Design, Synthesis, and In Vitro Evaluation of Hydroxybenzimidazole-Donepezil Analogues as Multitarget-Directed Ligands for the Treatment of Alzheimer’s Disease
Abstract
1. Introduction
2. Results and Discussion
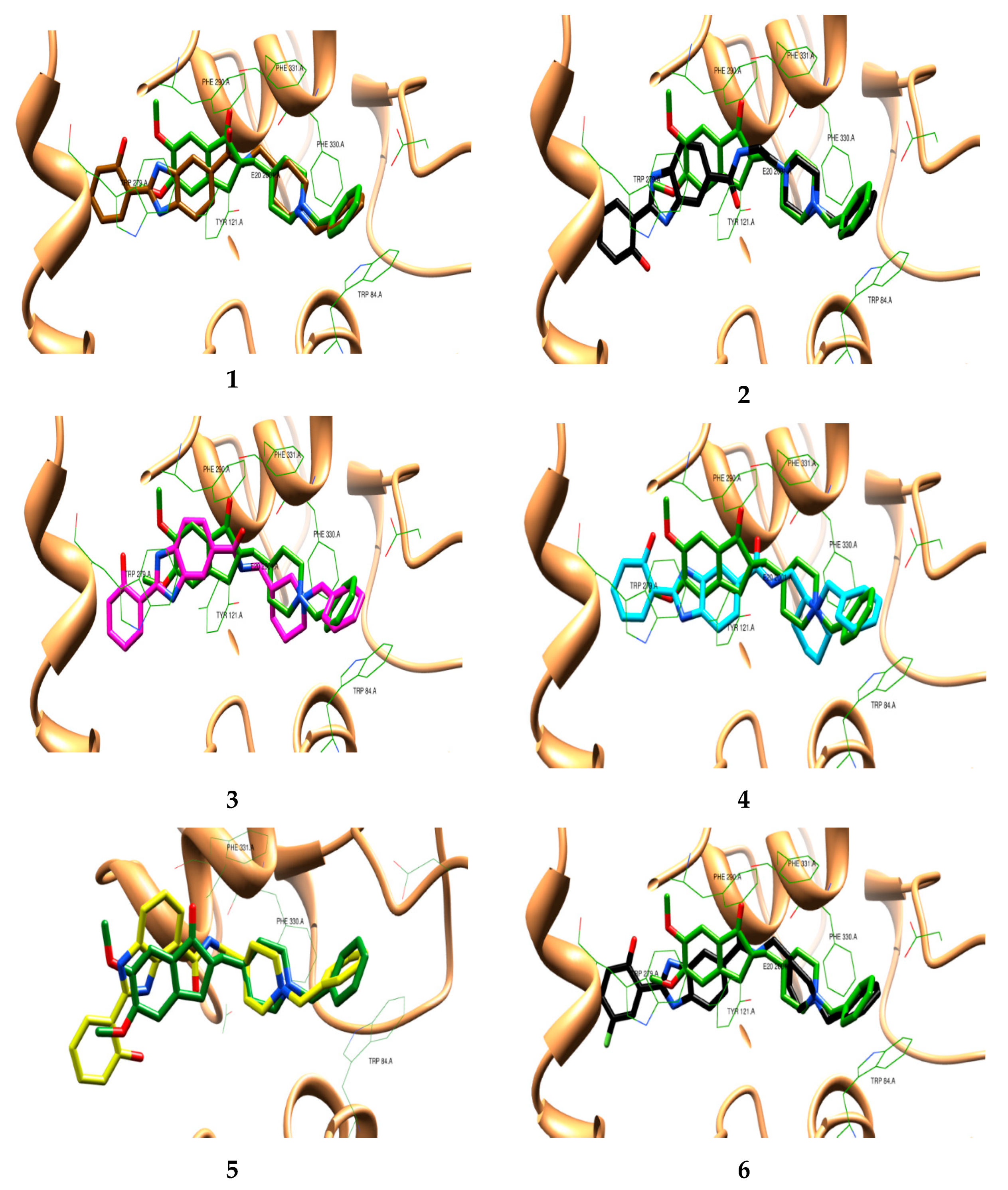
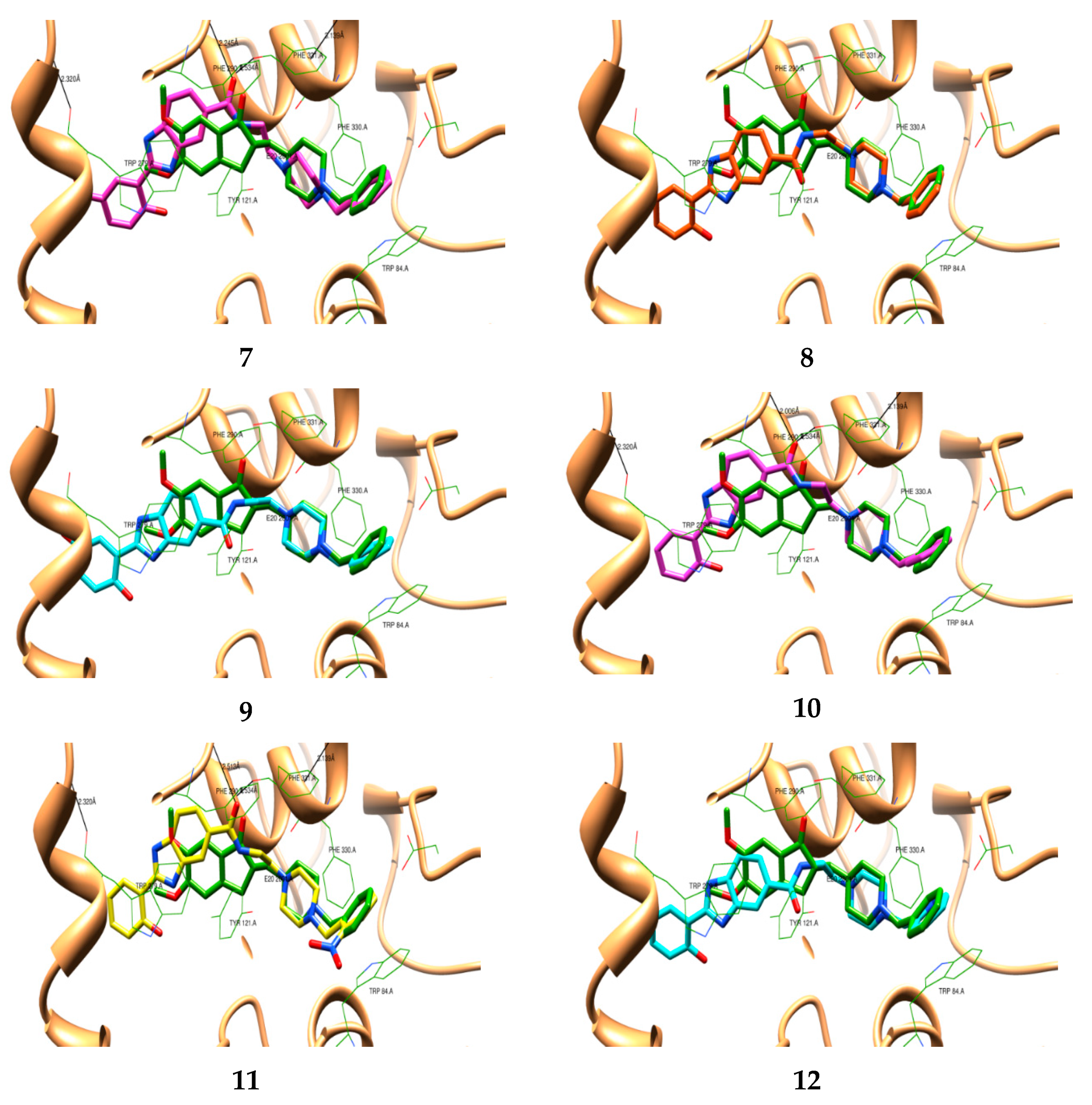
2.1. Molecular Design and Docking
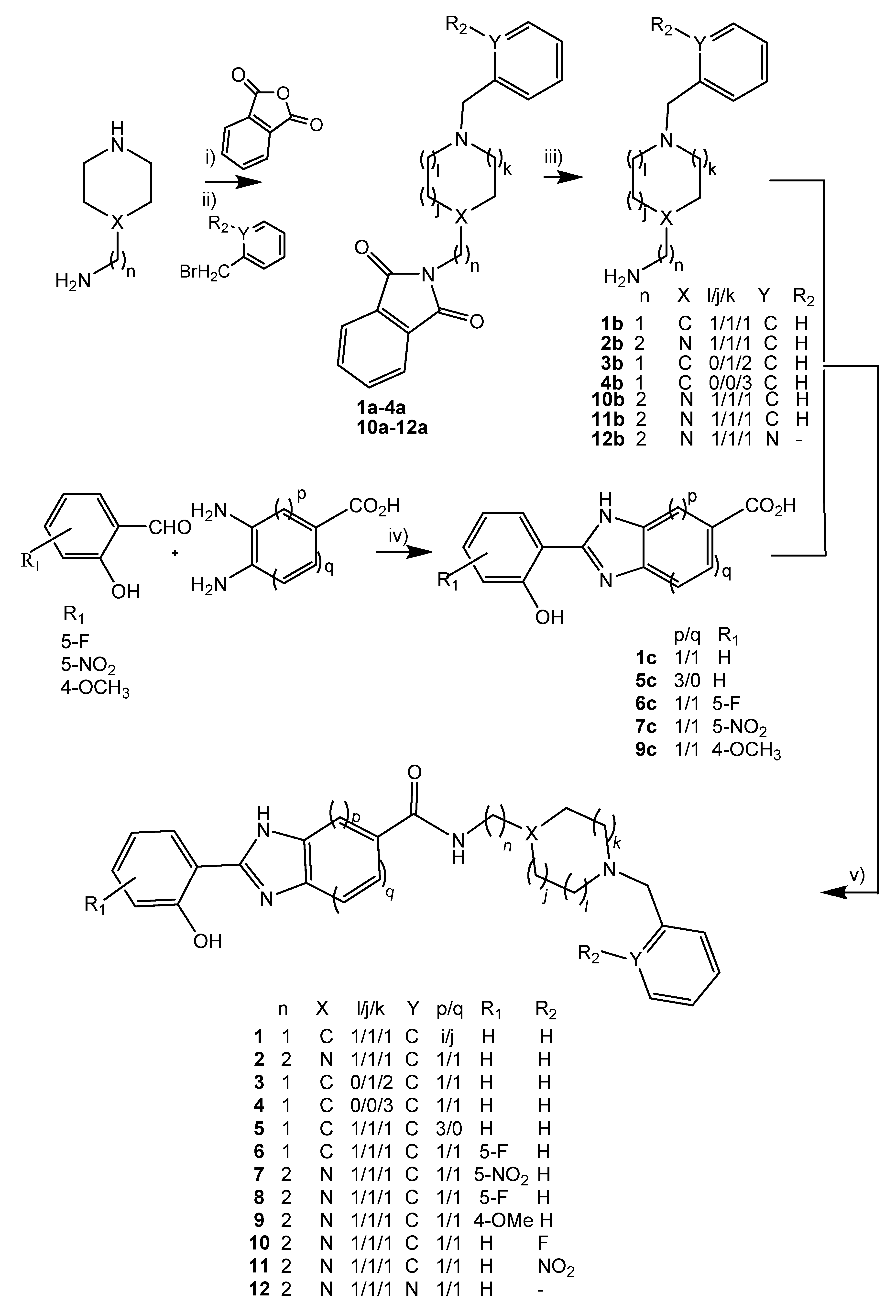
2.2. Synthesis
2.3. Metal Chelation
2.4. Biological Studies in Solution
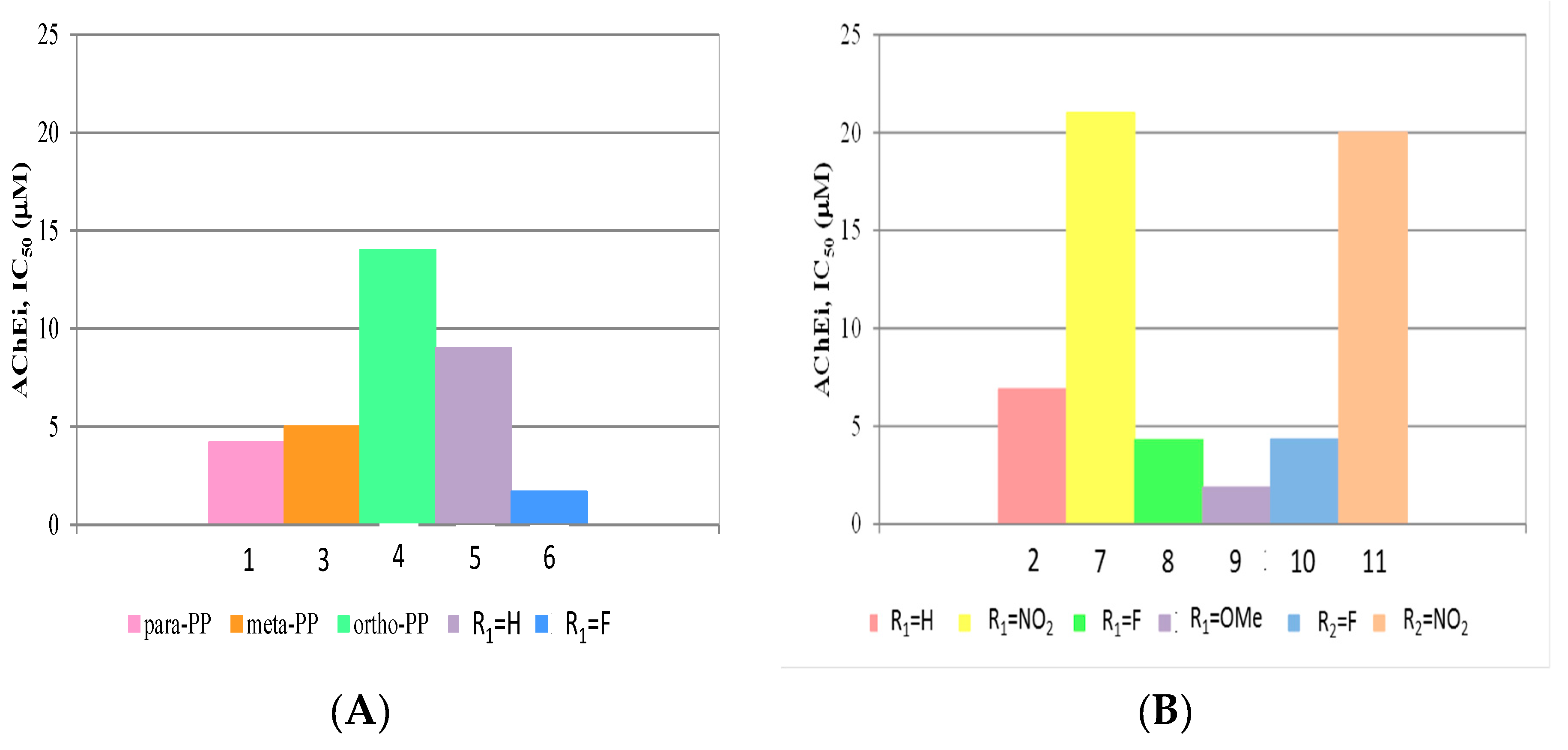
2.4.1. Acetylcholinesterase (AChE) Inhibition
2.4.2. Inhibition of Aβ1–42 Aggregation
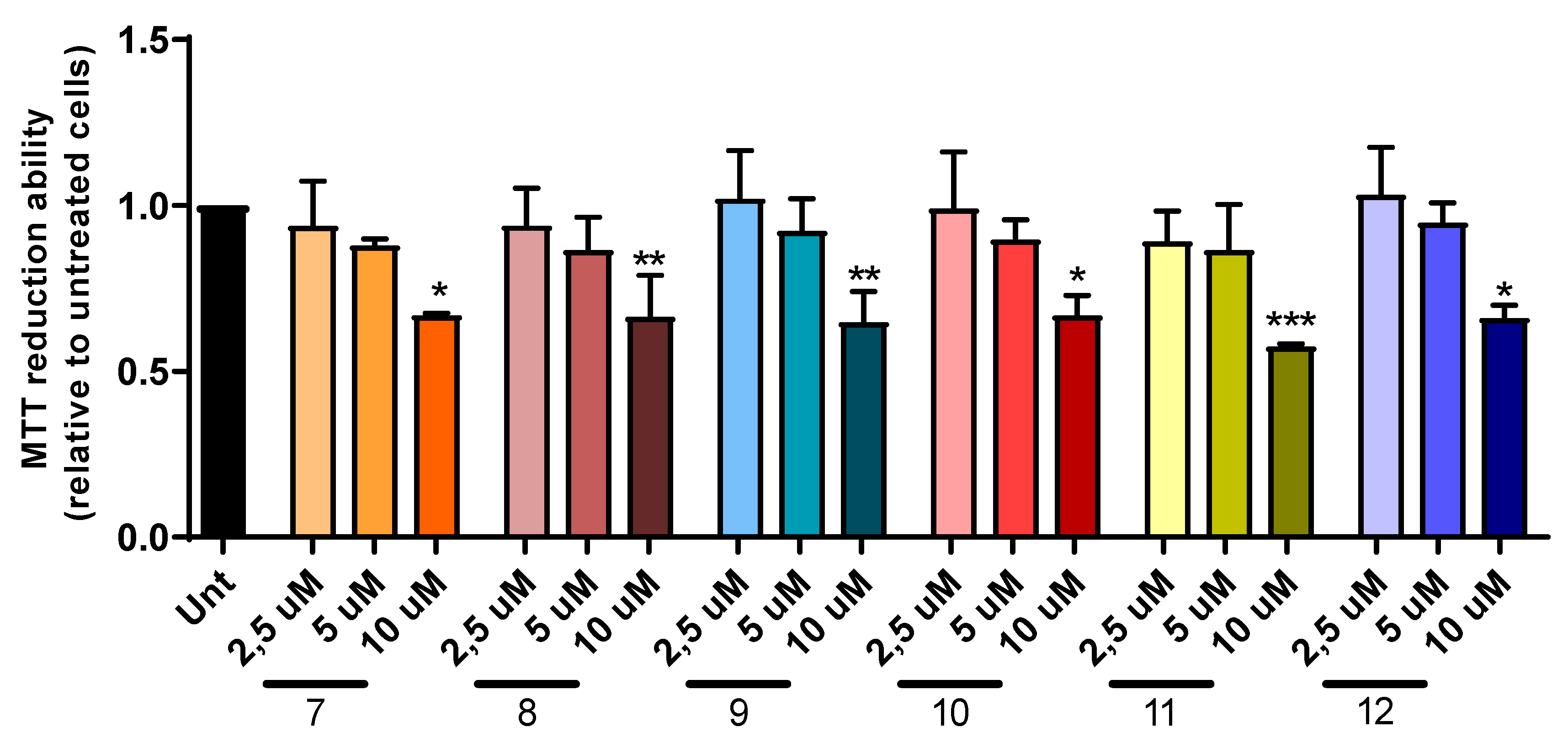
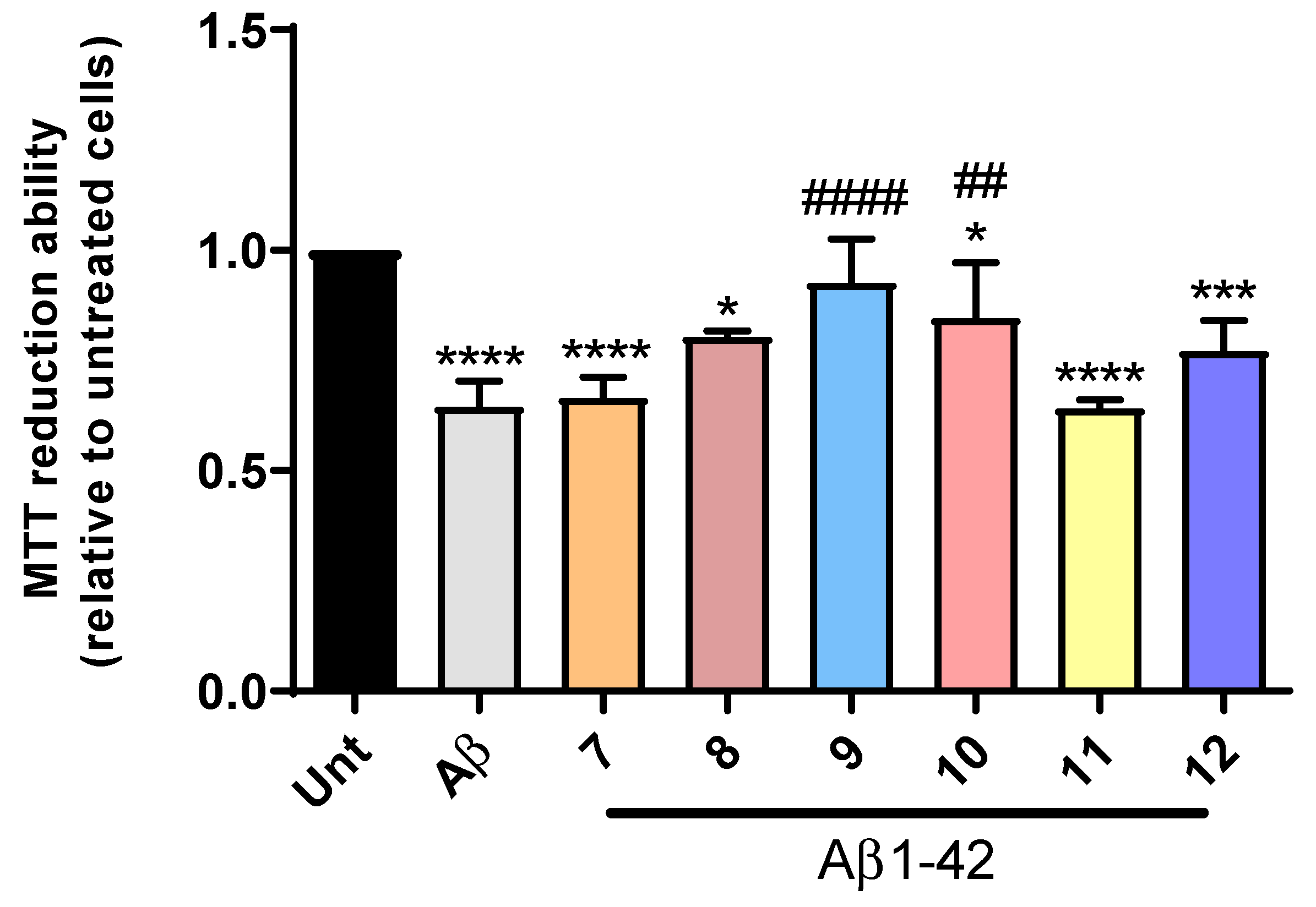
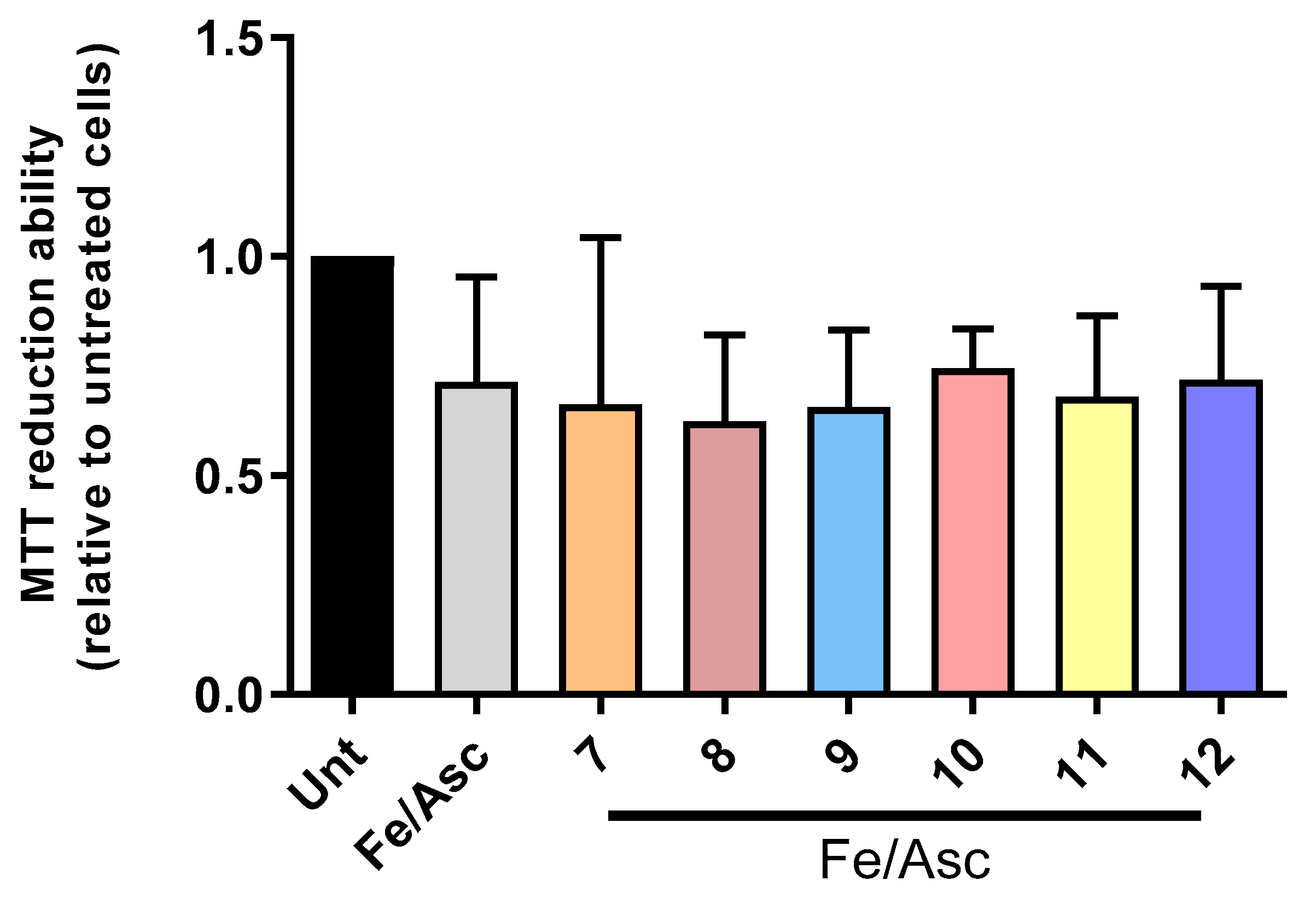
2.5. Cell Viability and Neuroprotection
2.6. Prediction of Pharmacokinetic Properties
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of the Compounds
3.2.1. Synthesis of the Benzyl-Piperidine as Well as Benzyl- and Pyridinyl-Piperazine Alkylamino Derivatives (1–4b,10–12b)
3.2.1.1. Synthesis of the N-benzyl Compounds with Isoindoline-1,3-Dione Protection (1–4a, 10–12a)
General Procedure
3.2.1.2. Deprotection of Primary Amine from the Isoindoline-1,3-Diones (1b−4b,10b−12b)
General Procedure
3.2.2. Synthesis of 2-(X-2-hydroxyphenyl)-1H-benzo[d]imidazole-5-carboxylic Acids (X = 5-F, 5-NO2, 4-OMe)
General Procedure (Method B)
3.2.3. Synthesis of the Final Conjugates (1–12)
General Procedure
3.3. Molecular Modeling: Docking and Pharmacokinetics Studies
3.4. Bio-Analytical Procedures
3.4.1. Inhibition of Acetylcholinesterase
3.4.2. Inhibition of Self- and Cu-Mediated Aβ1–42 Aggregation
3.4.3. Metal Chelation
3.4.4. Cell Viability and Neuroprotection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res. 2014, 6, 37. [Google Scholar] [CrossRef]
- Anderson, R.M.; Hadjichrysanthou, C.; Evans, S.; Wong, M.M. Why do so many clinical trials of therapies for Alzheimer’s disease fail? Lancet 2017, 390, 2327–2329. [Google Scholar] [CrossRef]
- Tricco, A.C.; Ashoor, H.M.; Soobiah, C.; Rios, P.; Veroniki, A.A.; Hamid, J.S.; Ivory, J.D.; Khan, P.A.; Yazdi, F.; Ghassemi, M.; et al. Comparative effectiveness and safety of cognitive enhancers for treating Alzheimer’s disease: Systematic review and network metaanalysis. J. Am. Geriatr. Soc. 2018, 66, 170–178. [Google Scholar] [CrossRef]
- Savelieff, M.; Nam, G.; Kang, J.; Lee, H.J.; Lee, M.; Lim, M.H. Development of multifunctional molecules as potential therapeutic candidates for Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis in the last decade. Chem. Rev. 2019, 119, 1221–1322. [Google Scholar] [CrossRef]
- Marco-Contelles, J. Facts, results and perspectives of the current Alzheimer’s disease research. ACS Chem. Neurosci. 2019, 10, 1127–1128. [Google Scholar] [CrossRef]
- Piemontese, L.; Loiodice, F.; Chaves, S.; Santos, M.A. The therapy of Alzheimer’s disease: Towards a new generation of drugs. Front. Clin. Drug Res. Alzheimer Dis. 2019, 8, 33–80. [Google Scholar]
- Prati, F.; Bottegoni, G.; Bolognesi, M.L.; Cavalli, A. BACE-1 Inhibitors: From recent single-target molecules to multitarget compounds for Alzheimer’s disease. J. Med. Chem. 2018, 61, 619–637. [Google Scholar] [CrossRef]
- Guzior, N.; Wieckowska, A.; Panek, D.; Malawska, B. Recent development of multifunctional agents as potential drug candidates for the treatment of Alzheimer’s disease. Curr. Med. Chem. 2015, 22, 373–404. [Google Scholar] [CrossRef]
- Santos, M.A.; Chand, K.; Chaves, S. Recent progress in repositioning Alzheimer’s disease drugs based on a multitarget strategy. Fut. Med. Chem. 2016, 8, 2113–2142. [Google Scholar] [CrossRef]
- Dudley, J.; Berliocchi, L. Drug Repositioning: Approaches and Applications for Neurotherapeutics; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 9781315373669. [Google Scholar]
- Castro, A.A.; Cunha, E.F.F.; Pereira, A.F.; Soares, F.V.; Leala, D.H.S.; Kuca, K.; Ramalho, T.C. Insights into the drug repositioning applied to the Alzheimer’s disease treatment and future perspectives. Curr. Alzheimer Res. 2018, 15, 1–18. [Google Scholar] [CrossRef]
- Mezeiova, E.; Spilovska, K.; Nepovimova, E.; Gorecki, L.; Soukup, O.; Dolezal, R.; Malinak, D.; Janockova, J.; Jun, D.; Kuca, K.; et al. Profiling donepezil template into multipotent hybrids with antioxidant properties. J. Enz. Inhib. Med. Chem. 2018, 33, 583–606. [Google Scholar] [CrossRef]
- Mezeiova, E.; Chalupova, K.; Nepovimova, E.; Gorecki, L.; Prchal, L.; Malinak, D.; Kuca, K.; Soukup, O.; Korabecny, J. Donepezil Derivatives Targeting Amyloid-β Cascade in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 772–800. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C.; Berthoumieu, O. Role of metal ions in the self-assembly of the Alzheimer’s amyloid Ab peptide. Inorg. Chem. 2013, 52, 12193–12206. [Google Scholar] [CrossRef]
- Nam, G.; Lim, M.H. Intertwined Pathologies of Amyloid-β and Metal Ions in Alzheimer’s Disease: Metal-Amyloid-β. Chem. Lett. 2019, 48, 951–960. [Google Scholar] [CrossRef]
- Sales, T.A.; Prandi, I.G.; de Castro, A.A.; Leal, D.H.S.; Cunha, E.F.F.; Kuca, K.; Ramalho, T.C. Recent Developments in Metal-Based Drugs and Chelating Agents for Neurodegenerative Diseases Treatments. Int. J. Mol. Sci. 2019, 20, 1829. [Google Scholar] [CrossRef]
- Santos, M.A.; Chand, K.; Chaves, S. Recent progress in multifunctional metal chelators as potential drugs for Alzheimer’s disease. Coord. Chem. Rev. 2016, 327–328, 287–303. [Google Scholar] [CrossRef]
- Chand, K.; Rajeshwari; Chaves, S.; Santos, M.A. Tacrine–deferiprone hybrids as multi-target-directed metal chelators against Alzheimer’s disease: A two-in-one drug. Metallomics 2018, 10, 1460–1475. [Google Scholar] [CrossRef]
- Gaia, F.; Karam, C.; Tomás, D.; Orlandini, E.; Piemontese, L.; Silva, D.F.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Novel Tacrine–Benzofuran hybrids as potential multi-target drug candidates for the treatment of Alzheimer’s Disease. J. Enz. Inhib. Med. Chem. 2020, 35, 211–226. [Google Scholar]
- Hiremathad, A.; Keri, R.S.; Esteves, A.R.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Novel Tacrine-Hydroxyphenylbenzimidazole hybrids as potential multitarget drug candidates for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 148, 255–267. [Google Scholar] [CrossRef]
- Piemontese, L.; Tomás, D.; Hiremathad, A.; Capriati, V.; Candeias, E.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s drug candidates. J. Enzym. Inhib. Med. Chem. 2018, 33, 1212–1224. [Google Scholar] [CrossRef]
- Kryger, G.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept): Implications for the design of new anti-Alzheimer drugs. Struct. Fold. Des. 1999, 7, 297–307. [Google Scholar] [CrossRef]
- Piemontese, L.; Sergio, R.; Rinaldo, F.; Brunetti, L.; Perna, F.M.; Santos, M.A.; Capriati, V. Deep eutectic solvents as effective reaction media for the synthesis of 2-hydroxyphenylbenzimidazole-based scaffolds en route to Donepezil-like Compounds. Molecules 2020, 25, 574. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Liptak, M.D.; Gross, K.C.; Seybold, P.G.; Feldgus, S.; Shields, G.C. Absolute pKa determinations for substituted phenols. J. Am. Chem. Soc. 2002, 124, 6421–6427. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Bibliography. In Critical Stability Constants; Plenum Press: New York, NY, USA; London, UK, 1989; Volume 6, p. 493. [Google Scholar]
- Smith, R.M.; Martell, A.E. Azines. In Critical Stability Constants; Plenum Press: New York, NY, USA; London, UK, 1989; Volume 6, p. 258. [Google Scholar]
- Walba, H.; Isensee, R.W. Acidity constants of some arylimidazoles and their cations. J. Org. Chem. 1961, 26, 2789–2791. [Google Scholar] [CrossRef]
- Costa, M.; Josselin, R.; Silva, D.F.; Cardoso, S.M.; May, N.V.; Chaves, S.; Santos, M.A. Donepezil-Based Hybrids as Multifunctional Anti-Alzheimer’s Disease Chelating Agents: Effect of Positional Isomerization. J. Inorg. Biochem. 2020. under publication. [Google Scholar] [CrossRef]
- Raymond, K.N.; Carrano, C.J. Coordination chemistry and microbial iron transport. ACC Chem. Res. 1979, 12, 183–190. [Google Scholar] [CrossRef]
- Chaves, S.; Hiremathad, A.; Tomás, D.; Keri, R.S.; Piemontese, L.; Santos, M.A. Exploring the chelating capacity of 2-hydroxyphenyl-benzimidazole based hybrids with multi-target ability as anti-Alzheimer’s agents. New J. Chem. 2018, 42, 16503–16515. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Tan, C.C.; Yu, J.T.; Wang, H.F.; Tan, M.S.; Meng, X.F.; Wang, C.; Jiang, T.; Zhu, X.C.; Tan, L. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 41, 615–631. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Bolognesi, M.L.; Cavalli, A.; Melchiorre, C.; Andrisano, V. Insight into the kinetic of amyloid beta (1-42) peptide self-aggregation: Elucidation of inhibitors’ mechanism of action. ChemBioChem 2007, 8, 2152–2161. [Google Scholar] [CrossRef]
- Chao, X.; He, X.; Yang, Y.; Zhou, X.; Jin, M.; Liu, S.; Cheng, Z.; Liu, P.; Wang, Y.; Yu, J.; et al. Design, synthesis and pharmacological evaluation of novel tacrine-caffeic acid hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2012, 22, 6498–6502. [Google Scholar] [CrossRef]
- Mold, M.; Ouro-Gnao, L.; Wieckowski, B.; Exley, C. Copper prevents amyloid-β1–42 from forming amyloid fibrils under near-physiological conditions in vitro. Sci. Rep. 2013, 3, 1256. [Google Scholar] [CrossRef]
- Tahmasebinia, F.; Emadi, S. Effect of metal chelators on the aggregation of beta-amyloid peptides in the presence of copper and iron. Biometals 2017, 30, 285–293. [Google Scholar] [CrossRef]
- Gestwicki, J.E.; Ranke, A. Structure-activity relationships of amyloid beta-aggregation inhibitors based on curcumin: Influence of linker length and flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar]
- Faller, P.; Hureau, C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-β peptide. Dalton Trans. 2009, 7, 1080–1094. [Google Scholar] [CrossRef]
- Silva, D.F.; Selfridge, J.E.; Lu, E.L.; Cardoso, S.M.; Swerdlow, R.H. Mitochondrial abnormalities in Alzheimer’s disease: Possible targets for therapeutic intervention. Adv. Pharmacol. 2012, 64, 83–126. [Google Scholar]
- QikProp, Version 2.5; Schrödinger LLC: New York, NY, USA, 2005.
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–26. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann; Oxford Press: Oxford, UK, 1999. [Google Scholar]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Maestro, Version 9.3; Schrödinger Inc.: Portland, OR, USA, 2012.
- Hassinen, T.; Peräkylä, M. New energy terms for reduced protein models implemented in an off-lattice force field. J. Comput. Chem. 2001, 22, 1229–1242. [Google Scholar] [CrossRef]
- Acton, A.; Banck, M.; Bréfort, J.; Cruz, M.; Curtis, D.; Hassinen, T.; Heikkilä, V.; Hutchison, G.; Huuskonen, J.; Jensen, J.; et al. Ghemical Version 3.0; Bioinformatics.org: Biglerville, PA, USA, 2011. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Quintanova, C.; Keri, R.S.; Marques, S.M.; Fernandes, M.G.; Cardoso, S.M.; Serralheiro, M.L.; Santos, M.A. Design, synthesis and bioevaluation of tacrine hybrids with cinnamate and cinnamylidene acetate derivatives as potential anti-Alzheimer drugs. MedChemComm 2015, 6, 1969–1977. [Google Scholar] [CrossRef]
- Šebestík, J.; Marques, S.M.; Falé, P.L.; Santos, S.; Arduíno, D.M.; Cardoso, S.M.; Oliveira, C.R.; Serralheiro, M.L.M.; Santos, M.A. Bifunctional phenolic-choline conjugates as anti-oxidants and acetylcholinesterase inhibitors. J. Enzym. Inhib. Med. Chem. 2011, 26, 485–497. [Google Scholar] [CrossRef]
- Hiremathad, A.; Chand, K.; Esteves, A.R.; Cardoso, S.M.; Ramsay, R.R.; Chaves, S.; Keri, R.S.; Santos, M.A. Tacrine-allyl/propargylcysteine-benzothiazole trihybrids as potential anti-Alzheimer’s drug candidates. RSC Adv. 2016, 6, 53519–53532. [Google Scholar] [CrossRef]
- Rossotti, F.J.C.; Rossotti, H. Potentiometric titrations using Gran plots: A textbook omission. J. Chem. Ed. 1965, 42, 375–378. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

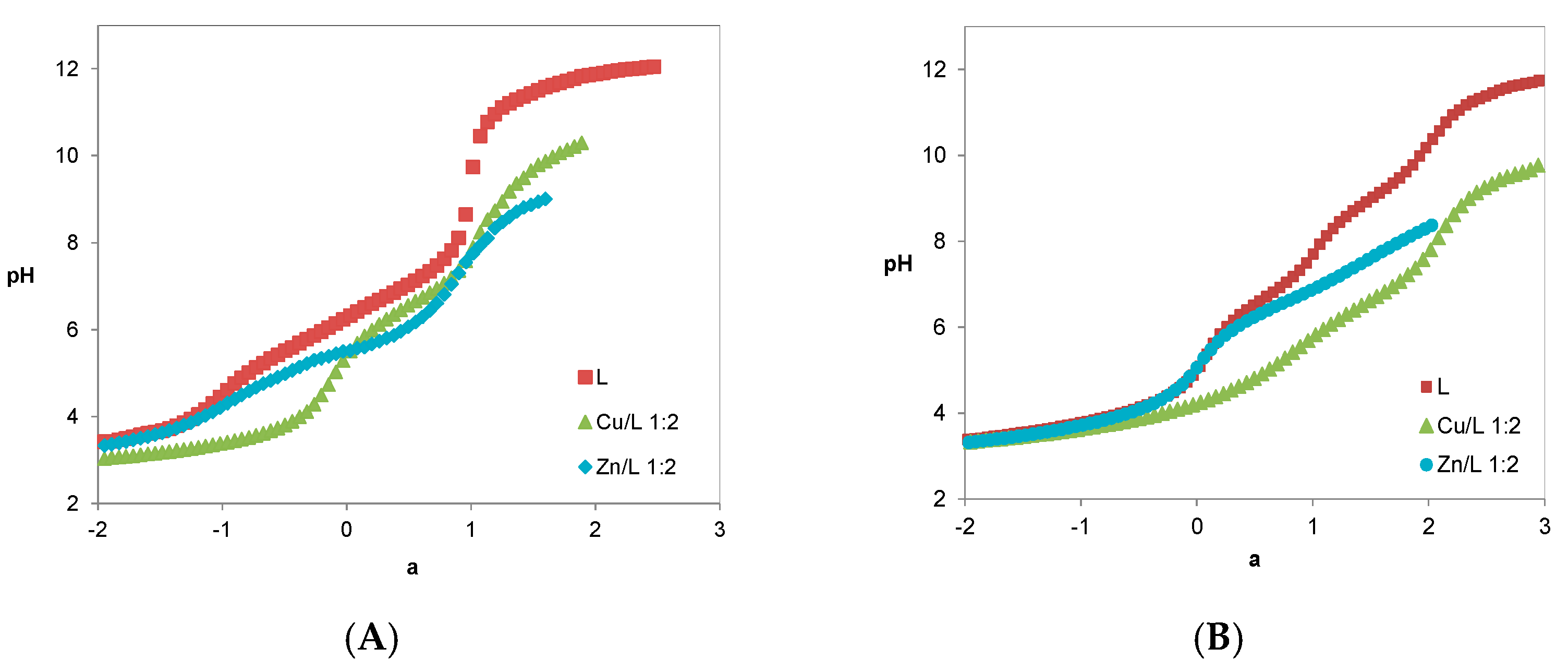
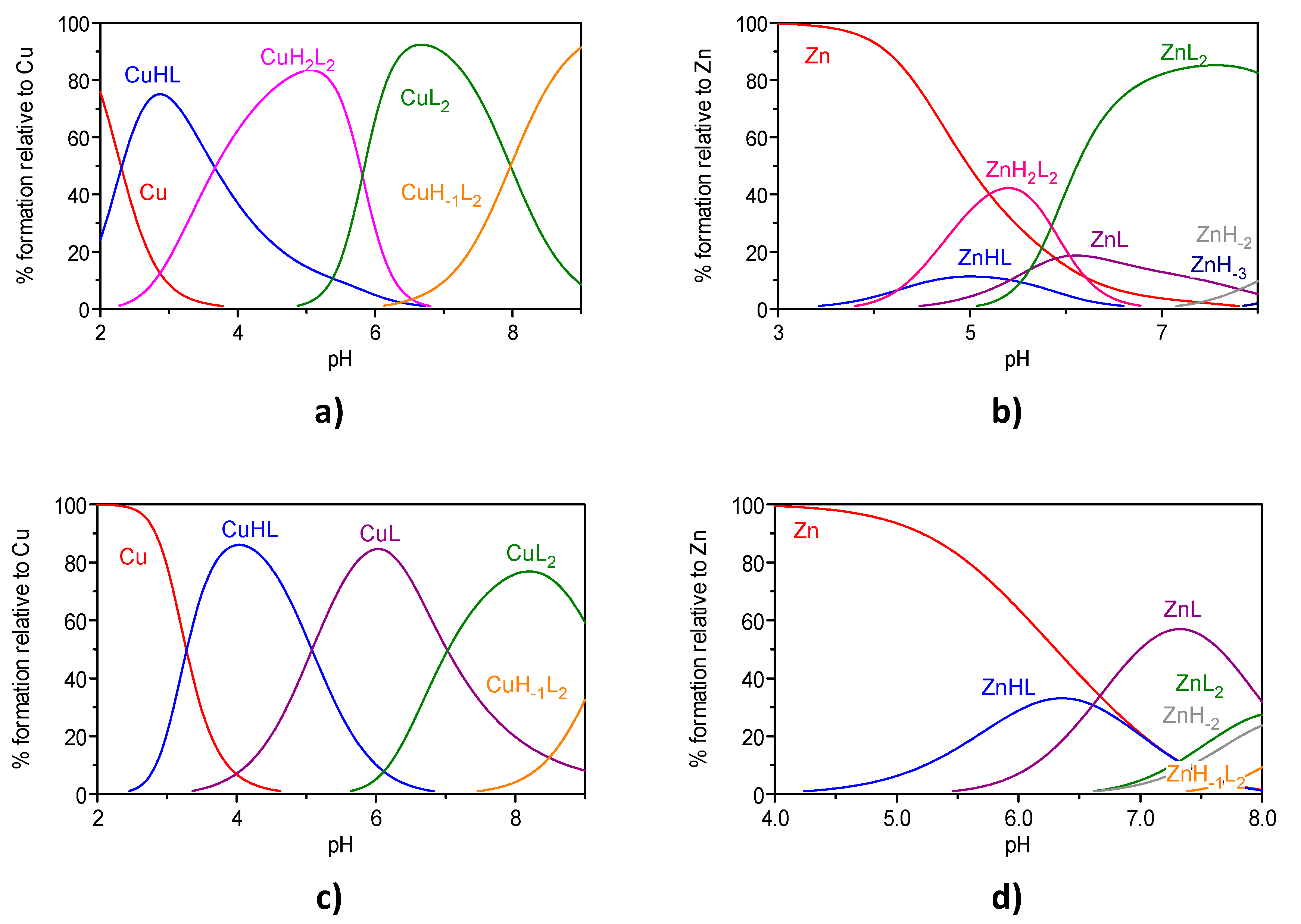

| Compound | MmHhLl (mhl) | log Ki | ||
|---|---|---|---|---|
 7 | (011) (021) (031) (111) (101) (122) (102) (1–12) pM | 6.99(2) 5.51(5) 2.95(6) | 14.23(5) - 26.90(7) 15.27(8) 7.30(9) 10.9 | 10.40(8) 4.98(8) 21.92(7) 10.22(8) - 6.5 |
 12 | (011) (021) (031) (041) (111) (101) (102) (1–12) pM | 9.09(3) 6.44(4) 3.64(5) 3.17(6) | 16.26(3) 11.18(5) 17.14(7) 7.88(7) 10.4 | 12.58(4) 6.26(5) 10.54(5) 2.07(7) 6.3 |
 1c | (011) (021) (031) pM | 8.671(1) c 7.39(1) c 3.21(1) c | 10.7/ | 6.3 |
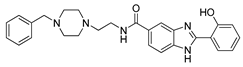 2c | (011) (021) (031) pM | 9.04(3) 6.76(6) 3.37(7) | 10.7 | 6.3 |
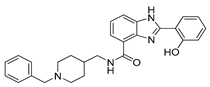 5d | (011) (021) (031) pM | 8.98(2) 7.78(4) 2.86(5) | 14.3 | 6.4 |
| Compound | TcAChE Inhibition a IC50 (μM) | Aβ1–42 Aggregation Inhibitionb,c (%) | |
|---|---|---|---|
| Self-Induced | Cu-Induced | ||
| 1 | 4.2 ± 0.8 d | 48.8 e | 69.0 e |
| 2 | 6.9 ± 0.6 d | 25.3 d | 37.7 d |
| 3 | 5.0 ± 0.5 | 71.4 | 81.8 |
| 4 | 14 ± 1 | 47.6 | 55.9 |
| 5 | 9 ± 1 e | 38.6 e | 58.9 e |
| 6 | 1.67 ± 0.05 | 51.2 | 61.0 |
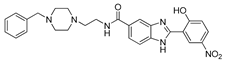
| 7 | 21 ± 3 | 57.6 | 71.4 |
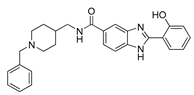
| 8 | 4.3 ± 0.3 | 50.6 | 55.2 |
| 9 | 1.87 ± 0.8 | 43.7 | 66.0 |
| 10 | 4.32 ± 0.03 | 39.1 | 75.8 |
| 11 | 20 ± 1 | 22.7 | 43.5 |
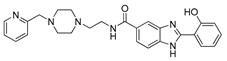
| 12 | >102 | 30.1 | 44.4 |
| DNPf | 0.026 | - | - |
| Comp. | MW (Da) | clog P | log BB | Caco-2 Permeability (nm/s) | CNS | Violations of Lipinski’s Rule |
|---|---|---|---|---|---|---|
| 1 | 440.54 | 4.386 | −0.730 | 194 | - | 0 |
| 2 | 455.55 | 2.587 | −0.097 | 48 | +/− | 0 |
| 3 | 440.54 | 4.122 | −0.825 | 155 | - | 0 |
| 4 | 440.54 | 4.202 | −0.981 | 121 | - | 0 |
| 5 | 440.54 | 4.352 | −0.638 | 225 | +/− | 0 |
| 6 | 458.53 | 4.513 | −0.663 | 177 | +/- | 0 |
| 7 | 516.55 | 2.421 | −1.148 | 7 | - | 1 |
| 8 | 473.54 | 3.490 | −0.147 | 61 | +/− | 0 |
| 9 | 485.58 | 2.840 | −0.029 | 79 | - | 0 |
| 10 | 473.54 | 3.455- | −0.385 | 45 | +/− | 0 |
| 11 | 500.55 | 2.604 | −1.641 | 6 | - | 1 |
| 12 | 456.54 | 2.686 | −0.597 | 34 | +/− | 0 |
| DNP | 379.50 | 4.269 | 0.132 | 893 | + | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, S.; Resta, S.; Rinaldo, F.; Costa, M.; Josselin, R.; Gwizdala, K.; Piemontese, L.; Capriati, V.; Pereira-Santos, A.R.; Cardoso, S.M.; et al. Design, Synthesis, and In Vitro Evaluation of Hydroxybenzimidazole-Donepezil Analogues as Multitarget-Directed Ligands for the Treatment of Alzheimer’s Disease. Molecules 2020, 25, 985. https://doi.org/10.3390/molecules25040985
Chaves S, Resta S, Rinaldo F, Costa M, Josselin R, Gwizdala K, Piemontese L, Capriati V, Pereira-Santos AR, Cardoso SM, et al. Design, Synthesis, and In Vitro Evaluation of Hydroxybenzimidazole-Donepezil Analogues as Multitarget-Directed Ligands for the Treatment of Alzheimer’s Disease. Molecules. 2020; 25(4):985. https://doi.org/10.3390/molecules25040985
Chicago/Turabian StyleChaves, Sílvia, Simonetta Resta, Federica Rinaldo, Marina Costa, Romane Josselin, Karolina Gwizdala, Luca Piemontese, Vito Capriati, A. Raquel Pereira-Santos, Sandra M. Cardoso, and et al. 2020. "Design, Synthesis, and In Vitro Evaluation of Hydroxybenzimidazole-Donepezil Analogues as Multitarget-Directed Ligands for the Treatment of Alzheimer’s Disease" Molecules 25, no. 4: 985. https://doi.org/10.3390/molecules25040985
APA StyleChaves, S., Resta, S., Rinaldo, F., Costa, M., Josselin, R., Gwizdala, K., Piemontese, L., Capriati, V., Pereira-Santos, A. R., Cardoso, S. M., & Santos, M. A. (2020). Design, Synthesis, and In Vitro Evaluation of Hydroxybenzimidazole-Donepezil Analogues as Multitarget-Directed Ligands for the Treatment of Alzheimer’s Disease. Molecules, 25(4), 985. https://doi.org/10.3390/molecules25040985








