Chemical Constituents from Albiziae Cortex and Their Ability to Ameliorate Steatosis and Promote Proliferation and Anti-Oxidation In Vitro
Abstract
:1. Introduction
2. Results and Discussion
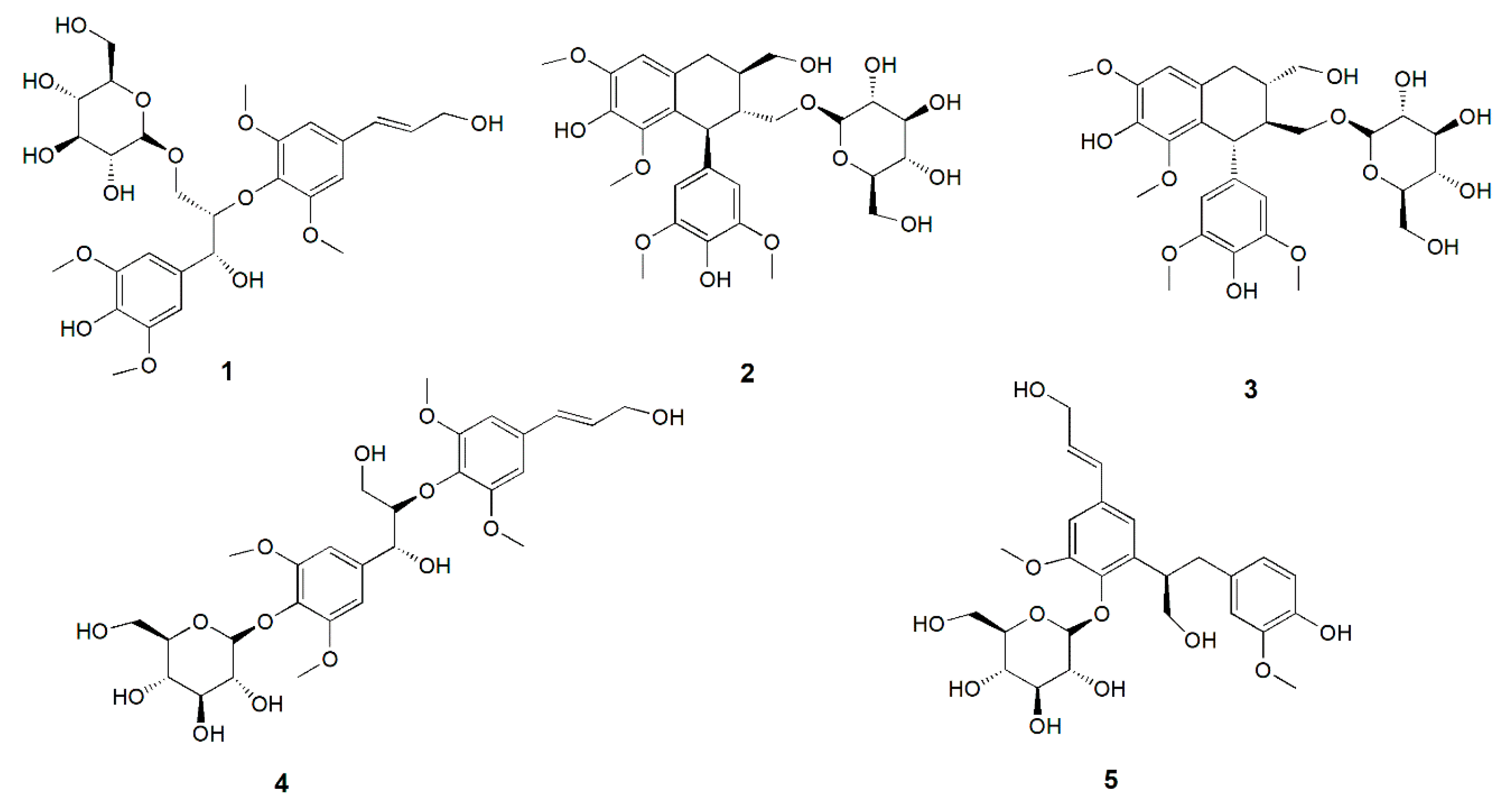
2.1. Compounds Isolated from Albiziae Cortex
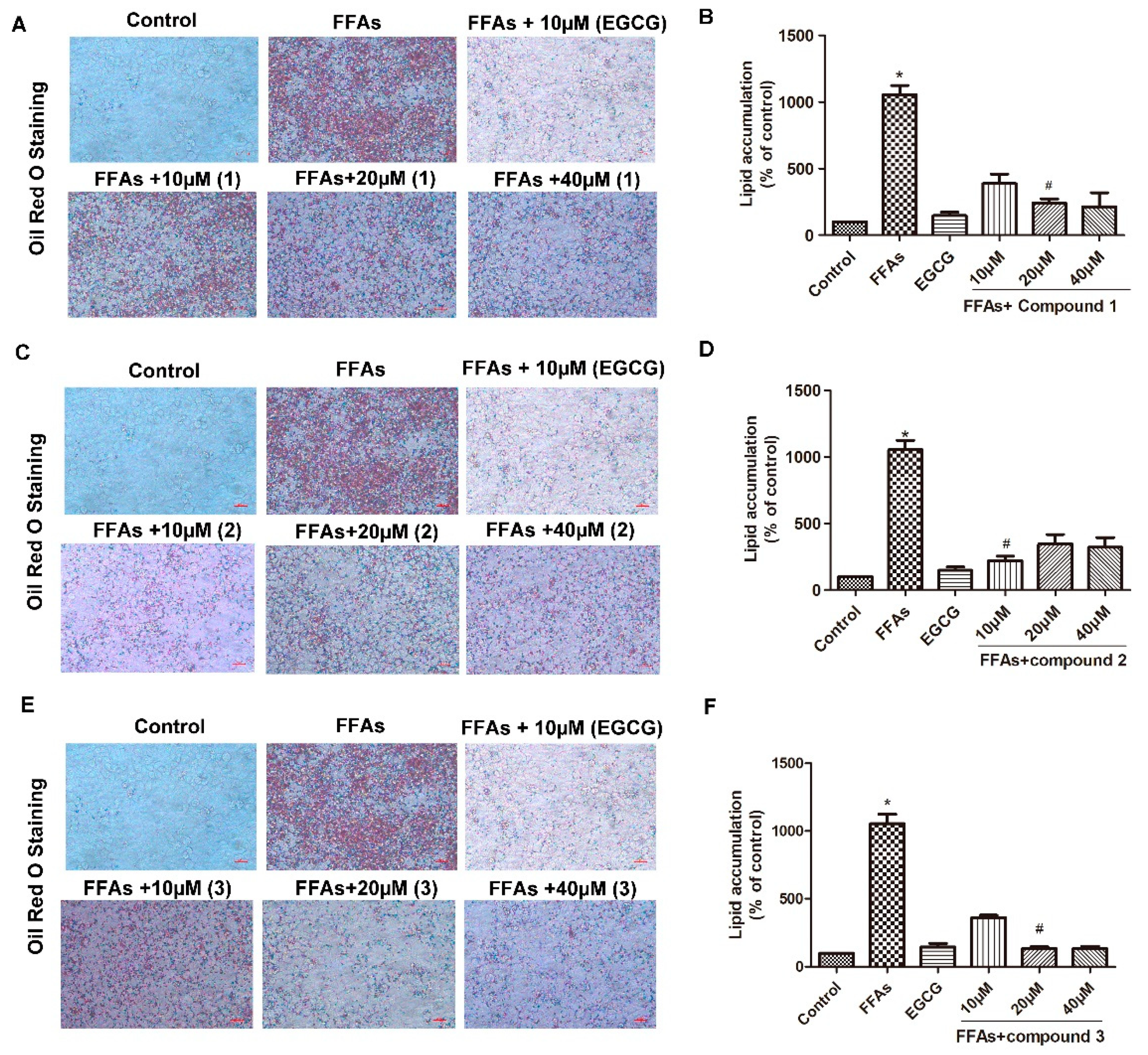
2.2. Effects of Compounds 1–5 on FFA-Induced Macro-Lipid Droplets and Steatosis
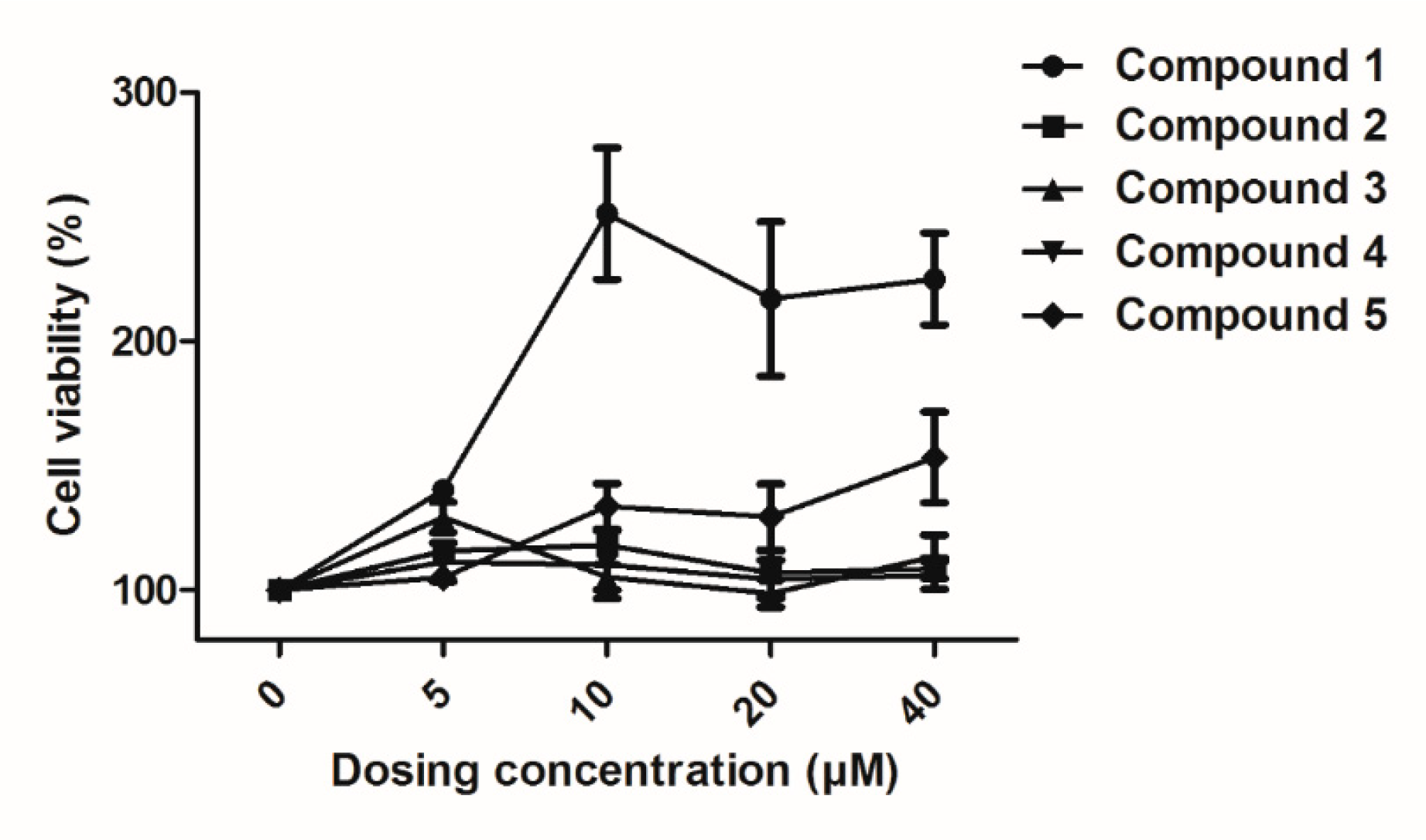
2.3. Effects of Compounds 1–5 on HUVEC Proliferation
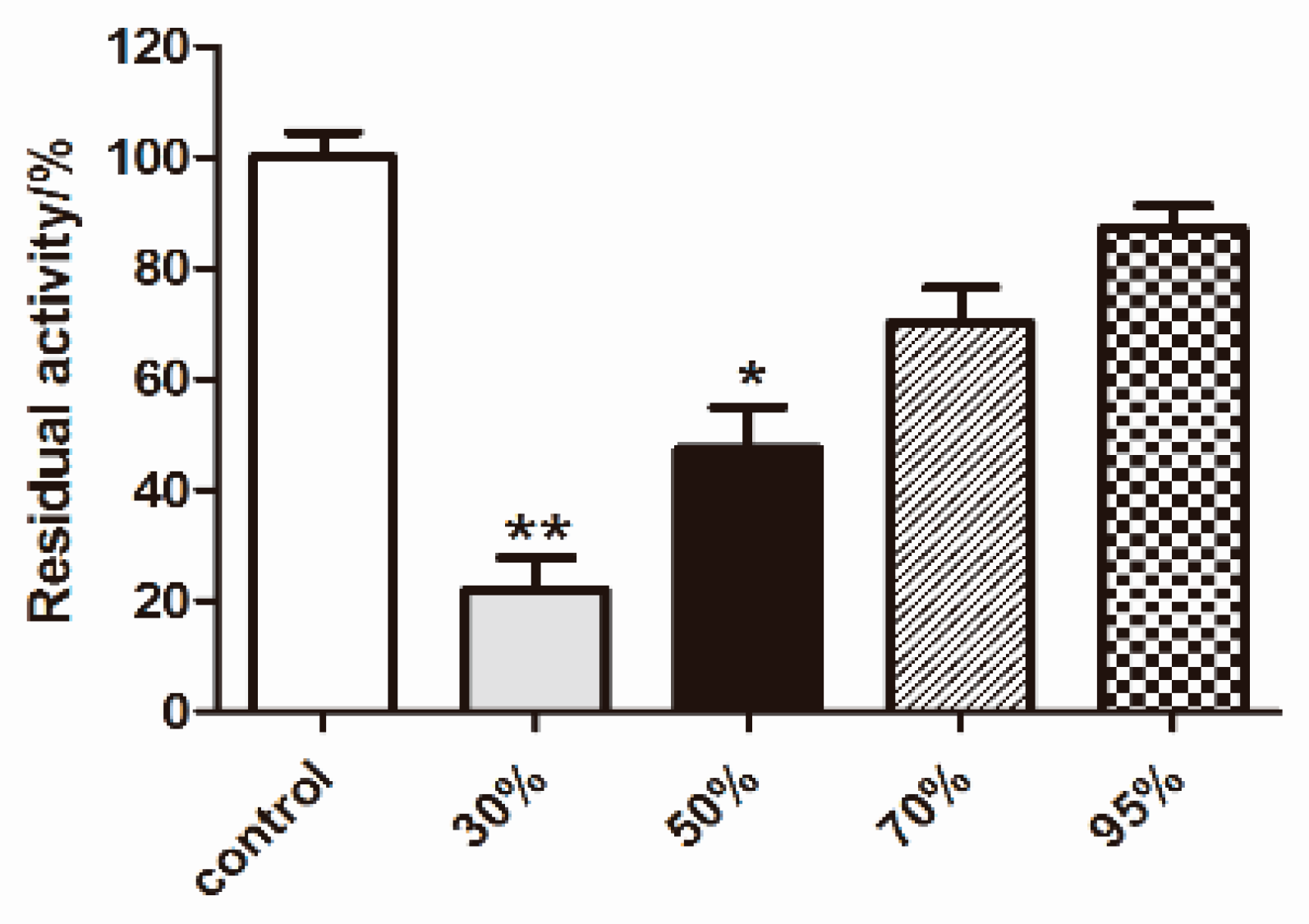
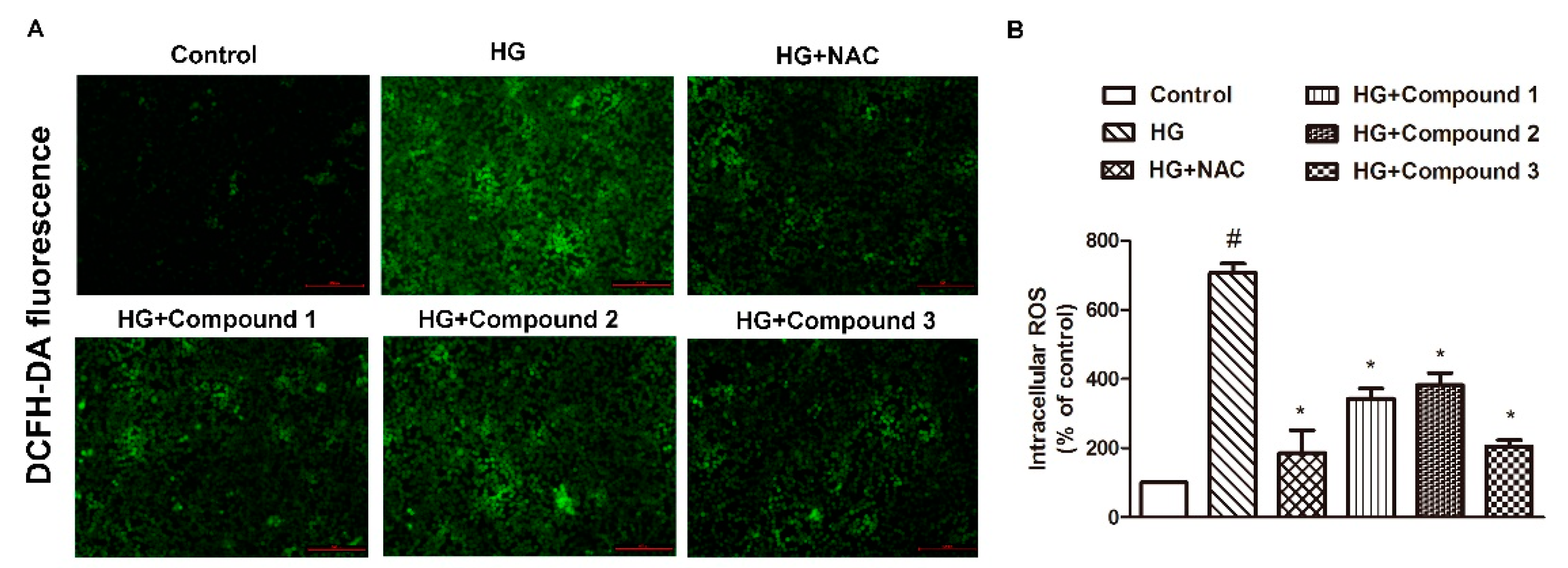
2.4. Effects of Compounds 1–5 on HG-Induced Oxidative Stress
3. Experimental Section
3.1. General
3.2. Reagents
3.3. Material
3.4. Extraction and Isolation
3.5. Spectral Data
3.6. Cell Culture and Treatments
3.7. Oil Red O Staining
3.8. Cytotoxicity Evaluation and Cell Viability Assay
3.9. Intracellular Reactive Oxygen Species Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Xu, W.; Zhai, T.; You, J.; Chen, Y. Silibinin ameliorates hepatic lipid accumulation and oxidative stress in mice with non-alcoholic steatohepatitis by regulating CFLAR-JNK pathway. Acta Pharm. Sin. B 2019, 9, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.T.; Francque, S.; Staels, B. Pathophysiology and Mechanisms of Nonalcoholic Fatty Liver Disease. Annu. Rev. Physiol. 2016, 78, 181–205. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Zhao, Y.; Feng, Q.; Peng, J.; Hu, Y. Advances in medicine active ingredients of fatty liver prevention. Chin. J. Integr. Tradit. West. Med. Liver Dis. 2011, 21, 185–187. [Google Scholar]
- Zheng, Y.; Tong, Y.; Wang, X.; Zhou, J.; Pang, J. Studies on the Design and Synthesis of Marine Peptide Analogues and Their Ability to Promote Proliferation in HUVECs and Zebrafish. Molecules 2018, 24, 66. [Google Scholar] [CrossRef] [PubMed]
- Hyder, S.M.; Stancel, G.M. Regulation of angiogenic growth factors in the female reproductive tract by estrogens and progestins. Mol. Endocrinol. 1999, 13, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.A.; Yaqoob, M.M.; Harwood, S.M. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J. Nutr. Biochem. 2005, 16, 705–713. [Google Scholar] [CrossRef] [PubMed]
- National Pharmacopoeia Commission. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2010; pp. 134–135. [Google Scholar]
- Li, Q.; Feng, L.; Shi, J. Screening of Effect Anti-Tumor Angiogenesis Substances in Albiziae Cortex. Chin. Tradit. Pat. Med. 2011, 34, 744–747. [Google Scholar]
- Machida, K.; Sakamoto, S.; Kikuchi, M. Structure elucidation and NMR spectral assignments of four neolignan glycosides with enantiometric aglycones from Osmanthus ilicifolius. Magn. Reson. Chem. 2008, 46, 990–994. [Google Scholar] [CrossRef]
- Dong Gun Lee, H.J.J.; Eun-Rhan, W. Antimicrobial Property of (+)-Lyoniresinol-3 -O- -D-Glucopyra noside Isolated From the Root Bark of Lycium chinense Miller Against Human Pathogenic Microorganisms. Arch. Pharm. Res. 2005, 28, 1031–1036. [Google Scholar]
- Ohashi, K.; Watanabe, H.; Okumura, Y.; Uji, T.; Kitagawa, I. Indonesian Medicinal Plants. XII. Four Isomeric Lignan-Glucosides from the Bark Aegle marmelos (Rutaceae). Chem. Pharm. Bull. 1994, 42, 1924–1926. [Google Scholar] [CrossRef]
- Kazuko, Y.; Saori, S.; Shigenobu, A. Phenylpropanoids and other Secondary Metabolites from Fresh Fruits of Picrasma Quassioides. Phytocheraistry 1995, 40, 253–256. [Google Scholar]
- Li, T.; Bin, W.; Yu, Y.Z. A Lignan Glucoside from Bupleurum scorzonerifolium. Chin. Chem. Lett. 2004, 25, 1053–1056. [Google Scholar]
- Grunt, T.W. Interacting Cancer Machineries: Cell Signaling, Lipid Metabolism, and Epigenetics. Trends Endocrinol. Metab. 2018, 29, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.P.; Lim, Y.C.; Kim, Y.M.; Ha, K.S. C-peptide activates AMPKalpha and prevents ROS-mediated mitochondrial fission and endothelial apoptosis in diabetes. Diabetes 2013, 62, 3851–3862. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Lei, Y.; Liu, Y.; Tan, F.; Li, S.; Wang, X.; Xu, M.; Cai, W.; Du, B.; Xu, F.; et al. Vaccarin prevents ox-LDL-induced HUVEC EndMT, inflammation and apoptosis by suppressing ROS/p38 MAPK signaling. Am. J. Transl. Res. 2019, 11, 2140–2154. [Google Scholar] [PubMed]
- Wu, D.-P.; Chen, B.; Qin, M.-E.; Huang, J.-I. Study of two kinds of Alzheimer’s disease tree shrew models. Chin. Pharmacol. Bull. 2017, 33, 1622–1626. [Google Scholar]
- Lei, Y.; Gong, L.; Tan, F.; Liu, Y.; Li, S.; Shen, H.; Zhu, M.; Cai, W.; Xu, F.; Hou, B.; et al. Vaccarin ameliorates insulin resistance and steatosis by activating the AMPK signaling pathway. Eur. J. Pharmacol. 2019, 851, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Du, B.; Xie, F.; Cai, W.; Liu, Y.; Li, Y.; Feng, L.; Qiu, L. Vaccarin attenuates high glucose-induced human EA*hy926 endothelial cell injury through inhibition of Notch signaling. Mol. Med. Rep. 2016, 13, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lei, Y.; Tan, F.; Gong, L.; Gong, H.; Yang, W.; Chen, T.; Zhang, Z.; Cai, W.; Hou, B.; et al. Vaccarin protects human microvascular endothelial cells from apoptosis via attenuation of HDAC1 and oxidative stress. Eur. J. Pharmacol. 2018, 818, 371–380. [Google Scholar] [CrossRef]
- Wu, D.B.; Chen, J.F.; Xu, Q.; Lin, J.Q.; Liao, J.Q.; Wu, W. Exogenous hydrogen sulfide inhibits high-glucose-induced injuries via regulating leptin/leptin receptor signaling pathway in human umbilical vein endothelial cells. Nan Fang Yi Ke Da Xue Xue Bao 2016, 36, 1055–1061. [Google Scholar]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Li, Z.; Cai, W.; Liu, Y.; Li, S.; Ai, M.; Sun, J.; Hou, B.; Ni, L.; Qiu, L. Chemical Constituents from Albiziae Cortex and Their Ability to Ameliorate Steatosis and Promote Proliferation and Anti-Oxidation In Vitro. Molecules 2019, 24, 4041. https://doi.org/10.3390/molecules24224041
Shi X, Li Z, Cai W, Liu Y, Li S, Ai M, Sun J, Hou B, Ni L, Qiu L. Chemical Constituents from Albiziae Cortex and Their Ability to Ameliorate Steatosis and Promote Proliferation and Anti-Oxidation In Vitro. Molecules. 2019; 24(22):4041. https://doi.org/10.3390/molecules24224041
Chicago/Turabian StyleShi, Xuelin, Zhongjie Li, Weiwei Cai, Yixiao Liu, Shuangshuang Li, Min Ai, Jiangnan Sun, Bao Hou, Lulu Ni, and Liying Qiu. 2019. "Chemical Constituents from Albiziae Cortex and Their Ability to Ameliorate Steatosis and Promote Proliferation and Anti-Oxidation In Vitro" Molecules 24, no. 22: 4041. https://doi.org/10.3390/molecules24224041
APA StyleShi, X., Li, Z., Cai, W., Liu, Y., Li, S., Ai, M., Sun, J., Hou, B., Ni, L., & Qiu, L. (2019). Chemical Constituents from Albiziae Cortex and Their Ability to Ameliorate Steatosis and Promote Proliferation and Anti-Oxidation In Vitro. Molecules, 24(22), 4041. https://doi.org/10.3390/molecules24224041





