Application of Octanohydroxamic Acid for Salting out Liquid–Liquid Extraction of Materials for Energy Storage in Supercapacitors
Abstract
1. Introduction
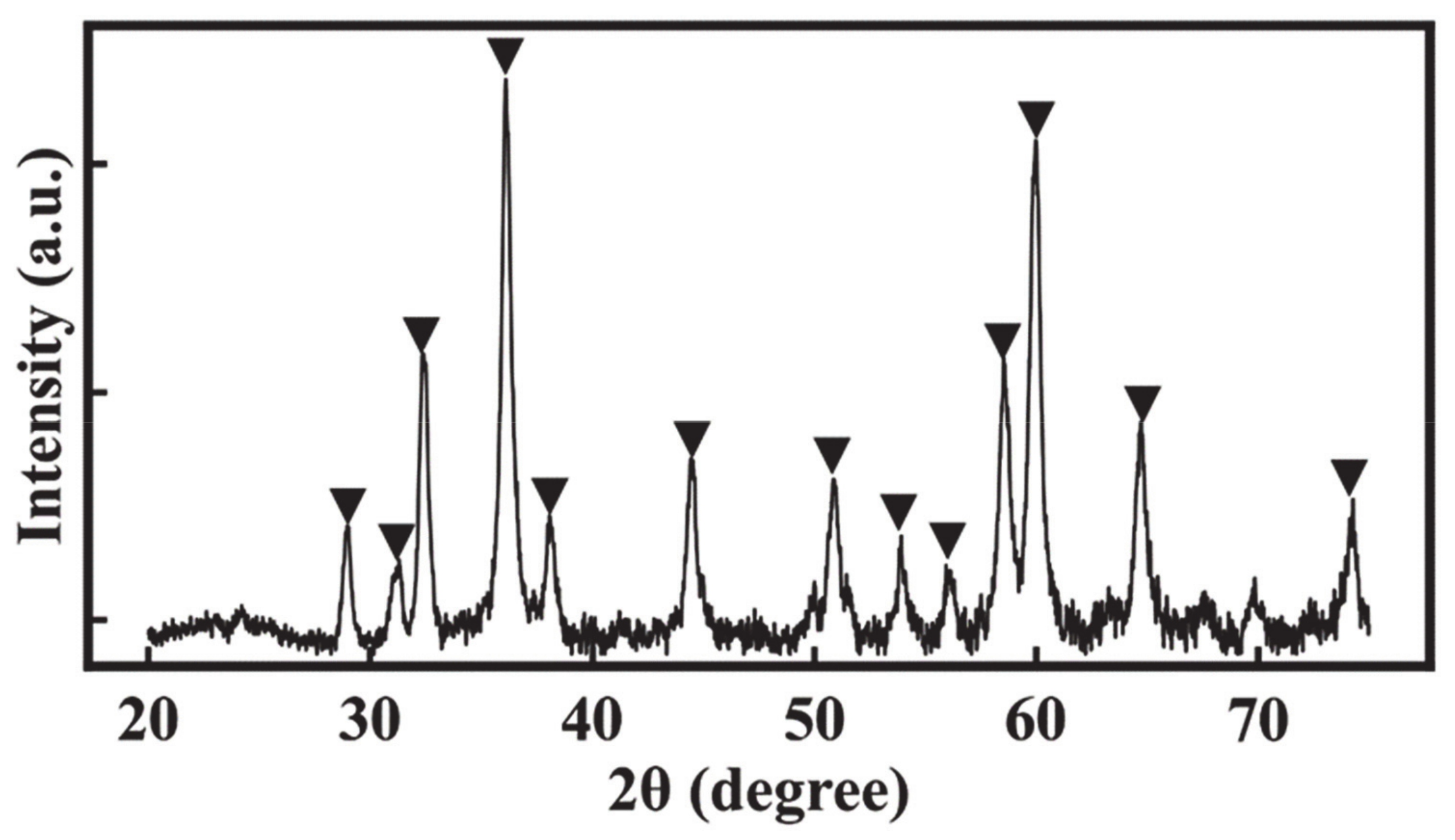
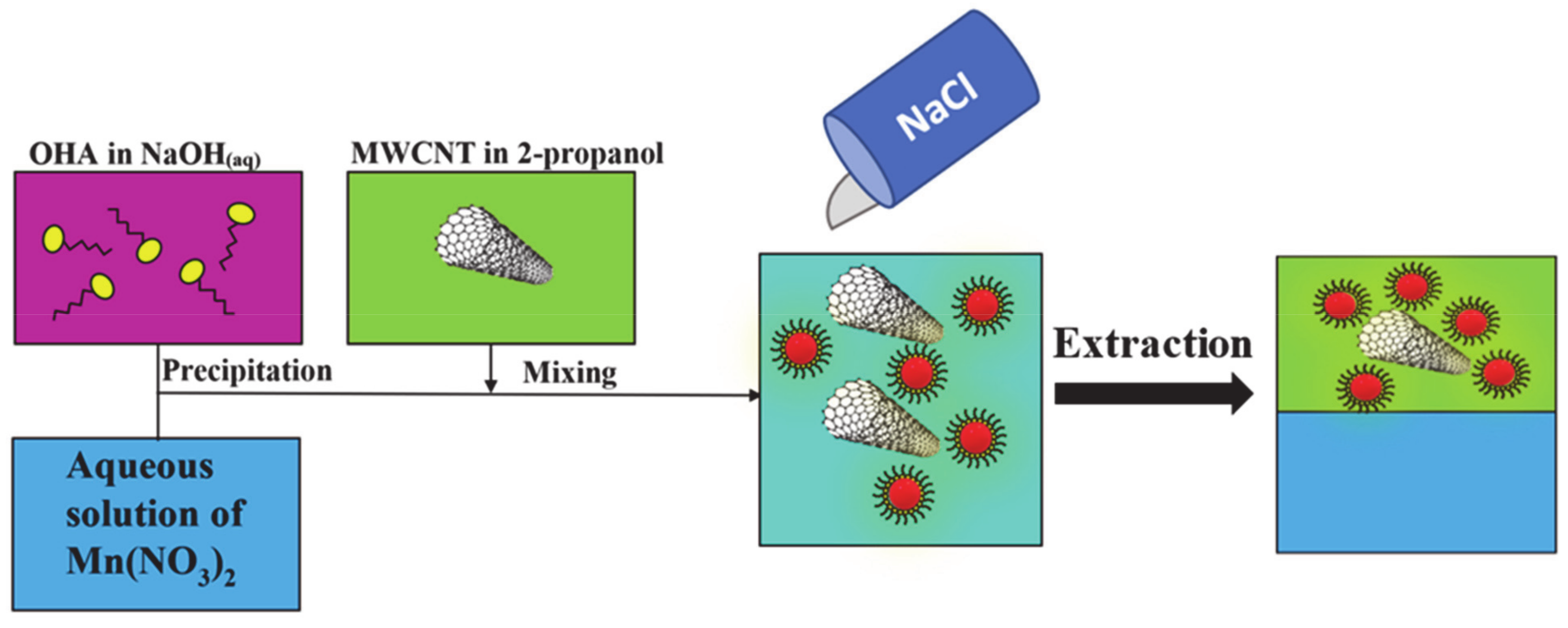
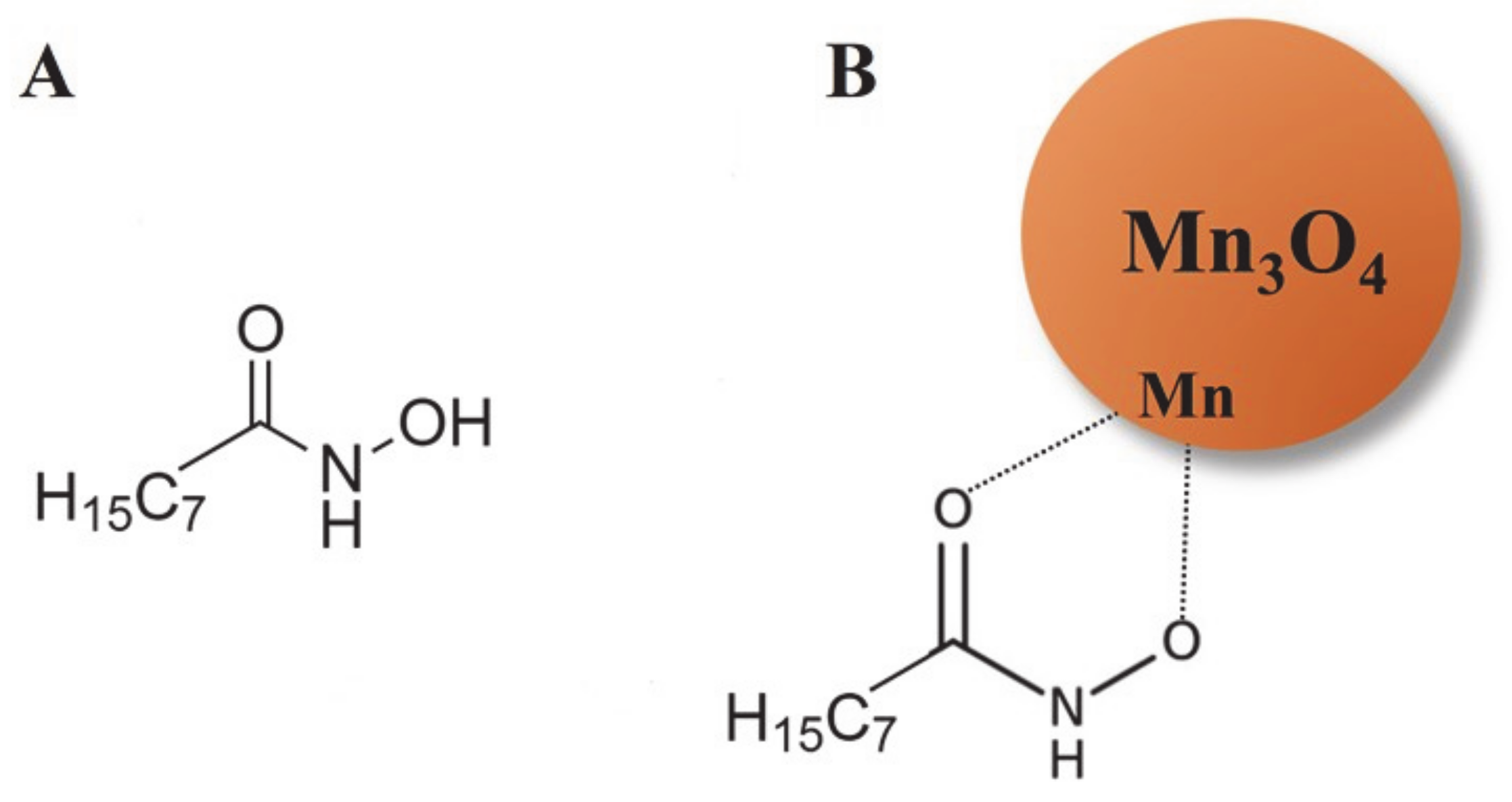
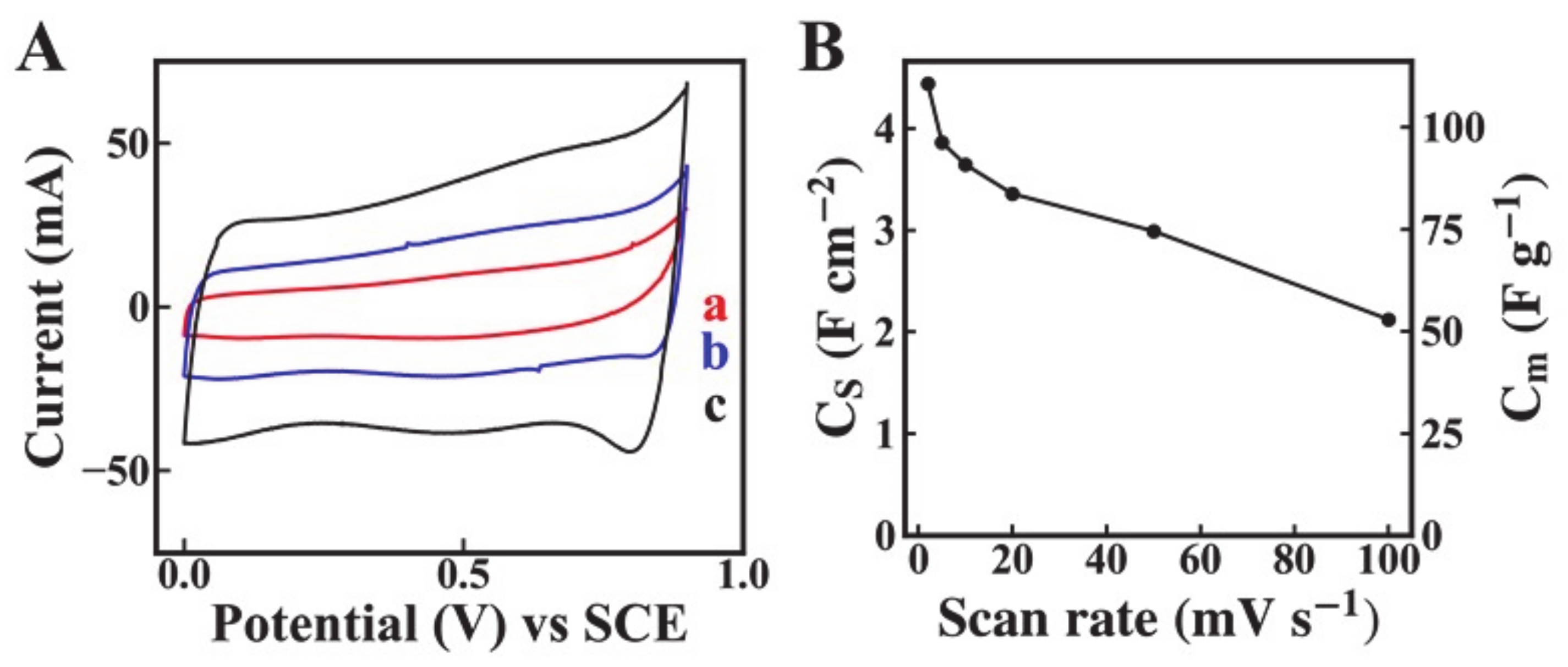
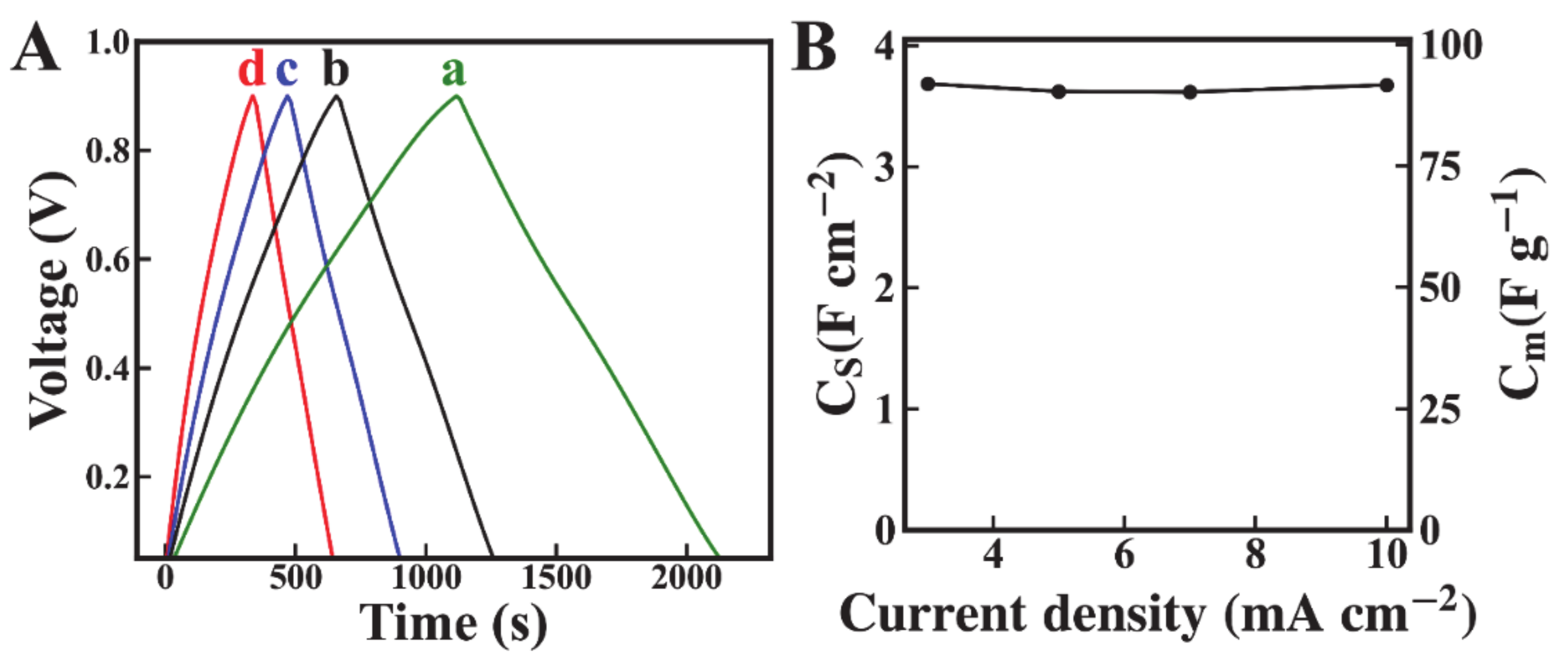
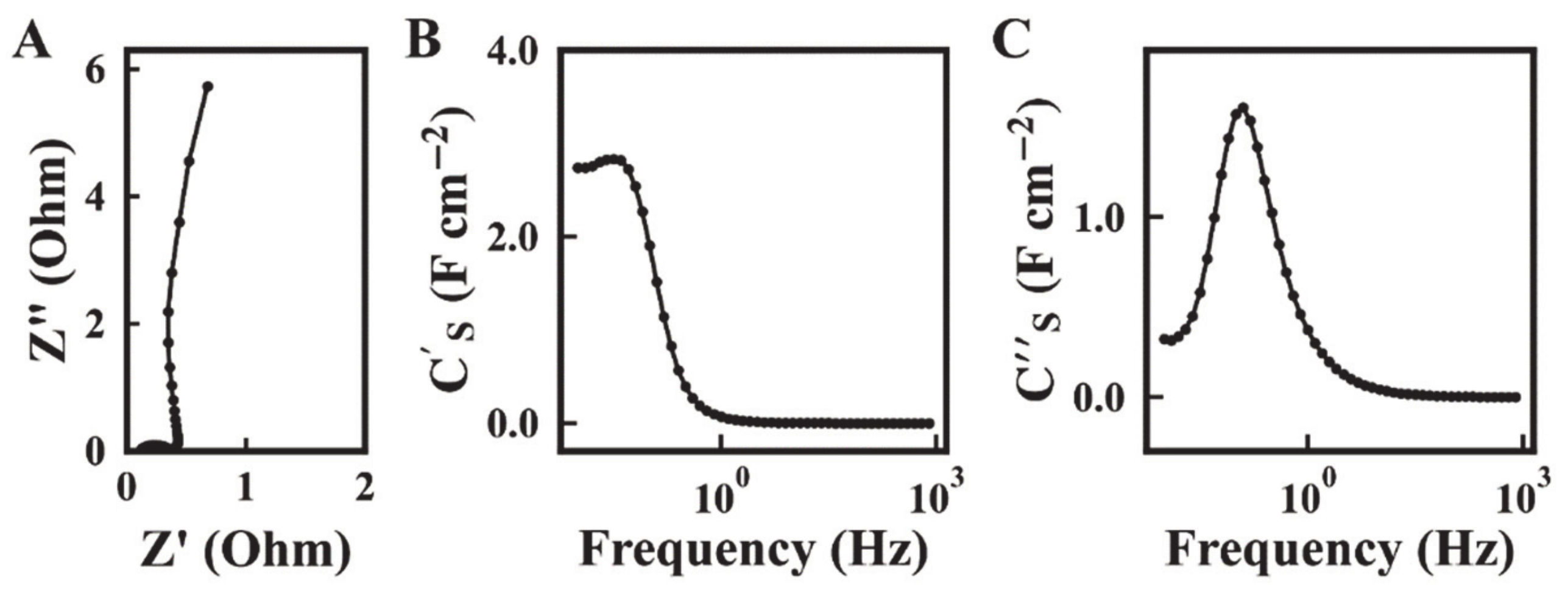
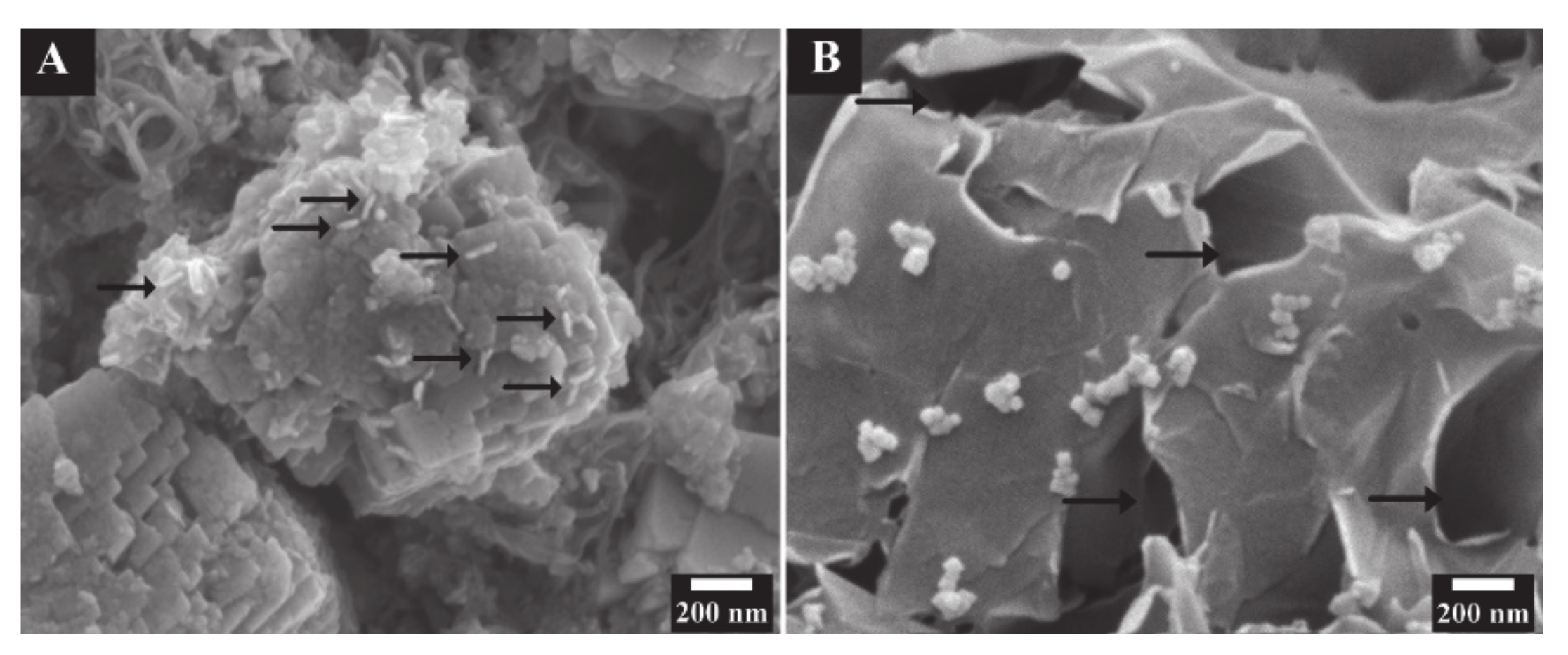
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attias, R.; Sharon, D.; Borenstein, A.; Malka, D.; Hana, O.; Luski, S.; Aurbach, D. Asymmetric supercapacitors using chemically prepared MnO2 as positive electrode materials. J. Electrochem. Soc. 2017, 164, A2231–A2237. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D. A Hybrid Fe3O4-MnO2 capacitor in mild aqueous electrolyte. Electrochem. Solid State Lett. 2003, 6, A244–A248. [Google Scholar] [CrossRef]
- Kierzek, K.; Gryglewicz, G. Activated Carbons and their evaluation in electric double layer capacitors. Molecules 2020, 25, 4255. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Pan, G.-T.; Hong, Z.-Y.; Hsu, C.-T.; Chong, S.; Yang, T.C.-K.; Huang, C.-M. Biomass-derived porous carbons derived from soybean residues for high performance solid state supercapacitors. Molecules 2020, 25, 4050. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, Y.-S.; Kwac, L.K.; Shin, H.K. Characterization of activated carbon paper electrodes prepared by rice husk-isolated cellulose fibers for supercapacitor applications. Molecules 2020, 25, 3951. [Google Scholar] [CrossRef]
- Li, J.; Zhitomirsky, I. Cathodic electrophoretic deposition of manganese dioxide films. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 248–253. [Google Scholar] [CrossRef]
- Yoon, S.; Kang, E.; Kim, J.K.; Lee, C.W.; Lee, J. Development of high-performance supercapacitor electrodes using novel ordered mesoporous tungsten oxide materials with high electrical conductivity. Chem. Commun. 2011, 47, 1021–1023. [Google Scholar] [CrossRef]
- Zhang, W.; Mu, B.; Wang, A. Halloysite nanotubes induced synthesis of carbon/manganese dioxide coaxial tubular nanocomposites as electrode materials for supercapacitors. J. Solid State Electrochem. 2015, 19, 1257–1263. [Google Scholar] [CrossRef]
- Ivol, F.; Porcher, M.; Ghosh, A.; Jacquemin, J.; Ghamouss, F. Phenylacetonitrile (C6H5CH2CN) ionic liquid blends as alternative electrolytes for safe and high-performance supercapacitors. Molecules 2020, 25, 2697. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Pant, H.R.; Park, M. Fe1−xS Modified TiO2 NPs embedded carbon nanofiber composite via electrospinning: A Potential electrode material for supercapacitors. Molecules 2020, 25, 1075. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Song, X.; Du, W.; Zhao, X.; Zhang, D. Review on Carbon/Polyaniline Hybrids: Design and Synthesis for Supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Electrophoretic nanotechnology of graphene–carbon nanotube and graphene–polypyrrole nanofiber composites for electrochemical supercapacitors. J. Colloid Interface Sci. 2013, 407, 474–481. [Google Scholar] [CrossRef]
- Xu, X.; Tang, J.; Qian, H.; Hou, S.; Bando, Y.; Hossain, M.S.A.; Pan, L.; Yamauchi, Y. Three-Dimensional Networked Metal–Organic Frameworks with Conductive Polypyrrole Tubes for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 38737–38744. [Google Scholar] [CrossRef]
- Conway, B.E.; Pell, W.G. Power limitations of supercapacitor operation associated with resistance and capacitance distribution in porous electrode devices. J. Power Sources 2002, 105, 169–181. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, K.; Zhitomirsky, I. Polypyrrole coated carbon nanotubes for supercapacitor devices with enhanced electrochemical performance. J. Power Sources 2014, 268, 233–239. [Google Scholar] [CrossRef]
- Athouël, L.; Moser, F.; Dugas, R.; Crosnier, O.; Bélanger, D.; Brousse, T. Variation of the MnO2 birnessite structure upon charge/discharge in an electrochemical supercapacitor electrode in aqueous Na2SO4 electrolyte. J. Phys. Chem. C 2008, 112, 7270–7277. [Google Scholar] [CrossRef]
- Haldorai, Y.; Giribabu, K.; Hwang, S.-K.; Kwak, C.H.; Huh, Y.S.; Han, Y.-K. Facile synthesis of α-MnO2 nanorod/graphene nanocomposite paper electrodes using a 3D precursor for supercapacitors and sensing platform to detect 4-nitrophenol. Electrochim. Acta 2016, 222, 717–727. [Google Scholar] [CrossRef]
- He, W.; Wu, B.; Lu, M.; Li, Z.; Qiang, H. Fabrication and Performance of Self-Supported Flexible Cellulose Nanofibrils/Reduced Graphene Oxide Supercapacitor Electrode Materials. Molecules 2020, 25, 2793. [Google Scholar] [CrossRef]
- Shi, K.; Yang, X.; Cranston, E.D.; Zhitomirsky, I. Efficient lightweight supercapacitor with compression stability. Adv. Funct. Mater. 2016, 26, 6437–6445. [Google Scholar] [CrossRef]
- Chang, Y.; Zhou, W.; Wu, J.; Ye, G.; Zhou, Q.; Li, D.; Zhu, D.; Li, T.; Nie, G.; Du, Y. High-performance flexible-film supercapacitors of layered hydrous RuO2/poly (3, 4-ethylenedioxythiophene)-poly (styrenesulfonate) through vacuum filtration. Electrochim. Acta 2018, 283, 744–754. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, X.; Ma, Y.; Song, H.; Pi, C.; Zheng, Y.; Gao, B.; Fu, J.; Chu, P.K. In-Situ Synthesis of Heterostructured Carbon-Coated Co/MnO Nanowire Arrays for High-Performance Anodes in Asymmetric Supercapacitors. Molecules 2020, 25, 3218. [Google Scholar] [CrossRef] [PubMed]
- Appadurai, T.; Subramaniyam, C.M.; Kuppusamy, R.; Karazhanov, S.; Subramanian, B. Electrochemical Performance of Nitrogen-Doped TiO2 Nanotubes as Electrode Material for Supercapacitor and Li-Ion Battery. Molecules 2019, 24, 2952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shao, Y.; Kong, X.; Jiang, M.; Lei, X. Hierarchical carbon-decorated Fe3O4 on hollow CuO nanotube array: Fabrication and used as negative material for ultrahigh-energy density hybrid supercapacitor. Chem. Eng. J. 2018, 349, 491–499. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The Development of Pseudocapacitor Electrodes and Devices with High Active Mass Loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Yang, S.; Wu, C.; Cai, J.; Zhu, Y.; Zhang, H.; Lu, Y.; Zhang, K. Seed-assisted smart construction of high mass loading Ni–Co–Mn hydroxide nanoflakes for supercapacitor applications. J. Mater. Chem. A 2017, 5, 16776–16785. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xie, Z.; Xu, X.; Yuan, Y.; Cheng, Z.; Liu, Y. A nanoporous MXene film enables flexible supercapacitors with high energy storage. Nanoscale 2018, 10, 9642–9652. [Google Scholar] [CrossRef]
- Han, D.; Jing, X.; Xu, P.; Ding, Y.; Liu, J. Facile synthesis of hierarchical hollow ε-MnO2 spheres and their application in supercapacitor electrodes. J. Solid State Chem. 2014, 218, 178–183. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y.; Kong, X.; Jiang, M.; Lei, D.; Lei, X. Facile fabrication of large-area hybrid Ni-Co hydroxide/Cu (OH)2/copper foam composites. Electrochim. Acta 2016, 218, 294–302. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, J.; Wang, R.; Wang, H.; Ji, S. Manganese dioxide core–shell nanostructure to achieve excellent cycling stability for asymmetric supercapacitor applications. RSC Adv. 2017, 7, 33635–33641. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Fabrication of Polypyrrole-Coated Carbon Nanotubes Using Oxidant–Surfactant Nanocrystals for Supercapacitor Electrodes with High Mass Loading and Enhanced Performance. ACS Appl. Mater. Interfaces 2013, 5, 13161–13170. [Google Scholar] [CrossRef] [PubMed]
- Ata, M.S.; Poon, R.; Syed, A.M.; Milne, J.; Zhitomirsky, I. New developments in non-covalent surface modification, dispersion and electrophoretic deposition of carbon nanotubes. Carbon 2018, 130, 584–598. [Google Scholar] [CrossRef]
- Ata, M.; Liu, Y.; Zhitomirsky, I. A review of new methods of surface chemical modification, dispersion and electrophoretic deposition of metal oxide particles. RSC Adv. 2014, 4, 22716–22732. [Google Scholar] [CrossRef]
- Silva, R.M.E.; Poon, R.; Milne, J.; Syed, A.; Zhitomirsky, I. New developments in liquid-liquid extraction, surface modification and agglomerate-free processing of inorganic particles. Adv. Colloid Interface Sci. 2018, 261, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhou, R.; Duan, Z. Separation of isopropanol from aqueous solution by salting-out extraction. J. Chem. Technol. Biotechnol. 2001, 76, 757–763. [Google Scholar]
- Chung, N.H.; Tabata, M. Salting-out phase separation of the mixture of 2-propanol and water for selective extraction of cobalt (II) in the presence of manganese (II), nickel (II), and copper (II). Hydrometallurgy 2004, 73, 81–89. [Google Scholar] [CrossRef]
- Chung, N.H.; Tabata, M. Selective extraction of gold (III) in the presence of Pd (II) and Pt (IV) by salting-out of the mixture of 2-propanol and water. Talanta 2002, 58, 927–933. [Google Scholar] [CrossRef]
- Folkers, J.P.; Gorman, C.B.; Laibinis, P.E.; Buchholz, S.; Whitesides, G.M.; Nuzzo, R.G. Self-assembled monolayers of long-chain hydroxamic acids on the native oxide of metals. Langmuir 1995, 11, 813–824. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Azizi, D.; Larachi, F. Hydroxamic acid interactions with solvated cerium hydroxides in the flotation of monazite and bastnäsite—Experiments and DFT study. Appl. Surf. Sci. 2016, 387, 986–995. [Google Scholar] [CrossRef]
- Eisenlauer, J.; Matijević, E. Interactions of metal hydrous oxides with chelating agents. II. α-Fe2O3—low molecular and polymeric hydroxamic acid species. J. Colloid Interface Sci. 1980, 75, 199–211. [Google Scholar] [CrossRef]
- Natarajan, R.; Fuerstenau, D. Adsorption and flotation behavior of manganese dioxide in the presence of octyl hydroxamate. Int. J. Miner. Process. 1983, 11, 139–153. [Google Scholar] [CrossRef]
- Ata, M.S.; Milne, J.; Zhitomirsky, I. Fabrication of Mn3O4—carbon nanotube composites with high areal capacitance using cationic and anionic dispersants. J. Colloid Interface Sci. 2018, 512, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.; Zhitomirsky, I. Application of Cyrene as a solvent and dispersing agent for fabrication of Mn3O4-carbon nanotube supercapacitor electrodes. Colloid Interface Sci. Commun. 2020, 34, 100226. [Google Scholar] [CrossRef]
- Poon, R.; Zhao, X.; Ata, M.S.; Clifford, A.; Zhitomirsky, I. Phase transfer of oxide particles for application in thin films and supercapacitors. Ceram. Int. 2017, 43, 8314–8320. [Google Scholar] [CrossRef]
- Taberna, P.; Simon, P.; Fauvarque, J.-F. Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors. J. Electrochem. Soc. 2003, 150, A292–A300. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rorabeck, K.; Zhitomirsky, I. Application of Octanohydroxamic Acid for Salting out Liquid–Liquid Extraction of Materials for Energy Storage in Supercapacitors. Molecules 2021, 26, 296. https://doi.org/10.3390/molecules26020296
Rorabeck K, Zhitomirsky I. Application of Octanohydroxamic Acid for Salting out Liquid–Liquid Extraction of Materials for Energy Storage in Supercapacitors. Molecules. 2021; 26(2):296. https://doi.org/10.3390/molecules26020296
Chicago/Turabian StyleRorabeck, Kaelan, and Igor Zhitomirsky. 2021. "Application of Octanohydroxamic Acid for Salting out Liquid–Liquid Extraction of Materials for Energy Storage in Supercapacitors" Molecules 26, no. 2: 296. https://doi.org/10.3390/molecules26020296
APA StyleRorabeck, K., & Zhitomirsky, I. (2021). Application of Octanohydroxamic Acid for Salting out Liquid–Liquid Extraction of Materials for Energy Storage in Supercapacitors. Molecules, 26(2), 296. https://doi.org/10.3390/molecules26020296



