, 1249, 1175, 999, 826; 1H NMR (CDCl3, 500 MHz) δ ppm: 4.04 (s, 2H, H3), 5.19 (s, 2H, PhCH2), 7.11 (d, 2H, H3′,5′, J = 8.0 Hz), 7.13 (s, 1H, H4), 7.38–7.50 (m, 7H, Ar), 7.58–7.72 (m, 2H, Hv,6), 7.88 (d, 1H, H7, J = 7.5 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 31.8, 69.8, 115.3, 124.5, 126.6, 126.8, 127.7, 127.9, 128.1, 128.5, 132.1, 136.2, 136.9, 137.6, 137.9, 139.7, 147.9, 160.1, 194.8. Anal. Calcd for C23H17ClO2: C, 76.56; H, 4.75; O, 8.87. Found: C, 76.59; H, 4.72; O, 9.04.
3.2.23. (E)-2-[4-(Benzyloxy)benzylidene]-6-chloro-2,3-dihydroinden-1-one 29
Yield 77%; m.p. 186–187 °C; IR (ZnSe) cm−1: 3065, 1690, 1622, 1596, 1509, 1463, 1254, 988; 1H NMR (CDCl3, 500 MHz) δ ppm: 3.97 (d, 2H, H3, J = 2.0 Hz), 5.08 (s, 2H, PhCH2), 7.26–7.36 (m, 8H, Ar), 7.53 (d, 2H, H3′,5′, J = 8.6 Hz), 7.56 (dd, 1H, H5, J = 8.0, 2.1 Hz), 7.62 (t, 1H, Hv, J = 2.0 Hz), 7.86 (d, 1H, H7, J = 2.1 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 31.3, 70.2, 115.6, 122.7, 127.5, 128.4, 128.8, 128.7, 131.0, 132.9, 133.0, 133.5, 135.8, 136.3, 141.1, 145.5, 160.6, 192.0; Anal. Calce for C23H17ClO2: C, 76.56; H, 4.75; O, 8.87. Found: C, 76.43; H, 4.70; O, 8.97.
3.2.24. (E)-2-[4-(Benzyloxy)benzylidene]-4,6-dichloro-2,3-dihydroinden-1-one 30
Yield 67%; m.p. 202–203 °C; IR (ZnSe) cm−1: 1698, 1621, 1595, 1451, 1246, 833, 741; 1H NMR (CDCl3, 500 MHz) δ ppm: 3.92 (s, 2H, H3), 5.14 (s, 2H, PhCH2), 7.07 (d, 2H, H2′,6′, J = 8.5 Hz), 7.35 (t, 1H, H4″, J = 7.5 Hz), 7.39–7.45 (m, 4H, Ar), 7.58 (d, 1H, H5, J = 2.0 Hz), 7.64 (d, 2H, H3′,5′, J = 8.5 Hz), 7.68 (s, 1H, Hv), 7.66 (d, 1H, H7, J = 2.0 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 31.3, 70.2, 115.6, 122.7, 127.5, 127.8, 128.2, 128.7, 131.0, 132.9, 133.0, 133.5, 134.8, 135.8, 136.3, 141.1, 145.5, 160.6, 192.0. Anal. Calcd for C23H16Cl2O2: C, 69.89; H, 4.08; O, 8.10. Found: C, 70.05; H, 4.11; O, 8.25.
3.2.25. (E)-2-[4-(Benzyloxy)benzylidene]-5,7-difluoro-2,3-dihydroinden-1-one 31
Yield 67%; m.p. 204–206 °C; IR (ZnSe) cm−1: 1690, 1621, 1594, 1256, 847, 827, 697; 1H NMR (CDCl3, 500 MHz) δ ppm: 4.05 (d, 2H, H3, J = 1.5 Hz), 5.14 (s, 2H, PhCH2), 6.78 (td, 1H, H4, J = 9.0, 2.0 Hz), 7.01 (dd, 1H, H6, J = 9.0, 2.0 Hz), 7.05 (d, 2H, H2′,6′, J = 9.0 Hz), 7.36 (t, 1H, H4, J = 7.5 Hz), 7.39–7.45 (m, 4H, Ar), 7.59 (d, 2H, H2′,6′, J = 9.0 Hz), 7.61 (t, 1H, Hv, J = 1.5 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 32.7, 70.2, 103.7 (q, J = 23 Hz), 109.1 (d, J = 17.6 Hz), 115.5, 127.5, 128.0, 128.2, 128.7, 131.4, 132.6, 134.1, 136.4, 136.6, 153.3, 160.3, 163.6, 189.4. Anal. Calcd for C23H16F2O2: C, 76.23; H, 4.45; O, 8.83. Found: C, 76.31; H, 4.47; O, 8.92.
3.2.26. (E)-2-[4-(Benzylthio)benzylidene]-3,4-dihydronaphthalen-1(2H)-one 32
Yield 82%; m.p. 99–100 °C; IR (ZnSe) cm−1: 3018, 1656, 1608, 1584, 1512, 1439, 1260, 893; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.92 (t, 2H, H4, J = 6.7 Hz), 3.09 (t, 2H, H3, J = 6.5 Hz), 4.17 (s, 2H, PhCH2), 7.22–7.35 (m, 11H, Ar), 7.48 (t, 1H, Ar, J = 7.4 Hz), 7.79 (s, 1H, Hv), 8.11 (d, 1H, H8, J = 7.67 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.4, 28.9, 38.3, 127.1, 127.5, 128.2, 128.3, 128.5, 128.7, 128.9, 130.5, 133.4, 133.5, 133.6, 135.3, 136.2, 137.0, 138.0, 143.2, 187.8. Anal. Calca for C24H20OS: C, 80.86; H, 5.65; S, 8.99. Found: C, 81.01; H, 5.66; S, 9.18.
3.2.27. (E)-2-[4-(Benzylthio)benzylidene]-3,4-dihydro-5-methoxynaphthalen-1(2H)-one 33
Yield 74%; m.p. 87–89 °C; IR (ZnSe) cm−1: 3119, 1661, 1612, 1563, 1490, 1265, 831; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.90 (t, 2H, H4, J = 6.4 Hz), 3.05 (t, 2H, H3, J = 6.0 Hz), 3.85 (s, 3H, OCH3), 4.17 (s, 2H, PhCH2), 7.03 (dd, 1H, H5, J = 8.2, 1.2 Hz), 7.25–7.33 (m, 9H, Ar), 7.73 (dd, 1H, H8, J = 8.2, 1.2 Hz), 7.75 (s, 1H, Hv); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 21.5, 26.7, 38.3, 55.8, 114.4, 120.0, 127.3, 127.5, 128.5, 128.7, 128.9, 130.5, 132.3, 133.6, 134.5, 135.3, 135.9, 137.0, 137.9, 156.4, 188.1. Anal. Calce for C25H22O2S: C, 77.69; H, 5.74; S, 8.30. Found: C, 77.73; H, 5.77; S, 8.26.
3.2.28. (E)-2-[4-(Benzylthio)benzylidene]-3,4-dihydro-6-methoxynaphthalen-1(2H)-one 34
Yield 68%; m.p. 122–124 °C; IR (ZnSe) cm−1: 3137, 1651, 1610, 1590, 1435, 1301, 1260; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.89 (t, 2H, H4, J = 6.9 Hz), 3.07 (t, 2H, H3, J = 6.7 Hz), 3.86 (s, 3H, OCH3) 4.16 (s, 2H, PhCH2), 6.69 (d, 1H, H5, J = 2.5 Hz), 6.86 (dd, 1H, H7, J = 8.6, 2.5 Hz), 7.27–7.33 (m, 9H, Ar), 7.76 (t, 1H, Hv, 1.5 Hz), 8.09 (d, 1H, H8, J = 8.6 Hz); 13C NMR (CDCl3, 67.9 MHz) δ: 27.4, 29.3, 38.4, 55.6, 112.3, 113.4, 127.1, 127.4, 128.6, 128.7, 128.9, 130.4, 130.9, 133.7, 135.4, 135.5, 137.0, 137.7, 145.7, 163.7, 186.7. Anal. Calca for C25H22O2S: C, 77.69; H, 5.74; S, 8.30. Found: C, 77.70; H, 5.75; S, 8.41.
3.2.29. (E)-2-[4-(Benzylthio)benzylidene]-3,4-dihydro-7-methoxynaphthalen-1(2H)-one 35
Yield 76%; m.p. 112–115 °C; IR (ZnSe) cm−1: 3100, 1648, 1604, 1571, 1437, 1329, 1293, 825; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.86 (t, 2H, H4, J = 7.0 Hz), 3.07 (t, 2H, H3, J = 6.8 Hz), 3.85 (s, 3H, OCH3) 4.16 (s, 2H, PhCH2), 7.04 (dd, 1H, H6, J = 8.4, 2.7 Hz), 7.14 (d, 1H, H5, J = 8.4 Hz), 7.24–7.35 (m, 9H, Ar), 7.59 (d, 1H, H8, J = 2.7 Hz), 7.77 (t, 1H, Hv, J = 1.5 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.6, 28.0, 38.3, 55.7, 110.4, 121.6, 127.5, 128.5, 128.7, 128.9, 129.5, 130.5, 133.5, 134.3, 135.3, 135.9, 136.3, 137.0, 158.7, 187.8. Anal. Calce for C25H22O2S: C, 77.69; H, 5.74; S, 8.30. Found: C, 77.90; H, 5.79; S, 8.53.
3.2.30. (E)-2-[4-(Benzyloxy)benzylidene]-3,4-dihydronaphthalen-1(2H)-one 36
Yield 66%; oil; IR (ZnSe) cm−1: 3117, 1661, 1610, 1584, 1523, 1487, 1265, 841; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.72 (t, 2H, H4, J = 6.5 Hz), 3.03 (t, 2H, H3, J = 6.5 Hz), 5.21 (s, 2H, PhCH2), 7.03 (d, 2H, H2′6′, J = 8.5 Hz), 7.14 (d, 1H, H5, J = 8.5 Hz), 7.32–7.53 (m, 9H, Ar), 7.81 (s, 1H, Hv), 8.14 (dd, 1H, H8, J = 8.5, 2.0 Hz); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.2, 28.8, 70.1, 110.6, 115.0, 127.1, 127.2, 128.0, 128.2, 128.4, 128.5, 131.7, 133.1, 133.6, 136.2, 136.7, 139.0, 143.1, 160.1, 189.4. Anal. Calcd for C24H20O2: C, 84.68; H, 5.92; O, 9.40. Found: C, 84.90; H, 5.87; O, 9.63.
3.2.31. (E)-2-[4-(Benzyloxy)benzylidene]-3,4-dihydro-5-methoxynaphthalen-1(2H)-one 37
Yield 47%; m.p. 67–69 °C; IR (ZnSe) cm−1: 3087, 1667, 1608, 1577, 1512, 1493, 1267, 829; 1H NMR (CDCl3, 270 MHz) δ ppm: 2.70 (t, 2H, H4, J = 6.2 Hz), 2.97 (t, 2H, H3, J = 6.0 Hz), 3.93 (s, 3H, OCH3), 5.22 (s, 2H, PhCH2), 7.04–7.19 (m, 7H, Ar), 7.26–7.41 (m, 3H, Ar), 7.77 (dd, 1H, H6, J = 8.4, 2.0 Hz), 7.90 (m, 2H, H8, Hv); 13C NMR (CDCl3, 67.9 MHz) δ ppm: 27.4, 28.6, 55.4, 69.8, 110.7, 114.1, 120.6, 127.9, 128.0, 128.2, 128.5, 130.4, 131.7, 133.7, 136.1, 136.2, 138.2, 139.7, 157.2, 160.1, 189.3. Anal. Calcd for C25H22O3: C, 81.06; H, 5.99; O, 12.96: Found: C, 80.92; H, 5.97; O, 13.03.
3.2.32. (E)-2-[4-(Benzyloxy)benzylidene]-3,4-dihydro-6-methoxynaphthalen-1(2H)-one 38
Yield 59%; m.p. 130–131 °C; IR (ZnSe) cm−1: 3109, 1665, 1600, 1579, 1510, 1270, 1142, 830; 1H NMR (CDCl3, 500 MHz) δ ppm: 2.91 (t, 2H, H4, J = 6.5 Hz), 3.12 (t, 2H, H3, J = 6.5 Hz), 3.87 (s, 3H, OCH3), 5.11 (s, 2H, PhCH2), 6.70 (d, 1H, H5, J = 2.0 Hz), 6.87 (dd, 1H, H7, J = 8.5, 2.0 Hz), 7.01 (d, 2H, H2′,6′, J = 8.5 Hz), 7.37 (t, 1H, H4″, J = 7.5 Hz), 7.38–7.45 (m, 6H, Ar), 7.81 (s, 1H, Hv), 8.11 (d, 1H, H8, J = 8.5 Hz); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 27.3, 29.2, 55.5, 70.1, 112.3, 113.3, 114.8, 127.2, 127.5, 128.1, 128.7, 128.8, 130.7, 131.7, 133.8, 135.9, 136.7, 145.6, 159.0, 163.5, 186.8. Anal. Calcd for C25H22O3: C, 81.06; H, 5.99; O, 12.96. Found: C, 80.93; H, 5.95; O, 13.16.
3.2.33. (E)-2-[4-(Benzyloxy)benzylidene]-3,4-dihydro-7-methoxynaphthalen-1(2H)-one 39
Yield 71%; m.p. 119–120 °C; IR (ZnSe) cm−1: 3121, 1662, 1603, 1582, 1508, 1495, 1254, 1286, 830; 1H NMR (CDCl3, 500 MHz) δ ppm: 2.89 (t, 2H, H4, J = 6.5 Hz), 3.12 (t, 2H, H3, J = 6.0 Hz), 3.87 (s, 3H, OCH3), 5.12 (s, 2H, PhCH2), 7.02 (d, 1H, H5, J = 8.5 Hz), 7.06 (dd, 2H, H6, J = 8.5, 2.0 Hz), 7.15 (d, 2H, H2′,6′, J = 8.0 Hz), 7.34 (t, 1H, H4″, J = 7.0 Hz), 7.39–7.45 (m, 6H, Ar), 7.62 (d, 1H, H8, J = 2.0 Hz), 7.83 (s, 1H, Hv); 13C NMR (CDCl3, 125.7 MHz) δ ppm: 27.4, 27.9, 55.6, 70.1, 110.4, 114.9, 121.3, 127.5, 128.1, 128.6, 128.7, 129.3, 131.7, 133.7, 134.5, 135.8, 136.7, 158.7, 159.2, 187.8. Anal. Calca for C25H22O3: C, 81.06; H, 5.99; O, 12.96. Found: C, 81.12; H, 6.05; O, 13.21.
3.3. Biology
3.3.1. Inhibition of β-Haematin Formation In Vitro
The assay was performed according to previously reported protocols [39,40]. Hemin chloride (50 μL, 4 mM) in DMSO (5.2 mg mL−1) was pipetted into 96-well microplates. Different concentrations (0.1–100 mM) of the compounds in DMSO were added in triplicate (50 μL) with a final concentration of 0.025 mM–25 mM/well. Water (50 μL) or DMSO (50 μL) were used as controls. Acetate buffer (100 μL, 0.2 M, pH 4.4) initiated the formation of β-hematin. Next, the plates were incubated at 37 °C for 48 h and subsequently centrifuged (4000 rpm × 15 min). The supernatant was washed twice with DMSO (200 μL) and resuspended in NaOH (200 μL, 0.2 N). The solubilized products were diluted (1:2) with NaOH (0.1 N), and the plates were read in an ELISA reader at 405 nm (Microplate Reader, BIORAD-550). The results are expressed as % IβHF.
3.3.2. Plasmodium Berghei and Experimental Host Maintenance
Male Balb-C mice with a weight of 18–22 g were fed with a commercial diet ad libitum under standard procedures of animal care following the aforementioned method approved by the Ethics Committee of the Institute of Immunology. The animals were infected with a rodent malaria strain of Plasmodium berghei ANKA. A million infected erythrocytes, in phosphate-buffered saline solution (PBS, 10 mM, pH 7.4, 0.1 mL), were inoculated ip to infect the animal. The parasitemia was inspected by continuous microscopic examination of Giemsa-stained smears [39].
3.3.3. Four-Day Suppressive Test
One million P. berghei, injected in the caudal vein i.v, were used to infect the mice (n = 6). After two hours of inoculation, the compounds that were active in vitro were dissolved in DMSO (0.1 M) and subsequently diluted with Saline-Tween 20 solution (2%), and administered ip for 4 days (20 mg kg−1). The parasite load was evaluated on the fourth day by examining Giemsa-stained smears. Chloroquine (20 mg kg−1) was used as the positive control and saline solution as the negative control. Non-treated mice were used as a baseline control for survival times [39,41]. The survival days and percentage of parasitemia were used to express the results.
3.3.4. Trypanocidal Activity
The trypanocidal activity of compounds 7–39 was evaluated on the viability of T. cruzi epimastigotes (Y Strain). Parasites were cultivated in LIT medium supplemented with 10% FBS. The MTT method was used with minor modifications [42]. Next, 2 × 106 parasites/mL were seeded in a 96-well plate, adding 50 μM of each derivative dissolved in DMSO (final concentration remained below 1%). The plate was incubated for 96 h at 29 °C. Then, 5 mg mL−1 of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was added and incubated in darkness for 4 h. After this time, acidic isopropanol (4N) was added and the plate was read at 540 nm in a spectrophotometer Synergy HT (Biotek). Benznidazole (50 µM) was used as a reference drug.
3.3.5. Cytotoxicity Assay
The in vitro evaluations of compounds toxicity by MTT in VERO cells were performed as described in brief: VERO cells culture in DMEM, BSF 10%, were counted in suspension, seeded at 2 × 104 cells/well (100 μL) into a 96-well microplate, and incubated at 37 °C in a humidified 5% CO2 incubator for 24 h. After the incubation period, compounds were added in different concentrations: 50, 100, 150, 200, and 500 μM. The plate was incubated for 72 h in the same conditions. Then, 5 mg mL−1 of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was added and incubated in darkness for 4 h. After this time, DMSO was added and the plate was read at 540 nm in a spectrophotometer Synergy HT (Biotek). The test was carried out in triplicate including untreated cells and reference drug controls [43].
4. Conclusions
In summary, we synthesized 33 compounds derived from benzocycloalkanones with final yields ranging from moderate to very good through a synthesis strategy that was very useful and feasible. The compounds were characterized by spectroscopic techniques. Only three compounds, 10, 11, and 12, showed an inhibitory effect greater than 50% on the formation of β-hematin in vitro. The results of the in vivo antimalarial evaluation show that 10, 11, and 12 reduced parasitemia marginally, and an insignificant increase in the days of survival of the mice was observed after the fourth day of infection. As trypanocidals, all benzocycloalkanones showed marginal activity as inhibitors of the proliferation of T. cruzi epimastigotes, except compound 33, which had an activity of 51.08 ± 3.4% compared to the activity shown by the reference compound benznidazole 59.99 ± 2.9%. The compounds appear to have little cytotoxic effect on VERO cells when compared to the value presented by benznidazole against these mammalian cells. In general, we can infer that benzylthiobenzocycloalkanone compounds would be candidates as possible antiprotozoal agents, but the best activity could be achieved when tetralone rings are incorporated, which would make them ideal hits for further optimization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28145569/s1. The following are available: 1H/13C NMR, DEPT 135°, COSY, HETCOR, HMQC, and HMBC.
Author Contributions
Conceptualization, A.M., H.R and E.F.-M.; methodology, A.M., Z.B. and N.P.-G.; validation, N.P.-G. and J.E.C.; formal analysis, H.R, E.F.-M. and J.E.C.; NMR investigation, S.E.-T. and J.E.C.; resources, H.R., E.F.-M., N.P.-G. and J.E.C.; writing—original draft preparation, H.R., Z.B. and J.E.C.; writing—review and editing, E.F.-M. and J.E.C.; supervision, N.P.-G. and J.E.C.; project administration, E.F.-M. and J.E.C. All authors have read and agreed to the published version of the manuscript.
Funding
The project was partially funded by the Escuela de Medicina, Universidad de Especialidades Espíritu Santo (UEES), 2022-MED-001, and Ministerio de Ciencia, Tecnología e Innovación de Venezuela 028/2022 and 055/2023.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institute of Biomedicine Committee on Animal Research.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article and Supplementary Materials.
Acknowledgments
The authors acknowledge the Instituto de Investigaciones Farmacéuticas de la Facultad de Farmacia, Universidad Central de Venezuela.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Centers for Disease Control and Prevention. March 2022. Available online: https://www.cdc.gov/parasites/about.html (accessed on 5 July 2023).
- Barrow, P.; Dujardin, J.C.; Fasel, N.; Greenwood, A.D.; Osterrieder, K.; Lomonossof, G.; Fiori, P.L.; Atterbury, R.; Rossi, M.; Lalle, M. Viruses of protozoan parasites and viral therapy: Is the time now right? Virol. J. 2020, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Geneva: World Health Organization; 2020. License: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/publications/i/item/9789240010352 (accessed on 5 June 2023).
- World Health Organization. Available online: http://www.who.int/chagas/en/ (accessed on 15 February 2023).
- Castillo-Riquelme, M. Chagas disease in non-endemic countries. Lancet Glob. Health 2017, 5, e379–e380. [Google Scholar] [CrossRef] [PubMed]
- World Malaria Report. An In-Depth Update on Global and Regional Malaria Data and Trends. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 (accessed on 15 February 2023).
- Short, E.E.; Caminade, C.; Bolaji, N.; Thomas, B.N. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect. Dis. Res. Treat. 2017, 10, 1178633617732296. [Google Scholar] [CrossRef] [PubMed]
- Ridley, R.G. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 2002, 415, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.; Dhorda, M.; Fairhurst, R.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.; Mao, S.; Sam, B.; et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommends Groundbreaking Malaria Vaccines for Children at Risk. 2021. Available online: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk (accessed on 2 May 2023).
- Mosha, J.F.; Kulkarni, M.A.; Lukole, E.; Matowo, N.S.; Pitt, C.; Messenger, L.A.; Mallya, E.; Jumanne, M.; Aziz, T.; Kaaya, R.; et al. Effectiveness and cost-effectiveness against malaria of three types of dual-active-ingredient long-lasting insecticidal nets (LLINs) compared with pyrethroid-only LLINs in Tanzania: A four-arm, cluster-randomized trial. Lancet 2022, 399, 1227–1241. [Google Scholar] [CrossRef]
- Njoroge, M.; Njuguna, N.M.; Mutai, P.; Ongarora, D.S.B.; Smith, P.W.; Chibale, K. Recent approaches to chemical discovery and development against malaria and the Neglected Tropical Diseases human African trypanosomiasis and schistosomiasis. Chem. Rev. 2014, 114, 11138–11163. [Google Scholar] [CrossRef]
- Njogu, P.M.; Guantai, E.M.; Pavadai, E.; Chibale, K. Computer-aided drug discovery approaches against the tropical infectious diseases malaria, tuberculosis, trypanosomiasis, and leishmaniasis. ACS Infect. Dis. 2016, 2, 8–31. [Google Scholar] [CrossRef] [PubMed]
- Goupil, L.; McKerrow, J. Introduction: Drug discovery and development for neglected diseases. Chem. Rev. 2014, 114, 11131–11137. [Google Scholar] [CrossRef] [PubMed]
- Devine, W.; Thomas, S.M.; Erath, J.; Bachovchin, K.A.; Lee, P.J.; Leed, S.E.; Rodriguez, A.; Sciotti, R.J.; Mensa-Wilmot, K.; Pollastri, M.P. Antiparasitic lead discovery: Toward optimization of a chemotype with activity against multiple protozoan parasites. ACS Med. Chem. Lett. 2017, 8, 350–354. [Google Scholar] [CrossRef]
- Noronha, M.; Pawar, V.; Prajapati, A.; Subramanian, R. A literature review on traditional herbal medicines for malaria. S. Afr. J. Bot. 2020, 128, 292–303. [Google Scholar] [CrossRef]
- Kapishnikov, S.; Hempelmann, E.; Elbaum, M.; Als-Nielsen, J.; Leiserowitz, L. Malaria pigment crystals: The achilles’ heel of the malaria parasite. ChemMedChem. 2021, 16, 1515–1532. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Arora, P.; Kumar, B.; Mishra, L.; Bhattacharya, A.; Awasthi, S.; Bhasin, V. Synthesis of novel substituted 1,3-diaryl propenone derivatives and their antimalarial activity in vitro. Eur. J. Med. Chem. 2008, 43, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.; Deng, L.; Carrera, C.; Adachi, S.; Cottam, H.; Carson, D. Rational design, synthesis, and structure-activity relationships of antitumor (E)-2-benzylidene-1-tetralones and (E)-2-benzylidene-1-indanones. Bioorg. Med. Chem. Lett. 2000, 10, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Beteck, R.M.; Legoabe, L.J.; Isaacs, M.; Khanye, S.D.; Laming, D.; Hoppe, H.C. Anti-Trypanosomal and antimalarial properties of tetralone derivatives and structurally related benzocycloalkanones. Medicina 2019, 55, 206. [Google Scholar] [CrossRef]
- Charris, J.; Domínguez, J.; Gamboa, N.; Rodrigues, J.; Angel, J. Synthesis and antimalarial activity of E-2-quinolinylbenzocycloalcanones. Eur. J. Med. Chem. 2005, 40, 875–881. [Google Scholar] [CrossRef]
- Charris, J.; Lobo, G.; Camacho, J.; Ferrer, R.; Barazarte, A.; Domínguez, J.; Gamboa, N.; Rodrigues, J.; Angel, J. Synthesis and antimalarial activity of (E) 2-(2′-Chloro-3′-quinolinylmethylidene)-5,7-dimethoxyindanones. Lett. Drug Des. Dis. 2007, 4, 49–54. [Google Scholar] [CrossRef]
- Ferrer, R.; Lobo, G.; Gamboa, N.; Rodrigues, J.; Abramjuk, C.; Jung, K.; Lein, M.; Charris, J. Synthesis of [(7-chloroquinolin-4-yl)amino]chalcones: Potential antimalarial and anticancer agents. Sci. Pharm. 2009, 77, 725–742. [Google Scholar] [CrossRef]
- Domínguez, J.; León, C.; Rodrigues, J.; Gamboa, N.; Gut, J.; Rosenthal, P. Synthesis of chlorovinyl sulfones as structural analogs of chalcones and their antiplasmodial activities. Eur. J. Med. Chem. 2009, 44, 1457–1462. [Google Scholar] [CrossRef]
- Bahekar, S.; Hande, S.; Agrawal, N.; Chandak, H.; Bhoj, P.; Goswami, K.; Reddy, M. Sulfonamide chalcones: Synthesis and in vitro exploration for therapeutic potential against Brugia malayi. Eur. J. Med. Chem. 2016, 124, 262–269. [Google Scholar] [CrossRef]
- Li, R.; Kenyon, G.; Cohen, F.; Chen, X.; Gong, B.; Domínguez, J.; Davidson, E.; Kurzban, G.; Miller, R.; Nuzum, E.; et al. In vitro antimalarial activity of chalcones and their derivatives. J. Med. Chem. 1995, 38, 5031–5037. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Balbhadra, S.; Choudhary, J.; Kohli, D. Exploring the pharmacological significance of chalcone scaffold: A review. Curr. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef]
- Mahapatra, D.; Bharti, S.; Asati, V. Chalcone scaffolds as anti-infective agents: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 101, 496–524. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.; Gomes, J.; Gomes, P. Falcipains, Plasmodium falciparum cysteine proteases as key drug targets against malaria. Curr. Med. Chem. 2011, 18, 1555–1572. [Google Scholar] [CrossRef] [PubMed]
- Go, M.; Liu, M.; Wilairat, P.; Rosenthal, P.; Saliba, K.; Kirk, K. Antiplasmodial chalcones inhibit sorbitol-induced hemolysis of Plasmodium falciparum-infected erythrocytes. Antimicrob. Agents Chemother. 2004, 48, 3241–3245. [Google Scholar] [CrossRef]
- Geyer, J.; Keenan, S.; Woodard, C.; Thompson, P.; Gerena, L.; Nichols, D.; Gutteridge, C.; Waters, N. Selective inhibition of Pfmrk, a Plasmodium falciparum CDK, by antimalarial 1,3-diaryl-2-propenones. Bioorg. Med. Chem. Lett. 2009, 19, 1982–1985. [Google Scholar] [CrossRef]
- Srivastava, S.; Joshi, S.; Singh, A.; Yadav, S.; Saxena, A.; Ram, V.; Chandra, S.; Saxena, J. Oxygenated chalcones and bischalcones as a new class of inhibitors of DNA topoisomerase II of malarial parasites. Med. Chem. Res. 2008, 17, 234–244. [Google Scholar] [CrossRef]
- Kumar, S.; Guha, M.; Choubey, V.; Maity, P.; Bandyopadhyay, U. Antimalarial drugs inhibiting hemozoin (beta-hematin) formation: A mechanistic update. Life Sci. 2007, 80, 813–828. [Google Scholar] [CrossRef]
- Le Bonniec, S.; Deregnaucourt, C.; Redeker, V.; Banerjee, R.; Grellier, P.; Goldberg, D.; Schrével, J. Plasmepsin II, an acidic hemoglobinase from the Plasmodium falciparum food vacuole, is active at neutral pH on the host erythrocyte membrane skeleton. J. Biol. Chem. 1999, 274, 14218–14223. [Google Scholar] [CrossRef]
- Ribeiro, F.F.; Junior, F.J.; da Silva, M.S.; Scotti, M.T.; Scotti, L. Computational and investigative study of flavonoids active against Trypanosoma cruzi and Leishmania spp. Nat. Prod. Commun. 2015, 10, 917–920. [Google Scholar] [PubMed]
- Magalhaes, E.P.; Barroso Gomes, N.D.; Araújo de Freitas, T.; Pinheiro Silva, B.; Rodrigues Ribeiro, L.; Queiroz Ameida-Neto, F.W.; Machado Marinho, M.; de Lima-Neto, P.; Silva Marinho, E.; Silva dos Santos, H.; et al. Chloride substitution on 2-hydroxy-3,4,6-trimethoxyphenylchalcones improves in vitro selectivity on Trypanosoma cruzi strain Y. Chem. Biol. Interact. 2022, 361, 109920. [Google Scholar] [CrossRef]
- Leitao Cavalcante, C.H.; Queiroz Almeida-Neto, F.W.; Nunes da Rocha, M.; Nogueira Bandeira, P.; Pessoa Bezerra de Menezes, R.R.; Magalhaes, E.P.; Lima Sampaio, T.; Silva Marinho, E.; Machado Marinho, M.; Costa Martins, A.M.; et al. Antichagasic evaluation, molecular docking and ADMET properties of the chalcone (2E)-3-(2-fluorophenyl)-1-(2-hydroxy- 3,4,6-trimethoxyphenyl)prop-2-en1-one against Trypanosoma cruzi. J. Biomol. Struct. Dyn. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, H.; Domínguez, J.; Fernandez-Moreira, E.; Rodrigues, J.; Rodríguez, m.; Charris, J.E. Synthesis of 4-benzylsulfanyl and 4-benzylsulfonyl chalcones. biological evaluation as antimalarial agents. Farmacia J. 2022, 70, 30–42. [Google Scholar] [CrossRef]
- Baelmans, R.; Deharo, E.; Muñoz, V.; Sauvain, M.; Ginsburg, H. Experimental conditions for testing the inhibitory activity of chloroquine on the formation of beta-hematin. Exp. Parasitol. 2000, 96, 243–248. [Google Scholar] [CrossRef]
- Peters, W.; Robinson, B. Parasitic Infection Models. In Handbook of Antimalarial Models of Infection; Zak, O., Sande, M., Eds.; Academic Press: London, UK, 1999; p. 757. [Google Scholar]
- Leañez, J.; Nuñez, J.; García-Marchan, Y.; Sojo, F.; Arvelo, F.; Rodriguez, D.; Buscema, I.; Alvarez-Aular, A.; Bello, J.; Kouznetsov, V.; et al. Anti-leishmanial effect of spiro dihydroquinoline-oxindoles on volume regulation decrease and sterol biosynthesis of Leishmania braziliensis. Exp. Parasitol. 2019, 198, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Martín, X.; Payares, G.; Mendoza-León, A. Glibenclamide, a blocker of K+ATP channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2006, 4, 4214–4216. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).