Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023)
Abstract
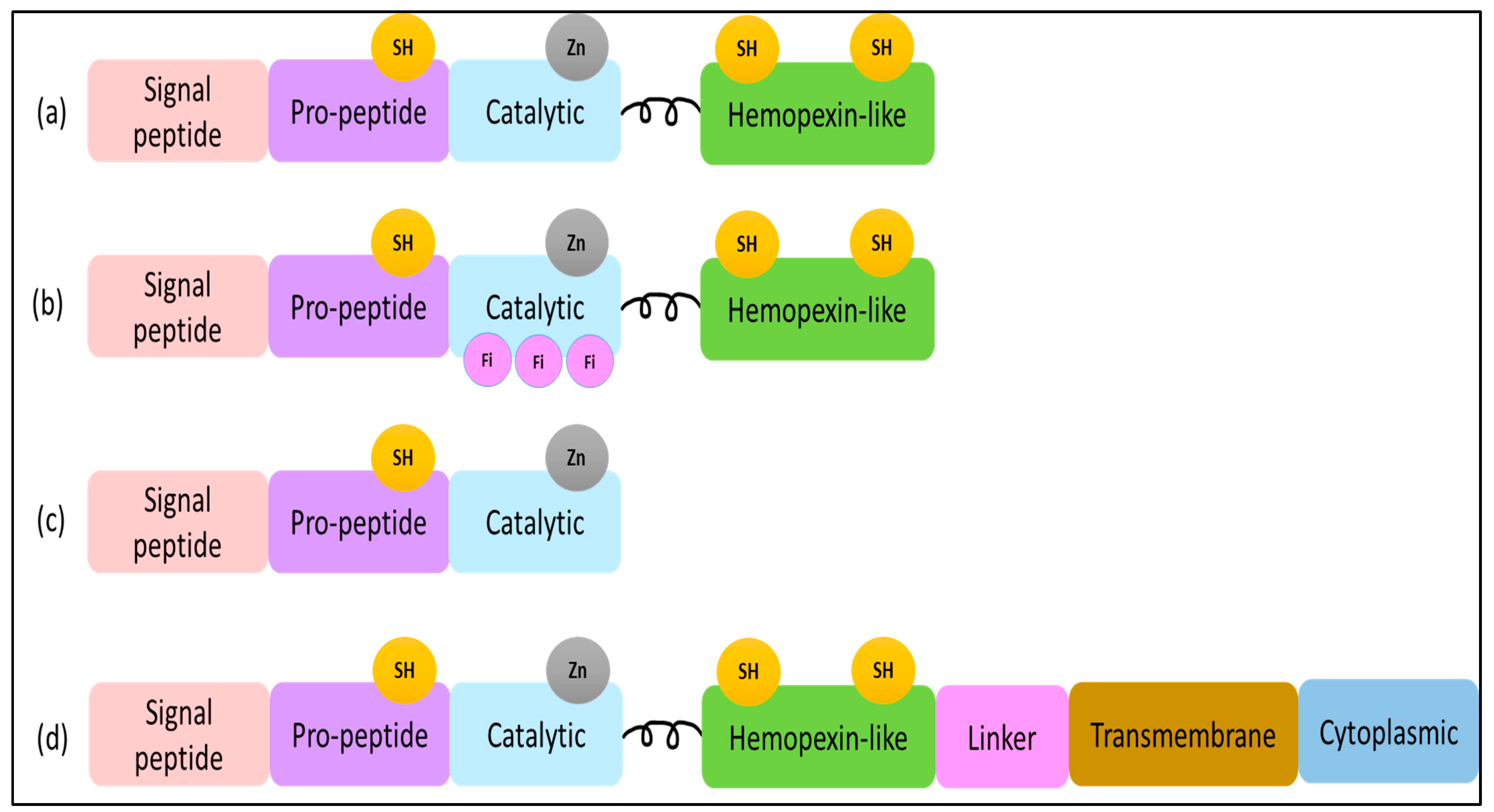
1. Introduction
2. Matrix Metalloprotease-1 (MMP-1)
2.1. Function and Localization
2.2. Role in Cancer
2.3. Structure of the Catalytic Domain
2.4. MMP-1 Inhibitors
3. Matrix Metalloprotease-2 (MMP-2)
3.1. Function and Localization
3.2. Role in Cancer
3.3. Structure of the Catalytic Domain
3.4. MMP-2 Inhibitors
| Compound | Potency | Chemical Structure | Reference |
|---|---|---|---|
| 6 | - |  | [89] |
| 7 | pIC50 (−logIC50) = 8.60“IC50, nM” |  | [97] |
| 8 | IC50 = 0.38 µM |  | [66] |
| 9 | IC50 < 5 µM |  | [96] |
| 10 | IC50 = 0.33 µM |  | [96] |
| 11 | IC50 = 0.21 µM |  | [98] |
| 12 | IC50 = 6.40 µM |  R1 = 4Br/R2= CH2C6H4 4-Nitrobenzyl | [99] |
| 13 | IC50 = 7.4 ± 0.8 µM |  | [91] |
| 14 | IC50 = 2.80 µM |  | [100] |
| 15 | IC50 = 0.70 ± 0.02 µM |  | [65] |
| 16 | IC50 = 0.376 µM |  | [67] |
| 17 | IC50 = 5.6 nM |  | [101] |
| 18 | - |  | [102] |
| 19 | Percent of inhibition = 73.3% |  | [102] |
| 20 | Percent of inhibition = 75.2% |  | [102] |
| 21 | IC50 = 31 ± 5 µM |  | [103] |
| 22 | IC50 = 21 nM |  | [104] |
| 23 | IC50 < 1nM |  | [104] |
4. Matrix Metalloprotease-3 (MMP-3)
4.1. Function and Localization
4.2. Role in Cancer
4.3. Structure of the Catalytic Domain
4.4. MMP-3 Inhibitors
5. Matrix Metalloprotease-7 (MMP-7)
5.1. Function and Localization
5.2. Role in Cancer
5.3. Structure of the Catalytic Domain
5.4. MMP-7 Inhibitors
6. Matrix Metalloprotease-12 (MMP-12)
6.1. Function and Localization
6.2. Role in Cancer
6.3. Structure of Catalytic Domain
6.4. MMP-12 Inhibitors
7. Matrix Metalloprotease-14 (MMP-14)
7.1. Function and Localization
7.2. Role in Cancer
7.3. Structure of the Catalytic Domain
7.4. MMP-14 Inhibitors
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Woessner, J.F. The Matrix Metalloproteinase Family. In Biology of Extracellular Matrix, Matrix Metalloproteinases; Parks, W.C., Mecham, R.P., Eds.; Academic Press: Cambridge, MA, USA, 1998; pp. 1–14. ISBN 9780125450904. [Google Scholar]
- Nosrati, R.; Kheirouri, S.; Ghodsi, R.; Ojaghi, H. The effects of zinc treatment on matrix metalloproteinases: A systematic review. J. Trace Elem. Med. Biol. 2019, 56, 107–115. [Google Scholar] [CrossRef]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef]
- Abdool, A.Y.; Abbas, L.; Sebaei, T.; Schmitt, E.; Sikora, A. How can we design an inhibitor with an enhanced binding affinity that is selective for MMP12? In Protein Modeling Reports 4; NSUWorks: Fort Lauderdale, FL, USA, 2021. [Google Scholar]
- Shi, Y.; Ma, X.; Fang, G.; Tian, X.; Ge, C. Matrix metalloproteinase inhibitors (MMPIs) as attractive therapeutic targets: Recent progress and current challenges. NanoImpact 2021, 21, 100293. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- DeClerck, Y.A.; Mercurio, A.M.; Stack, M.S.; Chapman, H.A.; Zutter, M.M.; Muschel, R.J.; Raz, A.; Matrisian, L.M.; Sloane, B.F.; Noel, A. Proteases, extracellular matrix, and cancer: A workshop of the path B study section. Am. J. Pathol. 2004, 164, 1131–1139. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. S1), 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lenci, E.; Cosottini, L.; Trabocchi, A. Novel matrix metalloproteinase inhibitors: An updated patent review (2014–2020). Expert Opin. Ther. Pat. 2021, 31, 509–523. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Butler, G.S.; Overall, C.M. Updated Biological Roles for Matrix Metalloproteinases and New “Intracellular” Substrates Revealed by Degradomics. Biochemistry 2009, 48, 10830–10845. [Google Scholar] [CrossRef] [PubMed]
- Seizer, P.; May, A.E. Platelets and matrix metalloproteinases. Thromb. Haemost. 2013, 110, 903–909. [Google Scholar] [CrossRef]
- Noël, A.; Perveen, Z.; Xiao, R.; Hammond, H.; Le Donne, V.; Legendre, K.; Gartia, M.R.; Sahu, S.; Paulsen, D.B.; Penn, A.L. Mmp12 Is Upregulated by in utero Second-Hand Smoke Exposures and Is a Key Factor Contributing to Aggravated Lung Responses in Adult Emphysema, Asthma, and Lung Cancer Mouse Models. Front. Physiol. 2021, 12, 704401. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgis, C.A.; Papaioannou, P.; Hadjipavlou-Litina, D.J. Matrix metalloproteinase inhibitors: A review on pharmacophore mapping and (Q) SARs results. Curr. Med. Chem. 2005, 12, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, A.; Beltrán-Debón, R.; Mulero, M.; Pujadas, G.; Garcia-Vallvé, S. Understanding the variability of the S1′ pocket to improve matrix metalloproteinase inhibitor selectivity profiles. Drug Discov. Today 2020, 25, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol. 2019, 56, 100–115. [Google Scholar] [CrossRef]
- Chelluboina, B.; Nalamolu, K.R.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. MMP-12, a promising therapeutic target for neurological diseases. Mol. Neurobiol. 2018, 55, 1405–1409. [Google Scholar] [CrossRef]
- Amălinei, C.; Căruntu, I.-D.; Bălan, R.A. Biology of metalloproteinases. Rom. J. Morphol. Embryol. 2007, 48, 323–334. [Google Scholar]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Ti, H.; Zhou, Y.; Liang, X.; Li, R.; Ding, K.; Zhao, X. Targeted treatments for chronic obstructive pulmonary disease (COPD) using low-molecular-weight drugs (LMWDs). J. Med. Chem. 2019, 62, 5944–5978. [Google Scholar] [CrossRef]
- Ramezani, M.; Shamsara, J. An integrated structure-and pharmacophore-based MMP-12 virtual screening. Mol. Divers. 2018, 22, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Visse, R.; Nagase, H.; Acharya, K.R. Crystal structure of an active form of human MMP-1. J. Mol. Biol. 2006, 362, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Baidya, S.K.; Banerjee, S.; Adhikari, N.; Jha, T. Selective Inhibitors of Medium-Size S1′ Pocket Matrix Metalloproteinases: A Stepping Stone of Future Drug Discovery. J. Med. Chem. 2022, 65, 10709–10754. [Google Scholar] [CrossRef] [PubMed]
- Hadler-Olsen, E.; Winberg, J.-O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, Y.K.; Singh, S.; Arunraja, S. Picrorhiza kurroa inhibits experimental arthritis through inhibition of pro-inflammatory cytokines, angiogenesis and MMPs. Phytother. Res. 2016, 30, 112–119. [Google Scholar] [CrossRef]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future SuccessesMatrix Metalloproteinase Inhibitors in Cancer Therapy. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
- Fabre, B.; Ramos, A.; de Pascual-Teresa, B. Targeting matrix metalloproteinases: Exploring the dynamics of the S1′ pocket in the design of selective, small molecule inhibitors: Miniperspective. J. Med. Chem. 2014, 57, 10205–10219. [Google Scholar] [CrossRef]
- Devy, L.; Dransfield, D.T. New strategies for the next generation of matrix-metalloproteinase inhibitors: Selectively targeting membrane-anchored MMPs with therapeutic antibodies. Biochem. Res. Int. 2011, 2011, 191670. [Google Scholar] [CrossRef]
- Mahasenan, K.V.; Bastian, M.; Gao, M.; Frost, E.; Ding, D.; Zorina-Lichtenwalter, K.; Jacobs, J.; Suckow, M.A.; Schroeder, V.A.; Wolter, W.R. Exploitation of conformational dynamics in imparting selective inhibition for related matrix metalloproteinases. ACS Med. Chem. Lett. 2017, 8, 654–659. [Google Scholar] [CrossRef]
- Hoseok, I.; Cho, J.-Y. Chapter Three—Lung Cancer Biomarkers. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 72, pp. 107–170. [Google Scholar]
- Ma, Y.; Iyer, R.P.; de Castro Brás, L.E.; Toba, H.; Yabluchanskiy, A.; Deleon-Pennell, K.Y.; Hall, M.E.; Lange, R.A.; Lindsey, M.L. Chapter 4—Cross Talk Between Inflammation and Extracellular Matrix Following Myocardial Infarction. In Inflammation in Heart Failure; Blankesteijn, W.M., Altara, R., Eds.; Academic Press: Boston, MA, USA, 2015. [Google Scholar]
- Lemaître, V.; D’Armiento, J. Matrix metalloproteinases in development and disease. Birth Defects Res. Part C Embryo Today Rev. 2006, 78, 1–10. [Google Scholar] [CrossRef]
- Hatfield, K.J.; Reikvam, H.; Bruserud, Ø. The crosstalk between the matrix metalloprotease system and the chemokine network in acute myeloid leukemia. Curr. Med. Chem. 2010, 17, 4448–4461. [Google Scholar] [CrossRef] [PubMed]
- Herrera, I.; Cisneros, J.; Maldonado, M.; Ramírez, R.; Ortiz-Quintero, B.; Anso, E.; Chandel, N.S.; Selman, M.; Pardo, A. Matrix metalloproteinase (MMP)-1 induces lung alveolar epithelial cell migration and proliferation, protects from apoptosis, and represses mitochondrial oxygen consumption. J. Biol. Chem. 2013, 288, 25964–25975. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [PubMed]
- Fischer, T.; Senn, N.; Riedl, R. Design and Structural Evolution of Matrix Metalloproteinase Inhibitors. Chemistry 2019, 25, 7960–7980. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; López-Otín, C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar]
- Chen, P.; Parks, W.C. Role of matrix metalloproteinases in epithelial migration. J. Cell. Biochem. 2009, 108, 1233–1243. [Google Scholar] [CrossRef]
- Limb, G.A.; Matter, K.; Murphy, G.; Cambrey, A.D.; Bishop, P.N.; Morris, G.E.; Khaw, P.T. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am. J. Pathol. 2005, 166, 1555–1563. [Google Scholar] [CrossRef]
- Galt, S.W.; Lindemann, S.; Allen, L.; Medd, D.J.; Falk, J.M.; McIntyre, T.M.; Prescott, S.M.; Kraiss, L.W.; Zimmerman, G.A.; Weyrich, A.S. Outside-in signals delivered by matrix metalloproteinase-1 regulate platelet function. Circ. Res. 2002, 90, 1093–1099. [Google Scholar] [CrossRef]
- Naito, S.; Shimizu, S.; Matsuu, M.; Nakashima, M.; Nakayama, T.; Yamashita, S.; Sekine, I. Ets-1 Upregulates Matrix Metalloproteinase-1 Expression through Extracellular Matrix Adhesion in Vascular Endothelial Cells. Biochem. Biophys. Res. Commun. 2002, 291, 130–138. [Google Scholar] [CrossRef]
- Said, A.H.; Hu, S.; Abutaleb, A.; Watkins, T.; Cheng, K.; Chahdi, A.; Kuppusamy, P.; Saxena, N.; Xie, G.; Raufman, J.P. Interacting post-muscarinic receptor signaling pathways potentiate matrix metalloproteinase-1 expression and invasion of human colon cancer cells. Biochem. J. 2017, 474, 647–665. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.D.; Steele, I.; Moore, A.R.; Murugesan, S.V.; Rakonczay, Z.; Venglovecz, V.; Pritchard, D.M.; Dimaline, R.; Tiszlavicz, L.; Varro, A.; et al. Gastrin stimulates MMP-1 expression in gastric epithelial cells: Putative role in gastric epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G78–G86. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Dier, U.; Melendez, J.A.; Hempel, N. Regulation of MMP-1 expression in response to hypoxia is dependent on the intracellular redox status of metastatic bladder cancer cells. Biochim. Biophys. Acta 2015, 1852, 2593–2602. [Google Scholar] [CrossRef]
- Bostrom, P.; Soderstrom, M.; Vahlberg, T.; Soderstrom, K.O.; Roberts, P.J.; Carpen, O.; Hirsimaki, P. MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer 2011, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.W.; Li, J.; Li, X.Q.; Wang, J.Q.; Huang, Y. Expression of STAT3, MMP-1 and TIMP-1 in gastric cancer and correlation with pathological features. Mol. Med. Rep. 2012, 5, 1438–1442. [Google Scholar]
- Shimizu, Y.; Kondo, S.; Shirai, A.; Furukawa, M.; Yoshizaki, T. A single nucleotide polymorphism in the matrix metalloproteinase-1 and interleukin-8 gene promoter predicts poor prognosis in tongue cancer. Auris Nasus Larynx 2008, 35, 381–389. [Google Scholar] [CrossRef]
- Trivedi, V.; Boire, A.; Tchernychev, B.; Kaneider, N.C.; Leger, A.J.; O’Callaghan, K.; Covic, L.; Kuliopulos, A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell 2009, 137, 332–343. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, J.; Yu, J.; Wu, Y.; Guo, J.; Xu, Z.; Sun, X. Knockdown of MMP-1 inhibits the progression of colorectal cancer by suppressing the PI3K/Akt/c-myc signaling pathway and EMT. Oncol. Rep. 2020, 43, 1103–1112. [Google Scholar] [CrossRef]
- Tao, Y.S.; Ma, X.Y.; Chai, D.M.; Ma, L.; Feng, Z.Z.; Cheng, Z.N.; Lai, M.D. Overexpression of MMP-1 and VEGF-C is associated with a less favorable prognosis in esophageal squamous cell carcinoma. Onkologie 2012, 35, 651–656. [Google Scholar] [CrossRef]
- Zamolo, G.; Grahovac, M.; Žauhar, G.; Vučinić, D.; Kovač, L.; Brajenić, N.; Grahovac, B. Matrix metalloproteinases MMP-1, MMP-2, and MMP-13 are overexpressed in primary nodular melanoma. J. Cutan. Pathol. 2020, 47, 139–145. [Google Scholar] [CrossRef]
- Becker, D.P.; Barta, T.E.; Bedell, L.J.; Boehm, T.L.; Bond, B.R.; Carroll, J.; Carron, C.P.; DeCrescenzo, G.A.; Easton, A.M.; Freskos, J.N.; et al. Orally Active MMP-1 Sparing α-Tetrahydropyranyl and α-Piperidinyl Sulfone Matrix Metalloproteinase (MMP) Inhibitors with Efficacy in Cancer, Arthritis, and Cardiovascular Disease. J. Med. Chem. 2010, 53, 6653–6680. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, W.; Wang, L.; Shan, L.; Li, H.; Huang, J.; Sun, Q.; Zhang, W. Synthesis of derivatives of methyl rosmarinate and their inhibitory activities against matrix metalloproteinase-1 (MMP-1). Eur. J. Med. Chem. 2013, 62, 148–157. [Google Scholar] [CrossRef]
- Umedera, K.; Yoshimori, A.; Bajorath, J.; Nakamura, H. Design of MMP-1 inhibitors via SAR transfer and experimental validation. Sci. Rep. 2022, 12, 20915. [Google Scholar] [CrossRef]
- Kaplancikli, Z.A.; Altintop, M.D.; Atli, O.; Sever, B.; Baysal, M.; Temel, H.E.; Demirci, F.; Ozdemir, A. Synthesis and Evaluation of A New Series of Thiazole Derivatives as Potential Antitumor Agents and MMP Inhibitors. Anticancer Agents Med. Chem. 2017, 17, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Massaro, A.; Calderone, V.; Fragai, M.; Luchinat, C.; Mordini, A. Discovery of a New Class of Potent MMP Inhibitors by Structure-Based Optimization of the Arylsulfonamide Scaffold. ACS Med. Chem. Lett. 2013, 4, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Asawa, Y.; Yoshimori, A.; Bajorath, J.; Nakamura, H. Prediction of an MMP-1 inhibitor activity cliff using the SAR matrix approach and its experimental validation. Sci. Rep. 2020, 10, 14710. [Google Scholar] [CrossRef] [PubMed]
- Hrabec, E.; Naduk, J.; Strek, M.; Hrabec, Z. [Type IV collagenases (MMP-2 and MMP-9) and their substrates--intracellular proteins, hormones, cytokines, chemokines and their receptors]. Postep. Biochem. 2007, 53, 37–45. [Google Scholar]
- Skiles, J.W.; Gonnella, N.C.; Jeng, A.Y. The design, structure, and therapeutic application of matrix metalloproteinase inhibitors. Curr. Med. Chem. 2001, 8, 425–474. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Murphy, G. Metalloproteinases, Matrix. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 90–97. [Google Scholar]
- Baidya, S.K.; Amin, S.A.; Jha, T. Outline of gelatinase inhibitors as anti-cancer agents: A patent mini-review for 2010-present. Eur. J. Med. Chem. 2021, 213, 113044. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Fang, H.; Hou, X. Design, synthesis and preliminary bioactivity evaluations of 8-hydroxyquinoline derivatives as matrix metalloproteinase (MMP) inhibitors. Eur. J. Med. Chem. 2019, 181, 111563. [Google Scholar] [CrossRef]
- Qiu, H.-Y.; Wang, Z.-C.; Wang, P.-F.; Yan, X.-Q.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. Design, synthesis, evaluation and 3D-QSAR analysis of benzosulfonamide benzenesulfonates as potent and selective inhibitors of MMP-2. RSC Adv. 2014, 4, 39214–39225. [Google Scholar] [CrossRef]
- Albelwi, F.F.; Teleb, M.; Abu-Serie, M.M.; Moaty, M.N.A.A.; Alsubaie, M.S.; Zakaria, M.A.; El Kilany, Y.; Aouad, M.R.; Hagar, M.; Rezki, N. Halting tumor progression via novel non-hydroxamate triazole-based mannich bases MMP-2/9 inhibitors; design, microwave-assisted synthesis, and biological evaluation. Int. J. Mol. Sci. 2021, 22, 10324. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Amin, S.A.; Jha, T. Inhibitors of gelatinases (MMP-2 and MMP-9) for the management of hematological malignancies. Eur. J. Med. Chem. 2021, 223, 113623. [Google Scholar] [CrossRef]
- Dofara, S.G.; Chang, S.-L.; Diorio, C. Gene Polymorphisms and Circulating Levels of MMP-2 and MMP-9: A Review of Their Role in Breast Cancer Risk. Anticancer Res. 2020, 40, 3619–3631. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol./Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.; Vacirca, J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 101–117. [Google Scholar] [CrossRef]
- Rodriguez Faba, O.; Palou-Redorta, J.; Fernández-Gómez, J.M.; Algaba, F.; Eiró, N.; Villavicencio, H.; Vizoso, F.J. Matrix Metalloproteinases and Bladder Cancer: What is New? ISRN Urol. 2012, 2012, 581539. [Google Scholar] [CrossRef]
- Jezierska, A.; Motyl, T. Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med. Sci. Monit. 2009, 15, Ra32–Ra40. [Google Scholar]
- Guo, C.B.; Wang, S.; Deng, C.; Zhang, D.L.; Wang, F.L.; Jin, X.Q. Relationship between matrix metalloproteinase 2 and lung cancer progression. Mol. Diagn. Ther. 2007, 11, 183–192. [Google Scholar] [CrossRef]
- Chen, W.; Huang, S.; Shi, K.; Yi, L.; Liu, Y.; Liu, W. Prognostic Role of Matrix Metalloproteinases in Cervical Cancer: A Meta-Analysis. Cancer Control 2021, 28, 10732748211033743. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Wang, H.; Xu, L.; Yu, Y. MMP-2 expression and correlation with pathology and MRI of glioma. Oncol. Lett. 2019, 17, 1826–1832. [Google Scholar] [PubMed]
- Liu, R.R.; Li, M.D.; Li, T.; Tan, Y.; Zhang, M.; Chen, J.C. Matrix metalloproteinase 2 (MMP2) protein expression and laryngeal cancer prognosis: A meta analysis. Int. J. Clin. Exp. Med. 2015, 8, 2261–2266. [Google Scholar] [PubMed]
- Al-Alem, L.; Curry, T.E., Jr. Ovarian cancer: Involvement of the matrix metalloproteinases. Reproduction 2015, 150, R55–R64. [Google Scholar] [CrossRef] [PubMed]
- Morgunova, E.; Tuuttila, A.; Bergmann, U.; Isupov, M.; Lindqvist, Y.; Schneider, G.; Tryggvason, K. Structure of Human Pro-Matrix Metalloproteinase-2: Activation Mechanism Revealed. Science 1999, 284, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Jani, M.; Tordai, H.; Trexler, M.; Bányai, L.; Patthy, L. Hydroxamate-based peptide inhibitors of matrix metalloprotease 2. Biochimie 2005, 87, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.C.; Silletti, S.; von Schalscha, T.L.; Friedlander, M.; Cheresh, D.A. Disruption of Angiogenesis by PEX, a Noncatalytic Metalloproteinase Fragment with Integrin Binding Activity. Cell 1998, 92, 391–400. [Google Scholar] [CrossRef]
- Choi, W.S.; Jeon, O.H.; Kim, H.H.; Kim, D.S. MMP-2 regulates human platelet activation by interacting with integrin αIIbβ3. J. Thromb. Haemost. 2008, 6, 517–523. [Google Scholar] [CrossRef]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Corcoran, M.L.; Hewitt, R.E.; Kleiner, D.E., Jr.; Stetler-Stevenson, W.G. MMP-2: Expression, activation and inhibition. Enzym. Protein 1996, 49, 7–19. [Google Scholar] [CrossRef]
- Yang, R.; Zhao, G.; Cheng, B.; Yan, B. Identification of potential matrix metalloproteinase-2 inhibitors from natural products through advanced machine learning-based cheminformatics approaches. Mol. Divers. 2022, 27, 1053–1066. [Google Scholar] [CrossRef]
- El-Hussieny, M.; Mansour, S.T.; Hashem, A.I.; Fouad, M.A.; Abd-El-Maksoud, M.A. Design, synthesis, and biological evaluation of new heterocycles bearing both silicon and phosphorus as potent MMP-2 inhibitors. J. Chin. Chem. Soc. 2022, 69, 1908–1923. [Google Scholar] [CrossRef]
- Sanyal, S.; Amin, S.A.; Adhikari, N.; Jha, T. Ligand-based design of anticancer MMP2 inhibitors: A review. Future Med. Chem. 2021, 13, 1987–2013. [Google Scholar] [CrossRef]
- Sanyal, S.; Amin, S.; Adhikari, N.; Jha, T. QSAR modelling on a series of arylsulfonamide-based hydroxamates as potent MMP-2 inhibitors. SAR QSAR Environ. Res. 2019, 30, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Ammazzalorso, A.; De Filippis, B.; Campestre, C.; Laghezza, A.; Marrone, A.; Amoroso, R.; Tortorella, P.; Agamennone, M. Seeking for non-zinc-binding MMP-2 inhibitors: Synthesis, biological evaluation and molecular modelling studies. Int. J. Mol. Sci. 2016, 17, 1768. [Google Scholar] [CrossRef]
- Zhong, Y.; Lu, Y.-T.; Sun, Y.; Shi, Z.-H.; Li, N.-G.; Tang, Y.-P.; Duan, J.-A. Recent opportunities in matrix metalloproteinase inhibitor drug design for cancer. Expert Opin. Drug Discov. 2018, 13, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-H.; Lin, C.-W.; Cheng, C.-W.; Wen, Y.-C.; Yang, S.-F. Matrix metalloproteinase-2 as a target for head and neck cancer therapy. Expert Opin. Ther. Targets 2013, 17, 203–216. [Google Scholar] [CrossRef]
- Fingleton, B. Matrix metalloproteinase inhibitors for cancer therapy: The current situation and future prospects. Expert Opin. Ther. Targets 2003, 7, 385–397. [Google Scholar] [CrossRef]
- Hoekstra, R.; Eskens, F.; Verweij, J. Matrix metalloproteinase inhibitors: Current developments and future perspectives. Oncologist 2001, 6, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Qiu, H.Y.; Baloch, S.K.; Gong, H.B.; Wang, Z.C.; Zhu, H.L. Synthesis, Biological Evaluation, and Docking of Dihydropyrazole Sulfonamide Containing 2-hydroxyphenyl Moiety: A Series of Novel MMP-2 Inhibitors. Chem. Biol. Drug Des. 2015, 86, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Turra, K.M.; Rivelli, D.P.; de Moraes Barros, S.B.; Pasqualoto, K.F.M. Predicting Novel Antitumor Agents: 3D-Pharmacophore Mapping of β-N-biaryl Ether Sulfonamide-Based Hydroxamates as Potentially MMP-2 Inhibitors. Mol. Inform. 2014, 9, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-Q.; Wang, Z.-C.; Li, Z.; Wang, P.-F.; Qiu, H.-Y.; Chen, L.-W.; Lu, X.-Y.; Lv, P.-C.; Zhu, H.-L. Sulfonamide derivatives containing dihydropyrazole moieties selectively and potently inhibit MMP-2/MMP-9: Design, synthesis, inhibitory activity and 3D-QSAR analysis. Bioorg. Med. Chem. Lett. 2015, 25, 4664–4671. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.K.; Mallick, S.; Shikha, D.; Saha, A.; Saha, K.D.; Jha, T. Design of dual MMP-2/HDAC-8 inhibitors by pharmacophore mapping, molecular docking, synthesis and biological activity. RSC Adv. 2015, 5, 72373–72386. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Shen, F.-Q.; Yang, M.-R.; You, L.-X.; Chen, L.-Z.; Zhu, H.-L.; Lu, Y.-D.; Kong, F.-L.; Wang, M.-H. Dihydropyrazothiazole derivatives as potential MMP-2/MMP-8 inhibitors for cancer therapy. Bioorg. Med. Chem. Lett. 2018, 28, 3816–3821. [Google Scholar] [CrossRef]
- Aouad, M.R.; Almehmadi, M.A.; Albelwi, F.F.; Teleb, M.; Tageldin, G.N.; Abu-Serie, M.M.; Hagar, M.; Rezki, N. Targeting the interplay between MMP-2, CA II and VEGFR-2 via new sulfonamide-tethered isomeric triazole hybrids; Microwave-assisted synthesis, computational studies and evaluation. Bioorg. Chem. 2022, 124, 105816. [Google Scholar] [CrossRef]
- Kreituss, I.; Rozenberga, E.; Zemītis, J.; Trapencieris, P.; Romanchikova, N.; Turks, M. Discovery of aziridine-triazole conjugates as selective MMP-2 inhibitors. Chem. Heterocycl. Compd. 2013, 49, 1108–1117. [Google Scholar] [CrossRef]
- Laghezza, A.; Luisi, G.; Caradonna, A.; Di Pizio, A.; Piemontese, L.; Loiodice, F.; Agamennone, M.; Tortorella, P. Virtual screening identification and chemical optimization of substituted 2-arylbenzimidazoles as new non-zinc-binding MMP-2 inhibitors. Bioorg. Med. Chem. 2020, 28, 115257. [Google Scholar] [CrossRef]
- Bertran, A.; Khomiak, D.; Konopka, A.; Rejmak, E.; Bulska, E.; Seco, J.; Kaczmarek, L.; Tarragó, T.; Prades, R. Design and synthesis of selective and blood-brain barrier-permeable hydroxamate-based gelatinase inhibitors. Bioorg. Chem. 2020, 94, 103365. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Dinesh, N.; Baskaran, S.; Wedekind, D.; Gavrilovic, J.; Murray, M.Y.; Bevan, D.; Kelm, S. Novel specific human and mouse stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) antibodies for biochemical and immunohistochemical analyses. Wound Repair Regen. 2019, 27, 309–323. [Google Scholar] [CrossRef]
- Adamcova, M.; Simko, F. Multiplex biomarker approach to cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1068–1072. [Google Scholar] [CrossRef]
- Honsawek, S.; Malila, S.; Yuktanandana, P.; Tanavalee, A.; Deepaisarnsakul, B.; Parvizi, J. Association of MMP-3 (-1612 5A/6A) polymorphism with knee osteoarthritis in Thai population. Rheumatol. Int. 2013, 33, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Kubota, S.; Kawata, K.; Mukudai, Y.; Uehara, J.; Ohgawara, T.; Ibaragi, S.; Sasaki, A.; Kuboki, T.; Takigawa, M. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Mol. Cell. Biol. 2008, 28, 2391–2413. [Google Scholar] [CrossRef] [PubMed]
- Si-Tayeb, K.; Monvoisin, A.; Mazzocco, C.; Lepreux, S.; Decossas, M.; Cubel, G.; Taras, D.; Blanc, J.F.; Robinson, D.R.; Rosenbaum, J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am. J. Pathol. 2006, 169, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. Biophys. Acta. Mol. Cell Res. 2017, 1864 Pt A, 2043–2055. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, G.; Li, X.; Qiu, X.; Ouyang, J.; Dai, J.; Min, S. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiation. Front. Physiol. 2021, 12, 663978. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Yune, T.Y. MMP-3 secreted from endothelial cells of blood vessels after spinal cord injury activates microglia, leading to oligodendrocyte cell death. Neurobiol. Dis. 2015, 82, 141–151. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Zamilpa, R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc. Ther. 2012, 30, 31–41. [Google Scholar] [CrossRef]
- Chao, P.Z.; Hsieh, M.S.; Cheng, C.W.; Lin, Y.F.; Chen, C.H. Regulation of MMP-3 expression and secretion by the chemokine eotaxin-1 in human chondrocytes. J. Biomed. Sci. 2011, 18, 86. [Google Scholar] [CrossRef]
- Christensen, J.; Shastri, V.P. Matrix-metalloproteinase-9 is cleaved and activated by cathepsin K. BMC Res. Notes 2015, 8, 322. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef]
- Argote Camacho, A.X.; Gonzalez Ramirez, A.R.; Perez Alonso, A.J.; Rejon Garcia, J.D.; Olivares Urbano, M.A.; Torne Poyatos, P.; Rios Arrabal, S.; Nunez, M.I. Metalloproteinases 1 and 3 as Potential Biomarkers in Breast Cancer Development. Int. J. Mol. Sci. 2021, 22, 9012. [Google Scholar] [CrossRef]
- Nagase, H. Chapter 158: Matrix Metalloproteinase-3/Stromelysin-1. In Handbook of Proteolytic Enzymes; Rawlings, N.D.S.G., Ed.; Elsevier: London, UK, 2013; pp. 763–774. [Google Scholar]
- Wang, X.X.; Tan, M.S.; Yu, J.T.; Tan, L. Matrix metalloproteinases and their multiple roles in Alzheimer’s disease. BioMed Res. Int. 2014, 2014, 908636. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, S.A.; Chan, S.C.; Rosli, R. Matrix Metallopeptidase 3 Polymorphisms: Emerging genetic Markers in Human Breast Cancer Metastasis. J. Breast Cancer 2020, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Maguire, T.M.; Hill, A.; McDermott, E.; O’Higgins, N. Metalloproteinases: Role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000, 2, 252–257. [Google Scholar] [CrossRef]
- Koujan, S.E.; Gargarib, B.P.; Khalili, M. Matrix Metalloproteinases and Breast Cancer. Thrita 2015, 4, e21959. [Google Scholar]
- Lynch, C.C.; Matrisian, L.M. Matrix metalloproteinases in tumor-host cell communication. Differentiation 2002, 70, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zheng, Z.; Bai, Z.; Ouyang, K.; Wu, Q.; Xu, S.; Huang, L.; Jiang, Y.; Wang, L.; Gao, J.; et al. Overexpression of angiogenic factors and matrix metalloproteinases in the saliva of oral squamous cell carcinoma patients: Potential non-invasive diagnostic and therapeutic biomarkers. BMC Cancer 2022, 22, 530. [Google Scholar] [CrossRef] [PubMed]
- Frieling, J.S.; Li, T.; Tauro, M.; Lynch, C.C. Prostate cancer-derived MMP-3 controls intrinsic cell growth and extrinsic angiogenesis. Neoplasia 2020, 22, 511–521. [Google Scholar] [CrossRef]
- Chen, W.; Ni, D.; Zhang, H.; Li, X.; Jiang, Y.; Wu, J.; Gu, Y.; Gao, M.; Shi, W.; Song, J.; et al. Over-expression of USP15/MMP3 predict poor prognosis and promote growth, migration in non-small cell lung cancer cells. Cancer Genet 2023, 272–273, 9–15. [Google Scholar] [CrossRef]
- Park, H.I.; Jin, Y.; Hurst, D.R.; Monroe, C.A.; Lee, S.; Schwartz, M.A.; Sang, Q.X. The intermediate S1′ pocket of the endometase/matrilysin-2 active site revealed by enzyme inhibition kinetic studies, protein sequence analyses, and homology modeling. J. Biol. Chem. 2003, 278, 51646–51653. [Google Scholar] [CrossRef]
- Amin, E.A.; Welsh, W.J. A preliminary in silico lead series of 2-phthalimidinoglutaric acid analogues designed as MMP-3 inhibitors. J. Chem. Inf. Model. 2006, 46, 2104–2109. [Google Scholar] [CrossRef]
- Huang, R.Z.; Liang, G.B.; Huang, X.C.; Zhang, B.; Zhou, M.M.; Liao, Z.X.; Wang, H.S. Discovery of dehydroabietic acid sulfonamide based derivatives as selective matrix metalloproteinases inactivators that inhibit cell migration and proliferation. Eur. J. Med. Chem. 2017, 138, 979–992. [Google Scholar] [CrossRef]
- Goyal, G.; Palaniappan, A.; Liedberg, B. Protease functional assay on membrane. Sens. Actuators B Chem. 2020, 305, 127442. [Google Scholar] [CrossRef]
- Moskalenko, M.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Polymorphisms of the matrix metalloproteinase genes are associated with essential hypertension in a Caucasian population of Central Russia. Sci. Rep. 2021, 11, 5224. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-Y.; Da, C.-M.; Liao, B.; Zhang, H.-H. Roles of matrix metalloproteinase-7 (MMP-7) in cancer. Clin. Biochem. 2021, 92, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Liu, L.; Chen, Z.; Kou, M.; Yang, X.; Sun, Y.; Di, S.; Wang, X.; Cai, J.; Guo, D. Characterization, mRNA expression profile, subcellular distribution and association analysis with piglet diarrhea of porcine matrix metallopeptidase 7 (pMMP7). Gene 2022, 821, 146319. [Google Scholar] [CrossRef]
- Ii, M.; Yamamoto, H.; Adachi, Y.; Maruyama, Y.; Shinomura, Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp. Biol. Med. 2006, 231, 20–27. [Google Scholar] [CrossRef]
- Piskór, B.M.; Przylipiak, A.; Dąbrowska, E.; Niczyporuk, M.; Ławicki, S. Matrilysins and stromelysins in pathogenesis and diagnostics of cancers. Cancer Manag. Res. 2020, 12, 10949. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef]
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell. Physiol. 2008, 217, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Luukkaa, H.; Klemi, P.; Hirsimäki, P.; Vahlberg, T.; Kivisaari, A.; Kähäri, V.-M.; Grénman, R. Matrix metalloproteinase (MMP)-7 in salivary gland cancer. Acta Oncol. 2010, 49, 85–90. [Google Scholar] [CrossRef]
- Dozier, S.; Escobar, G.; Lindsey, M. Matrix metalloproteinase (MMP)-7 activates MMP-8 but not MMP-13. Med. Chem. 2006, 2, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Abacherli, L.E.; Nadler, S.T.; Wang, Y.; Li, Q.; Parks, W.C. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting α2β1 integrin activation. PLoS ONE 2009, 4, e6565. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Lwin, S.; Fowler, J.; Drake, M.; Edwards, J.; Lynch, C.; Edwards, C. A loss of host-derived MMP-7 promotes myeloma growth and osteolytic bone disease in vivo. Mol. Cancer 2017, 16, 49. [Google Scholar] [CrossRef]
- Yamada, K.; Kadota, K.; Fujimoto, S.; Yoshida, C.; Ibuki, E.; Ishikawa, R.; Haba, R.; Yokomise, H.; Yajima, T. MMP-7 expression is associated with a higher rate of tumor spread through air spaces in resected lung adenocarcinomas. Lung Cancer 2023, 175, 125–130. [Google Scholar] [CrossRef]
- Liao, C.-H.; Chang, W.-S.; Hsu, W.-L.; Hu, P.-S.; Wu, H.-C.; Hsu, S.-W.; Wang, B.-R.; Yueh, T.-C.; Chen, C.-H.; Hsia, T.-C. Association of Matrix Metalloproteinase-7 Genotypes With Prostate Cancer Risk. Anticancer Res. 2023, 43, 381–387. [Google Scholar] [CrossRef]
- Wang, W.-S.; Chen, P.-M.; Wang, H.-S.; Liang, W.-Y.; Su, Y. Matrix metalloproteinase-7 increases resistance to Fas-mediated apoptosis and is a poor prognostic factor of patients with colorectal carcinoma. Carcinogenesis 2006, 27, 1113–1120. [Google Scholar] [CrossRef]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: They’re not just for matrix anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540. [Google Scholar] [CrossRef]
- Nomden, M.; Beljaars, L.; Verkade, H.J.; Hulscher, J.B.; Olinga, P. Current concepts of biliary atresia and matrix metalloproteinase-7: A review of literature. Front. Med. 2020, 7, 617261. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Miyamoto, S.; Maeda, H.; Ishii, G.; Hasebe, T.; Chiba, T.; Asaka, M.; Ochiai, A. Matrix metalloproteinase-7 degrades all insulin-like growth factor binding proteins and facilitates insulin-like growth factor bioavailability. Biochem. Biophys. Res. Commun. 2005, 333, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Huo, N.; Ichikawa, Y.; Kamiyama, M.; Ishikawa, T.; Hamaguchi, Y.; Hasegawa, S.; Nagashima, Y.; Miyazaki, K.; Shimada, H. MMP-7 (matrilysin) accelerated growth of human umbilical vein endothelial cells. Cancer Lett. 2002, 177, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Piskór, B.M.; Przylipiak, A.; Dąbrowska, E.; Sidorkiewicz, I.; Niczyporuk, M.; Szmitkowski, M.; Ławicki, S. Plasma concentrations of matrilysins MMP-7 and MMP-26 as diagnostic biomarkers in breast cancer. J. Clin. Med. 2021, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Koskensalo, S.; Louhimo, J.; Nordling, S.; Hagström, J.; Haglund, C. MMP-7 as a prognostic marker in colorectal cancer. Tumor Biol. 2011, 32, 259–264. [Google Scholar] [CrossRef]
- Palumbo, A., Jr.; Meireles Da Costa, N.; Pontes, B.; Leite de Oliveira, F.; Lohan Codeço, M.; Ribeiro Pinto, L.F.; Nasciutti, L.E. Esophageal cancer development: Crucial clues arising from the extracellular matrix. Cells 2020, 9, 455. [Google Scholar] [CrossRef]
- Van Doren, S.R. MMP-7 marks severe pancreatic cancer and alters tumor cell signaling by proteolytic release of ectodomains. Biochem. Soc. Trans. 2022, 50, 839–851. [Google Scholar] [CrossRef]
- Wattanawongdon, W.; Bartpho, T.S.; Tongtawee, T. Expression of Matrix Metalloproteinase-7 Predicts Poor Prognosis in Gastric Cancer. Biomed Res. Int. 2022, 2022, 2300979. [Google Scholar] [CrossRef]
- Impola, U.; Uitto, V.-J.; Hietanen, J.; Hakkinen, L.; Zhang, L.; Larjava, H.; Isaka, K.; Saarialho-Kere, U. Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2004, 202, 14–22. [Google Scholar] [CrossRef]
- Meng, F.; Yang, H.; Jack, C.; Zhang, H.; Moller, A.; Spivey, D.; Page, R.C.; Tierney, D.L.; Crowder, M.W. Biochemical characterization and zinc binding group (ZBGs) inhibition studies on the catalytic domain of MMP7 (cdMMP7). J. Inorg. Biochem. 2016, 165, 7–17. [Google Scholar] [CrossRef]
- Overall, C.M.; Kleifeld, O. Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 2006, 6, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Riedl, R. Development of a non-hydroxamate dual matrix metalloproteinase (MMP)-7/-13 inhibitor. Molecules 2017, 22, 1548. [Google Scholar] [CrossRef] [PubMed]
- Matt, C.; Hess, T.; Benlian, A. Digital transformation strategies. Bus. Inf. Syst. Eng. 2015, 57, 339–343. [Google Scholar] [CrossRef]
- Hitaoka, S.; Chuman, H.; Yoshizawa, K. A QSAR study on the inhibition mechanism of matrix metalloproteinase-12 by arylsulfone analogs based on molecular orbital calculations. Org. Biomol. Chem. 2015, 13, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, W.; Ali, M.A.; Schulz, R. Multifunctional intracellular matrix metalloproteinases: Implications in disease. FEBS J. 2021, 288, 7162–7182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, W.; Guo, Y.; Li, D.; Liu, Y.; Xu, S. MMP gene polymorphisms, MMP-1-1607 1G/2G,-519 A/G, and MMP-12-82 A/G, and ischemic stroke: A meta-analysis. J. Stroke Cerebrovasc. Dis. 2018, 27, 140–152. [Google Scholar] [CrossRef]
- Graff, J.W.; Powers, L.S.; Dickson, A.M.; Kim, J.; Reisetter, A.C.; Hassan, I.H.; Kremens, K.; Gross, T.J.; Wilson, M.E.; Monick, M.M. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS ONE 2012, 7, e44066. [Google Scholar] [CrossRef]
- Li, L.; Li, H. Role of microRNA-mediated MMP regulation in the treatment and diagnosis of malignant tumors. Cancer Biol. Ther. 2013, 14, 796–805. [Google Scholar] [CrossRef]
- Ren, X.-S.; Yin, M.-H.; Zhang, X.; Wang, Z.; Feng, S.-P.; Wang, G.-X.; Luo, Y.-J.; Liang, P.-Z.; Yang, X.-Q.; He, J.-X. Tumor-suppressive microRNA-449a induces growth arrest and senescence by targeting E2F3 in human lung cancer cells. Cancer Lett. 2014, 344, 195–203. [Google Scholar] [CrossRef]
- Kong, Q.; Shu, N.; Li, J.; Xu, N. miR-641 functions as a tumor suppressor by targeting MDM2 in human lung cancer. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 26, 735–741. [Google Scholar] [CrossRef]
- Fortunato, O.; Boeri, M.; Moro, M.; Verri, C.; Mensah, M.; Conte, D.; Caleca, L.; Roz, L.; Pastorino, U.; Sozzi, G. Mir-660 is downregulated in lung cancer patients and its replacement inhibits lung tumorigenesis by targeting MDM2-p53 interaction. Cell Death Dis. 2014, 5, e1564. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lei, H.; Xu, Y.; Tao, Z.-Z. miR-512-5p suppresses tumor growth by targeting hTERT in telomerase positive head and neck squamous cell carcinoma in vitro and in vivo. PLoS ONE 2015, 10, e0135265. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Garzon, R.; Cimmino, A.; Liu, Z.; Zanesi, N.; Callegari, E.; Liu, S.; Alder, H.; Costinean, S.; Fernandez-Cymering, C. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA 2007, 104, 15805–15810. [Google Scholar] [CrossRef]
- Amor, M.; Moreno-Viedma, V.; Sarabi, A.; Grün, N.G.; Itariu, B.; Leitner, L.; Steiner, I.; Bilban, M.; Kodama, K.; Butte, A.J. Identification of matrix metalloproteinase-12 as a candidate molecule for prevention and treatment of cardiometabolic disease. Mol. Med. 2016, 22, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J.O. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011, 278, 28–45. [Google Scholar] [CrossRef]
- Marchant, D.J.; Bellac, C.L.; Moraes, T.J.; Wadsworth, S.J.; Dufour, A.; Butler, G.S.; Bilawchuk, L.M.; Hendry, R.G.; Robertson, A.G.; Cheung, C.T. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat. Med. 2014, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Siddhartha, R.; Garg, M. Molecular and clinical insights of matrix metalloproteinases into cancer spread and potential therapeutic interventions. Toxicol. Appl. Pharmacol. 2021, 426, 115593. [Google Scholar] [CrossRef]
- Itoh, Y. Metalloproteinases in rheumatoid arthritis: Potential therapeutic targets to improve current therapies. Prog. Mol. Biol. Transl. Sci. 2017, 148, 327–338. [Google Scholar]
- Chelluboina, B.; Warhekar, A.; Dillard, M.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Veeravalli, K.K. Post-transcriptional inactivation of matrix metalloproteinase-12 after focal cerebral ischemia attenuates brain damage. Sci. Rep. 2015, 5, srep09504. [Google Scholar] [CrossRef]
- Chelluboina, B.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. Matrix metalloproteinase-12 induces blood–brain barrier damage after focal cerebral ischemia. Stroke 2015, 46, 3523–3531. [Google Scholar] [CrossRef]
- Chen, S.-S.; Song, J.; Tu, X.-Y.; Zhao, J.-H.; Ye, X.-Q. The association between MMP-12 82 A/G polymorphism and susceptibility to various malignant tumors: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10845. [Google Scholar]
- Ng, K.T.-P.; Qi, X.; Kong, K.-L.; Cheung, B.Y.-Y.; Lo, C.-M.; Poon, R.T.-P.; Fan, S.-T.; Man, K. Overexpression of matrix metalloproteinase-12 (MMP-12) correlates with poor prognosis of hepatocellular carcinoma. Eur. J. Cancer 2011, 47, 2299–2305. [Google Scholar] [CrossRef]
- Zeng, L.; Qian, J.; Zhu, F.; Wu, F.; Zhao, H.; Zhu, H. The prognostic values of matrix metalloproteinases in ovarian cancer. J. Int. Med. Res. 2020, 48, 0300060519825983. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Arii, S.; Gorrin-Rivas, M.J.; Mori, A.; Onodera, H.; Imamura, M. Human macrophage metalloelastase gene expression in colorectal carcinoma and its clinicopathologic significance. Cancer 2001, 91, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Jiang, F.H.; Zhao, L.M.; Dai, Q.; Yang, W.Y.; Zhu, L.M.; Wang, B.J.; Xu, C.; Bao, Y.J.; Zhang, Y.J. Human macrophage metalloelastase correlates with angiogenesis and prognosis of gastric carcinoma. Dig. Dis. Sci. 2010, 55, 3138–3146. [Google Scholar] [CrossRef]
- Klupp, F.; Neumann, L.; Kahlert, C.; Diers, J.; Halama, N.; Franz, C.; Schmidt, T.; Koch, M.; Weitz, J.; Schneider, M. Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in colon cancer patients. BMC Cancer 2016, 16, 494. [Google Scholar] [CrossRef]
- Li, Y.; Jia, J.-H.; Kang, S.; Zhang, X.-J.; Zhao, J.; Wang, N.; Zhou, R.-M.; Sun, D.-L.; Duan, Y.-N.; Wang, D.-J. The functional polymorphisms on promoter region of matrix metalloproteinase-12,-13 genes may alter the risk of epithelial ovarian carcinoma in Chinese. Int. J. Gynecol. Cancer 2009, 19, 129–133. [Google Scholar] [CrossRef]
- He, M.K.; Le, Y.; Zhang, Y.F.; Ouyang, H.Y.; Jian, P.E.; Yu, Z.S.; Wang, L.J.; Shi, M. Matrix metalloproteinase 12 expression is associated with tumor FOXP3(+) regulatory T cell infiltration and poor prognosis in hepatocellular carcinoma. Oncol. Lett. 2018, 16, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Cai, Q.; Shu, X.-O.; Gao, Y.-T.; Zheng, W. Genetic polymorphisms in the matrix metalloproteinase 12 gene (MMP12) and breast cancer risk and survival: The Shanghai Breast Cancer Study. Breast Cancer Res. 2005, 7, R506–R512. [Google Scholar] [CrossRef]
- Balaz, P.; Friess, H.; Kondo, Y.; Zhu, Z.; Zimmermann, A.; Büchler, M.W. Human macrophage metalloelastase worsens the prognosis of pancreatic cancer. Ann. Surg. 2002, 235, 519. [Google Scholar] [CrossRef]
- Bradbury, P.A.; Zhai, R.; Hopkins, J.; Kulke, M.H.; Heist, R.S.; Singh, S.; Zhou, W.; Ma, C.; Xu, W.; Asomaning, K. Matrix metalloproteinase 1, 3 and 12 polymorphisms and esophageal adenocarcinoma risk and prognosis. Carcinogenesis 2009, 30, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Kerkelä, E.; Ala-aho, R.; Klemi, P.; Grénman, S.; Shapiro, S.D.; Kähäri, V.M.; Saarialho-Kere, U. Metalloelastase (MMP-12) expression by tumour cells in squamous cell carcinoma of the vulva correlates with invasiveness, while that by macrophages predicts better outcome. J. Pathol. 2002, 198, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Kerkelä, E.; Ala-aho, R.; Jeskanen, L.; Rechardt, O.; Grénman, R.; Shapiro, S.D.; KaÈhaÈri, V.-M.; Saarialho-Kere, U. Expression of human macrophage metalloelastase (MMP-12) by tumor cells in skin cancer. J. Investig. Dermatol. 2000, 114, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Wang, J.; Wu, Y.; Chen, H.; Shen, X. Knockdown of MMP12 inhibits the growth and invasion of lung adenocarcinoma cells. Int. J. Immunopathol. Pharmacol. 2015, 28, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Hong, K.P.; Hong, S.H.; Kang, S.; Chung, K.Y.; Cho, S.H. MMP expression profiling in recurred stage IB lung cancer. Oncogene 2004, 23, 845–851. [Google Scholar] [CrossRef]
- Hofmann, H.-S.; Hansen, G.; Richter, G.; Taege, C.; Simm, A.; Silber, R.-E.; Burdach, S. Matrix metalloproteinase-12 expression correlates with local recurrence and metastatic disease in non–small cell lung cancer patients. Clin. Cancer Res. 2005, 11, 1086–1092. [Google Scholar] [CrossRef]
- Qu, P.; Yan, C.; Du, H. Matrix metalloproteinase 12 overexpression in myeloid lineage cells plays a key role in modulating myelopoiesis, immune suppression, and lung tumorigenesis. Blood J. Am. Soc. Hematol. 2011, 117, 4476–4489. [Google Scholar] [CrossRef]
- Morales, R.; Perrier, S.; Florent, J.-M.; Beltra, J.; Dufour, S.; De Mendez, I.; Manceau, P.; Tertre, A.; Moreau, F.; Compere, D. Crystal structures of novel non-peptidic, non-zinc chelating inhibitors bound to MMP-12. J. Mol. Biol. 2004, 341, 1063–1076. [Google Scholar] [CrossRef]
- Pickett, S.D.; Green, D.V.; Hunt, D.L.; Pardoe, D.A.; Hughes, I. Automated lead optimization of MMP-12 inhibitors using a genetic algorithm. ACS Med. Chem. Lett. 2011, 2, 28–33. [Google Scholar] [CrossRef]
- Gona, K.; Toczek, J.; Ye, Y.; Sanzida, N.; Golbazi, A.; Boodagh, P.; Salarian, M.; Jung, J.-J.; Rajendran, S.; Kukreja, G. Hydroxamate-based selective macrophage elastase (MMP-12) inhibitors and radiotracers for molecular imaging. J. Med. Chem. 2020, 63, 15037–15049. [Google Scholar] [CrossRef]
- Butsch, V.; Börgel, F.; Galla, F.; Schwegmann, K.; Hermann, S.; Schäfers, M.; Riemann, B.; Wünsch, B.; Wagner, S. Design, (radio) synthesis, and in vitro and in vivo evaluation of highly selective and potent matrix metalloproteinase 12 (MMP-12) inhibitors as radiotracers for positron emission tomography. J. Med. Chem. 2018, 61, 4115–4134. [Google Scholar] [CrossRef]
- Baggio, C.; Velazquez, J.V.; Fragai, M.; Nordgren, T.M.; Pellecchia, M. Therapeutic targeting of MMP-12 for the treatment of chronic obstructive pulmonary disease. J. Med. Chem. 2020, 63, 12911–12920. [Google Scholar] [CrossRef] [PubMed]
- Krarup, P.-M.; Eld, M.; Heinemeier, K.; Jorgensen, L.N.; Hansen, M.B.; Ågren, M.S. Expression and inhibition of matrix metalloproteinase (MMP)-8, MMP-9 and MMP-12 in early colonic anastomotic repair. Int. J. Color. Dis. 2013, 28, 1151–1159. [Google Scholar] [CrossRef]
- Le Quement, C.; Guenon, I.; Gillon, J.Y.; Valenca, S.; Cayron-Elizondo, V.; Lagente, V.; Boichot, E. The selective MMP-12 inhibitor, AS111793 reduces airway inflammation in mice exposed to cigarette smoke. Br. J. Pharmacol. 2008, 154, 1206–1215. [Google Scholar] [CrossRef]
- Nuti, E.; Panelli, L.; Casalini, F.; Avramova, S.I.; Orlandini, E.; Santamaria, S.; Nencetti, S.; Tuccinardi, T.; Martinelli, A.; Cercignani, G. Design, synthesis, biological evaluation, and NMR studies of a new series of arylsulfones as selective and potent matrix metalloproteinase-12 inhibitors. J. Med. Chem. 2009, 52, 6347–6361. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wu, Y.; Wu, J.; Hotchandani, R.; Cunningham, K.; McFadyen, I.; Bard, J.; Morgan, P.; Schlerman, F. A selective matrix metalloprotease 12 inhibitor for potential treatment of chronic obstructive pulmonary disease (COPD): Discovery of (S)-2-(8-(methoxycarbonylamino) dibenzo [b, d] furan-3-sulfonamido)-3-methylbutanoic acid (MMP408). J. Med. Chem. 2009, 52, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Wu, J.; Morgan, P.; Xu, X.; Rancati, F.; Vallese, S.; Raveglia, L.; Hotchandani, R.; Fuller, N. Discovery of potent and selective matrix metalloprotease 12 inhibitors for the potential treatment of chronic obstructive pulmonary disease (COPD). Bioorg. Med. Chem. Lett. 2012, 22, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864 Pt A, 1940–1951. [Google Scholar] [CrossRef]
- Vos, M.C.; van der Wurff, A.A.M.; van Kuppevelt, T.H.; Massuger, L. The role of MMP-14 in ovarian cancer: A systematic review. J. Ovarian Res. 2021, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Mignon, C.; Okada, A.; Mattei, M.G.; Basset, P. Assignment of the human membrane-type matrix metalloproteinase (MMP14) gene to 14q11-q12 by in situ hybridization. Genomics 1995, 28, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.; Zhou, L.; Yang, L.; Wu, X.; Tang, B.; Xie, S.; Fang, L.; Zheng, S.; Hong, T. Immune Infiltration of MMP14 in Pan Cancer and Its Prognostic Effect on Tumors. Front. Oncol. 2021, 11, 717606. [Google Scholar] [CrossRef]
- Zarrabi, K.; Dufour, A.; Li, J.; Kuscu, C.; Pulkoski-Gross, A.; Zhi, J.; Hu, Y.; Sampson, N.S.; Zucker, S.; Cao, J. Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer cell migration. J. Biol. Chem. 2011, 286, 33167–33177. [Google Scholar] [CrossRef]
- Spencer, H.L.M.; Shnyder, S.D.; Loadman, P.M.; Falconer, R.A. The role of MT1-MMP in the progression and metastasis of osteosarcoma. J. Cancer Metastasis Treat. 2022, 8, 2. [Google Scholar] [CrossRef]
- Knapinska, A.M.; Fields, G.B. The expanding role of MT1-MMP in cancer progression. Pharmaceuticals 2019, 12, 77. [Google Scholar] [CrossRef]
- Thakur, V.; Bedogni, B. The membrane tethered matrix metalloproteinase MT1-MMP at the forefront of melanoma cell invasion and metastasis. Pharmacol. Res. 2016, 111, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Nyalendo, C.; Sartelet, H.; Barrette, S.; Ohta, S.; Gingras, D.; Béliveau, R. Identification of membrane-type 1 matrix metalloproteinase tyrosine phosphorylation in association with neuroblastoma progression. BMC Cancer 2009, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Maniwa, Y.; Tanaka, Y.; Tane, S.; Hashimoto, S.; Ohno, Y.; Nishio, W.; Nishimura, Y.; Ohbayashi, C.; Okita, Y. MT1-MMP plays an important role in an invasive activity of malignant pleural mesothelioma cell. Exp. Mol. Pathol. 2011, 90, 91–96. [Google Scholar] [CrossRef]
- Liao, H.; Wang, Z.; Deng, Z.; Ren, H.; Li, X. Curcumin inhibits lung cancer invasion and metastasis by attenuating GLUT1/MT1-MMP/MMP2 pathway. Int. J. Clin. Exp. Med. 2015, 8, 8948. [Google Scholar]
- Markovic, D.; Vinnakota, K.; Chirasani, S.; Synowitz, M.; Raguet, H.; Stock, K.; Sliwa, M.; Lehmann, S.; Kälin, R.; Van Rooijen, N. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc. Natl. Acad. Sci. USA 2009, 106, 12530–12535. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Matrisian, L.M. Matrix metalloproteases in head and neck cancer. Head Neck J. Sci. Spec. Head Neck 2006, 28, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, H.o.; Yokota, K.y.; Kurokawa, Y.; Murakami, Y.; Nishitani, M.; Kagawa, S. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1998, 82, 1359–1366. [Google Scholar]
- Mimori, K.; Ueo, H.; Shirasaka, C.; Mori, M. Clinical significance of MT1-MMP mRNA expression in breast cancer. Oncol. Rep. 2001, 8, 401–403. [Google Scholar] [CrossRef]
- Sakata, K.; Shigemasa, K.; Nagai, N.; Ohama, K. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int. J. Oncol. 2000, 17, 673–754. [Google Scholar] [CrossRef]
- Ellenrieder, V.; Alber, B.; Lacher, U.; Hendler, S.F.; Menke, A.; Boeck, W.; Wagner, M.; Wilda, M.; Friess, H.; Büchler, M. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int. J. Cancer 2000, 85, 14–20. [Google Scholar] [CrossRef]
- Leeman, M.F.; Curran, S.; Murray, G.I. New insights into the roles of matrix metalloproteinases in colorectal cancer development and progression. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2003, 201, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Molina, J.; Gramolelli, S.; Liao, Z.; Carlson, J.W.; Ojala, P.M.; Lehti, K. MMP14 in Sarcoma: A Regulator of Tumor Microenvironment Communication in Connective Tissues. Cells 2019, 8, 991. [Google Scholar] [CrossRef]
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef]
- Pietraszek-Gremplewicz, K.; Karamanou, K.; Niang, A.; Dauchez, M.; Belloy, N.; Maquart, F.-X.; Baud, S.; Brézillon, S. Small leucine-rich proteoglycans and matrix metalloproteinase-14: Key partners? Matrix Biol. 2017, 75, 271–285. [Google Scholar] [CrossRef]
- Remacle, A.G.; Golubkov, V.S.; Shiryaev, S.A.; Dahl, R.; Stebbins, J.L.; Chernov, A.V.; Cheltsov, A.V.; Pellecchia, M.; Strongin, A.Y. Novel MT1-MMP small-molecule inhibitors based on insights into hemopexin domain function in tumor growth. Cancer Res. 2012, 72, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Youn, I.; Demissie, R.; Vaid, T.M.; Che, C.T.; Azar, D.T.; Han, K.Y. Identification of small molecule inhibitors against MMP-14 via High-Throughput screening. Bioorg. Med. Chem. 2023, 85, 117289. [Google Scholar] [CrossRef] [PubMed]
- Nuti, E.; Cantelmo, A.R.; Gallo, C.; Bruno, A.; Bassani, B.; Camodeca, C.; Tuccinardi, T.; Vera, L.; Orlandini, E.; Nencetti, S.; et al. N-O-Isopropyl Sulfonamido-Based Hydroxamates as Matrix Metalloproteinase Inhibitors: Hit Selection and in Vivo Antiangiogenic Activity. J. Med. Chem. 2015, 58, 7224–7240. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, D.; Nuti, E.; Gifford, V.; Ito, N.; Camodeca, C.; Tuccinardi, T.; Nencetti, S.; Orlandini, E.; Itoh, Y.; Rossello, A. Design, synthesis and biological evaluation of bifunctional inhibitors of membrane type 1 matrix metalloproteinase (MT1-MMP). Bioorg. Med. Chem. 2019, 27, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Sjøli, S.; Solli, A.I.; Akselsen, Ø.; Jiang, Y.; Berg, E.; Hansen, T.V.; Sylte, I.; Winberg, J.-O. PAC-1 and isatin derivatives are weak matrix metalloproteinase inhibitors. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 3162–3169. [Google Scholar] [CrossRef] [PubMed]
| Traditional Classification (Common Name) | Numerical Classification | Group of Substrates |
|---|---|---|
| Collagenases | ||
| Collagenase-1 | MMP-1 | Collagen (I, II, III, VII, VIII, X), casein, entactin, laminin, pro-MMP-1, -2, -9, and serpins |
| Collagenase-2 | MMP-8 | Collagen (I–III, V, VII, VIII, X), gelatin, aggrecan, fibronectin |
| Collagenase-3 | MMP-13 | Gelatin, collagen (IV–VI, X), elastin, fibronectin |
| Gelatinases | ||
| Gelatinase A | MMP-2 | Gelatin, collagens (IV, V, VII, X, XIV), elastin, fibrillin, osteonectin |
| Gelatinase B | MMP-9 | Gelatin, collagens (IV, V, VII, X, XIV), elastin, fibrillin, osteonectin |
| Stromelysins | ||
| Stromelysin-1 | MMP-3 | Laminin, aggrecan, gelatin, fibronectin |
| Stromelysin-2 | MMP-10 | Collagens (III–V), gelatin, casein, aggrecan, elastin, MMP-1,8 |
| Stromelysin-3 | MMP-11 | Fibronectin, laminin, aggrecan, gelatin |
| Matrilysins | ||
| Matrilysin-1 | MMP-7 | Collagen (IV–X), fibronectin, laminin, gelatin, aggrecan, pro-MMP-9 |
| Matrilysin-2 | MMP-26 | Gelatin, collagen IV, pro-MMP-9 |
| Macrophage Metalloelastase | ||
| Macrophage Metalloelastase | MMP-12 | Elastin, gelatin, collagen I, IV, fibronectin, laminin, vitronectin, proteoglycan |
| Membrane-type MMPs (MT-MM) | ||
| MT-MMP-1 | MMP-14 | Collagen (I, II, III), gelatin, fibronectin, laminin aggrecan, tenascin |
| MT-MMP-2 | MMP-15 | Fibronectin, laminin, aggrecan, perlecan |
| MT-MMP-3 | MMP-16 | Collagen III, gelatin, casein |
| MT-MMP-4 | MMP-17 | Fibrinogen, TNF precursor |
| MT-MMP-5 | MMP-24 | Proteoglycans |
| Compound | Potency | Chemical Structure | Reference |
|---|---|---|---|
| 1 | IC50 = 0.4 µM |  | [56] |
| 2 | IC50 = 0.034 µM |  | [57] |
| 3 | IC50 = 20 µg/mL (MCF-7) |  | [58] |
| 4 | Ki = 77 nM |  | [59] |
| 5 | IC50 = 0.18 µM |  | [60] |
| Compound | Potency | Chemical Structure | Reference |
|---|---|---|---|
| 24 | Ki = 43 nM |  | [38] |
| 25 | Ki = 19 nM |  | [38] |
| 26 | Ki = 15 nM |  | [38] |
| 27 | IC50 = 1 nM |  | [128] |
| 28 | IC50 = 0.4 µM |  | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023). Molecules 2023, 28, 5567. https://doi.org/10.3390/molecules28145567
Almutairi S, Kalloush HM, Manoon NA, Bardaweel SK. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023). Molecules. 2023; 28(14):5567. https://doi.org/10.3390/molecules28145567
Chicago/Turabian StyleAlmutairi, Shriefa, Hanin Moh’d Kalloush, Nour A. Manoon, and Sanaa K. Bardaweel. 2023. "Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023)" Molecules 28, no. 14: 5567. https://doi.org/10.3390/molecules28145567
APA StyleAlmutairi, S., Kalloush, H. M., Manoon, N. A., & Bardaweel, S. K. (2023). Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023). Molecules, 28(14), 5567. https://doi.org/10.3390/molecules28145567













