Shelf-Life Stability of Ready-to-Use Green Rooibos Iced Tea Powder—Assessment of Physical, Chemical, and Sensory Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Ingredients
2.1.1. Compatibility of Food Ingredients with IN50
2.1.2. Moisture Sorption Isotherms (MSIs)
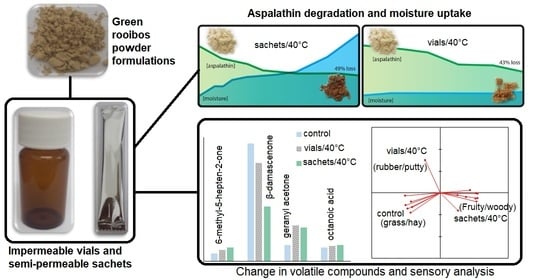
2.2. Physical Changes in Powders during Storage
2.3. Chemical Stability and Kinetic Modelling of the Degradation of Dihydrochalcones
2.4. Change in the Sensory Profile of M3 during Storage
2.5. Change in the Volatile Profile of M3 during Storage
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Spray-Drying and Microencapsulation
3.3. Preparation of Green Rooibos Powders
3.4. Storage Stability Testing and Kinetic Modelling of Aspalathin and Nothofagin Degradation
3.5. Physicochemical Analysis
3.6. Descriptive Sensory Analysis (DSA), PH, and Colour of Reconstituted Beverages
3.7. GC-TOF-MS Analysis of Reconstituted Beverages
3.7.1. Headspace-Solid Phase Micro-Extraction (HS-SPME) Procedure
3.7.2. GC-TOF-MS Conditions
3.7.3. Data Processing
3.8. Statistical Analyses
3.8.1. Rooibos Powder Stability Data
3.8.2. DSA and GC-TOF-MS Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals. Available online: https://books.google.co.za/books?id=-A4LDgAAQBAJ (accessed on 4 February 2021).
- Di Monaco, R.; Miele, N.A.; Cabisidan, E.K.; Cavella, S. Strategies to reduce sugars in food. Curr. Opin. Food Sci. 2018, 19, 92–97. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional beverages: The emerging side of functional foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- González, F.; García-Martínez, E.; Del Mar Camacho, M.; Martínez-Navarrete, N. Stability of the physical properties, bioactive compounds and antioxidant capacity of spray-dried grapefruit powder. Food Biosci. 2019, 28, 74–82. [Google Scholar] [CrossRef]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef]
- Muller, C.J.F.; Malherbe, C.J.; Chellan, N.; Yagasaki, K.; Miura, Y.; Joubert, E. Potential of rooibos, its major C-glucosyl flavonoids, and Z-2-(β-D-glucopyranosyloxy)-3-phenylpropenoic acid in prevention of metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2018, 58, 227–246. [Google Scholar] [CrossRef]
- Orlando, P.; Chellan, N.; Louw, J.; Tiano, L.; Cirilli, I.; Dludla, P.; Joubert, E.; Muller, C.J.F. Aspalathin-rich green rooibos extract lowers LDL-cholesterol and oxidative status in high-fat diet-induced diabetic vervet monkeys. Molecules 2019, 24, 1713. [Google Scholar] [CrossRef] [Green Version]
- De Beer, D.; Joubert, E.; Viljoen, M.; Manley, M. Enhancing aspalathin stability in rooibos (Aspalathus linearis) ready-to-drink iced teas during storage: The role of nano-emulsification and beverage ingredients, citric and ascorbic acids. J. Sci. Food Agric. 2012, 92, 274–282. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; Tobin, J.; Walczak, B.; van der Rijst, M.; Joubert, E. Phenolic composition of rooibos changes during simulated fermentation: Effect of endogenous enzymes and fermentation temperature on reaction kinetics. Food Res. Int. 2019, 121, 185–196. [Google Scholar] [CrossRef]
- Krafczyk, N.; Glomb, M.A. Characterization of phenolic compounds in rooibos tea. J. Agric. Food Chem. 2008, 56, 3368–3376. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, T.; Willenberg, I.; Glomb, M.A. Chemistry of color formation during rooibos fermentation. J. Agric. Food Chem. 2012, 60, 5221–5228. [Google Scholar] [CrossRef]
- Mertens, N.; Heymann, T.; Glomb, M.A. Oxidative fragmentation of aspalathin leads to the formation of dihydrocaffeic acid and the related lysine amide adduct. J. Agric. Food Chem. 2020, 68, 13111–13120. [Google Scholar] [CrossRef]
- Verbeke, W.; Scholderer, J.; Lähteenmäki, L. Consumer appeal of nutrition and health claims in three existing product concepts. Appetite 2009, 52, 684–692. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Qian, Y.P.L.; Qian, M.C. Flavor and chiral stability of lemon-flavored hard tea during storage. Food Chem. 2018, 239, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Kaack, K.; Christensen, L.P. Effect of packing materials and storage time on volatile compounds in tea processed from flowers of black elder (Sambucus nigra L.). Eur. Food Res. Technol. 2008, 227, 1259–1273. [Google Scholar] [CrossRef]
- Ortiz, J.; Kestur, U.S.; Taylor, L.S.; Mauer, L.J. Interaction of environmental moisture with powdered green tea formulations: Relationship between catechin stability and moisture-induced phase transformations. J. Agric. Food Chem. 2009, 57, 4691–4697. [Google Scholar] [CrossRef] [Green Version]
- De Beer, D.; Pauck, C.E.; Aucamp, M.; Liebenberg, W.; Stieger, N.; van der Rijst, M.; Joubert, E. Phenolic and physicochemical stability of a functional beverage powder mixture during storage- effect of the microencapsulant inulin and food ingredients. J. Sci. Food Agric. 2018, 98, 2925–2934. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Prebiotic capacity of inulin-type fructans. J. Nutr. 2007, 137, 2503S–2506S. [Google Scholar] [CrossRef] [Green Version]
- Joubert, E.; Viljoen, M.; De Beer, D.; Manley, M. Effect of heat on aspalathin, iso-orientin, and orientin contents and color of fermented rooibos (Aspalathus linearis) iced tea. J. Agric. Food Chem. 2009, 57, 4204–4211. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. Effects of xylitol as a sugar substitute on diabetes-related parameters in nondiabetic rats. J. Med. Food 2011, 14, 505–511. [Google Scholar] [CrossRef]
- Storey, D.; Lee, A.; Bornet, F.; Brouns, F. Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid. Eur. J. Clin. Nutr. 2007, 61, 349–354. [Google Scholar] [CrossRef]
- Schmitt, E.A.; Peck, K.; Sun, Y.; Geoffroy, J.M. Rapid, practical and predictive excipient compatibility screening using isothermal microcalorimetry. Thermochim. Acta 2001, 380, 175–184. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; McMinn, W.A.M.; Magee, T.R.A. Moisture sorption isotherm characteristics of food products: A review. Food Bioprod. Process. 2002, 80, 118–128. [Google Scholar] [CrossRef]
- Mauer, L.J.; Taylor, L.S. Water-solids interactions: Deliquescence. Annu. Rev. Food Sci. Technol. 2010, 1, 41–63. [Google Scholar] [CrossRef]
- Labuza, T.P.; Altunakar, L. Water activity prediction and moisture sorption isotherms. In Water Activity in Foods; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 109–154. ISBN 0813824087. [Google Scholar]
- Bott, R.F.; Labuza, T.P.; Oliveira, W.P. Stability testing of spray- and spouted bed-dried extracts of Passiflora alata. Dry. Technol. 2010, 28, 1255–1265. [Google Scholar] [CrossRef]
- Apelblat, A. Properties of citric acid and its solutions. In Citric Acid; Springer International Publishing: Cham, Switzerland, 2014; pp. 13–141. ISBN 9783319112336. [Google Scholar]
- Sun, C. Improving powder flow properties of citric acid by crystal hydration. J. Pharm. Sci. 2009, 98, 1744–1749. [Google Scholar] [CrossRef]
- Stoklosa, A.M.; Lipasek, R.A.; Taylor, L.S.; Mauer, L.J. Effects of storage conditions, formulation, and particle size on moisture sorption and flowability of powders: A study of deliquescent ingredient blends. Food Res. Int. 2012, 49, 783–791. [Google Scholar] [CrossRef]
- De Paepe, D.; Valkenborg, D.; Coudijzer, K.; Noten, B.; Servaes, K.; De Loose, M.; Voorspoels, S.; Diels, L.; van Droogenbroeck, B. Thermal degradation of cloudy apple juice phenolic constituents. Food Chem. 2014, 162, 176–185. [Google Scholar] [CrossRef]
- Beelders, T.; De Beer, D.; Joubert, E. Thermal degradation kinetics modeling of benzophenones and xanthones during high-temperature oxidation of Cyclopia genistoides (L.) Vent. plant material. J. Agric. Food Chem. 2015, 63, 5518–5527. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Taylor, L.S.; Mauer, L.J. The physical and chemical stability of amorphous (-)-epi-gallocatechin gallate: Effects of water vapor sorption and storage temperature. Food Res. Int. 2014, 58, 112–123. [Google Scholar] [CrossRef]
- Ortiz, J.; Ferruzzi, M.G.; Taylor, L.S.; Mauer, L.J. Interaction of environmental moisture with powdered green tea formulations: Effect on catechin chemical stability. J. Agric. Food Chem. 2008, 56, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Thorat, A.; Marrs, K.N.; Ghorab, M.K.; Meunier, V.; Forny, L.; Taylor, L.S.; Mauer, L.J. Moisture-mediated interactions between amorphous maltodextrins and crystalline fructose. J. Food Sci. 2017, 82, 1142–1156. [Google Scholar] [CrossRef]
- Joubert, E.; Viljoen, M.; De Beer, D.; Malherbe, C.J.; Brand, D.J.; Manley, M. Use of green rooibos (Aspalathus linearis) extract and water-soluble nanomicelles of green rooibos extract encapsulated with ascorbic acid for enhanced aspalathin content in ready-to-drink iced teas. J. Agric. Food Chem. 2010, 58, 10965–10971. [Google Scholar] [CrossRef] [PubMed]
- Jolley, B.; van der Rijst, M.; Joubert, E.; Muller, M. Sensory profile of rooibos originating from the Western and Northern Cape governed by production year and development of rooibos aroma wheel. S. Afr. J. Bot. 2017, 110, 161–166. [Google Scholar] [CrossRef]
- Koch, I.S.; Muller, M.; Joubert, E.; van der Rijst, M.; Næs, T. Sensory characterization of rooibos tea and the development of a rooibos sensory wheel and lexicon. Food Res. Int. 2012, 46, 217–228. [Google Scholar] [CrossRef]
- Muller, M.; De Beer, D.; Truzzi, C.; Annibaldi, A.; Carloni, P.; Girolametti, F.; Damiani, E.; Joubert, E. Cold brewing of rooibos tea affects its sensory profile and physicochemical properties compared to regular hot, and boiled brewing. LWT 2020, 132, 109919. [Google Scholar] [CrossRef]
- Lule, S.U.; Xia, W. Food phenolics, pros and cons: A review. Food Rev. Int. 2005, 21, 367–388. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.-P.; Campo, E.; Fernández-Zurbano, P.; Valentin, D.; Ferreira, V. An assessment of the effects of wine volatiles on the perception of taste and astringency in wine. Food Chem. 2010, 121, 1139–1149. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Sensory evaluation of bitterness and astringency sub-qualities of wine phenolic compounds: Synergistic effect and modulation by aromas. Food Res. Int. 2014, 62, 1100–1107. [Google Scholar] [CrossRef] [Green Version]
- Labbe, D.; Rytz, A.; Morgenegg, C.; Ali, S.; Martin, N. Subthreshold olfactory stimulation can enhance sweetness. Chem. Senses 2007, 32, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Habu, T.; Flath, R.A.; Mon, T.R.; Morton, J.F. Volatile components of rooibos tea (Aspalathus linearis). J. Agric. Food Chem. 1985, 33, 249–254. [Google Scholar] [CrossRef]
- Du Preez, B.V.P.; De Beer, D.; Moelich, E.I.; Muller, M.; Joubert, E. Development of chemical-based reference standards for rooibos and honeybush aroma lexicons. Food Res. Int. 2020, 127, 108734. [Google Scholar] [CrossRef]
- Song, N.E.; Kim, M.K.; Lee, K.G.; Jang, H.W. Analysis of volatile compounds in rooibos tea (Aspalathus linearis) using different extraction methods and their relationship with human sensory perception. Food Res. Int. 2021, 141, 109942. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-Q.; Wang, J.-Q.; Chen, J.-X.; Wang, F.; Yin, J.-F.; Zeng, L.; Shi, J.; Xu, Y.-Q. Effect of baking on the flavor stability of green tea beverages. Food Chem. 2020, 331, 127258. [Google Scholar] [CrossRef] [PubMed]
- Marteau, C.; Ruyffelaere, F.; Aubry, J.M.; Penverne, C.; Favier, D.; Nardello-Rataj, V. Oxidative degradation of fragrant aldehydes. Autoxidation by molecular oxygen. Tetrahedron 2013, 69, 2268–2275. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Bouwmeester, H.J.; Gershenzon, J.; Konings, M.C.J.M.; Croteau, R. Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway: I. Demonstration of enzyme activities and their changes with development. Plant Physiol. 1998, 117, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.; De Beer, D.; Aucamp, M.; Malherbe, C.J.; Joubert, E. Inulin as microencapsulating agent improves physicochemical properties of spray-dried aspalathin-rich green rooibos (Aspalathus linearis) extract with α-glucosidase inhibitory activity. J. Funct. Foods 2018, 48, 400–409. [Google Scholar] [CrossRef]
- Flexifoil Packaging PVT LTD. Available online: http://www.flexifoilpackaging.com/barrier-properties-films (accessed on 26 April 2019).
- Walters, N.A.; De Villiers, A.; Joubert, E.; De Beer, D. Improved HPLC method for rooibos phenolics targeting changes due to fermentation. J. Food Compos. Anal. 2017, 55, 20–29. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. Kinetic modeling of food quality: A critical review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 144–158. [Google Scholar] [CrossRef]
- Human, C.; De Beer, D.; Aucamp, M.; Marx, I.J.; Malherbe, C.J.; Viljoen-Bloom, M.; van der Rijst, M.; Joubert, E. Preparation of rooibos extract-chitosan microparticles: Physicochemical characterisation and stability of aspalathin during accelerated storage. LWT Food Sci. Technol. 2020, 117, 108653. [Google Scholar] [CrossRef]
- Pati, S.; Tufariello, M.; Crupi, P.; Coletta, A.; Grieco, F.; Losito, I. Quantification of volatile vompounds in wines by HS-SPME-GC/MS: Critical issues and use of multivariate statistics in method optimization. Processes 2021, 9, 662. [Google Scholar] [CrossRef]
- Næs, T.; Brockhoff, P.; Tomic, O. Statistics for Sensory and Consumer Science, 1st ed.; Wiley: New York, NY, USA, 2010; ISBN 9780470518212. [Google Scholar]










| Powder | Interaction Average Heat Flow Error (µW/g) 1 | Moisture Content (% Dry Basis) | aw | Parameters of GAB Model | Parameters of BET Model | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M02 | C | K | R2 | M0 | C | R2 | ||||
| GRE 3 | N/A 4 | 2.40 ± 0.14 b 5 | 0.176 ± 0.006 c | 3.41 | 10.1 | 1.2 | 0.9503 | 3.73 | 10.4 | 0.9981 |
| IN50 6 | 0.76 | 2.98 ± 0.10 a | 0.149 ± 0.004 d | 3.83 | 6.9 | 1.2 | 0.8690 | 3.80 | 9.2 | 0.9986 |
| M1 7 | 4.90 | 0.661 ± 0.036 c | 0.290 ± 0.013 b | 0.178 | 11.1 | 1.1 | 0.7799 | 0.224 | 7.1 | 0.9743 |
| M2 8 | 1.29 | 0.348 ± 0.035 e | 0.323 ± 0.011 a | 0.148 | 12.5 | 1.4 | 0.7745 | 0.263 | 5.5 | 0.9733 |
| M3 9 | 4.85 | 0.489 ± 0.035 d | 0.321 ± 0.010 a | 0.154 | 11.7 | 1.4 | 0.7877 | 0.244 | 6.7 | 0.9955 |
| M4 10 | 3.13 | 0.517 ± 0.012 d | 0.322 ± 0.022 a | 0.184 | 11.1 | 1.1 | 0.7989 | 0.240 | 7.4 | 0.9721 |
| M5 11 | 3.45 | 0.596 ± 0.016 cd | 0.333 ± 0.004 a | 0.154 | 10.0 | 1.4 | 0.7287 | 0.255 | 5.7 | 0.9849 |
| Powder | Sealed Glass Vials | Semi-Permeable Sachets | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 °C/65% RH | 40 °C/65% RH | 30 °C/65% RH | 40 °C/65% RH | |||||||
| % Decrease (5 Months) | % Decrease (12 Months) | K (months−1) | % Decrease (5 Months) | % Decrease (12 Months) | K (months−1) | % Decrease (5 Months) | K (months−1) | % Decrease (5 Months) | K (months−1) | |
| GRE 2 | 3.6 ± 0.5 c 3 | 6.7 ± 1.3 d | 4 | 6.2 ± 0.5 d | 17.8 ± 0.8 f | 0.019 ± 0.003 e | 4.9 ± 1.1 e | 4 | 18.5 ± 0.2 f | 0.035 ± 0.003 e |
| IN50 5 | 1.9 ± 1.6 c | 7.3 ± 0.3 d | 4 | 3.7 ± 0.4 d | 9.7 ± 0.4 g | 4 | 0.3 ± 0.7 f | 4 | 4.4 ± 0.7 g | 4 |
| M1 6 | 15.3 ± 1.5 b | 17.7 ± 1.6 c | 0.016 ± 0.003 c | 34.0 ± 2.0 c | 50.1 ± 1.0 e | 0.069 ± 0.005 d | 25.2 ± 2.1 c | 0.044 ± 0.008 b | 39.8 ± 2.8 e | 0.090 ± 0.018 d |
| M2 7 | 12.1 ± 2.9 b | 34.3 ± 3.0 b | 0.040 ± 0.002 b | 40.8 ± 2.3 b | 76.9 ± 0.6 d | 0.114 ± 0.007 c | 21.0 ± 2.1 d | 0.035 ± 0.005 b | 61.5 ± 0.8 c | 0.149 ± 0.014 b |
| M3 8 | 11.9 ± 2.6 b | 50.6 ± 2.3 a | 0.056 ± 0.006 a | 41.7 ± 1.5 b | 87.0 ± 0.5 b | 0.140 ± 0.003 b | 29.9 ± 1.9 b | 0.048 ± 0.012 b | 50.2 ± 2.4 d | 0.114 ± 0.006 c |
| M4 9 | 27.4 ± 1.4 a | 35.0 ± 2.7 b | 0.040 ± 0.003 b | 54.1 ± 1.3 a | 85.5 ± 0.7 c | 0.156 ± 0.0041a | 41.2 ± 2.6 a | 0.088 ± 0.011 a | 82.1 ± 1.1 a | 0.283 ± 0.010 a |
| M5 10 | 25.9 ± 3.6 a | 36.8 ± 3.0 b | 0.040 ± 0.004 b | 54.2 ± 5.3 a | 90.3 ± 0.4 a | 0.158 ± 0.012 a | 41.9 ± 1.4 a | 0.087 ± 0.011 a | 67.0 ± 2.7 b | 0.167 ± 0.003 b |
| Storage Condition | L* | a* | b* | C* | h | ΔE |
|---|---|---|---|---|---|---|
| ctrl | 54.48 ± 0.79 b 5 | 4.81 ± 0.18 e | 24.96 ± 0.28 e | 25.15 ± 0.28 e | 78.97 ± 0.40 a | 0.00 ± 0.00 e |
| s_30 °C | 55.83 ± 0.56 a | 5.54 ± 0.31 c | 27.65 ± 0.24 c | 28.20 ± 0.27 c | 78.68 ± 0.57 ab | 3.39 ± 0.32 c |
| s_40 °C | 51.01 ± 0.50 d | 9.93 ± 0.15 a | 36.04 ± 0.18 a | 37.38 ± 0.20 a | 74.60 ± 0.19 d | 12.93 ± 0.27 a |
| v_30 °C | 54.24 ± 0.54 b | 5.22 ± 0.11 d | 25.27 ± 0.35 d | 25.80 ± 0.36 d | 78.32 ± 0.11 b | 0.88 ± 0.42 d |
| v_40 °C | 53.53 ± 0.41 c | 6.83 ± 0.25 b | 28.33 ± 0.50 b | 29.04 ± 0.51 b | 77.33 ± 0.44 c | 4.10 ± 0.56 b |
| Peak No. | Compound Name | RIcal 3 | RIlit 4 | Aroma Description 5 |
|---|---|---|---|---|
| 1 | hexanal | 1060 | 1083 | green, grass, fatty |
| 2 | β-myrcene | 1129 | 1161 | spicy, balsamic, plastic |
| 3 | cumene | 1137 | 1180 | not available |
| 4 | limonene | 1161 | 1213 | citrus, camphor |
| 5 | 2-hexenal | 1196 | 1213 | green, apple |
| 6 | 2-pentylfuran | 1202 | 1231 | fruity, green, earthy |
| 7 | ethyl hexanoate | 1208 | 1233 | fruity, apple peel, pineapple |
| 8 | cymene | 1239 | 1275 | terpenic, fresh citrus, solvent, |
| 9 | octanal | 1264 | 1289 | aldehydic, fat, citrus, green |
| 10 | 1-octen-3-one | 1278 | 1300 | earthy, mushroom, metal |
| 11 | (Z)-2-heptenal | 1302 | 1322 | green, fat, rancid |
| 12 | 6-methyl-5-hepten-2-one | 1316 | 1338 | citrus, lemongrass, apple |
| 13 | (E)-2-octenal | 1408 | 1429 | fatty, fresh cucumber, green |
| 14 | 1-octen-3-ol | 1428 | 1450 | earthy, soap, plastic |
| 15 | 2-ethyl-1-hexanol | 1467 | 1491 | citrus, floral |
| 16 | (E)-2-nonenal | 1515 | 1534 | fatty, green cucumber |
| 17 | linalool | 1526 | 1547 | floral |
| 18 | 1-octanol | 1535 | 1557 | waxy, moss, nut |
| 19 | (E,Z)-2,6-nonadienal | 1566 | 1584 | green, cucumber, leaf green, fat |
| 20 | 6-methyl-3,5-heptadien-2-one | 1577 | 1602 | spicy, cinnamon, woody |
| 21 | 2,5,5,8α-tetramethyl-3,5,6,8α-tetrahydro-2H-chromene | 1585 | 1611 | not available |
| 22 | (E)-2-decenal | 1623 | 1644 | fatty, waxy, coriander, green |
| 23 | 1-nonanol | 1638 | 1660 | floral, fatty, dusty, oily |
| 24 | 2-undecenal | 1732 | 1751 | fruity-sweet, orange peel |
| 25 | (E)-β-damascenone | 1800 | 1823 | fruity, apple, honey, tobacco |
| 26 | hexanoic acid | 1824 | 1846 | fatty, sour, sweat |
| 27 | geranyl acetone | 1835 | 1859 | floral, citrus, magnolia |
| 28 | α-calacorene | 1892 | 1919 | woody |
| 29 | 3-(2,6,6-trimethyl-1-cyclohexen-1-yl-2-propenal) | 1902 | 1936 | not available |
| 30 | heptanoic acid | 1931 | 1950 | cheesy, rancid sour |
| 31 | octanoic acid | 2037 | 2060 | fatty, rancid, oily, vegetable |
| 32 | nonanoic acid | 2143 | 2171 | waxy, green, fatty |
| 33 | cadalene | 2200 | 2233 | not found |
| 34 | n-decanoic acid | 2250 | 2276 | fatty, rancid |
| 35 | 3,5-di-tert-butylphenol | 2291 | 2319 | not found |
| 36 | dodecanoic acid | 2462 | 2498 | fatty, coconut, metal |
| 37 | hexadecanoic acid | 2884 | 2915 | waxy, creamy |
| Ingredient | Rooibos Powders | ||||||
|---|---|---|---|---|---|---|---|
| GRE | IN50 | M1 | M2 | M3 | M4 | M5 | |
| GRE | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| IN50 | 0 | 100.00 | 5.54 | 5.54 | 5.54 | 5.54 | 5.54 |
| Sucrose | 0.00 | 0.00 | 94.46 | 0.00 | 0.00 | 0.00 | 0.00 |
| Xylitol | 0.00 | 0.00 | 0.00 | 94.46 | 92.58 | 94.15 | 92.26 |
| Ascorbic acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.31 | 0.31 |
| Citric acid | 0.00 | 0.00 | 0.00 | 0.00 | 1.89 | 0.00 | 1.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Human, C.; de Beer, D.; Muller, M.; van der Rijst, M.; Aucamp, M.; Tredoux, A.; de Villiers, A.; Joubert, E. Shelf-Life Stability of Ready-to-Use Green Rooibos Iced Tea Powder—Assessment of Physical, Chemical, and Sensory Properties. Molecules 2021, 26, 5260. https://doi.org/10.3390/molecules26175260
Human C, de Beer D, Muller M, van der Rijst M, Aucamp M, Tredoux A, de Villiers A, Joubert E. Shelf-Life Stability of Ready-to-Use Green Rooibos Iced Tea Powder—Assessment of Physical, Chemical, and Sensory Properties. Molecules. 2021; 26(17):5260. https://doi.org/10.3390/molecules26175260
Chicago/Turabian StyleHuman, Chantelle, Dalene de Beer, Magdalena Muller, Marieta van der Rijst, Marique Aucamp, Andreas Tredoux, André de Villiers, and Elizabeth Joubert. 2021. "Shelf-Life Stability of Ready-to-Use Green Rooibos Iced Tea Powder—Assessment of Physical, Chemical, and Sensory Properties" Molecules 26, no. 17: 5260. https://doi.org/10.3390/molecules26175260
APA StyleHuman, C., de Beer, D., Muller, M., van der Rijst, M., Aucamp, M., Tredoux, A., de Villiers, A., & Joubert, E. (2021). Shelf-Life Stability of Ready-to-Use Green Rooibos Iced Tea Powder—Assessment of Physical, Chemical, and Sensory Properties. Molecules, 26(17), 5260. https://doi.org/10.3390/molecules26175260