Enantioseparation and Determination of Penconazole in Rat Plasma by Chiral LC-MS/MS: Application to a Stereoselective Toxicokinetic Study
Abstract
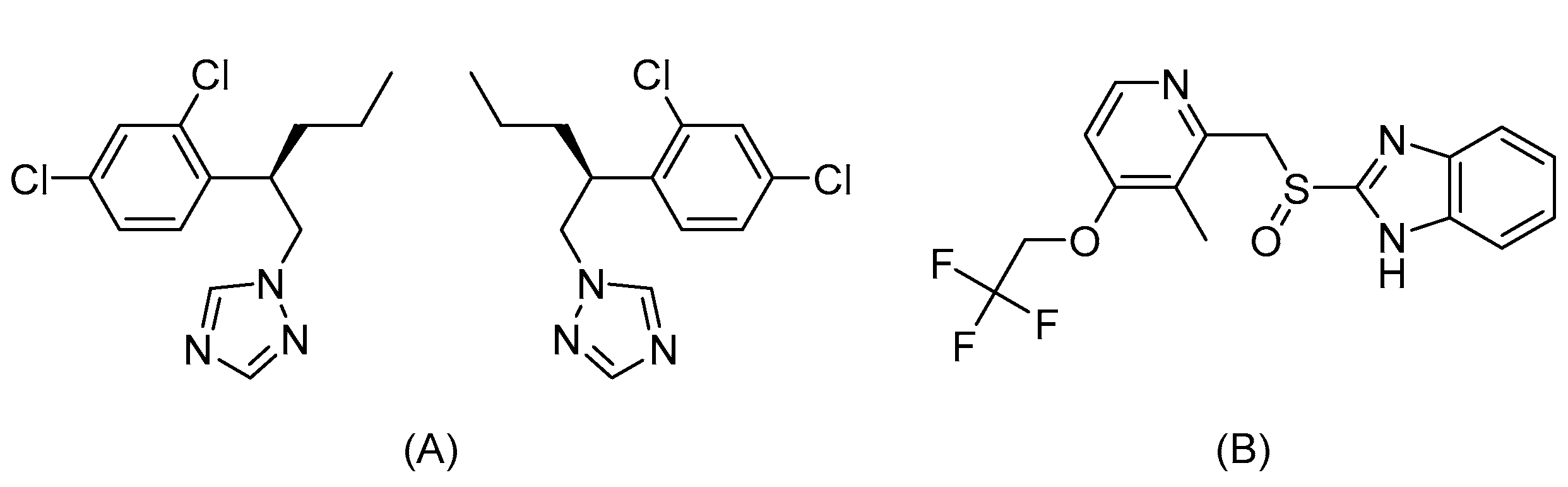
1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Preparation of Standard and Quality Control Solutions
2.3. Sample Preparation
2.4. Chiral LC-MS/MS Conditions
2.5. Enantioselective Toxicokinetic Study
3. Results and Discussion
3.1. Optimization of the Chiral Separation
3.2. Optimization of Mass Spectrometry Conditions
3.3. Optimization of Sample Pretreatment Procedures
3.4. Method Validation
3.4.1. Specificity and Carry-Over Effect
3.4.2. Linearity and the Lower Limit of Quantification (LLOQ)
3.4.3. Accuracy and Precision
3.4.4. Extraction Recovery and Matrix Effect
3.4.5. Stability
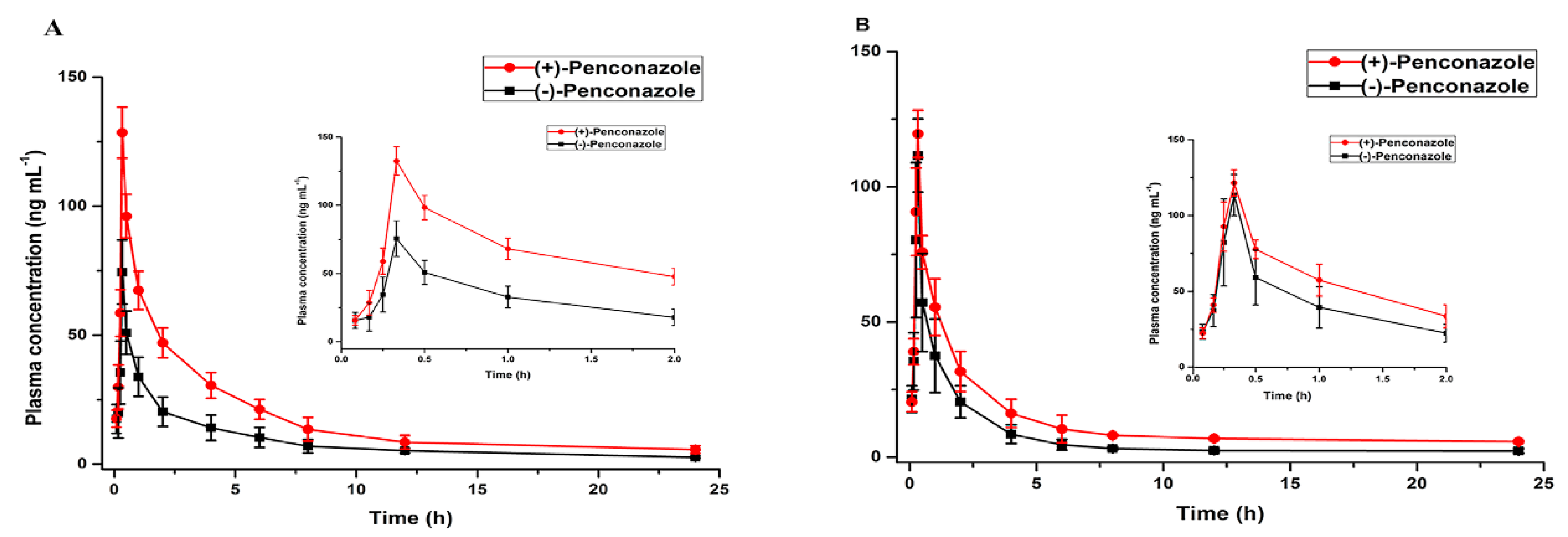
3.5. Application to Enantioselective Toxicokinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, P.F.; Li, S.; Chen, X.M.; Guo, X.J.; Zhao, L.S. Simultaneous enantiomeric analysis of six chiral pesticides in functional foods using magnetic solid-phase extraction based on carbon nanospheres as adsorbent and chiral liquid chromatography coupled with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 175, 112784. [Google Scholar] [CrossRef] [PubMed]
- Musarurwa, H.; Chimuka, L.; Pakade, V.E.; Tavengwa, N.T. Recent developments and applications of QuEChERS based techniques on food samples during pesticide analysis. J. Food. Comps. Anal. 2019, 84, 103314. [Google Scholar] [CrossRef]
- Zhao, J.; Meng, Z.R.; Zhao, Z.X.; Zhao, L.S. Ultrasound-assisted deep eutectic solvent as green and efficient media combined with functionalized magnetic multi-walled carbon nanotubes as solid-phase extraction to determine pesticide residues in food products. Food. Chem. 2020, 310, 125863. [Google Scholar] [CrossRef] [PubMed]
- Trösken, E.R.; Scholz, K.; Lutz, R.W.; Völkel, W.; Zarn, J.A.; Lutz, W.K. Comparative Assessment of the Inhibition of Recombinant Human CYP19 (Aromatase) by Azoles Used in Agriculture and as Drugs for Human. Endocr. Res. 2004, 30, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Sams, C.; Jones, K. Human volunteer studies investigating the potential for toxicokinetic interactions between the pesticides deltamethrin; Pirimicarb and chlorpyrifos-methyl following oral exposure at the acceptable daily intake. Toxicol. Lett. 2011, 200, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Duan, M.Y.; Chen, G.; You, X.H.; Liu, C.L.; Guo, X.J. An LC-MS/MS method for determination of novel fungicide pyraoxystrobin in rat plasma and tissues: Toxicokinetics and tissue distribution study. Talanta 2015, 136, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Jiang, J.Z.; Su, H.; Sun, M.J.; Wang, P.; Liu, D.H.; Zhou, Z.Q. Bioactivity, toxicity and dissipation of hexaconazole enantiomers. Chemosphere 2013, 93, 2523–2527. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Xu, H.Y.; Yao, S.J.; He, Y.W.; Zhang, H.C.; Yu, Y.L. Chiral triazole fungicide tebuconazole: Enantioselective bioaccumulation, bioactivity, acute toxicity, and dissipation in soils. Environ. Sci. Pollut. Res. 2018, 25, 25468–25475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hua, X.D.; Shi, H.Y.; Liu, J.S.; Tian, M.M.; Wang, M.H. Enantioselective bioactivity, acute toxicity and dissipation in vegetables of the chiral triazole fungicide flutriafol. J. Hazard. Mater. 2015, 284, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Wang, X.R.; Luo, F.J.; Sheng, H.S.; Zhou, L.; Zhong, Q.; Luo, Z.Y.; Sun, H.Z.; Yang, M.; Cui, X.; et al. Application and enantioselective residue determination of chiral pesticide penconazole in grape, tea, aquatic vegetables and soil by ultra performance liquid chromatography-tandem mass spectrometry. Ecotoxicol. Environ. Saf. 2019, 172, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Perdichizzi, S.; Mascolo, M.G.; Silingardi, P.; Morandi, E.; Rotondo, R.; Guerrini, A.; Prete, L.; Vaccari, M.; Colacci, A. Cancer-related genes transcriptionally induced by the fungicide penconazole. Toxicol. Vitro. 2014, 28, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Chaâbane, M.; Soudani, N.; Benjeddou, K.; Turki, M.; Makni, F.A.; Boudawara, T.; Zeghal, N.; Ghorbel, R.E. The protective potential of Nitraria retusa on penconazole-induced hepatic injury in adult rats. Toxicol. Environ. Chem. 2015, 97, 1253–1264. [Google Scholar] [CrossRef]
- El-Nisr, E.E.N. Testicular dysfunction induced by penconazole fungicide on male albino rats. Comp. Clin. Pathol. 2013, 22, 475–480. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, L.; Jia, M.; Li, R.; Yan, S.; Zhou, Z.; Zhu, W. Impacts of penconazole and its enantiomers exposure on gut microbiota and metabolic profiles in mice. J. Agric. Food Chem. 2019, 67, 8303–8311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Qi, P.P.; Zhang, H.; Xu, H.; Wang, X.Y.; Li, Z.; Wang, Z.W.; Wang, Q. Enantioselective Analysis and Dissipation of Triazole Fungicide Penconazole in Vegetables by Liquid Chromatography−Tandem Mass Spectrometry. J. Agric. Food Chem. 2014, 62, 11047–11105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.F.; Wang, Z.K.; Gao, X.; Guo, X.J.; Zhao, L.S. Simultaneous enantioselective determination of 22 chiral pesticides in fruits and vegetables using chiral liquid chromatography coupled with tandem mass spectrometry. Food Chem. 2019, 277, 298–306. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf (accessed on 6 June 2013).
Sample Availability: Samples of the compounds are not available from the authors. |
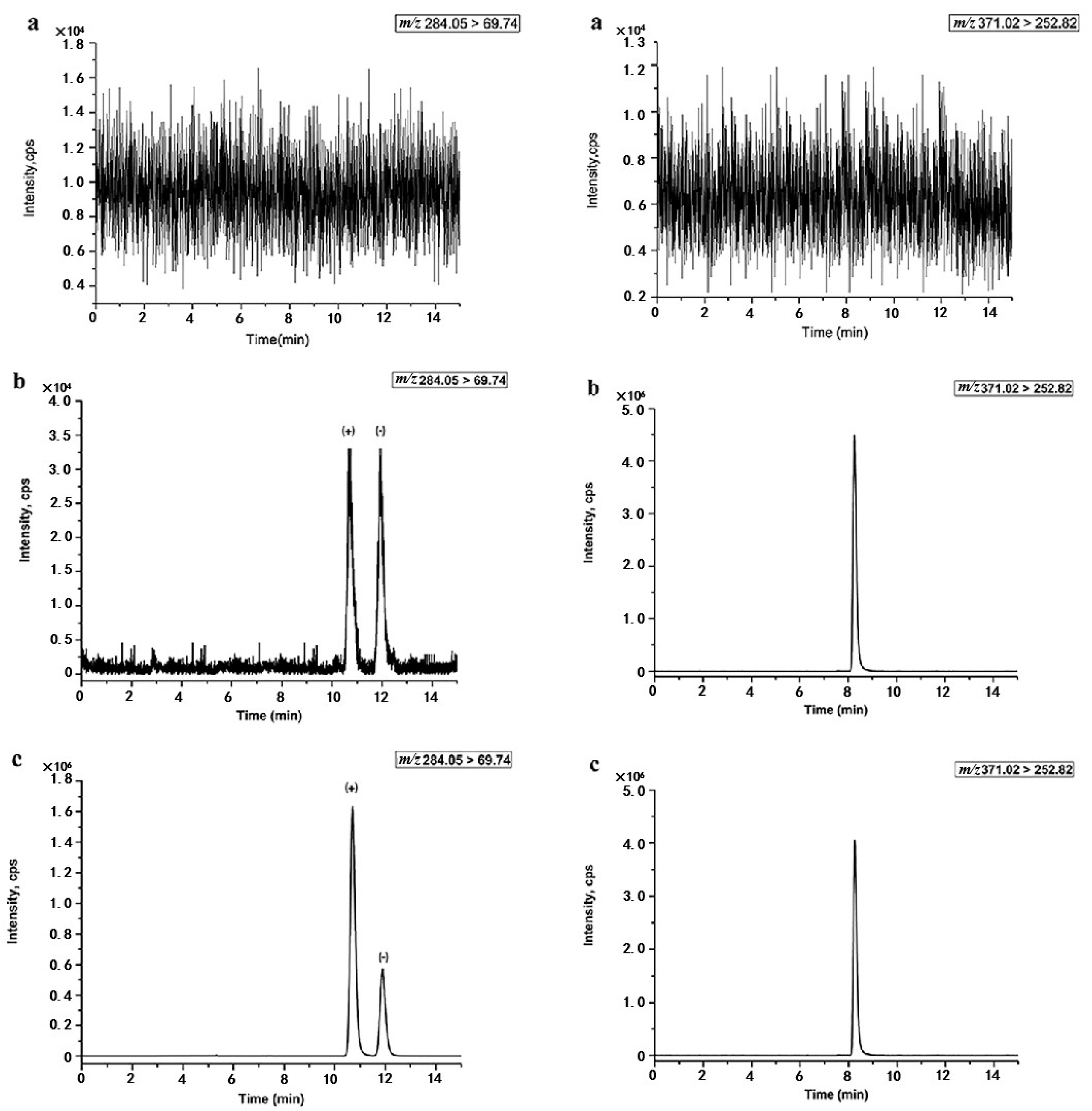
| Content of MeOH (%) | Retention Time (tR) | Resolution (Rs) | Content of CAN (%) | Retention Time (tR) | Resolution (Rs) |
|---|---|---|---|---|---|
| 90 | 8.3 | 0.32 | 90 | 6.2 | 0.96 |
| 80 | 15.3 | 0.63 | 80 | 10.7 | 2.21 |
| 70 | 24.5 | 1.02 | 70 | 15.3 | 3.45 |
| 60 | 35.4 | 1.43 | 60 | 24.9 | 4.87 |
| Analytes | Spiked Concentration (ng mL−1) | Intraday | Interday | ||||
|---|---|---|---|---|---|---|---|
| Measured Concentration (ng mL−1) | Precision (RSD, %) | Accuracy (RE, %) | Measured Concentration (ng mL−1) | Precision (RSD, %) | Accuracy (RE, %) | ||
| (+)-penconazole | 5.0 | 5.1 ± 0.2 | 3.9 | −0.7 | 5.0 ± 0.3 | 6.0 | −1.1 |
| 50.0 | 50.9 ± 2.4 | 4.7 | 1.4 | 51.0 ± 1.7 | 3.3 | 2.7 | |
| 200.0 | 196.9 ± 7.2 | 3.7 | 0.5 | 205.4 ± 2.5 | 1.2 | 0.9 | |
| (−)-penconazole | 5.0 | 5.1 ± 0.2 | 3.9 | 1.4 | 5.2 ± 0.2 | 3.8 | 3.2 |
| 50.0 | 50.1 ± 0.7 | 1.4 | −0.9 | 50.9 ± 1.2 | 2.4 | 1.6 | |
| 200.0 | 201.6 ± 4.8 | 2.4 | 0.3 | 198.8 ± 6.0 | 3.0 | −0.4 | |
| Analytes | Concentration (ng mL−1) | Recovery (%, Mean ± SD) | RSD | NMF (%, Mean ± SD) | RSD |
|---|---|---|---|---|---|
| (+)-penconazole | 5.0 | 95.3 ± 5.9 | 6.2 | 103.8 ± 4.1 | 3.9 |
| 50.0 | 96.3 ± 3.5 | 3.6 | 105.8 ± 3.7 | 3.5 | |
| 200.0 | 95.8 ± 1.0 | 1.0 | 103.6 ± 3.4 | 3.3 | |
| (−)-penconazole | 5.0 | 96.0 ± 6.0 | 6.3 | 109.1 ± 5.7 | 5.2 |
| 50.0 | 97.9 ± 2.5 | 2.6 | 104.9 ± 4.6 | 4.4 | |
| 200.0 | 94.8 ± 2.4 | 2.5 | 107.1 ± 1.8 | 1.7 | |
| IS | 100.0 | 90.9 ± 4.9 | 5.4 | 101.2 ± 2.8 | 2.8 |
| Parameters | Male Rats | Female Rats | ||||
|---|---|---|---|---|---|---|
| (+)-Penconazole | (−)-penconazole | (+)/(−)-penconazole | (+)-Penconazole | (−)-penconazole | (+)/(−)-penconazole | |
| Cmax (ng·mL−1) | 119.4 ± 8.69 | 111.14 ± 13.51 | 1.07 ± 0.21 | 132.47 ± 10.37 | 75.59 ± 13.09 | 1.75 ± 0.46 a |
| Tmax (h) | 0.31 ± 0.04 | 0.30 ± 0.04 | 1.03 ± 0.23 | 0.32 ± 0.09 | 0.30 ± 0.11 | 1.07 ± 0.57 |
| t1/2 (h) | 8.89 ± 3.28 | 8.07 ± 4.61 | 1.10 ± 0.66 | 9.29 ± 3.76 | 3.36 ± 0.71 | 2.76 ± 0.59 a |
| AUC0–24h (ng·h·mL−1) | 263.12 ± 32 | 187.90 ± 43 | 1.40 ± 0.48 a | 364.10 ± 79 | 175.64 ± 26 | 2.07 ± 0.19 a |
| AUC0–∞ (ng·h·mL−1) | 294.30 ± 28 | 199.32 ± 50 | 1.48 ± 0.49 a | 356.05 ± 89 | 185.54 ± 30 | 1.92 ± 0.19 a |
| MRT0–24 (h) | 4.82 ± 0.25 | 4.31 ± 1.47 | 1.12 ± 0.45 | 5.07 ± 1.36 | 2.58 ± 0.80 | 1.97 ± 0.50 a |
| CLz/F (L/h/kg) | 264,814 ± 16,328 | 779,535 ± 75,588 | 0.34 ± 0.00094 a | 147,374 ± 37,892 | 970,193 ± 50,180 | 0.15 ± 0.08 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Lun, J.; Liu, Y.; Jiang, Z.; Guo, X. Enantioseparation and Determination of Penconazole in Rat Plasma by Chiral LC-MS/MS: Application to a Stereoselective Toxicokinetic Study. Molecules 2020, 25, 2964. https://doi.org/10.3390/molecules25132964
Ma S, Lun J, Liu Y, Jiang Z, Guo X. Enantioseparation and Determination of Penconazole in Rat Plasma by Chiral LC-MS/MS: Application to a Stereoselective Toxicokinetic Study. Molecules. 2020; 25(13):2964. https://doi.org/10.3390/molecules25132964
Chicago/Turabian StyleMa, Siman, Jia Lun, Yanru Liu, Zhen Jiang, and Xingjie Guo. 2020. "Enantioseparation and Determination of Penconazole in Rat Plasma by Chiral LC-MS/MS: Application to a Stereoselective Toxicokinetic Study" Molecules 25, no. 13: 2964. https://doi.org/10.3390/molecules25132964
APA StyleMa, S., Lun, J., Liu, Y., Jiang, Z., & Guo, X. (2020). Enantioseparation and Determination of Penconazole in Rat Plasma by Chiral LC-MS/MS: Application to a Stereoselective Toxicokinetic Study. Molecules, 25(13), 2964. https://doi.org/10.3390/molecules25132964






