Determination of Mifepristone (RU-486) and Its Metabolites in Maternal Blood Sample after Pharmacological Abortion
Abstract
1. Introduction
Case History
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Blank Material
2.3. Working Solutions, Quiality Control Samples, Calibration Curve
2.4. Instrumentation
2.5. Sample Preparation
2.6. Optimization of Extraction Technique
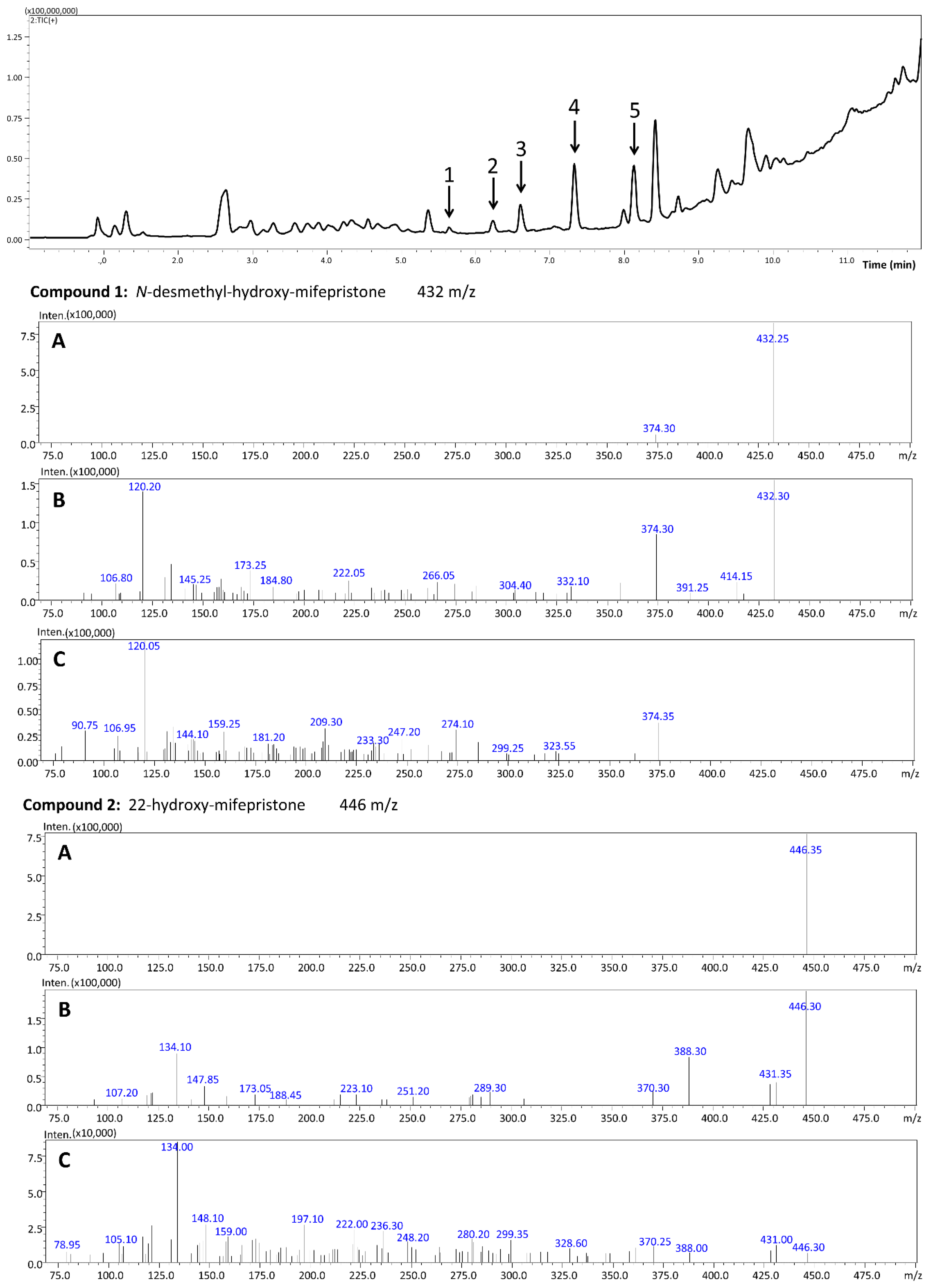
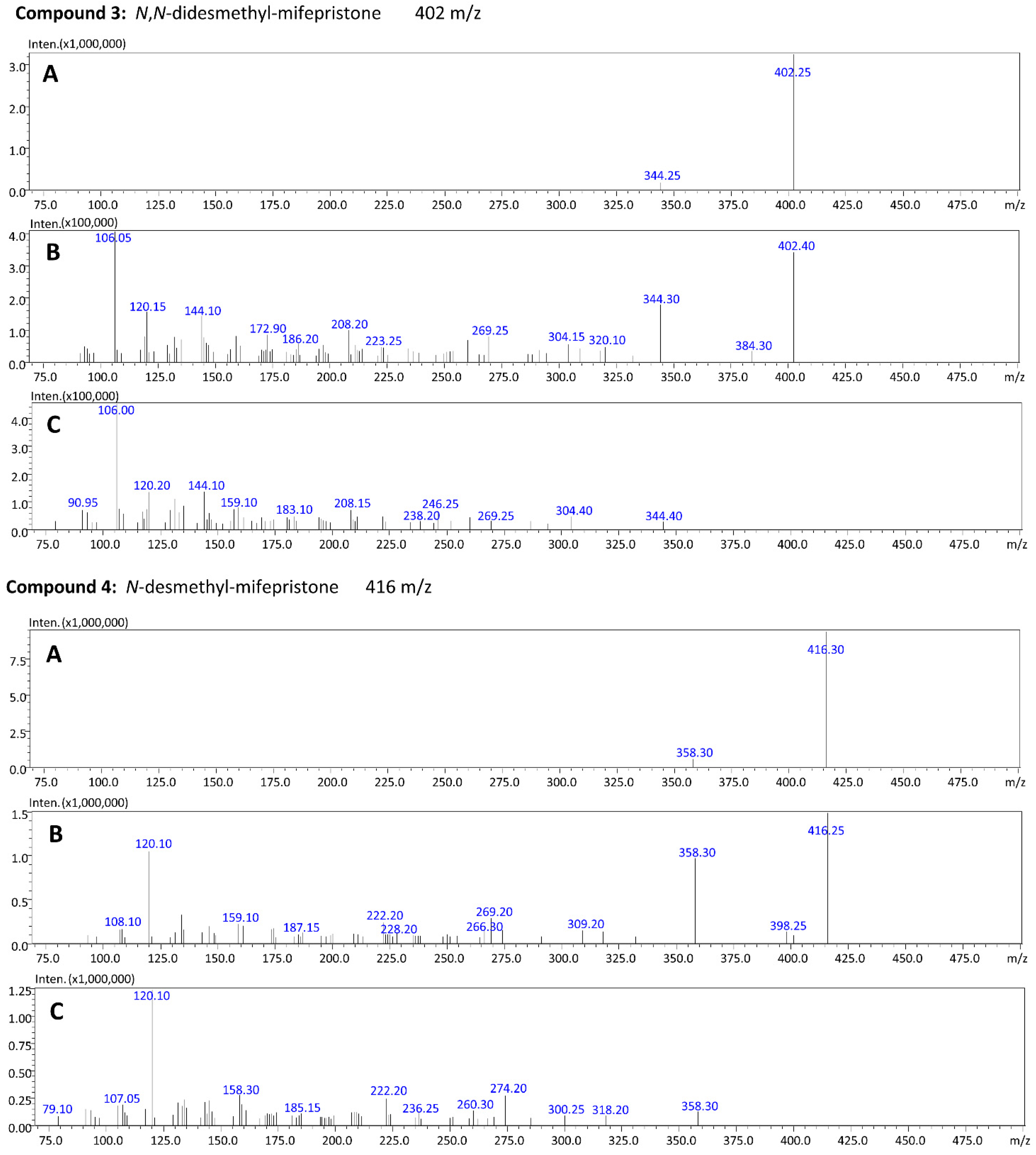
2.7. Identification of Metabolites
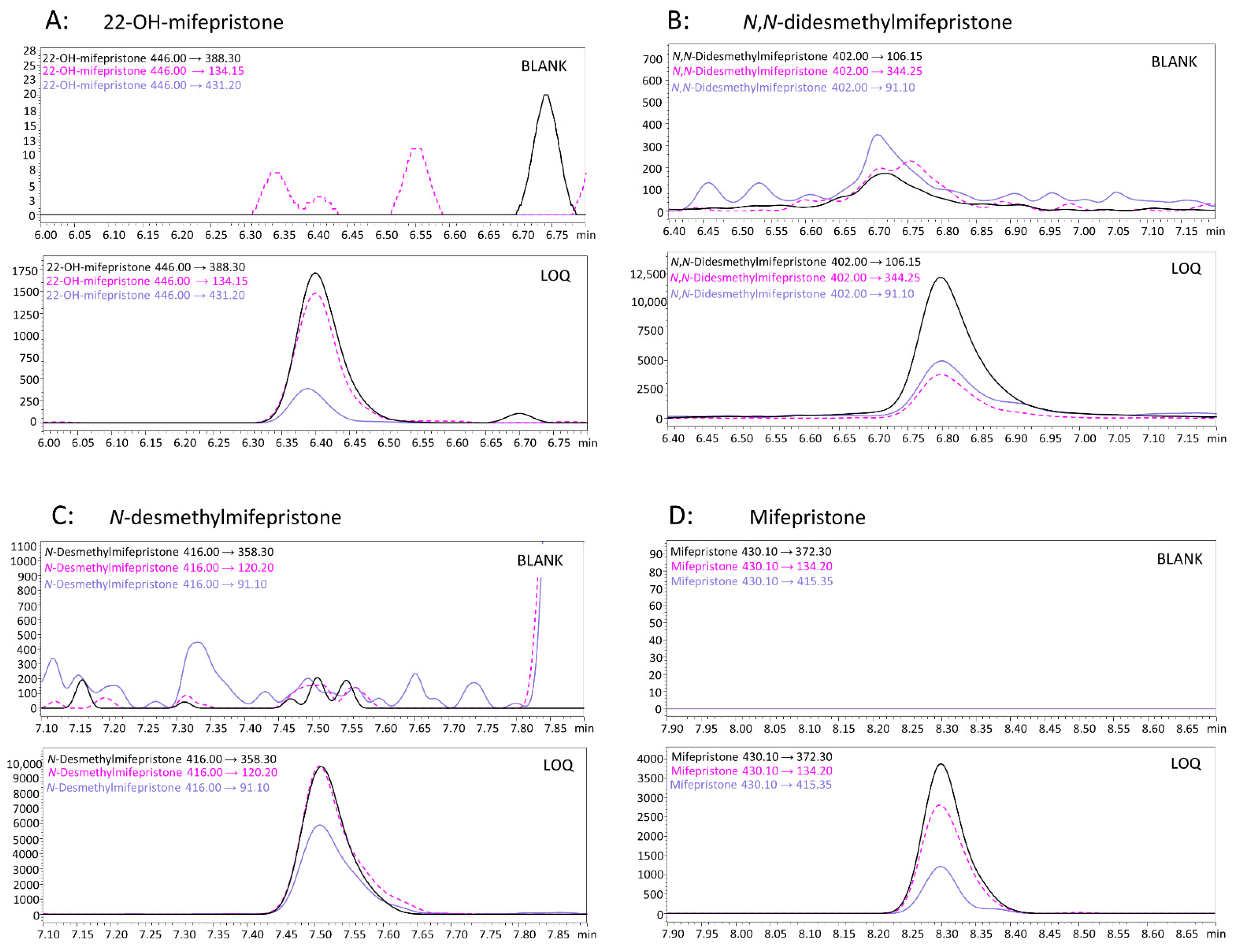
2.8. Method Validation
3. Results and Discussion
3.1. Method Development and Validation Results
3.2. Method Application
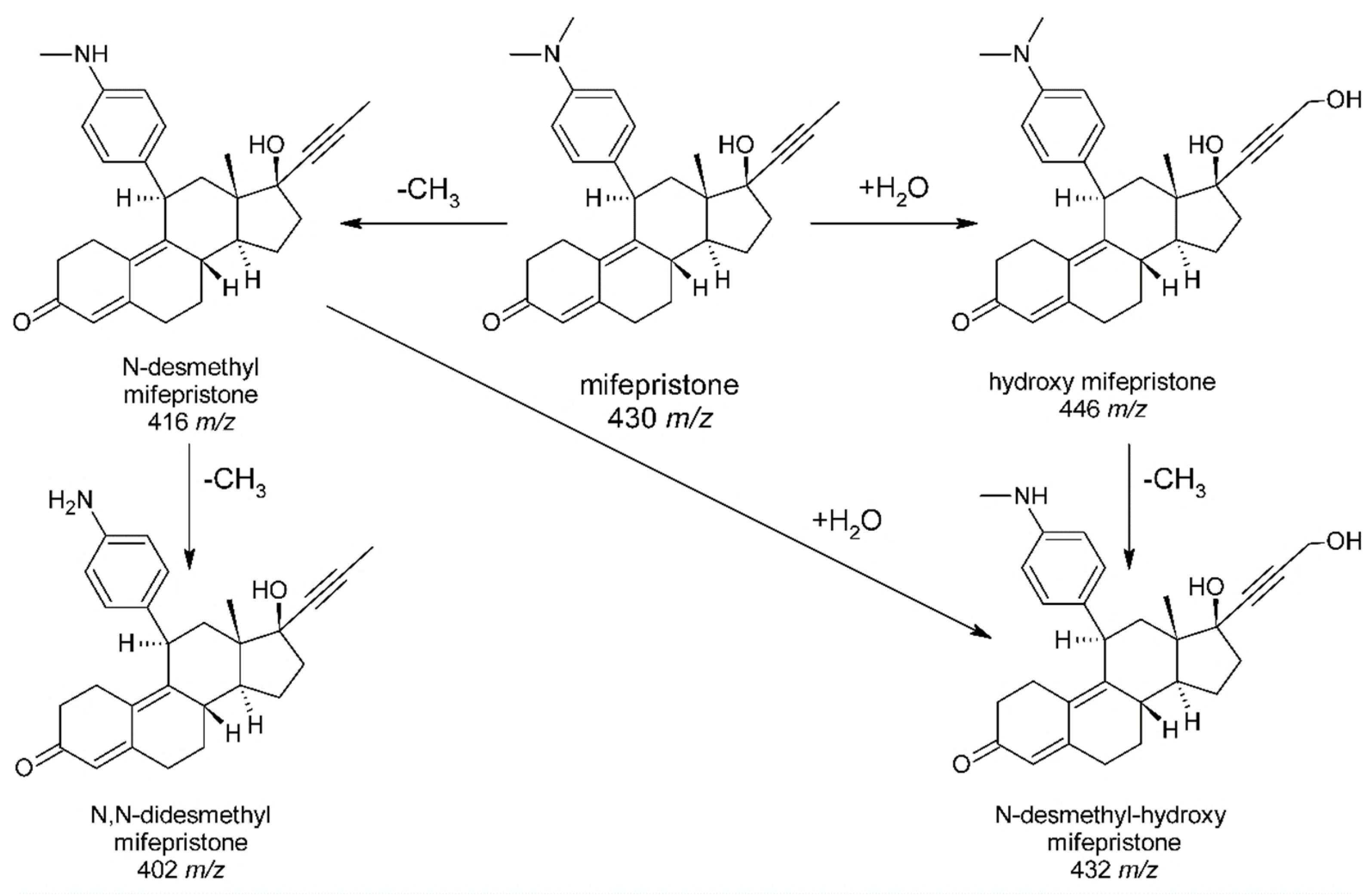
3.3. Metabolic Pathway of Mifepristone in Humans
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Baulieu, E.E. RU 486: An Antiprogestin Steroid with Contragestive Activity in Women. In The Antiprogestin Steroid RU 486 and Human Fertility Control. Reproductive Biology. Reproductive Biology; Baulieu, E.E., Segal, S.J., Eds.; Springer: Boston, MA, USA, 1985; pp. 1–25. [Google Scholar]
- Tao, A.; Zheng, X.; Weng, M. Clinical observation of 114 cases patients with midtrimester abortion treated by combining using of rivanol and mifepristone. Chin. J. Fam. Plan 2002, 10, 419. [Google Scholar]
- Fiala, C.; Gemzel-Danielsson, K. Review of medical abortion using mifepristone in combination with a prostaglandin analogue. Contraception 2006, 74, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, B.; Gerdts, C.; Rossier, C.; Johnson, B.R., Jr.; Tunçalp, Ö.; Assifi, A.; Sedgh, G.; Singh, S.; Bankole, A.; Popinchalk, A.; et al. Global, regional, and subregional classification of abortions by safety, 2010–2014: Estimates from a Bayesian hierarchical model. Lancet 2017, 90, 2372–2381. [Google Scholar] [CrossRef]
- Bearak, J.; Popinchalk, A.; Ganatra, B.; Moller, A.B.; Tunçalp, Ö.; Beavin, C.; Kwok, L.; Alkema, L. Unintended pregnancy and abortion by income, region, and the legal status of abortion: Estimates from a comprehensive model for 1990-2019. Lancet Glob. Health 2020, 8, e1152–e1161. [Google Scholar] [CrossRef]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Singh, S.; Maddow-Zimet, I. Facility-based treatment for medical complications resulting from unsafe pregnancy termination in the developing world, 2012: A review of evidence from 26 countries. BJOG 2016, 123, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Vlassoff, M.; Shearer, J.; Walker, D.; Lucas, H. Economic Impact of Unsafe Abortion-Related Morbidity and Mortality: Evidence and Estimation Challenges; IDS Research Reports 59; Institute of Development Studies: Brighton, UK, 2008. [Google Scholar]
- Lee, J.H.; Park, H.N.; Kim, N.S.; Park, H.J.; Park, S.; Shin, D.; Kang, H. Detection of illegal abortion-induced drugs using rapid and simultaneous method for the determination of abortion-induced compounds by LC–MS/MS. Chromatographia 2019, 82, 1365–1371. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, D.; Yin, G.; Wang, S.; Zhao, S.; Chu, Y.; Zhai, J. Simultaneous determination of rivanol and mifepristone in human plasma by a HPLC-UV method with solid-phase extraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 856, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Stith, C.; Delwar Hussain, M. Determination of mifepristone levels in wild canid serum using liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 794, 9–15. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, S.; Wei, D.; Zhai, J. Development of a high-performance liquid chromatographic method for the determination of mifepristone in human plasma using norethisterone as an internal standard: Application to pharmacokinetic study. Contraception 2007, 76, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chu, C.; Yin, G.; He, M.; Fu, K.; Wu, J. An HPLC method for the determination of ng mifepristone in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 832, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Bi, H.C.; Zhong, G.P.; Chen, X.; Huang, Z.Y.; Huang, M. Simultaneous determination of mifepristone and monodemethyl-mifepristone in human plasma by liquid chromatography-tandem mass spectrometry method using levonorgestrel as an internal standard: Application to a pharmacokinetic study. Biomed. Chromatogr. 2009, 23, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Zaitsu, K.; Kusano, M.; Asano, T.; Ogawa, T.; Hattori, H.; Seno, H. Identification and quantitation of mifepristone and its N-demethyl metabolite in the plasma of an aborted fetus by liquid chromatography–quadrupole–time-of-flight–mass spectrometry (LC–Q–TOFMS) and ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS–MS). Forensic. Toxicol. 2015, 33, 409–412. [Google Scholar] [CrossRef]
- Chambers, E.; Wagrowski-Diehl, D.M.; Lu, Z.; Mazzeo, J.R. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 852, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Szpot, P.; Wachełko, O.; Zawadzki, M. Forensic toxicological aspects of misoprostol use in pharmacological abortions. Molecules 2022, 27, 6534. [Google Scholar] [CrossRef] [PubMed]
- Szpot, P.; Wachełko, O.; Zawadzki, M. Diclofenac Concentrations in Post-Mortem Specimens—Distribution, Case Reports, and Validated Method (UHPLC-QqQ-MS/MS) for Its Determination. Toxics 2022, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.N.; McKown, L.A.; Moyer, M.D.; Johannsen, T.B.; Takacs, A.R. In vitro metabolism of mifepristone (RU-486) in rat, monkey and human hepatic S9 fractions: Identification of three new mifepristone metabolites. Xenobiotica 1999, 29, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Heikinheimo, O. Clinical pharmacokinetics of mifepristone. Clin. Pharmacokinet. 1997, 33, 7–17. [Google Scholar] [CrossRef] [PubMed]
| Substance | Precursor Ion [m/z] | Product Ion [m/z] | Dwell Time (msec) | Q1 Pre-Bias [V] | Collision Energy [V] | Q3 Pre-Bias [V] |
|---|---|---|---|---|---|---|
| 22-OH-mifepristone | 446.0 | 388.3 * | 9 | −11 | −23 | −17 |
| 134.15 | −11 | −30 | −24 | |||
| 431.2 | −11 | −26 | −30 | |||
| 22-OH-mifepristone-d6 | 452.0 | 394.25 * | 9 | −11 | −22 | −18 |
| 140.25 | −12 | −30 | −28 | |||
| 154.3 | −10 | −28 | −30 | |||
| N,N-didesmethyl-mifepristone | 402.0 | 106.15 * | 9 | −10 | −31 | −10 |
| 344.25 | −11 | −19 | −11 | |||
| 91.1 | −10 | −54 | −22 | |||
| N-desmethyl-mifepristone | 416.0 | 358.3 * | 2 | −11 | −20 | −16 |
| 120.2 | −11 | −30 | −21 | |||
| 91.1 | −10 | −55 | −19 | |||
| N-desmethyl-mifepristone-d3 | 419.0 | 123.2 | 2 | −11 | −27 | −24 |
| 361.3 * | −11 | −20 | −16 | |||
| 161.2 | −11 | −34 | −15 | |||
| Mifepristone | 430.1 | 372.3 * | 2 | −20 | −23 | −24 |
| 134.2 | −20 | −31 | −22 | |||
| 415.35 | −20 | −27 | −19 | |||
| Mifepristone-d3 | 433.1 | 375.3 * | 2 | −12 | −24 | −17 |
| 137.2 | −12 | −33 | −21 | |||
| 415.35 | −12 | −25 | −19 |
| Extraction Efficiency [%] | |||||
|---|---|---|---|---|---|
| Mifepristone | N-Desmethyl Mifepristone | N,N-Didesmethyl Mifepristone | 22-OH-Mifepristone | ||
| pH 7.4 | Ethyl acetate | 50.3 | 60.1 | 46.8 | 56.1 |
| n-Hexane | 20.7 | 2.9 | 8.9 | 0.2 | |
| DCM | 29.8 | 54.8 | 52.2 | 47.2 | |
| TBME | 55.9 | 68.5 | 69.0 | 89.0 | |
| pH 9 | Ethyl acetate | 49.0 | 65.6 | 61.7 | 78.5 |
| n-Hexane | 16.7 | 2.8 | 7.8 | 0.2 | |
| DCM | 20.0 | 36.9 | 55.9 | 64.0 | |
| TBME | 62.0 | 71.1 | 73.0 | 94.4 | |
| Substance | Calibration Curve | Validation Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Linear Concentration Range [ng/mL] | Internal Standard | Concentration Level [ng/mL] | Intra-Day | Inter-Day | Recovery [%] * | Matrix Effect [%] * | |||
| Precision [%] * | Accuracy [%] * | Precision [%] * | Accuracy [%] * | ||||||
| Mifepristone | 0.5–500 | Mifepristone-d3 | 5 | 2.6 | 0.1 | 7.4 | −8.4 | 103.4 | 3.4 |
| 100 | 7.8 | −5.5 | 2.6 | −2.4 | 101.1 | 1.1 | |||
| 500 | 5.5 | −13.2 | 6.2 | −4.2 | 97.7 | −2.3 | |||
| N-desmethyl mifepristone | 0.5–500 | N-desmethyl mifepristone-d3 | 5 | 2.3 | 0.5 | 3.4 | 1.6 | 108.0 | 8.0 |
| 100 | 9.8 | −0.7 | 9.2 | −2.8 | 97.0 | −3.0 | |||
| 500 | 2.0 | −5.0 | 9.8 | −7.5 | 110.2 | 10.2 | |||
| N,N-didesmethyl mifepristone | 0.5–1000 | N-desmethyl mifepristone-d3 | 5 | 2.4 | 3.6 | 7.6 | 11.3 | 103.6 | 3.6 |
| 100 | 6.1 | −5.3 | 7.4 | −10.4 | 100.1 | 0.1 | |||
| 500 | 1.1 | −8.7 | 3.3 | 1.1 | 114.7 | 14.7 | |||
| 22-OH-mifepristone | 0.5–500 | 22-OH-mifepristone-d6 | 5 | 0.0 | −2.1 | 11.7 | −3.0 | 96.3 | 6.3 |
| 100 | 3.8 | −7.2 | 8.7 | −8.8 | 100.0 | 0.0 | |||
| 500 | 6.5 | −12.2 | 7.2 | −4.8 | 104.8 | 4.8 | |||
| Matrix (Volume) | Sample Preparation | Method | Recovery/IS | LOQ [ng/mL] | References |
|---|---|---|---|---|---|
| Plasma (1000 µL) | SPE (Oasis HLB) | HPLC-UV | 95.1–105.8%/norethisterone | 10 | [10] |
| Plasma (500 µL) | SPE (Oasis HLB) | HPLC-QqQ-MS/MS | 94.5–103.7%/levonorgestrel | 5 | [14] |
| Serum (1000 µL) | SPE (Oasis HLB) | HPLC-UV | 92.7–104.3%/ mifepristone analogue | 10 | [11] |
| Plasma (1000 µL) | SPE (Oasis HLB) | HPLC-UV | 91.7–100.1%/norethisterone | 10 | [12] |
| Plasma (100 µL) | Protein precipitation with ACN | UPLC-QqQ-MS/MS | –/alfaxalone | 0.25–the lowest calibration level | [15] |
| Plasma (500 µL) | SPE (Oasis HLB) | HPLC-UV | 94.7–101.2%/– | 20 | [13] |
| Whole blood (200 µL) | LLE (pH 9; TBME) | UHPLC-QqQ-MS/MS | 97.7–103.4%/mifepristone-d3 | 0.5 | Presented method * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpot, P.; Wachełko, O.; Jurek, T.; Zawadzki, M. Determination of Mifepristone (RU-486) and Its Metabolites in Maternal Blood Sample after Pharmacological Abortion. Molecules 2022, 27, 7605. https://doi.org/10.3390/molecules27217605
Szpot P, Wachełko O, Jurek T, Zawadzki M. Determination of Mifepristone (RU-486) and Its Metabolites in Maternal Blood Sample after Pharmacological Abortion. Molecules. 2022; 27(21):7605. https://doi.org/10.3390/molecules27217605
Chicago/Turabian StyleSzpot, Paweł, Olga Wachełko, Tomasz Jurek, and Marcin Zawadzki. 2022. "Determination of Mifepristone (RU-486) and Its Metabolites in Maternal Blood Sample after Pharmacological Abortion" Molecules 27, no. 21: 7605. https://doi.org/10.3390/molecules27217605
APA StyleSzpot, P., Wachełko, O., Jurek, T., & Zawadzki, M. (2022). Determination of Mifepristone (RU-486) and Its Metabolites in Maternal Blood Sample after Pharmacological Abortion. Molecules, 27(21), 7605. https://doi.org/10.3390/molecules27217605








