Potentially Bioaccessible Phenolics from Mung Bean and Adzuki Bean Sprouts Enriched with Probiotic—Antioxidant Properties and Effect on the Motility and Survival of AGS Human Gastric Carcinoma Cells
Abstract
1. Introduction
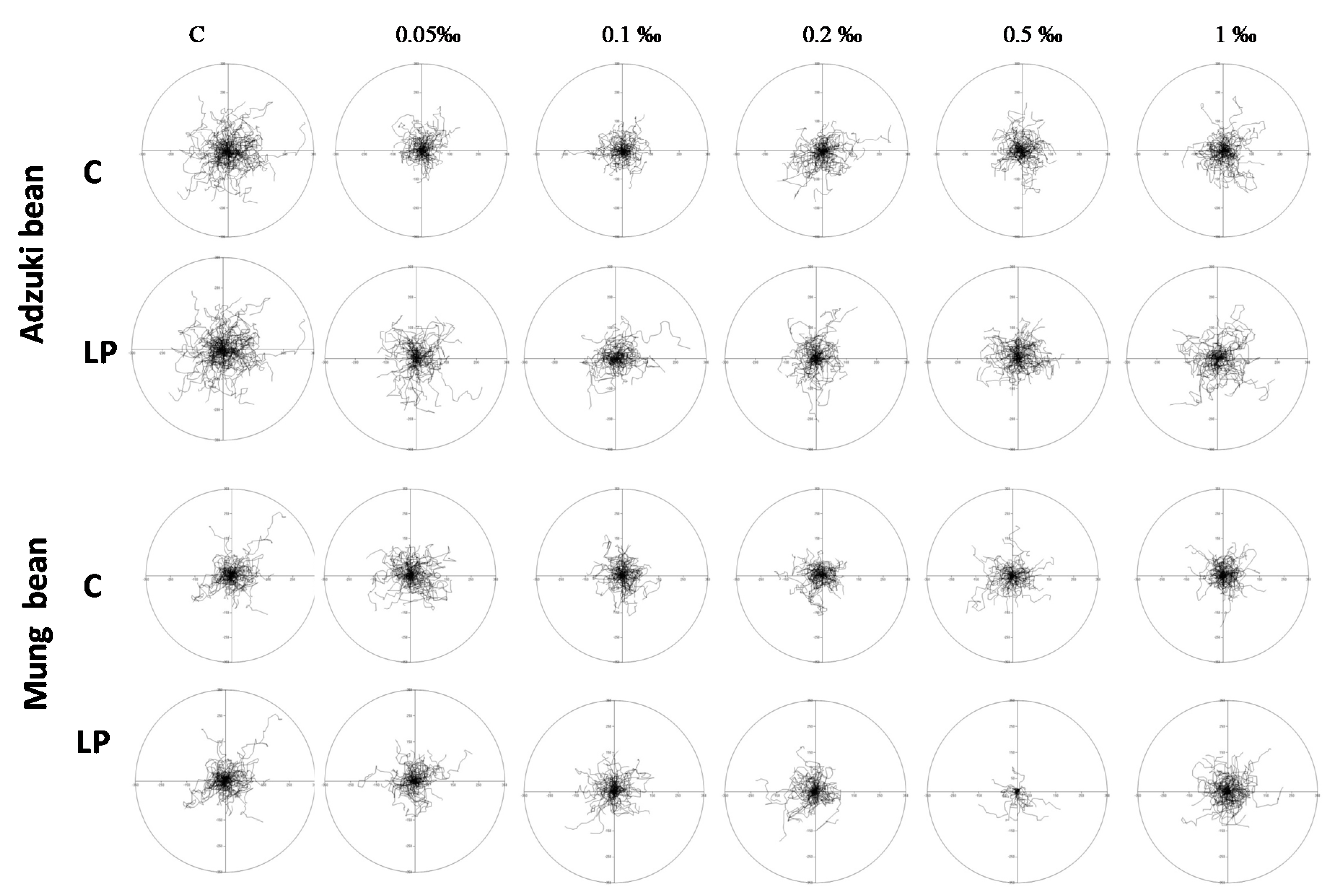
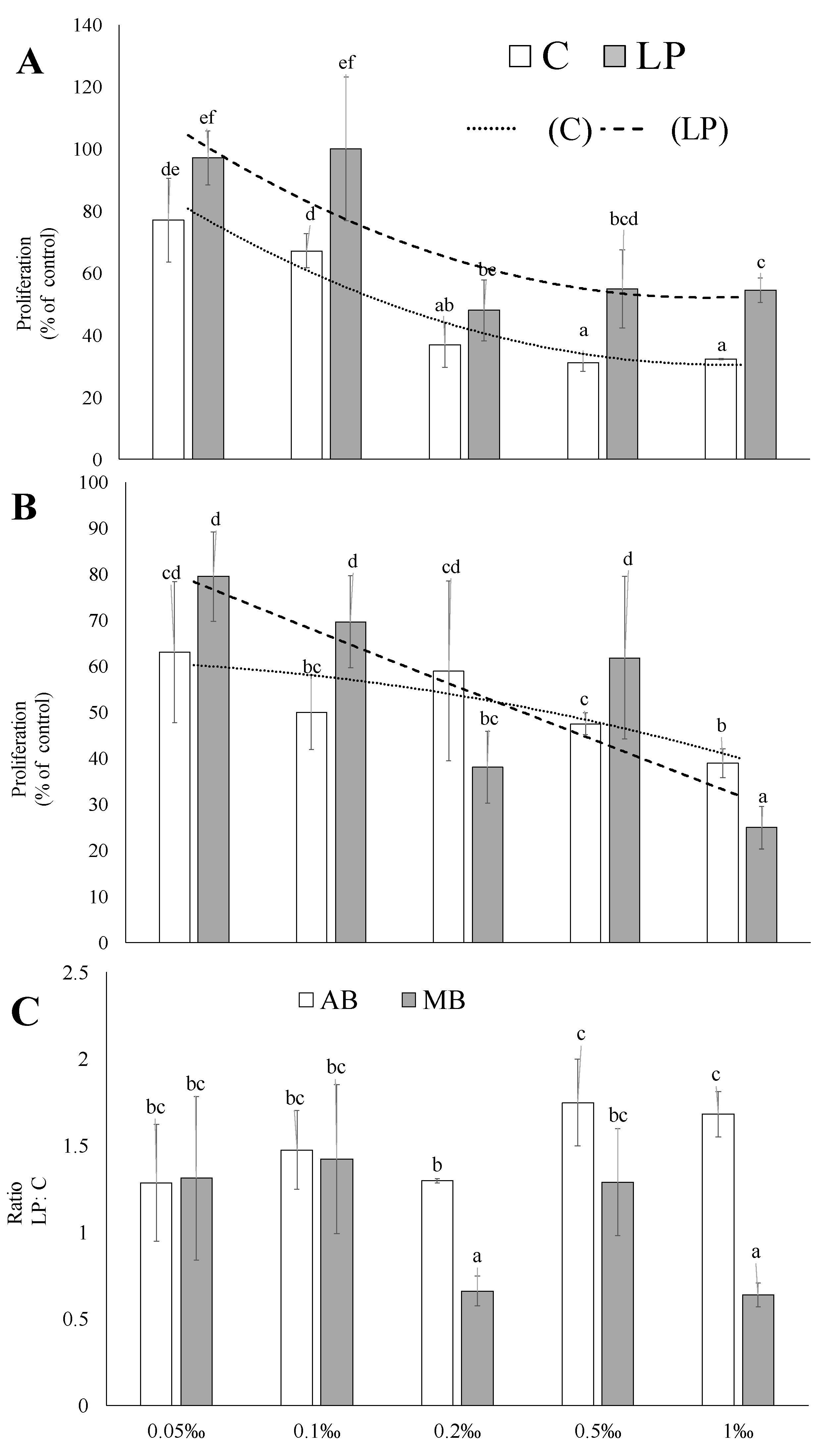
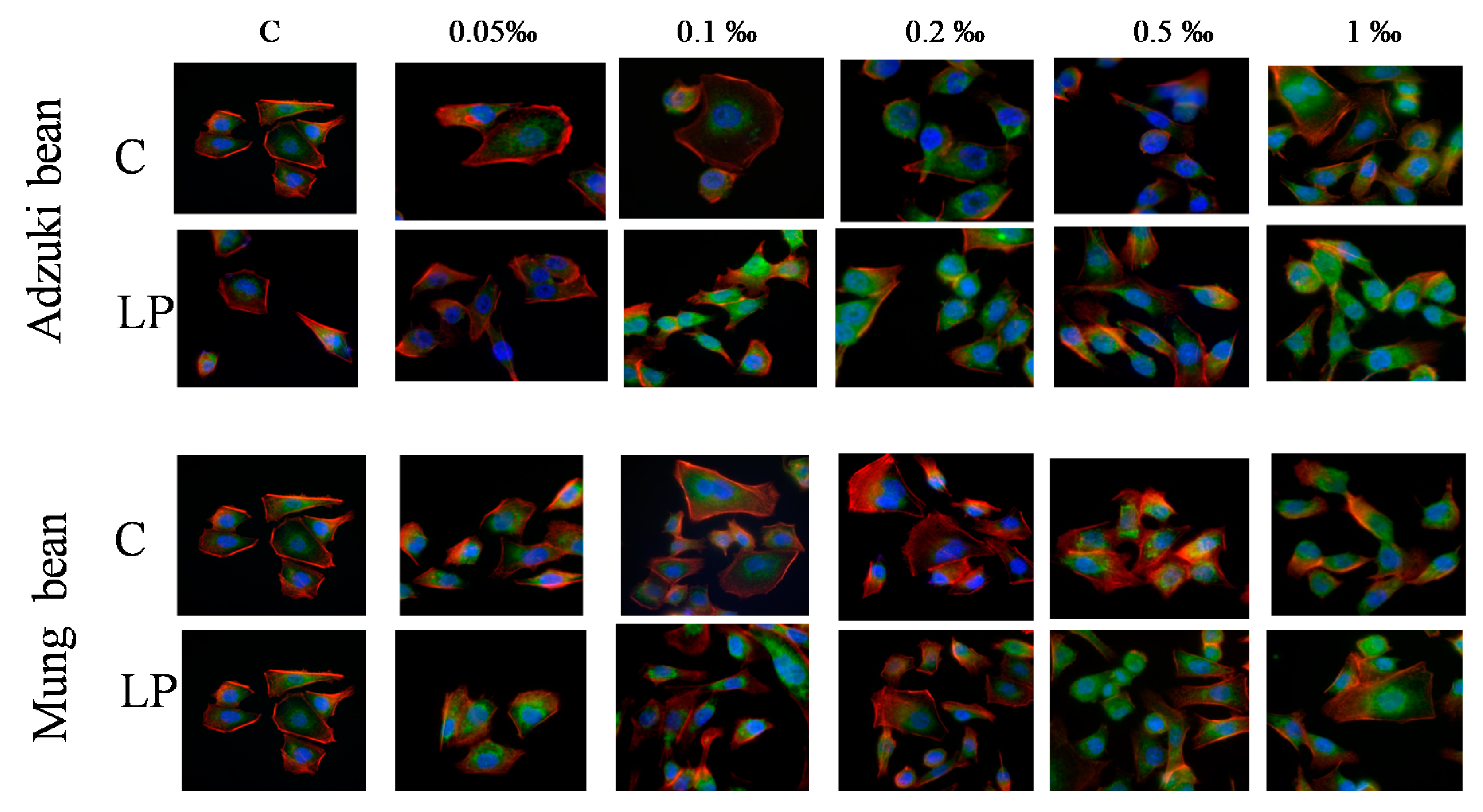
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Sprouting Conditions
3.3. In Vitro Digestion
3.4. Phenolic and Antioxidant Test
3.4.1. Phenolic Assay
3.4.2. Antioxidant Capacity
Reducing Power
Inhibition of Lipid Peroxidation
Ability to Chelate Metal Ions
Ability to Quench ABTS Radicals
Ability to Quench Hydroxyl (OH•) Radicals
Ability to Quench Superoxide Anion Radicals (O2–)
Total Antioxidant Activity Index (AI)
3.5. Effect on Human Stomach Cancer AGS and Human Colon Carcinoma HT-29 Cells
3.5.1. Culture Conditions
3.5.2. Proliferation Tests
3.5.3. Motility Tests
3.5.4. Cytoskeleton Architecture
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Golkar, L.; Ding, X.Z.; Ujiki, M.B.; Salabat, M.R.; Kelly, D.L.; Scholtens, D.; Fought, A.J.; Bentrem, D.J.; Talamonti, M.S.; Bell, R.H.; et al. Resveratrol inhibits pancreatic cancer cell proliferation through transcriptional induction of macrophage inhibitory cytokine-1. J. Surg. Res. 2007, 138, 163–169. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-Da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Gao, S.; Hu, M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini-Rev. Med. Chem. 2010, 10, 550–567. [Google Scholar] [CrossRef]
- Weng, C.J.; Yen, G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012, 31, 323–351. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chang, S.K.C. Effect of black soybean extract on the suppression of the proliferation of human AGS gastric cancer cells via the induction of apoptosis. J. Agric. Food Chem. 2011, 59, 4597–4605. [Google Scholar] [CrossRef] [PubMed]
- Swieca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Bochnak, J.; Baraniak, B. Lactobacillus plantarum 299V improves microbiological quality of legume sprouts and effectively survives in those carriers during cold storage and digestion in vitro. PLoS ONE 2018, 13, e0, 1–13. [Google Scholar]
- Swieca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Seczyk, L.; Złotek, U.; Kapusta, I. Nutritional and pro-health quality of lentil and adzuki bean sprouts enriched with probiotic yeast Saccharomyces cerevisiae var. boulardii. LWT Food Sci. Technol. 2019, 100, 220–226. [Google Scholar] [CrossRef]
- Zhao, K.; Xie, Q.; Xu, D.; Guo, Y.; Tao, X.; Wei, H.; Wan, C. Antagonistics of Lactobacillus plantarum ZDY2013 against Helicobacter pylori SS1 and its infection in vitro in human gastric epithelial AGS cells. J. Biosci. Bioeng. 2018, 126, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.B.; Moon, J.Y.; Cho, S.K. Quercetin suppresses CYR61-mediated multidrug resistance in human gastric adenocarcinoma AGS cells. Molecules 2018, 23, 209. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Arena, M.P.; Silvain, A.; Normanno, G.; Grieco, F.; Drider, D.; Spano, G.; Fiocco, D. Use of Lactobacillus plantarum strains as a bio-control strategy against food-borne pathogenic microorganisms. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Limón, R.I.; Peñas, E.; Martínez-Villaluenga, C.; Frias, J. Role of elicitation on the health-promoting properties of kidney bean sprouts. LWT Food Sci. Technol. 2014, 56, 328–334. [Google Scholar] [CrossRef]
- Luna-Vital, D.; González de Mejía, E. Peptides from legumes with antigastrointestinal cancer potential: Current evidence for their molecular mechanisms. Curr. Opin. Food Sci. 2018, 20, 13–18. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT Food Sci. Technol. 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Gulewicz, P.; Martinez-Villaluenga, C.; Kasprowicz-Potocka, M.; Frias, J. Non-nutritive compounds in fabaceae family seeds and the improvement of their nutritional quality by traditional processing–A review. Polish J. Food Nutr. Sci. 2014, 64, 75–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef]
- Tang, D.; Dong, Y.; Guo, N.; Ren, H. Metabolomic analysis of the polyphenols in germinating mung beans (Vigna radiata) seeds and sprouts. J. Sci. Food Agric. 2014, 94, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xu, Y.; Wang, B.; Li, S.; Guo, F.; Hua, H.; Zhao, Y.; Yu, Z. Comparison of phenolic compounds, antioxidant and antidiabetic activities between selected edible beans and their different growth periods leaves. J. Funct. Foods 2017, 35, 694–702. [Google Scholar] [CrossRef]
- Seczyk, L.; Swieca, M.; Gawlik-Dziki, U. Soymilk enriched with green coffee phenolics - Antioxidant and nutritional properties in the light of phenolics-food matrix interactions. Food Chem. 2017, 223, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, Y.S.; Shin, W.B.; Park, J.S.; Moon, S.H.; Jeon, B.T.; Park, P.J. Induction of caspase-mediated apoptosis using Alnus japonica extracts in AGS human gastric carcinoma cells. J. Appl. Biomed. 2018, 16, 198–207. [Google Scholar]
- Chen, J.; Chen, J.; Li, Z.; Liu, C.; Yin, L. The apoptotic effect of apigenin on human gastric carcinoma cells through mitochondrial signal pathway. Tumor Biol. 2014, 35, 7719–7726. [Google Scholar] [CrossRef] [PubMed]
- Lefort, É.C.; Blay, J. Apigenin and its impact on gastrointestinal cancers. Mol. Nutr. Food Res. 2013, 57, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, K.; Zhang, Q.; Mei, J.; Chen, C.; Feng, Z. Effects of quercetin on the apoptosis of the human gastric carcinoma cells. Toxicol. Vitr. 2012, 26, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Gawande, S.; Kale, A.; Kotwal, S. Effect of nutrient mixture and black grapes on the pharmacokinetics of orally administered (-)epigallocatechin-3-gallate from green tea extract: A human study. Phyther. Res. 2008, 22, 802–808. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Comparative study on antiproliferation properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Schantz, M.; Mohn, C.; Baum, M.; Richling, E. Antioxidative efficiency of an anthocyanin rich bilberry extract in the human colon tumor cell lines Caco-2 and HT-29. J. Berry Res. 2010, 1, 25–33. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrì, F.; Boutrou, R.; Corredig, F.M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Goupy, P.; Vulcain, E.; Caris-Veyrat, C.; Dangles, O. Dietary antioxidants as inhibitors of the heme-induced peroxidation of linoleic acid: Mechanism of action and synergism. Free Radic. Biol. Med. 2007, 43, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Su, X.Y.; Wang, Z.Y.; Liu, J.R. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 2009, 117, 681–686. [Google Scholar] [CrossRef]
- Swieca, M.; Sęczyk, L.; Gawlik-Dziki, U. Elicitation and precursor feeding as tools for the improvement of the phenolic content and antioxidant activity of lentil sprouts. Food Chem. 2014, 161, 288–295. [Google Scholar] [CrossRef]
- Daniel-Wójcik, A.; Misztal, K.; Bechyne, I.; Sroka, J.; Miekus, K.; Madeja, Z.; Czyz, J. Cell motility affects the intensity of gap junctional coupling in prostate carcinoma and melanoma cell populations. Int. J. Oncol. 2008, 33, 309–315. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Compounds | Identification | Content (µg/g of Flour) | |||
|---|---|---|---|---|---|
| Rt | (M − H)m/z | C | LP | ||
| min | MS | MS/MS | Adzuki bean | ||
| Kaempferol 3.7.4′-O-triglucoside | 3.63 | 771 | 447, 285 | 4.78 ± 0.24a | 5.12 ± 1.08a |
| Kaempferol 3-O-glucosyl-rhamnosyl-glucoside | 4.04 | 755 | 593, 285 | 10.48 ± 2.50a | 12.00 ± 0.62a |
| Quercetin 3.4′-O-diglucoside | 4.12 | 625 | 463, 301 | 26.4 ± 2.50a | 22.0 ± 3.6a |
| Quercetin 3-O-glucuronide | 4.2 | 477 | 301 | 7.16 ± 1.56a | 8.22 ± 1.68a |
| Kaempferol 3-O-rhamnosyl-rhamnosyl-glucoside | 4.42 | 739 | 577, 301 | 7.72 ± 1.13a | 9.06 ± 1.12b |
| Quercetin 3-O-galactoside 7-O-rhamnoside | 4.64 | 609 | 463, 301 | 11.0 ± 0.80a | 12.16 ± 1.92a |
| Quercetin 3-O-rutinoside (Rutin) | 4.76 | 609 | 463, 301 | 31.0 ± 0.2a | 25.4 ± 2.6b |
| Unidentified | 4.93 | 481 | 263 | 5.00 ± 0.08a | 6.00 ± 0.20b |
| Kaempferol 3-O-rutinoside | 5.09 | 593 | 431, 285 | <LOQ | <LOQ |
| Quercetin-3-O-acetylo-hexoside | 5.23 | 505 | 463, 301 | <LOQ | <LOQ |
| Unidentified | 5.32 | 575 | 271 | <LOQ | <LOQ |
| Unidentified | 6.66 | 863 | 269 | 3.63 ± 0.19b | 1.16 ± 0.23a |
| Unidentified | 6.77 | 287 | 136 | <LOQ | <LOQ |
| Total | 107 ± 9.3a | 102 ± 8.7a | |||
| Mung bean | |||||
| Kaempferol 3-O-glucoside | 4.21 | 447 | 285 | <LOQ | <LOQ |
| Kaempferol 3-O-galactoside | 4.35 | 447 | 285 | <LOQ | <LOQ |
| Apigenin 3-O-glucoside | 4.8 | 431 | 269 | 323 ± 23a | 364 ± 10b |
| Apigenin 3-O-rutinoside | 5.04 | 577 | 269 | 41.0 ± 11a | 59.6 ± 4.0a |
| Apigenin 3-O-acetylo-glucoside | 5.49 | 473 | 269 | 23.6 ± 4.6a | 30.1 ± 1.0b |
| Total | 388 ± 21a | 454 ± 18b | |||
| Adzuki Bean | Mung Bean | |||
|---|---|---|---|---|
| C | LP | C | LP | |
| Ability to quench O2− (mg TE/g of flour) | 2.56 ± 0.17b | 1.35 ± 0.10a | 2.63 ± 0.12b | 3.13 ± 0.13c |
| Ability to quench ABTS radicals (mg TE/g of flour) | 2.48 ± 0.06b | 2.50 ± 0.04b | 2.02 ± 0.08a | 1.74 ± 0.24a |
| Ability to chelate metal ions (µg EDTA/g of flour) | 4.62 ± 0.23b | 4.69 ± 0.16b | 3.57 ± 0.21a | 4.67 ± 0.15b |
| Ability to quench OH radicals (mg TE/g of flour) | 0.50 ± 0.025a | 0.55 ± 0.027a | 0.69 ± 0.034b | 0.90 ± 0.045c |
| Reducing potential (mg TE/g of flour) | 6.21 ± 0.36c | 9.30 ± 0.28d | 4.79 ± 0.06a | 5.12 ± 0.21b |
| Inhibition of lipids peroxidation (mg TE/g of flour) | 5.32 ± 0.19a | 6.01 ± 0.26b | 5.23 ± 0.26a | 4.80 ± 0.65a |
| Total antioxidantactivity index | 1.00 | 1.13 | 1.00 | 1.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świeca, M.; Herok, A.; Piwowarczyk, K.; Sikora, M.; Ostanek, P.; Gawlik-Dziki, U.; Kapusta, I.; Czyż, J. Potentially Bioaccessible Phenolics from Mung Bean and Adzuki Bean Sprouts Enriched with Probiotic—Antioxidant Properties and Effect on the Motility and Survival of AGS Human Gastric Carcinoma Cells. Molecules 2020, 25, 2963. https://doi.org/10.3390/molecules25132963
Świeca M, Herok A, Piwowarczyk K, Sikora M, Ostanek P, Gawlik-Dziki U, Kapusta I, Czyż J. Potentially Bioaccessible Phenolics from Mung Bean and Adzuki Bean Sprouts Enriched with Probiotic—Antioxidant Properties and Effect on the Motility and Survival of AGS Human Gastric Carcinoma Cells. Molecules. 2020; 25(13):2963. https://doi.org/10.3390/molecules25132963
Chicago/Turabian StyleŚwieca, Michał, Anna Herok, Katarzyna Piwowarczyk, Małgorzata Sikora, Patryk Ostanek, Urszula Gawlik-Dziki, Ireneusz Kapusta, and Jarosław Czyż. 2020. "Potentially Bioaccessible Phenolics from Mung Bean and Adzuki Bean Sprouts Enriched with Probiotic—Antioxidant Properties and Effect on the Motility and Survival of AGS Human Gastric Carcinoma Cells" Molecules 25, no. 13: 2963. https://doi.org/10.3390/molecules25132963
APA StyleŚwieca, M., Herok, A., Piwowarczyk, K., Sikora, M., Ostanek, P., Gawlik-Dziki, U., Kapusta, I., & Czyż, J. (2020). Potentially Bioaccessible Phenolics from Mung Bean and Adzuki Bean Sprouts Enriched with Probiotic—Antioxidant Properties and Effect on the Motility and Survival of AGS Human Gastric Carcinoma Cells. Molecules, 25(13), 2963. https://doi.org/10.3390/molecules25132963









