Abstract
Nitrogen-rich porous networks with additional polarity and basicity may serve as effective adsorbents for the Lewis electron pairing of iodine molecules. Herein a carbazole-functionalized porous aromatic framework (PAF) was synthesized through a Sonogashira–Hagihara cross-coupling polymerization of 1,3,5-triethynylbenzene and 2,7-dibromocarbazole building monomers. The resulting solid with a high nitrogen content incorporated the Lewis electron pairing effect into a π-conjugated nano-cavity, leading to an ultrahigh binding capability for iodine molecules. The iodine uptake per specific surface area was ~8 mg m−2 which achieved the highest level among all reported I2 adsorbents, surpassing that of the pure biphenyl-based PAF sample by ca. 30 times. Our study illustrated a new possibility for introducing electron-rich building units into the design and synthesis of porous adsorbents for effective capture and removal of volatile iodine from nuclear waste and leakage.
1. Introduction
To overcome the energy shortages and environmental concerns originated from fossil fuels, nuclear power, the only mature technology, is considered a possible approach for providing electricity on a large scale with little greenhouse gases emission [1]. However, the treatment of nuclear waste and the emergency response for nuclear leakage, cause consternation in the increasing development of the nuclear industry [2]. The 129I and 131I atoms originated from the uranium fission are the two main ingredients of nuclear waste, especially 129I, which has an ultra-long radioactive half-life (t1/2 = 15.7 × 106 years) [3,4]. Because the enrichment and toxic effects in organisms, effective methods for the capture and removal of radiological iodine aroused strong concerns. To date, several strategies have been proposed, including dry dedusting [5,6], chemical precipitation [7], and physical adsorption [8,9,10]. Among them, the physical adsorption method has specific advantages of high adsorption efficiency, low cost, simple operation, and high recyclability [11,12].
Porous aromatic frameworks (PAFs) composed of covalently bonded light atoms (H, B, C, N, and O), have superb thermal and chemical stability, high surface area, and tunable pore size, which make them ideal candidates for iodine capture from the nuclear waste stream containing volatile iodine radionuclides [13,14,15,16]. In the past few decades, PAF solids with tunable pore properties including surface area, volume, and size distribution were demonstrated to play important roles for the physical adsorption for guest molecules [17,18,19,20]. However, pure carbon-based PAFs with a micropore cavity do not show an excellent capacity and fast kinetics for I2 matter adsorption. For instance, PAF-1 with an exceptionally high surface area (5600 m2 g−1) and micropore volume (0.89 cm3 g−1) exhibits a low iodine vapor capture capability with 186 wt% at 298 K per 40 Pa [21]. It is obvious that the adsorption capacity of the adsorbent for iodine is not only related to the surface area and pore size, but the effective adsorption sites on the accessible surface may possess a more important role to interact with volatile iodine gases. A detailed investigation should be conducted to reveal the relationship between the chemical features of PAFs and iodine molecules, which provides significant advantages and opportunities of PAFs for the development of next-generation porous adsorptions.
Based on the polarization effect, active sites transform the speciation of iodine molecules into multiple oxidation states (−1, 0, +1, +3, +5, and +7), primarily as molecular iodine (I2), iodide (I−), iodate, or organic iodine (org-I) [22,23,24,25]. A nitrogenous fragment possesses lone pair electrons, thereby revealing highly negative charge to enhance the binding affinity for the polarizable electron cloud of I2 molecules [26]. Herein, 2,7-dibromocarbazole was adopted as the functional building monomer to prepare a carbazole-containing PAF network through a one-step Sonogashira-Hagihara coupling reaction. Consequently, the resulting PAF sample with the electron-rich system exhibits an outstanding performance for the capture of a volatile iodine with an uptake of 2.10 g g−1. The results of this study provide useful guidance for the development of new porous adsorbents for the removal of radioactive iodine.
2. Results and Discussion
LNU-13 was synthesized through the Sonogashira-Hagihara coupling of 2,7-dibromocarbazole and 1,3,5-triethynylbenzene (Figure 1a). As determined by the Fourier transform infrared spectroscopy (FTIR, Figure 2a), the C-Br stretching vibration of 2,7-dibromocarbazole at 495 cm−1 and the C–H stretching vibration of the terminal alkyne (1,3,5-triethynylbenzene) at 3270 cm−1 disappeared from the IR spectrum of LNU-13, verifying the completeness of the Sonogashira-Hagihara coupling reaction. The structural integrity of LNU-13 was further confirmed by 13C NMR (Figure 2b). The main peaks observed in the range of 120–150 ppm were attributed to the substituted carbon of the aromatic ring connected to the benzene ring; and the resonance around 90 ppm was assigned to the carbons originated from the –C≡C– group.
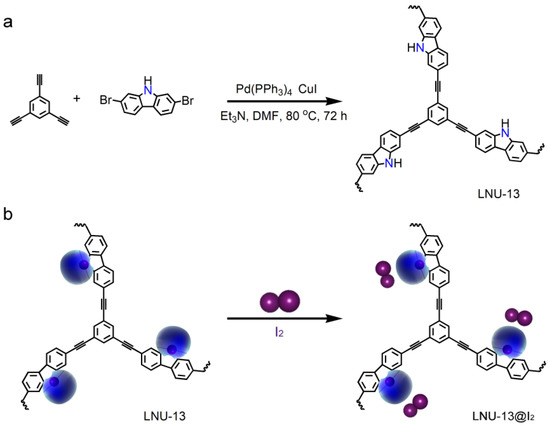
Figure 1.
(a) Synthesis of LNU-13 polymer; (b) schematic diagram of PAF solid for I2 sorption.
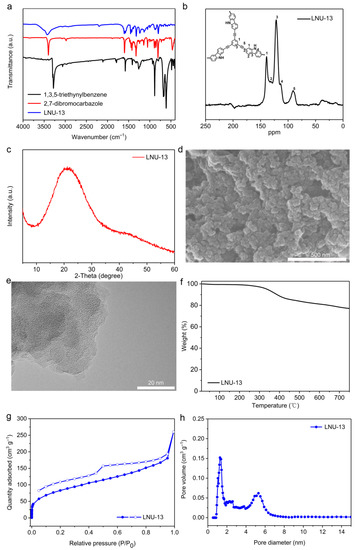
Figure 2.
(a) FTIR spectra of 2,7-dibromocarbazole, 1,3,5-triethynylbenzene, and LNU-13; (b) solid-state 13C NMR spectrum of LUN-13; (c) powder X-ray diffraction pattern of LNU-13. (d) SEM image of LNU-13; (e)TEM image of LNU-13; (f) TGA plot of LNU-13 at N2 condition with a ramp rate of 5 °C min−1; (g) N2 adsorption-desorption isotherm of LNU-13; (h) pore size distribution of LNU-13.
Powder X-ray diffraction (XRD) pattern of LNU-13 shows a characteristic broad peak, indicating they are amorphous in nature (Figure 2c). It seems that the formation of the stacked layer structure by the ordered connection among the building blocks is otherwise difficult [13,27]. Scanning electron microscopy (SEM) analysis demonstrated the stacked spherical structures of LNU-13, as shown in Figure 2d. Transmission electron microscopy (TEM) clearly confirmed the amorphous structure of LNU-13 (Figure 2e). As illustrated by thermogravimetric analysis (TGA, Figure 2f), the LNU-13 material begins to degrade at 350 °C and the weight loss is about 20% at 750 °C under a purified nitrogen atmosphere, indicating that LNU-13 possesses good thermal stability. All the results demonstrate that LNU-13 retains its intact skeleton under a variety of harsh conditions.
The porosity of the resulting PAF material was probed using N2 adsorption-desorption isotherms at 77 K up to 1 bar. The adsorption curve combined the features of type-I and type-IV adsorption isotherms, indicating the co-existence of a micro- and meso-pore system (Figure 2g). The BET surface area of LNU-13 was determined to be 255 m2 g−1. LNU-13 possessed wide pore size distribution in the range of 1–6 nm calculated using a nonlocalized DFT (NL-DFT) (Figure 2h). This hierarchical porous structure made the PAF solid an excellent scaffold for the access of the I2 guest into the internal space of LNU particle [28,29].
The iodine uptake measurement of LUN-13 was conducted by placing the PAF powder into a sealed vessel filled with iodine vapor at 348 K under normal atmosphere. As shown in Figure 3a, the iodine adsorption capacity increased significantly with the prolonging of the contact time. In the first 5 h, the adsorption capacity of LNU-13 was very fast with a value of 1.75 g g−1. No further change in iodine loading was observed after 48 h exposure, indicating that LNU-13 was basically saturated (2.10 g g−1). A significant color change in the powder from brown to black was observed (Figure 3a inset). Calculated by the BET surface area (255 m2 g−1), the iodine uptake per specific surface area was ~ 8 mg m−2 which achieved the highest level among silver-containing zeolite [30], metal-organic frameworks (MOFs), and conjugated microporous polymers (CMPs), etc., reported by the same adsorption method, surpassing that of PAF-1 by ca. 30 times (Figure 4). Moreover, it also has a certain competitiveness compared with other forms of adsorbent, such as carbon foam, fiber adsorbent, carbon cloth, aerogel, etc., including BN foam (2.12 g g−1) [31], PE/PP-g-PNVP fibers (1.2378 g g−1) [32], C60-CC-PNP (2.4 g g−1) [33], CC-PNP (1.02 g g−1) [33], ENTDAT dried gel (1.8 g g−1) [34], G-TP5 (0.67 g g−1) [35] and G-TP6 (0.58 g g−1) [35].
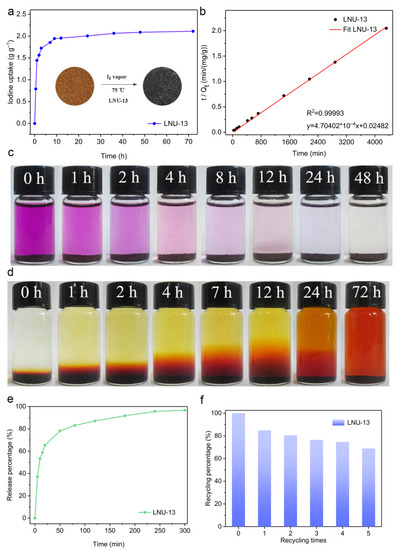
Figure 3.
(a) I2 adsorption curve of LNU-13 at 348 K. Inset: the photographs reveal the color change in LNU-13 before and after iodine adsorption; (b) curve-fitting for the I2 adsorption process; (c) photographs showing the iodine-adsorbed process in n-hexane; (d) photographs showing the iodine-released process of LNU-13@I2 in ethanol; (e) I2 release curve of LNU-13@I2 at 398 K; (f) recycling experiment of LNU-13.
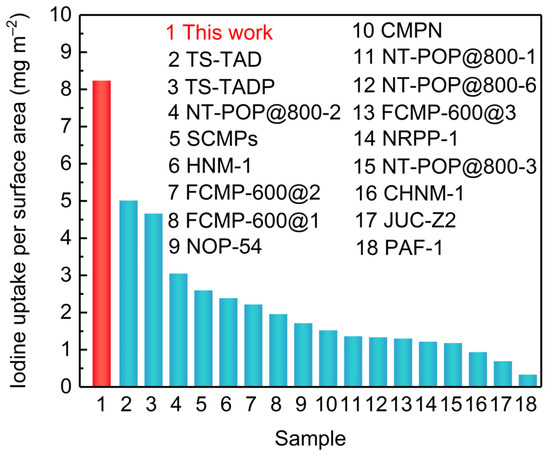
Figure 4.
Iodine uptake capacities of different adsorbents.
The adsorption mechanism of iodine vapor in LNU-13 was studied through PXRD, Raman, and FT-IR spectroscopy. Curve-fitting for the I2 adsorption isotherm was based on pseudo-second-order kinetics (Figure 3b), a high correlation coefficient (R2 = 0.99993) suggested the chemical adsorption process of LNU-13. As shown in Figure 5a, there were no characteristic peaks of I2 crystal diffraction peaks observed in the iodine-loaded LNU-13 (LNU-13@I2). This phenomenon proved the monodispersed iodine species in the form of molecular or ionic states in the PAF architecture [27]. Raman spectroscopy of LNU-13@I2 presented a series of bands centered at 110 and 170 cm−1 (Figure 5b). The characteristic bands in the region of 100–120 cm−1 were assigned to the symmetric stretching of the I3− species, while the band located at 170 cm−1 was ascribed to the higher polyiodide anions, i.e., I5− [36,37]. Comparing the FTIR spectra of pristine LNU-13 and LNU-13@I2 (Figure 5c,d), the aromatic rings were centered at 1555 cm−1 in LNU-13 vs. 1612 cm−1 in I2@LNU-13. A similar shift was also observed for the band assigned to vC–N (str) bond vibration (1234 cm−1 for LNU-13 and 1262 cm−1 for I2@LNU-13). In addition, the peak at 731 cm−1 belonged to the characteristic signal for iodine molecules. All these results indicate that the lone pair electron of the carbazole nitrogen polarizes the iodine molecule into an ionic state, and then achieves the excellent adsorption property for an iodine guest [38,39].
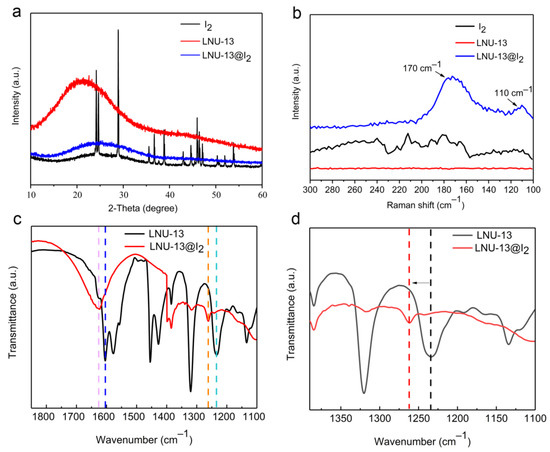
Figure 5.
(a) PXRD spectra of I2, LNU-13, and LNU-13@I2; (b) Raman spectra of I2, LNU-13, and LNU-13@I2; (c,d) FTIR spectra of LNU-13 and LNU-13@I2.
In order to evaluate the ability of LNU-13 for the capture of elemental iodine from the solution, LNU-13 powder was immersed into a closed vial containing a pre-prepared iodine elemental n-hexane solution (300 mg L−1). As depicted in Figure 3c, the color of the initial solution originated from iodine elemental substance changed from purple to colorless over time; after exposure for 24 h, the n-hexane solution containing both LNU-13 and iodine molecules became transparent and colorless, which proved that LNU-13 powder captured iodine from a n-hexane solution.
The recyclability for I2 capture is also a key parameter in practical usage. The iodine-loaded LNU-13 powder can be activated by both thermal desorption and solvent elution. The iodine adsorbed in the PAF cavity is easily released in polar organic solvents including methanol and ethanol. After immersion in an ethanol solution for 72 h, the color of the mixture gradually changes from colorless to dark brown, correspondingly, the color of the solid varies from black to brown (Figure 3d). These results manifest that guest iodine is gradually released from the PAF structure into the organic solvent. As shown in Figure 3e, the release efficiency of LNU-13@I2 is as high as 97% after the solid is heated in air at 398 K for 320 min. In addition, the LNU-13 sample withstands multiple adsorption-desorption cycles, and the adsorption capacity reaches 69% of the initial capacity after five cycles of iodine adsorption (Figure 3f).
3. Materials and Methods
3.1. Materials
2,7-Dibromocarbazole was purchased from Energy Chemical, Shanghai, China and 1,3,5-triethynylbenzene was received from TCI, Tokyo, Japan. Copper iodide and tetrakis (triphenylphosphine) palladium were obtained from Sigma-Aldrich, St. Louis, MO, USA. Other chemicals and solvents were purchased from commercial suppliers and used without further purification. All reactions were performed under a purified nitrogen atmosphere.
3.2. Synthesis of LNU-13
The 2,7-Dibromocarbazole (649 mg, 1.9976 mmol), 1,3,5-triethynylbenzene (200 mg, 1.3317 mmol), tetrakis (triphenylphosphine) palladium (30 mg), and copper (I) iodide (10 mg) were added into a round-bottom flask. The mixture was degassed through a N2 bubbling process for 30 min; after that, 20 mL of anhydrous N,N-dimethylformamide (DMF) and 8 mL of anhydrous triethylamine (TEA) were added into the system. Then, the reaction mixture was heated to 80 °C for 72 h under N2 gas atmosphere. Cooling to room temperature, the precipitate was washed with each DMF, tetrahydrofuran (THF), and acetone solvents for several times to obtain a crude product. Further purification of the product was carried out via Soxhlet extraction with THF, dichloromethane, and methanol in turns for 72 h. The product was dried in a vacuum for 10 h at 90 °C to obtain LNU-13.
3.3. Iodine Adsorption and Release
3.3.1. Iodine Adsorption from Volatile Iodine
The iodine adsorption capacity was analyzed according to the gravimetric measurements. The LNU-13 powder (30.0 mg) was loaded into a small weighing bottle, which was then placed in a closed system at 348 K (75 °C) and ambient pressure, along with excess non-radioactive solid iodine. After certain time intervals, the bottle was taken out, cooled down to room temperature and weighted, and then loaded back into the vapor of iodine to continue iodine adsorption [40,41]. The weight percentage of captured iodine was calculated using the following formula:
where m2 and m1 are the masses of PAF powder after and before iodine intake, respectively.
3.3.2. Iodine Adsorption from Solution
To evaluate the adsorption of dissolved iodine in cyclohexane, LNU-13 samples were immersed in n-hexane solution (300 mg L−1) containing iodine for 24 h, the adsorption process of iodine was photographed at selected time intervals.
3.3.3. Iodine Desorption in Solution
Ethanol was used as the extraction solvent to evaluate the reversibility of PAF materials iodine adsorption. Pouring five milliliters of ethanol to five milligrams of iodine-loaded polymer, the release process of iodine was photographed at selected time intervals.
4. Conclusions
In summary, a carbazole-based porous aromatic framework was successfully synthesized through a one-step Sonogashira-Hagihara cross-coupling polymerization. Based on the Lewis electron pairing effect, the resulting solid achieved the highest value of iodine uptake per specific surface area. The iodine uptake per specific surface area far surpassed that of silver-containing zeolite, MOFs, and CMPs, etc. Our study firmly demonstrated the important role of electron-rich units in the open architecture for capture and the removal of iodine substance, which opened a gate for the design and synthesis of porous adsorbents for remediation of radioactive iodine to address environmental issues.
Author Contributions
Z.Y., L.X. and N.B. designed and planned the project. B.C. and H.Z. conducted all of the experiments. T.Z. and Y.L. helped to characterize the samples. J.X. and N.L. helped to synthesize the materials. Z.Y. and Y.Y. helped to explain the mechanism. Z.Y., Y.Y. and B.C. analyzed the data and wrote the paper. Z.Y., L.X., N.B. and Y.Y. revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31972522, 21704037, 21671089), National Key Research and Development Project of China (2018YFC1801200), Major Science and Technology Project of Liaoning Province (2019JH1/10300001), the Scientific Research Fund of Liaoning Provincial Education Department (2020-YKLH-22), LiaoNing Revitalization Talents Program (XLYC2007032), Scientific Research Fund of Liaoning Provincial Education Department (LQN202003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to this study are presented in this publication.
Acknowledgments
All individuals appreciate the partial support of Liaoning University.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples are not available from the author.
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Hoffert, M.I.; Caldeira, K.; Benford, G.; Criswell, D.R.; Green, C.; Herzog, H.; Jain, A.K.; Kheshgi, H.S.; Lackner, K.S.; Lewis, J.S. Advanced Technology Paths to Global Climate Stability: Energy for a Greenhouse Planet. Science 2002, 298, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Zhu, Z.Q.; Qian, X.; Liang, W.D.; Mu, P.; Sun, H.X.; Liu, J.H.; Li, A. Novel thiophene-beared conjugated microporous polymer honeycomb-liked porous spheres with ultrahigh Iodine uptake. Chem. Commun. 2016, 52, 9797–9800. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.J.; Yuan, Y.; Tian, Y.Y.; Zhang, D.M.; Zhu, G.S. Highly efficient enrichment of volatile iodine by charged porous aromatic frameworks with three sorption sites. Angew. Chem. Int. Ed. 2015, 54, 12733–12737. [Google Scholar] [CrossRef]
- Liang, S.Y.; Fan, Z.Y.; Zhang, W.D.; Guo, M.; Cheng, F.Q.; Zhang, M. Controllable growth of Na2CO3 fibers for mesoporous activated alumina ball modification towards the high-efficiency adsorption of HCl gas at low temperature. RSC Adv. 2017, 7, 53306–53315. [Google Scholar] [CrossRef] [Green Version]
- Lan, Y.S.; Tong, M.M.; Yang, Q.Y.; Zhong, C.L. Computational screening of covalent organic frameworks for the capture of radioactive iodine and methyl iodide. CrystEngComm 2017, 19, 4920–4926. [Google Scholar] [CrossRef]
- Lei, Y.; Zhan, Z.S.; Saakes, M.; Weijden, R.D.; Buisman, C.J.N. Electrochemical recovery of phosphorus from acidic cheese wastewater: Feasibility, quality of products, and comparison with chemical precipitation. ACS EST Water 2021, 1, 1002–1013. [Google Scholar]
- Ighaloa, J.O.; Adeniyia, A.G.; Adelodun, A.A. Recent advances on the adsorption of herbicides and pesticides from polluted waters: Performance evaluation via physical attributes. J. Ind. Eng. Chem. 2021, 93, 117–137. [Google Scholar] [CrossRef]
- Cui, P.; Jing, X.F.; Yuan, Y.; Zhu, G.S. Synthesis of porous aromatic framework with Friedel–Crafts alkylation reaction for CO2 separation. Chin. Chem. Lett. 2016, 27, 1479–1484. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, Y.; Sun, F.X.; Zhu, G.-S. Targeted synthesis of novel porous aromatic frameworks with selective separation of CO2/CH4 and CO2/N2. Chin. Chem. Lett. 2014, 25, 1407–1410. [Google Scholar] [CrossRef]
- Rabbani, M.G.; Islamoglu, T.; El-Kaderi, H.M. Benzothiazole- and benzoxazole-linked porous polymers for carbon dioxide storage and separation. J. Mater. Chem. A 2017, 5, 258–265. [Google Scholar] [CrossRef]
- Muhammad, R.; Mohanty, P. Cyclophosphazene based hybrid nanoporous materials as superior metal-free adsorbents for gas sorption applications. Langmuir 2018, 34, 2926–2932. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Yuan, Y.; Xu, Y.M.; Zheng, G.Y.; Zhang, Q.; Jiang, Y.Q.; Wang, Z.Y.; Bu, N.S.; Xia, L.X.; Yan, Z.J. Fine-regulating ultramicropore of porous carbon via a selfsacrificial template route for high-performance supercapacitors. Nanoscale 2021, 13, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhu, G. Porous aromatic frameworks as a platform for multifunctional applications. ACS Cent. Sci. 2019, 5, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, A.G.; Jawad, K.; Yousif, E.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S. Synthesis of telmisartan organotin(IV) complexes and their use as carbon dioxide capture media. Molecules 2019, 24, 1631. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Yang, Y.J.; Zhu, G. Molecularly Imprinted Porous Aromatic Frameworks for Molecular Recognition. ACS Cent. Sci. 2020, 6, 1082–1094. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I.Y.; Lakhi, K.S.; Joseph, S.; Srivastava, P.; Naidu, R.; Vinu, A. Heteroatom functionalized activated porous biocarbons and their excellent performance for CO2 capture at high pressure. J. Mater. Chem. A 2017, 5, 21196–21204. [Google Scholar] [CrossRef] [Green Version]
- Pelech, I.; Sibera, D.; Staciwa, P.; Narkiewicz, U.; Cormia, R. Pressureless and low-pressure synthesis of microporous carbon spheres applied to CO2 adsorption. Molecules 2020, 25, 5328. [Google Scholar] [CrossRef]
- Muhammad, R.; Chaudhary, M.; Mohanty, P. Harnessing electron-rich framework in cyclophosphazene derived hybrid nanoporous materials for organocatalytic C-C bond formation and gas sorption applications. J. CO2 Util. 2018, 25, 302–309. [Google Scholar] [CrossRef]
- Maya, E.M.; Valverde-Gonzalez, A.; Iglesias, M. Conversion of CO2 into chloropropene carbonate catalyzed by iron (II) phthalocyanine hypercrosslinked porous organic polymer. Molecules 2020, 25, 4598. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Li, H.-K.; Zhu, Q.-Q.; Yuan, R.; He, H. An intriguing electrochemical impedance aptasensor based on a porous organic framework supported silver nanoparticles for ultrasensitively detecting theophylline. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Yadav, H.; Vinodkumar, M.; Limbachiya, C.; Vinodkumar, P.C.; Mason, N.J. Low energy electron interactions with Iodine molecule (I2). J. Quant. Spectrosc. Radiat. Transf. 2020, 250, 107035–107045. [Google Scholar] [CrossRef]
- Yeager, C.M.; Amachi, S.; Grandbois, R.; Kaplan, D.I.; Xu, C.; Schwehr, K.A.; Santschi, P.H. Microbial Transformation of Iodine: From Radioisotopes to Iodine Deficiency. Adv. Appl. Microbiol. 2017, 101, 83–136. [Google Scholar]
- Alizadeh, N.; Dehghanikhah, S. Spectrophotometric study of the charge transfer complexes of 4′-nitrobenzo-15-crown-5 and benzo-15-crown-5 with iodine in nonaqueous solvents. Chin. Chem. Lett. 2011, 22, 587–590. [Google Scholar] [CrossRef]
- Hu, K.K.; Huang, W.X.; Su, Y.H.; Hu, R.Z. Simultaneous determination of fluorine and iodine in urine by ion chromatography with electrochemical pretreatment. Chin. Chem. Lett. 2009, 20, 1483–1486. [Google Scholar] [CrossRef]
- Xiong, S.H.; Tang, X.; Pan, C.Y.; Li, L.; Tang, J.T.; Yu, G.P. Carbazole-bearing porous organic polymers with mulberry-like morphology for efficient iodine capture. ACS Appl. Mater. Interfaces 2019, 11, 27335–27342. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Murmu, N.C.; Banerjee, P. Tailor-made synthesis of a melamine-based aminal hydrophobic polymer for selective adsorption of toxic organic pollutants: An initiative towards wastewater purification. RSC Adv. 2019, 9, 7469–7478. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.Y.; Chen, L.H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Dai, Z.F.; Meng, X.J.; Xiao, F.S. Porous polymer catalysts with hierarchical structures. Chem. Soc. Rev. 2015, 44, 6018–6034. [Google Scholar] [CrossRef]
- Shen, D.Z.; Cai, T.T.; Zhu, X.L.; Ma, X.L.; Kong, L.Q.; Kang, Q. Monitoring iodine adsorption onto zeolitic-imidazolate framework-8 film using a separated-electrode piezoelectric sensor. Chin. Chem. Lett. 2015, 26, 1022–1025. [Google Scholar] [CrossRef]
- Li, G.; Huang, Y.; Lin, J.; Yu, C.; Liu, Z.Y.; Fang, Y.; Xue, Y.M.; Tang, C.C. Effective capture and reversible storage of iodine using foam-like adsorbents consisting of porous boron nitride microfibers. Chem. Eng. J. 2020, 382, 122833–122842. [Google Scholar] [CrossRef]
- Ye, F.; Huang, C.; Jiang, X.H.; He, W.; Gao, X.; Ma, L.; Ao, J.X.; Xu, L.; Wang, Z.Q.; Li, Q.G.; et al. Reusable fibrous adsorbent prepared via Co-radiation induced graft polymerization for iodine adsorption. Ecotoxicol. Environ. Saf. 2020, 203, 111021–111028. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, R.; Attia, N.F.; Cho, S.; Park, J.; Jung, M.; Chung, J.; Oh, H. Exploitation of surface heterogeneity and textural properties in nanoporous carbon fabrics for efficient iodine capture. Thin Solid Films 2020, 706, 138049–138057. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, T.; Han, Y.N.; Chen, Q.; Li, C.P.; Xue, P.C. Multi-responsive fluorescent switches and iodine capture of porous hydrogen-bonded self-assemblies. J. Mater. Chem. C 2021, 9, 9932–9940. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Huang, X.Y.; Dai, L.; Cui, L.; Li, J.; Jia, X.S.; Li, C.J. Terphen[n]arenes and quaterphen[n]arenes (n = 3–6): One-pot synthesis, self-assembly into supramolecular gels, and iodine capture. Angew. Chem. Int. Ed. 2019, 58, 3885–3889. [Google Scholar] [CrossRef]
- Xu, M.Y.; Wang, T.; Zhou, L.; Hu, D.B. Fluorescent conjugated mesoporous polymers with N,N-diethylpropylamine for the efficient capture and real-time detection of volatile iodine. J. Mater. Chem. A 2020, 8, 1966–1974. [Google Scholar] [CrossRef]
- Hu, X.W.; Wang, H.G.; Faul, C.F.J.; Wen, J.; Wei, Y.; Zhu, M.F.; Liao, Y.Z. A crosslinking alkylation strategy to construct nitrogen-enriched tetraphenylmethane-based porous organic polymers as efficient carbon dioxide and iodine adsorbents. Chem. Eng. J. 2020, 382, 12299–12307. [Google Scholar] [CrossRef]
- Geng, T.M.; Chen, G.F.; Xia, H.Y.; Zhang, W.Y.; Zhu, Z.M.; Cheng, B.S. Poly{tris[4-(2-thienyl)phenyl]amine} and poly[tris(4-carbazoyl-9-ylphenyl)amine] conjugated microporous polymers as absorbents for highly efficient iodine adsorption. J. Solid State Chem. 2018, 265, 85–91. [Google Scholar] [CrossRef]
- Hassan, A.; Alam, A.; Ansari, M.; Das, N. Hydroxy functionalized triptycene based covalent organic polymers for ultra-high radioactive iodine uptake. Chem. Eng. J. 2022, 427, 130950–130959. [Google Scholar] [CrossRef]
- Mehlana, G.; Ramon, G.; Bourne, S.A. A 4-fold interpenetrated diamondoid metal-organic framework with large channels exhibiting solvent sorption properties and high iodine capture. Microporous Mesoporous Mater. 2016, 231, 21–30. [Google Scholar] [CrossRef]
- Chen, D.Y.; Fu, Y.; Yu, W.G.; Yu, G.P.; Pan, C.Y. Versatile Adamantane-based porous polymers with enhanced microporosity for efficient CO2 capture and iodine removal. Chem. Eng. J. 2018, 334, 900–906. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).