Abstract
Novel 1,2,3-triazolo-linked-1,5-benzodiazepinones were designed and synthesized via a Cu(I)-catalyzed 1,3-dipolar alkyne-azide coupling reaction (CuAAC). The chemical structures of these compounds were confirmed by 1H NMR, 13C NMR, HMBC, HRMS, and elemental analysis. The compounds were screened for their in vitro antibacterial and antifungal activities. Several compounds exhibited good to moderate activities compared to those of established standard drugs. Furthermore, the binding interactions of these active analogs were confirmed through molecular docking.
1. Introduction
The development of new therapeutic agents is one of the major goals in medicinal chemistry research [1]. Generally, evidence that agents are modulating more than one target may develop a wider field of therapeutic applications compared to single-target drugs [2,3]. Hence, the actual increase in interest in agent discovery is already addressing multiple biological targets for many therapeutic treatments [4,5].
One of the privileged structures that have been recently updated by Patchett et al. [6,7] is the 1,5-benzodiazepine (BZD) derivatives that have been repeatedly reported to display tranquilizing, muscular relaxant, anticonvulsant, hypnotic, and sedative effects [8,9,10]. Actually, the use of this class of scaffolds is not only limited to anxiety and stress conditions but also seemingly minor changes in their structures that can produce a host of different biological activities [11]. Accordingly, polycyclic BZD derivatives A, B, and C have proven their bioactivity against peptides hormone (A), interleukin converting enzymes (B), and potassium blockers (C) [12,13,14] (Figure 1).
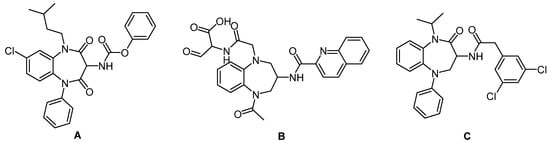
Figure 1.
Polycyclic BZD derivatives A, B, and C.
Moreover, in previous work, our research group has reported the production and subsequent determination of photoluminescence properties of an understudied family of 1,5-benzodiazepin-2-one derivatives. Furthermore, the recent work published by Chiraz Ismail et al. reports on the synthesis of some fluorescent N-triazolo-1,5-benzodiazepine-2-ones [15,16].
Because the N-functionalization of benzodiazepines is highly desired for the development of novel powerful molecular targets [17], it appears that the N-1,2,3-triazolo-1,5-benzodiazepine scaffold has great importance due to the remarkable biological relevance of such combination [18,19]. Triazoles belong to an important class of heterocycles. They display an ample spectrum of biological activities and are widely employed as pharmaceuticals and agrochemicals [20,21,22]. More particularly, the 1,2,3-triazole derivatives that exhibit favorable physicochemical properties interact with different biological targets through hydrogen bonding and dipole interactions, improving both the potency and specificity of the resulting analogs [23,24].
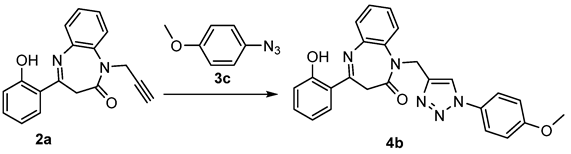
Thus, and as a continuation of our ongoing research to synthesize novel 1,5-benzodiazepines derivatives bearing a triazole moiety [25], we turn our attention to designing novel hybrid conjugates of 1,2,3-triazoles tethered to 1,5-benzodiazepines namely the N-triazolo-1,5-benzodiazepin-2-ones. On the other hand, the click chemistry methodology is one of the most used strategies for simple access to these compounds, particularly the Cu(I)-catalyzed 1,3-dipolar alkyne-azide coupling reaction (CuAAC) [26]. In addition, derivatives 4a–i and 6a–c were evaluated for their antibacterial and antifungal potentials, and further molecular docking of synthesized compound 6 was also performed.
2. Results and Discussion
Our synthetic strategy for building the N-triazolo-1,5-benzodiazepine scaffolds is based on the CuAAC reaction and involves the preparation of N-alklynic benzodiazepine 2a–c reacted with aromatic azides 3a–d. Thus, the key intermediate BZD 2 was primarily prepared following the method of E. Latteman et al. [27,28]. We treated compound 1a–c with propargyl bromide in the presence of sodium hydride as a base in N,N-dimethylformamide at 0 °C. Eventually, DMF was found to be especially effective in this reaction for weakening the bromine-carbon bond [29]. Under these experimental conditions, the reaction monitored by TLC showed the formation of a single product that was identified, on the basis of its spectral data, as the N-prop-2-yn-1,5-benzodiazpin-2-one 2a–c. Note here that compound 2a has already been prepared by our research team [13]. In addition, H. Ahabchane and co-workers have prepared BZD derivatives but are limited in substitution patterns [30].
The 1H NMR spectrum of compound 2a–c recorded at 300 MHz in CDCl3 exhibited characteristic signals from which chemical shifts and multiplicities we were able to assign the propargyl group. Thus, for compound 2b, taken as an example, the spectrum showed a doublet at 4.25 ppm (J = 2.4 Hz) corresponding to the methylene group at C-1″ coupled with the acetylenic proton H-3″ which appears as a triplet at 2.30 ppm (J = 4.80 Hz).
Taking notes that the propargylation of the benzodiazepine could obviously occur either on the hydroxyl or on the amide function [31], the presence of the deshielded phenolic hydrogen singlet observed at~13.95 ppm excluded from the beginning the obtention of the O-prop-2-yn-1,5-benzodiazepin-2-one (Scheme 1). Particularly in 2b, the non-equivalence of the methylenic protons H-3 (a pair of two doublets at 3.00 ppm (J = 12.6 Hz) and 4.70 ppm(J = 16.8 Hz)) is undoubtedly consistent with partial non-planarity of the heptatomic ring. Similarly, this result is also cited in our previously described N-isopropylated-1,5-benzodiazepine-2-one [32,33].
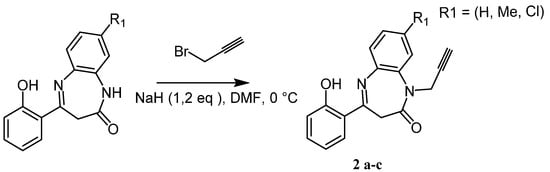
Scheme 1.
Synthesis of alkynes 2a–c.
The reluctance of the hydroxyl group to react was rationalized in terms of a steric hindrance due to a strong intermolecular hydrogen bonding between the hydroxyl group (13.95 ppm) and the nitrogen of the imine C=N functionality at the 5-position of the diazepine ring [34].
The analysis of the 13C NMR spectra recorded at 75.47 MHz came comforting the obtention of the N-propargyl-1,5-benzodiazepinone 2b that showed a peak at 37.1 ppm (C-1″), 72.0 ppm (C-3″) as well as 78.1 ppm (C-2″) of the propargyl group.
The corresponding azides 3a–c were prepared according to the reported method via a devastation reaction of p-substituted aniline using NaNO2 and a diluted solution of HCl in ethanol at 0 °C followed by treatment with NaN3 [35].
The coupling of azides 3a–c and the N-propargyl-1,5-benzodiazepin-2-ones 2a–c was carried out in DCM at room temperature using CuI as catalyst and triethylamine as an additive base. Very interesting pentacyclic compounds 4a–I were then isolated in suitable yields (Scheme 2). The reaction parameters were optimized using the N-propargyl-1,5-benzodiazepines 2a, the azides 3c, and the CuI as catalysts. The reaction did occur whatever the solvent used. Replacing acetonitrile with toluene increased the yields owing to a better solubility of the starting materials (entries 2 and 4). The reaction resulted in comparable yields when performed at room temperature or under gentle heating. On the other hand, an increase in the amount of the catalyst (from 5 to 10 mol%) did not modify the yield (entries 6 and 7).
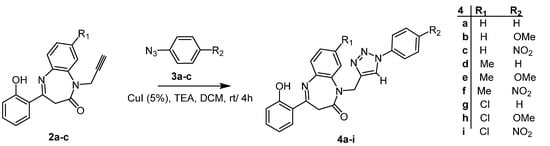
Scheme 2.
Copper-catalyzed click reactions of azide 3a–c with N-propargylbenzodiazepine 2a–c.
However, the excess of CuI probably caused a decrease in the performance due to the deposition of copper species on the dipole and the low solubility of cuprous iodide in triethylamine (entries 8). DCM proved to be by far the most suitable solvent at room temperature (entries 6) (Table 1)

Table 1.
Optimization of Cu(I)-catalyzed 1,3-dipolar cyclization for the synthesis of 1,2,3-triazoles 4a–i a.
Thus, one can state that the use of 1 m mole N-propargyl-1,5-benzodiazepine 2a–c, aromatic azide 3a–c (2 eq) at rt for 4 h in DCM as a solvent with CuI (5 mol%) as catalyst and triethylamine (2.5 eq) as an additive [36] are the best experimental conditions to generate the series of new N-triazolyl-1,5-benzodiazepin-2-one 4a–i in suitable yield (Scheme 2 (Table 2)).

Table 2.
One-pot synthesis of 1,4-disubstituted 1,2,3-triazole 4a–i.
In particular we have observed that the solubility of the 1,2,3-triazole-BZD conjugates 4a–i is enhanced in most of the organic solvents. This may be attributed to the new functionalities present in these novel conjugates.
Unambiguous proofs for the obtained products 4a–i were obtained from their 1H/13C NMR and 2D NMR spectra, which were consolidated by HRMS and elemental analysis (see Supplementary Materials).
As mentioned in the introduction, the effects of such benzodiazepines on the nervous system are abundantly described in the literature [37]. Moreover, interesting biological activities are observed with some analog derivatives, but their very low hydrosolubility can restrict their applications. Generally, when glycopyranosyl is attached to the nitrogen of the heptatomic ring systems, it can increase the water solubility and confer amphiphilic properties.
Obviously, there is no single function for oligosaccharides. Perhaps their most important function is to serve as recognition markers. Additionally, oligosaccharides have the ability to alter the intrinsic properties of the molecules to which they are attached [38].
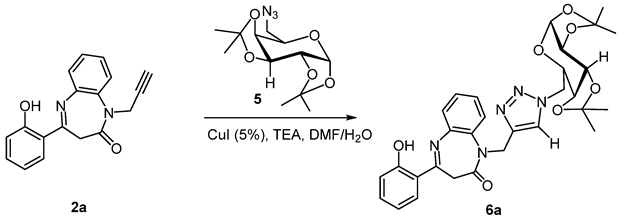
Accordingly and encouraged by the above interesting result, we have extended this method to the synthesis of novel N-galactopyranosyl-N-triazolo-1,5-benzodiazepines. Therefore, we screened an azido galactpyranosyl [39], a choice that was not fortuitous insofar as our research team has used it for the synthesis of some optically active pyrazolines [40,41,42,43].
As exemplified in (Scheme 3), the reaction proceeded smoothly to completion, and the corresponding N-galactopyranosyl-N-triazolo-1,5-benzodiazepinones products 6a–c were obtained after 8 h with excellent yields and with high purity (Table 3).
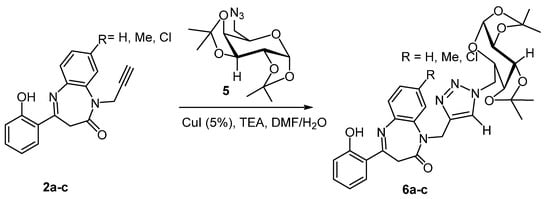
Scheme 3.
Copper-catalyzed reactions of galactopyranoseazide 5 with N-propargylbenzodiazepine 2a–c.

Table 3.
One-pot synthesis of 1,4-disubstituted 1,2,3-triazoles 6a–c.
To find the optimal experimental conditions for the reaction, the cycloaddition reaction was firstly carried out in different solvents: DCM, acetonitrile, toluene, and a mixture of DMF/H2O at room temperature and under reflux. Finally, it was found that DMF/H2O(8:2) was the most suitable solvent (Table 4).

Table 4.
Optimization of Cu(I)-catalyzed 1,3-dipolar cyclization for the synthesis of 1,2,3-triazoles 6a a.
The use of both dimensional and bidimensional NMR spectroscopy techniques allowed one to deduce unambiguously the exclusive formation of the regioisomeric species, namely the 1,4-triazoles (as exemplified for 6b).
Four singlets integrating three hydrogens each and corresponding to the methyl of the galactopyranose part appeared at 1.34, 1.36, 1.47, and 1.50. A peak integrating one proton was also observed at 7.69 ppm and assigned as the characteristic H-5″triazolic hydrogen.
A minor influence of the triazole group was observed on the proton H-1″ in front of the triazole ring at C-1, which has a chemical shift of 4.25 ppm in the precursor 2b and a two doublet at 4.75 ppm and 5.09 ppm in 6b. Furthermore, a modest downfield shift was observed for the galactose H-6′′′ signal due to the influence of the triazole ring at C-4, changing from 3.55 ppm in azide5 to approximately 4.52 ppm in the triazole products for 6 series.
The resulting 1,4-regioisomers were evidenced by the presence in the NOESY spectrum of an NOE between the triazolic proton H-5″ and the H-5′′′ proton of the galactopyranosyl moiety, in addition to another NOE between the proton H-5′′′ and H-6′′′. Such regiospecificity agrees with that cited in the literature [44].
To our knowledge, the obtention of these N-galactopyranosyl-N-triazolo-1,5-benzodiazepinones conjugates 6a–c is very demanded given the interesting pharmacological properties of some analogs so far reported by I. Carvalho et al. As a matter of fact, they proved to be moderate to weak TcTS (Trypanosoma cruzi and its cell surface trans-sialidase) inhibitors in vitro [45].
Most prepared 1,5-benzodiazepin-2-ones were evaluated for antibacterial and antifungal activity in order to survey the possible biological activities of this class of compounds [46,47].
2.1. Biological Activity
2.1.1. Antibacterial Activity
Were tested in vitro for antibacterial activity against an array of eight bacteria using streptomycin as a control, with the findings expressed as MIC in g/mL. (Table 5). The obtained data revealed that all the tested compounds 4a–i showed suitable inhibition against all strains. Particularly compounds 4d (R1 = Me, R2 = H) and 4e (R1 = Me, R2 = OMe) in series 1 might be the major active compounds, and they all showed a similar activity potential, especially against S. epidermidis (MIC = 32 µg/mL) showing values better than the reference antibiotic. Most of the tested compounds displayed poor activity against E. coli and S. typhimurum. Further, toward B. cereus, derivatives 4e seems to contribute better (MIC = 32 μg/mL) than the other analogs followed by 4d (MIC = 64 μg/mL) whereas, against S. aureus, compound 4f (R1= Me, R2= NO2) displayed the highest activity (MIC= 32 μg/mL). Toward M. luteus, derivative 4e was found to be the most active compound, followed by 4d and 4g. Moreover, compounds 4d then 4e due to hydrogen atom and methoxy group in the phenyl para-position, respectively, showed the best values for the antibacterial activity compared to other analogs against E. fecalis. Furthermore, toward L. monocytogenes, also 4d and 4e displayed noticeable antibacterial activity. On the other hand, as depicted in Table 5, the obtained data demonstrate that all the tested compounds 6a–c showed better values of the antibacterial potential compared to compounds 4a–i. Finally, these findings clearly showed the importance of the added fragments to the 1,5-benzodiazepine 1 via the methylene linker to confer activity, essentially the nature of the aromatic system and the galactopyranosyl attached to the triazole ring in the activity.

Table 5.
Antibacterial activities of 4a–i and 6a–b: minimum inhibitory concentration (MIC).
2.1.2. Antifungal Activity
The target compounds 4a–i and 6a–c were assayed for inhibitory activity against clinically important pathogenic fungi such as the Candida albicans and the Aspergillus flavus. Ketoconazole was used as the reference drug (Table 6). All the titled compounds showed good to moderate inhibition against the tested fungal pathogens. Particularly, compound 4d (R1 = Me, R2 = H) revealed excellent activity against both the Candida albicans and the Aspergillus flavus.

Table 6.
Antifungal activities of 2a–c, 4a–i, and 6a–b: minimum inhibitory concentration (MIC).
Furthermore, the tested compounds 6a (R = H) and 6b (R = OMe) showed suitable activity against Aspergillus flavus with MIC = 64 µg/mL, which was significantly more potent than Ketoconazole. Toward Candida albicans, 6b (MIC = 32 µg/mL) seems to be the most active, followed by its analogs 6a and 6c (R = Cl). These obtained results suggest that the galactopyranosyl part on the C-4 triazole ring of compound 6b is favorable for enhancing antifungal activity.
2.2. Molecular Docking Studies
A molecular docking study of the newly synthesized compound of series 1 (4a–i) and series 2 (6a–c)was conducted to gain insights into its probable mechanism of action. Indeed, the crystallized structure of Staphylococcus epidermidis TcaR in complex with streptomycin (PDB code: 4EJW) was taken as the target receptor, and the binding pocket was validated by performing redocking of the ligand (Streptomycin). The binding pocket and the interaction of the ligand in complex with the target receptor are shown in Figure 2. Molecular docking calculations of all the test compounds were carried out with Auto Dock vina software. The docked ligand with the lowest binding free energy was used for analysis in Table 7.
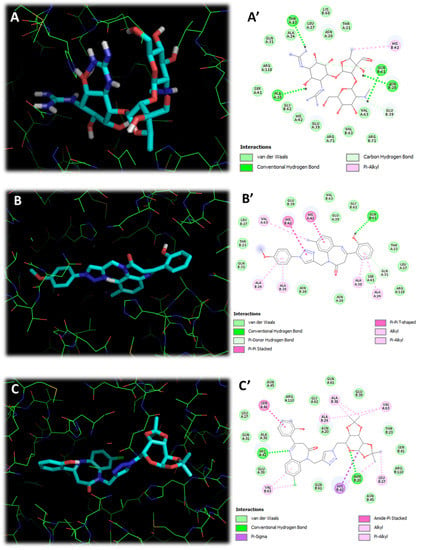
Figure 2.
(A) is the 3D docking picture of reference ligand «Streptomycin» (the cyan one), (A’) is the 2D docking picture of reference ligand «Streptomycin», (B) is the 3D docking picture of the most active compound in series 1 (the cyan one), (B’) is the 2D docking picture of the most active compound in series 1, (C) is the 3D docking picture of the most active compound in series 2 (the cyan one), (C’) is the 2D docking picture of the most active compound in series 2.

Table 7.
Docking binding energies (kcal mol−1) of promising antibacterial agents.
As can be seen from the results, the molecular docking for the representative compounds: the most active derivative in series 1 is BZD 4e, the most active derivative in series 2 is compound 6c, and the redocked «streptomycin» showed that the ligands were well oriented toward the active site gorge. Thus, 4e formed a conventional hydrogen bond with GLN-B-61 through its hydroxyl group besides a Pi-Donor hydrogen bond with GLN-A-31. In addition, the ligand 4e was oriented to a hydrophobic pocket composed of ALA-A-24 and ALA-A-38 with Pi-Alkyl interactions. The methylbenzodiazepine ring contributed to shaping interaction with HIS-A-42 and Alkyl interaction with VAL-A-63. The methoxytriazole moiety formed Pi-Alkyl interactions with ALA-B-24 and ALA-B-38 besides a stacking interaction with HIS-B-42 (Figure 2B,B′).
On the other hand, ligand 6c set up H-bonds with ASN-B-20 and HIS-A-42 through its N-galactopyranosyl and BZD pharmacophores, respectively. This finding demonstrates the crucial role of the N-galactopyranosyl in series 2 (compounds 6a–c) linked to the triazole ring, which took the place of the aryl group in series 1(compounds 4a–i). Furthermore, 6c formed some interesting Alkyl interactions with residues: VAL-A-63, ALA-B-24, LEU-B-27, ALA-B-38, and HIS-B-42 via its N-galactopyranosyl fragment, which displayed a Pi-Sigma interaction with HIS-B-42. In addition, derivative 6c showed Amide-Pi stacked with SER-A-41 and hydrophobic Pi-Alkyl and Alkyl interactions with VAL-B-63. (Figure 2C,C′).
From these results, it can be inferred that docked compound, especially derivative 6c, probably showed its antibacterial activity in a similar way as that of the Streptomycin antibiotic (Figure 2A,A′) by interfering with the functioning of epidermidis TcaR in complex with streptomycin receptor.
3. Materials and Methods
3.1. Instruments and Methods
Toluene and methylene chloride (DCM) were obtained from MBRAUN′s MB SPS-800 apparatus and dried according to conventional protocols. Unless otherwise specified, cyclohexane, ethyl acetate (EtOAc), acetonitrile (CH3CN), and diethyl ether (OEt2) were acquired in ACS-grade quality and utilized without additional purification. Unless otherwise noted, commercially available reagents were utilized without further purification.
1H and 13C NMR spectra were recorded with an AC-300 Bruker spectrometer with tetramethylsilane as an internal reference. Chemical shifts are reported in parts per million. Two-dimensional NMR experiments were performed with an Avance-300 Bruker spectrometer. Multiplicities are described as s (singlet), d (doublet), dd, dd, etc. (doublet of doublets), t (triplet), and m (multiplet). High-resolution mass spectra of compounds 4b, 4e, and 4g were performed within a Hewlett-Packard 5890/5970 GC mass spectrometer. Elemental analysis was recorded on a PERKIN–ELMER 240B microanalyzer.
All the reactions were followed by TLC using aluminum sheets of Merck silica gel 60 F254, 0.2 mm. The spots were visualized through illumination with a UV lamp (λ = 254 nm) and/or staining with KMnO4. Column chromatography purifications were performed on silica gel (40–63 μm) carried out on Merck DC Kiesel gel 60 F-254 aluminum sheets. The starting material 1a–c was prepared according to the literature [8]. Melting points of benzodiazepines 2a–c, 4a–l, and 6a–c were determined on a Buchi 510 capillary melting point apparatus.
3.2. Synthesis of N-Propargyl-1,5-benzodiazepinones (2a–c)
NaH (60% in mineral oil, 0.88 g, 2.4 mmol, 1.2 equiv.) was added to a solution of 4-(2′-hydroxypheny1)-1,5-benzodiazepin-2-one 1a–c (2 mmol) in DMF (15 mL) at 0 °C under nitrogen. Before the mixture was stirred for 10 to 15 min and propargyl bromide, 1.2 equiv was added. The reaction mixture was maintained at room temperature for 6 h. The reaction mixture was kept at room temperature. The raw ingredient was poured into distilled water, and dichloromethane was used to extract it. The organic layers were mixed together and dried over anhydrous MgSO4, then filtered and concentrated under reduced pressure. The crude substance was purified using silica gel column chromatography (80:20 hexane/EtOAc).
3.2.1. 1-prop-2-ynyl-4-(2-Hydroxyphenyl)-3H-1,5-benzodiazepin-2-one (2a)
This compound was preparedaccordingtotheliteraturemethod [13].
3.2.2. 1-prop-2-ynyl-4-(2-Hydroxyphenyl)-8-methyl-3H-1,5-benzodiazepin-2-one (2b)
Yield 377 mg (62%). Yellow solid, m.p. 136–138 °C. 1H NMR (300 MHz, CDCl3) δH 2.30 (t, 1H, H-3″), 3.00 (d, 1H, H-3b, J = 12.3 Hz), 4,25 (d, 2H, CH2-1″, J = 2.4 Hz), 4.70 (d, 1H, H-3a, J = 17.1 Hz), 6.93 (t, 1H, H-5′), 7.01 (d, 1H, H-3′, J = 7.5 Hz), 7.15 (dd, 1H, H-7), 7.23 (d, 1H, H-6, J = 3.9 Hz), 7.40 (t, 1H, H-4′), 7.51 (s, 1H, H-9), 7.83 (d, 1H, H-6, J = 6.6 Hz), 13.95 (s, 1H, OH). 13C NMR (75.47 MHz, CDCl3) δC 20.7 (CH3-8a), 37.0 (C-3), 38.1 (C-1″), 72.0 (C-3″), 78.4 (C-2″), 117.7 (C-3′), 118.6 (C-1′), 121.0 (C-5′), 121.7 (C-9), 126.5 (C-7), 126.7 (C-4′), 127.7 (C-6), 131.7 (C-6′), 133.4 (C-9a), 135.5 (C-5a), 137.7 (C-8), 161.6 (C-2′), 163.5 (C-2), 164.5 (C-4). Anal. Calcd for C19H16N2O2 (304.12): C, 74.98; H, 5.30; N, 9.20 found: C, 74.90; H, 5.27; N, 9.18.
3.2.3. 1-prop-2-ynyl-4-(2-Hydroxyphenyl)-8-chloro-3H-1,5-benzodiazepin-2-one (2c)
Yield 452 mg (60%). Yellow solid, m.p 176–178 °C. 1H NMR (300 MHz, CDCl3) δH2.37 (t, 1H, H-3″), 3.03 (d, 1H, H-3a, J = 12.3 Hz), 4.27 (d, 2H, CH2-1″, J = 2.4 Hz), 4.74 (d, 1H, H-3b, J = 17.1 Hz), 6.98 (t, 1H, H-5′), 7.04 (d, 1H, H-3′, J = 8.4 Hz), 7.34 (d, 1H, H-7, J = 8.7 Hz), 7.44 (t, 1H, H-4′), 7.46 (s, 1H, H-9), 7.69 (d, 1H, H-6,J = 8.7 Hz), 7.89 (d, 1H, H-6′, J = 7.8 Hz), 13.74 (s, 1H, OH).13C NMR (75.47 MHz, CDCl3) δC20.7 (C-8a), 37.0 (C-3), 38.1 (C-2″), 72.0 (C-4″), 78.4 (C-3″), 117.7 (C-3′), 118.6 (C-1′), 121.0 (C-5′), 121.7 (C-9), 126.5 (C-7), 126.7 (C-8), 127.7 (C-6), 131.7 (C-6′), 134.0 (C-4′), 135.5 (C-9a), 137.7 (C-5a), 161.6 (C-2′), 163.5 (C-2), 164.5 (C-4).Anal. Calcd for C18H13ClN2O2 (324.07): C, 66.57; H, 4.03; N, 8.63 found: C, 66.01; H, 4.12; N, 8.69.
3.3. General Procedure for the Synthesis of Compounds (4a–i)
CuI (5.0 mg, 0.025 mmol, 5 mol percent) and the corresponding phenyl azide 3a–e derivative were added to a mixture of compounds 2a–c (0.5 mmol, 1 eq) and Et3N (2.0 eq, 134 l, 1 mmol) in DCM (20 mL) (1.0 mmol, 2.0 eq).At room temperature, the reaction mixture was stirred for 4 h. The filtrate was concentrated under reduced pressure after the crude reaction was filtered using Celite®. Flash column chromatography on silica gel (Cyclohexane/EtOAcfrom 100:0 to 90:10) was usedtopurifythecrudesubstance, yielding pure 4a–oin 77–89% yields.
3.3.1. 4-(2-Hydroxyphenyl)-1-((1-phenyl)-1H-1,2,3-triazol-4-yl)methyl)-1,5-benzodiazepin-3H-2-one (4a)
Yield 178 mg (87%). Yellow solid, m.p. 201–203 °C.1H NMR (300 MHz, CDCl3) δH 3.00 (d, 1H, H-3a, J = 12.00 Hz), 4.25 (d, 1H, H-3b, J = 2.4 Hz), 4.90 (d, 1H, H-1a″, J = 14.7 Hz), 5.25 (d, 1H, H-1b″, J = 15 Hz), 6.96 (t, 1H, H-7), 7.05 (d, 1H, H-6, J = 8.4 Hz), 7.32 (d, 1H, H-9, J = 7.5 Hz), 7.41–7.45 (m, 4H, H-4′, H-5′, H-4′′′, H-8), 7.48 (t, 2H, H-3′′′, H-5′′′), 7.70 (d, 2H, H-2′′′, H-6′′′, J = 7.5 Hz), 7.86 (d, 1H, H-3′, J = 8.1 Hz), 8.11 (s, 1H, H-5″), 8.13 (d, 1H, H-6′, J = 8.1 Hz).13C NMR (75.47 MHz, CDCl3) δC 38.2 (C-3), 44.8 (C-1″), 117.9 (C-1′), 118.3 (C-6), 119.1 (C-2′′′, C-6′′′), 120.4 (C-6′), 122.5 (C-5″), 126.1; 126.7; 127.6; 134.2 (C-4′, C-5′, C-8, C-4′′′), 128.8 (C-3′), 129.3 (C-3′′′, C-5′′′), 129.7 (C-9a), 135.2 (C-5a), 136.9 (C-1′′′), 138.2 (C-4″), 162.1 (C-2′), 164.9 (C-4), 165.1 (C-2). Anal. Calcd for C24H19N5O2 (409.45): C, 70.40; H, 4.68; N, 17.10; found: C, 70.22; H, 4.37; N, 17.16.
3.3.2. 4-(2-Hydroxyphenyl)-1-((1-(4-metoxyphenyl))-1H-1,2,3-triazol-4-yl)methyl)-1,5-benzodiazepin-3H-2-one (4b)
Yield 189 mg (86%). Yellow solid, m.p. 174–176 °C.1H NMR (300 MHz, CDCl3) δH 3.02 (d, 1H, H-3a, J = 12.00 Hz), 3.88 (s, 3H, OCH3), 4.27 (d, 1H, H-3b, J = 12.00 Hz), 4.96 (d, 1H, H-1a″, J = 15.00 Hz), 5.27 (d, 1H, H-1b″, J = 15.30 Hz), 6.98–7.09 (m, 4H, H-arom), 7.34–7.49 (m, 4H, H-arom), 7.62 (d, 2H, H-3′′′, H-5′′′, J = 7.80 Hz), 7.88 (d, 1H, H-arom, J = 8.1 Hz), 8.05 (s, 1H, H-5″′), 8.16 (d, 1H, H-6′, J = 8.10 Hz), 13.95 (1s, 1H, OH). 13C NMR (75.47 MHz, CDCl3) δC21.08 (CH3-4a′′′), 38.2 (C-3), 44.8 (C-1″), 55.6 (OCH3), 114.7 (C-arom), 118.0 (C-1′), 118.3 (C-arom), 119.1 (C-arom), 122,0 (C-2′′′), 122.6 (C-5″), 123.3 (C-arom), 126.1 (C-arom), 126.9 (C-arom), 127.6 (C-arom), 129.3 (C-arom), 130.4 (C-9a), 134.1 (C-arom), 135.3 (C-5a), 138.2 (C-1′′′), 144.2 (C-4″) 159.8 (C-4′′′), 162.2 (C-2′), 164.0 (C-4), 165.0 (C-2). Anal. Calcdfor C25H21N5O2 (439.16): C, 68.33; H, 4.82; N, 15.94; found: C, 68.12; H, 4.89; N, 16.06. HRMS (ESI+): calcd. for C25H21N5NaO2[M+Na]+: 460.1749; found: 460.1763.
3.3.3. 4-(2-Hydroxyphenyl)-1-((1(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1,5-benzodiazepin-3H-2-one (4c)
Yield 165 mg (78%). Yellow solid, m.p. 225–227 °C. 1H NMR (300 MHz, CDCl3) δH 3.04 (d, 1H, H-3a, J = 12 Hz), 4.28 (d, 1H, H-3b, J = 12 Hz), 5.05 (d, 1H, H-1a″, J = 15 Hz), 5.26 (d, 1H, H-1b″, J = 15,3 Hz), 6.99 (t, 1H, H-arom), 7.08 (d,1H, H-arom, J = 8.10 Hz), 7.34–7.50 (m, 4H, H-arom), 7.88 (d, 1H, H-arom, J = 7.80 Hz), 7.95 (d, 2H, H-3′′′, H-5′′′, J = 9.00 Hz), 8.07 (d, 1H, H-6′, J = 8.10 Hz), 8.19 (s, 1H, H-5″), 8.41 (d, 2H, H-2′′′, H-6′′′, J = 9.00 Hz).13C NMR (75.47 MHz, CDCl3) δ= 38.2 (C-3), 44.6 (C-1′′), 117.9 (C-1′), 118.3 (C-arom), 119.2 (C-arom), 120.4 (C-arom), 122.3 (C-arom), 123.1 (C-5″), 125.5 (C-arom), 126.3 (C-arom), 127.0 (C-arom), 127.6 (C-arom), 129.3 (C-arom), 134.3 (C-9a), 134.9 (C-1′′′), 138.4 (C-5a), 141.0 (C-4″), 147.3 (C-4′′′) 162.2 (C2′), 164.1 (C-4), 165.2 (C-2). Anal. Calcd for C24H18N6O4 (454.14): C, 63.43; H, 3.99; N, 18.49; found: C, 63.15; H, 4.09; N, 18.23.
3.3.4. 4-(2-Hydroxyphenyl)-8-methyl-1-((1-phenyl)-1H-1,2,3-triazol-4-yl)methyl)-1,5-benzodiazepin-3H-2-one (4d)
Yield 173 mg (82%). Yellow solid, m.p. 202–204 °C. 1H NMR (300 MHz, CDCl3) δH 2.40 (s, 3H, CH3-8a), 2.99 (d, 1H, H-3a, J = 12.00 Hz), 4.23 (d, 1H, H-3b, J = 12.30 Hz), 4.91 (d, 1H, H-1a″, J = 15.30 Hz), 5.22 (d, 1H, H-1b″, J = 15.30 Hz), 6.94 (t, 1H, H-arom), 7.03 (d, 1H, H-arom, J = 8,4 Hz), 7.22 (s, 1H, H-9), 7.39 (t, 2H, H-arom), 7.48 (t, 2H, H-arom), 7.69 (d, 2H, H-arom, J = 8,4 Hz), 7.84 (dd, 1H, H-arom, J = 7,8 Hz), 7.96 (d, 1H, H-arom, J = 8.4 Hz), 8.07 (s, 1H, H-5″). 13C NMR (75.47 MHz, CDCl3) δC 20.7 (CH3-8a), 38.2 (C-3), 44.7 (C-1″), 118.2 (C-1′), 119.1 (C-arom), 118.1 (C-arom), 120.4. (C-arom), 122.4 (C-5″), 123.0 (C-arom) 126.9 (C-arom), 128.6 (C-arom), 128.7 (C-arom), 129.3 (C-arom), 129.7 (C-arom), 132.8 (C-9a), 134.0 (C-arom), 135.1 (C-5a), 136.1 (C-8), 138.0 (C-1′′′), 144.5 (C-4″), 162.2 (C-2′), 163.8 (C-4), 164.9 (C-2). Anal. Calcd for C25H21N5O2 (423.17): C, 70.91; H, 5.00; N, 16.54; found: C, 70.51; H, 5.09; N, 16.24.
3.3.5. 4-(2-Hydroxyphényl)-8-méthyl-1-((1-métoxyphényl)-1H-1,2,3-triazol-4-yl)méthyl)-1,5-benzodiazépin-3H-2-one (4e)
Yield 187 mg (83%). Yellow solid, m.p. 184–186 °C.1H NMR (300 MHz, CDCl3) δH 2.40 (s, 6H, CH3-8a), 2.98 (d, 1H, H-3a, J = 12.00 Hz), 3.85 (s, 3H, OCH3-4′′′), 4.22 (d, 1H, H-3b, J = 12.30 Hz), 4.92 (d, 1H, H-1a″, J = 15.00 Hz), 5.21 (d, 1H, H-1b″, J = 15.30 Hz), 6.94–7.05 (m, 4H, H-arom), 7.18 (m, 1H, H-arom), 7.21 (s, 1H, H-9), 7.39 (t, 1H, H-arom), 7.58 (d, 2H, H-2′′′, H-6′′′, J = 9.00 Hz), 7.84 (d, 1H, H-6′, J = 8.10 Hz), 7.97 (s, 1H, H-5″), 13.95 (1s, 1H, OH). 13C NMR (75.47 MHz, CDCl3) δC 19.7 (CH3-8a), 20.0 (CH3-4a′′′), 38.2 (C-3), 44.6 (C-1″), 117.0 (C-1′), 117.2 (C-arom), 118.0 (C-arom), 121.5 (C-arom), 122.3 (C-5″), 125.9 (C-arom), 126.9 (C-arom), 128.2 (C-arom), 129.4 (C-arom), 131.8 (C-arom), 133.0 (C-arom) 135.1 (C-9a), 137.0 (C-5a), 143.3 (C-4″), 158.8 (C-1′′′), 161.2 (C-4′′′), 162.3 (C-2′), 162.8 (C-4), 163.9 (C-2). Anal. Calcd for C26H23N5O3 (453.18): C, 68.86; H, 5.11; N, 15.44; found: C, 69.12; H, 5.29; N, 15.46; HRMS (ESI+): calcd. for C26H24N5O3 [M+H]+: 454.1879; found: 454.1879.
3.3.6. 4-(2-Hydroxyphenyl)-8-methyl-1-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1,5-benzodiazépin-3H-2-one (4f)
Yield 185 mg (79%). Yellow solid, m.p. 235–237 °C. 1H NMR (300 MHz, CDCl3) δH 2.41 (s, 6H, CH3-8a), 3.01 (d, 1H, H-3a, J = 12.00 Hz), 4.23 (d, 1H, H-3b, J = 12.00 Hz), 5.03 (d, 1H, H-1a″, J = 15.00 Hz), 5.24 (d, 1H, H-1b″, J = 15.00 Hz), 6.95 (t, 1H, H-arom), 7.00 (d, 1H, H-arom, J = 8.10 Hz), 7.16 (m, 1H, H-arom), 7.22 (s, 1H, H-9), 7.41 (t, 1H, H-arom), 7.80 (m, 2H, H-arom), 7.90 (d, 2H, H-2′′′, H-6′′′, J = 9.30 Hz), 8.12 (s, 1H, H-5″), 8.37 (d, 2H, H-3′′′, H-5′′′, JJ = 9.00 Hz).13C NMR (75.47 MHz, CDCl3) δC 19.7 (CH3-8a), 37.2 (C-3), 43.5 (C-1″), 117.3 (C-1′), 118.1 (C-arom), 119.4 (C-arom), 121.1 (C-arom), 121.8 (C-5″), 124.4 (C-arom), 125.9 (C-arom), 127.6 (C-arom), 128.2 (C-arom), 131.5 (C-arom), 133.1 (C-9a), 135.4 (C-5a), 137.2 (C-8), 140.0 (C-1′′′), 144.4 (C-4″), 146.2 (C-4′′′), 161.2 (C-2′), 162.9 (C-4), 164.0 (C-2). Anal. Calcd for C25H20N6O4 (468.15): C, 64.10; H, 4.30; N, 17.94; found: C, 63.85; H, 4.19; N, 17.64.
3.3.7. 4-(2-Hydroxyphényl)-8-chloro-1-((1-phényl)-1H-1,2,3-triazol-4-yl)méthyl)-1,5-benzodiazépin-3H-2-one (4g)
Yield 173 mg (78%). Yellow solid, m.p. 246–248 °C. 1H NMR (300 MHz, CDCl3) δH 2.99 (d, 1H, H-3a, J = 12.30 Hz), 4.29 (d, 1H, H-3b, J = 12.30 Hz), 4.85 (d, 1H, H-1a″, J = 13.50 Hz), 5.27 (d, 1H, H-1b″, J = 14.10 Hz), 6.99 (t, 1H, H-arom), 7.07 (d, 1H, H-arom, J = 8.40 Hz), 7.41–7.50 (m, 4H, H-arom), 7.53 (t, 1H, H-arom), 7.74 (d, 2H, H-2′′′, H-6′′′, J = 9.00 Hz), 7.88 (d, 1H, H-arom, J = 7.20 Hz), 8.19 (s, 1H, H-5″), 13.64 (1s, 1H, OH). 13C NMR (75.47 MHz, CDCl3) δC 38.4 (C-3), 44.7 (C-1″), 117.8 (C-1′), 118.4 (C-arom), 119.3 (C-arom), 120.5 (C-arom), 124.6 (C-arom), 126.4 (C-arom), 127.6 (C-arom), 128.9 (C-arom), 129.4 (C-arom), 129.8 (C-arom), 131.3 (C-8), 133.9. (C-4″), 134.5 (C-arom), 139.2 (C-4′′′), 162.2 (C-2′), 164.6 (C-4), 164.9 (C-2). Anal. Calcd for C24H18ClN5O2 (443.11): C, 64.94; H, 4.09; N, 15.78; found: C, 65.24; H, 3.89; N, 16.08.HRMS (ESI+): calcd. for C24H18BrClN5O2[M+Br]+: 522.1324; found: 522.1324.
3.3.8. 4-(2-Hydroxyphenyl)-8-chloro-1-((1-metoxyphényl)-1H-1,2,3-triazol-4-yl)methyl)-1,5-benzodiazepin-3H-2-one (4h)
Yield 191 mg (81%). Yellow solid, m.p. 236–238 °C.1H NMR (300 MHz, CDCl3) δH 2.99 (d, 1H, H-3a, J = 12.30 Hz), 3.89 (s, 3H, OCH3-4a′′′), 4.28 (d, 1H, H-3b, J = 12.30 Hz), 4.86 (d, 1H, H-1a″, J = 15.00 Hz), 5.25 (d, 1H, H-1b″, J = 15.00 Hz), 6.98–7.09 (t, 4H, H-arom), 7.28 (dd, 1H, H-arom, J = 8.7 Hz), 7.37 (s, 1H, H-9), 7.44 (t, 1H, H-arom), 7.63 (d, 2H, H-2′′′, H-6′′′, J = 9.00 Hz), 7.86 (d, 1H, H-arom, J = 8.10 Hz), 8.05 (s, 1H, H-5″), 8.28 (d, 1H, H-arom, J = 7,8 Hz), 13.80 (1s, 1H, OH). 13C NMR (75.47 MHz, CDCl3) δC 37.2 (C-3), 43.8 (C-1″), 54.6 (OCH3-4a′′′), 113.7 (C-arom), 116.8 (C-1′), 117.3 (C-arom), 118.2 (C-arom), 121.0 (C-arom), 122.2 (C-5″), 125.4 (C-arom), 127.0 (C-arom), 128.3 (C-arom), 129.3 (C-arom), 131.8 (C-8), 133.3 (C-9a), 134.9 (C-1′′′), 135.8 (C-4″), 158.8 (C-4′′′), 161.1 (C-2′), 163.1 (C-4), 163.6 (C-2). Anal. Calcd for C25H20ClN5O2 (473.13): C, 63.36; H, 4.25; N, 15.29; found: C, 65.07; H, 4.46; N, 14.78.
3.3.9. 4-(2-Hydroxyphényl)-8-chloro-1-((1-phényl)-1H-1,2,3-triazol-4-yl)méthyl)-1,5-benzodiazépin-3H-2-one (4i)
Yield 178 mg (77%). Yellow solid, m.p.>250 °C. 1H NMR (300 MHz, CDCl3) δH 3.01 (d, 1H, H-3a, J = 12.00 Hz), 4.29 (d, 1H, H-3b, J = 12.30 Hz), 4.91 (d, 1H, H-1a″, J = 15.30 Hz), 5.25 (d, 1H, H-1b″, J = 15.00 Hz), 7.00 (t, 1H, H-arom), 7.07 (d, 1H, H-arom, J = 8.10 Hz), 7.38 (dd, 1H, H-arom, J = 9.00 Hz), 7.46 (m, 2H, H-arom), 7.86 (dd, 1H, H-arom, J = 8.10 Hz), 7.97 (d, 2H, H-3′′′, H-5′′′, J = 9.00 Hz), 8.09 (d, 1H, H-arom, J = 8.70 Hz), 8.24 (s, 1H, H-5′′), 8.42 (d, 2H, H-2′′′, H-6′′′, J = 9.00 Hz), 13.64 (1s, 1H, OH). 13C NMR (75.47 MHz, CDCl3) δC 38.3 (C-3), 44.6 (C-1″), 117.6 (C-1′), 118.4 (C-arom), 119.3 (C-arom), 120.5 (C-arom), 122.6 (C-arom), 124.4 (C-arom), 125.5 (C-arom), 126.5 (C-arom), 127.6 (C-arom), 129.4(C-arom), 131.5 (C-8), 133.6 (C-9a), 134.7 (C-H), 139.3 (C-1′′′), 140.9 (C-4″), 147.3 (C-4′′′), 162.2 (C-2′), 164.8 (C-4), 164.9 (C-2). Anal. Calcdfor C24H17ClN6O4 (488.10): C, 58.96; H, 3.51; N, 17.19; found: C, 59.26; H, 3.41; N, 17.00.
3.4. General Procedure for the Synthesis of Compounds (6a–c)
CuI (5.0 mg, 0.025 mmol, 5 mol percent) and the suitable galactopyranose azide 5 (1 mmol, 2 eq,) were added to a combination of compounds 2a–c (0.5 mmol, 1 eq) and Et3N (2 eq, 134 µL, 1 mmol) in DMF/H2O (8/2). For 8 h, the reaction mixture was stirred at room temperature. The raw material was put into distilled water and extracted with dichloromethane after being filtered through Celite®. Flash column chromatography on silica gel (Cyclohexane/EtOAcfrom 100:0 to 90:10) was used to purify the crude substance, yielding pure 6a–c in 80–85% yields.
3.4.1. 4-(2-Hydroxyphényl)-1-(1-(3aR, 5R, 5aS, 8aS, 8bR)-2,2,7,7-tetraméthyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)méthyl)-1H-1,2,3-triazolo-4-yl)méthyl)-1H-1,5-benzodiazépin-2-one (6a)
Yield 202 mg (85%). Yellow solid, m.p. 156–158 °C. 1H NMR (300 MHz, CDCl3) δH 1.29; 1.37; 1.42; 1.50 (s, 3H, CH3), 2.97 (d, 1H, H-3a, J = 12.00 Hz), 4.12 (m, 3H, H-3b, H-3′′′, H-5′′′), 4.29 (m, 1H, H-2′′′), 4.45 (m, 1H, H-4′′′), 4.56 (m, 2H, CH2-6′′′), 4.86 (d, 1H, H-1a″, J = 15.00 Hz), 5.21 (d, 1H, H-1b″, J = 15.30 Hz), 5.51 (s, 1H, H-1′′′), 6.96–8.19 (m, 8H, H-arom), 8.06 (s, 1H, H-5′′), 13.95 (1s, 1H, OH). 13C NMR (75.47 MHz, CDCl3) δC23.9; 24.3; 25.4; 31.0 (CH3-Sucre), 37.7 (C-3), 44.0 (C-1″), 50.3 (C-6′′′), 61.5 (C-2′′′), 67.1 (C-3′′′), 70.3 (C-4′′′), 70.7 (C-5′′′), 96.2 (C-1′′′), 108.5 (C-isop), 109.4 (C-isop), 117.5 (C-1′), 117.7 (C-3′), 118.2 (C-arom), 119.0 (C-arom), 123.2 (C-arom), 125.8 (C-arom), 126.9 (C-arom), 127.5 (C-arom), 128.8 (C-quat), 129.3 (C-arom), 133.5 (C-quat), 134.0 (C-arom), 134.8 (C-8) 137.9 (C-4″), 161.7 (C-2′), 163.8 (C-4), 164.2 (C-2). Anal. Calcd for C30H33N5O7 (575.24): C, 62.60; H, 5.78; N, 12.17; found: C, 63.75; H, 6.36; N, 11.68.
3.4.2. 4-(2-Hydroxyphényl)-8-méthyl-1-(1-(3aR, 5R, 5aS, 8aS, 8bR)-2,2,7,7-tetraméthyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)méthyl)-1H-1,2,3-triazolo-4-yl)méthyl)-1H-1,5-benzodiazépin-2-one (6b)
Yellow solid, yield 247 mg (84%), m.p.140–142 °C. 1H NMR (300 MHz, CDCl3) δH 1.34; 1.36; 1.47; 1.50 (s, 3H, CH3), 2.41 (s, 3H, CH3-8a), 2.95 (d, 1H, H-3a, J = 12.00 Hz), 4.12 (m, 3H, H-3b, H-3′′′, H-5′′′), 4.25 (m, 1H, H-2′′′), 4.36 (m, 1H, H-4′′′), 4.52 (m, 2H, CH2-6′′′), 4.75 (d, 1H, H-1a″, J = 15.00 Hz), 5.09 (d, 1H, H-1b″, J = 15.30 Hz), 5.40 (s, 1H, H-1′′′), 6.87–7.95 (m, 7H, H-arom), 7.69 (s, 1H, H-5″), 13.95 (1s, 1H, OH). 13C NMR (75.47 MHz, CDCl3)δC 14.1(CH3-8a), 24.4; 24.9; 25.9; 31.5 (CH3-Sucre), 38.2 (C-3), 44.8 (C-1″), 50.2 (C-6′′′), 66.8 (C-2′′′), 67.1 (C-3′′′), 70.2 (C-4′′′), 70.7 (C-5′′′), 96.2 (C-1′′′), 109.9 (C-isop), 109.9 (C-isop), 118.0 (C-1′), 118.2 (C-3′), 119.0 (C-5′), 123.0 (C-arom), 126.7 (C-5″), 126.9 (C-9), 127.0 (C-9a), 129.3 (C-6′), 133.9 (C-arom), 134.0 (C-5a), 135.9 (C-8) 137.9 (C-4′′), 162.2 (C-2′), 163.8 (C-4) 164.9 (C-2). Anal. Calcd for C31H35N5O7 (589.25): C, 63.15; H, 5.98; N, 11.88; found: C, 62.65; H, 5.49; N, 12.15.
3.4.3. 4-(2-Hydroxyphényl)-8-chloro-1-(1-(3aR, 5R, 5aS, 8aS, 8bR)-2,2,7,7-tetraméthyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)méthyl)-1H-1,2,3-triazolo-4-yl)méthyl)-1H-1,5-benzodiazépin-2-one (6c)
Yield 240 mg (80%). Yellow solid, m.p. 172–174 °C. 1H NMR (300 MHz, CDCl3) δH1.32; 1.39; 1.45; 1.52 (s, 3H, CH3,), 2.97 (d, 1H, H-3a, J = 12.00 Hz), 4.17 (m, 3H, H-3b, H-3′′′, H-5′′′), 4.32 (m, 1H, H-2′′′), 4.49 (m, 1H, H-4′′′), 4.59 (m, 1H, CH2-6′′′), 4.86 (d, 1H, H-1a″, J = 15.00 Hz), 5.21 (d, 1H, H-1b″, J = 15.30 Hz), 5.54 (s, 1H, H-1′′′), 6.98–8.21 (m, 8H, H-arom), 8.08 (s, 1H, H-5″), 13.95 (1s, 1H, OH). 13C NMR (75.47 MHz, CDCl3) δC23.8; 24.4; 25.2; 29.9 (CH3-Sucre), 37.7 (C-3), 42.0 (C-1″), 50.2 (C-6′′′), 60.3 (C-2′′′), 66.0 (C-3′′′), 70.1 (C-4′′′), 70.4 (C-5′′′), 96.0 (C-1′′′), 107.5 (C-isop), 108.3 (C-isop), 117.5 (C-1′), 117.7 (C-3′), 120.3 (C-arom), 121.0 (C-arom), 123.9 (C-arom), 126.5 (C-arom), 127.8 (C-arom), 128.3 (C-arom), 128.8 (C-quat), 129.7 (C-arom), 133.5 (C-quat), 134.5 (C-arom), 134.8 (C-8) 137.9 (C-4″), 161.7 (C-2′), 163.8 (C-4) 164.2 (C-2).Anal. Calcd for C30H32ClN5O7 (609.20): C, 59.06; H, 5.29; N, 11.48; found: C, 58.65; H, 6.01; N, 12.00.
3.5. Bioactivity
The in vitro antimicrobial activity of the structurally promising 4a–I and 6a–c against Gram-positive (B. subtilis and S. aureus…) and Gram-negative (E. coli and P. aeruginosa…) bacteria were investigated using microdilution assays along with reference drug streptomycin for comparison. H2O was used as a negative control.
3.5.1. Antibacterial Tests
Microbial Inhibitory Concentration
Microdilution assay The MICs of the compounds were determined by microdilution [48] using standard inocula of 2 × 106 CFU/mL. Serial dilutions of the test compounds were prepared in DMSO. A bacterial fluid (1 mL of 0.5 McFarland standard) was added to each tube. The MIC was visually determined after incubation for 18 h at 37 °C.
3.5.2. Antifungal Activity
The antifungal activity of compounds 4a–I and 6a–c was tested against two fungal species, namely: Aspergillus flavus and Candida albicans. These fungi were obtained from the (Department of Clinical biology, Laboratory of Analysis, Treatment and valorization of Pollutants of the Environment and Products, Faculty of Pharmacy of Monastir).They were cultured at 25 °C on potato dextrose agar (PDA) medium one week before use.
3.5.3. Molecular Docking Procedure
Molecular docking simulations were performed by Auto Dock 4.2 program package [49]. The optimization of all the geometries of compounds was carried out using ACD (3D viewer) software (http://www.filefacts.com/acd3d-viewer-freeware-info, accessed on 25 March 2022). The three-dimensional structure of PDB (PDB: 4EJW) was obtained from the RSCB protein data bank [50]. First, the water molecules were eliminated, and the missing hydrogens and Gasteiger charges were added to the system during the preparation of the receptor input file. Then, AutoDock Tools were used for the preparation of the corresponding ligand and protein files (PDBQT). Subsequently, pre-calculation of the grid maps was performed using Auto Grid to save much time during docking. Next, the docking calculation was carried out using a grid per map with 40 × 40 × 40 A˚ points of (PDB: 4EJW) in addition to a grid-point spacing of 0.375 A, ˚ which was centered on the receptor in order to determine the active site. The visualization and analysis of interactions were performed using Discovery Studio 2017R2 (https://www.3dsbiovia.com/products/collaborative-science/biovia–discovery-studio/, accessed on 25 March 2022).
4. Conclusions
In our study, novel conjugates N-triazolo-1,5-benzodiazepinones 4a–i and 6a–c were designed and synthesized. In fact, we have incorporated 1,2,3-triazole at the first position of the heptatomic ring with either linkage employing the Cu(I)-catalyzed 1,3-dipolar alkyne-azide coupling reaction (CuAAC). Compounds synthesized by this method are of high quality, allowing for simple purification and screening in a high throughput manner. Some of them were screened for their antimicrobial activity and have shown good to moderate antibacterial and antifungal activities. Even though the inhibition levels are only at µM levels, we believe these novel classes of N-triazolo-1,5-benzodiazepin-2-ones could find applications in biology. Our strategy, therefore, lays the foundations for the future exploration of more potent and selective N-fonctonalized-1,5-benzodiazepinones. To understand the mechanism of antibacterial activity and binding mode of these novel derivatives inside the binding pocket of the crystallized structure of Staphylococcus epidermidis TcaR in complex with streptomycin and to confirm the experimental results, molecular docking studies were performed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27134015/s1, Figure S1: NMR spectra 1H (300 MHz, CDCl3) of compound 4b; Figure S2: NMR spectra 13C (75,47 MHz, CDCl3) of compound 4b; Figure S3: DEPT 135 of compound 4b; Figure S4: CHcorr spectra of compound 4b; Figure S5: NMR spectra 1H (300 MHz, CDCl3) of compound 6b; Figure S6: NMR spectra 1H (300 MHz, CDCl3) of compound 6b; Figure S7: NOESY Spectra of compound 6b; Figure S8: COSY 1H-1H spectra of compound 6b; Figure S9: COSY 1H-1H spectra of compound 6b; Figure S10: NMR spectra 1H (300 MHz, CDCl3) of compound 6b; Figure S11: DEPT 135 (75,47 MHz, CDCl3) of compound 6b; Figure S12:CHcorr Spectra of compound 6b; Figure S13: COSY Spectra of compound 6b; Figure S14: NOESY Spectra of compound 6b; Figure S15: HRMS Spectra of compound 4b; Figure S16: HRMS Spectra of compound 4g; Figure S17: NMR spectra 1H (300 MHz, CDCl3) of compound 2c; Figure S18: DEPT 135 of compound 2c; Figure S19: NMR spectra 1H (300 MHz, CDCl3) of compound 4d; Figure S20: NMR spectra 13C (75,47 MHz, CDCl3) of compound 4d; Figure S21: NMR spectra 1H (300 MHz, CDCl3) of compound 4e; Figure S22: NMR spectra 13C (75,47 MHz, CDCl3) of compound 4e; Figure S23: NMR spectra 1H (300 MHz, CDCl3) of compound 4f; Figure S24: NMR spectra 13C (75,47 MHz, CDCl3) of compound 4f; Figure S25: NMR spectra 1H (300 MHz, CDCl3) of compound 4h; Figure S26: NMR spectra 13C (75,47 MHz, CDCl3) of compound 4h; Figure S27: NMR spectra 1H (300 MHz, CDCl3) of compound 4i; Figure S28: NMR spectra 13C (75,47 MHz, CDCl3) of compound 4i.
Author Contributions
Conceptualization, R.G.; methodology, S.C. and H.A.-G.; formal analysis, H.M.; data curation, A.N.; supervision, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers supporting project number (PNURSP2022R95), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers supporting project number (PNURSP2022R95), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors are grateful to Amna Benzarti and Nadia Msaddek, NMR service at the Faculty of Science of Monastir, University of Monastir, for the NMR analysis and to the Ministry of Higher Education and Scientific Research of Tunisia for financial support (LR11ES39). We gratefully acknowledge Sadok Khouaja for the antimicrobial activities evaluation (Laboratory of analysis, treatment, and valorization of environmental and products of pollutants, Faculty of Pharmacy of Monastir).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 4a–h, 6a–c are not available from the authors.
References
- Greenblatt, D.J.; Shader, R.I. Benzodiazepines in Clinical Practice; Raven: New York, NY, USA, 1974. [Google Scholar]
- Mandoli, A. Recent Advances in Recoverable Systems for the Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction (CuAAC). Molecules 2016, 21, 1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Olvera, R.; Urquiza-Castro, C.I.; Negron-Silva, G.E.; Angeles-Beltran, D.; Lomas-Romero, L.; Gutierrez-Carrillo, A.; Lara, V.H.; Santillan, R.; Morales-Serna, J.A. Cu–Al mixed oxide catalysts for azide–alkyne 1, 3-cycloaddition in ethanol–water. RSC Adv. 2016, 6, 63660–63666. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Yu, S.; Cornilleau, T.; Ruiz, J.; Salmon, L.; Astruc, D. Design and Applications of an Efficient Amphiphilic “Click” CuI Catalyst in Water. ACS Catal. 2016, 6, 5424–5431. [Google Scholar] [CrossRef]
- Kaffy, J.; Pontiks, R.; Carrez, D.; Croisy, A.; Monnereta, C.; Floreta, J.C. Isoxazole-type derivatives related to combretastatin A-4, synthesis and biological evaluation. Bioorg. Med. Chem. 2006, 14, 4067–4077. [Google Scholar] [CrossRef]
- Bakshi, R.K.; Hong, Q.; Tang, R.; Kalyani, R.N.; MacNeil, T.; Weinberg, D.H.; Ploeg, L.H.T.; Patchett, A.A.; Nargund, R.P. Optimization of a privileged structure leading to potent and selective human melanocortin subtype-4 receptor ligands. Bioorg. Med. Chem. Lett. 2006, 16, 1130. [Google Scholar] [CrossRef]
- Patchett, A.A.; Nargund, R.P. Exploring privileged structures: The combinatorial synthesis of cyclic peptides. Annu. Rep. Med. Chem. 2000, 35, 289. [Google Scholar]
- Sternbach, L.H. 1, 4-benzodiazepines. Chemistry and some aspects of the structure—activity relationship. Angew. Chem. Int. Ed. 1971, 13, 34–43. [Google Scholar] [CrossRef]
- Boyd, G.V. Six Membered and Larger Hetero Rings with Maximum Unsaturation; Schauman, E., Ed.; Houben-Weyl: New York, NY, USA, 1998; Volume 26, p. 299. [Google Scholar]
- Fryer, R.I.; Walser, A. Chemistry of Heterocyclic Compounds: Bicyclic Diazepines: Diazepines with an Additional Ring. Chem. Heterocycl. Compd. 1991, 50, 20. [Google Scholar]
- Lu, X.; Shi, L.; Shang, H.; Jiang, Y.; Ma, D. Assembly of N-substituted pyrrolo [2, 1-c][1, 4] benzodiazepine-5, 11-diones via copper catalyzed aryl amination. Tetrahedron 2010, 66, 5714–5718. [Google Scholar] [CrossRef]
- Soares Meinej, R. Novel functionalized 1,2,3-triazole derivatives promote antileishmanial activity, increase in total and mitochondrial-R and depolarization of mitochondrial membrane potential, Chemico-Biological Interactions of Leishmania amazonensis. Chem.-Biol. Interact. 2020, 315, 108850. [Google Scholar] [CrossRef]
- Tranquillini, M.E.; Cassara, P.G.; Corsi, M.; Curotto, G.; Donati, D.; Finizia, G.; Pentassuglia, G.; Polinelli, S.; Tarzia, G.; Ursini, A.; et al. Effect of aryl-carbamic substituents at the C-3 position together with halogen substitution on the benzo-fused ring. Arch. Der Pharm. 1997, 330, 353. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, M.J.; Bebbington, D.; Bemis, G.W.; Fridman, W.H.; Gillespie, R.J.; Golec, J.M.C.; Lauffer, D.J.; Livingston, D.J.; Matharu, S.S.; Mullican, M.D.; et al. Inhibitors of interleukin -1 beta. Converting enzyme inhibitors. U. S. Pat. 2002, 6, 423–840. [Google Scholar]
- Mtiraoui, H.; Gharbi, R.; Msaddek, M.; Bretonnière, Y.; Andraud, C.; Sabot, C.; Renard, P.Y.J. 1,5-Benzodiazepin-2-ones: Investigation of a family of photoluminescent materials. Org. Chem. 2016, 81, 4720–4727. [Google Scholar] [CrossRef] [PubMed]
- Ismail, C.; Mtiraoui, H.; Winum, J.Y.; Msaddek, M.; Gharbi, R. Design, synthesis and photoluminescent studies of new 1, 5-benzodiazepines derivatives: Towards new ESIPT compounds. Tetrahedron 2021, 86, 132078. [Google Scholar] [CrossRef]
- Wejdane, A.; Mansour, Z.; Anne, R.; Hichem, B.J.; Delphine, D.; Rafik, G. Synthesis of S-mono-and S, O-bis-1, 2, 3-triazole linked 1, 5-benzodiazepine conjugates and evaluation of their cytotoxic, anti-tyrosinase, and anti-cholinesterase activities. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 835–844. [Google Scholar]
- Richardson, K.; Whittle, P.J. human secreted proteins, Eur Pat App Ep 115:416. Chem. Abstr. 1984, 101, 230544. [Google Scholar]
- de las Heras, F.G.; Alonso, R.; Alonso, G. Alkylating nucleosides. 1. Synthesis and cytostatic activity of N-glycosyl (halomethyl)-1, 2, 3-triazoles. A new type of alkylating agent. J. Med. Chem. 1979, 22, 496. [Google Scholar] [CrossRef]
- Hardman, J.; Limbird, L.; Gilman, A. Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 9th ed.; McGraw-Hill: New York, NY, USA, 1996; p. 988. [Google Scholar]
- Gennaro, A.R.; Easton, P.A.M. The Science and Practice of Pharmacy; Mack Publishing Company: London, UK, 1995; p. 1327. [Google Scholar]
- Richardson, K.; Whittle, P.J. 17 human secreted proteins. Eur. Pat. Appl. 1984, 115, 416. [Google Scholar]
- Horne, W.S.; Stout, C.D.; Ghadiri, M.R. A heterocyclic peptide nanotube. J. Am. Chem. Soc. 2003, 125, 9372–9376. [Google Scholar] [CrossRef]
- Horne, W.S.; Yadav, M.K.; Stout, C.D.; Ghadiri, M.R. Heterocyclic peptide backbone modifications in an α-helical coiled coil. J. Am. Chem. Soc. 2004, 126, 15366–15367. [Google Scholar] [CrossRef] [Green Version]
- Mtiraoui, H.; Gharbi, R.; Msaddek, M.; Bretonnière, Y.; Andraud, C.; Renard, P.Y.; Sabot, C. Solution and solid-state fluorescence of 2-(2′-hydroxyphenyl)-1, 5-benzodiazepin-2-one (HBD) borate complexes. RSC Adv. 2016, 6, 86352–86360. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase:[1, 2, 3]-triazoles by regiospecific copper (I)-catalyzed 1, 3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Tornoe, C.W. Cu-catalyzed azide− alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Lattman, E.; Sattayasai, J.; Billington, D.C.; Poyner, D.R.; Puapairaj, P.; Tamkao, S.; Airarat, W.; Singh, H.; Offel, M. Synthesis and evaluation of N1-substituted-3-propyl-1,4-benzodiazepine-2-ones as cholecystokinin (CCK2) receptor ligands. J. Pharm. Pharmacol. 2002, 54, 827–834. [Google Scholar] [CrossRef]
- Rahmouni, A.; Romdhane, A.; Guérineau, V.; Touboul, D.; Jannet, H.B. Synthesis of novel isoxazolines and isoxazoles of N-substituted pyrazolo[3,4-d] pyrimidin-4(5H) ones derivatives through [3+2] cycloaddition. Arab. J. Chem. 2014, 12, 1974–1982. [Google Scholar] [CrossRef] [Green Version]
- Mabrour, M.; Bougrin, K.; Benhida, R.; Loupy, A.; Soufiaoui, M. An efficient one-step regiospecific synthesis of novel isoxazolines and isoxazoles of N-substituted saccharin derivatives through solvent-free microwave-assisted [3+ 2] cycloaddition. Tetrahedron Lett. 2007, 48, 443–447. [Google Scholar] [CrossRef]
- Ahabchane, H.; Essasi, E.M. synthèse de nouveaux dérivés de la 1,5-bromo-1H-indole-2,3-dione à visée thérapeutique. J. Tun. Chem. Soc. 2000, 8, 753. [Google Scholar]
- Mtiraoui, H.; Nsira, A.; Msaddek, M.; Renard, P.Y.; Sabot, C. Regioselective synthesis of o-triazolyl-1,5-benzodiazepin-2-ones and o-isoxazolyl-1,5-benzodiazepin-2-ones via copper-catalyzed 1,3-dipolar cycloaddition reactions. C. R. Chim. 2017, 7, 747–757. [Google Scholar] [CrossRef]
- Nsira, A.; Tekaya, A.; Gharbi, R.; Msaddek, M. Chemoselectivity of 1,3-dipolar cycloaddition of some diazoalkanes with 1,5-benzodiazepines derivatives. J. Chem. Res. 2012, 36, 152–156. [Google Scholar] [CrossRef]
- Gharbi, R.; Youssef, M.B.; Martin, M.T.; Mighri, Z. Reactivity studies on a novel 4-(2-hydroxyphenyl)-1,3-dihydro1,5-benzodiazepine-2-thione. J. Chem. Res. 2005, 2005, 257. [Google Scholar] [CrossRef]
- Kamalraj, V.R.; Senthil, S.; Kannan, P. One-pot synthesis and the fluorescent behavior of 4-acetyl-5-methyl-1, 2, 3-triazole regioisomers. J. Molec. Struc. 2008, 892, 210. [Google Scholar] [CrossRef]
- Chouaïb, K.; Romdhane, A.; Delemasure, S.; Dutartre, P.; Elie, N.; Touboul, D.; Jannet, H.B. Regiospecific synthesis by copper and ruthenium catalysezd azide-alkyne 1,3-dipolar cycloaddition anti-cancer and anti-inflammatory of oleanolic acid triazoles derivatives. Arab. J. Chem. 2015, 12, 1–15. [Google Scholar]
- Ifuku, S.; Wada, M.; Morimoto, M.; Saimoto, H. Preparation of highly regioselective chitosan derivatives via click chemistry. Carbohydr. Polym. 2011, 85, 653–657. [Google Scholar] [CrossRef]
- Saeedi, M.; Mahdavi, M.; Foroumadi, A.; Shafiee, A. Synthesis of novel fused 4, 5-dihydro-1, 2, 3-triazolo [1, 5-a][1, 4] benzodiazepine derivatives via four-component Ugi–Smiles-type reaction. Tetrahedron 2013, 69, 3506–3510. [Google Scholar] [CrossRef]
- Gabius, H.J.; Siebert, H.C.; André, S.; Jiménez-Barbero, J.; Rudiger, H. Chemical biology of the sugar code. ChemBioChem 2004, 5, 740–764. [Google Scholar] [CrossRef]
- Dweck, R.A. Glycobiology: Toward understanding the function of sugars. Chem. Rev. 1996, 96, 683–720. [Google Scholar] [CrossRef]
- Mendonca-Previato, L.; Todechini, A.R.; Heise, N.; Agrellos, O.A.; Dias, W.B.; Previato, J.O. Chemical structure of major glycoconjugates from parasites. Curr. Org. Chem. 2008, 12, 926. [Google Scholar] [CrossRef]
- Hamadi, N.B.; Msaddek, M. Synthesis and reactivity of N-sugar-maleimides: An access to novel highly substituted enantiopure pyrazolines. Tetrahedron Assymetry 2012, 23, 1689–1693. [Google Scholar] [CrossRef]
- Rammah, M.M.; Gati, W.; Mtiraoui, H.; Rammah, M.E.B.; Ciamala, K.; Knorr, M.; Rousselin, Y.; Kubicki, M.M. Synthesis of isovazoles and 1,2,3-triazole-isoindoles derivarives via silver and copper catalyzed 1,3-dipolar cycloaddition, reactions. Molecules 2016, 21, 307. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, I.; Andrade, P.; Campo, V.L.; Guedes, P.M.; Sesti-Costa, R.; Silva, J.S.; Schenkman, S.; Dedola, S.; Hill, L.; Rejzek, M.; et al. ‘Click chemistry’ synthesis of a library of 1,2,3-triazole-substituted galactose derivatives and their evaluation against Trypanosoma cruzi and its cell surface trans-sialidase. Bioorg. Med. Chem. 2010, 18, 2412–2427. [Google Scholar] [CrossRef] [PubMed]
- Marmonier, A. Antibiotiques technique de diffusion en gélose méthode des disques. In Bactériologie Médicale Techniques Usuelles; SIMEP SA: Paris, France, 1987; Volume 4, pp. 237–243. [Google Scholar]
- Barry, A.L.; Thornsberry, C. Manual of Clinical Microbiology; Ballows, A., Hausler, W.J., Jr., Herrman, K.L., Isenberg, H.D., Shadomy, H.J., Eds.; American Society for Microbiology Press: Washington, DC, USA, 1991; pp. 1117–1125. [Google Scholar]
- Zhang, F.F.; Gan, L.L.; Zhou, C.H. Synthesis, antibacterial and antifungal activities of some carbazole derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.M.; Chen, C.K.M.; Ko, T.P.; Chang-Chien, M.W.; Wang, A.H.J. Structural analysis of the antibiotic-recognition mechanism of MarR proteins. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1138–1149. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).