Abstract
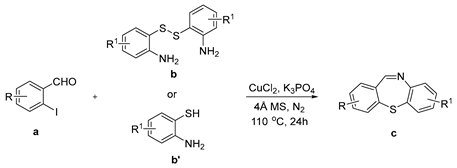
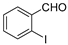
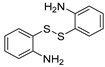
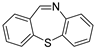
The efficient “One-pot” CuCl2-catalyzed C–S bond coupling reactions were developed for the synthesis of dibenzo[b,f][1,4]thiazepines and 11-methy-ldibenzo[b,f][1,4]thiazepines via 2-iodobenzaldehydes/2-iodoacetophenones with 2-aminobenzenethiols/2,2′-disulfanediyldianilines by using bifunctional-reagent N, N′-dimethylethane-1,2-diamine (DMEDA), which worked as ligand and reductant. The reactions were compatible with a range of substrates to give the corresponding products in moderate to excellent yields.
1. Introduction
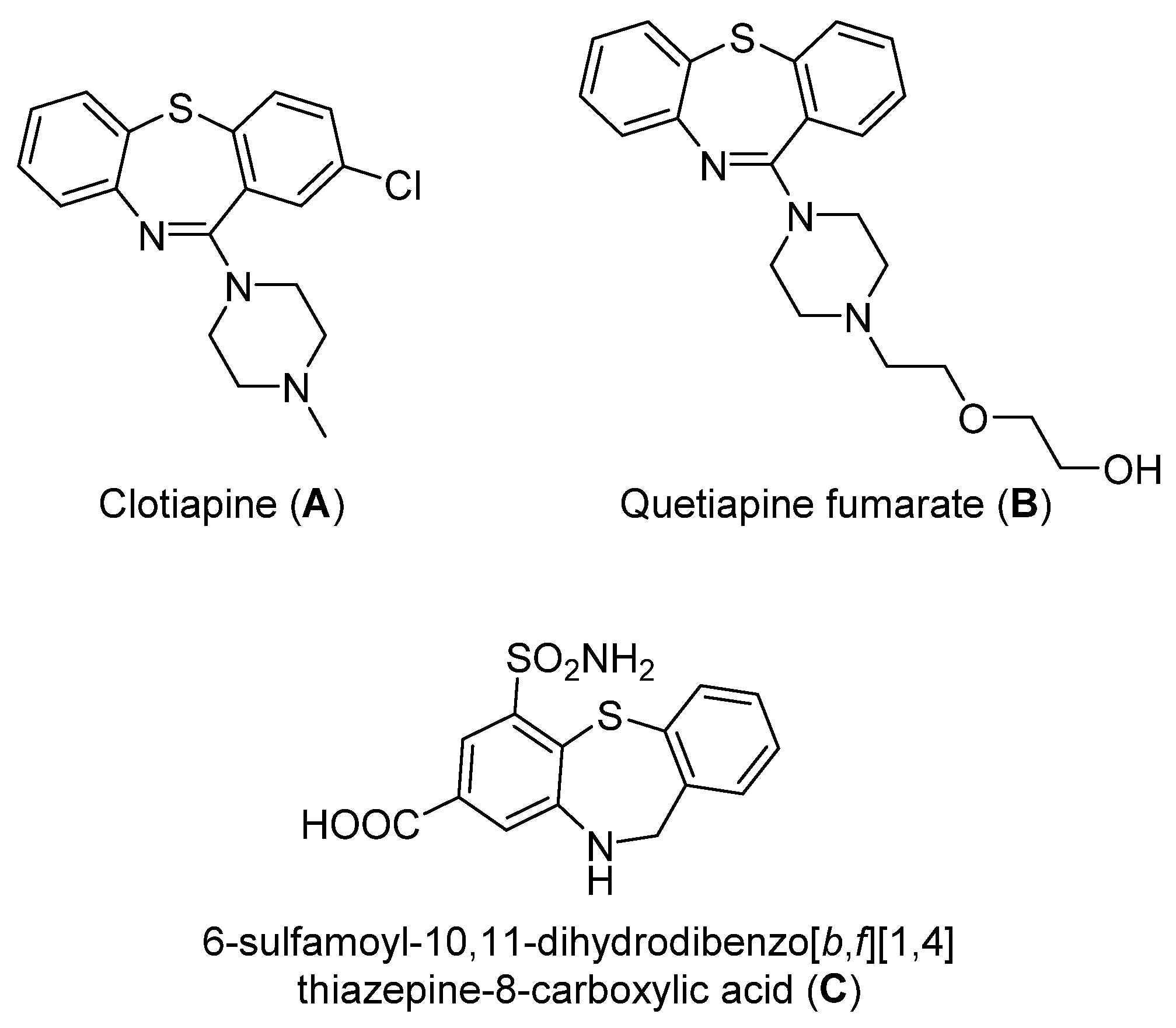
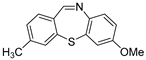
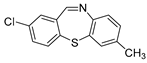
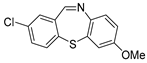
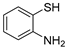
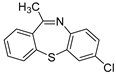
Dibenzothiazepine derivatives are a class of molecules with important biological and pharmaceutical activities [1,2,3,4]. For example, Clotiapine (A) has good anti-hallucination, the delusion and the anti-excited restlessness function [5]. Quetiapine fumarate (B) is effective for both positive symptoms and negative symptoms of schizophrenia, it can also reduce the emotional symptoms associated with schizophrenia, such as depression, anxiety and cognitive deficits [6,7,8]. The 6-Sulfamoyl-10,11-dihydrodibenzo[b,f][1,4]thiazepine-8-carboxylic acid (C) is a structural analogue of nitroxazepine with high activity, indicating its potential medicinal value (Figure 1) [9].
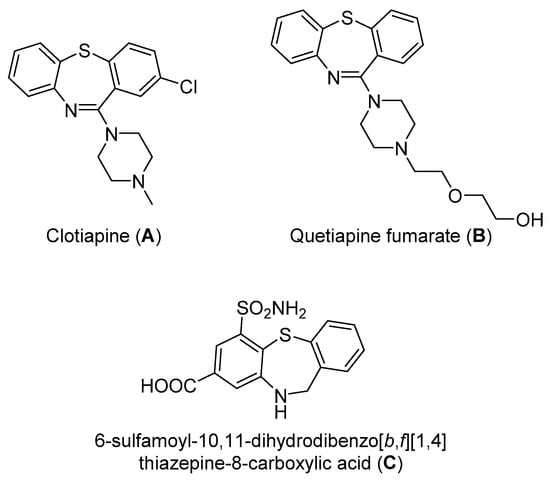
Figure 1.
Some biological and pharmaceutical active dibenzothiazepines.
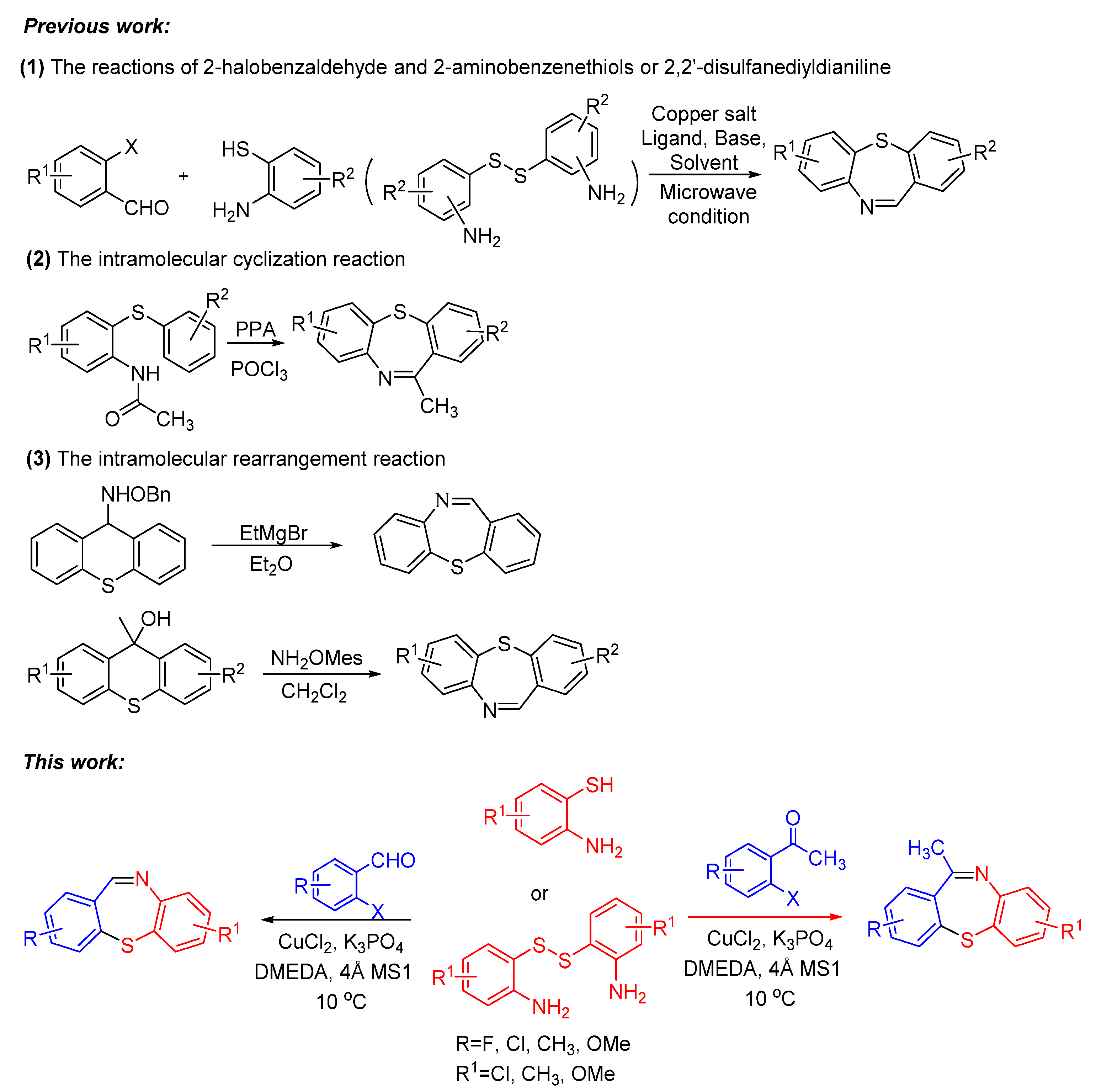
Due to its wide applications, it is meaningful to find some simple, efficient and practical methods for the synthesis of dibenzothiazepines. In past studies, several typical methods have been developed, including the reactions of 2-halobenzaldehyde and 2-aminobenzenethiols or 2,2′-disulfanediyldianiline [10,11,12,13,14], the intramolecular cyclization reactions [9,15], the intramolecular rearrangement reactions [16], and some other methods (Scheme 1) [17]. The previous reports have mainly focused on the classic coupling reactions of 2-halogenated benzaldehyde with 2-aminobenzenethiols or 2,2′-disulfanediyldianiline because it is direct and effective. However, the classic copper-catalyzed coupling reactions for the synthesis of dibenzothiazepine are complicated, often required the copper salt, ligand, base and solvent and the substrate application scopes were limited [18]. For example, in 2009, Reiko Yanada et al. employed a Pd(OAc)2-catalyzed one-pot reaction to synthesize dibenzo[b,f][1,4]thiazepines by microwave-accelerated tandem process of 2-brmobenzaldehyde and 2-aminobenzenethiols [19]. In 2015, Yie-jia Cherng et al. also reported the microwave-assisted strategy to assemble dibenzo[b,f][1,4]thiazepines [10]. In the same year, Anuj Sharma et al. utilized a standard base-catalyzed condensation of amines with aldehydes to afford dibenzo[b,f][1,4] thiazepines [11]. Recently, Yun Luo and Jiaxi Xu et al. synthesized these heterocyclic molecules using K2CO3 and ethane-1,2-diol. [20] Copper-catalyzed coupling reactions is a powerful tool for the construction of C–X (X = S, N, O, etc.) bonds and can be applied in the efficient synthesis of heterocyclic compounds in organic synthesis [21,22,23,24,25,26,27]. Simplifying the copper-catalyzed reaction conditions is also an irreplaceable advantage for the application of the reaction. Our group has been working on the copper-catalyzed coupling reactions to synthesize heterocyclic compounds, in continuation of our works to modify the copper-catalyzed cross-coupling reactions [28,29]. Here, we reported an “One-pot” CuCl2-catalyzed C–S bond coupling reactions for the synthesis of dibenzo[b,f][1,4]thiazepines and 11-methyldibenzo[b,f][1,4]thiazepines via 2-iodobenzaldehydes/2-iodoacetophenones with 2-aminobenzenethiols/2,2′-disulfanediyldianilines by using bi-functional-reagent DMEDA. Compared to the previous reports of synthesizing dibenzothiazepines in entry 1, our research used DMEDA in small quantities as a bifunctional reagent, which worked as ligand and reductant; the substrate scope is wide (the reaction substrates 2-iodobenzaldehydes/2-iodoacetophenones and 2-aminobenzenethiols/2,2′-disulfanediyldianilines could cross-react with each other) and the reaction conditions were simplified, making the reaction conditions more suitable for large-scale production.
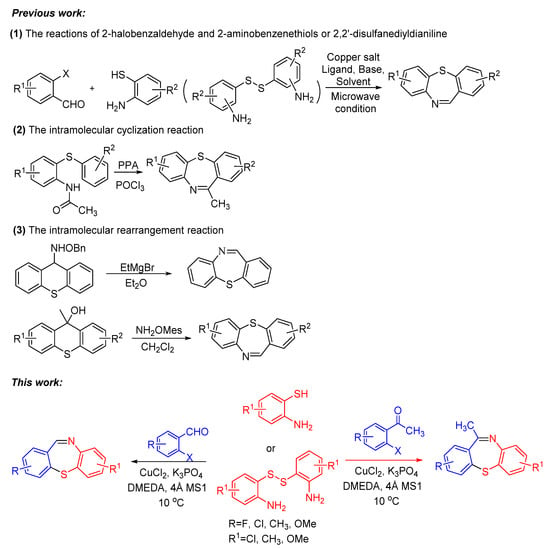
Scheme 1.
Previous work and this work to synthesize dibenzothiazepine derivatives.
2. Results
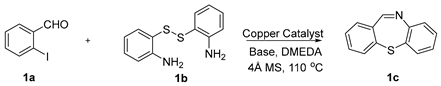
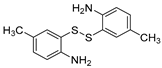
First, 2-iodobenzaldehyde (1a) and 2,2′-Disulfanediyldianiline (1b) were selected to optimize the reaction conditions (Table 1). The reaction was conducted with CuCl2 (15 mol%), 1a (0.3 mmol), 1b (0.15 mmol), Cs2CO3 (0.6 mmol) and 4 Å molecular sieve (25 mg) in DMEDA (0.50 mL) at 110 °C under N2 atmosphere for 24 h, the product 1c was produced in 73% (Table 1, entry 1). When we decreased the amount of DMEDA, 0.25 mL showed the best results (Table 1, entries 1–3). There was a significant decrease without inorganic base Cs2CO3 (Table 1, entry 4). When K3PO4 (0.6 mmol) was used for the reaction, 1c was improved in 82% yield (Table 1, entries 5–6). Then, the reaction temperature was screened, it was found that higher reaction temperature did not change the reaction yield; 110 °C was the best choice (Table 1, entries 7–8). Finally, other copper salts, such as Cu(OAc)2, CuSO4·5H2O, CuI, were surveyed under the conditions, the yields of 1c were not increased (Table 1, entries 9–11). Without the 4 Å molecular sieves, the reaction yield was also reduced (Table 1, entry 12). A gram-scale reaction was also conducted, the yield of 1c was the same as entry 5 (Table 1, entry 13). From the above results we could conclude that the bi-functional reagent DMEDA was necessary for the reaction.

Table 1.
Optimization of the reaction conditions a.
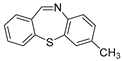
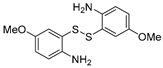
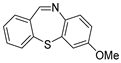
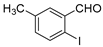
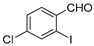
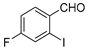
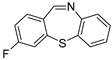
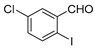
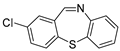
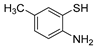
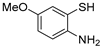
With the optimized reaction conditions in hand, the reaction scope was investigated (Table 2). Our initial studies were focused on the reaction of 2-iodobenzaldehydes a, with 2,2′-disulfanediyldianilines b, and the products c could be isolated in moderate-to-good yields. The 2-Bromobenzaldehyde and 2-chlorobenzaldehyde were used to react with 1b, and the yields decreased. When 2,2′-disulfanediyldianilines bearing electron-donating and electron-withdrawing groups were used to react with 1a, the products were obtained in good yields (Table 2, entries 2–4). Substituted 2-iodobenzaldehydes were also used to react with 1b, and the reactions yields were basically kept in good yields (Table 2, entries 5–9). Some cross-reactions were tested, and moderate-to-excellent yields were obtained. (Table 2, entries 10–12). The above results indicated that the reaction yields of 2-iodobenzaldehydes a with 2,2′-disulfanediyldianilines b were not influenced significantly by the electronic effect and steric effect. Subsequently, we examined the reaction of 2-iodobenzaldehydes a with 2-aminobenzenethiols b′; the reactions proceeded smoothly, and the reaction yields were obtained in moderate-to-good yields (Table 2, entries 16–22).

Table 2.
Scope of the CuCl2-catalyzed condensation/C–S bond coupling reaction of 2-iodobenzaldehydes and 2,2′-disulfanediyldianilines/2-aminobenzenethiols in DMEDA a.
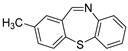
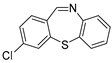
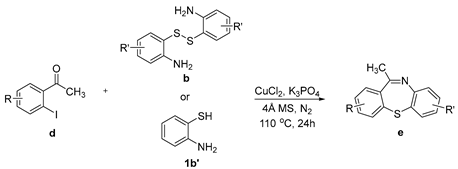
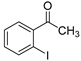
The scope of 2′-iodoacetophenones d and 2,2′-disulfanediyldianilines b/2-aminobenzenethiol 1b′ was also investigated (Table 3). The 2′-Iodoacetophenone 1d and 2,2′-disulfanediyldianiline 1b were used under the optimal conditions, and 75% yield of 1e was isolated (Table 3, entry 1). We used 2′-iodoacetophenones 1d and substituted 2,2′-disulfanediyldianilines 2b–5b to do the reaction, and the products e were isolated in moderate-to-good yields (Table 3, entries 2–5). Then, 2,2′-disulfanediyldianiline 1b was replaced by 2-aminobenzenethiols 1b′ and reacted with 2′-iodoacetophenone 1d to obtain the desired product in 95% yield (Table 3, entry 6). Finally, (2-iodophenyl)(phenyl)methanone 3d was used, and it could not react with 1b to generate the product 6e (Table 3, entry 7).

Table 3.
Scope of the CuCl2-catalyzed condensation/C–S bond coupling reaction of 2′-iodoacetophenones and 2,2′-disulfanediyldianilines/2-aminobenzenethiol in DMEDA a,b.
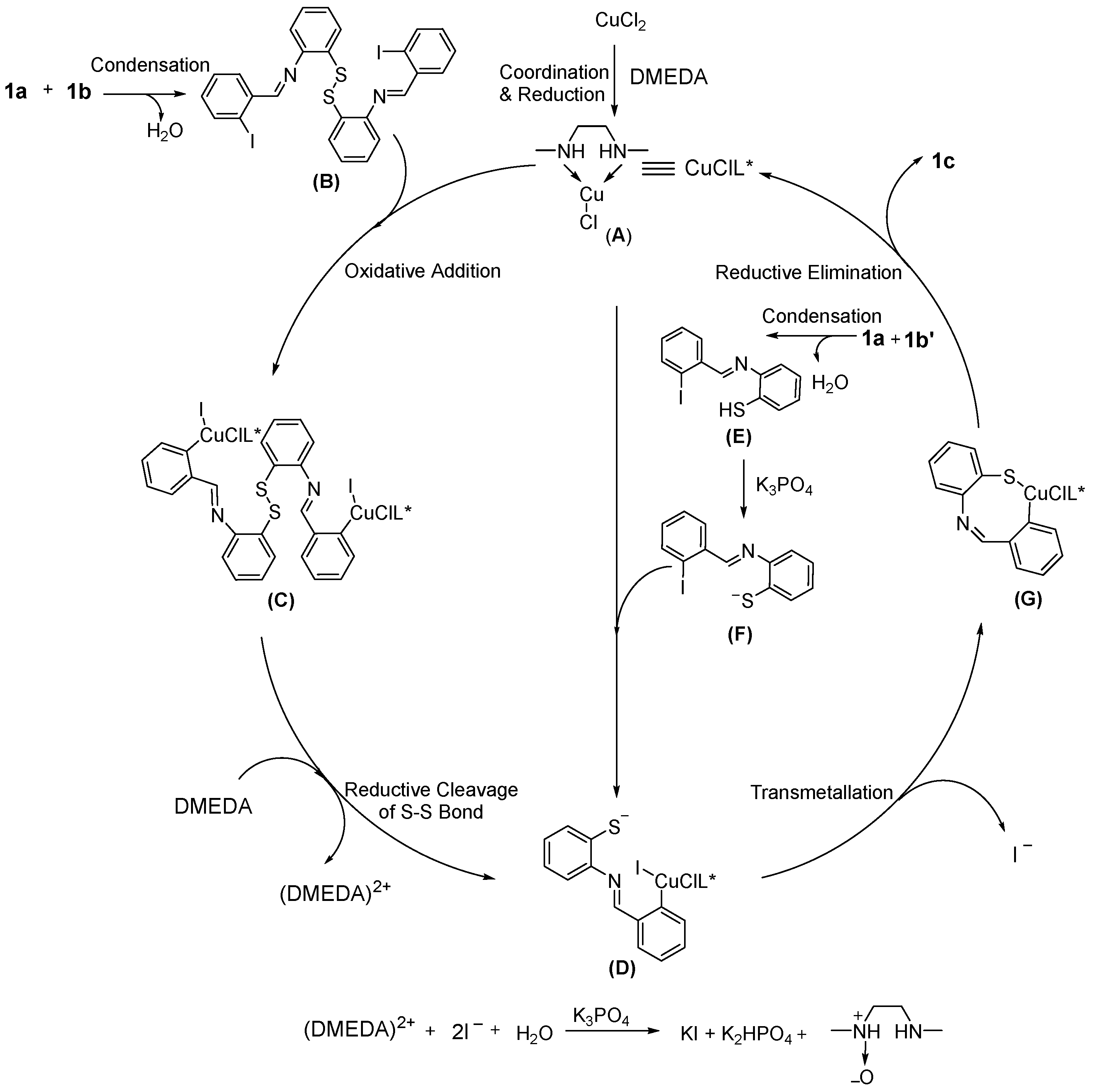
Finally, a possible mechanism was proposed for the reaction based on the experimental results (Scheme 2). Firstly, CuCl2 coordinates with DMEDA and is reduced to generate the Cu(I) complex (A). At the same time, the starting material 1a could react with 1b to form the intermediate (B) through the intermolecular condensation. The intermediate (C) could be generated via the oxidative addition of (A) and (B). Then, (C) was converted to the intermediate (D), and DMEDA might act as the reductant. For the substrate 1a and 1b′, the intermediate (D) is also generated during the reaction process. After the transmetallation/reductive elimination reaction, the product 1c could be formed. From the possible mechanism, we found that the bifunctional reagent DMEDA worked as ligand and reductant.
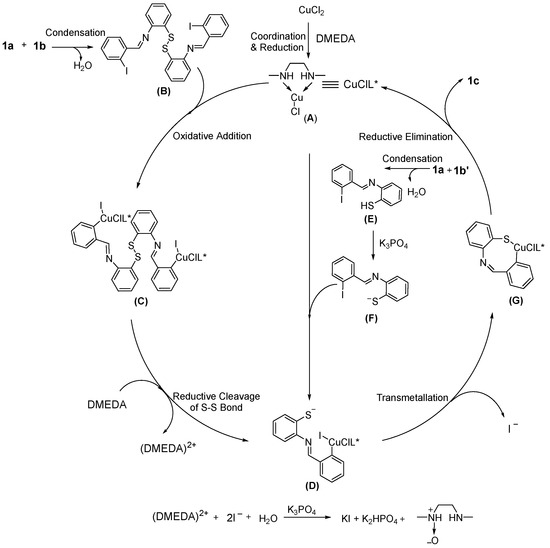
Scheme 2.
Possible mechanism.
3. Experimental Section
3.1. General
1H NMR and 13C NMR spectra were recorded 500 MHz (Bruker, Kanton Zug, Switzerland) instrument; CDCl3 (δH = 7.26 ppm, δC = 77.16 ppm) was used as the internal standard. Chemical shifts were reported in ppm. Multiplicity was recorded: s (singlet), d (doublet), t (triplet), q (quartet), dd (doublet of doublets), dt (doublet of triplets), m (multiplet). The direct used reagents and solvents were pure analytical grade and purchased from commercial sources, if not stated otherwise. The starting substrates were synthesized according to the known literature. Column chromatography was hand packed with silica gel (200–300 mesh). The melting points were uncorrected. High-resolution mass spectra (HRMS) were recorded on a Q-TOF Premier (ESI, Waters, Milford, CT, USA). The silica gel plates (GF254, 0.2 mm thick) were used for TLC testing.
3.2. General Procedure for the Synthesis of Dibenzo[b,f][1,4]thiazepines (1c–12c) Catalyzed by CuCl2 in DMEDA
An oven-dried 25 mL flask equipped with a rubber stopper was charged with a magnetic stir bar, CuCl2 (15 mol%, 0.045 mmol), 2-iodobenzaldehyde a (0.3 mmol), 2,2′-disulfanediyldianilines b (0.15 mmol)/ 2-aminobenzenethiols b′ (0.3 mmol), K3PO4 (0.6 mmol), 4 Å molecular sieve (25 mg) and DMEDA (0.5 mL). The reaction mixture was stirred at 110 °C for 24 h. The reaction was monitored by TLC. When benzaldehydes a was consumed, the reaction was stopped and cooled to room temperature, the crude reaction mixture was diluted with 20 mL water, extracted with ethyl acetate (20 mL × 3), combined with organic phase, then washed organic phase with brine (20 mL), dried organic phase with anhydrous Mg2SO4. The organic phase was concentrated and the residue was purified directly by column chromatography on silica gel using petrol/EtOAc as eluent to give the pure products c.
3.3. General Procedure for the Synthesis of 11-Methyldibenzo[b,f][1,4]thiazepines (1e–5e) Catalyzed by CuCl2 in DMEDA
An oven-dried 25 mL flask equipped with a rubber stopper was charged with a magnetic stir bar, CuCl2 (15 mol%, 0.045 mmol), 1-(2-iodophenyl)ethan-1-ones d (0.3 mmol), 2,2′-disulfanediyldianilines b (0.15 mmol)/2-aminobenzenethiols b′ (0.3 mmol), K3PO4 (0.6 mmol), 4 Å molecular sieve (25 mg) and DMEDA (0.5 mL). The reaction mixture was stirred at 110 °C for 24 h. The reaction was monitored by TLC. When benzaldehydes d was consumed, the reaction was stopped and cooled to room temperature, the crude reaction mixture was diluted with 20 mL water, extracted with ethyl acetate (20 mL × 3), combined with organic phase, then washed organic phase with brine (20 mL), dried organic phase with anhydrous Mg2SO4. The organic phase was concentrated and the residue was purified directly by column chromatography on silica gel using petrol/EtOAc as eluent to give the pure products e. For the NMR spectrum of compounds see the Supplementary Materials.
3.4. Characterization Data
The dibenzo[b,f][1,4]thiazepine 1c (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent). Yellow solid; mp: 126–128 °C (Lit: m.p. 124 °C) [30]; 52.0 mg; 82% yield (1a and 1b were used); 51.3 mg; 81% yield (1a and 1b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.90 (s, 1H), 7.44–7.30 (m, 7H), 7.19–7.15 (m, 1H). 13C NMR (125 MHz, CDCl3/TMS): δ 162.4, 148.7, 139.5, 137.4, 132.9, 131.8, 131.6, 129.5, 129.4, 129.0, 128.4, 127.3, 127.1.
The 7-methyldibenzo[b,f][1,4]thiazepine 2c (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent). Yellow solid; mp: 115–117 °C; 54.0 mg; 80% yield (1a and 2b were used); 54.0 mg; 80% yield (1a and 2b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.85 (s, 1H), 7.42 (d, J = 7.5 Hz, 1H), 7.39–7.33 (m, 3H), 7.24–7.20 (m, 2H), 7.13 (d, J = 8.0 Hz, 1H), 2.30 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 161.8, 146.3, 139.3, 137.6, 137.5, 133.2, 131.7, 131.5, 130.2, 129.5, 128.5, 128.3, 127.0, 20.7.
The 7-methoxydibenzo[b,f][1,4]thiazepine 3c (Flash column chromatography on silica gel using petrol/EtOAc (5:1, v:v) as eluent). Yellow solid; mp: 101–102 °C; 62.2 mg; 86% yield (1a and 3b were used); 62.2 mg; 86% yield (1a and 3b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.80 (s, 1H), 7.43–7.34 (m, 4H), 7.24 (s 1H), 6.95 (d, J = 3.0 Hz, 1H), 6.88 (dd, J1 = 9.0 Hz, J2 = 3.0 Hz, 1H), 3.79 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 160.8, 159.1, 142.4, 138.6, 137.5, 131.7, 131.5, 129.6, 129.4, 128.5, 128.4, 117.0, 115.6, 55.7. HRMS (ESI): m/z calcd for C14H12NOS [M + H]+: 242.0634, found: 242.0641.
The 7-chlorodibenzo[b,f][1,4]thiazepine 4c (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent). Yellow solid; mp: 75–76 °C; 65.6 mg; 89% yield (1a and 4b were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.87 (s, 1H), 7.44–7.36 (m, 5H), 7.28 (dd, J1 = 8.5 Hz, J2 = 2.5 Hz, 1H), 7.23 (d, J = 8.5 Hz, 1H). 13C NMR (125 MHz, CDCl3/TMS): δ 162.7, 147.2, 138.6, 137.2, 132.8, 132.3, 131.90, 131.88, 130.3, 129.6, 129.5, 128.7, 128.0.
The 3-methyldibenzo[b,f][1,4]thiazepine 5c (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent) Yellow solid; mp: 93–94 °C; 56.7 mg; 84% yield (2a and 1b were used); 52.8 mg; 78% yield (2a and 1b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.86 (s, 1H), 7.42–7.40 (m, 1H), 7.33–7.28 (m, 3H), 7.20–7.14 (m, 3H), 2.33 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 162.5, 148.7, 138.5, 137.2, 136.2, 132.8, 132.4, 131.6, 130.1, 129.29, 129.28, 127.2, 127.0, 21.1.
The 2-methyldibenzo[b,f][1,4]thiazepine 6c (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent). Yellow solid; mp: 92–94 °C; 49.3 mg; 73% yield (3a and 1b were used); 56.1 mg; 83% yield (3a and 1b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.86 (s, 1H), 7.42–7.40 (m, 1H), 7.33–7.28 (m, 3H), 7.20–7.14 (m, 3H), 2.34 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 162.5, 148.8, 138.5, 137.2, 136.3, 132.8, 132.4, 131.6, 130.1, 129.33, 129.28, 127.2, 127.1, 21.1.
The 3-chlorodibenzo[b,f][1,4]thiazepine 7c (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent). White solid; mp: 107–109 °C; 63.3 mg; 86% yield (4a and 1b were used); 64.7 mg; 88% yield (4a and 1b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.84 (s, 1H), 7.45 (d, J = 1.0 Hz 1H), 7.41 (dd, J1 = 7.5 Hz, J2 = 1.0 Hz, 1H), 7.37–7.29 (m, 4H), 7.19 (td, J1 = 7.0 Hz, J2 = 1.5 Hz, 1H). 13C NMR (125 MHz, CDCl3/TMS): δ 161.2, 148.6, 141.1, 137.8, 135.7, 133.0, 131.6, 130.5, 129.7, 128.6, 128.2, 127.6, 127.2.
The 3-fluorodibenzo[b,f][1,4]thiazepine 8c (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent). Yellow solid; mp: 54–56 °C; 53.5 mg; 78% yield (5a and 1b were used); 61.1 mg; 89% yield (5a and 1b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.84 (s, 1H), 7.42–7.30 (m, 4H), 7.22–7.15 (m, 2H), 7.05 (td, J1 = 8.5 Hz, J2 = 3.0 Hz, 1H). 13C NMR (125 MHz, CDCl3/TMS): δ 164.4 (d, J = 253.6 Hz), 161.2, 148.6, 141.8 (d, J = 8.3 Hz), 133.7 (d, J = 3.4 Hz), 133.0, 131.4 (d, J = 9.3 Hz), 129.7, 128.2, 127.5, 127.1, 118.8 (d, J = 22.3 Hz), 115.6 (d, J = 21.8 Hz).
The 2-chlorodibenzo[b,f][1,4]thiazepine 9c (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent). Yellow solid; mp: 111–112 °C; 61.2 mg; 83% yield (6a and 1b were used); 58.9 mg; 80% yield (6a and 1b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.82 (s, 1H), 7.41 (dd, J1 = 8.0 Hz, J2 = 1.5 Hz, 1H), 7.37–7.29 (m, 5H), 7.18 (td, J1 = 7.5 Hz, J2 = 1.5 Hz, 1H). 13C NMR (125 MHz, CDCl3/TMS): δ 160.7, 148.5, 138.5, 138.0, 134.7, 133.0, 132.9, 131.5, 129.7, 129.3, 128.5, 127.6, 127.1.
The 7-methoxy-3-methyldibenzo[b,f][1,4]thiazepine 10c (Flash column chromatography on silica gel using petrol/EtOAc (5:1, v:v) as eluent). Yellow solid; mp: 128–129 °C; 56.8 mg; 74% yield (2a and 3b were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.76 (s, 1H), 7.29 (d, J = 8.5 Hz, 1H), 7.23 (d, J = 8.5 Hz, 1H), 7.18 (d, J = 7.0 Hz, 2H), 6.94 (d, J = 3.0 Hz, 1H), 6.87 (dd, J1 = 8.5 Hz, J2 = 2.5 Hz, 1H), 3.78 (s, 3H), 2.33 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 160.9, 159.0, 142.5, 138.6, 137.3, 135.2, 132.3, 131.6, 130.1, 129.7, 128.4, 116.9, 115.5, 55.7, 21.1. HRMS (ESI): m/z calcd for C15H14NOS [M + H]+: 256.0791, found: 256.0794.
The 2-chlorodibenzo[b,f][1,4]thiazepine 11c (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent). Gray solid; mp: 110–111 °C; 65.4 mg; 84% yield (6a and 2b were used); 60.8 mg; 78% yield (6a and 2b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.77 (s, 1H), 7.36–7.32 (m, 3H), 7.24–7.19 (m, 2H), 7.16–7.13 (m, 1H), 2.30 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 160.1, 146.1, 138.5, 137.9, 137.8, 134.6, 133.2, 132.9, 131.4, 130.5, 129.3, 128.0, 127.1, 20.7. HRMS (ESI): m/z calcd for C14H11ClNS [M + H]+: 260.0295, found: 260.0284.
The 2-chloro-7-methoxydibenzo[b,f][1,4]thiazepine 12c (Flash column chromatography on silica gel using petrol/EtOAc (5:1, v:v) as eluent). Yellow solid; mp: 111–112 °C; 62.0 mg; 75% yield (6a and 3b were used); 66.1 mg; 80% yield (6a and 3b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 8.71 (s, 1H), 7.34 (s, 3H), 7.25 (d, J = 8.7 Hz, 1H), 6.94 (d, J = 2.8 Hz, 1H), 6.90 (dd, J1 = 2.8 Hz, J2 = 8.8 Hz, 1H), 3.79 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 159.3, 159.0, 142.2, 138.6, 137.0, 134.8, 133.0, 131.4, 129.3, 128.9, 128.6, 117.1, 115.8, 55.8. HRMS (ESI): m/z calcd for C14H11ClNOS [M + H]+: 276.0244, found: 276.0235.
The 11-methyldibenzo[b,f][1,4]thiazepine 1e (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent). Yellow solid; mp:75–76 °C (Lit: m.p. 76 °C) [31]; 50.0 mg; 75% yield (1d and 1b were used); 64.2 mg; 95% yield (1d and 1b′ were used); 1H NMR (500 MHz, CDCl3/TMS): δ 7.46–7.45 (m, 1H), 7.42–7.40 (m, 2H), 7.34–7.30 (m, 2H), 7.28–7.24 (m, 1H), 7.18 (dd, J1 = 1.3 Hz, J2 = 8.0 Hz, 1H), 7.05 (td, J1 = 1.3 Hz, J2 = 7.5 Hz, 1H), 2.66 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 169.9, 148.8, 140.0, 139.5, 132.5, 132.0, 130.8, 129.2, 128.9, 128.5, 128.0, 125.6, 125.4, 29.6.
The 7,11-dimethyldibenzo[b,f][1,4]thiazepine 2e (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent) Yellow solid; mp: 115–117 °C; 53.1 mg; 74% yield (1d and 2b were used); 1H NMR (500 MHz, CDCl3/TMS): δ 7.46–7.43 (m, 1H), 7.42–7.38 (m, 1H), 7.33–7.29 (m, 2H), 7.23 (s, 1H), 7.09–7.05 (m, 2H), 2.65 (s, 3H), 2.27 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 169.3, 146.4, 139.9, 139.5, 135.6, 132.8, 131.9, 130.7, 130.1, 128.40, 128.39, 128.0, 125.3, 29.6, 20.7.
The 7-methoxy-11-methyldibenzo[b,f][1,4]thiazepine 3e (Flash column chromatography on silica gel using petrol/EtOAc (5:1, v:v) as eluent). Yellow solid; mp: 130–132 °C; 52.9 mg; 69% yield (1d and 3b were used); 1H NMR (500 MHz, CDCl3/TMS): δ 7.46–7.43 (m, 1H), 7.42–7.39 (m, 1H), 7.34–7.30 (m, 2H), 7.12 (d, J = 8.9Hz, 1H), 6.96 (d, J = 2.9 Hz, 1H), 6.83 (dd, J1 = 2.8 Hz, J2 = 8.8 Hz, 1H), 3.76 (s, 3H), 2.64 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 168.5, 157.6, 142.5, 139.6, 139.4, 132.0, 130.7, 129.2, 128.5, 128.0, 126.6, 116.6, 115.7, 55.7, 29.5. HRMS (ESI): m/z calcd for C15H14NOS [M + H]+: 256.0791, found: 256.0794.
The 7-chloro-11-methyldibenzo[b,f][1,4]thiazepine 4e (Flash column chromatography on silica gel using petrol/EtOAc (6:1, v:v) as eluent). Yellow solid; mp: 115–117 °C; 47.5 mg; 61% yield (1d and 4b were used); 1H NMR (500 MHz, CDCl3/TMS): δ 7.47–7.44 (m, 1H), 7.43–7.39 (m, 2H), 7.36–7.33 (m, 2H), 7.21 (dd, J1 = 2.3 Hz, J2 = 8.6 Hz, 1H), 7.10 (d, J = 8.5 Hz, 1H), 2.65 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 170.4, 147.4, 139.3, 139.2, 132.2, 131.9, 131.0, 130.8, 130.1, 129.3, 128.8, 128.0, 126.4, 29.6. HRMS (ESI): m/z calcd for C14H11ClNS [M + H]+: 260.0295, found: 260.0275.
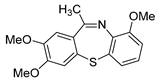
The 2,3,9-trimethoxy-11-methyldibenzo[b,f][1,4]thiazepine 5e (Flash column chromatography on silica gel using petrol/EtOAc (3:1, v:v) as eluent). Yellow solid; mp: 52–54 °C; 72.9 mg; 77% yield (2d and 5b were used); 1H NMR (500 MHz, CDCl3/TMS): δ 7.10 (d, J = 8.8 Hz, 1H), 6.95–6.90 (m, 2H), 6.86 (s, 1H), 6.83 (dd, J1 = 2.8 Hz, J2 = 8.8 Hz, 1H), 3.87 (d, J = 10.5 Hz, 6H), 3.76 (s, 3H), 2.62 (s, 3H). 13C NMR (125 MHz, CDCl3/TMS): δ 167.9, 157.5, 150.9, 149.3, 142.5, 132.0, 130.9, 129.7, 126.5, 116.4, 115.6, 114.3, 110.7, 56.3, 56.2, 55.7, 29.3. HRMS (ESI): m/z calcd for C17H18NO3S [M + H]+: 316.1002, found: 316.1012.
4. Conclusions
In summary, this paper reported a ‘one-pot’ CuCl2 catalyzed synthesis of dibenzo[b,f][1,4]thiazepines and 11-methyldibenzo[b,f][1,4]thiazepines via the condensation/C–S bond coupling reactions of 2-iodobenzaldehydes/2-iodoacetophenones with 2-aminobenzenethiols/2,2′-disulfanediyldianilines in moderate-to-good yields. The reaction is easy to operate, uses readily available and bi-functional-reagent DMEDA working as ligand and reductant, and exhibits functional group tolerance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217392/s1, Figure S1: 1H NMR (CDCl3) spectrum of Compound (1c); Figure S2: 13C NMR (CDCl3) spectrum of Compound (1c); Figure S3: 1H NMR (CDCl3) spectrum of Compound (2c); Figure S4: 13C NMR (CDCl3) spectrum of Compound (2c); Figure S5: 1H NMR (CDCl3) spectrum of Compound (3c); Figure S6: 13C NMR (CDCl3) spectrum of Compound (3c); Figure S7: 1H NMR (CDCl3) spectrum of Compound (4c); Figure S8: 13C NMR (CDCl3) spectrum of Compound (4c); Figure S9: 1H NMR (CDCl3) spectrum of Compound (5c); Figure S10: 13C NMR (CDCl3) spectrum of Compound (5c); Figure S11: 1H NMR (CDCl3) spectrum of Compound (6c); Figure S12: 13C NMR (CDCl3) spectrum of Compound (6c); Figure S13: 1H NMR (CDCl3) spectrum of Compound (7c); Figure S14: 13C NMR (CDCl3) spectrum of Compound (7c); Figure S15: 1H NMR (CDCl3) spectrum of Compound (8c); Figure S16: 13C NMR (CDCl3) spectrum of Compound (8c); Figure S17: 1H NMR (CDCl3) spectrum of Compound (9c); Figure S18: 13C NMR (CDCl3) spectrum of Compound (9c); Figure S19: 1H NMR (CDCl3) spectrum of Compound (10c); Figure S20: 13C NMR (CDCl3) spectrum of Compound (10c); Figure S21: 1H NMR (CDCl3) spectrum of Compound (11c); Figure S22: 13C NMR (CDCl3) spectrum of Compound (11c); Figure S23: 1H NMR (CDCl3) spectrum of Compound (12c); Figure S24: 13C NMR (CDCl3) spectrum of Compound (12c); Figure S25: 1H NMR (CDCl3) spectrum of Compound (1e); Figure S26: 13C NMR (CDCl3) spectrum of Compound (1e); Figure S27: 1H NMR (CDCl3) spectrum of Compound (2e); Figure S28: 13C NMR (CDCl3) spectrum of Compound (2e); Figure S29: 1H NMR (CDCl3) spectrum of Compound (3e); Figure S30: 13C NMR (CDCl3) spectrum of Compound (3e); Figure S31: 1H NMR (CDCl3) spectrum of Compound (4e); Figure S32: 13C NMR (CDCl3) spectrum of Compound (4e); Figure S33: 1H NMR (CDCl3) spectrum of Compound (5e); Figure S34: 13C NMR (CDCl3) spectrum of Compound (5e).
Author Contributions
Synthesis and investigation, D.W., Q.L., Z.L., C.F. and R.L.; writing—original draft, G.S.; conceptualization and writing, G.S.; Project administration, B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to by the Shandong Provincial Natural Science Foundation (ZR2019QB022) and Liaocheng University fund (318051403) for financial support of this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Saha, D.; Jain, G.; Sharma, A. Benzothiazepines: Chemistry of a privileged scaffold. RSC Adv. 2015, 5, 70619–70639. [Google Scholar] [CrossRef]
- Geyer, H.M., 3rd; Watzman, N.; Buckley, J.P. Effects of a tranquilizer and two antidepressants on learned and unlearned behaviors. J. Pharm. Sci. 1970, 59, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, S.; Kinoshita, M.; Ishikawa, H.; Kagoshima, T.; Katori, R.; Ishikawa, K.; Hirota, Y. Efficacy and safety of clentiazem in patients with essential hypertension: Results of an early pilot test. Clin. Cardiol. 1991, 14, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.C.; Salvino, J.M.; Labaudiniere, R.F.; Herpin, T.F. Novel solid-phase synthesis of 1,5-benzothiazepine-4-one derivatives. Tetrahedron Lett. 2000, 41, 3029–3033. [Google Scholar] [CrossRef]
- Geller, V.; Gorzaltsan, I.; Shleifer, T.; Belmaker, R.H.; Bersudsky, Y. Clotiapine compared with chlorpromazine in chronic schizophrenia. Schizophr. Res. 2005, 80, 343–347. [Google Scholar] [CrossRef]
- Agarwal, S.; HariKumar, S.L.; Negi, P.; Upadhyay, N.; Garg, R. Quetiapine Fumarate Loaded Nanostructured Lipid Carrier for Enhancing Oral Bioavailability: Design, Development and Pharmacokinetic Assessment. Curr. Drug Deliv. 2021, 18, 184–198. [Google Scholar] [CrossRef]
- Hopenwasser, J.; Mozayani, A.; Danielson, T.J.; Harbin, J.; Narula, H.S.; Posey, D.H.; Shrode, P.W.; Wilson, S.K.; Li, R.; Sanchez, L.A. Postmortem distribution of the novel antipsychotic drug quetiapine. J. Anal. Toxicol. 2004, 28, 264–268. [Google Scholar] [CrossRef]
- Nyavanandi, D.; Kallakunta, V.R.; Sarabu, S.; Butreddy, A.; Narala, S.; Bandari, S.; Repka, M.A. Impact of hydrophilic binders on stability of lipid-based sustained release matrices of quetiapine fumarate by the continuous twin screw melt granulation technique. Adv. Powder Technol. 2021, 32, 2591–2604. [Google Scholar] [CrossRef]
- Guo, R.N.; Gao, K.; Ye, Z.S.; Shi, L.; Li, Y.Q.; Zhou, Y.G. Iridium-catalyzed asymmetric hydrogenation of dibenzo[b,f][1,4]thiazepines. Pure. Appl. Chem. 2013, 85, 843–849. [Google Scholar] [CrossRef]
- Lin, Y.C.; Li, N.C.; Cherng, Y.J. Microwave-Assisted Synthesis of Substituted Dibenzo[b,f][1,4]thiazepines, Dibenzo[b,f][1,4]oxazepines, Benzothiazoles, and Benzimidazoles. J. Heterocyclic. Chem. 2014, 51, 808–814. [Google Scholar] [CrossRef]
- Saha, D.; Wadhwa, P.; Sharma, A. A sequential synthetic strategy towards unexplored dibenzo[b,f][1,4]thiazepine carboxamides: Copper catalysed C-S cyclisation followed by Ugi type 3CC cascade. RSC Adv. 2015, 5, 33067–33076. [Google Scholar] [CrossRef]
- De Munck, L.; Sukowski, V.; Vila, C.; Munoz, M.C.; Pedro, J.R. Catalytic enantioselective aza-Reformatsky reaction with seven-membered cyclic imines dibenzo[b,f][1,4]oxazepines. Org. Chem. Front. 2017, 4, 1624–1628. [Google Scholar] [CrossRef]
- Saha, D.; Kaur, T.; Sharma, A. Facile Construction of Imidazo-benzothia-/oxazepines by a Quick and Efficient van Leusen Protocol. Asian J. Org. Chem. 2017, 6, 527–533. [Google Scholar] [CrossRef]
- Shimotori, Y.; Hoshi, M.; Murata, M.; Ogawa, N.; Miyakoshi, T.; Kanamoto, T. Synthesis of dibenzothiazepine analogues by one-pot S-arylation and intramolecular cyclization of diaryl sulfides and evaluation of antibacterial properties. Heterocycl. Commun. 2018, 24, 219–230. [Google Scholar] [CrossRef]
- Balakrishna, B.; Bauza, A.; Frontera, A.; Vidal-Ferran, A. Asymmetric Hydrogenation of Seven-Membered C=N-containing Heterocycles and Rationalization of the Enantioselectivity. Chem. Eur. J. 2016, 22, 10607–10613. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Hao, W.; Yoshimura, T. New method for the preparation of dibenzo[b,f][1,4]thiazepines. Heteroatom. Chem. 2004, 15, 246–250. [Google Scholar] [CrossRef]
- Creed, T.; Leardini, R.; McNab, H.; Nanni, D.; Nicolson, I.S.; Reed, D. Gas-phase cyclisation reactions of 1-(2-arylthiophenyl)alkaniminyl and 2-(aryliminomethyl)thiophenoxyl radicals. J. Chem. Soc. Perkin Trans. 1 2001, 9, 1079–1085. [Google Scholar] [CrossRef]
- Evano, G.; Blanchard, N.; Toumi, M. Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem. Rev. 2008, 108, 3054–3131. [Google Scholar] [CrossRef]
- Okamoto, N.; Sakurai, K.; Ishikura, M.; Takeda, K.; Yanada, R. One-pot concise syntheses of benzimidazo[2,1-a]isoquinolines by a microwave-accelerated tandem process. Tetrahedron Lett. 2009, 50, 4167–4169. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, J. Annulation of Diaryl(aryl)phosphenes and Cyclic Imines to Access Benzo-δ-phospholactams. Org. Lett. 2020, 22, 7780–7785. [Google Scholar] [CrossRef]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions: Copper makes a difference. Angew. Chem. Int. Ed. 2008, 47, 3096–3099. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kim, J.Y.; Kwak, J.; Chang, S. Recent advances in the transition metal-catalyzed twofold oxidative C-H bond activation strategy for C-C and C-N bond formation. Chem. Soc. Rev. 2011, 40, 5068–5083. [Google Scholar] [CrossRef] [PubMed]
- Surry, D.S.; Buchwald, S.L. Diamine ligands in copper-catalyzed reactions. Chem. Sci. 2010, 1, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-Type Coupling Reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef]
- Ma, D.W.; Cai, Q.A. Copper/Amino Acid Catalyzed Cross-Couplings of Aryl and Vinyl Halides with Nucleophiles. Accounts. Chem. Res. 2008, 41, 1450–1460. [Google Scholar] [CrossRef]
- Dai, W.P.; Shi, H.Y.; Zhao, X.H.; Cao, S. Sterically Controlled Cu-Catalyzed or Transition-Metal-Free Cross-Coupling of gem-Difluoroalkenes with Tertiary, Secondary, and Primary Alkyl Grignard Reagents. Org. Lett. 2016, 18, 4284–4287. [Google Scholar] [CrossRef]
- Ke, J.; Tang, Y.L.; Yi, H.; Li, Y.L.; Cheng, Y.D.; Liu, C.; Lei, A.W. Copper-Catalyzed Radical/Radical C-sp3-H/P-H Cross-Coupling: Alpha-Phosphorylation of Aryl Ketone O-Acetyloximes. Angew. Chem. Int. Ed. 2015, 54, 6604–6607. [Google Scholar] [CrossRef]
- Shen, G.D.; Yang, B.C.; Huang, X.Q.; Hou, Y.X.; Gao, H.; Cui, J.C.; Cui, C.S.; Zhang, T.X. Copper- and Palladium-Catalyzed Cross-Coupling Reactions for the Synthesis of N-Fused Benzo[4,5]imidazo[2,1-b]thiazole Derivatives via Substituted trans-1,2-Diiodoalkenes, 1H-Benzo[d]imidazole-2-thiols, and Halobenzenes. J. Org. Chem. 2017, 82, 3798–3805. [Google Scholar] [CrossRef]
- Shen, G.D.; Lu, Q.C.; Wang, Z.Y.; Sun, W.W.; Zhang, Y.L.; Huang, X.Q.; Sun, M.M.; Wang, Z.M. Environmentally Friendly and Recyclable CuCl2-Mediated C-S Bond Coupling Strategy Using DMEDA as Ligand, Base, and Solvent. Synthesis 2022, 54, 184–198. [Google Scholar] [CrossRef]
- Narasimhan, N.S.; Chandrachood, P.S. Synthetic Application of Lithiation Reactions; X. Synthesis of Dibenzo[b,f][1,4]oxazepine, Dibenzo(b,f][1,4]thiazepine, and 5H-Dibenzo[b,e][1,4]-diazepine. Synthesis 1979, 8, 589–590. [Google Scholar] [CrossRef]
- Jílek, J.O.; Pelz, K.; Pavlíčková, D.; Protiva, M. Neurotrope und psychotrope Substanzen IV. Über einige neue Derivate des Dibenzo(b,f)(1,4)thiazepins. Collect. Czechoslov. Chem. Commun. 1965, 30, 1676–1683. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).