Theoretical Insights on ORR Activity of Sn-N-C Single-Atom Catalysts
Abstract
1. Introduction
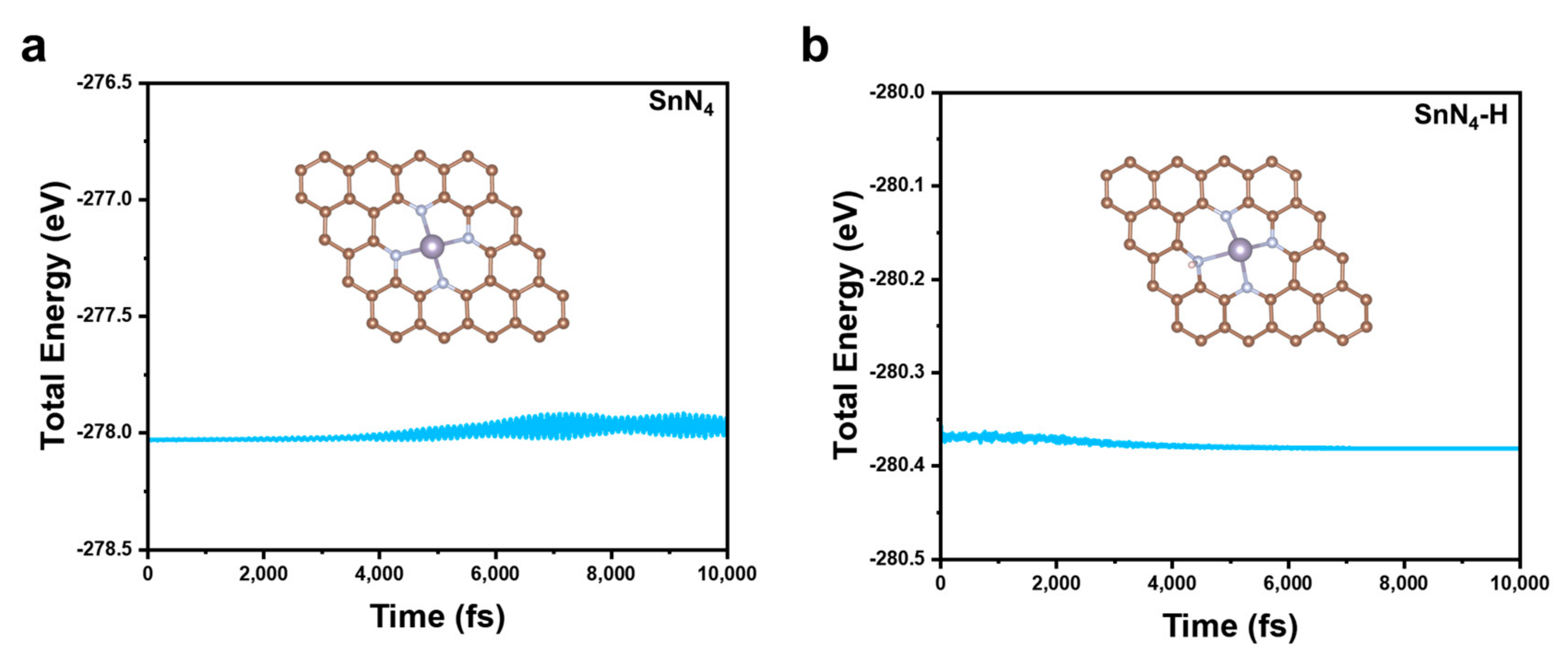
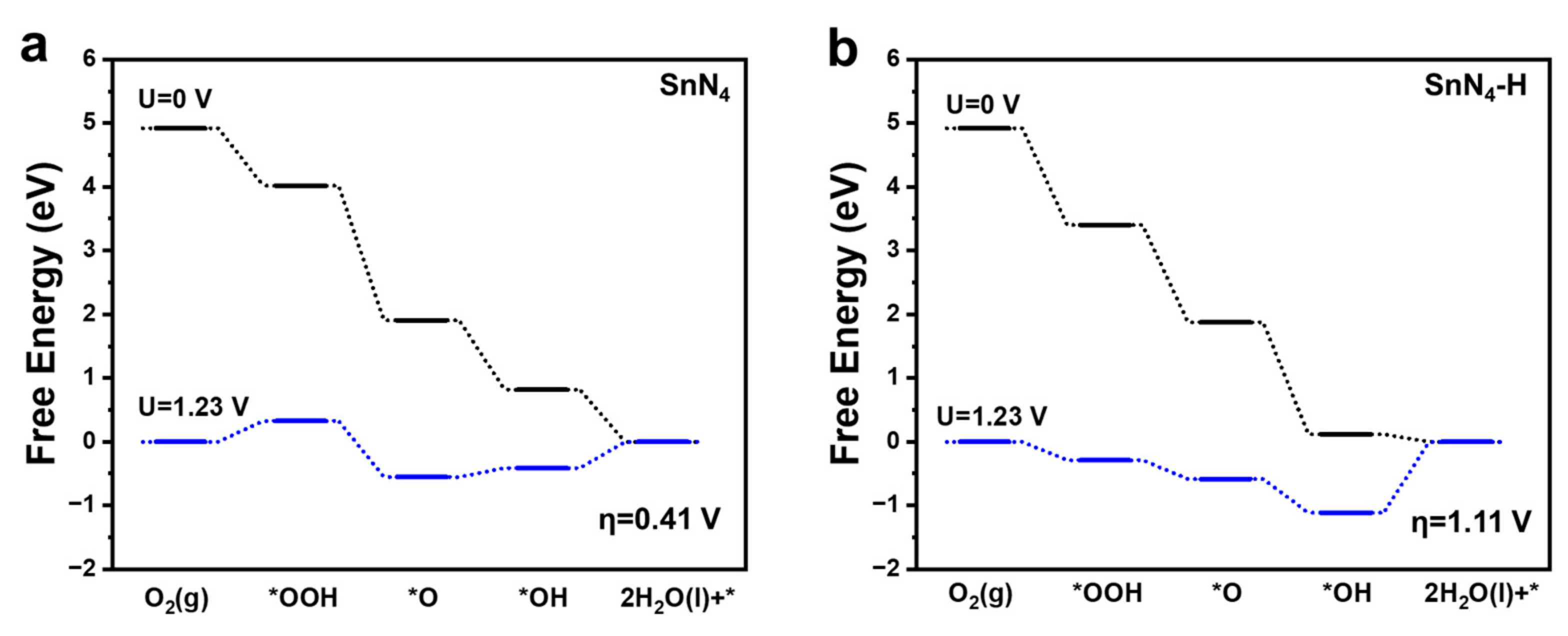
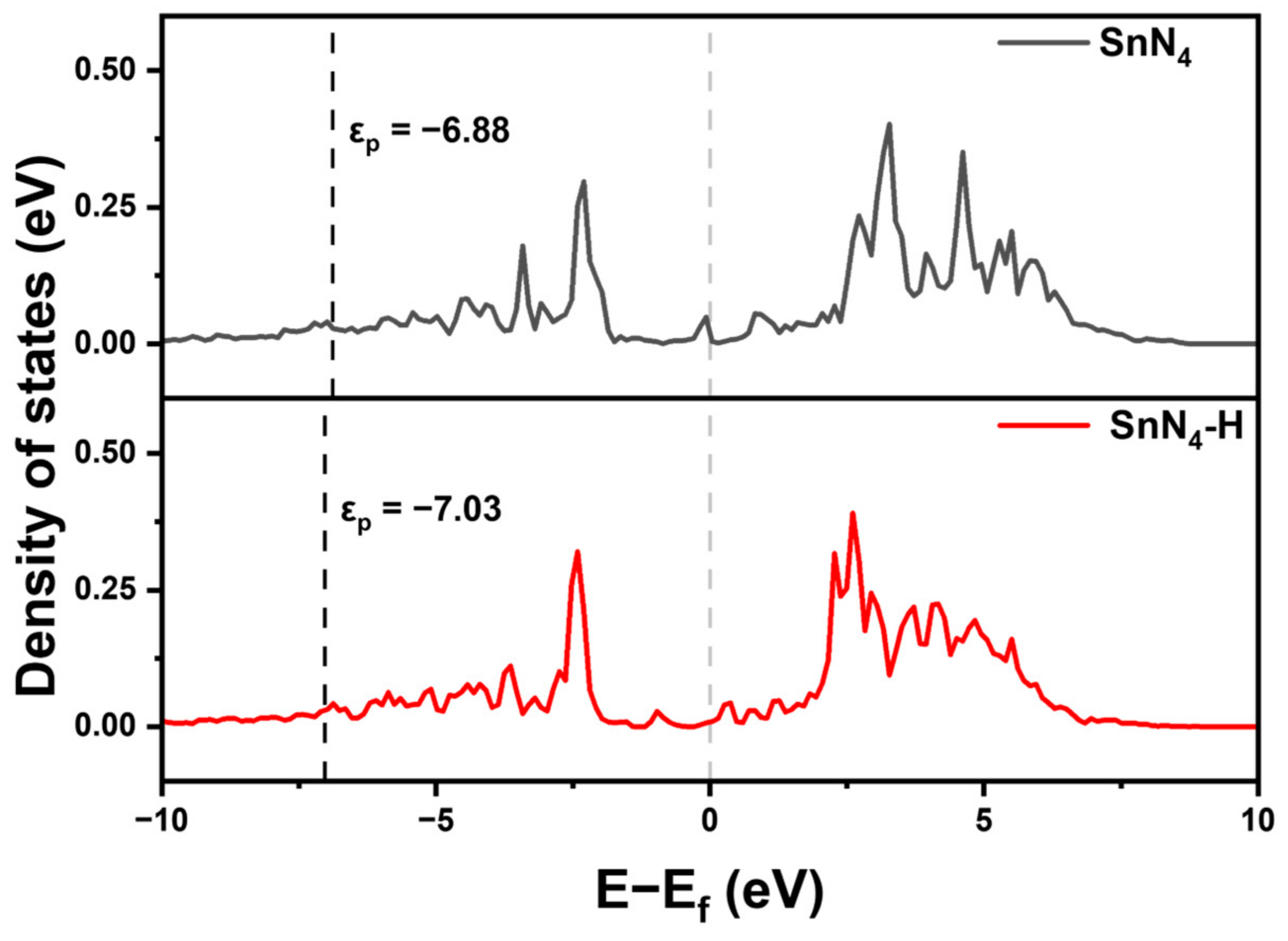
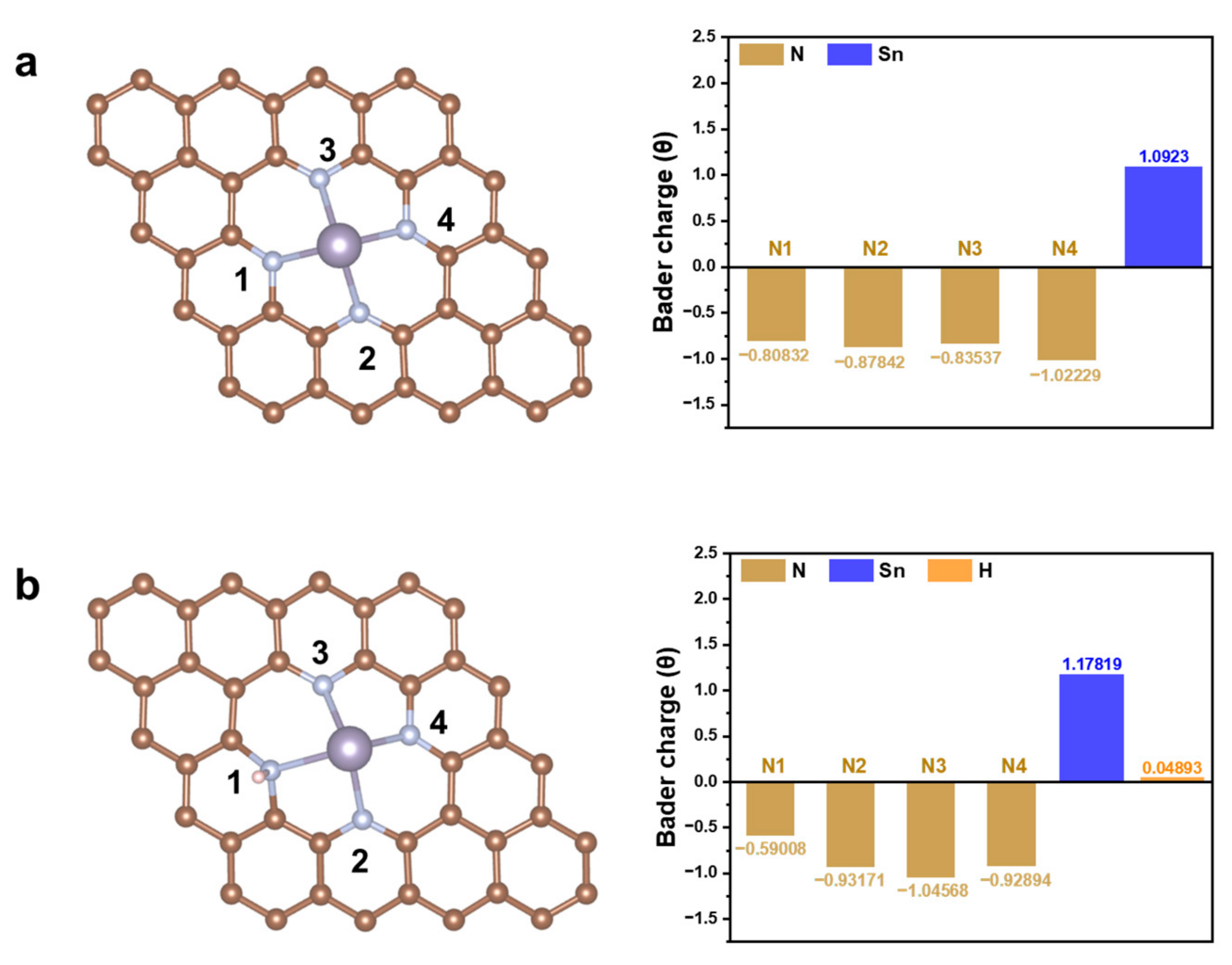
2. Results and Discussion
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dilpazir, S.; He, H.; Li, Z.; Wang, M.; Lu, P.; Liu, R.; Xie, Z.; Gao, D.; Zhang, G. Cobalt Single Atoms Immobilized N-Doped Carbon Nanotubes for Enhanced Bifunctional Catalysis toward Oxygen Reduction and Oxygen Evolution Reactions. ACS Appl. Energy Mater. 2018, 1, 3283–3291. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Yang, C.; Tao, S.; Huang, N.; Zhang, X.; Duan, J.; Makiura, R.; Maenosono, S. Heteroatom-Doped Carbon Electrocatalysts Derived from Nanoporous Two-Dimensional Covalent Organic Frameworks for Oxygen Reduction and Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 5481–5488. [Google Scholar] [CrossRef]
- Khan, K.; Khan Tareen, A.; Li, J.; Khan, U.; Nairan, A.; Yuan, Y.; Zhang, X.; Yang, M.; Ouyang, Z. Facile synthesis of tin-doped mayenite electride composite as a non-noble metal durable electrocatalyst for oxygen reduction reaction (ORR). Dalton Trans. 2018, 47, 13498–13506. [Google Scholar] [CrossRef]
- Li, B.; Fang, J.; Xu, D.; Zhao, H.; Zhu, H.; Zhang, F.; Dong, Z. Atomically Dispersed Co Clusters Anchored on N-doped Carbon Nanotubes for Efficient Dehydrogenation of Alcohols and Subsequent Conversion to Carboxylic Acids. ChemSusChem 2021, 14, 4536–4545. [Google Scholar] [CrossRef]
- Zhuo, H.-Y.; Zhang, X.; Liang, J.-X.; Yu, Q.; Xiao, H.; Li, J. Theoretical Understandings of Graphene-based Metal Single-Atom Catalysts: Stability and Catalytic Performance. Chem. Rev. 2020, 120, 12315–12341. [Google Scholar] [CrossRef]
- Liang, X.; Ke, Q.; Zhao, X.; Chen, X. Graphene-Supported Tin Single-Atom Catalysts for CO2 Hydrogenation to HCOOH: A Theoretical Investigation of Performance under Different N Coordination Numbers. ACS Appl. Nano Mater. 2023, 6, 4489–4498. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, F.; Tang, Q. The active structure of p-block SnNC single-atom electrocatalysts for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2022, 24, 27302–27311. [Google Scholar] [CrossRef]
- Shen, Z.; Hong, L.; Zheng, B.; Wang, G.; Zhang, B.; Wang, Z.; Zhan, F.; Shen, S.; Yun, R. Highly Efficient and Chemoselective Hydrogenation of Nitro Compounds into Amines by Nitrogen-Doped Porous Carbon-Supported Co/Ni Bimetallic Nanoparticles. Inorg. Chem. 2021, 60, 16834–16839. [Google Scholar] [CrossRef]
- Chen, Y.N.; Zhang, X.; Zhou, Z. Carbon-Based Substrates for Highly Dispersed Nanoparticle and Even Single-Atom Electrocatalysts. Small Methods 2019, 3, 1900050. [Google Scholar] [CrossRef]
- Li, B.; Zhao, H.; Fang, J.; Li, J.; Gao, W.; Ma, K.; Liu, C.; Yang, H.; Ren, X.; Dong, Z. Ru nanoparticles anchored on porous N-doped carbon nanospheres for efficient catalytic hydrogenation of Levulinic acid to gamma-valerolactone under solvent-free conditions. J. Colloid Interface Sci. 2022, 623, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, L.; Zhao, M.; Huang, H.; Ding, X.; Wu, C.; Gates, I.D.; Gao, Z. Theoretical prediction of graphene-based single-atom iron as a novel catalyst for catalytic oxidation of Hg0 by O2. Appl. Surf. Sci. 2020, 508, 145035. [Google Scholar] [CrossRef]
- Yuan, K.; Lutzenkirchen-Hecht, D.; Li, L.; Shuai, L.; Li, Y.; Cao, R.; Qiu, M.; Zhuang, X.; Leung, M.K.H.; Chen, Y.; et al. Boosting Oxygen Reduction of Single Iron Active Sites via Geometric and Electronic Engineering: Nitrogen and Phosphorus Dual Coordination. J. Am. Chem. Soc. 2020, 142, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, R.; Li, J.; Zhao, H.; Ma, J.; Dong, Z. Atomically dispersed Co-N4 sites anchored on N-doped carbon for aqueous phase transfer hydrogenation between nitroarenes and saturated N-heterocycles. Appl. Catal. B Envrion. 2021, 299, 120681. [Google Scholar] [CrossRef]
- Yun, R.; Zhang, B.; Zhan, F.; Du, L.; Wang, Z.; Zheng, B. Cu Nanoclusters Anchored on the Metal-Organic Framework for the Hydrolysis of Ammonia Borane and the Reduction of Quinolines. Inorg. Chem. 2021, 60, 12906–12911. [Google Scholar] [CrossRef]
- Luo, F.; Roy, A.; Silvioli, L.; Cullen, D.A.; Zitolo, A.; Sougrati, M.T.; Oguz, I.C.; Mineva, T.; Teschner, D.; Wagner, S.; et al. P-block single-metal-site tin/nitrogen-doped carbon fuel cell cathode catalyst for oxygen reduction reaction. Nat. Mater. 2020, 19, 1215–1223. [Google Scholar] [CrossRef]
- Ni, W.; Gao, Y.; Lin, Y.; Ma, C.; Guo, X.; Wang, S.; Zhang, S. Nonnitrogen Coordination Environment Steering Electrochemical CO2-to-CO Conversion over Single-Atom Tin Catalysts in a Wide Potential Window. ACS Catal. 2021, 11, 5212–5221. [Google Scholar] [CrossRef]
- Xing, T.; Zheng, Y.; Li, L.H.; Cowie, B.C.C.; Gunzelmann, D.; Qiao, S.Z.; Huang, S.; Chen, Y. Observation of Active Sites for Oxygen Reduction Reaction on Nitrogen-Doped Multilayer Graphene. ACS Nano 2014, 8, 6856–6862. [Google Scholar] [CrossRef]
- Li, F.; Noh, H.J.; Che, W.; Jeon, J.P.; Han, G.F.; Shin, T.J.; Kim, M.G.; Wang, Y.; Bu, Y.; Fu, Z.; et al. Tin Nanoclusters Confined in Nitrogenated Carbon for the Oxygen Reduction Reaction. ACS Nano 2022, 16, 18830–18837. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Spendelow, J.S.; Wu, G. Atomically Dispersed Metal Catalysts for Oxygen Reduction. ACS Energy Lett. 2019, 4, 1619–1633. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, R.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Cao, W.; Zheng, B.; Wang, Z.; Yun, R. Iron-Based Active Sites Encapsulated in Carbon Nanotubes for an Efficient Hydride Process. ACS Appl. Nano Mater. 2023, 6, 3218–3225. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, B.; Su, Y. Theoretical Insights on ORR Activity of Sn-N-C Single-Atom Catalysts. Molecules 2023, 28, 5571. https://doi.org/10.3390/molecules28145571
Zhang Y, Li B, Su Y. Theoretical Insights on ORR Activity of Sn-N-C Single-Atom Catalysts. Molecules. 2023; 28(14):5571. https://doi.org/10.3390/molecules28145571
Chicago/Turabian StyleZhang, Yuhui, Boyang Li, and Yaqiong Su. 2023. "Theoretical Insights on ORR Activity of Sn-N-C Single-Atom Catalysts" Molecules 28, no. 14: 5571. https://doi.org/10.3390/molecules28145571
APA StyleZhang, Y., Li, B., & Su, Y. (2023). Theoretical Insights on ORR Activity of Sn-N-C Single-Atom Catalysts. Molecules, 28(14), 5571. https://doi.org/10.3390/molecules28145571







