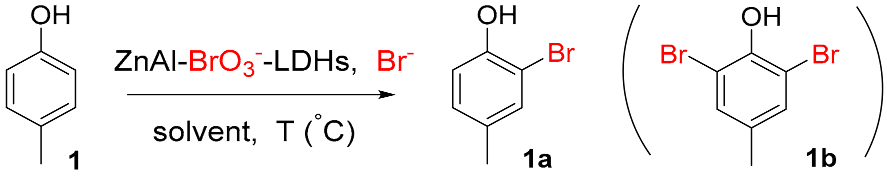
Abstract
The regioselective mono-bromination of phenols has been successfully developed with KBr and ZnAl–BrO3−–layered double hydroxides (abbreviated as ZnAl–BrO3−–LDHs) as brominating reagents. The para site is much favorable and the ortho site takes the priority if para site is occupied. This reaction featured with excellent regioselectivity, cheap brominating reagents, mild reaction condition, high atom economy, broad substrate scope, and provided an efficient method to synthesize bromophenols.
1. Introduction
Bromophenols are versatile starting materials in a carbon–carbon bond coupling reaction [1,2,3,4,5]. They are also important constituents of naturally occurring compounds that possess a range of biological activities [6,7,8,9], such as antioxidant, antimicrobial and anticancer effects. Cheap and active molecular bromine is a traditional brominating reagent despite it being corrosive, having a low atom economy (up to 50% in substitution reaction) and low selectivity [10,11,12]. Safer N-bromosuccinimide based analogues are developed as preferable brominating reagents [13,14,15,16,17,18,19]. However, these brominating reagents employ Br2 in preparation and produce useless organic waste [20,21]. Oxidative bromination is another approach to brominated substrates [22,23,24,25,26]. This brominating reagent is generated in situ from bromide ion in the presence of oxidants [27]. Various oxidants such as H2O2, DMSO, O2, bromate, oxone, persulfate, etc., have been applied in oxidative bromination [28,29,30,31,32,33,34].
Due to the high activity and multiple substituted sites of phenols, over-bromination frequently occurs when phenol derivatives undergo bromination [35,36]. It is usually hard to obtain mono-brominated product, as it is usually a mixture of mono- and multi-brominated substrate (Figure 1a). Numerous strategies for selective mono-bromination of phenols have been reported in literature.
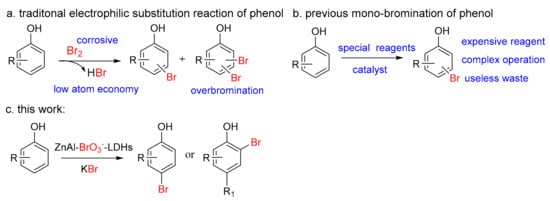
Figure 1.
Three bromination of phenols; (a) traditional electrophilic substitution reaction of phenol; (b) previous mono-bromination of phenol; (c) this work.
Some methods use special brominating reagents or additives (Figure 1b), such as o-xylylene bis (triethyl ammonium tribromide) [35], bromotrimethylsilane and di-4-chlorophenyl-sulfoxide [37], (diacetoxyiodo)benzene and AlBr3 [38], p-toluenesulfonic acid [39,40,41], methanol [39,42], polyvinylpolypyrrolidone-Br2 [43], 1,3-di-n-butylimidazolium tribromide [44], ethylenebis(N-methylimidazolium) ditribromide [45], N-benzyl-triethylenediamine tribromide [46], 1-butyl-3-methylpyridiniumtribromide [47], ZrBr4/diazene [48], β-cyclodextrin [49] and tetrabromobenzene-1,3-disulfonylamide [50]. There are also some methods requiring a catalyst, including thioamide [36], Cu−Mn spinel oxide [51], bromoperoxidase [52], Rhenium-promoted mesoporous zirconia [53], amberlyst-15 [54], mesoporous silica supported sulfated zirconia [55], copper icons [56,57], UV–vis light irradiation [58] and ammonium acetate [59].
However, some of these methods are associated with disadvantages such as special and expensive reagents, demand for catalysts, generation of a large amount of waste, and harsh conditions. Therefore, the development of simple, efficient approaches to bromophenol is still highly desirable.
Layered double hydroxides (LDHs) is a layered structure material that consists of interlayer anions and positively charged layers [60]. LDHs have been applied in various fields [61,62,63,64,65]. In our early work, bromate has been successfully intercalated into LDHs and it is named ZnAl-BrO3−-layered double hydroxides (abbreviated as ZnAl–BrO3−–LDHs). 0.93 g ZnAl-BrO3--LDHs is equivalent to 1 mmol bromate according to the indirect iodometric method [66]. Please see the specific synthetic procedure and calculation of iodometric method in the Supplementary Materials. We have utilized ZnAl–BrO3−–LDHs to achieve bromination of olefins and anilines [67,68,69]. Herein, we proposed a mild and efficient KBr and ZnAl–BrO3−–LDHs system for selective mono-bromination of phenols.
2. Results
4-Methylphenol was chosen as the starting reactant and the optimization result is listed in Table 1. At first, the reaction was carried on at room temperature in presence of ZnAl–BrO3––LDHs, lithium bromide and acetic acid. Maintaining the amount of ZnAl–BrO3−–LDHs at 0.2 equivalents, the amount of lithium bromide was changed from 1.4 to 0.8 equivalents. (Table 1, entry 1–4). The yield of mono-brominated product 1a altered from low to high then low and got the maximum 76% (entry 3). Then several different bromide salts (entry 5–7) were tested and potassium bromide gave the best yield in 83%. The brominated product was not observed in several common organic solvents which cannot provide acid environment (entry 8–11). This result demonstrates that acid is indispensable for our oxidative bromination system. Acetic acid plays a dual role as a solvent and acid provider. After adding a small amount of water, the reaction rate increased significantly and the yield slightly increased to 86% (entry 12). The possible reason is bromide salt and released bromate have good solubility in this mixed solvent. By screening reaction temperatures (entry 13–16), we determine 35 °C is sufficient to drive the reaction to completion and higher temperature cannot offer better yield.

Table 1.
Optimization of bromination.1
4-Methylphenol has two equal substituted sites, so double dosages of ZnAl–BrO3−–LDHs and potassium bromide were reasonable (entry 17). Surprisingly, the yield of desired di-brominated product 1b was only 17%, and 1a was still the main product 76% (entry 17). We conducted a control experiment that directly used potassium bromate (0.4 equiv.) and potassium bromide (2.0 equiv.) as brominating reagents. Not like the former result, 1a was not dominant and 1b was produced in large quantities in 61% (entry 18). Using potassium bromate (0.2 equiv.) and potassium bromide (1.0 equiv.) also produced 1b in 28% yield (entry 19). Without the addition of ZnAl-BrO3−-LDHs, brominated product was not obtained (entry 20). Our brominating system could make the bromination stop at monobromination stage by controlling the dosage of bromine. This is presumably due to the fact that open bromate produces large amounts of brominating reagents in a short period of time, while the special structure of LDHs can achieve slow release of BrO3− under acetic acid conditions. This series of experiments demonstrate that our reagents can achieve mono-bromination in good yield.
3. Discussion
To extend the substrate scope, some similar para-substituted phenols were chosen as substances (Figure 2). Our reagents show good tolerance for electron-withdrawing and electron-donating group. Phenol derivatives bearing methoxy and tert-butyl substituent were mono-brominated in ortho site to offer 2a and 3a in 82% and 71% yield. The bromination of phenol derivatives, which contain weak electronic withdrawing fluoro, chloro and bromo substituent at the para position, produced corresponding mono-brominated 4a–6a in 73%, 81%, and 84% yields, respectively. The inert 4-nitrophenol provided 7a in 51% yield.
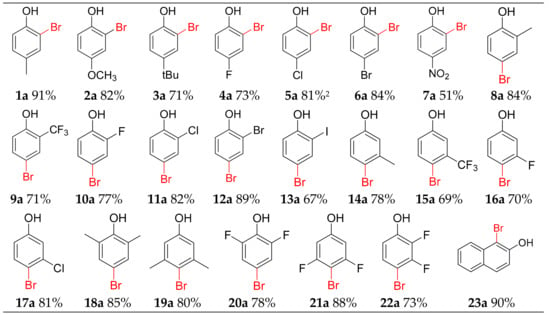
Figure 2.
Scope of bromination.
Several ortho-substituted phenol derivatives performed well producing bromophenols. Phenol derivatives bearing methyl, trifluoromethyl were all mono-brominated to furnish selective para-brominated products 8a–9a in 71%–84% yield. The halogen-containing derivatives could also be regioselectively brominated at para site to offer 10a–13a (67–89%). These ortho-substituted phenol derivatives all have two different substituted sites, but para-substituted product was dominant, the and ortho- and multi-brominated products were not observed. This result confirmed our method could achieve mono-bromination with an excellent yield and high regioselectivity.
Starting from meta-substituted derivatives 3-methyl, 3-trifluoromethyl, 3-fluoro and 3-chlorophenol, 78%, 69%, 70% and 81% yield were obtained for 14a–17a. The para site is still the main substituted position. Other para-open phenol derivatives 2,6-dimethylphenol, 3,5-dimethylphenol, 2,6-difluorophenol, 3,5-difluorophenol and 2,3-difluorophenol, were mono-brominated at the para site to offer 18a–22a in high yield (78%–90%). The bromination of 2-naphthol afforded 23a in a 90% yield.
On the basis of the previous reports, the possible mechanism of monobromination of phenols is proposed in Figure 3. Bromate is stored in LDHs and bromide is open and abundant in solvent, thus the bromide is the excess in the redox reaction between them. Released bromate reacts with bromide to produce Br2. Polarization and heterolytic cleavage occurred immediately in solvent. Then, the electrophilic substitution reaction is executed. Bromide and hydrogen that detached from phenol are used for next oxidative cycle. ZnAl-BrO3−–LDHs achieve smooth and slow release of brominating reagent, avoiding a high brominating concentration and violent reaction in the beginning.
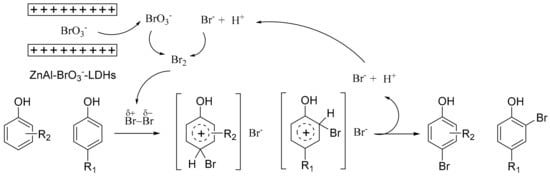
Figure 3.
Proposed mechanism of bromination.
4. Materials and Methods
4.1. General Information
All reagents and solvents were purchased from commercial suppliers and were not purified. The crude products were purified by column chromatography on silica gel (Aladdin Industrial Corporation, Shanghai, China). Melting points of the compounds were recorded on X-4 melting point detector (Beijing Tech Instrument Co. Ltd., Beijing, China) and are uncorrected. 1H NMR spectra were recorded on a Bruker 500 MHz (Beijing, China) and a 400 MHZ spectrometer (Beijing, China) in deuterated chlororoform using tetramethylsilane as the internal standard. 13C-NMR spectra (Beijing, China) were recorded at 126 MHz and 101 MHz spectrometer in deuterated chlororoform solutions. Chemical shifts (δ) and coupling constants (J) were expressed in ppm and Hz, respectively. The high-resolution mass analyses were recorded on an Agilent high resolution mass spectrometer (Shanghai, China).
4.2. General Procedure for the Bromination
0.11 g (1.0 mmol) of 4-methylphenol, 5 mL of acetic acid, 0.5 mL of water, and 0.12 g (1.0 mmol) potassium bromide were placed in round-bottomed flask. Then, 0.19 g (0.2 mmol) ZnAl–BrO3––LDHs was added in the flask under stirring at 35 °C. After the addition, stirring was continued to the end of reaction (monitored by thin layer chromatography). The residual ZnAl–BrO3−–LDHs were removed by centrifugation. The product was extracted with 3 × 10 mL dichloromethane. The combined extract was washed with sodium sulfite solution, brine, and dried (Na2SO4). Evaporation of the solvent left the crude product. The crude product was purified by column chromatography over silica gel (ethyl acetate-petroleum ether) to obtain pure product.
5. Conclusions
In conclusion, we developed a selective and mild mono-bromination of phenols with ZnAl-BrO3−–LDHs and potassium bromide as brominating reagents. Our strategy selectively produced para-brominated phenols, unless the para-position is substituted. As for para-substituted phenols, ortho-brominated products are obtained. The mild condition, simple experiment operation, high bromide atom economy, cheap brominating reagents, as well as no catalyst, make the present strategy prospective for the mono-bromination of phenols.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/25/4/914/s1, general procedure for ZnAl-BrO3--LDHs, characterization data, 1H NMR and 13C NMR Spectra of products.
Author Contributions
Conceptualization, L.W. and J.H.; data curation, C.F. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Thanks to Qingbao Song for the suggestions for this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Du, F.; Zhou, Q.; Liu, D.; Fang, T.; Shi, Y.; Du, Y.; Chen, G. Dimerization of aromatic aompounds using Palladium-carbon-catalyzed Suzuki-Miyaura cross-coupling by one-pot synthesis. Synlett 2018, 29, 779–784. [Google Scholar] [CrossRef]
- Harada, K.; Arioka, C.; Miyakita, A.; Kubo, M.; Fukuyama, Y. Efficient synthesis of neurotrophic honokiol using Suzuki-Miyaura reactions. Tetrahedron Lett. 2014, 55, 6001–6003. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Schmidt, B.; Riemer, M. Suzuki-Miyaura coupling of halophenols and phenol boronic acids: Systematic investigation of positional isomer effects and conclusions for the synthesis of phytoalexins from pyrinae. J. Org. Chem. 2014, 79, 4104–4118. [Google Scholar] [CrossRef] [PubMed]
- Schmoger, C.; Szuppa, T.; Tied, A.; Schneider, F.; Stolle, A.; Ondruschka, B. Pd on porous glass: A versatile and easily recyclable catalyst for Suzuki and Heck reactions. Chemsuschem 2008, 1, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lumb, J.-P. Phenol-directed C-H functionalization. Acs. Catal. 2019, 9, 521–555. [Google Scholar] [CrossRef]
- Jesus, A.; Correia-da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and potential biological applications of haloaryl secondary metabolites from macroalgae. Mar. Drugs 2019, 17. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Oztaskin, N.; Cetinkaya, Y.; Taslimi, P.; Goksu, S.; Gulcin, I. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg. Chem. 2015, 60, 49–57. [Google Scholar] [CrossRef]
- Ghorpade, P.V.; Pethsangave, D.A.; Some, S.; Shankarling, G.S. Graphene oxide promoted oxidative bromination of anilines and phenols in water. J. Org. Chem. 2018, 83, 7388–7397. [Google Scholar] [CrossRef]
- Jiang, P.-P.; Yang, X.-J. A quick, mild and efficient bromination using a CFBSA/KBr system. Rsc Adv. 2016, 6, 90031–90034. [Google Scholar] [CrossRef]
- Su, P.; Fan, C.; Yu, H.; Wang, W.; Jia, X.; Rao, Q.; Fu, C.; Zhang, D.; Huang, B.; Pan, C.; et al. Synthesis of Ti-Al binary oxides and their catalytic application for C-H halogenation of phenols, aldehydes and ketones. Mol. Catal. 2019, 475. [Google Scholar] [CrossRef]
- Sim, J.; Jo, H.; Viji, M.; Choi, M.; Jung, J.-A.; Lee, H.; Jung, J.-K. Rapid, Operationally simple, and metal-free NBS mediated one-pot synthesis of 1,2-Naphthoquinone from 2-naphthol. Adv. Synth. Catal. 2018, 360, 852–858. [Google Scholar] [CrossRef]
- Sengupta, G.; Pandey, P.; De, S.; Ramapanicker, R.; Bera, J.K. A bromo-capped diruthenium(I, I) N-heterocyclic carbene compound for in situ bromine generation with NBS: catalytic olefin aziridination reactions. Dalton T. 2018, 47, 11917–11924. [Google Scholar] [CrossRef] [PubMed]
- Anjaiah, B.; Prameela, K.; Srinivas, P.; Rajanna, K.C. Synthesis, kinetics, and mechanism of bromophenols by N-bromophthalimide in aqueous acetic acid. Int. J. Chem. Kinet. 2018, 50, 804–812. [Google Scholar] [CrossRef]
- Jereb, M.; Gosak, K. Acid-promoted direct electrophilic trifluoromethylthiolation of phenols. Org. Biomol. Chem. 2015, 13, 3103–3115. [Google Scholar] [CrossRef]
- Ishihara, K. Development of Highly selective organic transformation reactions using halogen lewis acids. J. Syn. Org. Chem. Jpn. 2014, 72, 137–148. [Google Scholar] [CrossRef]
- Andersh, B.; Murphy, D.L.; Olson, R.J. Hydrochloric acid catalysis of N-bromosuccinimide (NBS) mediated nuclear aromatic brominations in acetone. Synth. Commun. 2000, 30, 2091–2098. [Google Scholar] [CrossRef]
- Oberhauser, T. A new bromination method for phenols and anisoles: NBS/HBF4 center dot Et2O in CH3CN. J. Org. Chem. 1997, 62, 4504–4506. [Google Scholar] [CrossRef]
- Zysman-Colman, E.; Arias, K.; Siegel, J.S. Synthesis of arylbromides from arenes and N-bromosuccinimide (NBS) in acetonitrile - A convenient method for aromatic bromination. Can. J. Chem. 2009, 87, 440–447. [Google Scholar] [CrossRef]
- Khazaei, A.; Rostami, A.; Raiatzadeh, A. N-bromosuccinimide (NBS): a mild and efficient catalyst for tetrahydropyranylation of alcohols and Phenols under solvent-free conditions. J. Chin. Chem. Soc. 2007, 54, 1029–1032. [Google Scholar] [CrossRef]
- Semwal, R.; Ravi, C.; Kumar, R.; Meena, R.; Adimurthy, S. Sodium salts (NaI/NaBr/NaCl) for the halogenation of imidazo-fused heterocycles. J. Org. Chem. 2019, 84, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Sun, X.; Li, X.; Yuan, Y.; Jiao, N. Efficient and practical oxidative bromination and iodination of arenes and heteroarenes with DMSO and hydrogen halide: a mild protocol for late-Stage functionalization. Org. Lett. 2015, 17, 2886–2889. [Google Scholar] [CrossRef] [PubMed]
- Karki, M.; Magolan, J. Bromination of olefins with HBr and DMSO. J. Org. Chem. 2015, 80, 3701–3707. [Google Scholar] [CrossRef] [PubMed]
- Barhate, N.B.; Gajare, A.S.; Wakharkar, R.D.; Bedekar, A.V. Simple and practical halogenation of arenes, alkenes and alkynes with hydrohalic acid/H2O2 (or TBHP). Tetrahedron 1999, 55, 11127–11142. [Google Scholar] [CrossRef]
- Bora, U.; Bose, G.; Chaudhuri, M.K.; Dhar, S.S.; Gopinath, R.; Khan, A.T.; Patel, B.K. Regioselective bromination of organic substrates by tetrabutylammonium bromide promoted by V2O5-H2O2: An environmentally favorable synthetic protocol. Org. Lett. 2000, 2, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.; Gayen, K.S.; Khamarui, S.; Chatterjee, N.; Maiti, D.K. Addition of halide to π-bond directly from aqueous NaX solution: A general strategy for installation of two different functional groups. Chem. Commun. 2011, 47, 6933–6935. [Google Scholar] [CrossRef]
- Truchan, N.; Jandl, C.; Pothig, A.; Breitenlechner, S.; Bach, T. Access to biphenyls by Palladium-catalyzed oxidative coupling of phenyl carbamates and phenols. Synthesis 2019, 51, 3060–3076. [Google Scholar] [CrossRef]
- Khatun, R.; Biswas, S.; Ghosh, S.; Islam, S.M. Polymer-anchored Fe(III)Azo complex: An efficient reusable catalyst for oxidative bromination and multi-components reaction for the synthesis of spiropiperidine derivatives. J. Organomet. Chem. 2018, 858, 37–46. [Google Scholar] [CrossRef]
- Song, S.; Huang, X.; Liang, Y.-F.; Tang, C.; Li, X.; Jiao, N. From simple organobromides or olefins to highly value-added bromohydrins: a versatile performance of dimethyl sulfoxide. Green Chem. 2015, 17, 2727–2731. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Chen, B.; Lu, T.; Yuan, Y.; Yuan, G. A sustainable process for catalytic oxidative bromination with molecular oxygen. Chemsuschem 2013, 6, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Rohitha, C.N.; Kulkarni, S.J.; Narender, N. Bromination of aromatic aompounds using ammonium bromide and oxone (R). Synthesis 2010, 1629–1632. [Google Scholar] [CrossRef]
- Adimurthy, S.; Ramachandraiah, G.; Bedekar, A.V.; Ghosh, S.; Ranu, B.C.; Ghosh, P.K. Eco-friendly and versatile brominating reagent prepared from a liquid bromine precursor. Green Chem. 2006, 8, 916–922. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Khodykin, S.V.; Krylov, I.B.; Ogibin, Y.N.; Nikishin, G.I. A convenient synthesis of 2,2-dibromo-1-arylethanones by bromination of 1-arylethanones with the H2O2-HBr system. Synthesis 2006, 1087–1092. [Google Scholar] [CrossRef]
- Hemati, R.; Shahvelayati, A.S.; Yadollahzadeh, K. o-Xylylene Bis(Triethyl Ammonium Tribromide) as a mild and ecyclable reagent for rapid and regioselective bromination of anilines and phenols. Lett. Org. Chem. 2018, 15, 682–687. [Google Scholar] [CrossRef]
- Bovonsombat, P.; Teecomegaet, P.; Kulvaranon, P.; Pandey, A.; Chobtumskul, K.; Tungsirisurp, S.; Sophanpanichkul, P.; Losuwanakul, S.; Soimaneewan, D.; Kanjanwongpaisan, P.; et al. Regioselective monobromination of aromatics via a halogen bond acceptor-donor interaction of catalytic thioamide and N -bromosuccinimide. Tetrahedron 2017, 73, 6564–6572. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Jiang, M.; Wang, M.; Tang, L.; Wei, M.; Zhou, Q. Mild and regioselective bromination of phenols with TMSBr. Eur. J. Org. Chem. 2019, 2019, 4593–4596. [Google Scholar] [CrossRef]
- Satkar, Y.; Ramadoss, V.; Nahide, P.D.; García-Medina, E.; Juárez-Ornelas, K.A.; Alonso-Castro, A.J.; Chávez-Rivera, R.; Jiménez-Halla, J.O.C.; Solorio-Alvarado, C.R. Practical, mild and efficient electrophilic bromination of phenols by a new I(iii)-based reagent: the PIDA–AlBr3 system. RSC Adv. 2018, 8, 17806–17812. [Google Scholar] [CrossRef]
- Georgiev, D.; Saes, B.W.H.; Johnston, H.J.; Boys, S.K.; Healy, A.; Hulme, A.N. Selective and efficient generation of ortho-brominated para-substituted phenols in ACS-grade methanol. Molecules 2016, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Bovonsombat, P.; Ali, R.; Khan, C.; Leykajarakul, J.; Pla-on, K.; Aphimanchindakul, S.; Pungcharoenpong, N.; Timsuea, N.; Arunrat, A.; Punpongjareorn, N. Facile p-toluenesulfonic acid-promoted para-selective monobromination and chlorination of phenol and analogues. Tetrahedron 2010, 66, 6928–6935. [Google Scholar] [CrossRef]
- Alinezhad, H.; Tavakkoli, S.M.; Salehian, F. Efficient, rapid, and regioselective bromination of phenols and anilines with N-bromosaccharin using tungstophosphoric acid as a heterogeneous recyclable catalyst. Synth. Commun. 2010, 40, 3226–3232. [Google Scholar] [CrossRef]
- Narender, N.; Naresh, M.; Arun Kumar, M.; Mahender Reddy, M.; Swamy, P.; Nanubolu, J. Fast and efficient bromination of aromatic compounds with ammonium bromide and oxone. Synthesis 2013, 45, 1497–1504. [Google Scholar] [CrossRef]
- Mokhtary, M.; Lakouraj, M.M. Polyvinylpolypyrrolidone–bromine complex: Mild and efficient polymeric reagent for bromination of activated aromatic compounds. Chinese Chem. Lett. 2011, 22, 13–17. [Google Scholar] [CrossRef]
- Borikar, S.P.; Daniel, T.; Paul, V. Mild, Efficient, and regioselective monobromination of arylamines and phenols using [BBIm]Br3 as a new reagent. Synth. Commun. 2010, 40, 647–653. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Tajbakhsh, M.; Mohadjerani, M.; Lasemi, Z. Efficient and regioselective Bromination of aromatic compounds with ethylenebis(N-methylimidazolium) ditribromide (EBMIDTB). Synth. Commun. 2010, 40, 868–876. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Zargarani, D. Regioselective bromination of aromatic amines and phenols using N-benzyl-DABCO tribromide. Synth. Commun. 2009, 39, 4212–4220. [Google Scholar] [CrossRef]
- Borikar, S.P.; Daniel, T.; Paul, V. An efficient, rapid, and regioselective bromination of anilines and phenols with 1-butyl-3-methylpyridinium tribromide as a new reagent/solvent under mild conditions. Tetrahedron Lett. 2009, 50, 1007–1009. [Google Scholar] [CrossRef]
- Stropnik, T.; Bombek, S.; Kočevar, M.; Polanc, S. Regioselective bromination of activated aromatic substrates with a ZrBr4/diazene mixture. Tetrahedron Lett. 2008, 49, 1729–1733. [Google Scholar] [CrossRef]
- Suresh, P.; Annalakshmi, S.; Pitchumani, K. Regioselective monobromination of substituted phenols in the presence of β-cyclodextrin. Tetrahedron 2007, 63, 4959–4967. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Jalili, H. Mild and regioselective bromination of aromatic compounds with N,N,N′,N′-tetrabromobenzene-1,3-disulfonylamide and poly(N-bromobenzene-1,3-disulfonylamide). Synthesis 2005, 1099–1102. [Google Scholar] [CrossRef]
- Singh, P.P.; Thatikonda, T.; Kumar, K.A.A.; Sawant, S.D.; Singh, B.; Sharma, A.K.; Sharma, P.R.; Singh, D.; Vishwakarma, R.A. Cu-Mn Spinel oxide catalyzed regioselective halogenation of phenols and N-heteroarenes. J. Org. Chem. 2012, 77, 5823–5828. [Google Scholar] [CrossRef] [PubMed]
- Wischang, D.; Hartung, J. Bromination of phenols in bromoperoxidase-catalyzed oxidations. Tetrahedron 2012, 68, 9456–9463. [Google Scholar] [CrossRef]
- Chen, A.-J.; Wong, S.-T.; Hwang, C.-C.; Mou, C.-Y. Highly efficient and regioselective halogenation over well dispersed Rhenium-promoted mesoporous zirconia. ACS Catal. 2011, 1, 786–793. [Google Scholar] [CrossRef]
- Baharfar, R.; Alinezhad, H.; Azimi, S.; Salehian, F. Regioselective and high-yielding bromination of phenols and anilins using N-bromosaccharin and amberlyst-15. J. Chil. Chem. Soc. 2011, 56, 863–865. [Google Scholar] [CrossRef]
- Chen, A.-J.; Chen, X.-R.; Mou, C.-Y. Highly Regioselective oxybromination in an aqueous system using SBA-15 supported sulfated zirconia catalyst. J. Chin. Chem. Soc. 2010, 57, 820–828. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Li, J.-H. An efficient copper-catalysed aerobic oxybromination of arenes in water. Green Chem. 2010, 12, 2124–2126. [Google Scholar] [CrossRef]
- Bhatt, S.; Nayak, S.K. Copper(II) Bromide: A simple and selective monobromination reagent for electron-rich aromatic compounds. Synth. Commun. 2007, 37, 1381–1388. [Google Scholar] [CrossRef]
- Chhattise, P.K.; Ramaswamy, A.V.; Waghmode, S.B. Regioselective, photochemical bromination of aromatic compounds using N-bromosuccinimide. Tetrahedron Lett. 2008, 49, 189–194. [Google Scholar] [CrossRef]
- Das, B.; Venkateswarlu, K.; Majhi, A.; Siddaiah, V.; Reddy, K.R. A facile nuclear bromination of phenols and anilines using NBS in the presence of ammonium acetate as a catalyst. J. Mol. Catal. A- Chem. 2007, 267, 30–33. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Bromide-assisted oxidation of substituted phenols with hydrogen peroxide to the corresponding p-quinol and p-quinol ethers over WO42--exchanged layered double hydroxides. Angew. Chem. Int. Edit. 2005, 44, 310–313. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, Z.; Jiang, B.; Sun, Y.; Chen, Z.; Gao, X.; Yang, N. Ni-Al layered double hydroxides (LDHs) coated superhydrophobic mesh with flower-like hierarchical structure for oil/water separation. Appl. Surf. Sci. 2019, 490, 145–156. [Google Scholar] [CrossRef]
- Wang, B.; Shang, J.; Guo, C.; Zhang, J.; Zhu, F.; Han, A.; Liu, J. A general method to ultrathin bimetal-MOF nanosheets arrays via in situ transformation of layered double hydroxides arrays. Small 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Na, W.; Kim, J.; Lee, S.; Jang, J. Improved electrochemical performances of MOF-derived Ni-Co layered double hydroxide complexes using distinctive hollow-in-hollow structures. J. Mater. Chem. A 2019, 7, 17637–17647. [Google Scholar] [CrossRef]
- Guo, D.; Song, X.; Tan, L.; Ma, H.; Sun, W.; Pang, H.; Zhang, L.; Wang, X. A facile dissolved and reassembled strategy towards sandwich-like rGO@NiCoAl-LDHs with excellent supercapacitor performance. Chem. Eng. J. 2019, 356, 955–963. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Huang, R.; Zhou, Y.; Wu, Y.; Hu, Y.; Ostrikov, K. Ni-Co hydroxide nanosheets on plasma-reduced Co-based metal-organic nanocages for electrocatalytic water oxidation. J. Mater. Chem. A 2019, 7, 4950–4959. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, F.; Jiang, C.; Ni, Z. New reagent system for bromination built with Zn/Al-BrO3-- -LDHs as carrier. J. Chin. Cera. Soc. 2015, 43, 672–677. [Google Scholar]
- Wang, L.; Yu, Q.; Feng, C.; Zhang, Y.; Hu, J. Efficient synthesis of dibromoalkanes and iodoacetates from olefins using ZnAl-XO3(-)-LDHs/LiX (X= Br, I) as halogen sources. Chin. J. Org. Chem. 2019, 39, 1787–1793. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Yu, Q.; Feng, C.; Hu, J. Green synthesis of haloformates from olefins using formic acid as reactant, protonic acid, and solvent. Synlett 2018, 29, 1611–1616. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Zhang, H.; Yu, Q. Selective oxidative bromination of anilines using potassium bromide and ZnAl-BrO3--LDHs. Chin. J. Org. Chem. 2017, 37, 3186–3190. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).