Cytotoxic Activity of Organotin(IV) Derivatives with Triazolopyrimidine Containing Exocyclic Oxygen Atoms
Abstract
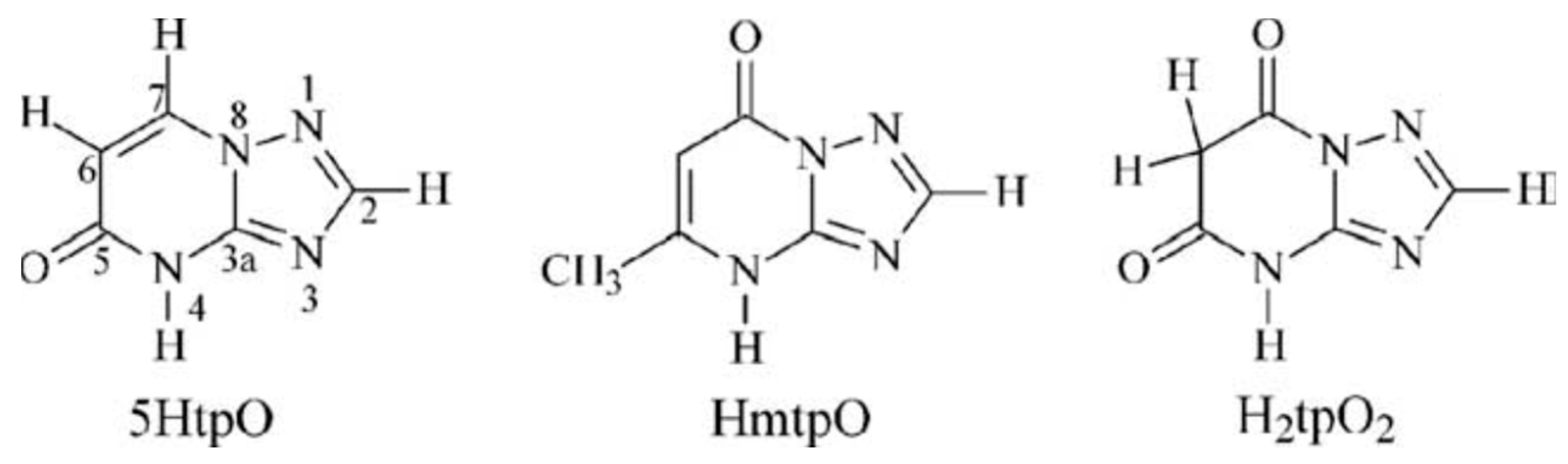
1. Introduction
2. Results and Discussion
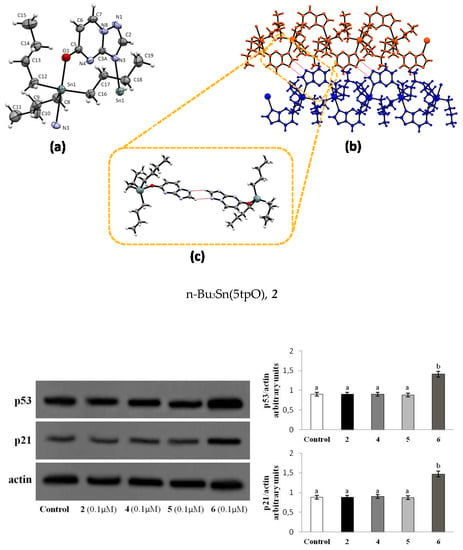
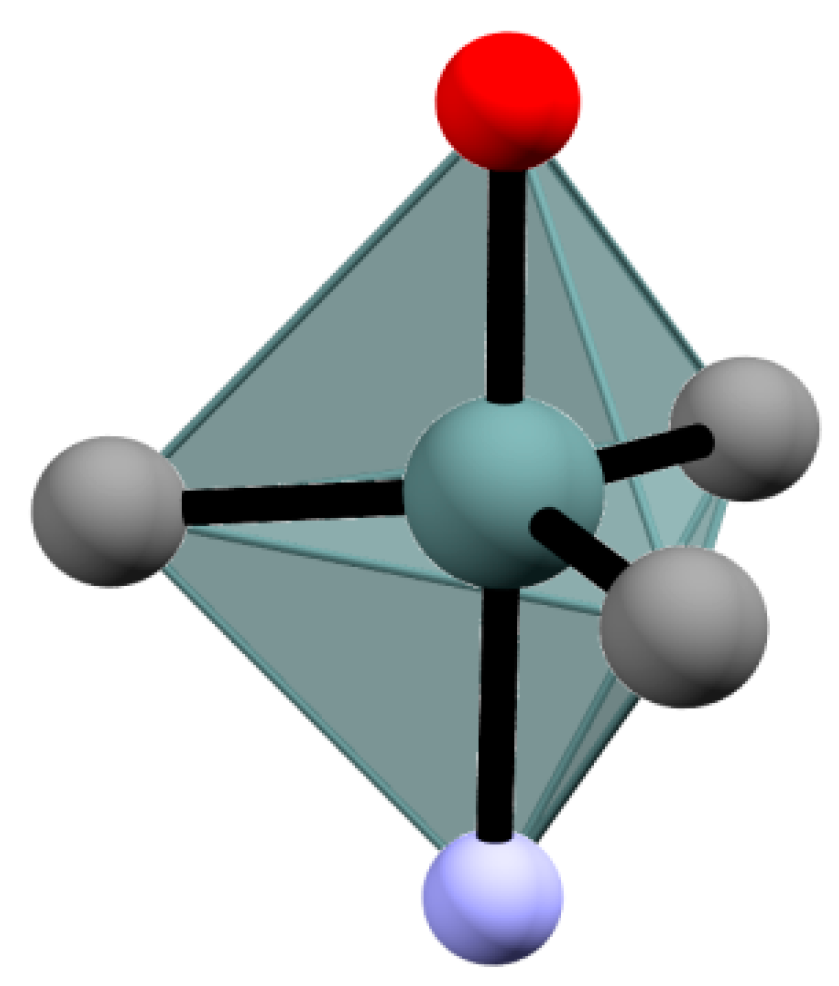
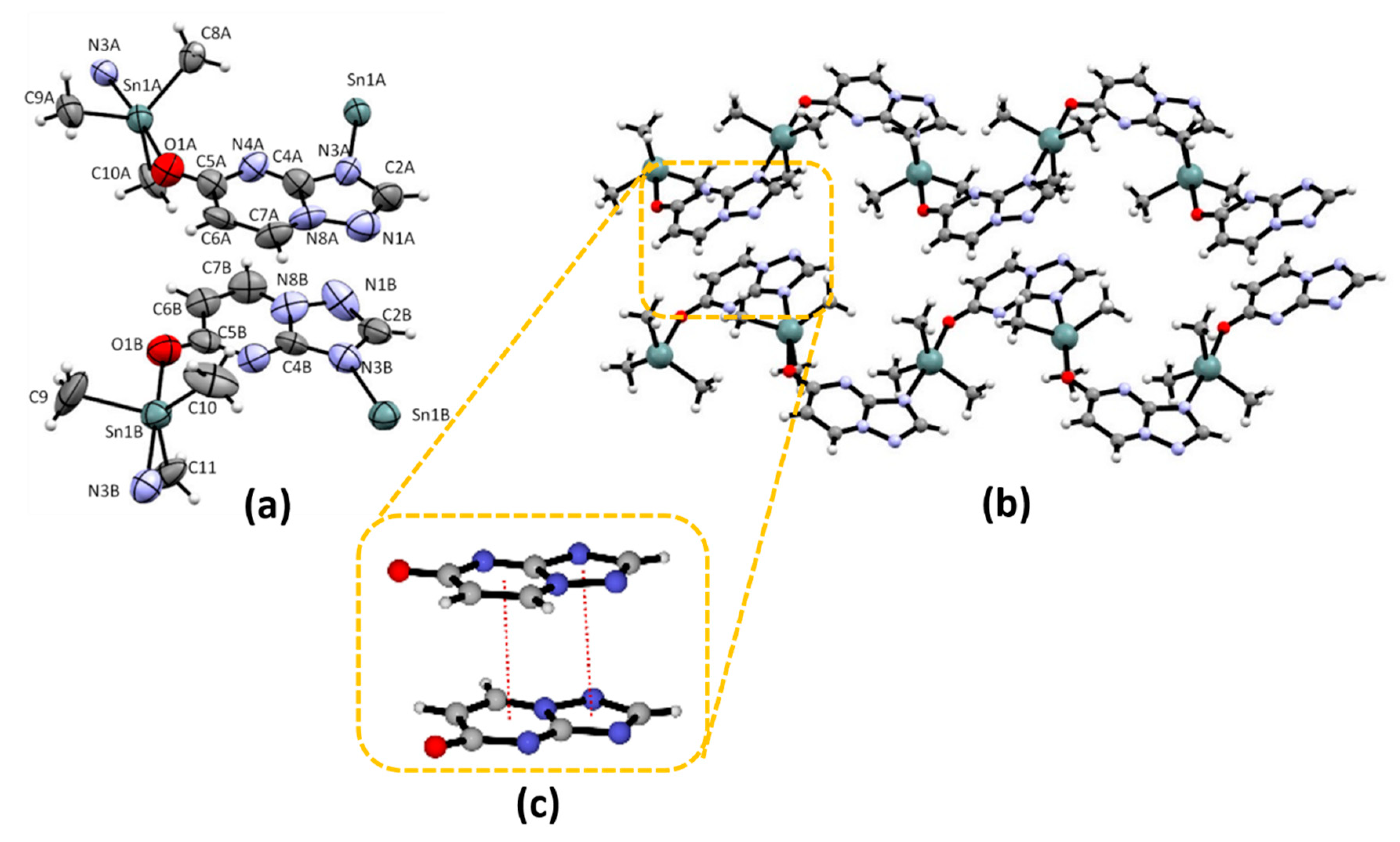
2.1. Crystal Structure of Me3Sn(5tpO), 1 and n-Bu3Sn(5tpO), 2
2.2. Biology
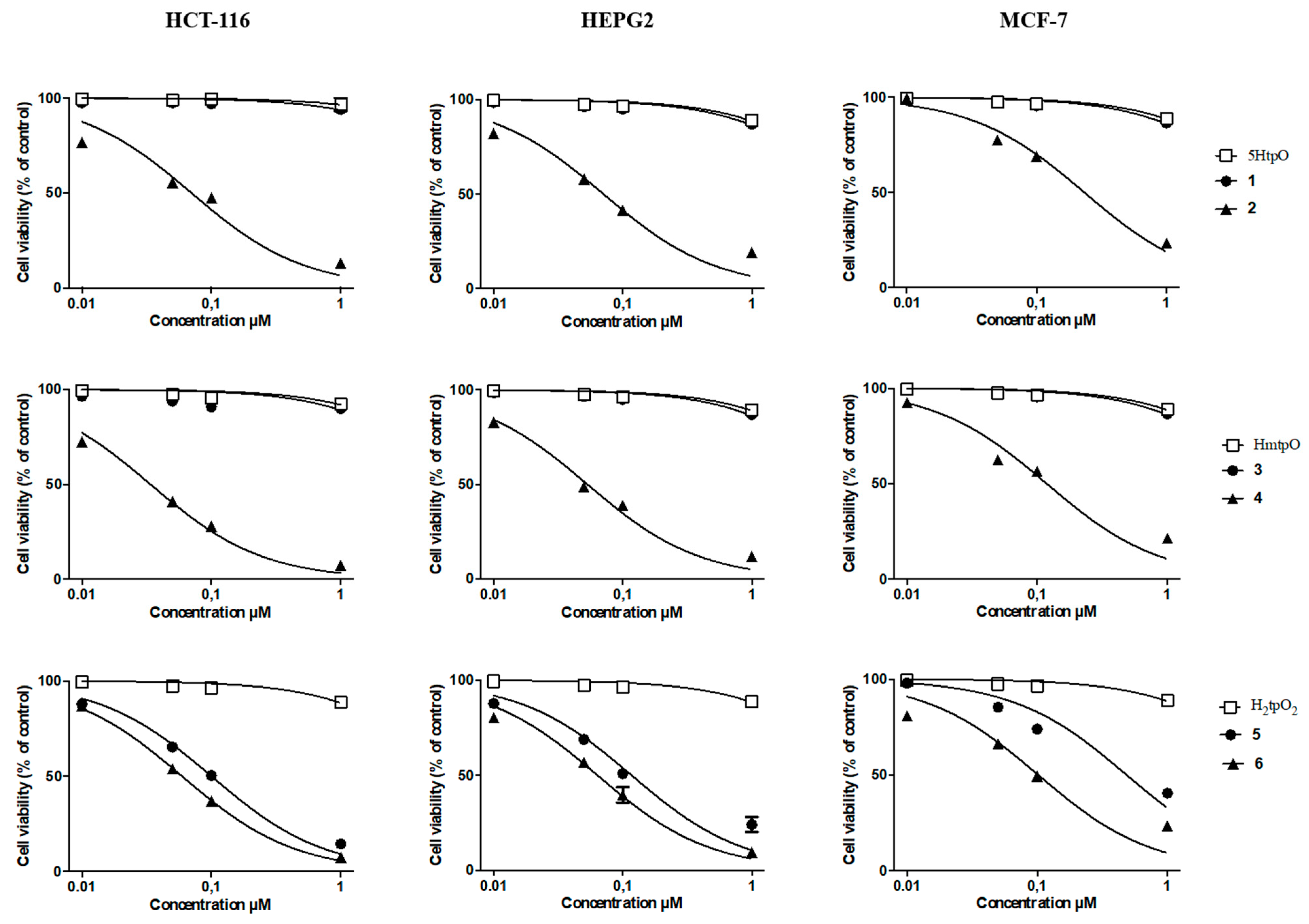
2.2.1. Cytotoxicity Assay
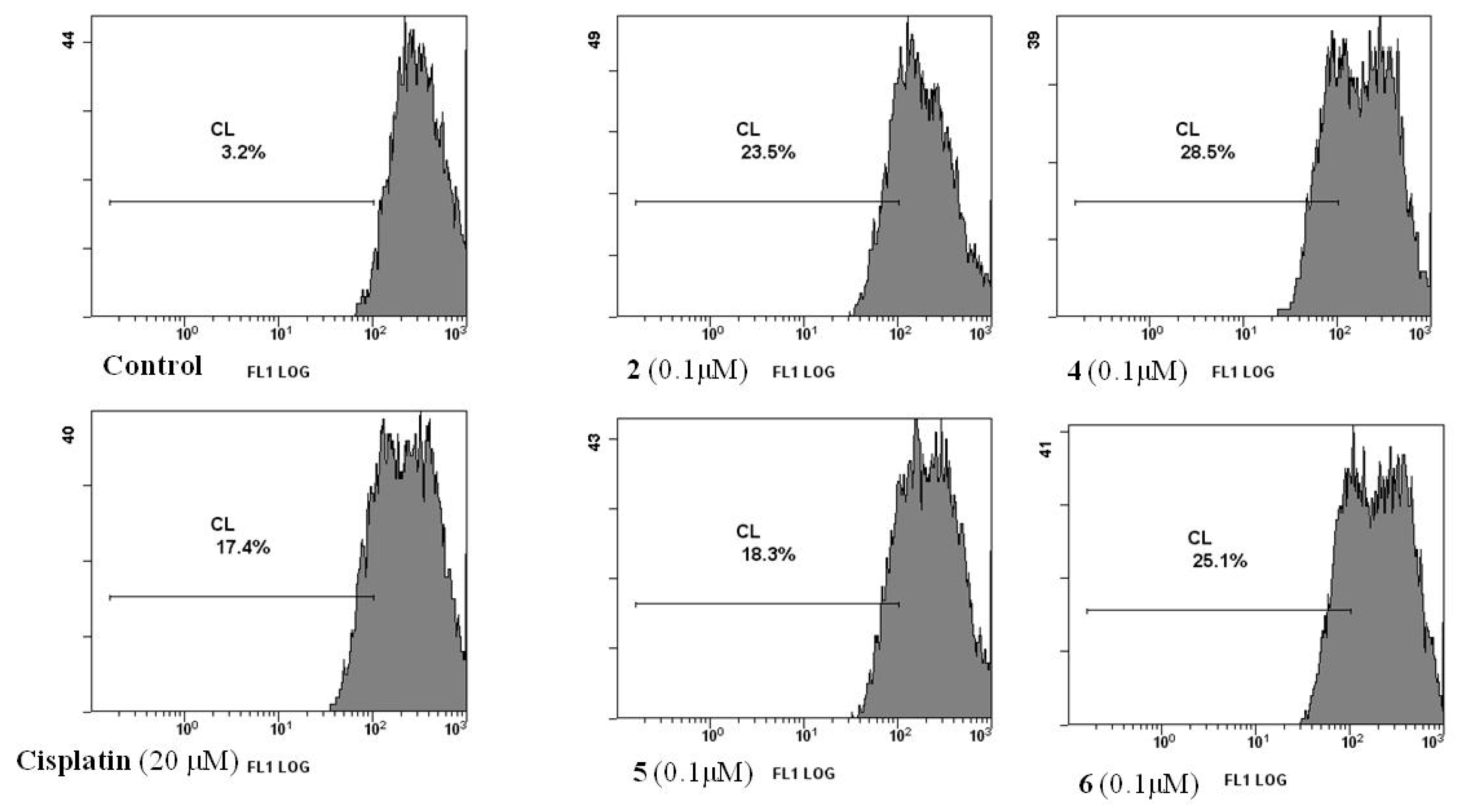
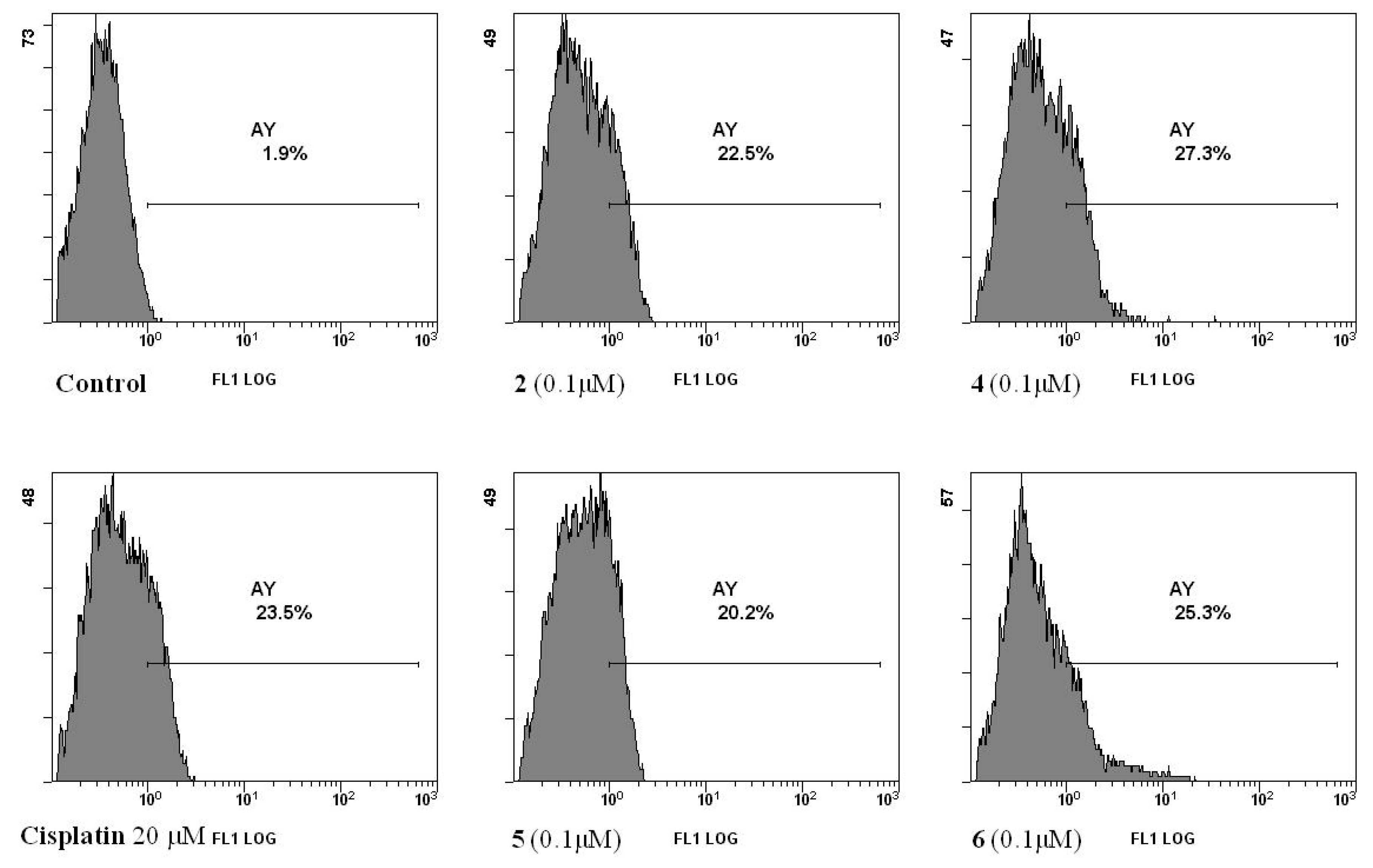
2.2.2. Cell Death
2.2.3. Mitochondrial Dysfunction
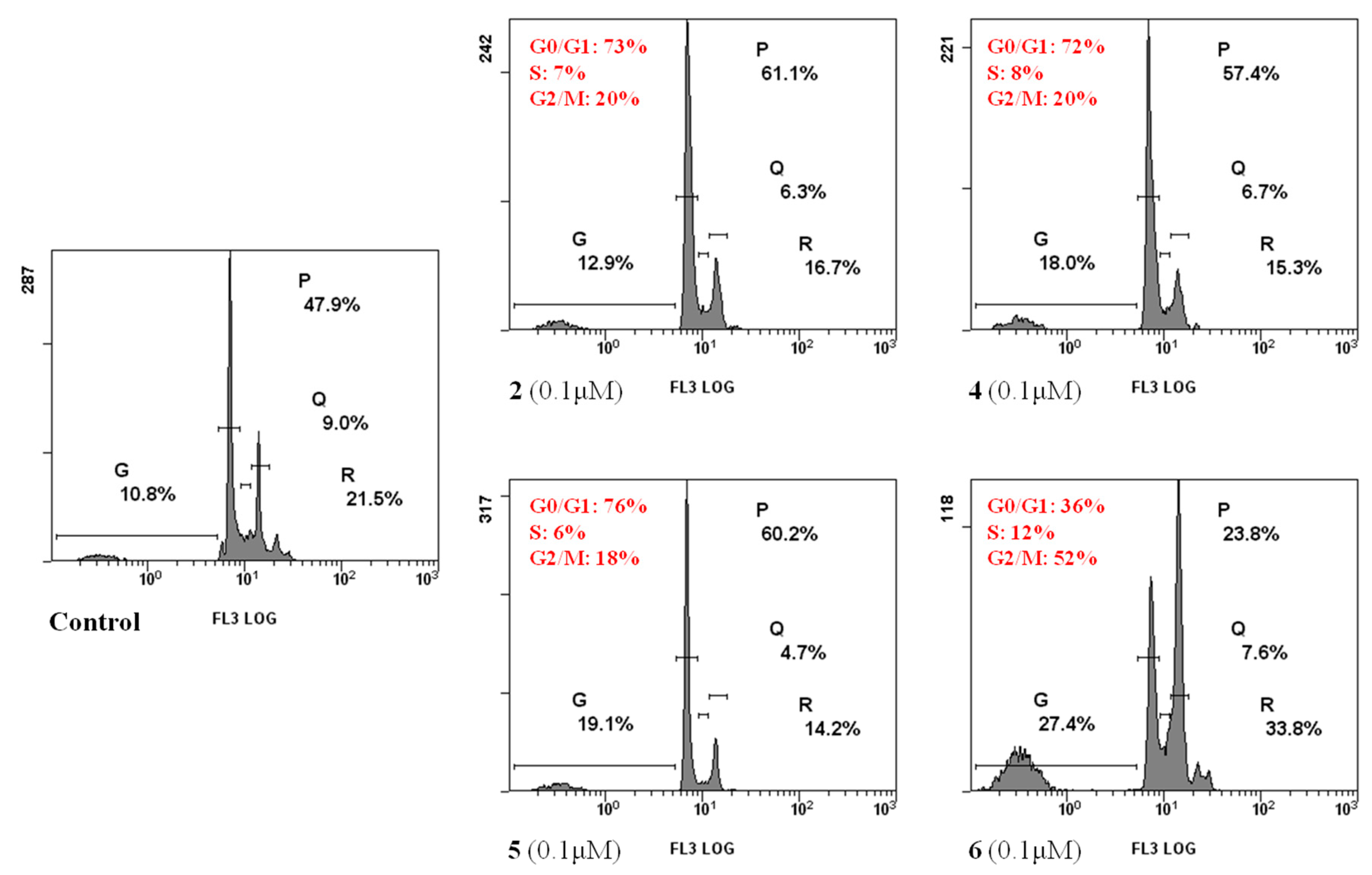
2.2.4. Cell Cycle Analysis
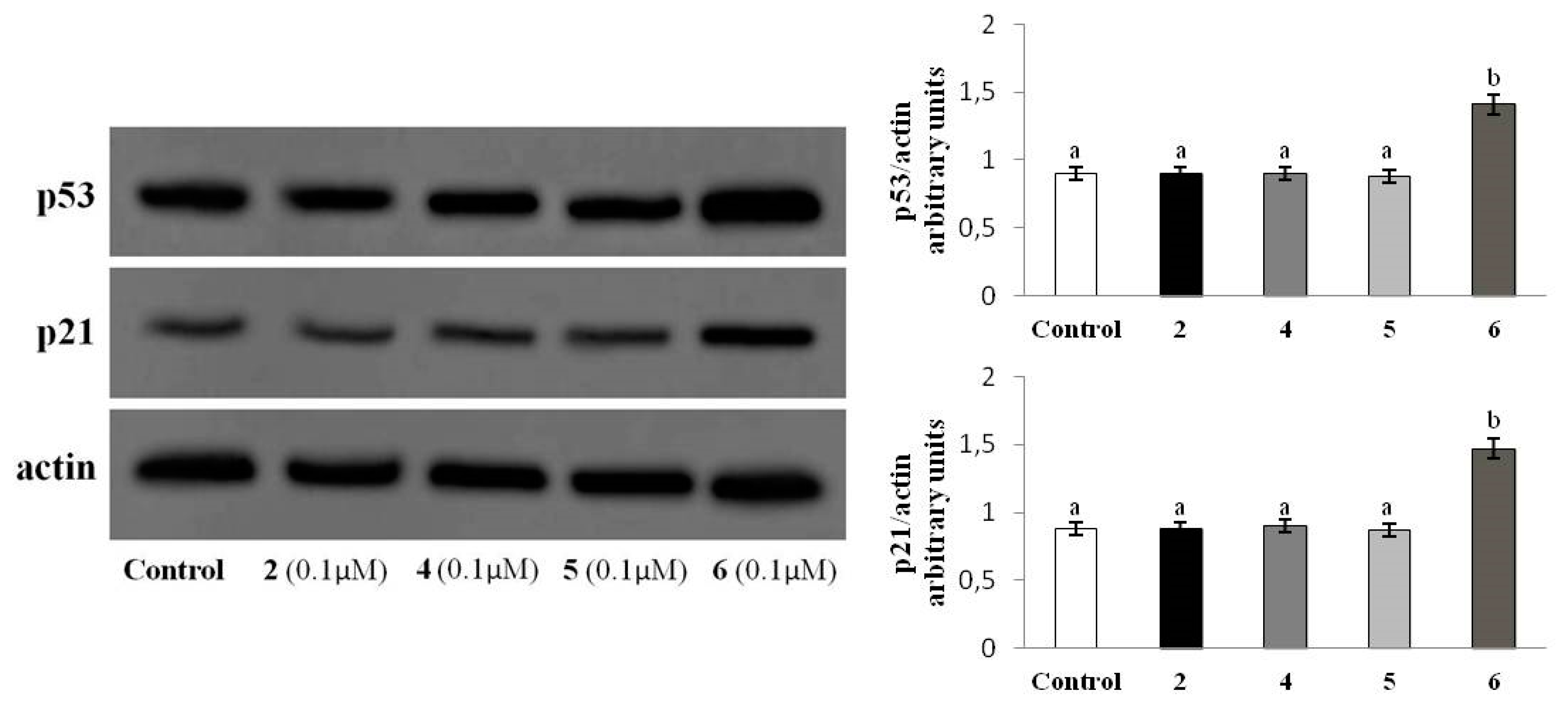
2.2.5. p53-p21WAF1 Protein Levels
3. Materials and Methods
3.1. Synthesis of Triorganotin(IV) Compounds
3.2. X-ray Crystallography of Me3Sn(5tpO), 1 and n-Bu3Sn(5tpO), 2
3.3. Biological Studies
3.3.1. Viability Assay
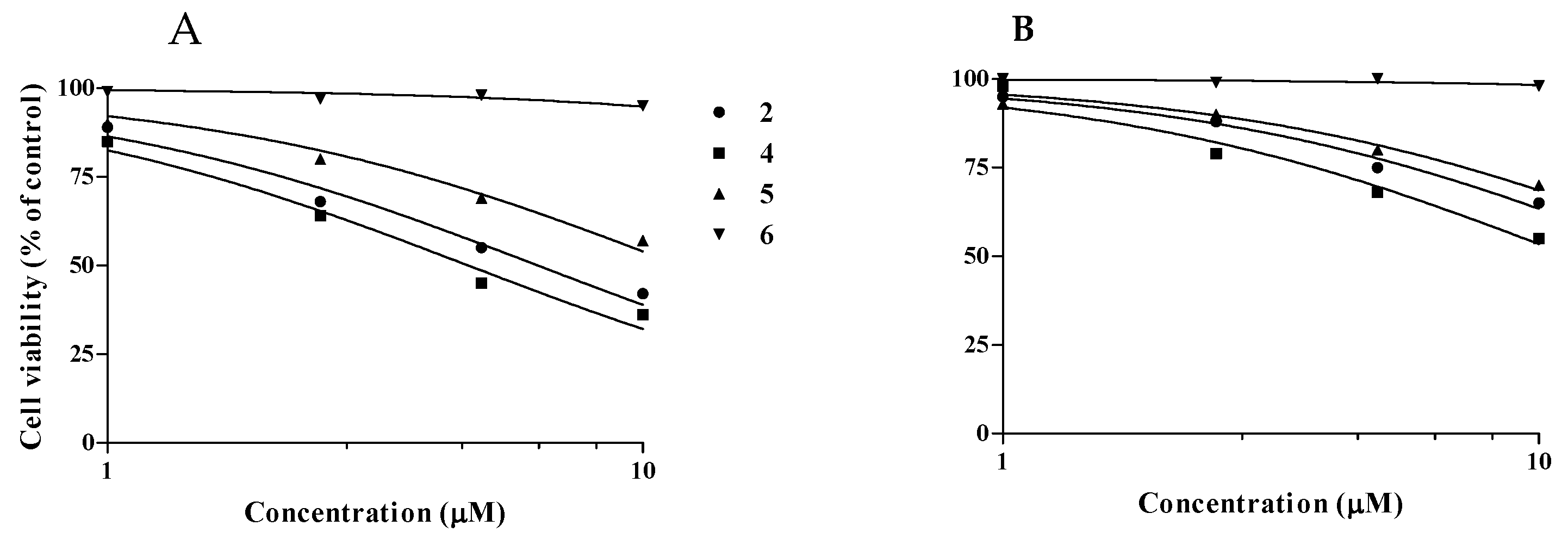
3.3.2. Selectivity Index (SI)
3.3.3. Measurement of Phosphatidylserine Exposure
3.3.4. Measurement of Mitochondrial Transmembrane Potential
3.3.5. Measurement of Intracellular Reactive Oxygen Species (ROS)
3.3.6. Cell Cycle Analysis
3.3.7. Western Blot Analysis
3.3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banti, C.N.; Hadjikakou, S.K.; Sismanoglu, T.; Hadjiliadis, N. Anti-proliferative and antitumor activity of organotin(IV) compounds. An overview of the last decade and future perspectives. J. Inorg. Biochem. 2019, 194, 114–152. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Shahzadi, S. Imtiaz-ud-Din Anticarcinogenicity and Toxicity of Organotin(IV) Complexes: A Review Iran. J. Sci. Technol. Trans. Sci. 2018, 42, 505–524. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S. Organotin(IV) carboxylates as promising potential drug candidates in the fiels of cancer chemotherapy. Curr. Pharm. Des. 2016, 22, 6665–6681. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.; Yadav, J. Recent Advancements in Organotin(IV) Complexes as Potential Anticancer Agents. Anti-Cancer Agents Med. Chem. 2018, 18, 335–353. [Google Scholar] [CrossRef]
- Carraher, C.E.; Roner, M.R. Organotin polymers as anticancer and antiviral agents. J. Organomet. Chem. 2014, 751, 67–82. [Google Scholar] [CrossRef]
- Arjmand, F.; Parveen, S.; Tabassum, S.; Pettinari, C. Organo-tin antitumor compounds: Their present status in drug development and future perspectives. Inorg. Chim. Acta 2014, 423, 26–37. [Google Scholar] [CrossRef]
- Deo, K.M.; Ang, D.L.; McGhie, B.; Rajamanickam, A.; Dhiman, A.; Khoury, A.; Holland, J.; Bjelosevic, A.; Pages, B.; Gordon, C.; et al. Platinum coordination compounds with potent anticancer activity. Coord. Chem. Rev. 2018, 375, 148–163. [Google Scholar] [CrossRef]
- Florea, A.-M.; Busselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Rubino, S.; Pibiri, I.; Minacori, C.; Alduina, R.; Di Stefano, V.; Orecchio, S.; Buscemi, S.; Girasolo, M.A.; Tesoriere, L.; Attanzio, A. Synthesis, structural characterization, anti-proliferative and antimicrobial activity of binuclear and mononuclear Pt(II) complexes with perfluoroalkyl-heterocyclic ligands. Inorg. Chim. Acta 2018, 483, 180–190. [Google Scholar] [CrossRef]
- Latsis, G.K.; Banti, C.N.; Kourkoumelis, N.; Papatriantafyllopoulou, C.; Panagiotou, N.; Tasiopoulos, A.; Douvalis, A.; Kalampounias, A.G.; Bakas, T.; Hadjikakou, S.K. Poly Organotin Acetates against DNA with Possible Implementation on Human Breast Cancer. Int. J. Mol. Sci. 2018, 19, 2055. [Google Scholar] [CrossRef]
- Salas, J.M.; Romero, M.A.; Sánchez, M.P.; Quirós, M. Metal complexes of [1,2,4]triazolo-[1,5-a]pyrimidine derivatives. Coord. Chem. Rev. 1999, 193, 1119–1142. [Google Scholar] [CrossRef]
- Łakomska, I.; Fandzloch, M. Application of 1,2,4-triazolo[1,5-a]pyrimidines for the design of coordination compounds with interesting structures and new biological properties. Coord. Chem. Rev. 2016, 221–241. [Google Scholar] [CrossRef]
- Ruisi, G.; Canfora, L.; Bruno, G.; Rotondo, A.; Mastropietro, T.F.; Debbia, E.A.; Girasolo, M.A.; Megna, B. Triorganotin(IV) derivatives of 7-amino-2-(methylthio)[1,2,4]triazolo[1,5-a]pyrimidine-6-carboxylic acid. Synthesis, spectroscopic characterization, in vitro antimicrobial activity and X-ray crystallography. J. Organomet. Chem. 2010, 695, 546–551. [Google Scholar] [CrossRef]
- Girasolo, M.A.; Canfora, L.; Sabatino, P.; Schillaci, D.; Foresti, E.; Rubino, S.; Ruisi, G.; Stocco, G. Synthesis, characterization, crystal structures and in vitro antistaphylococcal activity of organotin(IV) derivatives with 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidine. J. Inorg. Biochem. 2012, 106, 156–163. [Google Scholar] [CrossRef]
- Girasolo, M.A.; Attanzio, A.; Sabatino, P.; Tesoriere, L.; Rubino, S.; Stocco, G. Organotin(IV) derivatives with 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidine and their cytotoxic activities: The importance of being conformers. Inorg. Chim. Acta 2014, 423, 168–176. [Google Scholar] [CrossRef]
- Łakomska, I.; Fandzloch, M.; Wojtczak, A. Dimeric ruthenium-triazolopyrimidine complex: Synthesis and structural characterization. Inorg. Chem. Commun. 2014, 49, 24–26. [Google Scholar] [CrossRef]
- Rubino, S.; Di Stefano, V.; Attanzio, A.; Tesoriere, L.; Girasolo, M.A.; Nicolò, F.; Bruno, G.; Orecchio, S.; Stocco, G. Synthesis, spectroscopic characterization and antiproliferative activity of two platinum(II) complexes containing N-donor heterocycles. Inorg. Chim. Acta 2014, 418, 112–118. [Google Scholar] [CrossRef]
- Łakomska, I.; Jakubowski, M.; Barwiołek, M.; Muzioł, T. Different bonding of triazolopyrimidine to platinum(IV). Structural and in vitro cytotoxicity studies. Polyhedron 2019, 160, 123–129. [Google Scholar] [CrossRef]
- Fandzloch, M.; Dobrzańska, L.; Jezierska, J.; Filip-Psurska, B.; Wiśniewska, J.; Wietrzyk, J.; Salas, J.M.; Łakomska, I. In search of new anticancer drug – Dimethylsulfoxide ruthenium(III) complex with bulky triazolopyrimidine derivative and preliminary studies towards understanding the mode of action. Polyhedron 2018, 141, 239–246. [Google Scholar] [CrossRef]
- Méndez-Arriaga, J.; Esteban-Parra, G.; Juárez, M.; Rodríguez-Diéguez, A.; Sánchez-Moreno, M.; Isac-García, J.; Salas, J. Antiparasitic activity against trypanosomatid diseases and novel metal complexes derived from the first time characterized 5-phenyl-1,2,4-triazolo[1,5-a]pyrimidi-7(4H)-one. J. Inorg. Biochem. 2017, 175, 217–224. [Google Scholar] [CrossRef]
- Esteban-Parra, G.M.; Méndez-Arriaga, J.M.; Rodríguez-Diéguez, A.; Quirós, M.; Salas, J.M.; Sánchez-Moreno, M. High antiparasitic activity of silver complexes of 5,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidine. J. Inorg. Biochem. 2019, 201, 110810. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.B.; Rodriguez-Dieguez, A.; Quirós, M.; Salas, J.M.; Huertas, O.; Ramírez-Macías, I.; Olmo, F.; Marín, C.; Chaves-Lemaur, G.; Gutierrez-Sánchez, R.; et al. Triazolopyrimidine compounds containing first-row transition metals and their activity against the neglected infectious Chagas disease and leishmaniasis. Eur. J. Med. Chem. 2014, 85, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Abul-Haj, M.A.; Salas, J.; Quirós, M.; Molina, J.; Faure, R. 5-Oxo and 7-oxo derivatives of [1,2,4]triazolo-[1,5-a]pyrimidine: characterization and theoretical study. J. Mol. Struct. 2000, 519, 165–172. [Google Scholar] [CrossRef]
- Magán, R.; Marín, C.; Salas, J.M.; Barrera-Pérez, M.; Rosales, M.J.; Sánchez-Moreno, M. Cytotoxicity of three new triazolo-pyrimidine derivatives against the plant trypanosomatid: Phytomonas sp. isolated from Euphorbia characias. Mem. Inst. Oswaldo Cruz 2004, 99, 651–656. [Google Scholar] [CrossRef]
- Girasolo, M.A.; Di Salvo, C.; Schillaci, D.; Barone, G.; Silvestri, A.; Ruisi, G. Synthesis, characterization, and in vitro antimicrobial activity of organotin(IV) complexes with triazolo-pyrimidine ligands containing exocyclic oxygen atoms. J. Organomet. Chem. 2005, 690, 4773–4783. [Google Scholar] [CrossRef]
- Ruiz, J.; Villa, M.D.; Cutillas, N.; López, G.; De Haro, C.; Bautista, D.; Moreno, V.; Valencia, L. Palladium(II) and Platinum(II) Organometallic Complexes with 4,7-dihydro-5-methyl-7-oxo[1,2,4]triazolo[1,5-a]pyrimidine. Antitumor Activity of the Platinum Compounds. Inorg. Chem. 2008, 47, 4490–4505. [Google Scholar] [CrossRef]
- Łakomska, I.; Fandzloch, M.; Wojtczak, A.; Szłyk, E. Platinum(IV) coordination compounds containing 5-methyl-1,2,4-triazolo[1,5-a]pyrimidin-7(4H)-one as nonleaving ligand. Molecular and cytotoxicity in vitro characterization. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2011, 79, 497–501. [Google Scholar] [CrossRef]
- Caballero, A.B.; Marín, C.; Rodriguez-Dieguez, A.; Ramírez-Macías, I.; Barea, E.; Sanchez-Moreno, M.; Salas, J.M. In vitro and in vivo antiparasital activity against Trypanosoma cruzi of three novel 5-methyl-1,2,4-triazolo[1,5-a]pyrimidin-7(4H)-one-based complexes. J. Inorg. Biochem. 2011, 105, 770–776. [Google Scholar] [CrossRef]
- Orihuela, S.; Sánchez, M.; Quirós, M.; Molina, J.; Faure, R. 4,5,6,7-tetrahydro-5,7-dioxo-[1,2,4]triazolo-[1,5-a]pyrimidine: characterisation and theoretical study. J. Mol. Struct. 1997, 415, 285–292. [Google Scholar] [CrossRef]
- Orihuela, S.; Sánchez, M.P.; Quirós, M.; Martin, D.; Faure, R. First transition row metal complexes with 4,5,6,7-tetrahydro-5,7-dioxo-[1,2,4]triazolo-[1,5-a]pyrimidine. Polyhedron 1998, 17, 2477–2481. [Google Scholar] [CrossRef]
- Abul-Haj, M.; Quirós, M.; Salas, J.M. Diaquabis(4,5-dihydro-1,2,4-triazolo[1,5-a]pyrimidin-5-one-N3)bis-(thiocyanato-N)nickel(II). Acta Cryst. 2000, C56, 934–935. [Google Scholar]
- Abul-Haj, M.; Quirós, M.; Salas, J.M.; Faureb, R. Silver complexes with triazolopyrimidine ligands containing an exocyclic oxygen atom: X-ray evidence for an unusual tautomeric form. J. Chem. Soc. Dalton Trans. 2001, 1798–1801. [Google Scholar]
- Abul-Haj, M.; Quirós, M.; Salas, J.M. Dinuclear Pd(II) complexes with the anionic form of 4,5-dihydro-1,2,4-triazolo[1,5-a]pyrimidine-5-one. Polyhedron 2004, 23, 2373–2379. [Google Scholar]
- Caballero, A.B.; Rodríguez-Dieguez, A.; Lezama, L.; Salas, J.M. Toward a New Type of Multifunctional Metal–Organic Systems Based on Nucleobase Analogues: First Results Derived From The Use of Aliphatic α,ω-Dicarboxylates. Cryst. Growth Des. 2012, 12, 3583–3593. [Google Scholar] [CrossRef]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer. Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Riley, T.; Sontag, E.; Chen, P.; Levine, A. Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell Boil. 2008, 9, 402–412. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97, Program for Crystal Structure Solution and Refinement; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0– new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Sun, D.; Lennernas, H.; Welage, L.S.; Barnett, J.L.; Landowski, C.P.; Foster, D.; Fleisher, D.; Lee, K.-D.; Amidon, G.L. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm. Res. 2002, 19, 1400–1416. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Ullo, S.; Attanzio, A.; Spanò, V.; Cascioferro, S.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Cirrincione, G.; Diana, P. New Tripentone Analogs with Antiproliferative Activity. Molecules 2017, 22, 2005. [Google Scholar] [CrossRef]
- Allegra, M.; D’Acquisto, F.; Tesoriere, L.; Attanzio, A.; Livrea, M. Pro-oxidant activity of indicaxanthin from Opuntia ficus indica modulates arachidonate metabolism and prostaglandin synthesis through lipid peroxide production in LPS-stimulated RAW 264.7 macrophages. Redox Boil. 2014, 2, 892–900. [Google Scholar] [CrossRef][Green Version]
- Girasolo, M.A.; Tesoriere, L.; Casella, G.; Attanzio, A.; Capobianco, M.L.; Sabatino, P.; Barone, G.; Rubino, S.; Bonsignore, R. A novel compound of triphenyltin(IV) with N-tert-butoxycarbonyl-l-ornithine causes cancer cell death by inducing a p53-dependent activation of the mitochondrial pathway of apoptosis. Inorg. Chim. Acta 2017, 456, 1–8. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Me3Sn(5tpO) (1), n-Bu3Sn(5tpO) (2), Me3Sn(mtpO) (3), n-Bu3Sn(mtpO) (4), n-Bu3Sn(HtpO2) (5), Ph3Sn(HtpO2) (6) are available from the authors. |


| Me3Sn(5tpO) 1 | n-Bu3Sn(5tpO) 2 | |
|---|---|---|
| Sn1···O1 (Å) | 2.230(1)/2.310(1) | 2.210(2) |
| Sn1···N3 (Å) | 2.370(8)/2.348(8) | 2.445(3) |
| Sn1···C (Å) | 2.06(3)–2.17(2) | 2.141(5)–2.152(5) |
| C=O (Å) | 1.210(2)/1.241(2) | 1.282(5) |
| O-Sn-N (°) | 174.5(4)/178.9(5) | 176.9(1) |
| Sn1···N3 (Å) | 113.2(1)–129.1(1) | 115.1(2)–123.3(2) |
| Sn1···C (Å) | 84.3(6)–97.2(5) | 91.7(1)–95.1(1) |
| C=O (Å) | 86.8(7)–91.5(6) | 83.7(1)–88.1(1) |
| Compounds | IC50 (nM ± SD) | ||
|---|---|---|---|
| HCT-116 | HepG2 | MCF-7 | |
| (1) Me3Sn(5tpO) | >1000 | >1000 | >1000 |
| (2) n-Bu3Sn(5tpO) | 70 ± 8 | 70 ± 6 | 233 ± 21 |
| (3) Me3Sn(mtpO) | >1000 | >1000 | >1000 |
| (4) n-Bu3Sn(mtpO) | 34 ± 3 | 53 ± 6 | 118 ± 10 |
| (5) n-Bu3Sn(HtpO2) | 101 ± 11 | 117 ± 10 | 487 ± 47 |
| (6) Ph3Sn(HtpO2) | 60 ± 5 | 63 ± 5 | 102 ± 9 |
| Cisplatin | 46 × 103 ± 3 × 103 | 65 × 103 ± 2 × 103 | 10 × 103 ± 1 × 103 |
| Normal-Like Intestinal Cells LC50 (μM) | SI | 16-HBE Cells LC50 (μM) | SI | |
|---|---|---|---|---|
| 2 | 6 ± 0.4 | 91 | 17 ± 1 | 240 |
| 4 | 5 ± 0.4 | 118 | 11 ± 1 | 323 |
| 5 | 12 ± 1 | 116 | 22 ± 1 | 217 |
| 6 | 184 ± 9 | >1000 | 580 ± 22 | >1000 |
| 1 | 2 | |
|---|---|---|
| Empirical formula | C8H12N4O1Sn | C17H30N4O1Sn |
| Formula weight | 298.90 | 425.16 |
| Temperature (K) | 300 | 200 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21 | C2/C |
| Z | 2 | 8 |
| a (Å) | 6.9546(7) | 26.570(4) |
| b (Å) | 11.0787(9) | 11.6290(4) |
| c (Å) | 15.539(1) | 17.403(2) |
| α (deg) | 90 | 90 |
| β (deg) | 91.205(9) | 131.45(2) |
| γ (deg) | 90 | 90 |
| Volume (Å3) | 1196.9(2) | 4030.5(9) |
| ρcalc (g/cm3) | 1.659 | 1.401 |
| μ (mm−1) | 2.212 | 1.277 |
| Measured reflections | 6727 | 8816 |
| Independent reflections | 4070 | 4032 |
| R1[on F02, I > 2σ(I)] | 0.0746 | 0.0331 |
| wR2 (all data) | 0.2409 | 0.0615 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attanzio, A.; D’Agostino, S.; Busà, R.; Frazzitta, A.; Rubino, S.; Girasolo, M.A.; Sabatino, P.; Tesoriere, L. Cytotoxic Activity of Organotin(IV) Derivatives with Triazolopyrimidine Containing Exocyclic Oxygen Atoms. Molecules 2020, 25, 859. https://doi.org/10.3390/molecules25040859
Attanzio A, D’Agostino S, Busà R, Frazzitta A, Rubino S, Girasolo MA, Sabatino P, Tesoriere L. Cytotoxic Activity of Organotin(IV) Derivatives with Triazolopyrimidine Containing Exocyclic Oxygen Atoms. Molecules. 2020; 25(4):859. https://doi.org/10.3390/molecules25040859
Chicago/Turabian StyleAttanzio, Alessandro, Simone D’Agostino, Rosalia Busà, Anna Frazzitta, Simona Rubino, Maria Assunta Girasolo, Piera Sabatino, and Luisa Tesoriere. 2020. "Cytotoxic Activity of Organotin(IV) Derivatives with Triazolopyrimidine Containing Exocyclic Oxygen Atoms" Molecules 25, no. 4: 859. https://doi.org/10.3390/molecules25040859
APA StyleAttanzio, A., D’Agostino, S., Busà, R., Frazzitta, A., Rubino, S., Girasolo, M. A., Sabatino, P., & Tesoriere, L. (2020). Cytotoxic Activity of Organotin(IV) Derivatives with Triazolopyrimidine Containing Exocyclic Oxygen Atoms. Molecules, 25(4), 859. https://doi.org/10.3390/molecules25040859