1. Introduction
While migration from (and safety of) plastic materials in contact with food has been studied extensively for several decades, and plastic food contact materials (FCM) are subject to specific EU legislation (no 10/2011), research on migration from fiber-based FCMs is still in its infancy. Furthermore and, possibly as a consequence, so far there has been no specific EU-wide harmonized regulation on paper and board (P&B) FCM. Instead, on a national level, certain regulations do exist, such as the internationally recognized Recommendation XXXVI for paper and board, intended for food contact of the German Federal Institute for Risk Assessment (BfR). These recommendations are based on scientific evidence, where available, as well as largely on conventions, due to a lack of sufficient scientific data concerning migration under realistic conditions; hence, exposure from P&B FCM constituents. Such data and knowledge are indispensable as a basis for future legislation and rules for the paper packaging industry.
Less than 3.5% of all food packaging in direct contact with food are made from uncoated and untreated paper and board; thus, consumer exposure is estimated to be equally low. In addition, the foods that are in direct contact with this type of material are mainly dry foods (approximately 50%) and foods that need to be peeled or washed before their consumption (approximately 30%), so in these cases, the migration of substances is also expected to be low. [
1]. One of the critical properties of uncoated and untreated P&B for food packaging applications is its low resistance to high moisture content, which can cause disintegration of the P&B material in such applications [
1,
2]. To overcome this, technical solutions are the use of coatings or plastic layers for direct contact with food [
1]. Another solution could be hydrophobization of the fibers by introducing chemicals as additives into the pulp stock. This phenomenon, known as sizing of paper, allows improving its capacity to resist a certain degree on wetting with liquids, besides improving their dimensional stability and the quality of the surface [
1,
2,
3].
There are two main methods for applications of different conventional sizing: to add it in the paper machine at the wet end, known as internal sizing, or using a size press, which is surface sizing [
2]. The main internal sizing chemicals currently in use around the world are based on emulsions of rosin, alkyl ketene dimer (AKD), and alkenyl succinic anhydride (ASA). In all three cases, the objective is establishing a barrier against penetration and spreading of the liquid through the porous structure of the paper by protecting the hydroxyl groups in the sheet of the paper. This also improves the printing capacity of the paper and reduces the effect of aqueous fountain solution on loss of paper strength [
3].
AKD has become the most widely used internal sizing agent in the world since it was introduced to the papermaking industry in the 1950s due to its high efficiency and ease of use [
4,
5,
6]. Commercial AKDs are prepared from natural fatty acid sources; the stearic acid is mainly used for this purpose. Therefore, chemically, AKD sizing agents are typically mixtures with varying chain lengths according to the fatty acid fraction used for the synthesis. [
7].
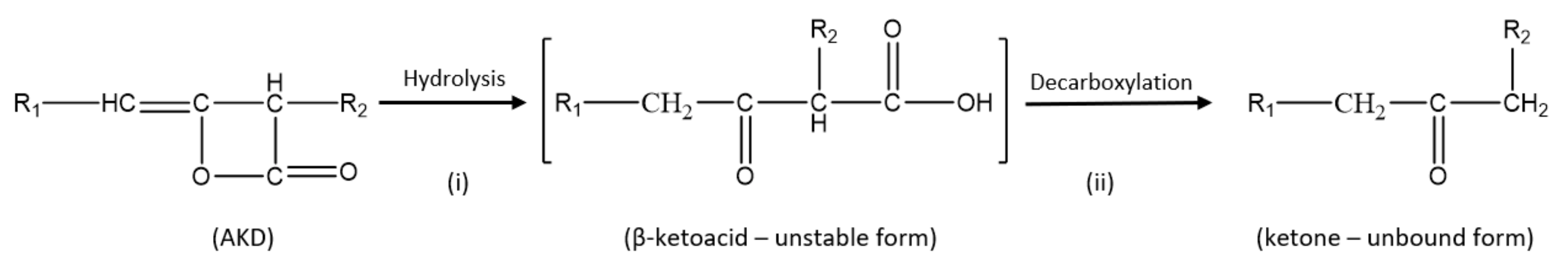
In general, it is assumed that the sizing mechanism of AKD involves the reaction of its β-lactone ring with the hydroxyl groups of cellulose molecule to form a covalent β-keto ester bond (
Figure 1); hence, making the paper resistant to liquids. A study by Lindström and Söderberg (1986) [
8] showed that the sizing effect depends mainly on the quantity of chemically reacted (bound) AKD in paper. However, some AKD may undergo hydrolysis in the presence of water molecules producing an unstable β-keto acid, which spontaneously decarboxylates to form the corresponding dialkylketones (DAK) (
Figure 2). This portion of AKD remains non-reacted, in the sense of not bound to cellulose and its reaction products, the DAK, absorb into the fibers. AKD molecules may also react together to form oligomers [
4].
Consequently, these DAK are present in many P&B-based food contact materials and are introduced in the recycling loop. DAK were identified, for instance, in extracts of recycled paper products and in the isooctane extract of a hot-cup starch lined by gas chromatography with mass spectrometry (GC-MS) [
9,
10]. They can be considered as a non-intentionally added substance (NIAS) to the packaging, since they originate from AKD degradation processes. These AKD based DAK molecules are characterized by a central carbonyl group and two long aliphatic lateral chains whose length depends on the original fatty acids involved in decarboxylation process. These two long aliphatic chains make the DAK highly lipophilic, similar as linear alkanes with the same C number. Their nomenclature refers to the total carbon atoms present in the two lateral aliphatic chains, minus the one lost in the decarboxylation process, together with the numerical position of the carbonyl group [
11]. Accordingly, the DAK with two stearyl chains (C17COC17) is named ‘stearone’ with an estimated log Po/w = 16 [
12]. Based on the typical average molecular weight of dialkylketones (for stearone: m.w. = 507 g/mol), their volatility is quite low. Consequently, their migration into food will take place only in case of intimate contact with the food, or will strongly depend on the temperature in case of indirect contact through the gas phase.
From a regulatory point of view in Europe, the German Federal Institute for Risk Assessment (BfR) allows the use of AKD as sizing agents for the manufacture of paper and board intended to come into contact with foodstuffs, in accordance with the Recommendation XXXVI “Paper and board for food contact”. However, in order to protect the consumer´s health, the following provision is established there: “Di-alkyl(C10-C22)diketenes, which can contain up to 65% isoalkyl groups, max 1.0%. The transfer of dialkylketones, that are produced by hydrolysis, into foodstuff may not exceed 5 mg/kg foodstuff” [
13]. The Food and Drug Administration (FDA) of the United States, in its Code of Federal Regulations (CFR) title 21, part 176.120, allows the use the alkyl ketene dimers as an adjuvant in the manufacture of paper and paperboard, but with the condition that the alkyl ketene dimers and their hydrolysis products dialkylketones do not exceed 0.4 percent by weight of the paper and paperboard [
14]. To control these restrictions, in particular the BfR specific migration limit of 5 ppm, the contact of paper and board with fatty food matrices must be especially taken into consideration due to the high lipophilicity and, hence, fat solubility of DAK. Liquid simulants, such as vegetable oils or isooctane, soak the P&B samples during contact and act, therefore, more or less extractive. The main packaging application of this type of P&B materials is (semi)solid foods. For liquid contact, coated or otherwise impregnated P&B materials would be needed. Solid or semi-solid real fatty foods, such as cheese, sausages, or fatty bakery products, show a different interaction with the P&B; however, migration data on real foods are not available. The 95% ethanol showed lower transfer than olive oil and isooctane [
15], which can be explained by lower solubility of the DAK in ethanol.
Interestingly, DAK molecules are not only relevant as potential migrants from P&B FCM, but were also identified as a new important class of compounds, newly found in the unsaponifiable fraction of vegetable oils that had undergone interesterification [
11]. In those processes, DAK are formed as by-products. Interesterification of fats is a common industrial practice in order to redistribute fatty acids in triglycerides under the influence of a chemical catalyst or using an enzyme [
16]. Those fats are used e.g., in margarine, chocolate, and bakery products [
11,
17]. It should be noted that the total DAK content determined in interesterified coconut oil, shea oil, and palm oil samples ranged between 80 and 2900 ppm [
11].
Analytical methods to determine the DAK in paper samples are summarized in [
3]. Analytical methods for quantification of DAK in oil are published by Derra and Jung [
15] and Santoro et al. [
11].
The two major objectives of this work were: (i) to establish a convenient sample work up-procedure and an analytical method based on the methodology published by Santoro et al. [
11] for the determination of DAK in fatty foodstuffs. Briefly, the method proposed by Santoro et al. [
11] involves an alkaline saponification with a 2.0 N ethanolic KOH solution, followed by an extraction with petroleum ether, including several wash cycles; then, the resulting extract was reconstituted in methylene chloride for direct analysis by gas chromatography (GC) and in heptane for further solid phase extraction (SPE) purification of the unsaponifiable fraction. The preferred analytical instrumentation should be gas chromatography coupled with flame ionization detection (GC-FID) as an inexpensive and robust tool available in any laboratory. (ii) To apply the developed method for comparative extraction/migration measurements of DAK from P&B-based food contact samples into food simulants versus some fatty foodstuffs for verification of the specific migration limit of 5 mg/kg food set by German BfR. The selection of the foods to be included in this study was based on two criteria: fatty foods, since they are able to dissolve lipophilic DAK, and fatty foods that can be in contact with in P&B articles. Therefore, we selected olive oil, sunflower oil, croissants, salami sausage, Gouda cheese, and cheddar cheese.
3. Materials and Methods
3.1. Reagents and Chemicals
All reagents were of analytical grade. Ethanol absolute p.A., ACS, Ph Eur., USP ≤ 99%; n-heptane p.A. ≤ 99%; n-hexane p.A. ≤ 99%; isooctane p.A. ≤ 99.5% and dichloromethane p.A. ≤ 99.8% were obtained from Chemsolute (Th. Geyer GmbH & Co. KG; Renningen, Germany).
The dialkylketone standards 18-pentatriacontanone > 95% (Stearone, DSK; CAS n° 504-53-0, MW 506.94 g/mol) and 16-hentriacontanone > 95% (Palmitone, DPK; CAS n° 502-73-8, MW 450.84 g/mol) were supplied by Tokyo Chemical Industry Deutschland GmbH (TCI; Tokyo, Japan). The internal standard (IS) tetracosane 99% (CAS n° 646-31-1) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Potassium hydroxide pellets p.A. and boiling chips granules were purchased from Merck (Darmstadt, Germany). The cellulose filters for ASE 200 were purchased from Restek (USA).
Stock solutions with a concentration of 1000 mg/L were prepared by dissolving both dialkylketone standard substances (palmitone and stearone) in the solvent n-heptane, after sonication with temperature. A mix standard solution series in the concentration range from 0.2 to 20 mg/L were prepared in n-heptane and were stored at 4 °C until analysis. The tetracosane was added as an internal standard at the 5 µg/mL final concentration. The calibration curve was used for the quantification of the dialkylketones and to verify GC-FID performance.
3.2. Food Simulants, Foods, and Paperboard Sample
Olive oil (composed of refined olive oils and virgin olive oils), sunflower oil, salami sausage (fat content 26 g/100 g), young cheddar cheese (fat content 33 g/100 g), young gouda cheese (fat content 27.9 g/100 g), and mini butter croissants (fat content 11 g/100 g) were purchased in local supermarkets (Freising, Germany) and stored in a refrigerator, according to specifications written on the label. The fat content of the foods was taken from the label and was verified by determination of extractable fat, giving results that fit for all samples.
For the migration study, paperboard packaging trays from Mare GmbH, with an area weight of 500 g/m2, were used.
3.3. Instrumentation
The experiments were performed on a Trace GC 2000 Series ThermoQuest CE Instruments (Austin, TX, USA) gas chromatograph, equipped with a model A200S autosampler (Carlo Erba Instruments, Egelsbach, Germany) and coupled to flame ionization detection.
The GC-MS system used for confirmation was an Agilent 6890 Series gas chromatograph, equipped with a HP 7673 autosampler (Hewlett Packard) and a Thermo Finnigan SSQ mass spectrometer.
The extraction of cheese fat was carried out using an Accelerated Solvent Extractor (ASE) 200 Dionex. A rotavapory vacuum evaporator Büchi rotavapor R-114 coupled to a Büchi vacuum system B-180 and a Büchi waterbath B-480 (Flawil, Switzerland) was used to evaporate the extracts. Freeze-drying of the samples was performed using a Heto Drywinner freeze-dryer model DW 6-85. A water bath with sonication (Bandelin Sonorex, Berlin, Germany) was used to dissolve the standards.
3.4. Extraction of DAK from Oil and Foods
3.4.1. Extraction of the Fat in Solid Food Samples
For removal of water, the weighed food samples (except oil) were frozen with liquid nitrogen and freeze-dried for 24 h, at −100 °C and 0.1 mbar.
The extraction of the fat was carried out using an Accelerated Solvent Extractor (ASE). The stainless steel extraction cells of 22 mL were previously cleaned with n-hexane. The lyophilized sample was placed in the stainless steel extraction cell with a filter of cellulose in each side to avoid the blockage of the stainless frit. The extraction cell was placed in the carousel and the samples were extracted under the conditions: 100 bar system pressure, three static cycles, and 15 min-static times/cycle (3 × 15 min static time) at an oven temperature of 120 °C. At the end, the extraction cell was flushed with solvent (flush volume 60%) and purged with nitrogen (purge time 120 s). Hexane was selected as extraction solvent. Finally, the solvent was evaporated using a rotavapor at 60 °C. Residual solvent was removed at 60 °C for 2 h in an oven. Total extractable fat was weighed and determined.
3.4.2. Extraction of the Unsaponifiable Fraction from Fat or Oil
The extraction procedure established in this study was based on the procedure described by Santoro et al. [
11] with some simplifying modifications. 2 g of oil or 2 g of the extracted fat (from the first step) was weighed and placed in a flask equipped with a reflux condenser together with 50 mL 2 N potassium hydroxide solution in ethanol 90%. The sample was heated under reflux for 30 min using boiling chips granules to make it boil more calmly. After cooling down, the extract was transferred from the flask into a separatory funnel. The flask was rinsed two times each with 25 mL deionized water, which was transferred (50 mL in total) into the separatory funnel. After addition of 50 mL n-heptane the closed funnel was shaken vigorously. After phase separation, 20 mL of the organic phase was concentrated using a rotatory evaporator to a volume of 2 mL, a volume of 500 µL of 20 mg/L of internal standard (tetracosane) in heptane was added, obtaining 5 mg/L final concentration in the extract. An aliquot of this extract was encapsulated in a glass vial to be analyzed by means of GC-FID or GC-MS. Recovery experiments showed that repeated extraction or additional washing steps were not necessary (recovery from olive oil: 85.5–90%).
3.5. Extraction/Migration Tests of Paperboard Sample
Extraction tests were carried out using non-polar solvents, isooctane as food simulant according to the European Standard DIN EN 15519 [
19], and additionally, under more severe conditions dichloromethane.
3.5.1. Extraction with Dichloromethane
One gram of the paperboard sample was extracted with 25 mL dichloromethane for 3 days at 40 °C (in duplicate). After this time, 500 µL of the internal standard was added to an aliquot of the extract and analyzed by GC-FID. The quantification was obtained by external calibration in the range of 0.5–12. 5 µg/g for both dialkylketones in n-heptane.
3.5.2. Extraction with Isooctane
One gram of the paperboard sample was extracted with 25 mL isooctane for 1 day at 20 °C (in duplicate). After this time, 500 µL of the internal standard was added to an aliquot of the extract and analyzed by GC-FID. The quantification in the extraction solution was done using the external calibration curve of both dialkylketones in n-heptane.
3.5.3. Migration into Olive Oil
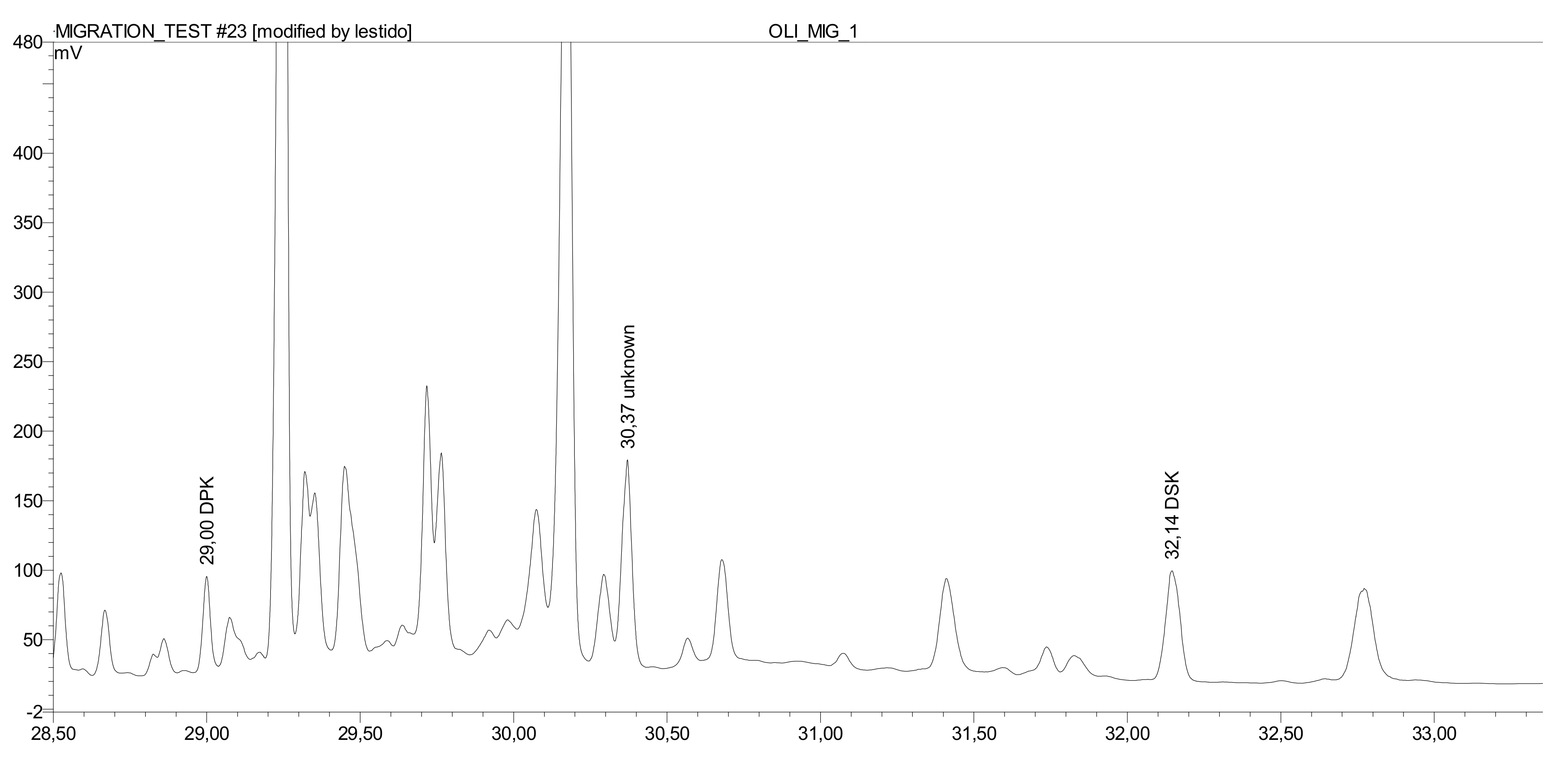
The 10 cm × 10 cm test specimens (1 dm2 single side area) of the paperboard sample were cut in small pieces, weighed, and totally immersed in 60 g olive oil for 10 days at 20 °C (in duplicate). Once this time has elapsed, the above described extraction method was applied to obtain the dialkylketones from oil in the unsaponifiable fraction.
3.5.4. Migration into Sunflower Oil
The 10 cm × 10 cm test specimens (1 dm2 single side area) of the paperboard sample were cut in small pieces, weighed, and totally immersed in 60 g sunflower oil for one week (7 days) at 10 °C (in duplicate). Once this time has elapsed, the above described extraction method was applied to extract the dialkylketones from oil after saponification.
3.5.5. Migration into Cheddar Cheese, Gouda Cheese, and Salami Sausage
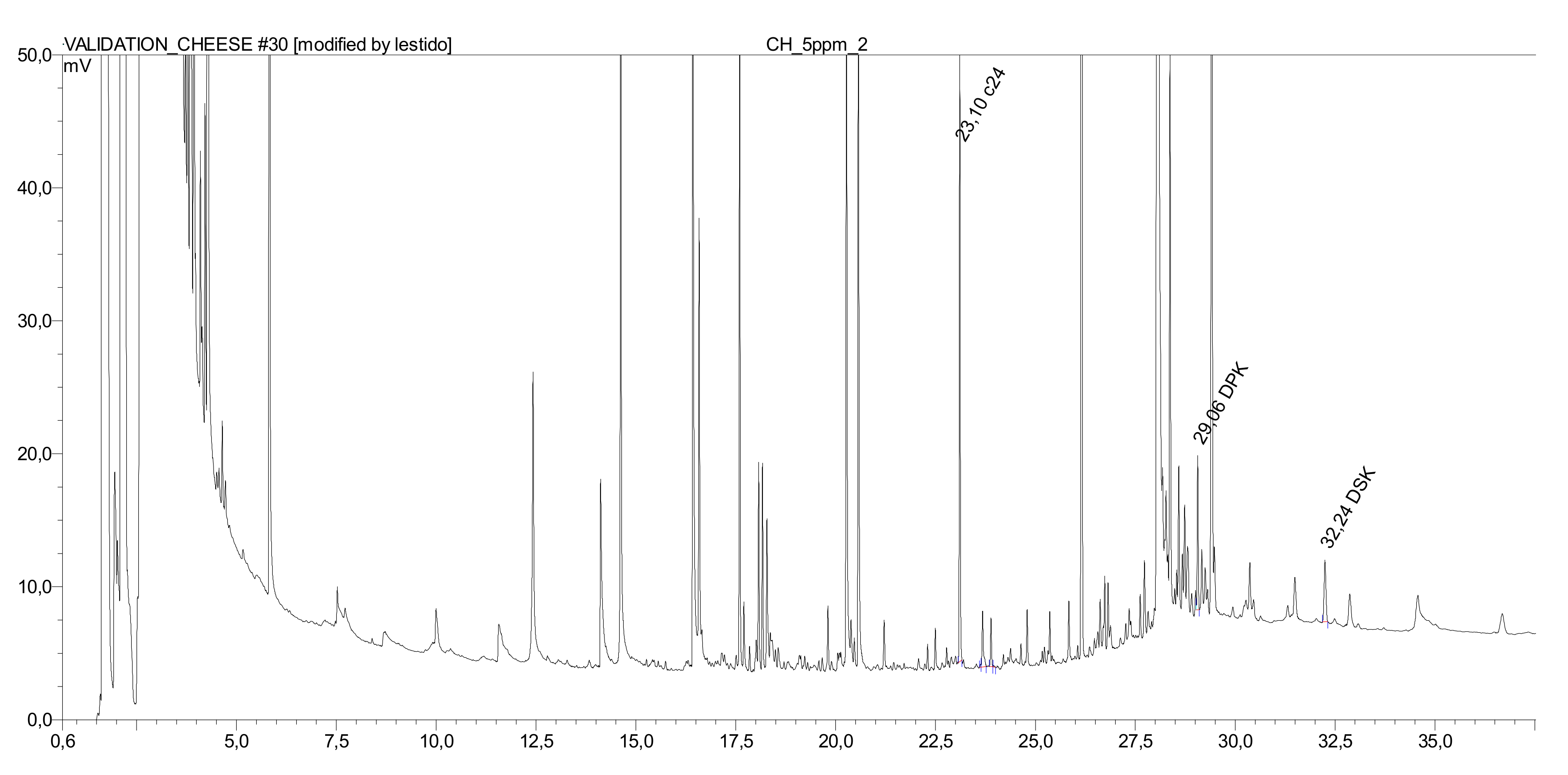
The 1 dm2 of the food contact side of the paperboard sample was put in contact with 1 dm2 of slices (2–3 mm thickness) of the food samples (cheddar cheese/Gouda cheese/salami). Each experiment was carried out in triplicate. The food slices were pressed on the paperboard to ensure intimate contact. Then these samples were wrapped in aluminum foil in order to eliminate possible contaminations and to minimize a possible loss of the analytes, and stored at 10 °C for 7 days in a controlled climate chamber. The food samples were controlled gravimetrically before and after the migration study, being 25 g of cheddar cheese, 27 g of Gouda cheese, and 8 g of salami. Once this time has elapsed, the paperboard was removed. The fat of the cheese and salami was extracted and the DAK from the fat after saponification, as described above.
3.5.6. Migration with Croissant
The 1 dm2 of the food contact side of the paperboard sample was put in contact with 1 dm2 of the external side of several croissants cut horizontally (in triplicate). The food slices were pressed on the paperboard to ensure intimate contact. Then these samples were wrapped in aluminum foil and were maintained at room temperature for 24 h (1 day). The sample was also controlled gravimetrically before and after the migration study. Once this time has elapsed, the paperboard was removed, the fat of the croissant was extracted, and then the DAK from the fat after saponification, as described above.
3.6. GC-FID Analysis
The analyses were carried out by gas chromatography with flame ionization detection (GC-FID). GC was carried out using a DB-1 capillary column with 30 m length, 0.25 mm inner diameter, and 0.25 µm film thickness. The conditions were as follows: injection temperature 300 °C, injection volume 1 µL in split mode (15 mL/min), flow rate of helium carrier gas 1.5 mL/min. Temperature program: initial temperature 50 °C for 2 min isotherm, ramp to 320 °C with a heating rate of 10 °C/min, and then a final isotherm at 320 °C of 10 min. FID transfer line temperature of 325 °C.
The data were acquired and processed with Chromeleon software (version 6.8). The identification of components was based on comparison of their GC retention time with those obtained for the dialkylketone authentic standards.
Quantification was obtained by external calibration using the ratio of analyte and internal standard area. Olive oil and cheddar cheese calibration was carried out by adding the palmitone and stearone standards to the oil or the extracted fat and performing the whole saponification/extraction procedure. The other foods were quantified using calibration in heptane.
3.7. GC-MS Analysis
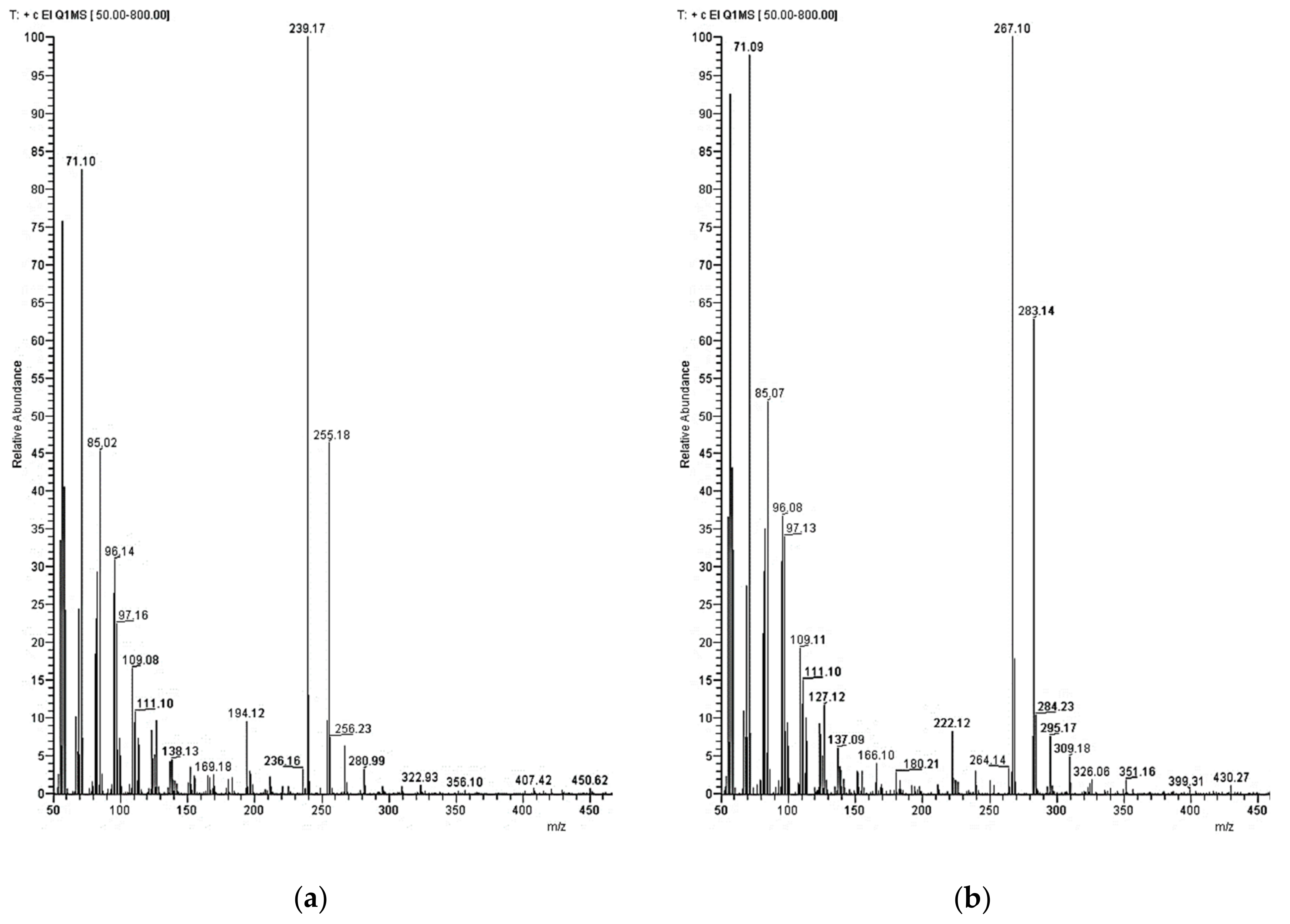
GC was carried out using an Optima-5MS column with 30 m length, 0.25 mm inner diameter, and 0.25 µm film thickness. The conditions were as follow: injection temperature 320 °C, injection volume 2 µL in split mode (1:10), flow rate of helium carrier gas 1.5 mL/min. Temperature program: initial temperature 50 °C for 2 min isotherm, then heating rate of 10 °C/min, and a final isotherm at 340 °C of 30 min. Mass detector conditions: transfer line temperature 320 °C and EI ionization mode (70 eV) with selective ion monitoring (SIM). The SIM masses selected were m/z 239 and 255 for palmitone and m/z 267 and 283 for stearone.
The data were acquired and processed with Xcalibur software. The identification of components was based on comparison of their GC retention time with those obtained for the dialkylketone authentic standards and using the National Institutes of Standards and Technology (NIST) mass spectra library. For quantification, the sum of both mass fragments per substance was used.
3.8. Method Validation
The analytical characteristics of the developed method for these dialkylketones were evaluated, including linearity, sensitivity, precision, and trueness.
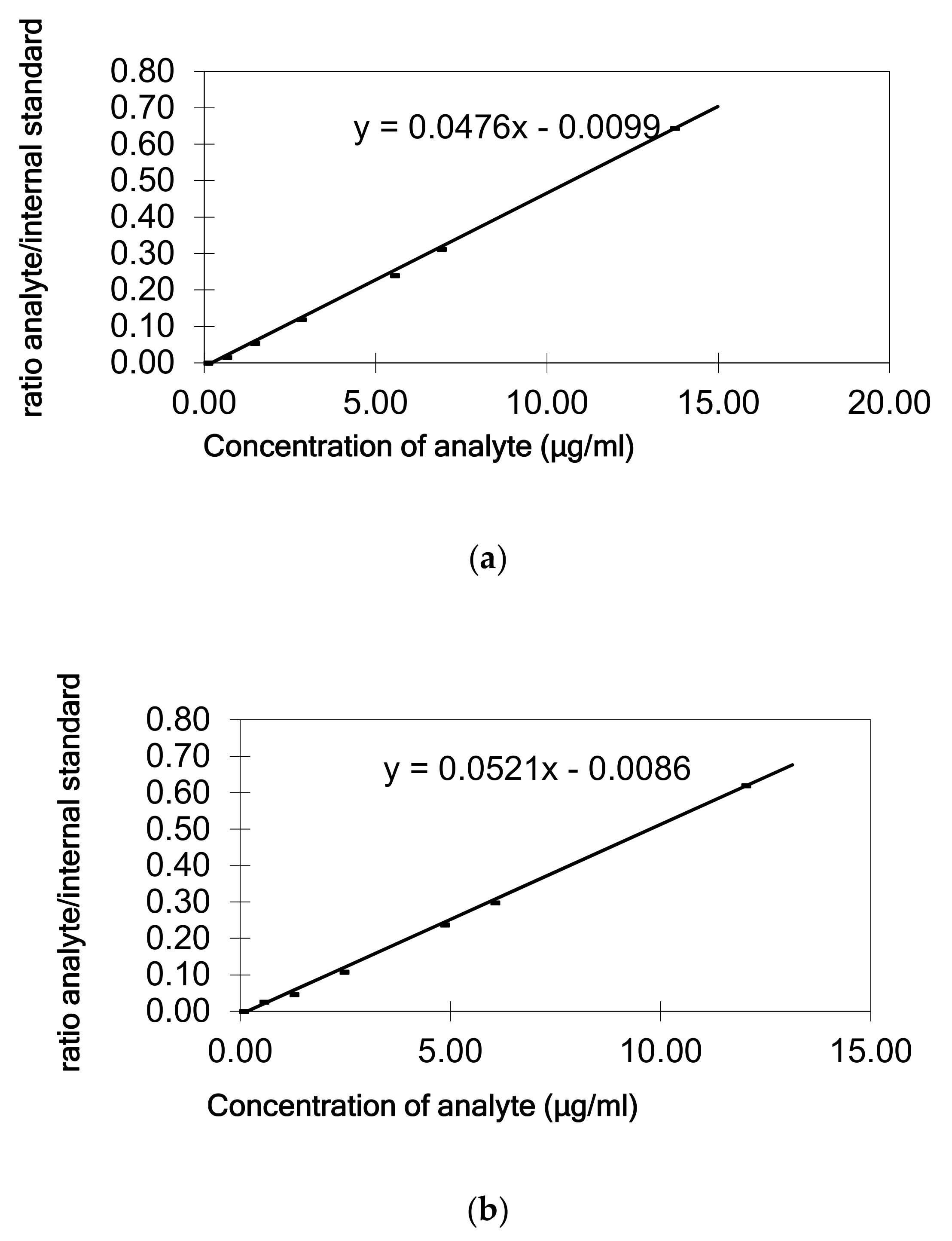
Linearity of the detector response was verified with standard solutions in heptane at six concentrations (0.2, 0.5, 1.0, 2.0, 2.5, and 5.0 µg/mL heptane corresponding to 0.5, 1.25, 2.5, 5, 6.25, and 12.5 µg/g of fat/oil). As internal standard tetracosane (5 µg/mL) was used for quantitative analysis in order to compensate instrumental variability of GC-FID. Calibration curves were constructed by plotting the standard concentration versus the peak area/internal standard area ratio obtained from GC-FID.
The quantification of dialkylketones in olive oil and the fat of the cheddar cheese were done using the calibration curve in the oil or cheese fat, respectively. Heptane standard solutions containing five different concentration levels of palmitone and stearone (0.5, 1.25, 2.5, 5, and 12.5 µg/g fat) were added to the oil/fat. Each level was done in duplicate and the sample without spiking was analyzed too. The quantification was based on calibration curves generated by plotting the peak area/internal standard ratio versus the amount spiked of each standard. In parallel standards of palmitone and stearone directly in heptane were measured, and calibration curves calculated. The trueness of the extraction and quantification method developed has been established in terms of recovery (by comparing the slopes of the calibration line obtained from the spiked olive oil/fat cheddar cheese and the calibration in n-heptane). The efficiency of the cheddar cheese fat extraction was also evaluated through recovery studies from spiked cheddar cheese samples with known amounts of dialkylketones (1 and 5 µg/g) in duplicate, before the cheese was subject to the freeze-drying process. Extraction and GC analysis were conducted using the same sample procedure.
Recoveries of the other foods (croissants, salami, and Gouda cheese) were determined by fortifying the extracted fat with 500 µL of mix standard solutions at two concentration levels (corresponding to 1 and 5 µg/g fat) in duplicate.
The sensitivity of the method was studied by determining the limit of detection (LOD) and limit of quantification (LOQ) for individual compounds in standard solutions taking into account the noise in the chromatographic analyses. The examination of a blank was always carried out in parallel. The limit of detection was considered as the lowest concentration, which provides a signal-to-noise ratio of 3, while the limit of quantification was considered as a signal-to-noise ratio of 10.
The precision of the extraction and measurement procedure was determined as repeatability for both standard compounds by extraction two spiked olive oil, and the cheddar cheese fat samples, at 3 different days, at a concentration of 2.5 µg/g (n = 6) and expressed as relative standard deviations (RSDs %).
4. Conclusions
A convenient and cost-effective method was developed and validated in this study for the extraction and determination of dialkylketones in edible oils and real fatty food samples using the gas chromatography technique with flame ionization detection (GC-FID). In addition, a gas chromatography with mass spectrometry (GC-MS) method was established for confirmation and quantification purposes when there was any interference in the food sample.
Based on this method, extraction tests of a paper-based food contact article with conventional food simulant solvent isooctane and extraction solvent dichloromethane, as well as extractive migration into edible oils (olive oil, sunflower oil) were carried out and compared with migration tests into real fatty food samples (croissants, salami, Gouda cheese, and cheddar cheese). As a result, it was found that the simulating tests, including the edible oil extraction tests, gave migration values exceeding the specific migration limit (SML) of 5 mg/kg, established by BfR largely. On the other hand, the migration results with the food samples were two to three orders of magnitude lower, and in any case, largely below the SML.
Our results, with the example of DAK as migrants, indicate one of the weaknesses in P&B migration testing, where extraction tests are dominating in opposite to migration testing of plastics: simulation of migration from P&B articles with conventional solvent based food simulants can be heavily misleading when the intention is to verify a given SML, or to provide data for exposure estimation. Clearly, isooctane extractions of P&B samples for verifying compliance with the DAK SML give ‘migration’ values far above realistic values obtainable when testing migration into foods.
When comparing the results obtained between the different fatty food samples after the contact with paperboard, it appears—although this is not proven and still needs further studies—that the extent of the migration of the dialkylketones present in the paper and board-based food packaging may depend on the fat content of the food, and on the intensity of the contact between food and P&B material.
We acknowledge that our studies are not fully comprehensive, and see the need for further studies with more P&B materials and other migrants, as well as other foods, including kinetic studies. However, the results presented here for DAK confirm, to a large degree, what one can expect for migration of P&B constituents into foods: in many cases such migration values will be lower and even considerably lower than those results obtained by solvent extraction tests. Clearly, the level of migration and, hence, the closeness of the results from the extraction versus into food migration will depend on the chemical nature and volatility of the migrant. Future research in this topic will further contribute to a more exposure-orientated assessment of migration of paper additives and their components into foodstuffs.